Abstract
An exacerbated inflammatory response questions biomaterial biocompatibility, but on the other hand, inflammation has a central role in the regulation of tissue regeneration. Therefore, it may be argued that an ‘ideal’ inflammatory response is crucial to achieve efficient tissue repair/regeneration. Natural killer (NK) cells, being one of the first populations arriving at an injury site, can have an important role in regulating bone repair/regeneration, particularly through interactions with mesenchymal stem/stromal cells (MSCs). Here, we studied how biomaterials designed to incorporate inflammatory signals affected NK cell behaviour and NK cell–MSC interactions. Adsorption of the pro-inflammatory molecule fibrinogen (Fg) to chitosan films led to a 1.5-fold increase in adhesion of peripheral blood human NK cells, without an increase in cytokine secretion. Most importantly, it was found that NK cells are capable of stimulating a threefold increase in human bone marrow MSC invasion, a key event taking place in tissue repair, but did not affect the expression of the differentiation marker alkaline phosphatase (ALP). Of significant importance, this NK cell-mediated MSC recruitment was modulated by Fg adsorption. Designing novel biomaterials leading to rational modulation of the inflammatory response is proposed as an alternative to current bone regeneration strategies.
Keywords: tissue regeneration, inflammation, biomaterials, natural killer cells, mesenchymal stem cells
1. Introduction
The burden associated with the incidence of bone disorders and conditions demands an increased effort to improve current tissue regeneration strategies for bone repair/regeneration. Thus, restorative biomaterials and therapies are being developed, but are still expensive and with limited efficiency. Recent studies have pointed towards a decisive role of inflammation in triggering bone repair/regeneration [1], while at the same time, it is accepted that an exacerbated inflammatory response may lead to rejection of an implant [2]. Thus, it is crucial to understand and regulate the degree of inflammation elicited by biomaterials used for bone repair/regeneration.
NK cells are one of the first cell populations to arrive at an injury site [3]. Their unique capacity to lyse target cells, to secrete immunoregulatory cytokines and to interact with other cells, including multi-potent mesenchymal stem/stromal cells (MSCs), makes them capable of regulating other cell populations during inflammation [4]. MSCs have capacity to differentiate into different lineages, including osteoblasts, chondroblasts, adipocytes, fibroblasts and myoblasts and are immunossupressive [5–7]. A bi-directional interaction between NK cells and MSCs has been reported in the last decade. Freshly isolated NK cells show an increase in interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) secretion upon interaction with bone marrow (BM) stromal cells but low or no cytotoxicity against MSCs [8,9]. Other studies suggested that NK cells are capable of lysing MSCs [8,10–14], but, on the other hand, proliferation, cytokine secretion and the cytotoxic capability of cytokine-activated NK cells are inhibited by MSCs [5,12,13,15–18]. The outcome of NK–MSC interactions has been studied with both allogeneic and autologous donors and it does not depend on major histocompatibility class (MHC) class I matching [5,8,10,12,13]. And since the immunomodulatory capacity of MSCs does not seem to depend on the donor, clinical strategies using allogeneic cells are currently being envisaged for different applications, including treatment of autoimmune diseases and tissue regeneration strategies [19].
The inflammatory response occurring upon injury can regulate both MSC differentiation and homing, which are essential for tissue repair [20]. For example, activated T cells [21,22] and macrophages [23] are capable of promoting MSC osteogenesis. Interestingly, the cytokines TNF-α and IFN-γ, which can be produced by NK cells, are important for intramembranous and endochondral bone repair [24,25] and can stimulate MSC differentiation in osteoblasts [26]. However, there are no reports on whether NK cells affect differentiation of MSCs. MSCs express different chemokine receptors and are chemotatic responsive [27] and NK cells produce chemokines known to be involved in recruitment of other cells [28]. Nevertheless, it remains unknown whether NK cells can stimulate MSC recruitment.
Adsorption of proteins is the first event to take place upon implantation of biomaterials influencing subsequent cellular responses. Fg is a plasma protein whose adsorption to the surface of the implanted biomaterial has been correlated with platelet adhesion and activation [29]. Fg is also important in wound healing and repair, and an intermediate poly(ethylene glycol)/Fg ratio induces extensive bone formation, while correlating with a favourable degree of inflammation [30]. Nearly all NK cells express Mac-1 (also called CR3 or CD11b/CD18), an adhesion molecule that recognizes, among other molecules, Fg [31–33]. Importantly, immobilized Fg readily binds Mac-1 while soluble Fg is a relatively poor ligand [34]. Chitosan (Ch) is a natural biodegradable polysaccharide and its application in tissue regeneration has been proposed, namely for osteoarticular applications [35,36], owing to its enzymatic degradability, similarity of structure to extracellular matrix glycosaminoglycans, versatility of processing without using toxic solvents, and easy functionalization [37–39]. Ch has thus been thoroughly studied, in particular, for protein adsorption [40], recruitment of inflammatory cells [41] and as a scaffold to engineer endochondral bone [42].
Here, we studied how adsorption of Fg to Ch ultrathin films affects NK cell responses and how NK cells affect MSC differentiation and invasion. It was found that Fg leads to a higher adhesion of NK cells, and to an increased capacity to recruit MSCs. This investigation contributes to elucidating the positive role of Fg in accelerating bone formation, indicating that designing biomaterials with ability to selectively adsorb Fg may be an alternative route to currently employed strategies.
2. Material and methods
2.1. Preparation of chitosan/fibrinogen substrates
Ch films were spin-coated on pre-washed glass coverslips prior to Fg adsorption. Firstly, coverslips were submerged in 65 per cent nitric acid (Sigma) overnight and washed with Milli-Q water three times for 5 min followed by three times for 20 min. Coverslips were then dried overnight at 220°C.
Ch solution (0.5% w/v) was prepared by dissolving purified Ch (France-Chitine, degree of N-acetylation (DA) 11–12%, molecular weight (MW) 324 ± 27 × 103 and endotoxin-free) in 0.2 M acetic acid (Sigma) overnight at 4°C under constant stirring. The Ch DA and MW were determined by infrared spectroscopy and size-exclusion chromatography, respectively, as previously described [43]. Ch solution was degasified under vacuum and filtered through 0.2 µm pore size filters. Ch two-dimensional films were prepared in glass coverslips with 5 mm (Thermo) or 13 mm diameter (VWR) by spin coating the Ch solution (20 or 50 µl, respectively) for 2 min at 9000 r.p.m. Films were neutralized with NaOH 0.1 M (Sigma) for 5 min and washed twice, 5 min each time, with ultrapure water (Milli-Q). Films were left drying at 37°C for 24 h, sterilized with 70 per cent ethanol and washed twice with filtered phosphate-buffered saline (PBS; Sigma). Films with a thickness of 23.9 ± 4.1 nm were prepared by this method. The surface thickness was determined by imaging ellipsometry, as previously described for other surfaces [44].
Human Fg (Sigma) was adsorbed to the films by incubating them in 50 µl (or 200 µl) of Fg solutions in PBS for 2 h at room temperature (RT). The Fg concentration used was 100 µg ml−1. Control films, with no Fg adsorption, were incubated with PBS. Finally, films were washed twice with filtered PBS and incubated for 1 h with the appropriate cell culture medium prior to adding the cells.
2.2. Characterization of chitosan/fibrinogen substrates
Adsorption of Fg to Ch films was estimated using Fg labelling with the radioisotope 125I as detailed in the electronic supplementary material.
