Abstract
Studying salt stress is an important means to the understanding of plant ion homeostasis and osmo-balance. Salt stress research also benefits agriculture because soil salinity significantly limits plant productivity on agricultural lands. Decades of physiological and molecular studies have generated a large body of literature regarding potential salt tolerance determinants. Recent advances in applying molecular genetic analysis and genomics tools in the model plant Arabidopsis thaliana are shading light on the molecular nature of salt tolerance effectors and regulatory pathways.
Introduction
Plants need essential mineral nutrients to grow and develop. However, excessive soluble salts in the soil are harmful to most plants. In fact, no toxic substance restricts plant growth more than does salt on a world scale. It is estimated that salinity affects at least 20% of world's arable land and more than 40% of irrigated land to various degrees (Rhoades and Loveday, 1990). In extreme cases, productive agricultural land could no longer sustain agricultural production and had to be abandoned. This may have contributed to the decline of some human civilizations in history. In coastal areas periodic invasions of seawater directly add salts to the soil. Soils in other semi-arid or arid regions, especially those with ineffective drainage, accumulate salts as irrigation water evaporates, leaving deposits of soluble salts.
Based on their capacity to grow on high salt medium, plants are traditionally classified as glycophytes or halophytes (Flowers et al., 1977). Halophytes are tolerant to high concentrations of NaCl; some can withstand salts that are more than twice the concentration of seawater. Most plants, including the majority of crop species, are glycophytes and cannot tolerate high salinity. For glycophytes, salinity imposes ionic stress, osmotic stress, and secondary stresses such as nutritional disorders and oxidative stress (Zhu, 2001a). Sodium toxicity represents the major ionic stress associated with high salinity. Additionally, some plant species are also sensitive to chloride, the major anion found in saline soils. In certain saline soils, ion toxicity is worsened by alkaline pH. The low osmotic potential of saline solutions hampers plant water uptake, resulting in “physiological drought.” For halophytic plants that are tolerant of sodium toxicity, osmotic stress may be the main cause of growth inhibition.
Understanding the mechanisms of plant salt tolerance will lead to effective means to breed or genetically engineer salt tolerant crops. Salt tolerance research also represents an important part of basic plant biology, contributing to our understanding of subjects ranging from gene regulation and signal transduction to ion transport, osmoregulation and mineral nutrition. Additionally, some aspects of salt stress responses are intimately related to drought and cold stress responses (Zhu, 2001b). Plant salt tolerance studies thus contribute to understanding mechanisms of cross-tolerance of abiotic stresses.
Salt stress studies generally fall into the following four categories: 1) Physiology of salt toxicity and salt tolerance. This includes cellular and metabolic responses to salt (Bohnert and Sheveleva, 1998; Hasagewa et al., 2000), as well as whole plant responses (Flowers et al., 1997; Greenway and Munns, 1980; Yeo, 1998). 2) Mechanisms of salt transport across cellular membranes and over long distances in plants. This includes physiological and molecular characterization of various ion transporters involved in salt uptake, extrusion, compartmentalization, and in the control of long distance transport (Blumwald et al. 2000; Schachtman and Liu, 1999). 3) Survey of genes whose expression is regulated by salt stress (Zhu et al., 1997; Xiong and Zhu, 2001; Shinozaki and Yamaguchi-Shinozaki, 1997; Ingram and Bartels, 1996; Bray, 1997; Bohnert et al., 1995). More recently, research in this aspect is being accelerated by using gene chips and cDNA microarrays (Seki et al., 2001; Kawasaki et al., 2001; Bohnert et al., 2001). 4) Mutational analysis of salt tolerance determinants and salt stress signaling (Zhu, 2000; 2001a, b; Xiong and Zhu, 2001). This overview will emphasize recent progress in the genetic analysis of salt tolerance in Arabidopsis.
Salt Stress and Arabidopsis
Arabidopsis is a glycophytic plant that is sensitive to growth inhibition and damage by salt stress. Although salt sensitivity among Arabidopsis ecotypes/accessions may exist, a systematic comparison among different ecotypes has not been reported. Given that the Arabidopsis genome has been fully sequenced, this comparison between ecotypes may localize salt tolerance QTLs (quantitative trait loci) and potentially lead to the molecular identification of some major loci, if relatively large differences in salt tolerance exist between ecotypes. Since Arabidopsis is a relatively new model for salt tolerance studies, and is not an ideal subject for physiological analysis, information on salt tolerance in Arabidopsis is rather limited. Research with other glycophytic plant species has shown that upon exposure to high salinity, plants may exhibit a reduced growth rate, accelerated development and senescence or death if the stress is severe or prolonged (reviewed by Lazof and Bernstein, 1999). Like many other glycophytes, the sensitivity of Arabidopsis to salt stress is exhibited at all stages of development. For example, a brief (8 hr) treatment with 150 mM NaCl during seed development stage resulted in callose deposition and abnormal changes in ovule and embryo structures indicative of cell death (Sun and Hauser, 2001). However, in Arabidopsis salt sensitivity is most evident at the seed germination and seedling stages. Even after cold stratification of imbibed seeds to break dominancy, Arabidopsis seed germination was greatly impaired at salt concentrations at or above 75 mM (Figure 1). After germination, seedling growth is also very sensitive to NaCl. Although lower concentrations of NaCl (< 50 mM NaCl) may have a slightly stimulating effect on fresh weight gain in culture media, higher than 50 mM of NaCl clearly inhibits plant growth (Figure 2) and eventually kills the plants.
Figure 1.
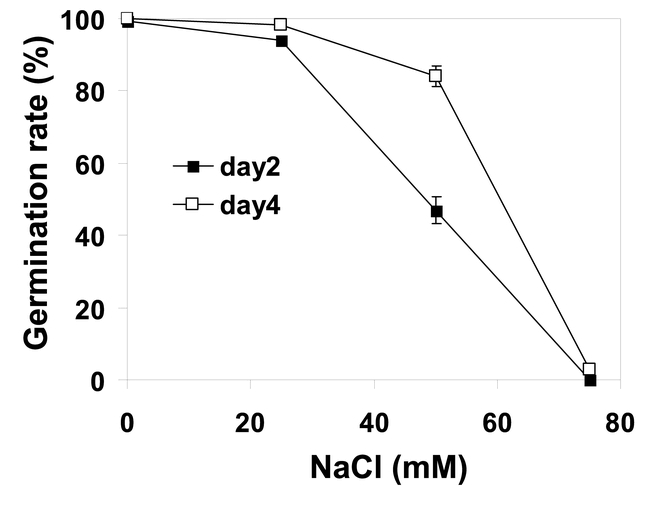
Germination of Arabidopsis seeds is sensitive to NaCl. Seeds of the ecotype C24 (one of the salt sensitive ecotypes) were planted on filter paper saturated with indicated concentrations of NaCl and incubated at 4oC for 2 days before being moved to room temperature (23oC) under white light for germination. Germination (radical emergence) was scored 2 (closed squares) or 4 (open squares) days after the plates being incubated at room temperature.
Figure 2.

Sensitivity of Arabidopsis plants to salt during the vegetative stage. Two-week-old seedlings (ecotype Columbia) growing in the soil were irrigated with 0, 50, 75, and 100 mM NaCl, respectively. Pictures were taken three weeks (upper panel) and four weeks (low panel) after the treatment. Note anthancyanin accumulation in leaves, the bleaching of leaves, and retarded growth and delayed development of seedlings treated with higher concentrations of the salt.
Arabidopsis As A Model Plant To Study Salt Tolerance
With their high levels of salt tolerance, halophytes would appear to be the plants of choice to search for tolerance genes and to study salt tolerance mechanisms. A number of research groups have been studying various halophytes in an effort to clone the genes responsible for salt tolerance (Bohnert et al., 1995). However, because of the complexity of salt tolerance mechanisms and the lack of a well-defined genetic system, the potential of halophytes in revealing salt tolerance mechanisms largely remains to be realized.
Because Arabidopsis is a glycophyte and is very sensitive to salt, one might assume that this plant is not suitable for studying the mechanisms of salt tolerance. However, previous studies with cultured glycophytic plant cells indicated that these cells could be adapted to tolerate high concentrations of salt that would kill un-adapted cells. For instance, normal tobacco cells cannot grow in the presence of 100 mM NaCl. After salt adaptation, some tobacco cells are able to tolerate five times that much salt (Hasegawa et al., 1994). Similarly, alfalfa cells as well as intact plants have been adapted to tolerate very high levels of NaCl (Winicov, 1991). In fact, like cold acclimation, whole plants can also become salt tolerant by being exposed to lower non-lethal concentrations of salt (e.g., Amzallag et al., 1990). Although salt adaptation process did not receive as much attention as cold acclimation did, presumably because of technical difficulties in quantitatively implementing the ‘salt acclimation’ treatments. Nevertheless, the fact that adaptation can increase plant salt tolerance suggests that glycophytes do have salt tolerance machinery that may not be operating effectively in un-adapted conditions. Therefore, the difference in salt tolerance between glycophytes and halophytes appears to be quantitative rather than qualitative, and basic salt tolerance mechanisms are probably conserved in all plant species. The difference in salt sensitivity/tolerance may have resulted from differences in regulatory circuits or from gene alleles coding for key salt tolerance effectors. For example, the vacuolar Na+/H+ antiporter gene AtNHX1 is not as highly inducible in Arabidopsis as its homologous gene is in halophytes, and high level AtNHX1 expression driven by the strong CaMV 35S promoter could significantly improve Arabidopsis salt tolerance (Apse et al., 1999; Hamada et al., 2001; Shi and Zhu, 2002).
The success of Arabidopsis as a genetic model plant for developmental and disease resistance studies has attracted investigators to test its feasibility as a model to study plant salt tolerance. Screening for mutants that are tolerant to high concentrations of salt during germination represented the first such attempt. The germination salt tolerant mutants were named as rs (Saleki et al., 1993), rss (Werner and Finkelstein, 1995), or san (Quesada et al., 2000). Other mutant screens used the inhibitory effect of salts on root growth (sos mutants) (Zhu, 2000) or general seedling growth (e.g., Tsugane et al., 1999). The identification of Salt Overly Sensitive (SOS) loci has uncovered an important regulatory pathway that controls ion homeostasis and salt tolerance (Zhu, 2000). More sophisticated genetic screens such as the one using a firefly luciferase reporter driven by a salt stress-responsive promoter have been successful in identifying salt tolerance determinants that are difficult to isolate from growth inhibition-based screens (Ishitani et al., 1997). It is expected that the use of Arabidopsis as a model will continue to unravel the salt tolerance mechanisms in higher plants (Sander, 2000; Zhu, 2000).
Mechanisms of Salt Entry into Root Cells
Sodium ions are not required for the growth of most land plants. Land plants do not seem to have transport systems specifically for Na+ uptake. However, Na+ can still enter plant cells via several routes. Since the concentration of Na+ in the soil solution is usually much higher than that in the cytosol of root cells, Na+ movement into root cells is passive. Current evidence suggests that Na+ enters root cells mainly through various cation channels. These channels could be voltage-dependent cation channels or voltage-independent cation channels (VIC). Among them, VIC channels are considered the major route for Na+ entry into plant cells (Amtmann and Sanders, 1999; Schachtman and Liu, 1999; Tyerman and Skerrett, 1999; White, 1999). Whereas the molecular identities of the VIC type non-selective cation channels are not known, those of voltage-dependent ion channels that facilitate Na+ entry have been understood to some extent. Due to the similarity between Na+ and K+, voltage-dependent K+ inward rectifiers or outward rectifiers appear to be one path for Na+ entry into root cells (reviewed in Blumwald et al., 2000). For example, in wheat roots, the K+ transporter HKT1 may function as a low affinity Na+ transporter at high external Na+ (Rubio et al., 1995). The homolog in Arabidopsis, AtHKT1, mediated Na+ uptake when expressed in Saccharomyces cerevisiae or Xenopus oocytes (Uozumi et al., 2000). However, the function of AtHKT1 in planta is unclear. In a screen for second site suppressor mutations of salt sensitive phenotypes of sos3 (see later sections), one of the suppressors was identified as a lesion in AtHKT1 (Rus et al., 2001). The hkt1 suppressor mutant has a lower Na+ content, implying that AtHKT1 mediates Na+ uptake into plants. This study also suggests that wild type SOS3, likely by acting together with SOS2, may repress the activity of AtHKT1 in allowing Na+ entry into root cells (Figure 3). It is thus expected that hkt1 mutations would also suppress the salt sensitive phenotype of sos2.
Figure 3.
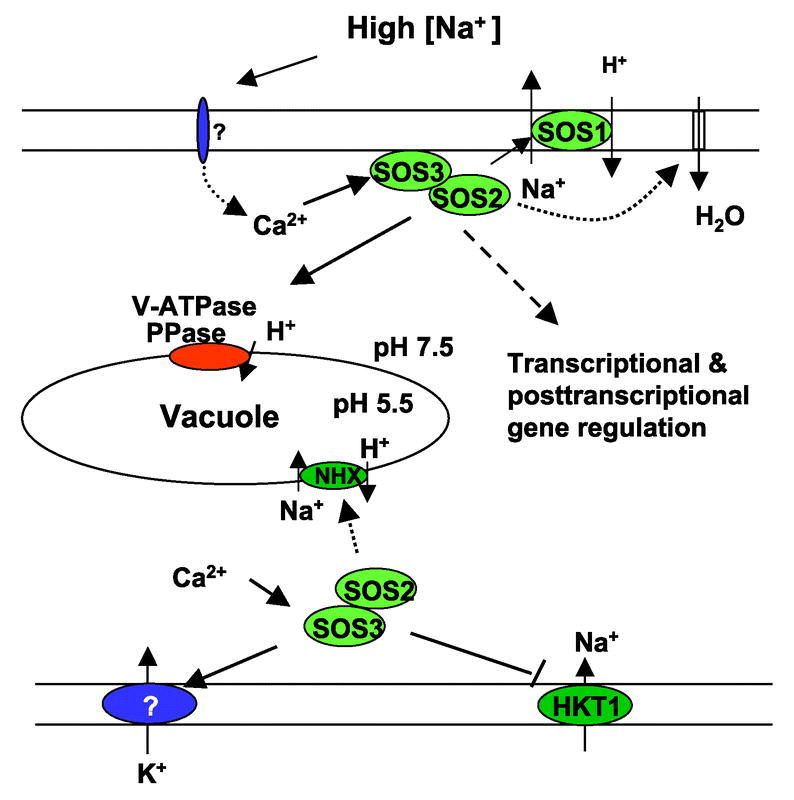
The SOS pathway functions in ion homeostasis under salt stress. High extracellular concentrations of salt elicit a rise in cytosolic Ca2+ concentrations. The Ca2+ sensor SOS3 upon the perception of this Ca2+ signal interacts with and activates the protein kinase SOS2. Activated SOS2 then regulates the activities of ion transporters or transcriptional activators to regulate ion homeostasis or gene expression. The SOS2 targets include the SOS1 Na+/H+ antiporter, the vacuolar Na+/H+ exchangers NHX, and the Na+ transporter HKT1. Other potential targets include tonoplast ATPase and pyrophosphtases, water channels, and potassium transporters.
