Abstract
Microsurgical techniques for the treatment of large peripheral nerve injuries (such as the gold standard autograft) and its main clinically approved alternative—hollow nerve guidance conduits (NGCs)—have a number of limitations that need to be addressed. NGCs, in particular, are limited to treating a relatively short nerve gap (4 cm in length) and are often associated with poor functional recovery. Recent advances in biomaterials and tissue engineering approaches are seeking to overcome the limitations associated with these treatment methods. This review critically discusses the advances in biomaterial-based NGCs, their limitations and where future improvements may be required. Recent developments include the incorporation of topographical guidance features and/or intraluminal structures, which attempt to guide Schwann cell (SC) migration and axonal regrowth towards their distal targets. The use of such strategies requires consideration of the size and distribution of these topographical features, as well as a suitable surface for cell–material interactions. Likewise, cellular and molecular-based therapies are being considered for the creation of a more conductive nerve microenvironment. For example, hurdles associated with the short half-lives and low stability of molecular therapies are being surmounted through the use of controlled delivery systems. Similarly, cells (SCs, stem cells and genetically modified cells) are being delivered with biomaterial matrices in attempts to control their dispersion and to facilitate their incorporation within the host regeneration process. Despite recent advances in peripheral nerve repair, there are a number of key factors that need to be considered in order for these new technologies to reach the clinic.
Keywords: peripheral nerve conduit, topographical guidance, molecular therapy, Schwann cells, stem cells, neurotrophic factors
1. Introduction: peripheral nerve injury and repair
Peripheral nerve injury is a large-scale problem annually affecting more than one million people worldwide. These injuries often result in painful neuropathies owing to reduction in motor function and sensory perception. Peripheral nerve injuries are common in both civil and military environments and are primarily the result of transection injuries or burns, but may also arise from degenerative conditions [1,2]. Over relatively short nerve gaps, spontaneous natural regeneration may occur. However, over larger gaps, microsurgical repair is essential for nerve repair [3–5].
Currently, there are a variety of microsurgical repair methods available, including direct repair, autograft/allograft transplantation and the use of hollow nerve guidance conduit (NGC) repair [3–5]. Direct nerve repair (also known as end-to-end suturing, end–end repair, end-to-end neurorrhaphy or end-to-end coaptation) is the preferred method of treatment for peripheral nerve repair [6]. This method of treatment, however, is limited to the treatment of short nerve defects requiring tension-free suturing of the injury site [6]. For optimal regeneration, the nerve stumps must be correctly aligned and repaired with minimal tissue damage, using the minimal number of sutures. This repair method is limited to nerve gaps shorter than 5 mm [7]. Beyond this relatively short gap, injuries are precluded from primary repair, and alternative tissue engineering strategies are the main option.
1.1. Autograft: the limited gold standard
For patients precluded from direct repair, autograft is the current gold standard and has remained so for the last 50 years [7–9]. These grafts being taken primarily from the sural nerve of the treated patient and have demonstrated a success rate of only 50 per cent on patients treated [6,10]. These grafts are primarily sensory, owing to the unavailability of motor nerves, limiting their potential to repair pure motor nerve deficits (tibial) and mixed nerve injuries (sciatic) and may be one of the primary reasons for the poor functional recovery rates associated with autografts [11,12]. The use of sensory nerves for the treatment of motor nerve deficits causes morphometric mismatches in the native environments, mismatch in axonal size, distribution and alignment [12,13]. Motor neurons are primarily in the range of 3–20 µm, whereas sensory neurons range from 0.2 to 15 µm [14]. If sensory nerve grafts are therefore used to treat a pure motor nerve injury, there is a great potential for size mismatch, potentially limiting regeneration. Secondary to this limitation, the use of autograft has a number of disadvantages, including donor site morbidity, the requirement for a second surgical site, a very limited supply, donor site mismatch and the possibility of painful neuroma formation and scarring [15]. The use of autograft also requires secondary removal of degenerated axons and myelin by the host from the graft itself, increasing the healing time [16]. Similarly in recent studies, it has been shown that sensory and motor neurons have different Schwann cell (SC) modalities and if placed in the incorrect microenvironment, may limit their regenerative ability [17].
Autograft use is currently limited to a critical nerve gap of approximately 5 cm in length and beyond this distance requires the use of allograft [2]. Allograft however requires the use of extensive immune suppression up to 18 months post implantation, and patients become susceptible to opportunistic infections, occasionally resulting in tumour formation [18]. The combinatorial effects of these limitations may be the primary cause for the limited recovery associated with autograft and allograft treatment. In efforts to address the limitations of these nerve grafting techniques, the primary alternative is the use of hollow NGCs.
