Abstract
The stem cell-like characteristics of tumor cells are not only essential for tumor development and malignant progression, but also significantly contribute to therapy resistance. However, it remains poorly understood how cancer cell differentiation or stemness is regulated in vivo. We investigated the role of the stem cell gene DLK1, or delta-like 1 homolog (Drosophila), in the regulation of cancer cell differentiation in vivo using neuroblastoma (NB) xenografts as a model. We found that loss-of-function mutants of DLK1 significantly enhanced NB cell differentiation in vivo likely by increasing the basal phosphorylation of MEK and ERK kinases, a mechanism that has been shown to facilitate neuronal differentiation. We also found that DLK1+ cells are preferentially located in hypoxic regions. These results clearly demonstrate that DLK1 plays an important role in the maintenance of undifferentiated, stem cell-like phenotypes of NB cells in vivo.
Keywords: cancer stem cell, differentiation, DLK1, hypoxia, neuroblastoma
1. Introduction
Tumor development and malignant progression are sustained by tumor-initiating cells (TIC) or cancer stem cells (CSC) that are capable of self-renewal. CSCs/TICs show many similarities with normal stem or progenitor cells. Several stem cell genes have been shown to play important roles in tumorigenesis [1; 2; 3; 4]. Because stem-like cancer cells appear to be more resistant to conventional therapies [5; 6; 7] and because high-grade tumors exhibit a stem cell gene expression signature [8], understanding molecular mechanisms that regulate cancer stem cell-like characteristics would have significant impact on the development of more effective cancer therapeutics. Currently, it remains largely unknown how stem cell genes regulate tumor cell growth and differentiation in vivo in established tumors or cell lines.
Previously, we characterized DLK1, or delta-like 1 homologue (Drosophila), as a bona fide stem cell gene in neuronal tumors [9]. DLK1, a type-1 transmembrane protein, is highly expressed in immature embryonic tissues, but not in differentiated adult tissues [10], suggesting a role of DLK1 in the regulation of stem cells and/or progenitors. We found that DLK1 was robustly expressed in undifferentiated, but not in differentiated, tumor cells [9]. Inhibition of DLK1 enhances spontaneous neuronal differentiation and decreases clonogenicity or the colony-forming potential, whereas DLK1 overexpression inhibits differentiation and enhances clonogenicity [9]. Interestingly, hypoxia strongly induced DLK1 expression, suggesting that DLK1 may play an important role in the regulation of cancer cell stemness within the tumor microenvironment [6; 9; 11].
Our current study has examined the role of DLK1 in the regulation of tumor cell differentiation in vivo using neuroblastoma (NB) xenografts as a model. In xenograft tumors derived from stable NB cell lines with overexpression of a DLK1 mutant (cytoplasmic domain deletion or mutations of Tyrosine-339 and Serine-355 in the cytoplasmic domain), expression of differentiation markers was significantly increased, suggesting that the DLK1 cytoplasmic domain is required for maintaining tumor cells in their undifferentiated state. Mechanistically, deletion of or loss-of-function mutations in the DLK1 cytoplasmic domain increased steady-state ERK phosphorylation, which is consistent with the finding that ERK activation facilitates neuronal differentiation [12; 13; 14]. Our data have clearly demonstrated that DLK1 plays an essential role in the maintenance of the undifferentiated cancer stem cell-like phenotype.
2. Materials and Methods
2.1. Plasmids
Retroviral vectors expressing DLK1-FL (full-length DLK1), DLK1-ΔCyto (deletion of DLK1 cytoplasmic domain), and DLK1-DM (Y339F/S355A mutations in DLK1 cytoplasmic domain) have been described in our previous publication [9].
2.2. Cell lines and tumor xenografts
SK-N-BE(2)C [BE(2)C] and SK-N-ER (ER) NB cell lines were maintained in Minimum Essential Medium and F12 (1:1). Cells were transduced with retrovirus (DLK1-FL, DLK1-ΔCyto, DLK1-DM or vector control) and then purified by FACS for the expression of green fluorescent protein (GFP).
Athymic mice were fed ad libitum with food and water and were maintained under pathogen-free conditions. All animal protocols were reviewed and approved by the Yale Institutional Animal Care and Use Committee. Tumor xenografts were generated from the above NB cells via subcutaneous implantation on the back of NU/J mice (6-wk-old males; Jackson Laboratory) as previously described [9]. To label hypoxic regions, pimonidazole hydrochloride (Hypoxyprobe-1, HPI, Inc. Burlington, MA) was injected intraperitoneally at 60 mg/kg body weight one hour before harvesting the tumors according to manufacturer’s recommended protocol.
2.3. Western blot
Western blots were analyzed as described previously [9] with the following antibodies: polyclonal rabbit anti-DLK1 (1:2,000; Millipore, Billerica, MA); polyclonal rabbit anti-phosphoMEK1/2 (1:1,000; Cell Signaling, Danvers, MA), polyclonal rabbit anti-phosphoERK1/2 (1:1,000; Cell Signaling), polyclonal rabbit anti-total ERK1/2 (1:1,000; Cell Signaling), polyclonal rabbit anti-phosphoAKT-Ser473 (1:1,000; Cell Signaling); polyclonal rabbit anti-total AKT (1:1,000; Cell Signaling), and mouse anti-glyceraldehyde 3-phosphate dehydrogenase (anti-GAPDH, clone 6C5, 1:5,000; Abcam, Cambridge, MA).
2.4. Cell proliferation assay
FACS-sorted BE(2)C cells with DLK1-DM, DLK1-ΔCyto, or vector control were seeded at a density of 1 × 104 cells per well in 96-well plates. After overnight incubation, cells were treated with or without 1 nM of retinoic acid (RA). Cell growth was then analyzed every day post RA-treatment using the MTS assay kit (Promega, Madison, WI) according to the manufacturer’s recommended protocol.
2.5. Immunohistochemistry (IHC)
Paraffin-embedded sections (5 µm) were de-waxed and rehydrated. Antigen retrieval was done by heating tumor sections in a citrate buffer (10mM Citric Acid, 0.05% Tween 20, pH 6.0) for 10 min at 95°–100°C. Endogenous peroxidase activities were quenched in 1% hydrogen peroxide for 30 minutes and non-specific binding was blocked in 5% horse serum for 60 minutes. Slides were then incubated with the following antibodies: anti-Ki67 (1:100 dilution; Biocare Medical CRM-325), anti-neurofilament (NF, 1:4 dilution; DakoCytomation N-1591, clone 2F11), anti-glial fibrilar acidic protein GFAP (1:100 dilution; Thermo, clone ASTRO6), or anti-von Willebrand Factor (vWF, 1:4000 dilution; DakoCytomation A0082) overnight at 4°C, followed by incubation with a horseradish peroxidase (HRP)-conjugated secondary antibody (DAKO Envision kit). Antigen-antibody reactions were visualized with 0.2% diaminobenzidine tetrahydrochloride and hydrogen peroxide. Slides were counterstained with Mayer's hematoxylin.
