Abstract
The inner ear is one of the most morphologically elaborate tissues in vertebrates, containing a group of mechanosensitive sensory organs that mediate hearing and balance. These organs are arranged precisely in space and contain intricately patterned sensory epithelia. Here, we review recent studies of inner ear development and patterning which reveal that multiple stages of ear development – ranging from its early induction from the embryonic ectoderm to the establishment of the three cardinal axes and the fine-grained arrangement of sensory cells – are orchestrated by gradients of signaling molecules.
Keywords: Otic placode, Morphogen, Inner ear, Cochlea, Otocyst
Introduction
The vertebrate inner ear has emerged as a fascinating model system for exploring how cell fate is controlled spatially and temporally during development. The vertebrate ear, which is often called a labyrinth owing to its interconnected and spatially complex system of fluid-filled ducts and chambers, produces at least six different sensory organs precisely positioned in space (Fig. 1) that mediate different aspects of hearing and balance. Angular acceleration is detected by three sensory organs called cristae that detect fluid motion in the three orthogonal semicircular canals (see Glossary, Box 1). Additional sensory organs, the maculae (see Glossary, Box 1), detect linear acceleration due to gravity. Finally, the organ of Corti (see Glossary, Box 1; also known as the papilla in non-mammals), is suspended across the cochlea (see Glossary, Box 1) and is specialized for hearing. The inner ear also generates its own population of neurons that innervate these sensory organs in a topographically precise fashion.
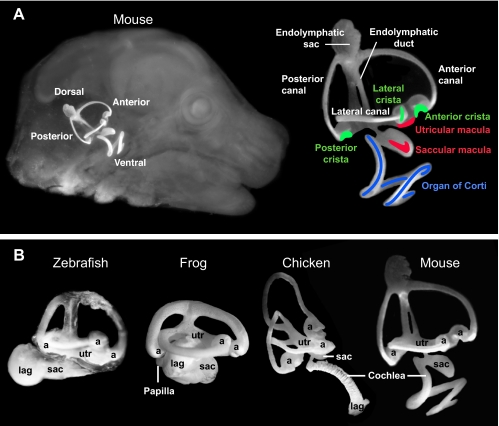
Fig. 1.
Inner ear anatomy. The interconnected fluid cavities of the inner ear can be revealed following injection of white paint in fixed and cleared specimens. (A) The mouse ear viewed in situ (left) and in detail (right) at E15.5. The approximate locations of sensory patches are indicated: organ of Corti, blue; maculae, red; cristae, green. (B) Lateral views of zebrafish, frog, chicken and mouse inner ears with the major chambers labeled. The orthogonal placement of semicircular canals dorsally is a shared feature of inner ears, although basal vertebrates such as lampreys have two, rather than the full complement of three, canals (not shown). At the end of each canal is an enlarged space, the ampulla (a). The endolymphatic duct projects dorsally and enlarges into a sac (shown only for mouse). Vestibular macular sensory organs are located in the utricle (utr), saccule (sac) and lagena (lag), although in fish the saccular macula has been co-opted to sense sound. In all species, the ventral ear houses a hearing organ, with frequency sensitivity that varies systematically along its length. In mammals, hearing is subserved by the organ of Corti located in the coiled cochlea. In archosaurs, lizards and amphibians, elongated sensory organs called papillae have evolved to sense hearing.
Box 1. Glossary
Basilar membrane. The thin and mechanically sensitive membrane that is suspended across the fluid-filled cochlear duct and runs along its length. The membrane vibrates in response to sound waves impinging on the fluid compartments of the cochlear duct.
Cochlea. The portion of the inner ear that is responsible for the detection of sound waves in amniotes. It forms as a fluid-filled epithelial tube emanating from the ventral portion of the otocyst.
Crista. One of three sensory organs of the vestibular system responsible for detecting angular acceleration in response to motion of fluid in the semicircular canals. The three cristae are oriented at right angles to each other and reside within epithelium-lined chambers called ampullae.
Endolymphatic duct. A portion of the vestibular apparatus that connects an endolymph-filled sac to the rest of the inner ear. Endolymph is the specialized, potassium-rich fluid that fills the inner ear.
Epibranchial placodes and ganglia. Ectodermal patches that will differentiate into ganglia responsible for mediating a variety of sensory modalities in the head, such as taste, touch, blood pressure, oxygen tension and pH.
Kölliker's organ. A nonsensory region of the cochlear duct lying adjacent to the organ of Corti that will ultimately give rise to the nonsensory inner sulcus. This region is sometimes referred to as the neural side of the cochlear duct, as it is located closest to the spiral (VIIIth) cranial ganglion that innervates cochlear hair cells.
Macula. A sensory organ of the vestibular apparatus usually specialized for detecting acceleration due to gravity. Two maculae are oriented at right angles to each other in epithelium-lined chambers called the utricle and saccule.
Neural plate. The primordium of the neural tube and future spinal cord and brain. It is derived from embryonic ectoderm.
Organ of Corti. A region of sensory tissue located on the basilar membrane that contains the sensory hair cells responsible for detecting sound.
Otic placode. The primordium of the inner ear, composed of a pseudostratified epithelium that remains thick as the epithelium surrounding it begins to thin. It is derived from cranial embryonic ectoderm and will either invaginate or cavitate after induction to form the otocyst.
Otocyst. The sphere of epithelial cells derived from the otic placode, and from which the sensory ganglion neurons and the entire endolymph compartment of the inner ear will derive.
Otolith or otoconial membrane. A dense aggregation of protein and calcified material that lies above macular organs and provides inertial mass on which gravitational or acceleration forces can act to stimulate vestibular hair cells. A single stone, called the otolith, is found above each macular organ in fish, whereas a suspension of otoconial crystals embedded in a protein matrix is suspended above the maculae in frogs, birds and mammals.
Outer sulcus. A nonsensory region of the cochlear duct lying adjacent to the organ of Corti. This region is sometimes referred to as the abneural side of the cochlear duct, as it is located furthest away from the spiral (VIIIth) cranial ganglion that innervates cochlear hair cells.
Rhombomere. A segmented division of the embryonic hindbrain, delineated first by unique segmental patterns of gene expression and later by distinct anatomical boundaries and nerve fiber tracts. The hindbrain typically contains eight rhombomeres, and the inner ear arises in the vicinity of rhombomeres 4-6, depending on the species.
Saccule. An epithelial out-pocketing of the inner ear containing one of the sensory maculae responsible for detecting gravitational acceleration. See also utricle.
Semicircular canal. One of three fluid-filled toroidal-shaped ducts that terminate at one end in an enlargement called the ampulla, which houses a sensory organ known as a crista. Motion of fluid in the canal in response to angular acceleration stimulates sensory hair cells in the associated crista.
Utricle. An epithelial out-pocketing of the inner ear containing one of the sensory maculae responsible for detecting gravitational acceleration. See also saccule.
Vestibular apparatus. The portion of the inner ear responsible for detecting gravity and angular acceleration.
The fate decisions that establish the sensory and nonsensory regions of the ear begin at an early stage of development, when the ear consists of a simple epithelial sphere termed the otocyst (see Glossary, Box 1). Evidence has emerged in the past few years that a variety of known signaling molecules, such as fibroblast growth factors (FGFs), Wnts, bone morphogenetic proteins (BMPs), sonic hedgehog (Shh) and retinoids, are deployed at different times and places to regulate differentiation of the inner ear. The expression of, or response to, some of these secreted molecules appears to occur in gradients, suggesting that they might function as classical morphogens (see Box 2), although definitive evidence that they do so is not yet available in most cases. This review will summarize recent findings on the identity of these signaling and morphogen molecules and how they are coordinated in space and time to generate the tissue and cellular architecture of the ear.
Box 2. Secreted signals as potential morphogens
A hallmark of a morphogen is that it is distributed as a spatial gradient across a field of equipotential cells to endow the cells with positional information. Exposure to specific concentrations of the morphogen (thresholds) can instruct a subset of cells to change their fates. Often, the readout of a fate change is evident by a relatively abrupt variation in gene expression. In this way, an embryonic field can be segregated into discrete subdomains. Morphogens can arise as point sources (such as in the center of a field) or as line sources (at the edge of a field or compartment) to influence patterning and cell fates. Diffusible morphogens typically act over distances not exceeding a few hundred microns, which may be the theoretical limit of morphogen action (Lander et al., 2009). A spatial gradient can also function as a vector to polarize cells towards or away from a morphogen source. Finally, spatial molecular gradients may be directly responsible for systematic but gradual changes in structural properties across a field of cells. The frequency axis of the vertebrate cochlea might offer one of the most striking examples of such a pattern.
From placode to ear – how graded signals turn ectoderm into the inner ear
The entire inner ear and its associated sensory ganglia derive from the otic placode (see Glossary, Box 1). It is one of a series of cranial placodes that collectively give rise to all craniofacial sensory organs, the lens and a subset of cranial ganglia (Baker and Bronner-Fraser, 2001; Schlosser, 2010). The precursors of different placodes are distributed in an anterior-posterior (AP) sequence around the rostral neural plate (see Glossary, Box 1) termed the pre-placodal domain (PPD) (Schlosser, 2006; Streit, 2007). The PPD is induced by signals, such as FGFs, from both the neural plate and the underlying cranial mesoderm, and inhibition of both Wnt and BMP signaling is required to correctly position the PPD at the neural plate border and to prevent it from extending into the embryonic trunk (Kwon et al., 2010; Litsiou et al., 2005).
It is now well established that FGF signals are both necessary and sufficient to induce early otic placode markers from competent pre-placodal ectoderm (Fig. 2) (Ohyama et al., 2007; Schimmang, 2007), although both the location of the FGF signals and the specific FGFs responsible for otic induction vary from species to species. The earliest markers of the future inner ear that are induced in response to FGF signaling are members of the paired homeobox-containing Pax2/5/8 transcription factor family – Pax8 in anamniotes and Pax2 in amniotes (Groves and Bronner-Fraser, 2000; Heller and Brandli, 1999; Ohyama and Groves, 2004; Pfeffer et al., 1998). Fate-mapping studies in mice and chick have suggested that although the Pax2-expressing domain is likely to contain all the progenitors of the inner ear, it also harbors cells that will give rise to the epibranchial placodes (see Glossary, Box 1) and epidermis (Ohyama and Groves, 2004; Ohyama et al., 2007; Streit, 2001). This Pax2-expressing progenitor domain has been termed the pre-otic field or the otic-epibranchial progenitor domain (OEPD) (Ladher et al., 2000; Freter et al., 2008).
