Abstract
Parasitic plants invade host plants in order to rob them of water, minerals and nutrients. The consequences to the infected hosts can be debilitating and some of the world's most pernicious agricultural weeds are parasitic. Parasitic genera of the Scrophulariaceae and Orobanchaceae directly invade roots of neighboring plants via underground structures called haustoria. The mechanisms by which these parasites identify and associate with host plants present unsurpassed opportunities for studying chemical signaling in plant-plant interactions. Seeds of some parasites require specific host factors for efficient germination, thereby insuring the availability of an appropriate host root prior to germination. A second set of signal molecules is required to induce haustorium development and the beginning of heterotrophy. Later stages in parasitism also require the presence of host factors, although these have not yet been well characterized. Arabidopsis is being used as a model host plant to identify genetic loci associated with stimulating parasite germination, haustorium development, and parasite support. Arabidopsis is also being employed to explore how host plants respond to parasite attack. Current methodologies and recent findings in Arabidopsis – parasitic plant interactions will be discussed.
Introduction
While an oft-taught dogma in biology curricula is that plants differ from animals in being able to generate organic nutrients from inorganic raw materials and sunlight, some plants obtain nutritional resources by feeding off other plants. For example mistletoes, the arboreal parasites closely associated with Christmas, are dependent on host plants for water, minerals, and to varying degrees, fixed carbon (Parker and Riches, 1993; Press and Graves, 1995). Like other parasitic plants, mistletoes directly invade their hosts and rob them of nutritional resources through specialized organs called haustoria (Kuijt, 1969). The direct attachment and penetration of host tissues make parasitic plants distinct from saprophytic plants, like Monotropa (Indian pipes), that use ectomycorrhizal fungi as physiological bridges to connect with host plants (Leake, 1994).
Plant parasitism has originated multiple times during angiosperm evolution, and consequently, parasitic genera vary considerably in their habits and host ranges (Kuijt, 1969; Nickrent et al., 1998). Viscaceae and Loranthaceae (mistletoe families) parasitize aerial parts of woody plants, Cuscutaceae (dodder family) are parasitic vines that entwine herbs and shrubs, and Scrophulariaceae (figwort family) and the closely related Orobanchaceae are subterranean parasites that invade roots of nearby plants. Some parasitic plants are completely dependent upon host resources while others can survive in the absence of host contacts. Holoparasites, which lack the ability to photosynthesize, typically have reduced chloroplast genomes that have undergone extensive deletions and rearrangements (dePamphilis and Palmer, 1990). Vegetative parts of evolutionarily advanced parasites can be dramatically reduced: Rafflesia, a bizarre, leafless and rootless parasitic plant, consists of little more than the world's biggest flower. An excellent collection of parasitic plant photographs and data regarding their phylogenic relationships can be found at The Parasitic Plant Connection web site (Nickrent, 2001).
The impact of parasitism on host plants can be debilitating and some of the world's worst agricultural pests are parasitic weeds (Parker and Riches, 1993). The North American forestry industry is plagued by dwarf mistletoe (Arceuthobium), a Viscaceae holoparasite closely related to the more obvious leafy mistletoes (Columbia, 1995). It has been estimated that approximately 15 million cubic meters of lumber are lost each year in western US and Canadian forests to dwarf mistletoe, and in many areas dwarf mistletoes are considered the single most damaging pathogen of coniferous forest trees (Hawksworth and Wiens, 1996). Another family of parasitic angiosperms, the dodders, plague agricultural systems worldwide. Dodders are twining parasites that invade the aerial parts of crop plants and generally appear like tangles of yellow yarn when viewed at a distance (Healy et al., 2001).
But the most notorious of the parasitic weeds are the root parasites in the Scrophulariaceae and Orobanchaceae families. Striga, commonly known as witchweed, is a root parasite that aggressively attacks both legume and cereal crops (Healy et al., 2001). The Striga problem is most pronounced in sub-Saharan Africa where yield losses caused by Striga can be devastating to poor farmers. All crop grasses cultivated in Africa, e.g. maize, sorghum, rice, millet sugarcane and fonio, are parasitized by one or more Striga species and in West Africa alone about forty million hectares are heavily infested with Striga (Berner et al., 1995). The Food and Agriculture Organization of the United Nations estimates that the livelihood of some 300 million people in Africa is affected by Striga (Lagoke, 1991). Another root parasite with significant agricultural importance is Orobanche, commonly known as broomrape (Healy et al., 2001). Orobanche causes yield and quality losses to many agricultural crops including, Leguminosae, Solanaceae, Compositae and Cruciferae species mainly in the Mediterranean region (Parker and Riches, 1993). The identification of germplasm resistant to these parasitic weeds is a high priority for breeding programs catering to farms in affected areas.
Parasitic Scrophulariaceae and Orobanchaceae
The parasitic Scrophulariaceae and Orobanchaceae have received considerable attention because of their relevance in world agriculture. These families are interesting for evolutionary studies as well because they encompass closely related parasites with vastly different host requirements. While most Scrophulariaceae, such as Antirrhinum, are photosynthetic autotrophs, about a third of the 290 genera listed in the Kew Garden Database are able to parasitize other plants (Nickrent, 2001). Of these, the majority are facultative hemiparasites that are photosynthetically competent and able to grow, albeit poorly, in the absence of host plants. In contrast, some Scrophulariaceae and all Orobanchaceae are obligate parasites that require association with a host plant within a few days after germination.
Scrophulariaceae and Orobanchaceae have traditionally been recognized as distinct families based on several morphological characters, notably those associated with heterotrophic growth. For example, Orobanchaceae has achlorophyllous shoots and leaves while most Scrophulariaceae are able to photosynthesize, though typically at greatly reduced efficiencies (Stewart and Press, 1990). However, parsimony analysis of sequence data obtained from both plastid and nuclear DNA places the parasitic Scrophulariaceae and Orobanchaceae on a single phylogenetic clade (dePamphilis et al., 1997). This indicates that parasitism within the Scrophulariaceae and Orobanchaceae had a single evolutionary origin, presumably linked to the origin of invasive haustoria. Subsequent to the evolutionary origin of parasitism, there were multiple independent losses of photosynthesis. Therefore, while parasitism arose one time in Scrophulariaceae and Orobanchaceae, holoparasitism had multiple origins (dePamphilis et al., 1997). Because obligate holoparasites represent a more advanced state of parasitism than facultative hemiparasites, parasitic features that are shared by facultative and obligate parasites are more primitive.
The parasitic genera studied by the authors represent extremes on the continuum from facultative to obligate heterotrophy. Triphysaria is a facultative parasite common to grassland stands throughout the Pacific coast (Hickman, 1993). Triphysaria is fully photosynthetic and can grow to maturity without attaching to a host. Indeed, many wildflower enthusiasts never realize that this common springtime annual is a root parasite. Triphysaria will, however, parasitize the roots of other plants and in naturalized fields is almost always parasitic on nearby plants. Orobanche, in contrast, is a non-photosynthetic, obligate parasite that completes most of its lifecycle as a subterranean heterotroph. The life cycles of Triphysaria and Orobanche are contrasted in Figure 1. Additional photographs of these parasites can be found at the Parasitic Plant Connection (Nickrent, 1997) and at the Yoder Lab Homepage (http://veghome.ucdavis.edu/Faculty/Yoder/Lab/index.html.
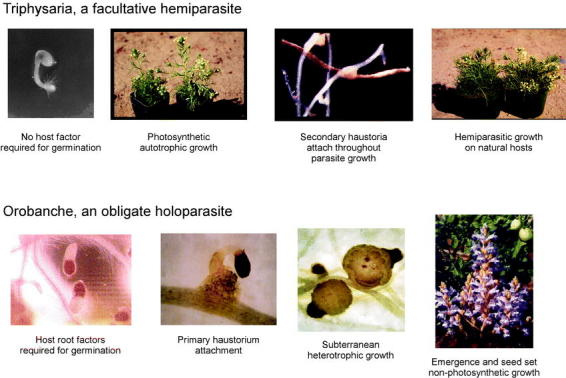
Figure 1: Life cycles of parasitic Scrophulariaceae and Orobanchaceae
In general the host range of facultative parasitic plants is broader than that of obligate parasites. In naturalized fields Triphysaria has been found associated with at least 27 host families (Thurman, 1966). Triphysaria parasitizes both monocots and dicots, including maize and Arabidopsis (Yoder, 1997). In the field, Triphysaria typically encounters roots of several different host species and ecological advantages may be achieved by parasitizing multiple hosts (Marvier, 1998). In contrast, the host range of obligate parasites is more specialized. Orobanche is restricted to dicot hosts and Striga, with the exception of S. gesnerioides, to monocots (Parker and Riches, 1993). Race specificity is best defined for S. gesnerioides where five races have been identified based on differential susceptibility of cowpea cultivars (Ouedraogo et al., 2001).
