Abstract
Clinical documents frequently contain a list of a patient’s medications. Missing information about the dosage, route, or frequency of a medication impairs clinical communication and may harm patients. We examined 253 medication lists. There were 181 lists (72%) with at least one medication missing a dose, route, or frequency. Missing information was judged to be potentially harmful in 47 of the lists (19% of 253) by three physician reviewers (kappa=0.69). We also observed that many lists contained additional information included as annotations, prompting a secondary thematic analysis of the annotations. Fifty-five of the 253 lists (22%) contained one or more annotations. The most frequent types of annotations were comments about the patient’s medical history, the clinician’s treatment plan changes, and the patient’s adherence to a medication. Future development of electronic medication reconciliation tools to improve medication list completeness should also support annotating the medication list in a flexible manner.
Introduction
Clinical encounters, such as outpatient visits and inpatient admissions and discharges, are documented in the form of a clinical note. These notes customarily include a medication list, which records the medications a patient is actively taking. Following numerous published studies on medication errors,1,2 policy makers, such as the Joint Commission, have focused on improving the quality of medication list documentation and communication through the process of medication reconciliation.
Medication reconciliation employs a systematic approach to comprehensively review all of a patient’s medications at each transition of care. This process helps ensure that an accurate list is maintained as clinicians add, change, or discontinue medications. Medication reconciliation may be viewed as a three-step process3,4,5:
Verification: Collect an accurate medication history, including dose, route, and frequency for each medication.
Clarification: Confirm that each medication and dose is appropriate for the patient.
Reconciliation: Document any changes to the medication list.
We have previously reported on the implementation of an electronic medication reconciliation process6. The goal of this study was to build on that work by examining the first step of the medication reconciliation process, namely, medication verification. Building on the work of other investigators, we attempted to measure the completeness of medication lists in terms of medication name, dose, route, and frequency. For medication lists that were incomplete, we evaluated the harm potential associated with the missing information. Improved understanding of medication list completeness can contribute to the development of more effective medication reconciliation tools and processes to improve patient safety in accordance with the Joint Commission’s National Patient Safety Goals.
Background
Most previous research on medication reconciliation has focused on the third step of the medication reconciliation process by looking for unintentional discrepancies between the medication list generated by clinicians and a “gold-standard” medication list. The percentage of patients with at least one discrepancy has ranged from 48–87% in the emergency department7,8 and 22–54% on hospital admission9,10,11. At hospital discharge, one study found that 41% of patients had at least one actual unintentional discrepancy12. In the outpatient setting the discrepancy rate has ranged from 22–82%13,14,15,16.
Some studies have attempted to estimate the clinical significance of discrepancies by having clinical experts rate the degree of potential harm posed by the discrepancy. Two studies have reported a rate of Potential Adverse Drug Events (PADEs) caused by medication discrepancies ranging from 1.05 to 1.44 PADEs/patient17,18. Other investigators have reported a percentage of discrepancies that were judged to be potentially harmful, with this percentage ranging from 12–39%9,10,11,12.
A study by Nassaralla, et al.15 is one of the few to focus on the first step of medication reconciliation, collecting a complete medication list, including medication name, dose, route, and frequency. The authors drew a distinction between medication list “completeness” and medication list “correctness.” In this context, “completeness” referred to whether each listed medication included the name, dose, route, and frequency. On the other hand, “correctness” measured the consistency between lists and a lack of discrepancies with what the patient was truly taking. In their study of 230 outpatient encounters, Nassaralla found that even after the introduction of electronic documentation and a process improvement campaign, only 19% of medications lists were complete. Most of the incomplete medications were due to missing route and frequency information. The Nassaralla study did not evaluate the clinical significance or harm potential of the missing information.
Methods
This study was conducted with electronic notes generated at two inpatient facilities and six community-based primary care clinics in northern Manhattan. The electronic notes were predominantly written as free-text using a locally-developed application, though some were entered using a commercial EHR documentation system, which the institution was deploying during the data collection period. Electronic notes authored over a two year period were collected for a random sample of 100 patients who had the following sequence of consecutive clinical encounters: an outpatient visit, an inpatient admission, an inpatient discharge, and a second outpatient visit. Each encounter was expected to generate a note, for a total of four notes per patient. Institutional review board approval was obtained prior to conducting the study.