2.6. Immunofluorescence
For double immunofluorescence staining of DLK1 and neurofilament, frozen tumor sections (7µm) were fixed for 20 minutes with ice-cold acetone and air-dried. After 60-minute incubation in 10% horse serum, tumor sections were incubated overnight at 4°C with rabbit anti-DLK1 (AB3511, 1:100; Millipore) and mouse anti-neurofilament-H (clone RMdO 20, 1:400; Cell Signaling), followed by incubation with Alexa 594-conjugated goat-anti-rabbit IgG (1:500; Invitrogen, Carlsbad, CA) and Alexa 488-conjugated donkey-anti-mouse IgG (1:500; Invitrogen), respectively. Hoechst 33342 (2 µg/mL) was used for nuclear staining.
For double immunofluorescence staining of DLK1 or Hypoxyprobe with blood vessels in frozen sections, DLK1 was detected using rabbit anti-DLK1 and Alexa 488-conjugated goat-anti-rabbit IgG (1:500 Invitrogen); while tumor hypoxia was detected by incubating tumor sections overnight with rabbit anti-pimonidazole (PAb2627, HPI, Inc.) followed by incubation with Alexa 488–conjugated donkey-anti-mouse IgG. Blood vessels were stained using rat anti-mouse CD31 (1:25, BD Pharmingen, San Diego, CA) followed by Alexa-594-conjugated anti-rat IgG.
2.7. Statistics
Statistical differences between two groups were analyzed using the two-tailed, unpaired Student's t test using Prizm 3.0 (GraphPad Software, Inc.)
3. Results
3.1. DLK1 is required for maintaining NB cells in undifferentiated states in vivo
As we previously found [9], knocking down DLK1 resulted in spontaneous differentiation of NB cells in vitro, as well as reduced tumorigenicity and tumor growth in vivo, which led to the hypothesis that DLK1 is required for maintaining the undifferentiated phenotype of NB cells in vivo. Toward this end, we examined xenograft tumors derived from stable NB cell lines expressing wild-type or mutant DLK1. For stable cell lines from BE(2)C cells with high endogenous DLK1 expression, we used DLK1-DM (Y339F/S355A mutations at the putative phosphorylation sites of the cytoplasmic domain) and DLK1-ΔCyto (without the cytoplasmic domain) to suppress the functions of endogenous DLK1. Using Western blots, we found that the overall levels of DLK1 protein were increased in stable BE(2)C cell lines with DLK1-DM or DLK1-ΔCyto, albeit the endogenous DLK1 protein levels were lower in DLK1-ΔCyto cells than in controls (Supplementary Figure 1). In addition, we established a stable cell line with overexpression of the full-length DLK1 (DLK1-FL) in SK-N-ER (ER) cells with low endogenous DLK1 expression. Our previous study showed that stable BE(2)C cell lines expressing DLK1-DM or DLK1-ΔCyto exhibited lower tumor-take rates and slower growth of subcutaneous xenografts in immune-compromised mice [9], suggesting an inhibitory effect of DLK1-DM and DLK1-ΔCyto on endogenous DLK1.
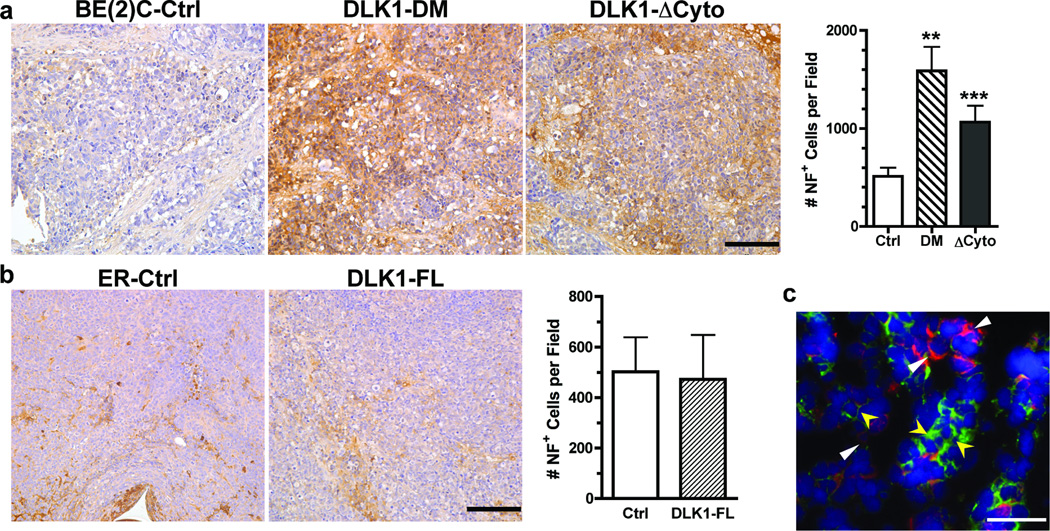
Because NB cells can undergo neuronal and glial differentiation [15; 16], we examined the impact of DLK1 on differentiation of NB cells in xenografts by analyzing the expression of neurofilament (NF, neuronal differentiation) and glial fibrilar acidic protein (GFAP, glial differentiation). As shown in Figure 1a, xenograft tumors derived from BE(2)C cells with either DLK1-DM or DLK1-ΔCyto showed robust staining by an anti-NF antibody compared to the control cells, suggesting that the cytoplasmic domain, especially the two putative phosphorylation sites Y339 and S355, is required for maintaining BE(2)C cells in an undifferentiated state. Furthermore, we found that DLK1 and neurofilament were expressed in a mutually exclusive manner in vivo (Figure 1c), further indicating that down-regulation of DLK1 is required for NB cell differentiation in vivo.
Figure 1. DLK1 loss-of-function in NB cells increases the potential for neuronal differentiation in vivo.
