INTRODUCTION
The assimilation of inorganic nitrogen into organic form has marked effects on plant productivity, biomass, and crop yield (Hageman and Lambert, 1988; Lawlor et al., 1989). As such, a tremendous amount of biochemical and physiological studies have been performed on nitrogen assimilatory enzymes from a variety of plant species, especially crops and legumes. Summaries of such biochemical studies can be found in several comprehensive reviews (Miflin and Lea, 1976, 1977; Miflin, 1980; Miflin and Lea, 1980; Miflin and Lea, 1980, 1982; Miflin and Cullimore, 1984; Poulton et al., 1989). Although these biochemical studies have provided a solid groundwork, a complete picture of the N-assimilation process and its regulation in a single plant is still lacking for a number of reasons. The existence of multiple isoenzymes for each step in nitrogen assimilation has confounded biochemical purification as have the technical difficulties of isoenzyme purification and organelle isolation. As the mechanisms controlling intra- and intercellular transport of inorganic and organic nitrogen in plants are still under investigation, it is impossible to predict the in vivo function of nitrogen assimilatory enzymes localized in distinct cells or subcellular compartments based on in vitro biochemistry. Plant mutants have provided a mechanism to dissect the process of N-assimilation in vivo (Lea and Forde, 1994) (Lam et al., 1996). The aim of this chapter is to specifically highlight and update examples where molecular, genetic, and biochemical analyses of N-assimilation genes and mutants in Arabidopsis have begun to define the in vivo roles of individual isoenzymes in plant nitrogen assimilation and to uncover the mechanisms regulating this process.
In all higher plants, inorganic nitrogen is first reduced to ammonia prior to its incorporation into organic form (Lea, 1993). For a review of the regulation of Nitrate assimilation and reduction in Arabidopsis see (Crawford and Forde, 2002). Ammonia is assimilated into organic form as glutamine and glutamate, which serve as the nitrogen donors in the biosynthesis of essentially all amino acids, nucleic acids, and other nitrogen-containing compounds such as chlorophyll (Lea, 1993). The individual iso-enzymes of GS, GOGAT or GDH have been proposed to play roles in three major ammonium assimilation processes: (i) primary nitrogen assimilation; (ii) reassimilation of photorespiratory ammonia; and (iii) reassimilation of “recycled” nitrogen. For a review of these processes see (Stewart et al., 1980; Lea, 1993). Glutamine and glutamate can then be used to form aspartate and asparagine, and these four amino acids are used to translocate organic nitrogen from sources to sinks (Lea and Miflin, 1980; Peoples and Gifford, 1993). The enzymes involved in the primary assimilation of ammonium into these four N-transport amino acids Glu/Gln and Asp/Asn are shown in Fig. 1 and include; glutamine synthetase (GS), glutamate synthase (GOGAT), aspartate aminotransferase (AAT) and asparagine synthetase (AS). While most studies of nitrogen metabolism have previously been performed in legumes and crop species, HPLC analyses of Arabidopsis has demonstrated that these same four amino acids can account for 60–64% of the total free amino acids present in Arabidopsis leaves and are also transported in the vascular tissues (Fig. 2) (Schultz, 1994; Lam et al., 1995). Thus, Arabidopsis appears to be a suitable model plant for the study of nitrogen assimilation into primary amino acids and the results should have impact on less genetically tractable crop plants. It is noteworthy that each enzyme for GS, GOGAT, AspAT and AS exists as multiple isoenzymes, encoded by multiple genes, even in Arabidopsis (Fig. 1). Table 1 lists the enzymes, the genes, and available mutants for these N-assimilatory enzymes in Arabidopsis. Below, we review how studies of the genes and mutants for these isoenzymes in Arabidopsis have helped to illuminate the role of specific genes in this N-assimilation pathway.
Figure 1.
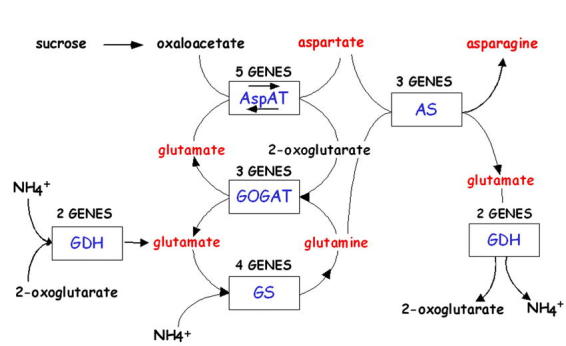
Nitrogen Assimilatory Pathway
Figure 2.
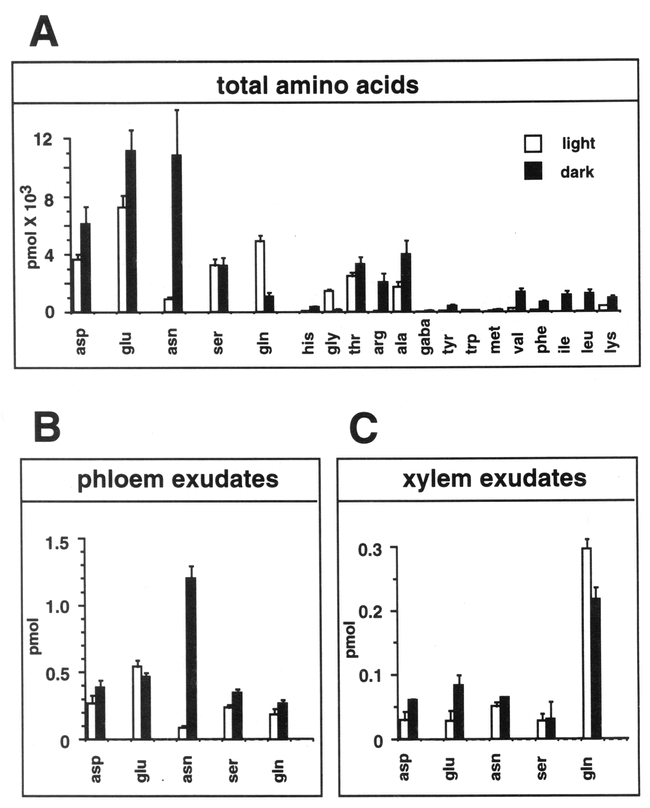
Amino Acid Levels in Light-Grown and Dark-Adapted Wild-Type Arabidopsis Plants. Amino acids levels in Arabidopsis plants grown in light (empty boxes) or subsequently dark adapted for 24 hr (filled boxes). The standard three letter code is used for all amino acids. (A) Average total free amino acid content. Each sample represents the average of three different plants (two leaves/plant). Gaba, γ-amino butyric acid. (B) Average free amino acid content in phloem exudates of three independent plants (one leaf/plant). (C) Average free amino acid content of xylem sap collected from cut hypocotyls of three independent plants. Data are from Schultz (1994). From: Hon-Ming Lam, Karen Coschigano, Carolyn Schultz, Rosana Melo-Oliveira, Gabrielle Tjaden, Igor Oliveira, Nora Ngai, Ming-Hsiun Hsieh, and Gloria Coruzzi (1995). Use of Arabidopsis Mutants and Genes to Study Amide Amino Acid Biosynthesis. Plant Cell 7, 887–898.
Table 1.
N-assimilation-Enzymes, Genes, Mutants.
Glutamine Synthetase (Gs, E.C.6.3.1.2)
GS, which has a very high affinity for ammonia (Km 3–5 µM), catalyzes the ATP-dependent conversion of glutamate into glutamine by incorporating a molecule of ammonia (Lea et al., 1990). GS is an octomeric holoenzyme, and two classes of GS isoenzymes were originally identified by ion-exchange chromatography: cytosolic GS1 and chloroplastic GS2 (Lea et al., 1990). The distinct physiological roles of chloroplast GS2 and cytosolic GS1 has been suggested primarily based on their organ-specific distribution. For example, as chloroplastic GS2 is the predominant isoenzyme in leaves, it has been proposed to function in primary assimilation of ammonia reduced from nitrate in chloroplasts and/or in the reassimilation of photorespiratory ammonia (Miflin and Lea, 1980). As cytosolic GS1 is the predominant isoenzyme in roots, it has been proposed to function in root nitrogen assimilation, although root plastid GS2 has also been implicated in this process (Miflin, 1974). The expression and localization of GS1 in vascular bundles of number of species further supports the notion that cytosolic GS1 functions to generate glutamine for intercellular nitrogen transport (Edwards and Coruzzi, 1990; Carvalho et al., 1992; Kamachi et al., 1992). This data, along with molecular-genetic studies described below in Arabidopsis have helped to assign in vivo functions to each GS isoenzyme.
Arabidopsis like all other higher plants contains a single nuclear gene for chloroplast GS2 (GLN2) and multiple genes for cytosolic GS1 (GLN1) (Peterman and Goodman, 1991; Oliveira and Coruzzi, 1999). Gene expression studies in Arabidopsis have shown that GLN2 is expressed predominantly in leaves, and its expression is regulated by light via phytochrome (Peterman and Goodman, 1991; Oliveira and Coruzzi, 1999). By contrast, the GLN1 genes (GLN1.1–1.3) encoding cytosolic GS1 isoenzymes are expressed at higher levels in roots (Oliveira and Coruzzi, 1999) (Peterman and Goodman, 1991). These organ-specific expression patterns suggest that GLN2 functions in leaf specific processes such as primary N-assimilation, and photorespiration. By contrast, the cytosolic GS1 isoenzymes are likely to function in primary N-assimilation in roots, as N is exported from roots via xylem as Gln (Fig. 2C). Gene expression studies also showed regulation of GS mRNA levels by light, carbon and nitrogen metabolites (Oliveira and Coruzzi, 1999). In particular, GS expression is induced by light or sucrose and repressed by applications of Glu and Gln (Fig. 3A). These gene regulation patterns mimic changes in levels of GS enzyme activity (Oliveira and Coruzzi, 1999) (Fig. 3B). This analysis suggests that N-assimilation into Gln is active when levels of carbon backbones are high (in the light), and is repressed when levels of Gln are high (Oliveira and Coruzzi, 1999; Oliveira et al., 2001). This negative regulation of GS expression by levels of Glu/Gln is reminiscent of the Ntr system of GS in bacteria (Magasanik, 1994). In fact, a component of the Ntr system in bacteria (PII) has been identified in Arabidopsis, and there is some evidence that it may be involved in sensing C:N ratios, and in regulating N-assimilation in plants (Hsieh et al., 1998; Smith et al., 2002; Smith et al., 2003).
Figure 3.
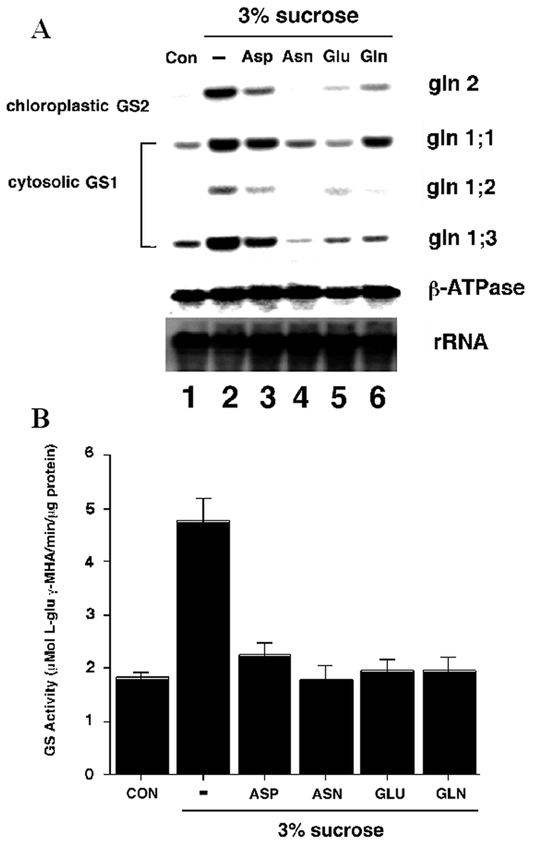
C:N Regulation of GS mRNA levels and GS Activity. (A) Amino acids and carbon reciprocally regulate GS mRNA accumulation and enzyme levels. Arabidopsis plants were grown semihydroponically and dark-adapted. The dark-adapted Arabidopsis plants were transferred in the dark to fresh low-nitrogen MS medium with no carbon supplementation (Con, lane 1), to fresh low-nitrogen MS medium with 3% Suc (lane 2), or to fresh low-nitrogen MS medium with 3% Suc in addition of either 10 mM Asp (lane 3), 10 mM Asn (lane 4), 10 mM Glu (lane 5), or 10 mM Gln (lane 6). After transfer, the plants were further incubated for 12 h in the dark. (B) Same as A except that an aliquot of each sample was collected for determination of total GS activity. A representative experiment of two repetitions is shown (results are GS activity per milligram of total protein ± SE of three independent determinations). From: Igor C. Oliveira and Gloria M. Coruzzi (1999). Carbon and Amino Acid Reciprocally Modulate the Expression of Glutamine Synthetase in Arabidopsis. Plant Physiology 121, 301–309.
