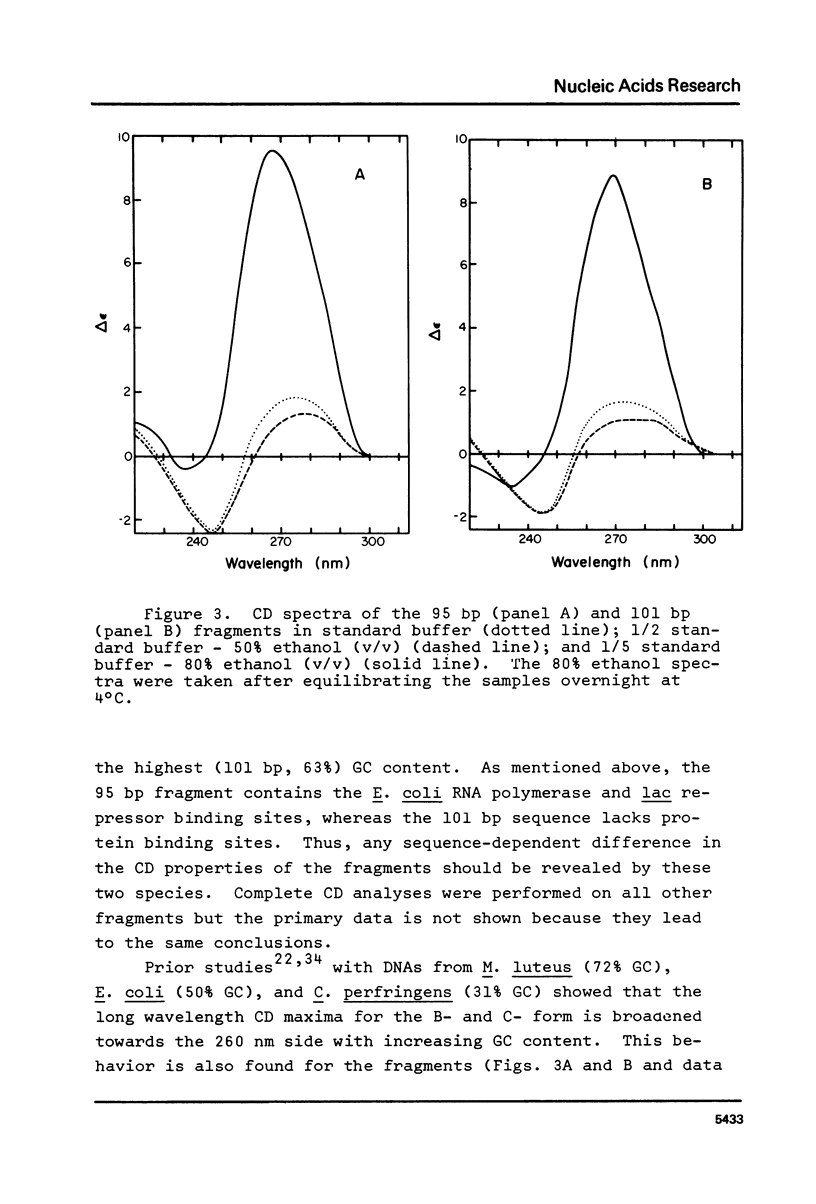
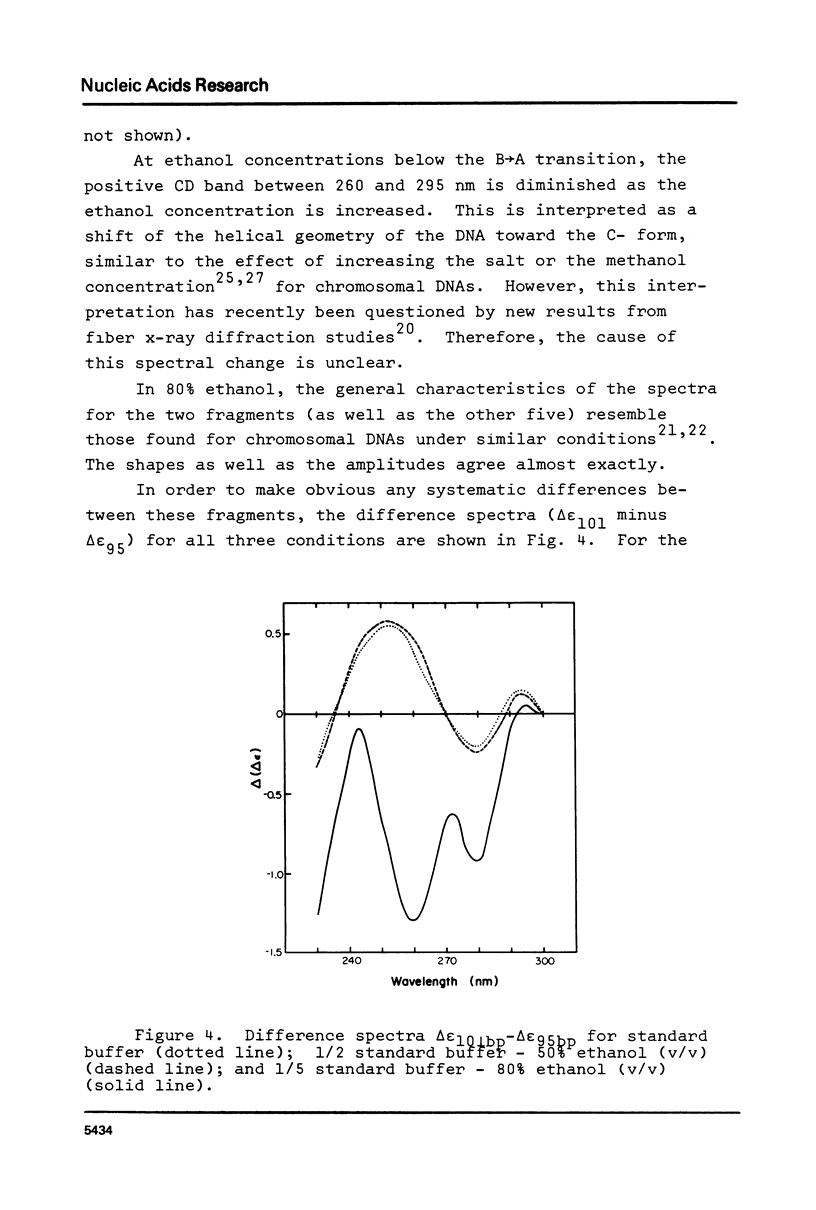
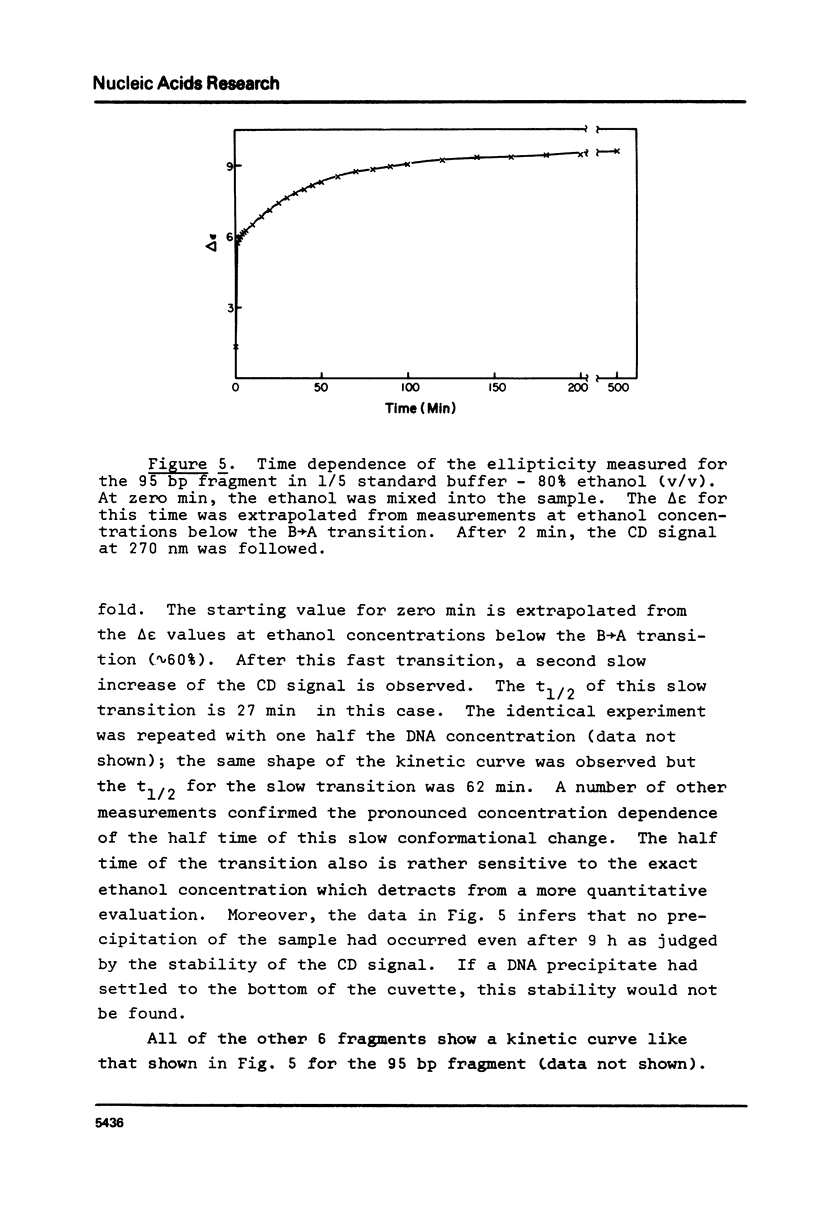
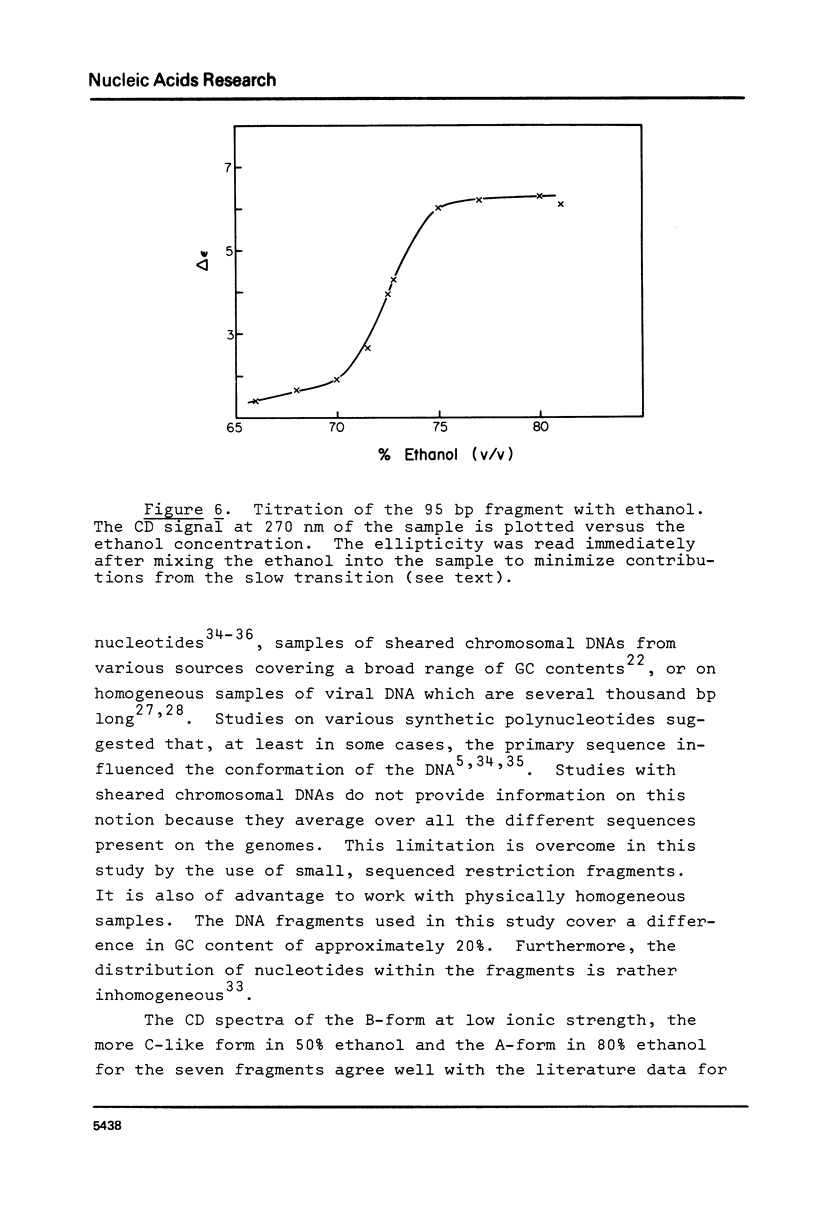
Abstract
The B goes to A conformational transition caused by high ethanol concentrations was studied for seven DNA restriction fragments with overlapping and known sequences. Since the DNAs are homogeneous and range in GC content from 44-63%, they permit an evaluation of the influence of DNA sequence and base composition on the B goes to A transition. Moreover, their small size (80-301 bp) minimizes precipitation artifacts. The B- form spectra (in low salt) and the transition toward the C- form (in ethanol concentrations below the B goes to A transition) agree with prior measurements on chromosomal DNAs and are similar for all seven DNAs. At higher ethanol concentrations (80%), all fragments undergo a transition to the A- form as judged by the large increase of the positive CD band at 270 nm. Difference spectra among the fragments reveal minor differences between the A- form spectra. The ethanol concentration necessary to cause this transition is 72 +/- 2% for all fragments, thus excluding a preference of the CAP-, E. coli RNA polymerase-, or lac repressor-binding sequences for the A- form. The kinetics of the B goes to A transition in 80% ethanol are biphasic; the initial rapid transition is an intramolecular B goes to A form shift and the slower transition is an aggregation (but not precipitation) of the DNA