1.2. The development of nerve guidance conduits
The use of hollow NGCs was originally proposed for use for nerve repair as early as 1881 with the first successful application occurring in 1882, where a hollow bone tube was used to bridge a 30 mm nerve gap in a dog [19]. Today, the use of hollow NGCs is the clinically approved alternative to autograft repair. These conduits have a number of advantages for nerve repair, including limited myofibroblast infiltration, reduced neuroma and scar formation, reduction in collateral sprouting and no associated donor site morbidity, and facilitates the accumulation of a high concentration of neurotrophic factors; ultimately guiding regenerating nerves to their distal targets [20]. However, the use of hollow NGCs is currently limited to a critical nerve gap of approximately 4 cm [21]. These NGCs allow the creation of a controlled microenvironment for the regeneration of nerve fibres and have shown some clinical success [22,23]. Current clinically translated NGCS are primarily made from synthetic materials such as poly-glycolic acid (PGA), polylactide-caprolactone (PLCL), various combinations of the PGA or PLCL, or from animal extracted collagen (table 1) [6,22,23].
Table 1.
Current clinically approved and upcoming nerve guidance conduits.
| product | company | composition | degradation time | max length |
|---|---|---|---|---|
| Neurogen | Integra Neurosciences, Plainsboru, NJ, USA | collagen type I | 4 years | 3 cm |
| NeuraWrap | Integra Neurosciences, Plainsboru, NJ, USA | collagen type I | 4 years | 4 cm |
| Neuromend | Collagen Matrix, Inc., Franklin Lakes, NJ, USA | collagen type I | 4–8 months | 2.5 cm |
| Neuromatrix/Neuroflex | Collagen Matrix, Inc., Franklin Lakes, NJ, USA | collagen type I | 4–8 months | 2.5 cm |
| Neurotube | Synovis Micro Companies Alliance, Birmingham, AL, USA | woven polyglycolic acid (PGA) | 6–12 months | 3 cm |
| Neurolac | Polyganics Inc., The Netherlands | poly(dl-lactic-co-ɛ-caprolactone) (PLCL) | 2–3 years | 3 cm |
| Salubridge/Hydrosheath or Salutunnel | Salumedica LLC, Atlanta, GA, USA | Salubria—polyvinyl alcohol (PVA) hydrogel | non-biodegradable | 6.35 cm |
| Surgisis Nerve Cuff/Axoguard | Cook Biotech Products, West Lafayette, IN, USA | porcine small intestinal submucosa (SIS) matrix | not reported | 4 cm |
| AxonScaff/Cellscaff/ StemScaff (filing for CE and FDA approval) | Axongen, Umeå, Sweden | polyhydroxybuturate (PHB) | not reported | not reported |
Despite some success in nerve repair, these hollow NGCs fail to match the regenerative levels of autograft and show poor functional recovery [24]. Early attempts of improvements for NGCs involved variations in material design and fulfilling a number of criteria for the ideal hollow conduit. These criteria included: (i) limiting scar infiltration, while allowing diffusion of nutrients into the conduit and wastes to exit the conduit; (ii) providing sufficient mechanical properties for structural support; (iii) exhibiting a low immune response; and (iv) biodegradability, to remove the need for secondary surgery and to prevent chronic inflammation and pain caused by nerve compression due to the eventual collapse of implanted NGCs [25]. For the first criterion, adequate nutrient exchange and waste removal in an NGC can be achieved, if the material is permeable with a molecular weight limit of approximately 50 kDa [26–28]. For the remaining criteria, a number of different materials both biological (e.g. collagen, small intestinal submucosa) and synthetic (e.g. polyhydroxybuturate, polyvinyl alcohol, PGA) have been considered throughout the years [3,5,29,30]. These past studies have shown some improvements in nerve regeneration and functional recovery; however, certain key elements are missing with the use of hollow NGCs alone.
1.3. Regeneration within a hollow nerve guidance conduit
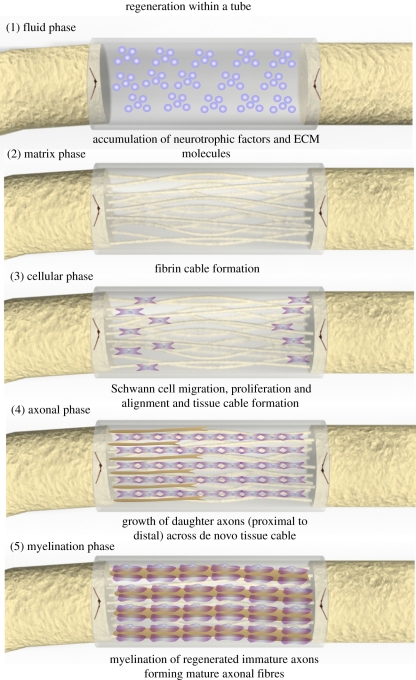
Understanding the natural regenerative process occurring within hollow NGCs is a prerequisite for improved nerve regeneration. Briefly this regenerative process can be divided into five main phases: (i) the fluid phase; (ii) the matrix phase; (iii) the cellular migration phase; (iv) the axonal phase; and (v) the myelination phase (figure 1) [7]. In the initial fluid phase, there is an influx of plasma exudate from both the proximal and distal nerve stumps, which is filled with neurotrophic factors and extracellular matrix (ECM) precursor molecules (e.g. fibrinogen and factor XIII), which peak in concentration after 3–6 h; the time-course mentioned refers to nerve conduit repair occurring within a rat model across a non-critical 10 mm gap [1,7,31]. This initial fluid phase is followed by the formation of an acellular fibrin cable between the proximal and distal stumps, formed from the former influxed ECM precursor molecules [7,31,32]. This fibrin cable usually forms within one week of NGC repair and forms an ECM bridge for the next stage of regeneration. During the second week of repair, SCs from the proximal and distal nerve stumps, as well as some endothelial cells and fibroblasts, migrate along this fibrin cable [1,7]. These SCs subsequently proliferate and align, forming an aligned SC cable, i.e. the glial bands of Büngner. This biological tissue cable provides a trophic and topographical tissue cable for the axonal phase of repair. During this axonal phase of repair, new regenerative axonal sprouts, guided by their individual growth cones, use this biological cable tissue as a guidance mechanism to ultimately reach their distal target.
