Abstract
Detailed taxonomic, cytological, and phylogenetic accounts of Arabidopsis are presented. As currently delimited, the genus consists of nine species all of which are indigenous to Europe, with the ranges of two species extending into northern and eastern Asia and North American into central United States. A survey of chromosome numbers in the genus is presented, and the country of origin for each count is given. Detailed descriptions of all species and subspecies and keys to all taxa are provided. Generic assignments are updated for the 50 species previously included in Arabidopsis. A cladogram of the species of Arabidopsis based on molecular phylogenetic studies by the authors is given.
Introduction
The significance of Arabidopsis in every aspect of plant biology is known to all biologists and therefore needs no introduction here. The interested reader should consult Meyerowitz and Somerville (1994) and articles and references therein for leads. The present contribution attempts to provide an up-to-date historical, taxonomic, cytological, and phylogenetic information on the genus not found in any other single publication.
Nomenclature and Generic Limits
Nomenclatural history of Arabidopsis
The name Arabidopsis was first proposed by de Candolle (1821) as a section of the genus Sisymbrium Linnaeus, and the seven species he assigned to that section were S. bursifolium Linnaeus [now Rorippa dentata (Linnaeus) O. Bolós & Vigo], S. pinnatifidum (Lamarck) de Candolle [now Murbeckiella pinnatifida (Lamarck) Rothmaler], S. erysimoides Desfontaines [same], S. ramulosum Delile [now S. irio Linnaeus], S. cinereum Desfontaines [now Ammosperma cinereum (Desfontaines) J.D. Hooker], S. torulosum Desfontaines [now Neotorularia torulosa (Desfontaines) Hedge & J. Léonard], and S. contortuplicatum Willdenow [now N. contortuplicata (Willdenow) Hedge & J. Léonard]. When Heynhold (in Holl and Heynhold, 1842) raised Arabidopsis to the generic rank, he listed A. thaliana as the sole member of the genus. Prior to the Berlin International Code of Botanical Nomenclature (Greuter et al., 1988), many botanists took A. thaliana as the type of the genus. However, such typification is erroneous because A. thaliana was not one of the original species that de Candolle (1821) assigned to section Arabidopsis. According to the Code (for the latest version, see Greuter et al., 2000), the genus must be typified by the type of the sect. Arabidopsis. However, de Candolle (1821) did not typify the section, and none of the original seven species he placed in it is now retained in Arabidopsis. Celakovsky (1872) retained one of the seven original de Candollean species, S. bursifolium, in Arabidopsis, thereby automatically lectotypfying the genus. However, this action would mean that Arabidopsis should be reduced to synonymy of the earlier published Rorippa Scopoli. The choice of any of the other six original de Candollean species as the type of Arabidopsis would mean that either Arabidopsis be reduced to synonymy of Sisymbrium, or Ammosperma J.D. Hooker, Murbeckiella Rothmaler, or Neotorularia Hedge & J. Léonard be reduced to synonymy of the earlier published Arabidopsis. Because of the importance of Arabidopsis thaliana in plant biology, taxonomists wanted to find a permanent solution to maintain this name regardless to the complicated nomenclatural past. The only solution was to conserve Arabidopsis with a conserved type, as proposed Stepánek (1983) and discussed by Al-Shehbaz (1988). The Berlin Code (Greuter et al. 1988) did just that.
Generic limits of Arabidopsis
The generic limits of Arabidopsis were expanded considerably by Busch (1909), and the five species he recognized in the genus (A. thaliana, A. pumila, A. toxophylla, A. verna, A. huetii) are currently placed in five different genera see the list of species excluded from Arabidopsis). Schulz (1924) accepted the first three species of Busch in Arabidopsis, but he expanded the limits of the genus further and added eight other species, of which one (A. parvula) was transferred by Al-Shehbaz and O'Kane (1995) to Thellungiella and the remaining seven to Crucihimalaya (Al-Shehbaz et al., 1999). Many other authors transferred to Arabidopsis dozens of species that were previously placed in the genera Arabis, Braya, Cardaminopsis, Cymatocarpus, Halimolobos, Microsisymbrium, Nasturtiopsis, Neotorularia, or Torularia As pointed out by Al-Shehbaz (1988), the limits of Arabidopsis became highly artificial because of the transfer of many species (with linear fruits and branched trichomes) that did not fit appropriately in other genera. As a result, Arabidopsis became so heterogeneous morphologically (see for example, the accounts of Hedge, 1968; Jafri, 1973; Ball, 1993) that it became almost impossible to delimit it.
The artificially delimited boundaries of Arabidopsis are reflected by numerous other cases in the mustard family (Brassicaceae). We believe that principal reasons for such artificiality is the heavy reliance by taxonomists on a few fruit and seed characters that show high convergence, and their placement of much less value on aspects of flowers, trichomes, and vegetative characters. In fact, convergence in the family is so common in almost every morphological character that superficially very similar genera might prove to be unrelated or remotely related. Because fruit and seed morphology has traditionally been used in taxonomy of the Brassicaceae, the problem becomes more acute among the numerous genera with apparently similar linear fruits and branched trichomes.
The pioneering molecular work on the genus and its presumed relatives (Price et al., 1994) stimulated subsequent studies on the taxonomy and phylogeny of the genus. Initial taxonomic attempts by Al-Shehbaz (1994) and Al-Shehbaz and O'Kane (1995, 1997) to exclude some of the anomalous species from Arabidopsis have paved the way for thorough morphological studies that led to the recognition of only nine species in this genus (O'Kane and Al-Shehbaz, 1997) and to the exclusion of the remaining species to several other genera (Al-Shehbaz et al., 1999). The latter two taxonomic studies went in parallel with extensive molecular phylogeny (O'Kane and Al-Shehbaz, in press) on the genus and almost all the species previously assigned to it. Subsequent molecular studies (e.g., Koch et al., 1999, 2000) provided full support to the taxonomic conclusions reached by O'Kane and Al-Shehbaz (1997) and Al-Shehbaz et al. (1999).
Distinguishing characters
The genus Arabidopsis can be distinguished from the related genera (see also the key below) by the presence of short petiolate but never auriculate or amplexicaul stem leaves, an indumentum of simple trichomes mixed with few-forked but never stellate ones, usually welldefined basal rosettes, white to lavender or rarely purple but never yellow flowers, at least slightly torulose, glabrous, compressed or rarely terete fruits with a distinct midvein, uniseriate, wingless or rarely winged seeds, and accumbent or rarely incumbent cotyledons. Arabidopsis can easily be confused with several genera, and the following key should readily separate the genus from those that currently house species previously placed in Arabidopsis.
Key to the genera with species previously placed in Arabidopsis
1a. Plants completely glabrous; leaves and stems glaucous; plants often restricted to strongly saline soils
1b. Plants sparsely to densely hairy; leaves and stems usuaaly not glaucous; plants (except Pseudoarabiopsis) typically on other soil types.
2a. Trichomes sessile and completely appressed, malpighiaceous and/or stellate with unbranched rigid straight rays
2b. Trichomes short or long stalked, simple or branched, if stellate and sessile then rays slender and/or branched.
3a. Scapose annuals without stem leaves; fruiting pedicel nearly as thick as fruit
3b. Nonscapose annuals, biennials, or perennials with few to many stem leaves; very rarely perennials without stem leaves; fruiting pedicels much narrower than fruit (except some Neotorularia).
4a. Fruits flattened; cotyledons accumbent.
5a. Stem leaves short petiolate, neither sagittate nor auriculate at base; trichomes simple and 2- or 3(or 4)-forked, never dendritic or stellate; fruit valves with a prominent midvein; seeds usually wingless
5b. Stem leaves sagittate or auriculate at base, rarely petiolate; at least some of leaf trichomes dendritic or stellate; fruit valve without or with obscure midvein; seeds often winged
4b. Fruits terete or 4-angled; cotyledons incumbent.
6a. Racemes rachis flexuous; leaves divided into 3 or 5 filiform to narrowly linear segments
6b. Racemes rachis not flexuous; leaves various but never divided into filiform or narrowly linear segments.
7a. Flowers yellow.
8a. Stem leaves petiolate; fruit apex strongly recurved or contorted: fruiting pedicels stout, nearly as thick as fruit
8b. Stem leaves auriculate, rarely sessile; fruit apex neither recurved nor contorted; fruiting pedicels slender, narrower than fruit
7b. Flowers white, lavender, or purple, very rarely creamy white.
9a. Fruits glabrous.
10a. Stem leaves petiolate; branched trichomes forked, rays always simple.
11a. Fruiting pedicels slender, narrower than fruit; seeds mucilaginous when wetted; fruits straight
11b. Fruiting pedicels stout, nearly as thick as fruit; seeds not mucilaginous when wetted; fruits often twisted
10b. Stem leaves sessile, often auriculate, sagittate, or amplexicaul, if short petiolate then plants canescent; at least some of the trichomes dendritic or stellate with some branched rays.
12a. All branched trichomes sessile; petals (6–)6.5–8(–9) mm; fruit short stipitate
12b. At least some of the branched trichomes distinctly stalked; petals 1.5–4(–5) mm; fruit sessile.
13a. Seeds uniseriate; Himalayas
13a. Seeds biseriate; North and South America, Russian Far East
14b. Perennial with caudex and persistent basal leaf rosette; stem leaves entire or rarely minutely toothed; Russian Far East, Alaska, N Canada, and northern USA
14b. Biennials without caudices or persistent basal leaf rosette; stem leaves coarsely dentate to sinuate; USA, Central and South America
9b. Fruit pubescent.
15a. Racemes bracteate at least along lower half
15b. Racemes ebracteate.
16a. Fruit with submalpighiaceous or short-stalked to subsessile stellate trichomes; septum lacking or often perforated
16b. Fruit with other trichome types; septum complete.
17a. Seeds not mucilaginous when wetted, uniseriate; fruit often strongly torulose and/or twisted; stems often decumbent; predominantly Old World
17b. Seeds mucilaginous when wetted, mostly biseriate; fruit often smooth; stems not decumbent; exclusively New World
Cytology
This section includes a summary of the chromosome counts made thus far on the species presently assigned to Arabidopsis. Matters relating to meiotic irregularities can easily be obtained by consulting the references cited in Table 1, and the space herein does not permit full coverage of that and the meiosis in artificial interspecific hybrids.
Table 1.
Chromosome count in Arabidopsis species

Table 1.
Chromosome count in Arabidopsis species (continued)
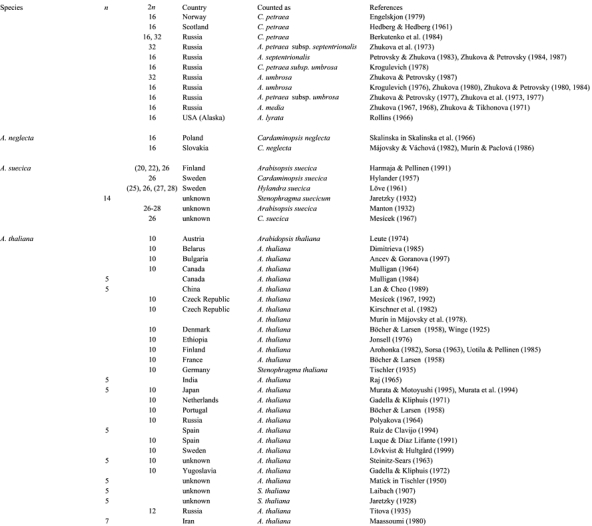
Haploid chromosome numbers n = 5, 8, 13, and 16 have been reported in the genus (Table 1). Only Arabidopsis thaliana is known to have n=5. The count n=13 characterizes A. suecica, an amphidiploid species originated through hybridization between A. thaliana and A. arenosa. Diploid and tetraploid counts based on x=8 are known in A. neglecta and the various subspecies of A. lyrata and A. arenosa. All three subspecies of A. halleri show 2n=16, but additional counts are needed, especially from populations in eastern Asia. The remaining species of Arabidopsis, A. cebennensis, A. croatica, and A. pedemontana, have not yet been investigated cytologically. Counts deviating from the norm (see Table 1) probably represent rare aberrations. Only the normal diploid counts are listed under each species.
Taxonomy
The present formal taxonomic account of Arabidopsis includes full descriptions of the genus and all of its nine species and eight subspecies, keys to all taxa, distributions, and habitats. It also includes a list of the species previously included in Arabidopsis but currently assigned to other genera, with full citations of the original publications of those species and their current assignments.
Arabidopsis (DC.) Heynhold in Holl & Heynhold, Fl. Sachsen 1: 538. 1842; nom. cons. Type: A. thaliana (L.) Heynhold, typ. cons.
Cardaminopsis (C. A. Meyer) Hayek, Fl. Steiermark 1: 477. 1908. Basionym: Arabis sect. Cardaminopsis C. A. Meyer in Ledeb., Fl. Altaic. 3: 19. 1831. Type not designated.
Hylandra Á. Löve, Svensk Bot. Tidskr. 55: 214. 1961. Type: H. suecica (Fries) Á. Löve.