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler A. P., Revzin A., von Hippel P. H. Molecular parameters characterizing the interaction of Escherichia coli lac repressor with non-operator DNA and inducer. Biochemistry. 1977 Nov 1;16(22):4757–4768. doi: 10.1021/bi00641a001. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Erfurth S. C., Bond P. J., Peticolas W. L. Characterization of the A in equilibrium B transition of DNA in fibers and gels by laser Raman spectroscopy. Biopolymers. 1975 Jun;14(6):1245–1257. doi: 10.1002/bip.1975.360140613. [DOI] [PubMed] [Google Scholar]
- Eyster J. M., Prohofsky E. W. On the B to A conformation change of the double helix. Biopolymers. 1977 May;16(5):965–982. doi: 10.1002/bip.1977.360160503. [DOI] [PubMed] [Google Scholar]
- Girod J. C., Johnson W. C., Jr, Huntington S. K., Maestre M. F. Conformation of deoxyribonucleic acid in alcohol solutions. Biochemistry. 1973 Dec 4;12(25):5092–5096. doi: 10.1021/bi00749a011. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Edmondson S. P., Lang D., Vaughan M. The circular dichroism and X-ray diffraction of DNA condensed from ethanolic solutions. Nucleic Acids Res. 1979;6(6):2089–2107. doi: 10.1093/nar/6.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. M., Taylor T. N., Lang D. Dehydrated circular DNA: circular dichroism of molecules in ethanolic solutions. Biopolymers. 1978 Jan;17(1):145–157. doi: 10.1002/bip.1978.360170111. [DOI] [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon S., Brudno S., Wu T. T., Wolf B. Structural transitions of deoxyribonucleic acid in aqueous electrolyte solutions. I. Reference spectra of conformational limits. Biochemistry. 1975 Apr 22;14(8):1648–1660. doi: 10.1021/bi00679a017. [DOI] [PubMed] [Google Scholar]
- Hardies S. C., Hillen W., Goodman T. C., Wells R. D. High resolution thermal denaturation analyses of small sequenced DNA restriction fragments containing Escherichia coli lactose genetic control loci. J Biol Chem. 1979 Oct 25;254(20):10128–10134. [PubMed] [Google Scholar]
