Abstract
Molecular chaperone–peptide complexes extracted from tumors (heat shock protein [HSP] vaccines) have been intensively studied in the preceding two decades, proving to be safe and effective in treating a number of malignant diseases. They offer personalized therapy and target a cross-section of antigens expressed in patients' tumors. Future advances may rely on understanding the molecular underpinnings of this approach to immunotherapy. One property common to HSP vaccines is the ability to stimulate antigen uptake by scavenger receptors on the antigen-presenting cell surface and trigger T-lymphocyte activation. HSPs can also induce signaling through Toll-Like receptors in a range of immune cells and this may mediate the effectiveness of vaccines.
Keywords: antigen presentation, dendritic cell, heat shock protein, molecular chaperone, T lymphocyte, Toll-like receptor, vaccine
Although tumors differ from their tissues of origin in terms of protein expression and express tumor-associated antigens, it is apparent that they are able to evade the immune response that their deviation may provoke [1]. Such resistance to immune killing involves mechanisms including anergy or apoptosis of T lymphocytes within tumors, downregulation of MHC class I complex molecules, escape from immunosurveillance and development of tolerance to tumor antigens [1–3]. Tolerance occurs when malignant cells present tumor antigens on their cell surface in the absence of a costimulatory signal such as that produced by a professional antigen-presenting cell (APC). Vaccination with antigenic materials obtained from the tumor is one way in which such resistance can be reversed. In this way, an immune response including generation of abundant tumor-specific cytotoxic T lymphocytes (CTLs) can be generated. Vaccination with antigenic materials from the tumor can stimulate the proliferation of T lymphocytes and activate them to kill the tumor in a specific antigen-dependent manner. Antigenicity of cancer is due to re-expressed embryonic antigens and proteins bearing alterations [4,5]. However, the nature of most of these alterations is unknown and probably differs between individuals even with tumors of similar histology. Optimal vaccines would then be individualized and built around the antigenic repertoire of the individual patient. A number of approaches offer this possibility and heat shock protein (HSP; for the purpose of this article, HSP is the general term for heat shock proteins, while individual HSPs are abbreviated as Hsp) vaccines are notable members of this group [6–8]. One question with regard to this is whether a polyvalent vaccine containing multiple, but relatively diluted, epitopes such as Gp96 purified from a tumor would be more desirable than a vaccine prepared by binding one single antigenic determinant, in high concentration to the HSP. Polyvalent vaccines against micro-organisms or human tumors have been shown to have increased effectiveness compared with vaccines with a single antigenic determinant [9–11]. However, this issue remains controversial and it has been shown that multiantigen cocktails can be inferior to preparations containing a single determinant [12]. In order for such preparations to stimulate significant immunity, HSP immunotherapy approaches need to ensure three main effects, including [13]:
A significant population of tumor responsive lymphocytes in the host under treatment
Activated T cells capable of penetrating the tumor microvasculature and encountering tumor cells
A population of fully activated T lymphocytes armed for killing tumor cells
We aim here to examine how HSP vaccines might meet these criteria.
Heat shock proteins are major components of the intracellular protein quality control pathways that protect client proteins from loss of structure and inappropriate interactions [14,15]. They are intrinsic to cellular life and are highly conserved [14]. HSPs are observed most readily in cells undergoing response to acute heat shock, when a fraction [16,17] of the proteome becomes disordered and HSP expression undergoes a massive increase to compensate for the stress. However, the deployment of the response is probably more subtle in multicellular organisms that can buffer temperature fluctuation [14,18]. Their importance is indicated by their role in deterring tissue degeneration and aging by inhibiting accumulation of damaging protein inclusions that occur in an age-dependent manner [19]. In addition, HSP expression increases in cancer in order to chaperone the overexpressed and mutant proteins that drive tumor progression [16,17].
HSP genes can be sorted into a number of families, most notably those encoding the small heat shock proteins (Hsp27 or hspb family), chaperonin 60 (hspd family), heat shock protein 70 (Hsp70 or hspa) and heat shock protein 90 (Hsp90 or hspc), which play collaborative roles in protein folding (Table 1) [20]. These HSPs can be further classified into two main groups on the basis of intracellular location, the cytoplasmic/nuclear hspa, hspb, hspc and the endoplasmic reticulum (ER) localized group (Grp78, Grp94 [also known as Gp96] and Grp170), known as glucose regulated proteins (GRPs) (Table 1) [21,22]. Although there is minimal sequence similarity between hspa and hspc gene families, the expressed proteins have each been associated with powerful immune properties [17,23,24]. Whether this is due to the role of the HSPs in chaperoning other oncogenic proteins, their high abundance and inducibility by stress or other properties is not clear: it does not, however, seem to reflect structural similarities.
Table 1.
Heat shock proteins with immune properties.
| Gene family | Common name of protein | Cellular compartment |
|---|---|---|
| hspa | Hsp70 | Cytoplasm/nucleus |
| Grp78 | ER | |
| Hsp110 | Cytoplasm | |
| Grp170 | ER | |
|
| ||
| hspb | Hsp27 | Cytoplasm |
|
| ||
| hspc | Hsp90 | Cytoplasm |
| Grp94 (Gp96) | ER | |
|
| ||
| hspd | Hsp60 | Mitochondria |
HSP vaccines: studies in the past decade
It has been apparent for a number of years that HSPs possess powerful immune properties [25]. Highly effective anticancer vaccines, that can lead to tumor regression in mice have been prepared by extracting HSP from tumors and immunizing hosts with these preparations [17,26–31]. The resulting immunogenicity was ascribed to the ability of these molecular chaperones to bind intracellular antigenic peptides and transport them into the cytoplasm of APCs which can present the antigens to CD8+ T cells [26,32,33]. HSP vaccines in their purest form are prepared by purification of Gp96 or Hsp70 peptide complexes from patients and deploying them in immunotherapy in an autologous mode [34,35]. This procedure permits direct targeting of the multiply deviated proteome that characterizes each individual neoplasm, provided the vaccine retains a faithful cross-section of the tumor antigens during HSP isolation [6,32]. This approach appears to be relatively safe in cancer therapy as indicated by Phase I and II clinical trials, and indeed recent Phase III trials of Gp96 vaccines show a response to the vaccine under more extensive courses of treatment [36,37]. The Gp96 vaccine appears successful in treatment of melanoma and renal carcinoma [36]. When compared with the results of a number of immunotherapy trials for treatment of metastatic cancer, these results are relatively encouraging [38].
Molecular chaperone mechanisms in tumor immunity
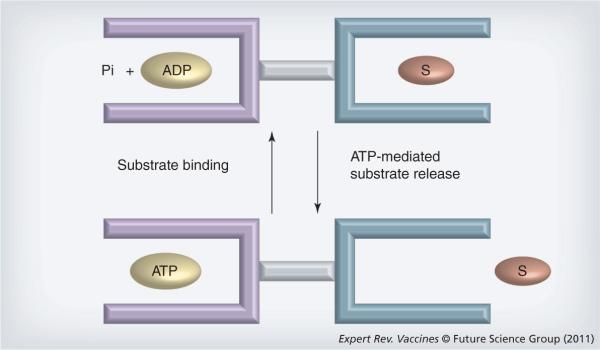
During the past decade much was learned regarding the mechanisms through which HSP vaccines function. Most immunogenic HSPs of the hspa and hspc families are able to bind polypeptides through a dedicated peptide-binding domain (Figure 1). An adjacent adenosine nucleotide-binding domain regulates the binding activities of these domains. The proteins have allosteric properties in that substrate binding mediates hydrolysis/release of adenosine nucleotides and ATP binding causes substrate release [39]. The structure of the substrate-binding domain of Hsp70 resembles the peptide-binding domain in MHC class I molecules by the criteria of sequence analysis and modeling [29]. However, structural studies of the substrate-binding domains of Hsp70 family proteins do not support the notion of a high degree of similarity [40,41]. Binding of ATP to HSP releases the substrate, while hydrolysis of ATP and ADP association with the nucleotide-binding domain causes tight binding to substrates [15,39]. HSPs can thus be thought of as peptide-binding modules that can transport antigens capable of binding MHC class I. Indeed, the studies of Binder and Srivastava indicate that Hsp70, Gp96, Hsp90 and ER stress protein calreticulin carry between them the whole of the intracellular peptide repertoire required for cross-priming T cells against ovalbumin or β-galactosidase [42].
Figure 1. Functional domains in heat shock protein A and heat shock protein C family members.
Heat shock protein (HSP)A (Hsp70, large HSP) and HSPC (Hsp90, GP96) each possess two major domains including a nucleotide (ATP, ADP) binding domain and a polypeptide (substrate) binding domain. When ATP binds to the HSP, the polypeptide-binding domain opens and cargo dissociates. When ADP binds to the nucleotide-binding domain, polypeptide cargo is bound and in this form HSP may capture and chaperone tumor antigens. These two domains, although similar in function, may differ considerably between individual HSPs. Pi: Phosphate; S: Substrate.
Other HSPs have also been attributed immune functions. Hsp60 can stimulate cytokine production when encountering monocytes and macrophages and can activate Tregs, while the small HSP family member Hsp27 appears to be immunosuppressive and reduces tumor immunity [43–46]. Little is known regarding the ER-resident HSPA family member Grp78.
HSP binding to proteins & peptides
As discussed earlier, class A and class C HSPs include peptide-binding domains that interact with infolded sequences in client proteins [15,29]. Such sequences are generally enriched in hydrophobic residues that become available when the hydrophobic cores of proteins move to the surface during the process of denaturation [47]. These interactions are probably transient and end when the proteins are refolded. Hsp70 is thought to bind 7-mer peptide sequences containing hydrophobic residues [48,49]. The degree to which residues not contained in the peptide-binding domain can `hang out' in solution is not clear although in an in vitro proteomic study, Grossman et al. showed that Hsp70 binds preferentially to peptides of 8–26-mer length [50]. This issue is discussed at length in a previous review [29]. Larger members of the HSPA family such as Hsp110 and Grp170 are evidently equipped with peptide-binding domains of greater capacity and can chaperone whole proteins in a 1:1 ratio [31,51,52]. HSPC family member Hsp90 can also bind to larger polypeptides and contributes to immunity by chaperoning peptides in vitro that can enter the pathways of antigen processing [53]. For molecular chaperone-based vaccines to be effective, the HSP must bind tenaciously to antigenic peptides. The HSP must bind to polypeptides during antigen processing in the tumor cells with sufficient avidity to form a relatively stable complex that can survive extraction during vaccine preparation, injection of HSP vaccine into host, uptake by APC and transport of the HSP–peptide complex into the sites of antigen processing/presentation during cross-priming. Peptides are known to bind tenaciously to Hsp90 during endogenous antigen processing and such polypeptides are thus protected from endopeptidases that would otherwise digest them, prior to entering the antigen presentation pathways [53–56]. One open question for the field is therefore the mechanism by which molecules such as molecular chaperones that interact transiently with clients during protein folding can form long-term complexes with peptides and perform the chaperone function within the HSP vaccine. However, HSPs have been shown to chaperone a number of self-tumor antigens such as MUC1, Melan A, Mart1, Gp100, CEA and Her2/neu[30,57–59]. It should be noted that important exceptions to this general mechanism for chaperone–substrate interaction, involving nucleotide-dependent peptide acquisition (Figure 1), exist and it has been shown that the nucleotide-binding domain of Gp96/GRP94 appears to be dispensable for peptide/client binding [60,61].