2.3. Isolation of peripheral blood human natural killer cells, T cells, macrophages and peripheral blood lymphocytes
NK cells, T cells and macrophages were obtained from human buffy coats or whole blood samples (for T cell isolation) from healthy donors, kindly provided by Instituto Português do Sangue (IPS), as detailed in the electronic supplementary material. Briefly, a peripheral blood mononuclear cell (PBMCs) suspension was prepared by density gradient centrifugation and NK cells were purified by negative selection using the EasySep human NK cell enrichment kit (StemCell Technologies), according to the manufacturer's instructions. The percentage of CD56+ CD3− cells was greater than 95 per cent unless otherwise stated. T cells were purified by negative selection using the RosetteSep human T cell enrichment cocktail (StemCell Technologies). The percentage of isolated CD3+/CD56− cells was greater than 95 per cent. Populations of macrophages were differentiated from monocyte-enriched populations obtained from PBMCs by adherence. The purity of monocyte-derived macrophages was assessed by examining cells morphology by microscopy and analysing CD14 and human leukocyte antigen (HLA)-DR expression by flow cytometry, and was found to vary between 40 and 70 per cent.
Peripheral blood lymphocytes (PBLs) were obtained by culturing 30 × 106 PBMCs in 10 ml of RPMI 1640 (Invitrogen) with 10 per cent heat inactivated foetal bovine serum (FBS; Lonza) and 50 U ml−1 penicillin–streptomycin (Invitrogen) for 3 h at 37°C/5 per cent CO2 in 90 mm culture plates, and collecting the non-adherent cells. The population thus obtained consisted on average of 57 per cent CD3+CD56− cells, 13 per cent CD56+CD3+ cells, 15 per cent CD56+CD3− cells, 6 per cent CD19+ cells and 12 per cent putative monocytes.
2.4. Isolation and culture of primary human bone marrow mesenchymal stem cells
MSCs were isolated from human BM by density gradient centrifugation and selection of adherent cells. BM was collected from discarded bone tissues of patients undergoing total hip arthroplasty, less than 50 years old and who did not suffer from known inflammatory diseases. After Lymphoprep gradient density centrifugation at 1100g for 30 min, at 20°C, and with no break, nucleated cells were collected and plated at approximately 180 000 cells cm−2 in MSC growth medium (Dulbecco's modified Eagle's medium (DMEM) with low glucose and with Glutamax plus 10% selected inactivated FBS and 1% penicillin/streptomycin (all from Invitrogen)). Cells were incubated at 37°C/5 per cent CO2, and after 72 h non-adherent cells were removed and new medium was added. The medium was changed twice per week until cells reached approximately 80 per cent confluence. For expansion, cells were detached by treatment with 0.05 per cent trypsin/ethylenediaminetetraacetic acid (EDTA; Invitrogen) and replaced in 150 cm2 tissue culture flasks (BD Falcon).
Isolation of MSCs was confirmed by surface staining of CD105, CD73, CD90, CD45, CD34, CD14, CD19 and HLA-DR and by testing the cells capacity to differentiate in osteoblasts, chondroblasts or adipocytes (electronic supplementary material).
Prior to each experiment, frozen aliquots of MSCs were thawed and cultured in MSC growth medium. Cells were grown and after reaching about 80 per cent confluence were detached by treatment with 0.05 per cent trypsin/EDTA. All essays described were performed with cells in passages 5 to 11.
2.5. Monoclonal antibodies
The following monoclonal antibodies (mAbs) were used in this study: fluorescein isothiocyanate (FITC)-labelled anti-human CD3 (clone MEM-57, used at 2 : 50 µl), phycoerythrin (PE)-labelled anti-human CD45 (clone MEM-28, 2 : 50 µl), FITC-labelled anti-human CD14 (clone MEM-15, 2 : 50 µl), PE-labelled anti-human CD19 (clone LT19, 2 : 50 µl), all from Immunotools; PE-labelled anti-CD56 (clone AF12-7H3, 4 : 40 µl), allophycocyanin-labelled anti-IFN-γ (clone 45-15, 7 : 45 µl), from MiltenyiBiotec; APC-labelled anti-human alkaline phosphatase (ALP; clone B4-78, 3 : 50 µl), from R&D Systems. The isotype controls FITC-labelled IgG2a (clone PPV-04), PE-labelled IgG1 (clone PPV-06), FITC-labelled IgG1 (clone PPV-06) and APC-labelled IgG1 (clone PPV-06), all from Immunotools were used at the corresponding concentrations.
2.6. Adhesion assay
Freshly isolated NK cells were resuspended at 105 cells per 100 µl in NK cell medium. Cells were incubated in different substrates (Ch films and Ch films with adsorbed Fg) in 96-well plates for 1 h, at 37°C/5 per cent CO2. Three replicates for each model surface were used. Wells were carefully rinsed twice with PBS to remove weakly attached cells. Samples were then stained with the Hemacolor kit (Merck). Briefly, cells were fixed with solution 1 for 3 min, washed twice with water and stained with solution 2 for 3 min, followed by 1 min with solution 3. Finally, cells were washed twice with water and were visualized using an inverted microscope (Axiovert, Zeiss). To determine the number of cells bound to each surface, five areas per well were analysed using the module Mark and Find from Axiovision (Zeiss). Mark and Find automatically relocates to different positions. Thus, five points were selected for each well, while maintaining the relative location of each point the same for every well, in such a way that the choice of fields did not depend on the experimenter. One image was then captured for each point and finally cells were counted with ImageJ.
2.7. Examining natural killer cell morphology
Cell morphology in the different substrates was determined by visualizing distribution of F-actin. To this purpose, NK cells were resuspended at 105 per 100 µl in NK cell medium and incubated in different substrates (Ch films and Ch films with adsorbed Fg) in a 24-well plate for 1 h, at 37°C/5 per cent CO2. Cells were carefully washed with PBS, fixed for 15 min at RT with 4 per cent paraformaldehyde and washed. Cells were then permeabilized with PBS/0.1 per cent Triton X-100 for 15 min at RT and washed with PBS three times for 5 min. Cells were incubated in blocking buffer (1% bovine serum albumin (BSA)/PBS) for 60 min at RT to minimize non-specific binding. Then, cells were stained with Alexafluor 488-phalloidin (Invitrogen) at 1 : 40 in 1 per cent BSA/PBS for 1 h in the dark, at RT, and washed with PBS three times, 5 min each wash. Finally, coverslips were mounted with Vectashield with 4′,6-diamidino-2-phenylindole (DAPI) (Vector laboratories). Brightfield and fluorescence images were captured with a ×63 lens, using an inverted laser scanning confocal microscope (Leica SP2 AOBS) or an inverted fluorescence microscope (ZeissAxiovert).
As a measure of cell polarity, the aspect ratio was determined by dividing the length by the width of the cell. The length corresponds to the major axis of the cell and the width to an axis perpendicular to the major one, crossing the cell in the centre of the nucleus.
2.8. Analysing natural killer cell motility
Freshly isolated NK cells were allowed to interact for 1 h with Ch and Ch/Fg films in a 24-well plate. Loosely adherent cells were removed by carefully washing with warm PBS, NK cell medium was added, and the remaining cells were imaged every minute with an inverted microscope (Axiovert, Zeiss), at 37°C/5 per cent CO2, for 2 h.