Host specialization in obligate parasites may be associated with their need to identify and invade the correct host root within days of germination in order to survive. As discussed below, obligate parasites identify potential host roots as a prerequisite to germination. Hemiparasites are under less stringent deadlines and host attachment may occur at both early and later stages in their lifecycles. While many hosts can be infected by facultative parasites, some hosts, notably nitrogen rich legumes, are preferred (Gibson and Watkinson, 1989). Secondary metabolites produced by host plants can further enhance their attractiveness to parasites; alkaloids produced by Lupinus albus and taken up by parasitizing Castilleja plants decrease herbivore damage and increase pollination in the parasite (Adler, 2000). On the other hand, pot studies have shown that some hosts inhibit parasite performance compared to growth without a host, suggesting detrimental metabolites in the host (Atsatt and Strong, 1970). These different selective pressures on Triphysaria and Orobanche are reflected in differences in their host identification and invasion mechanisms (Figure 1).
Parasite responses to host factors: germination stimulants
One of the most intriguing abilities of parasitic plants is their ability to identify host plants through chemical signals released by hosts into the rhizosphere. The obligate parasites Striga and Orobanche have evolved sophisticated host detection systems that ensure the presence of nearby host roots before germination. Subsequent to a preconditioning period of suitable moisture and temperature conditions, parasite seeds need to be exposed to specific germination stimulants released by potential host roots in order to germinate. By using host factors as germination cues, the parasite ensures that host roots are nearby before committing to germination.
Germination stimulants have been identified from a number of host roots, notably sorghum (Chang et al., 1986; Hauck et al., 1992), maize (Siame et al., 1993) and legumes (Müller et al., 1992; Yokota et al., 1998). The most common class of germination stimulants are sesquiterpene lactones, collectively referred to as strigolactones (Wigchert and Zwanenburg, 1999) (Figure 2). Strigolactones stimulate germination at low concentrations (10−8 to 10−12 M) and structural studies of natural and synthetic analogs revealed that bioactivity resides in the C and D rings with their absolute configuration being critical (Thuring et al., 1997). Together these results suggest that Striga perceives these molecules through a receptor-mediated process (Mangus and Zwanenburg, 1992). Another class of germination factors identified in host exudates is the dihydrosorgoleone referred to as SXSg (sorghum xenognosin of Striga germination) (Chang et al., 1986). The active hydroquinone of SXSg is rapidly oxidized to inactive sorgoleone and it has been hypothesized that this may explain why Striga seeds need to be close to host roots in order to germinate (Fate et al., 1990). The concentration of SXSg needed for half maximal germination of Striga seed is at least three orders of magnitude higher than that of strigolactone, so it is likely that these two classes of germination factors are perceived differently (Wigchert and Zwanenburg, 1999).
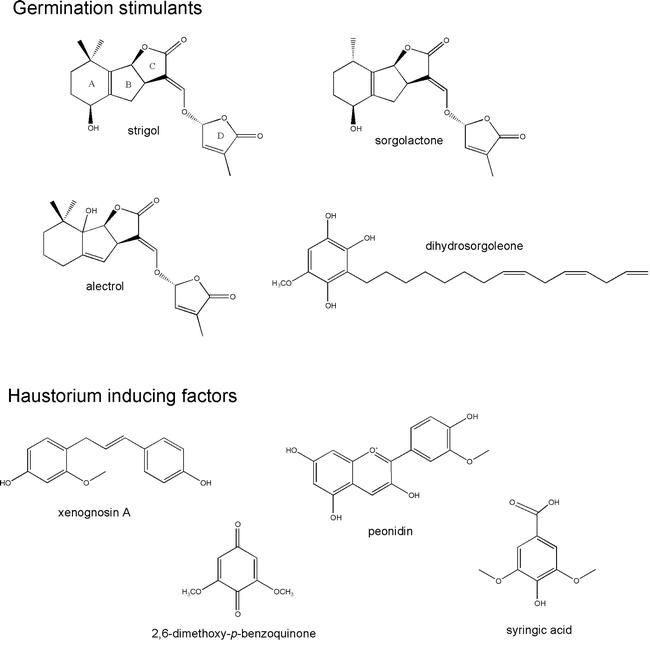
Figure 2: Host recognition factors The naturally occurring strigolactone germination stimulants strigol, sorgolactone, and alectra are shown (Wigchert et al., 1999). Another sorgolactone stimulant for Orobanche germination is orobanchol, and apparent isomer of strigol (Yokota, 1998). Another class of natural germination stimulants is represented by dihydrosorgoleone (Fate and Lynn, 1996). Haustoria are induced by a variety of plant phenolics including flavonoids (Lynn et al., 1981; Albrecht et al., 1999), quinones (Chang and Lynn, 1986), and phenolic acids (Riopel and Timko, 1995).
The requirement of Striga and Orobanche for host germination factors is one component of host specificity and has been employed in developing crop resistance (or avoidance) to these parasites. A single recessive mutation that reduces germination stimulant production in sorghum confers some resistance against Striga (Vogler et al., 1996). While the resistant line still stimulates germination, an in vitro bioassay demonstrated that the maximum distance from the sorghum root that Striga seeds germinated was significantly lower in the resistant lines. Most importantly, this line shows good resistance under field conditions and it is currently being planted in at least twelve African countries (Ejeta et al., 1997). Variability in the production of Orobanche germination stimulant has been observed in tobacco, pepper and faba been, but not yet genetically characterized (Racovitza, 1973; Aalders and Pieters, 1986; Hershenhorn et al., 1996; Alonso, 1998).
Germination factors are not sufficiently specific to distinguish host from non-host roots. In fact, the first stimulant identified, strigol, was originally obtained from roots of the non-host cotton (Cook et al., 1966). The production of germination factors by non-host crops can in principle be exploited in agricultural situations by using these ‘false hosts’ or ‘trap crops’ to germinate parasite seed banks without allowing them a real host on which to grow. Interestingly, some Orobanche-resistant species of Vicia release particularly active germination factors that can enhance parasitism on neighboring susceptible plants (Zaitoun and ter Borg, 1994; Goldwasser et al., 1997).
Parasite responses to host factors: haustoria inducing factors
After germination a second set of host recognition factors is required to trigger the transition from autotrophic to parasitic growth. This transition is characterized by the development of haustoria, parasite-specific organs that attach to and invade host tissues. Haustoria also function as physiological bridges through which host resources are translocated into the parasite and therefore function at multiple stages in parasitism. Because all parasitic plants make haustoria and they fulfill parasite-specific functions, haustoria “embody the very idea of parasitism” (Kuijt, 1969). In facultative parasites that germinate without host factors, haustorium development is the primary step in host recognition.
Haustorium development is different in facultative and obligate parasites, again a reflection of the urgency with which the obligate parasite must invade a host (Figure 1). Obligate parasites form haustoria at the tips of the emerging radical. These have been traditionally called primary haustoria and result from a terminal differentiation of the radical meristem (Kuijt, 1969; Riopel and Baird, 1987). The primary haustorium grows into a largely undifferentiated tubercle that eventually produces its own root-like structures and shoots, but only after host contact and penetration.
Unlike primary haustoria, secondary haustoria develop slightly proximal to the root tips and do not terminate the radical. All parasitic Scrophulariaceae develop secondary haustoria, though in obligate species only after the primary haustorium has penetrated a host. In Triphysaria, secondary haustoria develop most readily just behind the root tip, although haustoria can also develop from more proximal, mature root regions. The root system of a single mature Triphysaria plant collected from the field may have hundreds of haustoria attached to multiple host roots.
It has been known for several years that root parasites typically develop haustoria only when the emerging radicals contact the host root (Saunders, 1933; Atsatt et al., 1978; Riopel and Musselman, 1979). Two flavonoids, xenognosin A and B, and the quinone 2,6-dimethoxybenzoquinone (DMBQ) were isolated from host extracts as inducers of haustoria (Chang and Lynn, 1986; Steffens et al., 1986). In vitro studies demonstrated that an assortment of phenolic acids, quinones, and flavonoids can initiate haustorium development when applied to parasite roots (Riopel and Timko, 1995; Albrecht et al., 1999) (Figure 2). A model to explain the bioactivity of structurally distinct molecules is that haustorium signaling is triggered by redox cycling between electrochemical states of the inducers. In this model, the electrical potential of the inducer is a determining factor in its activity (Keyes et al., 2000).