Regarding mutant screens in Arabidopsis, thus far no GS mutants have been reported. This is surprising, as GS mutants have been uncovered in photorespiratory mutants screens in other species (barley) (Wallsgrove et al., 1987). While identical screens for plant mutants unable to survive in photorespiratory conditions were conducted in Arabidopsis (Somerville and Ogren, 1980) mutants specifically defective in GS2 were identified only in the barley screen (Wallsgrove et al., 1987). A dramatic result of this mutant study in barley is the finding that a GS2 isoenzyme located in the chloroplast is essential for the reassimilation of photorespiratory ammonia that is released in mitochondria. Paradoxically, the barley GS2 mutants were unable to reassimilate photorespiratory ammonia released in the mitochondria even though they contained normal levels of cytosolic GS1 (Wallsgrove et al., 1987). This paradox has been resolved by studies on the cell-specific expression patterns of genes for chloroplastic GS2 and cytosolic GS1. Studies of GS-promoter-GUS fusions revealed that chloroplastic GS2 and cytosolic GS1 of pea are expressed in non-overlapping cell types in transgenic tobacco (Edwards et al., 1990). Chloroplastic GS2 is expressed predominantly in leaf mesophyll cells, where photorespiration occurs, while cytosolic GS1 is expressed exclusively in the phloem (Forde et al., 1989; Edwards et al., 1990). These promoter-GUS fusion results were later confirmed by studies of the native cytosolic GS1 proteins in rice and tobacco (Carvalho et al., 1992; Kamachi et al., 1992). In those studies, antibodies specific to cytosolic GS1 were used in in situ immunolocalization experiments to show that all cytosolic GS1 proteins are expressed solely in vascular tissues. Thus, this vascular-specific expression pattern may explain why cytosolic GS1 cannot compensate for the loss of chloroplastic GS2 in mesophyll cells of the barley GS2 mutants.
One piece of the GS isoenzyme puzzle that is outstanding is fact that the screens for photorespiratory mutants in Arabidopsis failed to uncover any mutants defective in GS, either chloroplastic GS2 or cytosolic GS1. There are several possible explanations for this finding: (i) The Arabidopsis photorespiratory screen was not saturating. This is unlikely as multiple alleles for many enzymes in the photorespiratory pathway were isolated in that screen, including 58 mutants affecting Fd-GOGAT (Somerville and Ogren, 1980; Artus, 1988). (ii) Both chloroplastic GS2 and cytosolic GS1 are expressed in mesophyll cells, so that a mutation in one gene is masked. (iii) There is more than one gene for chloroplastic GS2 in Arabidopsis. This is not possible as the complete sequence of the Arabidopsis genome (Bevan et al., 2001) has revealed a single gene for chloroplastic GS2. (iv) A mutation in chloroplastic or cytosolic GS is lethal in Arabidopsis, preventing the isolation of mutants.
Glutamate Synthase/(Nadh-Gogat: E.C.1.4.1.14; FD-Gogat: E.C.1.4.7.1)
Glutamate synthase (GOGAT) functions in a cycle with glutamine synthetase (GS), catalyzing the transfer of the amide group from glutamine (generated by GS) to 2-oxoglutarate to form two molecules of glutamate (Fig. 1). In higher plants, there are two forms of GOGAT, a Fd- and an NADH-dependent GOGAT, each of which is chloroplast localized (Lea, 1993). Fd-GOGAT is uniquely found in photosynthetic organisms and biochemical studies in Arabidopsis have shown that Fd-GOGAT is the major isoenzyme in leaves accounting for 95% of the GOGAT activity, while NADH-GOGAT activity is a minor component in leaves accounting for 5% (Somerville and Ogren, 1980) (Table 2). These organ-specific distribution patterns were used to infer in vivo function. For Fd-GOGAT, the high levels in leaves suggested that it plays a role in N-assimilation processes in photosynthetic tissues such as primary N-assimilation and/or photorespiration. By contrast, biochemical studies on other species showed that NADH-GOGAT is located primarily in plastids of non-photosynthetic tissues such as roots (Matoh and Takahashi, 1982; Suzuki and Gadal, 1984) where it is proposed to function in primary assimilation or reassimilation of ammonia released during amino acid catabolism (Miflin and Lea, 1980). The molecular and genetic studies of GOGAT genes and mutants in Arabidopsis outlined below have helped to clarify the relative in vivo roles of Fd- vs. NADH-GOGAT in primary N-assimilation vs. the reassimilation of photorespiratory ammonium.
Table 2.
Relative GOGAT activity levels in wild-type and Fd-GOGAT mutants (gls1).
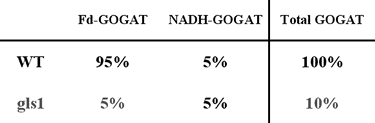
Arabidopsis mutants in Fd-GOGAT were uncovered in 1980 during a screen for photorespiratory mutants (Somerville and Ogren, 1980). In the three Arabidopsis gluS (aka gls) mutants initially characterized, leaf Fd-GOGAT activity was reduced to less than 5% of wild type levels, whereas NADH-GOGAT - which contributes about 5% of the total GOGAT activities in normal conditions-remained unchanged (Somerville and Ogren, 1980) (Table 2). The gluS mutants had a conditional phenotype: they were chlorotic when grown in air (photorespiratory conditions), but could be rescued when grown in conditions under which photorespiration was suppressed (1% CO2) (Fig. 4). This finding suggested that Fd-GOGAT plays a major role in the reassimilation of photorespiratory ammonium released in mitochondria. This gluS mutant revealed a number of surprises that could not have been determined based on traditional biochemical studies. 1. It showed that photorespiratory ammonium released in the mitochondria is reassimilated primarily in the chloroplast by Fd-GOGAT. Thus, despite the fact that glutamate dehydrogense (GDH) is located in the mitochondria, these studies on the gluS mutants suggested GDH plays a minor role, if any, in the reassimilation of photorespiratory ammonium. 2. The gluS mutant studies showed that Fd-GOGAT plays a major role in photorespiration, and that the normal low levels of NADH-GOGAT in leaves could not compensate for the loss of Fd-GOGAT. 3. The finding that the gluS mutants grew normally in high CO2 (non-photorespiratory conditions) was paradoxical, and suggested that Fd-GOGAT, the major GOGAT enzyme in leaves, was dispensable in primary N assimilation. Molecular analysis of the genes for Fd-GOGAT in Arabidopsis, and further studies of the gluS mutants helped to clarify this latter point. Coschigano et al identified two expressed genes for Fd-GOGAT from Arabidopsis, GLU1 and GLU2 (Coschigano et al., 1998). This was paradoxical, as the gluS mutants suggested the existence of a single genetic locus for Fd-GOGAT. Further studies resolved this issue. Gene expression studies showed that GLU1 was the major expressed gene in leaves, and that its mRNA was light regulated. By contrast, GLU2 was expressed primarily in roots (Fig. 5). Studies further showed that GLU1 is genetically linked to the gluS mutation, while GLU2 mapped to a separate chromosome (Coschigano et al., 1998). Thus, it appears that mutations in the highly expressed GLU1 gene are able to cause a mutant photorespiratory phenotype, and that the low levels of Fd-GOGAT from GLU2, and the normally low levels of NADH-GOGAT in leaves are unable to compensate for the loss of GLU1, encoding the major Fd-GOGAT isoenzyme in Arabidopsis. A similar situation has been seen in the Arabidopsis pathway of tryptophan synthesis, where a mutation in a highly expressed gene for tryptophan synthase leads to auxotrophy, even though a second less highly expressed tryptophan synthase gene was unaffected (Last et al., 1991).
Figure 4.
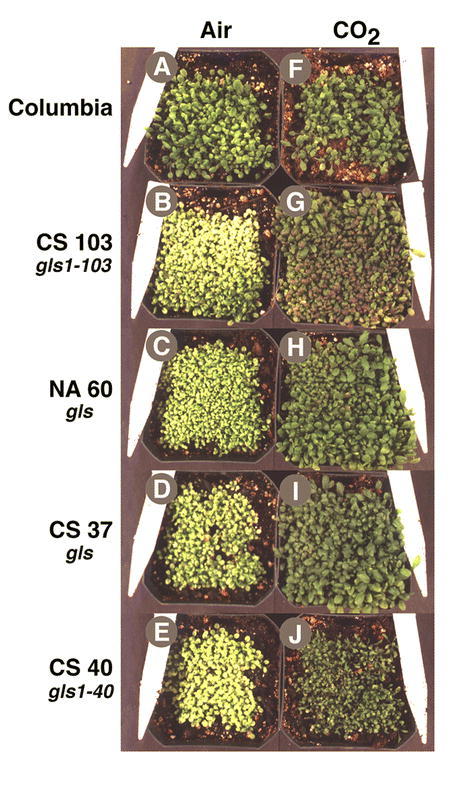
Photorespiratory Phenotype of gls Mutants Defective in Fd-GOGAT Activity. In (A) – (E), Arabidopsis plants were grown in air; in (F) – (J), plants were grown in 2% CO2 Genotypes: (A) and (F) Arabidopsis wild-type Columbia. (B) and (G) Photorespiratory gls mutant CS103. (C) and (H) NA60. (D) and (I) CS37. (E) and (J) CS340. From: Karen T. Coschigano, Rosana Melo-Oliveira, Jackie Lam, and Gloria Coruzzi (1998). Arabidopsis gls Mutants and Distinct Fd-GOGAT Genes: Implications for Photorespiration and Primary Nitrogen Assimilation. Plant Cell 10, 741–752.
Figure 5.
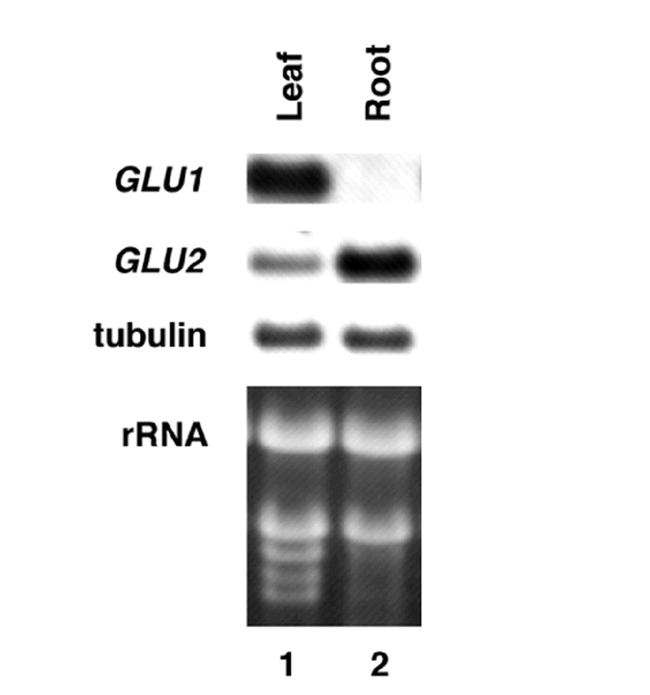
Levels of mRNA for Fd-GOGAT genes GLU1 & GLU2 in leaves vs. roots. GLU1 is the major mRNA in leaves, while GLU2 predominates in roots. Control RNA is tubulin. From: Karen T. Coschigano, Rosana Melo-Oliveira, Jackie Lam, and Gloria Coruzzi (1998). Arabidopsis gls Mutants and Distinct Fd-GOGAT Genes: Implications for Photorespiration and Primary Nitrogen Assimilation. Plant Cell 10, 741–752.