Besides being regulated by the SOS module (see later sections), Na+ uptake or exclusion and hence plant salt tolerance are also directly or indirectly modulated by many other factors. These factors include membrane potential, acidity of the external solution, variety and concentration of salts, and plant hormones (see below) (reviewed in Xiong and Zhu, 2002a). Here we briefly discuss the effect of one of these factors, membrane potential, on plant salt tolerance.
Under normal conditions, the plasma membrane potential (MP) of root cells is maintained at ∼ -130 mV. Apparently, a more negative potential would facilitate entry of the positively charged Na+ into cells. MP in plant cells is generated and maintained by P-type ATPases, which pump H+ out of the cell and create an electrochemical potential facilitating the uptake of solutes. Some transporters affect salt sensitivity indirectly by altering MP as a result of regulation of ion flux. For example, in yeast trk1/trk2 mutants that are defective in K+ uptake, the plasma membrane becomes hyperpolarized and this greatly enhances the uptake of cations and rendered the mutants more sensitive to Na+, Li+, and low pH (Serrano et al., 1999). An interesting observation was reported also in yeast where a deletion in the PMP3 gene resulted in hyperpolarization of the membrane as measured by methyl-ammonium uptake. The yeast mutant was more sensitive to salt stress due to increased uptake of Na+ (Navarre and Goffeau, 2000). As expected, this effect on uptake was not specific to Na+. The pmp3 mutation also suppressed the increased K+ requirement in the trk1 and trk2 mutants, presumably because membrane hyperpolarization enabled K+ uptake through other transporters. Interestingly, Ca2+ can reverse the salt sensitivity in the pmp3 mutant. PMP3 is a small hydrophilic protein predicted to be localized on the plasma membrane. It is not known how this protein can regulate membrane potential. PMP3 is homologous to the plant proteins RCI2A and RCI2B in Arabidopsis (Medina et al., 2001; Nylander et al., 2001) and BLT101 in barley (Goddard et al., 1993). In fact, the Arabidopsis RCI2A can complement the yeast pmp3/sna1 salt sensitive phenotype (Navarre and Goffeau, 2000; Nylander et al., 2001). Interestingly, the expression of RCI2A and RCI2B genes in Arabidopsis was transiently induced by low temperature or salt stress (Medina et al., 2001; Nylander et al., 2001). However, Nylander et al. (2001) reported that a mutation in the yeast homologous gene SNA1, which is the same gene as PMP3, makes the yeast sensitive specifically to NaCl, since the mutant was not sensitive to either 1 M KCl or 0.6 M LiCl (Nylander et al., 2001). It would be interesting to see whether mutations in Arabidopsis RCI2A and RCI2B also yield similar salt sensitive phenotypes. Future studies on the mechanism of RCI2 function might shed light on salt stress regulation by membrane potentials.
Dealing with Ion Toxicity
Restoring ion homeostasis in plants disturbed by salt stress represents an acute response, both in terms of time and the importance in dealing with overall salt stress effects. Plants employ several ways to combat ionic stress imposed by high salinity. These include restricting salt uptake, increased extrusion and compartmentalization, and controlled long distance transport to aerial parts. Additionally, to avoid cellular damage and nutrient deficiency, plant cells need to maintain adequate K+ nutrition and a favourable K+/Na+ ratio in the cytosol (Niu et al., 1995; Serrano et al., 1999).
Excessive sodium ions at the root surface may disrupt plant potassium nutrition that is vital for the maintenance of cell turgor, membrane potential, and the activities of many enzymes (reviewed by Lazof and Bernstein, 1999). Because of the similar physic-chemical properties of sodium and potassium ions, sodium at a high concentration has a strong inhibitory effect on potassium uptake by the root. For example, K+ uptake via Arabidopsis KUP1 (Kim et al., 1998; Fu and Luan, 1998), which mediates both high and low affinity K+ transport, was inhibited by 5 mM or higher concentrations of NaCl (Fu and Luan, 1998). Plants use both low and high affinity systems for potassium uptake. Sodium ions may have a more damaging effect on the low affinity system that has low potassium/sodium selectivity. Under sodium stress, it is necessary for plants to operate the more selective high affinity potassium uptake system in order to maintain adequate potassium nutrition. It is a general phenomenon that salt treatment of plants causes a decrease in cellular K+ content (Figure 4), which may be partly responsible for reduced growth and vigour under salt stress.
Figure 4.
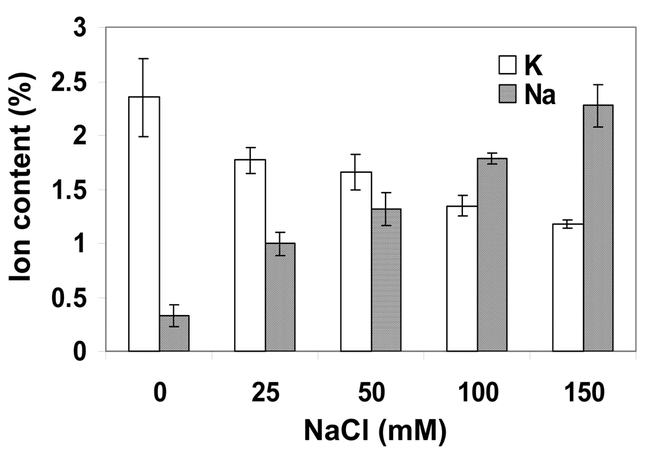
Salt stress impairs K nutrition in Arabidopsis. With increased concentration of NaCl in the culture medium, Na+ content in plants increases whereas K content decreases. Arabidopsis seedlings (ecotype Columbia) growing in half strength of the MS solution (pH 5.3) for two weeks were treated with NaCl by adding NaCl into the culture solution to the indicated final salt concentrations. Seedlings were allowed to grow for 3 days before harvesting and analyzing ion contents (dry weight basis). Data are means ± SE (n=3).
Sodium, once enters into the cytoplasm, has a strong inhibitory effect on the activity of many enzymes. This inhibition is also dependent on potassium level in the cytoplasm: a high sodium/potassium ratio is most damaging. Even for halophytes that accumulate large quantities of sodium inside the cell, their cytosolic enzymes are just as sensitive to sodium as enzymes of glycophytes. This implies that halophytes have to compartmentalize sodium into the vacuole, away from cytosolic enzymes, which is precisely what has been found (Flowers et al., 1977).
An important determinant for plant salt tolerance that is particularly relevant to sodium and potassium homeostasis is calcium. It has long been observed both in solution culture and in soils that increased calcium supply has a protective effect on plants under sodium stress. Yet these experiments did not distinguish whether Ca2+ acted extracellularlly or intracellularly. Recently, experiments with altered cellular Ca2+ homeostasis imply that internal/cytosolic Ca2+ is important to salt sensitivity regulation. For example, when the Arabidopsis ACA4 gene that codes for a vacuolar Ca2+-ATPase was expressed in yeast, it increased the salt tolerance of the yeast cells (Geisler et al., 2001). Arabidopsis vacuolar Ca2+/H+ antiporter gene CAX1, when overexpressed in tobacco, led to increased sensitivity to ionic stress in the transgenics. These transgenic plants appeared to be Ca2+-deficient despite a higher total Ca2+ content (Hirschi, 1999). Similarly, overexpression of the Arabidopsis ionotropic glutamate receptor GluR2 reduced the utilization efficiency of Ca2+ and resulted in Ca2+-deficient and salt sensitive phenotypes (Kim et al., 2001). The resulting physiological deficiency of Ca2+ may have been due to an alteration in the threshold requirement for this cation. Since different organelles within the cell may have different tolerance to Na+, it is of interest to know whether the tolerance has to do with Ca2+ concentration in organelles.
Calcium also sustains potassium transport and potassium/sodium selectivity in sodium-challenged plants. The underlying mechanism for maintaining at least a threshold level of cytosolic potassium in plant cells under sodium stress seems to be similar to that operating in the unicellular yeast Saccharomyces cerevisiae. Sodium stress is sensed by an as yet unknown receptor(s). An early detectable response to sodium stress is a rise in the cytosolic free calcium concentration (Knight, 2000). This calcium signal serves as a second messenger that is believed to turn on machineries that control, for example, potassium uptake and potassium/sodium discrimination. In Arabidopsis, genetic studies suggested that the sensor protein for this salt-induced calcium signature is the Ca2+-binding protein SOS3. A loss-of-function mutation in this protein renders the plant hypersensitive to salt stress. SOS3 is functionally similar to the B subunit of the protein phosphatase calcineurin (Liu and Zhu, 1998), although SOS3 does not function through a phosphatase (Halfter et al., 2000). In yeast cells, calcineurin upon activation by calcium modifies potassium transporters (Mendoza et al., 1994). As a result, low affinity potassium transport that cannot sufficiently discriminate potassium from sodium is turned off, while high affinity potassium transport is turned on. High affinity potassium transporters have the ability to take up potassium in the presence of higher concentrations of Na+. Although it has not been demonstrated experimentally, similar processes are believed to occur in plant cells following calcium activation of SOS3. Besides the regulation of potassium transport, the SOS3 pathway in plants or calcineurin pathway in yeast is also important in activating transporters that remove sodium from cells. Such transporters in Arabidopsis include for example, sodium/proton antiporters such as SOS1. Consistent with this, the Na+ efflux rate in sos3 was much slower than that in the wild type (Elphick et al., 2001).
Extrusion of sodium out of the cell is a straightforward way to avoid Na+ accumulation in the cytosol. It is expected that sodium extrusion may be more important in certain cells, for example, root epidermal cells. This is because most other cells in the plants are surrounded by neighbouring cells and sodium extruded by one cell would become a problem for its neighbouring cells. Indeed, SOS1 promoter-GUS analysis showed that the plasma membrane Na+/H+ antiporter gene is preferentially expressed in epidermal cells surrounding the root tip, and in parenchyma cells bordering the xylem throughout the plant (Shi et al., 2002a).
Sodium extrusion is achieved by sodium/proton antiporters on the plasma membrane. In Arabidopsis, this is accomplished by the plasma membrane-localized Na+/H+ antiporter SOS1 (Shi et al., 2000; 2002a). Mutations in SOS1 rendered the mutant plants very sensitive to Na+ (Wu et al., 1996). Recent studies (Qiu et al., 2002) indicate that plasma membrane vesicles from Arabidopsis plants have a Na+/H+ antiporter activity. This activity is enhanced by pre-treatment with salt stress. More importantly, the sos1 mutation reduces the antiporter activity drastically, demonstrating that SOS1 does function as a plasma membrane Na+/H+ antiporter.
To further explore the role of SOS1 in combating salt stress, the gene was overexpressed in wild type Arabidopsis. It was found that plants overexpressing SOS1 have markedly improved tolerance to salt stress (H. Shi and J.-K. Zhu, unpublished). Analysis of the transgenic plants revealed that these lines had a lower Na+ content in the shoot upon treatment with Na+ (H. Shi and J.-K. Zhu, unpublished). These results provide evidence that SOS1 as a Na+ efflux carrier is vital for plant salt tolerance.
Salt Compartmentalization and Long Distance Transport
Another important mechanism to reduce accumulation of cytosolic Na+ is to store it in the vacuole where the Na+ no longer contacts cytosolic enzymes. Compartmentalization of sodium into the vacuole not only separates Na+ from cytosolic enzymes, it also helps balance against the low extracellular osmotic potential created by salt stress. The ability to compartmentalize Na+ in the vacuole is likely the principle determinant of salt tolerance capacity in most plant species. Vacuolar compartmentalization of Na+ is achieved by the action of Na+/H+ antiporters on the tonoplast - the vacuolar membrane. The proton gradient that drives the antiporter is generated by tonoplast H+-ATPases and pyrophosphatases (Figure 3). Transgenic Arabidopsis plants overexpressing the vacuolar H+- pyrophosphatase AVP1 showed increased tolerance to salt stress presumably by increasing the sequestration of salt in vacuoles (Gaxiola et al., 2001). Similarly, overexpression of one of the vacuolar Na+/H+ antiporters, AtNHX1, increases the salt tolerance of the transgenic Arabidopsis plants (Apse et al., 1999).
The ability to restrict Na+ transport from the root to the aerial parts is another important determinant of salt tolerance. It was thought that growth inhibition is mainly restricted to the shoot under moderate salt stress (Lazof and Bernstein, 1999). One related process in the aerial parts is to retain Na+ in older leaves, which reduces transport to young organs. Amongst several steps that control the long distance transport of Na+ are the radial transport of Na+ across the root cortical cells and the loading of Na+ into the xylem vessel. One component of radial transport of Na+ in Arabidopsis root is SAS1. Mutations in SAS1 resulted in several times increased Na+ content in aerial parts while the Na+ content in roots remained unchanged (Nublat et al., 2001). Cloning of the SAS1 locus has not been reported. Another component of Na+ xylem loading is SOS1. In sos1 mutants, Na+ concentration in the xylem sap is higher than that in the wild type, suggesting that SOS1 controls Na+ loading into and/or retrieval from the xylem, in addition to its role in extruding Na+ in root epidermal cells (Shi et al., 2002a). SOS1 is expressed around vascular tissues, consistent with its function in Na+ loading/retrieval (Shi et al., 2002a).