The haustorium initially adheres to the host root by a mucilaginous substance secreted from haustorial hairs (Baird and Riopel, 1985; Joel and Losner-Gosher, 1994). Following attachment, invasive cells within the haustoria push their way through the host epidermis and cortex, apparently facilitated by pectolytic enzymes (Losner-Goshen et al., 1998). Studies of germinating Orobanche seedlings in the presence of various substrates indicate that the parasite secretes pectin methylesterase (PME), polygalacturonase, and endocellulase, but not exocellulase, b-glucosidase or xylanase (Losner-Goshen, et al., 1998; Shomer-Ilan, 1993; Ben-Hod et al., 1993). These observations have been supported in situ by immunocytochemical studies that demonstrate the presence of PME in Orobanche haustorial cells and the apoplast of adjacent host tissue (Losner-Goshen et al., 1998). At the same time, histochemical staining and immunolabeling of pectins have demonstrated a decrease in methylated pectins in the host middle lamella near the parasite intrusive cells. A similar study with the parasite Rhamphicarpa fistulosa supports this finding (Neumann et al., 1999).
The haustorium develops further once it has entered into host tissue. One of the most obvious events is the development of a xylem tube connecting host and parasite vascular systems (Figure 3). Xylem formation only occurs upon contact with the host stele and is not observed in the absence of host contact (Yoder, 1998). Although electron micrographs indicate sieve pore connections between host and Orobanche sieve elements (Dörr, 1996), the route of solute transport from host to parasite remains an incomplete picture. The parasites Orobanche and Striga appear to capture water and other resources by establishing a strong osmotic pull on the host. This is aided by biosynthesis of polyhydric alcohols such as mannitol in the parasites, which helps maintain a low water potential in the parasite relative to the host (Harloff and Wegmann, 1993; Press and Graves, 1995; Robert et al., 1999). Orobanche accumulates a wide variety of compounds present in the host phloem, including sugars (primarily sucrose) and translocated herbicides (Müller and Distler, 1991; Nandula et al., 1999).
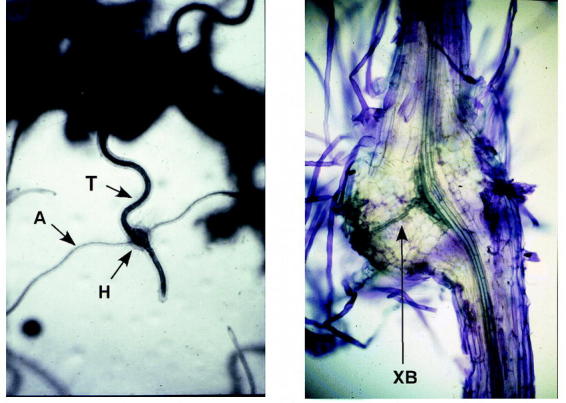
Figure 3: Triphysaria haustoria on Arabidopsis root The photo on the left shows a haustorium (H) forming on the root of Triphysaria (T) upon contact with an Arabidopsis root (A) in vitro. The photo on the right is a free hand section through a haustorium and shows the xylem bridge (XB) (photo by Hugette Albrecht). The Arabidopsis root has been detached from the haustorium and is not seen in this section.
Host responses to plant parasitism
The host response to parasitism is complex, apparently consisting of efforts to defend itself even as its metabolism is redirected to feed the attached parasite. With respect to defense responses, little definitive work has been published, perhaps in part due to the scarcity of resistant germplasm in crops. Nevertheless, some important clues to host response can be derived from these studies. Microscopic examination of resistant host varieties has found an accumulation of electron dense material in association with parasite growth arrest in the root cortex or at the barrier of the endodermis i.e. (Olivier et al., 1991; Neumann et al., 1999). In vetch (Vicia spp.), resistance to O. aegyptiaca is associated with accumulation of a reddish substance in the apoplastic space between parasite cells and those of the host. This observation is supported by biochemical analysis of host roots as the induced resistance response was correlated with higher concentrations of phenolics and lignin, and greater peroxidase activity than in the susceptible species (Goldwasser et al., 1999). Although PAL activity was not induced by parasitism, constitutive activity was five-fold higher in the resistant vetch genotype compared to the susceptible vetch genotype. Furthermore, a study of constitutive PAL activity in ten non-parasitized genotypes belonging to three Vicia species showed a correlation between PAL activity and the degree of resistance (Goldwasser et al., 1997). This biochemical analysis suggests that resistance may depend on quantitative rather than qualitative defense responses.
Alternatively, induction of lignification and phytoalexin biosynthetic pathways may accompany other, as yet unidentified, responses that play a more direct role in effective defense responses. For example, accumulation of colored material in association with resistance in a S. hermonthica-Sorghum bicolor interaction was attributed to a consequence of resistance, occurring after parasite development had been effectively arrested (Arnaud et al., 1999). Also, the hypersensitive response (HR), has been proposed as a mechanism of cowpea (Vigna unguiculata) resistance to S. gesnerioides based on localized host root necrosis at the site of parasite penetration (Lane et al., 1993). A study of S. asiatica parasitism of marigold (Tagetes erecta), a non-host of Striga (Gowda et al., 1999), demonstrated induction of a gene (NRSA-1) with homology to R genes, that was expressed in roots by following parasitism. However, the role of NRSA-1 in defense is not clear, as its structure suggests that it is not involved in pathogen recognition but may be part of the signal transduction cascade.
At the level of gene expression, transgenic tobacco bearing chimeric promoter-GUS fusions have been used to demonstrate the localized induction of defense related genes in compatible interactions with O. aegyptiaca. A key gene in the isoprenoid pathway, 3-hydroxy-3-methylglutaryl CoA reductase (HMG2), was induced following parasitism (Westwood et al., 1998) in a manner consistent with its role in local wound/pathogen response (Weissenborn et al., 1995). This suggestion of induction of genes associated with phytoalexin biosynthesis has been supported by recent work with genes from isoprenoid (Griffitts et al., 2001) and phenylpropanoid pathways (Griffitts, Cramer, and Westwood, unpublished data). In addition, Joel et al. (Joel and Portnoy, 1998) found that O. aegyptiaca induced expression of PRB-1b, a basic pathogenesis related (PR) protein expressed in response to such diverse stimuli as wounding, ethylene, SA, TMV, and darkness (Sessa et al., 1995). These results indicate that the parasitic plant is recognized as an invader that triggers certain defense pathways, even in a fully susceptible host. The question of why these host defense pathways are not effective in repelling parasitism, and what accounts for successful host defense in other cases, remains one of the pressing issues in protecting crops from parasitic plants.
In addition to defense responses, parasites have other impacts on host physiology. Using tobacco plants carrying a farnesyltransferase gene promoter fused to GUS, we have observed strong GUS expression in association with O. aegyptiaca tubercles (Westwood, Zhou, and Cramer, unpublished data). Expression of the farnesyltransferase promoter is associated with cell cycle control and nutrient partitioning but is not induced in defense situations (Qian et al., 1996). The expression of this gene in association with O. aegyptiaca parasitism is consistent with the parasite acting as a strong sink and suggests that the parasite has some sophistication in its ability to manipulate host resource allocation.
In summary, the identification and parasitism of host roots by parasitic plants offers an unsurpassed system for investigating molecular mechanisms that mediate plant-plant interactions. The agricultural destructiveness of parasitic weeds has further driven efforts to dissect the genetic components regulating host plant recognition. Naturally, Arabidopsis has been turned to as a model to identify host factors essential for successful parasitism. Arabidopsis populations are being examined for variability in stimulating parasite germination, haustorium development, and parasite support. Arabidopsis is also being employed to explore how host plants respond to parasite attack and Arabidopsis genome data will help determine which genetic factors are associated with parasitism. We predict that studies of parasite recognition, invasion, and host defense using Arabidopsis will lead to novel biological strategies for controlling parasitic weeds in agriculture.
Germination of Orobanche by Arabidopsis factors
The germination of Orobanche seeds can be monitored in the presence of Arabidopsis by various in vitro methods, three of which are given in Methods. The growth of Arabidopsis and Orobanche together in polyethylene bags allows the observation of Orobanche germination and subsequent parasitism development stages including attachment, tubercle and floral shoot formation on Arabidopsis roots (Figure 4) (Parker and Dixon., 1983). Orobanche germination in response to host factors can also be monitored on filter paper and agar, the latter having been adapted to a 96 well format to allow screening of mutagenized populations. In all cases, the synthetic germination stimulant GR24 can be used as a positive control for germination. Negative controls are obtained by mock-treating seeds with water to determine spontaneous germination rates in the absence of host factors.

Figure 4: In vitro assay of Orobanche parasitism of Arabidopsis The polyethylene bag method is used to monitor the germination, attachment and invasion of O. aegyptiaca into Arabidopsis roots.
Considerable variability exists between different Orobanche species in their ability to germinate in the presence of an Arabidopsis root. In filter paper germination assays, Arabidopsis stimulated germination of O. aegyptiaca, O. minor, and O. ramosa seeds at levels approximately 60–80% of those obtained with the host plants carrot and tobacco, or with GR24 (Westwood, 2000; Goldwasser and Yoder, 2001). Seeds of O. crenata, O. cumana, and O. cernua, were not stimulated to germinate at levels over background. These findings are consistent with the known host range of Orobanche species on agricultural crops because Cruciferae, notably mustard, cabbage, and rapeseed, are susceptible to O. aegyptiaca and O. ramosa but not O. crenata and O. cumana (Parker and Riches, 1993).
