CUTIN, THE STRUCTURAL POLYESTER OF THE CUTICLE
The aerial surfaces of vascular plants are covered with a continuous extracellular layer called the cuticle that overlays the cell wall of epidermal cells. The major structural component of the cuticle is cutin, a biopolyester mainly composed of interesterified hydroxy, and epoxy-hydroxy fatty acids with a chain length of 16 or/and 18 carbons (C16 and C18 class). Cutin is embedded and over-layered by intracuticular and epicuticular waxes, complex mixtures of hydrophobic material containing very long-chain fatty acids and their derivatives (chapter on waxes). The combination of cutin, waxes and possibly polysaccharides, forms the cuticle (Jeffree, 1996).
The ultrastructure of the cuticle
The ultrastructure of cuticles visualized by transmission electron microscopy (TEM) reflects the composition and deposition pattern of the different polymeric components in a manner that is not well understood. Cuticles may be of lamellate, recticulate, or amorphous appearance; a feature that has been used for their classification (Holloway, 1982). The thickness and ultrastructure of the cuticle change markedly during growth and development of organs and these changes are correlated to changes in cutin composition (Riederer and Schönherr, 1988).
In Arabidopsis, the cuticular structures of leaves and stems have been analyzed (Sieber et al., 2000). Both cuticles belong to the amorphous type, as described for the cuticles of the leaves of Brassica species (Jeffree, 1996). The thickness of the cuticles of Arabidopsis differs between organs, being 20 - 25 nm in leaves (Figure 1A) and 50 - 80 nm in stems (Figure 2A).
Figure 1.
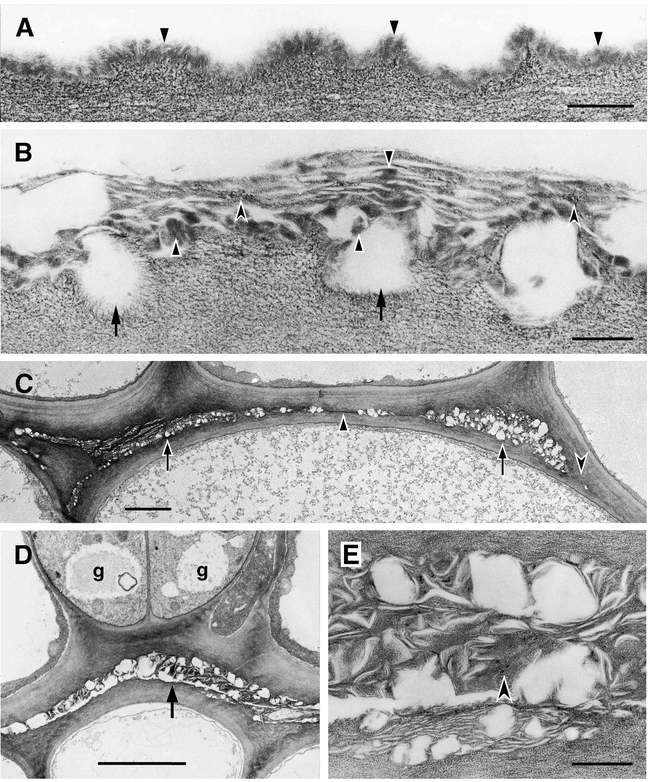
Ultrastructure of the cuticle of the stem epidermis of wild-type plants and cutinase-expressing transgenic Arabidopsis plants. (A) Columbia Col-0/gl1. Outer wall of epidermal cell. Cuticle of amorphous appearance (small arrowheads) overlaying the cell wall polysaccharides. Bar = 200 nm. (B) Cutinase-expressing Columbia, Col-0/gl1. In contrast to the cuticle of wild type plants, amorphous material of cuticular origin (solid arrowheads) is interspersed with polysaccharide microfibrils (concave arrowheads) in a loosely structured cuticle. Cuticular material accumulates to highly variable degrees, varying from apparently nothing to thick aggregates of mixed composition. The contact zone between the cell wall polysaccharides and the cuticle is interrupted since hydrophobic material of low molecular weight is at least partly extracted during dehydration and embedding procedures (arrows). Bar = 200 nm. (C) Cutinase-expressing Columbia Col-0/gl1. Organ fusion between two stems, overview. Apparently empty spaces surrounded by polysaccharide cell wall materials characterize the fusion zone at most places (arrows). Areas containing low amounts of cuticular material can also been seen (solid arrowhead). At some places the cuticle is interrupted and the cell walls of both epidermal cell come into direct contact (concave arrowhead). Bar = 2 micrometers. (D) Cutinase-expressing Columbia Col-0/gl1. Organ fusion between two stems. Two stomatal guard cells (g) are present in fused epidermal cell layers. Apparently empty spaces surrounded by polysaccharide cell wall materials characterize this fusion zone (arrow). Bar = 5 micrometers. (E) Cutinase-expressing Columbia Col-0/gl1. Enlarged view of detail in (D). At this magnification it can be seen that a large part of the materials in the fusion zone has a fibrillar ultrastructure and resembles cell wall polysaccharides (concave arrowhead). Waxy compounds are at least partly extracted during the dehydration and embedding procedures. Bar = 500 nm.
Figure 2.
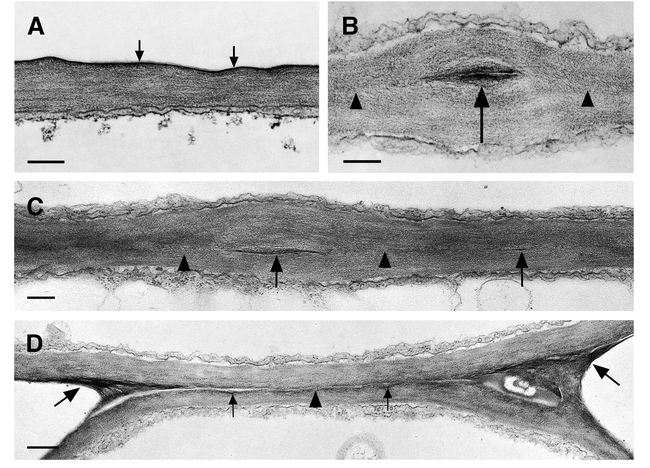
Ultrastructure of the cuticle of the leaf epidermis of wild type and structural variations of the cuticle in fusion zones between leaves of cutinase-expressing transgenic Arabidopsis plants. (A) Columbia Col-0/gl1. A thin electron-dense cuticle of amorphous structure (arrows) overlays the cell wall polysaccharides in leaves. Bar = 250 nm. (B) and (C) Cutinase-expressing Columbia Col-0/gl1. The fusion zone between leaves is characterized by stretches of a direct contact of the polysaccharides (arrowheads) of the two epidermal cells and the local occurrence of interspersed cuticular material (arrow). Tilting the specimen (C) in the regions of a direct contact of polysaccharides confirmed the absence of detectable amounts of cuticular material or any structural change at the position were both cell walls come into contact (arrowheads). Bars = 250 nm. (D) Cutinase-expressing Columbia Col-0/gl1. The fusion zone is characterized by stretches of low amounts of cuticular material (small arrows) that is partially missing (arrowhead). In contrast to (C), the position where the cell walls of both epidermal cells come into contact is still visible. At the positions of the cell corners where the fusion is tearing apart, electron-opaque polysaccharide material accumulates (large arrows). Bar = 500 nm.
Analysis of the composition of cutin
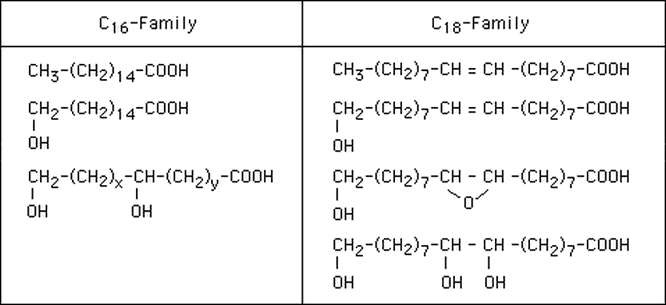
The standard procedure for the identification of the cutin monomer composition is based on the isolation of the cuticle by digestion of the tissue with cellulases and pectinases. The cuticle is then fractionated into chloroform-soluble material, called waxes, and chloroform-insoluble material, called cutin. After extensive solvent extraction, the cutin biopolyester can be depolymerized by procedures cleaving ester bonds, e.g. alkaline hydrolysis, transesterification with methanol containing boron trifluoride or sodium methoxide, as well as reductive cleavage with lithium aluminium hydride (Walton and Kolattukudy, 1972; Kolattukudy, 1981). The liberated cutin monomers may be first methylated or are directly converted into trimethylsilyl derivatives (with BFTSA) before subjecting them to gas chromotography/mass spectrometry (GC/MS). The monomers are identified by their characteristic fragmentation pattern (Walton and Kollatukudy, 1972). Typical cutin monomers of the C16 and C18 classes are listed in Table 1. Dihydroxypalmitic acid with a hydroxy group in mid-chain position and at the ω-end is a diagnostic monomer for the C16-cutin, while 9,10,18-trihydroxystearic acid and 9,10-epoxy,18-hydroxystearic acid are characteristic for the C18-cutin. Minor monomers may also be fatty acids, fatty alcohols, aldehydes, ketones, diacids as well as hydroxycinnamic acids.
