Abstract
The Central Indiana Beacon Community leads efforts for improving adherence to oral hypoglycemic agents (OHA) to achieve improvements in glycemic control for patients with type 2 diabetes. In this study, we explored how OHA adherence affected hemoglobin A1C (HbA1c) level in different racial groups. OHA adherence was measured by 6-month proportion of days covered (PDC). Of 3,976 eligible subjects, 12,874 pairs of 6-month PDC and HbA1c levels were formed between 2002 and 2008. The average HbA1c levels were 7.4% for African-Americans and 6.5% for Whites. The average 6-month PDCs were 40% for African-Americans and 50% for Whites. In mixed effect generalized linear regression analyses, OHA adherence was inversely correlated with HbA1c level for both African-Americans (−0.80, p<0.0001) and Whites (−0.53, p<0.0001). The coefficient was −0.26 (p<0.0001) for the interaction of 6-month PDC and African-Americans. Significant risk factors for OHA non-adherence were race, young age, non-commercial insurance, newly-treated status, and polypharmacy.
BACKGROUND
Non-adherence to oral hypoglycemic agents (OHA) is an important risk factor for suboptimal glycemic control and for both high morbidity and mortality rates among patients with type 2 diabetes.1–2 It is estimated that non-adherence to medications is as high as 60% among patients with type 2 diabetes and many patients do not adhere to their medication after the first six months of therapy.3 In addition, information about rates of medication adherence are usually not available to providers in clinical practice and few interventions have attempted to improve medication adherence for diabetic patients.4–5
Recently, major organizations have called for quality improvement interventions aimed at increasing medication adherence.6 However, assessing medication adherence through patient self-report is difficult and measures of adherence based on claims records are subject to a number of potential biases.7–9 Moreover, it is hard to collect and deliver the information to the provider when it is of the most use: at the time of the clinical encounter. Health information technology (HIT) and health information exchange (HIE), which support key components of chronic disease management including diabetes, may be useful in this regard.10–11 However, very few HIT/HIE systems have been applied to either documenting or intervening in medication adherence. A recent study reported that, among more than 7,000 articles about HIT interventions, only 13 had described HIT components on medication adherence.5 Boosting health data infrastructure and HIT that support medication adherence interventions should be a high priority for health care reform.12
HIE and HIT systems have good potential to establish integrated and multifaceted intervention strategies to improve medication adherence in several domains.13–14 First, the rich health information and data integration of the HIE can generate aggregated, complete, and longitudinal patient data across different health care facilities over time. If complete, patient medication history generated from an HIE could ensure an accurate data-driven medication adherence measure. Second, a computerized clinical decision support system (CDSS) could inexpensively remind providers or patients in routine care about medication refills, and perhaps could predict risk factors for medication non-adherence and thereby provide recommendations for overcoming barriers to adherence. Third, the nature of multidirectional communication in the HIE could allow adherence data to be shared among physicians, pharmacists, and care managers, and thus support the interactions needed for good care coordination. Fourth, widespread HIE connectivity could ensure monitoring and following up of patient adherence over time. In addition, the ability of an HIE to communicate adherence patterns to patients could encourage better patient engagement in medication-taking behavior.15–18
The American Recovery and Reinvestment Act promotes HIT/HIE innovations to achieve meaningful, measurable improvement in health care quality, safety, and efficiency in selected Beacon Communities.19 The Central Indiana Beacon Community (CIBC) leads efforts to increase medication adherence to improve diabetic patients’ glycemic and lipid control. Using clinical data captured from various sources, the CIBC will measure patient adherence and deliver this information to providers through our local well-established HIE infrastructure.20 In order to document medication adherence issues in type 2 patients and to demonstrate their impact, we conducted two studies, using HIE data, showing that medication adherence to OHA and antihyperlipidemic agents are significantly associated with measurable health outcomes.21–22 In order to better target these interventions, we explored a variety of patient factors that might be associated with glycemic control. The relationships of race, medication adherence, and subsequent glycemic control are inconsistent in previous different studies.23–24
The primary objective was to determine if medication adherence could be assessed using HIE, analyze how patterns of OHA adherence differ among racial groups, and the subsequent impact on glycemic control. The secondary objective was to identify the risk factors for OHA non-adherence. This study would provide valuable information in identifying patients at high risk of medication non-adherence and suboptimal glycemic control, in order to further construct patient-specific interventions in a rich HIE/HIT environment.