Mechanisms underlying HSP-mediated immunity: receptors, antigen cross-presentation, autoimmunity & engulfment
As mentioned earlier, HSP vaccines exploit the molecular chaperone properties of these proteins to bind antigenic peptides in vivo and carry them to APCs in vivo after injection (Figure 2). The effects of extracellular HSP on immune cells are largely mediated through cell surface receptors although some recent studies suggest the possibility of nonreceptor-mediated interactions [62,63]. It seems likely that surface receptors mediate at least two processes in HSP vaccine-mediated immunity including: first, HSP–peptide complex (HSP.PC) uptake by APCs and cross-priming of CTLs and, second, immune/inflammatory signaling. Whether these processes are carried out by one receptor or by multiple receptors specializing in endocytosis, signaling or other processes is not clear [62,64]. A family of cell surface receptors that have recently emerged as key structures in HSP binding and initiating CTLs are the scavenger receptors (SRs), including SR-A, SR-F1 (SCARF1/SREC-1), stabilin-1 and LOX-1 [33,62,65]. Hsp70 has been shown to bind SREC-I, LOX-1 and Stabilin-1 when overexpressed in CHO cells previously null for Hsp70 binding [33,62,65]. These receptors were characterized originally in endothelial cells as binding proteins for chemically modified forms of lipoproteins, such as oxidized and acetylated low-density lipoprotein in endothelial cells, and are emerging as key proteins in immunity, particularly through engulfment of apoptotic host cells and prokaryotic cells [66,67]. The different SR family members could operate in parallel in synergistic activation of immune response to HSP vaccines or may perform different cellular events in immunomodulation [68]. Indeed, recent studies indicate that both LOX-1 and SREC-I play roles in cross-presentation of ovalbumin antigens [33,64]. In addition, another group of stress protein receptors shares structural homology with LOX-1. These are the c-type lectins and include important receptors in the immune response such as Dectin-1, DC-SIGN, NKG2D and CD94 [69–74]. Hsp70 binds avidly to c-type lectins NKG2D and CD94 expressed in natural killer (NK) cells [65].
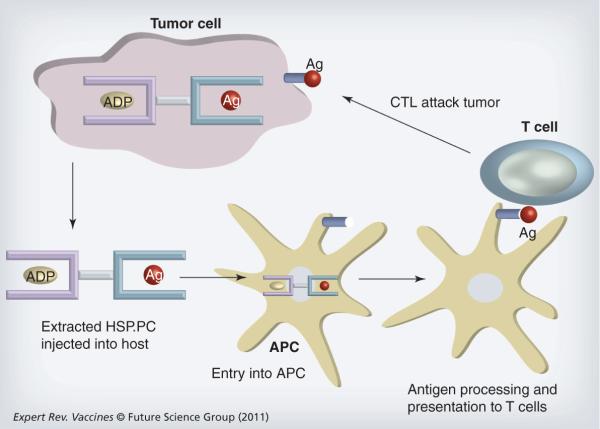
Figure 2. Heat shock protein vaccines prepared from tumor lysates.
Heat shock protein (HSP)A and HSPC family members chaperone intracellular antigens in tumor cells (see Figure 1). When tumor cells are lysed, HSP-peptide complexes (HSP.PC) can be isolated biochemically and form the basis for development of HSP vaccines. The vaccines are then injected into the periphery of tumor bearing hosts where they encounter APCs. APCs are able to take up the HSP.PC and transport them to the sites of antigen processing where the peptide antigens are proteolytically processed, loaded onto MHC class I molecules and presented on the APC surface. APCs (usually dendritic cells [DCs]) then traffic to the efferent lymph nodes where they encounter cognate CD8+ lymphocytes that recognize the tumor antigen-MHC class I complex on the DC surface through their T-cell receptors. T cells then become activated into proliferating CTLs and traffic to the tumor where, under the correct inflammatory circumstances they can extravasate, enter the tumor milieu and kill tumor cells expressing surface MHC class I–antigen complexes with high specificity.
Ag: Antigen; APC: Antigen-presenting cell; CTL: Cytotoxic T lymphocyte.
Some studies also suggest that HSPs can bind to CD91, also known as LRPs and the receptor for α2 macroglobulin. Indeed it was suggested that CD91/LRP is the common receptor for all extracellular HSPs [22]. However, although this receptor is highly expressed on macrophages, dendritic cells (DCs) do not show profound CD91 distribution [64]. In addition, later studies demonstrated that CD91 does not bind avidly to Hsp70 when overexpressed in CD91 null cells, although its role in antigen presentation and T-cell maturation appears to be profound [65,75]. It is notable that both SREC-I and CD91 possess structural homology with the Caenorhabditis elegans engulfing receptor CED-1, characterized as a receptor for apoptotic bodies [76,77]. Engulfment is likely to be an important property of receptors involved in antigen uptake by APCs and the primary step in antigen cross-presentation. It is also notable that a more closely related homolog of CED-1 has recently been discovered (MEGF10) and it will be interesting to discover whether this protein also acts as an HSP receptor [76].
Multiple HSP species were reported to interact with members of the Toll-like receptor (TLR) family [78–80]. TLRs are pattern recognition receptors that respond to exogenous danger signals, generally products of viruses or bacteria, and trigger innate immunity [16,23]. TLR activation triggers DC maturation, leading to a mature form that expresses costimulatory molecules that can interact in a productive manner with T lymphocytes [24]. Costimulatory molecules on DCs including CD80 (B7-1) and CD86 (B7.2) bind to counter-receptor CD28 on T cells simultaneously with MHC class I–T-cell receptor binding and are in most cases essential for productive T-cell activation (Figure 3) [81,82]. However, CD28 on the T-cell surface is competed for by an inhibitory molecule, CTLA-4, that is also expressed on Tregs [82]. Previous reports suggested that HSP could induce DC maturation, induce costimulatory molecules and mediate T-cell toxicity [17]. However, skepticism exists regarding some of the experiments carried out using exposure of monocytes, macrophages or DCs to recombinant HSP due to the exquisite ability of HSP to bind endotoxins, potent stimulators of innate immunity [83] (for a review, see [84]). In addition, recent reports suggest that Hsp70 can induce antigen presentation without inducing innate immunity [85]. Nonetheless we have shown that the activity of a Hsp70-based vaccine prepared from tumor–DC fusions (Hsp70.PC-F) is entirely dependent on intact TLR-2/TLR-4 signaling and that knockout of the tlr2 and tlr4 genes as well as downstream TLR pathway intermediate myd88 blocks vaccine-induced CD8+ T-cell proliferation and CTL production in MC38 tumors [86]. CTL activity could be restored to tlr2/tlr4 knockout mice by immunization with wild-type DCs that had been pulsed in vitro by Hsp70.PC-F and target cells were killed in a SREC-I-dependent and antigen (MUC1)-specific manner [86]. In addition, Massa et al. showed that endotoxin-free Hsp70 secreted from murine cells could induce DC maturation [87]. Interestingly, this effect was dependent on the presence of NK cells [87]. We have shown that Hsp70 binds avidly to NK-D receptors that are present on NK cells [65]. Additional receptors were shown to interact with mycobacterial Hsp70, including chemokine receptor CCR5 and CD40 in both DC and CD4+ T cells [88]. Mammalian Hsp70 can bind to CD40, a member of the TNF receptor family, which plays a major role in the maturation of APCs and has implications in cytokine release [89]. It will be interesting to examine the potential roles of these receptors in responses to mammalian cell-derived HSP vaccines, although recent studies seem to suggest that CD40 is not a major Hsp70 receptor [90].
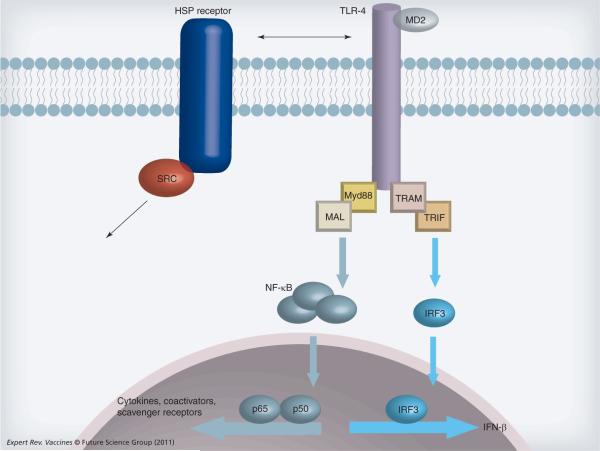
Figure 3. Heat shock protein receptors and Toll-like receptor 4 signaling.
TLR-4 (and other TLR family members) can be activated in antigen-presenting cells and other immune cells by extracellular HSP either directly or indirectly through a primary receptor such as SREC-I or LOX-1. Ligand binding then triggers the complex TLR signaling cascade. We show a highly simplified cartoon. Ligand-activated TLR-4 engages a range of intracellular adaptor proteins (Myd88, TRAM, MAL, TRIF shown here) that regulate the various arms of TLR-4 signaling. Here we show activation of transcription factors NF-κB and IRF3. NF-κB complex proteins p65 and p50 can bind the promoters of cytokine genes, co-activators and scavenger receptors to modulate the phenotype of the antigen-presenting cell and influence other cells through receptor expression and cytokine signaling. TLR-4 activation can influence other transcriptional events such as activation of IFN-β through IRF3.
HSP: Heat shock protein; IRF: Interferon regulatory factor; TLR: Toll-like receptor.
In addition to interaction with DCs, exposure to HSP modulates immune functions in other cell types. Hsp60 binds and activates TLR-2 in CD4+CD25+ regulatory T cells and binds to TLR-4 in B cells [44,91]. It is not clear whether these effects are direct or indirect and a number of studies have indicated that, at least in the case of Hsp70, there is minimal direct binding to TLR-2 or TLR-4 [64,65]. Indeed TLR-2 signaling has been shown to be activated downstream of both LOX-1 and SRECI [92]. Activation of TLR-4 in Treg cells, whether direct or indirect, appears to be a sturdy finding and not attributable to endotoxins as Hsp60 was introduced as a plasmid and expressed in situ [93].
