Abstract
Prostate cancer is one of the most prevalent cancers in men. Replication-competent oncolytic herpes simplex virus (oHSV) vectors are a powerful anti-tumor therapy that can exert at least two effects: direct cytocidal activity that selectively kills cancer cells and induction of anti-tumor immunity. In addition, oHSV vectors can also function as a platform to deliver transgenes of interest. In these studies, we have examined the expression of a xenogeneic homologue of the prostate cancer antigen, prostatic acid phosphatase (PAP), with the goal of enhancing virotherapy against PAP-expressing tumors. PAP has already been used for cancer vaccination in prostate cancer patients. Here we show that treatment with oHSV bPΔ6 expressing xenogeneic human PAP (hPAP) significantly reduces tumor growth and increases survival of C57/BL6 mice bearing mouse TRAMP-C2 prostate tumors, whereas expression of syngeneic mouse PAP (mPAP) from the same oHSV vector did not enhance anti-tumor activity. Treatment of mice bearing metastatic TRAMP-C2 lung tumors with oHSV expressing hPAP resulted in fewer tumor nodules. To our knowledge, this is the first report of oncolytic viruses being used to express xenoantigens. This data lends support to the concept of combining oncolytic and immunogenic therapies as a way to improve therapy of metastatic prostate cancer.
Keywords: HSV, TRAMP, virotherapy, cancer therapy
INTRODUCTION
Prostate cancer is the most common cancer amongst men in developed countries and in the United States alone over 180,000 new cases were diagnosed in 2008 1. Treatment options for prostate cancer include surgery with radical prostatectomy, hormone therapy, chemotherapy 2 and radiation 3. However, severe secondary effects are common amongst patients and there is no curative treatment once the primary tumor metastasizes 4. The introduction of genetically-engineered oncolytic viruses as a new therapeutic avenue in the battle against cancer began with the use of oncolytic herpes simplex viruses (oHSV) 5. Typically, oHSV carry mutations in the viral genome that enable them to replicate in and kill cancer cells, without harming normal tissue 6. Amongst the benefits of oHSV vectors are that they are easily manipulated, can carry transgene inserts 7 and have already been safely used in human subjects with a variety of cancers 8, 9. Another advantage of oHSV is that it can interact synergistically with other therapeutic modalities, including chemotherapeutic agents 10, 11 and radiotherapy 12, thereby promoting better therapeutic outcomes by targeting more then one aspect of cancer biology.
Previous work from our laboratory and others has shown that oHSVs can be used as an in situ vaccine to generate a tumor-specific host immune response 13-16. Prostatic acid phosphatase (PAP) is a prostate-specific antigen that is expressed in both prostate cells and prostate cancer cells 17 as well as other adenocarcinomas 18. However, as it is a self-antigen it is not immunogenic due presumably to tolerance. It has previously been shown that immunization of rats with human PAP (hPAP) generates a CTL response leading to tissue-specific prostatitis 19. Furthermore, immunization of metastatic prostate cancer patients with recombinant mouse PAP (mPAP) loaded dendritic cells has been shown to result in an anti-tumor immune response and clinical stabilization of the disease, as indicated by a decreased rise of serum PSA levels 18. This suggests that vaccination with a xenogeneic homolog can break tolerance to a self-antigen, human PAP (hPAP), inducing a Th1-type cytokine response to both mouse and human PAP antigens. Other studies have confirmed this concept of using a xenogenic form of an antigen to break anti-tumor tolerance to other proteins, such as: prostate stem cell antigen, where DNA vaccination induced anti-tumor immune responses against TRAMP-C1 tumors 19, prostate-specific membrane antigen (PSMA) 20, gp100 in melanoma 21, HER2 expressed from a replication-deficient adenovirus vector in breast cancer 22, and EGFR 23. We therefore hypothesized that an oHSV expressing a xenogenic PAP could be used to treat prostate cancer.
In the present study we have combined the ability of oHSV to specifically replicate in and kill cancer cells with the immunogenic effects of in situ vaccination with a xenogeneic PAP protein to create a more powerful therapeutic strategy for prostate cancer. Our results show that this combined approach achieves improved inhibition of established tumor growth and animal survival compared to oHSV alone or when expressing syngeneic mPAP. These results represent an encouraging step towards the development of a viable therapy for prostate cancer and describe a novel approach that should be applicable to other oncolytic viruses.