In addition to the hydrolysable cutin polymer, the cuticle of some plants may also contain a non-hydrolysable fraction, that can be characterized by different techniques, including 13C-nuclear magnetic resonance (NMR) and Fourier transform infrared (FT-IR) spectroscopy as well as ozonolysis. This non-ester fraction contains a network of aliphatic compounds linked by ether bonds in which linolenic acid is preferentially incorporated (Villena et al., 1999). If this fraction should still be called cutin or should be named cutan is still under discussion (Kolattukudy, 1996).
The general protocol for the analysis of the cutin polymer has not been successfully applied in Arabidopsis because the cuticle is thin and fragile. Preliminary results on some characteristic cutin monomers have been obtained by hydrolysis of the polymeric material of total stem segments resistant to extraction with methanol-chloroform and subsequent methanolysis. The monomers identified by GC-MS characterize the cutin of Arabidopsis as a C16-cutin containing palmitic acid, 16-hydroxypalmitic acid and 9, 16- dihydroxypalmitic acid (Lukas Schreiber and Christiane Nawrath, unpublished results). However, the particular low amounts of the hydrolyzed monomers that can only be detected sometimes lead to the question whether the cutin of Arabidopsis contains high amounts of a non-hydrolysable cutin fraction (“cutan”) (Lukas Schreiber and Christiane Nawrath, unpublished results). Thus, a reliable method for the analysis of cutin has not yet been established in Arabidopsis.
Cutin biosynthesis
The cuticle and their constituents are synthesized by epidermal cells during the growth period of the different organs of the aerial part of the plant (Kolattukudy, 1970; Bowen and Walton, 1988; Hoffman-Benning and Kende, 1994). The monomers of cutin of the C16 and C18 classes are synthesized from the CoA esters of palmitic acid (16:0) and oleic acid (18:1), respectively.
The general cutin biosynthetic pathway was largely discovered by the group of Kolattukudy in the early 70's (reviewed in Kolattukudy, 1981). Early experiments showed that cutin monomers are synthesized by multiple hydroxylation and epoxidation reactions. These reations are all catalyzed by oxygen and NADP-dependent enzyme systems that are inhibited by CO, a typical characteristic of cytochrome P450-dependent enzymes. The research on plant cytochrome P450s has much advanced during the recent years (Kahn and Durst, 2000). Different cytochrome P450-dependent enzymes have been characterized that catalyze the internal as well as the ω-hydroxylation of fatty acids and some of the corresponding genes have been cloned (Pinot et al., 1992, 1998; Cabello-Hurtado et al., 1998; Beneviste et al., 1998; Tijet et al., 1998). However, none of them has been demonstrated to be involved in cutin biosynthesis.
In addition, a lipoxygenase/peroxygenase/epoxide hydrolase pathway has been proposed for the synthesis of cutin monomers (Blée and Schuber, 1993). A peroxygenase may catalyze a hydroxyperoxide-dependent epoxidation of unsaturated fatty acids after the action of a lipoxygenase (Blée and Schuber, 1990; Hamberg and Hamberg, 1990; Blée and Schuber, 1993). The cis-epoxy group formed by the peroxygenase may then be hydrated in the trans position by an epoxide hydrolase resulting in a threo-diol in mid-chain position of the cutin monomers (Blée and Schuber, 1992; 1995; Pinot et al., 1997; Moriseau et al., 2000).
The order of the ω-hydroxylation and the mid-chain epoxidation/hydroxylation reactions may depend on the enzyme system identified. Some ω-hydroxylases fatty acids prefer epoxdized fatty acids as substrates and others not, while some peroxygenases prefer ω-hydroxylated fatty acids as substrates (Pinot et al., 1992; Pinot et al., 1997; Blée and Schuber, 1993).
How the cutin monomers are transported to the place of polymerization remains to be elucidated. For the transport of monomers to the plasma membrane, a vesicle-dependent transport has been proposed that is then followed by the action of a “flippase” that transfers the cutin monomers to the outer layer of the lipid bilayer (Daleke and Lyles, 2000). The transport through the cell wall may involve lipoproteins. This model has been proposed after proteins with the activity to transport lipids in vitro (Lipid-transfer proteins; LTP) had been localized in the cell wall (Kader, 1996). Although some circumstantial evidence for this function of the LTPs has been collected (Pyee and Kolattukudy, 1995; Hollenbach et al., 1997), the involvement of LTPs in cutin biosynthesis has still to be substantiated.
In the cuticle, the cutin biopolymer is formed by cross-linking hydroxylated fatty acids via intermolecular ester bonds leading to a 3-dimensional structure. The cutin monomers bound to the CoA as cofactors are transferred to free hydroxy groups present in the cutin polymer (Croteau and Kolattukudy, 1973; 1975; Kolattukudy, 1981). A hydroxyl-CoA:cutin transacylase has been detected in a crude extract that needs ATP for the reaction as well as cutin polymer as primer. However, the transacylase has not been purified and no genes encoding the enzyme have been identified. Recently, a putative acyl-CoA:cutin transferase may have been purified from Agave epidermis. After partial protein sequencing a gene has been isolated that encodes a novel small valine-rich protein with a putative HxxxE domain present in other acyltransferases (Reina and Heredia, 2001).
The role of cutin in the cuticle
The cuticle constitutes the contact zone between the plant and the environment. Its physical properties are closely related to the functions of the epidermis. The cuticle is involved in many processes that have been reviewed in detail and only some of them will be mentioned here (Kerstiens, 1996a,c). The cuticle plays a major role as a barrier for water and solutes and regulates gas exchange when stomata are closed or are not present (Kerstiens, 1996b). The cuticle is also regarded as a reservoir for hydrophobic compounds and regulates the uptake of non-volatile chemicals deposited on the epidermis (Schönherr and Baur, 1996). In addition, the cuticle protects the plant against mechanical and irradiation damage as well as herbivore and pathogen attacks (Grace and Gardingen, 1996; Kerstiens, 1996a). For many functions of the cuticle it is not known which cuticular component contributes certain physical properties, or if both wax and cutin act in a concerted action. However, there exist a number of examples where the wax layer is particularly important for the interaction of the plants with insects (Eigenbrode, 1996), while cutin is thought to be important for the interaction with pathogenic fungi (Mendgen, 1996; Kerstiens, 1996a, c). Experiments with reconstituted wax layers also showed that wax and not cutin is the major diffusion barrier in cuticles (Riederer and Schreiber, 1995; Schreiber et al., 1996).
Cutinase, an enzyme produced by different phytopathogenic fungi, has been shown to degrade the cutin polymer (Kolattukudy, 1984; 1985). Thus, the cutinase contributes to the array of strategies used by fungi to attack plants. The importance of cutinases for the successful penetration of the fungus has been demonstrated for some plant-pathogen interactions (Dickman et al., 1989; Rogers et al., 1994; Davies et al., 2000); in others they do not play a crucial role (Stahl and Schäfer, 1992). Cutinase may be not only be involved in the direct penetration of the cuticle but also in the adhesion of spores to the plant surface (Deising et al., 1992).
Cutin monomers produced by the action of cutinase have been shown to be important as signals. In fungi, cutin monomers induce the expression of the cutinase gene (Kolattukudy, 1995) and contribute to the induction of fungal appressoria (Francis et al., 1996; Gilbert et al., 1996). In plants, treatment with exogenous cutinase or cutin monomers lead to resistance of plants to certain pathogens (Namai et al., 1993; Schweizer et al., 1994; 1996a). Although the resistance mechanism remains to be elucidated, several experiments demonstrated that cutin monomers are perceived by the plants to induce defense reactions, such as alkalinization of the medium of potato cell cultures and H2O2 production in cucumber hypocotyls (Schweizer et al., 1996b; Fauth et al., 1998; Kauss et al., 1999). Cutin monomers may also contribute to resistance by inhibiting the germination of fungal spores (Wang et al., 2000). These results show that cutin acts as a physical and chemical barrier in plant-pathogen interactions and that its monomers and catabolic products may act as signaling and defense molecules.