The fact that all of the Fd-GOGAT deficient mutants isolated were chlorotic and eventually died when grown in atmospheric conditions promoting photorespiration (air), established an essential role for Fd-GOGAT in photorespiration. However, since the Fd-GOGAT deficient mutants recovered and were viable when grown in conditions where photorespiration was suppressed (high carbon dioxide or low oxygen), Fd-GOGAT appeared at first glance to be dispensable for non-photorespiratory roles, such as in primary nitrogen assimilation. This conclusion was paradoxical as most primary assimilation occurs in leaves, where Fd-GOGAT activity predominates (95% of total GOGAT activity) and NADH-GOGAT is a minor component (5% of total GOGAT activity) (Somerville and Ogren, 1980). How could Fd-GOGAT, the major GOGAT activity in leaves be dispensable for primary nitrogen assimilation? To address this question, Coschigano et al measured levels of chlorophyll as an indicator of N-status (Delgado et al., 1994) in wild-type and gluS mutant plants grown under non-photorespiratory conditions (Fig. 6). These studies suggest that the primary gluS mutants in fact have defects in N-assimilation (Coschigano et al., 1998). However, the gluS mutants are viable, suggesting that the normal low level expression of GLU2 in the mutants (and potentially NADH-GOGAT) is sufficient to support primary N-assimilation.
Figure 6.
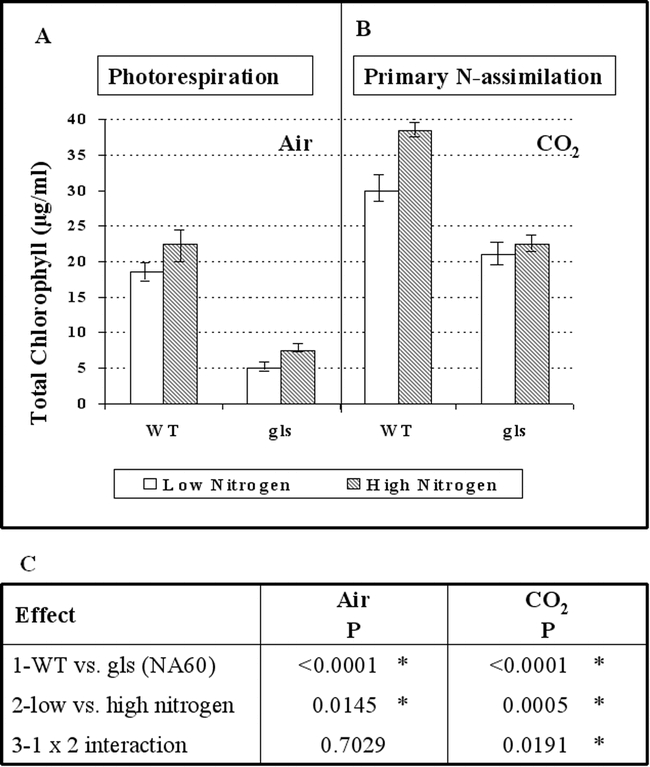
A gls Mutant Displays a Defect in the Assimilation of Inorganic Nitrogen under Nonphotorespiratory Conditions. (A) and (B) Plants grown in air and plants in 1% CO2, respectively. Wild-type Columbia (WT) and a gls mutant (NA60) were grown for 12 days on nitrogen-free MS media supplemented with either low levels of inorganic nitrogen (2 mM ammonium and 4 mM nitrate; open bars) or high levels of inorganic nitrogen (20 mM ammonium and 40 mM nitrate; hatched bars). Mean values of total chlorophyll measurements ± SE are shown. (C) Two-way ANOVA of (1) the wild type (WT) versus a gls mutant in air and CO2, (2) low versus high nitrogen conditions in air and CO2, and (3) interactions of 1 and 2 as given above. Statistically significant results are indicated by an asterisk. From: Karen T. Coschigano, Rosana Melo-Oliveira, Jackie Lam, and Gloria Coruzzi (1998). Arabidopsis gls Mutants and Distinct Fd-GOGAT Genes: Implications for Photorespiration and Primary Nitrogen Assimilation. Plant Cell 10, 741–752.
In contrast to Fd-GOGAT, NADH-GOGAT appears to be present primarily in non-green tissues such as roots. Arabidopsis has a single gene for NADH-GOGAT, GLT1, and recently, a T-DNA knock out mutant in this gene has been identified (Lancien et al., 2002). The GLT1 gene is expressed at low constitutive levels in leaves, and at higher levels in roots. The T-DNA mutant in the GLT1 gene (glt1-T) was null, and had no detectable levels of NADH GOGAT (Lancien et al., 2002). The glt1-T mutant was thus used to study the in vivo role of NADH GOGAT by scoring the mutants for defects related to N-assimilation, compared to wild-type and to a mutant in Fd-GOGAT (gls113) (Lancien et al., 2002). This analysis showed that the glt1-T mutant shows defects in fresh weight and chlorophyll accumulation, and glutamate production specifically when the plants are grown under non-photorespiratory conditions. These findings suggest that NADH-GOGAT plays a significant role in primary nitrogen assimilation in plants grown under non-photorespiratory conditions. The models for the respective roles of NADH vs. Fd-GOGAT are shown in Fig. 7.
Figure 7.
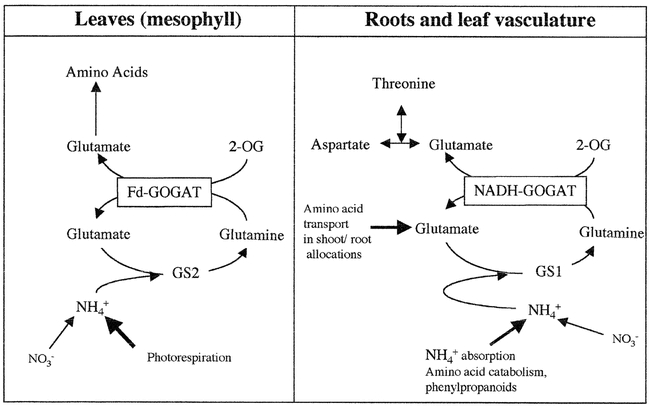
A Model for the Non-Redundant Roles of GOGAT Isoenzymes in Leaves and Roots. Model based on analysis of Arabidopsis GOGAT mutants in Fd-GOGAT (gls) or NADH-GOGAT (glt1-T) (Somerville and Ogren, 1980), (Coschigano et al., 1998), (Lancien et al., 2002). Fd-GOGAT in leaves encoded by Glu1 is primarily responsible for assimilation of photorespiratory ammonia while NADH-GOGAT is responsible for glutamate synthesis in roots. From: Muriel Lancien, Melinda Martin, Ming-Hsiun Hsieh, Tom Leustek, Howard Goodman and Gloria M. Coruzzi (2002). Arabidopsis glt1-T mutant defines a role for NADH-GOGAT in the non-photorespiratory ammonium assimilatory pathway. Plant Journal 29(3), 347–358.
Glutamate Dehydrogenase (Nadh-Gdh: E.C.1.4.1.2; Nadph-Gdh: E.C.1.4.1.4)
Glutamate dehydrogenase (GDH) is an enzyme that can catalyze forward and reverse biochemical reactions: the amination of 2-oxoglutarate into glutamate (biosynthetic) or the deamination of glutamate into ammonia and 2-oxoglutarate (catabolic) (Fig. 1) (Lea et al., 1990). Two major forms of GDH have been reported, an NADH-dependent form (NADH-GDH) that is found in the mitochondria (Day et al., 1988; Loulakakis and Roubelakis-Angelakis, 1990), and an NADPH-dependent form (NADPH-GDH) localized to the chloroplast (Lea and Thurman, 1972). GDH is a hexameric enzyme (Srivastava and Singh, 1987). In Arabidopsis, the GDH enzymes can be resolved into seven isoenzymes by enzyme activity staining of non-denaturing gel electrophoresis (Cammaerts and Jacobs, 1983; Melo-Oliveira et al., 1996) (Fig. 8). These seven GDH activity bands are the result of the random association of two types of subunits into a hexameric complex. The most anodal homohexamer (GDH1) is thought to catalyze the anabolic reaction, while the cathodal most homohexamer (GDH2) is thought to be primarily catabolic (Cammaerts and Jacobs, 1985). Under this model, the heterohexamers of GDH1 and GDH2 would have intermediate catabolic and anabolic activities depending on the proportion of GDH1 versus GDH2 subunits. It has been proposed that two non-allelic genes are responsible for the synthesis of the GDH1 and GDH2 subunits (Cammaerts and Jacobs, 1985), and the discovery of two GDH genes: GDH1 and GDH2, and gdh1-1 mutant of Arabidopsis supports this conclusion, as discussed below (Melo-Oliveira et al., 1996; Turano et al., 1997). GDH isoenzymes have also been used to study the effect of nitrogen on GDH in Arabidopsis (Jacobs and Joukas, 1978)
Figure 8.
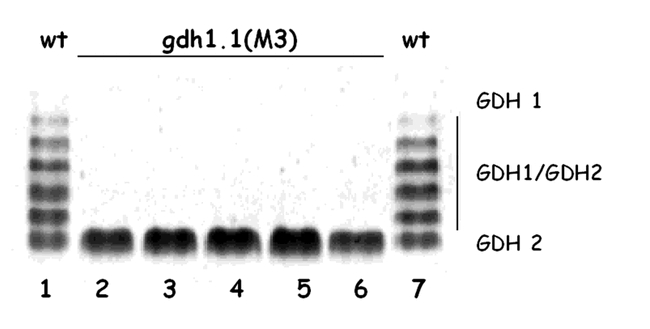
GDH Activity in Wild-Type Arabidopsis and gdh 1-1 Mutant. Crude leaf protein extracts were made from rosette leaves of 21-day-old Arabidopsis plants, separated by electrophoresis on a nondenaturing polyacrylamide gel, and stained for GDH activity. Lanes 1 and 7, extract of wild-type Arabidopsis (Columbia). The seven holoenzymes result from the formation of two homohexamers (GDH1 and GDH2), and five heterohexamers of GDH are indicated on the right (GDH1/GDH2). M3 individuals from a selfed gdh 1-1mutant display only the GDH2 homohexamer (lanes 2–6). From: Rosana Melo-Oliveira, Igor Cunha Oliveira, and Gloria M. Coruzzi (1996). Arabidopsis mutant analysis and gene regulation define a nonredundant role for glutamate dehydrogenase in nitrogen assimilation. Proc. Natl. Acad. Sci. 93, 4718–4723.