RESULTS AND DISCUSSION
Characterization of oHSVs expressing mouse and human PAP transgenes
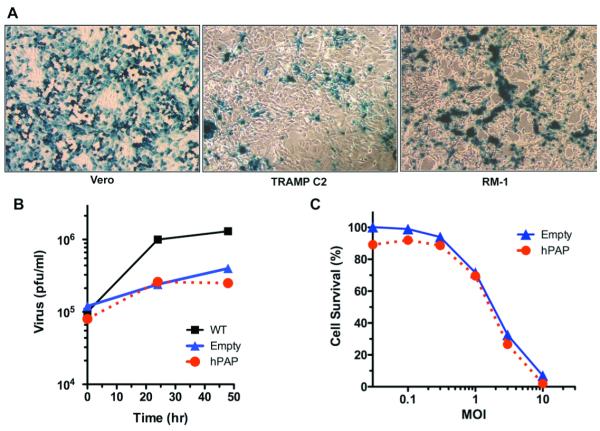
To construct oHSVs expressing human PAP (bPΔ6-hPAP) or the mouse homolog (bPΔ6-mPAP), we used the Flip-Flop HSV-BAC technology 7. A third construct, lacking a transgene (bPΔ6-empty), was also generated to be used as a control (Supplementary Fig. 1). The backbone for these vectors was HSV-1 strain Patton, from which PΔ6-BAC was constructed by inserting the BAC cassette into the ICP6 (UL39) gene, as previously described 7. The genomic structure of the vectors was confirmed by restriction endonuclease digestion and gel electrophoresis and the recombinant vectors all express β-galactosidase, which is detectable after X-gal histochemistry (data not shown). While mouse prostate cancer cells are much less susceptible to oHSV replication than human prostate cancer cells (compare 24 and 25) or Vero cells, they still replicate and spread in mouse TRAMP-C2 and RM-1 prostate cancer cells (Fig 1A). The transgene expressing viruses replicate less well than the parental wild-type strain Patton (WT, Fig 1B), likely due to the ICP6 mutation, however, there is no difference between bPΔ6-hPAP and bPΔ6- empty (Fig 1B). A dose response curve for killing TRAMP-C2 cells is illustrated in Fig 1C. Again there is no difference between bPΔ6-hPAP and bPΔ6-empty, demonstrating that expression of hPAP does not alter the in vitro replication or cytoxicity of bPΔ6.
Figure 1. Characterization of bPΔ6-transgene vectors.
(A) bPΔ6-hPAP viruses infect and spread in Vero and mouse prostate cancer cells. Vero (African Green Monkey Kidney) cells (American Type Culture Collection, Manassas, VA) were cultured in DMEM with glucose (4.5 g/L; Mediatech, Inc.,Herndon, VA) supplemented with 10% calf serum. TRAMP-C2 28, 34, obtained from Dr. N. Greenberg (Fred Hutchinson Cancer Research Center, Seattle, WA), and RM-1 35, obtained from Dr. T. C. Thompson (Baylor College of Medicine, Houston, TX), were cultured as previously described. Cells were seeded at 80% confluency and 24 hours later infected with bPΔ6-hPAP at a multiplicity of infection (MOI)=1. Cultures were fixed 18 hours post-infection and stained for β-galactosidase expression with X-Gal (1 mg/ml), 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM magnesium chloride in PBS for 4 hours at 37°C. Cells were washed with PBS, counter-stained with neutral red solution and β-galactosidade expression and viral spread visualised under a light microscope. (B) Virus replication assays. Vero cells were seeded at 1×105 cells/well in 12-well plates and 24 hours later infected with either bPΔ6-hPAP, bPΔ6-empty or wt viruses at MOI=1. Two hours post-infection the inoculum was removed and replaced with medium (DMEM/1% inactivated FCS). Cells and medium were harvested at indicated times post-infection, processed with freeze/thaw cycles and sonication, and titered on Vero cells. Virus yield is plotted as plaque-forming units (pfu)/ml. (C) Cell viability assays. TRAMP-C2 cells were seeded into 96-well plates at 5000 cells/well and 24 hours later infected with bPΔ6-hPAP (IC50=2.5) or bPΔ6-empty (IC50=2.9) at 3-fold serial dilutions (from 0.01 to 10 PFU/cell). Cell viability was assessed 4 days post-infection with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma) according to the manufacturer’s instructions.
