Abstract
Osseointegration, the direct contact between an implant surface and bone tissue, plays a critical role in interfacial stability and implant success. Analysis of interfacial zones at the micro- and nano-levels is essential to determine the extent of osseointegration. In this paper, a series of state-of-the-art microscopy techniques are used on laser-modified implants retrieved from humans. Partially laser-modified implants were retrieved after two and a half months' healing and processed for light and electron microscopy. Light microscopy showed osseointegration, with bone tissue growing both towards and away from the implant surface. Transmission electron microscopy revealed an intimate contact between mineralized bone and the laser-modified surface, including bone growth into the nano-structured oxide. This novel observation was verified by three-dimensional Z-contrast electron tomography, enabling visualization of an apatite layer, with different crystal direction compared with the apatite in the bone tissue, encompassing the nano-structured oxide. In conclusion, the present study demonstrates the nano-scale osseointegration and bonding between apatite and surface-textured titanium oxide. These observations provide novel data in human specimens on the ultrastructure of the titanium–bone interface.
Keywords: dental implant, laser, focused ion beam, transmission electron microscopy, electron tomography, bone
1. Introduction
Today, dental implants are a routine treatment for anchoring prostheses in edentulous jaws. The osseointegration of these implants with the surrounding bone tissue strongly influences the success and longevity of the implant. Clinically, a wide range of implant surfaces are available with different surface topography and chemistry.
It has been suggested that rougher surfaces, compared with the original turned implant, have a tendency to improve bone formation [1] and increase biomechanical fixation [2]. However, the number of randomized controlled clinical trials comparing different dental implants are few, and no particular implant surface could be concluded to be superior [3]. Further, a limited number of ultrastructural analyses of clinically retrieved implants have been published, leaving a lack of understanding of the bone-bonding process.
A novel partially laser-modified implant combines the best-documented turned implant surface with a roughened surface to increase early bone fixation. Experimental studies in rabbits have shown a 250 per cent increase in the biomechanical retention after eight weeks' healing [4], and more than 170 per cent higher removal torque after six months' healing [5], compared with turned implants. No adverse tissue reaction was detected around the implants and no major difference was found at light optical resolution when evaluating the histology [4,5]. Further, on an ultrastructural level, an intimate contact between mineralized bone tissue and the laser-modified surface was found, where calcium and phosphorus were documented in the nano-structured surface oxide [5].
The aim of this study was to evaluate two partially laser-modified implants retrieved from the human mandible after two and a half months' healing. Light optical and transmission electron microscopy (TEM) resolution analyses were performed to evaluate the interfacial bone-bonding ability on both the micro- and nano-level.
2. Material and methods
2.1. Case report
Six implants (BioHelix; Brånemark Integration AB, Sweden) with a length of 13 mm and a diameter of 3.75 mm were installed in the lower jaw of a 66-year-old male edentulous patient. The bone quality was judged as class II bone with a moderate density [6]. The patient was treated in a two-stage procedure according to the original Brånemark protocol with a shortened healing period of two and a half months. Owing to anatomical and topical reasons, only four implants were used in the supraconstruction, and, to prevent future hygienic problems, two fixtures were removed (from regions 32 and 43) at the time of the second-stage surgery. The fixtures were removed under local anaesthesia with a trephine drill with a diameter of 4.25 mm and sent to Biobank 513 at Sahlgrenska Academy at the University of Gothenburg, Göteborg, Sweden.
2.2. Surface analysis
Implant surface analysis was performed using a Leo Ultra 55 (Leo Electron Microscopy Ltd, UK) scanning electron microscope (SEM) operated at 5 kV.
2.3. Histological evaluation
The implants with surrounding tissue were processed for histology and ground sections were prepared by sawing and grinding [7]. In brief, after fixation in formalin, dehydration in a graded series of ethanol and embedding in plastic resin, a central ground section was prepared by sawing along the long axis of the implant. The final ground sections (15–20 µm in thickness) were stained with toluidine blue prior to histological examination in an optical microscope (Nikon Eclipse E600). Quantitative histomorphometry was performed by measuring the amount of bone in direct contact with the implant and the bone area within the threads. All threads along the entire implant were considered for the quantification.