METHODS
Data sources and settings
The setting for this retrospective study is the Indiana Network of Patient Care (INPC) which is an operational regional HIE of now more than 60 hospitals and more than 100 geographically distributed clinics that has served Indianapolis for more than fifteen years. This system mediates medical record and claims data from hospitals, laboratories, imaging centers, pharmacies, and physician offices. Information includes registration records, laboratory tests, radiology reports, diagnoses, and administrative data.25 Medical records in the INPC are linked at the patient level through a robust linking algorithm. Medication dispensing records typically include medication refill dates, days of supply, dose, and frequency. Environmental factors (at the county level), such as median household income, adult obesity rate, and adult diabetes rate were obtained from publicly accessible US Department of Agriculture (USDA) data and were mapped to each patient by county information. Patient identifiers were removed before data analysis, in order to protect the study participants’ confidentiality. This study was approved by the Institutional Review Board of Indiana University and the INPC management committee.
Eligibility Criteria
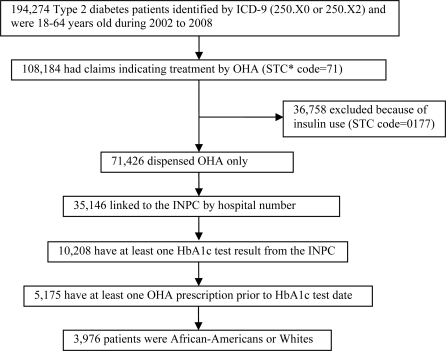
The study cohort was selected from patients whose data are contained in the INPC system. To be included, subjects had to be 18 years or older during the study period January 1, 2002 through December 31, 2008. They were either African-Americans or Whites and were diagnosed as having type 2 diabetes based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes of 250.X0 and 250.X2. These type 2 patients were additionally confirmed by their OHA history, according to the First Databank Standard Therapeutic Code (STC: 71) for OHAs: Biguanides, Sulfonylurea (SU), Thiazolidinedione (TZD) and other OHAs (Meglitinides and α-glucosidase) during the study period. Since the focus of this study was OHA use, patients who used insulin (STC: 0177) were excluded. To be included in the cohort, the patient had to have at least one HbA1c test result from the INPC and at least one pharmacy claim for an OHA six months prior to the HbA1c test date. (Figure 1)
Figure 1.
Patient selection
Measurements
Patient OHA medication adherence was calculated using a standard measurement: proportion of days covered (PDC). The PDC is the medication adherence measurement used by the Pharmacy Quality Assurance project conducted by the National Committee for Quality Assurance (NCQA).26 The PDC focuses on persistence or continuation at the time of prescribed treatment. It is defined as the total number of medication covered days divided by the number of days in a certain time period. Claims data, including refill date, days of supply, dosage, and frequency are used to calculate PDC.27 For patients who took multiple OHAs, we calculated the combined average 6-month PDC grouped by each OHA therapeutic class. OHA medication adherence was used as the independent variable in the analysis of its association with HbA1c control. It was also used as the dependent variable in a secondary analysis about predictors of OHA adherence. In the secondary analysis, OHA adherence was classified into two levels: 1) adherence to OHA if the 6-month PDC was equal to or greater than 80%; and 2) non-adherence to OHA if the 6-month PDC was less than 80%.
Patients HbA1c levels were retrieved from the INPC using a set of standard laboratory test terminologies that were mapped to the LOINC® codes 17855-8, 17856-6 and 8548-4. The HbA1c test date was defined as the index date, and we calculated the PDC for a 6-month period before each HbA1c laboratory test result for each patient. The patient’s HbA1c level was used as the dependent variable in the primary analysis.