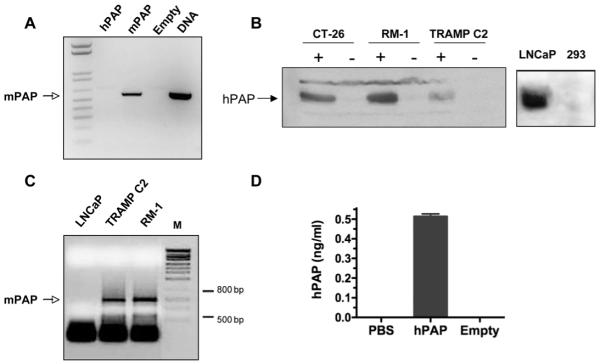
Next we confirmed that the viruses express the inserted transgenes. The CMV IE promoter was used to drive transgene expression, as we have described before 26, 27, because it provides high level expression. Vero cells were infected with either bPΔ6-hPAP, bPΔ6-mPAP or bPΔ6-empty and RT-PCR analysis was carried out for mPAP, using mouse transgene specific primers, and a band corresponding to the expected mPAP RNA was only seen in bPΔ6-mPAP infected Vero cells (Fig 2A). Expression of the hPAP transgene in mouse tumor cell lines was detected with human-specific anti-PAP antibody. Western blot analysis reveals a band corresponding to hPAP in all lanes where cells were infected with bPΔ6-hPAP (+, Fig 2B). As a control, we show the expression of endogenous hPAP in human LNCaP prostate cells but not in 293 cells (Fig 2B, right). In order to test the immunogenic effects of the oHSV PAP vectors it was necessary that the mouse prostate tumor model expressed mPAP. RT-PCR analysis confirmed the presence of mPAP RNA in both TRAMP-C2 and RM-1 prostate cancer cells (Fig. 2C). Ultimately, TRAMP-C2 was selected as our experimental tumor cell line since previous data from our lab has shown that these cells are responsive to oHSV treatment in both primary and metastatic tumor models 25, 28. Finally, in order to verify that bPΔ6-hPAP expresses human PAP protein in vivo, we implanted TRAMP-C2 cells subcutaneously into C57/BL6 immunocompetent mice. Once tumors were established, the mice were injected intra-neoplastically with bPΔ6-hPAP or bPΔ6-empty. Forty-eight hours post-injection, hPAP was detected in tumors treated with bPΔ6-hPAP, but not bPΔ6-empty (Fig 2D).
Figure 2. PAP expression.