Plants annual, biennial, or perennial herbs with stolons or woody caudex. Trichomes unicellular, eglandular, simple mixed with stalked 1–3-forked or rarely 4-rayed trichomes. Roots forming a taproot system, very rarely adventitious. Stems erect to ascending or decumbent, 1 or frequently several to many from base, few branched above, always leafy, usually glabrous above. Basal leaves petiolate, forming well-developed rosettes, simple, entire toothed, lyrate, to pinnately lobed. Stem leaves petiolate to subsessile and cuneate to attenuate at base, never auriculate or sagittate, entire, dentate, or rarely lyrate. Racemes few to several flowered, dense or lax, ebracteate, corymbose, elongated considerably in fruit; rachis straight. Fruiting pedicels slender, terete, ascending to divaricate or slightly reflexed. Sepals oblong, free, erect to ascending, glabrous or pubescent, equal, margin membranous or not, base of inner pair slightly saccate or not saccate. Petals white, pink, or purple, erect at base with flaring blade, longer than sepals; blade obovate to spatulate or oblanceolate, apex obtuse to emarginate, entire; claw obscurely differentiated from blade or distinct, glabrous, entire. Stamens 6, exserted, erect, tetradynamous; filaments filiform, wingless, unappendaged, glabrous, base slightly dilated or not dilated; anthers oblong, rounded to sagittate at base, obtuse at apex, not coiled after dehiscence. Nectar glands confluent and subtending bases of all stamens; median nectaries sometimes distinct and not confluent; lateral nectaries lunar or annular. Ovules 15–80 per ovary. Fruit dehiscent, capsular siliques, linear, terete or latiseptate (flattened parallel to the septum), not inflated, short stipitate to rarely subsessile, unsegmented; valves papery, veinless or with a distinct midvein, glabrous, not keeled, smooth or somewhat torulose, wingless, unappendaged; gynophore to 1 mm long; replum rounded; septum complete, membranous; style obsolete or to 1 mm long, cylindric, persistent, glabrous; stigma capitate, entire. Seeds numerous per locule, uniseriate, wingless or margined, oblong to ellipsoid, plump or flattened; seed coat minutely reticulate, mucilaginous or not mucilaginous when wetted; cotyledons accumbent or rarely incumbent.
Number of species and distribution: nine species distributed primarily in Europe, with two extending into northern and eastern Asia and one into northern North America. Arabidopsis thaliana is naturalized throughout the world. The distribution of the European taxa follows closely the account of Jalas & Suominen (1994), that of North American taxa follows Hopkins (1937) and Rollins (1941, 1993), and that of Asian taxa follows Berkutenko (1988), Busch (1939), and Tolmachew (1975).
Key to the species of Arabidopsis
1a. Fruits terete; petals 2–3.5(-4) mm long; seeds 0.3–0.5 mm long, usually plump; cotyledons incumbent
1b. Fruits strongly flattened, rarely subterete (A suecica); petals (4-)5–10 mm long; seeds longer, usually flattened; cotyledons accumbent, rarely obliquely incumbent (A. suecica).
2a. Replum constricted between seed; plants stoloniferous perennials; basal leaves orbicular or often with an orbicular terminal lobes
2b. Replum not constricted between seeds; plants annuals or almost always non-stoloniferous perennials with or without branched caudices; basal leaves lanceolate, spatulate, oblanceolate, obovate, or rarely suborbicular, without orbicular terminal lobes.
3a. Lower and middle stem leaves distinctly petiolate, suborbicular to ovate, nearly as long as wide, dentate with few large teeth.
4a. Petals 7–10 mm long, violet or very rarely white; plants (25-)40–80 cm tall, sparsely pubescent; basal leaves acute at apex, longer than wide
4b. Petals 6–7 mm long, white; plants 10–30 cm tall, plants usually glabrous; basal leaves obtuse at apex, “as long as wide
3b. Lower and middle stem leaves subsessile or attenuate to a narrow base, much longer than broad, entire, dentate, lyrate, to pinnatifid.
5a. Basal and stem leaves similar in shape and size (rarely stem leaves larger than basal)
5b. Basal and stem leaves dissimilar in shape and size, basal leaves always larger.
6a. Stems glabrous or rarely subglabrous; fruits horizontally spreading to slightly reflexed; petals becoming deep lilac; alpine areas in the Carpathian Mountains (Czech Republic, Slovakia, Romania, Poland, and adjacent Ukraine)
6b. Stems sparsely to densely hairy at least below; fruits erect-ascending to subdivaricate; petals white or rarely pale lilac; sea level to various altitudes in Eurasia and North America.
7a. Fruits weakly compressed to subterete; petals white, 4–5(-6) mm long; cotyledons obliquely incumbent
7b. Fruits strongly compressed; petals white to lilac, (5-)6–8 mm long; cotyledons accumbent.
8a. Petals with two small lateral teeth on the claw; basal leaves pinnatisect to pinnatipartite
8b. Petals without lateral teeth on the claw; basal leaves lyrate-pinnatifid, lyrate, dentate, or entire
1. Arabidopsis thaliana (Linnaeus) Heynhold in Holl & Heynhold,. Fl. Sachsen 1: 538. 1842.
Synonyms: Arabis thaliana Linnaeus, Sp. Pl. 2: 665. 1753; Conringia thaliana (Linnaeus) Reichenbach, Fl. Germ. Excurs. Il. 686. 1826; Crucifera thaliana (Linnaeus) E.H.L. Krause, in Sturm, Fl. Deutschland, ed. 2, 6: 86. 1902; Erysimum thalianum (Linnaeus) Kittel, Taschenb. Fl. Deutschl. ed. 2, 899. 1844; Hesperis thaliana (Linnaeus) Kuntze, Rev. Gen. 2: 935. 1891; Pilosella thaliana (Linnaeus) Kosteletzky, Ind. Pl. Hort. Prag. 104. 1844; Sisymbrium thalianum (Linnaeus) J. Gay & Monnard, Ann. Sci. Nat. Bot., ser. 1, 7: 399. 1826; Stenophragma thalianum (Linnaeus) Celakovsky, Kvet. Ok. Prazs. 75. 1870. Type: “Hab. in Europae septentrionalis sabulosis” (lectotype, LINN 842.5).
Cardamine pusilla A. Richard, Tent. Fl. Abyss. 1: 18. 1847; Arabidopsis thaliana var. pusilla (A. Richard) O.E. Schulz in Engler, Pflanzenr. IV. 105(Heft 86): 274. 1924; Sisymbrium pumilio, Oliver, Fl. Trop. Afr. 1: 64. 1868; Sisymbrium thalianum (Linnaeus) Gay & Monnard var. pusillum (A. Richard) T. Durand & Schinz, Consp. Fl. Afr. 1(2): 99. 1898; Stenophragma thaliana (Linnaeus) Celakovsky var. pusilla (A. Richard) Engler, Hochgebirgsfl. Trop. Afr. 226. 1895. Type: Ethiopia, Begemdir, Schimper II.1311 (holotype, P; isotype, K).
Annual herbs (2–)5–30(–60) cm tall. Stems erect, 1 to many from the base, simple or branched above, basally pilose with predominantly simple trichomes, apically glabrous. Basal leaves shortly petiolate, rosulate; leaf blade obovate, spatulate, ovate, or elliptic, 0.8–3.5(–4.5) × (0.1–)0.2–1(–1.5) cm, adaxially with predominantly simple and stalked 1-forked trichomes, margin entire, repand, serrulate, or dentate, apex obtuse. Stem leaves subsessile or sessile, usually few; blade lanceolate, linear, oblong, or elliptic, middle ones (0.4–)0.6–1.8(–2.5) × 0.1–0.6(–1) cm, margin entire or rarely sparsely dentate, apex acute to obtuse. Fruiting pedicels slender, divaricate, straight, 3–10(–15) mm long. Sepals 1–2(–2.5) mm long, glabrous or distally sparsely pubescent with simple trichomes, lateral pair not saccate. Petals white, spatulate, 2–3.5(–4) × 0.5–1.5 mm, apex obtuse, base attenuate to a short claw. Filaments white, 1.5–2 mm long. Ovules 40–70 per ovary. Siliques linear, terete, smooth, (0.8–)1–1.5(–2) cm × 0.5–0.8 mm; valves with a distinct midvein; style to 0.5 mm long. Seeds ellipsoid, plump, light to reddish brown, 0.3–0.5 mm long; cotyledons incumbent. Flowering and fruiting January–June(–October). 2n=10.
Distribution: native range almost all Europe to central Asia, now naturalized worldwide. Throughout Europe (excluding Iceland), most of Asia (excluding Bahrain, Bangladesh, Bhutan, Cambodia, Iraq, Israel, Jordan, Kuwait, Kyrgyzstan, Laos, Malaysia, Myanmar, Nepal, New Guinea, Oman, Philippines, Qatar, Sri Lanka, Taiwan, Thailand, Turkmenistan, United Arab Emirates, Vietnam, Yemen), Australia and New Zealand, Africa (Algeria, Eritrea, Ethiopia, Kenya, Libya, Morocco, South Africa, Tanzania, Tunisia, Uganda, Zaire), North America (Canada, U.S.A.), and South America (Argentina, Uruguay).
Habitats: open or disturbed habitats, sandy soil, river banks, roadsides, rocky slopes, waste places, cultivated ground, meadows, slightly alkaline flats, under shrubs, open areas; sea level to 4250 m.
2. Arabidopsis suecica (Fries) Norrlin, Meddel. Soc. Fauna Fl. Fenn. 2: 12. 1878.
Synonyms: Arabis suecica Fries, Summ. Veget. Scand. I, 30, 147. 1846; A. arenosa (Linnaeus) Scopoli subsp. suecica (Fries) Ahlfvengren in Neuman & Ahlfvengren, Sveriges Flora 453. 1901; A. arenosa var. suecica (Fries) Cajander in Mela, Suomenkasvio, ed. 5, 295. 1906; Cardaminopsis suecica (Fries) Hiitonen ex Hylander, Forteckn. Skand. Vaxt., 1. Karlvaxter, 62, 139. 1941; Hylandra suecica (Fries) Á. Löve, Svensk Bot. Tidskr. 55: 215. 1961; Sisymbrium suecicum (Fries) Nyman, Consp. Fl. Europ. 44. 1878; Stenophragma suecicum (Fries) Celakovsky ex Prantl in Engler & Prantl, Pflanzenfam. 3(2): 192. 1891. Type: Sweden. Prov. Södermanland: Strängnäs, Fries s. n. (lectotype, UPS; see Löve, 1961).
Annual herbs (5–)10–45(–60) cm tall. Stems erect, 1-5-8) from the base, usually branched above, basally pilose with simple trichomes to 1 mm long, apically glabrous. Basal leaves rosulate; petiole (0.3-)0.6–2(-2.8) cm long, ciliate; leaf blade obovate to oblanceolate, (0.3-)0.7–3(–4) × (0.1–)0.2–1.4(–1.7) cm, sparsely to densely pubescent with simple and short-stalked forked trichomes, rarely subglabrous, margin lyrate to pinnatifid on each side with 3–6 lateral lobes much smaller than terminal one, rarely dentate, apex obtuse to subacute. Stem leaves 3–8, short petiolate or uppermost sessile; blade lanceolate, oblong, or linear, middle ones (0.3–)1–2.5(–4) × 0.1–0.8(–1.3) cm, margin dentate on lower leaves, entire on upper ones, apex acute to obtuse. Fruiting pedicels slender, divaricate, straight, 5–10(–15) mm long. Sepals 1.5–2.2(–2.5) mm long, glabrous or distally sparsely pilose with simple trichomes, lateral pair saccate. Petals white to lavender, spatulate, 4–5(-6) × 1.5–2.5 mm, apex obtuse or truncate, base attenuate to a short claw. Filaments white, 2.5–4 mm long. Ovules 40–70 per ovary. Siliques linear, subterete to slightly compressed, smooth to slightly torulose, (2–)2.5–3.5(–4.5) cm × 0.5–0.8 mm; valves with a distinct or obscure midvein; style to 0.5 mm long; gynophore to 0.5 mm long. Seeds oblong, slightly compressed, light to reddish brown, 0.5–0.8 mm long; cotyledons obliquely incumbent. Flowering and fruiting May–July. 2n = 26.
Distribution: Fennoscandinavia and the Baltic region but perhaps native only in Finland, adventive in adjacent northwestern Russia, Estonia, northeastern Denmark, Sweden, Norway, and north Germany (see Jalas & Suominen, 1994).
Habitats: fields, roadsides, slopes, moist forest margins, grassy areas; near sea level to 600 m.
Arabidopsis suecica is an amphidiploid produced since the last glacial maximum from the hybridization of A. thaliana and diploid A. arenosa (Mummenhoff and Hurka, 1994, 1995; O'Kane et al., 1997, and references therein).
3. Arabidopsis arenosa (Linnaeus) Lawalrée, Bull. Soc. Roy. Bot. Belgique 92: 242. 1960.
Biennial or short-lived perennial with a caudex, (5–)8–80(–100) cm tall. Stems erect, simple or with few to many branches from the base, usually branched above, basally pilose to subhirsute with simple trichomes to 1 mm long, apically glabrous. Basal leaves rosulate; petiole (0.3–0.7–2(-3) cm long, pilose; leaf blade obovate to oblanceolate, 1–4.5(–6.5) × 0.5–1.3(–1.7) cm, sparsely to densely pubescent with simple and short-stalked forked trichomes, margin pinnatifid to lyrate-pinnatifid and with 3–11 lateral lobes on each side, rarely dentate, apex obtuse to subacute; terminal lobe considerably larger or nearly as large as adjacent lateral lobes. Stem leaves 3–10, short petiolate or uppermost sessile; blade lanceolate to narrowly oblong, middle ones 1–4 (–5) × 0.3–1.5 cm, margin pinnatifid to dentate on lower leaves, dentate or entire on uppermost ones, apex acute to obtuse. Fruiting pedicels slender, divaricate, straight, 4–10(–15) mm long. Sepals 2.2–3 mm long, glabrous or distally sparsely pilose with simple trichomes, lateral pair saccate. Petals white to lavender, obovate, 5–8 × 2.5–4 mm, apex obtuse or truncate, base attenuate to a short claw often apically with a pair of minute teeth. Filaments white, 3–4 mm long. Ovules 28–74 per ovary. Siliques linear, distinctly compressed, smooth to slightly torulose, (1–)2–4(–5) cm × (0.8–)1–1.5 mm; valves with a distinct midvein; style to 0.5 mm long; gynophore to 0.5 mm long. Seeds oblong, compressed, light to reddish brown, (0.6–)0.8–1.2 mm long, obscurely to prominently winged distally; cotyledons accumbent. Flowering and fruiting April–July or rarely to August. 2n=16, 32.
