Abstract
The clinical outcome for patients with chronic myeloid leukemia (CML) has changed dramatically in the past 15 years. This has been due to the development of tyrosine kinase inhibitors (TKI), compounds which inhibit the activity of the oncogenic BCR-ABL1 protein. Imatinib was the first TKI developed for CML, and it led to high rates of complete cytogenetic responses and improved survival for patients with this disease. However, about 35% of patients in chronic phase treated with imatinib will develop resistance or intolerance to this drug. The recognition of the problem of imatinib failure led to the design of 2nd-generation TKI (dasatinib, nilotinib and bosutinib). These drugs are highly active in the scenario of imatinib resistance or intolerance. More recently, both nilotinib and dasatinib were approved for frontline use in patients with chronic phase CML. Ponatinib represents the last generation of TKI, and this drug has been developed with the aim of targeting a specific BCR-ABL1 mutation (T315I) which arises in the setting of prolonged TKI therapy and leads to resistance to all commercially available TKI. Parallel to the development of specific drugs for treating CML, major advances were made in the field of disease monitoring and standardization of response criteria. In this review we summarize how therapy with TKI for CML has evolved over the last decade.
Keywords: Chronic Myelogenous Leukemia, BCR-ABL1, Tyrosine Kinase Inhibitors, Imatinib, Dasatinib, Nilotinib, Bosutinib, Ponatinib
Introduction
Chronic myelogenous leukemia (CML) is a myeloproliferative disorder characterized by the presence of translocation t(9;22)(q34;q11) which generates the Philadelphia (Ph) chromosome and the associated fusion gene BCR-ABL11. BCR-ABL1 encodes the chimeric protein BCR-ABL1 which has deregulated tyrosine kinase activity and leads to increased cellular proliferation, resistance to apoptosis and genetic instability1. BCR-ABL1 is at the center of CML pathogenesis, as attested by mouse models which replicate the disease2. CML classically follows a triphasic course, with most patients being diagnosed in an initial, oligosymptomatic chronic phase (CP) which eventually progress into a more advanced accelerated phase (AP) and culminates into a blast phase (BP), which is similar to an acute leukemia.
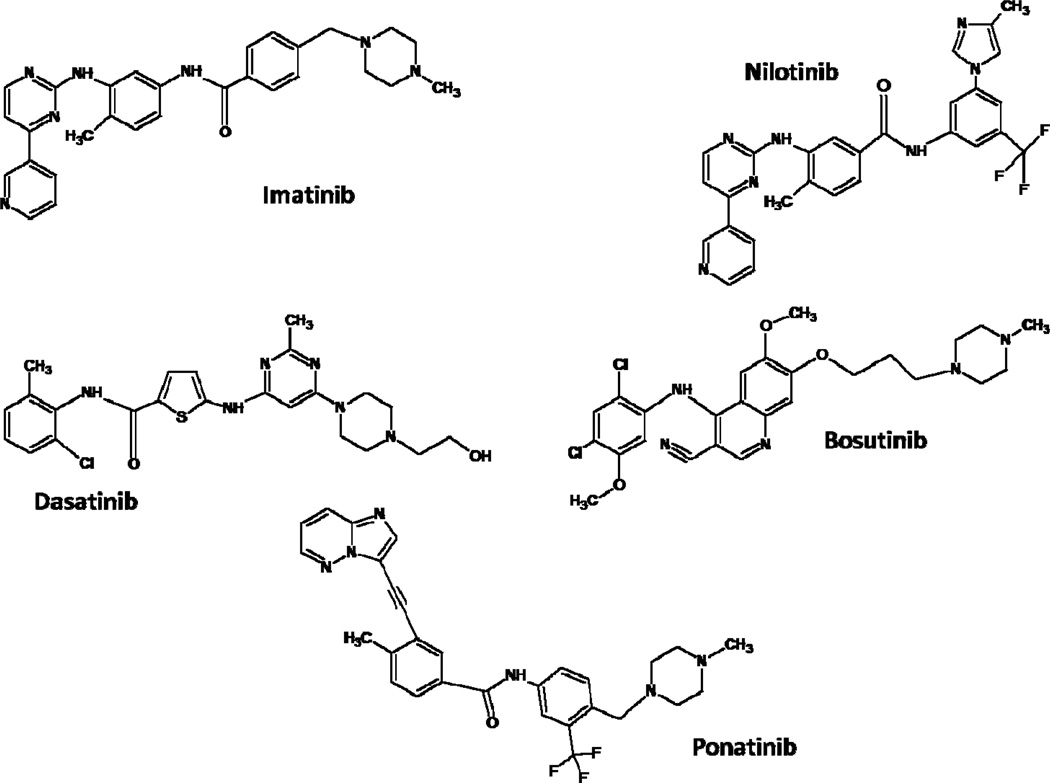
CML, once considered a fatal disease, is now essentially a chronic disorder, and most patients can enjoy long-term survival3. This history of success has been the result of development of tyrosine kinase inhibitors (TKI), compounds which suppress the abnormal tyrosine kinase (TK) activity of the BCR-ABL1 protein (Figure 1). In this article we review the rationale and development of three generations of TKI for therapy of CML.
Figure 1.
Three Generations of Tyrosine Kinase Inhibitors. First-generation: Imatinib; Second-generation: Nilotinib, Dasatinib and Bosutinib; Third-generation: Ponatinib
Therapy for CML in the pre-imatinib era
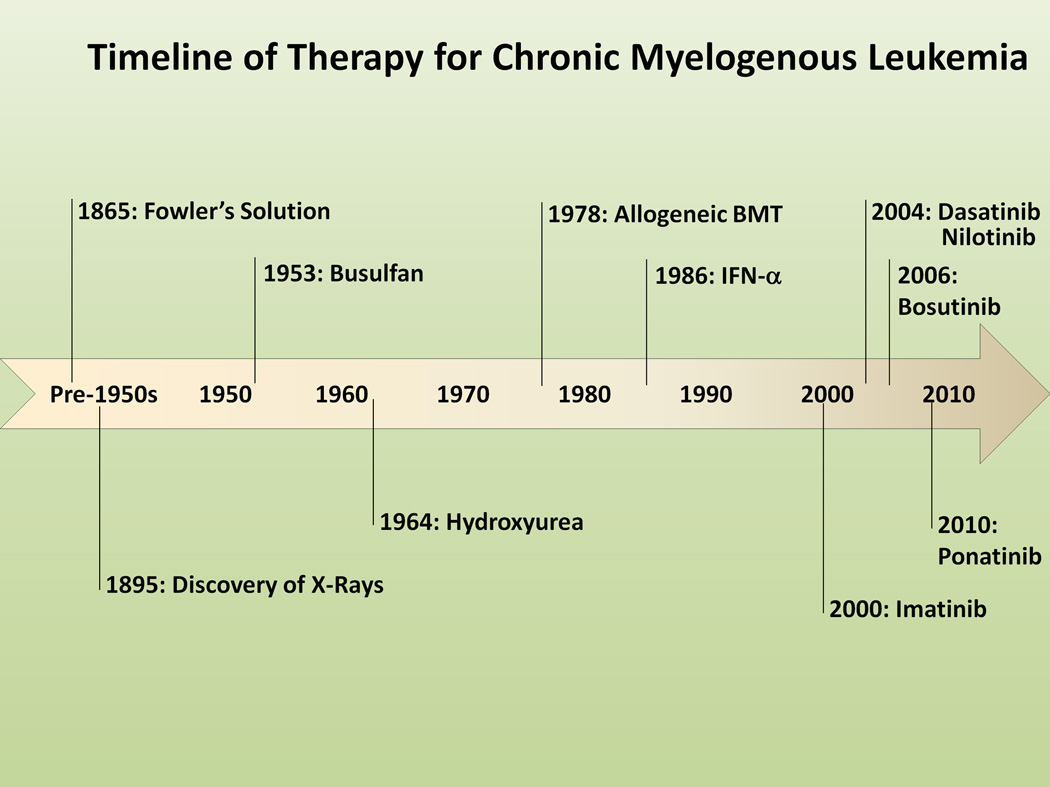
Historically, the first treatment for CML was Fowler’s solution, a 1% solution of arsenic trioxide, used for therapy of CML back in 18654 (Figure 2). Following the discovery of X-rays by Roentgen in 1895, radiation therapy was incorporated into the armamentarium of CML therapy in the first half of the 20th century, used mainly to alleviate symptoms caused by splenomegaly4. With the development of chemotherapy in the 1950s, busulphan and hydroxyurea became the main therapeutic options for several decades5. While these drugs could effectively control the WBC, they did not eradicate the leukemic clone or altered disease progression6. The arise of interferon-α (IFN-α) in the 1980s was a great advance, since the drug could induce hematologic and cytogenetic remissions and improvements in survival, but it was poorly tolerated due to frequent and serious side effects7. The use of IFN-α brought for the first time the possibility of eliminating the malignant clone as represented by the elimination of the Ph chromosome. A complete cytogenetic remission (CCyR; 0% Ph+-metaphases) was achieved in a small but significant percentage of patients, and it was recognized that patients who achieved CCyR had longer survival than those who failed to meet this endpoint, thus indicating that cytogenetic response was a surrogate for improved survival and the gold standard for optimal response to therapy8. Hematopoietic stem cell transplantation (HSCT) was developed parallel to drug therapy and it has proven curative potential for CML, but it is applicable in only a fraction of patients, mainly younger patients with a matched donor and is associated with considerable morbidity and mortality, although great progress has been made to ameliorate both9. Thus, for most patients with CML, therapy was limited to a few available drugs and the possibility of facing the risks of a HSCT.
Figure 2.