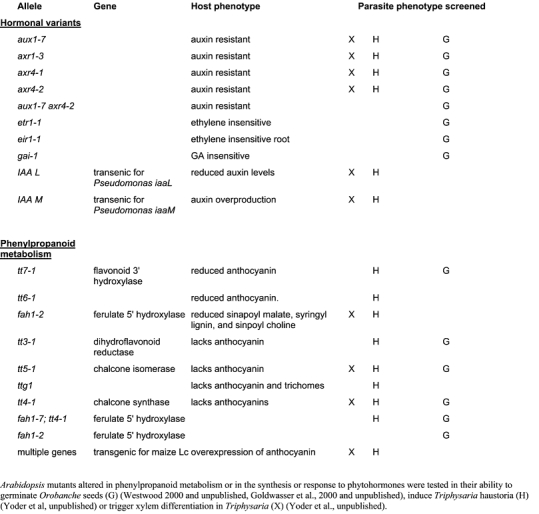
A total of 327 ecotypes representing populations from 23 countries throughout the globe were screened for their ability to germinate and support O. aegyptiaca (Westwood and Foy, 1998; Goldwasser et al., 2000). To date there have been no significant differences in germination rates between the ecotypes with all ecotypes inducing germination of O. aegyptiaca. Orobanche germination was also monitored in the presence of Arabidopsis defective in different steps of phenylpropanoid metabolism or altered in hormone sensitivity (Table 1). Again, all mutants examined to date have stimulated Orobanche germination ((Westwood, 2000) and unpublished).
We screened 13,000 M2 seedlings (representing 1,625 M1 parent families) from a fast neutron mutated population and recovered 34 individuals that did not stimulate germination. Progeny analysis showed that at least some M3 progeny stimulated O. ramosa germination for all M2 candidates but Chi Square analyses indicated significant differences in the number of M3 that induced germination between different M2 lines. There was a significant correlation between germination rate and the maximum distance that O. ramosa germination occurred from the Arabidopsis root (MGD). The low germination-stimulating Arabidopsis induced less total O. ramosa germination and only in seeds close to the root. Limited segregation analyses suggest that variation in germination stimulant production is quantitatively inherited (Goldwasser and Yoder, 2001)
Because all Arabidopsis mutants examined have stimulated germination to some degree, it is likely that there are either multiple stimulant molecules, and/or a redundancy in the genes encoding enzymes involved in stimulant synthesis.
Haustorium development in Triphysaria exposed to Arabidopsis factors
A different set of host recognition factors is used by the parasite to initiate haustorium development. Haustorium development in Triphysaria can be monitored in vitro by exposing parasite seedlings to host plant roots, host root exudates, or purified haustoria inducing factors (see Methods).
Exudates obtained from hydroponically grown Arabidopsis induce haustoria development when applied to aseptically grown Triphysaria seedlings. Haustorium development in response to maize root exudates is illustrated in Matvienko et al. (2001) and as a time lapse reconstruction at the Yoder web site; a similar ontogeny is observed when treated with Arabidopsis exudates. Elongation of the parasite root tip stops within thirty minutes of exposure to host exudates. Within four to six hours epidermal cells near the root tip elongate, forming haustorial hairs. At about the same time, cortical cells within the haustorium begin to swell, resulting in a noticeable swelling of the root near the tip. After about 24 hr, haustorium development terminates, the root apparently reverts to its more typical growth program and a normal appearing root emerges from the secondary haustorium. Development of primary haustoria is similar except there is no reversion to normal root growth until host contact is made (Riopel and Timko, 1995). Haustorium development in response to Arabidopsis root factors is synchronous, rapid, and morphologically obvious. As such, it is an amenable system for investigating how plants respond to chemical signals released by Arabidopsis roots.
Different Arabidopsis were tested as potential hosts for T. versicolor by growing seedlings of each together in agar culture and examining the parasite roots for haustoria formation after several weeks (Figure 3). We assayed fifty-nine ecotypes of Arabidopsis and all ecotypes stimulated haustorium development. We also examined haustoria development in the presence of Arabidopsis bearing mutations in phenylpropanoid and phytohormones synthesis and responsiveness (Table 1). Haustoria formation was monitored over six weeks and in no case did we detect a change in susceptibility to T. versicolor. We further assayed approximately 6000 M2 Arabidopsis from an EMS mutagenized population, but all induced haustoria in T. versicolor to similar degrees. T. versicolor forms haustoria on all Arabidopsis lines examined to date.
Xylem development
Once the haustorium has attached and penetrated a host root, a vascular connection between the parasite and host plant vascular systems is established. Most striking is the development of xylem tubes that connect the two plants. Xylem formation within the Triphysaria haustorium is absolutely dependent on host contact (Yoder, 1998). Because auxin stimulates cortical cells to differentiate into xylem (Aloni, 1995), we were interested in testing the role of host auxin levels on Triphysaria xylem formation.
We grew Triphysaria together with transgenic Arabidopsis that were altered in overall auxin levels and monitored the formation of xylem elements within attached haustoria (Yoder et al., unpublished). Expression of the Pseudomonas savastanoi indoleacetic acid-lysine synthetase (iaaL) gene in Arabidopsis leads to reduced, but not eliminated, auxin levels by converting IAA to IAA-lysine (Romano et al., 1991). Reciprocally, Arabidopsis constitutively expressing the Agrobacterium tumefaciens tryptophan monooxygenase (iaaM) gene have elevated levels of auxin (Romano et al., 1995). Preliminary results indicate that while between 80–90% of the haustoria formed with non-transgenic or iaaM-bearing Arabidopsis have xylem elements, only about 30% of the haustoria formed on iaaL containing Arabidopsis have xylem (Figure 5). This suggests that the parasite might recruit host hormones to direct its own development.
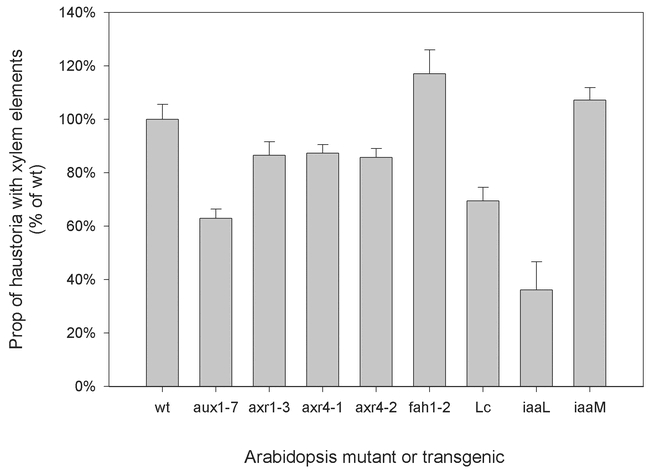
Figure 5: Xylem formation with Arabidopsis auxin variants Triphysaria were grown with different Arabidopsis mutants in agar. Haustoria that developed were cleared and examined for xylem bridges. The proportion of haustoria with xylem bridges is shown for each mutant host. The Standard Error was calculated as with n being the number of haustoria examined.
Post attachment interactions between Orobanche and Arabidopsis
As described above, germination of some Orobanche species is not stimulated by Arabidopsis; nevertheless, Arabidopsis can be parasitized if the Orobanche seed is artificially stimulated to germinate in the presence of the host root. For example, O. crenata does not normally germinate in the presence of Arabidopsis roots, but once germinated, would form normal connections and tubercles (Westwood, 2000). Orobanche development on Arabidopsis is similar to that on agricultural hosts (i.e. carrot, tomato, tobacco), although parasites developing on Arabidopsis are proportionately smaller at maturity than those developing on larger hosts. For example, whereas O. aegyptiaca growing on tomato may produce tens of flowers, this species growing on Arabidopsis will only produce 1–4 flowers (Figure 6). This plasticity in size and ability to adjust flowering in coordination with the amount of resources gained from the host is another remarkable aspect of parasitic plant biology.

Figure 6: Orobanche growth on Arabidopsis in pots Arabidopsis supporting a flowering O. aegyptiaca
A total of 309 ecotypes of Arabidopsis were tested for overall resistance to O. aegyptiaca. Young Arabidopsis plants were grown in the PE bag system described in Methods, inoculated with parasite seeds, and parasite attachment and growth monitored over time. Because plants of different ecotypes were grown side by side with no barriers to the diffusion of germination stimulants, the screen assessed tubercle formation in addition to germination. Under these conditions, all healthy Arabidopsis plants were parasitized and inspection of each parasite tubercle revealed normal development and root associations. In addition to ecotypes, several mutant lines of Arabidopsis have been assayed for ability to support O. aegyptiaca growth. These include members of the tt mutant class that are deficient in flavonoid metabolism and several hormone mutants (Table 1). If O. aegyptiaca were sensitive to host flavonoid or hormone levels, it would be expected that haustorial connections or tubercle growth would result in death or disruption of parasite growth, but no such visible symptoms were observed.