1a. Basal leaves with terminal lobe larger than the 1–6 lateral lobes on each side; seeds very narrowly winged distally or rarely wingless; ovules 36–74 per locule
1b. Basal leaves with terminal lobe subequal to or scarcely larger than the 4–11 lateral lobes on each side; seeds conspicuously winged distally; ovules 28–38 per locule
3a. Arabidopsis arenosa subsp. arenosa
Synonyms: Sisymbrium arenosum Linnaeus, Sp. Pl. 2: 658. 1753; Arabis arenosa (Linnaeus) Scopli, Fl. Carniol., ed. 2, 2: 32. 1772; Arabis petraea (Linnaeus) Lamarck var. arenosa (Linnaeus) Neilreich, Nachtr. Fl. Wein. 262. 1851; Cardamine arenosa (Linnaeus) Roth, Man. Bot. 291. 1830; Cardaminopsis arenosa (Linnaeus) Hayek, Fl. Steiermark 1: 478. 1908. Type: “In Germania, Helvetica” (holotype, LINN 836.22).
Arabis petrogena A. Kerner, Oesterr. Bot. Z. 13: 141. 1863; Arabis arenosa var. petrogena (A. Kerner) Borbás; Cardaminopsis arenosa subsp. petrogena (A. Kerner) Soó, Acta Bot. Acad. Sci. Hung. 16: 371. 1971; C. petrogena (A. Kerner) Mesicek, Preslia 42: 246. 1970. Type: “in locis petrosis mintium circa Budam; communissima in monte Adlersberg et in rupibus dolmaticis vallis Auwinkel” (syntypes, WU).
Arabis segetalis Schur, Enum. Pl. Transsilv. 45. 1866; Cardaminopsis arenosa var. segetalis (Schur) E.I. Nyárády in Savulescu, Fl. Reipubl. Popularis Roman. 3: 291. 1955. Type: “Auf Aeckern unter Saaten, an der Strasse nach Schellenberg bei Hermannstadt.” (holotype, not located).
Arabis arenosa var. simplex Neilreich, Fl. N.-Öster. 715. 1859; Cardaminopsis arenosa var. simplex (Neilreich) Hayek, Fl. Steiermark 1: 479. 1908. Type: “in ihrer typischen Gestalt am schönsten auf den Inseln und in den Auen dr Donau” (syntypes, W).
Cardaminopsis arenosa var. peregrina Lawalrée, Bull. Jard. Bot. État Bruxelles 26: 350. 1956; Arabidopsis arenosa var. runcinata (Godron) Lawalrée f. peregrina (Lawalrée) Lawalrée, Bull. Soc. Roy. Bot. Belgique 9: 243. 1960. Type: Belgium, Etterbeek, Etterbeek-Cinquantenaire, May 1924, E. Michel s.n (holotype, BR).
Plants annual or biennial, rarely perennial. Basal leaves pinnatisect, with 1–6 lateral lobes on each side, rarely dentate; terminal lobe considerably larger than adjacent lateral lobes. Petals white to lilac. Ovules 36–74 per locule. Seeds very narrowly winged at least distally or rarely wingless. 2n=16, 32; for aneuploid series, see Table 1.
Distribution: Europe: native in Austria, Belarus, Bosnia Herzegovina, Bulgaria, Croatia, Czech Republic, NE France, Germany, Hungary, N Italy, Latvia, Lithuania, Macedonia, Poland, Romania, Slovakia, Slovenia, Switzerland, Ukraine, and Yugoslavia; naturalized in Belgium, Denmark, Estonia, Finland, Holland, Norway, Russia and W Siberia, and Sweden; absent in Albania, Greece, C and S Italy, and Turkey.
Habitats: forest margins, roadsides, railroad tracks, river banks, disturbed areas, slopes, fields, grassy areas; near sea level to 2000 m.
This subspecies includes a multitude of chromosomal races that were recognized by Mesicek (1970) as distinct species. However, his taxa are weakly defined and were not legitimately published. Therefore, they have no taxonomic status. Subspecies arenosa is easily confused with Arabidopsis suecica but can be readily distinguished by having narrowly winged seeds, accumbent cotyledons, strongly flattened fruits, and petals with a pair of minute teeth between the blade and claw. By contrast, A. suecica differs by having wingless seeds, obliquely incumbent cotyledons, subterete to slightly flattened fruits, and toothless petals.
3b. Arabidopsis arenosa (Linnaeus) Lawalrée subsp. borbasii (Zapalowicz O'Kane & Al-Shehbaz, Novon 7: 325. 1997.)
Synonyms: Arabis arenosa (Linnaeus) Scopli subsp. borbasii Zapalowicz, Rozpr. Wydz. Mat.-Przyr. Acad. Umiejetn., Dzial B, Nauki Biol. 52: 31. 1912; Cardaminopsis arenosa (Linnaeus) Hayek subsp. borbasii (Zapalowicz Pawlowski ex H. Scholz, Willdenowia 3: 139. 1962; C. arenosa var. borbasii (Zapalowicz E.I. Nyárády in Savulescu, Fl. Reipubl. Popularis Roman. 3: 288. 1955; C. borbasii (Zapalowicz H. Hess, Landolt & Hirzel, Fl. Schweiz, Fl. Schweiz. 3: 778. 1972. Type: Poland. Carpathians, Babia Góra monte, 1285–1725 m, Zapalowicz s. n (lectotype, designated by O'Kane & Al-Shehbaz (1997), KRAM).
Arabis arenosa var. dependens Borbás, Math. Term. Közl. 15: 156. 1878; Cardaminopsis arenosa var. dependens (Borbás) Jávorka, Magyar Fl. 436. 1924; C. arenosa subsp. borbasii var. dependens (Borbás) Soó, Acta Bot. Acad. Sci. Hungar. 13: 304. 1967; C. dependens (Borbás) Kotov, comb. invalid.
Arabis arenosa var. multiceps Neilreich, Fl. N. Öster. 715. 1859; Arabis multiceps (Neilreich) Favarger & Rch., Abh. Zool. Bot. Ges. Wien 3: 31. 1905, not A. multiceps Greene, Leaflets Bot. Obs. 2: 76. 1910. Type: “Flussgebienten der Peisting, Schwarza, Traisen” (syntypes, W).
Arabis arenosa var. runcinata Godron, Fl. de Lorraine, ed. 2, 1: 60. 1857; Arabidopsis arenosa var. runcinata (Godron) Lawalrée, Bull. Soc. Roy. Bot. Belgique 92: 242. 1960. Type: “Nancy, aux Fonds Saint Barthelémy et à Liverdun (Soyer-Willemet); Pont-à-Mousson (Léré); Rosières-aux Salines; Lunéville” (syntypes, NCY).
Arabis arenosa var. sarmentosa Schur, Enum. Pl. Transsilv. 44. 1866. Type: “Auf dem Königstein bei Kronstadt, kalk., Aug., 6000'” (holotype, not located).
Arabis hispida Mygind var. intermedia Freyn, Österr. Bot. Z. 39: 133. 1889; A. freynii Brügger, Österr. Bot. Z. 39: 231. 1889. Type: Salzburg, J. Freyn s.n. (holotype, BRNM?).
Arabis multijuga Borbás, Linnaea, 41: 604. 1877; Arabis arenosa subsp. multijuga (Borbás) Kulczynski in Szafer, Fl. Polska 3: 162. 1927; A. arenosa var. multijuga (Borbás) Zapalowicz, Rozpr. Wydz. Mat.-Przyr. Acad. Umiejetn., Dzial B, Nauki Biol. 52: 35. 1912; Cardaminopsis multijuga (Borbás) Czerepanov, Plantae Vasculares URSS 127. 1981; C. arenosa var. multijuga (Borbás) Kotov in Klukov & E.D. Wissjulina, Fl. RSS Ucr. 5: 290. 1953. Type: Gutin, Borbás s.n (holotype, BP or BPU).
Arabis petraea (Linnaeus) Lamarck var. intermedia Neilreich, Nachtr. Fl. Wien. 262. 1851; Cardaminopsis arenosa var. intermedia (Neilreich) Hayek, Fl. Steiermark 1: 479. 1908. Type: “Gemein auf Felsen der kalkgebirge und in Sand subalpiner Bäche in der höhern Bergun Voralpenregion besonders im Flossgebiete des kaltenganges und der Schwarza steight night über 3000'” (syntypes, W).
Plants usually perennial. Basal leaves pinnatisect, with 4–11 lateral lobes on each side; terminal lobe about as large as adjacent lateral lobes. Petals bright lilac. Ovules 28–38 per locule. Seeds broadly winged at least distally. 2n=16, 32.
Distribution: E Belgium, Czech Republic, NE France, Germany, Hungary, Poland, Romania, Slovakia, Switzerland, Ukraine. Doubtfully occurring in Denmark.
Habitats: forest margins, slopes, stream banks, trails; 400–2100 m.
Unlike the many other segregates of Arabidopsis arenosa, subsp. borbasii is a well-defined taxon that can be consistently distinguished from subsp. arenosa (see key). Subspecies borbasii has variously been split into poorly defined races, but none of those merits recognition.
Zapalowicz (1912) indicated that interspecific hybridization between Arabidopsis (as Arabis) arenosa subsp. borbasii and A. halleri occurs in Tatra mountains (Poland).
4. Arabidopsis neglecta (Schultes) O'Kane & Al-Shehbaz, Novon 7: 326. 1997.
Synonyms: Arabis neglecta Schultes, Oestr. Fl. 2: 248. 1814; Arabis arenosa (Linnaeus) Scopli subsp. neglecta (Schultes) Zapalowicz, Rozpr. Wydz. Mat.-Przyr. Acad. Umiejetn., Dzial B, Nauki Biol. 52: 36. 1912; Cardaminopsis neglecta (Schultes) Hayek, Fl. Steiermark 1: 480. 1908. Type: Carpathians, ?Schultes s.n. (holotype, M).
Arabis floribunda Schur, Enum. Pl. Transsilv. 44. 1866. Type: “Auf steinigen Abhängen der Rodnaer Alpen, 6,500¢, in Gesellschaft von Lychn.” (holotype, not located)
Arabis glareosa Schur, Verh. Mitth. Siebenbürg. Vereins. Naturwiss. Hermannstadt 1: 106. 1850; Cardaminopsis neglecta subsp. glareosa (Schur) Soó, Acta Bot. Acad. Sci. Hung. 16: 371. 1971. Type: “Auf den Rodnaer Alpen von Herrn Albert Bielz, auf den Arpáscher Alpen von mir 1847” (holotype, not located).
Arabis transsilvanica Schur, Enum. Pl. Transsilv. 43. 1866. Type: “Auf feuchtem schlammigen Boden der Hochalpen, Glimmerschiefer, 6,000¢, an Quellen und kleinen Bächen der Arpaser Alpen” (holotype, not located).
Perennial herbs, with a distinct caudex, 5–15(–20) cm tall. Stems erect, simple or few branched, glabrous. Basal leaves rosulate; petiole to 2 cm long; leaf blade obovate to oblanceolate, 0.5–2 × 0.3–1 cm, fleshy, glabrous or sparsely pubescent with simple and fewer, short-stalked forked trichomes, margin subentire to lyrate-pinnatifid with up to 4 teeth or small lateral lobes on each side. Stem leaves 3–6, attenuate to a short petiole; blade ovate to oblanceolate, usually glabrous, entire or basally minutely 2-toothed. Fruiting pedicels slender, recurved or reflexed, 8–12 mm long. Sepals 2.5–3.5 mm long, glabrous or distally sparsely pilose with simple trichomes, lateral pair saccate. Petals purple, 5–6 × 2–3 mm, apex obtuse, base attenuate to a short claw. Filaments lavender, 3–4.5 mm long. Ovules ca. 30 per ovary. Siliques linear, distinctly compressed, smooth to slightly torulose, (1–)1.5–2.5(–3) cm × 1.2–1.8 mm; valves with a distinct midvein or obscurely veined; style to 1 mm long, slender. Seeds oblong, compressed, light brown, 1–1.2 mm long, wingless; cotyledons accumbent. Flowering and fruiting May–July. 2n=16, 32.
Distribution: Carpathian Mountains (Poland, Romania, Slovakia, and adjacent Ukraine).
Habitats: mountain slopes, forest margins; 1200–2660 m.
5. Arabidopsis croatica (Schott) O'Kane & Al-Shehbaz, Novon 7: 325. 1997.
Synonyms: Arabis croatica Schott, in Schott, Nyman, & Kotschy, Analect. Bot. 44. 1854.
Cardaminopsis croatica (Schott) Jávorka, Magyar Fl. 435. 1924. Type: Croatia, F. Maly s. n. (holotype, BP?).
Perennial herbs, with a distinct caudex, 8–18(–20) cm tall. Stems erect, divaricately branched, flexuous, glabrous. Basal leaves rosulate; petiole to 3 cm long; leaf blade obovate, 0.5–2 × 0.4–1.5 cm, glaucous, fleshy, glabrous or sparsely pubescent with simple and fewer, short-stalked forked trichomes, margin entire to denticulate, rarely lyrate-pinnatifid. Stem leaves similar to basal ones, sometimes larger. Fruiting pedicels slender, horizontal or reflexed, 7–10 mm long. Sepals 2.5–3.5 mm long, glabrous or distally sparsely pilose with simple trichomes, lateral pair saccate. Petals lavender to purplish, 4–6 × 2–2.5 mm, apex obtuse, base attenuate to a short claw. Filaments lavender, 2.5–3.5 mm long. Ovules ca. 50 per ovary. Siliques linear, compressed, smooth to slightly torulose, 1.8–5 cm × 0.9–1.1 mm; valves obscurely veined; style to 1 mm long, slender. Seeds oblong, compressed, light brown, ca. 1 mm long, wingless; cotyledons accumbent. Flowering and fruiting May–July.
Distribution: Bosnia, Croatia.
Habitats: Rocky areas, crevices.
Arabidopsis croatica is morphologically very close to and might represent a subspecies of the earlier published A. arenosa. Only a limited material was available for our study, and more collections are needed to understand the variation within the species. No chromosome counts have been made thus far.
6. Arabidopsis cebennensis (de Candolle) O'Kane & Al-Shehbaz, Novon 7: 325. 1997.
Synonyms: Arabis cebennensis de Candolle, Syst. Nat. 2: 234. 1821; Cardaminopsis cebennensis (de Candolle) Burdet in Greuter & Raus, Willdenowia 13: 86. 1983. Type: [France], “in locis asperis subumbrosis montium Cebennorum in horto Dei et Bramabiou” (holotype, G-DC).