Timeline of Development of Therapy for Chronic Myelogenous Leukemia
First Generation Tyrosine Kinase Inhibitor: Imatinib
Pre-Clinical Development
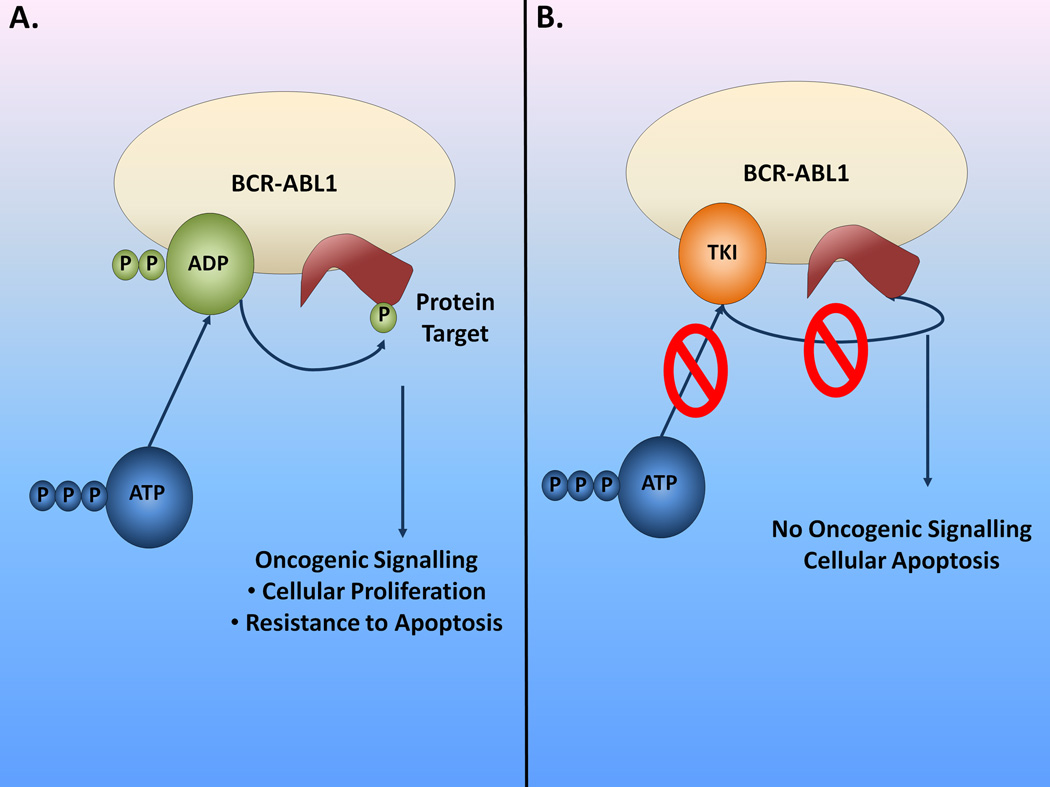
Imatinib (formerly known as CGP57148B, or STI-571; Novartis, Basel, Switzerland) was one of the first molecules developed belonging to a class of compounds, named ATP-mimetic kinase inhibitors, which compete with ATP for the ATP-binding pocket of the kinase, thus inhibiting further substrate phosphorylation by the enzyme10,11 (Figure 3). Imatinib can only bind the TK BCR-ABL1 in its inactive form and this is dependent on crucial interactions with several key amino acid residues10,11. Initial studies with purified enzyme based assays showed that imatinib had potent activity against the TK c-Abl (half-maximal inhibitory concentration [IC50]=0.2µM), including oncogenic BCR-ABL1 (IC50=0.25µM)12. Imatinib could also inhibit activity of TKs receptors PDGFRα/β(Platelet Derived Growth Factor Receptor-α and −β) and KIT13,14. Imatinib inhibited proliferation and induced apoptosis of BCR-ABL1 positive cells.12,15–17 Animal models of BCR-ABL1-positive leukemias confirmed its in vivo activity12,18.
Figure 3.
Mechanism of action of Imatinib and other TKI. A. The BCR-ABL1 oncogenic TK phosphorylates protein targets leading to activation of intracellular pathways associated with increased cellular proliferation and apoptosis resistance. ATP binds to BCR-ABL1 and supplies phosphate groups for the phosphorylation reaction. B. Current available TKI are ATP-mimetic compounds, competing with ATP for the ATP-binding site at BCR-ABL1. Binding of the TKI to BCR-ABL1 prevents phosphorylation of protein substrates, since no phosphate group is available for the reaction to occur. As a consequence, oncogenic signaling pathways are no longer activated and the cell undergoes apoptosis.
Clinical Studies
The phase I clinical trial of imatinib initially recruited patients with chronic phase (CP) CML who had failed therapy with IFN-α19. At doses greater than 300 mg, impressive clinical activity was observed, with 53 of 54 patients achieving a complete hematological response (CHR; disappearance of all signs and symptoms of the disease) and 31% achieving a major cytogenetic response (MCyR; 0–35% Ph+metaphases), including a CCyR rate of 13%. The dose of 400 mg once daily was chosen for future studies based on pharmacokinetic data showing that it achieved mean plasma through concentration greater than needed to inhibit BCR-ABL1. The phase I study then expanded to include patients with blast phase (BP) CML and patients with refractory/relapsed Ph+-acute lymphoblastic leukemia (Ph+-ALL)20. Therapy with imatinib led to a CHR in 11% of patients with myeloid BP (MBP) and 20% of patients with lymphoid phenotype (LBP). Another 10% and 15%, respectively, achieved reduction in blasts to < 5% but without peripheral blood count recovery. Unfortunately, responses were short-lived and most patients rapidly progressed after a few months.
The phase II clinical trials confirmed activity of imatinib in a much larger cohort of patients with CML in all stages21–23. Patients with CML in accelerated phase (AP)/BP were treated with imatinib at doses of 400–600 mg once daily, and among 235 patients with AP and 260 patients with myeloid BP, responses were seen in 82% and 52% and a CHR was obtained in 34% and 8%, respectively21,22. In patients with AP higher doses of imatinib (i.e. 600 mg vs. 400 mg) led to improved responses, and the Federal Drug and Administration (FDA) approved imatinib at a dose of 600 mg daily for therapy of patients with CML in AP/BP22. In the CP trial 454 patients were treated with imatinib 400 mg daily23. Response rates were CHR 95%, MCyR 60% and CCyR 41%. Side effects of imatinib were few and usually grade 1–2. Most common were edema (all grades 60%), nausea (all grades 55%), muscle cramps (all grades 49%), rash (all grades 32%) and diarrhea (all grades 29%). Grade 3–4 hematological side effects were anemia (7%), neutropenia (35%) and thrombocytopenia (20%). Only 2% of patients had to discontinue imatinib due to drug related side effects.
A large phase III randomized trial of imatinib versus IFN-α and low dose cytarabine (the standard of care at the time) was then launched for patients with newly diagnosed CP CML. The IRIS (International Randomized Study of Interferon and STI571) phase III trial randomized 1106 patients with newly-diagnosed CML to imatinib (400 mg daily) and IFN-α plus low dose cytarabine24. The initial report demonstrated superiority of imatinib, with a MCyR rate 87% vs. 35% with IFN-α +Ara-C (p<0.001), and a CCyR Rate of 76% vs. 15% (P<0.001), respectively. At 18 months transformation free survival (TFS) showed benefit of imatinib, 97% vs. 91.5% (p<0.001)24. Imatinib was much better tolerated than the combination of IFN-α+Ara-C. Only 3% of patients in the imatinib arm discontinued therapy due to side effects or crossed-over to the other arm due to intolerance, versus 30% of patients in the IFN-α+Ara-C arm. Side effects more commonly seen with imatinib included superficial edema, nausea, muscle cramps and rashes. Most were usually grade 1–2 events. Reported grade 3–4 cytopenias were anemia (3.1% [imatinib] vs. 4.3% [IFN-α+Ara-C]), neutropenia (14.3%[imatinib] vs. 25%{IFN-α+Ara-C]) and thrombocytopenia (7.8%[imatinib] vs. 16.5%[IFN-α+Ara-C])25.
Long term follow up of the IRIS trial have confirmed benefit of imatinib. After 8 years, 304 patients (55% of the original cohort) remain on use of imatinib26. The CCyR rate at 8 years is 83%, with 18% having lost CCyR and 3% having progressed to AP/BP. Event-free survival (EFS) is 81% and TFS is 92%. For patients who achieved a major molecular response (MMR, defined in the IRIS trial as a 3-log reduction in BCR-ABL1 transcripts from a standardized baseline value, assessed by real-time quantitative polymerase chain reaction [RT-PCR]) at 12 months, TFS was 100% at 8 years. The rate of progression to AP/BP decreased over time in the study, being 1.5% (1st year), 2.8% (2nd year), 1.6% (3rd year), 0.9% (4th year), 0.5% (5th year), 0% (6th and 7th year) and 0.4% (8th year). At 8 years, overall survival (OS) is 85% (93% considering only CML-related deaths). Since the design of the trial allowed for crossover, no difference in survival between arms was reported. However, several reports comparing cohorts of patients treated with imatinib with historical CML controls showed that imatinib clearly improved survival in patients with CML relative to the former standard, IFN-α and cytarabine27–29.
Monitoring Response to TKI Therapy
Achievement of cytogenetic response in patients with CML is associated with improved survival and decreased risk of transformation to AP/BP8. Furthermore, data from the imatinib studies showed that the prognostic impact of certain depth of response depended on the timing of their achievement. Patients who achieved a CCyR at 12 months of therapy had a 5-years EFS of 97%, versus 93% for those patients with a partial cytogenetic response (PCyR, 1–35% Ph+-metaphases) and 81% for those patients who had failed to achieve a MCyR altogether (p<0.001)3. Monitoring of BCR-ABL1 transcript levels by quantitative RT-PCR revealed itself as a method to further quantify residual disease, in patients already in CCyR. Clinical trials began to evaluate the clinical impact of achieving a molecular response. In the IRIS trial, patients who had a CCyR and a MMR, more recently defined as a BCR-ABL/ABL ≤0.1% in the international scale, at 18 months of therapy had a 5-year OS of 100%3. The European LeukemiaNet has published guidelines for monitoring patients with CML and criteria for optimal response, suboptimal response and failure to therapy with TKI (Tables 1–3)30.