2.4. Ultrastructural interface analysis
The remaining implant–tissue bloc was polished and coated with palladium prior to focused ion beam (FIB) (FEI Strata DB235 FIB/SEM; FEI Company, The Netherlands) machining. A total of four electron-transparent (approx. 100 nm) samples of the implant–tissue interface were prepared using an in situ lift-out system [8]: two from laser-modified areas and two from turned areas of the implant. The TEM analysis was performed using an FEI Tecnai F30 ST (FEI Company). Bright-field TEM and high-angle annular dark-field scanning TEM (HAADF-STEM) were used for imaging and elemental analysis was performed using energy-dispersive X-ray spectroscopy (EDS).
2.5. Electron tomography
Single-axis Z-contrast electron tomography was performed on one of the samples from the laser-modified surface using an FEI Titan 80–300 (FEI Company). Automated focusing, image shifting and acquisition of HAADF-STEM images over an angular range of 150° were achieved using the Inspect 3D software (FEI Company). A linear tilt scheme was employed, with image acquisition increments of 2° up to angles of ±60°, and 1° for further angles up to ±75°. The three-dimensional reconstructions were computed using a simultaneous iterative reconstruction technique in Inspect3D (FEI Company). Models for three-dimensional visualization were created in Amira Resolve RT FEI (Visage Imaging Inc., USA).
3. Results
3.1. Surface analysis
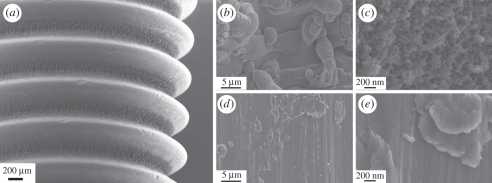
SEM analysis of the native implant showed the dual surface structure, a combination of the turned surface and the laser-modified surface. The turned surface was dominated by a rather smooth morphology and occasional machining debris. In contrast, the laser-modified area showed micro-sized spherical globules, originating from the melting and resolidification of material occurring during laser ablation. At higher magnification, a distinct nano-structure was observed (figure 1).
Figure 1.
Scanning electron micrographs of the implant. (a) Overview image showing the laser-modified valleys and the turned tops. (b,c) Higher magnification of the laser-modified areas. (d,e) Higher magnification of the turned areas.
3.2. Histological evaluation
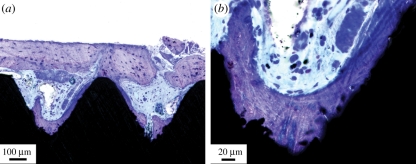
Histological analysis showed bone formation around and in direct contact with the implant surface. The hollow apex was filled with bone tissue, mainly trabecular bone and bone remnants from the surgery, undergoing active modelling and remodelling. Bone tissue had extended into the threads from the surrounding bone tissue, as well as formed in the bottom of the threads, appearing to extend outwards (figure 2). No morphological signs of adverse events, such as the presence of inflammatory infiltrates, were detected.
Figure 2.
Light micrographs of laser-modified threads showing bone formation in the bottom of the thread, as well as growing in from the surrounding bone tissue. (a) The old bone tissue outside the threads. (b) Higher magnification of the thread valley and the laser-modified area.
Quantitative histomorphometry showed that both retrieved partially laser-modified implants had about the same bone–implant contact, 32 and 31 per cent, respectively, while the bone areas within the threads around the implants had 25 and 31 per cent, respectively.
3.3. Ultrastructural interface analysis
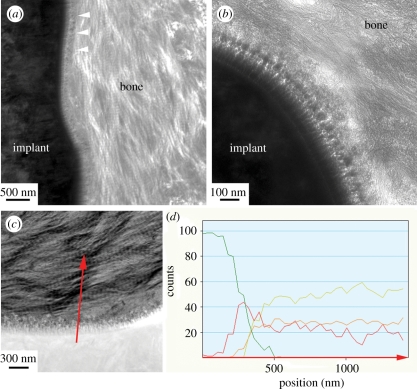
TEM micrographs showed mineralized bone tissue in direct contact with the laser-modified surface. Collagen banding was observed perpendicular and in close proximity to the surface oxide, indicating that the collagen fibres were laid parallel to the implant surface (figure 3a). At higher magnification, an intimate and entangled contact between the mineralized bone tissue and the 100–400 nm thick nano-structured surface oxide was observed. Bone tissue visible inside the oxide (figure 3b) was confirmed by elemental analysis, where the coexistence of calcium, phosphorus, titanium and oxygen was found over a range of 100 nm (figure 3c,d).