Perennial herbs (25-)40–80 cm tall. Stems erect, simple at base, branched above, pilose with simple trichomes mixed with stalked forked ones. Basal leaves rosulate; petiole (1-)1.5–4 cm long; leaf blade ovate to ovatelanceolate, 2–7 × 1.5–4.5 cm, sparsely to densely pubescent with stalked, forked or 3-rayed trichomes, base cuneate, margin coarsely and irregularly few toothed, apex acute. Stem leaves 7–15, similar to basal leaves, becoming smaller, short petiolate, and basally attenuate upward. Fruiting pedicels divaricate, slender, 6–12(-15) mm long. Sepals oblong, 2.5–3.5 mm long, lateral pair saccate. Petals deep purple, lavender, or very rarely white, obovate, 7–10 × 2.5–4 mm, slightly spreading. Filaments usually lavender, 3–4.5 mm long. Ovules 30–44 per ovary. Fruit linear, (2-)2.5–4.5(-5) cm × 1–1.5 mm, strongly compressed, torulose; valves with a distinct midvein; gynophore to 1 mm long; styles slender, 0.8–1.2 mm long. Seeds reddish brown, compressed, 1.2–1.6 mm long, narrowly winged distally; cotyledons accumbent. Flowering and fruiting June–August.
Distribution: SE France.
Habitats: slopes, forest margins; 600–2300 m.
A very rare and poorly known species for which no chromosome counts have been made. It is most closely related to the following species
7. Arabidopsis pedemontana (Boissier) O'Kane & Al-Shehbaz, Novon 7: 326. 1997.
Synonyms: Arabis pedemontana Boissier, Diagn. Pl. Orient. 1: 69. 1843; Arabis cebennensis de Candolle subsp. pedemontana (Boissier) P. Fournier, Quatre Fl. France 421. 1936; Cardaminopsis pedemontana (Boissier) Burdet in Greuter & Raus, Willdenowia 13: 86. 1983. Type: [Switzerland], “In regione alpina montium Pedemontii, legi Aug. supra limitem arborum in collo inter Crissolo et Luzerna sitio” (holotype, G).
Perennial herbs 10–30 cm tall. Stems erect, simple or branched above, glabrous or subglabrous. Basal leaves rosulate; petiole (1.5-)2.5–6(-7) cm long; leaf blade orbicular to broadly ovate-cordate, (1.5-)2–5 × (2-)2.5–5.5 cm, glabrous or very rarely sparsely pubescent with simple and stalked forked trichomes, base truncate to cordate, margin coarsely and irregularly few toothed or entire, apex obtuse to subacute. Stem leaves 3–6, smaller than basal ones, petiolate, broadly ovate or uppermost subsessile and lanceolate, gradually reduced in size upward, base cuneate, margin coarsely dentate, apex acute. Fruiting pedicels divaricate to ascending, slender, 7–12(-15) mm long. Sepals oblong, 2.5–3.5 mm long, lateral pair saccate. Petals white, obovate, 6–7 × 2–3.5 mm. Filaments white, 2.5–4 mm long. Ovules 20–30 per ovary. Fruit linear, 1.5–3 cm × 1–1.5 mm, strongly compressed, slightly torulose; valves with a distinct midvein; gynophore to 0.5 mm long; styles slender, to mm long. Seeds reddish brown, compressed, 0.9–1.4 mm long, broadly winged at least distally; cotyledons accumbent. Flowering and fruiting June–August.
Distribution: northwestern Italy and, presumably extinct, in adjacent SW Switzerland.
Habitats: mountain slopes, forest margins.
Arabidopsis pedemontana is morphologically similar to A. cebennensis except for being smaller (10–30 cm instead of (25-)40–80 cm) and glabrous or subglabrous instead of sparsely to densely pubescent. It has not yet been studied for chromosome numbers, and little is known about its morphological variation.
8. Arabidopsis lyrata (Linnaeus) O'Kane & Al-Shehbaz, Novon 7: 325. 1997.
Herbs biennial or perennial with a caudex and stolons, (5–)10–30(–45) cm tall. Stems erect or decumbent, 1 to numerous from base, usually branched and straight or flexuous above, basally pilose or subhirsute with simple and forked trichomes, apically glabrous. Basal leaves rosulate; petiole 0.4–2(–6) cm long; leaf blade oblanceolate or ovate, (0.5–)1–3(–8) × (0.3–)0.5–1.5 cm, adaxially with a mixture of simple and stalked, 1-forked trichomes, rarely glabrous, margin entire, dentate, lyrate, or pinnatifid with 1–3 lateral lobes on each side, apex obtuse. Stem leaves shortly petiolate and uppermost often sessile, several; blade of middle ones oblanceolate, (0.5–)1–3.5(–5.5) × (0.1–)0.2–0.7(–1.3) cm, entire, repand, or obscurely toothed, rarely lower ones lobed, gradually reduced in size upward. Fruiting pedicels slender, ascending to divaricate, straight, (0.4–)0.7–1.2(–1.5) cm long. Sepals 2–3 mm long, glabrous or densely pubescent, lateral pair saccate. Petals white, rarely lavender or purplish, spatulate or obovate, 4–8(-9) × 1.5–3(–3.5) mm. Filaments white, 2–3 mm long. Ovules 20–36 per ovary. Siliques linear, flattened, torulose, (1–)2–4.5(–6) cm × 0.5–1.5 mm; valves with a distinct midvein extending full length; gynophore to 0.5 mm long; style obsolete or to 1 mm long. Seeds oblong, flattened, light brown, 0.8–1.2 mm long, wingless, sometimes distally margined; cotyledons accumbent. Flowering and fruiting March–October. 2n=16, 32.
Arabidopsis lyrata is a circumboreal species divided here into three subspecies that can distinguished with some difficulty, especially in areas where their distribution ranges overlap. Busch (1939) and Mulligan (1995) treated the three subspecies of A. lyrata as distinct species, but as shown in the key above, the morphological differences between them do not support such a treatment.
1a. Basal leaves entire or toothed; older plants with an obvious, somewhat thickened, often branched caudex
1b. Basal leaves mostly lyrate to lyrate-pinnatifid; older plants with a slender, simple or rarely branched caudex.
2a. Basal leaves usually pubescent and sparsely hirsute on petiole margins; petals 6–8 mm long; fruit to 1 mm wide; style slender, 0.5–1 mm long
2b. Basal leaves glabrous to sparsely pubescent, petioles usually glabrous; petals (4-)5–6 mm long; fruit ca. 1.5 mm wide; style obsolete or rarely to 0.5 mm long
8a. Arabidopsis lyrata subsp. lyrata
Synonyms: Arabis lyrata Linnaeus, Sp. Pl. 2: 665. 1753; Cardaminopsis lyrata (Linnaeus) Hiitonen, Mem. Soc. Faun. Fl. Fenn. 25: 75. 1950. Type: Canada, D. Kalm s.n. (holotype, LINN 842.8).
Biennial or perennial herbs, with slender or rarely branched caudex. Basal leaves lyrate-pinnatifid; petioles pilose to subhirsute, often ciliate; lateral lobes 1–5 on each side, entire of few toothed; terminal lobe considerably larger than lateral lobes. Petals 6–8 mm long. Fruits 0.7–1 mm wide; styles slender, 0.5–1.1 mm long, distinctly longer than broad. 2n=16, 32.
Distribution: NE European Russia, Alaska, Canada (Ontario west into British Columbia), and southeastern and central United States (Vermont south into northern Georgia and Mississippi northward into Missouri and Minnesota).
Habitats: cliffs, calcareous ledges, rock crevices, sandy areas in open woods, river banks; near sea level to 2200 m.
8b. Arabidopsis lyrata subsp. petraea (Linnaeus) O'Kane & Al-Shehbaz, Novon 7: 326. 1997.
Synonyms: Cardamine petraea Linnaeus, Sp. Pl. 2: 654. 1753; Arabis arenosa (Linnaeus) Scopli subsp. petraea (L.) Celakovsky, Prodr. Fl. Böhm. 455. 1875; A. petraea (Linnaeus) Lamarck, Encycl. 1: 221. 1783; Cardaminopsis petraea (Linnaeus) Hiitonen in Hylander, Förteckn. Scand. Växt. 1, Kärlväxt., ed. 3, 62. 1941. Type: “In Angliae, Arvoniae, Mervinae, Sueciae, rupibus excelsis” (holotype, LINN-835.5).
Arabis amurensis N. Busch, Bot. Mater. Gerb. Glav. Bot. Sada RSFSR 3(3–4): 12. 1922; Cardaminopsis amurensis (N. Busch) O.E. Schulz in Engler & Prantl, Nat. Pflanzenfam. ed. 2, 17B: 541. 1936. Type: “Ad flumen Amur et ejus confluents (Schilka Kur) et in Sachalin (contra ostium fl. Amur)” (syntypes, LE).
Arabis crantziana Ehrhart, Beitr. Naturk. 5: 177. 1796
(nomen. nud.), Ehrhart ex Willdenow, Sp. Pl. 3: 535. 1802; A. petraea var. crantziana (Ehrhart) de Candolle, Syst. Nat. 2: 229. 1821. Type: “in monte Katzenstein,” Ehrhart 78 (holotype, not located).
Arabis hispida Mygind in Linnaeus, Syst. Veg., ed. 13, 501. 1774, not A. hispida Moench, Methodus 258. 1794; Cardaminopsis hispida (Mygind) Hayek, Fl. Steiermark 1: 478. 1908. Type: Austria (holotype, LINN-842.9).
Arabis media N. Busch, Bot. Mater. Gerb. Glav. Bot. Sada RSFSR 3(3–4): 11. 1922; Cardaminopsis media (Busch) O.E. Schulz in Engler & Prantl, Nat. Pflanzenfam., ed. 2, 17B: 541. 1936; C. petraea subsp. media (N. Busch) Hämet-Ahti, Ann. Bot. Fenn. 7: 293. 1970. TYPE: [East Siberia]. “Zona arctica et subarctica ab ostio Lenae usque ad Anadyr, montes Sajanenses orientales, montes cis-et transbaicalenses atque Jacutenses” (holotype, LE).
Arabis septentrionalis N. Busch, Bot. Mater. Gerb. Glav. Bot. Sada RSFSR 3(3–4): 10. 1922; A. petraea subsp. septentrionalis (N. Busch) Tolmachew & Bloomintal, Trudy Bot. Muz. 23: 204. 1931; Cardaminopsis septentrionalis (N. Busch) O.E. Schulz in Engler & Prantl, Nat. Pfanzenfam., ed. 2, 17B: 541. 1936. Type: “Zona arctica a Novaja Zemlja usque ad ostia Lenae fluminis” (holotype, LE).
Arabis umbrosa Turczaninow in Ledebour, Fl. Ross. 1: 120. 1842, not A. umbrosa Crantz, Stirp. Austr. Fasc. ed. 1, 1: 43. 1762, ed. 2, 1: 41. 1769; A. petraea subsp. umbrosa Tolmachew, Fl. Arctica URSS 7: 97. 1975; Cardaminopsis umbrosa Czerepanov, Plantae Vasculares URSS 127. 1981; C. petraea subsp. umbrosa Peschkova, Fl. Tsentral'noi Sibiri 1: 396. 1979. Type: “in sabulosis ad fl. Irkut,” Turczaninow s.n. (holotype, KW).
Perennial herbs, with stout, often several-branched caudex, rarely also stoloniferous. Basal leaves entire or dentate, rarely lyrate-dentate; petioles glabrous or pilose to subhirsute, often ciliate. Petals 4–7(-9) mm long. Fruits 1–1.5(-2) mm wide; styles slender to stout, to 0.6 mm long, slightly longer than broad. 2n=16, 32.
Distribution: Austria, Czech Republic, England, Germany, Hungary, Iceland, Ireland, N. Italy, Norway, Russia (NW Russia, Siberia and Far East), Scotland, Sweden, Ukraine, boreal North America (Alaska and Yukon). Apparently extinct in Poland.
Habitats: Rocky and gravelly ground, sandy creek beds or banks, tundra slopes, tundra tussocks, sand dunes; near sea level to 2000 m.
Beginning in central Siberia, the taxon is increasingly similar to subspecies kamchatica, and many collections can only doubtfully be assigned to one or the other taxon. North American plants of subsp. petraea have been placed in Arabis media by Mulligan (1995).
8c. Arabidopsis lyrata (Linnaeus) O'Kane & Al-Shehbaz subsp. kamchatica (Fischer ex de Candolle) O'Kane & Al-Shehbaz, Novon 7: 326. 1997.
Synonyms: Arabis l yrat a Linnaeus var. kamchat i ca Fischer ex de Candolle, Syst. Nat. 2: 231. 1821; A. kamchat i ca (Fischer ex de Candolle) Ledebour, Fl. Ross. 1: 121. 1842; A. lyrata subsp. kamchatica (Fischer ex de Candolle) Hultén, Fl. Aleut. Isl. 202. 1937;Cardami nopsi s kamchat i ca (Fischer ex de Candolle) O.E. Schulz in Engler & Prantl, Nat. Pflanzenfam., ed. 2, 17B: 541. 1936. TYPE: [Russia], Kamchatka, Fischer s.n. (holotype, LE).
Arabis ambigua de Candolle var. glabra de Candolle, Syst. Nat. 2: 231. 1821; A. kamchatica var. glabra (de Candolle) N. Busch, Fl. Sib. Orient. Est. 4: 468. 1926; A. lyrata Linnaeus var. glabra (de Candolle) M. Hopkins, Rhodora 39: 93. 1937. Type: “in Kamchatka et insulis Kurilensis” (holotype, G).
Arabis ambigua de Candolle var. intermedia de Candolle, Syst. Nat. 2: 231. 1821; A. kamchatica (Fischer ex de Candolle) Ledebour var. intermedia (de Candolle) N. Busch, Fl. Sib. Orient. Est. 4: 468. 1926; A. lyrata Linnaeus var. intermedia (de Candolle) Farwell, Annual Rep. Michigan Acad. Sci. 19: 256. 1917. Type: “insulis Kurilensibus,” (holotype, G).