Parafin-embedded sections of (a) BE(2)C xenografts (Ctrl, DLK1-DM or DLK1-ΔCyto) or (b) ER xenografts (Ctrl or DLK-FL) were incubated with an anti-neurofilament (NF) antibody as described in Materials and Methods. Bar = 100 µm. NF+ cells were counted from three random fields (mean ± sem, **p<0.001; ***p<0.0001 versus control). (c) Co-immunofluorescence of DLK1 (red) and neurofilament (green) was performed in frozen tumor sections as described in Materials and Methods (bar = 100 µm). DLK1+ cells (white triangles) do not express NF whereas NF+ cells (yellow arrowheads) do not express DLK1.
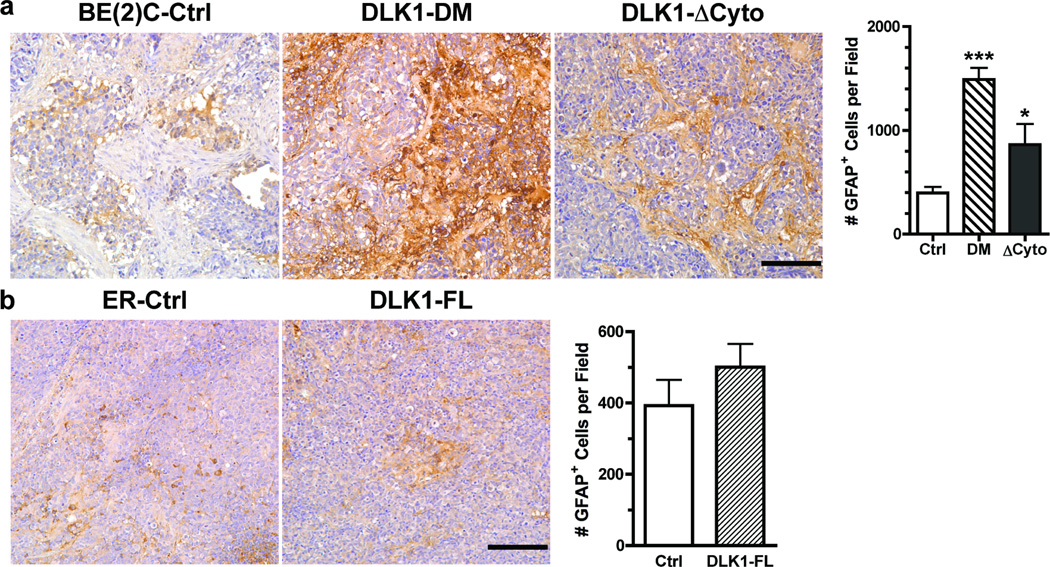
Similarly, tumors derived from BE(2)C cells with either DLK1-DM or DLK1-ΔCyto showed significantly higher levels of GFAP expression in vivo (Figure 2a), suggesting increased differentiation toward the glial lineage. It appears that BE(2)C cells with constitutive expression of DLK1-ΔCyto have higher tendency toward neuronal (Figure 1a) than glial (Figure 2a) differentiation; whereas DLK1-DM sensitizes BE(2)C cells to both neuronal and glial differentiation without much preference. These observations suggest that the DLK1 cytoplasmic domain with both Y339F and S355A mutations might still interact with yet unidentified proteins to regulate cell fate decisions. Consistent with these observations, we found that xenograft tumors derived from BE(2)C cells transduced with lentivirus containing a DLK1-specific shRNA or shDLK1 [9] also showed increased expression of neurofilament and GFAP (Supplemental Figure 2). However, overexpression of DLK1 in ER cells did not significantly affect either neuronal (Figure 1b) or glial (Figure 2b) differentiation in vivo, suggesting that overexpression of DLK1 alone is not sufficient to block spontaneous differentiation of NB cells in vivo.
Figure 2. DLK1 loss-of-function in NB cells enhances the potential for glial differentiation in vivo.
Parafin-embedded sections of (a) BE(2)C xenografts (Ctrl, DLK1-DM or DLK1-ΔCyto) or (b) ER xenografts (Ctrl or DLK-FL) were incubated with an anti-glial fibrilar acidic protein (GFAP) antibody as described in Materials and Methods. Bar = 100 µm. GFAP+ cells were counted from three random fields (mean ± sem, *p<0.01; ***p<0.0001 versus control).
3.2. DLK1 facilitates tumor growth by promoting proliferation in vivo
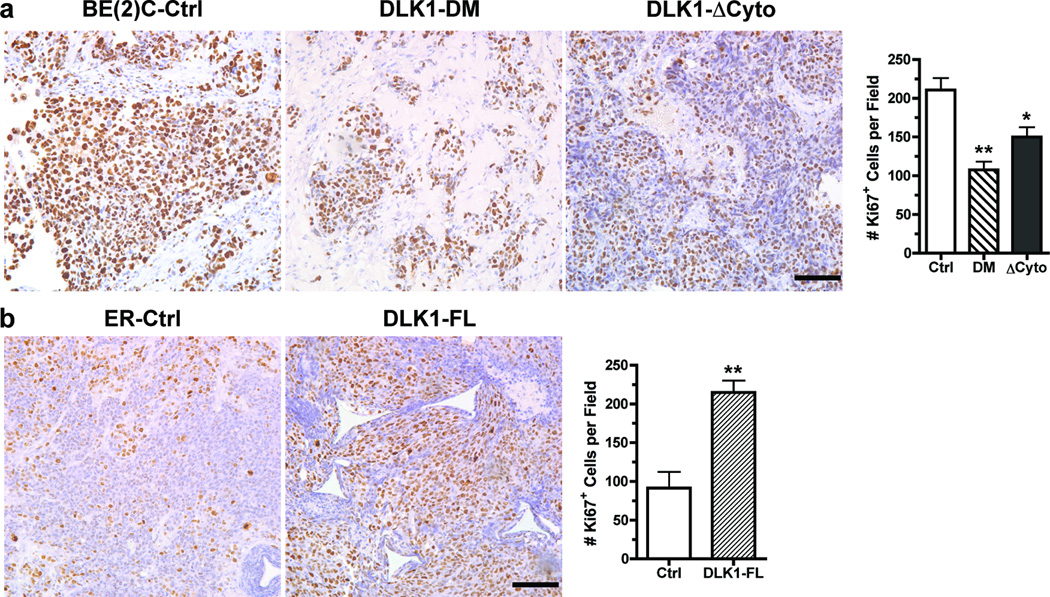
We examined the impact of DLK1 on NB cell proliferation in xenografts by analyzing the expression of the proliferation-associated nuclear antigen Ki67. As shown in Figure 3a, the percentages of Ki67+ cells were significantly reduced in xenografts derived from BE(2)C cells with either DLK1-DM or DLK1-ΔCyto. The reduced tumor cell proliferation is highly consistent with the increased spontaneous in vivo differentiation (Figures 1a and 2a) in these tumors. Similarly, tumors derived from BE(2)C cells transduced with shDLK1 lentivirus contained fewer Ki67+ cells per microscopic field (Supplemental Figure 2). In contrast, overexpression of the full-length DLK1 significantly enhanced the percentage of Ki67+ or proliferating tumor cells (Figure 3b). In light of the observations in Figures 1b and 2b, these findings suggest that DLK1 overexpression may have the potential to promote clonal expansion without significant impact on cell fate decisions in vivo.
