Abstract
Electronic prescribing can reduce certain types of medication errors but can also facilitate new types of errors. Internal prescription discrepancies arise when information in the structured (dose, frequency) fields conflicts with instructions given in the free-text field on the prescription, and are unique to electronic prescribing. It is not known whether internal prescription discrepancies lead to adverse events.
We have conducted a case-control study to determine whether internal discrepancies in warfarin prescriptions are associated with an increased risk of hemorrhage. We compared frequency of internal discrepancies in warfarin prescriptions between 573 patients admitted for a major hemorrhage and 1,719 controls. In multivariable analysis case patients had the odds of 0.61 of having an internal discrepancy in the most recent warfarin prescription (p = 0.045) compared to controls.
Consequences of EMR errors may not be obvious. Studies that directly examine clinical outcomes are necessary to identify categories of EMR errors likely to cause patient harm.
Background
Electronic medical records are widely promoted by independent and federal agencies for their potential to improve quality of patient care1,2. In particular, a number of researchers have demonstrated that electronic medical records can reduce certain types of medication errors and adverse drug events3–7. On the other hand, controversy remains whether this effect of electronic medical records is uniform. Studies have shown that suboptimal design and / or implementation of electronic medical records can lead to increases in medication error rates and even mortality8–10.
One particular unintended consequence of using electronic medical records to prescribe medications that our group and others have identified is internal prescription discrepancies11,12. These arise from conflicts between two main functional components of electronic prescribing interfaces: a) a set of structured fields (e.g. medication, dose, frequency) required for decision support and b) free-text instruction fields that allow flexibility needed to tailor prescriptions to the individual patients’ circumstances. The conflict arises when the information in structured fields contradicts information in the free-text field in the same prescription.
Internal prescription discrepancies are found in as many as 1 in 6 prescriptions with free-text fields. They are even more common in medications, such as warfarin, insulin and digoxin, that are associated with a particularly high risk for adverse drug events13. Internal prescription discrepancies could conceivably lead to adverse drug events if the patient follows the wrong set of instructions11. However, it is not known whether they actually result in adverse outcomes. We have therefore conducted this study to determine whether internal discrepancies in warfarin prescriptions are associated with an increased risk of major hemorrhage.
Methods
Design
We conducted a case-control study to establish whether internal discrepancies in EMR prescriptions for warfarin are associated with higher incidence of major hemorrhage. We compared the prevalence of internal discrepancies in warfarin prescriptions between patients who had a major hemorrhage during the study period (cases) and patients who did not (controls).
Study Cohort
Patients at the Brigham and Women’s Hospital and Massachusetts General Hospital who had warfarin on their active medication list between 2000 and 2008 were studied. Patients who were admitted to either hospital while having warfarin on their active medication list with an admission diagnosis of a hemorrhage were included in the case group. Controls were selected from among patients who were matched by age and the HEMORR2HAGES hemorrhage risk score14 to a case patient and had an active warfarin prescription at the time of the case patient’s hospitalization but did not have a hemorrhage during the study period. HEMORR2HAGES score includes patient age, creatinine level, history of liver disease, history of alcohol abuse, history of malignancy, platelet count, history of prior hemorrhage, history of hypertension, history of anemia, history of dementia or Parkinson’s disease and history of stroke. HEMORR2HAGES score can vary from 0 to 11 with most patients usually falling below 5. Patients were matched by HEMORR2HAGES score either if both had a score ≥ 5 or if their HEMORR2HAGES score was identical, consistent with the original stratification scheme used to validate the score14. Three control patients were selected for each case patient to increase the power of the analysis. If more than three patients matched a case patient by HEMORR2HAGES score, patients with a warfarin prescription dated closest to the case patient’s date of hospital admission were selected to reduce a possible temporal effect on hemorrhage risk.
Study Measurements
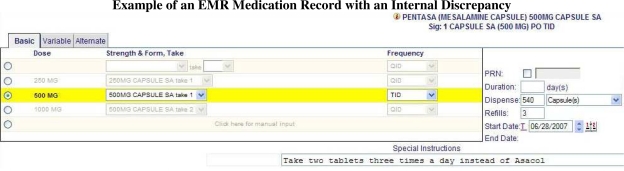
Presence of an internal discrepancy in the electronic warfarin prescription served as the primary outcome variable. A prescription was marked as having an internal discrepancy if the information contained in structured medication name, route, dose, frequency, strength and form contradicted the information in the free-text Special Instructions field as previously described11 (Figure 1). Discrepancies were identified through manual review of de-identified prescriptions by a trained senior pharmacy student who was blinded to the case / control assignments.