Arabis kawasakiana Makino, Bot. Mag. (Tokyo) 27: 24. 1913. Type: Japan. Prov. Ise: Yokkaichi, M. Kawasaki s. n. (holotype, MAK).
Arabis lyrata var. occidentalis S. Watson in A. Gray, Syn. Fl. N. Amer. 1: 159. 1895; A. occidentalis (S. Watson) A. Nelson, Univ. Wyoming Publ. 3: 111. 1937. Type: not designated.
Arabis morrisonensis Hayata, J. Coll. Sci. Imp. Univ. Tokyo 30(1): 29. 1911. Type: Taiwan, monte Morrison, 13,094 ft., Nov. 1905, S. Nagasawa 680 (holotype, TI).
Sisymbrium tilesii Ledebour, Mem. Acad. Imp. Sci. St. Pétersbourg Hist. Acad. 5: 548. 1815; Arabis tilesii (Ledebour) Karavaev ex V.N. Voroshilov, Byull. Glavn. Bot. Sada (Moscow) 134: 36. 1984. Type: Kamtschatka, 1806, Tilesius s.n. (holotype, LE?; isotype, MW).
Perennial herbs, with slender or rarely branched caudex. Basal leaves lyrate-pinnatifid; petioles glabrous or rarely very sparsely pilose; lateral lobes 1–5 on each side, entire of few toothed; terminal lobe considerably larger than lateral lobes. Petals 4–5(-5.5) mm long. Fruits (1-)1.2–1.5 mm wide; styles obsolete or distinct, stout, rarely to 0.5 mm long, distinctly wider than long. 2n= 32.
Distribution: boreal Alaska, Canada (Yukon, Mackenzie District, British Columbia, northern Saskatchewan), Aleutian Islands, eastern Siberia, the Russian Far East, Korea, northern China, Japan, and Taiwan.
Habitats: gravelly slopes, forests, alpine regions, roadsides, flooded areas; near sea level to 3500 m.
In the Russian Far East, subsp. kamchatica tends to grow closer to the sea than does subsp. petraea, which is a more continental taxon. Plants of subsp. kamchatica growing in sandy, ocean-side localities tend to have massive leaves and seem indistinguishable from those described as Arabis kawasakiana of Japan. Mulligan (1995) treated subsp. kamchatica as a species of Arabis, whereas Rollins (1993) treated it as a variety of Arabis lyrata.
9. Arabidopsis halleri (Linnaeus) O'Kane & Al-Shehbaz, Novon 7: 325. 1997.
Herbs perennial, stoloniferous, (10–)20–65(–80) cm tall. Stems decumbent, few from base, simple or branched above, basally with simple and 1-forked trichomes, apically glabrous. Basal leaves rosulate; petiole (5–)1–2.5(–5) cm long; leaf blade orbicular to broadly ovate, (1–)2–4.5(–9) × (0.5–)1–1.5(–2.5) cm, adaxially with a mixture of simple and stalked, 1- or 2-forked trichomes, margin pinnatifid to lyrate-pinnatifid and with (1 or)2–4(–7) suborbicular lateral lobes on each side; terminal lobe suborbicular, much larger than lateral ones, sometimes coarsely dentate, rarely entire or repand, apex obtuse. Stem leaves shortly petiolate, several; blade of middle and lower ones ovate to oblong or lanceolate, (0.5–)1–2.5(–8) × (0.2–)0.5–1.5(–3) cm, coarsely toothed or rarely lobed, gradually reduced in size upward. Fruiting pedicels slender, divaricate or slightly reflexed, straight, (0.5–)0.8–1.3(–2) cm long. Sepals 1.5–2 mm long, glabrous or apically with few simple trichomes, lateral pair saccate. Petals white, lilac, or purplish, obovate, 4–5(–6.5) × 2–2.5(–3.5) mm. Filaments white, 2–2.5 mm long. Ovules 14–18 per ovary. Siliques linear, flattened, torulose, (0.9–)1–2(–2.5) cm × 0.6–1 mm; replum constricted between seeds; valves not veined or with an obscure midvein; gynophore slender, to 0.7 mm long; style slender, to 1 mm long. Seeds oblong, flattened, light brown, 0.8–1.2 mm long, wingless; cotyledons accumbent. Flowering and fruiting June–August. 2n=16.
Arabidopsis halleri is widely distributed from central Europe into northern Russia, the Far East, and Japan south into Taiwan. It is highly variable in leaf morphology, flower color, and the degree of development of stolons. The three subspecies, however, are quite distinct.
1a. Basal leaves entire or with weakly developed lateral lobes; lower stem leaves orbicular to ovate, entire or crenate; flowering stems few, sparsely branched above; petals light purple to pale lavender
1b. Basal leaves pinnatifid or lyrate; lower stem leaves oblong and dentate; flowering stems numerous, branched at base and often also above; petals white or pale lavender.
2a. Basal leaves pinnatifid, with 1 7 lateral lobes; petals white or lavender; plants 20 45 cm tall; Europe
2b. Basal leaves lyrate; petals white; plants 40 80 cm tall; Russian Far East, northeastern China, Korea, Japan, and Taiwan
9a. Arabidopsis halleri subsp. halleri
Synonyms: Arabis halleri Linnaeus, Sp. Pl. ed. 2, 2: 929. 1763; Cardamine halleri (Linnaeus) Prantl, Exkursionflora v. Bayern, ed. 2, 229. 1884; Cardaminopsis halleri (Linnaeus) Hayek, FI. Steiermark 1: 479. 1908. Type: an epitype needs to be designated. O'Kane and Al-Shehbaz (1997) indicated that the type is LINN 842.11, but that typification is incorrect.
Arabis besseri Zapalowicz subsp. proseocarpatica Zapalowicz, Rozpr. Wydz. Mat.-Przyr. Acad. Umiejetn., Dzial B, Nauki Biol. 52: 31. 1912. Type: “in rupibus calcareis Carpatorum orientalium locis siccioribus: Budyowska Wielka, 1555 m,” Zapalowicz s.n. (lectotype, KRAM).
Arabis halleri Linnaeus var. pilifera Beck, FI. Nieder-Österreich 2: 458. 1892; Cardaminopsis halleri var. pilifera (Beck) E. Schmid in Hegi, Ill. FI. Mitel-Eur., ed. 1, 424. 1919. Type: Austria, Beck s.n. (holotype, PRC or W).
Arabis tenella Host, FI. Austriac. 2: 273. 1831. Type: “in Styria superiore in humidis alpinum judenburgensium” (holotype, W).
Plants with few stolons. Stems 20–45 cm tall, branched below and above. Basal leaves pinnatifid, with 1–7 lateral lobes on each side; lower stem leaves often oblong, dentate. Fruiting pedicels 5–10 mm long. Petals white or rarely lavender. 2n=16.
Distribution: Austria, Croatia, Czech Republic, Germany, N and C Italy, Poland, Romania, Slovakia, Slovenia, Switzerland, and S Ukraine. Probably introduced in N France and extinct in Belgium.
Habitats: montain slopes, forest margins, rocky crevices; 600–2200 m.
9b. Arabidopsis halleri subsp. ovirensis (Wulfen)
O'Kane & Al-Shehbaz, Novon 7: 325. 1997.
Synonyms: Arabis ovirensis Wulfen in Jacquin, Collectanea 1: 196. 1787; A. halleri Linnaeus subsp. ovirensis (Wulfen) Klástersky, Preslia 8: 30. 1929; Cardaminopsis halleri subsp. ovirensis (Wulfen) Hegi & E. Schmid in Hegi, Ill. FI. Mittel-Eur. 4(1): 424. 1919; C. ovirensis (Wulfen) Thellung ex Jávorka, Magyar FI. 436. 1924; C. ovirensis (Wulfen) O. Schwarz, Mitt. Thuring. Bot. Ges. 1(1): 101. 1949, comb. superfl. Type: [Austria] Ovirensis supra Ebriacum in Valle Junonia percurrerem alpes, Wulfen s.n. (holotype, W).
Arabis halleri Linnaeus subsp. transsilvanica Klástersky, Preslia 8: 29. 1929. Type: not designated, several syntypes cited.
Cardamine stolonifera Scopoli, FI. Carniol. ed. 2, 2: 22. 1772; Arabis stolonifera (Scopoli) Hornemann, Hort. Hafn. 2: 618. 1815. Type: “in Carnioliae distritu Vipaccensi” (holotype, LINN?).
Plants with numerous stolons. Stems 10–20 cm tall, few branched above. Basal leaves entire or with weakly developed lateral lobes; lower stem leaves orbicular to ovate, often entire. Fruiting pedicels 10–14 mm long. Petals lavender or purple. 2n=16.
Distribution: Albania, Austria, NE Italy, Romania, Slovakia, Slovenia, SW Ukraine, Yugoslavia.
Habitats: rocky slopes and crevices; 1200–2400m.
According to Jones & Akeroyd (1993), intermediates between subsp. halleri and subsp. ovirensis occur in the Tatra Mountains (Poland).
9c. Arabidopsis halleri subsp. gemmifera (Matsumura) O'Kane & Al-Shehbaz, Novon 7: 325. 1997.
Synonyms: Cardamine gemmifera Matsumura, Bot. Mag. (Tokyo) 13: 49. 1899; Cardaminopsis gemmifera (Matsumura) Berkutenko, Novosti Sist. Vyssh. Rast. 15: 154. 1978. Type: Japan. Prov. Shinano: monte Norikura, 1891, K. Fuji s.n. (holotype, TI).
Arabis coronata Nakai, Bot. Mag. (Tokyo) 28: 302. 1914. Type: Korea, “in silvis Laricis pede montis Paiktusan, Aug. 1913, T. Mori s.n. (holotype, TI).
Arabis gemmifera var. alpicola H. Hara, J. Jap. Bot. 12: 901. 1936. Type: Japan, Honshu, Prov. Omi, Mt. Ibuki, May 1914, G. Koidzumi s.n. (holotype, TI).
Arabis halleri Linnaeus var. senanensis Franchet & Savatier, Enum. Pl. Jap. 2: 279. 1879; A. senanensis (Franchet & Savatier) Makino, Bot. Mag. (Tokyo) 24: 224. 1910. Type: Prov. Senano, ubi Rein, Savatier 2802 (holotype, P).
Arabis maximowiczii N. Busch, Bot. Mater. Gerb. Glav. Bot. Sada RSFSR 3(3–4): 13. 1922; Cardaminopsis maximowiczii (N. Busch) O.E. Schulz in Engler & Prantl, Nat. Pflanzenfam., ed. 2, 17B: 541. 1936. Type: Manschuria, “Ussuri et ejus confluentes, Vladivostok, insula Putjatin,” Maximowicz s.n. (holotype, LE).
Cardamine greatrexii Miyabe & Kudo, Trans. Sapporo Nat. Hist. Soc. 6: 169. 1917; Arabis greatrexii (Miyabe & Kudo) Miyabe & Tatewaki, Trans. Sapporo Nat. Hist. Soc. 13: 379. 1934. Type: Japan, Hokkaido, Prov. Oshima, Hakodate, 10 June 1916, Greatrex s.n. (holotype, SAPA).
Plants with few to several stolons. Stems 40–65(–80) cm tall, branched below and above. Basal leaves often lyrate; lower stem leaves often ovate, dentate. Fruiting pedicels (8–)10–20 mm long. Petals white. 2n=16.
Distribution: Russian Far East, northeastern China, Korea, Japan, and Taiwan.
Habitats: gravelly or grassy slopes, forests, shaded and most areas; near sea level to 2600 m.
Generic placement of species excluded from Arabidopsis
The following species previously placed in Arabidopsis (italics) are currently considered as synonyms of those in boldface.
A. bactriana Ovczinnikov & Junussov, FI. Tadzhitskoi SSR 5: 626. 1978. No material has been seen, but according to the original description, the species cannot be assigned to Arabidopsis because it is pulvinate, scapose perennial with cylindric fruits, subbiseriate seeds, and leafless stems.
A. brevicaulis (Jafri) Jafri, FI. W. Pakistan 55: 272. 1973. = Crucihimalaya himalaica (Edgeworth) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 301. 1999.
A. bursifolia (de Candolle) Botschantsev, Not. Syst. Herb. Inst. Bot. Acad. Sci. URSS 19: 106. 1959. = Beringia bursifolia (de Candolle) R.A. Price, Al-Shehbaz & O'Kane, Novon 11: 2001 (in press).
A. campestris O.E. Schulz, Notizbl. Bot. Gart. Berlin-Dahlem 9: 1059. 1927. = Crucihimalaya wallichii (J.D. Hooker & Thomson) Al-Shehbaz, O'Kane & R.A. Price. Novon 9: 301. 1999.
A. dentata (Allioni) Dalla Torre, Alpenfl. 115. 1899. = Murbeckiella pinnatifida (Lamarck) Rothmaler, Bot. Notiser 1939: 469. 1939.
A. drassiana Naqshi & Javeid, J. Econ. Taxon. Bot. 7: 624. 1986. No material has been seen, and the species is excluded from Arabidopsis because it has sessile amplexicaul leaves (Naqshi and Javeid,1986).
A. erysimoides Hedge & Kit Tan, Pl. Syst. Evol. 156: 202. 1987. = Erysimum hedgeanum Al-Shehbaz, Novon 4: 1. 1994.
A. eseptata Hedge, FI. Iran. 57: 334. 1968. = Olimarabidopsis umbrosa (Botschantsev & Vvedensky) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 303. 1999.
A. gamosepala Hedge, FI. Iran. 57: 334. 1968. = Neotorularia gamosepala (Hedge) Al-Shehbaz & O'Kane, Novon 7: 93. 1997.
A. glauca (Nuttall ex Torrey & A. Gray) Rydberg, FI. Rocky Mt. 342. 1917. = Thellungiella salsuginea (Pallas) O.E. Schulz, Pflanzenr. IV. 105 (Heft 86): 252. 1924.