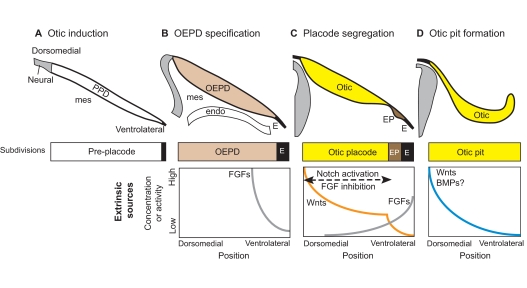
Fig. 2.
Otic induction from the pre-placode. (A) Otic induction requires extrinsic sources of secreted molecules originating from neural tissue, mesenchyme (mes) and pharyngeal endoderm (endo). (B) FGFs act on the pre-placodal domain (PPD) to specify the otic-epibranchial placode domain (OEPD) as separate from ectoderm (E). (C) The OEPD field is further segregated into the otic placode (Otic) and the epibranchial placodes (EP). The otic placode forms under the influence of high Wnts and low FGF signaling. This begins with a Wnt gradient that develops a sharp transition point through feedback loops involving Notch activation and FGF inhibition. (D) By the time the otic pit begins to invaginate, the dorsomedial domain may already be receiving higher Wnt and BMP signals from the adjacent hindbrain, thereby initiating both dorsal-ventral (DV) and mediolateral patterning (not shown). Note that in this and subsequent figures, illustrations of gradients of signaling proteins or their resulting activities are not based on direct observation but rather are speculative, taking into consideration results from experimental embryology, known gene expression patterns and/or phenotypes resulting from perturbations of signaling pathways using drugs or in mutant mice.
The refinement of the OEPD into distinct otic, epibranchial and epidermal territories is regulated by graded Wnt signals emanating from the midline (Fig. 2) (Ohyama et al., 2006). Examination of the cranial region of Wnt reporter mice revealed that OEPD cells closest to the neural plate, which are fated to form the otic placode, receive high levels of Wnt signaling, whereas more lateral OEPD cells, fated to form epidermis and the epibranchial placodes, receive far less or no Wnt signals (Ohyama et al., 2006). Candidates that might establish this signaling gradient include Wnt8 expressed in rhombomere 4 (see Glossary, Box 1) and Wnt6 expressed at the neural plate-epidermis boundary (Jayasena et al., 2008; Ladher et al., 2000; Ohyama et al., 2006; Urness et al., 2010). Loss-of-function studies in mouse and chick, in which Wnt signaling was inhibited in the OEPD by conditional deletion of β-catenin or by overexpression of secreted Wnt inhibitors, revealed a substantial reduction in otic fate and an expansion of markers for the epidermal/epibranchial region (Freter et al., 2008; Ohyama et al., 2006). Conversely, gain-of-function studies, in which Wnt signaling was constitutively activated throughout the OEPD, demonstrated an expansion in otic fate at the expense of epidermis (Freter et al., 2008; Ohyama et al., 2006). Together, these studies support a model in which graded Wnt signals subdivide the OEPD into otic and non-otic territories.
The occurrence of gradients of cell signaling molecules in many developing systems raises the question of how cells translate a continuously varying, or ‘analog’, signal into distinct ‘digital’ cell fates. In the case of the otic-epidermal fate choice in the OEPD, at least two different strategies are used to convert the Wnt gradient into otic and non-otic fates. First, the Notch signaling pathway is deployed to further amplify Wnt signaling in future otic regions, but not in non-otic regions. This is achieved by high levels of Wnt signaling that upregulate components of the Notch signaling pathway, such as the Notch ligand Jag1 (Jayasena et al., 2008). Jag1 signaling through the Notch1 receptor feeds back to augment Wnt signaling in these regions, but not in regions receiving low levels of Wnt signaling where Jag1 is not activated. Thus, a smooth gradient of Wnt signaling is turned into a discontinuous pattern, with high Wnt/high Notch signaling regions differentiating into otic tissue and low Wnt/low Notch signaling regions differentiating into epidermis (Fig. 2).
A second system that acts early in ear induction uses negative feedback from FGF signaling to distinguish between otic and non-otic fates (Fig. 1). Differentiating otic placode tissue rapidly upregulates negative regulators of the FGF signaling pathway, such as Sprouty (Spry) genes and the dual-specificity ERK phosphatase MKP3 (Dusp6) (Chambers and Mason, 2000; Urness et al., 2008). This rapid attenuation of FGF signaling is required for the subsequent differentiation of otic tissue, as forced activation of FGF signaling in the OEPD blocks the appearance of later otic markers (Freter et al., 2008). Moreover, combined loss of mouse Spry1 and Spry2 leads to alterations in placode size (Mahoney Rogers et al., 2011). By contrast, the epidermal/epibranchial region of OEPD does not express FGF inhibitors such as the Sprouty genes (Mahoney Rogers et al., 2011), and sustained FGF signaling in this region is compatible with differentiation into epidermis and epibranchial ganglia (Freter et al., 2008). Together, these two feedback and amplification systems partition the OEPD into a future otic region (high Wnt, high Notch, low FGF signaling) and a future epidermal and epibranchial region (low Wnt, low Notch and high FGF signaling).
Setting up the cardinal axes of the inner ear
The inner ear has an elaborate morphology with clear polarity in all three axes (Fig. 1), and much evidence suggests that this polarity begins to be established early in ear development. After the otic placode has been induced it undergoes invagination (in amniotes) or cavitation (in fish) to form a spherical otocyst. At this stage, asymmetries in gene expression can already be observed in the otocyst (Brigande et al., 2000; Fekete and Wu, 2002). For example, the production of auditory and vestibular neurons tends to occur in ventral and anterior regions of the ear and is preceded by the expression of proneural genes such as Ngn1 in the anteroventral otocyst (Raft et al., 2004). However, these early gene expression patterns show considerable plasticity, and rotation of the otocyst about one of its three axes is capable of reprogramming these expression patterns (Bok et al., 2011; Wu et al., 1998). Over time, the three axes of the ear become firmly established and can no longer be respecified by surgical manipulation. The fixing of each axis occurs at different times, with AP fates becoming permanent before dorsal-ventral (DV) fates (Wu et al., 1998), suggesting that different signals might be involved in the specification of each axis.
DV patterning of the inner ear primordium
The amniote inner ear has an obvious DV polarity, with the vestibular apparatus (see Glossary, Box 1) located dorsally and the sound-detecting cochlea emerging as a ventral protrusion of the otocyst (Fig. 1 and Fig. 4). A number of studies in the 1930s and 1940s showed that this basic DV pattern could be disrupted by manipulating the hindbrain, suggesting that signals from the neural tube might specify this axis of the inner ear (reviewed by Groves, 2005). Subsequent investigations of mouse mutants with hindbrain defects (such as the Kreisler mouse) (Choo et al., 2006; Deol, 1964; McKay et al., 1996) confirmed this idea. The discovery that signals from the notochord and ventral neural tube impart DV patterning information to the central nervous system (van Straaten et al., 1989) led to the proposition that similar signals might be used to pattern the otic vesicle, which develops next to the hindbrain (Fekete, 1996). The first confirmation of this idea was provided by Giraldez (Giraldez, 1998), who showed that neural tube signals are required for the proper regional expression of the Lim homeobox transcription factor Lmx1 in the dorsal and lateral regions of the otocyst. Specific surgical ablation or rotation of the notochord and ventral neural tube have also demonstrated that signals from these ventral structures are necessary for ventral patterning of the chick otocyst and can imbue dorsal ear tissue with ventral identity (Bok et al., 2005; Liang et al., 2010).
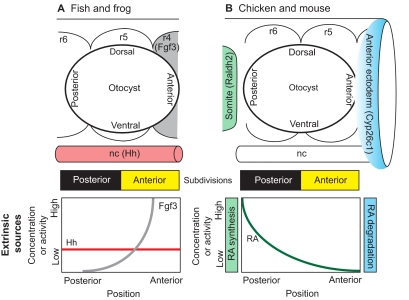
Fig. 4.
Anterior-posterior axial patterning in the inner ear. Extrinsic sources of signaling molecules that pattern the otic placode/vesicle differ between anamniotes and amniotes. (A) Zebrafish and Xenopus otic vesicles begin with a mirror-image symmetric prepattern of otic tissue that is centered along the anterior-posterior (AP) axis adjacent to rhombomere (r) 5. The symmetric template is then independently endowed with posterior identity via hedgehog (Hh) signaling originating from the notochord (nc) and anterior identity via Fgf3 signaling originating from rhombomere 4. (B) In chicken and mouse, the otocyst is centered next to the r5/r6 boundary. AP positional information is influenced by an extrinsic gradient of retinoic acid (RA). This gradient arises because of asymmetry in the synthesis and degradation of RA by enzymes (Raldh2 and Cyp26c1) present in the somatic mesoderm and anterior ectoderm, respectively. There are no lineage data to suggest that the otic field becomes fully subdivided into anterior and posterior halves, as shown schematically by the yellow and black bars for both fish/frog and chicken/mouse. However, the observation of mirror-image symmetry along this axis in mutants or following experimental manipulations supports the idea that there are two distinct compartments.
Shh produced by the notochord and floor plate acts as a morphogen to pattern the DV neural tube (Dessaud et al., 2008), and this diffusible signal also acts directly on the developing amniote otocyst to confer DV patterning (Riccomagno et al., 2002; Bok et al., 2007b; Whitfield and Hammond, 2007). Shh effectors, such as the Gli transcription factor family, and direct targets of Shh signaling, such as the Shh receptor patched 1 (Ptc1, or Ptch1), are expressed in the otocyst epithelium in a dorsal-to-ventral gradient, indicative of a graded response to Shh (Bok et al., 2007c; Brown and Epstein, 2011). Perturbation of Shh signaling with antibodies or in Shh mutant mice leads to a loss or reduction of transcription factors expressed in the ventral regions of the otocyst with a concomitant (but not complete) expansion of dorsal markers, and this loss of ventral patterning information is translated into a loss or reduction of ventral structures, including the cochlea and cochleovestibular ganglion (Bok et al., 2005; Riccomagno et al., 2002). Conversely, the Shh P1 transgenic mouse line, in which Shh is aberrantly expressed in the dorsal half of the otocyst, shows a loss of dorsal vestibular structures and an upregulation of ventral Shh targets in the dorsal otocyst (Riccomagno et al., 2002).