Mechanisms of HSP-mediated antigen cross-presentation
An important property of HSP.PC binding to receptors in APCs is the ability of this interaction to foster antigen cross-presentation to T lymphocytes [23,24,42,64,94,95]. This process takes place in APCs such as DCs and macrophages and involves extracellular antigens accessing the intracellular pathways for antigen processing and subsequently eliciting CD8+ T-cell responses [86,96–98]. Antigen cross-presentation utilizes some of the mechanisms involved in the presentation of endogenous antigens. Endogenous antigen processing and presentation involves digestion of damaged or unsuccessfully translated polypeptides by the proteasome in the cytosol, recruitment of resultant peptides by transporter associated with antigen processing (TAP) in the ER and delivery to the cell surface bound to MHC class I through ER–Golgi–cell surface anterograde vesicular trafficking [99–103]. In the cytosolic pathway of antigen cross-presentation, exogenous antigens penetrate this intracellular pathway. Exogenous antigens enter this pathway after being taken up into phagosomes, transported out of the phagosomes by Sec61 and then delivered to the cytosol for proteasomal processing [104,105]. Peptides are then re-imported by TAP molecules within the phagosomes and become bound to MHC class I in this compartment [105,106]. An alternative cross-presentation pathway also exists – the phagosome/endosome to cytosol pathway – catalyzed by proteases such as cathepsin S within phagosomes and endosomes [105,106]. Processed antigensthen encounter MHC class I in recycling endosomes or phagosomes and can be loaded onto MHC class I in this compartment. This pathway excludes a requirement for antigens to cross membrane-bound vesicles and is thus potentially more rapid.
The recent findings of receptors for extracellular HSPs may point the way to novel mechanisms for HSP-mediated antigen cross-presentation. As mentioned earlier, some SR family members bind avidly to HSP and can mediate their internalization [62,65,86]. LOX-1 is expressed in DCs, binds Hsp70 and can deliver Hsp70-bound tumor-specific antigens to MHC class I, mediate antigen specific cross-presentation and activate CTLs [21]. We have found similar properties for SREC-I [33,86]. However, SR-A behaves differently to LOX-1 and SREC-I in that it represses HSP-mediated immunity [107].
HSP & the MHC class II pathway
The major mechanism for the processing of extracellular antigens is the MHC class II pathway in which antigens are engulfed and processed through the endosomal and lysosomal compartments leading to encounter of antigens with MHC class II molecules and subsequent peptide loading. Antigens may be engulfed by phagocytosis or taken up as antigen–antibody complexes through Fc receptors. Interestingly, a number of studies suggest that Gp96 and Hsp70.PC-F can facilitate tumor antigen presentation through the MHC class II pathway and activate CD4+ T cells [86,108]. Stimulation of antigen presentation through the class II pathway in DCs by HSPs may involve the participation of SREC-I or the combination of LOX-1 and CD91 [86,109]. HSP-mediated entry of antigens through the class II pathway may be important in skewing the CD4+ T-cell response towards a Th1 phenotype that may support tumor cell killing by CTLs. In addition, earlier studies indicate a role for activated CD4+ T cells in `licensing' antigen cross-presentation [110–112]. This effect is observed when both CD8+ and CD4+ T cells interact with the same APC and involves the binding of CD40 ligand on the CD4+ T cell to CD40 on the DC [110]. This phenomenon may enhance the effectiveness of HSP vaccines that are likely to be rich in both CD4+ and CD8+ peptides. Although some studies suggest that Hsp70 can bind directly to CD40, our investigations were at variance with this finding and indicated minimal Hsp70 binding to HEK293 cells transfected with CD40 expression vector [89,113].
HSP, autoimmunity & tumor immunity
HSPs can also cause autoimmunity largely due to mimicry of immunogenic prokaryotic HSPs and activation of adaptive immunity to HSP-derived epitopes [114–116]. However, mammalian or pathogen-derived HSPs can be autoregulatory due to induction of T-cell tolerance, deviation of the cytokine response from a Th1- to a Th2-promoting pattern, suppression of the inflammatory cytokine IL-17, apoptosis of CTLs and activation and expansion of Treg CD4+CD25+ cells [117–119]. Tregs inhibit immune responses through production of the inhibitory cytokine IL-10, which reduces the activity of CTLs as well as expressing the co-inhibitory molecule CTLA-4 that prevents DC maturation or kills mature DCs leading to reduced antigen presentation [120]. The outcome of HSP interaction with the immune response is thus highly complex. This complexity was anticipated in earlier studies on HSP vaccines, which indicated a biphasic HSP dose–immune response relationship with, for instance, induction of immunity by lower doses of Gp96 while higher doses elicited immune suppression [27,121]. Recent studies indicate that Gp96–HBV peptide complexes activate both a CTL response and induction of CD4+CD25+FoxP3 Tregs in a coordinate manner with the balance shifting towards Tregs with increasing chaperone dose [122]. The complexity of the immune response to HSP vaccines may thus reflect contrasting regulatory immune responses elicited by the carrier (HSP) and immunogenic responses to cargo (chaperoned peptide). Relative effectiveness of HSP-based immunotherapy may depend on the relative amounts and ratio of carrier and cargo. Recent studies by the laboratory of Richard Vile demonstrate the complexity of responses to elevated Hsp70 expression in different tissues. They have attempted to exploit common properties shared by autoimmunity and tumor immunity, by killing normal cells under inflammatory (high Hsp70) conditions that lead to autoimmune killing and measuring growth response of transplanted syngeneic tumors [123,124]. They were able to show that elevation of Hsp70 levels in tissue undergoing melanocyte killing led to inhibition of melanoma growth in a CD8+ T-lymphocyte-dependent manner but not to long-term immunity [123,125]. The transient nature of immunity induced by this protocol was due to a slightly delayed generation of Treg cells and CD8+ T-cell responses were prolonged by Treg depletion [123]. This protocol was particularly effective when applied to prostate tissue where normal prostatic cell killing by fusogenic viruses combined with Hsp70 plasmid led to immune killing of prostate tumor cells unaccompanied by Treg generation [126]. Tumor resolution was correlated with the induction of cytokines IL-6 and TGF-β and subsequent induction of IL-17. By contrast, a similar approach was ineffective in the pancreas in which fusogenic virus combined with Hsp70 elevation led to potent Treg generation and failed to destroy pancreatic tumors [98]. This discrepancy appears to be due to lack of IL-6 generation by cells in the pancreas under Hsp70 stimulation compared with contrasting effects in the prostate [98,126]. Generation of IL-6 in tissues undergoing cell death in the presence of Hsp70 thus appears to bias the immune response towards inflammatory, IL-17-dependent killing by CTLs and away from Treg generation. In studies on adjuvant arthritis it has been shown that expression of self HSPs Hsp70 and Hsp90 from injected expression plasmids can downregulate the arthritogenic T-cell response and that this effect is due to triggering release of Hsp60, a master regulator of autoimmunity, into the circulation [127]. The effects of HSPs on tumor and autoimmunity are thus both tissue and context-dependent and may involve cascades of HSP expression and release. Responses may depend on the spectrum of cytokines produced by exposure to HSP, with IFN-g and IL-12 favoring a Th1-type response, TGF-β favoring Treg production, and a combination of TGF-β and IL-6 leading to a Th17-type response [120].
HSP-induced stimulation of bystander engulfment
As discussed earlier, HSPs can bind tumor antigens and transport them into APCs [7]. In addition to this remarkable property, HSPs also appear to have the ability to stimulate the engulfment of bystander antigens that may be contained in dying mammalian cells or bacteria [87,128–130]. Indeed Todryk et al. suggested that a major role for Hsp70 in tumor immunity was to deter DC maturation and increase the engulfing capacity of these APCs [131]. This effect has not been widely studied, although Wang et al. suggest a key role for the TLR-7 pathway in stimulation of engulfment by Hsp70 [130]. As key engulfing receptors, roles for SR or CD91 in this process might be anticipated.
Present studies on HSP vaccines
HSP vaccines derived from tumor–DC fusion vaccines
We have concentrated on optimizing peptide loading in an Hsp70-based vaccine involving tumor–DC fusion and targeting breast cancer (Hsp70.PC-F or Hsp70 peptide complex from tumor–DC fusion cells). The rationale behind the Hsp70.PC-F approach is that fusion of tumor cells to DCs leads to optimal antigen processing by professional APC components and that the vaccine will contain a cross-section of tumor antigens that do not need to be further characterized [59]. Tumor–DC fusion is a commonly used approach to vaccine preparation [132]. The Hsp70.PC-F vaccine also contains coprecipitating Hsp90, which is essential for tumor regression [59]. We have also improved peptide association with the molecular chaperones in the vaccine by a rapid and gentle isolation approach. Our studies showed that rapid immunoprecipitation of Hsp70, followed by elution of the Hsp70 complexes, leads to an Hsp70 vaccine with superior immunogenicity compared with standard affinity chromatography approaches [59,86]. However, the use of the vaccine in an autologous mode may be limited due to the finite amounts of tumor tissue available and the difficulty in obtaining large amounts of DCs from cancer patients. Next we attempted to move towards developing a common Hsp70 vaccine based on the existence of overlapping antigens between tumors. Hsp70.PC-F prepared from fusions of breast cancer cells and pooled human DC contained abundant breast cancer antigens including MUC1 and Her2/neu, stimulated T-cell proliferation and killed heterologous targets provided the target shared surface antigens with the source cells for the fusion vaccine [30]. Indeed Hsp70.PC-F from human ovarian carcinoma cells was capable of killing human breast cancer SKBR3 cells that share expression of HLA-A11 [30]. Although much remains to be learned regarding the antigenic repertoires of individual tumors, our experiments suggest that bulk quantities of Hsp70.PC-F could be prepared from pooled or individual tissue culture cells (with partially characterized antigenic repertoires) fused with human DCs [30]. Another advantage of this technique is the possibility of targeting individual tumor cell populations such as cancer stem cells (CSCs; discussed later) [133,134]. We have shown the principle of targeting such a population in ovarian carcinoma [20]. We found that CD44 is a useful surface marker for CSCs in ovarian carcinoma and that T cells induced by DC–CSC fusion vaccine preferentially killed ovarian CSCs as well as resistant cells surviving radiation that are enriched in CSCs [20]. The effectiveness of Hsp70.PC-F derived from DC–CSC fusion vaccines in targeting the CSC subpopulation is currently under investigation.
Large HSPs
HSPA protein family members Hsp110 and Grp170 appear to be promising candidates for anticancer vaccines [58]. These proteins are the larger cousins of the 70 kDa Hsp70s and are characterized by the ability to bind whole proteins as well as smaller peptides in their client-binding domains. Antigens can be efficiently and quantitatively loaded onto these large HSPs and induce an impressive antitumor response [135].