Figure 3. DLK1 promotes NB cell proliferation in vivo.
Parafin-embedded sections of (a) BE(2)C xenografts (Ctrl, DLK1-DM or DLK1-ΔCyto) or (b) ER xenografts (Ctrl or DLK-FL) were incubated with an anti-Ki67 antibody as described in Materials and Methods. Bar = 100 µm. Ki67+ cells were counted from three random fields (mean ± sem, *p<0.01; **p<0.001 versus control).
3.3. NB cell-expressed DLK1 regulates tumor angiogenesis
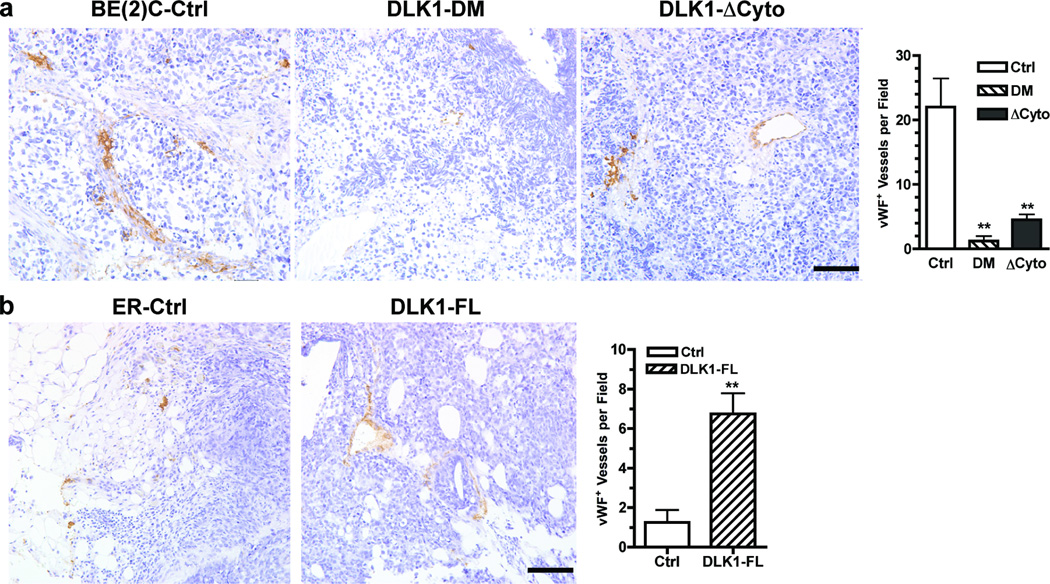
The observations that DLK1 loss-of-function enhances NB cell differentiation in vivo suggest that the DLK1 status in NB cells may regulate tumor-host interactions and thus the development of tumor microenvironment. Using immunohistochemical staining of NB xenografts with anti-von Willebrand Factor (vWF) to detect blood vessels, we found that expression of DLK1-DM or DLK1-ΔCyto in BE(2)C cells significantly reduced blood vessel densities in xenografts as compared to control (Figure 4a). Tumors derived from lentiviral shDLK1-transduced BE(2)C cells also had reduced blood vessel density (Supplemental Figure 2). In comparison, overexpression of the full-length DLK1 significantly increased angiogenesis in NB xenografts (Figure 4b). However, by quantitative RT-PCR, we found that the expression of vascular endothelial growth factor (VEGFA), angiopoietin (Ang1 and Ang2) and placental growth factor (PlGF) was not significantly changed in DLK1-DM, DLK1-ΔCyto cells or DLK1-FL cells (data not shown). The observed changes in angiogenesis in vivo may result from different rates of tumor growth.
Figure 4. DLK1 enhances angiogenesis in NB xenografts.
Parafin-embedded sections of (a) BE(2)C xenografts (Ctrl, DLK1-DM or DLK1-ΔCyto) or (b) ER xenografts (Ctrl or DLK-FL) were incubated with an anti-von Willebrand Factor (vWF) antibody as described in Materials and Methods. Bar = 100 µm. vWF+ cells were counted from three random fields (mean ± sem, **p<0.001 versus control).