2.9. Interferon-γ intracellular staining
After isolation, NK cells were resuspended at a concentration of 2 × 106 cells ml−1 in 50 µl of NK cell medium and were incubated in different substrates (Ch films and Ch films with adsorbed Fg) in flat bottom 96-well plates for 2 h at 37°C/5 per cent CO2. Then, MSCs were added at an NK:MSC ratio of 1 : 1 and NK cell medium was added up to 100 µl. As a positive control, NK cells were stimulated with 4 µl of 10 ng ml−1 phorbol-12-myristate-13-acetate (PMA, Sigma) and 1 µl of 500 ng ml−1 ionomycin (Sigma). As a negative control, NK cells were cultured only with medium. Cells were cultured for 5 h at 37°C/5 per cent CO2 in the presence of Brefeldin A (Sigma) at 10 µg ml−1. After incubation, the plate was centrifuged to remove the supernatant and conjugates were separated by incubating for 30 min on ice in PBS/0.5 per cent BSA/5 mM EDTA. Cells were washed with cold PBS and stained for the surface NK cell marker CD56-PE on ice for 30 min. Cells were washed and fixed with 4 per cent paraformaldehyde for 15 min at RT. Cells were washed again and permeabilized with 0.5 per cent BSA/0.2 per cent Tween/PBS on ice for 15 min, and spun. Cells were stained for intracellular IFN-γ by incubating with antibody diluted in 0.5 per cent BSA/0.2 per cent Tween/PBS for 30 min. Finally, cells were washed and 10 000 events were acquired in a flow cytometer (FACSCalibur, Becton Dicksinson) and analysed with FlowJo.
2.10. Evaluation of alkaline phosphatase expression by flow cytometry
MSCs were plated at 15 000 cells/well alone or in co-culture with NK cells (% of CD56+CD3− in this population was higher than 85%) at 75 000 cells/well (5 : 1 NK:MSC ratio) in 24-well plates with basal or osteogenic medium. The medium consisted of DMEM with low glucose and with glutamax supplemented with 10 per cent FBS tested for osteogenesis (PAA) and with penicillin/streptomycin, without (basal medium) or with added osteogenic supplements (100 nM dexametasone, 10 mM β-glycerophosphate (Sigma), 0.05 × 10−3 M ascorbic acid—osteogenic medium). Cells were incubated at 37°C/5 per cent CO2 and medium was changed after 3–4 days. After 7 days of incubation, cells were harvested with 0.05 per cent trypsin/EDTA and washed with cold PBS. Cells suspension was incubated on ice for 30 min with anti-human CD45 and anti-human ALP antibodies diluted in PBS/0.2 per cent BSA/0.01 per cent azide. Appropriate isotype controls were used. Cells were washed three times with cold PBS and fixed in 4 per cent paraformaldehyde. Flow cytometry analysis was performed using a FACSCalibur (Becton Dicksinson) and FlowJo analysis software.
2.11. Cell invasion assay
Studies on invasion of MSCs were performed using a transwell chamber system. Membrane filters with a pore size of 8 µm that had been coated with Matrigel (BD Biosciences) were used. The lower compartments of the invasion chamber were filled with 750 µl DMEM medium, as a negative control, or serum-free DMEM with immune cells at different effector:MSC ratios, or with NK cells on Ch or Ch/Fg films. Then, Matrigel-coated inserts that had been pre-incubated for 1 h with serum-free DMEM were placed in the wells, forming the upper compartment. MSCs (4 × 103 cells per well) in 500 µl serum-free DMEM medium were seeded into the upper compartment. The invasion chambers were incubated for 24 h at 37°C/5 per cent CO2. After incubation, inserts were washed with PBS and cells were fixed in 4 per cent paraformaldehyde for 15 min at RT. Inserts were washed with PBS and kept at 4°C until analysis. Cells on the top surfaces of filters were wiped off with cotton swabs and the membrane was carefully cut and mounted in a slide with Vectashield and DAPI. Cells that had migrated into the lower compartment and attached to the lower surface of the filter were counted in an inverted fluorescence microscope (ZeissAxiovert). Cell nuclei were counted in ten ×200 fields of view for each membrane. The number of migrated cells was estimated by taking into account the area of a field of view and the total area of the membrane.
2.12. Statistical analysis
Statistical differences between samples were determined with the non-parametric tests Mann–Whitney and Wilcoxon, which make no assumptions about the distributions of data: the Mann–Whitney test was used to compare two independent samples; the Wilcoxon test was performed to compare two related samples. Statistical analyses were performed with SPSS v. 17 for Windows.
3. Results
3.1. Characterization of chitosan thin films with adsorbed fibrinogen
Ultrathin films of Ch were used as substrates to test the effect of adsorbed human Fg on human NK cell responses. Ch matrices with 23.9 ± 4.1 nm thickness were prepared by spin-coating and the amount of adsorbed human Fg was quantified by protein radiolabelling with 125I. A surface concentration of 501 ± 63 ng of protein per square centimetre at the bulk concentration of 100 µg ml−1 was obtained. The percentage of Fg retained on the films surface remained high even after 7 days immersion in PBS (approx. 72%).
3.2. Adhesion of natural killer cells was higher on substrates with adsorbed fibrinogen
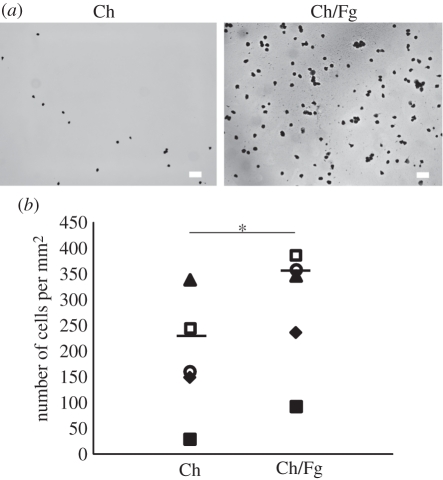
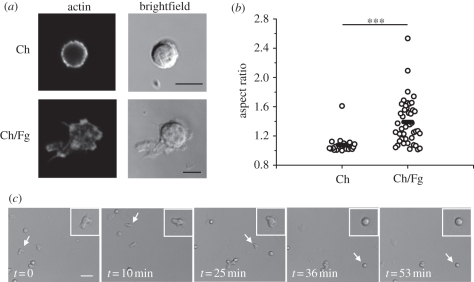
Fg is recognized by Mac-1, a receptor involved in mediating NK cell adhesion. Thus, it was determined how adsorption of Fg to thin films affects adhesion of freshly isolated human NK cells. Importantly, a statistically significant increase was found in the number of NK cells adhering after 1 h of incubation in Ch substrates with adsorbed Fg, as opposed to matrices without the adsorbed protein (figure 1). Also, cells adhering to films with adsorbed Fg showed a different morphology, more spread, than cells adhering to Ch films with no Fg (figure 2a) and in the presence of Fg the aspect ratio of the cells was bigger (figure 2b). In order to clarify whether polarized cells correlated with a migratory phenotype, cells were allowed to interact with Ch/Fg films and time-lapse movies were recorded. It was observed that polarized cells are indeed migratory, changing to a rounder shape when they stopped (figure 2c and electronic supplementary material, movie S1).
Figure 1.
Increased NK cell adhesion to Ch films with adsorbed Fg. NK cells were incubated for 1 h in Ch films without (Ch) and with adsorbed Fg (Ch/Fg), washed, fixed and stained. Cells were imaged in an inverted microscope and counted. (a) Representative images of cells adherent to films. Scale bar, 40 µm. (b) Summary of data obtained in five independent experiments. Each symbol represents the average number of cells in one experiment with three replicas. Bars show the average of the different experiments. Data are statistically significant (*p < 0.05; Wilcoxon test).
