Abstract
In this study, we retrieved 39 schizophrenia-related antipsychotic drugs from the DrugBank database. These drugs had interactions with 142 targets, whose corresponding genes were defined as drug targeted genes. To explore the complexity between these drugs and their related genes in schizophrenia, we constructed a drug-target gene network. These genes were overrepresented in several pathways including: neuroactive ligand-receptor pathways, glutamate metabolism, and glycine metabolism. Through integrating the pathway information into a drug-gene network, we revealed a few bridge genes connected the sub-networks of the drug-gene network: GRIN2A, GRIN3B, GRIN2C, GRIN2B, DRD1, and DRD2. These genes encode ionotropic glutamate receptors belonging to the NMDA receptor family and dopamine receptors. Haloperidol was the only drug to directly interact with these pathways and receptors and consequently may have a unique action at the drug-gene interaction level during the treatment of schizophrenia. This study represents the first systematic investigation of drug-gene interactions in psychosis.
Introduction
Schizophrenia is a severely debilitating psychiatric disease affecting approximately 1% of the population worldwide1–2. Prior studies suggested genetic factors and disruptions in molecular physiology as its primary etiology, yet no fully cohesive understanding of schizophrenia’s cause exists1–3. The diagnosis of this disorder relies on the presence of two distinct psychological symptoms: “positive” and “negative”. Disordered thought, hallucinations, and delusions comprise the positive symptom-set, while flattened emotional affect, generalized apathy, and an inability to experience pleasure represent its negative symptoms2,3. Traditional antipsychotic medications target positive symptoms and bear unfavorable side effects. Concurrently, ‘atypical’ antipsychotics mitigate both symptom types with fewer initial secondary effects2,4,6, but may harbor deleterious long-term consequences4,6. Moreover, physicians frequently prescribe multiple medications to achieve an acceptable patient response, unintentionally fostering additional drug-drug interactions2–4,6–7. So far, there have been many drugs tested in the treatment of schizophrenia, as well as other psychiatric disorders, but it remains largely unclear for the researchers and clinicians the underlying molecular mechanisms. In particular, there lacks a systems view of the interactions between those drugs and between the drugs and their targeted genes (more accurately, proteins encoded by those genes. For simplicity, we use genes and their proteins interchangeably hereafter). Such knowledge is critical in improving the treatment of schizophrenia and in the design and modification of future drugs.
Recently, we performed a pilot study by investigating the features of five major atypical antipsychotic drugs (clozapine, quetiapine, olanzapine, risperidone, and ziprasidone) through their related genes at the pathway level11. That study highlighted the features of those drugs involved in the serotonin receptor pathway. We constructed a drug-serotonin network including the five atypical antipsychotic drugs, serotonin receptors, and their relationships. The network showed that clozapine had more interactions and more complicated connections with serotonin receptors than the other four drugs. Clozapine also was found to have a relationship with the gene HTR2A, a schizophrenia susceptibility gene. Here, we substantially expand our previous work by including all the available drugs for schizophrenia. Specifically, we investigated drug-gene interactions of all the 39 schizophrenia-related drugs from DrugBank13–14 including 22 FDA currently approved drugs.
Rapid advances in biotechnologies including genomics, transcriptomics, and pharmacogenomics have triggered an exponential rise in pharmacological literature identifying candidate genes for complex disease like schizophrenia. Early clinical findings show that changes in glutamatergic transmission produced by antagonists for the N-methyl-D-aspartate (NMDA) subtype of ionotropic glutamate receptors, results in a state of psychosis in humans17. By leveraging bioinformatics methodologies to evaluate existing antipsychotic and pharmacogenomic data, as well as their inter-relationships, we present an integrative molecular network and pathway model for exploring the antipsychotics used in the treatment of schizophrenia symptoms.
