INTRODUCTION - HISTORICAL ASPECTS
In all multicellular organisms extensive communication is required between cells and tissues in order to coordinate growth and development. Both plants and animals have signaling chemicals, traditionally termed hormones that mediate short- and long-distance communication. The first phytohormone to be discovered was auxin, a substance that has since been implicated in an increasingly wide variety of developmental processes. It has emerged from decades of studies that a unique property of auxin - its directional movement between cells (polar auxin transport) - is a crucial process in auxin biology. In this report, we will first review the key events in the history of auxin research and then explore the current concepts and latest findings of the mechanisms of polar auxin transport and the resulting asymmetric auxin distribution.
The history of auxin began in the late 19th century when Charles and Francis Darwin were studying the phototropic responses of canary grass coleoptiles. They demonstrated the existence of an "influence" which moved from the tip of the coleoptile to the region below where it controlled bending (Darwin and Darwin, 1881). In fact, this was the first experimental demonstration of a plant signaling molecule and its intercellular movement. Some years later, in 1913, Boysen-Jensen was able to pass this "influence" through an agar block, thus confirming the chemical nature of this growth-promoting substance. Another important and visionary experiment was performed by Frits Went in 1926. He demonstrated that agar blocks soaked with the growth-promoting substance, when placed off-center on the decapitated coleoptiles, were sufficient to promote their bending away from the side that the block was sitting (Went, 1926; Cholodny, 1927; Went and Thimann, 1937; Went, 1974). Since this substance promoted coleoptile elongation, it was named auxin (from the Greek word auxein, which means: to increase, to grow) (Kögl and Haagen-Smit, 1931). It was later identified as indole-3-acetic acid (IAA) (reviewed by Thimann, 1977). While IAA seems to be the most physiologically important form of auxin, other natural forms exist, such as indole-3-butyric acid (IBA) and 4-chloroindole-3-acetic acid (4-Cl-IAA). There are also commonly known and useful synthetic auxins, such as 1-naphthaleneacetic acid (1-NAA) or 2,4-dichlorophenoxy-acetic acid (2,4-D). The natural and synthetic auxins differ in their effective concentrations, metabolic stability and transport properties (reviewed by Normanly et al., 2004; Woodward and Bartel, 2005). These early studies not only identified different auxins as regulators of plant growth but also highlighted the significance of polar auxin transport and indicated that asymmetric auxin distribution may be important for the tropic growth responses.
The identification of substances that can inhibit auxin flow (Katekar and Geissler, 1977; Jacobs and Rubery, 1988) established that cellular auxin efflux is crucial for auxin transport and provided tools for further studies into the physiological importance of this process. Studies using these inhibitors combined with auxin transport experiments led, in the middle 1970s, to the formulation of the chemiosmotic hypothesis, which proposed a mechanism by which auxin could move from cell to cell. It postulated that auxin is transported into and out of the cell through the action of specific carrier proteins (Rubery and Sheldrake, 1974; Raven, 1975). Importantly, it also proposed that the strictly controlled directionality of auxin flow may be a result of the asymmetric cellular localization of auxin efflux carriers.
The chemiosmotic hypothesis gained significant support from genetic studies in Arabidopsis thaliana that led to the identification and characterization of molecular components of auxin influx (AUX1/LAX family) and auxin efflux (PIN family) (Bennett et al., 1996; Luschnig et al., 1998; Gälweiler et al., 1998), as well as the identification of several PGP proteins from the ABC transporter superfamily, which are also involved in transport of auxin across the plasma membrane (Petrášek et al., 2006; Geisler et al., 2005). Many of the molecular components of polar auxin transport (PAT), in particular PIN proteins, show asymmetric localization at the cell membrane within auxin transport-competent cells as predicted by the chemiosmotic hypothesis.
Progress into the characterization of the molecular components of auxin response and signaling (Ulmasov et al., 1997; Gray et al., 2001; Dharmasiri et al., 2005: Kepinski and Leyser, 2005) also allowed the presence of auxin to be indirectly observed in plant cells and tissues. By comparing indirect and direct auxin measurements, these studies identified local, asymmetric auxin distribution (auxin gradients), which are the result of PAT, and which underpin most of the auxin-mediated developmental processes (for overview see Tanaka et al., 2006). These findings also highlighted the morphogenic properties of auxin and stirred the old debate on the morphogen versus hormone characteristics of auxin (Friml, 2003; Bhalearo and Bennett, 2003).
Even though most of the information about the molecular mechanism of PAT and recent physiological insights came from studies in Arabidopsis, research conducted in other plant organisms (e.g. rice, maize, tobacco, Brassica) or suspension cultured cells (e.g. tobacco BY-2 cells), as well as the use of heterologous cell systems (e.g. yeast or human HeLa cells), also greatly contributed to our knowledge about auxin transport, often supporting and complementing studies from Arabidopsis.
TYPES OF AUXIN TRANSPORT
Although virtually all plant tissues appear to be capable of synthesizing auxin, most is normally produced in young developing parts of plant such as the shoot apex, emerging leaves and developing seeds (Ljung et al., 2001; reviewed by Ljung et al., 2002). Recent studies have also uncovered that auxin is also synthesized to some extent in roots, with the most prominent auxin source located in the meristematic zone of primary root tip and developing lateral roots (Ljung et al., 2005).
From places of synthesis, auxin is redistributed throughout all the plant body where it is required for a variety of developmental processes such as cell division and elongation, lateral root formation, apical dominance, leaf and flower development and tropic responses to environmental stimuli (reviewed by Davies, 2004). The mechanism by which auxin is transported throughout the plant has for a long time been of great interest to plant biologists. Based on the physiological, molecular and biochemical data collected so far, we know that auxin distribution throughout a plant is conducted through two physiologically distinct and spatially separated transport pathways: a fast, non-polar transport in the phloem and a slower, cell-to-cell polar auxin transport in various tissues (Fig. 1).
Figure 1.
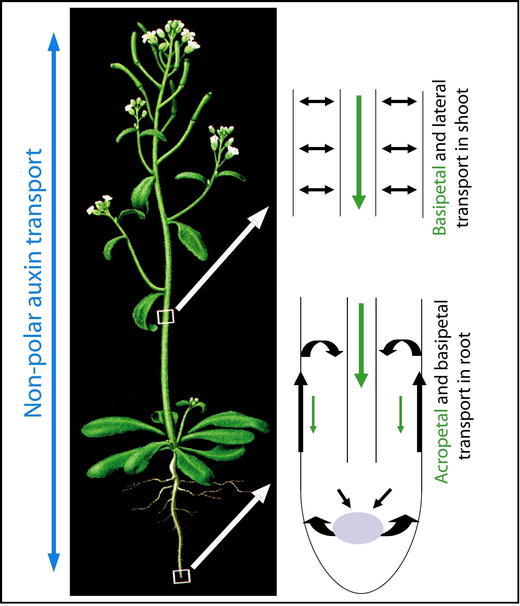
A scheme for non-polar and polar auxin transport in Arabidopsis thaliana. Auxin is redistributed throughout all aerial and underground plant body through fast, non-polar transport in phloem (marked by the blue arrows) and slow, cell-to-cell polar auxin transport (PAT; marked by the green and black arrows). Through PAT, auxin is transported polarly from the aerial apical tissues towards the base through the vasculature (basipetaly). This stream continues in the root to the root tip (acropetaly) where part of it is redirected upwards (basipetaly) through the outer layers. In the shoot, also existence of a short distance lateral transport between the main basipetal stream and outer cell layers is assumed. Adapted from Jones, A.M. (1998). Auxin transport: down and out and up again. Science 282, 2201–2203. - Figure 1. Readers may view, browse, and/or download material for temporary copying purposes only, provided these uses are for noncommercial personal purposes. Except as provided by law, this material may not be further reproduced, distributed, transmitted, modified, adapted, performed, displayed, published, or sold in whole or in part, without prior written permission from the publisher.
Non-Polar Auxin Transport
Experiments with radioactively labeled auxin established the existence of phloem transport (Morris and Thomas, 1978). Labeled auxin or tryptophan (precursor of IAA), when applied directly to source leaves were rapidly loaded into the phloem and passively translocated along a concentration gradient. This mass transport occurs relatively fast, with the molecules moving at the velocity of 5 to 20 cm/h. In contrast to the phloem, xylem does not seem to play any important role in long-distance auxin movement pathway. Only traces of endogenous IAA were found in this tissue (Baker, 2000). Auxin, together with other metabolites that are transported in the phloem sap, is gradually unloaded to the different sink organs and tissues where it is redistributed further by regulated, short-distance PAT (Fig. 1). The connection between phloem-based transport and PAT has been established by experiments in pea, which showed that labeled auxin, which was transported within the phloem, was later detected in the PAT system (Cambridge and Morris, 1996). Even though most of the physiological and genetic data highlight the importance of PAT in regulating plant development, the importance of auxin translocation via phloem should not be underestimated. It is likely that due to the higher capacity and velocity of phloem-based transport, the majority of long-distance auxin redistribution occurs in the phloem, which is especially significant in larger plant species.
Polar Auxin Transport
PAT has been found in bryophytes and throughout the higher plant species. It is characterized by its strictly controlled directionality, as designated by the name, polar auxin transport. From a multitude of studies in different species, the general properties of PAT and the way it is tightly regulated at many different levels have emerged: PAT occurs in a cell-to-cell manner, requires energy, is relatively slow (5-20 mm/h), saturable, specific for active free auxins, and sensitive to protein synthesis inhibitors. Although, generally two distinct types of PAT can be recognized: long-distance (along the whole plant body) and short-distance (delivering auxin to the precisely defined places within specific tissue), both of them equally meet the above described specifications and most likely use the same mechanism. Ordinarily, the major polar flow of auxin can be traced from apical tissues towards the base of the plant and further to the root tip (Fig.1; Morris et al., 2004). In the aerial part of a plant (stem), radiolabelled auxin was mainly detected in the vascular cambium and xylem parenchyma. Here, auxin was mainly transported down-wards, but it was also detected in the more peripheral cell layers suggesting that auxin can be redistributed laterally in stems (Fig. 1; Morris and Thomas, 1978). In the root, the auxin stream continues toward the root tip (acropetally) mainly through the vascular tissues. Once auxin reaches the tip of the root, part of it is redirected back upwards (basipetally) through the root epidermis into the root elongation zone (Rashotte et al., 2000) where it can be recycled back into the vasculature stream (Fig 1; Blilou et al., 2005). In other meristematic and differentiating tissues, such as the shoot apical meristem and lateral organ formation, a short distance PAT is operational.
A number of physiological and genetic studies have clearly demonstrated that PAT, and in particular the short distance PAT in meristematic tissues, is of crucial importance for many auxin-mediated developmental processes. This is because PAT is required for the formation and maintenance of local auxin distribution patterns - so called auxin gradients.
Local Auxin Distribution Patterns (Auxin Gradients)
A very puzzling and intriguing property of auxin is the fact that it can influence a wide variety of apparently unrelated plant developmental processes. Embryonic axis specification, organ formation and positioning, root meristem maintenance, vascular tissue differentiation, differential growth responses, fruit development, apical hook formation and apical dominance, all form part of a certainly still incomplete list (reviewed by Davies, 2004).
For a long time, the main barrier to a closer understanding of the role of auxin in plant development was the inability to visualize auxin distribution at a sufficient resolution in planta. An important breakthrough for monitoring spatial and temporal auxin distribution was achieved through the discovery of genes that are rapidly upregulated by auxin (reviewed by Hagen and Guilfoyle, 2002). Identifying the consensus sequence TGTCTC (auxin response element, AuxRE) within the promoters of these genes allowed the engineering of highly auxin-responsive synthetic promoters known as DR5 (or DR5rev, which is another variant with inverse repeats). The DR5 promoter consists of a multiple repeats of AuxRE and a minimal viral 35S promoter. The promoter design should confer high auxin sensitivity without preserving any other tissue-specific regulation and thus constitute a suitable tool for the indirect monitoring of endogenous auxin levels (Fig. 2). Indeed, the pattern of DR5 expression has been shown to correlate well with direct measurements of auxin content (Casimiro et al., 2001) and with auxin localization patterns as confirmed by the immunolocalization technique using anti-IAA antibodies (Avsian-Kretchmer et al., 2002; Benková et al., 2003; Friml et al., 2003b). In addition, exogenously applied auxin induces DR5 expression (Sabatini et al., 1999; Friml et al., 2003b), whereas the inhibition of auxin transport alters DR5 expression pattern (Sabatini et al., 1999; Friml et al., 2002a,b; Benková et al., 2003; Ottenschläger et al., 2003; Friml et al., 2003b; de Reuille et al., 2006). These results indicate that DR5-based reporters can be used as reasonable approximations of auxin accumulation in different cells of certain tissues, allowing insights into the spatio-temporal pattern of auxin distribution (Fig. 2).
Figure 2.
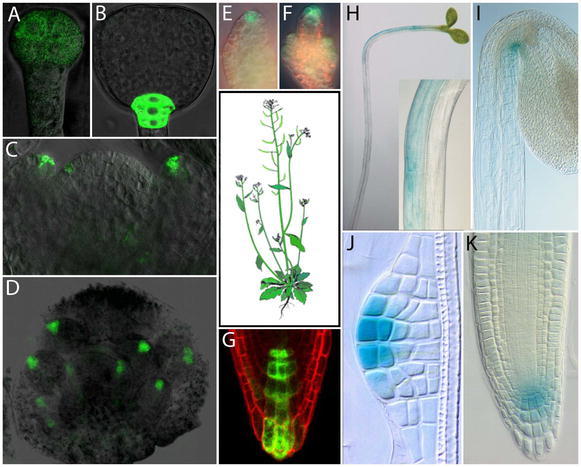
Examples of local auxin distribution in Arabidopsis thaliana marked by activity of auxin responsive promotor DR5. (A, B) DR5rev::GFP signal in preglobular and triangular stage embryos. (C) DR5rev::GFP signal at the position of incipient and developing flower primordia in the shoot apical meristem. (D) DR5rev::GFP signal in the developing flower bud. (E, F) DR5rev::GFP signal in a younger (E) and older (F) ovule primordium. (G) DR5rev::GFP in the columella initials region of a primary root. (H, I) Asymmetric expression of DR5::GUS in a hypocotyl of light stimulated seedling (H) and in the apical hook of an etiolated seedling (I). (J, K) DR5::GUS signal at the tip of a developing lateral root (J) and in the central meristem of a mature lateral root (K). A, B reprinted from Friml et al (2003b) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426, 147–153 - Figure 1 A, E; C, D, E, F, J, K reprinted from Benková et al (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell, 115, 591–602 - Figure 1 A, B; 6 A, C, L, N, Copyright 2003, with permission from Elsevier. H, I Reprinted from: Friml et al (2002b) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415, 806–809 - Figure 1 A, E.
Studies employing these methods have uncovered that auxin is involved in plant development from the initial stages of embryogenesis, accumulating first following zygote division in the apical cell, and later in the uppermost suspensor cells, which includes the future precursors of central root meristem, and at the tips of developing cotyledons (Fig 2; Friml et al., 2003b; Benková et al., 2003). During postembryonic plant development, auxin accumulates highly within the root meristem and at the initiation site of incipient organs in the root and shoot. In later stages of organ formation, a maximal point of DR5 activity is consistently visible at the tip of primordia including those of lateral roots, leaves, flowers, floral organs and ovules (Fig 2; Sabatini et al., 1999; Benková et al., 2003; Heisler et al., 2005). The asymmetric accumulation of auxin is also crucial for the directional growth of plants in response to environmental stimuli. As proposed by the Cholodny-Went hypothesis, organ bending results from differential growth mediated by asymmetric auxin distribution (Went, 1974). This hypothesized auxin redistribution was visualized and confirmed in various aerial and underground plant organs following light and gravity stimulation (Fig. 2; Li et al., 1991; Moctezuma, 1999; Friml et al., 2002b; Ottenschläger et al., 2003; Fusch et al., 2003; Swarup et al., 2005; Esmon et al., 2006). Thus, asymmetric auxin distribution has been found to accompany many auxin-dependent plant developmental processes.
The important question was how these auxin gradients arise. Conceptually, asymmetry in the distribution of active auxin can be achieved by local auxin biosynthesis, degradation, conjugation or by directional auxin transport. Numerous recent studies have demonstrated that pharmacological or genetic interference with PAT abolishes auxin gradients, which consequently arrests the programmed organogenesis and tropic responses (reviewed by Tanaka et al., 2006). Thus, it seems that PAT-dependent auxin accumulation is an important part of a conserved mechanism that mediates diverse patterning, organogenesis and differential growth responses in plants.
