Abstract
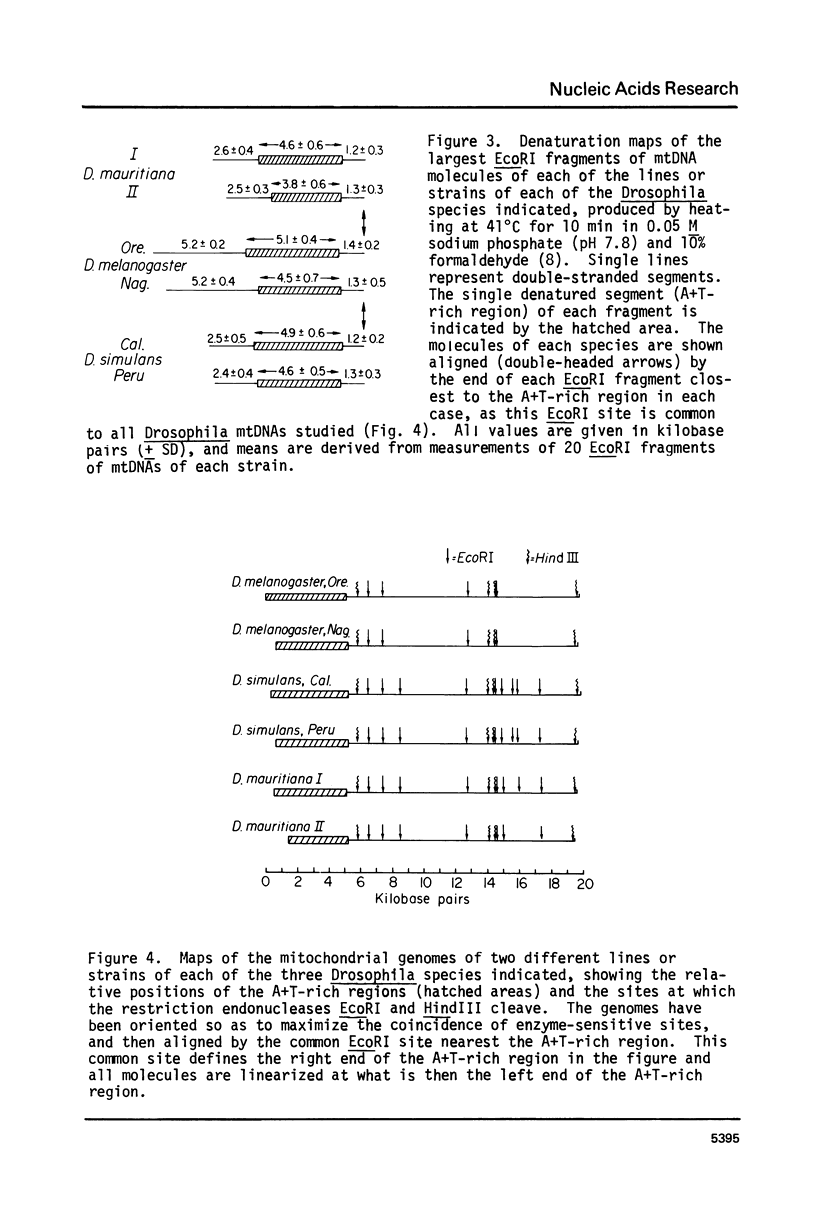
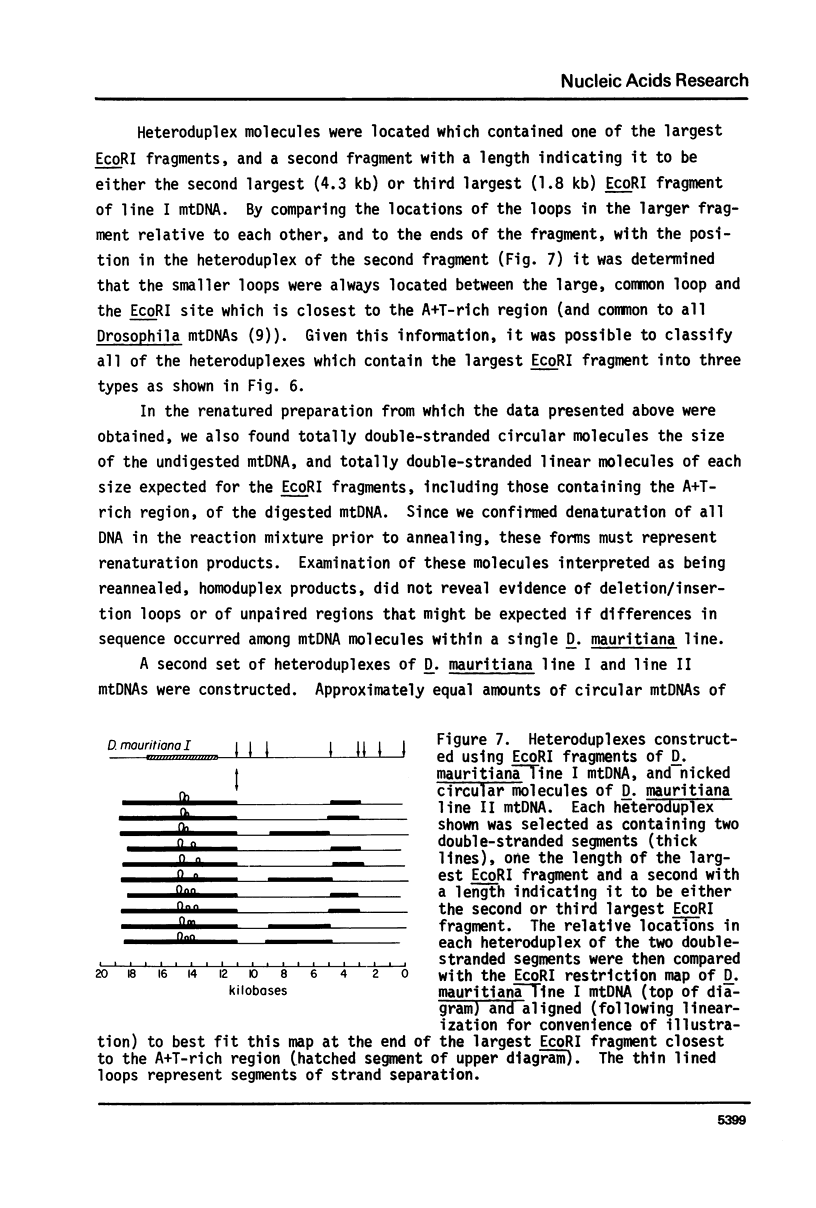
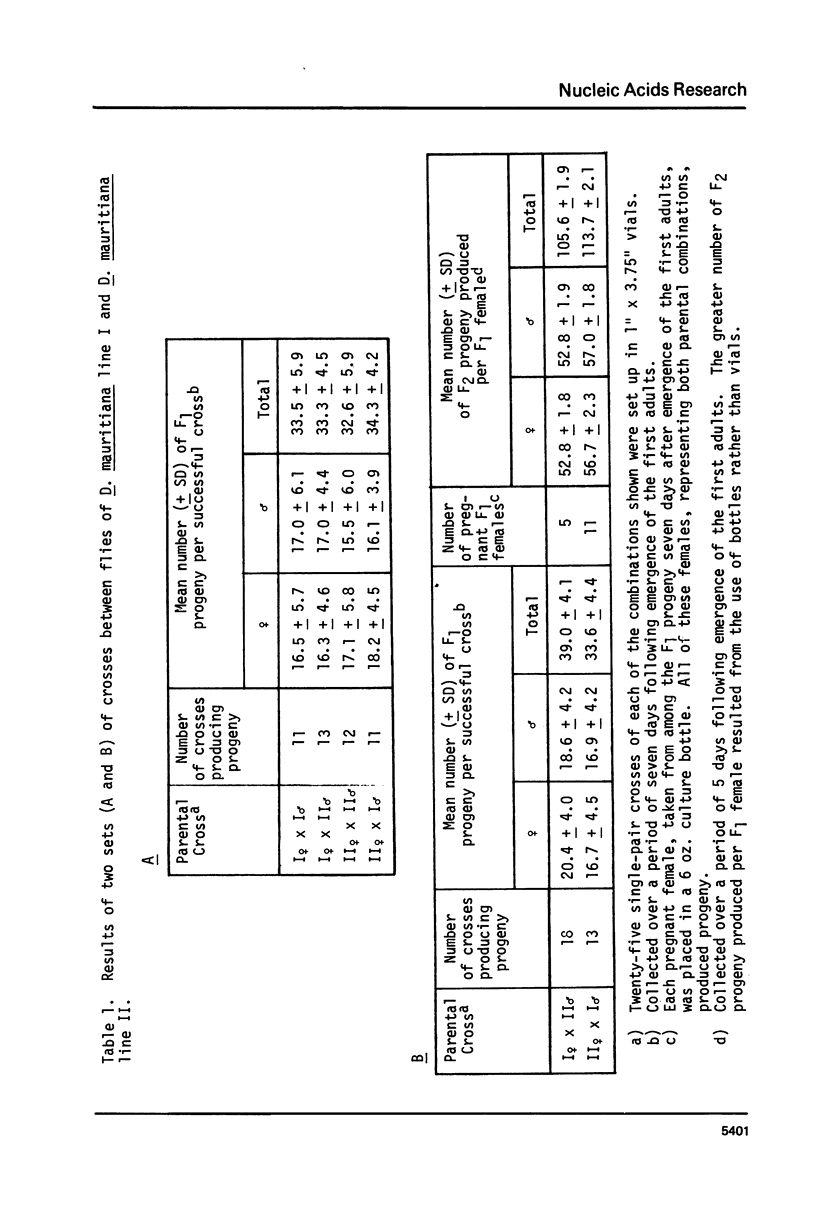
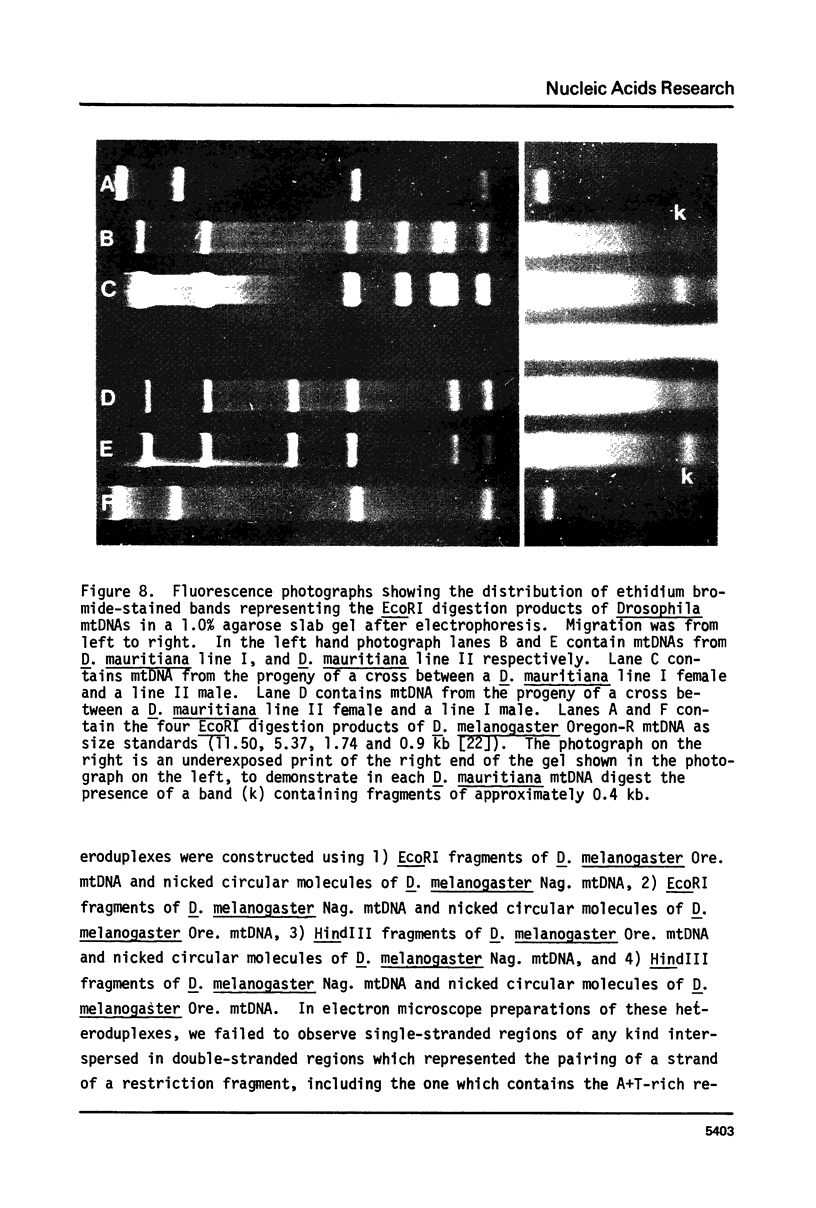
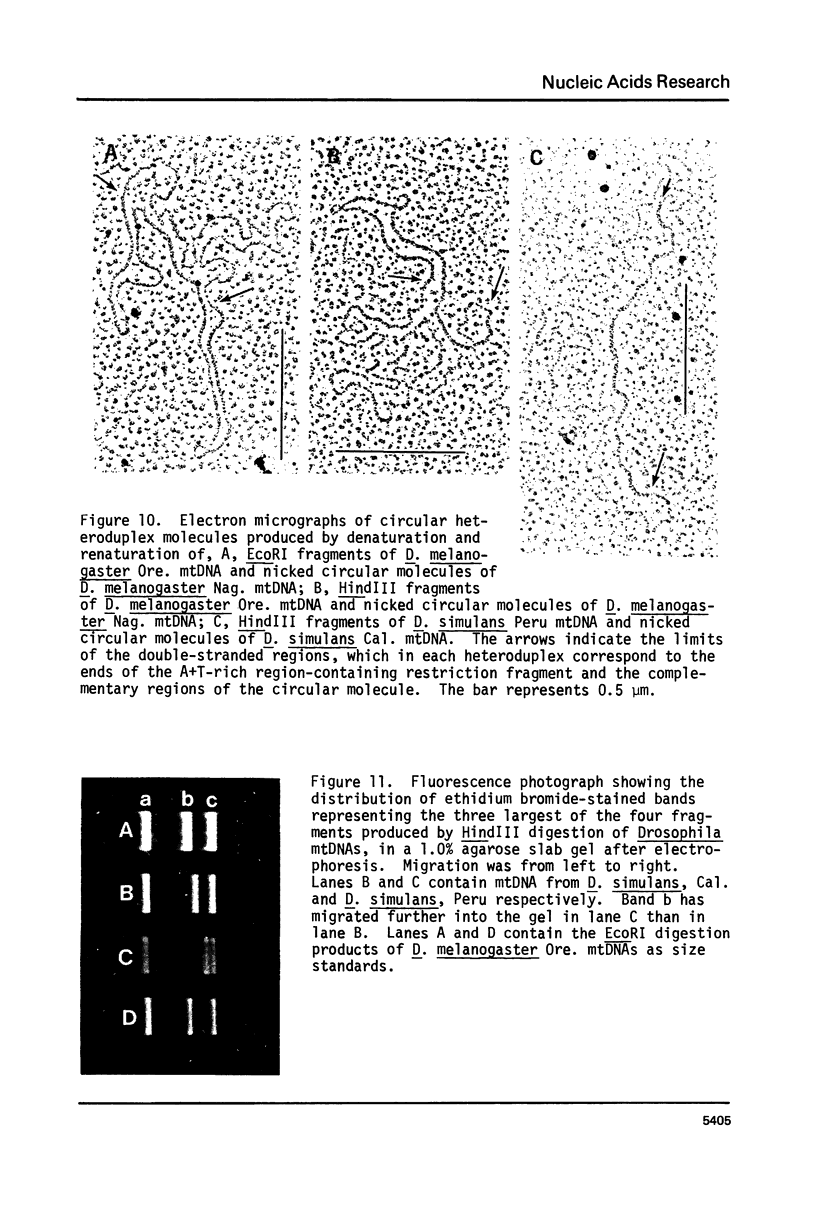
Mitochondrial DNA (mtDNA) molecules from Drosophila mauritiana, D. melanogaster, and D. simulans contain a single adenine + thymine (A+T)-rich region, which is similarly located in all molecules, but varies in size among these species. Using agarose gel electrophoresis and electron microscopy, a difference in occurrence of one EcoRI site, and a difference in size (approximately 0.7 kb) of the A+T-rich regions was found between mtDNA molecules of flies of two female lines of D. mauritiana. In heteroduplexes constructed between these two kinds of mtDNA molecules, two or three regions of strand separation, each comprising single strands of unequal length, were apparent near the center of the A+T-rich region. Using the structural differences between D. mauritiana mtDNA molecules it was demonstrated the mtDNA of this species is maternally inherited. Differences in length of A+T-rich regions were also found between mtDNA molecules of two geographically separated