The epidermis also has important functions during plant development. The unresponsiveness of the epidermal layer to contact ensures that organs develop normally and do not fuse when closely appressed (as in grafting) (Walker and Bruch, 1985). Thus, the cuticle may also be directly involved in maintaining organs separate from each other. Furthermore, the cuticle has been found to contribute to tissue stress in fast growing tissues due to limitation of the growth rate (Hoffmann-Benning and Kende, 1994).
Cutin mutants of Arabidopsis
The isolation of cutin mutants by a direct biochemical screen has been hampered by the low amount of cutin in a single plant. Until recently, the phenotypes and the viability of a plant with a disrupted cuticle have not been known (Sieber et al., 2000).
- Transgenic Arabidopsis with an altered cuticle structure
An indirect way of investigating the role of cutin was undertaken by generating transgenic Arabidopsis plants expressing of a fungal cutinase in the cell-wall space. These transgenic plants showed a disrupted integrity of the cuticle (Sieber et al., 2000). The cuticle of cutinase-expressing Arabidopsis is uneven in thickness and, when present, has a loose appearance. In stems, regions of mixed composition are interrupted by empty regions from which, most likely, cutin degradation products have been washed out during the fixation process of TEM (Figure 1B). The cuticle of cutinase-expressing plants has a higher permeability, (e.g. solutes are washed out easily from intercellular space) and plants are more sensitive to sudden changes in humidity. Molecules penetrate the cuticle more easily causing a high sensitivity of cutinase-expressing plants to herbicides. The altered surface composition and microclimate are most likely involved in the phenomenon of pollen germinating on leaves of cutinase-expressing plants. The most striking phenotype, however, is the formation of organ fusions early in development due to cross polymerization of pectin-rich cell wall material when organs come in close contact and the cuticle is not properly formed (Figures 1C–1E and 2B to 2D). These organ fusions are very strong so that organs do not separate during further growth, leading to distortions of the growth habit of the plant (Figure 3) (Sieber et al., 2000).
Figure 3.
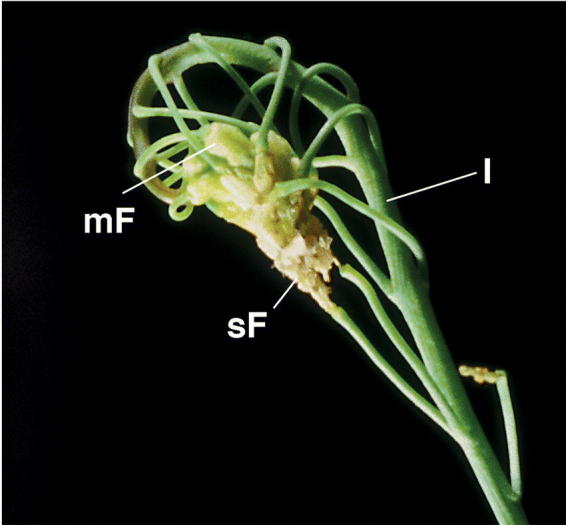
Visual phenotype of a cutinase-expressing transgenic Arabidopsis plant. Inflorescence with multiple organ fusion events between a series of flowers of different age and among flower organs. Inflorescence, I; mature flower, mF; senescing flower, sF. The points of cell contacts are hidden.
Thus, the analysis of cutinase-expressing plants showed that the integrity of the cutin polymer is involved in the function of the cuticle as a barrier to solutes either directly as barrier or indirectly as matrix for the deposition of waxes. Cutin not only has an impact on the interaction of the plant with its environment, but also in preventing organ fusions early during the development. The phenotypes of cutinase-expressing plants may help to identify novel mutants in cutin formation.
- Organ fusion mutants
Several mutants of Arabidopsis and maize suffer severe growth impairments during shoot development because of organ fusion (Lolle and Pruitt, 1999). Since cutinase-expressing Arabidopsis plants show a strikingly similar organ fusion phenotype, an overview is given here of the organ fusion mutants known to date and their characteristics, particularly what is known about their cuticle and the genes involved.
The fiddlehead (fdh) mutant is characterized by postgenital organ fusions in leaves and/or flower organs (Lolle et al., 1992; 1998; Lolle and Pruitt, 1999). In this mutant, organ fusion coincides with pollen germination on leaves and chlorophyll leaching as displayed in cutinase-expressing plants. In contrast to cutinase-expressing plants, an electron-dense cuticle is clearly visible at the points of organ fusion. However, a compositional analysis of the cell wall-bound lipids identified alterations in the fatty acid distribution (Lolle and Cheung, 1993; Lolle et al., 1997; 1998). Lately, it has also been found that Columbia plants carrying the fdh mutation have a significantly reduced number of trichomes which suggests a role of the fdh gene in trichome initiation (Yephremov et al., 1999). Whether this effect is a primary effect of the mutation or an indirect effect of possible modifications of the cuticle is not known. The cloning of the fdh gene has been reported by two independent groups (Yephremov et al., 1999; Pruitt et al., 2000). The FDH gene is highly expressed specifically in the epidermis of shoots of growing tissues and this location is, therefore, compatible with the idea that the FDH gene is involved in the proper formation of the cuticle. The FDH protein shows a significant homology to condensing enzymes, particularly those of the FATTY ACID ELONGATION (fae) family (Yephremov et al., 1999; Pruitt et al., 2000). Fatty acid elongation is known to be important for the formation of waxes rather than cutin. However, FDH may also be involved in the formation of an epidermis-specific fatty acid-derived signaling molecule still to be discovered and may not be directly involved in cuticle formation.
The Arabidopsis mutant lacerata (lcr) also displays strong organ fusions that form during early growth stages predominantly between leaves (Wellesen et al., 2001). The pleiotropic phenotype of the lcr mutant includes leaf deformations, pollen germination on the surface of leaves, stunted growth and reduced apical dominance. The cuticle is absent in the fusion zones in young organs, while mature organs have not been analyzed. The LCR gene encodes a cytochrome P450-dependent monooxygenase that has a high enzymatic activity with saturated and unsaturated fatty acids C14-C18 fatty acids when expressed in a yeast strain overexpressing a plant cytochrome P450 reductase. Thus, cuticle formation could well be disrupted in this mutant due to a block in the biosynthesis of cutin monomers. However, the direct involvement of LCR in cutin biosynthesis has not been shown.
Interestingly, only a few of the many Arabidopsis mutants affected in the wax layer (approx. 10%) show also organ fusions and an altered leaf morphology, such as wax1, cer10 and cer13 (for eceriferum) (Jenks et al., 1996; Yephremov et al., 1999). However, the exact block in the pathway has not been determined. It may also be possible that these mutants are not defective in a function specific for wax deposition but for both wax and cutin formation. Similarly, the adherent mutant (ad1) of maize shows leaf fusions at the same time as altered leaf reflectance and reduced wax load (Sinha and Lynch, 1998). In the ad1 mutant, the fusion zones have been shown to consist of a complex polysaccharide mixture (Sinha and Lynch, 1998).
Crinkly4 (cr4) is an organ fusion mutant of maize that is defective in a receptor kinase necessary for shoot development. Although CR4 controls the development of internal as well as epidermal tissues, the epidermal layer seems to be more sensitive to perturbations in CR4 signaling than other tissues (Becraft et al., 1996; Jin et al., 2000). Epidermal tissues show irregularities in shape, cell-wall thickness and structure, cuticle formation as well as vesicle trafficking. The cuticle may be particularly thick in some areas of the epidermis of the plant and missing in other areas. Organ fusions occur independently of the presence or the absence of the cuticle. The role of CR4 signaling on cutin and cuticle formation and organ fusion is, thus, not obvious. The corresponding gene/protein of Arabidopsis has not been characterized.
In addition to the mutants mentioned above, seven other organ fusion mutants have been identified in Arabidopsis (Lolle et al., 1998). These mutants show different phenotypes with respect to the strength of the fusion and the organ type involved, the fertility of the plants and the potential of pollen germination on their epidermal surfaces. The characterization of these mutants and their comparison to other organ fusion mutants of Arabidopsis resulted in the notion that these phenotypes are related biological processes but may also be independently inherited. The structure or components of the cuticle have not been characterized in these mutants.
SUBERIN, A TISSUE-SEALING POLYMER
Suberin is an extracellular matrix polymer that is deposited together with suberin-associated waxes at distinct locations during plant growth that is species-specific. Its typical function is to seal off the entire plant or one of its tissues against loss of water and solutes, but also to contribute to the strength of the cell wall. Suberin is constitutively present in the secondary growth periderm of aerial tissues and in several underground tissues, e.g. epidermis, hypodermis, peridermis and the Casparian strips of the root endodermis. It may be deposited in bundle sheets, the chalazea and absicission zone during seed development, and in secretory organs as well as fibers (Kolattukudy 1981). Suberin is also deposited in response to wounding and pathogen attack as a barrier against water and solute loss and as protection against opportunistic pathogen invasion. Despite functional, structural, and chemical similarities of suberin to cutin, suberin is characterized by a different composition and a specific location inside of the primary cell wall close to the plasma membrane. The latter feature is diagnostic for suberin.