MECHANISM OF POLAR AUXIN TRANSPORT
Chemiosmotic Hypothesis
The presumption that auxin flow may require carrier-mediated cell-to-cell transport came from the observation that known inhibitors of polar auxin transport such as 2,3,5-tri-iodobenzoic acid (TIBA) increase the accumulation of labeled IAA in maize coleoptile cells, which suggested that TIBA blocked efflux, rather then influx of auxin (Hertel and Leopold, 1963). This finding, together with other growing evidence about auxin flow, suggested that auxin-specific carrier proteins may exist, and led to the formulation of the chemiosmotic model for PAT (Fig. 3; Rubery and Sheldrake, 1974; Raven, 1975). In the fairly acidic environment of the cell wall (pH 5.5), some IAA exists in its protonated form (IAAH). Such a neutral, lipophilic molecule is able to pass the plasma membrane via simple diffusion. In the more alkaline cytosolic environment (pH 7.0) most IAA undergoes deprotonation giving rise to polar IAA− anions. Charged, deprotonated IAA− cannot easily depart from the cell, which consequently leads to the accumulation of IAA molecules inside the cell. IAA− can leave the cell only by active efflux, presumably mediated by specific efflux carriers. An asymmetric, cellular distribution of these carriers within each cell would explain the unidirectional (polar) feature of auxin flow. In other words, auxin efflux carriers being predominantly localized to one side of the cell would provide auxin transport in that direction (Fig. 3). In addition, the existence of specific auxin influx facilitating the uptake of IAA into cells was also proposed and demonstrated (Fig. 3; Goldsmith, 1977).
Figure 3.
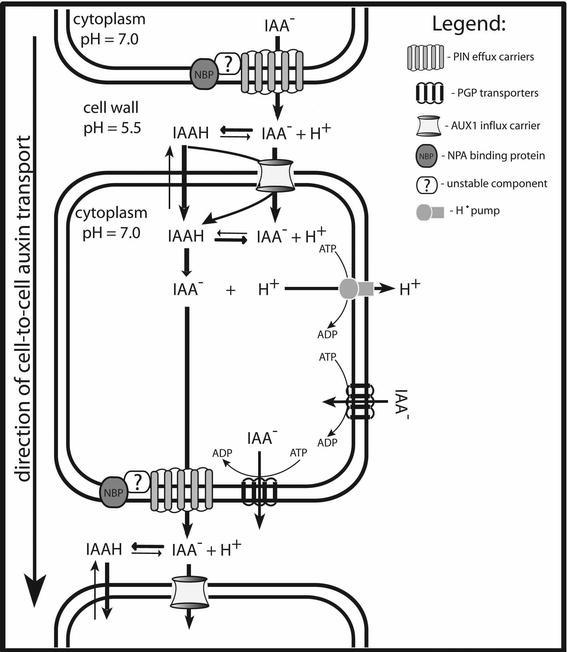
Model for polar auxin transport. According to the chemiosmotic hypothesis, the difference in pH between the relatively acidic cell wall and the cytoplasm (maintained by plasma membrane H+-ATPases) promotes the accumulation of IAA (entering by diffusion) inside the cell. At the more basic pH of the cytoplasm, the majority of IAAH undergoes deprotonation and become "trapped" inside the cell. Deprotonated IAA− can only leave the cell via active efflux, mediated by the specific efflux carriers. The asymmetric distribution of efflux carriers within each cell, promotes unidirectional (polar) auxin transport from cell to cell. Auxin efflux is presumably facilitated by a multi-component complex consisting of the efflux carrier (family of PIN proteins), the NPA-binding protein (NBP) and third unstable component. Auxin influx carrier (AUX1) facilitate auxin uptake into the cell. ABC transporters of the PGP family mediate additional efflux and influx.
This classical chemiosmotic model was strengthened 30 years later by the identification and characterization of proteins for auxin efflux carriers (PIN family) and auxin influx carriers (AUX1 family) (Bennett et al., 1996; Gälweiler et al., 1998; Luschnig et al., 1998). The corresponding mutant phenotypes were found to be impaired in auxin transport, and both AUX1 and PIN proteins have been shown to be asymmetrically localized at the cell membrane in accordance with known directions of auxin flux (Fig. 4; Gälweiler et al., 1998; Müller et al., 1998; Swarup et al., 2001; Friml et al., 2002a, b).
Figure 4.
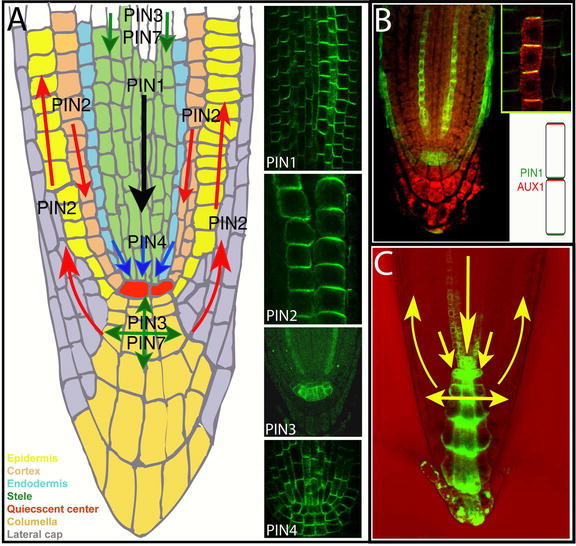
Polar localization of auxin efflux and influx carriers, and the direction of auxin flow in the Arabidopsis root. (A) A diagram representing the Arabidopsis root tip with arrows indicating the direction of auxin transport as mediated by PIN1, PIN2, PIN3, PIN4 and PIN7. Inset images show the subcellular polar localization of PIN1, PIN2, PIN3 and PIN4 (by immunolocalization). (B) AUX1 subcellular localization (by immunolocalization); inset: a close-up and scheme illustrating opposing PIN1 and AUX1 polar localization in protophloem cells. (C) A diagram showing the predominant directions of auxin flow and auxin accumulation in the root tip as visualized by DR5rev::GFP. B reprinted from Swarup et al (2001) Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 15, 2648–2653 -Figure 1B.
Recent support for the pH gradient-dependent mechanism of PAT came from experiments demonstrating that transgenic Arabidopsis plants overexpressing the H+-pyrophosphatase AVP1 (for Arabidopsis vacuolar pyrophosphatase) have a decreased apoplastic pH and exhibit an increased transport of IAA from the shoot tip to the root. Conversely, in the avp1 loss-of-function mutant, apoplastic pH was more alkaline and basipetal IAA transport was reduced (Li et al., 2005). These results show that apoplastic pH has an impact on PAT activity, which is in accordance with chemiosmotic model (Benjamins et al., 2005; Blakeslee et al., 2005).
Auxin Efflux
Our current knowledge about auxin efflux in plants was, to a large extent, acquired based on experiments with naturally occurring and synthetic substances that are able to inhibit auxin efflux from cells and hypocotyl segments (Katekar and Geissler, 1977; Jacobs and Rubery, 1988; reviewed by Rubery, 1990; Morris et al., 2004). These substances were later termed auxin efflux inhibitors (AEIs) and include substances with a weak structural similarity such as TIBA, 1-N-naphthylphthalamic acid (NPA) and 2-chloro-p-hydroxyfluorene-9-carboxylic acid. Proposed natural AEIs are flavonoids (e.g. quercetin). Finding that the Arabidopsis mutant transparent testa4 (tt4), which is deficient in flavonoid synthesis, exhibits increased auxin efflux (Murphy et al., 2000), supported the idea that flavonoids might be the natural inhibitors of auxin efflux carriers (Jakobs and Rubery, 1988).
Taking advantage of AEIs, it was possible to better physiologically characterize PAT and test the importance of auxin efflux on plant development. The collective work of many groups demonstrated that AEIs interfere with numerous developmental processes such as axis formation during embryogenesis (Liu et al., 1993; Hadfi et al., 1998), initiation of lateral roots (Casimiro et al., 2001) and aerial organs (Okada et al., 1991; Reinhardt et al., 2000), root meristem patterning (Kerk and Feldman, 1994; Ruegger et al., 1997; Sabatini et al., 1999), vascular patterning (Mattson et al., 1999), hypocotyl and root elongation in light (Jensen et al., 1998), apical hook formation (Lehman et al., 1996) and tropic responses (Katekar and Geissler, 1977; Li et al., 1991; Marchant et al., 1999).
Studies using AEIs also led to the idea that the auxin efflux carrier could be a multi-component complex (Morris et al., 1991). In an attempt to explain how AEIs work, the existence of a specific NPA-binding protein (NBP), which is a part of the auxin efflux complex was proposed and supported by the observation that protein extracts specifically bind NPA (Rubbery, 1990; Morris, 2000). Importantly, finding that TIBA does not compete with NPA for the binding site on the NBP suggested that different classes of AEI possess different binding sites. Despite decades of effort, our knowledge about the nature of a postulated NBP is still limited. Diverse attempts to visualize NBP in cells revealed different localizations. The first reports identified NBP as localized polarly at the basal side of cells (Jacobs and Gilbert, 1983). These studies, in fact, were the first ones which directly demonstrated that a protein putatively associated with PAT is localized polarly. Later on, it was reported that NBP is an integral membrane protein (Bernasconi et al., 1996), whereas others have demonstrated that it is a peripheral membrane protein, localized to the cytoplasmic face of the plasma membrane and associated with the cytoskeleton (Cox and Muday, 1994).
Experiments with the protein synthesis inhibitor cyclo-heximide (CHX) suggested the existence of a third component of the auxin efflux machinery. Short-term CHX treatment did not affect auxin efflux or the saturable binding of NPA to microsomal membranes, but it interfered with the inhibitory effect of NPA on efflux (Morris et al., 1991). This can be interpreted as NBP and the efflux carrier itself are separate proteins with a low turnover, which may interact through a third, short-lived, unstable protein (Delbarre et al., 1998; Morris, 2000; Luschnig, 2001; Muday and DeLong, 2001).
Further insights into the complexity of the auxin efflux carrier machinery came from experiments using inhibitors of intracellular protein trafficking such as monensin and brefeldin A (BFA). These compounds caused a rapid inhibition of auxin efflux from suspension-cultured tobacco cells and zucchini hypocotyl segments (Delbare et al., 1998; Morris and Robinson, 1998; Robinson et al., 1999; Morris, 2000). These results suggested that a catalytic component of the auxin efflux carrier system is targeted to the plasma membrane through the BFA-sensitive secretory pathway and it turns over rapidly without need for concurrent protein synthesis (Delbarre et al., 1998; Morris and Robinson, 1998; Robinson et al., 1999). As BFA does not affect NPA binding to microsomal preparations, it provides additional evidence that the NBP and auxin efflux carriers are distinct proteins (Robinson et al., 1999).
Despite the extensive use of NPA and other AEIs for physiological studies, the molecular identity of the proposed NPB remains unknown, although several candidates were presented along the way. In the middle 1990s, through the genetic screen on Arabidopsis for the NPA-insensitive roots, a mutant with reduced NPA-binding activity and auxin transport capacity, named transport inhibitor response3 (tir3) was isolated. The decrease in NPA binding to the extracts of the tir3 mutant suggested that TIR3 might encode the NBP or another functionally related protein (Ruegger et al., 1997). Unfortunately, the characterization of the TIR3 gene product as a homolog of the Drosophila CALOSSIN/PUSHOVER protein, termed BIG for its remarkable size, did not clarify its functional connection to auxin efflux or NPA binding (Gil et al., 2001). It looks as though BIG has something to do with the effect of AEIs and auxin on the trafficking of PIN auxin efflux carriers rather than directly mediating inhibition of auxin efflux by AEIs (Gil et al., 2001; Paciorek et al., 2005). Recently, biochemical approaches using Arabidopsis protein extracts have identified several diverse proteins which bind NPA with different affinities, such as aminopeptidase (AMP1) or members of multi-drug resistance (MDR) proteins of the ATP-binding cassette (ABC) transporters family (PGP1 and PGP19), which are also important components of auxin transport (Murphy et al., 2002; Geisler et al., 2005). However, the unequivocal identification of any of these proteins as a PAT-relevant NBP is still missing.
In summary, physiological studies have established that auxin efflux is crucial for PAT and many auxin-dependent developmental processes. In addition, they provide insight into the structure of the auxin efflux carrier depicting it as a highly dynamic, multi-component complex that is targeted by auxin efflux inhibitors such as NPA.
Auxin Influx
The existence of specific carriers mediating auxin influx was suggested by the observations that auxin uptake into suspension culture cells (Rubery and Sheldrake, 1974) and tissue segments (Davies and Rubery, 1978) is a saturable process, specific for auxins. Some years later, research on tobacco culture cells revealed that putative influx carriers readily transport not only naturally occurring auxin (IAA) but also its synthetic analog 2,4-D (Delbarre et al., 1996). Recently, modeling of IAA uptake also theoretically proved the requirement of an uptake carrier for auxin movement (Kramer and Bennett, 2006).
Further characterization of influx carriers and investigation of their influence on plant development was delayed for many years due to the lack of specific influx inhibitors. Only more recently were compounds such as 1-naph-thoxyacetic acid (1-NOA) and 3-chloro-4-hydroxypheny-lacetic acid (CHPAA) isolated and shown to specifically inhibit auxin uptake in suspension-cultured tobacco cells (Imhoff et al., 2000). Further findings that these inhibitors interfere with root growth and gravitropism (Parry et al., 2001), as well as leaf positioning (Stieger et al., 2002), demonstrated the functional importance of auxin influx in plant-wide processes. Thus despite the fact that carrier-mediated auxin uptake can be bypassed by diffusion through the PM, it is a physiologically significant process that also plays an important role in auxin distribution during tropisms and organogenesis.
MOLECULAR COMPONENTS OF POLAR AUXIN TRANSPORT
Physiological studies led to the postulation of the existence of auxin carrier proteins that are key components of the mechanism of polar auxin transport. However their identity came decades later from molecular genetic studies in Arabidopsis. With the use of diverse screening strategies, many different mutants affected in PAT or in responses to auxin or auxin transport inhibitors, were isolated and characterized. Three gene families that coded for important components of PAT emerged from these studies. These were: the PIN-FORMED (PIN) family, the AUXIN1 (AUX1)/LIKE AUX1 (LAX) genes and the POLYG-LYCOPROTEIN (PGP) subfamily of the MULTIDRUG RESISTANCE (MDR) superfamily.
PIN Proteins as Components of Auxin Efflux Machinery
In the early nineties, the classical Arabidopsis mutant, pin-formed1 (pin1) with its very characteristic needle-like inflorescence, was described and linked to defects in PAT (Okada et al., 1991). The characteristic phenotype was recognized to be very similar to wild type plants treated with auxin efflux inhibitors, and basipetal auxin transport in pin1 stem segments was significantly reduced, suggesting the function of PIN1 in auxin efflux (Okada et al., 1991). In the late nineties the PIN1 gene was identified as coding for a plant-specific transmembrane protein with a predicted topology reminiscent of carrier proteins (Gälweiler et al., 1998). Simultaneously, four different groups independently identified and analyzed a homologous gene, named PIN2/EIR1/AGR1, required for root gravitropism (Chen et al., 1998; Luschnig et al., 1998; Müller et al., 1998; Utsuno et al., 1998). Further work lead to the identification and characterization of additional homologous genes, named PIN3, PIN4 and PIN7 - required for tropism, root meristem patterning and early embryo development, respectively (Friml et al., 2002a, b; Friml et al., 2003b). In total, the Arabidopsis PIN gene family consists of eight members with as yet unknown physiological roles for PIN5, PIN6 and PIN8. Homologous PIN genes were also found in other monocot and dicot plant species (Paponov et al., 2005).