We studied several patient demographic, clinical, and health environmental characteristics which may influence HbA1c control and OHA adherence. The predictor selections were based on previous Type 2 diabetes management studies.28–29 We extracted patient demographic information such as age, gender, and race. Clinical characteristics included duration of OHA treatment, the number of HbA1c tests, and the number of concurrent OHA medications. The prescribed OHA drug classes included Biguanides, Sulfonylureas, Thiazolidinediones, other OHAs, and multiple classes. Selected common health environmental factors (at the county level) included adult diabetes rates, adult obese rate, recreation and fitness facilities per 1,000 population, median household income, and metropolitan status. These factors were analyzed as predictors for both HbA1c control and OHA adherence.
Statistical Analysis
Descriptive statistics of patient characteristics, OHA adherence, and average HbA1c levels were reported for black and white patients. Continuous variables were compared using t-tests and categorical variables were compared using χ2 tests.
We examined the associations between glycemic control and OHA adherence using mixed effect generalized linear regression models. Regression coefficients were used to quantify the associations between covariates and HbA1c control. Random subject effects were used in these models to accommodate the potential associations among observations contributed by the same study subjects. Stratified analyses by race were conducted to assess the relationship between covariates and HbA1c in black and white patients. Combined models including medication adherence (6 month PDC), races, and their interaction were used to examine the potentially differential effects of medication adherence in patients of difference races.
Secondarily, we explored predictors of medication adherence. In this analysis, we used dichotomized adherence outcomes. Odds ratios (OR) were used to quantify the magnitude of associations between potential predictors and OHA adherence. All analyses were implemented using SAS 9.1 (SAS Institute, Gary, North Carolina). P values less than 0.05 were considered significant.
RESULTS
Patients’ Characteristics
A total of 3,976 subjects (21% African-American and 79% White) met all inclusion and exclusion criteria. A total of 12,784 pairs of 6-month PDC and HbA1c level were formed across the study period. Patient demographic and clinical characteristics grouped by race are outlined in Table 1. Compared with White patients, African-American patients were younger (48 vs. 51), more female (65% vs. 51%), and a higher percentage proportion had noncommercial insurance (40% vs. 17%). African-Americans had higher average HbA1c levels (7.4% vs. 6.8%), a higher proportion of patients with uncontrolled HbA1c levels (48% vs. 35%), and a slightly higher average number of HbA1c tests (4.2 vs. 3.7). On the other hand, the African-American population had a lower average 6-month PDC (40% vs. 50%) and a lower proportion of patients with optimal OHA adherence (21% vs. 33%). Other patient factors, such as the age distribution, duration of treatment, number of concurrent OHA medications and OHA medication classes were similar among the two racial groups.
Table 1.
Patient demographic and clinical characteristics by race
|
African-American |
White |
|||
|---|---|---|---|---|
| Number | (Percentage) | Number | (Percentage) | |
| Number of Subject | 834 | (20.9%) | 3,142 | (79.1%) |
| Demographics | ||||
| Age at study entry (year) * | 48.65 | (48.0, 49.0) | 51.04 | (50.72, 51.36) |
| 18–30 | 34 | (4.1%) | 90 | (2.8%) |
| 31–40 | 131 | (15.7%) | 341 | (10.8%) |
| 41–50 | 279 | (33.5%) | 851 | (27.1%) |
| >51 | 390 | (46.7%) | 1,860 | (59.2%) |
| Female gender | 553 | (65.2%) | 1,171 | (54.0%) |
| Non-commercial insurance | 343 | (40.5%) | 525 | (16.6%) |
| Clinical Characteristics | ||||
| A1C Level (%) * | 7.38 | (7.26, 7.50) | 6.85 | (6.81, 6.91) |
| A1C <7% | 440 | (51.9%) | 2,056 | (64.9%) |
| Number of A1C test * | 4.15 | (4.03, 4.26) | 3.76 | (3.70, 3.83) |
| OHA 6-month PDC * | 40% | (39%, 43%) | 50% | (49%, 51%) |
| OHA 6-month PDC ≥ 80% | 177 | (21.2%) | 1,031 | (32.8%) |
| Duration of OHA treatment (Year) | ||||
| 0–3 | 696 | (83.5%) | 2,693 | (85.7%) |
| 3–6 | 133 | (15.9%) | 441 | (14.0%) |
| 6–9 | 5 | (0.6%) | 8 | (0.3%) |
| Number of concurrent OHA medications | ||||
| 1 | 593 | (71.1%) | 2,155 | (68.6%) |
| 2 | 198 | (23.7%) | 802 | (25.5%) |
| ≥ 3 | 43 | 5.2%) | 185 | (5.9%) |
| OHA medication classes | ||||
| Biguanide Only | 314 | (37.4%) | 1,316 | (42.1%) |
| Sulfonylurea Only | 173 | (20.6%) | 358 | (11.1%) |
| Thiazolidinedione Only | 41 | (4.9%) | 206 | (6.6%) |
| Other | 7 | (0.8%) | 38 | (1.2%) |
| Multiple classes | 304 | (36.2%) | 1,205 | (38.6%) |
Data are mean (95% CI). All values are statistically significant at p < 0.001.