Figure 2.
NK cells show a migratory phenotype in the presence of adsorbed Fg. (a) NK cells were incubated for 1 h in Ch films (Ch) or Ch films with adsorbed Fg (Ch/Fg), before staining for actin. Scale bar, 5 µm. (b) The aspect ratio, measured from images obtained after actin staining. Circles represent individual cells, bars represent the mean. Data shown were collected in five different experiments performed with NK cells from different donors. Statistical significance was determined with Mann–Whitney test (***p < 0.001). (c) Time-lapse movies were obtained by imaging freshly isolated NK cells on Ch/Fg films every 1 min for 2 h at 37°C/5%CO2, after 1 h of incubation. Snapshots taken at different time points of a representative movie are shown, with a white arrow pointing to an NK cell that exhibited different behaviours: migratory, from t = 0 to t = 35 min, and stopped from t = 36 min to t = 53 min. Scale bar, 20 µm.
3.3. Adsorbed fibrinogen did not affect natural killer cell interferon-γ production
As both NK cells and MSCs are recruited to an injury site and have been shown to interact with each other, it was tested whether adsorption of Fg to Ch matrices interferes with these interactions. MSCs were isolated from human BM by adherence and were positive for CD105, CD73 and CD90 surface expression, while not expressing CD45, CD34, CD14, CD19 or HLA-DR. Isolated MSCs had the capacity to differentiate in osteoblasts, chondroblasts or adipocytes upon appropriate stimuli, according to the criteria established to define MSCs by the International Society for Cellular Therapy [45] (electronic supplementary material, figure S2).
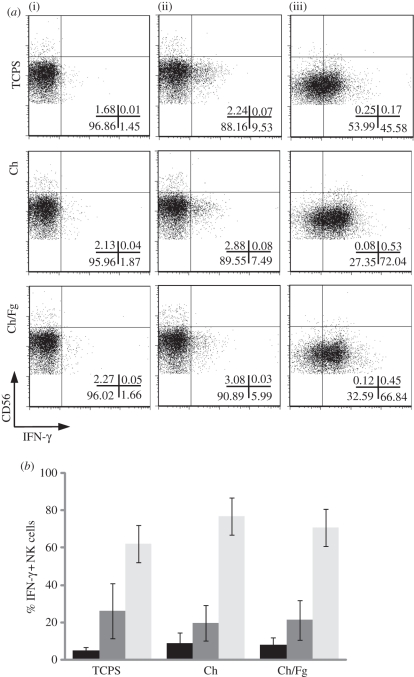
Importantly, freshly isolated NK cells incubated in the presence of MSCs consistently showed an increase in IFN-γ production (figure 3a, top), albeit to different extents with cells from different donors (data not shown). Incubating NK cells in the presence of different Ch matrices did not lead to significant differences in cytokine production (figure 3). Indeed, neither Ch in itself nor adsorbed Fg affected NK cell activation by MSCs. Also, IFN-γ production by NK cells stimulated with PMA and ionomycin or without any stimulus did not change significantly in the presence of adsorbed Fg (figure 3). Thus, Fg leads to augmented NK cell adhesion to matrices but did not interfere with cytokine secretion.
Figure 3.
Production of IFN-γ by NK cells is stimulated by MSCs but is not affected by Fg adsorbed to Ch films. (a) NK cells were incubated alone (i), in the presence of MSCs at a 1 : 1 ratio (ii) or with PMA + ionomycin (iii) in tissue culture polystyrene (TCPS), Ch or Ch/Fg substrates and stained for surface CD56 and intracellular IFN-γ and analysed by flow cytometry. NK cells were gated on the basis of size and CD56 positive staining. Gates were drawn from isotype control stainings. Plots show data from one experiment out of three. Percentage of cells in each quadrant is indicated. (b) The average ± s.e.m. percentage of IFN-γ positive NK cells was plotted for different substrates (n = 3). There were no significant differences between substrates (Wilcoxon test). Black bars, basal; dark grey bars, MSC; light grey bars, PMA.
3.4. Natural killer cells did not affect mesenchymal stem/stromal cell alkaline phosphatase expression
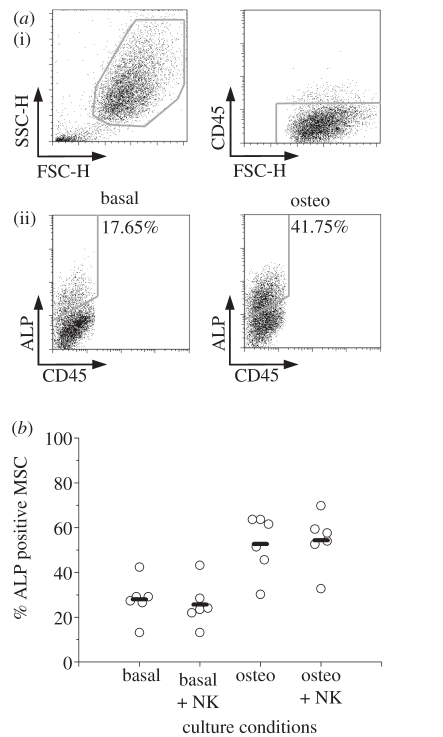
As upon co-incubation with MSCs NK cells secrete IFN-γ, which has been reported to regulate osteogenesis [26], it was tested whether NK cells affect MSC differentiation. For that, MSCs were incubated in the presence or absence of osteogenic factors (osteo and basal conditions) and in co-culture with NK cells. The percentage of MSCs expressing surface ALP, an early differentiation marker whose expression has been correlated with osteogenesis [46], was determined by flow cytometry (figure 4a). As expected, the percentage of ALP positive MSCs increased in osteogenic conditions, as opposed to basal conditions (figure 4a). The percentage of ALP positive MSCs correlated well with staining for ALP activity (data not shown). However, no significant effect was found of NK cells on MSC expression of ALP, neither in basal nor in osteogenic conditions (figure 4b), even though PBMCs induced an increase in the percentage of ALP expressing MSCs (data not shown).
Figure 4.
NK cells do not affect ALP expression by MSCs. MSCs were cultured in the absence or presence of osteogenic stimuli (basal or osteo) and in the absence or presence of NK cells, at a 5 : 1 NK:MSC ratio for 7 days, and stained for surface ALP and CD45. (a) (i) Dot plots show how MSCs were distinguished on the basis of size and negative expression of CD45. (ii) Osteogenic conditions lead to an increase in the percentage of MSCs expressing surface ALP. Numbers indicate the percentage of cells within the drawn gates (ALP positive). (b) Summary of data obtained in six independent experiments. Each circle shows the percentage of ALP positive MSCs in one experiment. Bars show the average of different experiments. Differences between basal and basal + NK or between osteo and osteo + NK conditions are not statistically significant (Wilcoxon test).