Table 1.
CML Response Criteria Definition (from30)
| Hematologic | Cytogenetic | Molecular |
|---|---|---|
| Complete: Normal complete blood cell count, non-palpable spleen and disappearance of all disease signs and symptoms | Complete: 0% Ph+-metaphases | Complete: Undetectable BCR-ABL1 transcripts on two consecutive quantitative RT-PCR or nested PCR assays (sensitivity at least 10−4) |
| Partial: 1–35% Ph+-metaphases | Major: Ratio BCR-ABL1:ABL1 transcripts ≤ 0.1% in the international scale by quantitative RT-PCR | |
| Major: 0–35% Ph+-metaphases | ||
| Minor: 36–65% Ph+-metaphases | ||
| Minimal: 66–95% Ph+-Metaphases | ||
| No Response: ≥ 96% Ph+-metaphases |
Table 3.
Criteria for Failure, Suboptimal Response and Optimal Response (from30)
| Time (mo) | Failure | Suboptimal | Optimal |
|---|---|---|---|
| 3 | No CHR | ≥ 96% Ph+ metaphases | CHR and Ph+ ≤ 65% |
| 6 | No CHR | > 35% Ph+ metaphases | < 35% Ph+ metaphases |
| ≥ 96% Ph+ metaphases | |||
| 12 | > 35% Ph+ metaphases | 1–35% Ph+ metaphases | 0% Ph+ metaphases |
| 18 | ≥ 1% Ph+ metaphases | No MMR | MMR |
| Any | Loss of CHR | Loss of MMR | Stable or improving MMR |
| Loss of CCyR | Mutation (intermediate sensitivity imatinib) |
||
| Mutation (poor sensitivity imatinib) |
|||
| Clonal Evolution |
Abbreviations: CCyR, Complete Cytogenetic Response; CHR, complete hematological Response; MMR, Major Molecular Response
The value of achieving a MMR in addition to a CCyR is still a matter of debate. Recently, Hughes et al evaluated the impact of achieving early MMR on outcomes based on data from the IRIS clinical trial31. At 18 months, patients who had not achieved a MMR had a significantly inferior 7-years EFS (95% vs. 75%; p<0.001) and TFS (99% vs. 90%; p<0.001). There was no statistically significant difference in OS (95% vs. 90%)31. However, when only the patients who achieved a CCyR are analyzed, achieving a MMR at 18 months is associated with lower rate of loss of CCyR (3% vs. 26%; p<0.001) and better 7-years EFS (95% vs. 86%), but no improvement in TFS (99% vs. 96%) or OS (95% vs. 96%). Thus, while achieving MMR is certainly beneficial, achieving CCyR must be considered the minimal acceptable response to be obtained by patients with CML and perhaps the gold standard of CML response as it is the only type of response criteria that is associated with an improvement in survival. It is also important to mention that failure to achieve a MMR or a complete molecular response (CMR; absence of BCR-ABL1 transcripts by quantitative RT-PCR) are not considered criteria for failure30. One report analyzed 116 patients who were in continuous CCyR and had increases in BCR-ABL1 transcript level by quantitative PCR on two or more occasions32. Only 11 patients (9.5%) had CML progression, and 10 of these were among 44 patients who had an increase >1 log in transcript levels and had either lost or never achieved MMR32. Thus, clinicians should refrain from making therapeutic changes based solely on BCR-ABL1 levels if the patient is still maintaining CCyR and the level of BCR-ABL1 increase is < 1 log, as there is no clinical evidence of the benefit of interventions in this setting, and even when improvements in transcript levels are reported (e.g., with increased doses of Imatinib) the long-term impact of such changes has not been demonstrated.
Mechanisms of Resistance to Imatinib
Despite these important clinical advances obtained with imatinib, it is clear that roughly 30–40% of patients will need additional therapy beyond imatinib. In the 8-year update of the IRIS trial, at least 37% of patients initially treated with imatinib had an unfavorable outcome: 17% failed to achieve CCyR, 15% lost CCyR and 5% had intolerance to imatinib26. Resistance to imatinib can be classified into primary (never had a response to frontline therapy with imatinib) or secondary (achieved a response but then lost it) 33. Resistance to imatinib has been defined by the European LeukemiaNet as failure to achieve predetermined milestones during therapy (table 3)30. While the incidence of resistance to imatinib in untreated CP CML is approximately 4% per year, it is much higher in patients with AP (40%) and BP (90%)3,34.
There are several distinct mechanisms of resistance to imatinib, conventionally divided into BCR-ABL1 dependent and independent mechanisms33. Among BCR-ABL1 dependent mechanisms, overexpression of the BCR-ABL1 gene and development of BCR-ABL1 mutations stand as the most relevant ones35,36. BCR-ABL1 mutations are found in 50–80% of patients with CML at time of development of resistance, and are more common in patients with AP/BP (particularly lymphoid BP) than patients remaining in CP37–40. Mutations cluster at the kinase domain and either disrupt contact points between imatinib and BCR-ABL1 or induce conformational changes from inactive to active, to which imatinib is unable to bind25,41. Some mutations are associated with a high level of resistance to imatinib, including P-Loop mutations (i.e. mutations in amino acid residues 244 to 255; the most common ones include Q252R/H, Y253F/H, E255K/V) and the gatekeeper T315I mutation36. The T315I mutation confers resistance to all commercially-available TKI, since it prevents the formation of an important hydrogen bond between the TKI and amino acid residue T315 of the BCR-ABL1 molecule36. This blocks binding of the TKI to the BCR-ABL1 protein. The T315I mutation is a common mechanism of resistance in CML patients evolving to AP and BP while on therapy with TKI37. Currently, screening for BCR-ABL1 mutations is recommended at the following time points: (1) at diagnosis, solely for patients who present with AP/BP; (2) during therapy with imatinib or other TKI for patients who have criteria for failure or suboptimal response42.
Non-BCR-ABL1 dependent resistance mechanisms are diverse and not well understood. Activation of other, BCR-ABL1 independent, signaling pathways is one potential avenue leukemic cells can exploit to escape inhibition by imatinib. Activation of Src family kinase (SFK) enzymes can lead to cell proliferation by a BCR-ABL1 independent pathway43. Variable activity of proteins responsible for imatinib transport across the cell membrane can influence intracellular concentrations of imatinib and its efficacy44. The human organic cationic transporter-1 (OCT1) is the main protein responsible for imatinib influx, and polymorphisms may influence expression of OCT145. Patients with CML who have low OCT1 activity have inferior rates of MMR (55% vs. 89% at 5 years; p=0.007), CMR (31% vs. 59%; p=0.038), EFS (5 years 48% vs. 74%; p=0.03) and OS (5 years 87% vs. 96%; p=0.031)46. Increasing the dose of imatinib might nullify the negative effect of low OCT1 activity, but this strategy needs to be evaluated prospectively47. One advantage of 2nd-generation TKI in comparison with imatinib is that neither dasatinib nor nilotinib need OCT1 for cell entry47,48.
Strategies for salvage of patients with CML who fail Imatinib: Imatinib Dose Escalation and Second-Generation TKI
Imatinib Dose Escalation
Currently, the European LeukemiaNet recommends imatinib dose escalation only for those patients who present with suboptimal response criteria30. Imatinib dose escalation seems to be more effective in patients who present with cytogenetic relapse (previous cytogenetic response to imatinib) and without signs of hematological relapse. Jabbour et al. reported on 84 patients with CP who had failure on standard dose imatinib and had dose increases to 600 mg or 800 mg49. Patients who had dose escalation due to cytogenetic failure had higher rates of CCyR (52%) compared to those who had dose escalation due to hematological failure or resistance (5%). Similarly, patients who had achieved a previous cytogenetic response to imatinib had a higher rate of CCyR compared to patients with primary cytogenetic resistance (73% vs. 0%). At 3 years, EFS (58% vs. 19%; p<0.001) and OS (83% vs. 56%; p=0.004) were superior for those patients who had dose escalation while on cytogenetic relapse only49.
Second-Generation TKI: Dasatinib
Dasatinib (formerly known as BMS354825; Sprycel; Bristol Myers Squibb, New York, NY) is an orally available TKI which is structurally unrelated to imatinib and is capable of binding BCR-ABL1 both in the active and in the inactive conformation50,51. Dasatinib is 325-fold more potent than imatinib against wild type BCR-ABL1 (IC50=0.8nM) and has activity against most imatinib-resistant BCR-ABL1 mutations and against SFK enzymes35,36. Dasatinib has no activity against the T315I BCR-ABL1 mutation36. Dasatinib is currently approved by the FDA for the frontline therapy of CML and for salvage of CML patients in all phases who are resistant or intolerant to imatinib.
In the phase I dose escalation study, 84 patients with imatinib-resistant/-intolerant CML or Ph+-ALL (CP=40, AP=11, MBP=23, LBP/Ph+-ALL=10) received therapy with dasatinib at doses ranging from 15–240 mg daily, administered in a once or twice daily schedule52. Most patients (86%) were resistant to imatinib. No maximum tolerated dose was determined, and pharmacokinetic and pharmacodynamic data supported a dose schedule of 70 mg twice daily in order to achieve constant TK inhibition. Most common toxicities included grade 3–4 neutropenia (CP: 45%; advanced CML: 89%), grade 3–4 thrombocytopenia (CP: 35%; advanced CML: 80%), grade 1–4 pleural effusion (18%), grade 1–2 diarrhea (23%), grade 1–2 edema (19%) and grade 1–2 headache (10%). Response data are summarized in Table 4. Briefly, patients with CP and AP had high rates of CHR (92% [CP] and 82% [AP]), MCyR (45% and 27%) and CCyR (35% and 18%). After a median follow-up of 12 and 5 months for patients with CP and AP, respectively, 95% and 82% were maintaining their response. Dasatinib was effective against all types of imatinib-resistant BCR-ABL1 mutations, with the exception of the T315I mutation.
