Abstract
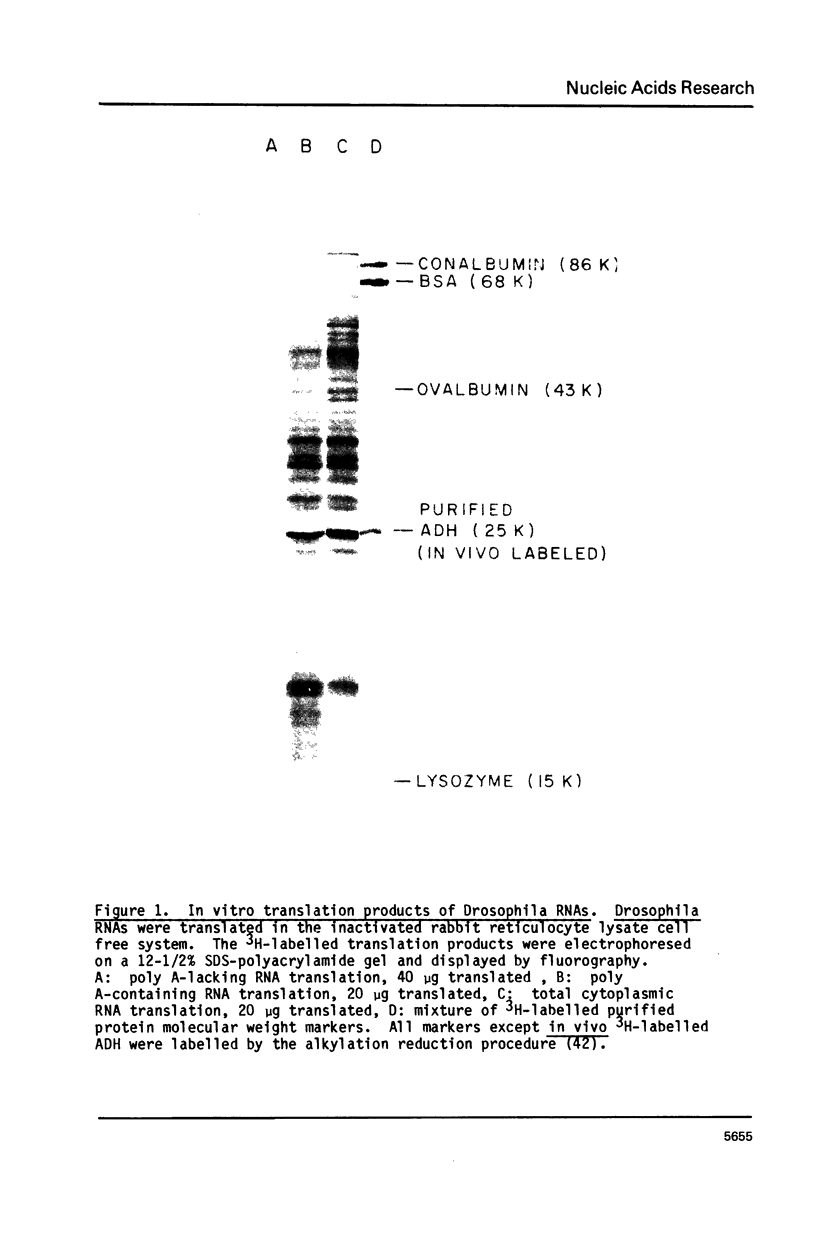
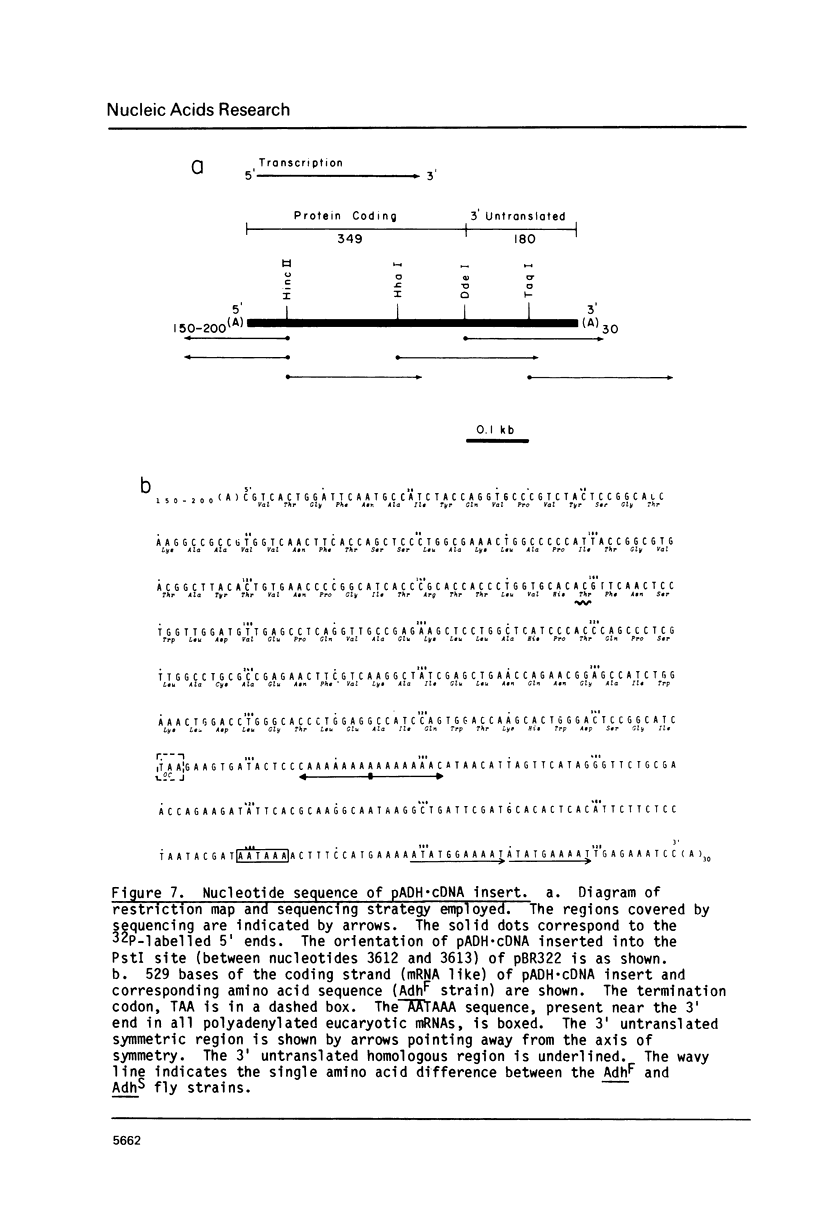
The mRNA for alcohol dehydrogenase (ADH) in D. melanogaster has been identified by translation in a cell-free system. The in vitro synthesized polypeptide, specifically precipitated by anti-ADH antibody, has identical subunit molecular weight (25,000 daltons) and tryptic peptide profile to the in vivo synthesized ADH. The poly A containing ADH-mRNA has been purified by specific precipitation of ADH-polysomes using anti-ADH antibody and S. aureus. Transformation of E. coli with the dA-tailed ADH-mRNA-complementary DNA hybrid annealed to the dT-tailed pBR322 yielded one plasmid which has been identified as the ADH-cDNA clone. The identification involved hybridization selection of ADH-mRNA and in vitro translation, in situ hybridization to the Adh locus on salivary gland polytene chromosomes and DNA sequencing. This ADH-cDNA plasmid contains 349 bases of the C-terminal protein coding and 180 bases of the 3' untranslated region.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell G. N., Wickens M. P., Payvar F., Schimke R. T. Synthesis of full length cDNAs from four partially purified oviduct mRNAs. J Biol Chem. 1978 Apr 10;253(7):2471–2482. [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher T. S., Ayala F. J., Thatcher D. R., Chambers G. K. Structural analysis of the ADHS electromorph of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5609–5612. doi: 10.1073/pnas.75.11.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E. Y., Rosbash M. The syntheiss of high yields of full-length reverse transcripts of globin mRNA. Nucleic Acids Res. 1977 Oct;4(10):3455–3471. doi: 10.1093/nar/4.10.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M., Adams J. M. Immunoprecipitation of specific polysomes using Staphylococcus aureus: purification of the immunoglobulin k chain messenger RNA from the mouse myeloma MPC11. Biochemistry. 1978 Dec 12;17(25):5560–5566. doi: 10.1021/bi00618a036. [DOI] [PubMed] [Google Scholar]