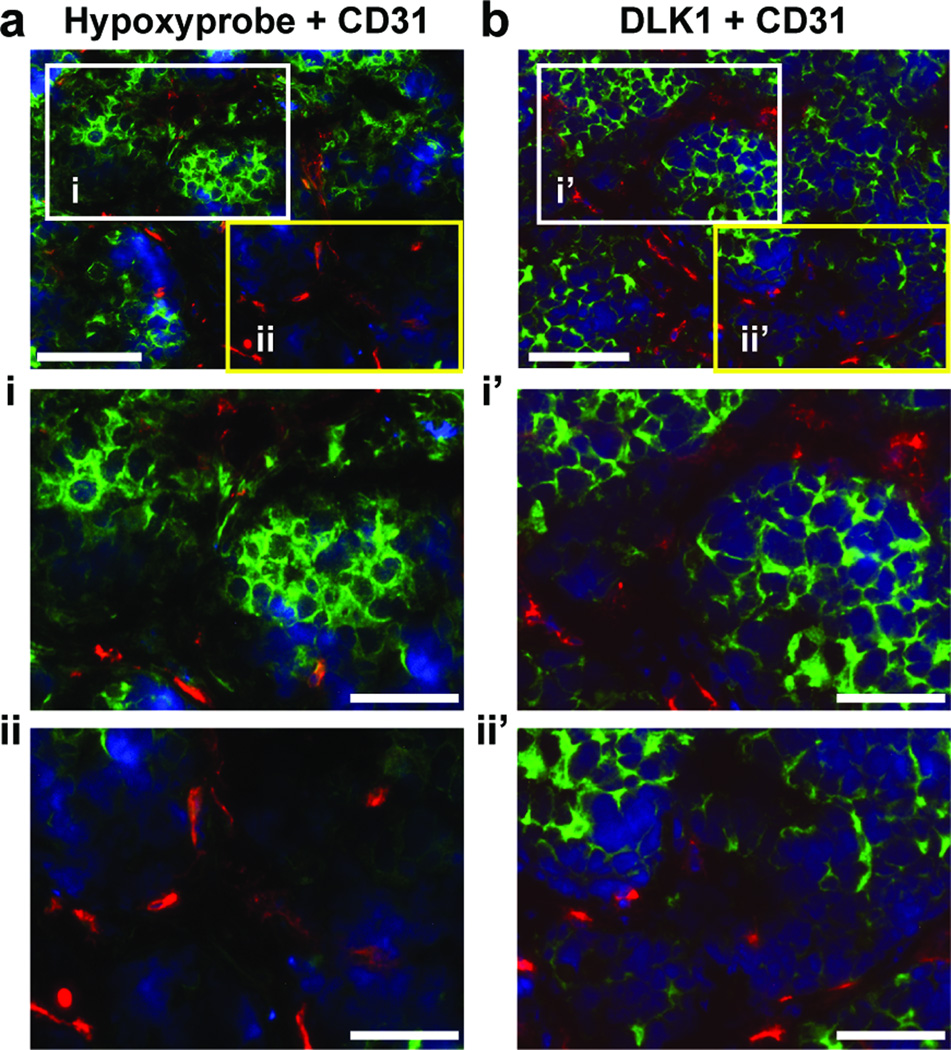
3.4. DLK1+ NB cells are preferentially localized in hypoxic regions
Previously, we found that DLK1 expression was strongly increased under hypoxia in a HIF-dependent manner [9]. Here, we examined the in vivo relationship between DLK1 expression and hypoxia in serial sections of BE(2)C xenografts (Figure 5). In hypoxic areas recognized by anti-Hypoxyprobe (Figure 5i), there was an enrichment of DLK1+ cells (Figure 5i’). In contrast, the well-oxygenated regions with little staining by anti-Hypoxyprobe (Figure 5ii) contained few DLK1+ cells (Figure 5ii’). It is possible that increased DLK1 expression in hypoxic cells likely results from a combination of hypoxia-enhanced DLK1 transcription [9] and hypoxia-suppressed differentiation [9; 17; 18; 19; 20]. Tumor cells located in well-oxygenated areas may be under strong stresses from host-derived factors to undergo differentiation, which may consequently result in loss of DLK1 expression. These observations strongly suggest that tumor hypoxia has the potential to inhibit tumor cell differentiation and maintain tumor cell stemness in vivo.
Figure 5. DLK1+ NB cells are preferentially localized in hypoxic regions.
Serial frozen sections of a BE(2)C xenograft tumor were subjected to double immunofluorescence staining of (a, i, ii) Hypoxyprobe (green) and CD31 (red) or b, I’, ii’) DLK1 (green) and CD31 (red) as described in Materials and Methods. Bars: 200 µm (a, b); 100 µm (i, ii, i’, ii’).
3.5. DLK1 intracellular domain regulates the ERK pathway
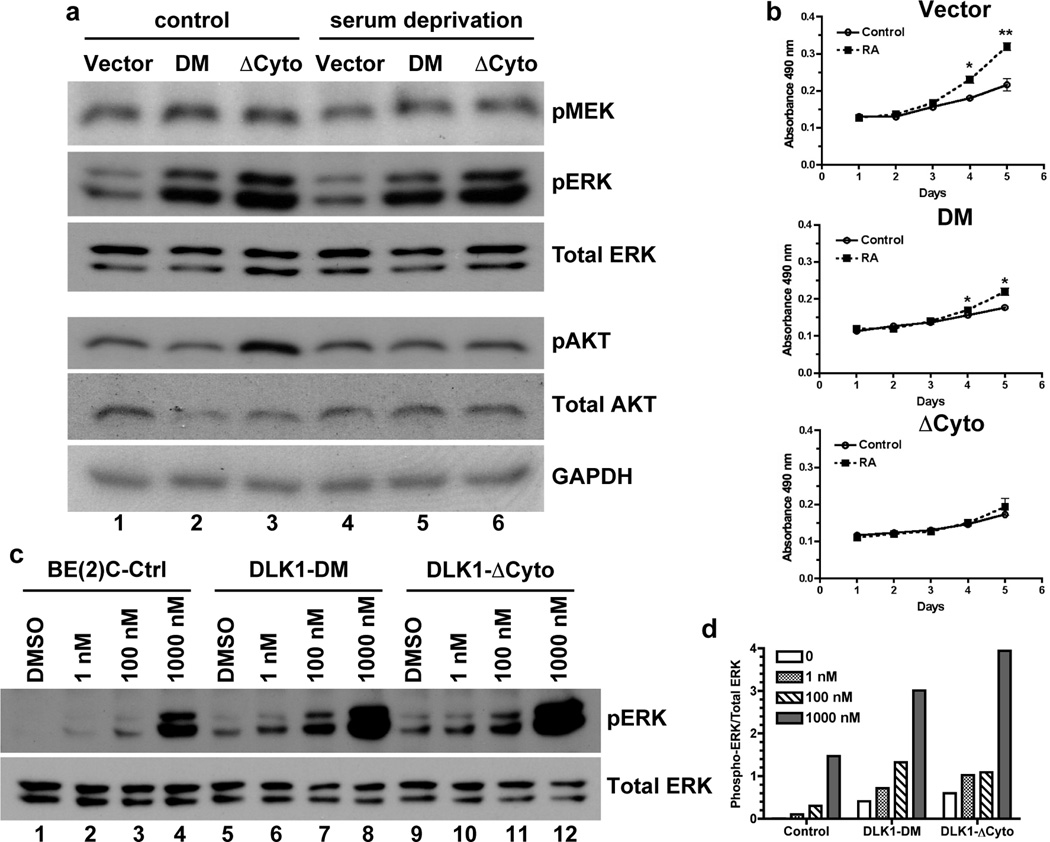
Our findings strongly suggest that the intracellular domain of DLK1 is potentially involved in intracellular signal transduction that regulates cell fate decisions. It has been previously shown that persistent activation of the MEK/ERK pathway promotes differentiation and decreases self-renewal of embryonal stem cells [12; 13; 14]. Increased ERK1/2 phosphorylation occurs during differentiation of neuronal progenitor cells [21]. Consistent with this observation, we found that DLK1-DM and DLK1-ΔCyto strongly increased the basal levels of phosphorylated ERK1/2 (p42 and p44) under both normal growth conditions (Lanes 1–3, Figure 6a) and serum deprivation (Lanes 4–6, Figure 6a). Levels of phosphorylated MEK1/2 were also slightly increased under the same conditions. In contrast, DLK1-DM or DLK1-ΔCyto did not significantly alter AKT phosphorylation under serum deprivation although they appear to have differential effects on AKT phosphorylation under normal growth conditions. Nonetheless, these data demonstrate that the DLK1 cytoplasmic domain, especially the putative phosphorylation sites Y339 and S355, play a critical role in regulating activation of the MEK-ERK pathway in NB cells.