(A) RT-PCR analysis of Vero cells infected with bPΔ6-mPAP shows mPAP mRNA expression. Cells were grown in 6-well plates and infected at 90-95% confluence with either bPΔ6-hPAP, bPΔ6-mPAP or bPΔ6-empty viruses at MOI=2. After 2 hrs, virus inoculum was removed and replaced with medium DMEM/1% inactivated FCS. Eight hours later the total RNA fraction was isolated from cell lysates using TRIZOL (Invitrogen, USA), and treated with RQ1 RNase-free DNase I (Promega, Madison, WI). Reverse transcription was carried out using SuperScriptIII First Strand Synthesis kit (Invitrogen, USA) and random hexamer primers. cDNAs were subjected to conventional PCR (5 min at 95°C then 28 cycles: 30 seconds at 95°C, 15 seconds at 68°C, 20 seconds at 72°C; and terminal elongation 2 min at 72°C) with specific primers: P1mPAP619 5′ – gcttcctggacaccttgtcgtcgctgtcg – 3′ and P2mPAP1214 5′ – attccgtccttggtggctgc – 3′, designed to generate a PCR product of 595 bp. As a positive control, plasmid DNA (pVec92-mPAP) was used and the PCR reaction was performed without the reverse transcription step. The reaction product was analyzed by 1.2% agarose gel electrophoresis and stained with ethidium bromide. (B) Western blot analysis of hPAP expression in CT-26 colorectal carcinoma 13, RM-1 and TRAMP-C2 cell lines after infection with bPΔ6-hPAP (indicated by +) or bPΔ6-empty (indicated by -) at MOI=2. Human prostate adenocarcinoma LNCaP cells 24 and human embryonic kidney 293 cells were used as controls. Cell lysates were prepared using RIPA buffer 36 24 hours post-infection. Each lysate was separated by SDS-polyacrylamide electrophoresis, transferred to PVDF membrane (BioRad), and immunoblotted with anti-hPAP (DAKO Rabbit anti-Human PAP, A0627) antibody (diluted 1:1000) using standard procedures. (C) Mouse prostate cancer cells express PAP. Total RNA was isolated from LNCaP, TRAMP-C2 and RM-1 cells and RT-PCR was performed using primers previously described 37. mPAP mRNA was observed in both TRAMP-C2 and RM -1 cells but not in human LNCaP prostate cancer cells. (D) In vivo expression of hPAP. TRAMP-C2 cells (5×106/mouse) were implanted subcutaneously into the flanks of male six to eight-week-old C57/BL6 mice (National Cancer Institute, Frederick, MD). Once tumors were established (50-10 mm3) mice were treated intraneoplastically once with bPΔ6-hPAP (2×107 pfu). After 48 hours, tumors were harvested, homogenized and centrifuged. Supernatants were assayed for hPAP using an ELISA Kit (R&D Systems, Minneapolis, MN) according to manufacturer’s instructions. Error bars represent the standard deviation of 3 measurements. All in vivo procedures were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
In vivo treatment with bPΔ6-hPAP leads to tumor reduction and increased animal survival
TRAMP-C2 cells were implanted subcutaneously in syngeneic C57/BL6 mice. Once tumors were established, mice were randomized into four treatment groups: bPΔ6-hPAP, bPΔ6-mPAP, bPΔ6-empty, and PBS only. Each mouse was treated four times intra-neoplastically with 1×107 PFU / injection over a period of 10 days and then monitored for tumor growth and survival. Mice treated with oHSV lacking transgene (bPΔ6-empty) or oHSV expressing a non-xenogenic form of PAP (bPΔ6-mPAP) showed a slower rate of tumor growth, with no difference between mPAP and empty (p=0.7) and not significantly different from the PBS-treated mice (PBS vs mPAP p=0.09, PBS vs empty p=0.08). However, mice treated with the xenogenic hPAP-expressing oHSV (bPΔ6-hPAP) showed a significant inhibition in tumor growth when compared to bPΔ6-mPAP (p=0.03), bPΔ6-empty (p=0.04) or PBS (p=0.002) groups (Fig 3A). This inhibition translated into a significant increase in survival (Fig. 3B) when compared to mice treated with bPΔ6-mPAP (p=0.01), bPΔ6-empty (p=0.01), or PBS only (p=0.0008), with the median survival increasing from 29 days for PBS to 39 for hPAP. In a separate experiment, 3 intra-tumoral injections of bPΔ6-hPAP resulted in significantly extended survival compared to bPΔ6-empty or PBS (data not shown). This indicates that expression of xenogenic hPAP is enhancing anti-tumor activity.
Figure 3. Mice treated with bPΔ6-hPAP show tumor reduction and increased survival.
C57/BL6 mice were implanted subcutaneously in the flanks with TRAMP-C2 cells (5×106/mouse). Once tumors were established (50-100 mm3) mice (N=8) were randomized and treated four times with 1×107 pfu of bPΔ6-empty, bPΔ6-mPAP, bPΔ6-hPAP or PBS over a period of 10 days by direct intra-tumoral inoculation. Tumor volumes were monitored using calipers and calculated with the formula v = [(length) × (width2)] / 2. Mice were sacrificed when tumors reached 1000 mm3. Animals treated with bPΔ6-hPAP showed a significant reduction in tumor volume (p=0.002, PBS vs hPAP: p=0.04, Empty vs hPAP: p=0.03, mPAP vs hPAP) (A) and a significant increase in survival (B) when compared with the bPΔ6-mPAP (p=0.01), bPΔ6-empty (0.01) or PBS (p=0.0008) groups. Analysis of tumor volumes (day 24, Student’s t-test) and survival (log-rank test) were performed with GraphPad Prism v.4 (San Diego, CA).