- Grell E. H., Jacobson K. B., Murphy J. B. Alcohol Dehydrogenase in Drosophila melanogaster: Isozymes and Genetic Variants. Science. 1965 Jul 2;149(3679):80–82. doi: 10.1126/science.149.3679.80. [DOI] [PubMed] [Google Scholar]
- Konkel D. A., Tilghman S. M., Leder P. The sequence of the chromosomal mouse beta-globin major gene: homologies in capping, splicing and poly(A) sites. Cell. 1978 Dec;15(4):1125–1132. doi: 10.1016/0092-8674(78)90040-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman G., Krauss S. W., Olsen A. S. Tryptic peptide analysis of normal and mutant forms of hypoxanthine phosphoribosyltransferase from HeLa cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):926–930. doi: 10.1073/pnas.74.3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell J., Gerace L., Leister F., Sofer W. Chemical selection of mutants that affect alcohol dehydrogenase in Drosophila. II. Use of 1-pentyne-3-ol. Genetics. 1975 Jan;79(1):73–83. doi: 10.1093/genetics/79.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell J., Mandel H. C., Krauss M., Sofer W. Genetic and cytogenetic analysis of the Adh region in Drosophila melanogaster. Genetics. 1977 Jul;86(3):553–566. doi: 10.1093/genetics/86.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Nucleic acid hybridization to the DNA of cytological preparations. Methods Cell Biol. 1975;10:1–16. doi: 10.1016/s0091-679x(08)60727-x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Reddy A. R., Pelliccia J. G., Sofer W. Adh-negative mutants: detection of an altered tryptic peptide in a mutant enzyme of Drosophila. Biochem Genet. 1980 Apr;18(3-4):339–351. doi: 10.1007/BF00484247. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Apr;3(4):863–877. doi: 10.1093/nar/3.