Figure 1.
Example of an EMR Medication Record with an Internal Discrepancy
Based on our previously published analysis of distribution of internal prescription discrepancies, we also determined whether each prescription was describing a complex regimen. A prescription was identified as describing a complex regimen if either the dose or frequency of the medication prescribed changed over time. Our previous analysis showed that this type of prescriptions was particularly prone to internal discrepancies11.
History of the conditions included in the calculation of the HEMORR2HAGES score was identified based on the ICD9-CM billing codes prior to the date of the case patient’s hospital admission for hemorrhage. At least one inpatient or two outpatient billing codes were required to establish each diagnosis. Highest creatinine level during the study period prior to the hemorrhage date was used for the score calculation.
The figure illustrates an example of an internal discrepancy between strength and form indicated in the structured Strength & Form field (“500MG CAPSULE SA take 1”) and the same information in the free-text Special Instructions field (“Take two tablets”).
Study Environment
The study was conducted at Partners HealthCare System. Partners HealthCare is an integrated healthcare delivery network in eastern Massachusetts that includes founding members Brigham and Women’s Hospital and Massachusetts General Hospital as well as several community hospitals and affiliated private practices. This study included medication records from Longitudinal Medical Record (LMR) – an internally developed CCHIT-certified EMR used by the majority of providers at Partners HealthCare. LMR medication screen includes a combination of structured fields (e.g. Dose, Frequency) and a free-text field (Special Instructions). Information in the structured fields is usually entered by selecting a medication-specific dictionary value from a dropdown list while the Special Instructions field places no restrictions on the text that can be entered. In addition to the Basic medication entry screen that assumes constant dose and frequency over the course of treatment, LMR also provides more advanced medication entry screens (e.g. Variable, Alternate and Sliding Scale) that allow to enter a regimen whose dose / frequency change over time. Warfarin prescriptions are written either by physicians or by clinical pharmacists in a central anticoagulation clinic under the supervision of a physician. Both physicians and clinical pharmacists prescribing warfarin have full access to the patients’ medical records. Patients primarily fill prescriptions in community pharmacies that are not affiliated with Partners HealthCare and do not have access to the patients’ electronic medical records.
Data Sources
Data were obtained from the electronic medical record system at Partners HealthCare. Demographic information, medication records, laboratory results and problem list were obtained from Quality Data Warehouse (QDW) at Partners HealthCare. Billing data and DRG codes were obtained from Research Patient Data Registry (RPDR) at Partners HealthCare.
Statistical Analysis
Summary statistics were constructed by using frequencies and proportions for categorical data and by using means, standard deviations, medians and ranges for continuous variables. Univariate associations between continuous variables were assessed using two-sided t-test. Univariate associations between categorical variables were evaluated using chi-square test.
To determine the relationship between major hemorrhage and internal discrepancies in warfarin prescriptions we constructed a multivariable conditional logistic regression model that included all of case and control patients. Case assignment (i.e. major hemorrhage) served as the primary outcome variable. Presence of internal discrepancy in the most recent warfarin prescription prior to the hemorrhage date served as the primary predictor variable. Presence of a “complex regimen” in the most recent warfarin prescription prior to the hemorrhage date served as the confounder variable. Risk factors for hemorrhage were not included in the model as the patients were already matched by the HEMORR2HAGES score. All analyses were performed with SAS statistical software, version 9.2 (SAS Institute, Cary, NC).
IRB
The study protocol was reviewed and approved by the Partners Human Research Committee.
Results
Study Cohort
We identified 573 patients on warfarin who were admitted for hemorrhage to Brigham and Women’s Hospital or Massachusetts General Hospital between 2000 and 2008. We subsequently selected 1,719 patients on warfarin who did not have an admission for hemorrhage during the study period into the control group. Characteristics of the patients in case and control groups are listed in Table 1. The mean difference between overall HEMORR2HAGES scores for case and corresponding control patients was 0.045 (standard deviation 0.27). Case patients were more likely to have a history of prior hemorrhage, or anemia, and less likely to have a history of dementia or Parkinson’s disease. Case patients also had slightly higher serum creatinine and were more likely to have Medicare or Medicaid as their health insurance. None of the other clinical or demographic characteristics showed a statistically significant difference. Gastrointestinal tract was the most common site of hemorrhage among the case patients (Table 2).
Table 1.