- Hardies S. C., Patient R. K., Klein R. D., Ho F., Reznikoff W. S., Wells R. D. Construction and mapping of recombinant plasmids used for the preparation of DNA fragments containing the Escherichia coli lactose operator and promoter. J Biol Chem. 1979 Jun 25;254(12):5527–5534. [PubMed] [Google Scholar]
- Hardies S. C., Wells R. D. Preparation and characterization of large amounts of restriction fragments containing the E. coli lac control elements. Gene. 1979 Sep;7(1):1–14. doi: 10.1016/0378-1119(79)90039-8. [DOI] [PubMed] [Google Scholar]
- Herbeck R., Yu T. J., Peticolas W. L. Effect of cross-linking on the secondary structure of DNA I. Cross-linking by photodimerization. Biochemistry. 1976 Jun 15;15(12):2656–2660. doi: 10.1021/bi00657a027. [DOI] [PubMed] [Google Scholar]
- Heyneker H. L., Shine J., Goodman H. M., Boyer H. W., Rosenberg J., Dickerson R. E., Narang S. A., Itakura K., Lin S., Riggs A. D. Synthetic lac operator DNA is functional in vivo. Nature. 1976 Oct 28;263(5580):748–752. doi: 10.1038/263748a0. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Minyat E. E., Frank-Kamenetskii M. D., Schyolkina A. K. The B to A transition of DNA in solution. J Mol Biol. 1974 Aug 25;87(4):817–833. doi: 10.1016/0022-2836(74)90086-2. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Schyolkina A. K., Poletayev A. I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12(1):89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- Kovacic R. T., van Holde K. E. Sedimentation of homogeneous double-strand DNA molecules. Biochemistry. 1977 Apr 5;16(7):1490–1498. doi: 10.1021/bi00626a038. [DOI] [PubMed] [Google Scholar]