Figure 1.
Regenerative sequence occurring within a hollow NGC. Figure adapted from Belkas et al. [7]. This regenerative process occurs in five main phases: (1) the fluid phase: plasma exudate fills the conduit resulting in accumulation of neurotrophic factors and ECM molecules; (2) the matrix phase: an acellular fibrin cable forms between the proximal and distal nerve stumps; (3) the cellular phase: Schwann cells, endothelial cells and fibroblasts migrate (from the proximal and distal nerve stumps), align and proliferate along the fibrin cable forming a biological tissue cable; (4) axonal phase: re-growing axons use this biological tissue cable to reach their distal targets; (5) myelination phase: Schwann cells switch to a myelinating phenotype and associated with regenerated axons forming mature myelinated axons.
These regenerating axons reach the aforementioned targets after approximately 2–4 weeks [1,7,31]. It is worth pointing out that during the weeks of the cellular and axonal phase, the fibrin cable, which has a degradation time of approximately two weeks, has most likely degraded, having fulfilled its role for cellular migration [33,34]. Following the axonal phase, SCs switch from the more proliferative ‘regenerative’ phenotype to a presumably more mature ‘myelinating’ phenotype [29]. These mature SCs subsequently wrap around the larger regenerated axons to form the myelin sheath (a mature myelinated axon), resulting in some functional repair of nerve fibres; this usually occurs 6–16 weeks after repair and longer in some larger animal models [35,36]. This regenerative sequence takes place within hollow NGCs up to a critical nerve gap of approximately 4 cm in humans and approximately 1.5 cm in a rat sciatic nerve model, after which regeneration is limited or absent [3,29,37,38]. Functional recovery however remains poor across all nerve gaps [38,39].
2. Guided nerve regeneration: the use of structural guidance cues
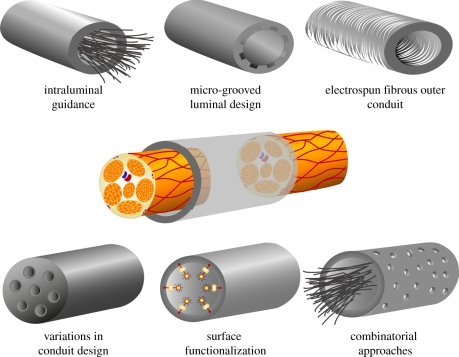
Insufficient levels of regeneration in a hollow NGC, especially across critical nerve gaps, may be attributed to the inadequate formation of ECM components during the initial stages of regeneration, i.e. the formation of the fibrin cable [13,40]. Without the formation of this aligned ECM bridge, there is a limited migration of native SCs into the site of the lesion, from both proximal and distal nerve stumps and consequently a reduction in the formation of glial bands of Büngner, the essential trophic and topographical guidance structure for regenerating axons [1,7,13,32]. In attempts to replace the support and guidance provided by this ECM tissue cable, a number of strategies for nerve repair have focused on the addition or manipulation of structure in NGCs (figure 2).
Figure 2.
Summarized schematic of the structural repair strategies used for improving existing hollow nerve guidance conduits. Repair strategies include the use of intraluminal guidance structures and micro-grooved luminal designs to provide additional structure support and topographical guidance to regenerating axons and migrating Schwann cells. A similar strategy involves using electrospun fibrous conduits with the advantages of high flexibility and porosity, a high surface area-volume and fibres that can be aligned for guided Schwann cell migration and proliferation and axonal growth. Variations in conduit design include the use of multi-channel conduits for control of axonal dispersion, as well as designs which optimize nutrient exchange or introduce external stimuli. These designs may be used alone or in combination, but also require further surface functionalization. These surface modifications can increase cell adhesion, migration, alignment and proliferation.