A. griffithiana (Boissier) N. Busch, FI. Cauc. Crit. 3(4): 457. 1909. = Olimarabidopsis pumila (Stephan) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 303. 1999.
A. himalaica (Edgeworth) O.E. Schulz, Pflanzenreich IV.105(Heft 86): 283. 1924. = Crucihimalaya himalaica (Edgeworth) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 301. 1999.
A. huetii (Boissier) N. Busch, Acta Hort. Petrop. 28: 389. 1908. = Murbeckiella huetii (Boissier) Rothmaler, Bot. Notiser 1939: 472. 1939.
A. kneuckeri (Bornmüller) O.E. Schulz, Pflanzenr. IV. 105(Heft 86): 277. 1924. = Crucihimalaya kneuckeri (Bornmüller) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 302. 1999.
A. korshinskyi Botschantsev, Novit. Syst. Pl. Vasc. Acad. Sci. URSS 1965: 272. 1965. = Olimarabidopsis cabulica (J.D. Hooker & Thomson) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 303. 1999.
A. lasiocarpa (J.D. Hooker & Thomson) O.E. Schulz, Pflanzenr. IV. 105(Heft 86): 282. 1924. = Crucihimalaya lasiocarpa (J.D. Hooker & Thomson) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 300. 1999.
A. minutiflora (J.D. Hooker & Thomson) N. Busch, FI Cauc. Crit. 3(4): 457. 1909 = Ianhedgea minutiflora (J.D. Hooker & Thomson) Al-Shehbaz & O'Kane, Edinb. J. Bot. 56: 322. 1999.
A. mollis (Hooker) O.E. Schulz, Bot. Jahrb. Syst. 66: 97. 1933. = Beringia bursifolia (de Candolle) R.A. Price, Al-Shehbaz & O'Kane, Novon 11: 2001 (in press).
A. mollissima (C.A. Meyer) N. Busch, FI. Sib. Or. Extr. 1: 136. 1913. = Crucihimalaya mollissima (C.A. Meyer) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 299. 1999. A. monachorum (W.W. Smith) O.E. Schulz, Pflanzenr. IV. 105(Heft 86): 282. 1924. = Crucihimalaya lasiocarpa (W.W. Smith) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 300. 1999.
A. mongolica (Botschantsev) Mesicek & Sojak, Folia Geobot. Phytotax. 30: 448. 1995. = Crucihimalaya mongolica (Botschantsev) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 300. 1999.
A. multicaulis Pampanini, Sped. Ital. DeDilippi Himal. etc. 1913–1914, Ser. 2, 11 (Agg. FI. Carac.): 160. 1934. = Arabis tibetica J.D. Hooker & Thomson, J. Linn. Soc., Bot. 5: 143. 1861.
A. novae-anglicae Britton in Britton & Brown, Illus. FI., ed. 2, 2: 176. 1913. = Neotorularia humilis (C.A. Meyer) Hedge & J. Léonard, Bull. Jard. Bot. Nat. Belg. 56: 394. 1986.
A. nuda (Bélanger) Bornmüller, Beih. Bot. Zentralbl. 33(2): 275. 1915. = Drabopsis nuda (Bélanger) Stapf, Denkschr. Akad. Wiss. Wien, Math.-Nat. Kl. 51(2): 298. 1886.
A. ovczinnikovii Botschantsev, in P. N. Ovczinnikov, FI. Tadzhitskoi SSR 5: 625. 1978. = Crucihimalaya mollissima (C. A. Meyer) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 299. 1999.
A. parvula (Schrenk) O.E. Schulz, Pflanzenr. IV. 105(Heft 86): 269. 1924. = Thellungiella parvula (Schrenk) Al-Shehbaz & O'Kane, Novon 5: 309. 1995.
A. pinnatifida (Lamarck) Ruprecht, Mém. Acad. Sci. St. Pétersb. Ser. 7, 15(2): 86. 1869. = Murbeckiella pinnatifida (Lamarck) Rothmaler, Bot. Notiser 1939: 469. 1939.
A. pumila (Stephan) N. Busch, FI. Cauc. Crit. 3(4): 457. 1909. = Olimarabidopsis pumila (Stephan) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 303. 1999.
A. qiranica Z.X. An, FI. Xinjiang. 2(2): 376. 1995. = Sisymbriopsis mollipila (Maximowicz) Botschantsev, Nov. Syst. Pl. Vasc. 3: 122. 1966.
A. richardsonii (Rydberg) Rydberg, FI. Rocky Mt. 341. 1917. = Neotorularia humilis (C.A. Meyer) Hedge & J. Léonard, Bull. Jard. Bot. Nat. Belg. 56: 394. 1986.
A. russeliana Jafri, Notes Roy. Bot. Gard. Edinb. 22: 97. 1956. = Crucihimalaya wallichii (J.D. Hooker & Thomson) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 301. 1999.
A. salsuginea (Pallas) N. Busch, FI. Sib. 1: 136. 1913. = Thellungiella salsuginea (Pallas) O.E. Schulz, Pflanzenr. IV. 105(Heft 86): 252. 1924.
A. sarbalica Naqshi & Javeid, J. Econ. Taxon. Bot. 7: 621. 1985 (1986). = Crucihimalaya wallichii (J.D. Hooker & Thomson) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 301. 1999.
A. schimperi (Boissier) N. Busch, FI. Cauc. Crit. 3(4): 457. 1909. = Robeschia schimperi (Boissier) O.E. Schulz, Pflanzenr. IV. 105(Heft 86): 360. 1924.
A. stenocarpa Rydberg, Torrea 7: 160. 1907. = Beringia bursifolia (de Candolle) R.A. Price, Al-Shehbaz & O'Kane subsp. virgata (Nuttall ex Torrey & A. Gray) R.A. Price, Al-Shehbaz & O'Kane Novon 11: 2001 (in press).
A. stewartiana Jafri, Notes Roy. Bot. Gard. Edinburgh 22: 96. 1956. = Olimarabidopsis pumila (Stephan) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 303. 1999.
A. stricta (Cambessèdes) N. Busch, FI. Cauc. Crit. 3(4): 457. 1909. = Crucihimalaya stricta (Cambessèdes) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 300. 1999.
A. taraxacifolia (T. Anderson) Jafri, FI. W. Pakistan, 55: 274. 1973. = Crucihimalaya wallichii (J.D. Hooker & Thomson) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 301. 1999.
A. tenuisiliqua (K.H. Rechinger & Köie) Jafri, FI. W. Pakistan 55: 171. 1973. = Arabis tenuisiliqua K.H. Rechinger & Köie, Anz. Math-Nat. Kl. Oesterr. Akad. Wiss. 7: 5. 1954. A. tibetica Naqshi & Javeid, J. Econ. Taxon. Bot. 7: 621. 1985 (1986). The name is illegitimate because it is a later homonym of the following species. = Crucihimalaya himalaica (Edgeworth) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 301. 1999.
A. tibetica (J.D. Hooker & Thomson) Lan & C. H. An ex K. C. Kuan, FI. Xizang. 2: 372. 1985. = Arabis tibetica J.D. Hooker & Thomson, J. Linn. Soc., Bot. 5: 143. 1861.
A. toxophylla (Bieberstein) N. Busch, FI. Cauc. Crit. 3(4): 457. 1909. = Pseudoarabidopsis toxophylla (Bieberstein) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 304. 1999.
A. trichocarpa R.F. Huang in S.W. Liu, FI. Qinghaica 1: 509. 1997. = Neotorularia humilis (C.A. Meyer) Hedge & J. Léonard, Bull. Jard. Bot. Nat. Belg. 56: 394. 1986.
A. trichopoda (Turczaninow) Botschantsev, Not. Syst. Herb. Inst. Bot. Acad. Sci. URSS 18: 104. 1957. = Beringia bursifolia (de Candolle) R.A. Price, Al-Shehbaz & O'Kane Novon 11: 2001 (in press).
A. tschuktschorum (Jurtsev) Jurtsev, Bot. Zhurn. 60: 240. 1975. = Beringia bursifolia (de Candolle) R.A. Price, Al-Shehbaz & O'Kane Novon 11: 2001 (in press).
A. tuemurnica K.C. Kuan & Z.X. An, Bull. Bot. Lab. North-East Forest Inst. 1980(8): 44. 1980. = Neotorularia humilis (C.A. Meyer) Hedge & J. Léonard, Bull. Jard. Bot. Nat. Belg. 56: 394. 1986.
A. verna (Koch) N. Busch, FI. Cauc. Crit. 3(4): 460. 1909. = Drabopsis nuda (Bélanger) Stapf, Denkschr. Akad. Wiss. Wien, Math.-Nat. Kl. 51(2): 298. 1886.
A. virgata (Nuttall ex Torrey & A. Gray) Rydberg, FI. Rocky Mt. 342. 1917. = Beringia bursifolia (de Candolle) R.A. Price, Al-Shehbaz & O'Kane subsp. virgata (Nuttall ex Torrey & A. Gray) R.A. Price, Al-Shehbaz & O'Kane Novon 11: 2001 (in press).
A. wallichii (J.D. Hooker & Thomson) N. Busch, FI. Cauc. Crit. 3(4): 457. 1909. = Crucihimalaya wallichii (J.D. Hooker & Thomson) Al-Shehbaz, O'Kane & R.A. Price, Novon 9: 301. 1999.
A. yadungensis K.C. Kuan & Z.X. An, FI. Xizang. 2: 375. 1985. =Ar abi spt eros per ma Edgeworth, Trans. Linn. Soc. London 20: 33. 1851.
Molecular phylogeny
Molecular studies on the nine species presently recognized in Arabidopsis and most of the 50 species previously assigned to the genus (O'Kane and Al-Shehbaz, 2001; Koch et al., 1999, 2000, 2001; Price et al. 1994) show beyond doubt that previous delimitation of the genus and the alleged phylogenetic relationships and tribal classifications (Schulz, 1924, 1936) are untenable. The artificiality of the tribal classifications of the family and the polyphyly of most tribes have been pointed out by many workers (e.g., Price et al., 1994; Koch et al., 2001) and need not be repeated here.
Arabidopsis forms a well-defined, monophyletic clade somewhat closely related to the recently segregated genus Olimarabidopsis (O'Kane and Al-Shehbaz, 2001; Price et al., 1994; Koch et al., 2001). The latter genus consists of three species distributed in Eurasia but mainly in central and southwestern Asia (Al-Shehbaz et al., 1999). Depending on the taxa studied, the various molecular phylogenies proposed thus far (Price et al., 1994; Koch, 1999, 2000, 2001; O'Kane and Al-Shehbaz, 2001) show different topologies and relationships between Arabidopsis and other genera, including Crucihimalaya, Pseudoarabidopsis, Capsella, Camelina, and Arabis sensu lato. However, the lack of comprehensive phylogenetic studies on the entire family makes it difficult to point out definitively what the nearest relatives of Arabidopsis really are. Because Arabidopsis is most highly diversified in Europe, it would be highly desirable to concentrate on the taxa there that share the same morphological and cytological aspects, especially those with base chromosome numbers of x=8, branched trichomes, and linear fruits. Species of the European Arabis sensu lato and Olimarabidopsis are good candidates to concentrate on. It should be kept in mind, however, that the molecular studies cited above clearly demonstrated the polyphyly of Arabis, and that concentrated efforts should be made to establish a monophyletic genus by assigning unrelated species to other, possibly new, genera. In fact, such attempts (Koch et al., 1999; Price et al., 2001) have already been made.
Molecular studies (Mummenhoff and Hurka, 1994, 1995; O'Kane et al., 1997) within Arabidopsis have demonstrated that A. sucecica (2n=26) is an allopolyploid formed by hybridization between the maternal parent A. thaliana (2n=10) and a diploid paternal parent not very different from A. arenosa or A. neglecta (2n=16). More recent work indicates that A. arenosa is in fact is the maternal parent (O'Kane, unpub. data) as indicated in Fig. 1. For taxonomic and experimental studies related to the origin of A. suecica, the interested reader should consult the references in the three works above.
Detailed molecular studies of the taxa of Arabidopsis, summarized in Fig. 1, based on DNA sequences of the internal transcribed spacers of nuclear ribosomal DNA (O'Kane and Al-Shehbaz, 2001) show that A. lyrata (and its subspecies) form a well supported clade in a sister group relationship to A. croatica. Arabidopsis lyrata is circumboreal with relict populations of subsp. kamchatica occurring at high elevations in Taiwan. Its presence on Taiwan is likely a consequence of southward range expansion during the last major glaciation when a land bridge was formed with mainland Asia (Voris, 2000). Arabidopsis halleri subsp. ovirensis is an alpine plant of the southeastern Alps, the Carpathians, and the northern Balkan Peninsula. From this subspecies evolved the lowland, stolon-producing subspecies halleri, which is distributed in central Europe and northern Asia. The species is represented in the eastern Asia (Russian Far East, eastern China, Korea, Japan, and Taiwan) by subsp. gemmifera. The latter evolved from subsp. halleri, and its migration into Taiwan apparently followed the same pattern as that of A. lyrata subsp. kamchatica.
Arabidopsis arenosa and A. neglecta together form a monophyletic group, but each of these species is paraphyletic with respect to the other in one or more molecular analyses. One-step statistical parsimony analysis (Templeton et al., 1992), however, indicates that the alpine A. neglecta is directly ancestral to A. arenosa, a species with weedy tendencies that grows at elevations below the alpine. These two species must have diverged so recently that paraphyly in the nrDNA ITS gene tree is still seen, i.e., not enough time has elapsed for drift and fixation to convert the lineage from paraphyletic to monophyletic (Rieseberg and Brouillet, 1994). It appears that evolutionary innovation in these two species, as in the subspecies of A. halleri, progressed from high to low elevations. As shown in Fig. 1, the broad-leaved A. cebennensis is basal to the clade including the rest of the species of the genus excluding A. thaliana. (Arabidopsis pedemontana was not included in the molecular analyses for lack of adequate material.)