3.5. Adsorption of fibrinogen leads to increased natural killer cell-mediated mesenchymal stem/stromal cell recruitment
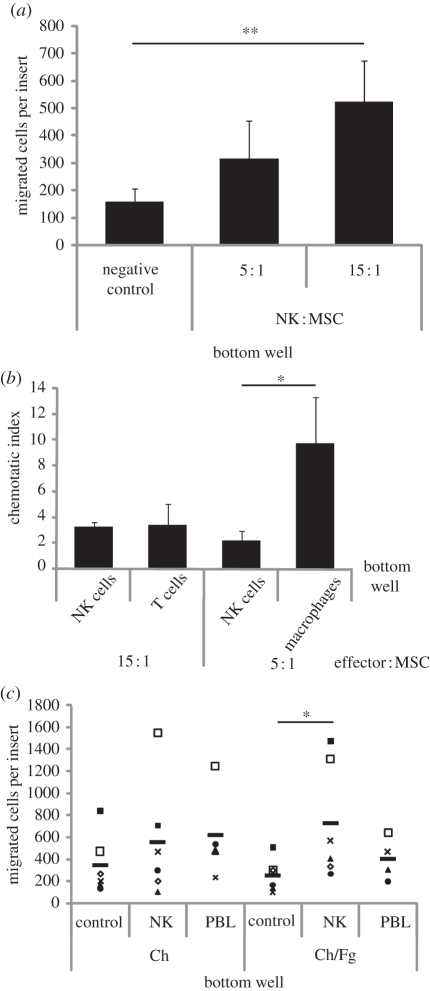
Recruitment of MSCs is an essential step in bone regeneration. MSCs express several chemokine receptors and respond to chemokines, some of which may be produced by NK cells [27,28]. Thus, it was tested whether NK cells affect MSC invasion through Matrigel-coated transwells by incubating MSCs on the top insert of a chamber system without or with NK cells on the bottom. Importantly, it was found that MSC migration increased in the presence of NK cells, in a dose dependent way (figure 5a). As a comparison, NK cells stimulated MSC recruitment to a similar degree of T cells, while macrophages were even stronger inducers of MSC invasion (figure 5b).
Figure 5.
Increased MSC invasion through Matrigel in the presence of NK cells. MSC invasion through filters with 8 µm pores and coated with Matrigel was determined after 24 h incubation. The number of migrated cells per insert was estimated upon counting 10 fields of view per sample (200×). (a) The lower compartment of the invasion chamber was filled with serum-free medium, as a negative control, or with serum-free medium with NK cells at an NK : MSC ratio of 5 : 1 or 15 : 1. Graph shows the mean ± s.e.m. of five to 10 independent experiments. (b) MSCs invasion in the presence of NK cells, T cells or macrophages. Chemotatic index refers to the number of migrated MSCs in the presence of the indicated cell type, divided by the number of migrated MSCs in the negative control. Graph shows the mean ± s.e.m. of experiments performed with at least four independent blood donors. (*p < 0.05; Mann–Whitney test). (c) The lower compartment of the chamber was filled with serum-free medium, as a negative control, with serum-free medium with NK cells, or with PBLs at an effector : MSC ratio of 15 : 1, either seeded on Ch or Ch/Fg films. Each symbol corresponds to data from one experiment. Different symbols correspond to data obtained with cells from different donors. Bars show the average of the different experiments. (*p < 0.05, **p < 0.01; Wilcoxon test).
Invasion of MSCs towards NK cells cultured in different Ch matrices was then analysed. Interestingly, NK cells seeded on Ch matrices did not consistently lead to a higher recruitment than Ch matrices in itself. But remarkably, the same number of NK cells in contact with adsorbed Fg lead to a consistent and significantly higher invasion of MSCs, an effect that was not observed with PBLs (figure 5c).
In summary, it was shown that adsorption of human Fg to Ch films leads to an increased human NK cell adhesion but not to NK cell cytokine secretion. Also, NK cells can become activated by MSCs and on the other hand stimulate MSC invasion, a crucial event in tissue regeneration.
4. Discussion
In order to develop suitable materials for tissue regeneration, it is crucial to understand cell–material interactions and cell–cell interactions. Upon biomaterial implantation, proteins quickly adsorb on surfaces, which will determine subsequent cellular responses. In this study, it was shown that Fg adsorption to Ch films leads to an increased human NK cell adhesion but not to NK cell cytokine secretion. It was also found that NK cells were capable of attracting BM MSCs but did not affect ALP expression. Thus, in the presence of Fg, not only will there be more NK cells adhering to the biomaterial, but also these cells will have a higher capacity to recruit MSCs. We propose that modulating inflammatory responses, and in particular NK cell responses, by Fg adsorption may provide a new way to manipulate and improve current tissue engineering strategies.
Previous studies have found anti-tumour effects of low MW Ch or oligochitosan, which were due to increased NK activity, mainly in intestinal sarcomas [47]. Furthermore, a Ch solution enhanced both humoral and cell-mediated immune responses to subcutaneous vaccination, one of the causes of this increase being the ability of Ch to stimulate NK cells and macrophages [48]. Here, using two-dimensional films, we found that Ch did not lead to NK cell activation on its own, probably owing to differences in MW.
Fg mediates inflammatory responses to biomaterials [49] and wound healing [50]. Fg binds to the integrin Mac-1, which is expressed by different leucocytes, including NK cells. Binding of Mac-1 by Fg alters the function of monocytes, macrophages and neutrophils, affecting cell migration, phagocytosis, production of cytokines and chemokines and other functions [51]. A murine model with a mutated form of Fg, which cannot bind Mac-1, has major defects in the host inflammatory response, providing strong evidence for Fg role as a regulator of the inflammatory response in vivo [51]. In this study, adsorbed Fg stimulated adhesion of NK cells to matrices but not an effector function. A possible explanation for this dichotomy is that, on NK cells, binding to Mac-1 in its resting state can mediate adhesion functions, but activation of the receptor is required for cytotoxicity [33].
It was found that NK cells seeded on Ch matrices with adsorbed Fg showed an asymmetrical, polarized morphology, which is correlated with a migratory phenotype (figure 2c, [52]). These asymmetrical kinapses are adhesive junctions that have been proposed to occur when immune cells are stimulated to migrate and do not receive a ‘stop’ signal, and allow for integration of signals while the cell moves over the surface of target cells [52,53]. Binding of different integrins is known to be involved and to precede cell transmigration through the endothelium. Thus, the different morphology observed with NK cells on ultrathin films of Ch with adsorbed Fg reflects both a capacity of the cells to adhere and to migrate on top of Ch films.
Freshly isolated NK cells were stimulated by MSCs to produce IFN-γ. Data in previous reports are somewhat contradictory, with some reports indicating that NK cells are able to secrete IFN-γ upon incubation with MSCs [8], while others have shown that IFN-γ can be secreted only with an additional stimulus [11]. We have found that NK cells would only become activated if cells were co-incubated with MSCs soon after NK cell isolation (data not shown). Thus, it is likely that differences in the NK cell isolation procedure and NK cell culture will affect NK–MSC interactions, even in the absence of cytokine stimulation. Also, the intensity of NK cell activation varied with different donors, which may reflect variability in receptor expression. CD56dim cells were the main producers of IFN-γ when cells were stimulated with MSCs, while PMA + ionomycin lead to cytokine production by all subsets. This is in agreement with previous studies that have shown that both CD56bright and CD56dim subsets produce IFN-γ upon stimulation with cytokines and PMA + ionomycin [54], while NK cells from the CD56dim subset are cytotoxic and produce IFN-γ upon stimulation with tumour target cells or with antibody-coated cells [55].