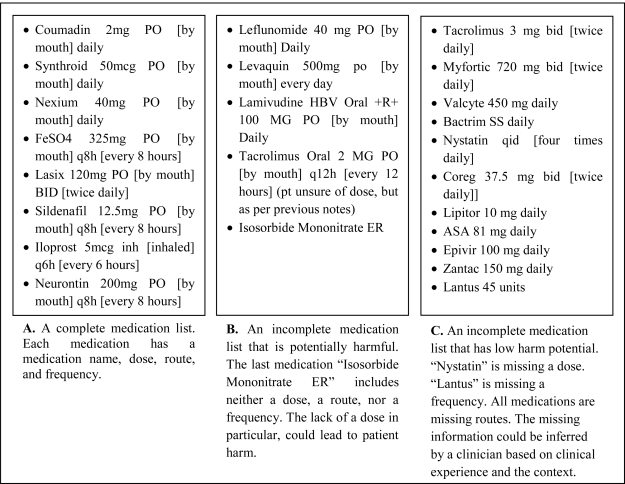
Each clinical note was reviewed by one of the authors (MO) to identify a medication list within the note. Any note that lacked a medication list or contained only a reference to see another note for the medication list was excluded from further analysis. Following the definition provided by Nassaralla et al., each medication list was categorized as “complete” or “incomplete.”15 A list was considered to be complete if it included a dose, route, and frequency for each medication (Figure 1, Panel A).
Figure 1.
Examples of complete and incomplete medication lists. Abbreviations are defined in brackets.
Medication lists deemed incomplete were independently reviewed and categorized as “potentially harmful” or “low harm potential” (Figure 1, Panels B and C) by three experienced physicians (MO, NC, and DC), who specialized in hospital medicine, ambulatory medicine, and critical care medicine, respectively. The physician reviewers were instructed to classify each incomplete medication list as “potentially harmful” if, in the opinion of the reviewer, the information missing from the list could lead to a prescribing error. If the missing information could likely be inferred by a practitioner with a similar background, then the medication list was classified as “low harm potential.” Inter-rater agreement was calculated using the Cohen’s kappa coefficient. If the three reviewers were not unanimous in their classifications, the classification chosen by a majority of the reviewers was used.
During the compilation of the medication lists for the study, it was observed that many lists contained comments or annotations separate from the dose, route, and frequency information. This observation prompted a secondary qualitative analysis of the medication lists based on a grounded theory approach. Thematic analysis19 was used to identify patterns in the content and meaning of the medication list annotations. Four candidate themes were proposed based on the initial review of the medication lists. The initial themes were “Source,” “Adherence,” “Reconciliation,” and “Certainty.” As each annotation was reviewed and categorized, the additional themes of “Historical Information” and “Pharmaceutical Information” were added.
Results
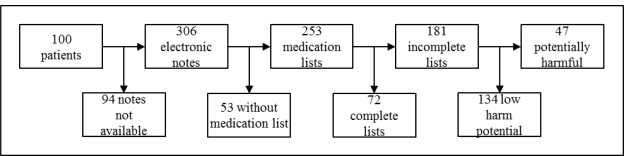
We searched for four notes from each of 100 patients and retrieved a total of 306 clinical notes that were available in electronic form (Figure 2). The notes that were not available were presumably documented using a paper medical record that was still in use at some locations during the study period. The notes contained 253 medication lists. Some notes did not include a medication list because the patient was taking no medications, the clinician referenced a separate medication list in another document, or the clinician commented on the patient’s medications in the plan section of the note without documenting a separate medication list. Of the 253 medication lists, 98 (38.7%) were from outpatient notes, 83 (32.8%) were from admission notes, and 72 (28.4%) were from discharge summaries. Of all notes with medication lists, 234 (92.5%) were entered as free-text documentation, and 19 (7.5%) were completed using structured documentation.
Figure 2.
Patient records were searched for cilinical notes. Each note was searched for a medication list. Medication lists were classified as complete or incomplete. Incomplete lists were evaluted for harm potential by clinical experts.
Of the 253 medication lists, 72 (28.5%) were complete, meaning all the medications on the list included a medication name, dose, route and frequency. There were 181 (71.5%) medication lists that were not complete.
All of the 181 medication lists that were not complete were examined by all three physician experts in order to evaluate whether the incomplete lists had the potential to cause harm. There was moderate agreement between the raters (kappa = 0.69) for the initial rating. After the review process, 134 (74.0%) of the incomplete lists had low harm potential, while 47 (26.0%) lists had the potential to cause harm (Table 1). A total of 206 (81.4%) medication lists were either complete or incomplete with low harm potential.