Table 4.
Studies with Dasatinib as Salvage Therapy for Imatinib Resistance or Intolerance
| Study | Disease Stage |
Dose | N | % Response | ||
|---|---|---|---|---|---|---|
| CHR | Cytogenetic Response | |||||
| MCyR | CCyR | |||||
| Phase I52 | CP | 15–240 mg daily |
40 | 92 | 45 | 35 |
| AP | 11 | 45 | 27 | 18 | ||
| MBP | 23 | 35 | 35 | 26 | ||
| LBP/Ph+ ALL | 10 | 70 | 80 | 30 | ||
| START-C53 | CP | 70 mg twice daily | 387 | 90 | 59 | 49 |
| START-A54 | AP | 70 mg twice daily | 174 | 45 | 39 | 32 |
| START-B55 | MBP | 70 mg twice daily | 109 | 27 | 33 | 27 |
| START-L55 | LBP | 70 mg twice daily | 48 | 29 | 52 | 46 |
| START-R57 | CP | 70 mg twice daily | 101 | 93 | 53 | 44 |
| Dose Optimization58 | CP | 100 mg daily | 167 | 92 | 63 | 50 |
Abbreviations: AP, advanced phase; BP, blast phase (myeloid [M] or lymphoid [L]); CCyR, Complete Cytogenetic Response; CHR, Complete Hematological Response; CP, Chronic Phase; MCyR, Major Cytogenetic Response Ph+-ALL, Philadelphia-Positive Acute Lymphoblastic Leukemia;
Following the results of the Phase I trial, several Phase II studies (START trials; Src-ABL1 Tyrosine Kinase Inhibition Activity Research Trials) were launched to evaluate the efficacy of dasatinib against all spectra of Ph-positive leukemias post intolerance or failure of imatinib: CP CML (START-C), AP CML (START-A), MBP (START-B) and LBP (START-L)53–55. Patients were treated with dasatinib at a dose of 70 mg twice daily. Results are summarized in Table 4. Overall, these phase II studies confirmed that dasatinib is a highly active TKI in the setting of imatinib failure or intolerance. Among patients treated with dasatinib in CP CML, the rate of MCyR and CCyR was 59% and 49%53. Cytogenetic responses were seen independently of duration of prior imatinib therapy, prior imatinib dose, presence of BCR-ABL1 mutations and prior CHR. Responses were durable, and after 15 months of follow up progression free survival (PFS) was 90% and OS was 96%53.
Two other studies with dasatinib deserve mention. In the START-R trial, patients with imatinib failure at doses of 400–600 mg daily were randomized in a 2:1 fashion to dasatinib (70 mg twice daily) or imatinib (800 mg daily)56,57. One hundred and fifty patients were enrolled. CHR rates were higher with dasatinib (93% vs. 82%; p=0.034), as well as MCyR (53% vs. 33%; p=0.017) and CCyR (44% vs. 18%; p=0.0025). Dasatinib also resulted in superior PFS (2 years 86% vs. 65%; p=0.0012) but was more toxic than high dose imatinib, with pleural effusion occurring in 17% of patients (versus 0%) and higher rates of grade 3–4 myelosuppression (neutropenia 61% vs. 39%; thrombocytopenia 56% vs. 14%)56,57.
A randomized phase III study evaluated the optimal dose and schedule of dasatinib in patients with CML in CP58. Six hundred and seventy patients were randomized among four different schedules of dasatinib: 100 mg once daily, 50 mg twice daily, 140 mg once daily and 70 mg twice daily. The rationale for this trial was that in the START-C study the median daily administered dose was 101 mg, lower than the approved 140 mg daily dose, but still with a significant response rate. After median treatment duration of 8 months, no difference was seen among the four treatment arms regarding CHR, MCyR, CCyR and PFS. However, the 100 mg once daily arm, compared to the approved schedule of 70 mg twice daily, was significantly less toxic, with lower rates of pleural effusion (all grades 7% vs. 16%; p=0.024), grade 3–4 thrombocytopenia (22% vs. 37%; p=0.004), grade 3–4 anemia (10% vs. 16%; p=0.07), treatment interruption, dose reductions and treatment discontinuation due to toxicity58. Dasatinib was then approved at 100 mg once daily for the treatment of imatinib-resistant/intolerant CML. A similar phase III study randomized patients with AP/BP or Ph+-ALL to dasatinib at two different schedules: 140 mg once daily or 70 mg twice daily. The 140 mg once daily arm led to similar response and survival outcomes but with improved toxicity59.
Major side effects of dasatinib include pleural effusions, myelosuppression and bleeding diathesis. Pleural effusions occur in 5–15% of patients receiving therapy with dasatinib, with a higher incidence among patients receiving high doses (140 mg daily), a twice daily schedule, in advanced stages of CML or with a previous history or cardiac disease60. Pleural effusions are usually managed with diuretics, dose reduction/interruption, corticosteroids and thoracocentesis60. Dasatinib can induce bleeding episodes in patients without coagulation abnormalities61. This might be secondary to dasatinib-induced platelet aggregation abnormalities, inhibiting aggregation in response to epinephrine and arachidonic acid62. Thus, concomitant use of dasatinib and platelet inhibitors should be avoided if possible. Myelosuppression is a relatively common side effect of dasatinib. In patients with CP CML receiving 100 mg once daily, incidence of grade 3–4 cytopenias are 10% (anemia), 33% (neutropenia) and 22% (thrombocytopenia)53. In patients with more advanced CML, who frequently start therapy with baseline cytopenias, grade 3–4 cytopenias are in the range of 80% (neutropenia), 82–88% (thrombocytopenia) and 50–69% (anemia)12. Cytopenias are usually managed with growth factor support and treatment interruption/dose reduction as needed63. Another peculiar hematological effect of dasatinib is the induction, in 30–46% of patients, of large granular cell (LGL) lymphocytosis64–66. These lymphocytes have been shown to be either T-cell or NK-cell, and represent expansion of pre-existing oligoclonal populations of T/NK lymphocytes67. Development of T/NK-LGL lymphocytosis has been associated with development of colitis, pleuritis, with a higher incidence of CCyR and MMR and with improved survival64,65. This suggests that dasatinib might not only act through inhibition of BCR-ABL1 but also through modulation of the immune system in some patients.
Second- Generation TKI: Nilotinib
Modification of the methylpiperazinyl group of imatinib in order to improve its binding characteristics led to the development of nilotinib (formerly known as AMN107; Tasigna; Novartis, Basel, Switzerland), an orally available TKI which has 10–30 fold greater potency than imatinib against BCR-ABL1 (IC50 25 nM)68. Nilotinib also has activity against most imatinib-resistant mutations, but it fails to inhibit the T315I mutation36,68,69. Compared to imatinib, nilotinib has a relative increase in specificity against BCR-ABL1, showing reduced activity against TK PDGFRβ (IC50 57 nM) and KIT (IC50 160 nM)68. Similar to imatinib, nilotinib does not inhibit SFK. Nilotinib is currently approved as first line therapy of CML and for patients with CML in CP or AP who are intolerant or resistant to imatinib.
The phase I dose escalation study recruited 119 patients with CML (CP=17, AP=56, BP=33) or Ph+-ALL (N=13) who were treated with nilotinib at doses ranging from 50–1,200 mg once daily and 400–600 mg twice daily70. Maximum tolerated dose was 600 mg twice daily. Dose limiting toxicities (DLT) at that dose level included grade 3–4 bilirubin elevation (11%) and grade 3–4 lipase elevation (11%). Other non-hematological toxicities were (percentage of all patients): rash (all grades: 22%; grade 3–4: 2%), pruritus (all grades: 17%; grade 3–4: 2%), dry skin (all grades: 12%), constipation (all grades:8%), nausea/vomiting (all grades:8%), fatigue (all grades:6%)68. Grade 3–4 hematological side effects were thrombocytopenia (20%), neutropenia (13%) and anemia (6%). The half-life of nilotinib was 15 hours, and there was saturation of plasma levels when nilotinib was given at doses ≥400 mg once daily. Thus, a twice daily schedule was explored, and the mean through level at steady state with 400 mg twice daily was 1.700 nM, which far exceeds the IC50 value for inhibiting both wild-type BCR-ABL1 and most imatinib resistant mutations (IC50 19–709 nM)70.
Clinical efficacy of nilotinib was first shown in the Phase I trial (table 5)70. The hematologic response (HR; includes CHR, marrow response and return to CP) was 92% in CP, 74% in AP and 39% in BP. MCyR were seen in 18% of BP patients, 31% of AP patients and 53% of CP patients. Among 91 patients who had a mutation analysis at baseline, 37 patients were found to harbor 51 different mutations. Nilotinib had similar efficacy in patients with and without BCR-ABL1 mutations, except in the case of the T315I mutation.
Table 5.