The Gli family of zinc-finger transcription factors comprises known downstream effectors of Shh signaling (Ruiz i Altaba et al., 2002), and their graded activity is required for a dose-dependent transduction of Shh in the spinal cord (Dessaud et al., 2008). Both Gli1 and Gli2 typically act as transcriptional activators. By contrast, Gli3 is cleaved in the absence of Shh signaling to release an N-terminal domain (GliR) that actively represses Shh targets. In the presence of Shh signals, Gli3 remains stable and uncleaved (GliA) and directly activates Shh targets (Wang et al., 2000). This suggests a model in which a graded increase in Shh signaling gradually inhibits GliR activity and then progressively increases GliA function. The amount of Shh signal a given cell receives within an Shh gradient can thus be thought of as the net output of activator and repressor Gli protein activity. The most distal (i.e. most ventral) region of the cochlear duct requires Gli activator function, as it fails to form in Gli2–/–;Gli3–/– mutants or in the Gli3D699 mutant, which only expresses the N-terminal repressor fragment of Gli3 (Bok et al., 2007c). More proximal regions of the cochlear duct and the saccule (see Glossary, Box 1), which is the most ventrally located part of the vestibular system in mammals, are located further from the midline source of Shh and are assumed to require lower levels of Shh signaling for their formation, which would arise from a balance of Gli activator and repressor activities. Accordingly, the proximal cochlear duct and saccule are missing in Shh mutants (which lack Gli activator function but possess expanded Gli3 repressor function), but are restored in Shh–/–;Gli3–/– mice (which lack both Gli activator and Gli3 repressor function) (Bok et al., 2007c). Finally, Gli3–/– mice (in which Gli3 activator function is compensated for by Gli2 and Gli1, but which lack any Gli3 repressor activity) have malformed dorsal structures, suggesting a requirement for a precise level of Gli3 repressor activity (Bok et al., 2007c). Together, these observations demonstrate that the smooth Shh signaling gradient in the ear is not interpreted by a correspondingly smooth response of a single transcription factor, but rather by competing activating and repressive transcriptional mechanisms (Fig. 3).
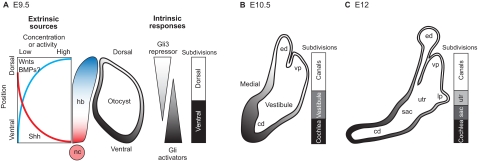
Fig. 3.
Dorsoventral axial patterning during inner ear development. (A) At E9.5, the mouse otocyst is positioned to receive secreted signals that originate asymmetrically from the adjacent hindbrain (hb) and notochord (nc). During otocyst development, gradients of sonic hedgehog (Shh) target genes are observed along the DV axis (gray shading). A working model is shown in which the otic epithelium experiences opposing gradients of Gli activators (high ventrally) and the Gli3 repressor (high dorsally) to establish positional information across the entire DV axis. Wnt signaling is also a major contributor to the patterning of dorsal inner ear structures. BMPs are also present but have not yet been shown to have a role in DV axis specification of the inner ear. (B,C) Between E10.5 and E12, the mouse otocyst enlarges and becomes progressively segregated along the DV axis into chambers and canals. Transcription factor expression domains mark dorsal versus ventral territories at E9.5-10.5. However, prior to overt morphogenesis, the lack of markers for a transitional domain (the vestibule) or for the saccular (sac) and utricular (utr) chambers, means that the diagrammatic representation of the sequential subdivision of these regions is speculative. Note that the 3-fold expansion in the size of the inner ear during these stages is not illustrated. cd, cochlear duct; ed, endolymphatic duct; lp, lateral canal plate; vp, vertical canal plate.
The importance of repressing hedgehog (Hh) signaling for the development of dorsal otic structures was recently confirmed by studies in zebrafish. The fish inner ear contains all the components of the vestibular system present in amniotes, but lacks a cochlea. Hammond and colleagues characterized a series of mutants of different inhibitors of the Hh pathway – ptc1, ptc2, suppressor of fused (sufu), DAZ-interacting protein 1 (dzip1) and hedgehog-interacting protein (hhip). Combinations of these mutant genes yield embryos with progressively stronger Hh signaling in the dorsal otocyst (Hammond et al., 2010). Markers of the dorsal and lateral otocyst were progressively reduced with increased Hh signaling, whereas ventral and medial markers became progressively expanded. This gradual change in otic patterning was reflected in a gradual reduction or loss of dorsal and lateral structures, including the cristae, semicircular canal pillars and the endolymphatic duct (see Glossary, Box 1).
It should be stressed that both the mouse and fish Hh signaling pathway loss-of-function studies involved manipulations that affect the entire embryo, not just the inner ear. Since Hh signaling is known to influence DV patterning of both neural and mesenchymal tissue (Chiang et al., 1996; Fan et al., 1995), it is formally possible that Shh is regulating inner ear DV patterning through both direct effects on the otic epithelium and indirect effects on the patterning of tissues adjacent to the otocyst. A recent study in which the Shh receptor smoothened (Smo) was conditionally inactivated in the otocyst suggests that both mechanisms pattern the otocyst (Brown and Epstein, 2011). In Smo conditional mouse mutants, the ventral inner ear (cochlea and saccule) is absent, but dorsal components of the inner ear (semicircular canal, endolymphatic duct, cristae and utricle; see Glossary, Box 1) develop normally. These changes are preceded by earlier patterning alterations in the otocyst. These data suggest that Shh acts on the ventral otocyst directly to regulate cochlear development, and that dorsal development can be regulated by signals from tissues adjacent to the otocyst that require Shh signaling for their normal development.
Just as in the spinal cord, the ventralizing effects of Shh are complemented by dorsalizing signals, again located in the midline. Wnt reporter mice have revealed that the initial mediolateral gradient of Wnt signaling seen in the otic placode, which is likely to be due to Wnt8 in the hindbrain (Urness et al., 2010) or Wnt6 at the hindbrain/placode boundary (Jayasena et al., 2008), is preserved as a DV gradient as the placode invaginates and closes to form the otocyst (Ohyama et al., 2006; Riccomagno et al., 2005). At this stage, both Wnt1 and Wnt3a are also expressed in the dorsal region of the neural tube (Riccomagno et al., 2005) (Fig. 3). Mouse mutants of either gene develop normal inner ears, but Wnt1;Wnt3a double mutants completely lack dorsal inner ear structures and the remaining cochlea is a severely malformed stub (Riccomagno et al., 2005). Accordingly, some dorsal otocyst markers are absent from Wnt1;Wnt3a double-mutant ears. Activation of Wnt signaling in explanted otocysts causes many dorsal markers to expand ventrally, and these same markers are reduced by ablation of the dorsal neural tube (Riccomagno et al., 2005).
It is tempting to suggest a simple model of DV patterning of the inner ear in which opposing gradients of Shh and Wnt signaling regulate the spatial localization of transcription factors, ultimately leading to the differentiation of a correctly patterned inner ear. In reality, however, the picture is more complicated, and much evidence suggests that Wnt and Shh signals regulate different inner ear genes in different ways. For example, although markers of the dorsal otocyst such as Dlx5 are expanded ventrally in Shh mutants, these markers rarely expand to occupy the entire otocyst, and the inner ear never becomes completely dorsalized (Riccomagno et al., 2002). Conversely, ventral markers of the otocyst, such as Pax2 and Otx2, do not expand dorsally in the otocysts of Wnt1;Wnt3a double mutants (Riccomagno et al., 2005). Some markers of the dorsal and dorsolateral regions of the otocyst, such as Wnt2b and Hmx3, respectively, are generally unaffected in Shh mutants or Wnt1;Wnt3a double mutants, or in tissues in which Wnt signaling has been constitutively activated (Ohyama et al., 2006; Riccomagno et al., 2002; Riccomagno et al., 2005), suggesting that they are regulated by signals other than Wnts and Shh. The ventral marker Six1 is also unaffected by loss of Shh signals, despite the fact that Six1 mutants share some phenotypic similarities with Shh mutants (Ozaki et al., 2004; Zheng et al., 2003).
Such evidence suggests that other signaling pathways also impart DV patterning information to the inner ear independently of Wnt and Shh. Among the candidates are BMPs, which are also expressed in the dorsal midline and serve to pattern the dorsal neural tube. At present, there is limited evidence to suggest that a gradient of BMP signaling patterns the inner ear at the otocyst stage, although Lmx1b appears to be positively regulated by BMP4 or BMP7 (Abello et al., 2010). Furthermore, as Bmp4 is itself expressed in many differentiating sensory patches in the ear, and as Bmp2 is expressed in semicircular canal primordia (Chang et al., 2004; Morsli et al., 1998; Wu and Oh, 1996), it is hard to separate an extrinsic role of BMPs in early ear axial patterning from that of BMPs expressed within the ear epithelium, as discussed below.
Patterning the AP axis of the ear
Although genes expressed in dorsal parts of the inner ear are some of the earliest to be expressed in the otic placode, embryonic manipulations of the developing ear reveal that its AP axis becomes fixed first (Wu et al., 1998). The AP axis of the embryo, and that of the nervous system, is established early in development, and some of these axial signals are also used to impart AP identity to the developing ear. Below, we describe candidates for these diffusible signals, first for fish and amphibians, and then for amniotes (Fig. 4).
A number of zebrafish mutants that affect the development of the hindbrain cause AP patterning defects in the developing ear but do not significantly affect the initial induction of the otic placode. For example, mutations in the zinc-finger transcription factor mafb gene cause a loss of rhombomeres 5 and 6 and an expansion of rhombomere 4 markers such as fgf3. These mutants have an expansion of anterior otic markers, whereas the fgf3 mutant lim-absent (lia) displays a partial loss of anterior otic markers (Hammond and Whitfield, 2011; Kwak et al., 2002). Exposure of zebrafish embryos to the FGF receptor inhibitor SU5402 after the formation of the otic placode causes a dramatic loss of anterior markers and duplication of posterior otocyst markers, leading to an inner ear with two mirror-image posterior domains (Hammond and Whitfield, 2011). Conversely, heat-shock activation of fgf3 in embryos containing ten somites or more led to the opposite phenotype – a downregulation of posterior otic markers and an inner ear bearing two mirror-image anterior domains (Hammond and Whitfield, 2011). Since the anterior regions of the zebrafish otocyst lie immediately adjacent to rhombomere 4, it is likely that Fgf3 and other FGF family members provide anterior patterning signals to the otocyst.
Posterior otic identity requires Hh signaling in zebrafish and Xenopus (Hammond et al., 2003; Waldman et al., 2007; Whitfield and Hammond, 2007). First, reduction of Hh signaling in the zebrafish contf18b or smub481 mutant, or by injection of Patched mRNA, leads to a loss of posterior structures and partial mirror-image duplications of anterior structures (Hammond et al., 2003). Such mirror-image anterior duplications are also seen in Xenopus embryos overexpressing the Hh inhibitor Hhip (Waldman et al., 2007). Second, strong activation of Hh signaling in zebrafish causes a loss of anterior character and partial mirror-image duplication of posterior structures (Hammond et al., 2003).