Although GDH exists in plant tissues at high levels, there is an ongoing debate as to its physiological role in higher plants. Originally, GDH was proposed to be the primary route for the assimilation of ammonia in plants. However, this biosynthetic role of GDH has been challenged by the discovery of an alternative pathway for ammonia assimilation via the GS/GOGAT cycle (Miflin and Lea, 1976). Moreover, the fact that the GDH enzyme has a high Km for ammonia argues against a role in primary nitrogen assimilation (Stewart et al., 1980). Studies have shown that GDH enzyme activity can be induced in plants exposed to high levels of ammonia, and as such GDH has been proposed to be important specifically for ammonia-detoxification purposes. Mitochondrial GDH has also been proposed to be involved in the assimilation of high levels of photorespiratory ammonia released in mitochondria (Yamaya and Oaks, 1987). However, the isolation of photorespiratory mutants defective in chloroplastic GS2 (in barley) (Wallsgrove et al., 1987) or Fd-GOGAT (in barley and in Arabidopsis) (Somerville and Ogren, 1980; Kendall et al., 1986; Blackwell et al., 1987) have argued against the importance of GDH in photorespiration (Wallsgrove et al., 1987). Furthermore, treatment of plants with the GS inhibitor MSO prevents the incorporation of ammonia into glutamate and glutamine, even though both GDH activity and ammonia levels remain high (Lea et al., 1990). Altogether these results argue against a biosynthetic role for GDH. Instead a catabolic role for GDH has been invoked. Biochemical evidence for such a role is the fact that GDH activity is induced during germination and senescence, two periods where amino acid catabolism occurs (Stewart et al., 1980; Lea et al., 1990). To date, however, biochemical studies have failed to uncover the true in vivo role for GDH. The Arabidopsis gdh mutants described below have helped to define an in vivo role for GDH in plants.
Arabidopsis contains two genes for GDH: GDH1 and GDH2 (Melo-Oliveira et al., 1996; Turano et al., 1997). The predicted protein sequences suggest that they each encode NADH-dependent enzymes that are likely to be associated with the mitochondria. Gene expression studies have shown that each gene is subject to regulation by light and metabolites (Turano et al., 1997). Arabidopsis mutants deficient in GDH were identified in the M2 generation of EMS- and NMU-mutagenized Arabidopsis in a brute force screen using a GDH activity stain on crude leaf protein extracts following electrophoresis on native gels (Melo-Oliveira et al., 1996). A single Arabidopsis GDH mutant, gdh1-1, has been identified which has an altered pattern of GDH activity: it possesses a single GDH2 holoenzyme and is missing the GDH1 holoenzyme as well as the GDH1:GDH2 heterohexamers (Fig. 8) (Melo-Oliveira et al., 1996). The Arabidopsis gdh1-1 mutant displays an impaired growth phenotype compared to wild-type, specifically when plants are grown under conditions of high inorganic nitrogen (Fig. 9). This conditional phenotype suggests a non-redundant role for GDH in the assimilation of ammonia under conditions of inorganic nitrogen excess. A similar GDH-deficient mutant has been previously described in Zea mays, a C4 plant, which also appears to be affected in the GDH1 gene product (Pryor, 1974, 1979). Preliminary studies showed that the maize GDH mutant displays a growth phenotype only under low night temperatures (Pryor, 1990). Moreover, it has been reported that the maize GDH1 mutant shows a 10 to 15-fold lower total GDH activity when compared to wild type maize (Magalhaes et al., 1990). As the photorespiratory rate is very low or non-existent in a C4 plant, the maize GDH1 mutant cannot be used to assess the role of GDH in photorespiration. Therefore, the Arabidopsis gdh1-1 mutant will be valuable to assess the function of this enzyme in photorespiration in a C3 plant. It should be noted that neither the maize nor the Arabidopsis gdh mutants are null for GDH, as they each possess a second GDH2 gene.
Figure 9.
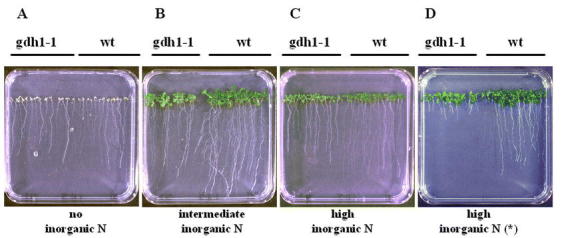
Growth Phenotype of the gdh 1-1 Mutant. Growth of wild-type Arabidopsis versus the gdh 1-1 mutant seedlings was measured in a vertical root length assay. Wild-type (wt) and M3 seeds of the gdh 1-1 mutant were sown side-by-side on ammonia-free/nitrate-free MS media containing vitamins and 3% sucrose supplemented with either: (A) no organic nitrogen (0 mM ammonia, 0 mM nitrate); (B) intermediate levels of inorganic nitrogen (2 mM ammonia, 4 mM nitrate); or (C) high levels of inorganic nitrogen (20 mM ammonia, 40 mM nitrate). (D) Plants grown on MS media supplemented with 3% sucrose containing high levels of inorganic nitrogen (20 mM ammonia, 40 mM nitrate) without the vitamin supplement (*). Plates were incubated vertically for 12 days and grown under a normal day/night cycle. From: Rosana Melo-Oliveira, Igor Cunha Oliveira, and Gloria M. Coruzzi (1996). Arabidopsis mutant analysis and gene regulation define a nonredundant role for glutamate dehydrogenase in nitrogen assimilation. Proc. Natl. Acad. Sci. 93, 4718–4723.
Aspartate Aminotransferase (Aspat: E.C. 2.6.1.1)
Aspartate aminotransferase (AspAT) is a pyridoxal phosphate-dependent enzyme that catalyzes the reversible transfer of an amino group from glutamate to oxaloacetate to generate aspartate and 2-oxoglutarate (Fig. 1). The AspAT holoenzyme is a homodimer consisting of two subunits (Lea, 1993). In addition to its role in aspartate synthesis and catabolism, AspAT plays an important role in C3 plants, as part of the malate-aspartate shuttle which allows the transfer of reducing equivalents from mitochondria and chloroplasts into the cytoplasm (Ireland and Joy, 1985). Accordingly, bio-chemical studies show that AspAT exists as distinct isoenzymes found in different subcellular locations such as the cytosol, mitochondria, chloroplasts, glyoxysomes or peroxisomes. For examples see: (Weeden and Gottlieb, 1980; Wadsworth et al., 1993; Schultz and Coruzzi, 1995; Taniguchi et al., 1995). The subcellular compartmentation of AspAT isoenzymes suggests that the different forms of AspAT might serve distinct roles in plant metabolism. The isolation of Arabidopsis mutants in genes for distinct AspAT isoenzymes has helped to elucidate the role of these isoenzymes in plant N-assimilation.
Arabidopsis contains a family of five genes encoding distinct AspAT isoenzymes, ASP1-5 (Schultz and Coruzzi, 1995; Wilkie et al., 1995). ASP1 encodes mitochondrial AAT1, ASP2 and ASP4 encode cytosolic isoenzymes, ASP5 encodes chloroplastic AAT3, and ASP3 encodes a putative peroxisomal isoenzyme (Fig. 10) (Schultz and Coruzzi, 1995; Wilkie et al., 1995; Schultz et al., 1998). AAT isoenzymes of Arabidopsis were identified by native gel analysis. Three major isoenzymes were detected in crude leaf extracts, and identified by subcellular fractionation (Schultz and Coruzzi, 1995; Schultz et al., 1998). The two major isoenzymes are AAT2 (cytosolic) and AAT3 (chloroplastic) (Fig.10). Mitochondrial AAT1 was also detected when the gels were overloaded. This native gel assay was used in a brute force screen to identify plant mutants deficient in one of the two major AAT isoenzymes; cytosolic AAT2 and chloroplastic AAT3 (Fig. 11) (Schultz et al., 1998; Miesak and Coruzzi, 2002), as described below.
Figure 10.
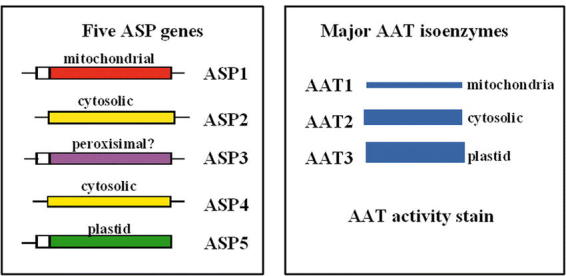
ASP Genes and Major AAT Isoenzymes in Arabidopsis. Five ASP genes encoding AAT isoenzymes: ASP1 encodes mitochondrial AAT1, ASP2 encodes cytosolic AAT2, ASP5 encodes chloroplastic AAT3, ASP3 encodes a putative peroxisomal AAT, and ASP4 encodes a minor cytosolic AAT.
Figure 11.
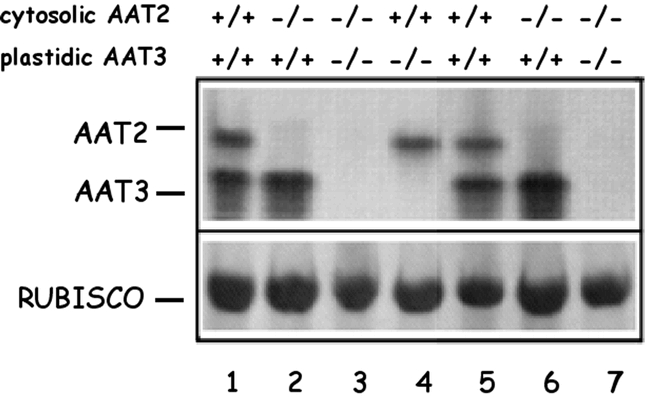
AspAT Activity Gels of Wild-Type, Single and Double aat Mutants. AspAT activity gel of seven different F2 individuals from the cross aat2-1/aat2-1 X aat3-2/aat3-2 showing individuals which are homozygous wild-type for both cytosolic and chloroplastic activity (lanes 1 and 5); homozygous mutant for cytosolic AAT2 but wild-type for chloroplastic AAT3 (lanes 2 and 6); homozygous mutant for both cytosolic and chloroplastic AspAT (lanes 3 and 7); and wild-type for cytosolic AAT2 but homozygous mutants for chloroplastic AAT3 (lane 4). The genotypes of the F2 individuals shown are as follows: AAT2-1/AAT2-1, AAT3-2/AAT3-2 (lanes 1 and 5); aat2-1/aat2-1, AAT3-2/AAT3-2 (lanes 2 and 6); aat2-1/aat2-1, aat3-2/aat3-2 (lanes 3 and 7); AAT2-1/AAT2-1, aat3-2/aat3-2 (lane 4). The amount of rubisco protein was determined by Coomassie staining of the gel after activity staining. From: Schultz CJ (1994) A molecular and genetic dissection of the aspartate aminotransferase isoenzymes of Arabidopsis thaliana. A Ph.D. thesis. New York University, New York, NY
To help determine the in vivo function(s) of each AspAT isoenzyme, a screen for Arabidopsis mutants defective in either cytosolic AAT2 or chloroplastic AAT3 was performed. M2 seedlings were screened for alterations in AAT profiles using the native gel screen on crude leaf extracts (Schultz et al., 1998; Miesak and Coruzzi, 2002). Screens of EMS-mutagenized Arabidopsis seeds and T-DNA lines uncovered two genetic loci: aat2 mutants defective in cytosolic AAT2, and aat3 mutants defective in chloroplastic AAT3 (Fig. 11). The ASP genes and aat mutants were mapped and the defective ASP genes sequenced in the aat mutants (Schultz et al., 1998; Miesak and Coruzzi, 2002). By analyzing the effects of these mutations on the growth phenotypes and the free amino acid pools in the aat mutant plants, the in vivo importance of each AAT isoenzyme was determined. Initial analyses show that a mutation in the cytosolic ASP2 gene results in a retarded growth phenotype and a decrease in the pools of free aspartate (Schultz et al., 1998). Moreover, the aat2 mutants also showed dramatic decreases in asparagine in dark-adapted plants (Fig. 12). These studies indicate that cytosolic AAT2 is responsible for the synthesis of pools of aspartate in the light that are used for asparagine synthesis in the dark. Further analysis of additional aat2 alleles, including a T-DNA mutant in ASP2 revealed additional information on the cytosolic AAT2 isoenzyme (Miesak and Coruzzi, 2002). For example, in an attempt to determine whether the growth deficiency of aat2 was due to an aspartate deficiency, aat2 mutants were supplied with exogenous aspartate. Surprisingly, the aat2 mutants exhibited an aspartate sensitivity (compared to wild-type) suggesting that the aat2 mutation led not only to an inability to synthesize aspartate, but also an inability to catabolize aspartate (Miesak and Coruzzi, 2002). These studies indicate that cytosolic AAT2 plays a non-redundant function compared to the other 4 ASP genes. Further HPLC studies on siliques of aat2 mutants revealed deficiencies in Asp and Asn in siliques, suggesting that cytosolic AAT2 plays an important role in N-transport to siliques (Miesak and Coruzzi, 2002).