Acknowledgments
We are grateful to the National Science Foundation for the generous support of this research through the grant DEB-9208433 to I.A.S.
Footnotes
Citation: Al-Shehbaz I.A., and O'Kane, Jr S.L. (2002) Taxonomy and Phylogeny of Arabidopsis (Brassicaceae). The Arabidopsis Book 1:e0001. doi:10.1199/tab.0001
elocation-id: e0001
Published on: September 30, 2002
Figure 1.
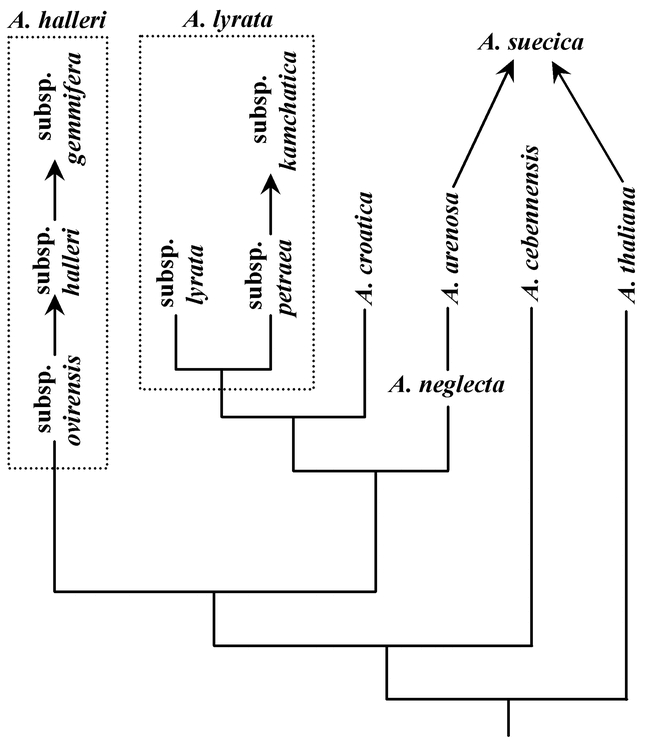
Phylogenetic relationships within Arabidopsis based on ITS data.
References
- Al-Shehbaz I. A. The genera of Sisymbrieae (Cruciferae: Brassicaceae) in the southeastern United States. J. Arnold Arb. 1988;691(1):213–237. [Google Scholar]
- Al-Shehbaz I. A. Erysimum hedgeanum (Brassicaceae), a new name replacing Arabidopsis erysimoides. Novon. 1994;41(1):1–2. [Google Scholar]
- Al-Shehbaz I. A., O'Kane S. L., Jr Placement of Arabidopsis parvula in Thellungiella (Brassicaceae). Novon. 1995;51(1):309–310. [Google Scholar]
- Al-Shehbaz I. A., O'Kane S. L., Jr Arabidopsis gamosepala and A. tuemurnica belong to Neotorularia (Brassicaceae). Novon. 1997;71(1):93–94. [Google Scholar]
- Al-Shehbaz I. A., O'Kane S. L., Jr, Price R. A. Generic placement of species excluded from Arabidopsis. Novon. 1999;91(1):296–307. [Google Scholar]
- Ancev M. E., Goranova V. Reports (855–872). 1997;71(1):246. In Mediterranean chromosome number reports-7, Kamari, G., Felver, F., and Gabari, F., eds, Flora Mediterranea. [Google Scholar]
- Arohonka T. Chromosome counts of vascular plants of the island Seili in Nauvo, SW Finland. Turun Yliopiston Biologian-Laitoksen Julkaisuja. 1982;31(1):1–12. [Google Scholar]
- Baksay L. Cytological studies on the flora of Hungary. Ann. Hist.-Nat. Mus. Natl. Hungarici. 1956;71(1):321–334. n.s. [Google Scholar]
- Ball P. W. Arabidopsis. 1993;11(1):322–323. In Flora Europaea, ed. 2, T.G. Tutin, N.A. Burges, A.O. Chater, J.R. Edmondson, V.H. Heywood, D.M. Moore, D.H. Valentine, S.M. Walters, and D.A. Webb, eds, (Cambridge Univ. Press, Cambridge) [Google Scholar]
- Berkutenko A. N. Brassicaceae. 1988;31(1):38–115. In Plantae Vasculares Orientis Extremi Sovietici, S.S. Charkevicz [Kharkevich], ed, (Academy Press, Leningrad) [Google Scholar]
- Berkutenko A. N., Tzytlenok S. I., Pulkina S. V. Chromosome numbers and dispersal of the Brassicaceae family in the Magadan District. Bot. Zhurn (Moscow & Leningrad) 1984;691(1):75–80. [Google Scholar]
- Böcher T. W. Experimental and cytological studies on plant species. IX. Some Arctic and montane crucifers. Biol. Skr. 1966;141(7)(1):1–74. [Google Scholar]
- Böcher T. W. Further studies in Arabis holboellii and allied species. Bot. Tidsskr. 1969;641(1):141–161. [Google Scholar]
- Böcher T. W., Larsen K. Chromosome numbers of some arctic or boreal flowering plants. Meddel. Grrnland. 1950;1471(6)(1):1–32. [Google Scholar]
- Böcher T. W., Larsen K. Experimental and cytological studies on plant species. IV. Further studies in short-lived herbs. Biol. Skr. 1958;101(2)(1):1–24. [Google Scholar]
- Burdet H. M. Contribution à l'étude caryologique des genres Cardaminopsis, Turritis et Arabis en Europe. Candollea. 1967;221(1):107–156. [Google Scholar]
- Busch N. A. Arabidopsis. 1909;31(4)(1):456–467. In Flora Caucasica Critica, N. Kusnezow, N. Busch, and A. Fomin, eds, (Technographia Mattesena, Yureev) [Google Scholar]
- Busch N. A. Arabis. 1939;81(1):172–197. In Fl. URSS, V.L. Komarov, ed, (Academy of Sciences Press, Leningrad) [Google Scholar]
- Buttler K. P. Chromosomenzahlen von Gefässpflanzen aus Hessen (und angrenzenden Ländern), 3. Folge. Hess. Florist. Briefe. 1985;341(1):37–42. [Google Scholar]
- Candolle A. Pde. Regni Vegetabilis Systema Naturale. 1821. Treuttel and Würtz, Paris.
- Dawe J. C., Murray D. F. In IOPB Chromosome number reports LXIII, A. 1979;281(1):265–279. Löve, ed, Taxon. [Google Scholar]
- Dmitrieva S. A. Chromosome numbers in some representatives of the families Apiaceae, Brassicaceae, Caryophyllaceae and Cyperaceae in the Byelorussian flora. Bot. Zhurn. 1985;701(1):994–996. (Moscow & Leningrad) [Google Scholar]
- Dmitrieva S. A., Parfenov V. I., Schvec I. V. The karyological characterization of some species of the Byelorussian flora. Vesci. Adad. Navuk Belarusk. SSR, ser. Bijal. Selskagasp Navuk. 1977;191(1):82–91. [Google Scholar]
- Dobes C., Hahn B. In IOPB chromosome data 11, C.A. Newslett. Int. Organ. Pl. Biosyst. (Oslo) 1997;261(1):15–18. Stace, ed. /27. [Google Scholar]
- Engelskjrn T. Chromosome numbers in vascular plants from Norway, including Svalbard. Opera Bot. 1979;521(1):1–38. [Google Scholar]
- Fedrov A. A. Chromosome numbers of flowering plants. 1969. Academy of Sciences of USSR, Leningrad.
- Gadella T. W. J., Kliphuis E. Chromosome numbers of flowering plants in the Netherlands, V. Proc. Kon. Ned. Akad. Weten. C. 1971;741(1):335–343. [Google Scholar]
- Gadella T. W. J., Kliphuis E. Studies in Chromosome numbers of Yugoslavian angiosperms. Acta Bot. Croat. 1972;311(1):91–103. [Google Scholar]
- Greuter W., Burdet H. M., Chaloner W. G., Demoulin V., Grolle R., Hawksworth D. L., Nicolson D. H., Silva P. C., Stafleu F. A., McNeill J. International Code of Botanical Nomenclature adopted by the Fourteenth International Botanical Congress, Berlin July–August. 1988. Koeltz Sci., Königsein, Germany.
- Greuter W., McNeill J., Barrie F. R., Burdet H. M., Demoulin V., Filgueiras T. S., Nicolson D. H., Silva P. C., Skog J. E., Trehane P., Turland N. J., Hawksworth D. L. International Code of Botanical Nomenclature (Saint Louis Code). 2000. Koeltz Sci., Königsein, Germany.
- Hämet-Ahti L., Virrankoski V. Chromosome numbers of some vascular plants of north Finland. Ann. Bot. Fenn. 1970;71(1):177–181. [Google Scholar]
- Harmaja H., Pellinen K. Three different chromosome numbers from Finnish Arabidopsis suecica, Brassicaceae. Ann. Bot. Fenn. 1991;271(1):335–336. [Google Scholar]
- Hedberg I., Hedberg O. Chromosome counts in British vascular plants. Bot. Not. 1961;1141(1):397–399. [Google Scholar]
- Hedge I. C. Arabidopsis. 1968;571(1):328–334. In Flora Iranica, K.H. Rechinger, ed., (Akademische Druck-u. Verlagsandstalt, Graz) [Google Scholar]
- Hopkins M. Arabis in eastern and central North America. Rhodora. 1937;391(1):63–186. [Google Scholar]
- Hsu C-C. Preliminary chromosome studies on the vascular plants of Taiwan (II). Taiwania. 1968;141(1):11–27. [Google Scholar]
- Hylander N. Cardaminopsis suecica (Fr.) Hiit., A northern amphidiploid species. Bull. Jard. Bot. État. Bruxelles. 1957;271(1):591–604. [Google Scholar]
- Holl C. F., Heynhold G. Flora von Sachsen. Vol. 1. 1842. Verlag von Justus Naumann, Dresden.
- Jalas J., Suominen J. Atlas Florae Europaeae: Distribution of Vascular Plants in Europe. 1994;101(1):1–224. (Helsinki University Printing House, Helsinki) [Google Scholar]
- Jafri S. M. H. Brassicaceae. 1973;551(1):1–308. In Flora of West Pakistan, E. Nasir and S.I. Ali, eds, (Ferozsons, Karachi) [Google Scholar]
- Jones B. M. G., Akeroyd J. R. Cardaminopsis. 1993;11(1):351–352. In Fl. Europaea, T.G. Tutin et al., eds, (Cambridge, UK.), ed. 2. [Google Scholar]
- Jaretzky R. Untersuchungen über Chromosomen und Phylogenie bei einigen Cruciferen. Jahrb. Wissenschaft. Bot. 1928;681(1):1–45. [Google Scholar]
- Jaretzky R. Beziehungen zwischen Chromosomenzahl und Systematik bei den Cruciferen. Jahbr. Wissenschaft. Bot. 1932;761(1):485–527. [Google Scholar]
- Johnson A. W., Packer J. G. Chromosome numbers in thee flora of Ogotoruk Creek, N.W. Alaska. Bot. Not. 1968;1211(1):403–456. [Google Scholar]
- Jonsell B. Some tropical African Cruciferae. Chromosome numbers and taxonomic comments. Bot. Not. 1976;1291(1):123–130. [Google Scholar]
- Kirschner J., Stepánek J., Stepanková J. IOPB chromosome number reports LXXVI, A. 1982;311(1):574–598. Löve, ed, Taxon. [Google Scholar]
- Knaben G. Chromosome numbers of Scandinavian Arcticalpine plant species, I. Blyttia. 1950;81(1):129–155. [Google Scholar]
- Knaben G. Chromosome numbers of Flowering plants from central Alaska. Nytt. Mag. Bot. 1968;151(1):240–254. [Google Scholar]
- Koch M., Bishop J., Michell-Olds T. Molecular systematics and evolution of Arabidopsis and Arabis. Plant Biol. 1999;11(1):529–537. [Google Scholar]
- Koch M., Haubold B., Mitchell-Olds T. Comparative evolutionary analysis of chalcone synthase and alcohol dehydrogenase loci in Arabidopsis, Arabis, and related genera (Brassicaceae). Mol. Biol. Evol. 2000;171(1):1483–1498. doi: 10.1093/oxfordjournals.molbev.a026248. [DOI] [PubMed] [Google Scholar]
- Koch M., Haubold B., Mitchell-Olds T. Molecular systematics of the Brassicaceae: evidence from coding plastidic matK and nuclear Chs sequences. Amer. J. Bot. 2001;881(1):534–544. [PubMed] [Google Scholar]
- Krogulevich R. E. Chromosome numbers of plant species from the Tunkinsky Alpes (East Sayan). News Sib. Dept. Ac. Sci. USSR, Biol. 1976;151(3)(1):46–52. [Google Scholar]
- Krogulevich R. E. Kariologicheskij analiz vidov flory Vostochnogo Sjana. V. Flora Pribajkal'ja. 19–48. 1978. Nauka, Novosibirsk.