Together, these studies suggest a model in which high levels of FGF signaling specify anterior fate in the anamniote ear, whereas Hh signaling specifies posterior fates (Fig. 4). Although FGF3 is a good candidate to induce anterior fates based on its localization, it is less clear how Hh signaling acts only on the posterior otocyst, as it is expressed in the notochord and the floor plate along the midline with no obvious AP difference in localization. One obvious possibility is that FGF signaling in the anterior region of the otocyst actively inhibits Hh signaling, restricting its influence to the posterior domain. However, Hh signaling, as revealed by Patched gene expression, still localizes to the ventromedial domain in the absence of FGF signaling; similarly, FGF signaling, as revealed by pea3 expression, remains localized to the anterior domain of the otocyst in the absence of Hh signaling (Hammond and Whitfield, 2011). Loss of both FGF and Hh signaling in zebrafish leads to a grossly abnormal inner ear that lacks most sensory cells and exhibits vestigial semicircular canals, and to a loss of most markers of anterior and posterior identity (Hammond and Whitfield, 2011). This suggests that neither signaling pathway is epistatic to the other, and that neither anterior nor posterior fate is a ‘default’ identity. However, these otocysts that are deficient in both FGF and Hh signaling do initially show a symmetric polarity – for example, at both poles of the otocyst they develop small otoliths (see Glossary, Box 1) and groups of ciliated tether cells, which are thought to be precocious sensory hair cells (Riley et al., 1997; Tanimoto et al., 2011) – but they never go on to manifest clear AP patterning.
The notion that the anamniote ear possesses an initially symmetric pre-pattern that then develops anterior and posterior identity is supported by several lines of evidence. First, the development of ciliated tether cells at either end of the zebrafish ear is prefigured by the symmetric expression of delta and atonal 1b at both poles (Haddon et al., 1998; Millimaki et al., 2007). Second, the mirror-image or enantiomorphic inner ears that develop when either FGF or Hh signaling is disrupted in zebrafish and amphibians suggest that both halves of the early otocyst are equally competent to respond to either anteriorizing (FGF) or posteriorizing (Hh) signals, but they do so independently rather than forming a single large anteriorized or posteriorized ear (Hammond et al., 2003; Hammond and Whitfield, 2011; Waldman et al., 2007). Finally, surgical manipulation of the amphibian ear, either by rotation of the otic vesicle or partial ablation of the otic placode, can sometimes result in mirror-image duplications of either anterior or posterior halves (Harrison, 1936; Waldman et al., 2007). These results suggest the presence of organizing centers at either pole of the otocyst that might confer polarity to the developing components of the inner ear. Since some cells bearing primary cilia are known to act as signaling sources (Quinlan et al., 2008), an attractive hypothesis is that the ciliated tether cells present at each pole of the otocyst help propagate polarity signals after the two halves of the ear receive FGF and Hh signals.
Unlike AP patterning in zebrafish, the AP patterning of the amniote inner ear does not appear to require signals from the hindbrain (Bok et al., 2005; Choo, 2007; Liang et al., 2010). Instead, recent work suggests that signals present in the ectoderm surrounding the inner ear confer correct AP patterning information (Bok et al., 2011). For example, 180° rotation of the otic cup along the AP axis in chick embryos, while leaving the other axes unchanged, typically results in normally patterned ears (Bok et al., 2007c). However, inclusion of adjacent ectoderm in these rotation experiments led to a greatly increased incidence of AP reversals (Bok et al., 2011). Retinoic acid (RA) is known to posteriorize the embryonic body axis, and the boundaries of expression of RA-synthesizing enzymes, such as retinaldehyde dehydrogenase 2 (Raldh2, or Aldh1a2), and of RA-degrading enzymes, such as Cyp26c1, appear to coincide with the axial level of the developing inner ear. This led Wu and colleagues to examine the role of RA in AP patterning of the chick and mouse ear (Bok et al., 2011). They identified a developmental period after otic placode induction during which the otocyst received RA signals, first throughout the ear and then only in its posterior half. This time period was particularly sensitive to manipulation of RA levels, as treatment with RA caused an expansion of posterior character in both mouse and chick otocysts, and pharmacological inhibition of RA production generated ears with expanded anterior character. Interestingly, manipulation of retinoid signaling led to similar mirror-image duplications of inner ear structures and regional markers to those seen in zebrafish after manipulation of FGF or Shh signaling, with twinned posterior ears developing after RA treatment (Bok et al., 2011).
How can we reconcile the seemingly disparate AP patterning mechanisms in frogs and fish with those in amniotes? Two issues need to be resolved: first, that Hh signaling appears to specify DV fates in amniotes but in fish and amphibians it appears to play a much more significant role in AP patterning (Bok et al., 2007b; Whitfield and Hammond, 2007); and second that RA signaling, rather than Hh signaling, appears to specify AP identity in amniotes. As discussed above, recent evidence suggests that repression or reduction of Hh signaling in fish is necessary for correct dorsal differentiation of the inner ear (Hammond et al., 2010), suggesting that at least some aspects of DV patterning in fish require appropriate regulation of Hh signals. RA has recently been shown to regulate posterior markers of the otocyst in fish, including Tbx1 and Her9, and to repress anterior markers in a similar manner to that seen in amniotes (Radosevic et al., 2011). However, although manipulation of retinoid signaling in fish can alter AP patterning, it does not appear to result in mirror-image duplications, suggesting that RA signals might operate independently of signals that specify polarity in the two halves of the zebrafish ear. RA and Hh signaling have been shown to interact in a variety of developmental systems (Bertrand and Dahmane, 2006), and in many instances RA is necessary for Hh signaling to occur (Niederreither and Dolle, 2008). It can achieve this by direct transcriptional regulation of the Shh gene, by direct transcriptional regulation of Shh effectors such as Gli2, or by RA receptors regulating target genes in concert with Shh effectors by occupying adjacent binding sites on target enhancers (Ribes et al., 2009; Ribes et al., 2008; Ribes et al., 2006). However, at present it is unclear whether any of these mechanisms operates during the patterning of the developing otocyst.
Another way to reconcile the patterning differences observed across taxa is to examine the functional and evolutionary relationships between sensory organs of the ear in different vertebrate groups. In fish, two sensory regions of the ear, the saccule and the lagena, arise from the ventral region of the ear and extend posteriorly from the rest of the vestibular apparatus (Popper and Fay, 1999). Both the saccule and lagena can serve a rudimentary auditory function in fish, and it has been proposed that a true auditory organ, the basilar papilla, arose as an elaboration from the posterior region of the saccule or lagenar recess (Fritzsch, 2003; Fritzsch et al., 2011; Smotherman and Narins, 2004). Thus, the amniote cochlea might have arisen from a posterior sensory region, but later developed as a ventral extension of the otocyst. Although this idea is attractive, it is not congruent with recent data from chick showing that RA treatment creates a mirror-image duplicated ear with two posterior halves that either contain no cochlea at all or a rudimentary cochlear duct with no sensory tissue (Bok et al., 2011). At present, we have no truly specific early markers of the cochlea in amniotes and so we do not yet know exactly where the cochlear anlage originates, nor if such markers are also present in the posterior regions of the fish otocyst.
Establishing gradients and boundaries in the developing cochlea
The auditory sensory epithelium (the papilla or the organ of Corti) and the fluid-filled cochlea in which it is housed are both long and narrow in lizards, birds and mammals. The organs rest on the basilar membrane (see Glossary, Box 1), which is ∼25 mm long in humans and extends to over 60 mm in elephants and whales (Parks et al., 2007; Ulehlova et al., 1987; West, 1985). By contrast, the width of the sensory epithelium is narrow, ranging from only ∼0.1-0.3 mm in mammals and birds (Gleich et al., 2004; Wever et al., 1971). The dimensions of the orthogonal axes of the cochlea thus place specific temporal demands on molecular diffusion as a patterning mechanism owing to theoretical limits on the size of effective morphogen gradients (see Box 2). For example, if a point source of a diffusible morphogen forms a gradient that mediates positional information along the cochlea then it probably acts very early, when the duct is well below 1 mm in length. By contrast, the radial dimensions of vertebrate cochleae are appropriately sized to make use of diffusion-based gradients for a more extended period of their developmental history. Below, we discuss how the cochlea becomes patterned during inner ear development, focusing first on patterning along its longitudinal axis.
In adults, the longitudinal axis has significant physiological relevance: frequency selectivity in the cochlea is systematically arrayed from low (basal) to high (apical). Just prior to cochlear duct outgrowth (E11 in the mouse), it is the DV axis that corresponds to the future basal-apical longitudinal axis (compare Fig. 3B with 3C). At this time, the expression of both Ptc1 and Gli1 is graded from ventral (stronger) to dorsal (weaker) (Bok et al., 2007c). Thus, Shh signaling could impart longitudinal positional information to the progenitors of the cochlear duct many days before any overt morphological manifestations of this specification are apparent. As a result, it has been suggested that early gradients of longitudinal positional information are likely to be crude and become refined over time by more local cell-cell interactions (Mann and Kelley, 2011). This hypothesis remains to be tested.
Although longitudinal positional information is manifested as anatomical gradients in the hearing organs of birds and mammals, this is not always true among lizards. Striking evolutionary divergence among lizard basilar papillae has given rise to abrupt anatomical partitions along the longitudinal axis, the radial axis, or both (Manley, 2004). Sharp versus gradual variations in patterns are likely to utilize different mechanisms for interpreting morphogen gradients (see Box 2). With the exception of Shh, as evidenced by gradients of downstream Gli activator activity, no other morphogens have yet emerged as candidates to mediate the acquisition of positional information along the longitudinal axis.
Both gradual and abrupt morphological patterns are observed across the radial axis (the width) of the cochlear chamber in different vertebrate groups. The sharp transitions from nonsensory flanks to hair-cell-bearing sensory organs arise gradually during development. Within the sensory compartment, radial transitions between cell types can be either gradual (such as in the bird basilar papilla) or abrupt (such as in the mammalian organ of Corti, with its distinctive tunnel flanked by rows of hair cells). As discussed above, both types of pattern could be mediated through diffusible morphogens. In fact, a number of different morphogen family members make an appearance in the developing mouse cochlea as the distinct radial domains and cell rows emerge (Fig. 5).
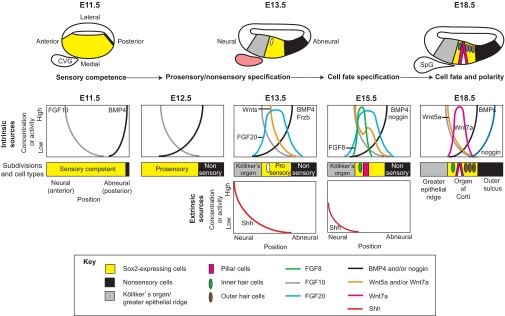
Fig. 5.