2.1. Intraluminal guidance structures: replacing or supporting the fibrin cable
One current strategy for nerve repair is the addition of structural intraluminal guidance cues, which may act as a replacement for the unformed or incomplete fibrin cable, or act as an additional anchor for its formation [38,41]. These intraluminal guidance channels act as a platform for SC migration and proliferation and simultaneously can provide additional topographical guidance cues to regenerating axons. Ultimately, the addition of intraluminal channels aim to recapitulate the hierarchical organization and biological function of the native ECM [13].
In early studies by Matsumoto et al. [36], the addition of laminin-coated collagen fibres to a PGA NGC was shown to bridge a gap of 8 cm within a canine peroneal nerve model far exceeding that of a critical nerve gap. However, functional recovery was not characterized. This concept was further explored by Yoshii and co-workers [42,43], using bundles of collagen fibres alone without the use of an external conduit structure. Gaps of 20 and 30 mm were consecutively bridged in successive studies; however, functional recovery remained poor. Despite this, the addition of intraluminal fillers clearly showed the ability to extend the regeneration limits of hollow NGCs [42,43]. Over the years, a number of variations of these intraluminal guidance structures have been used within hollow NGCs in attempts to bridge a critical nerve gap or to enhance functional recovery (table 2). Similar studies by Ngo et al. highlighted the importance of ‘packing density’ (or ‘void fraction’) [36], as well as the distribution of intraluminal structures, as essential considerations for their incorporation within hollow NGC [39,41,52]. Another study observed that high densities (approx. 15–30% of the cross-sectional area) of poly(l-lactide) (PLLA) microfilaments inhibited nerve regeneration [47]. At lower densities (approx. 3.75–7.5% of the cross-sectional area), regeneration was increased and the ability to bridge a critical nerve gap in a rat sciatic nerve model was demonstrated. These were taken as the optimal packing densities for the introduction of intraluminal structures, as lower densities resulted in the fibres settling to the bottom of the conduit, while higher densities resulted in the inhibition of regenerating nerves [47]. This inhibition was similarly seen by Stang et al. [52], where the addition of a dense collagen sponge within a hollow NGC was shown to inhibit regeneration entirely.
Table 2.
Examples of intraluminal guidance structures.
| structure | model | gap | time | significant outcome | references |
|---|---|---|---|---|---|
| laminin/YIGSR collagen fibres (θ, 100–150 µm) | rat sciatic | 15 mm | eight weeks | laminin/YIGSR coated fibre groups significantly increased axonal density versus uncoated fibres. | Itoh et al. [44] |
| 2000 × collagen filaments (θ, 20 µm) | rat sciatic | 20 mm | four, eight weeks | critical gap bridged. No significant difference versus autograft at eight weeks. | Yoshii & Oka [45] |
| 80 × laminin-coated collagen fibres (θ, 50 µm)/sponge | canine peroneal | 80 mm | 12 months | no significant difference in nerve regeneration or functional recovery seen between groups. | Toba et al. [46] |
| various densities of PLLA (θ, 40–100 µm) microfilaments | rat sciatic | 10, 14, 18 mm | 10 weeks | high filament densities inhibited nerve regeneration. Low filament densities increased nerve regeneration. | Ngo et al. [47] |
| 2000 × PGA (θ, 14 µm) filaments | dog sciatic | 30 mm | six months | critical gap bridged with similar functional recovery to autograft. | Wang et al. [48] |
| collagen gel | rat peroneal | 15 mm | 12 weeks | a critical nerve gap bridged without the addition of neurotrophic factors. | Lee et al. [49] |
| 1/3 PAN-MA fibrous film configurations (θ, 400–600 nm). | rat tibial | 14 mm | six, 13 weeks | functional nerve regeneration was significantly greater in the 1 film conduit versus that of the 3 film conduit. | Clements et al. [39] |
| fibrous (θ, 2–20 µm) keratin hydrogel | mouse tibial | 4 mm | six weeks | keratin group showed significantly greater conduction delay than autograft group. | Sierpinski et al. [50] |
| 1000 × PLGA fibres (θ, 14 µm) + MSCs | dog sciatic | 50 mm | six months | critical gap bridged. Functional recovery significantly greater than a hollow conduit and less than autograft. | Ding et al. [51] |
Ngo et al. [47] demonstrated that axonal regeneration was further reduced when intraluminal fibres were juxtaposed. One instance showed that fibres clustered in the centre of the conduit resulted in complete regeneration failure. This result highlights the necessity for the correct positioning of intraluminal fillers within a hollow NGC and when used with the appropriate material combinations, as seen in a later study where PLLA intraluminal filaments were incorporated into a permeable poly(lactic acid) (PLA) NGC. A further increase in their regenerative potential was seen in vivo in contrast to that of the original impermeable silicone NGC [53].
A number of similar studies were carried out using different combinations of intraluminal guidance structures and outer conduit materials and are summarized in table 2. These intraluminal guidance structures include gels, sponges, films, filaments and fibres, which have been used alone, or in combination with a number of supportive factors. One approach taken is the addition of nano-scale guidance cues to micrometre-scale intraluminal guidance structures.