Figure 3.
Transmission electron micrographs of the laser-modified surface and bone. Bone tissue was observed in intimate contact with the implant surface. (a) Characteristic collagen banding was observed close to the implant (white arrow heads). (b) Higher magnification of the interface showing the surface oxide and bone tissue. (c) HAADF-STEM micrograph with a corresponding EDS line scan (red arrow). (d) Element distribution where an overlap zone was observed where calcium (yellow) and phosphorus (orange) increased simultaneously as the titanium (green) decreased and oxygen (red line) peaked, indicating osseointegration on the nano-level.
In contrast, the turned surface showed the mineralized bone tissue at a distance from the surface separated by a plastic resin-filled crack, probably an artefact from the embedding process. Elemental analysis did confirm the presence of calcium and phosphorus at the turned titanium surface. These remnants indicated that the mineralized bone had been in contact with the surface prior to the artefact created during sample preparation.
3.4. Electron tomography
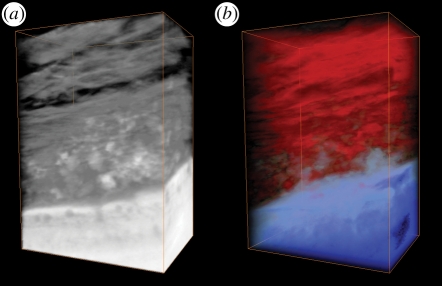
Electron tomography of the interface between the laser-modified surface and bone was performed. Hydroxyapatite (HA) formation in the collagenous matrix appeared aligned with the fibre direction, while a denser HA was visible close to the implant surface, encompassing the nano-structured titanium oxide (figure 4a). By using coloration of the different contrast levels, the three-dimensional nano-structures of the HA-encompassed titanium oxide could be highlighted and clearly differentiated from the bulk (figure 4b).
Figure 4.
Three-dimensional reconstructions of the electron tomograms. From the bottom: bulk titanium, surface oxide, apatite layer and bone tissue. The size of the reconstruction is roughly 700 nm high, 550 nm wide and 200 nm thick. (a) The reconstructed area in greyscale. (b) The same region with histogram contrast changes to highlight interior features.
4. Discussion and conclusion
Histological and ultrastructural characterization of osseointegration is important for a deepened understanding of bone tissue bonding to implant surfaces. An additional value is obtained by analysing osseointegrated implants retrieved from humans.
Consistent with experimental animal models of similar surface treatments, light optical histomorphometry exhibited bone tissue growth directly at the implant's modified surface and significantly higher bone–implant contact in the laser-modified thread valleys compared with turned surfaces [4], indicating the osteoconductive property of the altered surface topography and chemistry. Similar values of bone–implant contact for retrieved screw-shaped dental implants have been reported in the literature for turned implants [9]. Further, it has been shown that surface properties influence cell behaviour in vivo, resulting in a significant upregulation of gene expression for bone remodelling on oxidized implants [10,11].
On the ultrastructural level, the present results in humans are in agreement with previous findings for the same surface treatments in experimental animal models [5,12]. Intimate contact was demonstrated between the laser-modified surface and mineralized bone, while separation and embedding artefacts prevented the preparation of an intact TEM sample of the turned surface. It has been postulated that the difficulties associated with FIB techniques on smoother implant interfaces is probably partly due to volume reduction of the tissue, related to fixation and dehydration, and partly due to minimal biomechanical integration needed to withstand tensions at the immediate interface. However, a small amount of biological tissue is always present at the immediate interface of FIB-produced specimens [5,13], confirming a preparation artefact rather than a lack of osseointegration on the nano-level. For the laser-modified surface, intact samples could be readily prepared by FIB, and showed an intimate contact between the surface oxide and bone tissue using TEM. By the use of site-specific chemical analysis in the TEM, elemental information showed a mineralized implant–bone interface. This observation stands in contrast to earlier TEM studies using other preparation techniques that usually showed an amorphous zone closest to the surface of retrieved oral implants from humans [9]. Other interfaces suggested in the literature involve a direct bone–implant contact at the TEM level [14,15], bonding through a cement line-like layer [16] or a 20–50 nm electron-dense layer at the interface [17].