Cochlear patterning. The medial wall of the mouse cochlea undergoes sequential subdivisions under the influence of morphogen family members. Between E11.5 and E13.5, Sox2-expressing sensory-competent cells (yellow) become confined to a central domain, called the prosensory domain, and cease cell division. By E18.5, this region differentiates into the organ of Corti, which is composed of a single row of inner hair cells (green), a pair of pillar cells (pink) and three rows of outer hair cells (brown) that lie above Sox2-expressing supporting cells (yellow). The organ of Corti is flanked by two nonsensory regions: Kölliker's organ (gray) on the neural side and the outer sulcus (black) on the abneural side. Genes for several morphogens and signaling molecules, including FGFs, Wnts and BMPs, are expressed in discrete territories within the epithelium (intrinsic sources), from which they could diffuse to form gradients to mediate positional information in adjacent cells. Other signals, such as Shh, arise from nearby tissues (extrinsic sources). The cochleovestibular ganglion (CVG), for example, is an extrinsic source of Shh, which is presumed to diffuse into the epithelium to repress sensory differentiation of Kölliker's organ. The CVG will separate into a vestibular ganglion (not shown) and the spiral ganglion (SpG) of the cochlea. Shh expression is transient and is not present in the SpG on E18.5. Predicted gradients for these intrinsic and extrinsic signals are shown for the mid-base, reflecting a longitudinal location about one-quarter of the distance to the tip of the cochlea.
At E11.5, when a transverse section through the cochlear duct shows a flattened tube with a thickened medial wall that will become the sensory side, Sox2 is uniformly expressed in the medial wall of the duct under the influence of Notch signaling (Dabdoub et al., 2008). Sox2 marks a sensory-competent region (Kiernan et al., 2005) and we can borrow nomenclature used in the chicken to orient its radial dimension: the anterior edge is close to the forming cochleovestibular ganglion and is defined as the neural side, whereas the opposite, posterior edge, is defined as the abneural side. Already, asymmetric gene expression is observed, with a narrow strip of Bmp4 abneurally and a gradient of Fgf10 that peaks at the neural edge (Ohyama et al., 2010). Over the next few days, the Bmp4 domain expands and will become the outer sulcus (see Glossary, Box 1) (Morsli et al., 1998), while the Fgf10 domain sharpens, is confined to Kölliker's organ (see Glossary, Box 1), and will subsequently become the greater epithelial ridge. The region sandwiched in the middle will exit the cell cycle before the rest of the cochlear tissue to form the organ of Corti proper (Lee et al., 2006), which is composed of a single row of inner hair cells, a pair of pillar cells and three rows of outer hair cells that are nurtured by Sox2-expressing supporting cells. As we discuss below and as summarized in Fig. 5, recent studies have shown that transcripts for members of the BMP, Shh, FGF and Wnt signaling families show regional expression during key stages of subdivision and cell fate specification in the mammalian cochlea.
BMP4 and subdivision boundaries
The asymmetry in Bmp4 transcript expression within the early cochlear duct presents an opportunity for the secreted ligand to function as a morphogen. Genetic reduction of the copy number of two BMP receptors, or treatment of organ cultures with BMP activators or inhibitors, offers insight into the responsiveness of cochlear progenitors to variations in BMP signaling (Ohyama et al., 2010). Culturing E11.5 mouse cochlear explants in high concentrations of BMP4 (50 ng/ml) induces genes that mark outer sulcus fate, whereas low or no BMP signaling induces markers of Kölliker's organ. Notably, intermediate levels of BMP4 (10 ng/ml) can increase hair cell numbers. Phosphorylated Smad proteins, which are transducers of BMP signaling, show a graded pattern (high abneurally) across the abneural half of the cochlea by E13.5. Together, these data support a model in which progenitors require exposure to a moderate threshold concentration of BMP4 to acquire a prosensory fate and to a higher threshold BMP4 concentration to acquire an outer sulcus fate. If so, this would indicate that BMP4 indeed functions as a morphogen in this context. Although it is desirable to test this idea by direct removal of Bmp4 in intact animals, ubiquitously doing so causes embryonic lethality, and, unfortunately, the Foxg1cre driver commonly used to restrict conditional gene deletion to otocyst derivatives fails to prevent Bmp4 transcription in the cochlea (Chang et al., 2008). However, conditional deletion of Bmp4 in the vestibular domains of the inner ear revealed an important role in patterning both sensory and nonsensory regions of the crista ampullaris, a vestibular sensory organ (Chang et al., 2008). Thus, the jury is still out as to whether BMP4 acts to specify the prosensory domain in the cochlea, either alone or in combination with other factors.
How long can BMP4 exert its effect on regionalization of the cochlea? Hair cell numbers are modestly increased when the E12.5 cochlea is cultured for 6 days in the presence of intermediate concentrations of BMP4 (20 ng/ml) (Liu et al., 2011). Treatment of E15.5 cochlear cultures with beads soaked in a high concentration of BMP4 (40 μg/ml) locally doubles the number of outer hair cell rows, whereas treatment with noggin (Nog), a secreted BMP antagonist, represses outer hair cell differentiation (Puligilla et al., 2007), a finding suggestive of ongoing plasticity in the precise placement of the sensory/outer sulcus border. Specifically, additional outer hair cells could be explained by sliding the boundary abneurally. However, this result is counterintuitive if a high threshold of BMP4 is responsible for setting the boundary, as proposed above. Complicating the interpretation is the appearance of Nog transcripts in the outer sulcus domain by E15.5 (Bok et al., 2007a; Hwang et al., 2010), together with crossveinless 2 (or BMP-binding endothelial regulator, Bmper), which can either enhance or attenuate BMP signaling (Ambrosio et al., 2008; Serpe et al., 2008; Zhang et al., 2008). BMP4 also positively regulates its own transcription in the cochlea (Ohyama et al., 2010). Because Nog should repress BMP4 efficacy or its realm of activity, Nog removal offers an alternative approach to examine BMP gain-of-function effects. Mutant mice with conditional deletion of Nog (Nog–/–) have additional rows of both inner and outer hair cells (Hwang et al., 2010), again suggesting that the prosensory domain might be enlarged when BMP signaling is enhanced. Alternatively, extra rows can be a consequence of reduced convergence extension (Wang et al., 2005), and, indeed, the Nog–/– cochlea is 30% shorter than normal. Importantly, the number of inner and outer hair cells is decreased by 11% and 24%, respectively, in Nog–/– mice (Hwang et al., 2010), which implies the formation of a smaller prosensory domain. A reduced ratio of outer hair cells to inner hair cells further suggests that excessive BMP activity might have a greater impact on positioning the outer sulcus border than the Kölliker's organ border. Two possibilities are that the Nog gradient does not reach the border with Kölliker's organ, or that this border is already fixed when Nog is first expressed. In summary, the diffusion gradients of both BMP4 and its inhibitors may combine to fix the outer sulcus border, but it remains plastic at least until E15.5.
Shh and subdivision boundaries
On E13.5, an extrinsic source of Shh arises in the cochleovestibular ganglion, which lies just beneath the neural domain. In cultured cochleae, excess Shh reduces hair cell numbers and obviously disrupts patterning within the sensory zone and along its edges, if treatment begins before E15.5. Moreover, decreasing Shh signaling (with Gli3-truncating mutations) mildly expands the Sox2 prosensory domain at E13 (Driver et al., 2008). Whether the prosensory domain is influenced by a spatial gradient of Shh signaling that is stronger on the neural half, as shown schematically in Fig. 5, is not yet confirmed. Curiously, Gli3 mutation or treatment with Shh inhibitors yields ectopic (vestibular-type) sensory patches within Kölliker's organ, which nonetheless continues to be recognizable as a separate domain. This latter observation argues that Shh is not solely responsible for setting the boundary between Kölliker's organ and the prosensory domain. Validation of this conclusion will require analysis of mice with a conditional knockout of Shh in the ganglion, in contrast to the complete knockdown approaches currently reported.
FGFs and subdivision boundaries
The early and asymmetric expression of Fgf10 on the neural side places it in the correct position to contribute to formation of the neural boundary of the prosensory domain (Fig. 5). Furthermore, abrogation of FGF signaling yields undulating borders between the sensory domain and its nonsensory flanks, as demonstrated in the Fgfr1 knockout (Pirvola et al., 2002). However, FGF10 appears to be dispensable because Fgf10 knockout mice show unblemished cellular patterning in the cochlea (Pauley et al., 2003).
Recently, FGF20 has emerged as another ligand available to interact with FGFR1 in the developing cochlea (Hayashi et al., 2008). Unlike Fgf10 localization, Fgf20 mRNA is confined to the emerging prosensory domain from its earliest appearance on E13.5 (Hayashi et al., 2010), perhaps in response to lower concentrations of flanking morphogens (Fig. 5). In this central location, FGF20 could positively promote prosensory identity. This was suggested by treatment of cochlear explants with FGF20 function-blocking antibodies, which mimics the Fgfr1 knockout, although significant numbers of inner hair cells and pillar cells are able to differentiate under these conditions (Hayashi et al., 2008). These data are also consistent with a model in which FGF20 normally acts in opposition to the prosensory repressors on its flanks to regulate the width of the prosensory domain. In this context, it will be interesting to see whether there are spatial changes across the cochlea in BMP or Shh activities (as shown by appropriate activity reporters, for example) in Fgf20 or Fgfr1 knockout mice.
Wnts and subdivision boundaries
Wnt signaling has been implicated in regulating sensory organ size in the cochlea. Enhanced canonical Wnt signaling in the chicken cochlea generates enlarged sensory organs and the profound overproduction of hair cells (Stevens et al., 2003). However, this phenotype might result from overproliferation within a predetermined prosensory domain, rather than from a change in the position of the sensory/nonsensory boundaries. On the other hand, ectopic (vestibular) hair cells can be induced beyond the neural edge of the sensory domain by viral transduction of activated β-catenin, revealing a cell-autonomous response that might implicate Wnt signaling as instructive for sensory fate. A reporter mouse line (Lgr5EGFP/+) reveals upregulation of a Wnt target gene, leucine rich repeat containing G protein coupled receptor 5 (Lgr5), in Kölliker's organ at E15.5 in the apex (Chai et al., 2011). Development of the apex is delayed by ∼2 days relative to the mid-base (which is used as a reference point for Fig. 5). Thus, the reporter line is expected to show Wnt signaling in Kölliker's organ in the mid-base by E13.5. This overlaps with the expression domain of Wnt5a, while Wnt7a transcripts extend further into the prosensory domain (Dabdoub et al., 2003; Qian et al., 2007). At the same time, the Wnt inhibitor frizzled-related protein (Frzb) is expressed in the future outer sulcus domain (Qian et al., 2007) and might serve to steepen a Wnt activity gradient across the radial axis of the cochlea. Wnt7a transcripts are confined to the pillar cells by E16 and thus may be responsible for a concomitant shift of Wnt reporter activity to the prosensory domain (Chai et al., 2011; Dabdoub et al., 2003). To date, there is no direct evidence that a Wnt gradient helps to establish or maintain the boundaries of the prosensory domain. Instead, current evidence supports a later role for Wnt5a, Wnt7a and Frzb in the orientation of hair cell stereociliary bundles (Dabdoub et al., 2003; Dabdoub and Kelley, 2005; Qian et al., 2007).