Figure 12.
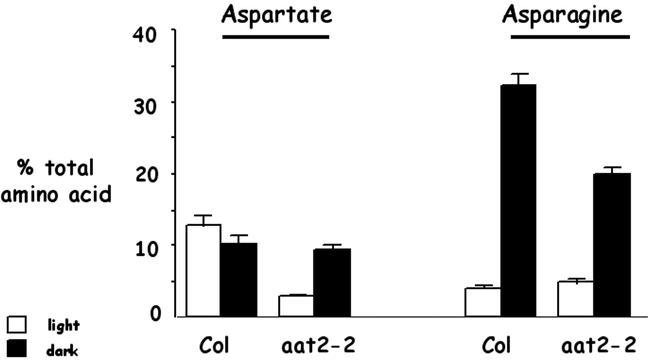
aat2-2 mutants have specific reductions in levels of aspartate in light-grown plants and asparagine in dark-adapted plants. The relative proportions of aspartate and asparagine in the phloem exudates from wild-type Columbia (Col) and aat2-2 mutant plants grown in light (unshaded boxes) or dark adapted (shaded boxes). Each sample is the average of a single leaf from three representative plants. Plants were grown in soil in a normal day/night cycle (16 hr light/8 hr dark) for 3 wk and either light adapted (unshaded box) or dark adapted (shaded box) for 24 hr. Error bars represent the standard error of the mean. From: Carolyn J. Schultz, Meier Hsu, Barbara Miesak and Gloria Coruzzi (1998). Arabidopsis Mutants Define an in Vivo Role for Isoenzymes of Aspartate Aminotransferase in Plant Nitrogen Assimilation. Genetics 149; 491–499.
Asparagine Synthetase (AS: E.C.6.3.5.4)
Asparagine was the first amino acid discovered, as it was crystallized from asparagus almost 200 years ago (Vauquelin and Robiquet, 1806). Despite this historical placement, the mechanism of asparagine biosynthesis in plants has been elucidated in plants only recently. While three possible routes for asparagine synthesis have been proposed (Sieciechowicz et al., 1988), the glutamine-dependent asparagine synthetase enzyme is now generally accepted as the major route for asparagine biosynthesis in plants (Lea et al., 1990). In an ATP-dependent reaction, AS catalyzes the transfer of an amino group of glutamine to a molecule of aspartate to generate a molecule of glutamate and asparagine (Fig. 1). Glutamine is the preferred substrate for nearly all of the AS enzymes studied in higher plants (Sieciechowicz et al., 1988). However, ammonia was also reported to be a possible AS substrate, particularly in the case of maize roots (Oaks and Ross, 1984). Traditional biochemical studies of AS in higher plants were hampered by the fact that AS is a very unstable enzyme in vitro and only partially purified AS enzymes have been isolated from various plant species (Lea and Miflin, 1980). Compared to glutamine, asparagine is relatively inert and carries more nitrogens per carbon. Thus, asparagine is used to store and transport nitrogen in many higher plant species including legumes, crop plants and also in Arabidopsis. While no higher plant mutants in AS exist to date, molecular and reverse genetic studies on ASN genes in Arabidopsis have begun to provide the first insights into the regulation of asparagine biosynthesis in plants.
Arabidopsis contains three genes for asparagine synthetase, ASN1, ASN2, and ASN3. ASN1 was cloned by homology to pea AS1 (Lam et al., 1994), while ASN2 and ASN3 were cloned functionally by complementation of a yeast asparagine auxotroph (Lam et al., 1998). The AS polypeptides encoded in each of these ASN cDNA clones contains a purF-type glutamine-binding domain (Richards and Schuster, 1992), suggesting that glutamine is the preferred substrate. Phylogenetic grouping suggests that ASN1 forms a separate clade from the ASN2 and ASN3 genes of Arabidopsis, suggesting the possibility that these enzymes may play distinct roles in vivo (Lam et al., 1998). The ASN2 & 3 genes group with monocot AS enzymes. Studies in maize suggest this form of AS encodes in form of the enzyme that uses Gln or ammonia as a substrate in vitro (Oaks and Ross, 1984).
The ASN genes also differ in expression patterns. ASN1 appears to be the major expressed gene in Arabidopsis seedlings (Lam et al., 1998). Expression of ASN3 is very low, and ASN2 expression while higher, is distinct from ASN1 suggesting that they serve distinct functions. ASN1 is expressed at highest levels in dark-adapted plants, and light inhibits its transcription (Fig. 13) and (Lam et al., 1994; Lam et al., 1998). By contrast, ASN2 is expressed at higher levels in the light (Fig. 13). The light repression of ASN1 and the light induction of ASN2 occur with 30 minutes and are kinetically reciprocal, suggesting distinct functions for the encoded enzymes (Lam et al., 1998). There are several pieces of evidence suggesting that ASN1 controls the major levels of free asparagine synthesized in Arabidopsis. 1. Levels of ASN1 mRNA parallel changes in levels of asparagine (Fig. 13), and 2. Transgenic manipulation of ASN1 (overexpression or antisense) affect levels of asparagine in plants (Lam et al., 2003)
Figure 13.
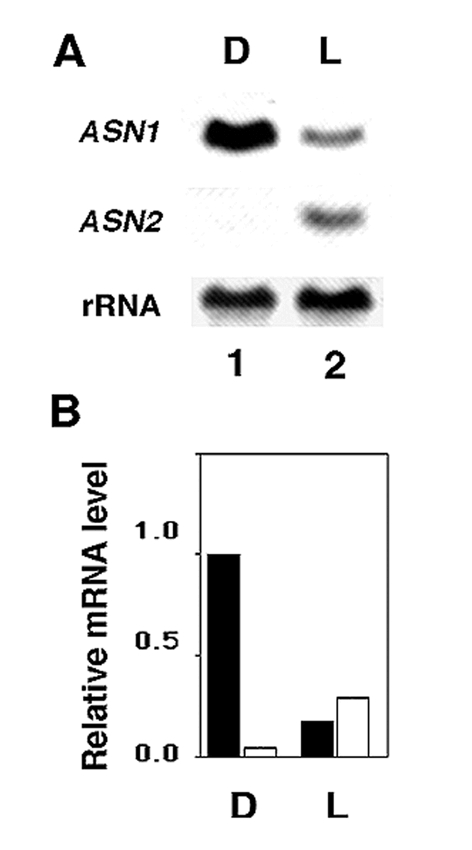
Relative levels of ASN1 and ASN2 mRNA in dark-adapted versus light-treated plants. Sixteen-day-old Arabidopsis seedlings were grown on tissue culture plates (MS plus 3% sucrose) and dark-adapted (D, lane 1) or light-treated (L, lane 2) for 48 h. Ten µg of total RNA was subjected to Northern blot analysis and hybridized with ASN1 or ASN2. ASN1 and ASN2 gene-specific probes were approximately the same specific activity and X-ray exposure times were identical. 18S rRNA was detected on a replicate blot as a loading control. Following normalization to the corresponding rRNA, the highest level of mRNA (ASN1, lane 1) was set at 1.0. The bar graph (b) represents the relative levels of ASN1 and ASN2 transcripts in (a). From: Hon-Ming Lam, Ming-Hsiun Hsieh and Gloria Coruzzi (1998). Reciprocal regulation of distinct asparagine synthetase genes by light and metabolites in Arabidopsis thaliana. Plant Journal 16(3), 345–353.
Light also plays a major role in regulating asparagine levels in Arabidopsis, and this appears to be controlled at the level of transcription of the ASN1 gene. Levels of asparagine are low in light grown plants, and high in dark adapted plants. Similarly, levels of ASN1 mRNA are low in light-grown plants and high in dark-adapted plants (Fig. 13). This light repression of ASN1 is mediated in part by phytochrome, and in part by light-induced changes in levels of photosynthate (Lam et al., 1994). More recently we have shown that blue light is also involved in repressing ASN1 expression (Thum et al., 2003). Studies have shown that sucrose (or glucose) can mimic the repressive effects of light on ASN1 expression when supplied to dark-adapted plants (Lam et al., 1998). Finally, why do plants use light and carbon to repress ASN1 transcription to affect asparagine biosynthesis? Because asparagine carries more nitrogens per carbon compared to glutamine, asparagine is used to transport nitrogen when levels of carbon are low. Hence, ASN1 is transcriptionally active in the dark, when levels of carbon are low, and transcription is repressed in the light, when carbon levels are high. Understanding the mechanisms by which light and carbon transcriptionally repress ASN1 is significant, because this transcriptional mechanism controls asparagine biosynthesis in Arabidopsis.
Light and Metabolic Control of Nitrogen Assimilation into amino acids
A significant amount of evidence has been accumulated to show that the genes regulating nitrogen assimilation into amino acids is subject to control at the transcriptional level. The signals affecting the transcription of N-assimilatory genes are light, carbon metabolites, and nitrogen metabolites. Below is a brief review of these three forms of regulation, as well as evidence for interactions between these signals. While effects of light on N-assimilation have been studied largely at the transcriptional level, they are also reflected at the level of amino acids. For example, the repression of ASN1 transcription by light (Lam et al., 1998), results in the specific accumulation of asparagine in dark-adapted plants (Lam et al., 1995). By contrast, Gln levels are higher in light-grown plants (Lam et al., 1995), and this is reflective of the induction of GLN2 transcription by light (Oliveira and Coruzzi, 1999). In Arabidopsis, the reciprocal control of GLN2 versus ASN1 by light at the mRNA level has been shown to reflect similar light-induced changes in the levels of glutamine and asparagine. Glutamine levels are higher in light-grown plants, while asparagine levels are highest in dark-adapted plants (Schultz, 1994; Lam et al., 1995). Under light conditions, nitrogen is assimilated into metabolically active glutamine and glutamate, and transported as such for use in anabolic reactions in plants. Under dark conditions (low carbon concentration relative to organic nitrogen), the plants direct the assimilated nitrogen into inert asparagine for long distance transport or long-term storage (Fig. 14).
Figure 14.
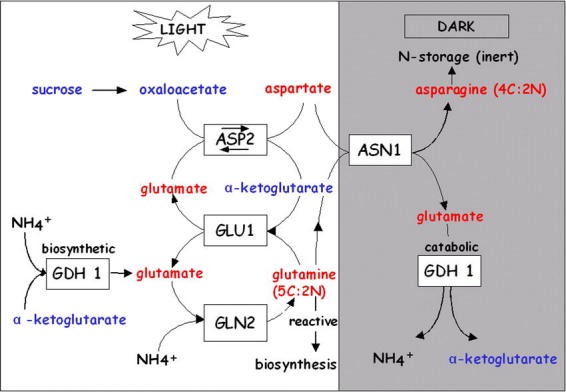
N-assimilation into Amino Acids is regulated by light. Cytosolic AAT2 controls the synthesis of aspartate in the light, which is converted to asparagine in the dark. A model is depicted for the metabolic flow of nitrogen assimilation into the nitrogen-transport amino acids glutamate, glutamine, aspartate, and asparagine in the light and dark. In the light, inorganic nitrogen is assimilated initially into glutamate and glutamine by the combined actions of the plastid enzymes: chloroplastic glutamine synthetase (GS2, encoded by GLN2), and ferredoxin-dependent glutamate synthase (Fd-GOGAT, encoded by GLU1; Oliveira et al. 1997; Coschigano et al. 1998). The conversion of glutamate into aspartate in the light is controlled by cytosolic AAT2. In the dark, this pool of aspartate is converted into asparagine by asparagine synthetase (ASN1) (Lam et al. 1994, 1995). From: Carolyn J. Schultz, Meier Hsu, Barbara Miesak and Gloria Coruzzi (1998). Arabidopsis Mutants Define an in Vivo Role for Isoenzymes of Aspartate Aminotransferase in Plant Nitrogen Assimilation. Genetics 149; 491–499.