Dealing with Osmotic Stress and Salt Stress Injuries
When plants are challenged with hyperosmolarity, accumulation of ions such as Na+ in the vacuoles can serve as a means to lower osmotic potential of the cells, and this process is perhaps cost-effective with regard to the amount of energy and resources spent (Yeo, 1983). A related strategy used to lower the osmotic potential of the cell cytosol is to accumulate compatible osmolytes. For glycophytes, the capacities for Na+ compartmentalization and accumulation of osmolytes are both limited. Various compatible osmolytes such as proline, glycine betaine, and polyols can greatly reduce stress damage to plant cells. An increased production of osmolytes is a general phenomenon found in all plants in response to salt stress. This is clearly an adaptive strategy, and transgenic plants with increased osmolyte production or reduced degradation (e.g., Nanjo et al., 1999) show improved salt tolerance. Besides reducing the osmotic potential of the cytosol to facilitate water uptake, many compatible osmolytes have additional functions such as protecting proteins from misfolding and alleviating the toxic effect of reactive oxygen species generated by salt stress (Smirnoff and Cumber, 1989; for reviews see Nuccio et al, 1998; Hong et al., 2000). In fact, the direct protective effect of osmolytes on cellular structures against oxidative stress or protein misfolding may outweigh their role in osmotic adjustment, since their concentration in the transgenic plants overexpressing osmolyte biosynthetic genes is generally very low and thus not very significant for osmotic adjustment (Zhu, 2000).
The beneficial role of compatible osmolytes was suggested by early studies showing that the activities of certain enzymes were less inhibited by excessive salts in the presence of compatible osmolytes (Greenway and Munns, 1980). The major compatible osmolyte in Arabidopsis is proline. One interesting Arabidopsis mutant with altered proline accumulation is eskimo1 that exhibits increased freezing tolerance (Xin and Browse, 1998). The eskimo1 mutant has a high content of proline, resulting from both enhanced production and reduced degradation (Xin and Browse, 1998). It was not reported in that study whether this mutant also has enhanced tolerance to salt stress. Nonetheless, the role of osmolytes in plant salt tolerance was supported with experimental evidence that salt tolerance was increased to various extents in transgenic plants engineered to produce new osmolytes absent in the parental lines or in plants that overexpressed the genes whose products limit the production of these osmolytes (Nuccio et al., 1999; Bohnert and Sheveleva, 1998).
As osmolytes have general protective effects, the benefit seen in transgenic plants goes beyond salt stress tolerance. For example, expression of a bacterial choline oxidase gene CodA in Arabidopsis conferred accumulation of glycinebetaine and increased tolerance to cold and salt stress (Hayashi et al., 1997). In another example, Holmstrom et al. (2000) expressed bacterial glycine betaine biosynthesis genes in tobacco, a species that does not accumulate glycine betaine. The transgenic plants accumulated glycine betaine, and were more tolerant to salt stress, to photoinhibition by high light, as well as to low temperature stress. Nevertheless, it should be pointed out that an enhanced production of osmolytes is not the whole story of salt tolerance. As discussed in earlier sections, there are many other determinants that are also important. In fact, it is likely that osmolyte accumulation may initially result from cellular damage (Delauney and Verma, 1993). For example, it was found that there is an increased accumulation of proline in the sos1 mutant under salt stress, which probably reflects increased cellular damage by salt stress in the mutant (Liu and Zhu, 1997).
Whereas the types of osmolytes that plants produce are plant species-dependent, the magnitude of the accumulation is influenced by other factors including environmental conditions such as salt stress. Although the signal transduction pathways leading to the production of osmolytes are not understood at the moment (Hare et al., 1999), it is possible that these pathways are similar to those operating in yeast, i.e., the HOG1 pathway. Upregulation by salt or drought stress of genes in proline biosynthesis such as P5CS was widely reported (Savoure et al. 1997; Strizhov et al., 1997; Yoshiba et al., 1999; Xiong et al., 2001c). In yeast cells, genes that encode glycerol transporters are also down regulated by osmotic stress with an outcome of reduced glycerol loss. In Arabidopsis, genes that encode enzymes in proline catabolism are also down regulated by osmotic stress. For example, expression of the proline dehydrogenase gene is up-regulated by proline and down-regulated by dehydration (Kiyosue et al., 1996; Reymond et al., 2000). Additionally, the production of osmolytes is modulated by feedback regulation. For example, the biosynthesis of proline in plants involves a feedback inhibitory regulation. Under salt stress, the P5CS protein, which is responsible for the limiting step in proline biosynthesis, may lose this negative regulation due to conformational changes in the protein. Tobacco plants expressing a mutated form of P5CS protein that disabled this negative regulation accumulated more proline under both stress and unstressed conditions and showed increased salt tolerance (Hong et al., 2000).
Secondary Stresses
The ionic and osmotic stresses imposed by high salinity on plants may create secondary stresses. These secondary or derived stresses include for example the accumulation of toxic or unwanted compounds, perturbance in cellular metabolism, and nutritional disorders. Among these, oxidative stress is an important constraint for salt tolerance.
Studies implicated that salt stress could generate reactive oxygen species (ROS) in plants (e.g., Burdon et al., 1996; Shen et al., 1997; Tsugane et al., 1999; Hong et al., 2000), which include, for example, hydrogen peroxide, hydroxyl radicals, and superoxide anions. The mechanism responsible for the generation of ROS upon abiotic stress in plants is not clear. In phagocytic cells, H2O2 generation is via NADPH oxidase that is triggered by growth factors (Rhee et al., 2000). In plants, NADPH oxidases are likely also responsible for the generation of H2O2 in hypersensitive responses (Kawasaki et al., 1999). ROS can have damaging effects on cellular structures and macromolecules such lipids, enzymes and DNA. Detoxification of these compounds therefore contributes to salt and other stress tolerance. As mentioned above, one major function of compatible osmolytes is to alleviate oxidative stress damage, as suggested by transgenic studies (Reviewed in Bohnert et al., 1998; Nuccio et al., 1999). Thus, transgenic plants overexpressing ROS scavenging enzymes such as superoxide dismutase, catalase, and GST showed somewhat increased tolerance to salt stress and other stresses (reviewed in Bohnert et al., 1998). The role of ROS scavengers in plant salt tolerance is also supported by genetic evidence. For example, a genetic screen for salt tolerant growth in Arabidopsis resulted in the isolation of several salt tolerant pst mutants. The enhanced salt tolerance in pst1 mutants is associated with increased ROS detoxification as a result of activation of superoxide dismutase and ascorbate peroxidase (Tsugane et al., 1999).
Salt stress, like some other stresses, may also cause genotoxicity, i.e. DNA damage. In murine kidney cells, it was reported that hyperosmolarity from NaCl causes DNA double strand breaks within 15 min, which is not attributable to apoptosis (Kultz and Chakravarty, 2001). In plants, although direct observation on DNA damage caused by salt stress has not been reported, a connection between DNA damage and salt stress is demonstrated by the isolation of the Arabidopsis uvs66 mutant. This mutant is hypersensitive to UV-C and to the DNA damaging agents methyl methane sulfonate and mitomycin (Albinsky et al., 1999). Interestingly, root growth of the mutant seedlings is also sensitive to NaCl and ABA. The salt sensitivity is specific to NaCl and not to general osmotic stress. This suggests that the genotoxic signal pathway and salt stress signal pathways may use some common components, for example, the MAPK (mitogen activated protein kinase) module.
Studies in yeast and animal cells have implicated connections between oxidative stress and osmotic stress, both of which use common components of the MAPK module (for review, see Kyriakis and Avruch, 1996; Xiong and Zhu, 2002b). Similar connections were recently also revealed in Arabidopsis. A number of studies showed that several MAPK components are activated or their gene expression induced by salt and other stresses (reviewed in Meskiene and Hirt, 2000; Xiong and Zhu, 2001). The role of MAPK modules in plant tolerance to abiotic stresses was further illustrated by the study where a tobacco MAPK kinase kinase (MAPKK) ANP orthologue, NPK1, was expressed in an active form in Arabidopsis. NPK1 mediates H2O2-regulated gene expression in plants (Kovtun et al., 2000). The engineered Arabidopsis plants showed increased tolerance to freezing, heat shock and salt stress (Kovtun et al., 2000), yet the expression of the stress-responsive gene RD29A was not affected. This suggests that in addition to the connections among MAPK modules used by osmotic stress and ROS signaling, there is specificity in the individual pathways (Knight and Knight, 2001). For example, an Arabidopsis mutant defective in a MAPK kinase phosphatase exhibited hypersensitivity to UV-C, but not to salt stress (Ulm et al., 2001).
Salt Stress Regulation of Gene Expression
The expression of numerous plant genes is regulated by salt stress. Investigations of salt induced transcript changes in fact opened the era of molecular biology of plant salt stress. By studying which genes are regulated and how they are regulated, investigators are most interested in the functions of the gene products in plant stress tolerance. A general assumption has been that stress up-regulated genes may be important for stress tolerance. Early works employed several model plants such as the common ice plant, the resurrection plant, several crop plants, as well as Arabidopsis. These studies have been comprehensively reviewed (Skriver and Mundy, 1990; Ingram and Bartel, 1996; Bray, 1997; Zhu et al., 1997) and readers are referred to these reviews for details. Here we briefly outline the main findings in Arabidopsis and also discuss some recent progress.
Genes that are upregulated by salt stress mainly belong to several groups, based on their possible functionality. These genes encode the LEA proteins, enzymes (involved in the biosynthesis of osmolytes, hormones, detoxification, and general metabolism), transporters (ion transporters, ABC transporters, and aquaporins), and regulatory molecules such as transcription factors, protein kinase and phosphatases. The most common and widely reported genes that are stress-regulated are perhaps the LEAs or LEA-like genes. LEA genes encode late embryogenesis abundant proteins (Baker et al., 1988; Dure et al., 1989). These genes are highly expressed in seeds during the desiccation stage following maturation, and in vegetative tissues in response to water deficit. They have been described in many plant species. LEA-like genes are also found in bacteria, yeast and animals (Close and Lammers, 1993; Garay-Arroyo et al., 2000; Shen et al., 2001), suggesting that they may represent some common stress-responses since the LEA-like genes in these non-plant systems are also induced by osmotic stress (Garay-Arroyo et al., 2000). Despite their wide occurrence, the functions of this group of polypeptides are ill defined except in a few cases where overexpression of individual LEA genes resulted in some degree of stress protection (e.g. Xu et al., 1996).
One group of genes in Arabidopsis that are termed RD (responsive to dehydration), COR (cold-regulated) or LTI (low temperature-induced), or KIN (cold induced), are strongly induced by salt, drought, low temperature, and ABA. The products of many of these genes may not be structurally related to but share some features with LEA proteins and are therefore can be loosely classified as LEA-like proteins. These proteins are highly soluble and remain soluble even after being boiled. Because these genes are not expressed under normal (non-stress) growth conditions, they may not be required for normal cellular functions. Since they are strongly expressed following stress treatments such as salt, drought, or low temperature, their products are thought to have some function in protecting cellular structures under stress. One major hypothesis is that these proteins could prevent the denaturation of key proteins by acting as chaperones. The finding that enhanced expression of the transcription factors that regulate the expression of these LEA-like genes can increase the tolerance of transgenic plants to cold, drought, or salt stress demonstrates that these proteins do have a protective effect against abiotic stresses (Jaglo-Ottesen et al, 1998; Liu et al., 1998; Kasuga et al., 1999).
Traditional expression profiling approach to search for salt-regulated genes is being replaced with cDNA microarrays or oligo chips. Microarray analysis of gene expression under salt stress was reported for the yeast S. cerevisae (Yale and Bohnert, 2001; Rep et al., 2000; Posas et al., 2000). Microarray has the potential to monitor gene expression profiles on a genome scale, yet current application of this technology in Arabidopsis still falls short of this expectation, partly because only a limited number of cDNAs are available. Seki et al. (2001) examined Arabidopsis 1,300 genes under drought or cold stresses. Kawasaki et al. (2001) and Bohnert et al. (2001) examined the expression of more genes in rice and Arabidopsis and several other organisms, respectively. The significance of the results generated can only be appreciated with a careful consideration of the experimental conditions and after confirmatory experiments.
With the availability of the microarray technology, the large number of genes that are regulated may at first please the investigators. However, caution should be exercised in interpreting the results. For example, salt stress treatment results in a rapid decline in photosynthesis within minutes (e.g., Kawasaki et al., 2001). Therefore, genes that are regulated by photosynthetic activities may be affected but they are not regulated by salt stress per se. In an example, Reymond et al. (2000) studied the expression of 150 genes in response to wounding, pathogen inoculation and drought treatment. It was found that many of the wounding-induced genes are also up-regulated by drought treatment. Therefore, some of these genes may be primarily induced by drought stress instead of the mechanical stimulus intended. The dosage of stress and the time course of gene induction are also important factors to be considered. The primary stresses and secondary stresses may induce genes in different time frames.
ABA and Other Plant Hormones in Salt Stress Responses
The inhibitory effect of salt stress on plant growth is exhibited at several levels and involves an array of cellular processes. Salt stress affects the expression of cell cycle progression genes (e.g., Burssens et al., 2000) and thus affects cell division. A perturbed turgor may also affect cell expansion and inhibits growth. All these cellular processes may be regulated by an altered hormone homeostasis under salt stress. One well-documented observation is that abscisic acid (ABA) level increases upon salt stress. ABA may have to do with cell cycle regulation (e.g., Wang et al., 1998). However, a major physiological effect of ABA is the stimulation of stomatal closure. As a result, photosynthesis declines (e.g., Kawasaki et al., 2001) and photoinhibition and oxidative stress occur. Another factor that contributes to decreased photosynthesis is the inhibitory effect of salt stress on the efficiency of translocation and assimilation of photosynthetic products; both processes are regulated by plant hormones. Therefore, a perturbed growth, evidenced particularly by inhibition of cell expansion under salt stress, may be controlled to a large extent by hormone homeostasis. Accordingly, quite a number of reports have indicated that applications on crops of growth regulators, such gibberrelic acid and cytokinin, had some benefits in alleviating the adverse effects of salt stress.