Basic mechanisms involved in HSP vaccines
Much has been learned regarding the trafficking of HSP–peptide complexes into APCs and subsequent antigen cross-presentation. Many of the studies involve Hsp90 and chaperoning of ovalbumin-derived polypeptides [33,53,94]. Hsp90-mediated antigen cross-presentation involves at least three components including Hsp90, the chaperoned peptide antigen and the receptor and this is probably true for other HSPs. We have shown that Hsp90–Ova peptide complexes bind efficiently to SREC-I leading to rapid internalization via a newly characterized clathrin- and dynamin-independent endocytic pathway [33]. Other HSPs also bind this receptors, which may play a general role in binding and internalizing molecular chaperones [136,137]. Hsp90.PC-induced SREC-I uptake is cholesterol-dependent, CDC42-regulated and dynamin- and clathrin-independent in APCs [138]. These are known properties of the GEEC pathway characterized previously for GPI-anchored proteins such as CD59 and the folate receptor [139]. Our experiments showed that SREC-I mediates cross-presentation of Hsp90-bound Ova peptides after traversing this pathway. We have also shown that forced expression of SREC-I on the surface of CHO cells (normally unable to engulf or cross-present antigens) along with H2Kb (murine MHC class I) was sufficient for Ova antigen cross-presentation, confirming a specific role for SREC-I [33]. Another function for HSPs in the vaccines is the chaperoning of peptides and deterrence of proteolysis, and the studies of Shastri and Kunisawa show efficient chaperoning of Ova polypeptides by intracellular Hsp90 in the conventional pathway of antigen presentation [53].
Cell surface SREC-I appears to be localized to cell surface lipid raft domains upon ligand binding. These domains contain a number of GPI-anchored proteins and tyrosine kinases important in cell regulation. Hsp90 and other HSPs may thus carry out an additional function in antigen cross-presentation involving the triggering of signaling pathways that promote antigen uptake and other processes. Lipid raft localization suggested to us that src-family kinases might play a role in recruitment of SREC-I into the clathrin-independent pathway. Indeed Hsp90.PC was shown to activate c-src and lead to its co-localization with SREC-I [33]. In addition, src-family kinase inhibitor PP1 blocked Hsp90.PC internalization by SREC-I and subsequent antigen cross-presentation. Upon Hsp90 binding, SREC-I may thus mediate rapid formation of a signaling platform, which may increase the docking sites for other signaling molecules. MHC class I and SREC-I are relocated to the lipid raft domain compartment, marked by CD59, after Hsp90 exposure.
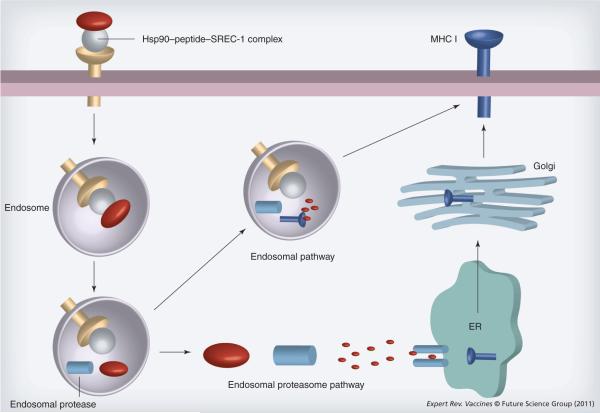
After internalization through the GEEC pathway, Hsp90–Ova complexes next traffic to early or recycling endosomes, where peptides can be loaded onto recycling MHC class I molecules [33]. Recent reports suggest that early/recycling endosomes can participate in peptide loading [94]. Our studies indicate that early endosomes and/or recycling endosomes are sites for Hsp90/SREC-I-mediated cross-presentation and endosomal cysteine protease cathepsin S was required for maximal Ova peptide generation and subsequent cross-presentation [33]. We showed that while proteasomal function and TAP are also required for efficient cross-presentation of the SIINFEKL peptide derived from full length Ova bound to Hsp90, smaller Ova peptides (C-terminally extended SIINFEKL) could be presented in a TAP-independent and lactacystin-insensitive manner. Our findings indicated complex antigen cross-presentation pathways for Hsp90-chaperoned Ova polypeptides that may depend on the size and structure of the chaperoned polypeptide (Figure 4).
Figure 4. Hsp90 and antigen cross-presentation.
Hsp90–peptide complexes bind to SREC-I and this receptor causes uptake of complexes into early endosomes. Peptides can then be processed within the endosome by proteases such as cathepsin S, encounter MHC class I molecules in recycling endosomes and transit to the cell surface. Polypeptides can also exit the endosome, be processed through the proteasome and enter the conventional pathway of antigen presentation requiring transporter associated with antigen presentation. We found that larger antigens such as full length Ova tend to utilize the latter pathway for processing. Hsp90 appears to chaperone peptides through the receptor binding step until the early endosome stage.
ER: Endoplasmic reticulum.
It was shown in vivo that in mice vaccinated with Hsp70.PC-F vaccine, SREC-I is induced and DCs expressing SREC-I traffic to the lymph nodes [86]. Vaccination led to SREC-I-dependent cross-presentation of MUC1, breaking of tolerance to this antigen and immunity to MUC1-expressing tumor cells. Under in vivo conditions, Hsp90.PC and other HSPs would probably interact with DCs expressing a range of SRs, many of which bind HSPs [64,65,136,137]. The outcome, in terms of immunity, will thus be an integral part of a number of cell surface HSP-receptor interactions; for instance, SRA-1 inhibits immune processes by blocking TLR-4 activation while SRECI and LOX-1 both activate CTL [130]. In addition, we have found that stabilin-1/FEEL 1 (a class H SR) is capable of binding to Hsp90 but fails to cross-present Ova peptide in CHO-K1 cells expressing FEEL-1 and H2Kb [Murshid A & Calderwood SK, Unpublished Data]. LOX-1 was shown previously to support cross-presentation of Ova antigens.
Future developments
Autologous anticancer vaccines
The next decade is likely to see the continued development of the autologous HSP vaccines prepared by affinity chromatography and improvements in deployment in cancer treatment. Clinical trials with Gp96-based vaccine vitespen are ongoing and suggest considerable promise for this nontoxic approach to therapy. Indeed a Gp96-based vaccine became the first cancer vaccine to be approved anywhere in the world (in Russia in this case), and is only one of the two cancer vaccines in use today [139].
The Hsp70.PC-F vaccine (described earlier) appears to be promising in preclinical studies and may offer increased tumor cell killing compared with the standard affinity purified HSP [30,59,86,140]. This is a highly flexible approach and may be used to target individual tumor populations such as CSCs [20]. Phase I clinical trials are at the planning stage. Another related approach to generating chaperone-based autologous vaccines utilizes chaperone-rich lysates (CRCLs) [141]. In this approach proteins are fractionated by isoelectric focusing and chaperone-rich fractions, which contain bound peptide antigens, are used as vaccines. The approach has the advantage of using multiple chaperones with perhaps overlapping specificities for antigen [Graner MW, Pers. Comm.]. CRCL vaccines are currently employed to treat stage IV cancer patients as part of an immunotherapy regimen conducted by Immunovative Therapies in conjunction with the National Cancer Institute of Thailand in Bangkok. These trials involve a `priming' phase using a product called AlloStim®, which contains highly activated allogeneic effector memory T cells that secrete large quantities of Th1 cytokines over a short half-life. Multiple intradermal injections of AlloStim provide a potent adjuvant stimulus that `awakens' the host immune system, leading to a Th2 to Th1 cytokine shift. Following an intravenous AlloStim dose, CRCL vaccines are included in the ensuing treatments, injecting the vaccine into the same site as the AlloStim, forming AlloVax®. The patients in this protocol have suffered no severe adverse events, have shown strong delayed-type hypersensitivity responses to CRCL, and at least one patient had stable disease despite metastatic progression up to the point of receiving the immunotherapy. Over the next 5 years, trials may be extended to a Phase II setting for metastatic breast cancer patients in Thailand, and a Phase I/II trial in the USA for patients with recurrent high-grade gliomas.
Large HSPs chaperoning common tumor antigens
Large HSP vaccines are also being developed for immunotherapy purposes. As mentioned earlier, these HSPA family members bind antigenic substrates with high affinity. For example, recombinant Hsp110 bound to Gp100 provides a highly concentrated vaccine (full length Gp100 that contains multiple MHC class I and class II determinants) and could be effective in a heterogeneous patient population, the large peptide repertoire potentially overcoming HLA restriction [31,142]. Use of this vaccine increases the possibility of polyepitope directed T- and B-cell responses. Hsp110 and Grp170 can strongly enhance the immunogenic effects of peptides and were shown to bind avidly to full length melanoma antigens TRP2 and Gp100 [135]. Use of recombinant proteins would theoretically permit an unlimited supply of vaccine. In addition, combination of chaperone complexes containing the two antigens provoked strongly enhanced inhibition of tumor growth compared with vaccines in which one antigen is chaperoned [135]. Future preclinical studies and clinical trials may thus involve the use of common tumor antigens complexed to these large HSPs and offer the use of large antigens in a highly concentrated form. A Phase I clinical trial using an Hsp110–Gp100 vaccine for treatment of melanoma in collaboration with the National Cancer Institute RAID program is imminent [Subjeck J, Pers. Comm.]. One drawback to this approach may be that such preparations would lack the personalized nature of the other approaches mentioned earlier and require some knowledge of the antigenic repertoire of target tumors. The relative merits of using a vaccine with one highly concentrated antigen as opposed to a polyvalent antigen were discussed earlier.
Combination of HSP vaccines with adjuvants & anti-CTLA antibodies
We may expect to see improvements in tumor responses by harnessing what we have learned about the biology. This may include stimulating the innate arm of the tumor immune response. Earlier studies suggested that chaperone-based vaccines may have unique properties due to the ability of HSP to chaperone antigens, stimulate immunity and activate DC maturation [7]. However, recent studies are not clear on this point and suggest that HSPs used as single agents might possess, at most, weak innate immune stimulation [79,83,143]. As DC activation in the absence of a maturation signal leads to anergy, loss of CTL activity and dominance of a Treg response, these studies might suggest combining HSP vaccine treatment with compounds with adjuvant activity to stimulate co-activator expression and CTL activity. Recently, bacterial/viral-derived oligonucleotides containing CpG motifs with PAMP activity have been shown to activate DCs through TLR-9 and play significant roles in cancer immunotherapy [144–148]. A number of reports suggest that HSP might be able to directly interact with PAMPs and stimulate innate immunity. Gp96 was shown to enhance interaction of TLR-2 ligand Pam3Cys or TLR-4 ligand LPS with DCs, stimulate synthesis of IL-12 and IL-6 and activate costimulatory molecules [149]. In addition, it has been shown recently that synthetic oligonucleotides containing CpG motifs that can activate TLR-9 bind to Hsp90 and induce synthesis of IFN-α [150]. However, binding of CpG to Hsp90 reduced synthesis of IL-6 and TNF-α, and failed to induce the DC maturation phenotype [150].