As PINs represent a plant-specific protein family, with only limited sequence similarity to transport facilitators from other kingdoms (Chen et al., 1998; Luschnig et al., 1998; Müller et al., 1998; Utsuno et al., 1998), their molecular role remained unclear for years. Nonetheless, several lines of evidence demonstrated a crucial role of PIN proteins in PAT and auxin efflux in particular:
1. As proposed by the chemiosmotic hypothesis with regard to the efflux carrier components (Rubery and Sheldrake, 1974; Raven, 1975), all PIN proteins display asymmetric (polar) localization at cell membranes in particular cell types, correlating well with the known directions of auxin flow (Fig. 4). PIN1 is expressed from the earliest developmental stages on, first in proembryo cells where it is plasma membrane localized in a non-polar manner, then becoming polarized to the basal (lower) side of provascular cells around the early globular stage (Steinmann et al. 1999; Friml et al., 2003b). Later, it marks future vascular cells from the tips of developing cotyledons towards the root pole with polarity pointing towards the root pole (Fig. 5). A similar pattern of PIN1 can be also found postembryonically in the root stele, and in the xylem parenchyma cells of more differentiated vasculature in the aerial parts of the plant (Gälweiler et al., 1998; Friml et al., 2002a; Scarpella et al., 2006). In the outer cell layers of the embryo and shoot apical meristem, highly dynamic PIN1 expression is associated with forming shoot-derived organ primordia with polarity pointing toward the apex of these organs (Benková et al., 2003; Reinhardt et al, 2003; Heisler et al., 2005). PIN2 was found to be expressed only postembryonically in the root tip, with the protein localized predominantly at the basal (towards the root apex) side of young cortex cells and at the apical (towards the shoot apex) side of epidermis and lateral root cap cells (Müller et al., 1998; Friml et al., 2003a). PIN3 protein is localized pre-dominantly to the inner baso-lateral side of shoot endodermis (starch sheath) cells or root pericycle cells and symmetrically in the columella cells of the root tip (Friml et al., 2002b). PIN4 expression was found from the middle globular stage on, in cells of the central root meristem, with the protein polarity pointing towards the quiescent center (QC) cells but without a clearly defined polarity in the quiescent cells themselves (Fig. 4; Friml et al., 2002a). The expression of PIN7 has been detected during embryogenesis in suspensor cells with polar protein localization at early stages pointing towards the proembryo and switching to the opposite side away from the proembryo during post-globular stages (Fig. 5; Friml et al., 2003b). Postembryonically, PIN7 exhibits a similar pattern to PIN3, in the columella and stele cells of the root meristem (Blilou et al., 2005).
Figure 5.
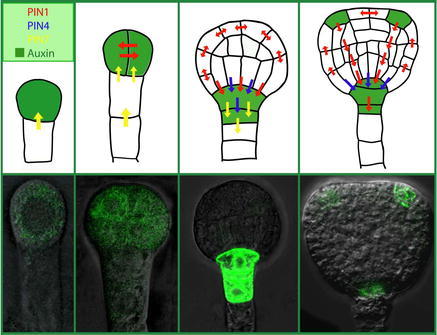
PIN-mediated auxin transport and distribution during Arabidopsis embryo development. The upper panel illustrates embryo development, auxin transport and distribution from the one-cell stage to the triangular-stage. PIN7 become expressed from the earliest stages of embryo development (marked by yellow arrow), later also PIN1 and PIN4 become present (marked by red and blue arrows, respectively). Notice that during embryo development the polarity of PIN localization changes. PIN expression correlates with the accumulation of auxin (highlighted in green) first in the apical cell and developing proembryo, and later at the basal part of the embryo with a maximum in the upper suspensor cell. The lower panel shows pictures of auxin accumulation as indirectly visualized by DR5rev::GFP. Images adapted from Friml et al (2003b) and Benkova et al (2003).
Importantly, the detected polar localization patterns of PIN proteins in all cases correlate with the known or presumptive directions of auxin flow, and local auxin accumulation patterns (auxin gradients) (Figs. 4 and 5).
2. All loss-of-function data from various single and multiple pin mutants show growth defects indicative of impaired PAT, which can be mimicked by the treatment of wild type plants with auxin efflux inhibitors (Okada et al., 1991; Müller et al., 1998; Friml et al., 2002a, b; Friml et al., 2003b; Benková et al., 2003; Blilou et al., 2005). In several pin mutants, direct measurements demonstrated a significantly reduced PAT, which directly correlated with the loss of PIN function in corresponding tissues (Okada et al., 1991; Rashotte et al., 2000). In addition, auxin distribution, as inferred from both the detection of auxin and the activity of auxin-responsive promoters is disrupted in many analyzed pin mutants (Luschnig et al., 1998; Friml et al., 2002a; Friml et al., 2003b; Benková et al., 2003; Ottenschläger et al., 2003).
3. The direct involvement of PIN proteins in auxin efflux from the cells has been demonstrated in tobacco (PIN4, PIN6 and PIN7) and Arabidopsis (PIN1) cultured cell lines, which were engineered to conditionally express particular PIN genes. Auxin transport measurements based on the accumulation of radioactively labeled auxins showed a strong stimulation of NPA-sensitive auxin (NAA, 2,4-D and IAA) efflux following the expression of Arabidopsis PINs. Remarkably, the amount of expressed PIN protein correlated with the level of efflux stimulation, demonstrating a rate limiting function of PIN proteins in auxin efflux. Also pin2 mutant root tips, when preloaded with radioactively labeled auxin, retained more radioactivity then similarly treated wild type roots consistent with a role of PINs in auxin efflux (Chen et al., 1998).
Although all above mentioned data from the plant systems clearly demonstrated the importance of PIN proteins in efflux-dependent auxin distribution, they could not distinguish whether the PINs functioned directly as auxin transporters or as positive regulators of auxin transport. This issue was addressed by introducing PINs into heterologous non-plant systems such as yeast and mammalian HeLa cells. Yeast cells overexpressing PIN2 showed an increased resistance to the toxic 5-fluoroindole (a structural auxin analog) (Luschnig et al., 1998) and retained less radioactively labeled auxin compared to control cells (Chen et al., 1998). Also direct measurements of the auxin efflux kinetics showed the stimulation of auxin efflux from yeast following the expression of PIN2 or PIN7 (Petrášek et al., 2006). Similarly, in HeLa cells, which do not contain PIN-related genes or an auxin-related system, the expression of PIN2 and PIN7 significantly stimulated the efflux of radioactively labeled IAA from the cells (Petrášek et al., 2006).
These results demonstrated that PIN proteins are capable (although with a decreased specificity) of stimulating auxin efflux without a need of additional plant-specific factors. Thus, it seems that PIN genes indeed code for catalytic components of the auxin efflux carrier complex (Petrášek et al., 2006).
AUX1 Protein as the Auxin Influx Carrier
The aux1 mutant was originally found in a screen for seedlings resistant to 2,4-D (Maher and Martindale, 1980). The auxin resistant and agravitropic root growth phenotype of aux1 mutants suggested a defect in auxin transport. However, similar phenotypes can be also observed in mutants defective in auxin signaling (Lincoln et al., 1990). Significantly, the resistance to auxin of aux1 mutants relates specifically to the membrane-impermeable auxin 2,4-D. Yet on the other hand, the aux1 root agravitropism can be rescued much more efficiently by the membrane-permeable auxin NAA than by the less permeable IAA or 2,4-D (Marchant et al., 1999). These data together suggest that AUX1 is involved in auxin uptake rather than auxin signaling. Furthermore, the re-establishment of normal root growth coincides with the restoration of basipetal auxin transport in the root, which is otherwise defective in aux1 mutants (Yamamoto and Yamamoto, 1998; Marchant et al., 1999). Additional evidence favoring AUX1 as a protein involved in auxin influx came from auxin uptake assays performed in aux1 and wild type roots. The experiments revealed that aux1 roots accumulated approximately 2-fold less radioactively labeled 2,4-D than roots of wild type seedlings. Importantly, this difference was not seen with the membrane permeable 1-NAA (Marchant et al., 1999). Moreover, growing wild type seedlings on media containing inhibitors of auxin influx such as 1-NOA or CHPAA, mimicked the aux1 phenotype (Parry et al., 2001). The characterization of the AUX1 gene as encoding protein with pronounced similarity to amino acid permeases, favored a role of AUX1 in the uptake of tryptophan-like IAA (Bennett et al., 1996; Swarup et al., 2004). The confirmation that AUX1 acts as an auxin uptake carrier came from studies using the Xenopus oocyte expression system. AUX1-expressing oocytes showed a considerably increased capacity of uptake of radioactively labeled IAA. Importantly, this uptake was reduced by specific auxin-influx inhibitors 1-NOA and 2-NOA, but not by auxin-efflux inhibitors NPA or TIBA. Moreover, the kinetic properties of auxin uptake, including substrate affinity, provided evidence that AUX1 is a specific, high affinity auxin influx carrier (Yang et al., 2006).
In Arabidopsis root tips, AUX1 is strongly expressed with cell membrane protein localization in protophloem, columella, lateral root cap and epidermal cells. Although in most of these tissues AUX1 appears to be uniformly distributed around all sides of the cells, in the protophloem, AUX1 was found to be enriched at the upper side of cells, that is, opposite to the PIN1 localization in the same cells (Fig. 4; Swarup et al., 2001). The plasma membrane localization of AUX1 was also confirmed by biochemical fractionation (Swarup et al., 2004). Given the fact that aux1 root tips contain less auxin than wild type roots - suggesting defect in the long-distance auxin distribution down into the root tip - the restricted expression of AUX1 to only a subset of tissues in the root tip was surprising. This discrepancy between the local AUX1 expression and long-distance effects, together with its expression in pro-tophloem cells put forward the hypothesis that AUX1 is involved in unloading auxin from the mature phloem via the protophloem into the PAT system of the root meristem (Swarup et al., 2001). In this scenario, an AUX1-dependent system would constitute a connection between phloem-based and PAT routes. AUX1 is a member of a small gene family in Arabidopsis consisting of three other LIKE AUX1 (LAX) genes (Swarup et al., 2000), but their functional characterization has not yet been reported.
MDR/PGP Sub-Family of ABC Transporters
The third group of candidate components of PAT is represented by members of the multi-drug resistance/P-glyco-protein (MDR/PGP) subfamily, belonging to the ATP-binding cassette (ABC) transporter superfamily (Martinoia et al., 2002). MDR1 (also known as PGP19) was originally identified in an Arabidopsis screen aiming to identify genes related to anion-channel functions, but analysis of both the mdr1 mutant and plants defective in its closest homologue, PGP1, connected these proteins instead with auxin transport (Noh et al., 2001). The single pgp1 and pgp19 mutants exhibit phenotypes indicative of altered auxin response and/or auxin transport such as epinastic cotyledons, abnormally wrinkled leaves, reduced apical dominance, partial dwarfism and reduced basipetal polar auxin transport in hypocotyls and inflorescences (Noh et al., 2001; Geisler et al., 2003). Double pgp1/pgp19 mutants show strongly enhanced defects suggesting overlapping functions (Noh et al., 2001; Geisler et al., 2003; Geisler et al., 2005). Interestingly, PGP1 and PGP19, as well as other family members PGP2, PGP4 and PGP10, were also isolated as proteins that bind the auxin transport inhibitor NPA in vitro (Noh et al., 2001; Murphy et al., 2002; Terasaka et al., 2005) or when expressed in yeast cells (Noh et al., 2001; reviewed by Muday and Murphy, 2002). This provided an additional link between MDR/PGP and auxin transport and suggests that these proteins could represent the so far molecularly uncharacterized NPA-binding proteins (NBP). On the other hand, membranes prepared from pgp19 mutants still exhibit up to 64% of the NPA binding activity in comparison to the wild type and still show a pronounced sensitivity of PAT to NPA. Thus, it is likely that the Arabidopsis genome also contains other proteins with NPA binding properties (Muday and Murphy, 2002) and the functional meaning of PGP binding to NPA still remains unclear. There is also an extensive body of evidence directly linking the function of several MDR/PGPs to auxin transport. It was shown that MDR1 and PGP1 proteins are capable of transporting auxins across the PM (Geisler et al., 2005; Petrášek et al., 2006). Importantly, pgp1 and pgp19 mutants exhibit a reduced net efflux of natural and synthetic auxins from Arabidopsis leaf protoplasts (Geisler et al., 2003; Geisler et al., 2005). Moreover, heterologous expression of PGP1 in yeast cells and mammalian HeLa cells resulted in increased cellular efflux of auxin and the cytotoxic IAA analog, 5-fluoro-indole, from cells (Geisler et al., 2005). Also, the inducible expression of PGP19 in tobacco BY-2 cells lead to a decrease in radioactively labeled NAA accumulation in these cells, confirming the role of this protein in auxin efflux (Petrášek et al., 2006). On the other hand, another member of the family, PGP4, seems to be involved in auxin import, as root tips of pgp4 mutants exhibit reduced auxin uptake and heterologous expression of this protein in HeLa cells resulted in an increase of auxin retention within the cells. Furthermore, expression of PGP4 in yeast cells increased their sensitivity to 5-fluoro-indole. All these data suggest the involvement of PGP4 in auxin influx (Terasaka et al., 2005; Santelia et al., 2005). The localization studies of PGP1 and PGP4 revealed that both genes are expressed in Arabidopsis roots. While the PGP1 protein is localized at the plasma membrane in a non-polar manner in the root tip, it is also polarly localized in the more proximal part of the root (on both sides of the cells in the elongation zone and enriched on upper sides of cells in the mature root) (Geisler et al., 2005). Similarly, PGP4 also exhibited non-polar localization within the root tip and polar localization in the more proximal part of the root (on upper sides of the cells in the elongation zone and lower sides of cells in mature root) (Terasaka et al., 2005). These contrasting localizations of PGP1 and PGP4 on the opposite sides of some cells are in a good accordance with different roles of PGP1 and PGP4 in auxin efflux and uptake, respectively.
Thus, the functional characterization of a number of the PGP proteins suggested that in addition to the proton gradient-driven movement of auxin across the plasma membrane, as described by the chemiosmotic hypothesis, an energized transport of auxin by ABC transporter family proteins operates in plants. It seems that PINs and PGPs define two functionally distinct auxin efflux systems (Petrášek et al., 2006), but it is hard to imagine that their roles in planta will be entirely independent. Indeed, an extensive and complicated functional interaction between PIN- and PGP-based transport systems has been recently demonstrated, but the developmental role and exact molecular basis of such interactions have been not fully clarified (Blakeslee et al., 2007).
Role of Potassium Transporter TRH1 in Polar Auxin Transport
Beside the already mentioned molecular components of PAT described above, recent data suggests the existence of additional proteins involved in auxin transport in Arabidopsis roots. A postulated carrier belonging to the KT/KUP/HAK multi-gene family is the TINY ROOT HAIR1 (TRH1) potassium transporter (Vicente-Agullo et al., 2004). The phenotype of trh1 mutant, which has affected root hair development and root gravitropism, is consistent with impaired auxin transport and can be rescued by auxin (Rigas et al., 2001; Vicente-Agullo et al., 2004). Additionally, trh1 mutants exhibit reduced efflux of radioactively labeled IAA from root segments, but increased efflux from the yeast cells expressing TRH1. TRH1 is expressed in columella and lateral root cap cells, where it is potentially involved in the redistribution of auxin important for root gravitropism and root hair development (Vicente-Agullo et al., 2004). Although the data collected so far indicate that this potassium transporter might be involved in auxin transport, the molecular mechanism of TRH1-dependent PAT still needs to be provided.
POLARITY OF AUXIN FLOW
The directionality of auxin flow is an important characteristic that distinguishes PAT from other transport processes in plants. A substantial body of evidence has demonstrated that, indeed, the polarity of PAT is a crucial feature in auxin-mediated plant development (reviewed by Friml, 2003; Tanaka et al., 2006). As mentioned, the chemiosmotic hypothesis proposed that the polarity of auxin flow is determined by the polar, subcellular localization of auxin efflux carriers (Rubery and Sheldrake, 1974; Raven, 1975). In fact, the polar subcellular localization of PIN auxin efflux carriers correlates with the known or predicted directions of auxin flow, supporting this hypothesis (Fig. 4 and 5; reviewed by Friml, 2003; Tanaka et al., 2006). In addition, the direct manipulation of PIN polarity has a clear impact on the direction of auxin transport, confirming that cellular PIN positioning is a determining factor in PAT directionality (Friml et al., 2004; Wiśniewska et al., 2006). Because comparable data for AUX1 and PGP proteins are lacking so far, the issue of PAT directionality is, at the moment, mostly about how the polar subcellular localization of PIN proteins is controlled.
Cell Type-Based Determinants of Cellular PIN Polarity
By looking at PIN expression and localization patterns it is clear that there must be cell type-specific determinants of PIN polar localization, because the same PIN proteins can be localized to various cell sides in different cell types. For example, in the primary root of Arabidopsis, PIN2 is localized predominantly at the basal (towards the root apex) side of young cortex cells, but strictly at the apical (towards the shoot apex) side of adjoining epidermis cells (Fig. 4; Müller et al., 1998; Friml et al., 2003a). Also, PIN1 is localized at the apical side of the outer cells of the shoot apical meristem, whereas it is at the basal side in vasculature cells (Gälweiler et al., 1998; Friml et al., 2002a; Benková et al., 2003; Reinhardt et al., 2003). The idea that cell type is important for PIN polar localization was further reinforced by the observation that during regeneration of the root meristem, PIN polarity only changes following respecification of cell types (Xu et al., 2006). All these observations clearly show that cell type influences the polarity of PIN localization. However, the nature of these cell type-specific determinants remains unknown (Fig. 4).