The ultrastructure of suberin
The ultrastructure of suberin is very characteristic having an alteration of lamellae of electron-opaque and electron-translucent materials when viewed perpendicularly to the orientation of the suberin layer in TEM. Whereas the electron-translucent lamellae have a rather consistent thickness, the thickness of the electron-opaque lamellae may be very varible (Ulrich Ryser, unpublished results). An explanation of the thickness of the suberin lamellae has been sought by growing cotton fibers in the presence of a specific fatty acid elongase inhibitor. However, the changes of the structure and composition of the suberin were too complex for a conclusive interpretation (Schmutz et al., 1996). Thus, the chemical basis of the lamellation is still under debate (Bernards and Lewis, 1998).
The structure of the suberin layer has been analyzed in Arabidopsis in the oldest part of the roots of soil-grown, seed-setting Arabidopsis. Suberization can be observed at the periderm during secondary growth and at the endodermis (Figure 4, Ulrich Ryser, unpublished results).
Figure 4.
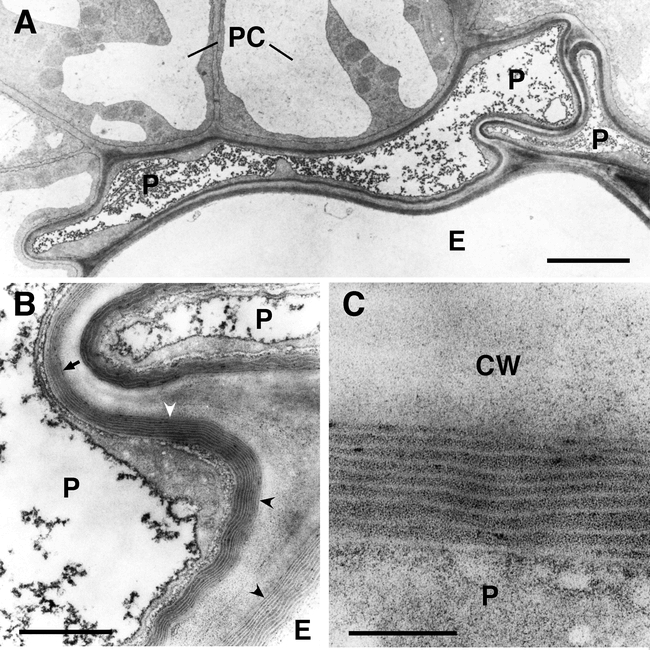
Ultrastructure of suberized roots tissues of wild type Arabidopsis plants at the beginning of the secondary thickening of the root. (A) Overview of suberized endodermal and peridermal cells in the root. The suberin deposition is visible as electron-opaque layer inside of the primary cell wall. The fully suberized peridermal cell layer typically collapses during the dehydration and embedding procedures necessary for TEM because of the low permeability of the suberized cell walls. Bar: 2.5 micrometers. (B) Enlargement of (A). Fine structure of suberin. The structure of the lamellae with an alternation of electron-opaque and electron-translucent layers of suberin is clearly visible when the specimen is cut perpendicularly to the suberin layers (concave arrowheads). However, the lamellate structure of suberin is barely visible when the specimen in not cut perpendicularly to the suberin layers (arrow). Bar: 500 nm. (C) Enlargement of (B). The thickness of the electron-opaque and electron-translucent layers of the suberin is very regular and characteristic for the tissue sample. Bar: 100 nm. P, peridermal cell; E, endodermal cell; PC, pericycle cell; CW, cell wall.
Analysis of the composition of suberin
The analysis of the composition of suberin is similar to this one of cutin. However, the analysis of suberin of most tissues is hampered by the fact that suberin cannot be isolated in a pure form and is always contaminated with some primary cell wall. Pure suberin could only be isolated from the bark of cork oak by a procedure combining different solvent extractions with several enzymatic digestions (Rocha et al., 2001).
For a long time, suberin has been thought to be solely a heteropolymer of aliphatic and phenolic compounds that are linked by ester bonds (Kolattukudy, 1981). Chemical depolymerization of suberin with similar methods as used for cutin (see above) yields significant amounts (roughly a third) of monomeric hydroxycinnamic acid, such as ferulic acid, cinnamic acid, p-coumaric acid or caffeic acid, in addition to aliphatic compounds. The aliphatic portion of the polymer consists mainly of ω-hydroxy fatty acids (C16-C28) and fatty α,ω-diacids (C16-C26), of which the latter are taken as diagnostic for suberin. Minor quantities of very-long chain fatty acids and alcohols (C18-C30) may also be present. The first tentative model of suberin proposed that the aliphatic constituents of suberin form a polyester to which the phenolic compounds that are cross-linked in a lignin-like manner are bound (Kolattukudy, 1981).
Recently the analysis of water soluble compounds of the depolymerization reaction confirmed earlier reports of glycerol being a principal monomer (20%) of suberin in oak, cotton, and potato (Moire et al., 1999; Graça and Pereira, 2000a, b). Partial methanolysis with calcium oxide as catalyst has identified that glycerol may be present as monoacylglycerol esters of alkanoic acids, α, ω-diacids, and ferulic acid (Graça and Pereira, 2000b), as well as diglycerol ester being linked to a α, ω-diacid at both ends (Graça and Pereira, 2000c). Thus, the hypothesis has been proposed that glycerol and α, ω-diacids may form the backbone of the suberin polymer and that thus suberin is a poly-(acylglycerol)-polyester (Graça and Pereira, 2000c). Glycerol may also cross-link the aromatic and aliphatic suberin components, corresponding to the electron-opaque and electron-translucent suberin lamellae to a three-dimensional network, while aliphatic and aromatic suberin monomers may only form a linear polymer on their own (Moire et al., 1999). Glycerol and glycerol-fatty acid esters are not only components of the suberin polymer but also of suberin-associated waxes in suberized cotton fibers (Schmutz et al., 1993; 1994).
Additional studies of the aromatic part of suberin with techniques used for the analysis of lignin, such as alkaline nitrobenzene oxidation, non-destructive solid-state NMR, and thioacidolysis, showed that the suberin of potato is a hydroxycinnamic acid-derived polymer, primarily comprised of ferulic acid and N-feruoyl tyramine. Thus, it is not a lignin-like polymer containing monolignols (Négrel et al., 1996; Bernards and Razem, 2001). However, other researchers reported a significant amount of monolignols in potato periderm investigated by solid-state NMR (Yan and Stark, 2000). In maize, feruoyl tyramine is not detectable in suberized endodermal and hypodermal cell walls of roots indicating that the aromatic part of the suberin might show compositional variations in different plants or/and different tissues (Schreiber et al., 1999). Furthermore, the observation that the phenolic and aliphatic parts occupy different domains in the suberin polymer lead to the hypothesis that suberin is composed of different polymers that are cross-linked to each other and whose aromatic part is also cross-linked to the primary cell wall (Bernards and Lewis, 1998).
The aliphatic portion of the suberin of Arabidopsis has been analyzed by total hydrolysis of roots using methanol with boron trifluoride as catalyst. The hydrolysate contained mainly ω-hydroxyacids from C16 to C24 and the C16 and C18:1 α, ω-fatty diacids. Minor compounds were C18-C24 fatty acids and fatty alcohols (Lukas Schreiber and Christiane Nawrath, unpublished results).
Suberin biosynthesis
The aliphatic monomers of suberin derive from the general fatty acid biosynthetic pathway, namely from palmitic (16:0), stearic (18:0) and oleic (18:1) acid. Fatty acid elongases catalyze the elongation of the carbon chain of stearate to different lengths, as found in wax biosynthesis (Domerque et al., 1998). Root-specific fatty acid elongases have been characterized from maize (Schreiber et al., 2000). The necessary hydroxylation steps are introduced by cytochrome P450-dependent enzymes. The characteristic formation of α, ω-diacids from ω-hydroxyacids is catalyzed by a ω-hydroxy fatty acid dehydrogenase (Agrawal and Kolattukudy, 1978a,b). Recently, a cytochrome P450 that oxidizes fatty acids to the corresponding ω-alcohols and subsequently to the α, ω-diacids was described (LeBouquin et al., 2001). While the major types of enzymes responsible for the synthesis of aliphatic suberin monomers are identified, none of them have been shown to be directly involved in suberin biosynthesis.