strains of D. melanogaster, and between mtDNA molecules of two geographically separated strains of D. simulans. However, in both cases, in heteroduplexes constructed between mtDNA molecules of different strains of one species, the A+T-rich regions appeared completely paired.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner J. J., Berninger M., Pardue M. L. Transcription of polytene chromosomes and of the mitochondrial genome in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):803–814. doi: 10.1101/sqb.1978.042.01.080. [DOI] [PubMed] [Google Scholar]
- Bultmann H., Laird C. D. Mitochondrial DNA from Drosophila melanogaster. Biochim Biophys Acta. 1973 Mar 19;299(2):196–209. doi: 10.1016/0005-2787(73)90342-0. [DOI] [PubMed] [Google Scholar]
- Buzzo K., Fouts D. L., Wolstenholme D. R. EcoRI cleavage site variants of mitochondrial DNA molecules from rats. Proc Natl Acad Sci U S A. 1978 Feb;75(2):909–913. doi: 10.1073/pnas.75.2.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J., Lemeunier F., Tsacas L., Bocquet C. Hybridation d'une nouvelle espèce, Drosophila mauritiana avec D. melanogaster et D. simulans. Ann Genet. 1974 Dec;17(4):235–241. [PubMed] [Google Scholar]
- Fauron C. M., Wolstenholme D. R. Extensive diversity among Drosophila species with respect to nucleotide sequences within the adenine + thymine-rich region of mitochondrial DNA molecules. Nucleic Acids Res. 1980 Jun 11;8(11):2439–2452. doi: 10.1093/nar/8.11.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauron C. M., Wolstenholme D. R. Structural heterogeneity of mitochondrial DNA molecules within the genus Drosophila. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3623–3627. doi: 10.1073/pnas.73.10.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouts D. L., Manning J. E., Wolstenholme D. R. Physicochemical properties of kinetoplast DNA from Crithidia acanthocephali. Crithidia luciliae, and Trypanosoma lewisi. J Cell Biol. 1975 Nov;67(2PT1):378–399. doi: 10.1083/jcb.67.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. M., Wolstenholme D. R. Origin and direction of replication in mitochondrial DNA molecules from Drosophila melanogaster. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3886–3890. doi: 10.1073/pnas.75.8.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. M., Wolstenholme D. R. Origin and direction of replication in mitochondrial DNA molecules from the genus Drosophila. Nucleic Acids Res. 1980 Feb 25;8(4):741–757. [PMC free article] [PubMed] [Google Scholar]