Studies with Nilotinib as Salvage Therapy for Imatinib Resistance or Intolerance
| Study | Disease Stage | N | % Response | |||
|---|---|---|---|---|---|---|
| Hematologic Response | Cytogenetic Response | |||||
| HR | CHR | MCyR | CCyR | |||
| Phase I70 | CP | 17 | 92 | 92 | 35 | 35 |
| AP | 56 | 74 | 51 | 27 | 14 | |
| BP | 33 | 39 | 6 | 18 | 6 | |
| Ph+-ALL | 10 | 10 | - | - | - | |
| Phase II | CP72 | 321 | NR | 76 | 59 | 44 |
| AP73 | 134 | 56 | 30 | 32 | 19 | |
| BP71 | 135 | 38 | 25 | - | - | |
| Ph+-ALL74 | 41 | 27 | 24 | - | - | |
Abbreviations: AP, advanced phase; BP, blast phase; CCyR, Complete Cytogenetic Response; CHR, Complete Hematological Response; CP, Chronic Phase; HR, Hematological Response; MCyR, Major Cytogenetic Response Ph+-ALL, Philadelphia-Positive Acute Lymphoblastic Leukemia;
Four different phase II studies were launched to evaluate the clinical efficacy of nilotinib (400 mg twice daily) in patients with CML in CP, AP, BP and patients with Ph+ ALL71–74. Results are summarized in Table 5. The study in CP recruited 321 patients with resistance (71%) or intolerance (29%) to imatinib. The MCyR, CCyR and MMR rates were 59%, 44% and 28%, respectively. The 2-years PFS was 64% and 2-years OS was 87%. In the AP trial, 138 patients were enrolled and the majority (80%) had imatinib resistance. The HR was 56% (CHR 30%), MCyR was 32% and CCyR was 19%. Median time to progression was 16 months, and OS at 1 year was 82%.
Overall, nilotinib is a very well tolerated drug. Myelosuppression is observed in 30–40% and usually comprises grade 3–4 neutropenia and thrombocytopenia, but these are easily managed with treatment interruption and or dose reductions72,73. The dose of 400 mg twice daily seems to be higher than the minimal required dose, as dose reductions of 2nd-generation TKI do not seem to impact the clinical outcomes of CML patients receiving these drugs as salvage or frontline therapy75. Grade 3–4 laboratorial abnormalities are relatively frequent in patients receiving nilotinib. Most common ones are lipase increase (17%), bilirubin increase (8%), hypophosphatemia (12–15%) and hyperglycemia (12%)72,73. Despite the high frequency of increase in lipase levels, clinically significant pancreatitis is uncommon (<1% of patients). Non-hematological clinical side effects are usually mild; most common ones (> 20%) include rash (all grades: 28%; grade 3–4: 3%), nausea (all grades: 24%; grade 3–4: 1%) and pruritus (all grades: 24%; grade 3–4: 1%). Prolongation of QTc interval has been reported, but is very uncommon, happening in 2.5% of patients72,73. However, there have been reports of sudden deaths in patients receiving therapy with nilotinib, and physician prescribing this drug should be aware of potential drug interactions that might increase the QTc interval70.
Second-Generation TKI: Bosutinib
Bosutinib (formerly known as SKI-606, Pfizer, New York, NY) is an orally available, 2nd-generation TKI with dual activity against Src and Abl kinases. Bosutinib is more potent than imatinib, with an IC50 for BCR-ABL1 of 13 nM, but has very limited activity against PDGFRβ (IC50 370 nM) and KIT (IC50 6,000 nM)76. In a recently published phase I/II trial, bosutinib was administered to 288 patients with CP CML who were intolerant (N=88) or resistant (N=200) to imatinib77. The MTD was determined to be 500 mg daily. After a median follow-up of 24 months, a CHR was achieved in 86% of patients, a MCyR in 53% and a CCyR in 41%. Two-year PFS and OS were 79% and 92%, respectively. Bosutinib had activity against all subtypes of patients with imatinib-resistance or intolerance, except for those harboring the T315I mutation. Bosutinib was very well tolerated. The most common grade 3–4 non-hematological toxicities were diarrhea (9%), rash (9%) and vomiting (3%). Grade 3–4 cytopenias were also uncommon and included anemia (13%), neutropenia (18%) and thrombocytopenia (23%). Another phase II trial evaluated bosutinib (500 mg/day) in 114 CP CML patients who had failed two TKIs, either imatinib/nilotinib or imatinib/dasatinib78. Rates of CHR, MCyR and CCyR were 73%, 32% and 22%, respectively, demonstrating that bosutinib has activity in this scenario.
Issues with 2nd-generation TKI in patients post-imatinib failure
Two points regarding therapy with 2nd-generation TKI post imatinib failure merit further discussion: (1) time to intervene post-imatinib failure; (2) prognostic factors for response and survival with 2nd-generation TKI.
One study sought to determine the answer to the first question79. Among 293 patients with resistance to imatinib and treated with dasatinib, 151 had only lost MCyR (but maintained CHR), 33 had lost both MCyR and CHR, and 109 had lost CHR and had never achieved MCyR. The rates of CCyR and MMR with dasatinib were higher in the first group (72% and 60%, respectively) versus the second group (42% and 29%) and the third group (26% and 26%). The EFS was also better for those patients who had only lost MCyR. Thus, the appropriate time to intervene and change therapy is when the patient loses a MCyR (or a CCyR) but remains in CHR, as intervening at later time points will lead to an inferior outcome.
It is important to determine which patients with imatinib resistant CML have a low probability of response to 2nd-generation TKI, since these patients could be considered for other therapeutic strategies such as allogeneic HSCT80. In one study, two variables were found to be associated with low EFS: lack of prior cytogenetic response to imatinib and performance status ≥181. Patients with both variables had an EFS of only 20%. In a subsequent study by the same group, the achievement of a CCyR after 3 months of therapy in CML-CP patients receiving 2nd-generation TKI post imatinib failure was the only variable associated with EFS (3-years: 74% vs. 43%) and OS (98% vs. 79%)82. Another prognostic model recently published identified the following 3 variables as prognostic: (1) best cytogenetic response achieved with imatinib; (2) Sokal risk score; (3) presence or absence of grade 3–4 neutropenia during treatment with imatinib necessitating dose reduction and/or growth factors83. With these 3 variables the authors built a prognostic score which could predict the rate of CCyR achieved with a 2nd-generation TKI. For those patients who present with mutations, the presence of intermediate-sensitivity BCR-ABL1 mutations (IC50 ≥3 nM for dasatinib and ≥150 nM for nilotinib) is associated with inferior response rates84,85. Mutations with a low response rate to dasatinib include F317L, Q252H and V299L. Mutations with a low response rate to nilotinib include Y253H, E255V/K and F359V/C. Clinicians should tailor therapy for patients with imatinib-resistant CML who present with BCR-ABL1 mutations in order to choose the most adequate TKI to eradicate mutant clones.
Second-Generation TKI as Frontline Therapy for CML
The higher potency of 2nd-generation TKI as compared to imatinib led to the investigation of their use in untreated patients with CML CP. Three phase 2 studies (2 with nilotinib and 1 with dasatinib) were published and demonstrated very high response rates of these drugs when used as frontline therapy (Table 6)86–88. For nilotinib, at 3 months the rate of CCyR was 78–90% and of MMR was 42–52%. At 12 months of therapy, rates of CCyR and MMR were 96% and 81–85%. Dasatinib led to a CCyR rate at 3 months of 82% and at 12 months of 98%; comparative rates of MMR were 24% and 71%. Thus, it appears that these drugs do not only lead to higher rates of response but also to faster responses compared to imatinib. While patients who achieve a late response appear to have a similar survival as patients who achieve an early response89, a drug that is able to lead to a higher response rate earlier in the treatment course might decrease the rate of evolution to AP/BP90.
Table 6.
Studies with 2nd-generation TKI as Frontline Therapy in CML
| Study | Drug | N | % Response at 12 months | |
|---|---|---|---|---|
| CCyR | MMR | |||
| Phase II-MDACC86 | Nilotinib 400 mg twice daily | 67 | 97 | 81 |
| Phase II-MDACC12 | Dasatinib 100 mg once daily | 62 | 98 | 71 |
| Phase II- GIMEMA88 |
Nilotinib 400 mg twice daily | 73 | 96 | 85 |
| Phase III- ENESTnd91 |
Nilotinib 300 mg twice daily | 282 | 80 | 55 |
| Nilotinib 400 mg twice daily | 281 | 78 | 51 | |
| Imatinib 400 mg once daily | 283 | 65 | 27 | |
| Phase III- DASISION93 |
Dasatinib 100 mg once daily | 259 | 83 | 46 |
| Imatinib 400 mg once daily | 260 | 72 | 28 | |
| Phase III-BELA95 | Bosutinib 500 mg once daily | 250 | 70 | 39 |
| Imatinib 400 mg once daily | 252 | 68 | 26 | |
Abbreviations: CCyR, Complete Cytogenetic Response; ENESTnd, Evaluation of Nilotinib Efficacy and Safety in clinical Trials-newly diagnosed patients; GIMEMA, Gruppo Italiano Malattie Ematologiche dell’Adulto; MDACC, M.D. Anderson Cancer Center; MMR, Major Molecular Response.
Two phase III studies were published in 2010 comparing nilotinib and dasatinib against standard dose imatinib, and a phase III trial comparing bosutinib against imatinib has been recently presented (Table 6). In the nilotinib trial, ENESTnd (Evaluating Nilotinib Efficacy and Safety in clinical trials-newly diagnosed patients), 846 patients were randomized in a 1:1:1 fashion to three treatment arms (nilotinib 300 mg twice daily, nilotinib 400 mg twice daily, imatinib 400 mg daily)91. The primary endpoint was the MMR at 12 months. Patients were equally distributed between three groups with no imbalance in pretreatment characteristics. Nilotinib was superior to imatinib regarding the primary endpoint, rendering a MMR rate of 44% (300 mg), 43% (400 mg) and 22% (imatinib) (P<0.0001 for both comparisons). The cumulative rate of CCyR was also higher with nilotinib: 80% (300 mg), 78% (400 mg) and 65% (imatinib). Response was achieved faster with nilotinib, as MMR rate at 6 months was 33% (nilotinib 300 mg), 30% (nilotinib 400 mg) and 12% (imatinib 400 mg). Longer follow-up continues to demonstrate improved response rate for patients treated with nilotinib, with a MMR at 24 months of 71% (300 mg), 67% (400 mg) and 44% (imatinib)92. There was also a higher rate of CMR (26% [300 mg], 21% [400 mg], 10% [imatinib]; p<0.0001). Importantly, nilotinib at both dose schedules decreased time to progression to AP or BP. At the time of first report, 11 patients (4%) on the imatinib had progressed to AP or BP, while only 2 patients (<1%) and 1 patient (<1%) had progressed on the nilotinib 300 mg and 400 mg arms, respectively. The majority of grade 3–4 nonhematological adverse events occurred in <1% of patients in all 3 arms. Grade 3–4 hematological side effects included thrombocytopenia (10%–12% [nilotinib] vs. 9% [imatinib]), neutropenia (10–12%[nilotinib] vs. 20% [imatinib]) and anemia (3% [nilotinib] vs. 5% [imatinib]). Grade 3–4 biochemical abnormalities which were more common with nilotinib than with imatinib included hyperbilirrubinemia (4–8% vs. <1%), hyperglycemia (4–6% vs. 0%), hyperlipasemia (6% vs. 3%) and increased ALT (4–9% vs. 2%).
