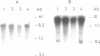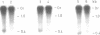Abstract
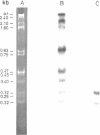
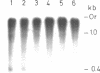
The macronuclear rRNA genes of Tetrahymena thermophila contain an 0.4 kb intervening sequence in the 26S rRNA coding region. The sequence is represented within the primary transcription product. We demonstrate in this paper that the enzyme activities necessary for the endonucleolytic cleavage as well as for the ligation of the transcript are associated with the isolated. The intervening sequence is excised as an unique molecule, which is stable in vitro. About 50% of the in vitro synthesized RNA is processed. Faithful in vitro transcription occurs in the presence of the divalent ions Mg2+, Mn2+ and Co2+ while processing takes place only in the presence of Mg2+. The absolute requirement for Mg2+ in the excision reaction enables us to synthesize labelled pre-rRNA in the presence of Mn2+ or Co2+. The synthesized RNA can be used as a substrate in studies of th processing enzymes in vitro.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson J. RNA processing and the intervening sequence problem. Annu Rev Biochem. 1979;48:1035–1069. doi: 10.1146/annurev.bi.48.070179.005131. [DOI] [PubMed] [Google Scholar]
- Blanchard J. M., Weber J., Jelinek W., Darnell J. E. In vitro RNA-RNA splicing in adenovirus 2 mRNA formation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5344–5348. doi: 10.1073/pnas.75.11.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Rio D. C. Localization of transcribed regions on extrachromosomal ribosomal RNA genes of Tetrahymena thermophila by R-loop mapping. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5051–5055. doi: 10.1073/pnas.76.10.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu N. H., Bruszewski W. B., Salzman N. P. Evidence for the role of double-helical structures in the maturation of simian virus-40 messenger RNA. Nucleic Acids Res. 1980 Jan 11;8(1):153–168. doi: 10.1093/nar/8.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. Split genes and RNA splicing. Science. 1979 Apr 20;204(4390):264–271. doi: 10.1126/science.373120. [DOI] [PubMed] [Google Scholar]
- Din N., Engberg J. Extrachromosomal ribosomal RNA genes in Tetrahymena: structure and evolution. J Mol Biol. 1979 Nov 5;134(3):555–574. doi: 10.1016/0022-2836(79)90367-x. [DOI] [PubMed] [Google Scholar]
- Din N., Engberg J., Kaffenberger W., Eckert W. A. The intervening sequence in the 26S rRNA coding region of T. thermophila is transcribed within the largest stable precursor for rRNA. Cell. 1979 Oct;18(2):525–532. doi: 10.1016/0092-8674(79)90069-2. [DOI] [PubMed] [Google Scholar]
- Dudov K. P., Dabeva M. D., Hadjiolov A. A. Simple agar--urea-gel electrophoretic fractionation of high molecular weight ribonucleic acids. Anal Biochem. 1976 Nov;76(50):250–258. doi: 10.1016/0003-2697(76)90283-9. [DOI] [PubMed] [Google Scholar]
- Engberg J., Andersson P., Leick V., Collins J. Free ribosomal DNA molecules from Tetrahymena pyriformis GL are giant palindromes. J Mol Biol. 1976 Jun 25;104(2):455–470. doi: 10.1016/0022-2836(76)90281-3. [DOI] [PubMed] [Google Scholar]
- Engberg J., Christiansen G., Leick V. Autonomous rDNA molecules containing single copies of the ribosomal RNA genes in the macronucleus of Tetrahymena pyriformis. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1356–1365. doi: 10.1016/0006-291x(74)90463-x. [DOI] [PubMed] [Google Scholar]
- Garber R. L., Gage L. P. Transcription of a cloned Bombyx mori tRNA2Ala gene: nucleotide sequence of the tRNA precursor and its processing in vitro. Cell. 1979 Nov;18(3):817–828. doi: 10.1016/0092-8674(79)90134-x. [DOI] [PubMed] [Google Scholar]