Patient Characteristics
| Variable | Cases | Controls | P-value |
|---|---|---|---|
| Study patients, n | 573 | 1,719 | |
| Age, mean (± SD), years | 71.7 (± 13.5) | 71.7 (± 13.5) | 1.0 |
| Women, n (%) | 272 (47.4) | 822 (47.8) | 0.92 |
| Ethnicity, n (%) | |||
| White | 455 (79.4) | 1,381 (80.3) | 0.27 |
| Black | 56 (9.8) | 133 (7.7) | |
| Hispanic | 28 (4.9) | 76 (4.4) | |
| Other (includes unknown) | 34 (5.9) | 129 (7.5) | |
| English is the primary language, n (%) | 521 (90.8) | 1,580 (91.9) | 0.51 |
| Health insurance, n (%) | |||
| Private | 110 (19.2) | 475 (27.6) | 0.0002 |
| Medicare | 414 (72.3) | 1,149 (66.8) | |
| Medicaid | 42 (7.3) | 83 (4.8) | |
| Uninsured | 7 (1.2) | 12 (0.7) | |
| HEMORR2HAGES score, mean (± SD) | 2.1 (1.5) | 2.1 (± 1.5) | 0.88 |
| Serum creatinine, mean (± SD), mg/dL | 2.3 (1.9) | 2.0 (± 1.6) | 0.0012 |
| Platelet count < 100,000 / μl, n (%) | 127 (22.2) | 360 (20.9) | 0.58 |
| Liver disease, n (%) | 17 (3.0) | 31 (1.8) | 0.13 |
| Alcohol abuse, n (%) | 9 (1.6) | 51 (3.0) | 0.097 |
| Malignancy, n (%) | 178 (31.1) | 587 (34.1) | 0.19 |
| Prior hemorrhage, n (%) | 28 (4.9) | 25 (1.5) | < 0.0001 |
| Uncontrolled Hypertension, n (%) | 11 (1.9) | 38 (2.2) | 0.81 |
| Anemia, n (%) | 215 (37.5) | 486 (28.3) | < 0.0001 |
| Dementia / Parkinson’s disease, n (%) | 50 (8.7) | 254 (14.8) | 0.0003 |
| Prior stroke, n (%) | 155 (27.1) | 479 (27.9) | 0.75 |
| Complex warfarin regimen, n (%) | 65 (11.3) | 193 (11.2) | 1.0 |
Table 2.
Distributions of Diagnoses among Patients Admitted for Major Hemorrhage
| Hemorrhage Site | N (%) |
|---|---|
| Intracranial | 123 (21.5) |
| Gastrointestinal | 387 (67.5) |
| Hemoptysis | 43 (7.5) |
| Other | 20 (3.5) |
Internal Discrepancies in Warfarin Prescriptions
Out of the 2,292 warfarin prescriptions reviewed, 254 were found to have a total of 259 internal discrepancies (four prescriptions had more than one discrepancy). The most common discrepancies involved a complex regimen (196 / 75.7%), and dose discrepancy (41 / 15.8%).
Major Hemorrhage and Internal Prescription Discrepancies
Internal discrepancies were found in 55 (9.6%) case and 199 (11.6%) control patient warfarin prescriptions (p = 0.22). In multivariable analysis adjusted for the prevalence of “complex regimen” prescriptions, case patients had the odds ratio of 0.61 of having an internal discrepancy in the most recent warfarin prescription (p = 0.045). There was a trend for higher frequency of hemorrhage in patients with complex regimen prescriptions (p = 0.12).
Discussion
In this large retrospective case-control study we have found that, contrary to our original expectations, patients who had suffered a major hemorrhage were less likely to have an internal discrepancy in their warfarin prescriptions. It is important to consider the implications of this finding both for the specific clinical scenario we analyzed and for the general approach to studying of effects of health information technology on patient safety.
Warfarin and insulin have been shown to place patients at a particularly high risk for adverse drug events13. These are also medications whose dosing frequently varies depending on the time of the day or the day of the week. Such dosing regimens may be difficult or time consuming to express in structured format provided by electronic prescribing interfaces. In these circumstances providers frequently resort to using free text instruction fields while often leaving default information in the structured prescription fields. It is therefore not surprising that these medications were also found to have a high rate of internal prescription discrepancies11. Prescription discrepancies could in turn lead to misunderstanding of the complicated instructions by the patient and ultimately to adverse drug events, either from under- or over-anticoagulation. However, our study showed evidence for the opposite effect.