ABA is involved in many aspects of plant responses to salt stress. Besides inducing the expression of many salt-responsive genes (Rock, 2000; Bray, 1997; Skriver and Mundy, 1990; Ingram and Bartel, 1996; Bray, 1997; Zhu et al., 1997; Chandler and Robertson, 1994), exogenous ABA application was shown in some experiments to increase salt tolerance of the treated plants or plant tissues. ABA may regulate plant salt responses at several levels. ABA synthesized in the root under drought or salt stress may be an endogenous signal that ascends with transpirational flow to regulate shoot growth, stomatal aperture and hence stress tolerance (reviewed in Davies and Zhang, 1991). At the whole plant level, ABA can relieve the growth inhibition caused by decreased water potential (Øw) under salt or drought stress. Under these conditions, root activity is very important for coordinating the whole plant to combat stress. Increased endogenous ABA levels were shown to be important to maintain maize primary root elongation at low Øw (Saab et al., 1990). This effect may have to do with ABA inhibition of ethylene production (Sharp, 2002). Spollen et al. (2000) reported that at a Øw of −1.6 MPa, inhibition of maize primary root elongation by the ABA synthesis inhibitor fluoridone was prevented by applying inhibitors of ethylene biosynthesis or of ethylene signaling. As in ABA deficient mutants, the production of ethylene in fluoridone-treated seedlings was increased. Re-application of ABA can reduce ethylene production back to the non-fluoridone-treated control levels. Similarly, ABA also stimulates leaf expansion and this is not related to the control of water potentials in the leaf, but instead to the control of ethylene production in the leaf, as was demonstrated with the tomato ABA deficient mutants flacca (Sharp et al., 2000). These results convincingly demonstrated that one role of ABA in promoting root and shoot growth at low Øw is to restrict the production of ethylene, and thereby reduce its inhibitory effect on root elongation and leaf expansion. In Arabidopsis, one interesting observation regarding the relation of ethylene production and root elongation was made with the sos4 mutants. While root and shoot growth of sos4 seedlings were more inhibited by salt stress than found in wild type, root growth of sos4 was more tolerant to mannitol (Shi et al., 2002b). Because SOS4 catalyzes the production of pyridoxal phosphate (Shi et al., 2002b), a co-factor required by ACC (aminocyclopropane-1-carboxylic acid) synthase in ethylene synthesis, sos4 mutant plants may have a reduced ethylene production relative to the wild type under the osmotic stress imposed by high concentrations of mannitol.
The role of ethylene in inhibiting primary root elongation may not be the whole story of ethylene in drought or salt stress responses. Root elongation obviously helps plants explore further soil regions which may have untapped water, and is therefore important against severe water shortages. However root hairs may be more relevant to combating less severe or temporary water shortages. Although ethylene inhibits primary root elongation, it also aids root hair differentiation and promotes root hair elongation. Interestingly, the sos4 mutation diminishes root hair elongation, possibly through diminishing ethylene production. In support of this concept, supplying the ethylene precursor ACC can overcome the short root hair phenotype in this mutant (J.-K. Zhu, unpublished). Further studies on the relationship between root hair elongation, primary root elongation and salt or drought tolerance by using sos4 may help understand the fine balance between root development and plant drought and salt tolerance.
The interaction between ABA and ethylene in vegetative growth as well as in seed germination was further demonstrated by genetic analysis of ABA signal transduction in Arabidopsis. Characterization of mutants with altered responses to ABA during germination and analysis of ethylene response mutants indicated that ethylene counteracts ABA signaling during seed germination, but it positively regulates ABA action in root inhibition (Beaudoin et al., 2000; Ghassemian et al., 2000; Gazzarrini and McCourt, 2001).
At the molecular level, ABA induces the expression of numerous plant genes (reviewed by Rock, 2000). Some of these genes encode various signal transduction components such as putative receptors, protein kinases/phosphatases and transcription factors that may participate in salt stress signaling; others encode effectors for stress tolerance. These effectors include, for example, H+-ATPases, P5CS, and aquaporins (e.g., Kaldenholf et al., 1993; Tyerman et al., 1999). ABA-regulated and salt stress-regulated pathways that lead to the activation of these genes are at least partially separate because the level of gene expression for combined treatment with ABA and salt could be additive (e.g. Chak et al., 2000). Additionally, these two pathways appear to interact at certain points since in some experiments the signaling output from combined treatment with ABA and salt is synergistic relative to individual treatments (Bostock and Quatrano, 1992; Xiong et al., 1999b; Xiong et al., 2001c).
Plant mutants defective in ABA and other hormone production or signal transduction are excellent tools for analyzing the function of the plant hormones in salt stress tolerance. In a genetic study of abiotic stress signal transduction, we recovered a group of mutants that exhibit reduced expression of the luciferase reporter gene under control of the ABA- and salt-stress responsive promoter RD29A (Ishitani et al., 1997; Xiong et al., 2001c). Expression of the endogenous RD29A and many other stress-responsive genes are also changed in these mutants. This is particularly evident in the los5 mutant. In los5, the expression of several genes such as RD29A, COR15, COR47, RD22, and P5CS under salt stress was severely reduced or even completely blocked (Xiong et al., 2001c). Surprisingly, several of these mutants are found to be ABA deficient (Xiong et al., 2001a; Xiong et al., 2002). When ABA was re-introduced, the expression of RD29A-LUC was restored to the wild type level, demonstrating that ABA-deficiency is responsible for blocking the expression of these stress responsive genes (Xiong et al., 2001c). Positional cloning of two of mutant loci, LOS6 and LOS5, identified that they are allelic to ABA DEFICIENT 1 (ABA1) and ABA3 respectively (Xiong et al., 2001c; Xiong et al., 2002). These findings suggest that salt induction of these stress-responsive genes is almost completely dependent on ABA, which contradicts with the previous model that osmotic stress signaling uses both ABA-dependent and ABA-independent pathways.
The studies on los5 and los6 mutants demonstrated a critical role of ABA in regulating salt-regulated gene expression. However, there has been ambiguity in the relationship between ABA and salt tolerance. Like other ABA-deficient or ABA-insensitive mutants, both los5 and los6 are more tolerant to NaCl during germination. This is because salt induces ABA biosynthesis, which contributes to the salt sensitivity of seed germination. In aba or abi mutants, the biosynthesis or the responses to ABA is defective, and hence salt stress has less inhibition on germination. Genetic screens that attempted to isolate salt tolerant mutants using the seed germination phenotype also support this notion, since some of the salt tolerant mutants are alleles of abi mutants (Quesada et al., 2000). The tolerance to salt found in mutants isolated by seed germination screening often does not extend to seedling or adult stages (Saleki et al., 1993; Quesada et al., 2000). Thus salt tolerance must involve some different processes at different stages of plant development.
In the aba mutants, salt stress responses are also complicated. Using los5/aba3 and los6/aba1 mutants, we tested their responses to salt in vegetative growth. We found little difference in root elongation between los6/aba1 and the wild type at various concentrations of NaCl. However, root elongation in los5/aba3 plants was more sensitive to NaCl. With prolonged exposure to high concentrations of salt (e.g. 100 mM NaCl for 3 weeks), los5/aba3 plants showed a higher sensitivity to NaCl, as indicated by leaf yellowing or eventual death after longer exposure (Xiong et al., 2001c), whereas los6/aba1 did not differ much from the wild type in appearance. The reason for the difference in salt sensitivity between los6/aba1 and los5/aba3 is not very clear, although both mutants have similar ABA contents as measured in bulk leaf samples. One possibility is that the ABA levels thus measured may not be accurate in reflecting cell or tissue-specific ABA contents, which may be important for salt tolerance. Another possibility is that los5/aba3 might have other roles unrelated to ABA biosynthesis (Xiong et al., 2001c). These additional roles could be related to the fact that the sulfurylated MoCo, the LOS5/ABA3 catalyzed product, is required both by aldehyde oxidases and xanthine dehydrogenase. It is known that aldehyde oxidases also participate in the synthesis of auxin and that xanthine dehydrogenases function in nitrogen metabolism. Currently, it is not clear whether these enzymatic functions affect salt stress signaling/tolerance or not. Additionally, it is likely that MoCo synthases may be required for the anchoring of certain receptors to membrane patches.
Like aba mutants, Arabidopsis abi mutants also showed an increased tolerance to NaCl during seed germination, yet their sensitivity or tolerance to salt stress at vegetative stages was not reported. Our study indicates that the salt responses in root and shoot growth in abi1 or abi2 mutants are rather complicated: both sensitivity and tolerance to salt relative to the wild type seedlings were observed, depending on the severity and duration of salt stress and seedling ages (J.-K. Zhu, unpublished).
Aspects of Salt Stress Signal Transduction in Arabidopsis
We refer to the processes involved in the perception of salt stress and subsequent initiation of physiological responses as salt stress signal transduction. For abiotic stresses such as low temperature, drought, and salinity, the nature of the primary signals that are recognized by plants is unclear. Salt stress may have either an ionic, osmotic, or a mechanical impact on plant cells. One possibility is that all these different signals have their own specific receptors (Xiong and Zhu, 2001). These receptors can either operate independently or cooperatively to initiate downstream signaling events. This multiplicity of signals originated from one stress may account for part of the complexity of the signaling pathway that is ascribed as ‘crosstalk’ or connectedness (Xiong and Zhu, 2001). For ionic signals, candidate receptors could be various ion transporters that harbor ion-binding sites on either the extracellular and/or intracellular side. Along with the primary ionic and osmotic signals, secondary signals such as stress hormones (ABA and ethylene) and ROS are generated. These secondary signals may act as ligands and bind to cognate receptors. These receptors include the receptor-like protein kinases, two-component sensor kinases, and G-protein coupled receptors (Xiong and Zhu, 2001). Despite this complexity, three types of signaling pathways are apparent. These are the ionic stress signaling pathway, the osmolyte accumulation pathways, and the stress-responsive LEA-like gene regulation pathways. Here we mainly discuss the ionic stress signal transduction pathway under salt stress.
It has been well documented that salt stress, like many other abiotic stresses, can elicit a transient increase in cytosolic Ca2+ (for review, see Knight, 2000; Sanders et al., 1999). Higher plants, like yeast, also use Ca2+ to signal and combat salt stress (Bressan et al., 1998). The source of this Ca2+ could be extracellular or intracellular, both of which have been demonstrated experimentally (Bush, 1995; Muir and Sander, 1997). For ligand-mediated intracellular Ca2+ release, the three ligands known in animal cells, i.e., inositol 1,4,5-trisphosphate (IP3), cyclic ADP-ribose (cADPR), and nicotinic acid adenine dinucleotide phosphate (NAADP) have now been implicated to release Ca2+ from intracellular stores in plant cells as they do in animal cells (NaVazio et al., 2000; Wu et al., 1997; Allen et al., 1995; Cote and Grain, 1994). Evidence suggests that intracellular Ca2+ transient upon hyperosmotic stress of plant cells involves inositol 1,4,5-trisphosphate (IP3) gated Ca2+ release from internal stores such as endoplasmic reticulum (ER) and vacuoles. It has been shown previously that exogenous IP3 was able to release Ca2+ from vacuolar vesicles or isolated vacuoles (e.g., Schumaker and Sze, 1987) and to mediate transient increases in cytosolic Ca2+ (e.g., Allen et al., 1995). Both IP3 and Ca2+ were implicated in ABA and environmental stress responses (for review, see Munnik et al., 1998; Sanders et al., 1999). In addition, the transcript levels of several enzymes involved in the generation of IP3 are induced by ABA, osmotic stress or cold (Hirayama et al., 1995). PIP2 levels as well as IP3 levels increased during salt stress of Arabidopsis plants and the time frame for these changes correlates with the transient increase in cytosolic Ca2+ (DeWald et al., 2001). Microinjection as well as pharmacological experiments showed that increased cytoplasmic Ca2+ can activate the expression of stress responsive genes such as RD29A, KIN1, and cas15 (Monroy and Dhindsa, 1995; Sheen, 1996; Wu et al., 1997). In animal cells, the d forms of phosphatide-specific phospholipase C (PLC), which are structurally similar to the plant PLCs, is activated by G-protein á subunit (Gá), resulting in the cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) to produce IP3. Whereas plant G-protein associated receptors are poorly characterized (Xiong and Zhu, 2001), recent identification of Arabidopsis gpa1 mutant with lesions in the prototypic Gá gene showing impaired ABA-regulated stomatal aperture (Wang et al., 2001) may provide a clue to the identification of G protein-associated receptors in plants. It has not been reported whether this sole prototypic Gá in Arabidopsis also mediates salt and other stress signal transduction.