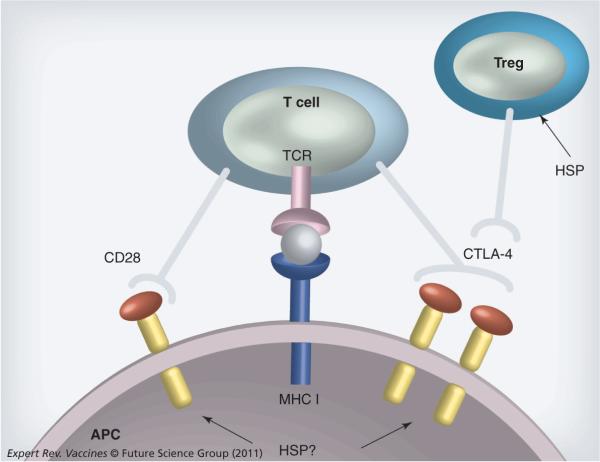
Another approach to increasing the activity of HSP vaccines might be to reduce the inhibitory effects of Tregs on APCs. Activation of a Treg response can accompany antigen cross-presentation and CTL activation, at least with Gp96-based vaccines [116,122]. Tregs have been shown to inhibit DC maturation at least partially through co-inhibitory molecule CTLA-4 that blocks DC maturation (Figure 5). Indeed a number of studies have employed anti-CTLA-4 antibodies to block the restraining influence of CTLA-4 and activate tumor immunity [151–153]. These agents, for example, Yervoy® (ipilimumab), have recently been approved for the treatment of late-stage melanoma, which may provide impetus for combining anti-Treg agents with vaccines.
Figure 5. Heat shock protein co-activation and co-repression.
Activation of a cytotoxic T lymphocyte response by APCs requires encounter of MHC class I–peptide complexes on APCs with the TCR. However, mature dendritic cells also express coreceptors that are usually required for full activation. These include Toll-like receptor-induced immunoglobulin family members CD80 and CD86 that encounter CD28 on the T-cell surface (arrows). However, T cells also express an inhibitory co-receptor CTLA-4 that can be induced by TCR ligation and inhibits cytotoxic T lymphocyte function by multiple mechanisms. Treg cells also express CTLA-4, which can disrupt APC–dendritic cell interaction by antagonizing the functions of CD80 and CD86 and blocking T-cell activation. HSPs may influence this process by altering co-activator expression or binding and triggering Tregs.
APC: Antigen-presenting cell; CTLA: Cytotoxic T-lymphocyte antigen; HSP: Heat shock protein; TCR: T-cell receptor.
Immune targeting of tumor subpopulations with HSP vaccines
It has become apparent that tumor heterogeneity is a reflection of both the cell biology and genetics of cancer. While it was accepted that tumors contain a pool of altered antigens due to an enhanced mutator phenotype, current studies suggest that tumor cell populations may contain a hierarchical ordering such as is observed in renewal tissues, including stem cells, progenitors and differentiated cells [154]. In at least some cancers, a tumor-initiating population resembling tissue stem cells (CSCs) exists often with marked resistance to standard therapies such as chemotherapy, radiotherapy and immunotherapy targeting antigens in the bulk cell population [134,155,156]. Immunotherapeutic approaches specifically targeting CSCs would thus be desirable. Recent studies indicate that CSCs from melanoma escape host immunosurveillance by stimulating immunoregulatory Treg cells, expressing increased levels of IL-10 and downregulating melanoma-associated antigens and/or MHC class I molecules [157,158]. HSP-based vaccines have the potential to target this population. We have shown that CSCs can be purified from the bulk population of ovarian or breast cancer cells using selection for surface markers then fused to DC prior to Hsp70.PC-F preparation [132]. Such Hsp70.PC-F vaccines may induce CTLs that preferentially kill cells enriched in CSC.
Combination of HSP-based immunotherapy with conventional cancer therapy
Immunotherapy is most effective in the context of minimal disease and is able to eradicate disseminated cancer, while localized treatments such as radiotherapy are highly effective in killing large numbers of cells in the primary tumor, but do not treat the metastases. Combination therapy might thus be indicated based on these criteria alone. However, localized radiation therapy offers additional attraction in being an immunogenic form of killing that can enhance the effects of immunotherapy [159,160]. Mechanisms are not currently clear but are believed to involve upregulation of MHC class I on the cancer cell surface and creation of an inflammatory environment in the tumor [161,162]. Radiation could thus reduce tolerance to tumor antigens, increase effectiveness of CD8+ CTLs, downmodulate Tregs within the tumor and permit CTLs to enter the tumor and kill malignant cells [160]. Interestingly, immunogenic killing by ionizing radiation involves upregulation of the ER stress protein calreticulin on the surface of tumor cells [163–165]. Calreticulin is a proinflammatory `eat me' signal, has been shown to carry antigenic peptides and can bind to SREC-I and SRA [136,166]. It is notable that cells surviving radiation therapy contain an expanded population of CSCs and can be targeted by Hsp70. PC-F prepared from the purified CSC [20]. We can thus envision a personalized vaccine that can target individual populations of a patient's tumor cells tailored towards enhancing the effectiveness of fractionated or large-dose radiotherapy. As mentioned earlier, HSP overexpression in tissues in the presence of cell death, at least in some tissue contexts (melanocyte, prostate cells), increases activity of CTLs, promotes CD4+ T cells and overcomes the inhibitory effects of Tregs on tumor cell killing in an IL-6-dependent manner [126]. These findings are thus encouraging for the use of combined therapy. Most individuals contain a population of CD4+ T lymphocytes that can respond to HSPs and differentiate towards an immunosuppressive Treg phenotype [25,122]. Such cells are important in regulating diseases such as arthritis, early onset diabetes and atherosclerosis [117]. Hsp70 expression in the presence of necrotic killing appears to be able to dampen the Treg response without giving rise to intolerable levels of autoimmunity [123].
Expert commentary & five-year view
Molecular chaperone–peptide complexes extracted from tumors (HSP vaccines) have been intensively studied in the preceding two decades, proving to be safe and effective in treating a number of malignant diseases. They offer personalized cancer therapy and bind a cross-section of antigens expressed in patients' tumors. Future advances will require further understanding of the molecular underpinnings of this approach to immunotherapy. One property common to HSP vaccines is the ability to stimulate antigen uptake by scavenger receptors on the APC surface and trigger T-lymphocyte activation by antigen cross-presentation. HSPs can also induce signaling through TLRs in a range of immune cells and this may mediate the effectiveness of vaccines. However, it is not clear to what degree the vaccines can activate innate immunity and induce APC maturation. Combination of molecular chaperone vaccines with adjuvants such as CpG DNA or with anti-CTLA-4 antibodies might therefore be effective in promoting APC maturation and reducing the activity of Treg cells. In addition, the vaccines may be used in combination with standard therapies such as radiotherapy. One of the hurdles in immunotherapy is the barrier to extravasation of CTLs in the tumor milieu and inflammatory killing in the tumor may provide the signals for CTLs to penetrate the tumor vasculature.
The next decade will see many of the approaches to chaperone based immunotherapy tested in clinical trials with or without accompanying combination therapies. This is likely to be an iterative process with progress or problems in the clinic provoking laboratory investigation and new laboratory findings regarding properties of molecular chaperones leading to modifications in approach to therapy.
Key issues
Heat shock protein (HSP) vaccines have undergone Phase I–III clinical trials and have proven to be safe and effective in treating a number of malignant diseases. They offer a personalized approach to therapy and target a cross-section of antigens expressed in the individual patients' tumors.
HSP vaccines could be enhanced by loading with high levels of antigens common to individual disease types or can be prepared from treatment-resistant tumor subpopulations such as cancer stem cells.
Preclinical studies indicate that HSP vaccines can stimulate effective cytotoxic lymphocytes that kill tumor cells and cause regression in vivo. However, HSP can cause accompanying activation of an HSP-selective Treg population that can limit responses. The use of chemical adjuvants such as CpG DNA or monoclonal antibodies targeting the co-inhibitor CTLA-4 may increase effectiveness in future studies.
HSP vaccines may be used in combination with other therapies such as locally applied ionizing radiation that have been shown in recent years to be immunogenic. Such standard therapies may decrease tumor burden and exert inflammatory responses in tumors that may increase vaccine effectiveness.
The cellular and molecular mechanisms underlying the effectiveness of HSP vaccines have not been fully elucidated. However, one property common to HSPs is the ability to stimulate antigen uptake by antigen-presenting cells and trigger productive interactions with T lymphocytes.
Although the effects of HSP vaccines appear to be exerted through surface receptors, no dedicated HSP receptor has been discovered. Instead a number of receptors with scavenging function appear to be involved with engulfment of HSP–peptide complexes. HSP can also induce signaling through Toll-like receptors in a range of immune cells, although such effects may be secondary to HSP binding to the scavenger receptor.
HSP can induce inflammatory cytokines in vivo in the presence of necrotic cell death, although the nature of the inflammatory cells involved is not clear.
Acknowledgements
The authors would like to thank the department of Radiation Oncology, Beth Israel Deaconess Medical Center for support and encouragement. They would also like to thank Pramod Srivastava, Michael Graner and John Subjeck for helpful discussions.