The DASISION trial randomized 519 patients (1:1) to dasatinib (100 mg once daily) or imatinib standard dose93. The primary endpoint was confirmed CCyR at 12 months. Dose escalation to imatinib 400 mg twice daily or dasatinib 140 mg once daily was allowed for patients with suboptimal responses. At 12 months, dasatinib led to superior rates of CCyR (83% vs. 72%; p<0.001) and MMR (46% vs. 28%; p<0.0001). After 18 months of follow-up, there was improvement in CCyR (85% vs. 80%) and MMR (57% vs. 41%; p=0.0002)94. CMR was attained in 13% of dasatinib patients versus 7% of imatinib patients. There were fewer events of transformation to AP or BP in the dasatinib arm (2.3% vs. 3.5%, p=non-significant). There was no difference in OS (12 months: 97% [dasatinib] vs. 99% [imatinib]). Drug related side effects were primarily grade 1–2. Dasatinib led to higher rates of grade 3–4 thrombocytopenia (19% vs. 10%), but similar rates of grade 3–4 neutropenia (21% vs. 20%). Dasatinib, led to more episodes of pleural effusion (10% vs. 0%); all were grade 1–2, while imatinib caused more superficial edema (36% vs. 9%). Incidence of other common non-hematological side effects, including diarrhea, nausea, vomiting, myalgia, rash and fatigue were more common with imatinib than with dasatinib.
The BELA trial randomized 502 CP CML patients to either bosutinib 500 mg/day (N=250) or imatinib 400 mg/day (N=252)95. The primary endpoint was rate of CCyR at 12 months. In the 18-month follow-up report, the 12 month CCyR rate was 70% (bosutinib) and 68% (imatinib). The cumulative 12-months CCyR rate was 79% for bosutinib and 75% for imatinib. The MMR rate at 1 year was higher for bosutinib, 39% vs. 26% (p=0.002). Time to CCyR and MMR were shorter with bosutinib compared to imatinib (p<0.001 for both comparisons). Transformation to AP or BP occurred in 2% of patients in bosutinib arm versus 4% of patients on imatinib arm (p=0.053). Grade 3–4 side effects with bosutinib included diarrhea (10%), vomiting (3%), pneumonia (3%) and dyspnea (2%). Grade 3–4 cytopenias included thrombocytopenia (14% [bosutinib], 14% [imatinib]) and neutropenia (9% [bosutinib], 21% [imatinib]). Despite the perceived better toxicity profile for bosutinib because of its narrower inhibition spectrum, discontinuation due to adverse events occurred in 22% of patients treated with bosutinib and 6% of those receiving imatinib. This probably reflects the lesser familiarity with the management of side effects induced by bosutinib among investigators and the early switch to alternative available treatment options.
Overall these phase III trials confirmed the superior efficacy of 2nd-generation TKI versus imatinib for the frontline therapy of CML, leading to faster and deeper responses, and with a similar or improved toxicity profile. The FDA has approved both nilotinib and dasatinib for the frontline therapy of patients with CML. Since the follow-up of these trials is still relatively short, the potential impact of these agents on PFS, EFS, and OS remain to be determined. One initial report from the ENESTnd trial has suggested that nilotinib may lower the incidence of BCR-ABL1 mutations, which occurred in only 2.3% of patients receiving nilotinib, versus 6% of patients receiving imatinib96. However, it must be emphasized that an important number of patients fail TKI therapy for reasons different from BCR-ABL1 mutations. Another potential concern is the outcome of patients after they progress while on 2nd-generation TKI. An analysis of 23 patients treated on two frontline phase 2 trials with nilotinib and dasatinib at M.D. Anderson Cancer Center has revealed that, in the majority of cases, failure to 2nd-generation TKI is related to toxicity or patient preference, and patients not infrequently respond to the alternative 2nd-generation TKI97.
Third-Generation TKI: Ponatinib
The T315I mutation of BCR-ABL1 is associated with a high level of resistance to all available TKI. The isoleucine side chain does not form a hydrogen bond with the TKI and prevents binding of the drug due to steric hindrance. Ponatinib (formerly known as AP24534, Ariad Pharmaceuticals, Cambridge, MA) is the first TKI to have potent and consistent activity against BCR-ABL1 with the T315I mutation98. Ponatinib was developed based on a scaffold that, unlike current available TKI, does not make a hydrogen bond with T315, and has a long and flexible ethynil tri-carbon linker which permits its accommodation in the catalytic domain even in the presence of the bulky side chain of isoleucine at residue 31598. Ponatinib inhibits both wild-type (IC50=0.37 nM) and T315I mutated (IC50=2.0 nM) BCR-ABL1, while having activity against several common BCR-ABL1 mutations such as E255K, Y253H and G250E. Ponatinib also inhibits other TK, including SFK, PDGFRα and KIT98. In vivo, ponatinib prolonged survival of mice injected with both wild-type and T315I BCR-ABL1 cells. In a cell based mutagenesis screen, 40nM of ponatinib, a concentration achieved in humans at doses above 30mg daily, completely abolished growth of resistant BCR-ABL1 mutations, suggesting that this drug may prevent the emergence of resistance mediated by BCR-ABL1 mutations98.
Results from a recently completed phase I study of ponatinib in patients with advanced hematological malignancies were recently presented99. Seventy-four patients (64 with refractory CML or Ph+-ALL) were recruited. Patients received ponatinib at doses ranging from 2–60 mg once daily. The most common side effects were thrombocytopenia (23%), rash (22%) and arthralgia (15%). The DLT was pancreatitis, and the MTD was set at 45 mg once daily99. Among 38 patients with CML in CP recruited into the trial, a CHR was obtained in 95%, a MCyR in 66% and a CCyR in 53%. Among 9 patients with the T315I mutation evaluable for response, 100% achieved a CHR and MCyR, and 89% achieved a CCyR. The phase II PACE study is currently evaluating further the efficacy of ponatinib in Ph+ leukemias.
Conclusions
Looking back, it is mesmerizing the impressive amount of progress made in the treatment of CML with TK inhibition strategies overall the last decade, first establishing the activity of imatinib, then recognizing and delineating several mechanisms of resistance to TKIs, and finally, developing 2nd and 3rd generation TKI for the management of imatinib resistance. Such fast pace of developments in the field of CML therapy is the direct result of the close collaboration between basic scientists, biochemists and physicians, which has produced a greater understanding of the pathophysiology of CML and the mechanisms of resistance to TKI. However, there is still room for improvement, particularly for the patient who progress to more advanced stages of the disease, where outcomes are still poor despite the use of potent TKI. Important advancements are still needed regarding our understanding of BCR-ABL1 independent mechanisms of resistance, the biology of primitive CML progenitors, which are resistant to TKI therapy, the possibility of stopping TKI therapy in patients with no evidence of residual disease, and the development of definite curative strategies. We can only hope that strengthening the collaboration between basic and translational investigators and with the invaluable collaboration of patients and their families we will be able to overcome all remaining obstacles in our quest to curing CML in the near future.
Table 2.