Although molecular and pharmacological studies have implicated phosphoinositides in many cellular responses in plants, genetic evidence on the involvement of phosphoinositide signaling in abiotic stress signal transduction has been lacking until recently. In a genetic screen using the stress-responsive RD29A promoter driven firefly luciferase as a reporter (Ishitani et al. 1997; Xiong et al., 1999a), we isolated a mutant, fiery1 (fry1) that exhibits much enhanced reporter gene expression in response to cold, salt, drought, and ABA (Xiong et al., 2001b). The FRY1 gene encodes an enzyme with both 3′(2′),5′-bisphosphate nucleotidase and inositol polyphosphate 1-phosphatase activities. This gene was identical to the previously characterized SAL1 gene that was isolated by its ability to confer increased salt tolerance when expressed in yeast cells (Quintero et al., 1996). Because the fry1 mutant plants did not show sulfur deficiency symptoms, the bisphospahte nucleotidase activity of FRY1 in sulfur assimilation appears dispensable. Therefore, it is hypothesized that lack of the inositol polyphosphate 1-phosphatase activity is responsible for the enhanced gene expression in response to stress and ABA treatments. In animal cells, in vitro studies suggest that IP3 is degraded through either a 5-phosphatase pathway or a 3-kinase pathway (Figure 5) (Majerus, 1992; Berridge and Irvine, 1989). These pathways in plant cells are not yet demonstrated. In a recent study, overexpression of an inositol polyphospahte 5-phosphatase resulted in reduced ABA- and stress-responsive gene expression in transgenic plants (Burnette et al., 2001). Two independent studies reported that feeding plants with [3H]-labeled IP3 yielded inositol 4,5-bisphosphate as the primary and immediate catabolite (Brearly et al., 1997; Drøbak et al., 1991), demonstrating that in these plant cells IP3 was likely first hydrolyzed through a 1-phosphatase pathway. However, the inositol polyphosphate 1-phosphatase responsible for this early termination of IP3 signal has not been isolated or characterized. Added to this complexity is the fact that the inositol polyphosphate 1-phosphatase characterized in animal cells is not active against IP3 (Inhorn et al., 1987; Majerus, 1992). Whereas the FRY1/SAL1 activity against Ins(1,4)P2 and Ins(1,3,4)P3 was demonstrated previously (Quintero et al., 1996), its activity on IP3 was not known. We measured the activity against IP3 of the FRY1 recombinant protein and found that it had a measurable although limited activity against IP3 (∼13% of that against Ins (1,4)P2 or Ins (1,3,4)P3) (Xiong et al., 2001b). The in vivo activity of FRY1 on IP3 and its significance in overall metabolism of IP3 are still unknown. Nevertheless, loss of FRY1 activities would inevitably slow down IP3 degradation (Figure 5) and lead to enhanced stress gene expression. To test the impact of fry1 mutations on IP3 metabolism, we measured IP3 levels in fry1 and wild type plants treated with ABA, and found that whereas the ABA-induced IP3 rise was transient in wild type plants, it was higher and more sustained in fry1 mutant plants (Xiong et al., 2001b).
Figure 5.
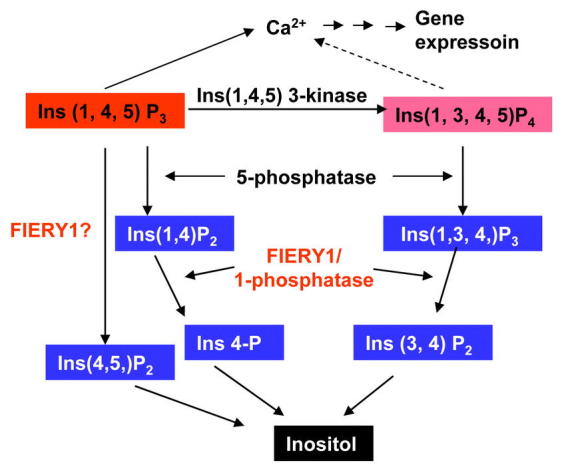
Pathways for the degradation of inositol 1,4,5-trisphosphate (IP3). In animal cells, IP3 can be catabolized by either Ins (1,4,5) 3-kinase or inositol polyphosphate 5-phosphatase (5-phosphatase). The products Ins (1,3,4,5)P4 and Ins(1,4)P2 are further dephosphorylated by FIERY1-like inositol polyphosphate 1-phosphatase (1-phosphatase) and finally to inositol. In plants, it is purposed that FIERY1 dephosphorylates both Ins (1,4)P2 and Ins (1,3,4)P3 and thus mediates the degradation of IP3. Additionally, there may be a 1-phosphatase(s) that can directly dephosphorylate the IP3 at the 1-position. The in vivo ability of FIERY1 in this direct catabolism of IP3 is yet to be determined. A higher and sustained level of IP3 may contribute to a higher level of gene expression.
Whatever the mechanisms involved, transient increases in cytosolic Ca2+ must be coupled with downstream signaling events to mediate stress adaptation. In Arabidopsis salt stress signaling, the Ca2+ signal is perceived by the calcineurin B-like Ca2+ sensor SOS3 (Liu et al., 2000). However, different from the calcineurin B in yeast that acts through activation of a protein phosphatase, SOS3 interacts with and activates the protein kinase SOS2 (Halfter et al., 2000). Thus, SOS3 in some aspects resembles an adaptor or scaffold protein that mediates the interaction of SOS2 with other proteins such as ion transporters. This property of SOS3 is suggested by the requirement of myristoylation for full function in salt tolerance (Ishitani et al., 2000). It appears that SOS3, by anchoring SOS2 to particular plasma membrane patches, may facilitate SOS2 phosphorylation of ion transporters or regulatory partners of transporters. This is further supported by the study that introducing SOS1, SOS2 and SOS3 all together into a yeast mutant deleted of endogenous Na+ transporters greatly improved salt tolerance of the yeast transformants, whereas expressing SOS1 alone only slightly improved salt tolerance of the yeast cells (Quintero et al., 2002).
To understand the interaction between SOS3 and SOS2, the functional domains of both proteins were analyzed. The regulatory and catalytic domains of SOS2 interact, resulting in autoinhibition of the kinase (Guo et al., 2001) (Figure 6). A 21 amino acid motif (FISL motif, refers to the conserved amino acid residues) in the regulatory domain mediates SOS2 interaction with SOS3. It seems that the interaction between SOS3 and SOS2 results in the release of autoinhibition and provides substrate access to the SOS2 kinase domain (Figure 6) (Guo et al., 2001). Presumably through phosphorylation, the activated SOS2 can modulate the activity of ion transporters such as SOS1 and HKT1 (Figure 3), as discussed before.
Figure 6.
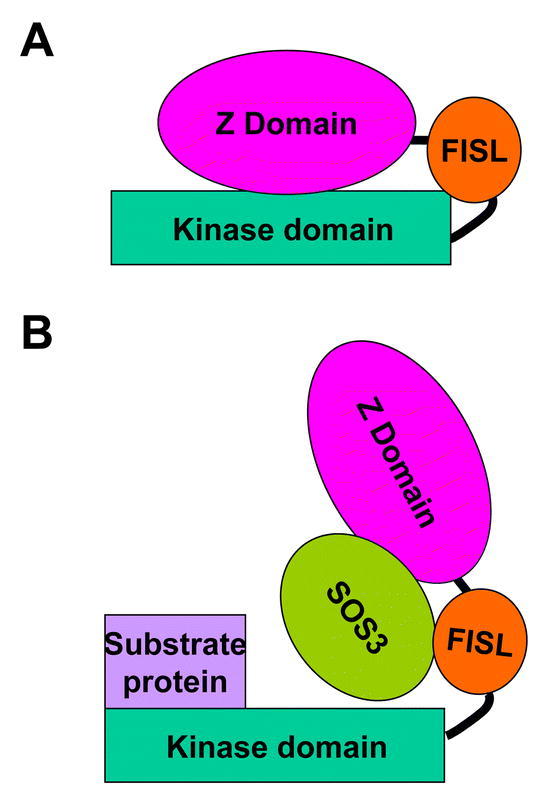
Activation of SOS2 protein kinase by SOS3 Ca2+ –binding protein. (A) In the absence of activated SOS3, the kinase activity of SOS2 is inhibited by interaction between the C-terminus and the kinase domain through the FISL motif. (B) Upon binding to Ca2+, SOS3 becomes active and then interacts with the FISL motif and releases its inhibition of SOS2 kinase activity. This also provides substrate accessibility to SOS2 kinase domain. Through protein phosphorylation, SOS2 regulates the activity of ion transporters and restore ion homeostasis under salt stress. By myristoylation, SOS3 may also help to recruit SOS2 to specific membrane localization (not shown). The Z domain of SOS2 does not have a defined function yet. Figure is a courtesy of Dr. Yan Guo.
Strategies for Finding Salt Tolerance Determinants in Arabidopsis
Several approaches are commonly used to identify genes important for plant salt tolerance (Xiong and Zhu, 2001). They can be classified as either biochemical, expression profiling or genetic. The biochemical approach relies on previous knowledge that a particular enzyme or biochemical pathway is important for salt tolerance. These studies are typically supported by transgenic studies where the genes of interest are overexpressed and/or underexpressed and the resultant transgenic plant phenotypes analyzed. The gene expression profiling approach has been the most popular one for the last decade and a half. In this approach, gene expression under stress conditions is compared to that in the absence of stress. Genes that show increased expression under stress are cloned by using a variety of differential or subtractive screening techniques. More recently, microarray or gene chip analysis is employed to probe gene expression on larger scales as described in earlier sections. A major limit of the expression profiling approach is that many genes critical for plant salt tolerance are not induced by salt stress. In addition, many of the induced genes may not be important for salt tolerance, e.g., their increased expression under salt stress may be a consequence of stress injury. Eventually, functional analysis through transgenic over-expression or under-expression, or through reverse genetics to identify knockout mutants is needed to establish whether an induced gene is functionally important for plant salt tolerance.
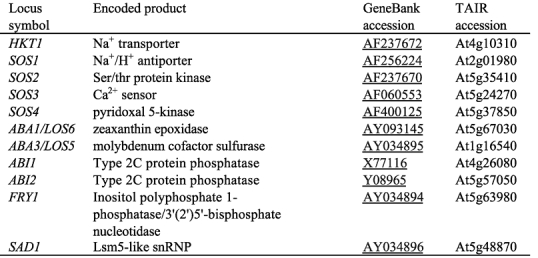
The genetic approach utilizes natural or induced variations in stress tolerance or stress gene regulation. Gene mutations in Arabidopsis plants can be easily created by using one of several mutagens such as ethylmethane sulfonate, fast neutrons, transposons or T-DNA. Mutations in salt tolerance genes are expected to cause decreased salt tolerance in plants, unless they are functionally redundant. Therefore, one can search for plant mutants that are hypersensitive to salt stress, and these mutants will lead to the identification of salt tolerance genes. The sos mutants were isolated in such a screen. Of course, screening for the opposite phenotype (i.e. increased salt tolerance) would identify negative regulators of stress tolerance or gain-of-function mutations. To overcome the shortage of salt stress-specific visible phenotypes, a stress-responsive promoter driven reporter can be used to facilitate genetic screening based on molecular phenotypes. Our studies have found this promoter-reporter approach to be efficient, and especially powerful in identifying gene mutations that have only subtle visible or tolerance phenotypes (Ishitani et al. 1997; Xiong et al., 1999a). In Table 1, we summarize some of the cloned genetic loci that affect plant sensitivity to salt stress.
Table 1.
Selected Arabidopsis genetic loci that affect plant responses to salt stress. Please refer to the text for details.
Several other methods such as transgenic plant analysis and yeast complementation can be used to complement mutational studies. RNAi knockout of candidate genes is a new and much more efficient way to suppress the expression of genes of interest, compared to the traditional antisense approach (Chuang and Meyerowitz, 2000; Smith et al., 2000).
Transgenic plants expressing candidate genes under the control of a constitutive promoter (such as CaMV 35S) have been widely used to examine gene function in salt and other stress tolerance. The use of inducible promoters or tissue-specific promoters may avoid the adverse side effects of constitutive or ubiquitous expression. Kasuga et al (1999) found that plants expressing CBF/DREB under the stress-responsive RD29A promoter were more stress-tolerant but were not compromised in growth, unlike CaMV 35S promoter-driven expression. These studies are exciting in providing leads for the genetic engineering of stress tolerance in crop plants. However, caution should be taken when the overexpression results are used to infer the native function of the endogenous plant genes. For example, overexpression of a transcription factor in a tissue or condition where it is normally not expressed may activate other target genes and thus lead to phenotypes unrelated to its normal function.
It is also worth noting that high expression of stress-responsive genes in some plant mutants does not necessarily mean that the mutants are more stress-tolerant. In our screen of signal transduction mutants using the RD29A-LUC reporter, not only mutants with reduced stress gene induction were more sensitive to respective stresses (e.g., los5, Xiong et al., 2001c), but also some mutants with a high level of stress gene induction are stress-sensitive. The latter mutants include hos1 (Ishitani et al., 1998; Lee et al., 2001), hos2 (Lee et al., 1999), hos5 (Xiong et al., 1999b), sad1(Xiong et al., 2001a), and fry1 (Xiong et al., 2001b). One explanation is that the stress gene products may in fact provide some protection against stress damage in the wild type background, but in the mutant, their high level of expression may be a compensatory response to reduced stress tolerance as a result of the primary mutation, and thus the enhanced stress gene expression response may be triggered by stress injury. In the mutant backgrounds, the protective effect of the stress gene products simply cannot overcome stress sensitivity caused by the primary lesions.
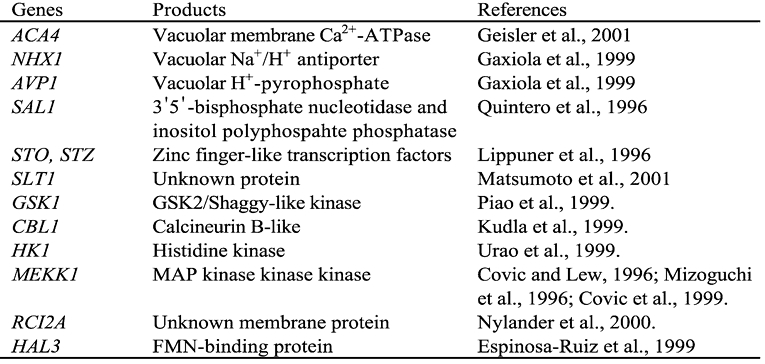
Yeast complementation often has been the method of choice in identifying plant genes with similar functions. This approach was very successful in the isolation of plant ion transporters, some of which are related to salt tolerance. Examples of putative plant salt tolerance determinants isolated by yeast complementation are presented in Table 2. Readers are referred to the references for details.
Table 2.