The aromatic moiety of suberin is synthesized via the general phenylpropanoid pathway with its key enzyme PAL (Kolattukudy, 1981). In poly (hydroxycinnamic acid)-like suberin, hydroxycinnamic acids may be incorporated as CoA esters by the action of hydroxycinnamoyl: CoA transferases (Bernards and Lewis, 1998). During wound-induced suberization, a hydroxycinnamoyl-CoA:tyramine N-hydroxycinnamoyl transferase (THT) and a hydroxycinnamoyl-CoA:ω-hydroxypalmitic acid O-hydroxycinnamoyl transferase (HHT) have been found in potato containing alkyl ferulyl esters in its suberin (Négrel et al., 1993; 1995; Hohlfeld et al., 1996). HHT enzymes have also been biochemically characterized in other plants (Bolwell et al., 1997; Lofty et al., 1995; 1996).
The aromatic constituents of suberin with a lignin-like monomer composition containing the alcohols derived from hydroxycinnamic acids, such as p-coumaryl alcohol, coniferyl alcohol, or sinapoyl alcohol, are thought to be synthesized as monolignols by a reduction of cinnamic acid involving a cinnamoyl-CoA reductase (CCR) and cinnamoyl alcohol dehydrogenase (CAD) (Sederoff et al., 1999) and/or possibly the recently discovered sinapoyl alcohol dehydrogenase (SAD) (Li et al., 2001).
Glycerol is likely to enter the suberin biosynthetic pathway as phospho-glycerol that might be synthesized from free glycerol by the glycerol-phosphokinase or from dihydroxyacetone phosphate by the glycerol-phosphodehydrogenase (Gurr and James, 1975).
Virtually nothing is known about the cellular site of suberin biosynthesis, the transport of monomers to the cell wall as well as the control of polymerization leading to the highly regular lamellar structure visible in TEM. However, it can be expected that the polymerization occurs in a organized manner, potentially involving so-called “dirigent” proteins that ensure regio-specific radical precursor coupling in lignin biosynthesis (Lewis, 2000; Davin and Lewis, 2000).
The only step characterized is the assembly of the aromatic moiety of suberin through a peroxidase/H2O2-mediated process analogous to lignin formation. Peroxidases that may be associated with suberization have been described from different species (Espelie et al., 1986; Roberts and Kolattukudy, 1989; Ouiroga et al., 2000). The anionic peroxidase from potato that is associated with suberization preferentially cross-links aromatic acids, and not monolignols in accordance with the finding of hydroxycinnamates in the potato wound periderm (Bernards et al., 1999). Since peroxidases need H2O2 for their catalytic reaction, H2O2-generating systems associated with suberization are currently under investigation and found to be an oxidase-like enzyme (Bernards and Razem, 2001).
Suberin mutants
No specific mutants in suberin biosynthesis and deposition have been described by now. Some plants show, however, an unusual suberin deposition. For example, cotton producing green fibers may show ectopic suberization causing their green color; this might indicate that cotton with white fibers is a mutant deficient in fiber suberization (Ryser, 1999).
The scarecrow mutant in Arabidopsis lacking the formation of an endodermis during root development has been shown to form a suberized precursor cell file during the primary root formation (Di Laurentio et al., 1996).
The elongation defective1 (eld1) mutant of Arabidopsis has a pleiotropic phenotype, including impairments in cell elongation, cell division in the root meristem, and vascular tissue differentiation as well as photomorphogenesis in the dark. Edl1 displays ectopic suberization of the twisted vascular bundles in root and hypocotyl tissues (Cheng et al., 2000).
Concluding remarks
The biosynthesis and deposition of cutin and suberin belong to the areas of plant biology about which very little is known in Arabidopsis. Neither a gene nor a mutant related specifically to these processes have been identified. The difficulties in Arabidopsis may be attributed to the fact that only small amounts of material are deposited, making the isolation of specific components challenging. These facts combined with the complex chemical isolation methods required in the analysis make direct biochemical screens for Arabidopsis mutants affected either in the quantity or quality of the cutin/suberin very difficult.
Although the generation of transgenic plants expressing a fungal cutinase led to plants with an altered cuticle and characteristic phenotypes, the information did not yet lead to the identification of mutants in cutin biosynthesis/deposition. Mutant candidates possibly having an altered cutin composition will remain speculative until the analytical difficulties in the determination of the cutin composition/deposition have been solved. Furthermore, a direct screening for specific mutant phenotypes may also be complicated by the redundancy in genes important for cutin biosynthesis, e.g. fatty acid hydroxylases and acyltransferases are large gene families. In addition, Arabidopsis plants might possibly cope with changes in the composition of cutin without displaying strong phenotypic changes or showing an abnormal phenotype only under very specific environmental conditions.
Recently, the quantitative and qualitative determination of the composition of suberin from Arabidopsis roots has become assessable (Christiane Nawrath and Lukas Schreiber, unpublished results). This will provide an opportunity to learn more about the biological function of suberin in Arabidopsis.
The analysis of the biochemical pathways for the synthesis of cutin and suberin monomers in other plants furthered our knowledge of the classes of enzymes involved in these processes. Tools in genomics that are now available in Arabidopsis are likely to lead to the identification of genes/proteins involved in cutin and suberin monomer biosynthesis in the near future. For example, the systematic knockout of genes together with a detailed analysis of the mutants, in particular regarding their cutin and suberin structure/composition may lead to advances in the field. However, unraveling the directed transport processes of cutin/suberin monomers to the site of polymer formation as well as the characterization of the monomer:polymer assembly itself will remain challenges for another decade.
Acknowledgments
I gratefully acknowledge Ulrich Ryser and Lukas Schreiber for contributing micrographs and communicating results prior to publication. I thank Jean-Pierre Métraux, Yves Poirier, Ulrich Ryser and Lukas Schreiber for critical reading of the manuscript.
Footnotes
Citation: Nawrath C. (2002) The Biopolymers Cutin and Suberin. The Arabidopsis Book 1:e0021. doi:10.1199/tab.0021
elocation-id: e0021
Published on: April 4, 2002
Table 1.
Structures of the major cutin monomers. Δ12-unsaturated monomers are also common in the C16-family. X=5, 6, 7, 8; y= 5, 6, 7, 8, x+y=13. (modified after Walton and Kolattukudy, 1972).
REFERENCES
- Agrawal V. P., Kolattukudy P. E. Purification and characterization of a wound-induced ω-hydroxy fatty acid:NADP oxidoreductase from potato tuber disks. Arch. Biochem. Biophys. 1978a;1911(1):452–465. doi: 10.1016/0003-9861(78)90384-3. [DOI] [PubMed] [Google Scholar]
- Agrawal V. P., Kolattukudy P. E. Mechanism of action of a wound-induced ω-hydroxy fatty acid:NADP oxidoreductase isolated from potato tubers (Solanum tuberosum L.). Arch. Biochem. Biophys. 1978b;1911(1):466–478. doi: 10.1016/0003-9861(78)90385-5. [DOI] [PubMed] [Google Scholar]