It has previously been reported that NK cells preferentially bind to cells in mitosis and that there is an increased NK cell degranulation in the presence of mitotic susceptible target cells [56]. It was also shown that autologous cells in mitosis triggered increased NK cell binding but did not lead to an increased NK cell activation, indicating that signals other than those provided by the cell cycle stage alone are also required to stimulate NK cell activation. Here, it was show that MSCs activate secretion of IFN-γ by NK cells. The percentage of MSCs in G2/Mitosis was approximately 5 per cent, as determined by staining DNA with propidium iodide and analysing by flow cytometry (data not shown). Thus, the effect of MSCs in NK cell cytokine secretion (up to 70% NK cells become activated) is most likely not solely owing to the small percentage of cells in division. However, it would be interesting to determine in future studies whether stimulating MSCs proliferation/division might determine NK cell surveillance and activation, and thus whether novel tissue engineering therapies designed to modulate MSC proliferation might impact on NK cell responses.
Other immune cell populations, including macrophages [23] and activated T cells [21,22], are known to have a role in MSCs osteogenesis, and IFN-γ has been shown to stimulate osteogenesis [26]. Therefore, MSCs were cultured in the presence of NK cells to investigate whether this lead to an increase in expression of ALP, a differentiation marker. Crucially, NK cells did not lead to a difference in expression of ALP, neither in basal conditions nor in the presence of osteogenic stimuli. Thus, in vitro, the presence of freshly isolated human NK cells per se did not lead to differentiation of MSCs, a process that must be regulated by other factors in the microenvironment surrounding the cells.
A crucial event in bone repair/regeneration is MSC recruitment, a process that is still not completely understood. MSCs express several chemokine receptors [27], including some whose expression is modulated by inflammatory mediators [57]. Among the chemokines able to recruit MSCs are chemokines secreted by NK cells [28]. Here, it was shown for the first time that NK cells lead to an increase in MSC invasion through a Matrigel matrix, even though this was to a lesser extent than macrophages. Interestingly, NK cells are distinct from other immune cell populations in that they stimulate MSC recruitment without affecting their differentiation. This capacity of NK cells to recruit MSCs was more evident in the presence of Fg adsorbed to Ch films than in the absence of the protein, correlating with increased NK cell adhesion and polarization in the presence of Fg. The mechanism behind this effect is currently under study and is likely to involve chemokines or other soluble mediators being released by NK cells.
Owing to their capacity to interact with other cell types, either directly or through cytokine secretion, NK cells have been proposed to have regulatory roles in inflammatory responses [4]. Furthermore, NK cells are recruited to the endometrium upon embryo implantation and are involved in uterine tissue remodelling. It has also been suggested that NK cells may contribute to the wound healing process [58]. Moreover, it has been shown that NK cells can trigger differentiation of monocytes into osteoclasts, which are crucial for bone remodelling [59]. However, a role in bone repair/regeneration has been overlooked. Here, it was reported for the first time that NK cells recruit MSCs, a crucial event in bone regeneration. In vivo, both NK cells and MSCs can be found in the BM, as well as at an injury site, where NK cells are one of the first cell populations to arrive. Thus, we propose that NK cells have an important role in regulating fracture healing by recruiting MSCs. Developing novel biomaterials that take into account these NK–MSC interactions may lead to improved strategies for bone repair/regeneration. In particular, we propose that biomaterials incorporating Fg will lead to an increase in the local concentration of NK cells and on the capacity of attracting MSCs to a regeneration site.
5. Conclusions
Here, we analysed how biomaterials designed to incorporate inflammatory signals affect NK cell responses and consequently MSC behaviour. It was found that 1.5 more NK cells adhered to Ch substrates with adsorbed Fg than to substrates without the adsorbed protein. Also, these cells exhibited a migratory phenotype, but were not activated to secrete IFN-γ. And most importantly, it was found that NK cells are capable of stimulating a threefold increase in human BM MSCs invasion, but did not affect the expression of the differentiation marker ALP. This NK cell-mediated MSCs recruitment was modulated by Fg adsorption.
Acknowledgements
Samples of BM were collected by Serviço de Ortopedia e Traumatologia from Hospital de S.João after ethics committee approval and informed consent. We thank Dr Cristina C. Barrias for size-exclusion chromatography, Dr M. Cristina L. Martins for help with the radiolabelling, Dr Trigo Cabral and Dr Nuno Neves (Serviço de Ortopedia e Traumatologia, Hospital de S.João) for BM samples and Instituto Português do Sangue for buffy coats. This work was supported by ‘COMPETE—Programa Operacional Factores de Competitividade’ (FEDER component) and by ‘Fundação para a Ciência e a Tecnologia’ (OE component—project reference PTDC/SAU-BEB/099954/2008). C.R.A. was funded by Fundação para a Ciência e a Tecnologia (SFRH/BPD/48533/2008).
References
- 1.Mountziaris P. M., Mikos A. G. 2008. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part B Rev. 14, 179–186 10.1089/ten.teb.2008.0038 (doi:10.1089/ten.teb.2008.0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson J. M., Rodriguez A., Chang D. T. 2008. Foreign body reaction to biomaterials. Semin. Immunol. 20, 86–100 10.1016/j.smim.2007.11.004 (doi:10.1016/j.smim.2007.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agaiby A. D., Dyson M. 1999. Immuno-inflammatory cell dynamics during cutaneous wound healing. J. Anat. 195, 531–542 10.1046/j.1469-7580.1999.19540531.x (doi:10.1046/j.1469-7580.1999.19540531.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. 2008. Functions of natural killer cells. Nat. Immunol. 9, 503–510 10.1038/ni1582 (doi:10.1038/ni1582) [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal S., Pittenger M. F. 2005. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822 10.1182/blood-2004-04-1559 (doi:10.1182/blood-2004-04-1559) [DOI] [PubMed] [Google Scholar]
- 6.Caplan A. I. 2007. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 213, 341–347 10.1002/jcp.21200 (doi:10.1002/jcp.21200) [DOI] [PubMed] [Google Scholar]
- 7.Pittenger M. F., et al. 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 10.1126/science.284.5411.143 (doi:10.1126/science.284.5411.143) [DOI] [PubMed] [Google Scholar]