Some Arabidopsis genes that compliment salt sensitive phenotypes of yeast mutants or increase yeast salt tolerance when expressed in respective yeast strains.
Salt Tolerance beyond Arabidopsis
What this review on salt tolerance is all about is Arabidopsis, a plant after all very sensitive to salt. With growing knowledge on salt tolerance in Arabidopsis, it is high time to tackle the salt tolerance mechanisms in halophytes. An ideal halophytic model organism has been sought after by many researchers. Commonly used salt tolerant organisms such as the common ice plant Mesembryanthemum crystallinum, and the unicellular alga Dunaliella salina are not amenable to genetic analysis. One genetically tractable halophyte is Thellungiella halophila that is native to the coastal regions of eastern China. This plant can complete its life cycle in the presence of 300 mM NaCl. More importantly, its genome size is small, and its cDNAs share over 90% identity to their counterparts from Arabidopsis (Zhu, 2001). Genetic analysis of Thellungiella halophila in comparison with that of Arabidopsis should shed light on how the ionic and osmotic homeostasis and detoxification mechanisms are fine tuned to achieve extreme salt tolerance in halophytic plants.
Acknowledgments
We would like to thank Dr. Andre Jagendorf for critical reading of the manuscript.
Footnotes
Citation: Xiong L., and Zhu J. (2002) Salt Tolerance. The Arabidopsis Book 1:e0048. doi:10.1199/tab.0048
elocation-id: e0048
Published on: September 30, 2002
References
- Albinsky D., Masson J. E., Bogucki A., Afsar K., Vass I., Nagy F., Paszkowski J. Plant responses to genotoxic stress are linked to an ABA/salinity signaling pathway. Plant J. 1999;171(1):73–82. [Google Scholar]
- Allen G. J., Muir S. R., Sanders D. Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science. 1995;2681(1):735–737. doi: 10.1126/science.7732384. [DOI] [PubMed] [Google Scholar]
- Amtmann A., Sanders D. Mechanisms of Na+ uptake by plant cells. Adv. Bot. Res. 1999;291(1):76–112. [Google Scholar]
- Amzallag G. N., Lemer H. R., Poljakoff-Mayber A. Induction of increased salt tolerance in Sorghum bicolor by NaCl treatment. J. Exp. Bot. 1990;411(1):29–34. [Google Scholar]
- Apse M. P., Aharon G. S., Snedden W. A., Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science. 1999;2851(1):1256–1258. doi: 10.1126/science.285.5431.1256. [DOI] [PubMed] [Google Scholar]
- Baker J., Steele C., Dure L., III Sequence and characterization of 6 Lea proteins and their genes from cotton. Plant Mol. Biol. 1988;111(1):277–291. doi: 10.1007/BF00027385. [DOI] [PubMed] [Google Scholar]
- Beaudoin N., Serizet C., Gosti F., Giraudat J. Interactions between abscisic acid and ethylene signaling cascades. Plant Cell. 2000;21(1):1103–1015. doi: 10.1105/tpc.12.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989;3411(1):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Blumwald E., Aharon G., Apse M. P. Sodium transport in plant cells. Biochim. Biophy. Acta. 2000;14651(1):140–151. doi: 10.1016/s0005-2736(00)00135-8. [DOI] [PubMed] [Google Scholar]
- Bostock R. M., Quatrano R. S. Regulation of Em gene expression in rice, interaction between osmotic stress and abscisic acid. Plant Physiol. 1992;981(1):1356–1363. doi: 10.1104/pp.98.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert H. J., Ayoubi P., Borchert C., Bressan R. A., Burnap R. L., Cushman J. C., Cushman M. A., Deyholos M., Fischer R., Galbraith D. W., Hasegawa P. M., Jenks M., Kawasaki S., Koiwa H., Kore-eda S., Lee B. H., Michalowski C. B., Misawa E., Nomura M., Ozturk N., Postier B., Prade R., Song C. P., Tanaka Y., Wang H., Zhu J. K. A genomics approach towards salt stress tolerance. Plant Physiol. Biochem. 2001;391(1):295–311. [Google Scholar]
- Bohnert H. J., Nelson D. E., Jensen R. G. Adaptations to environmental stresses. Plant Cell. 1995;71(1):1099–1111. doi: 10.1105/tpc.7.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert H. J., Sheveleva E. Plant stress adaptations, making metabolism move. Curr. Opinion Plant Biol. 1998;11(1):267–274. doi: 10.1016/s1369-5266(98)80115-5. [DOI] [PubMed] [Google Scholar]
- Bohnert H. J., Su H., Shen B. Molecular mechanisms of salinity tolerance. 1999;1(1):29–62. In Molecular responses to cold, drought, heat, and salt stress in higher plants, Shinozaki, K., and Yamaguchi-Shinozaki, K. (ed.). R.G. Landes Company, Austin, p. [Google Scholar]
- Bray E. A. Plant responses to water deficit. Trends in Plant Sci. 1997;21(1):48–54. [Google Scholar]
- Brearley C. A., Parmar P. N., Hanke D. E. Metabolic evidence for PtdIns(4,5)P2-directed phospholipase C in permeabilized plant protoplasts. Biochem J. 1997;3241(1):123–131. doi: 10.1042/bj3240123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan R. A., Hasegwa P. M., Pardo J. M. Plants use calcium to resolve salt stress. Trends Plant Sci. 1998;31(1):411–412. [Google Scholar]
- Burdon R. H., O'Kane D., Fadzillah N., Gill V., Boyd P. A., Finch R. R. Oxidative stress and responses in Arabidopsis thaliana and Oryza sativa subjected to chilling and salinity stress. Biochem. Soc. Trans. 1996;241(1):469–472. doi: 10.1042/bst0240469. [DOI] [PubMed] [Google Scholar]
- Burnette R., Gunesekara B., Ecertin M., Berdy S., Gillaspy G. A signal terminating gene from Arabidopsis can alter ABA signaling. 2001. The 12th International Meeting on Arabidopsis Research. June 23–27. Madison, WI. USA. Abstract No. 373.
- Burssens S., Himanen K., van de Cotte B., Beeckman T., van Montagu M., Inze D., Verbruggen N. Expression of cell cycle regulatory genes and morphological alterations in response to salt stress in Arabidopsis. Planta. 2000;2111(1):632–640. doi: 10.1007/s004250000334. [DOI] [PubMed] [Google Scholar]
- Bush D. S. Calcium regulation in plant cells and its role in signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995;461(1):95–122. [Google Scholar]
- Chak R. K. F., Thomas T. L., Quatrano R. S., Rock C. D. The genes ABI2 and ABI2 are involved in abscisic acid- and drought-inducible expression of the Daucus carota L. Dc3 promoter in guard cells of transgenic Arabidopsis thaliana (L.) Heynh. Planta. 2000;2101(1):875–883. doi: 10.1007/s004250050692. [DOI] [PubMed] [Google Scholar]
- Chandler P. M., Robertson M. Gene expression regulation by abscisic acid and its relation to stress tolerance. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1994;451(1):113–141. [Google Scholar]
- Chuang C. F., Meyerowitz E. M. Specific and heritable genetic interference by double-stranded RNA in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2000;971(1):4985–4990. doi: 10.1073/pnas.060034297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close T. J., Lammers P. J. An osmotic protein of cyanobacteria is immunologically related to plant dehydrins. Plant Physiol. 1993;1011(1):773–779. doi: 10.1104/pp.101.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote G. G., Crain R. Why do plants have phosphoinositides? BioEssays. 1994;161(1):39–46. [Google Scholar]
- Covic L., Lew R. R. Arabidopsis thaliana cDNA isolated by functional complementation shows homology to serine/threonine protein kinases. Biochim. Biophys. Acta. 1996;13051(1):125–129. doi: 10.1016/0167-4781(95)00233-2. [DOI] [PubMed] [Google Scholar]
- Covic L., Silva N. F., Lew R. R. Functional characterization of ARAKIN (ATMEKK1): a possible mediator in an osmotic stress response pathway in higher plants. Biochim. Biophys Acta. 1999;14511(1):242–252. doi: 10.1016/s0167-4889(99)00096-8. [DOI] [PubMed] [Google Scholar]
- Davies W. J., Zhang J. Root signals and the regulation of growth and development of plants in drying soil. Ann. Rev. Plant Physiol. 1991;421(1):55–73. [Google Scholar]
- Delauney A., Verma D. P. S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993;41(1):215–223. [Google Scholar]
- DeWald D. B., Torabinejad J., Jones C. A., Shope J. C., Cangelosi A. R., Thompson J. E., Prestwich G. D., Hama H. Rapid accumulation of phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate correlates with calcium mobilization in salt-stressed Arabidopsis. Plant Physiol. 2001;1261(1):759–769. doi: 10.1104/pp.126.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drøbak B. K., Watkins P. A. C., Chattaway J. A., Roberts K., Dawson A. P. Metabolism of inositol (1,4,5) trisphosphate by a soluble enzyme fraction from pea (Pisum sativum) roots. Plant Physiol. 1991;951(1):412–419. doi: 10.1104/pp.95.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dure L., III, Crouch M., Harada J., Ho T-H. D., Mundy J., Quatrano R., Thomas T., Sung Z. R. Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol. Biol. 1989;121(1):475–486. doi: 10.1007/BF00036962. [DOI] [PubMed] [Google Scholar]
- Elphick C. H., Sanders D., Maathuis F. J. M. Critical role of divalent cations and Na+ efflux in Arabidopsis thaliana salt tolerance. Plant Cell Environ. 2001;241(1):733–740. [Google Scholar]
- Espinosa-Ruiz A., Belles J. M., Serrano R., Gulianez-Macia F. A. Arabidopsis thaliana AtHAL3: a flavoprotein related to salt and osmotic tolerance and plant growth. Plant J. 1999;201(1):529–539. doi: 10.1046/j.1365-313x.1999.00626.x. [DOI] [PubMed] [Google Scholar]
- Flowers T. J., Troke P. F., Yeo A. R. The mechanism of salt tolerance in halophytes. Annu. Rev. Plant Physiol. 1977;281(1):89–121. [Google Scholar]
- Fu H. H., Luan S. AtKUP1: a dual-affinity K+ transporter from Arabidopsis. Plant Cell. 1998;101(1):63–74. doi: 10.1105/tpc.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay-Arroyo A., Colmenero-Flores J. M., Garciarrubio A., Covarrubias A. A. Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. J. Biol. Chem. 2000;2751(1):5668–5674. doi: 10.1074/jbc.275.8.5668. [DOI] [PubMed] [Google Scholar]
- Gaxiola R. A., Rao R., Sherman A., Grisafi P., Alper S. L., Fink G. R. The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc. Natl. Acad. Sci. USA. 1999;961(1):1480–1485. doi: 10.1073/pnas.96.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaxiola R. A., Li J., Undurraga S., Dang L. M., Allen G. J., Alper S. L., Fink G. R. Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc. Natl. Acad. Sci. USA. 2001;981(1):11444–11449. doi: 10.1073/pnas.191389398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzarrini S., McCourt P. Genetic interaction between ABA, ethylene and sugar signaling pathways. Curr. Opinion Plant Biol. 2001;41(1):387–391. doi: 10.1016/s1369-5266(00)00190-4. [DOI] [PubMed] [Google Scholar]
- Geisler M., Frange N., Gomes E., Martinoia E., Palmgren M. G. The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol. 2000;1241(1):1814–1827. doi: 10.1104/pp.124.4.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemian M., Nambara E., Cutler S., Kawaide H., Kamiya Y., McCourt P. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell. 2000;121(1):1117–1126. doi: 10.1105/tpc.12.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard N. J., Dunn M. A., Zhang L., White A. J., Jack P. L., Hughes M. A. Molecular analysis and spatial expression pattern of a low-temperature-specific barley gene, blt101. Plant Mol Biol. 1993;231(1):871–879. doi: 10.1007/BF00021541. [DOI] [PubMed] [Google Scholar]
- Greenway H., Munns R. Mechanisms of salt tolerance in non-halophytes. Ann. Rev. Plant Physiol. 1980;311(1):149–180. [Google Scholar]
- Guo Y., Halfer U., Ishitani M., Zhu J-K. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell. 2001;131(1):1383–1399. doi: 10.1105/tpc.13.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter U., Ishitani M., Zhu J. K. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. USA. 2000;971(1):3735–3740. doi: 10.1073/pnas.040577697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada A., Shono M., Xia T., Ohta M., Hayashi Y., Tanaka A., Hayakawa T. Isolation and characterization of a Na+/H+ antiporter gene from the halophyte Atriplex gmelini. Plant Mol. Biol. 2001;461(1):35–42. doi: 10.1023/a:1010603222673. [DOI] [PubMed] [Google Scholar]
- Hare P. D., Cress W. A., van Staden J. Proline synthesis and degradation: a model system for elucidating stress-related signal transduction. J. Exp. Bot. 1999;501(1):413–434. [Google Scholar]
- Hasegawa P. M., Bressan R. A., Nelson D. E., Samaras Y., Rhodes D. Tissue culture in the improvement of salt tolerance in plants. 1994;1(1):83–125. In: Soil Mineral Stresses. Approaches to crop improvement, Monographs on Theoretical and Applied Genetics (edited by Yeo, A.R.; Flowers, T.J.), Vol. 21, Springer-Verlag, Berlin, pp. [Google Scholar]
- Hasegawa P. M., Bressan R. A., Zhu J. K., Bohnert H. J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000;511(1):463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Alia, Mustardy L., Deshnium P., Ida M., Murata N. Transformation of Arabidopsis thaliana with the CodA gene for choline oxidase: accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant J. 1997;121(1):133–132. doi: 10.1046/j.1365-313x.1997.12010133.x. [DOI] [PubMed] [Google Scholar]
- Hirayama T., Ohto C., Mizoguchi T., Shinozaki K. A gene encoding a phosphatidylinositol-specific phospholipase C is induced by dehydration and salt stress in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 1995;921(1):3903–3907. doi: 10.1073/pnas.92.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi K. D. Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell. 1999;111(1):2113–2122. doi: 10.1105/tpc.11.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom K. O., Somersalo S., Mandal A., Palva T. E., Welin B. Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J. Exp. Bot. 2000;511(1):177–185. doi: 10.1093/jexbot/51.343.177. [DOI] [PubMed] [Google Scholar]