- Becraft P. W., Stinard P. S., McCarty D. R. CRINKLY4: A TNKR-like receptor kinase involved in epidermal maize differentiation. Science. 1996;2731(1):1406–1409. doi: 10.1126/science.273.5280.1406. [DOI] [PubMed] [Google Scholar]
- Beneviste I., Tijet N., Adas F., Philipps G., Salaun J. P., Durst F. CYP86A1 from Arabidopsis thaliana encodes a cytochrome P450-dependent fatty acid omega-hydroxylase. Biochem. Biophys. Res. Commun. 1998;2431(1):688–693. doi: 10.1006/bbrc.1998.8156. [DOI] [PubMed] [Google Scholar]
- Bernards M. A., Lewis N. G. The macromolecular aromatic domain in suberized tissue: a changing paradigm. Phytochemistry. 1998;471(1):915–933. doi: 10.1016/s0031-9422(98)80052-6. [DOI] [PubMed] [Google Scholar]
- Bernards M. A., Fleming W. D., Llewellyn D. B., Priefer R., Yang X., Sabatino A., Plourde G. L. Biochemical characterization of the suberization-associated anionic peroxidase of potato. Plant Physiol. 1999;1211(1):135–146. doi: 10.1104/pp.121.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards M. A., Razem F. A. The poly (phenolic) domain of potato suberin: a non-lignin cell wall bio-polymer. Phytochemistry. 2001;571(1):1115–1122. doi: 10.1016/s0031-9422(01)00046-2. [DOI] [PubMed] [Google Scholar]
- Blée E., Schuber F. Efficient epoxidation of unsaturated fatty acids by a hydroperoxide-dependent oxygenase. J. Biol. Chem. 1990;2651(1):12887–12894. [PubMed] [Google Scholar]
- Blée E., Schuber F. Occurance of fatty acid epoxide hydrolases in soybean (Glycine max). Biochem J. 1992;2821(1):711–714. doi: 10.1042/bj2820711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blée E., Schuber F. Biosynthesis of cutin monomers: involvement of a lipoxygenase/peroxygenase pathway. Plant J. 1993;41(1):113–123. [Google Scholar]
- Blée E., Schuber F. Stereocontrolled hydrolysis of the linoleic acid monoepoxide regioisomers catalyzed by soybean epoxide hydrolase. Eur. J. Biochem. 1995;2301(1):229–234. doi: 10.1111/j.1432-1033.1995.tb20555.x. [DOI] [PubMed] [Google Scholar]
- Bolwell G. P., Gerrish C., Salaün J-P. Changes in enzymes involved in suberization in elicitor-treated french bean cells. Phytochemistry. 1997;451(1):1351–1357. [Google Scholar]
- Bowen D. J., Walton T. J. Cutin monomer composition and biosynthesis during gibberellic acid-induced stem extension of Pisum sativum var. meteor. Plant Sci. 1988;551(1):115–127. [Google Scholar]
- Cabello-Hurtado F., Batard Y., Salaün J-P., Durst F., Pinot F., Werck-Reichart D. Cloning, expression in yeast, and functional characterization of CYP81B1, a plant cytochrome P450 that catalyzes in-chain hydroxylation of fatty acids. J. Biol. Chem. 1998;2731(1):7260–7267. doi: 10.1074/jbc.273.13.7260. [DOI] [PubMed] [Google Scholar]
- Cheng J. C., Lertpeiriyapong K., Wang S., Sung Z. R. The role of the Arabidopsis ELD1 gene in cell development and photomorphogenesis in darkness. Plant Physiol. 2000;1231(1):509–520. doi: 10.1104/pp.123.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau R., Kolattukudy P. E. Enzymatic biosynthesis of a hydroxy fatty acid polymer, cutin, by a particulate preparation from Vicia faba epidermis. Biochem. Biophys. Res. Commun. 1973;521(1):863–869. doi: 10.1016/0006-291x(73)91017-6. [DOI] [PubMed] [Google Scholar]
- Croteau R., Kolattukudy P. E. Biosynthesis of hydroxy fatty acid polymers. Enzymatic epoxidation of 18-hydroxyoleic acid to 18-hydroxy-cis-9,10-epoxystearic acid by a particular preparation from spinach (Spinacea oleracea). Arch. Biochem. Biophys. 1975;1701(1):61–72. doi: 10.1016/0003-9861(75)90097-1. [DOI] [PubMed] [Google Scholar]
- Daleke D. L., Lyles J. V. Identification and purification of aminophospholipid flippases. Biochim Biochem Acta. 2000;14861(1):108–127. doi: 10.1016/s1388-1981(00)00052-4. [DOI] [PubMed] [Google Scholar]
- Davies K. A., De Lorono I., Foster S. J., Li D., Johnston K., Ashby A. M. Evidence for a role of cutinase in pathogenicity of Pyrenopeziza brassicas. Physiol. Mol. Plant Pathol. 2000;571(1):63–75. [Google Scholar]
- Davin L. B., Lewis N. G. Dirigent proteins and dirigent sites explain the mystery of specifity of radical precursor coupling in lignan and lignin biosynthesis. Plant Physiol. 2000;1231(1):453–461. doi: 10.1104/pp.123.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deising H., Nicholson R. L., Haug M., Howard R. J., Mendgen K. Adhesion pad formation and the involvement of cutinase and esterases in the attachment of uredospores to the host cuticle. Plant Cell. 1992;41(1):1101–1111. doi: 10.1105/tpc.4.9.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman M. B., Podila G. K., Kolattukudy P. E. Insertion of a cutinase gene into a wound pathogen enables it to infect intact host. Nature. 1989;3421(1):446–448. [Google Scholar]
- Di Laurentio L., Wysocka-Diller J., Malamy J. E., Pysh L., Helariutta Y., Freshour G., Hahn M. G., Feldmann K. A., Benfey P. N. The SCARECROW gene regulates an asymetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell. 1996;861(1):423–433. doi: 10.1016/s0092-8674(00)80115-4. [DOI] [PubMed] [Google Scholar]
- Domergue F., Bessoule J-J., Moreau P., Lessire R., Cassagne C. Recent advances in plant fatty acid elongation. 1998;1(1):185–220. In “Plant Lipid biosynthesis” (J.L. Harwood, ed.) Vol. 67, pp. Cambridge University Press, Cambridge. [Google Scholar]
- Eigenbrode S. D. Plant surface waxes and insect behavior. 1996;1(1):201–222. In Plant Cuticles: An Integrated Functional Approach, G. Kerstiens, ed (BIOS Scientific Publishers Limited, Oxford, UK), pp. [Google Scholar]
- Espelie K. E., Franceschi V. R., Kolattukudy P. E. Immunocytochemical localization and time course of appearance of an anionic peroxidase associated with suberization in wound-healing potato tuber tissue. Plant Physiol. 1986;811(1):487–492. doi: 10.1104/pp.81.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth M., Schweizer P., Buchala A., Markstädter C., Riederer M., Kato T., Kauss H. Cutin monomers and surface wax constituents elicit H2O2 in conditioned cucumber hypocotyl segments and enhance the activity of other H2O2 elicitors. Plant Physiol. 1998;1171(1):1373–1380. doi: 10.1104/pp.117.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S. A., Dewey F. M., Gurr S. J. The role of cutinase in germling development and infection by Erysiphe graminis f. sp. hordei. Physiol. Mol. Plant Pathol. 1996;491(1):201–211. [Google Scholar]
- Gilbert R. D., Johnson A. M., Dean R. A. Chemical signals responsible for appressorium formation in rice blast fungus Magnaporte grisea. Physiol. Mol. Plant Pathol. 1996;481(1):335–346. [Google Scholar]
- Graça J., Pereira H. Methanolysis of bark suberins: analysis of glycerol and acid monomers. Phytochem. Anal. 2000a;111(1):45–51. [Google Scholar]
- Graça J., Pereira H. Suberin in potato periderm: Glycerol, long-chain monomers, and glyceryl and feruloyl dimers. J. Agric. Food Chem. 2000b;481(1):5476–5483. doi: 10.1021/jf0006123. [DOI] [PubMed] [Google Scholar]
- Graça J., Pereira H. Diglycerol alkenedioates in suberin: Building units of a poly(acylglycerol) polyester. Biomacromolecules. 2000c;11(1):519–522. doi: 10.1021/bm005556t. [DOI] [PubMed] [Google Scholar]
- Grace J., van Gardingen P. R. Plant cuticles under challenge. 1996;1(1):319–330. In Plant Cuticles: An Integrated Functional Approach, G. Kerstiens, ed (BIOS Scientific Publishers Limited, Oxford, UK), pp. [Google Scholar]
- Gurr M. I., James A. T. “Lipid biochemistry. An introduction”. Science Paperbacks/ED. 1975. Chapman and Hall, London.