- Gocke E., Leer J. C., Nielsen O. F., Westergaard O. Transcriptional properties of nucleoli isolated from Tetrahymena. Nucleic Acids Res. 1978 Nov;5(11):3993–4006. doi: 10.1093/nar/5.11.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Wyler T., Seebeck T., Braun R. Processing of ribosomal precursor RNAs in Physarum polycephalum. Nucleic Acids Res. 1980 Jun 25;8(12):2647–2664. doi: 10.1093/nar/8.12.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback D. B., Angerer L. M., Davidson N. Improved methods for the formation and stabilization of R-loops. Nucleic Acids Res. 1979 Jun 11;6(7):2499–2317. doi: 10.1093/nar/6.7.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer K. M., Gall J. G. The macronuclear ribosomal DNA of Tetrahymena pyriformis is a palindrome. J Mol Biol. 1976 Jun 25;104(2):421–453. doi: 10.1016/0022-2836(76)90280-1. [DOI] [PubMed] [Google Scholar]
- Knapp G., Beckmann J. S., Johnson P. F., Fuhrman S. A., Abelson J. Transcription and processing of intervening sequences in yeast tRNA genes. Cell. 1978 Jun;14(2):221–236. doi: 10.1016/0092-8674(78)90109-5. [DOI] [PubMed] [Google Scholar]
- Knapp G., Ogden R. C., Peebles C. L., Abelson J. Splicing of yeast tRNA precursors: structure of the reaction intermediates. Cell. 1979 Sep;18(1):37–45. doi: 10.1016/0092-8674(79)90351-9. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- Leer J. C., Tiryaki D., Westergaard O. Termination of transcription in nucleoli isolated from Tetrahymena. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5563–5566. doi: 10.1073/pnas.76.11.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Expression of ribosomal DNA insertions in Drosophila melanogaster. Cell. 1979 Dec;18(4):1185–1196. doi: 10.1016/0092-8674(79)90231-9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Cordell B., Valenzuela P., Rutter W. J., Goodman H. M. Structure and processing of yeast precursor tRNAs containing intervening sequences. Nature. 1978 Aug 3;274(5670):438–445. doi: 10.1038/274438a0. [DOI] [PubMed] [Google Scholar]
- Ogden R. C., Beckman J. S., Abelson J., Kang H. S., Söll D., Schmidt O. In vitro transcription and processing of a yeast tRNA gene containing an intervening sequence. Cell. 1979 Jun;17(2):399–406. doi: 10.1016/0092-8674(79)90166-1. [DOI] [PubMed] [Google Scholar]
- Peebles C. L., Ogden R. C., Knapp G., Abelson J. Splicing of yeast tRNA precursors: a two-stage reaction. Cell. 1979 Sep;18(1):27–35. doi: 10.1016/0092-8674(79)90350-7. [DOI] [PubMed] [Google Scholar]
- Snyder A. L., Kann H. E., Jr, Kohn K. W. Inhibition of the processing of ribosomal precursor RNA by intercalating agents. J Mol Biol. 1971 Jun 14;58(2):555–565. doi: 10.1016/0022-2836(71)90371-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Waring M. J. Drugs which affect the structure and function of DNA. Nature. 1968 Sep 28;219(5161):1320–1325. doi: 10.1038/2191320a0. [DOI] [PubMed] [Google Scholar]
- Westergaard O., Gocke E., Nielsen O. F., Leer J. C. Effect of lucanthone (miracil D) on transcription of ribosomal RNA genes from Tetrahymena in vivo and in vitro. Nucleic Acids Res. 1979 Jun 11;6(7):2391–2402. doi: 10.1093/nar/6.7.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. L., Hogness D. S. R loop mapping of the 18S and 28S sequences in the long and short repeating units of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):177–192. doi: 10.1016/0092-8674(77)90213-6. [DOI] [PubMed] [Google Scholar]
- Wild M. A., Gall J. G. An intervening sequence in the gene coding for 25S ribosomal RNA of Tetrahymena pigmentosa. Cell. 1979 Mar;16(3):565–573. doi: 10.1016/0092-8674(79)90030-8. [DOI] [PubMed] [Google Scholar]
- Wild M. A., Sommer R. Sequence of a ribosomal RNA gene intron from Tetrahymena. Nature. 1980 Feb 14;283(5748):693–694. doi: 10.1038/283693a0. [DOI] [PubMed] [Google Scholar]
- Yannarell A., Niemann M., Schumm D. E., Webb T. E. Proflavine sensitivity of RNA processing in isolated nuclei. Nucleic Acids Res. 1977 Mar;4(3):503–511. doi: 10.1093/nar/4.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaug A. J., Cech T. R. In vitro splicing of the ribosomal RNA precursor in nuclei of Tetrahymena. Cell. 1980 Feb;19(2):331–338. doi: 10.1016/0092-8674(80)90507-3. [DOI] [PubMed] [Google Scholar]