Recommendations for disease monitoring (from30)
| Exam | Frequency |
|---|---|
| Complete Blood Cell Count | Every 2 weeks until CHR, them every 3 months or as needed |
| Cytogenetic | At diagnosis, 3 months, 6 months and every 6 months until CCyR, then every 12 months if no molecular test available |
| At failure or unexpected myelosuppression | |
| Molecular | Every 3 months until MMR, then every 6 months |
| Mutation analysis | In case of failure, suboptimal response and before changing 2nd-generation TKI |
Abbreviations: CCyR, complete cytogenetic response; CHR, complete hematological response; MMR, major molecular response; TKI, tyrosine kinase inhibitor
Acknowledgments
Conflicts of interest: HK and JC have received research funding from Novartis and Bristol Myers Squibb; FPS has received research funding from Novartis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quintas-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood. 2009;113(8):1619–1630. doi: 10.1182/blood-2008-03-144790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gishizky ML, Johnson-White J, Witte ON. Efficient transplantation of BCR-ABL-induced chronic myelogenous leukemia-like syndrome in mice. Proc Natl Acad Sci U S A. 1993;90(8):3755–3759. doi: 10.1073/pnas.90.8.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 4.Goldman JM. Chronic myeloid leukemia: a historical perspective. Seminars in hematology. 2010;47(4):302–311. doi: 10.1053/j.seminhematol.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Galton DA. Myleran in chronic myeloid leukaemia; results of treatment. Lancet. 1953;264(6753):208–213. doi: 10.1016/s0140-6736(53)90885-x. [DOI] [PubMed] [Google Scholar]
- 6.Allan NC. Therapeutic options in chronic myeloid leukaemia. Blood Rev. 1989;3(1):45–52. doi: 10.1016/0268-960x(89)90024-6. [DOI] [PubMed] [Google Scholar]
- 7.Interferon alfa versus chemotherapy for chronic myeloid leukemia: a meta-analysis of seven randomized trials: Chronic Myeloid Leukemia Trialists' Collaborative Group. J Natl Cancer Inst. 1997;89(21):1616–1620. [PubMed] [Google Scholar]
- 8.Kantarjian HM, O'Brien S, Cortes JE, et al. Complete cytogenetic and molecular responses to interferon-alpha-based therapy for chronic myelogenous leukemia are associated with excellent long-term prognosis. Cancer. 2003;97(4):1033–1041. doi: 10.1002/cncr.11223. [DOI] [PubMed] [Google Scholar]
- 9.Biggs JC, Szer J, Crilley P, et al. Treatment of chronic myeloid leukemia with allogeneic bone marrow transplantation after preparation with BuCy2. Blood. 1992;80(5):1352–1357. [PubMed] [Google Scholar]
- 10.Nagar B, Bornmann WG, Pellicena P, et al. Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571) Cancer Res. 2002;62(15):4236–4243. [PubMed] [Google Scholar]
- 11.Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289(5486):1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- 12.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2(5):561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 13.Buchdunger E, Zimmermann J, Mett H, et al. Selective inhibition of the platelet-derived growth factor signal transduction pathway by a protein-tyrosine kinase inhibitor of the 2-phenylaminopyrimidine class. Proc Natl Acad Sci U S A. 1995;92(7):2558–2562. doi: 10.1073/pnas.92.7.2558. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Tuveson DA, Willis NA, Jacks T, et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20(36):5054–5058. doi: 10.1038/sj.onc.1204704. [DOI] [PubMed] [Google Scholar]
- 15.Beran M, Cao X, Estrov Z, et al. Selective inhibition of cell proliferation and BCR-ABL phosphorylation in acute lymphoblastic leukemia cells expressing Mr 190,000 BCR-ABL protein by a tyrosine kinase inhibitor (CGP-57148) Clin Cancer Res. 1998;4(7):1661–1672. [PubMed] [Google Scholar]
- 16.Gambacorti-Passerini C, le Coutre P, Mologni L, et al. Inhibition of the ABL kinase activity blocks the proliferation of BCR/ABL+ leukemic cells and induces apoptosis. Blood cells, molecules & diseases. 1997;23(3):380–394. doi: 10.1006/bcmd.1997.0155. [DOI] [PubMed] [Google Scholar]
- 17.Deininger MW, Goldman JM, Lydon N, Melo JV. The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of BCR-ABL-positive cells. Blood. 1997;90(9):3691–3698. [PubMed] [Google Scholar]
- 18.Wolff NC, Ilaria RL., Jr. Establishment of a murine model for therapy-treated chronic myelogenous leukemia using the tyrosine kinase inhibitor STI571. Blood. 2001;98(9):2808–2816. doi: 10.1182/blood.v98.9.2808. [DOI] [PubMed] [Google Scholar]
- 19.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 20.Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344(14):1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 21.Sawyers CL, Hochhaus A, Feldman E, et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood. 2002;99(10):3530–3539. doi: 10.1182/blood.v99.10.3530. [DOI] [PubMed] [Google Scholar]
- 22.Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002;99(6):1928–1937. doi: 10.1182/blood.v99.6.1928. [DOI] [PubMed] [Google Scholar]
- 23.Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346(9):645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 25.Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2(2):117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 26.Deininger M, O'Brien SG, Guilhot F, et al. International Randomized Study of Interferon Vs STI571 (IRIS) 8-Year Follow up: Sustained Survival and Low Risk for Progression or Events in Patients with Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase (CML-CP) Treated with Imatinib [abstract] Blood. 2009;114(22) Abstract 1126. [Google Scholar]
- 27.Kantarjian H, Talpaz M, O'Brien S, et al. Survival benefit with imatinib mesylate therapy in patients with accelerated-phase chronic myelogenous leukemia--comparison with historic experience. Cancer. 2005;103(10):2099–2108. doi: 10.1002/cncr.21032. [DOI] [PubMed] [Google Scholar]
- 28.Kantarjian HM, Talpaz M, O'Brien S, et al. Survival benefit with imatinib mesylate versus interferon-alpha-based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia. Blood. 2006;108(6):1835–1840. doi: 10.1182/blood-2006-02-004325. [DOI] [PubMed] [Google Scholar]
- 29.Kantarjian HM, O'Brien S, Cortes J, et al. Imatinib mesylate therapy improves survival in patients with newly diagnosed Philadelphia chromosome-positive chronic myelogenous leukemia in the chronic phase: comparison with historic data. Cancer. 2003;98(12):2636–2642. doi: 10.1002/cncr.11831. [DOI] [PubMed] [Google Scholar]
- 30.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes TP, Hochhaus A, Branford S, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS) Blood. 2010;116(19):3758–3765. doi: 10.1182/blood-2010-03-273979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kantarjian HM, Shan J, Jones D, et al. Significance of increasing levels of minimal residual disease in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in complete cytogenetic response. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(22):3659–3663. doi: 10.1200/JCO.2008.18.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apperley JF. Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8(11):1018–1029. doi: 10.1016/S1470-2045(07)70342-X. [DOI] [PubMed] [Google Scholar]
- 34.Lahaye T, Riehm B, Berger U, et al. Response and resistance in 300 patients with BCR-ABL-positive leukemias treated with imatinib in a single center: a 4.5-year follow-up. Cancer. 2005;103(8):1659–1669. doi: 10.1002/cncr.20922. [DOI] [PubMed] [Google Scholar]
- 35.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305(5682):399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 36.O'Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65(11):4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 37.Gorre ME, Ellwood-Yen K, Chiosis G, Rosen N, Sawyers CL. BCR-ABL point mutants isolated from patients with imatinib mesylate-resistant chronic myeloid leukemia remain sensitive to inhibitors of the BCR-ABL chaperone heat shock protein 90. Blood. 2002;100(8):3041–3044. doi: 10.1182/blood-2002-05-1361. [DOI] [PubMed] [Google Scholar]
- 38.Soverini S, Martinelli G, Rosti G, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: a study by the GIMEMA Working Party on Chronic Myeloid Leukemia. J Clin Oncol. 2005;23(18):4100–4109. doi: 10.1200/JCO.2005.05.531. [DOI] [PubMed] [Google Scholar]
- 39.Jabbour E, Kantarjian H, Jones D, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia. 2006;20(10):1767–1773. doi: 10.1038/sj.leu.2404318. [DOI] [PubMed] [Google Scholar]
- 40.Jones D, Luthra R, Cortes J, et al. BCR-ABL fusion transcript types and levels and their interaction with secondary genetic changes in determining the phenotype of Philadelphia chromosome-positive leukemias. Blood. 2008;112(13):5190–5192. doi: 10.1182/blood-2008-04-148791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293(5531):876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 42.Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118(5):1208–1215. doi: 10.1182/blood-2010-12-326405. [DOI] [PubMed] [Google Scholar]
- 43.Donato NJ, Wu JY, Stapley J, et al. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood. 2003;101(2):690–698. doi: 10.1182/blood.V101.2.690. [DOI] [PubMed] [Google Scholar]
- 44.Thomas J, Wang L, Clark RE, Pirmohamed M. Active transport of imatinib into and out of cells: implications for drug resistance. Blood. 2004;104(12):3739–3745. doi: 10.1182/blood-2003-12-4276. [DOI] [PubMed] [Google Scholar]
- 45.White DL, Saunders VA, Dang P, Engler J, Hughes TP. OCT-1 activity measurement provides a superior imatinib response predictor than screening for single-nucleotide polymorphisms of OCT-1. Leukemia. 2010;24(11):1962–1965. doi: 10.1038/leu.2010.188. [DOI] [PubMed] [Google Scholar]
- 46.White DL, Dang P, Engler J, et al. Functional activity of the OCT-1 protein is predictive of long-term outcome in patients with chronic-phase chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2010;28(16):2761–2767. doi: 10.1200/JCO.2009.26.5819. [DOI] [PubMed] [Google Scholar]
- 47.White DL, Saunders VA, Dang P, et al. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood. 2006;108(2):697–704. doi: 10.1182/blood-2005-11-4687. [DOI] [PubMed] [Google Scholar]
- 48.Hiwase DK, Saunders V, Hewett D, et al. Dasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implications. Clin Cancer Res. 2008;14(12):3881–3888. doi: 10.1158/1078-0432.CCR-07-5095. [DOI] [PubMed] [Google Scholar]
- 49.Jabbour E, Kantarjian HM, Jones D, et al. Imatinib mesylate dose escalation is associated with durable responses in patients with chronic myeloid leukemia after cytogenetic failure on standard-dose imatinib therapy. Blood. 2009;113(10):2154–2160. doi: 10.1182/blood-2008-04-154344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47(27):6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 51.Tokarski JS, Newitt JA, Chang CY, et al. The structure of Dasatinib (BMS-354825) bound to activated ABL kinase domain elucidates its inhibitory activity against imatinib-resistant ABL mutants. Cancer Res. 2006;66(11):5790–5797. doi: 10.1158/0008-5472.CAN-05-4187. [DOI] [PubMed] [Google Scholar]