One possible explanation involves discrepancies leading to an increased attention of the dispensing pharmacists to both prescriptions and the patients. Ordinarily patients frequently receive little or no counseling from the pharmacist about their prescriptions17 even though pharmacist counseling has been shown to decrease the rate of adverse drug events18. Prescriptions with internal discrepancies, on the other hand, commonly lead to a phone call from the pharmacist to the prescribing provider for clarification13. Subsequently the pharmacist would be more likely to discuss the confusing prescription with the patient, while prescriptions without discrepancies would not attract a similar level of attention. Our finding of a trend of higher risk for hemorrhage associated with complex prescriptions is consistent with this explanation. Further studies analyzing direct evidence of pharmacists’ communication with the patients will be necessary to confirm this as a possible mechanism.
Our findings also raise an important issue with respect to the methods used to study the effect of health information technology on patient care. Adverse drug events are rare19–23. Consequently many investigations of the effect of health information technology on patient safety are not powered to identify effects on actual adverse events and focus on hypothetical or potential adverse events24,25. Our findings show that potential adverse drug events may not always translate into real patient harm and unforeseen components of clinical workflow may even reverse the predicted association. It is therefore crucial to conduct studies that directly examine clinical outcomes in order to achieve a better understanding of the complex interplay between health information technology and patient care.
Our study has several limitations. It was focused on a particular type of electronic error for a specific medication (warfarin). Its findings may therefore not be applicable to other errors facilitated by health information technology, and each case will likely require a dedicated evaluation. The study was conducted in two academic medical centers that used an internally developed electronic medical record which could also limit its generalizability. However, our informal review showed that the majority of widely used commercial and open-source electronic medical record systems include the same fundamental combination of structured and free-text fields in their prescription interfaces, making them highly susceptible to internal prescription discrepancies. The overall clinical workflow of prescribers who have access to the patients’ records and community pharmacists who only have the prescription and must contact the patient’s physician to obtain more detailed information was similar to that in many other institutions and private practices in the U.S.. Our multivariable analysis was not adjusted for patient demographics. The reason for this approach was our focus on the proven risk factors for warfarin-associated hemorrhage. Most studies including the one that validated the risk score we utilized did not show an association between demographic characteristics other than age and incidence of hemorrhage for patients on warfarin14,26,27; age was already included in the risk score we used. Prothrombin time measurements were not included in the model because elevated prothrombin time was hypothesized to be a part of the mechanism for increased risk of hemorrhage (due to the patient’s misunderstanding of the prescription and subsequent warfarin overdose). There were several significant differences in the prevalence of individual hemorrhage risk factors between the case and control groups. However, the individual case-control pairs were very closely matched by the overall HEMORR2HAGES score, implying similar risk; differences in individual components of the score were likely random and unavoidable. Several components of the HEMORR2HAGES score were calculated using administrative data, which could have underestimated their prevalence. However, the original study that described the HEMORR2HAGES score utilized the same approach14. We therefore opted to duplicate their method to ensure the score validity.
Conclusions
Prescription discrepancies were common in patients on warfarin but against the original expectations were associated with a lower risk for major hemorrhage. This finding demonstrates that consequences of EMR errors may not be obvious. Studies that directly examine clinical outcomes are necessary to identify categories of EMR errors likely to cause patient harm.
Acknowledgments
We are grateful to Dayton Yuen for his assistance with record review. We would like to thank Quality Data Management and Research Patient Data Registry teams at Partners HealthCare for their input and assistance. This work was supported in part by funding from the Partners IS Research Council.