Among the selected environmental health factors (at the county level), African-Americans had lower median household income ($44,957 ± 6,367 vs. $52,297 ± 12,534) and higher rate of metropolitan resident (99% vs. 91%). Statistics for other factors were not significantly different between African-Americans and Whites: adult diabetes rate (8.94 ± 0.34 vs. 8.85 ± 0.77), adult obesity rate (28.46 ± 0.65 vs. 28.38 ± 1.28), and recreation and fitness facilities per 1,000 population (0.14 ± 0.01 vs. 0.10 ± 0.03).
Effects of OHA adherence and patient factors on HbA1c control
Table 2 summarizes the results of the mixed model that analyzed effects of OHA adherence and patient factors on HbA1c control. OHA adherence is significantly inversely correlated with patient HbA1c level: the coefficients were −0.80 (p<0.0001) for African-Americans, −0.53 (p<0.0001) for Whites, and −0.49 (p<0.0001) for the entire cohort. Other factors that were significantly inversely correlated with HbA1c level for all three models include noncommercial insurance, number of HbA1c tests, and number of concurrent OHA medications. Patients who were treated with multiple OHA classes had significantly higher HbA1c levels than patients who were treated with a single OHA class. In the combined model, African-Americans had higher HbA1c levels (0.55, p<0.0001); however, the coefficient for HbA1c and the interaction of the 6-month PDC and African-American race was −0.26 (p<0.0001). Patient age, duration of OHA treatment, and metropolitan status had no effects on HbA1c control. Environmental factors at the county level also were not significantly associated with HbA1c control (data not shown).
Table 2.
Association between A1C level and patient factors
| Predictors | African-American | White | Combined Model | |||
|---|---|---|---|---|---|---|
| N (observations) | 834 | (2,856) | 3,142 | (9,928) | 3,976 | (12,784) |
| 6-month PDC | −0.80 | ± 0.10 | −0.53 | ± 0.04 | −0.49 | ± 0.05 |
| Age | 0.02 | ± 0.06 ^ | 0.03 | ± 0.02 ^ | 0.02 | ± 0.02 ^ |
| African-American (AA) | -- | -- | -- | -- | 0.55 | ± 0.06 |
| 6-month PDC *AA | -- | -- | -- | -- | −0.26 | ±0.11 |
| Non-commercial insurance | −0.23 | ± 0.12 | −0.26 | ± 0.06 | −0.19 | ±0.06 |
| Female gender | 0.06 | ± 0.13 ^ | −0.24 | ± 0.04 | −0.21 | ± 0.04 |
| Duration of OHA treatment | −0.10 | ± 0.06 ^ | 0.15 | ± 0.30 | 0.12 | ± 0.03 |
| Number of A1C test | −0.10 | ± 0.02 | −0.04 | ± 0.01 | −0.06 | ± 0.01 |
| Number of OHA medication | −0.38 | ± 0.06 | −0.17 | ± 0.02 | −0.24 | ± 0.02 |
| OHA class | ||||||
| Biguanides Only | −1.25 | ± 0.15 | −0.80 | ± 0.05 | −0.95 | ± 0.05 |
| Sulfonylurea Only | −0.51 | ± 0.16 | −0.23 | ± 0.07 | −0.34 | ± 0.07 |
| Thiazolidinedione Only | −1.23 | ± 0.27 | −0.87 | ± 0.09 | −1.01 | ± 0.10 |
| Other | −1.47 | ± 0.58 | −0.68 | ± 0.20 | −0.90 | ± 0.22 |
| Multiple Classes | -- | -- | -- | -- | -- | -- |
| Metropolitan Status | −1.16 | ± 0.80 ^ | −0.06 | ± 0.07 ^ | −0.05 | ±0.09 ^ |
p-value >0.05
p-value for other coefficients are smaller than 0.0001
Predictors of OHA adherence