Materials and Methods
The DrugBank database (http://www.drugbank.ca/) offers detailed biochemical specifications and target information for each drug entry in the database13–14. It is currently one of the most comprehensive drug databases and offers an opportunity to study multiple genes and drugs related to one specific biological process. To identify the drugs related to schizophrenia, we used “schizophrenia” as a search term and retrieved 39 specific drugs, each of which was checked manually before we retrieve other drug related information. Each drug entry in the database contains specific information for that drug including the target genes affected by a particular drug. A target gene is defined as a gene that encodes for a protein to which a given drug binds. The entries also include other fields including specific identifications, taxonomy, pharmacology, pharmacoeconomics, properties, and interactions.
To obtain the drug-related targets, we downloaded a text file of the entire DrugBank database (version 2.0, downloaded on August 9, 2010). Using the unique drug ID identifier from the identification section, we developed a Perl script to extract drug names and their associated gene targets. This information was incorporated into a sorted associative data container, comparable to a map. The resulting pseudo-database contained rapidly searchable key/value pairs of drug name/drug information items. For each drug, we exported a list of every target gene that the drug interacts with. The results were concatenated into one large text file which contained all of the drug gene interactions for all of the schizophrenia related drugs. This file formed the basis of our network analysis.
To visualize the drug-gene pathways identified from the DrugBank database, the tab delimited file was imported into software Cytoscape21, which is an open source platform for complex network analysis. In this network analysis, nodes correspond to drugs and gene targets, while the edges indicate the relationship between drugs and their gene targets.
We used WebGestalt, a Web-based Gene Set Analysis Toolkit (http://bioinfo.vanderbilt.edu/webgestalt/)15, to identify all significant molecular pathways associated with the target genes identified in DrugBank. We first exported the SWISS_Prot ID number from DrugBank for each corresponding target gene identified above into a text file. This file was imported into WebGestalt for pathway analysis. Specifically, we used the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotations, since KEGG data is most frequently used in pathway analysis16. This analysis identified the pathways that are enriched with the given gene set. To identify the significant pathways for a gene set in examination, we compared the remaining genes in the human genome (i.e., reference gene set) by applying the hypergeometric statistical test. Then, we performed a Bonferroni multiple testing adjustment for assessing preliminary significance. Furthermore, we required at least 5% of the genes in our gene set to be included in each preliminary significant pathway, aiming to eliminate some stochastic effect in small size pathways.
Results
We obtained 39 antipsychotic drugs and 142 target genes from the DrugBank. The details of the 39 drugs, including their name, FDA approval status, and number of targets, are provided in Table 1. Among those target genes, 71 were associated with glutamate, 31 with glycine, and 34 were associated with neuroactive ligand-receptors (dopamine, cholinergic, histamine, serotonin, adrenergic, and solute carrier family receptors). To further assess the function of these target genes, we conducted the pathway enrichment analysis in WebGestalt. We found 8 KEGG pathways that were significantly enriched for these 142 genes (Table 2).
Table 1.
General Information of 39 Schizophrenia related drugs
| DrugBank ID | Generic Name | FDA Status | # Targets | DrugBank ID | Generic Name | FDA Status | # Targets |
|---|---|---|---|---|---|---|---|
| DB01238 | Aripiprazole | Approved | 3 | DB00313 | Valproic Acid | Approved | 3 |
| DB00477 | Chlorpromazine | Approved | 3 | DB00246 | Ziprasidone | Approved | 11 |
| DB00363 | Clozapine | Approved | 9 | DB01063 | Acetophenazine | Discontinued | 2 |
| DB00510 | Divalproex Sodium | Approved | 1 | DB01038 | Carphenazine | Discontinued | 3 |
| DB00843 | Donepezil | Approved | 2 | DB01239 | Chlorprothixene | Discontinued | 7 |
| DB00875 | Flupenthixol | Approved | 5 | DB00933 | Mesoridazine | Discontinued | 2 |
| DB00623 | Fluphenazine | Approved | 3 | DB01618 | Molindone | Discontinued | 1 |
| DB04842 | Fluspirilene | Approved | 1 | DB00372 | Thiethylperazine | Discontinued | 11 |
| DB00502 | Haloperidol | Approved | 3 | DB00508 | Triflupromazine | Discontinued | 5 |
| DB00408 | Loxapine | Approved | 2 | DB06288 | Amisulpride | Not approved | 2 |
| DB00334 | Olanzapine | Approved | 13 | DB01403 | Methotrimeprazine | Not approved | 11 |
| DB01267 | Paliperidone | Approved | 2 | DB00837 | Progabide | Not approved | 2 |
| DB00777 | Propiomazine | Approved | 14 | DB00409 | Remoxipride | Not approved | 1 |
| DB01224 | Quetiapine | Approved | 8 | DB06144 | Sertindole | Not approved | 8 |
| DB00734 | Risperidone | Approved | 6 | DB00391 | Sulpiride | Not approved | 1 |
| DB00679 | Thioridazine | Approved | 4 | DB01624 | Zuclopenthixol | Not approved | 3 |
| DB01622 | Thioproperazine | Approved | 0 | DB00145 | Glycine | Natural | 32 |
| DB01623 | Thiothixene | Approved | 0 | DB00142 | L-Glutamic Acid | Natural | 71 |
| DB00656 | Trazodone | Approved | 8 | DB00120 | L-Phenylalanine | Natural | 7 |
| DB00831 | Trifluoperazine | Approved | 4 | ||||
Table 2.