FGFs and BMPs in cell fate specification in the organ of Corti
On E13.5, as soon as the prosensory domain becomes postmitotic, a row of inner hair cells can be identified by molecular markers (Chen et al., 2002; Montcouquiol and Kelley, 2003). Two days later, Fgf8 is upregulated in these inner hair cells (Hayashi et al., 2007). FGF8 acts through the FGFR3 receptor to promote maturation of adjacent pillar cells at the expense of outer hair cell fates (Colvin et al., 1996; Jacques et al., 2007; Mueller et al., 2002; Puligilla et al., 2007; Shim et al., 2005). The repression of outer hair cell fate by FGF is antagonized by BMP signaling (Puligilla et al., 2007), thus revealing that opposing sources of secreted ligands can act in concert to regulate the fate of cells lying between them. Finally, FGF20 plays a permissive role in the differentiation of both outer hair cells and their associated supporting cells, as initially indicated by FGF20 function-blocking antibodies (Hayashi et al., 2008). Some of these alterations in cell fate induced by treatments with secreted factors or their inhibitors can also be explored for evidence of shifts in the boundaries between subdivisions or cell types, as discussed above.
Conclusions
A puzzling feature common to developmental systems is how a small number of signaling pathways can be used iteratively in different contexts within a tissue to provide patterning information, and the ear is no exception. For example, FGF signaling is first used to induce the otic placode, later to regulate the outgrowth of semicircular canals, and later still to regulate the appearance of specific cell types in the organ of Corti. The temporal separation of these events and the changes in the transcriptional and epigenetic states of inner ear cells that are likely to occur as development proceeds allow similar sets of signals to be interpreted in different ways. The use of intracellular inhibitory feedback, such as the upregulation of Sprouty genes and pathway-specific phosphatases in response to FGF signaling (Mahoney Rogers et al., 2011; Urness et al., 2008), also allows cells to rapidly cease responding to particular signals in preparation for their next developmental choice. Physical growth and morphogenesis also allow the creation of distinct subdivisions within the inner ear that can develop independently from one another or interact only at precise physical positions (Fekete and Wu, 2002). As a result, inducing signals that act across a similarly small number of cell diameters can regulate global fate choices at early stages (such as in the initial decision to induce the entire inner ear primordium) or the formation of a single row of pillar cells in the organ of Corti at later stages.
Another issue arising when considering signaling gradients is the problem of robustness – how a diffusible signal is able to produce predictable outcomes from embryo to embryo despite unpredictable fluctuations in conditions such as temperature, genetic polymorphisms or gene dosage. Many strategies to ensure reproducibility of developmental outcomes have been proposed (Lander, 2007), and some are observed in the ear. These include the induction of positive- and negative-feedback signals to reinforce boundaries (such as the action of Notch and Sprouty genes in placode induction), the generation of opposing gradients of signals (such as Shh and Wnts in the DV axis of the otocyst), or the separation of signal-producing and signal-degrading centers across a patterning region (as in the case of RA in AP patterning of the amniote otocyst). However, other features of signaling gradients in the ear remain poorly understood. For example, the expression of BMP4 on the abneural side of the cochlear duct is accompanied by the expression of at least two secreted antagonists of BMP4, Nog and crossveinless 2. It is not clear how the presence of these inhibitors, together with the known ability of BMP4 to positively regulate its own transcription, leads to the formation of a stable gradient of BMP signaling across the cochlear duct. Both Wnt ligands and their Frzb antagonists are also expressed in the cochlear duct, as are a series of FGF family members and Sprouty antagonists. An additional level of complexity is added with the realization that different signaling pathways are likely to interact not only at the level of intracellular cross-talk, but also in the reciprocal regulation of their receptors, secreted inhibitors and signal degradation mechanisms.
It will also be interesting to determine how patterning signals in the ear are coordinated with organ growth. For example, the otocyst increases dramatically in size in amniotes during the period in which the DV and AP axes of the ear are being established. In the case of the cochlea, the prosensory cells that will generate the organ of Corti exit the cell cycle well before other cells in the cochlear duct, and therefore patterning across this region occurs in the absence of cell division (Lee et al., 2006). However, cells of the prosensory domain undergo significant radial intercalation as the cochlear duct elongates, changing from an epithelium that is five to ten cells thick to one that is one to two cells thick over a period of several days. Signaling gradients regulating cell fate choices must therefore be integrated with simultaneous changes in cell shape and position.
It should be stressed that, in the ear, much of the evidence for signaling gradients determining cell fate in a dose-dependent fashion is largely circumstantial. The existence of dose-dependent responses to secreted signals has been demonstrated in other tissues by exposing tissue uniformly to different doses of signals, or by perturbing the responses to signaling gradients in a cell-autonomous fashion. For example, if the cochlear duct is truly responding to different levels of BMP signaling in a dose-dependent fashion, one might predict that creating patches of cells within Kölliker's organ that express a constitutively active BMPR1A receptor would lead to the upregulation of outer sulcus markers in these cells but nowhere else.
In summary, the emergence of otic identity and axial asymmetry of the inner ear is mediated by extrinsic inducers that form molecular gradients across the target field. By showcasing how secreted molecules may contribute to the sequential subdivision of the mammalian cochlear epithelium across its radial dimension, we offer a glimpse into a patterning strategy that might be applied elsewhere in the inner ear to fine-tune structural variations at the tissue and cellular levels. Future discoveries should soon be forthcoming to refine the details of how morphogens act, and interact, to establish positional information and mediate cell fates in the vertebrate labyrinth.
Note added in proof
The cochlear phenotype of the Fgf20 knockout mouse (Huh et al., 2011) bears a striking resemblance to that of the Fgfr1 knockout (Hayashi et al., 2008), with the organ of Corti dispersed into a series of isolated clusters of hair cells at E13.5-19.5. Moreover, Sox2-positive cells are present in the gaps between the sensory islands, and these cells are confirmed as prosensory because they can be pushed through the differentiation process by exogenous application of FGF9, a member of the FGF20 subfamily, if treatment starts at E13.5-14.5. However, normal numbers of outer hair cells and Prox1-positive supporting cells are not fully restored. This suggests that although a continuous prosensory domain is established in the absence of Fgf20, the pool of sensory progenitors is reduced by 30-50%. In other words, the prosensory domain is presumably compressed across the radial axis. This is consistent with a role for FGF20 in setting the width of the prosensory domain through an interaction with Fgfr1.
Acknowledgments
We thank Doris Wu and Tanya Whitfield for their comments on the manuscript; David Ornitz and Tanya Whitfield for sharing results prior to publication; and John Brigande for paint-filling the mouse ears.