The coexistence of titanium, oxygen, calcium and phosphorus signals at a distance of about 100 nm from the implant surface indicate a bone ingrowth in the nano-structured surface oxide. However, few techniques are available for further characterization of this zone between the implant and the characteristic banding patterns of the collagen fibres. A recent development in electron tomography enables three-dimensional imaging over the interfacial zone [18]. In the present study, the alignment of the HA crystals in the collagenous matrix was parallel to the collagen fibres, similar to recent observations for HA implants [18]. In addition, the formation of an apatite layer encompassing the nano-structured surface oxide could be clearly visualized by the three-dimensional imaging technique. However, the exact borders between apatite and the nano-irregularities of the surface oxide are difficult to distinguish without the use of elemental analysis in conjunction with electron tomography.
This is the first ultrastructural report on laser-modified retrieved human dental implants studied using both TEM and electron tomography. The intimate contact between bone apatite and implant surface oxide indicates that nano-scale osseointegration occurs between laser-modified surfaces and human bone.
Acknowledgements
Support from the VINNOVA VinnVäxt Programme Biomedical Development in Western Sweden, the Swedish Research Council (grant K2009-52X-09495-22-3), Dr Félix Neuberghs Stiftelse, LUA/ALF grant and BIOMATCELL Vinn Excellence Centre of Biomaterials and Cell Therapy is gratefully acknowledged.
References
- 1.Albrektsson T., Wennerberg A. 2004. Oral implant surfaces. I. Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 17, 536–543 [PubMed] [Google Scholar]
- 2.Wennerberg A., Ektessabi A., Albrektsson T., Johansson C., Andersson B. 1997. A 1-year follow-up of implants of differing surface roughness placed in rabbit bone. Int. J. Oral Maxillofac. Implants 12, 486–494 [PubMed] [Google Scholar]
- 3.Esposito M., Murray-Curtis L., Grusovin M. G., Coulthard P., Worthington H. V. 2007. Interventions for replacing missing teeth: different types of dental implants. Cochrane Database Syst. Rev. 4, CD003815. 10.1002/14651858.CD003815.pub3 (doi:10.1002/14651858.CD003815.pub3) [DOI] [PubMed] [Google Scholar]
- 4.Branemark R., Emanuelsson L., Palmquist A., Thomsen P. 2011. Bone response to laser-induced micro- and nano-size titanium surface features. Nanomedicine 7, 220–227 10.1016/j.nano.2010.10.006 (doi:10.1016/j.nano.2010.10.006) [DOI] [PubMed] [Google Scholar]
- 5.Palmquist A., Emanuelsson L., Branemark R., Thomsen P. 2011. Biomechanical, histological and ultrastructural analyses of laser micro- and nano-structured titanium implant after 6 months in rabbit. J. Biomed. Mater. Res. Part B Appl. Biomater. 97, 289–298 10.1002/jbm.b.31814 (doi:10.1002/jbm.b.31814) [DOI] [PubMed] [Google Scholar]
- 6.Lekholm U., Zarb G. A. 1985. Patient selection and preparation. In Tissue-integrated prostheses: osseointegration in clinical dentistry (eds Brånemark P.-I., Zarb G. A., Albrektsson T.). Chicago, IL: Quintessence Publishing Co. Inc [Google Scholar]
- 7.Donath K., Breuner G. 1982. A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage–Schliff (sawing and grinding) technique. J. Oral Pathol. 11, 318–326 10.1111/j.1600-0714.1982.tb00172.x (doi:10.1111/j.1600-0714.1982.tb00172.x) [DOI] [PubMed] [Google Scholar]