The light control of N-assimilatory genes such as ASN1 and GLN2 in Arabidopsis has been shown to operate via the plant photoreceptor phytochrome (Lam et al., 1994; Oliveira and Coruzzi, 1999) and by blue light (Thum et al., 2003). It appears that light also exerts an effect on the expression of other genes in the N-assimilatory pathway. For example, both GLN2 and the GLU1 gene encoding Fd-GOGAT are both induced by light (Coschigano et al., 1998) (Fig. 14). As the GS/GOGAT cycle affects N-assimilation, the coregulation of these genes by light serves to coordinate their expression with the production of C-skeletons during photosynthesis. Conversely, light has been shown to repress genes encoding ASN1 and GDH (Lam et al., 1994; Turano and Fang, 1998) (Fig. 14).
This coordinate regulation by light of N-assimilatory genes appears not only to involve phytochrome and blue light photoreceptors, but also reflects a coordination of N-assimilation by carbon availability. It has been shown that a carbon source such as glucose or sucrose can at least partially mimic the effects of light. In the case of GLN2, sucrose supplied to dark-adapted plants can at least partially mimic the inductive effects of light (Fig. 15) (Oliveira and Coruzzi, 1999; Thum et al., 2003). By contrast, the light repression of ASN1, can be at least partially mimicked by supplying sucrose to dark adapted plants (Fig. 15) (Lam et al., 1994; Thum et al., 2003). Studies on glucose control of gene expression in Arabidopsis suggest that the regulation of N-assimilation by carbon is mediated via a non hexokinase dependent pathway (Sheen et al., 1999).
Figure 15.
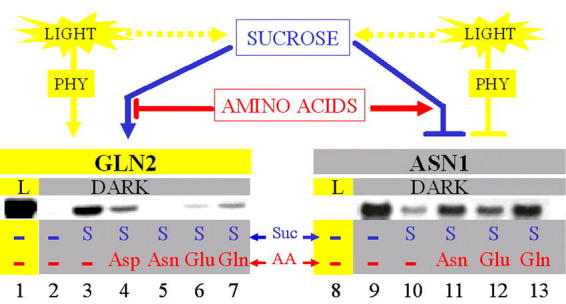
C:N Regulation of GLN2/ASN1 mRNA levels. Light and metabolites cause a reciprocal effect on Arabidopsis GLN2 and ASN1 gene expression. The mRNA levels of ASN1 are repressed to nearly undetectable levels in light-grown plants (lane 8) and are strongly enhanced in dark-adapted plants (lane 9). By contrast, the low, undetectable levels of GLN2 mRNA from plants grown in the dark (lane 2) are highly elevated in light-grown plants (lane 1). Sucrose can partially mimic the effects of light by causing the induction of GLN2 and repressing the expression of ASN1 mRNA in the dark (lane 10). In the dark, the light-mimicking effects of sucrose can be antagonized by treatment with amino acids (AA) (GLN2, lane 4 to 7 and ASN1, lanes 11 to 13) with each AA affecting the expression of GLN2 and ASN1 to different extents. The differential effects of the AA on both GLN2 and ASN1 mRNA levels may be explained by the possibility that each AA exerts its effects through different but partially overlapping pathways. However, one cannot rule out differences due to rate of uptake or metabolism. PHY, phytochrome; GLN2, Arabidopsis GS2 (glutamate synthetase) gene; ASN1, Arabidopsis gene AS1 (asparagine synthetase) gene. From: I.C. Oliveira, E. Brenner, J. Chiu, M.-H. Hsieh, A. Kouranov, H.-M. Lam, M.J.Shin and G. Coruzzi (2001). Metabolite and light regulation of metabolism in plants: lessons from the study of a single biochemical pathway. Brazillian Journal of Medical and Biological Research. 34: 567–575.
This regulation of nitrogen assimilatory genes by the cellular carbon status appears to coincide with the interrelationship between carbon and nitrogen metabolism in plants. On the one hand, carbon assimilation and nitrogen assimilation compete for reducing power and energy sources generated by photosynthesis. On the other hand, carbon metabolism provides the necessary carbon backbones for the biosynthetic process of nitrogen assimilation while the products, e.g. amino acids, are essential components of the photosynthetic apparatus. In addition to the control by carbon status in the cell, it has been proposed that the relative abundance of nitrogen pools also plays a significant role in regulating nitrogen assimilation (Coruzzi and Bush, 2001; Coruzzi and Zhou, 2001). In fact, some reports claim that the cellular carbon to nitrogen ratio is a major player in the metabolic control of nitrogen assimilation (Coruzzi and Zhou, 2001; Foyer et al., 2003). Plant glutamate receptors uncovered by (Lam et al., 1998) have also been implicated in sensing changes in Glu and in C/N levels (Kang and Turano, 2003). In the case of N-assimilation gene expression, the notion that plants sense C:N ratio is supported by the data which shows that the carbon induction of GLN2 is reversed by the addition of organic N, while the carbon repression of ASN1 is relieved by the addition of organic N (Lam et al., 1994; Oliveira and Coruzzi, 1999) (Fig. 15). In more recent gene chip experiments, Crawford's group showed that nitrate treatments affected the transcription of a host of genes involved in nitrogen assimilation including nitrate and nitrite reduction, as well as genes involved in N-assimilation such as Fd-GOGAT and asparagine syntetase (Wang et al., 2000). Data using Arabidopsis mutants or GS inhibitors suggest that either inorganic N (nitrate) (Crawford and Glass, 1998; Stitt, 1999) and organic N (Gln) (Rawat et al., 1999) may serve as signals to report on N-status and control the expression of genes involved in nitrogen uptake (e.g. the ammonium transporter AMT1) and/or assimilation.
In addition to its regulation by factors such as light, carbon and nitrogen, the pathway of N-assimilation genes such as ASN1 is further affected transcriptionally by other forms of regulation including regulation by circadian control (Harmer et al., 2001). Thus, the control of N-assimilation in plants is subject to complex control mechanisms. Both forward and reverse genetic approaches have been initiated in Arabidopsis in order to uncover the molecular mechanisms by which plants regulate gene expression in response to C and N metabolites. These studies are in their infancy, and this section is prime for expansion in the subsequent updates of this chapter.
PERSPECTIVES
Our lab has recently begun to use a systems approach to model and integrate the intersection of multiple signals that regulate the expression of N-assimilatory genes (Shasha et al., 2001; Thum et al., 2003). In one approach, we have employed a math tool called Combinatorial Design to design a parsimonious set of experiments that cover all combinations of treatments with light, carbon, and nitrogen sources (Shasha et al., 2001). By covering such experimental spaces, we have been able to use Boolean logic to model how signals interact. In another recent study, we covered an experimental space that allowed us to model how light sources (red, blue, far red, white) interact with C sources (sucrose) in the regulation of genes in the N-assimilation pathway (Thum et al., 2003) (Fig. 17). Modeling the regulation of this pathway and incorporating metabolic data, is a first step to the creating of a virtual plant which may be used in a predictive mode to allow changes that will enhance N-assimilation in plants.
Figure 17.
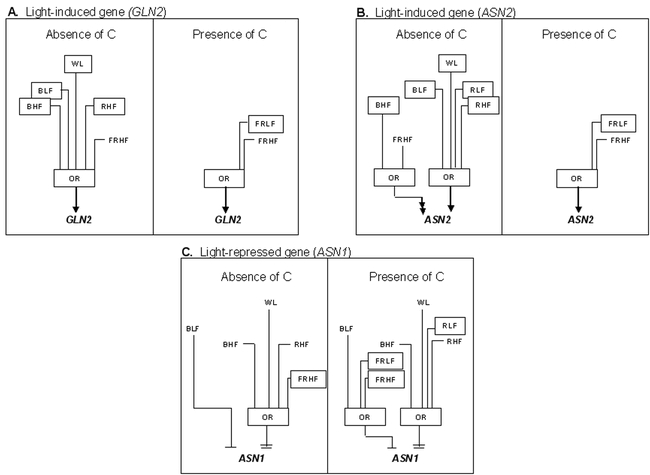
Boolean Circuits for Interaction of Light and Carbon (light grown). Boolean circuits model ASN1, ASN2 and GLN2 regulation by light and carbon in light-grown plants. A, B, and C Boolean circuits based on 16 experiments. A. GLN2 regulation by white, blue, red or far-red light when compared against a base-case of no light, no carbon or no light, carbon. B. ASN2 regulation by white, blue, red or far-red light when compared against a base-case of no light, no carbon or no light, carbon. C. ASN1 regulation by white, blue, red or far-red light when compared against a base-case of no light, no carbon or no light, carbon. The inputs are white light (WL), blue light low fluence (BLF), blue light high fluence (BHF), red light low fluence (RLF), red light high fluence (RHF), far-red light low fluence (FRLF), far-red light high fluence (FRHF). Low fluence is 2µµmol m−2 s−1, high fluence is 100µµmol m−2 s−1. The arrow or barred lines indicate the function of the inputs as either inductive or repressive. Double arrows or double bars denote super-induction or super-repression, respectively. For a Boolean OR, if any one of the inputs is active, the output will also be active. Differences in the input for Boolean circuits when comparing ‘absence of carbon’ to ‘presence of carbon’ are shown as boxed inputs. From: Thum KE, Shasha DE, Lejay L, Coruzzi G (2003) Light- and Carbon Signaling Pathways. Modeling Circuits of Interactions. Plant Physiology 132: 440–452.
Molecular and genetic analyses of N-assimilation have provided important tools to extend our knowledge of nitrogen assimilation that was originally based on biochemical studies. The cloned genes and Arabidopsis mutants in specific genes have helped to distinguish the physiological roles of specific nitrogen assimilatory isoenzyme. The mechanisms by which light and/or metabolic status (carbon and nitrogen) regulate nitrogen assimilation is beginning to be dissected using cloned genes and using genetic approaches. For example, some potential candidate regulatory genes have already been identified. In addition, specific screens for mutants in this process can be conducted in a genetic tractable system such as Arabidopsis. A combined molecular and genetic study of the regulatory network by which a gene responds to the metabolic status will lead to a better understanding of the genetic cross-talk between different carbon and nitrogen metabolic pathways. Basic research studies in these areas in Arabidopsis may also make significant contributions that can be applied to the improvement of nitrogen usage efficiency and crop yield in less genetically tractable systems.
Acknowledgments
This work was supported by the National Institute of Health (NIH) grant GM32877, Department of Energy (DOE) grant DE-FG02-92ER20071, and National Science Foundation (NSF) grant MCB-9817900. The structure and background sections of this chapter are expended on (Lam et al., 1996), which is an excellent review of research in Arabidopsis and other species. This chapter highlights advances in Arabidopsis genetics since that report was published. We thank Karen Coschigano, Hon-Ming Lam, Ming-Hsiun Hsieh, Muriel Lancien, Rosana Melo-Oliveira, Barbara Miesak, Igor Oliveira, Carolyn Schultz, and Maria Shamis for their respective contributions.
Footnotes
Citation: Coruzzi G.M. (2003) Primary N-assimilation into Amino Acids in Arabidopsis. The Arabidopsis Book 2:e0010. doi:10.1199/tab.0010
elocation-id: e0010
Published on: September 30, 2003
Figure 16.
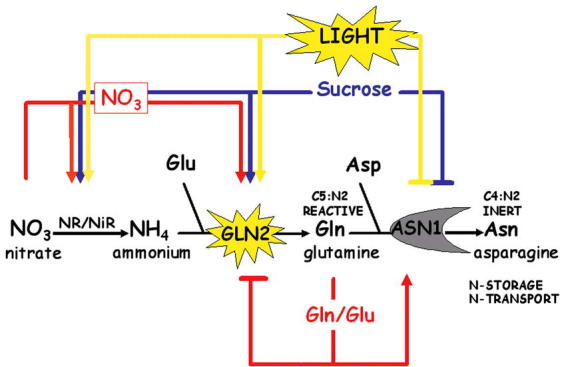
Multiple Input Regulation of N-assimilation genes. A simplified scheme depicting reciprocal regulation of GLN2 & ASN1 by Light, Carbon and Nitrogen. Also depicted are NR (nitrate reductase) and NiR (nitrite reductase).