- Hamberg M., Hamberg G. Hydroperoxide-dependent epoxidation of unsaturated fatty acids in the broad bean (Vicia faba L.). Arch. Biochem. Biophys. 1990;2831(1):409–416. doi: 10.1016/0003-9861(90)90662-i. [DOI] [PubMed] [Google Scholar]
- Hoffmann-Benning S., Kende H. Cuticle biosynthesis in rapidly growing internodes of deepwater rice. Plant Physiol. 1994;1041(1):719–723. doi: 10.1104/pp.104.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld H., Scheel D., Strack D. Purification of hydroxycinnmoyl-CoA:tyramine hydroxycinnamoyl transferase from cell suspension cultures of Solanum tuberosum L. cv. Datura. Planta. 1996;1991(1):166–168. [Google Scholar]
- Hollenbach B., Schreiber L., Hartung W., Dietz K-J. Cadmium leads to stimulated expression of the lipid transfer protein genes in barley: Implications for the involvement of lipid transfer proteins in wax assembly. Planta. 1997;2031(1):9–19. doi: 10.1007/s00050159. [DOI] [PubMed] [Google Scholar]
- Holloway P. J. Structure and histochemistry of plant cuticular membranes: An overview. 1982;1(1):1–32. In The Plant Cuticle, D.F. Cutler, K.L. Alvin, C.E. Price eds (Academic Press, London) pp. [Google Scholar]
- Jeffree C. E. Structure and ontogeny of plant cuticles. 1996;1(1):33–82. In Plant Cuticles: An Integrated Functional Approach, G. Kerstiens, ed (BIOS Scientific Publishers Limited, Oxford, UK), pp. [Google Scholar]
- Jenks M. A., Rashotte A. M., Tuttle H. A., Feldmann K. A. Mutants in Arabidopsis thaliana altered in epicuticular wax and leaf morphology. Plant Physiol. 1996;1101(1):377–385. doi: 10.1104/pp.110.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P., Guo T., Becraft P. W. The maize CR4 receptor-like kinase mediates a growth factor-like differentiation response. Genesis. 2000;271(1):104–116. doi: 10.1002/1526-968x(200007)27:3<104::aid-gene30>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kader J-C. Lipid-transfer proteins in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;471(1):627–654. doi: 10.1146/annurev.arplant.47.1.627. [DOI] [PubMed] [Google Scholar]
- Kahn R. A., Durst F. Function and evolution of plant cytochrome P450. 2000;1(1):151–189. In: Evolution of metabolic pathways, J.T. Romeo, R. Ibrahim, L. Varin, and V. De Luca, eds, (Recent Advances in Phytochemistry 34, Elsevier Science Ltd), pp. [Google Scholar]
- Kauss H., Fauth M., Merten A., Jeblick W. Cucumber hypocotyls respond to cutin monomers via both an inducible and a constitutive H2O2-generating system. Plant Physiol. 1999;1201(1):1175–1182. doi: 10.1104/pp.120.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstiens G. Plant Cuticles: An Integrated Functional Approach. 1996a. (BIOS Scientific Publishers Limited, Oxford, UK)
- Kerstiens G. Diffusion of water vapour and gases across cuticles and through stomatal pores presumed closed. 1996b;1(1):121–134. In Plant Cuticles: An Integrated Functional Approach, G. Kerstiens, ed (BIOS Scientific Publishers Limited, Oxford, UK), pp. [Google Scholar]
- Kerstiens G. Signalling across the divide: a wider perspective of cuticular structure-function relationships. Trends Plant Sci. 1996c;11(1):125–129. [Google Scholar]
- Kolattukudy P. E. Cutin biosynthesis in Vicia faba leaves. Effects of age. Plant Physiol. 1970;461(1):759–760. doi: 10.1104/pp.46.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy P. E. Structure, biosynthesis, and biodegradation of cutin and suberin. Ann. Rev. Plant. Physiol. 1981;321(1):539–567. [Google Scholar]
- Kolattukudy P. E. Cutinases from fungi and pollen. 1984;1(1):471–504. In Lipases. B. Borgström and H. Brockman, eds (Elsevier, Amsterdam, The Netherlands), pp. [Google Scholar]
- Kolattukudy P. E. Enzymatic penetration of the plant cuticle by fungal pathogens. Annu. Rev. Phytopathol. 1985;231(1):223–250. [Google Scholar]
- Kolattukudy P. E., Rogers L. M., Li D., Hwang C-S., Flaishman M. A. Surface signaling in pathogenesis. Proc. Natl. Acad. Sci. USA. 1995;921(1):4080–4087. doi: 10.1073/pnas.92.10.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolattukudy P. E. Biosynthetic pathways of cutin and waxes, and their sensitivity to environmental stresses. 1996;1(1):83–108. In Plant Cuticles: An Integrated Functional Approach, G. Kerstiens, ed (BIOS Scientific Publishers Limited, Oxford, UK), pp. [Google Scholar]
- Le Bouquin R., Skarbs M., Kahn R., Beneviste I., Salaün J-P., Schreiber L., Durst F., Pinot F. CYP94A5, a new cytochrome P450 from Nicotiana tabacum is able to catalyze the oxidation of fatty acids to the omega-alcohol and to the coresspondingcorresponding diacid. Eur. J. Biochem. 2001;2681(1):3083–3083. doi: 10.1046/j.1432-1327.2001.02207.x. [DOI] [PubMed] [Google Scholar]
- Lewis N. G. A 20th century roller coaster ride: a short account of lignification. Curr. Opin. Plant Biol. 2000;21(1):153–162. doi: 10.1016/S1369-5266(99)80030-2. [DOI] [PubMed] [Google Scholar]
- Li L., Cheng X. F., Leshkevich J., Umezawa T., Harding S. A. The last step of syringyl monolignol biosynthesis in angiosperms is regulated by a novel gene encoding sinapyl alcohol dehydrogenase. Plant Cell. 2001;131(1):1567–1585. doi: 10.1105/TPC.010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofty S., Javelle F., Négrel J. Distribution of hydroxycinnamoyl CoA:ω-hydroxypalmitic acid O-hydroxycinnamoyltransferase in higher plants. Phytochemistry. 1995;401(1):389–391. [Google Scholar]
- Lofty S., Javelle F., Négrel J. Purification and characterization of hydroxycinnamoyl-Coenzyme A:ω-hydroxypalmitic acid O-hydroxycinnamoyl transferase from tobacco (Nicotiana tabacum L.) cell-suspension cultures. Planta. 1996;1991(1):475–480. [Google Scholar]
- Lolle S. J., Cheung A. Y., Sussex I. M. Fiddlehead: An Arabidopsis mutant constitutively expressing an organ fusion program that involves interactions between epidermal cells. Develop. Biol. 1992;1521(1):383–392. doi: 10.1016/0012-1606(92)90145-7. [DOI] [PubMed] [Google Scholar]
- Lolle S. J., Cheung A. Y. Promiscuous germination and growth of wildtype pollen from Arabidopsis and related species on the shoot of the Arabidopsis mutant, fiddlehead. Develop. Biol. 1993;1551(1):250–258. doi: 10.1006/dbio.1993.1022. [DOI] [PubMed] [Google Scholar]
- Lolle S. J., Berlyn G. P., Engstrom E. M., Krolikowski K. A., Reiter W. D., Pruitt R. E. Developmental regulation of cell interactions in the Arabidopsis fiddlehead1 mutant: A role for the epidermal cell wall and cuticle. Develop. Biol. 1997;1891(1):311–321. doi: 10.1006/dbio.1997.8671. [DOI] [PubMed] [Google Scholar]
- Lolle S. J., Hsu W., Pruitt R. E. Genetic analysis of organ fusion in Arabiopsis thaliana. Genetics. 1998;1491(1):607–619. doi: 10.1093/genetics/149.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolle S. J., Pruitt R. E. Epidermal cell interactions: a case for local talk. Trends Plant Sci. 1999;41(1):14–20. doi: 10.1016/s1360-1385(98)01353-3. [DOI] [PubMed] [Google Scholar]
- Mendgen K. Fungal attachment and penetration. 1996;1(1):175–188. In Plant Cuticles: An Integrated Functional Approach, G. Kerstiens, ed (BIOS Scientific Publishers Limited, Oxford, UK), pp. [Google Scholar]
- Moire L., Schmutz A., Buchala A., Yan B., Stark R., Ryser U. Glycerol is a suberin monomer. New experimental evidence for an old hypothesis. Plant Physiol. 1999;1191(1):1137–1146. doi: 10.1104/pp.119.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C., Beetham J. K., Pinot F., Debernard S., Newman J. W., Hammock B. D. Cress and potato soluble epoxide hydrolases: purification, biochemical characterization, and comparison to mammalian enzymes. Arch. Biochem. Biophys. 2000;3781(1):321–332. doi: 10.1006/abbi.2000.1810. [DOI] [PubMed] [Google Scholar]
- Namai T., Kato T., Yamaguchi Y., Hirukawa T. Anti-rice blast activity and resistance induction of C-18 oxygenated fatty acids. Biosci. Biotech. Biochem. 1993;571(1):611–613. doi: 10.1271/bbb.57.283. [DOI] [PubMed] [Google Scholar]
- Négrel J., Javelle F., Paynot M. Wound-induced tyramine hydroxycinnamoyl transferase in potato (Solanum tuberosum) tuber discs. J. Plant Physiol. 1993;1421(1):518–524. [Google Scholar]
- Négrel J., Lofty S., Javelle F. Modulation of the activity of two hydroxycinnamoyl transferases in wound-healing potato tuber discs in response to pectinase or abscisic acid. J. Plant Physiol. 1995;1461(1):318–322. [Google Scholar]
- Négrel J., Pollet B., Lapierre C. Ether-linked ferulic acid amides in natural and wound periderms of potato tuber. Phytochemistry. 1996;431(1):1195–1199. [Google Scholar]