- 8.Poggi A., Prevosto C., Massaro A. M., Negrini S., Urbani S., Pierri I., Saccardi R., Gobbi M., Zocchi M. R. 2005. Interaction between human NK cells and bone marrow stromal cells induces NK cell triggering: role of NKp30 and NKG2D receptors. J. Immunol. 175, 6352–6360 10.175/10/6352[pii] (doi:10.175/10/6352[pii]) [DOI] [PubMed] [Google Scholar]
- 9.Rasmusson I., Ringden O., Sundberg B., Le Blanc K. 2003. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation 76, 1208–1213 10.1097/01.TP.0000082540.43730.80 (doi:10.1097/01.TP.0000082540.43730.80) [DOI] [PubMed] [Google Scholar]
- 10.Götherström C., Lundqvist A., Duprez I. R., Childs R., Berg L., le Blanc K. 2010. Fetal and adult multipotent mesenchymal stromal cells are killed by different pathways. Cytotherapy 13, 269–278.(doi:10.3109/14653249.2010.523077) [DOI] [PubMed] [Google Scholar]
- 11.Jewett A., Arasteh A., Tseng H. C., Behel A., Arasteh H., Yang W., Cacalano N. A., Paranjpe A. 2010. Strategies to rescue mesenchymal stem cells (MSCs) and dental pulp stem cells (DPSCs) from NK cell mediated cytotoxicity. PLoS ONE 5, e9874. 10.1371/journal.pone.0009874 (doi:10.1371/journal.pone.0009874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sotiropoulou P. A., Perez S. A., Gritzapis A. D., Baxevanis C. N., Papamichail M. 2006. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 24, 74–85 10.1634/stemcells.2004-0359 (doi:10.1634/stemcells.2004-0359) [DOI] [PubMed] [Google Scholar]
- 13.Spaggiari G. M., Capobianco A., Becchetti S., Mingari M. C., Moretta L. 2006. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 107, 1484–1490 10.1182/blood-2005-07-2775 (doi:10.1182/blood-2005-07-2775) [DOI] [PubMed] [Google Scholar]
- 14.Tseng H. C., et al. 2010. Increased lysis of stem cells but not their differentiated cells by natural killer cells; de-differentiation or reprogramming activates NK cells. PLoS ONE 5, e11590. 10.1371/journal.pone.0011590 (doi:10.1371/journal.pone.0011590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krampera M., et al. 2006. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24, 386–398 10.1634/stemcells.2005-0008 (doi:10.1634/stemcells.2005-0008) [DOI] [PubMed] [Google Scholar]
- 16.Pradier A., Passweg J., Villard J., Kindler V. 2010. Human bone marrow stromal cells and skin fibroblasts inhibit natural killer cell proliferation and cytotoxic activity. Cell Transplant. 10.3727/096368910X536545 (doi:10.3727/096368910X536545) [DOI] [PubMed] [Google Scholar]
- 17.Selmani Z., et al. 2008. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 26, 212–222 10.1634/stemcells.2007-0554 (doi:10.1634/stemcells.2007-0554) [DOI] [PubMed] [Google Scholar]
- 18.Spaggiari G. M., Capobianco A., Abdelrazik H., Becchetti F., Mingari M. C., Moretta L. 2008. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 111, 1327–1333 10.1182/blood-2007-02-074997 (doi:10.1182/blood-2007-02-074997) [DOI] [PubMed] [Google Scholar]
- 19.Salem H. K., Thiemermann C. 2010. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells 28, 585–596 10.1002/stem.269 (doi:10.1002/stem.269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimitriou R., Tsiridis E., Giannoudis P. V. 2005. Current concepts of molecular aspects of bone healing. Injury 36, 1392–1404 10.1016/j.injury.2005.07.019 (doi:10.1016/j.injury.2005.07.019) [DOI] [PubMed] [Google Scholar]
- 21.Rifas L. 2006. T-cell cytokine induction of BMP-2 regulates human mesenchymal stromal cell differentiation and mineralization. J. Cell. Biochem. 98, 706–714 10.1002/jcb.20933 (doi:10.1002/jcb.20933) [DOI] [PubMed] [Google Scholar]
- 22.Rifas L., Arackal S., Weitzmann M. N. 2003. Inflammatory T cells rapidly induce differentiation of human bone marrow stromal cells into mature osteoblasts. J. Cell. Biochem. 88, 650–659 10.1002/jcb.10436 (doi:10.1002/jcb.10436) [DOI] [PubMed] [Google Scholar]
- 23.Champagne C. M., Takebe J., Offenbacher S., Cooper L. F. 2002. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone 30, 26–31 10.1016/S8756-3282(01)00638-X (doi:10.1016/S8756-3282(01)00638-X) [DOI] [PubMed] [Google Scholar]
- 24.Gerstenfeld L. C., Cho T. J., Kon T., Aizawa T., Cruceta J., Graves B. D., Einhorn T. A. 2001. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs 169, 285–294 10.1159/000047893 (doi:10.1159/000047893) [DOI] [PubMed] [Google Scholar]
- 25.Gerstenfeld L. C., Cho T. J., Kon T., Aizawa T., Tsay A., Fitch J., Barnes G. L., Graves D. T., Einhorn T. A. 2003. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-α in endochondral cartilage resorption. J. Bone Miner. Res. 18, 1584–1592 10.1359/jbmr.2003.18.9.1584 (doi:10.1359/jbmr.2003.18.9.1584) [DOI] [PubMed] [Google Scholar]
- 26.Duque G., Huang D. C., Macoritto M., Rivas D., Yang X. F., Ste-Marie L. G., Kremer R. 2009. Autocrine regulation of interferon gamma in mesenchymal stem cells plays a role in early osteoblastogenesis. Stem Cells 27, 550–558 10.1634/stemcells.2008-0886 (doi:10.1634/stemcells.2008-0886) [DOI] [PubMed] [Google Scholar]
- 27.Honczarenko M., Le Y., Swierkowski M., Ghiran I., Glodek A. M., Silberstein L. E. 2006. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 24, 1030–1041 10.1634/stemcells.2005-0319 (doi:10.1634/stemcells.2005-0319) [DOI] [PubMed] [Google Scholar]
- 28.Robertson M. J. 2002. Role of chemokines in the biology of natural killer cells. J. Leukoc Biol. 71, 173–183 [PubMed] [Google Scholar]
- 29.Rodrigues S. N., Gonçalves I. C., Martins M. C. L., Barbosa M. A., Ratner B. D. 2006. Fibrinogen adsorption, platelet adhesion and activation on mixed hydroxyl-/methyl-terminated self-assembled monolayers. Biomaterials 27, 5357–5367 10.1016/j.biomaterials.2006.06.010 (doi:10.1016/j.biomaterials.2006.06.010) [DOI] [PubMed] [Google Scholar]
- 30.Peled E., Boss J., Bejar J., Zinman C., Seliktar D. 2007. A novel poly(ethylene glycol)-fibrinogen hydrogel for tibial segmental defect repair in a rat model. J. Biomed. Mater. Res. 80, 874–884 10.1002/jbm.a.30928 (doi:10.1002/jbm.a.30928) [DOI] [PubMed] [Google Scholar]
- 31.Bryceson Y. T., March M. E., Ljunggren H. G., Long E. O. 2006. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 214, 73–91 10.1111/j.1600-065X.2006.00457.x (doi:10.1111/j.1600-065X.2006.00457.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muto S., Vetvicka V., Ross G. D. 1993. CR3 (CD11b/CD18) expressed by cytotoxic T cells and natural killer cells is upregulated in a manner similar to neutrophil CR3 following stimulation with various activating agents. J. Clin. Immunol. 13, 175–184 10.1007/BF00919970 (doi:10.1007/BF00919970) [DOI] [PubMed] [Google Scholar]