- 52.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354(24):2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 53.Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22(6):1200–1206. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- 54.Apperley JF, Cortes JE, Kim DW, et al. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: the START a trial. J Clin Oncol. 2009;27(21):3472–3479. doi: 10.1200/JCO.2007.14.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cortes J, Kim DW, Raffoux E, et al. Efficacy and safety of dasatinib in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blast phase. Leukemia. 2008;22(12):2176–2183. doi: 10.1038/leu.2008.221. [DOI] [PubMed] [Google Scholar]
- 56.Kantarjian H, Pasquini R, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109(12):5143–5150. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 57.Kantarjian H, Pasquini R, Levy V, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily: two-year follow-up of a randomized phase 2 study (START-R) Cancer. 2009;115(18):4136–4147. doi: 10.1002/cncr.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26(19):3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 59.Saglio G, Hochhaus A, Goh YT, et al. Dasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice daily. Cancer. 2010;116(16):3852–3861. doi: 10.1002/cncr.25123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quintas-Cardama A, Kantarjian H, O'Brien S, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 2007;25(25):3908–3914. doi: 10.1200/JCO.2007.12.0329. [DOI] [PubMed] [Google Scholar]
- 61.Quintas-Cardama A, Kantarjian H, Ravandi F, et al. Bleeding diathesis in patients with chronic myelogenous leukemia receiving dasatinib therapy. Cancer. 2009;115(11):2482–2490. doi: 10.1002/cncr.24257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quintas-Cardama A, Han X, Kantarjian H, Cortes J. Tyrosine kinase inhibitor-induced platelet dysfunction in patients with chronic myeloid leukemia. Blood. 2009;114(2):261–263. doi: 10.1182/blood-2008-09-180604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quintas-Cardama A, De Souza Santos FP, Kantarjian H, et al. Dynamics and management of cytopenias associated with dasatinib therapy in patients with chronic myeloid leukemia in chronic phase after imatinib failure. Cancer. 2009;115(17):3935–3943. doi: 10.1002/cncr.24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim DH, Kamel-Reid S, Chang H, et al. Natural killer or natural killer/T cell lineage large granular lymphocytosis associated with dasatinib therapy for Philadelphia chromosome positive leukemia. Haematologica. 2009;94(1):135–139. doi: 10.3324/haematol.13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mustjoki S, Ekblom M, Arstila TP, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia. 2009;23(8):1398–1405. doi: 10.1038/leu.2009.46. [DOI] [PubMed] [Google Scholar]
- 66.Lee SJ, Jung CW, Kim DY, et al. Retrospective multicenter study on the development of peripheral lymphocytosis following second-line dasatinib therapy for chronic myeloid leukemia. Am J Hematol. 2011;86(4):346–350. doi: 10.1002/ajh.21980. [DOI] [PubMed] [Google Scholar]
- 67.Kreutzman A, Juvonen V, Kairisto V, et al. Mono/oligoclonal T and NK cells are common in chronic myeloid leukemia patients at diagnosis and expand during dasatinib therapy. Blood. 2010;116(5):772–782. doi: 10.1182/blood-2009-12-256800. [DOI] [PubMed] [Google Scholar]
- 68.Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7(2):129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Manley PW, Mestan J, Cowan-Jacob S, et al. AMN107: Inhibitory profile against non-mutated and mutated forms of the Bcr-Abl tyrosine kinase [abstract] Proc Am Assoc Cancer Res. 2005;46 Abstract 5985. [Google Scholar]
- 70.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354(24):2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 71.Giles FJ, Larson RA, Kantarjian HM, et al. Nilotinib in Patients (pts) with Philadelphia Chromosome-Positive (Ph+) Chronic Myelogenous Leukemia in Blast Crisis (CML-BC) Who Are Resistant or Intolerant to Imatinib [abstract] Blood. 2007;110(11) Abstract 1025. [Google Scholar]
- 72.Kantarjian HM, Giles FJ, Bhalla KN, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood. 2011;117(4):1141–1145. doi: 10.1182/blood-2010-03-277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.le Coutre PD, Giles F, Hochhaus A, et al. Nilotinib in Chronic Myeloid Leukemia Patients in Accelerated Phase (CML-AP) with Imatinib Resistance or Intolerance: 2-Year Follow-up Results of a Phase 2 Study [abstract] Blood. 2008;112(11) Abstract 3229. [Google Scholar]
- 74.Ottmann OG, Larson RA, Kantarjian HM, et al. Nilotinib in Patients (pts) with Relapsed/Refractory Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia (Ph+ALL) Who Are Resistant or Intolerant to Imatinib [abstract] Blood. 2007;110(11) Abstract 2815. [Google Scholar]
- 75.Santos FP, Kantarjian H, Fava C, et al. Clinical impact of dose reductions and interruptions of second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukaemia. Brit J Haematol. 2010;150(3):303–312. doi: 10.1111/j.1365-2141.2010.08245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puttini M, Coluccia AM, Boschelli F, et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res. 2006;66(23):11314–11322. doi: 10.1158/0008-5472.CAN-06-1199. [DOI] [PubMed] [Google Scholar]
- 77.Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive CML patients with resistance or intolerance to imatinib. Blood. 2011 doi: 10.1182/blood-2011-05-355594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brummendorf TH, Cortes J, Kantarjian H, et al. Bosutinib (BOS) as third-line therapy for chronic phase (CP) chronic myeloid leukemia (CML) following failure with imatinib (IM) and dasatinib (DAS) or nilotinib (NIL) J Clin Oncol (Abstract) 2011;29(15_suppl) Abstract 6535. [Google Scholar]
- 79.Quintas-Cardama A, Cortes JE, O'Brien S, et al. Dasatinib early intervention after cytogenetic or hematologic resistance to imatinib in patients with chronic myeloid leukemia. Cancer. 2009;115(13):2912–2921. doi: 10.1002/cncr.24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jabbour E, Cortes J, Santos FP, et al. Results of allogeneic hematopoietic stem cell transplantation for chronic myelogenous leukemia patients who failed tyrosine kinase inhibitors after developing BCR-ABL1 kinase domain mutations. Blood. 2011;117(13):3641–3647. doi: 10.1182/blood-2010-08-302679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jabbour E, Kantarjian H, O'Brien S, et al. Predictive factors for outcome and response in patients treated with second-generation tyrosine kinase inhibitors for chronic myeloid leukemia in chronic phase after imatinib failure. Blood. 2011;117(6):1822–1827. doi: 10.1182/blood-2010-07-293977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jabbour E, Kantarjian HM, Shan J, et al. The Achievement of a 3-Month Complete Cytogenetic Response (3-mo CCyR) to Second Generation (2nd) Tyrosine Kinase Inhibitors (TKI) Post Imatinib Failure Is the Only Predictive Factor for Event-Free (EFS) and Overall Survival (OS) [abstract] Blood. 2010;116(21) Abstract 2289. [Google Scholar]
- 83.Milojkovic D, Nicholson E, Apperley JF, et al. Early prediction of success or failure of treatment with second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukemia. Haematologica. 2010;95(2):224–231. doi: 10.3324/haematol.2009.012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muller MC, Cortes JE, Kim DW, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to preexisting BCR-ABL mutations. Blood. 2009;114(24):4944–4953. doi: 10.1182/blood-2009-04-214221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hughes T, Saglio G, Branford S, et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol. 2009;27(25):4204–4210. doi: 10.1200/JCO.2009.21.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cortes JE, Jones D, O'Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28(3):392–397. doi: 10.1200/JCO.2009.25.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cortes JE, Jones D, O'Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28(3):398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosti G, Palandri F, Castagnetti F, et al. Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 2009;114(24):4933–4938. doi: 10.1182/blood-2009-07-232595. [DOI] [PubMed] [Google Scholar]
- 89.Guilhot F, Larson RA, O'Brien SG, Gathmann I, Druker BJ. Time to Complete Cytogenetic Response (CCyR) Does Not Affect Long-Term Outcomes for Patients on Imatinib Therapy [abstract] Blood. 2007;110(11) Abstract 27. [Google Scholar]
- 90.Quintas-Cardama A, Kantarjian H, Jones D, et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myeloid leukemia in early chronic phase receiving high-dose or standard-dose imatinib therapy. Blood. 2009;113(25):6315–6321. doi: 10.1182/blood-2008-07-166694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 92.Hughes TP, Hochhaus A, Saglio G, et al. ENESTnd Update: Continued Superiority of Nilotinib Versus Imatinib In Patients with Newly Diagnosed Chronic Myeloid Leukemia In Chronic Phase (CML-CP) [abstract] Blood. 2010;116(21) Abstract 207. [Google Scholar]
- 93.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 94.Shah N, Kantarjian H, Hochhaus A, et al. Dasatinib Versus Imatinib In Patients with Newly Diagnosed Chronic Myeloid Leukemia In Chronic Phase (CML-CP) In the DASISION Trial: 18-Month Follow-up [abstract] Blood. 2010;116(21) Abstract 206. [Google Scholar]
- 95.Gambacorti-Passerini C, Cortes J, Kim D, et al. Bosutinib (BOS) versus imatinib (IM) in patients (pts) with chronic phase chronic myeloid leukemia (CP CML) in the BELA trial: 18-month follow-up. J Clin Oncol [abstract] 2011;29(15_suppl) Abstract 6509. [Google Scholar]
- 96.Hochhaus A, Saglio G, Larson RA, et al. Nilotinib Lowers the Incidence of BCR-ABL Mutations and Improves the Molecular Response Kinetics Compared with Imatinib in Patients (Pts) with Newly Diagnosed Chronic Myeloid Leukemia (CML) [abstract] Blood. 2010;116(21) Abstract 3431. [Google Scholar]
- 97.Eghtedar A, Kantarjian H, Jabbour E, et al. Outcome After Failure to Second Generation Thyrosine Kinase Inhibitors(TKI) Treatment as Frontline Therapy for Patients with Chronic Myeloid Leukemia (CML) In Chronic Phase(CP) [abstract] Blood. 2010;116(21) Abstract 3442. [Google Scholar]
- 98.O'Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16(5):401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cortes J, Talpaz M, Bixby D, et al. A Phase 1 Trial of Oral Ponatinib (AP24534) In Patients with Refractory Chronic Myelogenous Leukemia (CML) and Other Hematologic Malignancies: Emerging Safety and Clinical Response Findings [abstract] Blood. 2010;116(21) Abstract 210. [Google Scholar]