Systemically administrated bPΔ6-hPAP reduces tumor nodule burden in a metastatic tumor model
To investigate the therapeutic efficacy of bPΔ6-hPAP in a metastatic tumor model, we injected TRAMP-C2 cells intravenously via tail vein. TRAMP-C2 cells metastasize primarily to the lungs 28. Tumor bearing mice were systemically treated via tail vein injection with bPΔ6-hPAP, bPΔ6-mPAP, bPΔ6-empty, or PBS 4 times over a period of 10 days. Mice were sacrificed at day 60 and the lungs were stained with India ink to detect lung tumor nodules, which were measured and counted. Mice treated with bPΔ6-hPAP showed significantly fewer tumors per group then the bPΔ6-empty (p=0.015) or PBS (p= 0.017) groups and also a significant reduction in tumor burden when compared with bPΔ6-empty (p=0.034) (Table 1).
Table 1.
bPΔ6-hPAP treatment leads to fewer tumor nodules in a metastatic tumor model. TRAMP-C2 cells (5×105/mouse) were injected intravenously in the tail vain of C57/BL6 mice (N=8/group) and lung tumors established as previously described 28. Preliminary studies showed microscopic tumor nodules by day 25 and by day 60 animals showed signs of morbidity. Mice were treated 4 times with 3×107 pfu of bPΔ6-hPAP, bPΔ6-mPAP or bPΔ6-empty, or PBS over a period of 10 days. Animals were sacrificed at day 60 and the number of lung tumor nodules and tumor burden per mouse was analyzed as described previously 28. Animals treated with bPΔ6-hPAP viruses showed significantly fewer tumor nodules when compared with bPΔ6-mPAP or PBS groups (* p=0.02), as well as a decrease in tumor burden (** p=0.03 vs bPΔ6-empty). There was no statistical difference between tumor nodules in the bPΔ6-Empty versus PBS groups. Pairwise statistical analysis were performed using Student’s T-Tests.
| PBS | empty | mPAP | hPAP | |
|---|---|---|---|---|
|
Total tumor
nodules |
8 | 18 | 10 | 4* |
|
Mean tumor burden
per mouse (cm2) |
7.0 | 16.8 | 6.9 | 1.6** |
In an effort to improve the therapeutic potential of the oHSV vector system we combined oHSV with a xenoantigen transgene, designed to stimulate an auto-immune response against a prostate antigen, PAP. For these studies we developed a new oHSV vector using wild-type HSV-1 strain Patton as the backbone and Flip-Flop HSV-BAC technology 7 to insert the PAP transgenes into the ICP6 locus of the oHSV genome in a rapid fashion. Many oHSV vectors contain ICP6 mutations, which inactivates viral ribonucleotide reductase, impeding virus replication in normal quiescent cells except those lacking p16, such as many tumor cells 29. HrR3, with an insertion of E. coli LacZ inactivating ICP6 in the HSV-1 KOS backbone was the first such vector 30, 31. bPΔ6 has the same LacZ insertion into ICP6 as hrR3, but in a Patton backbone. Expression of PAP did not affect virus replication or tumor cell killing in vitro. To test the xenoantigen strategy, we needed a tumor model with endogenous mPAP expression that progressed slowly enough to allow the treated animals time to generate an immune response, and that was susceptible to oHSV. For these reasons we chose the TRAMP-C2 tumor cell line. Incorporating hPAP transgene into the oHSV vector bPΔ6, led to a reduction in tumor burden in subcutaneous and metastatic prostate tumor models that did not occur with mPAP. Because of limited oHSV replication in mouse prostate cancer cells, syngeneic mouse prostate tumors have been difficult to treat with oHSV, except with the addition of immune-modulatory transgenes, such as IL-12 25, 28.