Nine predictors of adherence are shown in Table 3. Six of them achieved statistical significance (p<0.001). Three negative predictors were African-American race (OR: 0.61), non-commercial insurance (OR: 0.62), and number of concurrent OHA medications (OR: 0.80). Positive predictors were age (OR: 1.32), number of A1C tests (OR: 1.10), and duration of OHA treatment (OR: 1.19). Patients treated with OHA monotherapy have significantly higher 6-month PDCs than patients treated by polypharmacy. Gender and metropolitan status had no effects on OHA medication adherence.
Table 3.
Predictors for OHA adherence
| Predictors | Odds Ratio | 95 % CI | p-value | |
|---|---|---|---|---|
| African-American | 0.61 | (0.50, 0.73) | < .0001 | |
| Female gender | 0.93 | (0.81, 1.01) | 0.28 | |
| Non-commercial insurance | 0.62 | (0.51, 0.76) | < .0001 | |
| Age | 1.32 | (1.20, 1.45) | < .0001 | |
| Number of HbA1c test | 1.10 | (1.06, 1.13) | < .0001 | |
| Duration of OHA treatment | 1.19 | (1.02, 1.38) | 0.02 | |
| Number of medication | 0.80 | (0.71, 0.89) | < .0001 | |
| OHA class | ||||
| Biguanides Only | 1.39 | (1.10, 1.76) | 0.005 | |
| Sulfonylurea Only | 1.24 | (1.04, 1.49) | 0.01 | |
| Thiazolidinedione Only | 1.56 | (1.20, 2.04) | 0.0008 | |
| Other | 0.25 | (0.09, 0.64) | 0.004 | |
| Multiple Classes | -- | -- | -- | -- |
| Metropolitan status | 0.90 | (0.71, 1.14) | 0.40 | |
DISCUSSION
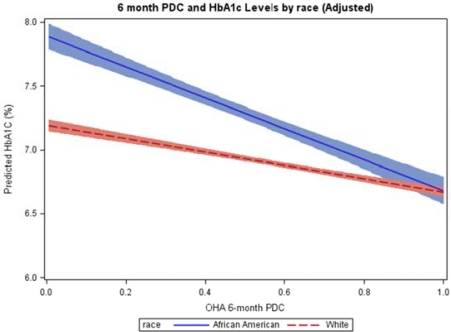
The primary purpose of this study was to determine whether medication adherence could be assessed using an HIE system and determine if there were racial differences that impacted glycemic control. We found significant differences in both OHA adherence and HbA1c control between African-Americans and Whites. The African-American population had a 10% lower average OHA adherence and a 0.5% higher HbA1c level. We additionally confirmed that OHA adherence is significantly and inversely associated with HbA1c levels. More interestingly, the larger magnitude of the slope of this relationship for African-Americans means that African-Americans who become compliant achieve the same level of HbA1c control as Whites (Figure 2). In the stratified mixed model, after adjusting for confounders, a 10% increased OHA adherence was associated with 0.08% lower HbA1c values in African-American and 0.05% lower HbA1c values in Whites. Similar findings have been reported from various studies.30–31 In the combined analysis, the significant interaction (−0.26, p<0.0001) between OHA adherence and race suggested potentially differential adherence effects in black and white patients. Although the racial difference in HbA1c control may or may not be biologically and genetically independent,32–33 our study identified a modifiable risk factor: OHA non-adherence. It is a consistent finding with one study that found no evidence of racial difference in glycemic control after controlling for adherence to OHA.34 To reduce the racial difference in long-term glycemic control, an emphasis on OHA adherence among African-American diabetic patients may be necessary.