Table 1.
All notes categorized by visit type (outpatient note, admission note, and discharge note) and completion type (complete, incomplete—low harm potential, and incomplete—potentially harmful).
| Note Type | Complete | Incomplete | Total | |
|---|---|---|---|---|
| Low Harm Potential | Potentially Harmful | |||
| Outpatient | 21 (21.4%) | 52 (53.1%) | 25 (25.5%) | 98 |
| Admission | 19 (22.9%) | 51 (61.4%) | 13 (15.7%) | 83 |
| Discharge | 32 (44.4%) | 31 (43.1%) | 9 (12.5%) | 72 |
| Total | 72 (28.5%) | 134 (53.0%) | 47 (18.6%) | 253 |
A total of 160 annotations were identified in 86 medication lists for 55 patients (Table 2). Annotations were categorized as relating to:
Historical Information: the indication for a particular medication, previous treatments tried, the name of the clinician who prescribed a medication, start and stop date, etc.
Reconciliation Information: instructions on medication changes such as “start,” “stop,” “hold,” etc.
Adherence Information: differences between how a medication was ordered or prescribed and how the patient was actually taking it or not taking it.
Pharmaceutical Information: the medication’s drug class, generic or trade name, etc.
Medication List Source: who supplied the information for the medication list, such as the patient, family member, pharmacy, previous note, etc.
Level of Certainty: how sure the clinician documenting the medication list was that the list was accurate.
Table 2.
The six annotations types that were observed, including the number of each type observed and the percent of all annotations. Examples are taken from actual medication lists. Latin abbreviations are defined in brackets.
| Annotation Type, Number Observed (%) | Definition | Examples | ||
|---|---|---|---|---|
| Historical, 44 (28%) | Indication, previous treatments, prescriber, start and stop dates | fluconazole 100 mg daily (for thrush, beginning mid September - thrush went away within 1.5 weeks) | Wellbutrin (started 2 days ago) | neurontin 300mg tid [three times daily] (by Pain clinic) |
| Reconciliation, 35 (22%) | Instructions regarding medication changes | Glucotrol 5mg PO [by mouth] daily (NEW) | Lasix 40mng Po [by mouth] daily (increased) | - HOLD Metformin 850 mg PO daily |
| Adherence, 28 (18%) | Differences between prescription and how the patient takes the medication | Glipizide 20 mg bid [twice daily] (pt reports only taking 10 mg daily) | fibercon 625 bid [twice daily] (pt does not take every day, states minimal effectiveness) | -lasix 40 daily (pt taking lasix 80 mg bid [twice daily]) |
| Medication Information, 24 (15%) | Drug class or generic name | Sitaglipitin 50mg (Januvia) po [by mouth] daily | Anama (some OTC herbal med) | Prograf (Tacrolimus) 2mg at 10am and 10pm |
| Source, 16 (10%) | Where did the medication list come from | pt brought pill bottles 8/31 | (patient does not know her medications, lists provided below per d/c summary 9/08) | (pt brought in list from PMD) |
| Certainty, 13 (8%) | How certain is the clinician that the medication list is accurate | Hydroxyzine 25 mg po [by mouth] ? Frequency | (pt does not know doses, family to bring meds in am) | Norvasc 5 mg po daily (may be 10 patient unsure.) |
Discussion
This study examined the completeness and safety of medication lists recorded in outpatient notes, admission notes, and discharge summaries. We found that 28.5% of notes were complete, including a medication name, dose, route, and frequency. Of the incomplete notes, 74.0% were judged to be safe by two clinical experts, and 26.0% were judged to be potentially harmful. We also examined the notes for annotations that supplied information in addition to the medication name, dose, route, and frequency. The annotations were categorized according to the type of information they contained.