- Maurizot J. C., Charlier M., Hélène C. Lac repressor binding to poly (d(A-T)). Conformational changes. Biochem Biophys Res Commun. 1974 Oct 8;60(3):951–957. doi: 10.1016/0006-291x(74)90406-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Potaman V. N., Bannikov Y. A., Shlyachtenko L. S. Sedimentation of DNA in ethanol-water solutions within the interval of B to A transition. Nucleic Acids Res. 1980 Feb 11;8(3):635–642. doi: 10.1093/nar/8.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M. T., Jr, deHaseth P. L., Lohman T. M. Interpretation of monovalent and divalent cation effects on the lac repressor-operator interaction. Biochemistry. 1977 Nov 1;16(22):4791–4796. doi: 10.1021/bi00641a005. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Scherer G. E., Walkinshaw M. D., Arnott S. A computer aided oligonucleotide analysis provides a model sequence for RNA polymerase-promoter recognition in E.coli. Nucleic Acids Res. 1978 Oct;5(10):3759–3773. doi: 10.1093/nar/5.10.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher C. A., Baase W. A., Johnson W. C., Jr Conformation and circular dichroism of DNA. Biopolymers. 1979 Apr;18(4):1009–1019. doi: 10.1002/bip.1979.360180418. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Barkley M. D., Bourgeois S. Measurements of unwinding of lac operator by repressor. Nature. 1974 Sep 20;251(5472):247–249. doi: 10.1038/251247a0. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Blakesley R. W., Hardies S. C., Horn G. T., Larson J. E., Selsing E., Burd J. F., Chan H. W., Dodgson J. B., Jensen K. F. The role of DNA structure in genetic regulation. CRC Crit Rev Biochem. 1977;4(3):305–340. doi: 10.3109/10409237709102561. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Hardies S. C., Horn G. T., Klein B., Larson J. E., Neuendorf S. K., Panayotatos N., Patient R. K., Selsing E. RPC-5 column chromatography for the isolation of DNA fragments. Methods Enzymol. 1980;65(1):327–347. doi: 10.1016/s0076-6879(80)65043-5. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E. Buoyant density studies on natural and synthetic deoxyribonucleic acids in neutral and alkaline solutions. J Biol Chem. 1972 Jun 10;247(11):3405–3409. [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]
- Wolf B., Hanlon S. Structural transitions of deoxyribonucleic acid in aqueous electrolyte solutions. II. The role of hydration. Biochemistry. 1975 Apr 22;14(8):1661–1670. doi: 10.1021/bi00679a018. [DOI] [PubMed] [Google Scholar]
- Yang J. T., Samejima T. Optical rotatory dispersion and circular dichroism of nucleic acids. Prog Nucleic Acid Res Mol Biol. 1969;9:223–300. doi: 10.1016/s0079-6603(08)60770-9. [DOI] [PubMed] [Google Scholar]
- Zavriev S. K., Minchenkova L. E., Frank-Kamenetskii M. D., Ivanov V. I. On the flexibility of the boundaries between the A-form and B-form sections in DNA molecule. Nucleic Acids Res. 1978 Jul;5(7):2657–2663. doi: 10.1093/nar/5.7.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C., Luck G. Conformation and reactivity of DNA. VI. Circular dichroism studies of salt-induced conformational changes of DNAs of different base composition. Biochim Biophys Acta. 1974 Aug 15;361(1):11–32. [PubMed] [Google Scholar]
- deHaseth P. L., Lohman T. M., Record M. T., Jr Nonspecific interaction of lac repressor with DNA: an association reaction driven by counterion release. Biochemistry. 1977 Nov 1;16(22):4783–4790. doi: 10.1021/bi00641a004. [DOI] [PubMed] [Google Scholar]