Footnotes
Financial & competing interests disclosure Stuart K Calderwood is supported by NIH research grants R01CA047407, R01CA094397 and R01CA119045. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Pardoll D. Does the immune system see tumors as foreign or self? Annu. Rev. Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 2.Chouaib S, Thiery J, Gati A, et al. Tumor escape from killing: role of killer inhibitory receptors and acquisition of tumor resistance to cell death. Tissue Antigens. 2002;60:273–281. doi: 10.1034/j.1399-0039.2002.600401.x. [DOI] [PubMed] [Google Scholar]
- 3.Moller P, Hammerling G. The role of surface HLA A, B, C molecules in tumor immunity. Cancer Surv. 1992;13:101–127. [PubMed] [Google Scholar]
- 4.Engelhard VH, Bullock TN, Colella TA, Sheasley SL, Mullins DW. Antigens derived from melanocyte differentiation proteins: self-tolerance, autoimmunity, and use for cancer immunotherapy. Immunol. Rev. 2002;188:136–146. doi: 10.1034/j.1600-065x.2002.18812.x. [DOI] [PubMed] [Google Scholar]
- 5.Disis ML, Cheever MA. Oncogenic proteins as tumor antigens. Curr. Opin. Immunol. 1996;8:637–642. doi: 10.1016/s0952-7915(96)80079-3. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava PK. Heat shock protein-based novel immunotherapies. Drug News Perspect. 2000;13:517–522. doi: 10.1358/dnp.2000.13.9.858479. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava PK, Amato RJ. Heat shock proteins: the `Swiss army knife' vaccines against cancers and infectious agents. Vaccine. 2001;19:2590–2597. doi: 10.1016/s0264-410x(00)00492-8. [DOI] [PubMed] [Google Scholar]
- 8.Calderwood SK, Gong J, Theriault JR, Mambula SS, Gray PJ., Jr. Cell stress proteins: novel immunotherapeutics. Novartis Found. Symp. 2008;291:115–131. doi: 10.1002/9780470754030.ch9. discussion 131–140. [DOI] [PubMed] [Google Scholar]
- 9.Bystryn JC, Zeleniuch-Jacquotte A, Oratz R, et al. Double-blind trial of a polyvalent, shed-antigen, melanoma vaccine. Clin. Cancer Res. 2001;7:1882–1887. [PubMed] [Google Scholar]
- 10.Sachdeva R, Banerjea AC, Malla N, Dubey ML. Immunogenicity and efficacy of single antigen Gp63, polytope and polytopeHSP70 DNA vaccines against visceral Leishmaniasis in experimental mouse model. PLoS ONE. 2009;4:e7880. doi: 10.1371/journal.pone.0007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young MD, Gooch WM, 3rd, Zuckerman AJ, et al. Comparison of a triple antigen and a single antigen recombinant vaccine for adult hepatitis B vaccination. J. Med. Virol. 2001;64:290–298. doi: 10.1002/jmv.1049. [DOI] [PubMed] [Google Scholar]
- 12.Willadsen P. Antigen cocktails: valid hypothesis or unsubstantiated hope? Trends Parasitol. 2008;24:164–167. doi: 10.1016/j.pt.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg SA. Shedding light on tumor immunotherapy of cancer. N. Engl. J. Med. 2004;350:1461–1463. doi: 10.1056/NEJMcibr045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindquist S, Craig EA. The heat shock proteins. Ann. Rev. Genet. 1988;22:631–637. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 15.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol. Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray PJ, Jr, Prince T, Cheng J, Stevenson MA, Calderwood SK. Targeting the oncogene and kinome chaperone CDC37. Nat. Rev. Cancer. 2008;8:491–495. doi: 10.1038/nrc2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noessner E, Gastpar R, Milani V, et al. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J. Immunol. 2002;169:5424–5432. doi: 10.4049/jimmunol.169.10.5424. [DOI] [PubMed] [Google Scholar]
- 18.Subjeck JR, Sciandra JJ, Johnson RJ. Heat shock proteins and thermotolerance; a comparison of induction kinetics. Br. J. Radiol. 1982;55:579–584. doi: 10.1259/0007-1285-55-656-579. [DOI] [PubMed] [Google Scholar]
- 19.Calderwood SK, Murshid A, Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging – a mini-review. Gerontology. 2009;55(5):550–558. doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weng D, Song B, Durfee J, et al. Induction of cytotoxic T lymphocytes against ovarian cancer-initiating cells. Int. J. Cancer. 2011 doi: 10.1002/ijc.25851. DOI: 10.1002/ijc.25851. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Kampinga HH, Hageman J, Vos MJ, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei J, Hendershot LM. Protein folding and assembly in the endoplasmic reticulum. EXS. 1996;77:41–55. doi: 10.1007/978-3-0348-9088-5_4. [DOI] [PubMed] [Google Scholar]
- 23.Singh-Jasuja H, Toes RE, Spee P, et al. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J. Exp. Med. 2000;191:1965–1974. doi: 10.1084/jem.191.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu. Rev. Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]; •• Summarizes the earlier studies on molecular chaperone vaccines and presents a lucid introduction to the field.
- 25.Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory roles of stress proteins. Trends Biochem. Sci. 2008;33:71–79. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava PK. Immunotherapy for human cancer using heat shock protein-peptide complexes. Curr. Oncol. Rep. 2005;7:104–108. doi: 10.1007/s11912-005-0035-8. [DOI] [PubMed] [Google Scholar]
- 27.Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J. Exp. Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]; • One of the seminal studies that established chaperone vaccines as a viable approach to cancer treatment.
- 28.Murshid A, Gong J, Calderwood SK. Heat-shock proteins in cancer vaccines: agents of antigen cross-presentation. Expert Rev. Vaccines. 2008;7(7):1019–1030. doi: 10.1586/14760584.7.7.1019. [DOI] [PubMed] [Google Scholar]
- 29.Calderwood SK, Theriault JR, Gong J. Message in a bottle: role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur. J. Immunol. 2005;35:2518–2527. doi: 10.1002/eji.200535002. [DOI] [PubMed] [Google Scholar]
- 30.Gong J, Zhang Y, Durfee J, et al. A heat shock protein 70-based vaccine with enhanced immunogenicity for clinical use. J. Immunol. 2010;184:488–496. doi: 10.4049/jimmunol.0902255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manjili MH, Henderson R, Wang XY, et al. Development of a recombinant HSP110-HER-2/neu vaccine using the chaperoning properties of HSP110. Cancer Res. 2002;62:1737–1742. [PubMed] [Google Scholar]
- 32.Srivastava PK, Maki RG. Stress-induced proteins in immune response to cancer. Curr. Top Microbiol. Immunol. 1991;167:109–123. doi: 10.1007/978-3-642-75875-1_7. [DOI] [PubMed] [Google Scholar]
- 33.Murshid A, Gong J, Calderwood SK. Heat shock protein 90 mediates efficient antigen cross-presentation through the scavenger receptor expressed by endothelial cells-I. J. Immunol. 2010;185:2903–2917. doi: 10.4049/jimmunol.0903635. [DOI] [PMC free article] [PubMed] [Google Scholar]; • One of the seminal studies that established chaperone vaccines as agents of antigen cross-presentation and details the endocytosis pathway taken by Hsp90–antigen–receptor complexes in dendritic cells leading to effcient presentation to CD8+ cells.
- 34.Belli F, Testori A, Rivoltini L, et al. Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein Gp96–peptide complexes: clinical and immunologic findings. J. Clin. Oncol. 2002;20:4169–4180. doi: 10.1200/JCO.2002.09.134. [DOI] [PubMed] [Google Scholar]
- 35.Mazzaferro V, Coppa J, Carrabba MG, et al. Vaccination with autologous tumor-derived heat-shock protein Gp96 after liver resection for metastatic colorectal cancer. Clin. Cancer Res. 2003;9:3235–3245. [PubMed] [Google Scholar]
- 36.di Pietro A, Tosti G, Ferrucci PF, Testori A. Heat shock protein peptide complex 96-based vaccines in melanoma: how far we are, how far we can get. Hum. Vaccin. 2009;5(11):727–737. [Google Scholar]
- 37.Srivastava PK. Therapeutic cancer vaccines. Curr. Opin. Immunol. 2006;18:201–205. doi: 10.1016/j.coi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 40.Morshauser RC, Wang H, Flynn GC, Zuiderweg ER. The peptide-binding domain of the chaperone protein Hsc70 has an unusual secondary structure topology. Biochemistry. 1995;34:6261–6266. doi: 10.1021/bi00019a001. [DOI] [PubMed] [Google Scholar]
- 41.Zhu X, Zhao X, Burkholder WF, et al. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binder RJ, Srivastava PK. Essential role of CD91 in re-presentation of Gp96-chaperoned peptides. Proc. Natl Acad. Sci. USA. 2004;101:6128–6133. doi: 10.1073/pnas.0308180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vabulas RM, Ahmad-Nejad P, da Costa C, et al. Endocytosed HSP60s use Toll-like receptor 2 (TLR2) and TLR4 to activate the Toll/interleukin-1 receptor signaling pathway in innate immune cells. J. Biol. Chem. 2001;276:31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- 44.Zanin-Zhorov A, Cahalon L, Tal G, et al. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J. Clin. Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Miller-Graziano CL, De A, Laudanski K, Herrmann T, Bandyopadhyay S. HSP27: an anti-inflammatory and immunomodulatory stress protein acting to dampen immune function. Novartis Found. Symp. 2008;291:196–208. 221–194. doi: 10.1002/9780470754030.ch15. discussion 208–111. [DOI] [PubMed] [Google Scholar]
- 46.Banerjee S, Lin CF, Skinner KA, et al. Heat shock protein 27 differentiates tolerogenic macrophages that may support human breast cancer progression. Cancer Res. 2010;71:318–327. doi: 10.1158/0008-5472.CAN-10-1778. [DOI] [PubMed] [Google Scholar]
- 47.Ellis RJ. Protein misassembly: macromolecular crowding and molecular chaperones. Adv. Exp. Med. Biol. 2007;594:1–13. doi: 10.1007/978-0-387-39975-1_1. [DOI] [PubMed] [Google Scholar]
- 48.Fourie AM, Sambrook JF, Gething MJ. Common and divergent peptide binding specificities of Hsp70 molecular chaperones. J. Biol. Chem. 1994;269:30470–30478. [PubMed] [Google Scholar]
- 49.Wu SJ, Wang C. Binding of heptapeptides or unfolded proteins to the chimeric C-terminal domains of 70kDa heaty shock cognate protein. Eur. J. Biochem. 1999;259:449–455. doi: 10.1046/j.1432-1327.1999.00073.x. [DOI] [PubMed] [Google Scholar]
- 50.Grossmann ME, Madden BJ, Gao F, et al. Proteomics shows Hsp70 does not bind peptide sequences indiscriminately in vivo. Exp. Cell Res. 2004;297:108–117. doi: 10.1016/j.yexcr.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 51.Park J, Easton DP, Chen X, et al. The chaperoning properties of mouse grp170, a member of the third family of Hsp70 related proteins. Biochemistry. 2003;42:14893–14902. doi: 10.1021/bi030122e. [DOI] [PubMed] [Google Scholar]
- 52.Wang XY, Kazim L, Repasky EA, Subjeck JR. Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity. J. Immunol. 2001;166:490–497. doi: 10.4049/jimmunol.166.1.490. [DOI] [PubMed] [Google Scholar]
- 53.Kunisawa J, Shastri N. Hsp90a chaperones large C-terminally extended proteolytic intermediates in the MHC class I antigen processing pathway. Immunity. 2006;24:523–534. doi: 10.1016/j.immuni.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 54.Lev A, Takeda K, Zanker D, et al. The exception that reinforces the rule: crosspriming by cytosolic peptides that escape degradation. Immunity. 2008;28:787–798. doi: 10.1016/j.immuni.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reits E, Neijssen J, Herberts C, et al. A major role for TPPII in trimming proteasomal degradation products for MHC class I antigen presentation. Immunity. 2004;20:495–506. doi: 10.1016/s1074-7613(04)00074-3. [DOI] [PubMed] [Google Scholar]
- 56.Yewdell JW. The seven dirty little secrets of major histocompatibility complex class I antigen processing. Immunol. Rev. 2005;207:8–18. doi: 10.1111/j.0105-2896.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- 57.Rivoltini L, Castelli C, Carrabba M, et al. Human tumor-derived heat shock protein 96 mediates in vitro activation and in vivo expansion of melanoma- and colon carcinoma-specific T cells. J. Immunol. 2003;171:3467–3474. doi: 10.4049/jimmunol.171.7.3467. [DOI] [PubMed] [Google Scholar]
- 58.Wang XY, Kazim L, Repasky EA, Subjeck JR. Immunization with tumor-derived ER chaperone grp170 elicits tumor-specific CD8+ T-cell responses and reduces pulmonary metastatic disease. Int. J. Cancer. 2003;105:226–231. doi: 10.1002/ijc.11058. [DOI] [PubMed] [Google Scholar]
- 59.Enomoto Y, Bharti A, Khaleque AA, et al. Enhanced immunogenicity of heat shock protein 70 peptide complexes from dendritic cell–tumor fusion cells. J. Immunol. 2006;177:5946–5955. doi: 10.4049/jimmunol.177.9.5946. [DOI] [PubMed] [Google Scholar]
- 60.Nicchitta CV, Carrick DM, Baker-Lepain JC. The messenger and the message: Gp96 (GRP94)–peptide interactions in cellular immunity. Cell Stress Chaperones. 2004;9:325–331. doi: 10.1379/CSC-62.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosser MF, Trotta BM, Marshall MR, Berwin B, Nicchitta CV. Adenosine nucleotides and the regulation of GRP94–client protein interactions. Biochemistry. 2004;43:8835–8845. doi: 10.1021/bi049539q. [DOI] [PubMed] [Google Scholar]
- 62.Calderwood SK, Theriault J, Gray PJ, Gong J. Cell surface receptors for molecular chaperones. Methods. 2007;43:199–206. doi: 10.1016/j.ymeth.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 63.Jockheck-Clark AR, Bowers EV, Totonchy MB, et al. Re-examination of CD91 function in GRP94 (glycoprotein 96) surface binding, uptake, and peptide cross-presentation. J. Immunol. 2010;185:6819–6830. doi: 10.4049/jimmunol.1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Delneste Y, Magistrelli G, Gauchat J, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]; •• Very thorough and convincing study showing that scavenger receptor LOX-1 can bind Hsp70–peptide complexes in antigen-presenting cells and mediate antigen cross-presentation.