REFERENCE
- Artus NN. Mutants of Arabidopsis thaliana that either require or are sensitive to high atmospheric CO2 concentrations: A Ph. D. dissertation. 1988. Michigan State University, E. Lansing.
- Bevan M, Mayer K, White O, Eisen JA, Preuss D, Bureau T, Salzberg SL, Mewes HW. Sequence and analysis of the Arabidopsis genome. Curr Opin Plant Biol. 2001;42(1):105–110. doi: 10.1016/s1369-5266(00)00144-8. [DOI] [PubMed] [Google Scholar]
- Blackwell RD, Murray AJS, Lea PJ. Inhibition of photosynthesis in barley with decreased levels of chloroplastic glutamine synthetase activity. Journal of Experimental Botany. 1987;382(1):1799–1809. [Google Scholar]
- Cammaerts D, Jacobs M. A study of the polymorphism and the genetic control of the glutamate dehydrogenase isozymes in Arabidopsis thaliana. Plant Science Letters. 1983;312(1):65–73. [Google Scholar]
- Cammaerts D, Jacobs M. A study of the role of glutamate dehydrogenase in the nitrogen metabolism of Arabidopsis thaliana. Journal of Heredity. 1985;1632(1):517–526. doi: 10.1007/BF00392709. [DOI] [PubMed] [Google Scholar]
- Carvalho H, Pereira S, Sunkel C, Salema R. Detection of cytosolic glutamine synthetase in leaves of Nicotiana tabacum L. by immunocytochemical methods. Plant Physiology. 1992;1002(1):1591–1594. doi: 10.1104/pp.100.3.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G, Bush DR. Nitrogen and carbon nutrient and metabolite signaling in plants. Plant Physiol. 2001;1252(1):61–64. doi: 10.1104/pp.125.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi GM, Zhou L. Carbon and nitrogen sensing and signaling in plants: emerging ‘matrix effects’. Curr Opin Plant Biol. 2001;42(1):247–253. doi: 10.1016/s1369-5266(00)00168-0. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Melo-Oliveira R, Lim J, Coruzzi GM. Arabidopsis gls Mutants and Distinct Fd-GOGAT Genes: Implications for Photorespiration and Primary Nitrogen Assimilation. The Plant Cell. 1998;102(1):741–752. doi: 10.1105/tpc.10.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford NM, Forde BG. Molecular and Developmental Biology in Inorganic Nitrogen Nutrition. 2002. In CRSaEM Meyerowitz, ed, The Arabidopsis Book, Vol doi/10.1199/tab.0011. American Society of Plant Biologists, Rockville, MD. [DOI] [PMC free article] [PubMed]
- Crawford NM, Glass ADM. Molecular and physiological aspects of nitrate uptake in plants. Trends in plant science. 1998;32(1):389–395. [Google Scholar]
- Day DA, Salom CL, Azcon-Bieto J, Dry IB, Wiskich JT. Glutamate Oxidation by Soybean Cotyledon and Leaf Mitochondria. Plant Cell Physiology. 1988;292(1):1193–1200. [Google Scholar]
- Delgado E, Mitchell RAC, Parry MA, Driscoll SP, Mitchell VJ, Lawlor DW. Interacting effects of CO2 concentration, temperature and nitrogen supply on the photosynthesis and composition of winter wheat leaves. Plant, Cell and Environment. 1994;172(1):1205–1213. [Google Scholar]
- Edwards JW, Coruzzi GM. Cell-specific gene expression in plants. Annu Rev Genet. 1990;242(1):275–303. doi: 10.1146/annurev.ge.24.120190.001423. [DOI] [PubMed] [Google Scholar]
- Edwards JW, Walker EL, Coruzzi GM. Cell-specific expression in transgenic plants reveals nonoverlapping roles for chloroplast and cytosolic glutamine synthetase. Proc Natl Acad Sci U S A. 1990;872(1):3459–3463. doi: 10.1073/pnas.87.9.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde BG, Day HM, Turton JF, Shen W-j, Cullimore JV, Oliver JE. Two glutamine synthetase genes from Phaseolus vulgaris L. display contrasting developmental and spatial patterns of expression in transgenic Lotus corniculatus plants. The Plant Cell. 1989;12(1):391–401. doi: 10.1105/tpc.1.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C, M P, G N. Markers and signals associated with nitrogen assimilation in higher plants. J Exp Bot. 2003;542(1):585–593. doi: 10.1093/jxb/erg053. [DOI] [PubMed] [Google Scholar]
- Hageman RH, Lambert RJ. The use of physiological traits for corn improvement. 1988;2(1):431–461. In GF Sprague, JW Dudley, eds, Corn and corn improvement, Ed 3. American Society of Agronomy and Soil Society of America, Madison, pp. [Google Scholar]
- Harmer SL, Panda S, Kay SA. Molecular bases of circadian rhythms. Annu Rev Cell Dev Biol. 2001;172(1):215–253. doi: 10.1146/annurev.cellbio.17.1.215. [DOI] [PubMed] [Google Scholar]
- Hsieh MH, Lam HM, van de Loo FJ, Coruzzi G. A PII-like protein in Arabidopsis: putative role in nitrogen sensing. Proc Natl Acad Sci U S A. 1998;952(1):13965–13970. doi: 10.1073/pnas.95.23.13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland RJ, Joy KW. Plant transaminases. 1985;2(1):376–384. In P Christen, DE Metzler, eds, Transaminases. John Wiley & Sons, New York, pp. [Google Scholar]
- Jacobs M, Joukas K. Glutamate dehydrogenase in Arabidopsis thaliana: polymorphism properties and effect of nitrogen source on enzyme activity [proceedings]. Arch Int Physiol Biochim. 1978;862(1):434–435. [PubMed] [Google Scholar]
- Kamachi K, Yamaya T, Hayakawa T, Mae T, Ojima K. Vascular bundle-specific localization of cytosolic glutamine synthetase in rice leaves. Plant Physiology. 1992;992(1):1481–1486. doi: 10.1104/pp.99.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Turano F. The putative glutamate receptor 1.1 (AtGLR1.1) functions as a regulator of carbon and nitrogen metabolism in Arabidopsis thaliana. Proc Natl Acad Sci. 2003;1002(1):6872–6877. doi: 10.1073/pnas.1030961100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall AC, Wallsgrove RM, Hall NP, Turner JC, Lea PJ. Carbon and nitrogen metabolism in barley (Hordeum vulgare L) mutants lacking ferredoxin-dependent glutamate synthase. Planta. 1986;1682(1):316–323. doi: 10.1007/BF00392355. [DOI] [PubMed] [Google Scholar]
- Lam HM, Chiu J, Hsieh MH, Meisel L, Oliveira IC, Shin M, Coruzzi G. Glutamate-receptor genes in plants. Nature. 1998;3962(1):125–126. doi: 10.1038/24066. [DOI] [PubMed] [Google Scholar]
- Lam HM, Coschigano K, Oliveira IC, Melo-Oliveira R, Coruzzi G. The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Ann.Rev.Plant Physiol.and Plant Mol.Biol. 1996;472(1):596–593. doi: 10.1146/annurev.arplant.47.1.569. [DOI] [PubMed] [Google Scholar]
- Lam HM, Coschigano K, Schultz C, Melo-Oliveira R, Tjaden G, Oliveira I, Ngai N, Hsieh MH, Coruzzi G. Use of Arabidopsis mutants and genes to study amide amino acid biosynthesis. Plant Cell. 1995;72(1):887–898. doi: 10.1105/tpc.7.7.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H-M, Coschigano KT, Oliveira IC, Melo-Oliveira R, Coruzzi GM. The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;472(1):569–593. doi: 10.1146/annurev.arplant.47.1.569. [DOI] [PubMed] [Google Scholar]
- Lam HM, Hsieh MH, Coruzzi G. Reciprocal regulation of distinct asparagine synthetase genes by light and metabolites in Arabidopsis thaliana. Plant J. 1998;162(1):345–353. doi: 10.1046/j.1365-313x.1998.00302.x. [DOI] [PubMed] [Google Scholar]
- Lam HM, Peng SS, Coruzzi GM. Metabolic regulation of the gene encoding glutamine-dependent asparagine synthetase in Arabidopsis thaliana. Plant Physiol. 1994;1062(1):1347–1357. doi: 10.1104/pp.106.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H-M, Wong P, Chan H-K, Yam K-M, Chen L, Chow C-M, Coruzzi GM. Overexpression of the ASN1 gene enhances nitrogen status in seeds of Arabidopsis thaliana. Plant Physiol. 2003. in press. [DOI] [PMC free article] [PubMed]
- Lancien M, Martin M, Hsieh MH, Leustek T, Goodman H, Coruzzi GM. Arabidopsis glt1-T mutant defines a role for NADH-GOGAT in the non-photorespiratory ammonium assimilatory pathway. Plant J. 2002;292(1):347–358. doi: 10.1046/j.1365-313x.2002.01218.x. [DOI] [PubMed] [Google Scholar]
- Last RL, Bissinger PH, Mahoney DJ, Radwanski ER, Fink GR. Tryptophan mutants in Arabidopsis: The consequences of duplicated tryptophan synthase beta genes. The Plant Cell. 1991;32(1):345–358. doi: 10.1105/tpc.3.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DW, Kontturi M, Young AT. Photosynthesis by flag leaves of wheat in relation of protein, ribulose bisphosphate carboxylase activity and nitrogen supply. Journal of Experimental Botany. 1989;402(1):43–52. [Google Scholar]
- Lea PJ. Nitrogen Metabolism. 1993;2(1):155–180. In PJ Lea, RC Leegood, eds, Plant biochemistry and Molecular Biology. John Wiley & sons, New York, pp. [Google Scholar]
- Lea PJ, Forde BG. The use of mutants and transgenic plants to study amino acid metabolism. Plant, Cell and Environment. 1994;172(1):541–556. [Google Scholar]
- Lea PJ, Miflin BJ. Transport and metabolism of asparagine and other nitrogen compounds within the plant. 1980;2(1):569–607. In BJ Miflin, ed, The biochemistry of plants. Vol. 5: amino acids and derivatives. Academic Press, New York, pp. [Google Scholar]
- Lea PJ, Robinson SA, Stewart GR. The enzymology and metabolism of glutamine, glutamate, and asparagine. 1990;2(1):121–159. In BJ Miflin, PJ Lea, eds, The Biochemistry of Plants. Vol. 16. Academic Press, New York, pp. [Google Scholar]
- Lea PJ, Thurman DA. Intracellular Location and Properties of Plant L-Glutamate Dehydrogenases. Journal of Experimental Botany. 1972;232(1):440–449. [Google Scholar]
- Loulakakis CA, Roubelakis-Angelakis KA. Intracellular Localization and Properties of NADH-Glutamate Dehydrogenase from Vitis vinifera L: Purification and Characterization of the Major Leaf Isoenzyme. Journal of Experimental Botany. 1990;412(1):1223–1230. [Google Scholar]
- Magalhaes JR, Ju GC, Rich PJ, Rhodes D. Kinetics of 15NH4+ assimilation in Zea mays Preliminary studies with a glutamate dehydrogenase (GDH1) null mutant. Plant Physiology. 1990;942(1):647–656. doi: 10.1104/pp.94.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B. The regulation of nitrogen utilization in enteric bacteria. Journal of Cellular Biochemistry. 1994;512(1):34–40. doi: 10.1002/jcb.240510108. [DOI] [PubMed] [Google Scholar]
- Matoh T, Takahashi E. Changes in the activites of ferredoxin and NADH-glutamate synthase durig seedling development of peas. Planta. 1982;1542(1):289–294. doi: 10.1007/BF00393905. [DOI] [PubMed] [Google Scholar]
- Melo-Oliveira R, Oliveira IC, Coruzzi G. Arabidopsis mutant analysis and gene regulation defines a non-redundant role for glutamate dehydrogenase in nitrogen assimilation. Proc.Natl.Acad.Sci.USA. 1996;932(1):4718–4723. doi: 10.1073/pnas.93.10.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo-Oliveira R, Oliveira IC, Coruzzi GM. Arabidopsis mutant analysis and gene regulation define a nonredundant role for glutamate dehydrogenase in nitrogen assimilation. Proc Natl Acad Sci U S A. 1996;932(1):4718–4723. doi: 10.1073/pnas.93.10.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesak BH, Coruzzi GM. Molecular and physiological analysis of Arabidopsis mutants defective in cytosolic or chloroplastic aspartate aminotransferase. Plant Physiol. 2002;1292(1):650–660. doi: 10.1104/pp.005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin BJ. The location of nitrite reductase and other enzymes related to amino acid biosynthesis in the plastids of root and leaves. Plant Physiology. 1974;542(1):550–555. doi: 10.1104/pp.54.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miflin BJ. The Biochemistry of Plants, Volume 5, Amino acids and Derivatives. 1980. Academic Press, Inc., New York.