Figure 6. DLK1 loss-of-function increases ERK phosphorylation.
(a) Cell lysates were prepared from BE(2)C-Ctrl, DLK1-DM and DLK1-ΔCyto cells either under normal growth conditions (control) or after 16-hour serum deprivation. Phosphorylated and total proteins were examined using Western blots as described in Materials and Methods. One of three independent experiments is shown. (b) BE(2)C cells with stable expression of DLK1-DM, DLK1-ΔCyto or vector control were seeded at a density of 1 × 104 cells per well in 96-well plates in the presence or absence of 1 nM retinoic acid (RA). Cell growth was analyzed by the MTS assay (Promega). Data shown are mean ± sem from three independent experiments. *p<0.02; **p<0.007 versus control at each time point. (c) BE(2)C cells with stable expression of DLK1-DM, DLK1-ΔCyto or vector control were incubated with 1nM, 100nM, 1µM RA for 48 hours. Phosphorylated and total ERK proteins were examined using Western blots. (d) Ratios of phophorylated to total p42 ERK protein were calculated using density measurements by NIH Image J. One of three experiments was shown.
Although retinoic acid (RA) causes growth arrest and induces cell differentiation at pharmacological (µM) concentrations [22; 23], it can promote cell proliferation at physiological (low nM) concentrations [24; 25; 26; 27]. We found (Figure 6b) that 1 nM RA robustly increased growth of the vector control BE(2)C cells (Vector), but by and large failed to enhance the growth of cells expressing DLK1-DM (DM) or DLK1-ΔCyto (ΔCyto). Consistent with these observations, we found that at a low concentration (1 nM) RA did not significantly affect ERK phosphorylation in control BE(2)C cells; whereas a high concentration of RA induced robust ERK phosphorylation (Figure 6c,d). In contrast, ERK phosphorylation was more readily induced even at 1–100 nM RA in BE(2)C cells expressing DLK1-DM or DLK1-ΔCyto (Figure 6c and d). These results are consistent with the observations that increased ERK1/2 phosphorylation is associated with differentiation of neuronal progenitor cells [21] and decreased self-renewal of embryonal stem cells [12; 13; 14]. Our data also indicate that wild-type DLK1 may play an important role promoting self-renewal and/or clonal expansion of NB cells.
4. Discussion
Tumor cell stemness is not only essential for tumor initiation, growth, and malignant progression, but also promotes resistance to conventional therapy [5]. The important concept of the CSC/TIC paradigm is that tumor cells can lose their clonogenic and tumorigenic potentials via differentiation. Therefore, identifying genes and molecular pathways that regulate cancer cell stemness will facilitate the development of effective therapy, as well as further our understanding of mechanisms of malignant tumor progression.
DLK1 is a member of the epidermal growth factor (EGF)-like homeotic supergene family with homologies to members of the notch/delta/serrate family [28]. Also known as pref-1, fetal antigen (FA1), pG2 and ZOG, DLK1 is a developmentally regulated gene with strong expression in immature embryonic cells [10; 29; 30], suggesting an important role of DLK1 in stem cells and progenitors. Elevated expression of DLK1 is also found in a variety of tumor cells, including neuroblastoma, gliomas, breast cancer, colon cancer, pancreatic cancer, small-cell lung carcinoma, and leukemia [31; 32; 33; 34; 35; 36; 37]. Studies have shown that DLK1 is capable of inhibiting in vitro cell differentiation, including mesenchymal progenitor cells [29; 38; 39] and hematopoietic stem cells [34; 35]. The role of DLK1 in regulating tumor cell differentiation in vitro has also been reported in glioma cells [33] and hematopoietic tumors [34]. Nonetheless, the role of DLK1 in the regulation of tumor cell differentiation in vivo had not previously been investigated.
In this study, we have demonstrated that DLK1 plays a critical role in regulating tumor cell differentiation in vivo using xenografts derived from NB cell lines stably expressing either the wild-type full-length DLK1 or one of the following dominant-negative DLK1 mutants: DLK1-ΔCyto without the cytoplasmic domain and DLK1-DM with Y339F/S355A mutations in the cytoplasmic domain. Y339 and S355 are two putative phosphorylation sites highly conserved among mammals [9]. Here we found that xenograft tumors derived from NB cells with stable expression of DLK1-ΔCyto or DLK1-DM showed significantly higher expression of the neuronal differentiation marker neurofilament and the glial differentiation marker GFAP. Interestingly, NB cells express DLK1 and neurofilament in a mutually exclusive manner. These observations suggest that loss of DLK1 function sensitizes NB cells to undergo differentiation in vivo. However, it should be noted that the increased expression of neurofilament and/or GFAP in NB cells simply suggests that these tumor cells have engaged in the processes of differentiation. It does not mean that NF+ and/or GFAP+ NB cells have undergone terminal differentiation like normal neuronal stem/progenitor cells because tumor cells do not follow the exact pathways of normal cell differentiation. Nonetheless, increased expression of differentiation markers strongly correlate with decreased numbers of proliferating (Ki67+) cells and vWF+ blood vessel density, demonstrating that increased differentiation likely accounts for the reduced tumor growth in vivo. Interestingly, overexpression of DLK1 does not significantly alter cell fate decision in vivo, suggesting that DLK1 alone is not sufficient to overcome the pro-differentiation stresses in the tumor microenvironment.
Consistent with our findings, a recent mouse genetic study has shown that DLK1 is required for self-renewal of neuronal stem cells [40]. Together, these findings strongly suggest that DLK1 plays a significant role in the maintenance of stemness, perhaps by functioning as a gatekeeper at “differentiation checkpoints.” Loss or inhibition of wild-type DLK1 would sensitize stem cells to engage in differentiation induced by environmental stresses. The terminal lineage-specific differentiation is likely determined by additional differentiation signals. In this study, the increased expression of both neuronal and glial differentiation markers may simply reflect an altered differentiation program in NB cells. It is also possible that the DLK1 mutants sensitize NB cells to acquire a more differentiated progenitor phenotype before terminal differentiation.