KEGG terms that are significantly enriched in the 142 schizophrenia related drug target genes. The P-values were estimated by the hypergeometric test and then adjusted by Bonferroni multiple testing correction (Adj. P-values).
| KEGG ID | KEGG Pathway Description | # Genes (%) | P-value | Adj. P-value |
|---|---|---|---|---|
| hsa04080 | Neuroactive Ligand-receptor Interaction | 52 (36.62) | 7.36 × 10−82 | 1.03 × 10−80 |
| hsa01100 | Metabolic Pathways | 42 (29.58) | 3.02 × 10−34 | 4.23 × 10−33 |
| hsa00250 | Alanine, Aspartate and Glutamate Metabolism | 15 (10.56) | 2.09 × 10−30 | 2.93 × 10−29 |
| hsa04020 | Calcium Signaling Pathway | 21 (14.79) | 1.72 × 10−27 | 2.41 × 10−26 |
| hsa00260 | Glycine, Serine and Threonine Metabolism | 12 (8.45) | 4.75 × 10−23 | 6.65 × 10−22 |
| hsa00330 | Arginine and Proline Metabolism | 10 (7.04) | 9.71 × 10−16 | 1.36 × 10−14 |
| hsa05014 | Amyotrophic Lateral Sclerosis (ALS) | 8 (5.63) | 4.47 × 10−12 | 6.26 × 10−11 |
| hsa04720 | Long-term Potentiation | 8 (5.63) | 4.56 × 10−11 | 6.38 × 10−10 |
The very low P-values ranging from 6.38×10−10 to 1.03×10−80 for the eight biological pathways indicate that the genes grouped into these sets are not random, but specific to the pathway they are clustered with. For example, 52 genes from our gene set were identified to be neuroactive ligand-receptor genes. Among the eight pathways in Table 1, we excluded from further discussion the “metabolic pathways” because it is too general, and the “Amyotrophic lateral Sclerosis (ALS)” pathway because it is a specific disease pathway distinct from schizophrenia. Among the remaining six pathways, two pathways, neuroactive ligand–receptor interactions and long-term potentiation are directly related to neurodevelopment, which has been considered for decades being involved in schizophrenia. The long-term potentiation pathway is important for synaptic plasticity development and related to schizophrenia22. Three pathways were directly related to amino acid metabolism pathways. They are “alanine, aspartate and glutamate metabolism”, “glycine, serine and threonine metabolism”, and “arginine and proline metabolism”. Among these amino acids, aspartate, glutamate and glycine act as neurotransmitters to transmit signals from a neuron to a target cell across a synapse. The calcium signaling pathway also stood out in the enriched pathway list. This pathway has been demonstrated to be affected by antipsychotic drugs and might be involved in the central unifying molecular pathology in schizophrenia23. The genes involved in these pathways were often investigated as schizophrenia candidate genes.