These nano-scale features were successfully incorporated into both film [39,54] and filament guidance structures [13]. The use of aligned polymeric fibrous films serves as one interesting alternative to the use of intraluminal fibres/filaments. A critical nerve gap of approximately 17 mm was bridged using aligned electrospun thin films of poly(acrylonitrile-co-methylacrylate; PAN-MA) fibres [54]. These aligned sub-micrometre-scale fibres (400–600 nm in diameter) showed a significant increase in nerve regeneration in contrast to that of control unaligned films and in later studies showed the ability to be arranged into a variety of configurations.
These electrospun films have the advantages of a high surface area-to-volume ratio, a compact aligned topography, controlled packing configurations and a low packing density (approx. 0.6% of the NGC cross-sectional area) [39]. These intraluminal films allow controlled positioning of guidance structures, eliminating the problem of fibre overlap associated with the use of intraluminal fibres/filaments. Despite the advantages of such a concept, a single film placed along the midline of the conduit showed the most promising results. The addition of further films (in various configurations) limited regeneration-creating areas devoid of axonal growth. The author noted the disadvantage of creating zones within the conduit itself. These zones allowed symmetrical mismatches of migration of supportive cells from the proximal and distal nerve stumps. SCs could be seen migrating in the upper zone proximally, while distilling migrating in a lower zone of the configuration. This misalignment resulted in the incorrect formation of an aligned tissue cable [39]. The use of such a system therefore requires careful positioning of each film within the conduit to create a controlled environment for repair.
The use of nano-scale topographies was similarly achieved by Koh et al. [13] through the use of micrometre-scale intraluminal filaments that were composed of aligned electrospun nanofibrous yarns. These intraluminal filaments consisted of poly(lactic-co-glycolic acid; PLGA) nanofibres (between 200 and 600 nm in diameter) and have a diameter of approximately 25 µm (approx. 10% of the NGC cross-sectional area). These filaments, combined with surface functionalization and growth factor delivery, successfully bridged a nerve gap of approximately 15 mm after a period of 12 weeks. Further such intraluminal structures combined with a bi-layered outer conduit were shown to achieve similar levels of regeneration and functional recovery to that of autograft across a large critical nerve gap [13].
2.2. Luminal wall guidance features: enhancing porosity and increasing guided cell migration
A number of physical alterations to the luminal wall have also been considered to introduce physical guidance signals within a hollow NGC. These physical guidance features range from micrometre-scale features to the more biomimetic nano-scale topographies (table 3) [4] and primarily involve either the incorporation of longitudinal micro-channels on the inner lumen of an NGC or luminal walls composed of orientated and non-orientated electrospun micrometre-scale to nano-scale fibres. It has been shown that the use of micrometre-scale features induces a guidance effect on neurites of re-growing neurons. These neurites show increasing alignment as features approach the size of regenerating axons, which are of approximately the same width as glial bands of Büngner or smaller [8]. Depending on nerve type and anatomical location, these axons may have a diameter of 2–5 µm (Aδ) or 15–20 µm (Aα) [8,14]. Overall, neurites show increasing alignment as features decrease in width from 500 to 5 µm [4,8,32,59,60]. To take advantage of the guidance effect of the aforementioned topographical features micro and nano-scale structures are currently being incorporated into a number of NGCs designs with the luminal walls displaying longitudinally ordered guidance structures to regenerating axons and similarly to that of migrating and proliferating SCs (table 3). One such example was shown by Rutkowski et al. [55] across a nerve gap of approximately 10 mm in a rat sciatic nerve model using a micro-patterned laminin-coated (poly (d,l-lactic acid)) PDLLA conduit. This study highlighted that over a non-critical nerve gap, the inclusion of micro-channels alone had no significant effect on the level of nerve regeneration, as against control hollow non-micro-grooved conduits. However, the addition of micro-channels, when assessed over a critical 1.5 cm nerve gap, exhibited a significant increase in nerve regeneration and functional recovery versus control NGCs [56]. Similarly, Hu et al., using a unidirectional freezing method, followed by freeze drying, produced a collagen–chitosan conduit with longitudinal orientated micro-channels, in the range of 25–55 µm, within the luminal wall. This produced a hollow NGC with topographical guidance features, while maintaining structural integrity and a high degree of porosity, and was successfully used to bridge a 15 mm critical nerve gap. This longitudinal micro-channelled conduit showed a similar level of regeneration and functional recovery to that of autografts at 12 weeks post implantation. It also showed the ability of an NGC with micro-scale topographical features to successfully bridge a critical nerve gap without the addition of neurotrophic factors, cells or similar molecular therapies [56].
Table 3.