Our data support the concept that better outcomes can be achieved by combining oncolytic viral therapy with immunotherapy than with oncolytic viral therapy alone. The ability of hPAP, but not mPAP, to enhance the anti-tumor activity of oHSV indicates that a specific immune response is at work, engendered by exposure to the xenogeneic PAP protein and resulting in a loss of tolerance to syngeneic PAP. Previous studies vaccinating rats with vaccinia virus or plasmid expressing xenogeneic human PAP transgenes demonstrated the induction of a MHC class I-dependent CTL-mediated anti-PAP auto-immune response, but not an antibody response 32, 33. In these studies the response differed to some extent between rat strains and the animals did not bear prostate tumors. To our knowledge, ours is the first report of an oncolytic virus expressing a xenoantigen for cancer vaccination. It is likely that the combination of hPAP with expression of other immunomodulatory molecules will further improve the efficacy of oHSV-mediated immunotherapy. As we have shown that oHSV expressing hPAP is efficacious in treating mouse prostate cancer, we hypothesize that oHSV expressing mPAP would be effective in treating human prostate cancer. This could be addressed in a clinical trial for the treatment of prostate cancer.
Supplementary Material
Supplementary Figure 1. Construction of PAP-expressing oHSV. Use of the Flip-Flop HSV-BAC system to construct recombinant oHSV vectors is illustrated in this cartoon and has been previously described 7, 26. Briefly, hPAP cDNA 38 was cloned into the shuttle vector pVec92 to generate pVec92-hPAP. pVec92-hPAP was inserted into bPΔ6-BAC via Cre/LoxP recombination. The bPΔ6-CMV-hPAP-shuttle plasmid was isolated and co-transfected into Vero cells with pCAGGS-Flp, a FLPe-expressing plasmid. Flp/Frt recombination removes the BAC, antibiotic resistance, GFP, and stuffer sequences so the viral genome can be efficiently packaged into virions, and the recombinant virus (bPΔ6-hPAP) harvested and plaque-purified.
ACKNOWLEDGMENTS
The authors would like to thank Dr M Crew for providing the mPAP cDNA and Dr Timothy C. Thompson for providing RM-1 cells. This work was supported in part by grants to RLM from the DOD (W81XWH-04-1-0254) and the NIH (R01 CA102139).
Footnotes
CONFLICT OF INTEREST The authors declare no conflict of interest.
REFERENCES
- 1.Moul JW, Mouraviev V, Sun L, Schroeck FR, Polascik TJ. Prostate cancer: the new landscape. Curr Opin Urol. 2009;19:154–160. doi: 10.1097/mou.0b013e328323f5d6. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell RE, Chang SS. Current controversies in the treatment of high-risk prostate cancer. Curr Opin Urol. 2008;18:263–8. doi: 10.1097/MOU.0b013e3282f9b37f. [DOI] [PubMed] [Google Scholar]
- 3.Sia M, Rosewall T, Warde P. Radiotherapy as primary treatment modality. Front Radiat Ther Oncol. 2008;41:15–25. doi: 10.1159/000139874. [DOI] [PubMed] [Google Scholar]
- 4.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8:497–511. doi: 10.1038/nrc2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–6. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 6.Varghese S, Rabkin SD. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002;9:967–978. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- 7.Kuroda T, Martuza RL, Todo T, Rabkin SD. Flip-Flop HSV-BAC: bacterial artificial chromosome based system for rapid generation of recombinant herpes simplex virus vectors using two independent site-specific recombinases. BMC Biotech. 2006;6:40. doi: 10.1186/1472-6750-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aghi M, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24:7802–16. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 9.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–17. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 10.Post DE, Fulci G, Chiocca EA, Van Meir EG. Replicative oncolytic herpes simplex viruses in combination cancer therapies. Curr Gene Ther. 2004;4:41–51. doi: 10.2174/1566523044577988. [DOI] [PubMed] [Google Scholar]