Decreased oxygenation, or hypoxia, is a common feature of the tumor microenvironment in solid tumors and is an independent prognostic factor for advanced disease progression and poor clinical outcome [41; 42; 43]. Our studies and those of others have shown that hypoxia inhibits cell differentiation [20; 44; 45] and is able to arrest progenitor cells in an undifferentiated state [18]. We found that hypoxia strongly increased DLK1 expression in neuronal tumor cells in vitro [9]. Here, we found that robust DLK1 expression correlated with tumor hypoxia. These observations suggest that DLK1 may synergize with hypoxia to repress tumor cell differentiation in vivo.
The requirement for the DLK1 cytoplasmic domain, especially the conserved Y339 and S355 residues, suggests that DLK1 is an important signal transducer or mediator for stem cell maintenance. As shown in this study, DLK1ΔCyto and DLK1-DM can increase the basal phosphorylation of ERKs, but not that of AKT. This result is consistent with the previous reports that increased ERK phosphorylation is associated with differentiation of neuronal progenitor cells [21] and that activation of the MEK/ERK pathway facilitates differentiation but reduces self-renewal of embryonal stem cells [12; 13; 14]. Since the MEK/ERK pathway can promote growth as well as differentiation, these findings strongly suggest that DLK1 can potentially play a critical role in regulating the MEK/ERK pathway in cell fate decision-making.
Together, our current work and our previous study [9] have provided strong evidence demonstrating a critical role of DLK1 in the maintenance of NB cell stemness. Since DLK1 is overexpressed in many types of cancers, it will be interesting to investigate whether CSCs/TICs from spontaneous tumors express higher levels of DLK1 than non-stem-like tumor cells do. In addition, our findings suggest that DLK1 may be further explored as a potential therapeutic target to induce differentiation of stem cell-like tumorigenic cells.
Supplementary Material
Acknowledgements
We thank Dr. Nai-Kong V. Cheung of Memorial Sloan-Kettering Cancer Center for SK-N-ER cells, Dr. Robert Ross of Fordham University for BE(2)C cells, Dr. Ravi Bhatia of City of Hope National Medical Center for full-length DLK1 and DLK1-ΔCyto. We would like to thank Amos A. Brooks (Research Histology Laboratory, Department of Pathology, Yale School of Medicine) for the IHC staining and Lisa Cabral for her excellent editorial assistance. This work was supported by a grant from the National Institutes of Health to ZY (R01CA125021). However, the granting agency had no other involvement in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None declared.
References
- 1.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 2.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 3.Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M. Stem cells and cancer; the polycomb connection. Cell. 2004;118:409–418. doi: 10.1016/j.cell.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 5.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 6.Lin Q, Yun Z. Impact of the hypoxic tumor microenvironment on the regulation of cancer stem cell characteristics. Cancer Biol Ther. 2010;9:949–956. doi: 10.4161/cbt.9.12.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagemaker G. Heterogeneity of radiation sensitivity of hemopoietic stem cell subsets. Stem Cells. 1995;13 Suppl 1:257–260. doi: 10.1002/stem.5530130731. [DOI] [PubMed] [Google Scholar]
- 8.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, Lin Q, Zelterman D, Yun Z. Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity. Cancer Research. 2009;69:9271–9280. doi: 10.1158/0008-5472.CAN-09-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Floridon C, Jensen CH, Thorsen P, Nielsen O, Sunde L, Westergaard JG, Thomsen SG, Teisner B. Does fetal antigen 1 (FA1) identify cells with regenerative, endocrine and neuroendocrine potentials? A study of FA1 in embryonic, fetal, and placental tissue and in maternal circulation. Differentiation. 2000;66:49–59. doi: 10.1046/j.1432-0436.2000.066001049.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y, Lin Q, Glazer PM, Yun Z. Hypoxic tumor microenvironment and cancer cell differentiation. Curr Mol Med. 2009;9:425–434. doi: 10.2174/156652409788167113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdon T, Stracey C, Chambers I, Nichols J, Smith A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Developmental Biology. 1999;210:30–43. doi: 10.1006/dbio.1999.9265. [DOI] [PubMed] [Google Scholar]
- 13.Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends in Cell Biology. 2002;12:432–438. doi: 10.1016/s0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 14.Casanova EA, Shakhova O, Patel SS, Asner IN, Pelczar P, Weber FA, Graf U, Sommer L, Burki K, Cinelli P. Pramel7 mediates LIF/STAT3-dependent self-renewal in embryonic stem cells. Stem Cells. 2011;29:474–485. doi: 10.1002/stem.588. [DOI] [PubMed] [Google Scholar]
- 15.Ross RA, Spengler BA, Domenech C, Porubcin M, Rettig WJ, Biedler JL. Human neuroblastoma I-type cells are malignant neural crest stem cells. Cell Growth Differ. 1995;6:449–456. [PubMed] [Google Scholar]
- 16.Walton JD, Kattan DR, Thomas SK, Spengler BA, Guo HF, Biedler JL, Cheung NK, Ross RA. Characteristics of stem cells from human neuroblastoma cell lines and in tumors. Neoplasia. 2004;6:838–845. doi: 10.1593/neo.04310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jogi A, Ora I, Nilsson H, Poellinger L, Axelson H, Pahlman S. Hypoxia-induced dedifferentiation in neuroblastoma cells. Cancer Lett. 2003;197:145–150. doi: 10.1016/s0304-3835(03)00095-8. [DOI] [PubMed] [Google Scholar]
- 18.Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281:30678–30683. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- 19.Pietras A, Gisselsson D, Ora I, Noguera R, Beckman S, Navarro S, Pahlman S. High levels of HIF-2α highlight an immature neural crest-like neuroblastoma cell cohort located in a perivascular niche. J Pathol. 2008;214:482–488. doi: 10.1002/path.2304. [DOI] [PubMed] [Google Scholar]