Luminal guidance features and variations in material design.
| feature | model | gap | time | significant results | references |
|---|---|---|---|---|---|
| micro-grooved/micro-channelled luminal features | |||||
| micro-channelled PDLLA NGC + SCs (groove width 10 µm, depth 4.3 µm) | rat sciatic | 10 mm | eight weeks | micro-channels had no significant effect on regeneration; addition of SCs increased functional recovery | Rutkowski et al. [55] |
| micro-channelled collagen–chitosan conduit (groove width 25–55 µm) | rat sciatic | 15 mm | four, 12 weeks | similar level of regeneration and functional recovery to autograft at 12 weeks. | Hu et al. [56] |
| electrospun nano- and micro-fibrous conduits | |||||
| electrospun PCLEEP fibrous conduit with GDNF (θ (3.96 ± 0.14) µm) | rat sciatic | 15 mm | 12 weeks | significant increase in functional recovery could be seen versus control conduits | Chew et al. [40] |
| silk fibroin poly(l-lactic acid-co-ε-caprolactone) (P(LLA-CL)) fibrous NGC | rat sciatic | 10 mm | four, eight weeks | significant increase in functional nerve regeneration versus P(LLA-CL) NGCs. | Wang et al. [57] |
| variations in conduit design: | |||||
| multi-channelled collagen conduit (1-, 2-, 4-, 7-channel conduits) | rat sciatic | 10 mm | 16 weeks | 4-channel conduits significantly decreased axonal dispersion versus control single channel conduits. | Yao et al. [58] |
| enhancing nutrient exchange and the introduction of external stimuli | |||||
| PLGA conduit with asymmetric/symmetric pores | rat sciatic | 10 mm | four, eight weeks | PLGA conduits with asymmetric pores showed higher nerve regeneration than PLGA conduits with symmetric pores. | Chang & Hsu [27] |
| bi-layered micro and nano porous PLGA/pluronic F127 NGC + US | rat sciatic | 10 mm | one, eight weeks | a bi-layered NGC + US increased nerve regeneration rates versus no US groups (0.72 mm d–1 V 0.48 mm d–1) | Park et al. [25] |
Another similar luminal wall guidance strategy involves the use of electrospun fibrous conduit (table 3) [25,27,40,55–58,61]. The use of these aligned electrospun tubes has a number of advantages over continuous tube strategies: (i) the materials are highly flexible and porous, and these are well adapted for use within biological systems; (ii) nano- and micro-scale fibres have a high surface area-to-volume ratio increasing the area available for protein absorption, SC migration and regeneration of axons; (iii) fibres that can be preferentially aligned resulting in increased SC alignment, proliferation and growth, and the promotion of guided axonal growth [13,40,62]. The use of wall guidance avoids the problem of uneven fibre distribution, seen in the use of low-density intraluminal guidance structures [40,47]. This eliminates the problem of potential growth inhibition from overlapping fibres or compartmentalization, which have adverse effects on nerve regeneration [39,47,52].
In an interesting study by Chew et al. [40], the use of micro-scale electrospun copolymer of caprolactone and ethyl ethylene phosphate (PCLEEP) fibres successfully bridged a 15 mm nerve gap. The aligned electrospun fibres showed an increase in functional recovery versus control non-fibrous PCLEEP conduits showing an increasing trend in nerve regeneration with the subsequent addition of exogenous growth factors [40]. Interestingly, in this study, regeneration occurred at both the periphery and at the centre of NGC lumen [40]. This was reported to be due to the slippage of PCLEEP fibres from the wall into the centre of the lumen and possibly highlights the need for additional intraluminal guidance structures [40]. In a recent in vitro study by Madduri et al. [4], the effects of topographical guidance of electrospun fibres is elegantly shown, through the use of silk fibroin nanofibres. These electrospun fibres, in the range of 400–500 nm, successfully encapsulated neurotrophic factors (glial-derived neurotrophic factor; GDNF and nerve growth factor; NGF) to provide synergistic topographical and trophic support to re-growing axons [4]. The silk fibroin membranes were subsequently assessed with chick dorsal root ganglion cells (primarily sensory neurons and SCs) and chicken embryonic spinal cord explants (primarily motor neurons and SCs) [4]. Interestingly, it was shown that there was a significant increase in neurite length and alignment, and promotion of glial cell migration and alignment, in the case of aligned electrospun nano-scale fibres [4]. This combination of topography and trophic support shows potential for the treatment of critical nerve gaps and increasing functional recovery. It also highlights the different modality of SCs and axons that need to be targeted for mixed nerve repair.
However, it seems that luminal wall guidance alone does not exhibit similar levels of axonal guidance as do intraluminal fillers when bridging a critical nerve gap [40]. To complement these luminal wall guidance features and increase regeneration across a critical nerve gap, a number of approaches need to be considered. A study by Koh et al. [13] combined a number of strategies within their conduit design. A bi-layered laminin-coated PLLA conduit, used in combination with intraluminal PLGA fibres, was demonstrated to enhance the modified outer NGC. This bi-layered conduit consisted of an outer layer of randomly aligned electrospun nanofibres and an inner layer of longitudinally aligned nanofibres that were in the range 250–1000 nm in diameter. It was proposed that the longitudinally aligned inner layer provided topographical cues for regenerating axons and migrating SCs, while the outer layer provided structural support to the conduit structure while maintaining the porosity of the tube. This conduit was successfully used to bridge a critical nerve gap of 15 mm and exhibited functional recovery comparable to autografts [13].