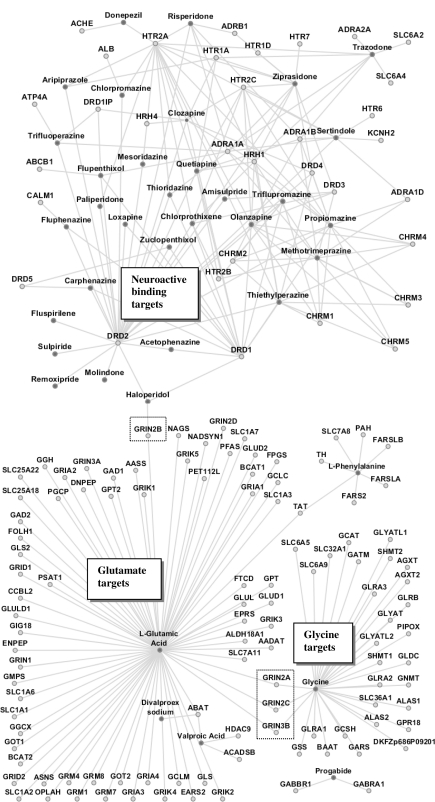
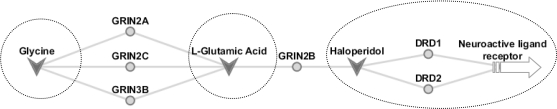
The three main clusters in Figure 1 correspond to neuroactive ligand-receptors, glutamate metabolism and glycine metabolism. The network contained six bridge genes: GRIN2A, GRIN3B, GRIN2C, GRIN2B, DRD1, and DRD2. The bridge between glycine metabolism and glutamate metabolism is through GRIN2A, GRIN3B, and GRIN2C. The bridge between glutamate metabolism and neuroactive ligand-receptor interactions is through GRIN2B. These bridge genes are ionotropic glutamate receptors belonging to the receptor family NMDA. The genes DRD1 and DRD2, from the dopamine receptor family, act as secondary bridge nodes connecting to the neuroactive binding cluster of gene targets. For clear presentation of the network features, we simplified Figure 1 to show only cluster drugs and bridge genes (Figure 2).
Figure 1.
Drugs and their target gene network. Nodes in dark grey: drugs. Nodes in light grey: genes. Dashed boxes show notable bridge nodes.
Figure 2.
Bridge nodes associated with glycine, L-glutamic acid, and haloperidol – GRIN2A, GRIN2B, GRIN2C, GRIN3B, DRD1, and DRD2. Gene targets are light grey circles, while drugs are dark grey v-shapes. All of the ionotropic glutamate receptors are of the NMDA family. DRD1 and DRD2 are dopamine receptors. The dashed circles denote the corresponding gene network clusters. A more detailed network is shown in Figure 1.
After the pathway information was collected we then constructed the schizophrenia drug-target molecular network and identified clusters of genes related to these molecular pathways. In the network (Figure 1), the dark grey nodes represent specific antipsychotic drugs while light grey nodes represent target genes they act upon.
Discussion
The visualization of drug-drug interactions in Cytoscape provides a beginning visual aid for the complexity of schizophrenia drug interactions, but the interaction network did not address the hundreds of known side effects of schizophrenic drugs at the molecular level. Strikingly, in the drug-gene network, we found three main clusters, which were connected by six bridge genes (nodes). This observation, which is the first time in our best knowledge, suggests that the identified bridge genes play a role in the drug design and treatment for schizophrenia.
The network showed that haloperidol is the only schizophrenia related antipsychotic to interact with both dopamine and the glutamate ionotropic receptors based on the drugbank database. Haloperidol was developed in the late 1950’s and has strong effects on hallucinations, delusions, aggressiveness, impulsiveness and states of excitement18. Worldwide, haloperidol is still one of the most frequently prescribed antipsychotic drugs and is the benchmark by which all other antipsychotics are measured18,19. Haloperidol has long been associated with movement disorders including acute dystonia, akathisia, and parkinsonism.