The completeness rate in our sample of 28.5% was higher than that found by Nassaralla and colleagues15. In that study, the authors found that 7.7% of medication lists were complete before an intervention that included process improvement and the use of an electronic medication documentation tool. After the intervention, the percentage of notes that were complete increased to 18.5%. While the work by Nassaralla only examined outpatient notes, we examined both outpatient and inpatient notes, which may account for some of the difference in the rate of completeness between our findings and those from the previous study. In our study sample, the outpatient notes had a completeness rate of 21.4%, which is closer to the post-intervention findings of Nassaralla. Discharge summaries had the highest rate of completeness (44.4%), which is probably due to the amount of time and resources that are applied to obtaining a complete medication list during the course of a hospitalization. Admission notes had a lower rate of completeness (22.9%) than discharge summaries, which may reflect the lack of information available in many cases at the time of admission. For example, complete medication information may not be available if a patient presents to the emergency room unexpectedly and does not remember the details of his or her medications.
We extended the work of Nassaralla, et al. by evaluating the clinical significance of incomplete medication lists. Of the incomplete medication lists, 74.0% were judged to be safe and only 26.0% were judged to be potentially harmful. There was moderate agreement between raters on the initial rating of whether an incomplete medication list was safe or potentially harmful, indicating that this determination is somewhat subjective. The raters believe that their particular clinical experience and backgrounds impacted their individual ratings of incomplete lists. For example, a clinician who is very familiar with a particular medication through his or her daily practice may feel more comfortable inferring missing information for that medication (such as the dose, route, or frequency) than another clinician who does not routinely prescribe that medication. This issue is especially relevant for medications that are only, or at least very commonly, given in one dose, formulation, or frequency. For example, in our sample of medication lists, many patients were taking cardiovascular medications such as simvastatin. Simvastatin is routinely administered at bedtime and always by mouth. Thus, a clinician familiar with simvastatin would probably not find a medication list that omitted the route or frequency for simvastatin to be harmful because she could infer that the route would be oral and the frequency would be daily at bedtime. On the other hand, a clinician who is not familiar with simvastatin might perceive such as list as potentially harmful. Therefore, the risk of patient harm may be lower than what we measured when the medication list is used for transitions of care between clinicians with similar levels of clinical experience and backgrounds, such as a transplant team or other specialized care team.
We observed annotations within the medication list regarding historical information, reconciliation of medication changes, patient adherence, pharmaceutical information, medication list source, and medication list certainty. We are unaware of any other study that has reported the presence and type of annotations in medication lists. Over half of the patients in our sample had at least one medication list with one annotation. We did not separately rate the clinical significance of the annotations, but they are likely to be important for patient care. The clinicians who documented the medication lists purposefully added extra information in the form of these annotations because they are likely to have thought that the extra information was important. It is possible that the information in the annotations also existed in other sections of the medical record; however, the clinicians who recorded the annotations may have thought that the information within the annotations would be most helpful to future users of the documented medication list if the annotation was directly linked with the medication list. For example, the annotations “HOLD Metformin 850 mg PO daily” may contain information that is also present in the assessment and plan section of the note or in a separate medication reconciliation document. Still, the clinician who duplicated this information in the medication list may have done so to alert any future reader who might only look at the medication list and not carefully read the entire document. Thus, the absence of these annotations (for example, as institutions convert from free-text to structured medication documentation) could lead to potential patient harm. Future medication reconciliation tools should be designed to easily accommodate documentation of the annotation types that we observed.
Our study was limited to the evaluation of medication list completeness. We did not look for discrepancies between medication lists. Since the data for this study included a longitudinal series of notes (consecutive outpatient, admission, discharge, and outpatient notes for the same patient) future work will address discrepancies between the lists across the continuum of care. Also, our study of annotations was limited to the counting and categorizing of annotations. We did not have clinical experts rate the significance of the annotations. Future work could be directed at determining the clinical significance of medication list annotations.
Conclusion
The process of medication reconciliation, including medication verification by obtaining a complete medication list, is important for improving patient safety. In our sample of medication lists, we found that a total of 81.4% of medication lists were complete or incomplete with low harm potential. Most of the incomplete medication lists were still safe for patient care. We also observed several categories of medication list annotations. These annotations occurred in many different lengths and formats and covered a broad range of topics. While the structured nature of the medication name, dose, route, and frequency information needed for a complete medication list could be represented by structured fields in an EHR, a purely structured documentation tool for medication reconciliation would probably not support the current workflow that we observed of annotating the medication list. Future development of electronic medication reconciliation tools should support annotating the medication list in a flexible manner, possibly including shortcuts to document the categories of annotations that we observed.
Acknowledgments
This work was supported by the Agency for Healthcare Research and Quality (1 R03 HS018250-01) and the National Library of Medicine (5T15LM007079-19).