- Hong Z., Lakkineni K., Zhang Z., Verma D. P. S. Removal of feedback inhibition of D1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 2000;1221(1):1129–1136. doi: 10.1104/pp.122.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhorn R. C., Bansal V. S., Majerus P. Pathway for inositol 1,3,4-trisphosphate and 1,4-bisphosphate metabolism. Proc. Natl. Acad. Sci. USA. 1987;841(1):2170–2174. doi: 10.1073/pnas.84.8.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram J., Bartels D. The molecular basis of dehydration tolerance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;471(1):377–403. doi: 10.1146/annurev.arplant.47.1.377. [DOI] [PubMed] [Google Scholar]
- Ishitani M., Liu J., Halfter U., Kim C. S., Shi W., Zhu J. K. SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell. 2000;121(1):1667–1678. doi: 10.1105/tpc.12.9.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani M., Xiong L., Stevenson B., Zhu J-K. Genetic analysis of osmotic and cold stress signal transduction in Arabidopsis: Interactions and convergence of abscisic acid-dependent and abscisic acid-independent pathways. Plant Cell. 1997;91(1):1935–1949. doi: 10.1105/tpc.9.11.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitani M., Xiong L., Lee H., Stevenson B., Zhu J-K. HOS1, a genetic locus involved in cold-responsive gene expression in Arabidopsis. Plant Cell. 1998;101(1):1151–1161. doi: 10.1105/tpc.10.7.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo-Ottosen K. R., Gilmour S. J., Zarka D. G., Schabenberger O., Thomashow M. F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science. 1998;2801(1):104–106. doi: 10.1126/science.280.5360.104. [DOI] [PubMed] [Google Scholar]
- Kaldenholf R., Kolling A., Ritchter G. A novel blue light-inducible and abscisic acid-inducible gene of Arabidopsis thaliana encoding an intrinsic membrane protein. Plant Mol. Biol. 1993;231(1):1187–1198. doi: 10.1007/BF00042352. [DOI] [PubMed] [Google Scholar]
- Kasuga M., Liu Q., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcriptional factor. Nature Biotechol. 1999;171(1):287–291. doi: 10.1038/7036. [DOI] [PubMed] [Google Scholar]
- Kawasaki S., Borchert C., Deyholos M., Wang H., Brazille S., Kawai K., Galbraith D., Bohnert H. Gene expression profiles during the initial phase of salt stress in rice. Plant Cell. 2001;131(1):889–905. doi: 10.1105/tpc.13.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Henmi K., Ono E., Hatakeyama S., Iwano M., Satoh H., Shimamoto K. The small GTP-binding protein Rac is a regulator of cell death in plants. Proc. Natl. Acad. Sci. USA. 1999;961(1):10922–10926. doi: 10.1073/pnas.96.19.10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. J., Kwak J. M., Uozumi N., Schroeder J. I. AtKUP1, an Arabidopsis gene encoding high-affinity potassium transporter activity. Plant Cell. 1998;101(1):51–62. doi: 10.1105/tpc.10.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. A., Kwak J. M., Jae S. K., Wang M. H., Nam H. G. Overexpression of the AtGluR2 gene encoding an Arabidopsis homolog of mammalian glutamate receptors impairs calcium utilization and sensitivity to ionic stress in transgenic plants. Plant Cell Physiol. 2001;421(1):74–84. doi: 10.1093/pcp/pce008. [DOI] [PubMed] [Google Scholar]
- Kiyosue T., Yoshiba Y., Yamaguchi-Shinozaki K., Shinozaki K. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell. 1996;81(1):1323–1335. doi: 10.1105/tpc.8.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H. Calcium signaling during abiotic stress in plants. International Rev. Cytol. 2000;1951(1):269–325. doi: 10.1016/s0074-7696(08)62707-2. [DOI] [PubMed] [Google Scholar]
- Knight H., Knight M. R. Abiotic stress signaling pathways: specificity and cross-talk. Trends Plant Sci. 2001;61(1):262–267. doi: 10.1016/s1360-1385(01)01946-x. [DOI] [PubMed] [Google Scholar]
- Kovtun Y., Chiu W. L., Tena G., Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. USA. 2000;971(1):2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J., Xu Q., Harter K., Gruissem W., Luan S. Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals. Proc. Natl. Acad. Sci. USA. 1999;91(1):4718–4723. doi: 10.1073/pnas.96.8.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kultz D., Chakravarty D. Hyperosmolarity in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc. Natl. Acad. Sci. 2001;981(1):1999–2004. doi: 10.1073/pnas.98.4.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J. M., Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J. Biol. Chem. 1996;2711(1):24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- Lazof D. B., Bernstein N. The NaCl induced inhibition of shoot growth: the case for distributed nutrition with special consideration of calcium. Adv. Bot. Res. 1999;291(1):113–189. [Google Scholar]
- Lee H., Xiong L., Ishitani M., Stevenson B., Zhu J. K. Cold-regulated gene expression and freezing tolerance in an Arabidopsis thaliana mutant. Plant J. 1999;171(1):301–308. doi: 10.1046/j.1365-313x.1999.00375.x. [DOI] [PubMed] [Google Scholar]
- Lee H., Xiong L., Gong Z., Ishitani M., Stevenson B., Zhu J-K. The Arabidopsis HOS1 gene negatively regulates cold signal transduction and encodes a RING finger protein that displays cold-regulated nucleo-cytoplasmic partitioning. Genes Dev. 2001;151(1):912–924. doi: 10.1101/gad.866801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippuner V., Cyert M. S., Gasser C. S. Two classes of plant cDNA clones differentially complement yeast calcinerin mutants and increase salt tolerance of wild type yeast. J. Biol. Chem. 1996;2711(1):12859–12866. doi: 10.1074/jbc.271.22.12859. [DOI] [PubMed] [Google Scholar]
- Liu J., Zhu J-K. Proline accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis. Plant Physiol. 1997;1141(1):591–596. doi: 10.1104/pp.114.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhu J-K. A calcium sensor homolog required for plant salt tolerance. Science. 1998;2801(1):1943–1945. doi: 10.1126/science.280.5371.1943. [DOI] [PubMed] [Google Scholar]
- Liu J., Ishitani M., Halfter U., Kim C. S., Zhu J. K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA. 2000;971(1):3730–3734. doi: 10.1073/pnas.060034197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;101(1):1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Schachtman D. P., Zhang W. Partial deletion of a loop region in the high affinity K+ transporter HKT1 changes ionic permeability leading to increased salt tolerance. J. Biol. Chem. 2000;2751(1):27924–27932. doi: 10.1074/jbc.M002056200. [DOI] [PubMed] [Google Scholar]
- Majerus P. W. Inositol phosphate biochemistry. Annu. Rev. Biochem. 1992;611(1):225–250. doi: 10.1146/annurev.bi.61.070192.001301. [DOI] [PubMed] [Google Scholar]
- Matsumoto T. K., Pardo J. M., Takeda S., Bressan R. A., Hasegawa P. M. Tobacco and Arabidiopsis SLT1 mediate salt tolerance of yeast. Plant Mol Biol. 2001;451(1):489–500. doi: 10.1023/a:1010659207604. [DOI] [PubMed] [Google Scholar]
- Medina J., Catala R., Salinas J. Developmental and stress regulation of RCI2A and RCI2B, two cold-inducible genes of Arabidopsis encoding highly conserved hydrophobic proteins. Plant Physiol. 2001;1251(1):1655–1666. doi: 10.1104/pp.125.4.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza I., Rubio F., Rodriguez-Navarro A., Pardo J. M. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 1994;2691(1):8792–8796. [PubMed] [Google Scholar]
- Meskiene I., Hirt H. MAP kinase pathways: molecular plug-and-play chips for the cell. Plant Mol Biol. 2000;421(1):791–806. doi: 10.1023/a:1006405929082. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T., Irie K., Hirayam T., Hayashida N., Yamaguchi-Shinozaki K., Matsumoto K., Shinozaki K. A gene encoding a MAP kinase kinase kinase is induced simultaneously with genes for a MAP kinase and an S6 kinase by touch, cold and water stress in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 1996;931(1):765–769. doi: 10.1073/pnas.93.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy A. F., Dhindsa R. S. Low-temperature signal transduction: induction of cold acclimation-specific genes of alfalfa by calcium at 25 degrees C. Plant Cell. 1995;71(1):321–331. doi: 10.1105/tpc.7.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir S. R., Sanders D. Inositol 1, 4, 5-trisphospahte-sensitive Ca2+-releasing across nonvacuolar membrane in cauliflower. Plant Physiol. 1997;1141(1):1511–1521. doi: 10.1104/pp.114.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik T., Irvine R. F., Musgrave A. Phospholipid signaling in plants. Biochim. Biophy. Acta. 1998;13891(1):222–272. doi: 10.1016/s0005-2760(97)00158-6. [DOI] [PubMed] [Google Scholar]
- Nanjo T., Kobayashi M., Yoshiba Y., Kakubari Y., Yamaguchi-Shinozaki K., Shinozaki K. Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett. 1999;4611(1):205–210. doi: 10.1016/s0014-5793(99)01451-9. [DOI] [PubMed] [Google Scholar]
- NaVazio L., Bewell M. A., Siddique A., Dickinson G. D., Galione A., Sanders D. Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proc. Natl. Acad. Sci. 2000;971(1):8693–8698. doi: 10.1073/pnas.140217897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X., Bressan R. A., Hasegawa P. M., Pardo J. M. Ion homeostasis in NaCl stress environment. Plant Physiol. 1995;1091(1):735–742. doi: 10.1104/pp.109.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nublat A., Desplans J., Casse F., Berthomieu P. sas1, an Arabidopsis mutant overaccumulating sodium in the shoot, shows deficiency in the control of the root radial transport of sodium. Plant Cell. 2001;131(1):125–137. doi: 10.1105/tpc.13.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccio M. L., Rhodes D., McNeil S. D., Hanson A. D. Metabolic engineering of plants for osmotic stress resistance. Curr. Opin. Plant Biol. 1999;21(1):128–134. doi: 10.1016/s1369-5266(99)80026-0. [DOI] [PubMed] [Google Scholar]
- Nylander M., Heino P., Helenius E., Palva E. T., Ronne H., Welin B. V. The low-temperature- and salt- induced RCI2A gene of Arabidopsis complements the sodium sensitivity caused by a deletion of the homologous yeast gene SNA1. Plant Mol. Biol. 2001;451(1):341–352. doi: 10.1023/a:1006451914231. [DOI] [PubMed] [Google Scholar]
- Piao H. L., Pih K. T., Lim J. H., Kang S. G., Jin J. B., Kim S. H., Hwang I. An Arabidopsis GSK3/shaggy-like gene that complements yeast salt stress-sensitive mutants in induced by NaCl and abscisic acid. Plant Physiol. 1999;1191(1):1527–1534. doi: 10.1104/pp.119.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F., Chambers J. R., Heyman J. A., Hoeffler J. P., de Nadal E., Arino J. The transcriptional response of yeast to saline stress. J. Biol. Chem. 2000;2751(1):17249–17255. doi: 10.1074/jbc.M910016199. [DOI] [PubMed] [Google Scholar]
- Qiu Q. S., Guo Y., Dietrich M. A., Schumaker K. S., Zhu J. K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA. 2002;991(1):8436–8841. doi: 10.1073/pnas.122224699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada V., Ponce M. R., Micol J. L. Genetic analysis of salt-tolerant mutants in Arabidopsis thaliana. Genetics. 2000;1541(1):421–436. doi: 10.1093/genetics/154.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero F. J., Garciadeblas B., Rodriguez-Navarro A. The SAL1 gene of Arabidopsis, encoding an enzyme with 3′(2′), 5′-bisphosphate nucleotide and inositol polyphosphate 1-phosphatase activities, increases salt tolerance in yeast. Plant Cell. 1996;81(1):529–537. doi: 10.1105/tpc.8.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero F. J., Ohta M., Shi H., Zhu J. K., Pardo J. M. Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc. Natl. Acad. Sci. USA. 2002;991(1):9061–9066. doi: 10.1073/pnas.132092099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rep M., Krantz M., Thevelein J. M., Hohmann S. The transcriptional response of Saccharomyces cerevisiae to osmotic shock. Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J. Biol. Chem. 2000;2751(1):8290–8300. doi: 10.1074/jbc.275.12.8290. [DOI] [PubMed] [Google Scholar]
- Reymond P., Weber H., Damond M., Farmer E. E. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell. 2000;121(1):707–719. doi: 10.1105/tpc.12.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. G., Bae Y. S., Lee S. R., Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. 2000. Science's STKE, www.stke.org/cgi/content/full/OC_sigtrans;2000/53/pe1. [DOI] [PubMed]
- Rhoades J. D., Loveday J. Salinity in irrigated agriculture. 1990;1(1):1089–1142. in American Society of Civil Engineers, Irrigation of Agricultural Crops (Steward, B.A. and Nielsen, D.R., eds), Am. Soc. Agronomists, Monograph 30. [Google Scholar]
- Rhodes D., Hanson A. D. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993;441(1):357–384. [Google Scholar]
- Rock C. D. Pathways to abscisic acid-regulated gene expression. New Phytol. 2000;1481(1):357–396. doi: 10.1046/j.1469-8137.2000.00769.x. [DOI] [PubMed] [Google Scholar]
- Rubio F., Gassmann W., Schroeder J. I. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;2701(1):1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- Rubio F., Schwarz M., Gassmann W., Schroeder J. I. Genetic selection of mutations in the high affinity K+ transporter HKT1 that define functions of a loop site for reduced Na+ permeability and increased Na+ tolerance. J. Biol. Chem. 1999;2741(1):6839. doi: 10.1074/jbc.274.11.6839. [DOI] [PubMed] [Google Scholar]