- 11.Passer BJ, Castelo-Branco P, Buhrman JB, Varghese S, Rabkin SD, Martuza RL. Oncolytic herpes simplex virus vectors and taxanes synergize to promote killing of prostate cancer cells. Cancer Gene Ther. 2009;16:551–60. doi: 10.1038/cgt.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington KJ, Melcher A, Vassaux G, Pandha HS, Vile RG. Exploiting synergies between radiation and oncolytic viruses. Curr Opin Mol Ther. 2008;10:362–70. [PubMed] [Google Scholar]
- 13.Toda M, Rabkin SD, Kojima H, Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–93. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- 14.Thomas DL, Fraser NW. HSV-1 therapy of primary tumors reduces the number of metastases in an immune-competent model of metastatic breast cancer. Mol Ther. 2003;8:543–51. doi: 10.1016/s1525-0016(03)00236-3. [DOI] [PubMed] [Google Scholar]
- 15.Benencia F, Courrèges MC, Conejo-García JR, Mohamed-Hadley A, Zhang L, Buckanovich RJ, et al. HSV oncolytic therapy upregulates interferon-inducible chemokines and recruits immune effector cells in ovarian cancer. Mol Ther. 2005;12:789–80. doi: 10.1016/j.ymthe.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Dutuor A, Tao L, Fu X, Zhang X. Virotherapy with a type 2 herpes simplex virus-derived oncolytic virus induces potent antitumor immunity against neuroblastoma. Clin Cancer Res. 2007;13:316–22. doi: 10.1158/1078-0432.CCR-06-1625. [DOI] [PubMed] [Google Scholar]
- 17.Lin MF, DaVolio J, Garcia-Arenas R. Expression of human prostatic acid phosphatase activity and the growth of prostate carcinoma cells. Cancer Res. 1992;52:4600–7. [PubMed] [Google Scholar]
- 18.Fong L, Brockstedt D, Benike C, Breen JK, Strang G, Ruegg CL, et al. Dendritic cell-based xenoantigen vaccination for prostate cancer immunotherapy. J Immunol. 2001;167:7150–6. doi: 10.4049/jimmunol.167.12.7150. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad S, Casey G, Sweeney P, Tangney M, O’Sullivan G. Prostate stem cell antigen DNA vaccination breaks tolerance to self-antigen and inhibits prostate cancer growth. Mol Ther. 2009;17:1101–8. doi: 10.1038/mt.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregor PD, Wolchok JD, Turaga V, Latouche JB, Sadelain M, Bacich D, et al. Induction of autoantibodies to syngeneic prostate-specific membrane antigen by xenogeneic vaccination. Int J Cancer. 2005;116:415–21. doi: 10.1002/ijc.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gold JS, Ferrone CR, Guevara-Patiño JA, Hawkins WG, Dyall R, Engelhorn ME, et al. A single heteroclitic epitope determines cancer immunity after xenogeneic DNA immunization against a tumor differentiation antigen. J Immunol. 2003;170:5188–94. doi: 10.4049/jimmunol.170.10.5188. [DOI] [PubMed] [Google Scholar]
- 22.Gallo P, Dharmapuri S, Nuzzo M, Maldini D, Iezzi M, Cavallo F, et al. Xenogeneic immunization in mice using HER2 DNA delivered by an adenoviral vector. Int J Cancer. 2005;113:67–77. doi: 10.1002/ijc.20536. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Wei Y, Tian L, Zhao X, Yang L, Hu B, et al. Immunogene therapy of tumors with vaccine based on xenogeneic epidermal growth factor receptor. J Immunol. 2003;170:3162–70. doi: 10.4049/jimmunol.170.6.3162. [DOI] [PubMed] [Google Scholar]
- 24.Walker JR, McGeagh KG, Sundaresan P, Jorgensen TJ, Rabkin SD, Martuza RL. Local and systemic therapy of human prostate adenocarcinoma with the conditionally replicating herpes simplex virus vector G207. Hum Gene Ther. 1999;10:2237–43. doi: 10.1089/10430349950017211. [DOI] [PubMed] [Google Scholar]