- 33.Ross G. D., Vetvicka V. 1993. CR3 (CD11b, CD18): a phagocyte and NK cell membrane receptor with multiple ligand specificities and functions. Clin. Exp. Immunol. 92, 181–184 10.1111/j.1365-2249.1993.tb03377.x (doi:10.1111/j.1365-2249.1993.tb03377.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lishko V. K., Kudryk B., Yakubenko V. P., Yee V. C., Ugarova T. P. 2002. Regulated unmasking of the cryptic binding site for integrin alpha M beta 2 in the gamma C-domain of fibrinogen. Biochemistry 41, 12 942–12 951 10.1021/bi026324c (doi:10.1021/bi026324c) [DOI] [PubMed] [Google Scholar]
- 35.Mwale F., Iordanova M., Demers C. N., Steffen T., Roughley P., Antoniou J. 2005. Biological evaluation of chitosan salts cross-linked to genipin as a cell scaffold for disk tissue engineering. Tissue Eng. 11, 130–140 10.1089/ten.2005.11.130 (doi:10.1089/ten.2005.11.130) [DOI] [PubMed] [Google Scholar]
- 36.Prabaharan M., Jayakumar R. 2009. Chitosan-graft-beta-cyclodextrin scaffolds with controlled drug release capability for tissue engineering applications. Int. J. Biol. Macromol. 44, 320–325 10.1016/j.ijbiomac.2009.01.005 (doi:10.1016/j.ijbiomac.2009.01.005) [DOI] [PubMed] [Google Scholar]
- 37.Agnihotri S. A., Mallikarjuna N. N., Aminabhavi T. M. 2004. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control Release 100, 5–28 10.1016/j.jconrel.2004.08.010 (doi:10.1016/j.jconrel.2004.08.010) [DOI] [PubMed] [Google Scholar]
- 38.Barbosa M. A., Granja P. L., Barrias C. C., Amaral I. F. 2005. Polysaccharides as scaffolds for bone regeneration. ITBM-RBM 26, 212–217 10.1016/j.rbmret.2005.04.006 (doi:10.1016/j.rbmret.2005.04.006) [DOI] [Google Scholar]
- 39.Berger J., Reist M., Mayer J. M., Felt O., Gurny R. 2004. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Eur. J. Pharm. Biopharm. 57, 35–52 10.1016/S0939-6411(03)00160-7 (doi:10.1016/S0939-6411(03)00160-7) [DOI] [PubMed] [Google Scholar]
- 40.Amaral I. F., Cordeiro A. L., Sampaio P., Barbosa M. A. 2007. Attachment, spreading and short-term proliferation of human osteoblastic cells cultured on chitosan films with different degrees of acetylation. J. Biomater. Sci. Polym. Ed. 18, 469–485 10.1163/156856207780425068 (doi:10.1163/156856207780425068) [DOI] [PubMed] [Google Scholar]
- 41.Barbosa J. N., Amaral I. F., Aguas A. P., Barbosa M. A. 2010. Evaluation of the effect of the degree of acetylation on the inflammatory response to 3D porous chitosan scaffolds. J. Biomed. Mater. Res. 93A, 20–28 10.1002/jbm.a.32499 (doi:10.1002/jbm.a.32499) [DOI] [PubMed] [Google Scholar]
- 42.Oliveira S. M., Mijares D. Q., Turner G., Amaral I. F., Barbosa M. A., Teixeira C. C. 2009. Engineering endochondral bone: in vivo studies. Tissue Eng. Part A 15, 635–643 10.1089/ten.tea.2008.0052 (doi:10.1089/ten.tea.2008.0052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amaral I. F., Sampaio P., Barbosa M. A. 2006. Three-dimensional culture of human osteoblastic cells in chitosan sponges: the effect of the degree of acetylation. J. Biomed. Mater. Res. 76, 335–346 10.1002/jbm.a.30522 (doi:10.1002/jbm.a.30522) [DOI] [PubMed] [Google Scholar]
- 44.Goncalves R. M., Martins M. C., Almeida-Porada G., Barbosa M. A. 2009. Induction of notch signaling by immobilization of jagged-1 on self-assembled monolayers. Biomaterials 30, 6879–6887 10.1016/j.biomaterials.2009.09.010 (doi:10.1016/j.biomaterials.2009.09.010) [DOI] [PubMed] [Google Scholar]
- 45.Dominici M., et al. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 10.1080/14653240600855905 (doi:10.1080/14653240600855905) [DOI] [PubMed] [Google Scholar]
- 46.Siddappa R., Licht R., van Blitterswijk C., de Boer J. 2007. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J. Orthop. Res. 25, 1029–1041 10.1002/jor.20402 (doi:10.1002/jor.20402) [DOI] [PubMed] [Google Scholar]
- 47.Maeda Y., Kimura Y. 2004. Antitumor effects of various low-molecular-weight chitosans are due to increased natural killer activity of intestinal intraepithelial lymphocytes in sarcoma 180-bearing mice. J. Nutr. 134, 945–950 [DOI] [PubMed] [Google Scholar]
- 48.Zaharoff D. A., Rogers C. J., Hance K. W., Schlom J., Greiner J. W. 2007. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine 25, 2085–2094 10.1016/j.vaccine.2006.11.034 (doi:10.1016/j.vaccine.2006.11.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang L., Eaton J. W. 1993. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. J. Exp. Med. 178, 2147–2156 10.1084/jem.178.6.2147 (doi:10.1084/jem.178.6.2147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drew A. F., Liu H., Davidson J. M., Daugherty C. C., Degen J. L. 2001. Wound-healing defects in mice lacking fibrinogen. Blood 97, 3691–3698 10.1182/blood.V97.12.3691 (doi:10.1182/blood.V97.12.3691) [DOI] [PubMed] [Google Scholar]
- 51.Flick M. J., Du X., Degen J. L. 2004. Fibrin(ogen)-alpha M beta 2 interactions regulate leukocyte function and innate immunity in vivo. Exp. Biol. Med. (Maywood) 229, 1105–1110 10.229/11/1105[pii] (doi:10.229/11/1105[pii]) [DOI] [PubMed] [Google Scholar]
- 52.Culley F. J., et al. 2009. Natural killer cell signal integration balances synapse symmetry and migration. PLoS Biol. 7, e1000159. 10.1371/journal.pbio.1000159 (doi:10.1371/journal.pbio.1000159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dustin M. L. 2007. Cell adhesion molecules and actin cytoskeleton at immune synapses and kinapses. Curr. Opin. Cell Biol. 19, 529–533 10.1016/j.ceb.2007.08.003 (doi:10.1016/j.ceb.2007.08.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacobs R., Hintzen G., Kemper A., Beul K., Kempf S., Behrens G., Sykora K. W., Schmidt R. E. 2001. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur. J. Immunol. 31, 3121–3127 (doi:10.1002/1521-4141(2001010)31:10<3121::AID-IMMU3121>3.0.CO;2-4) [DOI] [PubMed] [Google Scholar]
- 55.Anfossi N., et al. 2006. Human NK cell education by inhibitory receptors for MHC class I. Immunity 25, 331–342 10.1016/j.immuni.2006.06.013 (doi:10.1016/j.immuni.2006.06.013) [DOI] [PubMed] [Google Scholar]
- 56.Nolte-'t Hoen E. N. M., Almeida C. R., Cohen N. R., Nedvetzki S., Yarwood H., Davis D. M. 2006. Increased surveillance of cells in mitosis by human NK cells suggests a novel strategy for limiting tumor growth and viral replication. Blood 109, 670–673 10.1182/blood-2006-07-036509 (doi:10.1182/blood-2006-07-036509) [DOI] [PubMed] [Google Scholar]
- 57.Hemeda H., Jakob M., Ludwig A. K., Giebel B., Lang S., Brandau S. 2010. Interferon-gamma and tumor necrosis factor-alpha differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev. 19, 693–706 [DOI] [PubMed] [Google Scholar]
- 58.Liippo J., Toriseva M., Kähäri V.-M. 2009. Natural killer cell in wound healing. In Natural killer cells—basic science and clinical application (eds Lotze M., Thomson A.), pp. 519–525 New York, NY: Academic Press [Google Scholar]
- 59.Soderstrom K., Stein E., Colmenero P., Purath U., Muller-Ladner U., de Matos C. T., Tarner I. H., Robinson W. H., Engleman E. G. 2010. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc. Natl Acad. Sci. USA 107, 13 028–13 033 10.1073/pnas.1000546107 (doi:10.1073/pnas.1000546107) [DOI] [PMC free article] [PubMed] [Google Scholar]