- 20.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPARγ 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 21.Berwick DC, Calissano M, Corness JD, Cook SJ, Latchman DS. Regulation of Brn-3a N-terminal transcriptional activity by MEK1/2-ERK1/2 signalling in neural differentiation. Brain Res. 2009;1256:8–18. doi: 10.1016/j.brainres.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Niles RM. Signaling pathways in retinoid chemoprevention and treatment of cancer. Mutat Res. 2004;555:81–96. doi: 10.1016/j.mrfmmm.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds CP, Matthay KK, Villablanca JG, Maurer BJ. Retinoid therapy of high-risk neuroblastoma. Cancer Lett. 2003;197:185–192. doi: 10.1016/s0304-3835(03)00108-3. [DOI] [PubMed] [Google Scholar]
- 24.Perri M, Pingitore A, Cione E, Vilardi E, Perrone V, Genchi G. Proliferative and anti-proliferative effects of retinoic acid at doses similar to endogenous levels in Leydig MLTC-1/R2C/TM-3 cells. Biochimica et Biophysica Acta. 2011;1800:993–1001. doi: 10.1016/j.bbagen.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Hu L, Lind T, Sundqvist A, Jacobson A, Melhus H. Retinoic acid increases proliferation of human osteoclast progenitors and inhibits RANKL-stimulated osteoclast differentiation by suppressing RANK. PLoS One. 2010;5:e13305. doi: 10.1371/journal.pone.0013305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zauli G, Visani G, Vitale M, Gibellini D, Bertolaso L, Capitani S. All-trans retinoic acid shows multiple effects on the survival, proliferation and differentiation of human fetal CD34+ haemopoietic progenitor cells. British Journal of Haematology. 1995;90:274–282. doi: 10.1111/j.1365-2141.1995.tb05147.x. [DOI] [PubMed] [Google Scholar]
- 27.Enomoto M, Pan H, Suzuki F, Takigawa M. Physiological role of vitamin A in growth cartilage cells: low concentrations of retinoic acid strongly promote the proliferation of rabbit costal growth cartilage cells in culture. J Biochem. 1990;107:743–748. doi: 10.1093/oxfordjournals.jbchem.a123119. [DOI] [PubMed] [Google Scholar]
- 28.Laborda J. The role of the epidermal growth factor-like protein dlk in cell differentiation. Histol Histopathol. 2000;15:119–129. doi: 10.14670/HH-15.119. [DOI] [PubMed] [Google Scholar]
- 29.Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- 30.Tornehave D, Jensen CH, Teisner B, Larsson LI. FA1 immunoreactivity in endocrine tumours and during development of the human fetal pancreas; negative correlation with glucagon expression. Histochem Cell Biol. 1996;106:535–542. doi: 10.1007/BF02473268. [DOI] [PubMed] [Google Scholar]
- 31.Van Limpt VA, Chan AJ, Van Sluis PG, Caron HN, Van Noesel CJ, Versteeg R. High delta-like 1 expression in a subset of neuroblastoma cell lines corresponds to a differentiated chromaffin cell type. Int J Cancer. 2003;105:61–69. doi: 10.1002/ijc.11047. [DOI] [PubMed] [Google Scholar]
- 32.Laborda J, Sausville EA, Hoffman T, Notario V. dlk, a putative mammalian homeotic gene differentially expressed in small cell lung carcinoma and neuroendocrine tumor cell line. J Biol Chem. 1993;268:3817–3820. [PubMed] [Google Scholar]
- 33.Yin D, Xie D, Sakajiri S, Miller CW, Zhu H, Popoviciu ML, Said JW, Black KL, Koeffler HP. DLK1: increased expression in gliomas and associated with oncogenic activities. Oncogene. 2006;25:1852–1861. doi: 10.1038/sj.onc.1209219. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Forman SJ, Bhatia R. Expression of DLK1 in hematopoietic cells results in inhibition of differentiation and proliferation. Oncogene. 2005;24:4472–4476. doi: 10.1038/sj.onc.1208637. [DOI] [PubMed] [Google Scholar]
- 35.Sakajiri S, O'Kelly J, Yin D, Miller CW, Hofmann WK, Oshimi K, Shih LY, Kim KH, Sul HS, Jensen CH, Teisner B, Kawamata N, Koeffler HP. Dlk1 in normal and abnormal hematopoiesis. Leukemia. 2005;19:1404–1410. doi: 10.1038/sj.leu.2403832. [DOI] [PubMed] [Google Scholar]
- 36.Khoury H, Suarez-Saiz F, Wu S, Minden MD. An upstream insulator regulates DLK1 imprinting in AML. Blood. 2010;115:2260–2263. doi: 10.1182/blood-2009-03-212746. [DOI] [PubMed] [Google Scholar]
- 37.Yanai H, Nakamura K, Hijioka S, Kamei A, Ikari T, Ishikawa Y, Shinozaki E, Mizunuma N, Hatake K, Miyajima A. Dlk-1, a cell surface antigen on foetal hepatic stem/progenitor cells, is expressed in hepatocellular, colon, pancreas and breast carcinomas at a high frequency. J Biochem. 2010;148:85–92. doi: 10.1093/jb/mvq034. [DOI] [PubMed] [Google Scholar]
- 38.Villena JA, Kim KH, Sul HS. Pref-1 and ADSF/resistin: two secreted factors inhibiting adipose tissue development. Horm Metab Res. 2002;34:664–670. doi: 10.1055/s-2002-38244. [DOI] [PubMed] [Google Scholar]
- 39.Chen L, Qanie D, Jafari A, Taipaleemaki H, Jensen CH, Sanz ML, Laborda J, Abdallah BM, Kassem M. Delta-like 1 / fetal antigen-1 (Dlk1/FA1) is a novel regulator of chondrogenic cell differentiation via inhibition of the AKT-dependent pathway. Journal of Biological Chemistry. 2011 doi: 10.1074/jbc.M111.230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferron SR, Charalambous M, Radford E, McEwen K, Wildner H, Hind E, Morante-Redolat JM, Laborda J, Guillemot F, Bauer SR, Farinas I, Ferguson-Smith AC. Postnatal loss of Dlk1 imprinting in stem cells and niche astrocytes regulates neurogenesis. Nature. 2011;475:381–385. doi: 10.1038/nature10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nordsmark M, Overgaard J. Tumor hypoxia is independent of hemoglobin and prognostic for loco-regional tumor control after primary radiotherapy in advanced head and neck cancer. Acta Oncol. 2004;43:396–403. doi: 10.1080/02841860410026189. [DOI] [PubMed] [Google Scholar]
- 42.Brizel DM, Dodge RK, Clough RW, Dewhirst MW. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53:113–117. doi: 10.1016/s0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 43.Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 44.Yun Z, Lin Q, Giaccia AJ. Adaptive myogenesis under hypoxia. Mol. Cell. Biol. 2005;25:3040–3055. doi: 10.1128/MCB.25.8.3040-3055.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.