Arabidopsis mutant lines have also been employed to investigate defense interactions, and O. aegyptiaca was grown on Arabidopsis mutants having altered defense responses, including greater disease resistance as compared to wild type plants. Arabidopsis lines studied included variants of the lesion simulating disease (lsd) mutants (Dietrich et al., 1994), which exhibit increased sensitivity to stimuli that trigger systemic resistance and decreased control over processes leading to cell death. Orobanche was able to parasitize Arabidopsis lsd mutants in a manner similar to wild type Columbia lines in both the number and mean weight of tubercles per host (Westwood, unpublished data). These findings suggest that O. aegyptiaca is not inducing the defense pathways affected by the lsd mutations, but so little information is available on defense response in roots of Arabidopsis that it is difficult to draw definitive conclusions from this lack of response.
In order to identify host genes that are induced in response to Orobanche parasitism, we have screened promoter-trap lines of Arabidopsis generated by transforming Arabidopsis with a T-DNA containing a promoterless GUS construct close to the right border (Barthels et al., 1997) (Westwood, unpublished data). These lines have been screened for GUS expression in response to O. aegyptiaca using the PE bag method and at least 33 different lines expressing GUS have been identified. The patterns of GUS expression observed among these lines represent a range of responses to the parasite. All the transgenics examined to date express GUS in tissues other than roots in addition to the parasite-induced response. For example, many of the patterns indicate specificity for the stele and may be associated with vascular tissue and nutrient distribution. It is also common to find expression associated with secondary root branch points. Identification, cloning, and characterization of the tagged genes are currently underway.
The extreme susceptibility of Arabidopsis to parasitism by O. aegyptiaca may be an advantage for Arabidopsis as a model host. The lack of resistant germplasm identified to date from among ecotypes and mutants demonstrates that the parasite is flexible in its ability to adapt and compensate for perturbations in the host system. Once resistance mechanisms are identified in Arabidopsis, it is likely that they will be informative in identifying critical factors involved in the host-parasite interaction.
Methods
Polyethylene bag assay of Orobanche parasitism of Arabidopsis
This is a non-destructive assay useful formonitoring multiple stages of parasitism and is illustrated in Figure 4.
Orobanche and Arabidopsis seeds are surface-sterilized by soaking in 70% ethanol for 1 minute and then in 1% sodium hypochlorite+0.1% Tween 20 for 15 minutes. The seeds are washed 3 times with sterile water. Surface-sterilized Orobanche seeds (0.01g) are sprinkled onto sterilized moist 14 by 12 cm glass-fiber sheets and placed in a 23°C growth chamber (14h light of 100 mE m2s−1). Arabidopsis seeds are placed on ? strength Murashige and Skoog salts (MS) (GibcoBRL, Grand Island, NY) agar in a 5 cm diameter Petri dish and put into the 23°C growth chamber. Six 2-week-old Arabidopsis seedlings are extracted from the agar and mounted on the top of each glass-fiber sheet. Each sheet is placed in a clear 25 by 18 cm polyethylene bag that is placed upright in a black box so that plant roots are in the dark box and their shoots project into the light above the box. Plants are covered with another polyethylene bag to retain moisture and the box is placed in the 23°C growth chamber. Bags are replenished with 1/4 strength Hoagland nutrient solution (Hoagland and Arnon, 1950) as needed. Observations regarding Arabidopsis growth and Orobanche seed germination, radical attachment and development are conducted periodically on either 2 by 2 cm root sections or on the whole root system using a stereoscopic microscope (X10-X60).
Filter paper germination assay
Surface sterilized seeds are vacuum dried on a Buchner apparatus and then sprinkled on four 5 cm diameter glass-microfiber filter discs (Whatman GF/A paper) wetted with 1 ml sterilized water and placed in 5 cm diameter Petri dish. The Petri dishes are sealed with Parafilm, packaged in aluminum foil and put in a growth chamber (23°C, 14h light of 100 mE m2s−1) for a preconditioning period. Two weeks later, Petri dishes are opened and sixty surface sterilized Arabidopsis seeds are placed on each 5 cm glass-microfiber disc. A positive control is prepared by not placing Arabidopsis seeds on the GF/A discs but applying 0.6 ml aqueous solution of the synthetic strigol analogue GR24 to each Orobanche-inoculated GF/A disc (5 ppm for O. aegyptiaca, O. ramosa and O. minor, 10 ppm for O. crenata and O. cumana). A negative control consists of treating the Orobanche seeds with sterilized water instead of the GR24 solution. All Petri dishes are resealed with Parafilm and returned to the growth chamber. Fourteen days after treatments, Orobanche seed germination rate is determined under a stereoscopic microscope by counting the number of seeds with and without an emerged radical.
96 well germination screening assay
This technique allows a single plant screening of Arabidopsis ecotypes, lines and mutants in order to detect a germination phenotype of interest (Figure 7).

Figure 7: High throughput screen for germination stimulants A single Arabidopsis seedling is added to agar containing preconditioned Orobanche seeds in a 96 well microtiter plate. Germination is scored under a low power microscope.
Arabidopsis seeds are surface sterilized, suspended in 0.1% noble agar and plated in 9 cm Petri dishes containing 1/4 strength MS, 0.75% sucrose and 0.4% Phytagar (GibcoBRL Life Technologies, Rockville, MD). Petri dishes are sealed with Parafilm, placed for 2 days at 4°C in the dark and then transferred to the 23°C growth chamber for 7 days. Orobanche seeds are surface sterilized, suspended in deionized sterile water and transferred by vacuum onto a 9 cm glass-fiber filter paper disc. The dry inoculated filter is then placed in a 9 cm Petri dish and wetted with 2.5 ml deionized sterile water. Petri dishes are sealed with Parafilm, wrapped with aluminum foil and placed in the 23°C growth chamber. O. ramosa seeds are preconditioned for 14 days and O. aegyptiaca seeds for seven.
Preconditioned O. ramosa seeds are suspended in deionized sterile water and 20 ml suspension containing 20–50 seeds added to each well. One hundred ml of 1/4 strength MS without sucrose containing 0.6 % Phytagar at 45°C is added on top of the Orobanche seeds in each well. One-week-old Arabidopsis plants are transferred from the germination Petri dishes into the 96 well plate, one plant per well. In each plate eight wells are planted with wild type Arabidopsis as a positive control, and eight wells are left without host plants as negative controls. Plates are sealed with Micropore surgical tape (3M Health Care, St. Paul, MN) and placed in the 23°C growth chamber. Germination of Orobanche seeds is recorded seven and fourteen days later by inverting the plates and examining the seeds under the stereoscopic microscope.
Petri dish/agar germination rate and maximum germination distance assay
This assay determines germination rate together with the maximum germination distance (MGD) that Orobanche germinates from the Arabidopsis root.
Six ml 0.7% Phytagar are poured into a 5 cm diameter Petri dish containing a 200 ml pipette tip placed in the center of the Petri dish. After hardening of the agar, the tip is removed leaving a 3–5 mm wide groove in the agar. A uniform and adjustable O. ramosa seed distribution is achieved by suspending Orobanche seeds in warm (42°C) 0.6% Phytagar. 350 ml of this suspension, containing approximately 100 seeds, is added into the groove in the agar. A one-day-old Arabidopsis seedling is then placed at one side of the groove at the edge of the Petri dish. The Petri dish is sealed with Parafilm, wrapped with aluminum foil and placed in a 23°C growth chamber. Seven days after planting, the germination rate of Orobanche seeds is determined and the MGD from Arabidopsis roots is recorded under a stereoscopic microscope. Negative controls are performed using Petri dish with Orobanche seeds but in the absence of Arabidopsis seedlings, while positive controls are achieved by an identical set up to which 50 µl GR24 is added.
In vitro assay for Triphysaria parasitism of Arabidopsis
Arabidopsis seeds are surface sterilized by rinsing them in 70% ethanol for five minutes, followed by 25% bleach and 0.01% triton for ten minutes. Sterilized seeds are plated in deep petri dishes containing 1/4 MS media with 0.75% sucrose and 0.4% Phytagar at pH 5.8. Triphysaria seeds are surface sterilized in 70% ethanol for 10 minutes followed by 50% bleach and 0.02% triton for 30 minutes. Triphysaria seeds are plated on deep petri dishes containing 1/4 Hoagland's media (1.25mM CaNO3, 1.25mM KNO3, 0.5mM MgSO4, 0.25mM KH2PO4, 1X micronutrient solution (50mM H3BO3, 9.0mM MnCl2?4H2O, 70nM ZnSO4?7H2O, 30nM CuSO4?5H2O and 10nM Na2MoO4?2H2O)) (Johnson, 1977) with 1.0% sucrose and 0.6% Phytagar at pH 6.1. Plates containing both Arabidopsis and Triphysaria are kept in the dark at 2°C for 7 to 21 days, and then the plates are transferred to 16°C with 12 hours of light.