The NMDA receptor is the predominant molecular device for controlling synaptic plasticity and memory function while the D1 receptors regulate neuronal growth and development, mediate some behavioral responses, and modulate dopamine receptor D2-mediated events. The last 50 years of antipsychotic drug development for schizophrenia has focused on the dopamine D2 receptor. Although the underlying pathophysiology of schizophrenia remains unknown, recently the NMDA receptor has been identified as a target for therapeutics17.
Administration of low doses of NMDA receptor antagonists in normal subjects have been shown to cause the negative symptoms, cognitive impairment and physiologic disturbances observed in schizophrenia. This supports the hypothesis that hypo-function of the NMDA receptor may contribute to the pathophysiology of schizophrenia20. Clinical findings have reported that changes in glutamatergic transmission produced by antagonists of NMDA ionotropic glutamate receptors resulted in a state of psychosis in humans with all major symptom clusters (positive, negative, and cognitive)17. Our finding that haloperidol has a direct link to these receptors and that the different targets clusters are connected through these receptors strengthen the hypothesis that NMDA receptors are implicated in the pathophysiology of schizophrenia.17 Because haloperidol was the only drug found to interact directly with an NMDA ionotropic glutamate receptor, this finding suggests that haloperidol might have a different action in the treatment process of schizophrenia.
Conclusion
In this study, we identified 39 anti-psychotic drugs for schizophrenia treatment that interact with 142 target genes through DrugBank. Our pathway analysis showed that these genes are associated with glycine metabolism, glutamate metabolism, and neuroactive ligand-receptor (dopamine, histidine, serotonin, adrenergic) binding. Our network analysis in software Cytoscape contained these groups and revealed specific genes that connect these pathways. All of the connecting genes belonged to the ionotropic glutamate receptor belonging to the NMDA receptor family. Importantly, the only drug to interact directly with this pathway and dopamine receptors was haloperidol. This drug is a typical antipsychotic that is used in the treatment of acute psychotic states and delirium. It has strong central anti-dopaminergic action. Notable side effects of haloperidol include dry mouth, lethargy, restlessness, muscle-stiffness, muscle-cramping, tremors, weight gain, and depression. These results indicate that haloperidol might have a different action during the treatment process of schizophrenia because of the interaction with ionotropic glutamate receptors.
One of the challenging tasks for a psychiatrist is to maximize the benefits of antipsychotic medication, while minimizing the associated side-effects for a given patient. An increased understanding of drug gene interactions can aid the psychiatrist in understanding how particular combinations of drugs lead to particular negative side effects. This study offers the psychiatrist a unique perspective on haloperidol and also highlights the role of ionotropic glutamate receptors during drug treatment for psychosis.
This approach can be applied with any disease in terms of drug-gene interaction, a research area which has not been well studied at the systems level but rapidly accumulating data during recent years has provided us a great opportunity. The purpose of this study was to investigate the drug-gene interactions. Future work on this project is needed to expand the scope to include drug-drug interactions to see how multiple dugs affect the expression of target genes. Furthermore, we would like to expand from schizophrenia related drugs to all antipsychotic drugs, since sub phenotypes in psychosis share many genetic factors and drugs are commonly developed for similar symptoms. It is important to investigate whether the drug-gene network features observed in this study will be similarly found in psychosis. Furthermore, the use of multiple drug and gene resources would enrich the number and types of drug-target gene interactions found. As more drugs are added to the database more drug-gene interactions will be identified. During the revision of this manuscript, we found four additional drugs have been classified with Schizophrenia in the Drugbank. They are: Imipramine, Cabergoline, Pergolide, and Bromocriptine. It is often for databases to expand over time. However, we believe, the most important drugs pertaining to schizophrenia have been included in our current study. The additions of a few drugs will unlikely invalidate our network, rather likely enhance it. As pharmacogenomic data becomes available through high throughput technologies, this kind of system wide network analysis will be an invaluable tool to help researchers unlock the molecular etiology of complex disease.