- 65.Theriault JR, Adachi H, Calderwood SK. Role of scavenger receptors in the binding and internalization of heat shock protein 70. J. Immunol. 2006;177:8604–8611. doi: 10.4049/jimmunol.177.12.8604. [DOI] [PubMed] [Google Scholar]; • Screen for Hsp70 receptors shows that scavenger receptors LOX-1, SREC-1 and Stablin-1 and c-type lectins NKG2D and CD94 bind Hsp70.
- 66.Pluddemann A, Hoe JC, Makepeace K, Moxon ER, Gordon S. The macrophage scavenger receptor A is host-protective in experimental meningococcal septicaemia. PLoS Pathog. 2009;5:e1000297. doi: 10.1371/journal.ppat.1000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pluddemann A, Neyen C, Gordon S. Macrophage scavenger receptors and host-derived ligands. Methods. 2007;43:207–217. doi: 10.1016/j.ymeth.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 68.Gray PJ, Jr, Stevenson MA, Calderwood SK. Targeting Cdc37 inhibits multiple signaling pathways and induces growth arrest in prostate cancer cells. Cancer Res. 2007;67:11942–11950. doi: 10.1158/0008-5472.CAN-07-3162. [DOI] [PubMed] [Google Scholar]
- 69.Cambi A, Figdor C. Necrosis: C-type lectins sense cell death. Curr. Biol. 2009;19:R375–R378. doi: 10.1016/j.cub.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 70.den Dunnen J, Gringhuis SI, Geijtenbeek TB. Innate signaling by the C-type lectin DC-SIGN dictates immune responses. Cancer Immunol. Immunother. 2009;58:1149–1157. doi: 10.1007/s00262-008-0615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dominguez-Soto A, Corbi AL. Myeloid dendritic cell lectins and their role in immune responses. Curr. Opin. Investig. Drugs. 2007;8:910–920. [PubMed] [Google Scholar]
- 72.Graham LM, Brown GD. The Dectin-2 family of C-type lectins in immunity and homeostasis. Cytokine. 2009;48:148–155. doi: 10.1016/j.cyto.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat. Immunol. 2006;7:1258–1265. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- 74.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol. Cell Biol. 2010;89:216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 75.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins Gp96, Hsp90, Hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]; • First report of a surface receptor for heat-shock proteins, the low-density lipoprotein receptor CD91.
- 76.Suzuki E, Nakayama M. MEGF10 is a mammalian ortholog of CED-1 that interacts with clathrin assembly protein complex 2 medium chain and induces large vacuole formation. Exp. Cell Res. 2007;313:3729–3742. doi: 10.1016/j.yexcr.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 77.Zhou Z, Hartwieg E, Horvitz HR. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell. 2001;104:43–56. doi: 10.1016/s0092-8674(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 78.Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat. Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 79.Vabulas RM, Ahmad-Nejad P, Ghose S, et al. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 80.Vabulas RM, Wagner H. Toll-like receptor-dependent activation of antigen presenting cells by Hsp60, Gp96 and Hsp70. In: Henderson B, Pockley AG, editors. Molecular Chaperones & Cell Signalling. Cambridge University Press; Cambridge, UK: 2005. pp. 113–133. [Google Scholar]
- 81.Reis e Sousa C. Dendritic cells in a mature age. Nat. Rev. Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 82.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao B, Tsan MF. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor α release by murine macrophages. J. Biol. Chem. 2003;278:174–179. doi: 10.1074/jbc.M208742200. [DOI] [PubMed] [Google Scholar]
- 84.Henderson B, Calderwood SK, Coates AR, et al. Caught with their PAMPs down? The extracellular signalling actions of molecular chaperones are not due to microbial contaminants. Cell Stress Chaperones. 2009;15(2):123–141. doi: 10.1007/s12192-009-0137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bendz H, Ruhland SC, Pandya MJ, et al. Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. J. Biol. Chem. 2007;282:31688–31702. doi: 10.1074/jbc.M704129200. [DOI] [PubMed] [Google Scholar]
- 86.Gong J, Zhu B, Murshid A, et al. T cell activation by heat shock protein 70 vaccine requires TLR signaling and scavenger receptor expressed by endothelial cells-1. J. Immunol. 2009;183(5):3092–3098. doi: 10.4049/jimmunol.0901235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Massa C, Melani C, Colombo MP. Chaperon and adjuvant activity of Hsp70: different natural killer requirement for cross-priming of chaperoned and bystander antigens. Cancer Res. 2005;65:7942–7949. doi: 10.1158/0008-5472.CAN-05-0377. [DOI] [PubMed] [Google Scholar]
- 88.Pido-Lopez J, Whittall T, Wang Y, et al. Stimulation of cell surface CCR5 and CD40 molecules by their ligands or by HSP70 up-regulates APOBEC3G expression in CD4+ T cells and dendritic cells. J. Immunol. 2007;178:1671–1679. doi: 10.4049/jimmunol.178.3.1671. [DOI] [PubMed] [Google Scholar]
- 89.Becker T, Hartl FU, Wieland F. CD40, an extracellular receptor for binding and uptake of Hsp70–peptide complexes. J. Cell Biol. 2002;158:1277–1285. doi: 10.1083/jcb.200208083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Binder RJ. CD40-independent engagement of mammalian Hsp70 by antigen-presenting cells. J. Immunol. 2009;182:6844–6850. doi: 10.4049/jimmunol.0900026. [DOI] [PubMed] [Google Scholar]
- 91.Cohen-Sfady M, Nussbaum G, Pevsner-Fischer M, et al. Heat shock protein 60 activates B cells via the TLR4–MyD88 pathway. J. Immunol. 2005;175:3594–3602. doi: 10.4049/jimmunol.175.6.3594. [DOI] [PubMed] [Google Scholar]
- 92.Jeannin P, Bottazzi B, Sironi M, et al. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 2005;22:551–560. doi: 10.1016/j.immuni.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 93.Quintana FJ, Cohen IR. Heat shock proteins regulate inflammation by both molecular and network cross-reactivity. Cambridge University Press; Cambridge, UK: 2005. [Google Scholar]
- 94.Kurotaki T, Tamura Y, Ueda G, et al. Efficient cross-presentation by heat shock protein 90–peptide complex-loaded dendritic cells via an endosomal pathway. J. Immunol. 2007;179:1803–1813. doi: 10.4049/jimmunol.179.3.1803. [DOI] [PubMed] [Google Scholar]
- 95.Arnold-Schild D, Hanau D, Spehner D, et al. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J. Immunol. 1999;162:3757–3760. [PubMed] [Google Scholar]
- 96.Arnold D, Faath S, Rammensee H, Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein Gp96. J. Exp. Med. 1995;182:885–889. doi: 10.1084/jem.182.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nicchitta CV. Biochemical, cell biological and immunological issues surrounding the endoplasmic reticulum chaperone GRP94/Gp96. Curr. Opin. Immunol. 1998;10:103–109. doi: 10.1016/s0952-7915(98)80039-3. [DOI] [PubMed] [Google Scholar]
- 98.Kottke T, Pulido J, Thompson J, et al. Antitumor immunity can be uncoupled from autoimmunity following heat shock protein 70-mediated inflammatory killing of normal pancreas. Cancer Res. 2009;69(19):7767–7774. doi: 10.1158/0008-5472.CAN-09-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 100.Ortmann B, Copeman J, Lehner PJ, et al. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science. 1997;277:1306–1309. doi: 10.1126/science.277.5330.1306. [DOI] [PubMed] [Google Scholar]
- 101.Rock KL, York IA, Saric T, Goldberg AL. Protein degradation and the generation of MHC class I-presented peptides. Adv. Immunol. 2002;80:1–70. doi: 10.1016/s0065-2776(02)80012-8. [DOI] [PubMed] [Google Scholar]
- 102.Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5:103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 103.Solheim JC, Carreno BM, Hansen TH. Are transporter associated with antigen processing (TAP) and tapasin class I MHC chaperones? J. Immunol. 1997;158:541–543. [PubMed] [Google Scholar]
- 104.Rock KL, Farfan-Arribas DJ, Shen L. Proteases in MHC class I presentation and cross-presentation. J. Immunol. 2010;184:9–15. doi: 10.4049/jimmunol.0903399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol. Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 106.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25:607–617. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 107.Wang XY, Facciponte J, Chen X, Subjeck JR, Repasky EA. Scavenger receptor-A negatively regulates antitumor immunity. Cancer Res. 2007;67:4996–5002. doi: 10.1158/0008-5472.CAN-06-3138. [DOI] [PubMed] [Google Scholar]
- 108.Doody AD, Kovalchin JT, Mihalyo MA, et al. Glycoprotein 96 can chaperone both MHC class I- and class II-restricted epitopes for in vivo presentation, but selectively primes CD8+ T cell effector function. J. Immunol. 2004;172:6087–6092. doi: 10.4049/jimmunol.172.10.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matsutake T, Sawamura T, Srivastava PK. High efficiency CD91- and LOX-1-mediated re-presentation of Gp96-chaperoned peptides by MHC II molecules. Cancer Immun. 2010;10:7. [PMC free article] [PubMed] [Google Scholar]
- 110.Bennett SR, Carbone FR, Karamalis F, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 111.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kurts C, Robinson BW, Knolle PA. Cross-priming in health and disease. Nat. Rev. Immunol. 2010;10:403–414. doi: 10.1038/nri2780. [DOI] [PubMed] [Google Scholar]
- 113.Theriault JR, Mambula SS, Sawamura T, Stevenson MA, Calderwood SK. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett. 2005;579:1951–1960. doi: 10.1016/j.febslet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 114.Blasi C. The autoimmune origin of atherosclerosis. Atherosclerosis. 2008;201:17–32. doi: 10.1016/j.atherosclerosis.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 115.Puga Yung GL, Fidler M, Albani E, et al. Heat shock protein-derived T-cell epitopes contribute to autoimmune inflammation in pediatric Crohn's disease. PLoS ONE. 2009;4:e7714. doi: 10.1371/journal.pone.0007714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rajaiah R, Moudgil KD. Heat-shock proteins can promote as well as regulate autoimmunity. Autoimmun. Rev. 2009;8:388–393. doi: 10.1016/j.autrev.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat. Rev. Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- 118.Wieten L, Berlo SE, Ten Brink CB, et al. IL-10 is critically involved in mycobacterial HSP70 induced suppression of proteoglycan-induced arthritis. PLoS ONE. 2009;4:e4186. doi: 10.1371/journal.pone.0004186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Alard JE, Dueymes M, Youinou P, Jamin C. Modulation of endothelial cell damages by anti-Hsp60 autoantibodies in systemic autoimmune diseases. Autoimmun. Rev. 2007;6:438–443. doi: 10.1016/j.autrev.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 120.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 2010;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chandawarkar RY, Wagh MS, Srivastava PK. The dual nature of specific immunological activity of tumor-derived Gp96 preparations. J. Exp. Med. 1999;189:1437–1442. doi: 10.1084/jem.189.9.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu Z, Li X, Qiu L, et al. Treg suppress CTL responses upon immunization with HSP Gp96. Eur. J. Immunol. 2009;39:3110–3120. doi: 10.1002/eji.200939593. [DOI] [PubMed] [Google Scholar]
- 123.Daniels GA, Sanchez-Perez L, Diaz RM, et al. A simple method to cure established tumors by inflammatory killing of normal cells. Nat. Biotechnol. 2004;22:1125–1132. doi: 10.1038/nbt1007. [DOI] [PubMed] [Google Scholar]; •• Fascinating paper showing that expression of Hsp70 in normal melanocytes in vivo, accompanied by necrotic killing of these cells can lead to killing of malignant cells (melanoma) sharing common antigens by cytotoxic T lymphocytes.