Sequence-Based Determinants of Cellular PIN Polarity
Experiments into the ectopic expression of different PINs in particular cell types indicated that PIN polar targeting is also determined by PIN-specific factors (Wiśniewska et al., 2006). The engineering of constructs with epitope-tagged PIN1, PIN2, PIN3 and PIN4 genes under transcriptional control of the PIN2 promoter revealed that in root epidermal cells, PIN1:HA was localized to the basal side of the cells, contrary to the localization of PIN2:HA or the endogenous PIN2 protein. PIN3:HA and PIN4:HA were mostly localized to both apical and basal cell sides (Wiśniewska et al., 2006). The insertion of the GFP coding sequence at a specific position in the PIN hydrophilic loop interfered with the basal PIN1 localization in the epidermal cells, and led to its reversion to the apical side. These results suggest that specific motif(s) exist within PIN sequences that contribute to the decision about PIN polar targeting. These PIN2::PIN1:HA and PIN2::PIN1:GFP lines, with the manipulated PIN1 polarity in root epidermal cells, were also used to examine the connection between PIN polarity and the direction of auxin flow. Normally, gravity stimulation in wild type roots induces PIN2-dependent unidirectional auxin flow from the root tip towards the elongation zone (Abas et al., 2006). However, while gravistimulated pin2 roots transformed with PIN1 protein localized at the apical side of the epidermal cells exhibited proper auxin translocation and gravity response, roots with PIN1 located at the basal side of epidermal cells showed neither auxin relocation, nor proper gravity response (Wiśniewska et al., 2006). Thus, the specific positioning of PIN1 at the apical side of epidermal cells was necessary to mediate upward auxin flow demonstrating a causal connection between PIN polarity and the direction of auxin flow at least for short-distance PAT. As will become apparent throughout later sections, the regulation of intercellular auxin flow by changes in PIN polarity in individual cells is a key property of PAT and allows rapid redirection of auxin fluxes by different internal and external signals.
PIN Polarity Changes Mediated by Developmental Cues
There are instances during plant development when PIN polarity rapidly changes in response to some, so far unidentified, developmental signals. These polarity changes seemingly redirect intercellular auxin flow and thus re-arrange local auxin distribution patterns during various developmental responses. For example, in the early stages of Arabidopsis embryo development, PIN7 is localized apically (towards the apical cell) in the suspensor and PIN1 is mostly non-polar in the proembryo whereas at later stages, PIN1 polarizes to the basal side and PIN7 changes its polarity from apical to basal (Fig. 5; Friml et al., 2003b). These PIN polarity rearrangements lead to the accumulation of auxin at the presumptive embryo root pole and are one of the necessary factors for root specification (Friml et al., 2003b). Similarly, during postembryonic organ formation PIN1 relocates from the anticlinal to the periclinal cell sides of developing lateral root primordia (Benková et al., 2003), and in the shoot apical meristem epidermis, its polarity is continually rearranged towards positions of incipient leaf or flower primordia (Reinhardt et al., 2003; Heisler et al., 2005). Thus, PIN polarity rearrangements are one of the earliest events in the regulation of different patterning and organogenesis processes.
PIN Polarity Changes Mediated by Environmental Cues
The polarity of at least some of PIN proteins can also be modified in response to environmental stimuli. Studies into Arabidopsis roots demonstrated that PIN3 protein relocates in response to gravistimulation (Friml et al., 2002b). Normally, PIN3 is localized uniformly at the plasma membrane of columella cells in the root tip. However, when the root is reoriented into a horizontal position, gravity-sensing statoliths in the columella cells sediment and PIN3 rapidly relocalizes to the new lower side of those cells. The asymmetric repositioning of PIN3 is followed by the redirection of auxin flow to the lower side, which then leads to auxin accumulation at the lower side of the gravistimulated root and consequently to downward root bending (Fig. 6; Friml et al., 2002b). It is also possible that a similar mechanism involving PIN relocations underlies the gravity response in shoots and phototropic responses.
Figure 6.
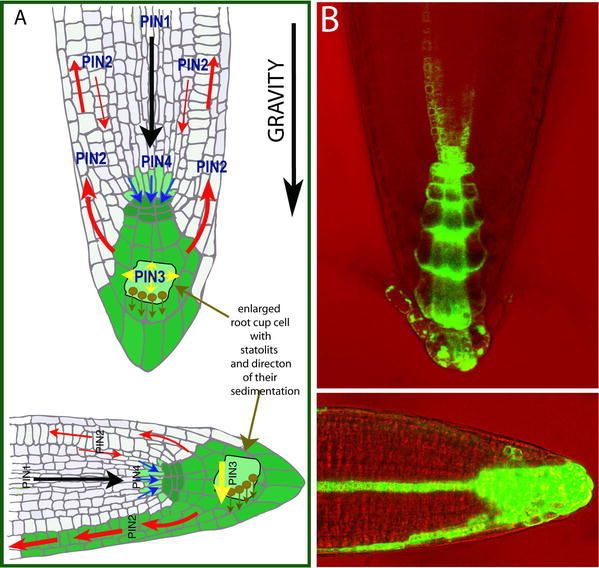
Auxin redistribution during Arabidopsis root gravity response. (A) A diagram illustrating the mature root with PIN-mediated auxin fluxes (marked by arrows) and resulting auxin accumulation (highlighted in green). Following gravistimulation, gravity-sensing statoliths sediment to the new bottom of columella cells, PIN3 relocates and mediates auxin flow downwards (yellow arrow) causing asymmetric auxin accumulation at the lower side of the root. (B) Root tips with auxin accumulation pattern indirectly marked by DR5rev::GFP before and after gravistimulation.
Phosphorylation in Control of Cellular PIN Polarity
The above mentioned examples document both the complex regulation and the considerable developmental importance of PIN polarity rearrangements. Despite this, very little is known about the molecular mechanisms that control PIN polar localization. So far, the only documented molecular component directly involved in the regulation of the subcellular localization of PIN proteins (Friml, et al., 2004) is the serine/threonine protein kinase PINOID (PID) (Christensen et al., 2000; Benjamins et al., 2001). It was shown that the overexpression of PID leads to the basal-to-apical shift of PIN1, PIN2 and PIN4 polar localization in Arabidopsis seedling roots. This apicalization of PIN localization to the upper side of root meristem cells causes auxin to flow away from the root tip, which consequently leads to the auxin depletion and root meristem collapse (Friml et al., 2004). Normal auxin accumulation and root organization can be largely restored by growing plants in the presence of auxin transport inhibitors such as NPA (Benjamins et al., 2001). In contrast to PID overexpression, pid loss-of-function alleles, both alone and to a greater degree in combination with enhancer of pinoid (enp), cause an opposite apical-to-basal shift in PIN1 polarity in the inflorescence apex and in embryonic cotyledons, which consequently leads to the depletion of auxin from the shoot apical meristem and embryo apex, causing strong organogenesis defects (Friml et al., 2004; Treml et al., 2005). Thus, it is likely that the level of PID-dependent phosphorylation influences the apical-versus-basal PIN polar targeting decision. High levels of phosphorylation cause apical targeting of PIN proteins, whereas low levels lead to basal PIN targeting, suggesting that PID acts as a binary switch regulating the direction of auxin flow (Friml et al., 2004). The manner by which PID regulates PIN polar targeting is still unknown. It may regulate the polar sorting machinery or directly phosphorylate cargos such as PIN proteins to change their affinity to apical or basal targeting pathways.
Other Factors Involved in Regulation of Polarity of PIN Localization
In addition to PINOID, several other proteins have been reported to be involved in the control of PIN localization, however in contrast to PID, more direct connections to PIN polarity control remain to be established.
An important factor for the delivery of PIN proteins to the plasma membrane is an endosomal regulator of the vesicle trafficking, GNOM, which encodes a GDP/GTP exchange factor for adenosyl ribosylation factors (ARF GEF) (Shevell et al., 1994; Geldner et al., 2001; Geldner et al., 2003). In gnom (also called emb30) mutant embryos, the coordinated polar localization of PIN1 is impaired (Steinmann et al., 1999). As a consequence, embryonic auxin gradients are not properly established (Friml et al., 2003b) and the creation of the apical-basal axis and cotyledon formation is defective (Mayer et al., 1991). A range of studies have determined GNOM as a crucial factor in the delivery of PIN proteins as well as other cargos to the plasma membrane. However, whether GNOM plays a specific role in decision on the polarity of PIN targeting remains to be seen.
Genetic and cell biological studies suggested that the proper localization of PIN proteins depends also on the sterol composition of plasma membranes (Willemsen et al., 2003; Grebe et al., 2003). The Arabidopsis mutant orc with mutations in the STEROL METHYLTRANSFERASE1 (SMT1) gene, which is involved in sterol biosynthesis, shows cell polarity defects including the impaired polar localization of PINs, accompanied with reduced auxin transport and auxin-related developmental defects (Willemsen et al., 2003). Furthermore, sterols and PIN proteins have been shown to have overlapping subcellular trafficking pathways (Grebe et al., 2003). Sterol-enriched plasma membrane microdomains, sometimes called lipid rafts, are important for various types of plasma membrane-based signaling processes and have been reported to be also present in higher plants (reviewed by Martin et al., 2005; Bhat and Panstruga, 2005). However, whether PIN proteins and/or related factors are directly associated with these structures, and what eventual functional relevance such associations have, remains unclear.
SUBCELLULAR DYNAMICS OF AUXIN CARRIERS
The classical chemiosmotic hypothesis had postulated that the PAT would be mediated by plasma membrane (PM)-resident carrier proteins (Fig. 3; Rubery and Sheldrake, 1974; Raven, 1975). However, physiological experiments suggested that efflux components of PAT undergo subcellular movement (Fig. 7; reviewed by Morris, 2000). Although hard to reconcile with the original models, the dynamic nature of auxin carriers was confirmed for PIN and AUX1 proteins by a variety of genetic and cell-biological studies.
Figure 7.
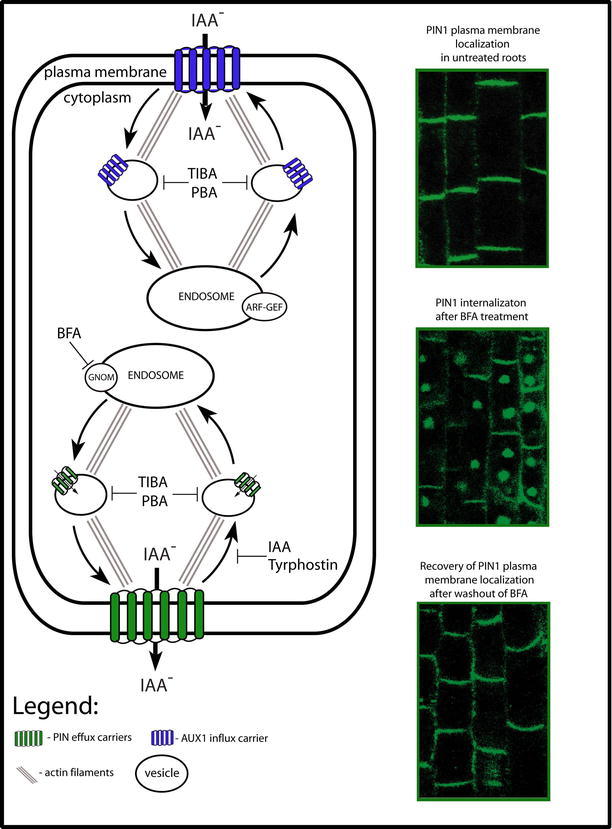
The subcellular dynamics of efflux (PINs) and influx (AUX1) carriers. This diagram illustrates a model for constitutive cycling of PIN and AUX1 proteins. Included are images of PIN1 subcellular localization (by immunolocalization). The constitutive cycling of PIN proteins between the endosomes and the plasma membrane (PM) is actin-dependent. BFA blocks the exocytosis of PINs by inhibiting GNOM, the ARF-GEF required for the vesicle budding at endosomes. This leads to the internalization of PINs into so called "BFA compartments." Endocytosis of PIN proteins is not affected by BFA, but is sensitive to clathrin pathway inhibitor Tyrphostin and IAA. Auxin efflux inhibitors such as TIBA or PBA interfere with both steps of PIN protein cycling. AUX1 also cycles constitutively between the endosomes and the PM along actin cytoskeleton. Although, AUX1 dynamics is sensitive to BFA treatment, its exocytosis is largely BFA-insensitive. TIBA and PBA interfere also with AUX1 cycling.
Constitutive Endocytic Cycling of PIN Proteins
An important tool for studies into PIN subcellular dynamics is the fungal toxin Brefeldin A (BFA), which is well known to interfere with various vesicle trafficking processes in cells. Molecular targets of BFA are GDP/GTP exchange factors for small G proteins of the ARF class (ARF-GEF). These types of ARF GEF proteins play a role in the recruitment of vesicle coats required for their budding and cargo selection in different subcellular compartments (reviewed by Donaldson and Jackson, 2000). In tobacco BY-2 cells, BFA primarily targets endoplasmatic reticulum (ER)-based ARF GEFs, which leads to the inhibition of ER-to-Golgi trafficking (Ritzenthaler et al., 2002). In Arabidopsis, on the other hand, one of the primary BFA targets is the endosomal ARF GEF, GNOM, and thus BFA preferentially inhibits endosomal recycling to the plasma membrane, whereas endocytosis from the plasma membrane remains operational (Geldner et al., 2003). This differential effect of BFA on exo- and endocytosis leads to an internal accumulation of plasma membrane proteins into so called "BFA compartments" (Fig. 7; Geldner et al., 2001; Geldner et al., 2003).
The incubation of Arabidopsis seedlings in BFA results in the rapid disappearance of PIN1 from the plasma membrane and their simultaneous aggregation inside the BFA compartments (Fig. 7; Steinmann et al., 1999). This effect also occurs in the presence of a protein synthesis inhibitor, indicating that the internalized PIN proteins come from the PM, and not as a result of de novo protein synthesis (Geldner et al., 2001). Moreover, this process seems to be fully reversible because washing BFA from the cells causes PIN proteins to relocalize to the PM (Fig. 7). Consistent with the high intracellular dynamics of PIN proteins, electron microscopy studies have detected PIN3 protein, not only at the PM, but also in intracellular vesicles (Friml et al., 2002b). Similarly in maize, PIN1 was found to internalize in response to BFA (Baluska et al., 2002; Boutte et al., 2006). These observations indirectly suggest that in several plant species PIN proteins undergo constitutive recycling between the endosomes and the plasma membrane (Geldner et al., 2001). Recent studies using photoconvertible (green-to-red) version of tagged PIN proteins directly demonstrated constitutive PIN cycling and showed that PIN internalization occurs via clathrin-dependent endocytic mechanism (Dhonukshe et al., 2007a).
Genetic evidence for intracellular trafficking of PIN proteins came with the analysis of the Arabidopsis mutant gnom (also called emb30) isolated in a screen for defects in early apical-basal patterning (Mayer et al., 1991; Patton et al. 1991). gnom mutants show a variety of developmental defects. Seedlings always lack a root and also show pronounced defects in the development of apical structures. The most severely affected gnom seedlings display a ball-like structure without a recognizable apical-basal axis (Mayer et al., 1991). These developmental defects are reminiscent of auxin- or auxin transport-related defects. GNOM encodes an ARF GEF, (Shevell et al., 1994; Busch et al., 1996; Steinmann et al., 1999) and the connection between the role of GNOM in vesicle trafficking and auxin biology was established by the observation that in gnom mutant embryos, the coordinated polar localization of PIN1 is affected (Steinmann et al., 1999). Similarly, in weak gnom alleles, PIN1 polar localization is defective in developing lateral root primordia (Geldner et al., 2004). These observations suggest that the GNOM protein is a BFA-sensitive regulator of PIN1 subcellular localization. Growing plants on medium supplemented with BFA affects root and hypocotyl gravitropism and elongation as well as lateral root initiation; all processes related to auxin transport (Geldner et al., 2001). A series of elegant experiments involving an engineered BFA-resistant version of GNOM, clearly established that GNOM is the endosome-localized regulator of PIN1 exocytic delivery from the endosomes to the PM (Geldner et al., 2003). However, it remains unclear whether GNOM is specifically involved in polar exocytosis or in a broader range of exocytic processes. In summary, these data showed that PIN proteins constitutively cycle between endosomes and the PM in a GNOM-dependent manner (Fig. 7). These findings nicely complemented earlier physiological studies on the importance of BFA-sensitive vesicle trafficking for the process of auxin transport (Delbare et al., 1998; Morris and Robinson, 1998).