Figure 2.
Effects of OHA adherence on glycemic control by race
Our secondary analysis indicated that independent risk factors for non-adherence to OHA are African-American race, non-commercial insurance status, higher number of OHA medications, less intensive HbA1c tests, and polypharmacy. Female gender and metropolitan status had no effects on OHA adherence. These risk factors are consistent with the results from most studies of mediation adherence.23–24,28–30 Conceptually, multiple OHAs have a stronger effect on HbA1c control; however, with the recognition that multiple OHA drugs is one of the risk factors of OHA non-adherence,35–36 effective interventions emphasizing OHA adherence should be applied to patients before adding more OHA drugs.
The study subjects were limited by these who have an HbA1c test record in the INPC. Interestingly, our data showed that increased number of HbA1c tests is associated with lower HbA1c level (−0.06, p<0.0001) and higher OHA adherence (OR: 1.10, p<0.0001). Possible explanations might be that patients who have their HbA1c tested may be more likely adhere to their medication to achieve better control and are more likely to get brief interventions during clinical visits. Other risk factors for OHA non-adherence generated from this analysis provide additional information to establish tailored and patient specific interventions.
In addition to OHA adherence, other patient behaviors such as physical activity, diet habits, education level, income, physical facility availability and soft drink consumption may also affect patient HbA1c control. However, this information usually is not available in an EMR/HIE system. In order to overcome this limitation, we linked the publically accessible USAD health environmental data. However, most environmental factors (which were only available at the county level) showed no significant association with patient HbA1c control, and very few showed marginal association in the analyses. Although county-level health profiles may capture important information, it may not provide sufficient information for the individual patients in our study. The reason might be a lack of variation in these measures because 90% of subjects were Indiana metropolitan residents.
Effective interventions in medication adherence combine convenient care, information, reminders, self-monitoring, and counseling.37 The emergence of HIE/HIT offers new approaches. CDSS’s provide faster and better clinical decisions and do reduce clinical errors caused by human limitations in processing data.38–39 A CDSS embedded in an HIE can easily and programmatically establish a data-driven assessment of medication adherence and identify patients who are not adhering to their medication. Recent success stories promoting adherence by HIT included Community Care of North Carolina (CCNC), Geisinger Health System, and Group Health Cooperative. Collecting patient medication history from multiple resources and assessing patient adherence status are the core components for each of these HIT interventions.12 Our study confirmed that data-driven measures of medication adherence and predicted risk factors using aggregated HIE data are associated with objective clinical outcomes in patients with type 2 diabetes. The evidence-based knowledge may therefore prove to be useful and should be introduced into routine clinical practices through CDSS’s.
Limitations
This study is an observational study, not an experiment. We should keep in mind that findings from this study demonstrated associations (not causation) between OHA adherence and HbA1c levels, as well as associations (not causation) between predictors and OHA adherence. This study was based on medication and HbA1c laboratory data that were captured from an operation HIE. Although we intended to maximally utilize patient data, we were unable to report on patients who had no records of an OHA dispensing event or HbA1c test. In addition, the patient data might be incomplete if a patient switched to an insurance plan which does not participate in our HIE.
CONCLUSIONS
Using longitudinal data from the Indiana Network of Patient Care (INPC), we identified significant differences in OHA adherence and HbA1c control between African-Americans and Whites in the Indiana population. Increased OHA adherence was significantly correlated with better glycemic control for both racial groups, and potentially eliminated the gap of HbA1c levels. Findings from this study support establishing HIE/HIT interventions that assess patient medication adherence using data captured from different data resources in an HIE. In addition, risk factors for OHA non-adherence identified from this study provide necessary knowledge to further establish more predictive, patient-specific, tailored, and team-based CDSS interventions regarding medication adherence, with continued efforts from the Central Indiana Beacon Community.