2.3. Optimizing conduit design and the introduction of external stimuli
Alternative strategies for enhancing nerve repair involve reconsidering the overall conduit design (table 3). These approaches have been used to limit axonal dispersion [58], optimize nutrient exchange [25,63] and to more closely resemble the micro-architecture of the peripheral nerve environment [64]. Some of these designs have been successfully combined with non-invasive clinical approaches (i.e. ultrasound) and have shown the potential to enhance peripheral nerve repair [25,61].
The use of a multi-channel conduit is one promising alternative for peripheral nerve repair [21,58,64,65]. A multi-channel PLGA was originally investigated as an alternative to conventional NGC, which was closely imitating native nerve's architecture [64]. Using a foam-processing technique, conduits with multiple micro-channels were manufactured. The primary premise for this design was the controlled introduction of allogenic SCs by increasing the overall surface area for SC adherence and distribution. From this basis, a five channel conduit was then successfully used to bridge a short 7 mm rat sciatic nerve gap. This design however had a very low cross-section available for nerve regeneration, making comparison with the control autograft group difficult [64]. It was later put forward by de Ruiter et al. [66] and by Yao et al. [58] that this multi-channel design could be used to limit axonal dispersion within NGC. It was later put forward by de Ruiter et al. that a single and seven channel PLGA NGCs were used to bridge a 10 mm nerve gap in a rat sciatic nerve model. At 12 weeks, there was no significant difference between single and multi-channel conduits with regard to nerve regeneration. However, using a simultaneous retrograde tracing technique, there was a significant decrease in axonal dispersion versus control single channel conduits. The use of this conduit however showed that these results in only 50 per cent of the groups assessed, primarily due to swelling of the PLGA tube, resulting in occlusion of a number of the channels and the consequences of these results were not definitive [66]. In order to improve this design, Yao et al. [58] showed that the use of multi-channelled collagen nerve conduits could similarly be used to bridge a 10 mm rat sciatic nerve gap, without the structural instability seen in previous studies. This study showed similar results for nerve regeneration to previous work; however there was a significant decrease in overall axonal dispersion/misdirection using this multi-channel design. Using this multi-channel design in combination with additional factors, such as guidance structures or molecular/cell-based therapies, could be an interesting approach for future nerve repair, and potentially could reduce significantly the misdirection of re-growing axons.
Another approach is the use of a bi-layered PLGA/pluronic F127 asymmetrically porous conduit that has been shown to have a number of features [25,63] to increase regeneration within a hollow nerve conduit. This conduit contains two distinct layers: an inner surface with nano-pores of 50 nm in diameter asymmetrically aligned, which allows the diffusion of nutrients and neurotrophic factors but reduces scar infiltration; and an outer surface consisting of micro-pores approximately 50 µm in diameter, which permits vascular ingrowth into the conduit [25,63]. The use of asymmetric pores over non-asymmetric pores had previously been shown to increase early stage nerve regeneration [27,63]. This, in combination with the pluronic F127 coating, increases the hydrophilicity of the conduit, resulting in an increase in the regeneration rate of regenerating axons versus that of control conduits [63]. In later studies, these bi-layered coated conduits were combined with external ultrasound stimulation (US), a novel non-invasive approach. The use of low-intensity US indicated a significant increase in nerve regeneration rates (0.72 mm d–1 in the US-treated group versus 0.48 mm d–1 in the non-treated group) [25]. Likewise, US resulted in increased myelination, axon diameter and thicker regenerative nerve cable [25]. The effects of US stimulation have exhibited comparable results in a number of studies and may hold potential to improve current clinical nerve therapies especially when used in combination with additional regenerative factors, i.e. neurotrophic factors, growth factors or cell-based therapies.
2.4. Surface modifications and peptide mimetics
The addition of topographical guidance cues and structural features to a conduit may require additional surface modifications of the biomaterial surface, depending on the base material. Numerous forms of surface modifications have been used with both synthetic biodegradable materials (polycaprolactone (PCL), PLA and PLLA) and numerous natural materials (collagen, chitosan and fibrin) [36,46,67–71]. These materials while they exhibit the required structural cues for guided cell growth, SC adhesion and migration, their surface characteristics may not be such as to induce the required effects; these materials tend to be hydrophilic or hydrophobic reducing their applicability for nerve repair [72]. Consequently, a number of surface modification techniques have been employed to increase cell adhesion, proliferation and migration. These modifications may take the form of full protein coatings, chemical and physical treatments, or the addition of protein mimetics onto the surface of the material [72].