- Rus A., Yokoi S., Sharkhuu A., Reddy M., Lee B-h, Matsumoto T. K., Koiwa H., Zhu J-K., Bressan R. A., Hasegawa P. M. AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proc. Natl. Acad. Sci. USA. 2001;981(1):14150–14155. doi: 10.1073/pnas.241501798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab I. N., Sharp R. E., Pritchard J., Voetberg G. S. Increased endogenous abascisic acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potential. Plant Physiol. 1990;931(1):1329–1336. doi: 10.1104/pp.93.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleki R., Young P., Lefebvre D. D. Mutants of Arabidopsis thaliana capable of germination under saline conditions. Plant Physiol. 1993;1011(1):839–845. doi: 10.1104/pp.101.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D., Brownlee C., Harper J. F. Communicating with calcium. Plant Cell. 1999;111(1):691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D. The salty tale of Arabidopsis. Curr. Biol. 2000;101(1):R486–488. doi: 10.1016/s0960-9822(00)00554-6. [DOI] [PubMed] [Google Scholar]
- Savoure A., Hua X-J., Bertauche N., Van-Montagu M., Verbruggen N. Abscisic acid-independent and abscisic acid dependent regulation of proline biosynthesis following cold and osmotic stresses in Arabidopsis thaliana. Mol. Gen. Genet. 1997;2541(1):104–109. doi: 10.1007/s004380050397. [DOI] [PubMed] [Google Scholar]
- Schachtman D., Liu W. Molecular pieces to the puzzle of the interaction between potassium and sodium uptake in plants. Trends Plant Sci. 1999;41(1):281–287. doi: 10.1016/s1360-1385(99)01428-4. [DOI] [PubMed] [Google Scholar]
- Schena M., Shalon D., Davis R. W., Brown P. O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;2701(1):467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. Inositol 1,4,5-trisphosphate releases Ca2+ from vacuolar membrane vesicles of oat roots. J. Biol. Chem. 1987;2621(1):3944–3946. [PubMed] [Google Scholar]
- Seki M., Narusaka M., Abe H., Kasuga M., Yamaguchi-Shinozaki K., Carninic P., Hayashizaki Y., Shinozaki K. Monitoring the expression pattern of 1300 Arabdiopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell. 2001;131(1):61–72. doi: 10.1105/tpc.13.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R., Mulet J. M., Rios G., Marguez J. A., de Larrinoa I. F., Leube M. P., Mendizabal I. M., Pascual-Ahuir A., Proft M., Ros R., Montesinos C. A glimpse of the mechanisms of ion homeostasis during salt stress. J. Exp. Bot. 1999;501(1):1023–1036. [Google Scholar]
- Sharp R. E., LeNoble M. E., Else M. A., Thorne E. T., Gherardi F. Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene. J. Exp. Bot. 2000;511(1):1575–1584. doi: 10.1093/jexbot/51.350.1575. [DOI] [PubMed] [Google Scholar]
- Sharp R. E. Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant, Cell Environ. 2002;251(1):213–224. doi: 10.1046/j.1365-3040.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Sheen J. Ca2+ dependent protein kinases and stress signal transduction in plants. Science. 1996;2741(1):1900–1902. doi: 10.1126/science.274.5294.1900. [DOI] [PubMed] [Google Scholar]
- Shen Q., Chen C-N., Brands A., Pan S-M., Ho T-H. D. The stress- and abscisic acid- induced barley gene HVA22: developmental regulation and homologues in diverse organisms. Plant Mol. Biol. 2001;451(1):327–340. doi: 10.1023/a:1006460231978. [DOI] [PubMed] [Google Scholar]
- Shen B., Jensen R. G., Bohnert H. Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol. 1997;1131(1):1177–1183. doi: 10.1104/pp.113.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Ishitani M., Kim C., Zhu J. K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA. 2000;971(1):6896–6901. doi: 10.1073/pnas.120170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Quintero F. J., Pardo J. M., Zhu J-K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long distance Na+ transport in plants. Plant Cell. 2002a;141(1):465–477. doi: 10.1105/tpc.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Xiong L., Stevenson B., Lu T., Zhu J-K. The Arabidopsis salt overly sensitive 4 mutants uncover a critical role for vitamin B6 in plant salt tolerance. Plant Cell. 2002b;141(1):575–588. doi: 10.1105/tpc.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Zhu J-K. Regulation of the vacuolar Na+/H+ antiporter gene AtNHX1 expression by salt stress and abscisic acid. Plant Mol. Biol. 2002. (in press) [DOI] [PubMed]
- Shinozaki K., Yamaguchi-Shinozaki K. Gene expression and signal transduction in water-stress response. Plant Physiol. 1997;1151(1):327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver K., Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990;21(1):503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N., Cumber Q. J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;281(1):1057–1060. [Google Scholar]
- Smith N. A., Singh S. P., Wang M. B., Stoutjesdijk P. A., Green A. G., Waterhouse P. M. Gene expression: Total silencing by intron-spliced hairpin RNAs. Nature. 2000;4071(1):319–320. doi: 10.1038/35030305. [DOI] [PubMed] [Google Scholar]
- Spollen W. G., LeNoble M. E., Samuel T. D., Bernstein N., Sharp R. E. Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiol. 2000;1221(1):967–976. doi: 10.1104/pp.122.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strizhov N., Abraham E., Okresz L., Blickling S., Zilberstein A., Schell J., Koncz C., Szabados L. Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J. 1997;121(1):557–569. doi: 10.1046/j.1365-313x.1997.00557.x. [DOI] [PubMed] [Google Scholar]
- Sun K., Hauser B. Salt stress induces anatomical changes in ovules and embryos, ultimately resulting in seed abortion. 2001. The 12th International Meeting on Arabidopsis Research. June 23–27. Madison, WI. USA. Abstract No. 420.
- Tarczynski M. C., Jensen R. G., Bohnert H. Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science. 1993;2591(1):508–510. doi: 10.1126/science.259.5094.508. [DOI] [PubMed] [Google Scholar]
- Tsugane K., Kobayashi K., Niwa Y., Ohba Y., Wada K., Kobayashi H. A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell. 1999;111(1):1195–1206. doi: 10.1105/tpc.11.7.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman S. D., Bohnert H. J., Maurel C., Steudle E., Smith J. A. C. Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. J. Exp. Bot. 1999;501(1):1055–1071. [Google Scholar]
- Tyerman S. D., Skerrett I. M. Root ion channels and salinity. Sci. Hort. 1999;781(1):175–235. [Google Scholar]
- Ulm R., Revenkova E., di Sansebastiano G-P., Bechtold N., Paszkowski J. Mitogen-activated protein kinase phosphatase is required for genotoxic stress relief in Arabidopsis. Genes Dev. 2001;151(1):699–709. doi: 10.1101/gad.192601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozumi N., Kim E. J., Rubio F., Yamaguchi T., Muto S., Tsuboi A., Bakker E. P., Nakamura T., Schroeder J. I. The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Physiol. 2000;1221(1):1249–1259. doi: 10.1104/pp.122.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao T., Yakubov B., Satoh R., Yamaguchi-Shinozakia K., Seki B., Hirayama T., Shinozaki K. A transmembrane hybrid-type histidine kinase in Arabidopsis functions as an osmosensor. Plant Cell. 1999;111(1):1743–1754. doi: 10.1105/tpc.11.9.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Qi Q., Schorr P., Cutler A. J., Crosby W. L., Fowke L. C. ICK1, a cyclin-dependent protein kinase inhibition from Arabidopsis thaliana interacts with both Cdc2a and CycD3, and its expression is induced by abscisic acid. Plant J. 1998;151(1):501–510. doi: 10.1046/j.1365-313x.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- Wang X. Q., Ullah H., Jones A. M., Assmann S. M. G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science. 2001;2921(1):2070–2072. doi: 10.1126/science.1059046. [DOI] [PubMed] [Google Scholar]
- Werner J., Finkelstein R. R. Arabidopsis mutants with reduced response to NaCl and osmotic stress. Physiol. Plant. 1995;931(1):659–666. [Google Scholar]
- White P. J. The molecular mechanisms of sodium influx to root cells. Trends Plant Sci. 1999;71(1):245–246. doi: 10.1016/s1360-1385(99)01435-1. [DOI] [PubMed] [Google Scholar]
- Winicov I. Characterization of salt tolerant alfalfa (Medicago sativa L) plants regenerated from salt tolerant cell lines. Plant Cell Reports. 1991;101(1):561–564. doi: 10.1007/BF00232511. [DOI] [PubMed] [Google Scholar]
- Wu S-J., Ding L., Zhu J-K. SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell. 1996;81(1):671–627. doi: 10.1105/tpc.8.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Kuzma J., Marechal E., Graeff R., Lee H. C., Foster R., Chua N. H. Abscisic acid signaling through cyclic ADP-ribose in plants. Science. 1997;2781(1):2126–2130. doi: 10.1126/science.278.5346.2126. [DOI] [PubMed] [Google Scholar]
- Xin Z., Browse J. eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proc. Natl. Acad. Sci. USA. 1998;951(1):7799–7804. doi: 10.1073/pnas.95.13.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., David L., Stevenson B., Zhu J-K. High throughput screening of signal transduction mutants with luciferase imaging. Plant Mol. Biol. Rep. 1999a;171(1):59–170. [Google Scholar]
- Xiong L., Ishitani M., Lee H., Zhu J. K. HOS5, a negative regulator of osmotic stress-induced gene expression in Arabidopsis thaliana. Plant J. 1999b;191(1):569–578. doi: 10.1046/j.1365-313x.1999.00558.x. [DOI] [PubMed] [Google Scholar]
- Xiong L., Ishitani M., Zhu J. K. Interaction of osmotic stress, temperature, and abscisic acid in the regulation of gene expression in Arabidopsis. Plant Physiol. 1999c;1191(1):205–211. doi: 10.1104/pp.119.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., Gong Z., Rock C. D., Subramanian S., Guo Y., Xu W., Galbraith D., Zhu J. K. Modulation of abscisic acid signal transduction and biosynthesis by an Sm-like protein in Arabidopsis. Dev. Cell. 2001a;11(1):771–781. doi: 10.1016/s1534-5807(01)00087-9. [DOI] [PubMed] [Google Scholar]
- Xiong L., Lee B. H., Ishitani M., Lee H., Zhang C., Zhu J. K. FIERY1 encoding an inositol polyphosphate 1-phosphatase is a negative regulator of abscisic acid and stress signaling in Arabidopsis. Genes Dev. 2001b;151(1):1971–1984. doi: 10.1101/gad.891901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., Ishitani M., Lee H., Zhu J-K. The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold and osmotic stress responsive gene expression. Plant Cell. 2001c;131(1):2063–2083. doi: 10.1105/TPC.010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., Zhu J-K. Abiotic stress signal transduction in plants: molecular and genetic perspectives. Physiol. Plant. 2001;1121(1):152–166. doi: 10.1034/j.1399-3054.2001.1120202.x. [DOI] [PubMed] [Google Scholar]
- Xiong L., Zhu J-K. Salt-stress signal transduction. 2002a;1(1):165–197. In: Scheel D and Wasternack C (eds) Plant Signal Transduction. Frontiers in Molecular Biology Series. Oxford University Press. pp. [Google Scholar]
- Xiong L., Zhu J-K. Molecular and genetic aspects of osmotic stress responses in plants. Plant, Cell Environ. 2002b;251(1):131–140. doi: 10.1046/j.1365-3040.2002.00782.x. [DOI] [PubMed] [Google Scholar]
- Xiong L., Lee H., Ishitani M., Zhu J. K. Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J Biol. Chem. 2002;2771(1):8588–8596. doi: 10.1074/jbc.M109275200. [DOI] [PubMed] [Google Scholar]
- Xu D., Duan X., Wang B., Hong B., Ho T. H. D., Wu R. Expression of a late embryogenesis abundant (LEA) protein gene, HvA1, from barley confers tolerance to drought and salinity in transgenic rice. Plant Physiol. 1996;1101(1):249–257. doi: 10.1104/pp.110.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yale J., Bohnert H. J. Transcript expression in Saccharomyces cerevisiae at high salinity. J. Biol. Chem. 2001;2761(1):15996–16007. doi: 10.1074/jbc.M008209200. [DOI] [PubMed] [Google Scholar]
- Yoshiba Y., Nanjo T., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. Stress-responsive and developmental regulation of D1-pyrroline-5-carboxylate synthetase 1 (P5CS1) gene expression in Arabidopsis thaliana. Biochem. Biophys. Res. Comm. 1999;2611(1):766–772. doi: 10.1006/bbrc.1999.1112. [DOI] [PubMed] [Google Scholar]
- Yeo A. R. Salinity resistance: Physiologies and prices. Physiol. Plant. 1983;581(1):214–222. [Google Scholar]
- Yeo A. Molecular biology of salt tolerance in the context of whole-plant physiology. J. Exp. Bot. 1998;491(1):913–929. [Google Scholar]
- Zhu J-K., Liu J., Xiong L. Genetic analysis of salt tolerance in Arabidopsis: Evidence for a critical role of potassium nutrition. Plant Cell. 1998;101(1):1181–1191. doi: 10.1105/tpc.10.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Davies W. J. Antitranspirant activity in xylem sap of maize plants. J. Exp. Bot. 1991;421(1):317–321. [Google Scholar]
- Zhu J-K. Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol. 2000;1241(1):941–948. doi: 10.1104/pp.124.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-K. Plant salt tolerance. Trends Plant Sci. 2001a;61(1):66–71. doi: 10.1016/s1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- Zhu J-K. Cell signaling under salt, water and cold stresses. Curr. Opinion Plant Biol. 2001b;41(1):401–406. doi: 10.1016/s1369-5266(00)00192-8. [DOI] [PubMed] [Google Scholar]
- Zhu J-K., Hasegawa P. M., Bressan R. A. Molecular aspects of osmotic stress in plants. Crit. Rev. Plant Sci. 1997;161(1):253–277. [Google Scholar]