Footnotes
Funding
Work from the authors' laboratories described in this review was funded by the National Institutes of Health. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
References
- Abello G., Khatri S., Radosevic M., Scotting P. J., Giraldez F., Alsina B. (2010). Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Dev. Biol. 339, 166-178 [DOI] [PubMed] [Google Scholar]
- Ambrosio A. L., Taelman V. F., Lee H. X., Metzinger C. A., Coffinier C., De Robertis E. M. (2008). Crossveinless-2 Is a BMP feedback inhibitor that binds Chordin/BMP to regulate Xenopus embryonic patterning. Dev. Cell 15, 248-260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. V., Bronner-Fraser M. (2001). Vertebrate cranial placodes I. Embryonic induction. Dev. Biol. 232, 1-61 [DOI] [PubMed] [Google Scholar]
- Bertrand N., Dahmane N. (2006). Sonic hedgehog signaling in forebrain development and its interactions with pathways that modify its effects. Trends Cell Biol. 16, 597-605 [DOI] [PubMed] [Google Scholar]
- Bok J., Bronner-Fraser M., Wu D. K. (2005). Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development 132, 2115-2124 [DOI] [PubMed] [Google Scholar]
- Bok J., Brunet L. J., Howard O., Burton Q., Wu D. K. (2007a). Role of hindbrain in inner ear morphogenesis: analysis of Noggin knockout mice. Dev. Biol. 311, 69-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok J., Chang W., Wu D. K. (2007b). Patterning and morphogenesis of the vertebrate inner ear. Int. J. Dev. Biol. 51, 521-533 [DOI] [PubMed] [Google Scholar]
- Bok J., Dolson D. K., Hill P., Ruther U., Epstein D. J., Wu D. K. (2007c). Opposing gradients of Gli repressor and activators mediate Shh signaling along the dorsoventral axis of the inner ear. Development 134, 1713-1722 [DOI] [PubMed] [Google Scholar]
- Bok J., Raft S., Kong K. A., Koo S. K., Drager U. C., Wu D. K. (2011). Transient retinoic acid signaling confers anterior-posterior polarity to the inner ear. Proc. Natl. Acad. Sci. USA 108, 161-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigande J. V., Kiernan A. E., Gao X., Iten L. E., Fekete D. M. (2000). Molecular genetics of pattern formation in the inner ear: do compartment boundaries play a role? Proc. Natl. Acad. Sci. USA 97, 11700-11706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. S., Epstein D. J. (2011). Otic ablation of smoothened reveals direct and indirect requirements for Hedgehog signaling in inner ear development. Development 138, 3967-3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai R., Xia A., Wang T., Jan T. A., Hayashi T., Bermingham-McDonogh O., Cheng A. G. (2011). Dynamic expression of lgr5, a wnt target gene, in the developing and mature mouse cochlea. J. Assoc. Res. Otolaryngol. 12, 455-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers D., Mason I. (2000). Expression of sprouty2 during early development of the chick embryo is coincident with known sites of FGF signalling. Mech. Dev. 91, 361-364 [DOI] [PubMed] [Google Scholar]
- Chang W., Brigande J. V., Fekete D. M., Wu D. K. (2004). The development of semicircular canals in the inner ear: role of FGFs in sensory cristae. Development 131, 4201-4211 [DOI] [PubMed] [Google Scholar]
- Chang W., Lin Z., Kulessa H., Hebert J., Hogan B. L., Wu D. K. (2008). Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet. 4, e1000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Johnson J. E., Zoghbi H. Y., Segil N. (2002). The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 129, 2495-2505 [DOI] [PubMed] [Google Scholar]
- Chiang C., Litingtung Y., Lee E., Young K. E., Corden J. L., Westphal H., Beachy P. A. (1996). Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383, 407-413 [DOI] [PubMed] [Google Scholar]
- Choo D. (2007). The role of the hindbrain in patterning of the otocyst. Dev. Biol. 308, 257-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo D., Ward J., Reece A., Dou H., Lin Z., Greinwald J. (2006). Molecular mechanisms underlying inner ear patterning defects in kreisler mutants. Dev. Biol. 289, 308-317 [DOI] [PubMed] [Google Scholar]
- Colvin J. S., Bohne B. A., Harding G. W., McEwen D. G., Ornitz D. M. (1996). Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat. Genet. 12, 390-397 [DOI] [PubMed] [Google Scholar]
- Dabdoub A., Kelley M. W. (2005). Planar cell polarity and a potential role for a Wnt morphogen gradient in stereociliary bundle orientation in the mammalian inner ear. J. Neurobiol. 64, 446-457 [DOI] [PubMed] [Google Scholar]
- Dabdoub A., Donohue M. J., Brennan A., Wolf V., Montcouquiol M., Sassoon D. A., Hseih J. C., Rubin J. S., Salinas P. C., Kelley M. W. (2003). Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development 130, 2375-2384 [DOI] [PubMed] [Google Scholar]
- Dabdoub A., Puligilla C., Jones J. M., Fritzsch B., Cheah K. S., Pevny L. H., Kelley M. W. (2008). Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc. Natl. Acad. Sci. USA 105, 18396-18401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deol M. S. (1964). The abnormalities of the inner ear in Kreisler Mice. J. Embryol. Exp. Morphol. 12, 475-490 [PubMed] [Google Scholar]
- Dessaud E., McMahon A. P., Briscoe J. (2008). Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development 135, 2489-2503 [DOI] [PubMed] [Google Scholar]
- Driver E. C., Pryor S. P., Hill P., Turner J., Ruther U., Biesecker L. G., Griffith A. J., Kelley M. W. (2008). Hedgehog signaling regulates sensory cell formation and auditory function in mice and humans. J. Neurosci. 28, 7350-7358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C. M., Porter J. A., Chiang C., Chang D. T., Beachy P. A., Tessier-Lavigne M. (1995). Long-range sclerotome induction by sonic hedgehog: direct role of the amino-terminal cleavage product and modulation by the cyclic AMP signaling pathway. Cell 81, 457-465 [DOI] [PubMed] [Google Scholar]
- Fekete D. M. (1996). Cell fate specification in the inner ear. Curr. Opin. Neurobiol. 6, 533-541 [DOI] [PubMed] [Google Scholar]
- Fekete D. M., Wu D. K. (2002). Revisiting cell fate specification in the inner ear. Curr. Opin. Neurobiol. 12, 35-42 [DOI] [PubMed] [Google Scholar]
- Freter S., Muta Y., Mak S. S., Rinkwitz S., Ladher R. K. (2008). Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development 135, 3415-3424 [DOI] [PubMed] [Google Scholar]
- Fritzsch B. (2003). The ear of Latimeria chalumnae revisited. Zoology (Jena) 106, 243-248 [DOI] [PubMed] [Google Scholar]
- Fritzsch B., Jahan I., Pan N., Kersigo J., Duncan J., Kopecky B. (2011). Dissecting the molecular basis of organ of Corti development: where are we now? Hear. Res. 276, 16-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez F. (1998). Regionalized organizing activity of the neural tube revealed by the regulation of lmx1 in the otic vesicle. Dev. Biol. 203, 189-200 [DOI] [PubMed] [Google Scholar]
- Gleich O., Fischer F. P., Köppl C., Manley G. A. (2004). Hearing organ evolution and specialization: archosaurs. In Evolution of the Vertebrate Auditory System (ed. Manley G. A., Popper A. N., Fay R. R.), pp. 224-255 New York: Springer Verlag; [Google Scholar]
- Groves A. K. (2005). The induction of the otic placode. In Development of the Inner Ear (ed. Popper A. N., Kelley M. W., Wu D. K.), pp. 10-42 New York: Springer Verlag; [Google Scholar]
- Groves A. K., Bronner-Fraser M. (2000). Competence, specification and commitment in otic placode induction. Development 127, 3489-3499 [DOI] [PubMed] [Google Scholar]
- Haddon C., Jiang Y. J., Smithers L., Lewis J. (1998). Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development 125, 4637-4644 [DOI] [PubMed] [Google Scholar]
- Hammond K. L., Whitfield T. T. (2011). Fgf and Hh signalling act on a symmetrical pre-pattern to specify anterior and posterior identity in the zebrafish otic placode and vesicle. Development 138, 3977-3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond K. L., Loynes H. E., Folarin A. A., Smith J., Whitfield T. T. (2003). Hedgehog signalling is required for correct anteroposterior patterning of the zebrafish otic vesicle. Development 130, 1403-1417 [DOI] [PubMed] [Google Scholar]
- Hammond K. L., van Eeden F. J., Whitfield T. T. (2010). Repression of Hedgehog signalling is required for the acquisition of dorsolateral cell fates in the zebrafish otic vesicle. Development 137, 1361-1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. G. (1936). Relations of symmetry in the developing ear of amblystoma punctatum. Proc. Natl. Acad. Sci. USA 22, 238-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Cunningham D., Bermingham-McDonogh O. (2007). Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev. Dyn. 236, 525-533 [DOI] [PubMed] [Google Scholar]
- Hayashi T., Ray C. A., Bermingham-McDonogh O. (2008). Fgf20 is required for sensory epithelial specification in the developing cochlea. J. Neurosci. 28, 5991-5999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Ray C. A., Younkins C., Bermingham-McDonogh O. (2010). Expression patterns of FGF receptors in the developing mammalian cochlea. Dev. Dyn. 239, 1019-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller N., Brandli A. W. (1999). Xenopus Pax-2/5/8 orthologues: novel insights into Pax gene evolution and identification of Pax-8 as the earliest marker for otic and pronephric cell lineages. Dev. Genet. 24, 208-219 [DOI] [PubMed] [Google Scholar]
- Huh S.-H., Jones J., Warchol M. E., Ornitz D. M. (2011). Differentiation of the lateral compartment of the cochlea requires temporally restricted FGF20 signal. PLoS Biol. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C. H., Guo D., Harris M. A., Howard O., Mishina Y., Gan L., Harris S. E., Wu D. K. (2010). Role of bone morphogenetic proteins on cochlear hair cell formation: analyses of Noggin and Bmp2 mutant mice. Dev. Dyn. 239, 505-513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques B. E., Montcouquiol M. E., Layman E. M., Lewandoski M., Kelley M. W. (2007). Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development 134, 3021-3029 [DOI] [PubMed] [Google Scholar]
- Jayasena C. S., Ohyama T., Segil N., Groves A. K. (2008). Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development 135, 2251-2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan A. E., Pelling A. L., Leung K. K., Tang A. S., Bell D. M., Tease C., Lovell-Badge R., Steel K. P., Cheah K. S. (2005). Sox2 is required for sensory organ development in the mammalian inner ear. Nature 434, 1031-1035 [DOI] [PubMed] [Google Scholar]
- Kwak S. J., Phillips B. T., Heck R., Riley B. B. (2002). An expanded domain of fgf3 expression in the hindbrain of zebrafish valentino mutants results in mis-patterning of the otic vesicle. Development 129, 5279-5287 [DOI] [PubMed] [Google Scholar]
- Kwon H. J., Bhat N., Sweet E. M., Cornell R. A., Riley B. B. (2010). Identification of early requirements for preplacodal ectoderm and sensory organ development. PLoS Genet. 6, e1001133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladher R. K., Anakwe K. U., Gurney A. L., Schoenwolf G. C., Francis-West P. H. (2000). Identification of synergistic signals initiating inner ear development. Science 290, 1965-1967 [DOI] [PubMed] [Google Scholar]
- Lander A. D. (2007). Morpheus unbound: reimagining the morphogen gradient. Cell 128, 245-256 [DOI] [PubMed] [Google Scholar]
- Lander A. D., Lo W. C., Nie Q., Wan F. Y. (2009). The measure of success: constraints, objectives, and tradeoffs in morphogen-mediated patterning. Cold Spring Harb. Perspect. Biol. 1, a002022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. S., Liu F., Segil N. (2006). A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development 133, 2817-2826 [DOI] [PubMed] [Google Scholar]