- Pinot F., Salaün J-P., Bosch H., Lesot A., Mioskowski C., Durst F. Omega-hydroxylation of Z9-octadecenoic, Z9,10-epoxistearic and 9, 10-dihydroxystearic acids by microsomal cytochrome P450 systems from Vicia sativa. Biochem. Biophys. Res. Commun. 1992;1841(1):183–193. doi: 10.1016/0006-291x(92)91176-q. [DOI] [PubMed] [Google Scholar]
- Pinot F., Bosch H., Salaün J. P., Durst F., Mioskowski C., Hammock B. D. Epoxide hydrolase activities in the microsomes and the soluble fraction from Vicia sativa seedlings. Plant Physiol. Biochem. 1997;351(1):103–110. [Google Scholar]
- Pinot F., Beneviste I., Salaün J. P., Durst F. Methyl jasmonate induces lauric acid omega-hydroxylase activity and accumulation of CYP94A1 transcripts but does not affect epoxide hydrolase activities in Vicia sativa seedlings. Plant Physiol. 1998;1181(1):1481–1486. doi: 10.1104/pp.118.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt R. E., Vielle-Calzada J-P., Ploense S. E., Grossniklaus U., Lolle S. J. FIDDLEHEAD, a gene required to suppress epidermal cell interactions in Arabidopsis, encodes a putative lipid biosynthetic enzyme. Proc. Natl. Acad. Sci. 2000;971(1):1311–1316. doi: 10.1073/pnas.97.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyee J., Kolattukudy P. E. The gene for the major cuticular wax-associated protein and three homologous genes from broccoli (Brassica oleracea) and their expression patterns. Plant Journal. 1995;71(1):49–59. doi: 10.1046/j.1365-313x.1995.07010049.x. [DOI] [PubMed] [Google Scholar]
- Quiroga M., Guerrero C., Botella M. A., Barcelo A., Amaya I., Medina M. I., Alonso F. J., de Forchetti S. M., Tigier H., Valpuesta V. A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol. 2000;1221(1):1119–1127. doi: 10.1104/pp.122.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina J. J., Heredia A. Plant cutin biosynthesis: the involvement of a new acyltransferase. Trends Plant Sci. 2001;61(1):296. doi: 10.1016/s1360-1385(01)02012-x. [DOI] [PubMed] [Google Scholar]
- Riederer M., Schönherr J. Development of plant cuticles: fine structure and cutin composition of Clivia miniata Reg. leaves. Planta. 1988;1741(1):127–138. doi: 10.1007/BF00394885. [DOI] [PubMed] [Google Scholar]
- Riederer M., Schreiber L. Waxes: The transport barriers of plant cuticles. 1995;1(1):131–156. In waxes: Chemistry, Molecular Biology and Functions, R.J. Hamilton, ed (Dundee, Scotland:Oily Press), pp. [Google Scholar]
- Roberts E., Kolattukudy P. E. Molecular cloning, nucleotide sequence, and abscisic acid induction of a suberization-associated highly anionic peroxidase. Mol. Gen. Genet. 1989;2171(1):233–232. doi: 10.1007/BF02464885. [DOI] [PubMed] [Google Scholar]
- Rocha S. M., Goodfellow B. J., Delgadillo I., Neto C. P., Gil A. M. Enzymatic isolation and structural characterization of polymeric suberin of cork from Quercus suber L. Int. J. Biol. Macromol. 2001;281(1):107–119. doi: 10.1016/s0141-8130(00)00163-x. [DOI] [PubMed] [Google Scholar]
- Rogers L. M., Flaishman M. A., Kolattukudy P. E. Cutinase disruption in Fusarium solani f sp pisi decreases its virulence on pea. Plant Cell. 1994;61(1):935–945. doi: 10.1105/tpc.6.7.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser U. Cotton fiber initiation and histodifferentiation. 1996;1(1):1–46. In: Cotton Fibers: developmental biology, quality improvement, and textile processing, A.S. Basra, ed (The Haworth Press, Inc, Binghampton, NY), pp. [Google Scholar]
- Sieber P., Schorderet M., Ryser U., Buchala A., Kolattukudy P. E., Métraux J-P., Nawrath C. Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organs. Plant Cell. 2000;121(1):721–737. doi: 10.1105/tpc.12.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr J., Baur P. Effects of temperature, surfactants and other adjuvants on rates of uptake of organic compounds. 1996;1(1):135–156. In Plant Cuticles: An Integrated Functional Approach, G. Kerstiens, ed (BIOS Scientific Publishers Limited, Oxford, UK), pp. [Google Scholar]
- Schmutz A., Jenny T., Amrhein N., Ryser U. Caffeic acid and glycerol are constituents of the suberin layers in green cotton fibers. Planta. 1993;1891(1):453–460. doi: 10.1007/BF00194445. [DOI] [PubMed] [Google Scholar]
- Schmutz A., Jenny T., Ryser U. A caffeoyl-fatty-acid glycerol ester from wax associated with green cotton fiber suberin. Phytochemistry. 1994;361(1):1343–1346. [Google Scholar]
- Schmutz A., Buchala A. J., Ryser U. Changing the dimensions of suberin lamellae of green cotton fibers with a specific inhibitor of the endoplasmatic reticulum-associated fatty acid elongases. Plant Physiol. 1996;1101(1):403–411. doi: 10.1104/pp.110.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber L., Skrabs M., Hartmann K., Becker D., Cassagne C., Lessire R. Biochemical and molecular characterization of corn (Zea mays L.) root elongases. Biochem. Soc. Trans. 2000;281(1):647–649. [PubMed] [Google Scholar]
- Schreiber L., Hartmann K., Skrabs M., Zeier J. Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. J. Exp. Botany. 1999;501(1):1267–1280. [Google Scholar]
- Schreiber L., Kirsch T., Riederer M. Diffusion through cuticles: principles and models. 1996;1(1):109–120. In Plant Cuticles: An Integrated Functional Approach, G. Kerstiens, ed (BIOS Scientific Publishers Limited, Oxford, UK), pp. [Google Scholar]
- Schweizer P., Jeanguénat A., Mösinger E., Métraux J-P. Plant protection by free cutin monomers in two cereal pathosystems. 1994;1(1):371–374. In Advances in Molecular Genetics of Plant-Microbe Interactions. M.J. Daniels, J.A. Downie and A.E. Osbourn, eds. (Kluwer Academic Publishers, Dordrecht, The Netherlands), pp. [Google Scholar]
- Schweizer P., Jeanguénat A., Whitacre D., Métraux J-P., Mösinger E. Induction of resistance in barley against Erysiphe graminis f. sp. hordei by free cutin monomers. Physiol. Mol. Plant Pathol. 1996a;491(1):103–120. [Google Scholar]
- Schweizer P., Felix G., Buchala A., Müller C., Métraux J-P. Perception of cutin monomers by plant cells. Plant J. 1996b;101(1):331–341. [Google Scholar]
- Sederoff R. R., MacKay J. J., Ralph J., Hatfield R. D. Unexpected variation in lignin. Curr. Opin. Plant Biol. 1999;21(1):145–152. doi: 10.1016/S1369-5266(99)80029-6. [DOI] [PubMed] [Google Scholar]
- Sinha N., Lynch M. Fused organs in the adherent1 mutant in maize show altered epidermal wall with no perturbations in tissue identities. Planta. 1998;2061(1):184–195. [Google Scholar]
- Stahl D. J., Schäfer W. Cutinase is not required for fungal pathogenicity. Plant Cell. 1992;41(1):621–629. doi: 10.1105/tpc.4.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijet N., Helvig C., Pinot F., Le Bouquin R., Lesot A., Durst F., Salaün J. P., Beneviste I. Functional expression in yeast and characterization of a clofibrate-inducible plant cytochrome P450 (CYP94A1) involved in cutin monomer synthesis. Biochem J. 1998;3321(1):583–589. doi: 10.1042/bj3320583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena J. F., Dominguez E., Stewart D., Heredia A. Characterization and biosynthesis of non-degradable polymers in plant cuticles. Planta. 1999;2081(1):181–187. doi: 10.1007/s004250050548. [DOI] [PubMed] [Google Scholar]
- Walker D. B., Bruck D. K. Incompetence of stem epidermal cells to dedifferentiate and graft. Can. J. Bot. 1985;631(1):2129–2132. [Google Scholar]
- Wang C., Chin C-K., Gianfanga T. Relationship between cutin monomers and tomato resistance to powdery mildew infection. Physiol. Mol. Plant Pathol. 2000;571(1):55–61. [Google Scholar]
- Walton T. J., Kolattukudy P. E. Determination of the structures of cutin monomers by a novel depolymerization procedure and combined gas-chromatography and mass spectrometry. Biochemistry. 1972;111(1):1885–1897. doi: 10.1021/bi00760a025. [DOI] [PubMed] [Google Scholar]
- Wellesen K., Durst F., Pinot F., Beneviste I., Nettesheim K., Wisman E., Steiner-Lange S., Saedler H., Yephremov A. Functional analysis of the LACERATA gene of Arabidopsis provides evidence for different roles of fatty acid omega-hydroxylation in development. Proc. Natl. Acad. Sci. 2001;981(1):9694–9699. doi: 10.1073/pnas.171285998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Stark R. E. Biosynthesis, molecular structure, and domain architecture of potato suberin: A 13C NMR study using isotopically labeled precursors. J. Agric. Food Chem. 2000;481(1):3298–3304. doi: 10.1021/jf000155q. [DOI] [PubMed] [Google Scholar]
- Yephremov A., Wisman E., Huijser P., Huijser C., Wellesen K., Saedler H. Characterization of the FIDDLEHEAD gene of Arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis. Plant Cell. 1999;111(1):2187–2201. doi: 10.1105/tpc.11.11.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]