- 25.Varghese S, Rabkin SD, Liu R, Nielsen PG, Ipe T, Martuza RL. Enhanced therapeutic efficacy of IL-12, but not GM-CSF, expressing oncolytic herpes simplex virus for transgenic mouse derived prostate cancers. Cancer Gene Ther. 2006;13:253–65. doi: 10.1038/sj.cgt.7700900. [DOI] [PubMed] [Google Scholar]
- 26.Fukuhara H, Ino Y, Kuroda T, Martuza RL, Todo T. Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by bacterial artificial chromosome-mediated system. Cancer Res. 2005;65:10663–8. doi: 10.1158/0008-5472.CAN-05-2534. [DOI] [PubMed] [Google Scholar]
- 27.Liu TC, Zhang T, Fukuhara H, Kuroda T, Todo T, Martuza RL, et al. Oncolytic HSV armed with platelet factor 4, an antiangiogenic agent, shows enhanced efficacy. Mol Ther. 2006;14(6):789–97. doi: 10.1016/j.ymthe.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Varghese S, Rabkin SD, Nielsen PG, Wang W, Martuza RL. Systemic oncolytic herpes virus therapy of poorly immunogenic prostate cancer metastatic to lung. Clin Cancer Res. 2006;12:2919–27. doi: 10.1158/1078-0432.CCR-05-1187. [DOI] [PubMed] [Google Scholar]
- 29.Aghi M, Visted T, Depinho RA, Chiocca EA. Oncolytic herpes virus with defective ICP6 specifically replicates in quiescent cells with homozygous genetic mutations in p16. Oncogene. 2008;27:4249–54. doi: 10.1038/onc.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein DJ, Weller SK. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988;62:196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mineta T, Rabkin SD, Martuza RL. Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutant. Cancer Res. 1994;54:3963–6. [PubMed] [Google Scholar]
- 32.Fong L, Ruegg CL, Brockstedt D, Engleman EG, Laus R. Induction of tissue-specific autoimmune prostatitis with prostatic acid phosphatase immunization: implications for immunotherapy of prostate cancer. J Immunol. 1997;159:3113–7. [PubMed] [Google Scholar]
- 33.Johnson LE, Frye TP, Chinnasamy N, Chinnasamy D, McNeel DG. Plasmid DNA vaccine encoding prostatic acid phosphatase is effective in eliciting autologous antigen-specific CD8+ T cells. Cancer Immunol Immunother. 2007;56:885–95. doi: 10.1007/s00262-006-0241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–30. [PubMed] [Google Scholar]
- 35.Hall SJ, Mutchnik SE, Chen S-H, Woo SLC, Thompson TC. Adenovirus-mediated Herpes Simplex Virus Thymidine kinase gene and ganciclovir therapy leads to systemic activity against spontaneous and induced metastasis in an orthotopic mouse model of prostate cancer. Int J Cancer. 1997;70:183–187. doi: 10.1002/(sici)1097-0215(19970117)70:2<183::aid-ijc8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 36.Davis L, Kuehl M, Battey J. Basic Methods in Molecular Biology. Appleton & Lange; Norwalk, CN: 1994. [Google Scholar]
- 37.Grossmann ME, Wood M, Celis E. Expression, specificity and immunotherapy potential of prostate-associated genes in murine cell lines. World J Urol. 2001;19:365–70. doi: 10.1007/pl00007104. [DOI] [PubMed] [Google Scholar]
- 38.Vihko P, Virkkunen P, Henttu P, Roiko K, Solin T, Huhtala ML. Molecular cloning and sequence analysis of cDNA encoding human prostatic acid phosphatase. FEBS Lett. 1988;236:275–81. doi: 10.1016/0014-5793(88)80037-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Construction of PAP-expressing oHSV. Use of the Flip-Flop HSV-BAC system to construct recombinant oHSV vectors is illustrated in this cartoon and has been previously described 7, 26. Briefly, hPAP cDNA 38 was cloned into the shuttle vector pVec92 to generate pVec92-hPAP. pVec92-hPAP was inserted into bPΔ6-BAC via Cre/LoxP recombination. The bPΔ6-CMV-hPAP-shuttle plasmid was isolated and co-transfected into Vero cells with pCAGGS-Flp, a FLPe-expressing plasmid. Flp/Frt recombination removes the BAC, antibiotic resistance, GFP, and stuffer sequences so the viral genome can be efficiently packaged into virions, and the recombinant virus (bPΔ6-hPAP) harvested and plaque-purified.