- Laibach F. Zur Frage nach der Individullität der Chromosomen im Pflanzenreich. Beih. Bot. Centralbl. 1907;221(1):191–210. [Google Scholar]
- Lan Y-Z., Cheo T-Y. Cytotaxonomical studies on Chinese species of Cruciferae. 1988;1(1):283. In D.-Y. Hong, ed, Plant Chromosome Research 1987. (Organizing Committee, Sino-Japanese Symposium on Plant Chromosomes, Beijing) [Google Scholar]
- Lee Y. N. Chromosome numbers of flowering plants in Korea (3). J. Korean Res. Inst. Better Living. 1970;51(1):127–129. [Google Scholar]
- Leute G-H. In IOPB chromosome number reports XLVI. 1974;231(1):801–812. A. Löve, ed, Taxon. [Google Scholar]
- Lippert W., Heubl G. R. Chromosomenzahlen von Pflanzen aus Bayern und angrenzenden Gebieten: [Teil 1]. Ber. Bayer. Bot. Ges. 1988;591(1):13–22. [Google Scholar]
- Löve A. Hylandra æ A new genus of Cruciferae. Sv. Bot. Tidskr. 1961;551(1):211–217. [Google Scholar]
- Löve A., Löve D. Chromosome numbers of northern plant species. Univ. Int. Appl. Sci. (Iceland), Dep. Agr. Reports B. 1948;31(1):9–131. [Google Scholar]
- Löve A., Löve D. Cytotaxonomical conspectus of the Icelandic flora. Acta Horti. Gotoburg. 1956;201(1):65–290. [Google Scholar]
- Löve A., Löve D. In IOPB Chromosome number reports LXXIV. 1982;311(1):119–128. A. Löve, ed, Taxon. [Google Scholar]
- Lövkvist B., Hultgåd U-M. Chromosome numbers in south Swedish vascular plants. Opera Bot. 1999;1371(1):1–42. [Google Scholar]
- Loon J. Cvan, Setten A. Kvan. In IOPB chromosome number reports LXXVI. 1982;311(1):574–598. A. Löve, ed, Taxon. [Google Scholar]
- Luque T., Díaz Lifante Z. Chromosome numbers of plants collected during Inter Mediterraneum I in the SE of Spain. Bocconea. 1991;11(1):303–364. [Google Scholar]
- Maassoumi A. A. R. Cruciferes de la flore d'Iran. Etude caryosystematiques. 1980. Thesis, Strasbourg, France.
- Májovsky J., Váchová M. Karyotaxonomischer Beitrag zu einigen Arten der Slowakischen Flora. Acta Facul. Rerum Nat. Univ. Comen. Bot. 1982;291(1):81–85. [Google Scholar]
- Májovsky J. Index of chromosome numbers of Slovakian flora (part 4). Acta Facul. Rerum. Nat. Univ. Comen. Bot. 1974;231(1):1–23. [Google Scholar]
- Májovsky J. Index of chromosome numbers of Slovakian flora (part 6). Acta Facul. Rerum. Nat. Univ. Comen. Bot. 1978;261(1):1–42. [Google Scholar]
- Manton I. Introduction to the general cytology of the Cruciferae. Ann. Bot. 1932;461(1):509–556. [Google Scholar]
- Mesicek J. The chromosome morphology of Arabidopsis thaliana (L.) Heynh. and some remarks on the problem of Hylandra suecica (Fr.) Löve. Folia Geob. Phytotax. 1967;21(1):433–436. [Google Scholar]
- Mesicek J. Chromosome counts in Cardaminopsis arenosa Agg. (Cruciferae). Preslia. 1970;421(1):225–248. [Google Scholar]
- Mesicek J. In List of Chromosome numbers of the Czech vascular Plants, J. 1992. Mesicek and V. Javcrková-Jarolímová, eds, (Academia, Praha)
- Mesicek J., Slavík B., Tomsovic P. Cardamine. 1992;31(1):116–122. In Kvetena Ceské Repbuliky, S. Henjy and B. Slavík,eds, (Academia, Praha) [Google Scholar]
- Meyerowitz E. M., Somerville C. R. Arabidopsis. 1994. Cold Spring Harbor Laboratory Press, New York.
- Mulligan G. A. Chromosome numbers of the family Cruciferae. I. Canad. J. Bot. 1964;421(1):1509–1519. [Google Scholar]
- Mulligan G. A. Chromosome numbers of some plants native and naturalized in Canada. Natur. Cand. 1984;1111(1):447–449. [Google Scholar]
- Mulligan G. A. Synopsis of the genus Arabis (Brassicaceae) in Canada, Alaska and Greenland. Rhodora. 1995;971(1):109–163. [Google Scholar]
- Mulligan G. A., Porsild A. E. Chromosome numbers of some plants from the unglaciated central Yukon Plateau, Canada. Canad. J. Bot. 1969;471(1):655–662. [Google Scholar]
- Mulligan G. A., Porsild A. E. In IOPB Chromosome number reports XXV. 1970;191(1):102–113. A. Löve, ed, Taxon. [Google Scholar]
- Mummenhoff K., Hurka H. Subunit Polypeptide composition of Rubisco and the origin of allopolyploid Arabidopsis suecica (Brassicaceae). Biochem. Syst. Ecol. 1994;221(1):807–811. [Google Scholar]
- Mummenhoff K., Hurka H. Allopolyploid origin of Arabidopsis suecica (Fries) Norrlin: evidence from chloroplast and nuclear genome markers. Bot. Acta. 1995;1081(1):449–456. [Google Scholar]
- Murata M., Motoyushi F. Floral chromosomes of Arabidopsis thaliana for detecting low-copy DNA sequences by fluorescence in situ hybridization. Chromosoma (Berlin) 1995;1041(1):39–43. doi: 10.1007/BF00352224. [DOI] [PubMed] [Google Scholar]
- Murata M., Ogura Y., Motoyoshi F. Centromeric repetitive sequences in Arabidopsis thaliana. Jap. J. Genet. 1994;691(1):361–370. doi: 10.1266/jjg.69.361. [DOI] [PubMed] [Google Scholar]
- Murín A., Paclová L. Karyological study of the Slovak flora IX. Acta Fac. Rerum Nat. Univ. Comen., Bot. 1986;331(1):45–48. [Google Scholar]
- Naqshi A. R., Javeid G. N. The genus Arabidopsis Heynh. (Brassicaceae) in J[ammu] & K[ashmir] State. J. Econ. Tax. Bot. 1985;71(1):617–627. [Google Scholar]
- O'Kane S. L., Jr, Al-Shehbaz I. A. A synopsis of Arabidopsis (Brassicaceae). Novon. 1997;71(1):323–327. [Google Scholar]
- O'Kane S. L., Jr, Schaal B. A., Al-Shehbaz I. The origins of Arabidopsis suecica (Brassicaceae) as indicated by nuclear rDNA sequences. Syst. Bot. 1997;211(1):559–566. [Google Scholar]
- O'Kane S. L., Jr, Al-Shehbaz I. A. Phylogenetic position and generic limits of Arabidopsis (Brassicaceae) based on sequences of nuclear ribosomal DNA. Syst. Bot. 2001. (submitted)
- Packer J. G., Witkus R. In IOPB Chromosome number reports LXXV. 1982;311(1):342–368. A. Löve, ed, Taxon. [Google Scholar]
- Pashuk K. T. Chromosome numbers in species of subalpine belt of Chernogora (Ukrainian Carpathians). Bot. Zhurn. 1987;721(1):1069–1074. (Moscow & Leningrad) [Google Scholar]
- Petrovsky V. V., Zhukova P. G. Polyploids and diploids in the vascular flora of the Wrangel Island. Bot. Zhurn. 1983;681(1):749–760. (Moscow & Leningrad) [Google Scholar]
- Polatschek A. Cytotaxonomische Beiträge zur Flora der Ostalpenländer, I. Österr. Bot. Z. 1966;1131(1):1–46. [Google Scholar]
- Polyakova T. F. Development of male and female gametophytes in Arabidopsis thaliana (L.) Heny. Genetic Investigations. 1964;21(1):125–134. Leningrad University. [Google Scholar]
- Price R. A., Palmer J. D., Al-Shehbaz I. A. Systematic relationships of Arabidopsis: a molecular and morphological perspective. 1994;1(1):7–19. In Arabidopsis, E.M. Meyerowitz and C.R. Somerville, eds, (Cold Spring Harbor Laboratory Press, NY), pp. [Google Scholar]
- Price R. A., Bailey C. D., Al-Shehbaz I. A. Transfer of the cupulate-flowered Arabis microsperma and A. tricornuta to Pennellia (Brassicaceae). Novon. 2001;11 (in press) [Google Scholar]
- Raj B. Chromosome numbers in some Indian angiosperms–II. Proc. Indian Adac. Sci. B. 1965;611(1):253–261. [Google Scholar]
- Rieseberg L. H., Brouillet L. Are many plant species paraphyletic? Taxon. 1994;431(1):21–32. [Google Scholar]
- Ritter J. Remarques caryologiques et phytosociologiques sur quelques taxons du Jura et des Alpes. Rev. Cytol. Biol. Veg. 1972;351(1):281–294. [Google Scholar]
- Rollins R. C. A monographic study of Arabis in western North America. Rhodora. 1941;431(1):289–325. [Google Scholar]
- Rollins R. C. Chromosome numbers of Cruciferae. Contr. Gray Herb. 1966;1971(1):43–65. [Google Scholar]
- Rollins R. C. The Cruciferae of continental North America. 1993. Stanford Univ. Press, Stanford.
- Ruíz de Clavijo E. Numeros cromosomicos para la Flora Española 748–763. Lagascalia. 1994;171(1):379–388. [Google Scholar]
- Skalinska M. Further studies in chromosome numbers of Polish angiosperms. Sixth Contribution. Acta Biol. Cracov., Bot. 1966;91(1):31–58. [Google Scholar]
- Skalinska M. Further studies in chromosome numbers of Polish angiosperms. Seventh Contribution. Acta Biol. Cracov., Bot. 1968;111(1):199–224. [Google Scholar]
- Smith R. H. Some chromosome numbers in the Cruciferae. Amer. J. Bot. 1938;251(1):220–221. [Google Scholar]
- Sorsa V. Chromosomen zahlen finnischer kormophyten II. Ann. Acad. Sci. Fenn. 1963;681(1):1–14. [Google Scholar]
- Steinitz-Sears L. M. Chromosome studies in Arabidopsis thaliana. Genetics. 1963;481(1):483–490. doi: 10.1093/genetics/48.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepánek J. Proposal to conserve 2999 Arabidopsis Heynh. (Cruciferae) with a conserved type. Taxon. 1983;321(1):649–650. [Google Scholar]
- Taylor R. L., Mulligan G. A. Flora of the Queen Charlotte Islands. Part 2. Cytological aspects of the vascular plants. Canad. Dept. Agr. Monogr. 1968;41(2)(1):1–148. [Google Scholar]
- Templeton A. R., Crandall K. A., Sing C. F. A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonuclease mapping and DNAsequence data. III. Cladogram estimation. Genetics. 1992;1321(1):619–633. doi: 10.1093/genetics/132.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppner H. Karyologie und Systematik einiger Gefasspflanzen der Ostalpen. Phyton (Horn) 1980;201(1):73–94. [Google Scholar]
- Tischler G. Die Bedeutung der Polyploidie für die Verbreitung der Angiospermen, erläutert an den Arten Schleswig-Holsteins, mit Ausblicken auf andere Florengebiete. Bot. Jahrb. Syst. 1935;671(1):1–36. [Google Scholar]
- Tischler G. Die Chromosomenzahlen der Gefässpflanzen Mitteleuropas. 1950. Uitgeverij Dr. W. Junk, Netherlands.
- Titova N. N. Recherche d'une Drosophila végétative (in Russian). Sov. Bot. 1935;19351(2)(1):61–67. [Google Scholar]
- Tolmatchew A. I. Cruciferae. 1975;71(1):38–167. In Flora Arctica URSS, A.I. Tolmatchew, ed, (Leningrad) [Google Scholar]
- Uotila P., Pellinen K. Chromosome numbers in vascular plants from Finland. Acta Bot. Fenn. 1985;1301(1):1–37. [Google Scholar]
- Váchová M., Májovsky J. In Chromosome number reports LXIX. 1980;291(1):703–730. A. Löve, ed, Taxon. [Google Scholar]
- Voris H. K. Maps of Pleistocene sea levels in Southeast Asia: shorlines, river systems and time durations. J. Biogeogr. 2000;271(1):1153–1167. [Google Scholar]
- Winge Ö. Contributions to the knowledge of chromosome numbers in plants. Cellule. 1925;351(1):303–324. [Google Scholar]
- Zapalowicz H. Conspectus flora Galiciae criticus. Rozpr. Wydz. Mat.-Przyr. Acad. Umiejetn., Dzial B, Nauki Biol. 1912;521(1):1–49. [Google Scholar]
- Zhukova P. G. Chromosome numbers in some species of plants of the northeastern part of the U.S.S.R., II. Bot. Zhurn. (Moscow & Leningrad) 1967;521(1):983–987. [Google Scholar]
- Zhukova P. G. Chromosome numbers in some plant species from the northeast of the U.S.S.R., III. Bot. Zhurn. (Moscow & Leningrad) 1968;531(1):365–368. [Google Scholar]
- Zhukova P. G. Chromosome numbers of some southern Chukotka plant species. Bot. Zhurn. (Moscow & Leningrad) 1980;651(1):51–59. [Google Scholar]
- Zhukova P. G., Petrovsky V. V. Chromosome numbers of some western Chukotka plant species. III. Bot. Zhurn. (Moscow & Leningrad) 1977;621(1):1215–1223. [Google Scholar]
- Zhukova P. G., Petrovsky V. V. Chromosome numbers and taxonomy of some species of the Anyui Mt. Bot. Zhurn. (Moscow & Leningrad) 1980;651(1):651–659. [Google Scholar]
- Zhukova P. G., Petrovsky V. V. A cytotaxonomical study of some species of the family Brassicaceae in northern Asia. Bot. Zhurn. (Moscow & Leningrad) 1984;691(1):236–240. [Google Scholar]
- Zhukova P. G., Petrovsky V. V. Chromosome numbers and taxonomy of some plant species from the northern Asia regions Bot. Zhurn. (Moscow & Leningrad) 1987;721(1):1617–1624. [Google Scholar]
- Zhukova P. G., Tikhonova A. D. Chromosome numbers of certain plant species indigenous to the Chukotskiy Province. Bot. Zhurn. (Moscow & Leningrad) 1971;561(1):868–875. [Google Scholar]
- Zhukova P. G., Petrovsky V. V., Plieva T. V. The chromosome numbers and taxonomy of some plant species from Siberia and Far East. Bot. Zhurn. (Moscow & Leningrad) 1973;581(1):1331–1342. [Google Scholar]
- Zhukova P. G., Korobkov A. A., Tikhanova A. D. Chromosome numbers of some plant species in the eastern Arctic Jakutia. Bot. Zhurn. (Moscow & Leningrad) 1977;621(1):229–234. [Google Scholar]