AUX1 Constitutive Cycling
The cell biological determinants of auxin influx have received less attention over the years than auxin efflux. However, with the finding that the AUX1 auxin influx carrier can also display polar, subcellular localization in certain cell types such as root protophloem cells (Fig. 4; Swarup et al., 2001) and shoot apical meristem (Reinhardt et al., 2003), the question of how AUX1 gets delivered to these destinations was addressed. AUX1 subcellular distribution, similar to that of PIN1, is sensitive to BFA treatment (Grebe et al., 2002). However, in contrast to PIN1, it seems that only the intracellular pool of AUX1 is BFA-sensitive, but the delivery of AUX1 to the PM is largely BFA insensitive and does not require the ARF GEF, GNOM (Fig. 7; Kleine-Vehn et al., 2006). Additionally, AUX1 and PIN1 trafficking vary in their sensitivities to different trafficking inhibitors (Kleine-Vehn et al., 2006) and only AUX1, but not PIN, trafficking is dependent on the novel endoplasmic reticulum-localized protein, AUXIN RESISTANT 4 (AXR4) (Dharmasiri et al., 2006). All these studies demonstrated that AUX1 trafficking utilizes a different mechanism from PIN proteins, providing an additional level for the cellular regulation of PAT.
Auxin Transport Inhibitors and Vesicle Trafficking
Auxin transport inhibitors (ATIs) have for decades been essential tools for manipulating plant development and studying mechanism of auxin transport. They seem to share only limited structural homology and may have several so far unidentified molecular targets (Katekar and Geissler, 1977). Some of the important representatives of this class of herbicides, such as TIBA or PBA, not only inhibit auxin efflux but also interfere with the subcellular trafficking of PIN and AUX1 proteins and, unexpectedly, with other PAT-unrelated trafficking processes (Geldner et al., 2001; Kleine-Vehn et al., 2006). PBA and TIBA interfere with organelle motility and the movement of individual vesicles probably by inhibiting actin cytoskeleton dynamics (Fig. 7; Dhonukshe et al., 2007b). The cell biological effects of these herbicides are not only restricted to plants but were also detected in yeast and mammalian cells, suggesting a common, evolutionarily conserved target (Dhonukshe et al., 2007b). The genetic manipulation of actin dynamics and the chemical stabilization of actin filaments mimic the ATI effect on actin cytoskeleton dynamics and also inhibit auxin efflux, auxin translocation and auxin transport-dependent physiological responses (Dhonukshe et al., 2007b). These results, together with the known importance of subcellular dynamics for auxin transport, suggest that the effects of ATIs on actin cytoskeleton dynamics underlie their effect on auxin efflux. The data are, however, so far not conclusive. The molecular target of ATIs that are relevant for the inhibition of auxin efflux has not been unequivocally identified, and the concentrations needed for the cell biological effects are higher than those required for the physiological effects on auxin transport (Dhonukshe et al., 2007b). Therefore, it is possible that effect of ATIs on actin dynamics and auxin efflux reflect two independent molecular targets.
Functional Importance of Constitutive Cycling
The finding that auxin transport components undergo intense subcellular dynamics was surprising and was not predicted by classical models of auxin transport, such as the chemiosmotic hypothesis (Fig. 3 and 7). A crucial question remains - what is the functional role of this constitutive cycling? One could envision several scenarios:
1. The first scenario assumes that constitutive trafficking of PIN proteins would provide a required flexibility for the fast repositioning of PIN carriers to other sides of the cell. This could allow for the rapid redirection of auxin flow in response to environmental or developmental cues (Friml, 2003; Morris, 2000). Indeed rapid PIN relocations were observed during embryonic development (Friml et al., 2003b), lateral root formation (Benková et al., 2003), phyllotaxis (Heisler et al., 2005) and during root gravity response (Friml et al., 2002b). In the latter, PIN relocation occurs within a few minutes after gravity stimulation, thus necessitating a rapid subcellular dynamic. However, the mechanism underlying the rapid PIN polarity changes is unknown and whether it utilizes transcytosis (internalization and subsequent retargeting to the other side of cell) remains to be demonstrated.
2. The second scenario proposes that components of PAT may have a double receptor/transporter function (Hertel, 1983) and cycling would be part of a mechanism for signal transduction and receptor regeneration, in analogy to ligand-dependent endocytosis known for receptors in the animal field (reviewed by Barnes, 2000; Foti et al., 2004; Holler and Dikic, 2004). Dual sensor and transport functions have already been proposed for sugar carriers in yeast and plants (Lalonde et al., 1999).
3. From non-plant models, it is known that constitutive cycling can be used to regulate protein activity by determining the amount of protein present at the cell surface (Royle and Murrell-Lagnado, 2003). This mechanism is known to regulate glucose uptake by insulin, and water uptake by vasopressin. Indeed, in plants, PIN recycling is modulated by auxin itself, thus providing a mechanism for the feedback regulation of auxin transport (Paciorek et al., 2005).
4. The fourth scenario proposes that auxin efflux could be mechanistically analogous to the transport of animal neurotransmitters. In this situation, components of auxin transport could constitutively recycle through the cell in vesicles and facilitate the loading and subsequent release of auxin from cells by polar exocytosis (Friml and Palme, 2002; Baluška et al., 2003). Indeed, it seems that PIN proteins are also functional inside the cell since yeast cells expressing an internalized version of PIN2 accumulate more auxin than control cells (Petrášek et al., 2006). It is also not clear exactly what the subcellular distribution of auxin is. Recent results based on immunolocalization of auxin indicated that at least some auxin can be found in BFA-sensitive subcellular compartments (Schlicht et al., 2006), however proper controls and independent confirmation for this important observation are lacking. On the other hand, in transport studies in cultured cells, the amount of PIN protein at the cell surface correlates with auxin efflux, favoring the scenario that PINs function at the plasma membrane (Paciorek et al., 2005). Thus the analogy with neurotransmitter release, however intriguing, is not supported so far by conclusive experimental data.
In summary, it seems likely that the constitutive recycling of auxin transport components may be important for the regulation of the directionality and throughput of auxin flow. Whether it is also a part of a neurotransmitter release-like mechanism and/or receptor-like function remains to be seen.
FEEDBACK REGULATIONS IN AUXIN TRANSPORT
Some physiological effects of auxin, especially those related to the differentiation of new vasculature such as leaf venation, vasculature regeneration and the flexible creation of new vasculature from places of organ formation, require de novo polarization of cells within the polarizing tissue. Attempts to explain how polarizing cells perceive their position within tissue, and how they could recognize their orientation relative to the rest of the plant body, led to the formulation of the canalization hypothesis (Sachs, 1981).
This theory suggests that in the population of initially homogenous, auxin-transport incapable cells, auxin would, by a positive feedback, induce the capacity and polarity of its own transport. This would gradually rearrange cell polarity and reinforce neighbor cells to repolarize in the same way. Ultimately, new auxin conductive channels could be established, determining the position of new vascular strands.
This intriguing hypothesis, and other auxin-dependent self-organizing models (de Reuille et al., 2006; Jönsson et al., 2006; Smith et al., 2006), however, strictly require the existence of positive feedback regulations between auxin signaling and the capacity and polarity of auxin transport. These feedback mechanisms can in theory involve auxin-dependent regulation of transcription, degradation and/or subcellular localization of auxin transport components (Fig. 8).
Figure 8.
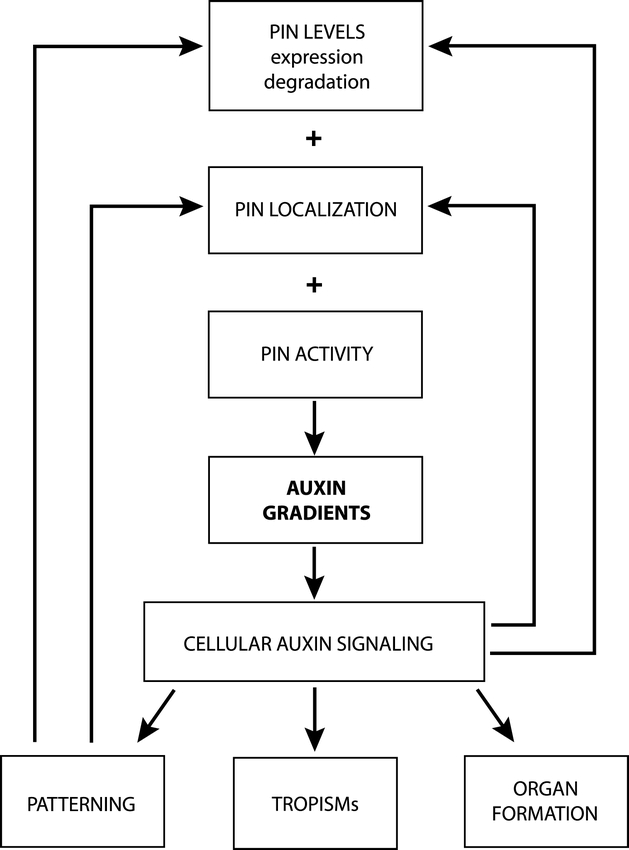
Possible feedback regulations in the generation of auxin distribution. PIN-based auxin transport (depending on PIN levels, cellular localization and activity) mediates asymmetric local auxin distribution. Auxin accumulation within cells is interpreted by cellular auxin signaling and translated into a given developmental response. Auxin feedback regulates its own transport at the levels of PIN transcription, PIN stability and PIN localization. In addition, cell type-based determinants influence PIN expression and polar localization. Adapted from Tanaka et al (2006).
Auxin-Dependent Regulation of PIN Transcription: Cross-Regulations in PIN Functional Redundancy
The analysis of the growth and development of various single and multiple pin mutants suggested that functional redundancy occurs between different members of the PIN gene family (Benková et al., 2003; Friml et al., 2003b; Blilou et al., 2005). This was, however, difficult to reconcile with the only partially overlapping expression patterns of most PIN genes. For example, comparisons of multiple pin mutants showed that pin1 3 4 7 early mutant embryos are more affected than pin1 7 embryos, even though PIN3 and PIN4 in wild type are not normally expressed during these early stages of embryogenesis (Friml et al., 2003b; Vieten et al., 2005). Such observations could not be explained by the conventional mechanism of genetic redundancy and suggested a functional cross-regulation between different PIN genes. Indeed, for example, the ectopic expression of PIN4 was detected in the normal PIN7 domain of early pin7 mutant embryos (Vieten et al., 2005). Studies of multiple pin mutant phenotypes and PIN expression in primary roots of pin mutants revealed a similar type of cross-regulation (Blilou et al., 2005; Vieten et al., 2005; Paponov et al., 2005). For example, in roots of pin2 mutants, PIN1 was ectopically expressed in vacant PIN2 domain, whereas in pin1 mutant roots, PIN2 and PIN4 were ectopically expressed in the PIN1 domain (Vieten et al., 2005). These results suggested that PIN function is directly or indirectly connected to the control of PIN expression. Since pin mutants also show altered patterns of auxin distribution (Luschnig et al., 1998; Friml et al., 2002a; Friml et al., 2003b), the cross-regulation of PIN expression may be related to the effect of auxin on PIN expression. Indeed, the treatments of Arabidopsis roots with auxin resulted in the ectopic up-regulation of PIN gene expression, suggesting that auxin itself can influence the transcription of PINs. The response to auxin treatment seems to be tissue-specific as well as time and concentration-dependent, and requires the Aux/IAA-ARF auxin signaling pathway (Vieten et al., 2005).
This feedback regulation of PIN expression by auxin itself provides a self-repairing backup mechanism in auxin distribution and fully explains the ectopic up-regulation of PIN genes in specific expression domains of pin mutants (Vieten et al., 2005).
Auxin-Dependent Post-Transcriptional Regulation of PIN Stability
It appears that auxin modulates PIN levels, not only on the transcriptional level but also post-transcriptionally, regulating the stability of PIN proteins (Fig. 8; Sieberer et al., 2000; Vieten et al., 2005). Whereas auxin, up to certain concentration, increases the level of PIN transcription, this effect is counteracted at higher auxin concentrations by a decrease in protein stability as shown for PIN1, PIN2 and PIN7 proteins (Sieberer et al., 2000; Vieten et al., 2005). Genetic and pharmacological studies indicated that the mechanism of regulated PIN degradation involves the activity of the proteosome and components of the ubiqui-tin ligation machinery, AUXIN RESISTANT 1 (AXR1). In vivo studies on root gravitropism suggested that a fine-tuned, auxin-mediated regulation of PIN2 stability is functionally involved. Gravity stimulation leads to asymmetric auxin flow at the root tip, which results in auxin accumulation at the lower side of roots when turned on their sides (for more details see Tropisms below). A detailed examination of PIN2 protein in cells during this process revealed that the protein was gradually internalized and depleted only at the upper side of the root tip. This differential proteosome-dependent regulation of PIN2 stability between the upper and lower sides of gravistimulated roots most likely leads to an increased capacity of PIN2-dependent auxin flow along the lower side of the root when compared with the upper side (Abas et al., 2006). Auxin treatment interferes with this differential PIN2 internalization and degradation indicating that asymmetry in auxin distribution following gravistimulation may mediate this effect (Abas et al., 2006). However the precise cellular and molecular mechanism remains unclear.
Auxin Effect on PIN Internalization: Feedback Regulation of Auxin Efflux
An unexpected mode of auxin action on PAT relates to its effect on subcellular protein trafficking. It has been shown that both exogenously applied and endogenously produced auxin specifically inhibits endocytosis, as evidenced by a reduced uptake of endocytic tracers or the inhibited internalization of several plasma membrane proteins including PINs (Paciorek et al., 2005). By inhibiting PIN internalization, auxin increases levels of PINs at the plasma membrane, and thus directly stimulates its own efflux from cells. This provides a mechanism for a positive feedback regulation of auxin transport by auxin itself. The inhibition of endocytosis by auxin requires the activity of a large Arabidopsis protein BIG (Gil et al., 2001), which is a single copy homologue of the Calossin/Pushover protein in Drosophila, where it regulates multiple cellular processes including synaptic transmission. It still remains unclear which pathway auxin acts through to exert this effect on endocytosis, and it is quite possible that it will not involve a known TIR1-Aux/IAA-ARF auxin-dependent gene expression pathway. It is intriguing to speculate that auxin may also influence other physiological responses, such as cell elongation, by targeting the subcellular trafficking of other proteins such as the plasma membrane H+-ATPase, and thereby mediating the acidification and loosening of the cell wall.
Auxin-Dependent Regulation of PIN Polar Targeting
A crucial aspect of the canalization hypothesis that is necessary in order to establish a new polarity of auxin flow in a field of unspecified cells, is a feedback regulation on the directionality of auxin flow by auxin itself (Sachs, 1981). A similar condition is also required by several mathematical models that describe phyllotactic patterning in shoot apical meristems (de Reuille et al., 2006; Jönsson et al., 2006; Smith et al., 2006). An obvious cellular mechanism for such an effect would be the auxin-dependent regulation of PIN polar targeting (Fig. 8). Indeed, remarkable rearrangements in PIN polarity have been observed during developmental processes involving the coordinated repolarization of cells within tissues such as vascular tissue differentiation and regeneration (Scarpella et al., 2006; Sauer et al., 2006), or lateral roots, leaf and flower initiation (Benkova et al., 2003; Reinhardt et al., 2003; Heisler et al., 2005).
An elegant study on auxin distribution and transport during the formation of vein patterns in developing Arabidopsis leaves revealed a fine interplay between auxin accumulation and the establishment of PIN polarity (Scarpella et al., 2006). PIN1 is first expressed in a broad zone at places of auxin accumulation, but as the leaf matured, this initially wide pattern progressively narrows down. The external application of IAA on the edge of a leaf is also sufficient to induce the strong up-regulation of PIN1 expression in the vicinity of auxin source, which also gradually narrows away from the source and coincides ultimately with the establishment of an ectopic vasculature (Scarpella et al., 2006). Along with a progressive formation of PIN1-expressing channels, the polarity of PIN1 protein within the cells was also gradually oriented away from the auxin source. Thus, it seems that auxin functions as the inductive signal, which, through the regulation of PIN1 expression and polarity, defines routes for the formation of new vasculature strands (Scarpella et al., 2006).