After ten days at 16°C, seedlings are transplanted into 24 well microtiter plates containing 2mL of 1/4 Hoagland's media in each well. Two Triphysaria and one Arabidopsis seedling are added to each well and the three plants grown together for 4 weeks. After that period, the wells are scored for the presence of haustoria by viewing the bottom under a light microscope with a magnification of 3X. A typical haustorial attachment is seen in Figure 3. Wells that do not contain haustoria are noted and checked again after 7 days.
Haustorium induction with Arabidopsis exudates
Triphysaria seeds are sterilized using a solution of 50% (v/v) Bleach (sodium hypochlorite 5.25%) and 0.1% (v/v) Triton X-100 (Sigma, St. Louis, MO), then thoroughly rinsed in 4 to 6 volumes of sterile de-ionized water. The seeds are then placed in round petri dishes (100 x 25 mm) containing 0.25X Hoagland's nutrient media and micronutrients, 1% (w/v) sucrose and 0.5 % (w/v) Phytagar. The plates are sealed with Parafilm and placed in a 16°C growth chamber under a 12-hour light regimen. The Triphysaria seeds germinate after seven to ten days under these conditions.
Approximately two weeks post-germination, Triphysaria seedlings are aseptically transferred to square petri plates containing 0.25x Hoagland's nutrient media, 1% (w/v) sucrose and 1% (w/v) Phytagar. In each plate, five seedlings are placed parallel to one another on the surface of the agar media. The plates are then wrapped with Micropore tape (3M Health Care, St. Paul, MN) and placed in racks, nearly vertical, to facilitate the growth of the root tips down the surface of the media. The seedlings are incubated in a 22°C growth chamber under a 16-hour light regimen for one week prior to induction.
Induction of Triphysaria root tips is achieved by adding 2mls of hydroponically isolated Arabidopsis root exudates directly to the root tips. Control seedlings are mock treated with sterile de-ionized water. Plates are kept horizontal for at least 30 minutes to allow absorption of the liquid inducer into the agar media. The number of haustoria and the number of root tips per plant is determined using a dissecting microscope at a magnification of 0.6 to 4X.
Preparation of Arabidopsis exudates
Arabidopsis seeds are surface sterilized in 70% ethanol (v/v) for 5 minutes, followed by 10 minutes in a solution of 30% (v/v) Bleach (sodium hypochlorite 5.25%) and 0.15% (v/v) Triton X-100 (Sigma, St. Louis, MO), then rinsed in 4 to 6 volumes of sterile de-ionized water. Using a sterile 1ml Pasteur pipette, approximately 30mg of seed are placed into 250 ml flasks containing 50 ml 0.5x MS salts, 1x Nitsch and Nitsch vitamin solution (Sigma Chemical Co., St. Louis, MO) and 0.075% (w/v) sucrose at pH 5.8. The flasks are placed on a shaker at 50 RPM in 22°C with 16 hours of light. After three weeks, the plants are removed from the media, which is filter sterilized through a 0.2mm filter (Nalge Nunc International, Rochester, NY) and stored at −20°C.
Parasitic weed research in the author's labs has been supported by grants from NSF #99-83053 (JIY), the Rockefeller Foundation (JIY), BARD research awards FI-272-98 (YG) and IS-3048-98 (JHW), and USDA #97-35315-4206 (JHW).
Footnotes
Citation: Goldwasser Y., Westwood J.H., and Yoder J.I. (2002) The Use of Arabidopsis to Study Interactions between Parasitic Angiosperms and Their Plant Hosts. The Arabidopsis Book 1:e0035. doi:10.1199/tab.0035
elocation-id: e0035
Published on: April 4, 2002
Table 1: Arabidopsis mutants examined for altered support of Triphysaria or Orobanche
References
- Aalders A. G., Pieters R. Plant vigor as a misleading factor in search for resistance in broad bean to Orobanche crenata. 1986;1(1):140–149. Proceedings of a workshop biology and control of Orobanche, Wageningen, pp. [Google Scholar]
- Adler L. S. Alkaloid uptake increases fitness in a hemiparasitic plant via reduced herbivory and increased pollination. Amer. Naturalist. 2000;1561(1):92–99. doi: 10.1086/303374. [DOI] [PubMed] [Google Scholar]
- Albrecht H., Yoder J. I., Phillips D. A. Flavonoids promote haustoria formation in the root parasite Triphysaria. Plant Physiol. 1999;1191(1):585–591. doi: 10.1104/pp.119.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R. The induction of vascular tissues by auxin and cytokinin. 1995;1(1):531–546. In Plant Hormones: Physiology, Biochemistry, and Molecular Biology. P. J. Davies, eds. (Dordrecht: Kluwer Academic Publishers), pp. [Google Scholar]
- Alonso C. A. Resistance to Orobanche and resistance breeding: a review. 1998;1(1):233–257. 4th Intl. Orobanche Workshop, Current Problems of Orobanche Researches, Inst. for Wheat and Sunflower, ‘Dobroudja’, Albena, Bulgaria, pp. [Google Scholar]
- Arnaud M-C., Véronési C., Thalouarn P. Physiology and histology of resistance to Striga hermonthica in Sorghum bicolor var. Framida. Aust. J. Plant Physiol. 1999;261(1):63–70. [Google Scholar]
- Atsatt P., Strong D. The population biology of annual grassland hemiparasites: I. The host environment. Evolution. 1970;241(1):278–291. doi: 10.1111/j.1558-5646.1970.tb01761.x. [DOI] [PubMed] [Google Scholar]
- Atsatt P. R., Hearn T. F., Nelson R. L., Heineman R. T. Chemical induction and repression of haustoria in Orthocarpus purpurascens (Scophulariaceae). Ann. Bot. 1978;421(1):1177–1184. [Google Scholar]
- Baird W. V., Riopel J. L. Surface characteristics of root haustorial hairs of parasitic Scrophulariaceae. Bot. Gazet. 1985;1461(1):63–69. [Google Scholar]
- Barthels N., van der Lee F. M., Klap J., Goddijn O. J. M., Karimi M., Puzio P., Grundler F. M. W., Ohl S. A., Lindsey K., Robertson L., Robertson W. M., Van Montagu M., Gheysen G., Sijmons P. C. Regulatory sequences of Arabidopsis drive reporter gene expression in nematode feeding structures. Plant Cell. 1997;91(1):2119–2134. doi: 10.1105/tpc.9.12.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hod G., Losner D., Joel D. M., Mayer M. Pectin methylesterase in calli and germinating seeds of Orobanche aegyptiaca. Phytochem. 1993;321(1):1399–1402. [Google Scholar]
- Berner D. K., Kling J. G., Singh B. B. Striga research and control: A perspective from Africa. Plant Disease. 1995;791(1):652–660. [Google Scholar]
- Chang M., Lynn D. G. The haustorium and the chemistry of host recognition in parasitic angiosperms. J. Chem. Ecol. 1986;121(1):561–579. doi: 10.1007/BF01020572. [DOI] [PubMed] [Google Scholar]
- Chang M., Netzly D. H., Butler L. G., Lynn D. G. Chemical regulation of distance: Characterization of the first natural host germination stimulant for Striga asiatica. J. Am. Chem. Soc. 1986;1081(1):7858–7860. doi: 10.1021/ja00284a074. [DOI] [PubMed] [Google Scholar]
- Columbia P. O. B. Dwarf Mistletoe Management Guidebook. 1995. British Columbia Ministry of Forests. http://www.for.gov.bc.ca/tasb/legsregs/fpc/fpcguide/dwarf/dwarftoc.htm#top.