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Sofer W. Alcohol dehydrogenase-negative mutants in Drosophila: defects at the structural locus? Genetics. 1976 May;83(1):125–136. doi: 10.1093/genetics/83.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofer W. H., Hatkoff M. A. Chemical selection of alcohol dehydrogenase negative mutants in drosophila. Genetics. 1972 Nov;72(3):545–549. doi: 10.1093/genetics/72.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofer W., Ursprung H. Drosophila alcohol dehydrogenase. Purification and partial characterization. J Biol Chem. 1968 Jun 10;243(11):3110–3115. [PubMed] [Google Scholar]
- Strobel E., Dunsmuir P., Rubin G. M. Polymorphisms in the chromosomal locations of elements of the 412, copia and 297 dispersed repeated gene families in Drosophila. Cell. 1979 Jun;17(2):429–439. doi: 10.1016/0092-8674(79)90169-7. [DOI] [PubMed] [Google Scholar]
- Thatcher D. R., Sawyer L. Secondary-structure prediction from the sequence of Drosophila melanogaster (fruitfly) alcohol dehydrogenase. Biochem J. 1980 Jun 1;187(3):884–886. doi: 10.1042/bj1870884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. O., Lee J. C. Integration of synthetic globin genes into an E. coli plasmid. Nucleic Acids Res. 1976 Aug;3(8):1961–1971. doi: 10.1093/nar/3.8.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff R. C., Ashburner M. The genetics of a small autosomal region of Drosophila melanogaster containing the structural gene for alcohol dehydrogenase. I. Characterization of deficiencies and mapping of ADH and visible mutations. Genetics. 1979 May;92(1):117–132. doi: 10.1093/genetics/92.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff R. C., Ashburner M. The genetics of a small autosomal region of Drosophila melanogaster containing the structural gene for alcohol dehydrogenase. II. Lethal mutations in the region. Genetics. 1979 May;92(1):133–149. doi: 10.1093/genetics/92.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]