A similar mechanism seems to operate during the regeneration of vasculature following wounding. The sub-cellular localization of PIN1 protein was monitored in pea epicotyls following partial wounding that bisected the central vasculature (Sauer et al., 2006). After wounding, PIN1 became ectopically expressed in an expanded area above the wound. This expression later gradually narrowed to a single cell file that bypassed the wound in order to establish a new vasculature pathway. Interestingly, the polarity of PIN1 localization progressively changed from lateral, above the wound, to basal at the level of the wound, and again lateral towards the old vasculature below the wound. The channel of PIN1-expressing cells, and the gradual establishment of PIN1 polarity, both preceded and determined the position and polarity of the regenerated vascular strand (Sauer et al., 2006). Auxin build-up seems to be the inductive signal, as additional auxin applied to the side of the epicotyl was sufficient to induce the ectopic expression of PIN1, with PIN1 protein polarized away from the source, ultimately leading to the formation of new vascula-ture (Sauer et al., 2006). Together, these data imply that, as predicted by the canalization hypothesis, an auxin-dependent effect on PIN expression and polarity occurs during de novo vasculature formation.
Additional studies employing auxin application onto the Arabidopsis root meristem revealed a time- and concentration-dependent repositioning of PIN1 protein from the basal to inner lateral sides of endodermis and pericycle cells. On the other hand, in cortex cells, endogenous PIN2 and ectopically expressed PIN1 relocated to the outer lateral side suggesting that the auxin effect on PIN polarity is modulated by cell type-specific factors (Sauer et al., 2006). This system was also used to address the molecular mechanism by which auxin influences PIN polarity. Genetic and pharmacological studies revealed that the Aux/IAA-ARF auxin signaling pathway is required for the auxin effects on PIN polarity, as, for example, in certain dominant stabilized aux/iaa mutants (e.g. axr3/iaa17), or in specific arf mutant combinations e.g. in arf 7,16,19 or arf 7,19/ARF17ox (Sorin et al., 2005), the auxin-induced retar-geting of PINs was impaired (Sauer et al., 2006). However, the components downstream of ARFs, which mediate the effect on PIN polarity, have not been identified so far.
In contrast, studies into Arabidopsis root meristem regeneration after the ablation of the root quiescent center (QC) did not suggest that auxin redistribution directly affects PIN polarity (Xu et al., 2006). The analysis of events happening after the disruption of auxin flow showed that auxin levels were rapidly up-regulated in cells above the ablated QC, which then induced cell-fate changes in this region. Several PIN genes, such as PIN1 and PIN4, were strongly down-regulated in the place where the new auxin maximum formed and their expression was shifted to the more proximally positioned cells with unchanged polarity. Only after renewed specification of the QC did PIN4 become expressed de novo, and the protein correctly polarized in these cells. Thus it seems that in the context of root meristem regeneration, auxin primarily influences cell-fate changes and only after proper cell specification, changes in PIN polarity are facilitated (Xu et al., 2006). These results suggest that classical canalization theory applies only to certain developmental situations in plants.
DEVELOPMENTAL ROLES OF LOCAL AUXIN DISTRIBUTION
PAT-dependent auxin distribution and the spatio-temporal accumulation of auxin in specific plant tissues underpins a number of plant developmental processes like embryonic axis formation, organogenesis, meristem maintenance, tropisms and many others. In this section a few selected examples of processes that depend on PIN-mediated auxin gradients will be presented to illustrate the developmental relevance of the above described mechanisms.
Transport-Dependent Auxin Distribution in Embryo Patterning
Embryogenesis is an elementary developmental process during which a single-celled zygote is transformed into a mature embryo that contains the principal elements of the future plant. The basic plant body is generated along the apical-basal axis of a developing embryo, with the shoot meristem forming at the apical end and the root meristem forming at the basal end (reviewed by Jürgens, 2001). The monitoring of auxin distribution and PIN expression, together with pharmacological and molecular genetic experiments in Arabidopsis, revealed that PIN-dependent auxin distribution significantly contributes to the crucial steps in embryo development, such as apical cell specification, root initiation and cotyledon formation (Friml et al., 2003b).
After the first asymmetric division of the zygote, PIN7 becomes expressed in the basal cell and its derivative suspensor cells. In these cells, PIN7 protein is apically localized and directs auxin flow towards the smaller apical cell, which accumulates more auxin than the suspensor cells and becomes specified as the founder of the proembryo. PIN1 is also present during early embryo stages but only in proembryo cells. Initially, the polarity of PIN1 is not obvious but newly synthesized PIN1 becomes localized into the inner cell membranes as they form. At the young globular stage, PIN1 localization becomes suddenly polarized towards the basal sides of provascular cells, mediating auxin flow towards the region of the future root pole. Simultaneously, PIN7 in the suspensor cells, changes its polarity from apical to basal, aiding PIN1 in redirecting auxin flow. Subsequently, PIN4 expression starts at the basal pole of the embryo. As a consequence of the rearrangements in PIN polarity, auxin accumulates at the basal part of embryo with a maximum in the upper sus-pensor cell which ultimately gives rise to the root meristem (Fig. 5; Friml et al., 2003b).
This coordinated PIN-dependent differential auxin distribution is crucial for proper embryo development. Pharmacological interference with PAT leads to changes in the pattern of auxin distribution and results in drastic developmental aberrations in Arabidopsis, Brassica and wheat embryos (Liu et al., 1993; Hadfi et al., 1998; Fischer-Iglesias et al., 2001; Friml et al., 2003b). Similarly, the genetic-based disruption of PIN-dependent auxin transport, which occurs in embryos of gnom or multiple pin mutants, leads to aberrant auxin distribution pattern and pronounced defects in apical-basal axis formation. In the strongest cases, no apical-basal axis is established (Friml et al., 2003b; Steinmann et al., 1999; Mayer et al., 1991; Weijers et al., 2005). Furthermore, overexpression of PINOID during embryogenesis leads to a basal-to-apical shift of PIN1 polarity and results in the accumulation of auxin uniformly in apical regions, instead of the root pole. As a consequence, strong embryo patterning defects including the incorrect specification of the root pole and cotyledons occur (Friml et al., 2004). These results demonstrate that PIN-dependent auxin flow, first to the apical and later to the basal pole of the embryo contribute to the specification of apical and later basal ends of the apical-basal axis (Fig. 5). It is clear, however, that other, so far unidentified signals, are contributing to these processes (Weijers et al., 2006).
Organogenesis
Embryo development results in the establishment of the basic body plan of the developing seedling, but the adult plant form largely depends on postembryonic organogenesis, such as the formation of new leaves, flowers, flower organs and lateral roots. The data collected so far indicate that PIN-dependent auxin transport and the resulting local auxin distributions also play an important role in these postembryonic events. The local accumulation of auxin was detected at the initiation sites of incipient organs in the shoot and root (Benková et al., 2003) and the local application of auxin was sufficient to trigger new leaf or flower formation in the shoot apex (Reinhardt et al., 2000), and the initiation of lateral roots (Laskowski et al., 1995). PIN expression and polar localization correlates with auxin accumulations patterns, and the disruption of auxin transport by genetic or pharmacological means blocks organ formation, indicating that PAT is critical for organ initiation (Okada et al., 1991; Benková et al., 2003). One very interesting aspect of leaves and flowers formation is their initiation in highly predictable phyllotactic patterns. It seems that PIN-dependent auxin transport is a mechanism underlying the generation of this pattern. PIN1 localized in the outermost layer (L1) of the shoot meristem undergoes dynamic polarity changes towards each new place of auxin accumulation (Reinhardt et al., 2003; Heisler et al., 2005). PIN1 then transports more auxin towards that point forming a temporary auxin maximum, which consequently triggers organ initiation (Benková et al., 2003; Reinhardt et al., 2003; Heisler et al., 2005; de Reuille et al., 2006; Smith et al., 2006). This also causes the temporary depletion of auxin around each emerging primordium, inhibiting the initiation of new primordia in the vicinity. In some models it is proposed that primordia can then only occur at points furthest from the absorbing influences of the immediately preceding organs (Reinhart et al., 2003), in others the regulated transport of auxin from the new primordia back toward the shoot meristem provides the location for the next primordium (Jönsson et al., 2006). In either case, the PIN-dependent generation of local auxin concentration gradients seems to be sufficient to create the distinctive phyllotactic patterns observed in plants (Reinhardt et al., 2003; Heisler et al., 2005). Interestingly, recent reports have demonstrated that computer simulations can generate phyllotactic patterns of auxin accumulation and organ formation using experimentally obtained parameters and the assumption that auxin distribution also influences PIN polarity (Smith et al., 2006; Jönsson et al., 2006).
In roots, auxin also accumulates by an auxin transport-dependent process in the presumptive founder cells of lateral root primordia, but the exact regulation of these events are still unknown (Benková et al., 2003). Once the site of organ initiation becomes selected and a primordium starts to form, PIN1 polar localization is rearranged forming a new direction of auxin flow that defines the growth axis of the developing organ. The creation and maintenance of the auxin gradient with its maximum at the tip of the new organ is crucial for proper primordium outgrowth (Fig. 2; Benková et al., 2003). In the aerial organs, auxin is transported through the outer layers towards the tip of the developing primordia and then into the interior part of the organ.
In lateral roots, however, the transport of auxin to the tip goes through the inner cells and then is distributed away through the primordium surface (Benková et al., 2003). Thus, despite the diversity of postembryonic plant organs, and differences in the direction of auxin circulation, PIN-dependent auxin flux and auxin distribution appears to be indispensable for the proper formation and outgrowth of all these organs.
Tropisms
Tropisms are continual growth responses to external, environmental stimuli such as light (phototropism) and gravity (gravitropism) generated by differential elongation rates on either side of the plant organ. The Cholodny-Went hypothesis, which was formulated in early years of the last century, proposed that this differential organ growth is caused by the asymmetric distribution of auxin. The accumulation of auxin on one side of an organ promotes (in shoots) or inhibits (in roots) growth and elongation leading to different bending responses (Went, 1974). Indeed, patterns of asymmetric auxin or auxin response distribution were observed in diverse plant organs, such as gravity stimulated tobacco shoots (Li et al., 1991), gravity-stimulated roots (Luschnig et al., 1998), light and gravity stimulated Arabidopsis hypocotyls (Fig. 2; Friml et al., 2002b) and developing peanut gynophores (Moctezuma et al., 1999). The pharmacological or genetic disruption of auxin transport inhibits both asymmetric auxin redistribution and plant tropic responses (Li et al., 1991; Luschnig et al., 1998; Friml et al., 2002b; Esmon et al., 2006) demonstrating that PAT mediates asymmetric auxin distribution for organ bending. In Arabidopsis, PIN3 is required for tropic responses as pin3 mutants are partially defective in shoot photo- and gravitropism and root gravitropism. PIN3 is localized predominantly at the inner lateral sides of endodermal cells of shoot, where its perfectly positioned to mediate lateral redistribution of auxin (Friml et al., 2002b). PIN3 also seems to link the gravity perception and lateral auxin redistribution during root gravitropism. In roots growing straight down, PIN3 protein is localized without any apparent polarity in columella cells, which contain gravity-sensing statoliths. During the gravistimulation, PIN3 follows the sedimenting statoliths and rapidly relocates to the new bottom side of the columella cells. This suggests that statolith sedimentation promotes the change in PIN3 polarity, and thus asymmetric auxin distribution (Fig. 6; Friml et al., 2002b). The precise connection between statolith sedimentation and PIN3 relocation remains unclear, but this process requires the adenosine kinase ADK1 (Young et al., 2006). Auxin accumulating at the new lower side of the root cap is further translocated basipetally to responsive cells in the root elongation zone by the combined action of auxin influx (AUX1) and efflux (PIN2) carriers (Müller et al., 1998; Luschnig et al., 1998; Swarup et al., 2001; Swarup et al., 2005). The forming auxin asymmetry is further reinforced by the asymmetric upregulation and stabilization of PIN2 along the basipetal route, whereas, at the upper root side, PIN2 becomes internalized in cells and is seemingly degraded (Fig. 6; Abas et al., 2006). Other components of auxin transport such as PGP4 and PGP19 ABC transporters are also involved in gravitropic responses, as pgp19 mutants show a hypergravitropic hypocotyl phenotype (Noh et al., 2003) and pgp4 mutants exhibit reduced gravitropic root bending (Terasaka et al., 2005). It still remains unclear whether these proteins are directly involved in asymmetric auxin distribution or mediate gravitropism by another mechanism (Terasaka et al., 2005; Geisler et al., 2005). Whatever the details of the process are, it seems clear that regulated auxin transport is a critical part of the mechanism by which external signals including gravity are translated into specific developmental responses via redirecting auxin fluxes and modulating auxin distribution patterns.
Footnotes
Citation: Michniewicz M., Brewer P.B., and Friml J. (2007) Polar Auxin Transport and Asymmetric Auxin Distribution. The Arabidopsis Book 5:e0108. doi:10.1199/tab.0108
elocation-id: e0108
Published on: August 21, 2007
REFERENCES
- Abas L., Benjamins R., Malenica N., Paciorek T., Wisniewska J., Moulinier-Anzola J. C., Sieberer T., Friml J., Luschnig C. Intracellular trafficking and pro-teolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 2006;85(1):249–256. doi: 10.1038/ncb1369. [DOI] [PubMed] [Google Scholar]
- Avsian-Kretchmer O., Cheng J. C., Chen L., Moctezuma E., Sung Z. R. Indole acetic acid distribution coincides with vascular differentiation pattern during Arabidopsis leaf ontogeny. Plant Physiol. 2002;1305(1):199–209. doi: 10.1104/pp.003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. A. Long-distance vascular transport of endogenous hormones in plants and their role in source: sink regulation. Israel J. Plant Sci. 2000;485(1):199–203. [Google Scholar]
- Baluška F., Hlavacka A., Samaj J., Palme K., Robinson D. G., Matoh T., McCurdy D. W., Menzel D., Volkmann D. F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol. 2002;1305(1):422–431. doi: 10.1104/pp.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F., Samaj J., Menzel D. Polar transport of auxin: carrier-mediated flux across the plasma membrane or neurotransmitter-like secretion? Trends Cell Biol. 2003;135(1):282–285. doi: 10.1016/s0962-8924(03)00084-9. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Jr. Intracellular trafficking of GABA (A) receptors. Life Sci. 2000;665(1):1063–1070. doi: 10.1016/s0024-3205(99)00469-5. [DOI] [PubMed] [Google Scholar]
- Benjamins R., Malenica N., Luschnig C. Regulating the regulator: the control of auxin transport. Bioessays. 2005;275(1):1246–1255. doi: 10.1002/bies.20322. [DOI] [PubMed] [Google Scholar]
- Benjamins R., Quint A., Weijers D., Hooykaas P., Offringa R. The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development. 2001;1285(1):4057–4067. doi: 10.1242/dev.128.20.4057. [DOI] [PubMed] [Google Scholar]
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;1155(1):591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- Bennett M. J., Marchant A., Green H. G., May S. T., Ward S. P., Millner P. A., Walker A. R., Schulz B., Feldmann K. A. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science. 1996;2735(1):948–950. doi: 10.1126/science.273.5277.948. [DOI] [PubMed] [Google Scholar]
- Bernasconi P., Patel B. C., Reagan J. D., Subramanian M. V. The N-1-naphthylphthalamic acid-binding protein is an integral membrane protein. Plant Physiol. 1996;1115(1):427–432. doi: 10.1104/pp.111.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalerao R. P., Bennett M. J. The case for mor-phogens in plants. Nat. Cell Biol. 2003;55(1):939–43. doi: 10.1038/ncb1103-939. [DOI] [PubMed] [Google Scholar]
- Bhat R. A., Panstruga R. Lipid rafts in plants. Planta. 2005;2235(1):5–19. doi: 10.1007/s00425-005-0096-9. [DOI] [PubMed] [Google Scholar]
- Blakeslee J. J., Peer W. A., Murphy A. S. Auxin transport. Curr. Opin. Plant Biol. 2005;85(1):494–500. doi: 10.1016/j.pbi.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Blakeslee J., Bandyopadhyay A., Lee O-K., Mravec J., Boosaree Titapiwatanakun B., Sauer M., Makam S., Cheng Y., Bouchard R., Adamec J., Geisler M., Nagashima A., Sakai T., Martinoia E., Friml J., Peer W., Murphy A. Interactions among PIN-FORMED and P-Glycoprotein Auxin Transporters in Arabidopsis. 2007. Plant Cell published on line 19. 1. 2007. [DOI] [PMC free article] [PubMed]
- Blilou I., Xu J., Wildwater M., Willemsen V., Paponov I., Friml J., Heidstra R., Aida M., Palme K., Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;4335(1):39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- Boutté Y., Crosnier M. T., Carraro N., Traas J., Satiat-Jeunemaitre B. The plasma membrane recycling pathway and cell polarity in plants: studies on PIN proteins. J. Cell Sci. 2006;1195(1):1255–1265. doi: 10.1242/jcs.02847. [DOI] [PubMed] [Google Scholar]
- Busch M., Mayer U., Jurgens G. Molecular analysis of the Arabidopsis pattern formation of gene GNOM: gene structure and intragenic complementation. Mol. Gen. Genet. 1996;2505(1):681–91. doi: 10.1007/BF02172979. [DOI] [PubMed] [Google Scholar]
- Cambridge A., Morris D. Transfer of exogenous auxin from the phloem to the polar auxin transport pathway in pea (Pisum sativum L.). Planta. 1996;1995(1):583–588. [Google Scholar]
- Casimiro I., Marchant A., Bhalerao R. P., Beeckman T., Dhooge S., Swarup R., Graham N., Inze D., Sandberg G., Casero P. J., Bennett M. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell. 2001;135(1):843–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Hilson P., Sedbrook J., Rosen E., Caspar T., Masson P. H. The Arabidopsis thaliana AGRAV-ITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc. Natl. Acad. Sci. U.S.A. 1998;955(1):15112–15117. doi: 10.1073/pnas.95.25.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholodny N. Wuchshormone und Tropismen bei den Pflanzen. Biol. Zentralbl. 1927;475(1):604–629. [Google Scholar]
- Christensen S. K., Dagenais N., Chory J., Weigel D. Regulation of auxin response by the protein kinase PINOID. Cell. 2000;1005(1):469–478. doi: 10.1016/s0092-8674(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Cox D. N., Muday G. K. NPA binding-activity is peripheral to the plasma membrane and is associated with the cytoskeleton. Plant Cell. 1994;65(1):1941–1953. doi: 10.1105/tpc.6.12.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C., Darwin F. The power of movement in plants.(German translation:Das Bewegungsvermögen der Pflanze). Darwins gesammelte Werke, Vol.13. Schweizer-bart'sche Verlagsbuchhandlung; Stuttgart: 1881. [Google Scholar]
- Davies P. J. Plant Hormones: biosynthesis, signal transduction, action! Kluwer Academic Publishers; Dordrecht, The Netherlands: 2004. [Google Scholar]
- Davies P. J., Rubery P. H. Components of auxin transport in stem segments of Pisum sativum. Planta. 1978;1425(1):211–219. doi: 10.1007/BF00388215. [DOI] [PubMed] [Google Scholar]
- de Reuille P. B., Bohn-Courseau I., Ljung K., Morin H., Carraro N., Godin C., Traas J. Computer simulations reveal properties of the cell-cell signaling network at the shoot apex in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 2006;1035(1):1627–1632. doi: 10.1073/pnas.0510130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre A., Muller P., Guern J. Short-lived and phosphorylated proteins contribute to carrier-mediated efflux, but not to influx, of auxin in suspension-cultured tobacco cells. Plant Physiol. 1998;1165(1):833–844. doi: 10.1104/pp.116.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbarre A., Muller P., Imhoff V., Guern J. Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta. 1996;1985(1):532–541. doi: 10.1007/BF00262639. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N., Dharmasiri S., Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;4355(1):441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- Dharmasiri S., Swarup R., Mockaitis K., Dharmasiri N., Singh S. K., Kowalchyk M., Marchant A., Mills S., Sandberg G., Bennett M. J., Estelle M. AXR4 is required for localization of the auxin influx facilitator AUX1. Science. 2006;3125(1):1218–1220. doi: 10.1126/science.1122847. [DOI] [PubMed] [Google Scholar]
- Dhonukshe P., Aniento F., Hwang I., Robinson D., Mravec J., Stierhof Y-D., Friml J. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr. Biol. 2007a. in press. [DOI] [PubMed]
- Dhonukshe P., Grigoriev I., Fischer R., Tominaga M., Robinson D., Hašek J., Paciorek T., Gadella T. W. J., Stierhof Y., Ueda T., Oiwa K., Akhmanova A., Brock R., Spang A., Friml J. Auxin transport inhibitors block vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes - submitted. 2007b.