- Cook C. E., Whichard L. P., Wall M. E., Egely G. H. Germination of witchweed (Striga lutea Lour.): Isolation and properties of a potent stimulant. Science. 1966;1961(1):1189–1190. doi: 10.1126/science.154.3753.1189. [DOI] [PubMed] [Google Scholar]
- dePamphilis C. W., Palmer J. D. Loss of photosynthetic and chlororespiratory genes from the plastid genome of a parasitic flowering plant. Nature. 1990;3481(1):337–339. doi: 10.1038/348337a0. [DOI] [PubMed] [Google Scholar]
- dePamphilis C. W., Young N. N., Wolfe A. D. Evolution of plastid gene rps2 in a lineage of hemiparasitic and holoparasitic plants: Many losses of photosynthesis and complex patterns of rate variation. Proceedings of the National Academy of Sciences USA. 1997;931(1):7367–7372. doi: 10.1073/pnas.94.14.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich R. A., Delaney T. P., Uknes S. J., Ward E. R., Ryals J. A., Dangl J. L. Arabidopsis mutants simulating disease resistance response. Cell. 1994;771(1):565–577. doi: 10.1016/0092-8674(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Dörr I. New results on interspecific bridges between parasites and their hosts. 1996;1(1):195–201. Advances in Parasitic Plant Research, Cordoba, Spain, Junta de Andalusia, pp. [Google Scholar]
- Ejeta G., Hermodson M., Tally S. Purdue, World Vision deliver life-saving seed to Africa. 1997. Purdue News. http://www.purdue.edu/UNS/html4ever/970314.Ejeta.striga.html.
- Fate G., Chang M., Lynn D. G. Control of germination in Striga asiatica: Chemistry of spatial definition. Plant Physiol. 1990;931(1):201–207. doi: 10.1104/pp.93.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson C. C., Watkinson A. R. The host range and selectivity of a parasitic plant: Rhinanthus minor L. Oecologia. 1989;781(1):401–406. doi: 10.1007/BF00379116. [DOI] [PubMed] [Google Scholar]
- Goldwasser Y. The basis for the differential response of vetch (Vicia spp.) to Egyptian broomrape (Orobanche aegyptiaca). 1998;1(1):86. Ph.D. thesis, The Hebrew University of Jerusalem, Israel. [Google Scholar]
- Goldwasser Y., Hershenhorn J., Plakhine D., Kleifeld Y., Rubin B. Biochemical factors involved in vetch resistance to Orobanche aegyptiaca. Physiol. Mol. Plant Pathol. 1999;541(1):87–96. [Google Scholar]
- Goldwasser Y., Kleifeld Y., Plakhine D., Rubin D. Variation in vetch (Vicia spp.) response to Orobanche aegyptiaca. Weed Sci. 1997;451(1):756–762. [Google Scholar]
- Goldwasser Y., Plakhine D., Yoder J. I. Arabidopsis thaliana susceptibility to Orobanche spp. Weed Sci. 2000;481(1):342–346. [Google Scholar]
- Goldwasser Y., Yoder J. I. Differential induction of Orobanche seed germination by Arabidopsis thaliana. Plant Science. 2001;1601(1):951–959. doi: 10.1016/s0168-9452(01)00331-4. [DOI] [PubMed] [Google Scholar]
- Gowda G. S., Riopel J., Timko M. P. NRSA-1: a resistance gene homolog expressed in roots of non-host plants following parasitism by Striga asiatica (witchweed). Plant J. 1999;201(1):217–230. doi: 10.1046/j.1365-313x.1999.00598.x. [DOI] [PubMed] [Google Scholar]
- Griffitts A. A., Cramer C. L., Westwood J. H. Characterization of host plant responses to parasitization by Orobanche aegyptiaca. 2001;1(1):178–181. 7th International Parasitic Weed Symposium, Nantes, France, Faculte des Sciences - Nantes, pp. [Google Scholar]
- Harloff H. J., Wegmann D. Evidence for a manitol cycle in Orobanche ramosa and Orobanche crenata. J. Plant Physiol. 1993;1411(1):513–520. [Google Scholar]
- Hauck C., Mueller S., Schildknecht H. A germination stimulant for parasitic flowering plants from Sorghum bicolor, a genuine host plant. J. Plant Physiol. 1992;1391(1):474–478. [Google Scholar]
- Hawksworth F. G., Wiens D. Dwarf Mistletoes: Biology, Pathology, and Systematics. 1996. In Agriculture Handbook 709. B. W. Geils and R. G. Nisley, eds.(Washington, DC: United States Department of Agriculture Forest Service), http://www.rms.nau.edu/publications/ah_709/frames.html.
- Healy E. A., Enloe S., DiTomaso J. M. Dodder. 2001. In ENCYCLOWEEDIA: Notes on Identification, Biology, and Management of Plants Defined as Noxious Weeds by California Law. California Department of Food and Agriculture). http://pi.cdfa.ca.gov/weedinfo/CUSCUTA2.html.
- Healy E. A., Enloe S., DiTomaso J. M. Orobanche. 2001. In ENCYCLOWEEDIA: Notes on Identification, Biology, and Management of Plants Defined as Noxious Weeds by California Law. California Department of Food and Agriculture). http://pi.cdfa.ca.gov/weedinfo/STRIGA2.html.
- Healy E. A., Enloe S., DiTomaso J. M. Striga. 2001. In ENCYCLOWEEDIA: Notes on Identification, Biology, and Management of Plants Defined as Noxious Weeds by California Law. California Department of Food and Agriculture). http://pi.cdfa.ca.gov/weedinfo/OROBANCH2.html.
- Hershenhorn J., Goldwasser Y., Plakhine D., Herzlinger G., Golan S., Russo R., Kleifeld Y. Role of pepper (Capsicum annum) as a trap and catch crop for control of Orobanche aegyptiaca and O. cernua. Weed Res. 1996;441(1):948–951. [Google Scholar]
- Hickman J. C. The Jepson Manual; Higher Plants of California. 1993. Berkeley, CA, University of California Press.
- Hoagland D. R., Arnon D. I. The water-culture method for growing plants without soil. Cal Ag Exp Stn Circ. 1950;3471(1):1–32. [Google Scholar]
- Joel D. M., Losner-Gosher D. Early host-parasite interaction: models and observations of host root penetration by the haustorium of Orobanche. 1994;1(1):237–247. Proceedings International Workshop on Orobanche and related Striga research, Amsterdam, Royal Tropical Institute, pp. [Google Scholar]
- Joel D. M., Portnoy V. H. The angiospermous root parasite Orobanche L. (Orobanchaceae) induces expression of a pathogenesis related (PR) gene in susceptible tobacco roots. Ann. Bot. 1998;811(1):779–781. [Google Scholar]
- Keyes W. J., O'Malley R. C., Kim D., Lynn D. G. Signaling organogenesis in parasitic angiosperms: Xenognosin generation, perception, and response. J Plant Growth Regul. 2000;191(1):217–231. doi: 10.1007/s003440000024. [DOI] [PubMed] [Google Scholar]
- Kuijt J. The Biology of Parasitic Flowering Plants. 1969;1(1):246. Berkeley: University of California Press. pp. [Google Scholar]
- Lagoke S. T. O. Second general workshop of the Pan-African Striga Control Network (PASCON). 1991. Nairobi, Kenya, Food and Agricultural Organization of the United Nations.
- Lane J. A., Bailey J. A., Butler R. C., Terry P. J. Resistance of cowpea (Vigna unguiculata (L.) Walp.) to Striga gesnerioides (Willd.) Vatke, a parasitic angiosperm. New Phytol. 1993;1251(1):405–412. doi: 10.1111/j.1469-8137.1993.tb03893.x. [DOI] [PubMed] [Google Scholar]
- Leake J. R. The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytol. 1994;1271(1):171–216. doi: 10.1111/j.1469-8137.1994.tb04272.x. [DOI] [PubMed] [Google Scholar]
- Losner-Goshen D., Portnoy V. H., Mayer A. M., Joel D. M. Pectolytic activity by the haustorium of the parasitic plant Orobanche L. (Orobanchaceae) in host roots. Ann. Bot. 1998;811(1):319–326. [Google Scholar]
- Lynn D. G., Steffens J. C., Kamat V. S., Graden D. W., Shabanowitz J., Riopel J. L. Isolation and characterization of the first host recognition substance for parasitic angiosperms. J Amer Chem Soc. 1981;1031(1):1868–1870. [Google Scholar]
- Mangus E. M., Zwanenburg B. Tentative molecular mechanism for germination stimulation of Striga and Orobanche seeds by strigol and its synthetic analogues. J. Agric. Food Chem. 1992;401(1):1066–1070. [Google Scholar]
- Marvier M. A. Parasite impacts on host communities: Plant parasitism in a California coastal prairie. Ecology. 1998;791(1):2616–2623. [Google Scholar]
- Matvienko M., Torres M., Yoder J. I. Transcriptional responses in parasitic plants to host plant signals. Plant Physiol. 2001;1271(1):272–282. doi: 10.1104/pp.127.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F., Distler B. Translocation of glyphosate in the host/parasite system Vicia faba and Orobanche crenata. 1991. Progress in Orobanche research, Eberhard-Karls-Univrersitat.