Acknowledgments
We would like to thank Roberta Ambuehl (senior data analyst) at Regenstrief Institute, Inc
REFERENCES
- 1.Ho PM, Rumsfeld, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, Magid DJ. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836–41. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 2.Jonathan Betz Brown GAN, Glauber Harry S, Bakst Alan, Pharmti Ten-year follow-up of antidiabetic drug use, nonadherence, and mortality in a defined population with type 2 diabetes mellitus. Clin Ther. 1999;21(13):1045–68. doi: 10.1016/S0149-2918(99)80023-X. [DOI] [PubMed] [Google Scholar]
- 3.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200–210. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 4.Ellis SSS, Sieber W, Rand C. Adherence to pharmacological interventions: Current trends and future directions. The Pharmacological Intervention Working Group. Control Clin Trials. 2000;21(5):218–225. doi: 10.1016/s0197-2456(00)00082-9. [DOI] [PubMed] [Google Scholar]
- 5.Alexander S, Misono SLC NKC, et al. Healthcare information technology interventions to improve cardiovascular and diabetes medication adherence. Am J Manag Care. 2010;16(12):82–92. [PubMed] [Google Scholar]
- 6.World Health Origanization Adherence to long-term therapies: Evidence for action. 2003.
- 7.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 8.Murri R, Ammassari A, Trotta MP, et al. Patient-reported and physician-estimated adherence to HAART: social and clinic center-related factors are associated with discordance. J Gen Intern Med. 2004;19:1104–10. doi: 10.1111/j.1525-1497.2004.30248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller LG, Liu H, Hays RD, et al. How well do clinicians estimate patients’ adherence to combination antiretroviral therapy? J Gen Intern Med. 2002;17:1–11. doi: 10.1046/j.1525-1497.2002.09004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorr D, Bonner LM, Cohen AN, et al. Informatics systems to promote improved care for chronic illness: A literature review. J Am Med Inform Assoc. 2007;14(2):156–166. doi: 10.1197/jamia.M2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchibroada JM. The impact of health information technology on collaborative chronic care management. J Manag Care Pharm. 2008;14(2):3–13. [PubMed] [Google Scholar]
- 12.David M, Cutler WE. Thinking outside the pillbox-medication adherence as a priority for health care reform. N Engl J Med. 2010;362(17):1553–5. doi: 10.1056/NEJMp1002305. [DOI] [PubMed] [Google Scholar]
- 13.Jan Berger BC, Stambaugh Tom, Tomcavage Janet. Benchmarks in improving medication adherence. Health Inteligence Network. 20102010 [Google Scholar]
- 14.Cutrona SL, Choudhry NK, Fischer MA, Fischer, et al. Modes of delovery for interventions to improve cardiovascular medication adherence. Am J Manag Care. 2010;16(12):929–42. [PMC free article] [PubMed] [Google Scholar]
- 15.Bates DW, Bitton A. The future of health information technology in the patient-centered medical home. Health Aff (Millwood) 2010;29(4):614–21. doi: 10.1377/hlthaff.2010.0007. [DOI] [PubMed] [Google Scholar]
- 16.Overhage JM, Evans L, Marchibroda J. Communities’ readiness for health information exchange: The national landscape in 2004. J Am Med Inform Assoc. 2005;12:107–13. doi: 10.1197/jamia.M1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedlin J, Dexter Paul R, Overhage JM. Details of a successful clinical decision support system. AMIA Annu Symp Proc; 2007. pp. 254–258. [PMC free article] [PubMed] [Google Scholar]
- 18.Simonaitis L, Belsito A, Overhage JM. Enhancing an ePrescribing system by adding medication histories and formularies: the Regenstrief medication Hub. AMIA Annu Symp Proc; 2008 Nov 6; 2008. pp. 677–81. [PMC free article] [PubMed] [Google Scholar]
- 19.2010. ONC Beacon Community Program: Improving health through health information technology.
- 20.Overhage JM. 2010. The Central Indiana Beacon Community.