- Donaldson J. G., Jackson C. L. Regulators and effectors of the ARF GTPases. Curr. Opin. Cell Biol. 2000;125(1):475–482. doi: 10.1016/s0955-0674(00)00119-8. [DOI] [PubMed] [Google Scholar]
- Esmon C. A., Tinsley A. G., Ljung K., Sandberg G., Hearne L. B., Liscum E. A gradient of auxin and auxin-dependent transcription precedes tropic growth responses. Proc. Natl. Acad. Sci. U.S.A. 2006;1035(1):236–241. doi: 10.1073/pnas.0507127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Iglesias C., Sundberg B., Neuhaus G., Jones A. M. Auxin distribution and transport during embryonic pattern formation in wheat. Plant J. 2001;265(1):115–129. doi: 10.1046/j.1365-313x.2001.01013.x. [DOI] [PubMed] [Google Scholar]
- Foti M., Moukil M. A., Dudognon P., Carpentier J. L. Insulin and IGF-1 receptor trafficking and signaling. Novartis Found Symp. 2004;2625(1):125–141. [PubMed] [Google Scholar]
- Friml J. Auxin transport - shaping the plant. Curr. Opin. Plant Biol. 2003;65(1):7–12. doi: 10.1016/s1369526602000031. [DOI] [PubMed] [Google Scholar]
- Friml J., Palme K. Polar auxin transport - old questions and new concepts? Plant Mol. Biol. 2002;495(1):273–284. [PubMed] [Google Scholar]
- Friml J., Benková E., Blilou I., Wisniewska J., Hamann T., Ljung K., Woody S., Sandberg G., Scheres B., Jürgens G., Palme K. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002a;1085(1):661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- Friml J., Wisniewska J., Benková E., Mendgen K., Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002b;4155(1):806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- Friml J., Benková E., Mayer U., Palme K., Muster G. Automated whole-mount localisation techniques for plants. Plant J. 2003a;345(1):115–24. doi: 10.1046/j.1365-313x.2003.01705.x. [DOI] [PubMed] [Google Scholar]
- Friml J., Vieten A., Sauer M., Weijers D., Schwarz H., Hamann T., Offringa R., Jürgens G. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003b;4265(1):147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- Friml J., Yang X., Michniewicz M., Weijers D., Quint A., Tietz O., Benjamins R., Ouwerkerk P. B., Ljung K., Sandberg G., Hooykaas P. J., Palme K., Offringa R. A PINOID-dependent binary switch in apical basal PIN polar targeting directs auxin efflux. Science. 2004;3065(1):862–865. doi: 10.1126/science.1100618. [DOI] [PubMed] [Google Scholar]
- Fuchs I., Philippar K., Ljung K., Sandberg G., Hedrich R. Blue light regulates an auxin-induced K+-channel gene in the maize coleoptile. Proc. Natl. Acad. Sci. U.S.A. 2003;1005(1):11795–11800. doi: 10.1073/pnas.2032704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gälweiler L., Guan C., Müller A., Wisman E., Mendgen K., Yephremov A., Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;2825(1):2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- Geisler M., Kolukisaoglu H. U., Bouchard R., Billion K., Berger J., Saal B., Frangne N., Koncz-Kalman Z., Koncz C., Dudler R., Blakeslee J. J., Murphy A. S., Martinoia E., Schulz B. TWISTED DWARF1, a unique plasma membrane-anchored immunophilin-like protein, interacts with Arabidopsis multidrug resistance-like transporters AtPGP1 and AtPGP19. Mol. Biol. Cell. 2003;145(1):4238–4249. doi: 10.1091/mbc.E02-10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M., Blakeslee J. J., Bouchard R., Lee O. R., Vincenzetti V., Bandyopadhyay A., Titapiwatanakun B., Peer W. A., Bailly A., Richards E. L., Ejendal K. F., Smith A. P., Baroux C., Grossniklaus U., Müller A., Hrycyna C. A., Dudler R., Murphy A. S., Martinoia E. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 2005;445(1):179–194. doi: 10.1111/j.1365-313X.2005.02519.x. [DOI] [PubMed] [Google Scholar]
- Geldner N., Anders N., Wolters H., Keicher J., Kornberger W., Muller P., Delbarre A., Ueda T., Nakano A., Jürgens G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003;1125(1):219–230. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Geldner N., Friml J., Stierhof Y. D., Jürgens G., Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;4135(1):425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- Geldner N., Richter S., Vieten A., Marquardt S., Torres-Ruiz R. A., Mayer U., Jurgens G. Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development. 2004;1315(1):389–400. doi: 10.1242/dev.00926. [DOI] [PubMed] [Google Scholar]
- Gil P., Dewey E., Friml J., Yhao Y., Snowden K. C., Putterill J., Palme K., Estelle M., Chory J. BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev. 2001;155(1):1985–1997. doi: 10.1101/gad.905201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith M. H. M. The polar transport of auxin. Annu. Rev. Plant Physiol. 1977;285(1):439–478. [Google Scholar]
- Gray W. M., Kepinski S., Rouse D., Leyser O., Estelle M. Auxin regulates SCF (TIR1)-dependent degradation of AUX/IAA proteins. Nature. 2001;4145(1):271–276. doi: 10.1038/35104500. [DOI] [PubMed] [Google Scholar]
- Grebe M., Friml J., Swarup R., Ljung K., Sandberg G., Terlou M., Palme K., Bennett M. J., Scheres B. Cell polarity signaling in Arabidopsis involves a BFA-sensitive auxin influx pathway. Curr. Biol. 2002;125(1):329–334. doi: 10.1016/s0960-9822(02)00654-1. [DOI] [PubMed] [Google Scholar]
- Grebe M., Xu J., Mobius W., Ueda T., Nakano A., Geuze H. J., Rook M. B., Scheres B. Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr. Biol. 2003;135(1):1378–1387. doi: 10.1016/s0960-9822(03)00538-4. [DOI] [PubMed] [Google Scholar]
- Hadfi K., Speth V., Neuhaus G. Auxin-induced developmental patterns in Brassica juncea embryos. Development. 1998;1255(1):879–887. doi: 10.1242/dev.125.5.879. [DOI] [PubMed] [Google Scholar]
- Hagen G., Guilfoyle T. Auxin-responsive gene expression: genes promoters and regulatory factors. Plant Mol. Biol. 2002;495(1):373–385. [PubMed] [Google Scholar]
- Heisler M. G., Ohno C., Das P., Sieber P., Reddy G. V., Long J. A., Meyerowitz E. M. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 2005;155(1):1899–1911. doi: 10.1016/j.cub.2005.09.052. [DOI] [PubMed] [Google Scholar]
- Hertel R. The mechanism of auxin transport as a model for auxin action. Z. Pflanzenphysiol. 1983;1125(1):53–67. [Google Scholar]
- Hertel R., Leopold A. C. Versuche zur Analyse des Auxintransports in der Koleoptile von Zea mays L. Planta. 1963;595(1):535–562. [Google Scholar]
- Holler D., Dikic I. Receptor endocytosis via ubiquitin-dependent and -independent pathways. Biochem. Pharmacol. 2004;675(1):1013–1017. doi: 10.1016/j.bcp.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Imhoff V., Muller P., Guern J., Delbarre A. Inhibitors of the carrier-mediated influx of auxin in suspension-cultured tobacco cells. Planta. 2000;2105(1):580–588. doi: 10.1007/s004250050047. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Gilbert S. F. Basal localization of the presumptive auxin transport carrier in pea stem cells. Science. 1983;2205(1):1297–1300. doi: 10.1126/science.220.4603.1297. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Rubery P. H. Naturally occurring auxin transport regulators. Science. 1988;2415(1):346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Jensen P. J., Hangarter R. P., Estelle M. Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol. 1998;1165(1):455–462. doi: 10.1104/pp.116.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. M. Auxin transport: down and out and up again. Science. 1998;2825(1):2201–2203. doi: 10.1126/science.282.5397.2201. [DOI] [PubMed] [Google Scholar]
- Jönsson H., Heisler M. G., Shapiro B. E., Meyerowitz E. M., Mjolsness E. An auxin-driven polarized transport model for phyllotaxis. Proc. Natl. Acad. Sci. U.S.A. 2006;1035(1):1633–1638. doi: 10.1073/pnas.0509839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens G. Apical-basal pattern formation in Arabidopsis embryogenesis. EMBO J. 2001;205(1):3609–3616. doi: 10.1093/emboj/20.14.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katekar G. F., Geissler A. E. Auxin Transport Inhibitors: III. Chemical requirements of a class of auxin transport inhibitors. Plant Physiol. 1977;605(1):826–829. doi: 10.1104/pp.60.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S., Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;4355(1):446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- Kerk N., Feldman L. The quiescent centre in roots of maize - initiation, maintenance, and role in organization of the root apical meristem. Protoplasma. 1994;1835(1):100–106. [Google Scholar]
- Kleine-Vehn J., Dhonukshe P., Swarup R., Bennett M., Friml J. A novel pathway for subcellular trafficking of AUX1 auxin influx carrier. Plant Cell. 2006;185(1):3171–3181. doi: 10.1105/tpc.106.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kögl F., Haagen-Smith A. J. über die Chemie des Wuchsstoffs K. Akad. Wetenschap. Amsterdam. Proc. Sect. Sci. 1931;345(1):1411–1416. [Google Scholar]
- Kramer E. M., Bennett M. J. Auxin transport: a field in flux. Trends Plant Sci. 2006;115(1):382–386. doi: 10.1016/j.tplants.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Lalonde S., Boles E., Hellmann H., Barker L., Patrick J. W., Frommer W. B., Ward J. M. The dual function of sugar carriers: transport and sugar sensing. Plant Cell. 1999;115(1):707–726. doi: 10.1105/tpc.11.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M., Williams M., Nusbaum H., Sussex I. Formation of lateral root meristem is a two-stage process. Development. 1995;1215(1):3303–3310. doi: 10.1242/dev.121.10.3303. [DOI] [PubMed] [Google Scholar]
- Lehman A., Black R., Ecker J. R. HOOK-LESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell. 1996;855(1):183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- Li J., Yang H., Peer W. A., Richter G., Blakeslee J., Bandyopadhyay A., Titapiwantakun B., Undurraga S., Khodakovskaya M., Richards E. L., Krizek B., Murphy A. S., Gilroy S., Gaxiola R. Arabidopsis H+- PPase AVP1 regulates auxin-mediated organ development. Science. 2005;3105(1):121–125. doi: 10.1126/science.1115711. [DOI] [PubMed] [Google Scholar]
- Li Y., Hagen G., Guilfoyle T. J. An auxin-responsive promoter is differentially induced by auxin gradients during tropisms. Plant Cell. 1991;35(1):1167–1176. doi: 10.1105/tpc.3.11.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln C., Britton J. H., Estelle M. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell. 1990;25(1):1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Xu Z., Chua N. H. Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell. 1993;55(1):621–630. doi: 10.1105/tpc.5.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K., Hull A. K., Celenza J., Yamada M., Estelle M., Normanly J., Sandberg G. Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell. 2005;175(1):1090–1104. doi: 10.1105/tpc.104.029272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K., Bhalerao R. P., Sandberg G. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. 2001;285(1):465–74. doi: 10.1046/j.1365-313x.2001.01173.x. [DOI] [PubMed] [Google Scholar]
- Ljung K., Hull A. K., Kowalczyk M., Marchant A., Celenza J., Cohen J. D., Sandberg G. Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Mol. Biol. 2002;505(1):309–332. doi: 10.1023/a:1016024017872. [DOI] [PubMed] [Google Scholar]
- Luschnig C. Auxin transport: Why plants like to think BIG. Curr. Biol. 2001;115(1):831–833. doi: 10.1016/s0960-9822(01)00497-3. [DOI] [PubMed] [Google Scholar]
- Luschnig C., Gaxiola R. A., Grisafi P., Fink G. R. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;125(1):2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher E. P., Martindale S. J. B. Mutants of Arabidopsis thaliana with altered responses to auxins and gravity. Biochem. Genet. 1980;185(1):1041–1053. doi: 10.1007/BF00484337. [DOI] [PubMed] [Google Scholar]
- Marchant A., Kargul J., May S. T., Muller P., Delbarre A., Perrot-Rechenmann C., Bennett M. J. AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J. 1999;185(1):2066–2073. doi: 10.1093/emboj/18.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. W., Glover B. J., Davies J. M. Lipid microdomains-plant membranes get organized. Trends Plant Sci. 2005;105(1):263–265. doi: 10.1016/j.tplants.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Martinoia E., Klein M., Geisler M., Bovet L., Forestier C., Kolukisaoglu U., Muller-Rober B., Schulz B. Multifunctionality of plant ABC transporters - more than just detoxifiers. Planta. 2002;2145(1):345–355. doi: 10.1007/s004250100661. [DOI] [PubMed] [Google Scholar]
- Mattsson J., Sung Z. R., Berleth T. Responses of plant vascular systems to auxin transport inhibition. Development. 1999;1265(1):2979–2991. doi: 10.1242/dev.126.13.2979. [DOI] [PubMed] [Google Scholar]
- Mayer U., Ruiz R. A. T., Berleth T., Misera S., Jurgens G. Mutations affecting body organization in the Arabidopsis embryo. Nature. 1991;3535(1):402–407. [Google Scholar]
- Moctezuma E. Changes in auxin patterns in developing gynophores of the peanut plant. Ann. Bot. 1999;835(1):235–242. doi: 10.1006/anbo.1998.0814. [DOI] [PubMed] [Google Scholar]
- Morris D. A. Transmembrane auxin carrier systems -dynamic regulators of polar auxin transport. Plant Growth Regul. 2000;325(1):161–172. doi: 10.1023/a:1010701527848. [DOI] [PubMed] [Google Scholar]
- Morris D. A., Robinson J. S. Targeting of auxin carriers to the plasma membrane: differential effects of brefeldin A on the traffic of auxin uptake and efflux carriers. Planta. 1998;2055(1):606–612. [Google Scholar]
- Morris D. A., Thomas A. G. A microautoradi-ographic study of auxin transport in the steam of intact pea seedlings. J. Exp. Bot. 1978;295(1):147–157. [Google Scholar]
- Morris D. A., Rubery P. H., Jarman J., Sabater M. Effects of inhibitors of protein synthesis on trans-membrane auxin transport in Cucurbita pepo L. hypocotyl segments. J. Exp. Bot. 1991;425(1):773–783. [Google Scholar]
- Morris D. A., Friml J., Zažímalová E. The transport of auxins. In: Davies P. J., editor. Plant Hormones: biosynthesis, signal transduction, action! 1. Vol. 5. Kluwer Academic Publishers; 2004. pp. 437–470. (ed.) [Google Scholar]
- Muday G. K., DeLong A. Polar auxin transport: controlling where and how much. Trends Plant Sci. 2001;65(1):535–542. doi: 10.1016/s1360-1385(01)02101-x. [DOI] [PubMed] [Google Scholar]