- Liang J. K., Bok J., Wu D. K. (2010). Distinct contributions from the hindbrain and mesenchyme to inner ear morphogenesis. Dev. Biol. 337, 324-334 [DOI] [PubMed] [Google Scholar]
- Litsiou A., Hanson S., Streit A. (2005). A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development 132, 4051-4062 [DOI] [PubMed] [Google Scholar]
- Liu S., Li W., Chen Y., Lin Q., Wang Z., Li H. (2011). Mouse auditory organ development required bone morphogenetic protein signaling. NeuroReport 22, 396-401 [DOI] [PubMed] [Google Scholar]
- Mahoney Rogers A. A., Zhang J., Shim K. (2011). Sprouty1 and Sprouty2 limit both the size of the otic placode and hindbrain Wnt8a by antagonizing FGF signaling. Dev. Biol. 353, 94-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley G. A. (2004). The lizard basilar papilla and its evolution. In Evolution of the Vertebrate Auditory System (ed. Manley G. A., Popper A. N., Fay R. R.), pp. 200-223 New York: Springer Verlag; [Google Scholar]
- Mann Z. F., Kelley M. W. (2011). Development of tonotopy in the auditory periphery. Hear. Res. 276, 2-15 [DOI] [PubMed] [Google Scholar]
- McKay I. J., Lewis J., Lumsden A. (1996). The role of FGF-3 in early inner ear development: an analysis in normal and kreisler mutant mice. Dev. Biol. 174, 370-378 [DOI] [PubMed] [Google Scholar]
- Millimaki B. B., Sweet E. M., Dhason M. S., Riley B. B. (2007). Zebrafish atoh1 genes: classic proneural activity in the inner ear and regulation by Fgf and Notch. Development 134, 295-305 [DOI] [PubMed] [Google Scholar]
- Montcouquiol M., Kelley M. W. (2003). Planar and vertical signals control cellular differentiation and patterning in the mammalian cochlea. J. Neurosci. 23, 9469-9478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsli H., Choo D., Ryan A., Johnson R., Wu D. K. (1998). Development of the mouse inner ear and origin of its sensory organs. J. Neurosci. 18, 3327-3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K. L., Jacques B. E., Kelley M. W. (2002). Fibroblast growth factor signaling regulates pillar cell development in the organ of corti. J. Neurosci. 22, 9368-9377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K., Dolle P. (2008). Retinoic acid in development: towards an integrated view. Nat. Rev. Genet. 9, 541-553 [DOI] [PubMed] [Google Scholar]
- Ohyama T., Groves A. K. (2004). Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis 38, 195-199 [DOI] [PubMed] [Google Scholar]
- Ohyama T., Mohamed O. A., Taketo M. M., Dufort D., Groves A. K. (2006). Wnt signals mediate a fate decision between otic placode and epidermis. Development 133, 865-875 [DOI] [PubMed] [Google Scholar]
- Ohyama T., Groves A. K., Martin K. (2007). The first steps towards hearing: mechanisms of otic placode induction. Int. J. Dev. Biol. 51, 463-472 [DOI] [PubMed] [Google Scholar]
- Ohyama T., Basch M. L., Mishina Y., Lyons K. M., Segil N., Groves A. K. (2010). BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J. Neurosci. 30, 15044-15051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Nakamura K., Funahashi J., Ikeda K., Yamada G., Tokano H., Okamura H. O., Kitamura K., Muto S., Kotaki H., et al. (2004). Six1 controls patterning of the mouse otic vesicle. Development 131, 551-562 [DOI] [PubMed] [Google Scholar]
- Parks S. E., Ketten D. R., O’Malley J. T., Arruda J. (2007). Anatomical predictions of hearing in the North Atlantic right whale. Anat. Rec. (Hoboken) 290, 734-744 [DOI] [PubMed] [Google Scholar]
- Pauley S., Wright T. J., Pirvola U., Ornitz D., Beisel K., Fritzsch B. (2003). Expression and function of FGF10 in mammalian inner ear development. Dev. Dyn. 227, 203-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer P. L., Gerster T., Lun K., Brand M., Busslinger M. (1998). Characterization of three novel members of the zebrafish Pax2/5/8 family: dependency of Pax5 and Pax8 expression on the Pax2.1 (noi) function. Development 125, 3063-3074 [DOI] [PubMed] [Google Scholar]
- Pirvola U., Ylikoski J., Trokovic R., Hebert J. M., McConnell S. K., Partanen J. (2002). FGFR1 is required for the development of the auditory sensory epithelium. Neuron 35, 671-680 [DOI] [PubMed] [Google Scholar]
- Popper A. N., Fay R. R. (1999). The auditory periphery in fishes. In Comparative Hearing: Fish and Amphibians (ed. Popper A. N., Fay R. R.), pp. 43-100 New York: Springer Verlag; [Google Scholar]
- Puligilla C., Feng F., Ishikawa K., Bertuzzi S., Dabdoub A., Griffith A. J., Fritzsch B., Kelley M. W. (2007). Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev. Dyn. 236, 1905-1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D., Jones C., Rzadzinska A., Mark S., Zhang X., Steel K. P., Dai X., Chen P. (2007). Wnt5a functions in planar cell polarity regulation in mice. Dev. Biol. 306, 121-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan R. J., Tobin J. L., Beales P. L. (2008). Modeling ciliopathies: primary cilia in development and disease. Curr. Top. Dev. Biol. 84, 249-310 [DOI] [PubMed] [Google Scholar]
- Radosevic M., Robert-Moreno A., Coolen M., Bally-Cuif L., Alsina B. (2011). Her9 represses neurogenic fate downstream of Tbx1 and retinoic acid signaling in the inner ear. Development 138, 397-408 [DOI] [PubMed] [Google Scholar]
- Raft S., Nowotschin S., Liao J., Morrow B. E. (2004). Suppression of neural fate and control of inner ear morphogenesis by Tbx1. Development 131, 1801-1812 [DOI] [PubMed] [Google Scholar]
- Ribes V., Wang Z., Dolle P., Niederreither K. (2006). Retinaldehyde dehydrogenase 2 (RALDH2)-mediated retinoic acid synthesis regulates early mouse embryonic forebrain development by controlling FGF and sonic hedgehog signaling. Development 133, 351-361 [DOI] [PubMed] [Google Scholar]
- Ribes V., Stutzmann F., Bianchetti L., Guillemot F., Dolle P., Le Roux I. (2008). Combinatorial signalling controls Neurogenin2 expression at the onset of spinal neurogenesis. Dev. Biol. 321, 470-481 [DOI] [PubMed] [Google Scholar]
- Ribes V., Le Roux I., Rhinn M., Schuhbaur B., Dolle P. (2009). Early mouse caudal development relies on crosstalk between retinoic acid, Shh and Fgf signalling pathways. Development 136, 665-676 [DOI] [PubMed] [Google Scholar]
- Riccomagno M. M., Martinu L., Mulheisen M., Wu D. K., Epstein D. J. (2002). Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 16, 2365-2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno M. M., Takada S., Epstein D. J. (2005). Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 19, 1612-1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley B. B., Zhu C., Janetopoulos C., Aufderheide K. J. (1997). A critical period of ear development controlled by distinct populations of ciliated cells in the zebrafish. Dev. Biol. 191, 191-201 [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Palma V., Dahmane N. (2002). Hedgehog-Gli signalling and the growth of the brain. Nat. Rev. Neurosci. 3, 24-33 [DOI] [PubMed] [Google Scholar]
- Schimmang T. (2007). Expression and functions of FGF ligands during early otic development. Int. J. Dev. Biol. 51, 473-481 [DOI] [PubMed] [Google Scholar]
- Schlosser G. (2006). Induction and specification of cranial placodes. Dev. Biol. 294, 303-351 [DOI] [PubMed] [Google Scholar]
- Schlosser G. (2010). Making senses development of vertebrate cranial placodes. Int. Rev. Cell Mol. Biol. 283, 129-234 [DOI] [PubMed] [Google Scholar]
- Serpe M., Umulis D., Ralston A., Chen J., Olson D. J., Avanesov A., Othmer H., O’Connor M. B., Blair S. S. (2008). The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev. Cell 14, 940-953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim K., Minowada G., Coling D. E., Martin G. R. (2005). Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev. Cell 8, 553-564 [DOI] [PubMed] [Google Scholar]
- Smotherman M., Narins P. (2004). Evolution of the amphibian ear. In Evolution of the Vertebrate Auditory System (ed. Manley G. A., Popper A. N., Fay R. R.), pp. 164-199 New York: Springer Verlag; [Google Scholar]
- Stevens C. B., Davies A. L., Battista S., Lewis J. H., Fekete D. M. (2003). Forced activation of Wnt signaling alters morphogenesis and sensory organ identity in the chicken inner ear. Dev. Biol. 261, 149-164 [DOI] [PubMed] [Google Scholar]
- Streit A. (2001). Origin of the vertebrate inner ear: evolution and induction of the otic placode. J. Anat. 199, 99-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A. (2007). The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int. J. Dev. Biol. 51, 447-461 [DOI] [PubMed] [Google Scholar]
- Tanimoto M., Ota Y., Inoue M., Oda Y. (2011). Origin of inner ear hair cells: morphological and functional differentiation from ciliary cells into hair cells in zebrafish inner ear. J. Neurosci. 31, 3784-3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulehlova L., Voldrich L., Janisch R. (1987). Correlative study of sensory cell density and cochlear length in humans. Hear. Res. 28, 149-151 [DOI] [PubMed] [Google Scholar]
- Urness L. D., Li C., Wang X., Mansour S. L. (2008). Expression of ERK signaling inhibitors Dusp6, Dusp7, and Dusp9 during mouse ear development. Dev. Dyn. 237, 163-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urness L. D., Paxton C. N., Wang X., Schoenwolf G. C., Mansour S. L. (2010). FGF signaling regulates otic placode induction and refinement by controlling both ectodermal target genes and hindbrain Wnt8a. Dev. Biol. 340, 595-604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Straaten H. W., Hekking J. W., Beursgens J. P., Terwindt-Rouwenhorst E., Drukker J. (1989). Effect of the notochord on proliferation and differentiation in the neural tube of the chick embryo. Development 107, 793-803 [DOI] [PubMed] [Google Scholar]
- Waldman E. H., Castillo A., Collazo A. (2007). Ablation studies on the developing inner ear reveal a propensity for mirror duplications. Dev. Dyn. 236, 1237-1248 [DOI] [PubMed] [Google Scholar]
- Wang B., Fallon J. F., Beachy P. A. (2000). Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100, 423-434 [DOI] [PubMed] [Google Scholar]
- Wang J., Mark S., Zhang X., Qian D., Yoo S. J., Radde-Gallwitz K., Zhang Y., Lin X., Collazo A., Wynshaw-Boris A., et al. (2005). Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat. Genet. 37, 980-985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. D. (1985). The relationship of the spiral turns of the cochlea and the length of the basilar membrane to the range of audible frequencies in ground dwelling mammals. J. Acoust. Soc. Am. 77, 1091-1101 [DOI] [PubMed] [Google Scholar]
- Wever E. G., McCormick J. G., Palin J., Ridgway S. H. (1971). Cochlea of the dolphin, Tursiops truncatus: the basilar membrane. Proc. Natl. Acad. Sci. USA 68, 2708-2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield T. T., Hammond K. L. (2007). Axial patterning in the developing vertebrate inner ear. Int. J. Dev. Biol. 51, 507-520 [DOI] [PubMed] [Google Scholar]
- Wu D. K., Oh S. H. (1996). Sensory organ generation in the chick inner ear. J. Neurosci. 16, 6454-6462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D. K., Nunes F. D., Choo D. (1998). Axial specification for sensory organs versus non-sensory structures of the chicken inner ear. Development 125, 11-20 [DOI] [PubMed] [Google Scholar]
- Zhang J. L., Qiu L. Y., Kotzsch A., Weidauer S., Patterson L., Hammerschmidt M., Sebald W., Mueller T. D. (2008). Crystal structure analysis reveals how the Chordin family member crossveinless 2 blocks BMP-2 receptor binding. Dev. Cell 14, 739-750 [DOI] [PubMed] [Google Scholar]
- Zheng W., Huang L., Wei Z. B., Silvius D., Tang B., Xu P. X. (2003). The role of Six1 in mammalian auditory system development. Development 130, 3989-4000 [DOI] [PMC free article] [PubMed] [Google Scholar]