References
- 1.IOM . Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 2.Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010 Aug 5;363(6):501–504. doi: 10.1056/NEJMp1006114. [DOI] [PubMed] [Google Scholar]
- 3.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. Jama. 1998 Oct 21;280(15):1311–1316. doi: 10.1001/jama.280.15.1311. [DOI] [PubMed] [Google Scholar]
- 4.Evans RS, Pestotnik SL, Classen DC, et al. A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med. 1998 Jan 22;338(4):232–238. doi: 10.1056/NEJM199801223380406. [DOI] [PubMed] [Google Scholar]
- 5.Bates DW, Teich JM, Lee J, et al. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc. 1999 Jul-Aug;6(4):313–321. doi: 10.1136/jamia.1999.00660313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullett CJ, Evans RS, Christenson JC, Dean JM. Development and impact of a computerized pediatric antiinfective decision support program. Pediatrics. 2001 Oct;108(4):E75. doi: 10.1542/peds.108.4.e75. [DOI] [PubMed] [Google Scholar]
- 7.King WJ, Paice N, Rangrej J, Forestell GJ, Swartz R. The effect of computerized physician order entry on medication errors and adverse drug events in pediatric inpatients. Pediatrics. 2003 Sep;112(3 Pt 1):506–509. doi: 10.1542/peds.112.3.506. [DOI] [PubMed] [Google Scholar]
- 8.Koppel R, Metlay JP, Cohen A, et al. Role of computerized physician order entry systems in facilitating medication errors. Jama. 2005 Mar;293(9)(10):1197–1203. doi: 10.1001/jama.293.10.1197. [DOI] [PubMed] [Google Scholar]
- 9.Han YY, Carcillo JA, Venkataraman ST, et al. Unexpected increased mortality after implementation of a commercially sold computerized physician order entry system. Pediatrics. 2005 Dec;116(6):1506–1512. doi: 10.1542/peds.2005-1287. [DOI] [PubMed] [Google Scholar]
- 10.Horsky J, Kuperman GJ, Patel VL. Comprehensive analysis of a medication dosing error related to CPOE. J Am Med Inform Assoc. 2005 Jul-Aug;12(4):377–382. doi: 10.1197/jamia.M1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palchuk MB, Fang EA, Cygielnik JM, et al. An unintended consequence of electronic prescriptions: prevalence and impact of internal discrepancies. J Am Med Inform Assoc. 2010 Jul-Aug;17(4):472–476. doi: 10.1136/jamia.2010.003335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh H, Mani S, Espadas D, Petersen N, Franklin V, Petersen LA. Prescription errors and outcomes related to inconsistent information transmitted through computerized order entry: a prospective study. Arch Intern Med. 2009 May 25;169(10):982–989. doi: 10.1001/archinternmed.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007 Dec 4;147(11):755–765. doi: 10.7326/0003-4819-147-11-200712040-00006. [DOI] [PubMed] [Google Scholar]
- 14.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006 Mar;151(3):713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73(3):751–754. [Google Scholar]
- 16.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Vol. 75. Biometrika Trust; 1988. pp. 800–802. [Google Scholar]
- 17.Svarstad BL, Bultman DC, Mount JK. Patient counseling provided in community pharmacies: effects of state regulation, pharmacist age, and busyness. J Am Pharm Assoc (2003) 2004 Jan-Feb;44(1):22–29. doi: 10.1331/154434504322713192. [DOI] [PubMed] [Google Scholar]
- 18.Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006 Mar 13;166(5):565–571. doi: 10.1001/archinte.166.5.565. [DOI] [PubMed] [Google Scholar]
- 19.Cullen DJ, Sweitzer BJ, Bates DW, Burdick E, Edmondson A, Leape LL. Preventable adverse drug events in hospitalized patients: a comparative study of intensive care and general care units. Crit Care Med. 1997 Aug;25(8):1289–1297. doi: 10.1097/00003246-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. Jama. 2001 Apr 25;285(16):2114–2120. doi: 10.1001/jama.285.16.2114. [DOI] [PubMed] [Google Scholar]
- 21.O’Neil AC, Petersen LA, Cook EF, Bates DW, Lee TH, Brennan TA. Physician reporting compared with medical-record review to identify adverse medical events. Ann Intern Med. 1993 Sep 1;119(5):370–376. doi: 10.7326/0003-4819-119-5-199309010-00004. [DOI] [PubMed] [Google Scholar]
- 22.Bates DW, Leape LL, Petrycki S. Incidence and preventability of adverse drug events in hospitalized adults. J Gen Intern Med. 1993 Jun;8(6):289–294. doi: 10.1007/BF02600138. [DOI] [PubMed] [Google Scholar]
- 23.Gurwitz JH, Field TS, Avorn J, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med. 2000 Aug 1;109(2):87–94. doi: 10.1016/s0002-9343(00)00451-4. [DOI] [PubMed] [Google Scholar]
- 24.Berger RG, Kichak JP. Computerized physician order entry: helpful or harmful? J Am Med Inform Assoc. 2004 Mar-Apr;11(2):100–103. doi: 10.1197/jamia.M1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhry B, Wang J, Wu S, et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006 May 16;144(10):742–752. doi: 10.7326/0003-4819-144-10-200605160-00125. [DOI] [PubMed] [Google Scholar]
- 26.Wells PS, Forgie MA, Simms M, et al. The outpatient bleeding risk index: validation of a tool for predicting bleeding rates in patients treated for deep venous thrombosis and pulmonary embolism. Arch Intern Med. 2003 Apr 28;163(8):917–920. doi: 10.1001/archinte.163.8.917. [DOI] [PubMed] [Google Scholar]
- 27.Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998 Aug;105(2):91–99. doi: 10.1016/s0002-9343(98)00198-3. [DOI] [PubMed] [Google Scholar]