- Miflin BJ, Cullimore JV. Nitrogen assimilation in the legume Rhizobium symbiosis: a joint endeavour. 1984;2(1):129–178. In DPS Verma, T Hohn, eds, Genes involved in microbe plant interactions. Springer, Vienna, pp. [Google Scholar]
- Miflin BJ, Lea PJ. The Pathway of nitrogen assimilation in plants. Phytochemistry. 1976;152(1):873–885. [Google Scholar]
- Miflin BJ, Lea PJ. Amino acid metabolism. Annual Review of Plant Physiology. 1977;282(1):299–329. [Google Scholar]
- Miflin BJ, Lea PJ. Ammonia Assimilation. The Biochemistry of Plants vol. 5. 1980. Academic Press, New York.
- Miflin BJ, Lea PJ. Ammonia assimilation. 1980;2(1):169–202. In BJ Miflin, EE Con, eds, The Biochemistry of Plants. Vol. 5: amino acids and derivatives. Academic Press, New York, pp. [Google Scholar]
- Miflin BJ, Lea PJ. Biosynthesis and metabolism of protein amino acids and proteins. 1982;2(1):5–64. In D Boulter, B Parthier, eds, Nucleic acid and proteins in plants I: Structure, biochemistry and physiology of proteins. Springer-Verlag, Berlin Heidelberg New York, pp. [Google Scholar]
- Oaks A, Ross DW. Asparagine synthetase in Zea mays. Canadian Journal of Botany. 1984;622(1):68–73. [Google Scholar]
- Oliveira IC, Brenner E, Chiu J, Hsieh MH, Kouranov A, Lam HM, Shin MJ, Coruzzi G. Metabolite and light regulation of metabolism in plants: lessons from the study of a single biochemical pathway. Braz J Med Biol Res. 2001;342(1):567–575. doi: 10.1590/s0100-879x2001000500003. [DOI] [PubMed] [Google Scholar]
- Oliveira IC, Coruzzi GM. Carbon and amino acids reciprocally modulate the expression of glutamine synthetase in Arabidopsis. Plant Physiol. 1999;1212(1):301–310. doi: 10.1104/pp.121.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples MB, Gifford RM. Long-distance transport of carbon and nitrogen from sources to sinks in higher plants. 1993;2(1):434–447. In DT Dennis, DH Turpin, eds, Plant physiology, biochemistry and molecular biology. John Wiley & Son, Inc., New York, pp. [Google Scholar]
- Peterman TK, Goodman HM. The glutamine synthetase gene family of Arabidopsis thaliana: light-regulation and differential expression in leaves, roots and seeds. Molecular and General Genetics. 1991;2302(1):145–154. doi: 10.1007/BF00290662. [DOI] [PubMed] [Google Scholar]
- Poulton JE, Romeo JT, Conn CC. Plant nitrogen metabolism. Recent advances in phytochemistry. Vol. 23. 1989. Plenum Press, New York.
- Pryor A. A maize glutamic dehydrogenase null mutant is cold temperature sensitive. Maydica. 1990;352(1):367–372. [Google Scholar]
- Pryor AJ. Allelic glutamic dehydrogenase isozymes in maize - a single hybrid isozyme in heterozygotes? Heredity. 1974;322(1):397–419. [Google Scholar]
- Pryor AJ. Mapping of glutamic dehydrogenase (Gdh) on chromosome 1, 20.1 recombination units distal to Adh 1. Maize Genet.Coop.Newsletter. 1979;532(1):25–26. [Google Scholar]
- Rawat S, Erner Y, Kronzucker H, Silim S, Schjoerring JK, Siddiqi MY, Glass A. AtAMT1 transcript abundance and high affinity ammonium influx in roots of Arabidopsis. 1999. submitted.
- Richards NGJ, Schuster SM. An alternative mechanism for the nitrogen transfer reaction in asparagine synthetase. FEBS Letters. 1992;3132(1):98–102. doi: 10.1016/0014-5793(92)81421-h. [DOI] [PubMed] [Google Scholar]
- Schultz CJ. A molecular and genetic dissection of the aspartate aminotransferase isoenzymes of Arabidopsis thaliana. A Ph.D. thesis. 1994. New York University, New York, NY.
- Schultz CJ, Coruzzi GM. The aspartate aminotransferase gene family of Arabidopsis encodes isoenzymes localized to three distinct subcellular compartments. Plant J. 1995;72(1):61–75. doi: 10.1046/j.1365-313x.1995.07010061.x. [DOI] [PubMed] [Google Scholar]
- Schultz CJ, Hsu M, Miesak B, Coruzzi GM. Arabidopsis mutants define an in vivo role for isoenzymes of aspartate aminotransferase in plant nitrogen assimilation. Genetics. 1998;1492(1):491–499. doi: 10.1093/genetics/149.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shasha DE, Kouranov AY, Lejay LV, Chou MF, Coruzzi GM. Using combinatorial design to study regulation by multiple input signals. A tool for parsimony in the post-genomics era. Plant Physiol. 2001;1272(1):1590–1594. [PMC free article] [PubMed] [Google Scholar]
- Sheen J, Zhou L, Jang JC. Sugars as signaling molecules. Curr Opin Plant Biol. 1999;22(1):410–418. doi: 10.1016/s1369-5266(99)00014-x. [DOI] [PubMed] [Google Scholar]
- Sieciechowicz KA, Joy KW, Ireland RJ. The metabolism of asparagine in plants. Phytochemistry. 1988;272(1):663–671. [Google Scholar]
- Smith C, AM W, GB M. Molecular properties of the putative nitrogen sensor PII from Arabidopsis thaliana. Plant Journal. 2003;332(1):353–360. doi: 10.1046/j.1365-313x.2003.01634.x. [DOI] [PubMed] [Google Scholar]
- Smith C, ST Z, DG M, GB M. Expression and purification of the chloroplast putative nitrogen sensor, PII, of Arabidopsis thaliana. Protein Expr Purif. 2002;252(1):342–347. doi: 10.1016/s1046-5928(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Somerville C, Ogren W. Photorespiration mutants of Arabidopsis deficient in serine-glyoxalate aminotransferase activity. Proc.Natl.Acad.Sci.USA. 1980;772(1):2684–2687. doi: 10.1073/pnas.77.5.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville CR, Ogren WL. Inhibition of photosynthesis in Arabidopsis mutants lacking leaf glutamate synthase activity. Nature. 1980;2862(1):257–259. [Google Scholar]
- Srivastava HS, Singh RP. Role and Regulation of L-Glutamate Dehydrogenase Activity in higher plants. Phytochemistry. 1987;262(1):597–610. [Google Scholar]
- Stewart GR, Mann AF, Fentem PA. Enzymes of glutamate formation: glutamate dehydrogenase, glutamine synthetase, glutamate synthase. 1980;2(1):271–327. In BF Miflin, ed, The biochemistry of plants. Vol. 5: Amino Acids and Derivatives. Academic Press, N.Y., pp. [Google Scholar]
- Stitt M. Nitrate regulation of metabolism and growth. Current Opinion in Plant Biology. 1999;22(1):178–186. doi: 10.1016/S1369-5266(99)80033-8. [DOI] [PubMed] [Google Scholar]
- Stitt M. Nitrate regulation of metabolism and growth. Curr Opin Plant Biol. 1999;22(1):178–186. doi: 10.1016/S1369-5266(99)80033-8. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Gadal P. Glutamate synthase: physicochemical and functional properties of different forms in higher plants and other organisms. Physiol.Veg. 1984;222(1):471–486. [Google Scholar]
- Taniguchi M, Kobe A, Kato M, Sugiyama T. Aspartate aminotransferase isoenzymes in Panicum miliaceum L., and NAD-malic enzyme-type C4 plant: comparison of enzymatic properties, primary structures, and expression patterns. Arch.Biochem.Biophys. 1995;3182(1):295–306. doi: 10.1006/abbi.1995.1233. [DOI] [PubMed] [Google Scholar]
- Thum KE, Shasha DE, Lejay L, Coruzzi G. Light- and Carbon Signaling Pathways, Modeling Circuits of Interactions. Plant Physiol. 2003;1322(1):440–452. doi: 10.1104/pp.103.022780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turano FJ, Fang TK. Characterization of Two Glutamate Decarboxylase cDNA clones from Arabidopsis. Plant Physiology. 1998;1172(1):1411–1421. doi: 10.1104/pp.117.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turano FJ, Thakkar SS, Fang T, Weisemann JM. Characterization and expression of NAD(H)-dependent glutamate dehydrogenase genes in Arabidopsis. Plant Physiology. 1997;1132(1):1329–1341. doi: 10.1104/pp.113.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turano FJ, Thakkar SS, Fang T, Weisemann JM. Characterization and expression of NAD(H)-dependent glutamate dehydrogenase genes in Arabidopsis. Plant Physiol. 1997;1132(1):1329–1341. doi: 10.1104/pp.113.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauquelin LN, Robiquet PJ. The discovery of a new plant principle in Asparagus sativus. Ann.Chim.(Paris) 1806;572(1):88–93. [Google Scholar]
- Wadsworth GW, Marmaras SM, Matthews BF. Isolation and characterization of a soybean cDNA clone encoding the plastid form of aspartate aminotransferase. Plant Molecular Biology. 1993;212(1):993–1009. doi: 10.1007/BF00023598. [DOI] [PubMed] [Google Scholar]
- Wallsgrove RM, Turner JC, Hall NP, Kendally AC, Bright SWJ. Barley mutants lacking chloroplast glutamine synthetase-biochemical and genetic analysis. Plant Physiology. 1987;832(1):155–158. doi: 10.1104/pp.83.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Guegler K, LaBrie ST, Crawford NM. Genomic analysis of a nutrient response in arabidopsis reveals diverse expression patterns and novel metabolic and potential regulatory genes induced by nitrate. Plant Cell. 2000;122(1):1491–1510. doi: 10.1105/tpc.12.8.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeden NF, Gottlieb LD. The genetics of chloroplast enzymes. Journal of Heredity. 1980;712(1):392–396. [Google Scholar]
- Wilkie SE, Roper J, Smith A, Warren MJ. Isolation, characterisation and expression of a cDNA clone encoding aspartate aminotransferase from Arabidopsis thaliana. Plant Molecular Biology. 1995;272(1):1227–1233. doi: 10.1007/BF00020897. [DOI] [PubMed] [Google Scholar]
- Yamaya T, Oaks A. Synthesis of glutamate by mitochondria - An anaplerotic function for glutamate dehydrogenase. Physiologia Plantarum. 1987;702(1):749–756. [Google Scholar]