- 21.Zhu VJ, Tu W, Rosenman MB, Overhage JM. Facilitating clinical research through the health information exchange: Lipid control as an example. AMIA Annu Symp Proc; 2010 Nov 1; 2010. pp. 947–51. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu VJ, Tu W, Rosenman MB, Overhage JM. Adherence to oral hypoglycemic agents and glycemic control among patients with Type 2 diabetes: Findings from the real world clinical perspective. Pharmacoepidemiology and Drug Safety. 2011 (Revising). [Google Scholar]
- 23.Kirk JK, Graves DE, Bell RA, et al. Racial and ethnic disparities in self-monitoring of blood glucose among US adults: A qualitative review. Ethn Dis. 2007;17(1):135–42. [PubMed] [Google Scholar]
- 24.Adams AS, Trinacty CM, Zhang F, et al. Medication adherence and racial differences in A1C control. Diabetes Care. 2008;31(6):916–22. doi: 10.2337/dc07-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald CJ, Overhage JM, Barnes M, et al. The Indiana network for patient care: A working local health information infrastructure. Health Aff (Millwood) 2005;24(5):1214–20. doi: 10.1377/hlthaff.24.5.1214. [DOI] [PubMed] [Google Scholar]
- 26.The National Committee for Quality Assurance 2008. Pharmacy Quality Alliance (PQA) Demonstration Project.
- 27.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11:449–57. [PubMed] [Google Scholar]
- 28.Yang Y, Thumula V, Pace PF, et al. Predictors of medication nonadherence among patients with diabetes in medicare part D programs: A retrospective cohort study. Clin Ther. 2009;31(11):2178–88. doi: 10.1016/j.clinthera.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Balkrishnan R, Rajagopalan R, Camacho FT, Huston SA, Murray FT, Anderson RT. Predictors of medication adherence and associated health care costs in an older population with Type 2 diabetes mellitus: A longitudinal cohort study. Clin Ther. 2003;25(11):2958–71. doi: 10.1016/s0149-2918(03)80347-8. [DOI] [PubMed] [Google Scholar]
- 30.Schectman JM, Nadkarni MM, Voss JD. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care. 2002;25(6):1015–21. doi: 10.2337/diacare.25.6.1015. [DOI] [PubMed] [Google Scholar]
- 31.Krapek K, King K, Warren SS. Medication adherence and associated hemoglobin A1c in Type 2 diabetes. Ann Pharmacother. 2004;38(9):1357–62. doi: 10.1345/aph.1D612. [DOI] [PubMed] [Google Scholar]
- 32.Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial differences in glycemic markers: A cross-sectional analysis of community-based data. Ann Intern Med. 2011;154(5):303–9. doi: 10.1059/0003-4819-154-5-201103010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandalia M, Grundy SM, Adams-Huet B, Abate N. Ethnic differences in the frequency of ENPP1/PC1 121Q genetic variant in the Dallas Heart Study cohort. J Diabetes Complications. 2007;21(3):143–8. doi: 10.1016/j.jdiacomp.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27(12):2800–5. doi: 10.2337/diacare.27.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yurgin N, Secnik K, Lage MJ. Antidiabetic prescriptions and glycemic control in German patients with type 2 diabetes mellitus: A retrospective database study. Clin Ther. 2007;29(2):316–25. doi: 10.1016/j.clinthera.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 36.Donnan PT, MacDonald TM, Morris AD. Adherence to prescribed oral hypoglycemic medication in a population of patents with Type 2 diabetes: A retrospective cohort study. Diabet Med. 2002;19(4):279–84. doi: 10.1046/j.1464-5491.2002.00689.x. [DOI] [PubMed] [Google Scholar]
- 37.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;16(2):CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 38.McDonald CJ. Protocol-based computer reminders, the quality of care and the non-perfectability of man. N Engl J Med. 1976;295(24):1351–5. doi: 10.1056/NEJM197612092952405. [DOI] [PubMed] [Google Scholar]
- 39.Overhage JM, Tierney WM, Zhou XH, McDonald CJ. A randomized trial of “corollary orders” to prevent errors of omission. J Am Med Inform Assoc. 1997;4(5):364–75. doi: 10.1136/jamia.1997.0040364. [DOI] [PMC free article] [PubMed] [Google Scholar]