References
- 1.Kohn LT, Corrigan JM, Donaldson MS. To err is human: building a safer health system. Washington, DC: National Academy Press; 2000. (Institute of Medicine). [PubMed] [Google Scholar]
- 2.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. JAMA. 1995;275:24–34. [PubMed] [Google Scholar]
- 3.5 Million Lives Campaign . Cambridge, MA: Institute for Healthcare Improvement; 2008. Getting started kit: prevent adverse drug events (medication reconciliation) how-to guide. (Available at www.ihi.org) [Google Scholar]
- 4.Rogers G, Alper E, Brunelle D, et al. Reconciling medications at admission: safe practice recommendations and implementation strategies. Joint Comm J Qual Patient Safety. 2006;32:37–50. doi: 10.1016/s1553-7250(06)32006-5. [DOI] [PubMed] [Google Scholar]
- 5.Greenwald JL, Halasyamani LK, Greene J, et al. Making inpatient medication reconciliation patient centered, clinically relevant, and implementable: a consensus statement on key principles and necessary first steps. Joint Comm J Qual Patient Safety. 2010;36:504–513. doi: 10.1016/s1553-7250(10)36074-0. [DOI] [PubMed] [Google Scholar]
- 6.Vawdrey DK, Chang N, Compton A, Tiase V, Hripcsak G. Impact of electronic medication reconciliation at hospital admission on clinician workflow. AMIA 2010 Proceedings; pp. 822–826. [PMC free article] [PubMed] [Google Scholar]
- 7.Caglar S, Henneman PL, Blank FS, Smithline HA, Henneman EA. Emergency department medication lists are not accurate. J Emergency Med. 2008 doi: 10.1016/j.jemermed.2008.02.060. corrected proof in press. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd G, Schwartz RB. Frequency of incomplete medication histories obtained at triage. Am J Health-Syst Pharm. 2009;66:65–9. doi: 10.2146/ajhp080171. [DOI] [PubMed] [Google Scholar]
- 9.Gleason KM, McDaniel MR, Feinglass J, et al. Results of the medications at transitions and clinical handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25:441–7. doi: 10.1007/s11606-010-1256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffey M, Mack L, Streitenberger K, Bishara T, De Faveri L, Matlow A. Prevalence and clinical significance of medication discrepancies at pediatric hospital admission. Academic Pediatrics. 2009;9:360–5. doi: 10.1016/j.acap.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Cornish PL, Knowles SR, Marchesano R, Tam V, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165:424–9. doi: 10.1001/archinte.165.4.424. [DOI] [PubMed] [Google Scholar]
- 12.Wong JD, Bajcar JM, Wong GG, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother. 2008;42:1373–9. doi: 10.1345/aph.1L190. [DOI] [PubMed] [Google Scholar]
- 13.Ernst ME, Brown GL, Klepser TB, Kelly MW. Medication discrepancies in an outpatient electronic medical record. Am J Health-Syst Pharm. 2001;58:2072–5. doi: 10.1093/ajhp/58.21.2072. [DOI] [PubMed] [Google Scholar]
- 14.Bedell SE, Jabbour S, Goldberg R, et al. Discrepancies in the use of medications: their extent and predictors in an outpatient practice. Arch Intern Med. 2000;160:2129–34. doi: 10.1001/archinte.160.14.2129. [DOI] [PubMed] [Google Scholar]
- 15.Nassaralla CL, Naessens JM, Chaudhry R, Hansen MA, Scheitel SM. Implementation of a medication reconciliation process in an ambulatory internal medicine clinic. Qual Saf Health Care. 2007;16:90–4. doi: 10.1136/qshc.2006.021113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner MM, Hogan WR. The accuracy of medication data in an outpatient electronic medical record. JAMIA. 1996;3:234–44. doi: 10.1136/jamia.1996.96310637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pippins JR, Gandhi TK, Hamann C, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23:1414–22. doi: 10.1007/s11606-008-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schnipper JL, Hamann C, Ndumele CD, et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster-randomized trial. Arch Intern Med. 2009;169:771–780. doi: 10.1001/archinternmed.2009.51. [DOI] [PubMed] [Google Scholar]
- 19.Aronson J. A pragmatic view of thematic analysis. Qualitative Report [Internet] 1994;2(1) Available from: http://www.nova.edu/ssss/QR/BackIssues/QR2-1/aronson.html. [Google Scholar]