- 124.Calderwood SK. Chaperones and slow death – a recipe for tumor immunotherapy. Trends Biotechnol. 2005;23:57–59. doi: 10.1016/j.tibtech.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 125.Sanchez-Perez L, Kottke T, Daniels GA, et al. Killing of normal melanocytes, combined with heat shock protein 70 and CD40L expression, cures large established melanomas. J. Immunol. 2006;177:4168–4177. doi: 10.4049/jimmunol.177.6.4168. [DOI] [PubMed] [Google Scholar]
- 126.Kottke T, Sanchez-Perez L, Diaz RM, et al. Induction of Hsp70-mediated Th17 autoimmunity can be exploited as immunotherapy for metastatic prostate cancer. Cancer Res. 2007;67:11970–11979. doi: 10.1158/0008-5472.CAN-07-2259. [DOI] [PubMed] [Google Scholar]
- 127.Van Eden W, Wick G, Albani S, Cohen I. Stress, heat shock proteins, and autoimmunity: how immune responses to heat shock proteins are to be used for the control of chronic inflammatory diseases. Ann. NY Acad. Sci. 2007;1113:217–237. doi: 10.1196/annals.1391.020. [DOI] [PubMed] [Google Scholar]
- 128.Massa C, Guiducci C, Arioli I, et al. Enhanced efficacy of tumor cell vaccines transfected with secretable Hsp70. Cancer Res. 2004;64:1502–1508. doi: 10.1158/0008-5472.can-03-2936. [DOI] [PubMed] [Google Scholar]
- 129.Wang R, Kovalchin JT, Muhlenkamp P, Chandawarkar RY. Exogenous heat shock protein 70 binds macrophage lipid raft microdomain and stimulates phagocytosis, processing, and MHC-II presentation of antigens. Blood. 2006;107:1636–1642. doi: 10.1182/blood-2005-06-2559. [DOI] [PubMed] [Google Scholar]
- 130.Wang R, Town T, Gokarn V, Flavell RA, Chandawarkar RY. HSP70 enhances macrophage phagocytosis by interaction with lipid raft-associated TLR-7 and upregulating p38 MAPK and PI3K pathways. J. Surg. Res. 2006;136:58–69. doi: 10.1016/j.jss.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 131.Todryk S, Melcher AA, Hardwick N, et al. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J. Immunol. 1999;163:1398–1408. [PubMed] [Google Scholar]
- 132.Gong J, Koido S, Calderwood SK. Cell fusion: from hybridoma to dendritic cell-based vaccine. Expert Rev. Vaccines. 2008;7(7):1055–1068. doi: 10.1586/14760584.7.7.1055. [DOI] [PubMed] [Google Scholar]
- 133.Kashyap V, Rezende NC, Scotland KB, et al. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev. 2009;18:1093–1108. doi: 10.1089/scd.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 135.Wang XY, Sun X, Chen X, et al. Superior antitumor response induced by large stress protein chaperoned protein antigen compared with peptide antigen. J. Immunol. 2010;184:6309–6319. doi: 10.4049/jimmunol.0903891. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Shows the efficacy of large heat-shock proteins coupled to antigenic proteins as effective vaccines in melanoma.
- 136.Berwin B, Delneste Y, Lovingood RV, Post SR, Pizzo SV. SREC-I, a type F scavenger receptor, is an endocytic receptor for calreticulin. J. Biol. Chem. 2004;279:51250–51257. doi: 10.1074/jbc.M406202200. [DOI] [PubMed] [Google Scholar]
- 137.Facciponte JG, Wang XY, Subjeck JR. Hsp110 and Grp170, members of the Hsp70 superfamily, bind to scavenger receptor-A and scavenger receptor expressed by endothelial cells-I. Eur. J. Immunol. 2007;37:2268–2279. doi: 10.1002/eji.200737127. [DOI] [PubMed] [Google Scholar]
- 138.Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol. Biol. Cell. 2004;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Srivastava PK, Callahan MK, Mauri MM. Treating human cancers with heat shock protein-peptide complexes: the road ahead. Expert Opin. Biol. Ther. 2009;9:179–186. doi: 10.1517/14712590802633918. [DOI] [PubMed] [Google Scholar]
- 140.Weng D, Calderwood SK, Gong J. Preparation of a heat shock protein 70-based vaccine from DC-tumor fusion cells. Methods Mol. Biol. 2011;787:255–265. doi: 10.1007/978-1-61779-295-3_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zeng Y, Graner MW, Katsanis E. Chaperone-rich cell lysates, immune activation and tumor vaccination. Cancer Immunol. Immunother. 2006;55:329–338. doi: 10.1007/s00262-005-0694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Manjili MH, Wang XY, Chen X, et al. HSP110-HER2/neu chaperone complex vaccine induces protective immunity against spontaneous mammary tumors in HER-2/neu transgenic mice. J. Immunol. 2003;171:4054–4061. doi: 10.4049/jimmunol.171.8.4054. [DOI] [PubMed] [Google Scholar]
- 143.Asea A, Rehli M, Kabingu E, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 144.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 145.Manegold C, Gravenor D, Woytowitz D, et al. Randomized Phase II trial of a Toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 2008;26:3979–3986. doi: 10.1200/JCO.2007.12.5807. [DOI] [PubMed] [Google Scholar]
- 146.Buhe V, Guerrier T, Youinou P, Berthou C, Loisel S. CpG ODN enhances the efficacy of rituximab in non-Hodgkin lymphoma. Ann. NY Acad. Sci. 2009;1173:858–864. doi: 10.1111/j.1749-6632.2009.04615.x. [DOI] [PubMed] [Google Scholar]
- 147.Saha A, Bhattacharya-Chatterjee M, Foon KA, Celis E, Chatterjee SK. Stimulatory effects of CpG oligodeoxynucleotide on dendritic cell-based immunotherapy of colon cancer in CEA/HLA-A2 transgenic mice. Int. J. Cancer. 2009;124:877–888. doi: 10.1002/ijc.24009. [DOI] [PubMed] [Google Scholar]
- 148.Zhou S, Kawakami S, Yamashita F, Hashida M. Intranasal administration of CpG DNA lipoplex prevents pulmonary metastasis in mice. Cancer Lett. 2010;287:75–81. doi: 10.1016/j.canlet.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 149.Warger T, Hilf N, Rechtsteiner G, et al. Interaction of TLR2 and TLR4 ligands with the N-terminal domain of Gp96 amplifies innate and adaptive immune responses. J. Biol. Chem. 2006;281:22545–22553. doi: 10.1074/jbc.M502900200. [DOI] [PubMed] [Google Scholar]
- 150.Okuya K, Tamura Y, Saito K, et al. Spatiotemporal regulation of heat shock protein 90-chaperoned self-DNA and CpG-oligodeoxynucleotide for type I IFN induction via targeting to static early endosome. J. Immunol. 2010;184:7092–7099. doi: 10.4049/jimmunol.1000490. [DOI] [PubMed] [Google Scholar]
- 151.Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin. Oncol. 2010;37:473–484. doi: 10.1053/j.seminoncol.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch. Dermatol. 2006;142:166–172. doi: 10.1001/archderm.142.2.166. [DOI] [PubMed] [Google Scholar]
- 153.Tarhini AA, Kirkwood JM. CTLA-4-blocking immunotherapy with ipilimumab for advanced melanoma. Oncology (Williston Park) 2010;24:1302, 1304. [PubMed] [Google Scholar]
- 154.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2010;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 155.Visvader JE. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23:2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Visvader JE, Smith GH. Murine mammary epithelial stem cells: discovery, function, and current status. Cold Spring Harb. Perspect. Biol. 2011:a004879. doi: 10.1101/cshperspect.a004879. DOI: 10.1101/cshperspect. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schatton T, Frank MH. Antitumor immunity and cancer stem cells. Ann. NY Acad. Sci. 2009;1176:154–169. doi: 10.1111/j.1749-6632.2009.04568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schatton T, Schutte U, Frank NY, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010;70:697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int. J. Radiat. Oncol. Biol. Phys. 2005;63:655–666. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McBride WH, Chiang CS, Olson JL, et al. A sense of danger from radiation. Radiat. Res. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- 161.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lugade AA, Sorensen EW, Gerber SA, et al. Radiation-induced IFN-γ production within the tumor microenvironment influences antitumor immunity. J. Immunol. 2008;180:3132–3139. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 163.Obeid M, Panaretakis T, Joza N, et al. Calreticulin exposure is required for the immunogenicity of γ-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–1850. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 164.Panaretakis T, Joza N, Modjtahedi, N, et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 2008;15:1499–1509. doi: 10.1038/cdd.2008.67. [DOI] [PubMed] [Google Scholar]
- 165.Tesniere A, Apetoh L, Ghiringhelli F, et al. Immunogenic cancer cell death: a key-lock paradigm. Curr. Opin. Immunol. 2008;20:504–511. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 166.Berwin B, Hart JP, Rice S, et al. Scavenger receptor-A mediates Gp96/GRP94 and calreticulin internalization by antigen-presenting cells. Embo J. 2003;22:6127–6136. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]