- Müller S., Hauck C., Schildknect H. Germination stimulants produced by Vigna unguiculata. J. Plant Growth Regul. 1992;111(1):77–84. [Google Scholar]
- Nandula V. K., Foy C. L., Orcutt D. M. Glyphosate for Orobanche aegyptiaca control in Vicia sativa and Brassica napus. Weed Sci. 1999;471(1):486–491. [Google Scholar]
- Neumann U., Vian B., Weber H. C., Sallé G. Interface between haustoria of parasitic members of the Scrophulariaceae and their hosts: a histochemical and immunocytochemical approach. Protoplasma. 1999;2071(1):84–97. [Google Scholar]
- Nickrent D. Parasitic plant connection. 1997. http://www.science.siu.edu/parasitic-plants/index.html.
- Nickrent D. The Parasitic Plant Connection. 2001. Southern Illinois University at Carbondale. http://www.science.siu.edu/parasitic-plants/index.html.
- Nickrent D. L., Duff R. J., Colwell A. E., Wolfe A. D., Young N. D., Steiner K. E., dePamphilis C. W. Molecular phylogenetic and evolutionary studies of parasitic plants. 1998;1(1):211–241. In Molecular Systematics of Plants II. DNA Sequencing. D. E. Soltis, P. S. Soltis and J. J. Doyle, eds. (Boston: Kluwer Academic Publishers), pp. [Google Scholar]
- Olivier A., Benhamou N., Leroux G. D. Cell surface interactions between sorghum roots and the parasitic weed Striga hermonthica: Cytochemical aspects of cellulose distribution in resistant and susceptible host tissues. Can. J. Bot. 1991;691(1):1679–1690. [Google Scholar]
- Ouedraogo J. T., Maheshwari V., Berner D. K., St-Pierre C. A., Belzile F., Timko M. P. Identification of AFLP markers linked to resistance of cowpea (Vigna unguiculata L.) to parasitism by Striga gesnerioides. Theor Appl Genet. 2001;1021(1):1029–1036. [Google Scholar]
- Parker C., Dixon N. The use of polyethylene bags in the culture study of Striga spp. and other organisms on crop roots. Ann. Appl. Biol. 1983;1031(1):485–488. [Google Scholar]
- Parker C., Riches C. R. Parasitic Weeds of the World: Biology and Control. 1993. Wallingford, CAB International.
- Press M. C., Graves J. D. Parasitic Plants. 1995. London, Chapman and Hall.
- Qian D., Zhou D., Rong J., Cramer C. L., Yang Z. Protein farnesyltransferase in plants: molecular characterization and involvement in cell cycle control. Plant Cell. 1996;81(1):2381–2394. doi: 10.1105/tpc.8.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racovitza A. Research on the susceptibility of various species of Nicotiana and various cultivars of tobacco to Orobanche ramosa. 1973. Proc. Symp. Parasitic Weeds, European Weed Research council, Wageningen.
- Riopel J., Musselman L. Experimental initiation of haustoria in Agalinis purpurea. Am. J. Bot. 1979;661(1):570–575. [Google Scholar]
- Riopel J. L., Baird W. V. Morphogenesis of the early development of primary haustoria in Striga asiatica. 1987;11(1):107–125. In Parasitic weeds in agriculture. L. J. Musselman, eds. (Boca Raton: CRC Press, Inc.) [Google Scholar]
- Riopel J. L., Timko M. P. Haustorial initiation and differentiation. 1995;1(1):39–79. In Parasitic Plants. M. C. Press and J. D. Graves, eds.(London: Chapman and Hall), pp. [Google Scholar]
- Robert S., Simier P., Fer A. Purification and characterization of mannose 6-phosphate reductase, a potential target for the control of Striga hermonthica and Orobanche ramosa. Aust. J. Plant Physiol. 1999;261(1):233–237. [Google Scholar]
- Romano C. P., Hein M. B., Klee H. J. Inactivation of auxin in tobacco transformed with the IAA-lysine synthetase gene of Pseudomonas savastanoi. Gene Develop. 1991;51(1):438–446. doi: 10.1101/gad.5.3.438. [DOI] [PubMed] [Google Scholar]
- Romano C. P., Robson P. R. H., Smith H., Estelle M., Klee H. Transgene-mediated auxin overproduction in Arabidopsis: Hypocotyl elongation phenotype and interactions with the hy6-1 hypocotyl elongation and axr1 auxin-resistant mutants. Plant Mol. Biol. 1995;271(1):1071–1083. doi: 10.1007/BF00020881. [DOI] [PubMed] [Google Scholar]
- Saunders A. R. Studies in phanerogamic parasitism with particular reference to Striga lutea. Science Bulletin / Union of South Africa, Deparment of Agriculture. 1933;1281(1):5–56. [Google Scholar]
- Sessa G., Yang X-Q., Raz V., Eyal Y., Fluhr R. Dark induction and subcellular localization of the pathogenesis-related PRB-1b protein. Plant Mol. Biol. 1995;281(1):537–547. doi: 10.1007/BF00020400. [DOI] [PubMed] [Google Scholar]
- Shomer-Ilan A. Germinating seeds of the root parasite Orobanche aegyptiaca Pers. excrete enzymes with carbohydrase activity. Symbiosis. 1993;151(1):61–70. [Google Scholar]
- Siame B. A., Weerasuriya Y., Wood K., Ejeta G., Butler L. G. Isolation of strigol, a germination stimulant for Striga asiatica, from host plants. J. Agric. Food Chem. 1993;411(1):1486–1491. [Google Scholar]
- Steffens J. C., Lynn D. G., Riopel J. L. An haustorial inducer for the root parasite Agalinis purpurea. Phytochem. 1986;251(1):2291. [Google Scholar]
- Stewart G. R., Press M. C. The physiology and biochemistry of parasitic angiosperms. Ann. Rev. Plant Phys. Plant Mol. Biol. 1990;411(1):127–151. [Google Scholar]
- Thuring J. W. J. F., Nefkens G. H. L., Zwanenburg B. Asymmetric synthesis of all stereoisomers of the strigol analogue GR24. Dependence of absolute configuration on stimulatory activity of Striga hermonthica and Orobanche crenata seed germination. J. Agric. Food Chem. 1997;451(1):2278–2283. [Google Scholar]
- Thurman L. D. Genecological studies in Orthocarpus subgenus Triphysaria. 1966;1(1):197. Berkeley, University of California, Berkeley. [Google Scholar]
- Vogler R. K., Ejeta G., Butler L. G. Inheritance of low production of Striga germination stimulant in Sorghum. Crop Sci. 1996;361(1):1185–1191. [Google Scholar]
- Weissenborn D. L., Denbow C. J., Laine M., Lang S., Yang Z., Yu X., Cramer C. L. HMG-CoA reductase and terpenoid phytoalexins: Molecular specialization within a complex pathway. Physiol. Plant. 1995;931(1):393–400. [Google Scholar]
- Westwood J. H. Characterization of the Orobanche-Arabidopsis system for studying parasite-host interactions. Weed Sci. 2000;1(1):742–748. V48. [Google Scholar]
- Westwood J. H., Foy C. L. Arabidopsis thaliana can be a model host for Orobanche research. 1998;1(1):155–160. Proc. of the 4th Intl. Orobanche Workshop, Inst. for Wheat and Sunflower, ‘Dobroudja’, Albena, Bulgaria, pp. [Google Scholar]
- Westwood J. H., Yu X., Foy C. L., Cramer C. L. Expression of a defense-related 3-hydroxy-3-methylglutaryl CoA reductase gene in response to parasitization by Orobanche spp. Mol. Plant. Microbe Interact. 1998;111(1):530–536. doi: 10.1094/MPMI.1998.11.6.530. [DOI] [PubMed] [Google Scholar]
- Wigchert S. C. M., Zwanenburg B. A critical account on the inception of Striga seed germination. J. Agric. Food Chem. 1999;471(1):1320–1325. doi: 10.1021/jf980926e. [DOI] [PubMed] [Google Scholar]
- Yoder J. I. A species-specific recognition system directs haustorium development in the parasitic plant Triphysaria (Scrophulariaceae). Planta. 1997;2021(1):407–413. doi: 10.1007/s004250050144. [DOI] [PubMed] [Google Scholar]
- Yoder J. I. Self and cross-compatibility in three species of the hemiparasite Triphysaria (Scrophulariaceae). Env. Exp. Bot. 1998;391(1):77–83. [Google Scholar]
- Yokota T., Sakai H., Okuno K., Yoneyama K., Takeuchi Y. Alectrol and orobanchol, germination stimulants for Orobanche minor, from its host red clover. Phytochem. 1998;491(1):1967–1973. [Google Scholar]
- Zaitoun F. M. F., ter Borg S. J. Resistance against Orobanche crenata in Egyptian and Spanish faba beans. 1994;1(1):264–275. Proceedings of the Third International Workshop on Orobanche and Related Striga Research, Amsterdam, The Netherlands, Royal Tropical Institute, pp. [Google Scholar]