Acknowledgments
We would like to thank Dr. Hua Xu and Dr. Naqi Khan for their valuable discussion in this project. This work was partially supported by the National Library of Medicine Training Grant 5T15LM007450-09, 2009 NARSAD Maltz Investigator Award to Z.Z., and 2010 NARSAD Young Investigator Award to J.S.
References
- 1.Danielyan A, Nasrallah HA. Neurological disorders in schizophrenia. Psychiatric Clinics of North America. 2009;32:719–57. doi: 10.1016/j.psc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Schultz SH, North SW, Shields CG. Schizophrenia: a review. Am Fam Physician. 2007;75:1821–9. [PubMed] [Google Scholar]
- 3.Sawa A, Snyder SH. Schizophrenia: Diverse Approaches to a Complex Disease. Science. 2002;296:692–5. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- 4.Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. CMAJ. 2005;172:1703–11. doi: 10.1503/cmaj.1041064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gejman PV, Sanders AR, Duan J. The role of genetics in the etiology of schizophrenia. Psychiatric Clinics of North America. 2010;33:35–66. doi: 10.1016/j.psc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds GP. Receptor mechanisms in the treatment of schizophrenia. J Psychopharmacol. 2004;18:340–5. doi: 10.1177/026988110401800303. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Jain S, Brahmachari SK, Kukreti R. Pharmacogenomics: a path to predictive medicine for schizophrenia. Pharmacogenomics. 2006;7:31–47. doi: 10.2217/14622416.7.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Arranz MJ, de Leon J. Pharmacogenetics and pharmacogenomics of schizophrenia: a review of last decade of research. Mol Psychiatry. 2007;12:707–47. doi: 10.1038/sj.mp.4002009. [DOI] [PubMed] [Google Scholar]
- 9.Basile VS, Masellis M, Potkin SG, Kennedy JL. Pharmacogenomics in schizophrenia: the quest for individualized therapy. Hum Mol Genet. 2002;11:2517–30. doi: 10.1093/hmg/11.20.2517. [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Maneen MJ, Stahl SM. Building a better antipsychotic: receptor targets for the treatment of multiple symptom dimensions of schizophrenia. Neurotherapeutics. 2009;6:78–85. doi: 10.1016/j.nurt.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J, Zhao Z. Pathway-assisted investigation of atypical antipsychotic drugs and serotonin receptors in schizophrenia. IEEE 2010 Annual ORNL Biomedical Science & Engineering Conference Proceedings; May 25–26, 2010; Oak Ridge, TN. IEEE Catalog Number: CFP1047GART. [Google Scholar]
- 12.Rietkerk T, Boks MPM, Sommer IEC, de Jong S, Kahn RS, Ophoff RA. Network analysis of positional candidate genes of schizophrenia highlights myelin-related pathways. Mol Psychiatry. 2009;14:353–5. doi: 10.1038/mp.2008.86. [DOI] [PubMed] [Google Scholar]
- 13.Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36:D901–6. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, Chang Z, Woolsey J. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2005;34:D668–72. doi: 10.1093/nar/gkj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:W741–8. doi: 10.1093/nar/gki475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeffrey Conn P, Lindsley Craig W. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacology Sci. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irving CB, Adams CE, Lawrie S. Haloperidol versus placebo for schizophrenia. Cochrane Database Syst Rev. 2006;(4) doi: 10.1002/14651858.CD003082.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willner P. The dopamine hypothesis of schizophrenia: current status, future prospects. International Clinical Pscyopharmacology. 1997;12:297–308. doi: 10.1097/00004850-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Joseph Coyle T. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cellular and Molecular Neurobiology. 2006 Jul;26 doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shannon P, Markiel A, Ozier O, Baliga NS, Want JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of bimolecular interaction networks. Genome Res. 2003;11:2498–504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo AY, Sun J, riley BP, Thiselton DL, Kendler KS, Zhao Z. The dystrobrevin-binding protein 1 gene: features and networks. Mol Psychiatry. 2009;14:18–29. doi: 10.1038/mp.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res Brain Res Rev. 2003;43:70–84. doi: 10.1016/s0165-0173(03)00203-0. [DOI] [PubMed] [Google Scholar]