- Goldring E. S., Peacock W. J. Intramolecular heterogeneity of mitochondrial DNA of Drosophila melanogaster. J Cell Biol. 1977 May;73(2):279–286. doi: 10.1083/jcb.73.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Newbold J. E., Potter S. S., Edgell M. H. Maternal inheritance of mammalian mitochondrial DNA. Nature. 1974 Oct 11;251(5475):536–538. doi: 10.1038/251536a0. [DOI] [PubMed] [Google Scholar]
- Klukas C. K., Dawid I. B. Characterization and mapping of mitochondrial ribosomal RNA and mitochondrial DNA in Drosophila melanogaster. Cell. 1976 Dec;9(4 Pt 1):615–625. doi: 10.1016/0092-8674(76)90044-1. [DOI] [PubMed] [Google Scholar]
- Lemeunier F., Ashburner M. A. Relationships within the melanogaster species subgroup of the genus Drosophila (Sophophora). II. Phylogenetic relationships between six species based upon polytene chromosome banding sequences. Proc R Soc Lond B Biol Sci. 1976 May 18;193(1112):275–294. doi: 10.1098/rspb.1976.0046. [DOI] [PubMed] [Google Scholar]
- Perotti M. E. The mitochondrial derivative of the spermatozoon of Drosophila before and after fertilization. J Ultrastruct Res. 1973 Aug;44(3):181–198. doi: 10.1016/s0022-5320(73)80055-3. [DOI] [PubMed] [Google Scholar]
- Polan M. L., Friedman S., Gall J. G., Gehring W. Isolation and characterization of mitochondrial DNA from Drosophila melanogaster. J Cell Biol. 1973 Feb;56(2):580–589. doi: 10.1083/jcb.56.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J. G., Thomas C. A., Jr Length polymorphisms, restriction site variation, and maternal inheritance of mitochondrial DNA of Drosophila melanogaster. Plasmid. 1980 Mar;3(2):109–115. doi: 10.1016/0147-619x(80)90102-x. [DOI] [PubMed] [Google Scholar]
- Shah D. M., Langley C. H. Inter- and intraspecific variation in restriction maps of Drosophila mitochondrial DNAs. Nature. 1979 Oct 25;281(5733):696–699. doi: 10.1038/281696a0. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Fauron C. M. A partial map of the circular mitochondrial genome of Drosophila melanogaster. Location of EcoRI-sensitive sites and the adenine-thymine-rich region. J Cell Biol. 1976 Nov;71(2):434–448. doi: 10.1083/jcb.71.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]