- Muday G. K., Murphy A. S. An emerging model of auxin transport regulation. Plant Cell. 2002;145(1):293–299. doi: 10.1105/tpc.140230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A., Guan C., Gälweiler L., Tänzler P., Huijser P., Marchant A., Parry G., Bennett M., Wisman E., Palme K. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;175(1):6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A. S., Hoogner K. R., Peer W. A., Taiz L. Identification, purification, and molecular cloning of N-1-naphthylphthalamic acid-binding plasma membrane associated aminopeptidases from Arabidopsis. Plant Physiol. 2002;1285(1):935–950. doi: 10.1104/pp.010519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A., Peer W. A., Taiz L. Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta. 2000;2115(1):315–324. doi: 10.1007/s004250000300. [DOI] [PubMed] [Google Scholar]
- Noh B., Bandyopadhyay A., Peer W. A., Spalding E. P., Murphy A. S. Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature. 2003;4235(1):999–1002. doi: 10.1038/nature01716. [DOI] [PubMed] [Google Scholar]
- Noh B., Murphy A. S., Spalding E. P. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin mediated development. Plant Cell. 2001;135(1):2441–2454. doi: 10.1105/tpc.010350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J., Slovin J. P., Cohen J. D. Auxin biosynthesis and metabolism. In: Plant Hormones: biosynthesis, transduction, action!. In: Davies P. J., editor. 1. Vol. 5. Kluwer Academic Publishers; 2004. pp. 36–62. (ed.) [Google Scholar]
- Okada K., Ueda J., Komaki M. K., Bell C. J., Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;35(1):677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottenschläger I., Wolff P., Wolverton C., Bhalerao R. P., Sandberg G., Ishikawa H., Evans M., Palme K. Gravity-regulated differential auxin transport from col-umella to lateral root cap cells. Proc. Natl. Acad. Sci. U.S.A. 2003;1005(1):2987–2991. doi: 10.1073/pnas.0437936100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T., Zažímalová E., Ruthardt N., Petrášek J., Stierhof Y. D., Kleine-Vehn J., Morris D. A., Emans N., Jürgens G., Geldner N., Friml J. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;4355(1):1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- Paponov I. A., Teale W. D., Trebar M., Blilou I., Palme K. The PIN auxin efflux facilitators: evolutionary and functional perspectives. Trends Plant Sci. 2005;105(1):170–177. doi: 10.1016/j.tplants.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Parry G., Marchant A., May S., Swarup R., Swarup K., James N., Graham N., Allen T., Martucci T., Yemm A., Napier R., Manning K., King G., Bennett M. Quick on the uptake: characterization of a family of plant auxin influx carriers. J. Plant Growth Regul. 2001;205(1):217–225. [Google Scholar]
- Patton D. A., Franzmann L. H., Meinke D. W. Mapping genes essential for embryo development in Arabidopsis thaliana. Mol. Gen. Genet. 1991;2275(1):337–47. doi: 10.1007/BF00273921. [DOI] [PubMed] [Google Scholar]
- Petrášek J., Mravec J., Bouchard R., Blakeslee J. J., Abas M., Seifertová D., Wisniewska J., Tadele Z., Kubeš M., Covanová M., Dhonukshe P., Skupa P., Benková E., Perry L., Krecek P., Lee O. R., Fink G. R., Geisler M., Murphy A. S., Luschnig C., Zažímalová E., Friml J. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;3125(1):914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- Rashotte A. M., Brady S. R., Reed R. C., Ante S. J., Muday G. K. Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol. 2000;1225(1):481–490. doi: 10.1104/pp.122.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J. A. Transport of indoleacetic acid in plant cells in relation to pH and electrical potential gradients and its significance for polar IAA transport. New Phytol. 1975;745(1):163–172. [Google Scholar]
- Reinhardt D., Pesce E. R., Stieger P., Mandel T., Baltensperger K., Bennett M., Traas J., Friml J., Kuhlemeier C. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;4265(1):255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- Reinhardt D., Mandel T., Kuhlemeier C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell. 2000;125(1):507–518. doi: 10.1105/tpc.12.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas S., Debrosses G., Haralampidis K., Vicente-Agullo F., Feldmann K. A., Grabov A., Dolan L., Hatzopoulos P. TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell. 2001;135(1):139–51. doi: 10.1105/tpc.13.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzenthaler C., Nebenfuhr A., Movafeghi A., Stussi-Garaud C., Behnia L., Pimpl P., Staehelin L. A., Robinson D. G. Reevaluation of the effects of brefeldin A on plant cells using tobacco Bright Yellow 2 cells expressing Golgi-targeted green fluorescent protein and COPI antisera. Plant Cell. 2002;145(1):237–261. doi: 10.1105/tpc.010237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. S., Albert A. C., Morris D. A. Differential effects of brefeldin A and cycloheximide on the activity of auxin efflux carriers in Cucurbita pepo L. J. Plant Physiol. 1999;1555(1):678–684. [Google Scholar]
- Royle S. J., Murrell-Lagnado R. D. Constitutive cycling: a general mechanism to regulate cell surface proteins. Bioessays. 2003;255(1):39–46. doi: 10.1002/bies.10200. [DOI] [PubMed] [Google Scholar]
- Rubery P. H. Phytotropins: receptors and endogenous ligands. Symp. Soc. Exp. Biol. 1990;445(1):119–146. [PubMed] [Google Scholar]
- Rubery P. H., Sheldrake A. R. Carrier-mediated auxin transport. Planta. 1974;1885(1):101–121. doi: 10.1007/BF00388387. [DOI] [PubMed] [Google Scholar]
- Ruegger M., Dewey E., Hobbie L., Brown D., Bernasconi P., Turner J., Muday G., Estelle M. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell. 1997;95(1):745–757. doi: 10.1105/tpc.9.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S., Beis D., Wolkenfelt H., Murfett J., Guilfoyle T., Malamy J., Benfey P., Leyser O., Bechtold N., Weisbeek P., Scheres B. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;995(1):463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- Sachs T. The control of patterned differentiation of vascular tissues. Adv. Bot. Res. 1981;95(1):151–262. [Google Scholar]
- Santelia D., Vincenzetti V., Azzarello E., Bovet L., Fukao Y., Duchtig P., Mancuso S., Martinoia E., Geisler M. MDR-like ABC transporter AtPGP4 is involved in auxin-mediated lateral root and root hair development. FEBS Lett. 2005;5795(1):5399–5406. doi: 10.1016/j.febslet.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Sauer M., Balla J., Luschnig C., Wisniewska J., Reinohl V., Friml J., Benková E. Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev. 2006;205(1):2902–11. doi: 10.1101/gad.390806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E., Marcos D., Friml J., Berleth T. Control of leaf vascular patterning by polar auxin transport. Genes Dev. 2006;205(1):1015–1027. doi: 10.1101/gad.1402406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht M., Strnad M., Scanlon M. J., Mancuso S., Hochholdinger F., Palme K., Volkmann D., Menzel D., Baluška F. Auxin Immunolocalization Implicates Vesicular Neurotransmitter-Like Mode of Polar Auxin Transport in Root Apices. Plant Signaling and Behaviour. 2006;15(1):122–133. doi: 10.4161/psb.1.3.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevell D. E., Leu W. M., Gillmor C. S., Xia G., Feldmann K. A., Chua N. H. EMB30 is essential for normal cell division, cell expansion, and cell adhesion in Arabidopsis and encodes a protein that has similarity to Sec7. Cell. 1994;775(1):1051–1062. doi: 10.1016/0092-8674(94)90444-8. [DOI] [PubMed] [Google Scholar]
- Sieberer T., Seifert G. J., Hauser M. T., Grisafi P., Fink G. R., Luschnig C. Post-transcriptional control of the Arabidopsis auxin efflux carrier EIR1 requires AXR1. Curr. Biol. 2000;105(1):1595–1598. doi: 10.1016/s0960-9822(00)00861-7. [DOI] [PubMed] [Google Scholar]
- Smith R. S., Guyomarch S., Mandel T., Reinhardt D., Kuhlemeier C., Prusinkiewicz P. A plausible model of phyllotaxis. Proc. Natl. Acad. Sci. U.S.A. 2006;1035(1):1301–1306. doi: 10.1073/pnas.0510457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorin C., Bussell J. D., Camus I., Ljung K., Kowalczyk M., Geiss G., McKhann H., Garcion C., Vaucheret H., Sandberg G., Bellini C. Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell. 2005;175(1):1343–1359. doi: 10.1105/tpc.105.031625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann T., Geldner N., Grebe M., Mangold S., Jackson C. L., Paris S., Gälweiler L., Palme K., Jürgens G. Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science. 1999;2865(1):316–318. doi: 10.1126/science.286.5438.316. [DOI] [PubMed] [Google Scholar]
- Stieger P. A., Reinhardt D., Kuhlemeier C. The auxin influx carrier is essential for correct leaf positioning. Plant J. 2002;325(1):509–517. doi: 10.1046/j.1365-313x.2002.01448.x. [DOI] [PubMed] [Google Scholar]
- Swarup R., Kargul J., Marchant A., Zadik D., Rahman A., Mills R., Yemm A., May S., Williams L., Millner P., Tsurumi S., Moore I., Napier R., Kerr I. D., Bennett M. J. Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell. 2004;165(1):3069–3083. doi: 10.1105/tpc.104.024737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R., Friml J., Marchant A., Ljung K., Sandberg G., Palme K., Bennett M. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 2001;155(1):2648–2653. doi: 10.1101/gad.210501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R., Kramer E. M., Perry P., Knox K., Leyser H. M., Haseloff J., Beemster G. T., Bhalerao R., Bennett M. J. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat. Cell Biol. 2005;75(1):1057–1065. doi: 10.1038/ncb1316. [DOI] [PubMed] [Google Scholar]
- Swarup R., Marchant A., Bennett M. J. Auxin transport: providing a sense of direction during plant development. Biochem. Soc. Trans. 2000;285(1):481–485. [PubMed] [Google Scholar]
- Tanaka H., Dhonukshe P., Brewer P. B., Friml J. Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell Mol. Life Sci. 2006;635(1):2738–54. doi: 10.1007/s00018-006-6116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaka K., Blakeslee J. J., Titapiwatanakun B., Peer W. A., Bandyopadhyay A., Makam S. N., Lee O. R., Richards E. L., Murphy A. S., Sato F., Yazaki K. PGP4, an ATP binding cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell. 2005;175(1):2922–2939. doi: 10.1105/tpc.105.035816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V. Hormone action in the whole life of plants. Amherst: The University of Massachusetts; 1977. [Google Scholar]
- Treml B. S., Winderl S., Radykewicz R., Herz M., Schweizer G., Hutzler P., Glawischnig E., Ruiz R. A. The gene ENHANCER OF PINOID controls cotyledon development in the Arabidopsis embryo. Development. 2005;1325(1):4063–4074. doi: 10.1242/dev.01969. [DOI] [PubMed] [Google Scholar]
- Ulmasov T., Murfett J., Hagen G., Guilfoyle T. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;95(1):1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsuno K., Shikanai T., Yamada Y., Hashimoto T. Agr, an Agravitropic locus of Arabidopsis thaliana, encodes a novel membrane-protein family member. Plant Cell Physiol. 1998;395(1):1111–1118. doi: 10.1093/oxfordjournals.pcp.a029310. [DOI] [PubMed] [Google Scholar]
- Vicente-Agullo F., Rigas S., Desbrosses G., Dolan L., Hatzopoulos P., Grabov A. Potassium carrier TRH1 is required for auxin transport in Arabidopsis roots. Plant J. 2004;405(1):523–535. doi: 10.1111/j.1365-313X.2004.02230.x. [DOI] [PubMed] [Google Scholar]
- Vieten A., Vanneste S., Wisniewska J., Benková E., Benjamins R., Beeckman T., Luschnig C., Friml J. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development. 2005;1325(1):4521–4531. doi: 10.1242/dev.02027. [DOI] [PubMed] [Google Scholar]
- Weijers D., Sauer M., Meurette O., Friml J., Ljung K., Sandberg G., Hooykaas P., Offringa R. Maintenance of embryonic auxin distribution for apical-basal patterning by PIN-FORMED-dependent auxin transport in Arabidopsis. Plant Cell. 2005;175(1):2517–26. doi: 10.1105/tpc.105.034637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D., Schlereth A., Ehrismann J. S., Schwank G., Kientz M., Jurgens G. Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Dev. Cell. 2006;105(1):265–270. doi: 10.1016/j.devcel.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Went F. W. On growth-accelerating substances in the coleoptile of Avena sativa. Proc. Kon. Ned. Akad. Wet. 1926;305(1):10–19. [Google Scholar]
- Went F. W. Reflections and speculations. Annu. Rev. Plant Physiol. 1974;255(1):1–26. [Google Scholar]
- Went F. W., Thimann K. W. Phytohormones. Macmillan; New York: 1937. [Google Scholar]
- Willemsen V., Friml J., Grebe M., Van den Toorn A., Palme K., Scheres B. Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYL-TRANSFERASE1 function. Plant Cell. 2003;155(1):612–625. doi: 10.1105/tpc.008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniewska J., Xu J., Seifertova D., Brewer P. B., Ruzicka K., Blilou I., Rouquie D., Benková E., Scheres B., Friml J. Polar PIN localization directs auxin flow in plants. Science. 2006;3125(1):883. doi: 10.1126/science.1121356. [DOI] [PubMed] [Google Scholar]
- Woodward A. W., Bartel B. Auxin: regulation, action, and interaction. Ann. Bot. 2005;955(1):707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Hofhuis H., Heidstra R., Sauer M., Friml J., Scheres B. A molecular framework for plant regeneration. Science. 2006;3115(1):385–388. doi: 10.1126/science.1121790. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Yamamoto K. T. Differential effects of 1-naphthaleneacetic acid, indole-3-acetic acid and 2,4-dichlorophenoxyacetic acid on the gravitropic response of roots in an auxin-resistant mutant of Arabidopsis, aux1. Plant Cell Physiol. 1998;395(1):660–664. doi: 10.1093/oxfordjournals.pcp.a029419. [DOI] [PubMed] [Google Scholar]
- Yang Y., Hammes U. Z., Taylor C. G., Schachtman D. P., Nielsen E. High-affinity auxin transport by the AUX1 influx carrier protein. Curr. Biol. 2006;165(1):1123–1127. doi: 10.1016/j.cub.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Young L. S., Harrison B. R., Narayana Murthy U. M., Moffatt B. A., Gilroy S., Masson P. H. Adenosine kinase modulates root gravitropism and cap morphogenesis in Arabidopsis. Plant Physiol. 2006;1425(1):564–73. doi: 10.1104/pp.106.084798. [DOI] [PMC free article] [PubMed] [Google Scholar]