- 8.Jarmar T., Palmquist A., Branemark R., Hermansson L., Engqvist H., Thomsen P. 2008. Technique for preparation and characterization in cross-section of oral titanium implant surfaces using focused ion beam and transmission electron microscopy. J. Biomed. Mater. Res. A 87A, 1003–1009 10.1002/jbm.a.31856 (doi:10.1002/jbm.a.31856) [DOI] [PubMed] [Google Scholar]
- 9.Sennerby L., Ericson L. E., Thomsen P., Lekholm U., Astrand P. 1991. Structure of the bone–titanium interface in retrieved clinical oral implants. Clin. Oral Implants Res. 2, 103–111 10.1034/j.1600-0501.1991.020302.x (doi:10.1034/j.1600-0501.1991.020302.x) [DOI] [PubMed] [Google Scholar]
- 10.Omar O., Svensson S., Zoric N., Lenneras M., Suska F., Wigren S., Hall J., Nannmark U., Thomsen P. 2010. In vivo gene expression in response to anodically oxidized versus machined titanium implants. J. Biomed. Mater. Res. A 92, 1552–1566 [DOI] [PubMed] [Google Scholar]
- 11.Omar O. M., Lenneras M. E., Suska F., Emanuelsson L., Hall J. M., Palmquist A., Thomsen P. 2011. The correlation between gene expression of proinflammatory markers and bone formation during osseointegration with titanium implants. Biomaterials 32, 374–386 10.1016/j.biomaterials.2010.09.011 (doi:10.1016/j.biomaterials.2010.09.011) [DOI] [PubMed] [Google Scholar]
- 12.Palmquist A., Lindberg F., Emanuelsson L., Branemark R., Engqvist H., Thomsen P. 2010. Biomechanical, histological, and ultrastructural analyses of laser micro- and nano-structured titanium alloy implants: a study in rabbit. J. Biomed. Mater. Res. A 92, 1476–1486 [DOI] [PubMed] [Google Scholar]
- 13.Palmquist A., Jarmar T., Emanuelsson L., Branemark R., Engqvist H., Thomsen P. 2008. Forearm bone-anchored amputation prosthesis: a case study on the osseointegration. Acta Orthop. 79, 78–85 10.1080/17453670710014806 (doi:10.1080/17453670710014806) [DOI] [PubMed] [Google Scholar]
- 14.Hemmerle J., Voegel J. C. 1996. Ultrastructural aspects of the intact titanium implant-bone interface from undecalcified ultrathin sections. Biomaterials 17, 1913–1920 10.1016/0142-9612(95)00244-8 (doi:10.1016/0142-9612(95)00244-8) [DOI] [PubMed] [Google Scholar]
- 15.Meyer U., Joos U., Mythili J., Stamm T., Hohoff A., Fillies T., Stratmann U., Wiesmann H. P. 2004. Ultrastructural characterization of the implant/bone interface of immediately loaded dental implants. Biomaterials 25, 1959–1967 10.1016/j.biomaterials.2003.08.070 (doi:10.1016/j.biomaterials.2003.08.070) [DOI] [PubMed] [Google Scholar]
- 16.Davies J. E., Nagai N., Takeshita N., Smith D. C. 1991. Deposition of cement-like matrix on implant materials. In The bone–biomaterial interface (ed. Davies J. E.). Toronto, ON: University of Toronto Press [Google Scholar]
- 17.Steflik D. E., Corpe R. S., Lake F. T., Young T. R., Sisk A. L., Parr G. R., Hanes P. J., Berkery D. J. 1998. Ultrastructural analyses of the attachment (bonding) zone between bone and implanted biomaterials. J. Biomed. Mater. Res. 39, 611–620 (doi:10.1002/(SICI)1097-4636(19980315)39:4<611::AID-JBM16>3.0.CO;2-9) [DOI] [PubMed] [Google Scholar]
- 18.Grandfield K., McNally E. A., Palmquist A., Botton G. A., Thomsen P., Engqvist H. 2010. Visualizing biointerfaces in three dimensions: electron tomography of the bone–hydroxyapatite interface. J. R. Soc. Interface 7, 1497–1501 10.1098/rsif.2010.0213 (doi:10.1098/rsif.2010.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]