Numerous ECM proteins have been considered as candidates for surface functionalization including collagen, fibronectin and laminin [13,73,74]. Laminin, a complex trimeric glycoprotein, is a major component of the basal lamina of SCs and has positive effects on neurite extension and SC adhesion, proliferation and migration, and overall have exhibited the ability to improve nerve regeneration [13,46,70]. This trimeric glycoprotein has been demonstrated to interact with SC integrins, which may result in activation of myelination needed for successful growth and repair [13]. Numerous studies have shown its ability to enhance Schwann proliferation and migration, as well as its direct effects on neurite outgrowth [13,36,70]. Laminin has been used most frequently for surface modification for NGC and for their respective structural components [13,36,46,55,68,70,74,75]. Other ECM molecules, such as collagen and fibronectin, have the ability to significantly increase SC adhesion as well as proliferation, and enhance neurite outgrowth however, although results have been shown to be significantly lower than that of laminin [13,73,74]. A number of studies have conjugated laminin to their respective material or used them to enhance the aforementioned intraluminal fillers [36,46,68,70,76]. Each respective study notably showed a significant increase in nerve regeneration compared with that of uncoated fibres [36,46,68,70]. Yu & Bellamkonda [74] presented a combination of laminin and slow-releasing NGF from an agarose hydrogel. The combined effect of these two factors yielded nerve regeneration and functional recovery similar to that of autograft [74]. Similarly, in a recent study by Koh et al. [13], the incorporation of a laminin coating, combined with PLGA intraluminal guidance structures, successfully bridged a critical nerve gap of 15 mm, and showed superior functional recovery to that of autograft. These same ECM molecules can similarly be used as a luminal wall coating, increasing cell adhesion and proliferation as well as increasing guided axonal outgrowth and may serve to enhance some of the luminal wall guidance features mentioned previously [55,67,77].
Large ECM molecules, such as laminin, have a large molecular weight (about 900 kDa), making them quite difficult to synthesize [67,70]. One alternative to the use of these large glycoproteins is the use of short chain protein peptide mimetics (table 4). These peptides have a number of advantages over large proteins, including (i) high stability; (ii) low immune response; (iii) high surface density and orientation for ligand–receptor interaction and cell adhesion; (iv) a relatively low molecular weight; and (v) the ability to be used in high concentrations [70,72]. A number of these peptides have been used in the context of peripheral nerve repair, including RGD (Arg–Gly–Asp), a peptide found in fibronectin, laminin and other ECM molecules; IKVAV (Ile–Lys–Val–Ala–Val) and YIGSR (Tyr–Ile–Gly–Ser–Arg) of the laminin β chain, RNIAEIIKDI (Arg–Asn–Ile–Ala–Glu–Ile–Ile–Lys–Asp–Ile) peptides of the laminin γ chain and the primary cell binding domains of laminin; as well as similar peptide sequences such as HAV (His–Ala–Val), a mimetic of the N-cadherin regulatory protein that is present on both neurons and glial cells [67,70–72,77].
Table 4.
Molecular therapies for the creation of a conductive microenvironment.
| NGC | delivery method | model | gap | time | significant results | references | |
|---|---|---|---|---|---|---|---|
| GDNF/NT-3 | EVA NGC | luminal release | rat facial | 5 mm | six weeks | NGC + GDNF showed highest nerve regeneration level. | Barras et al. [78] |
| VEGF | silicone NGC | suspension (matrigel) | rat sciatic | 10 mm | one, two, four and 26 weeks | VEGF increased angiogenesis, SC migration and nerve regeneration. | Hobson [79] |
| NGF | poly(phenylene ethynylene) (PPE) | PPE microsphere release | rat sciatic | 10 mm | 12 weeks | NGF increased nerve regeneration versus control conduits. | Xu et al. [80] |
| GDNF | collagen + PLGA NGC | luminal release (various rates) | rat peroneal | 3 mm | 12 weeks | number of myelinated fibres tripled for all rates versus no GDNF group. | Piquilloud et al. [81] |
| NGF | porous PCL conduit | suspension/luminal release | rat sciatic | 12 mm | four, eight weeks | NGF luminal release showed superior regeneration to suspension. | Chang [82] |
| GDNF + NGF | collagen + PLGA NGC | luminal release | rat sciatic | 10 mm | two weeks | significant increase in early peripheral nerve regeneration. | Madduri et al. [9] |
| GDNF/NGF | silicone NGC | affinity-based fibrin matrix | rat sciatic | 13 mm | four, eight, 12 weeks | 12 weeks motor recovery of GDNF greater than isograft. | Wood et al. [83] |
| CNTF | chitosan/PLGA NGC | luminal release | dog tibial | 25 mm | 12 weeks | functional recovery comparable to that of autograft. | Shen et al. [84] |
| NGF | PLLA-CL fibrous NGC | core shell nanofibrous release | rat sciatic | 10 mm | 12 weeks | NGC showed similar functional recovery regeneration to autograft. | Liu et al. [85] |
A range of these peptides was successfully assessed, in vitro and in vivo, by Schense et al. [71] within a fibrin matrix. These various peptide sequences exhibited a significant increase in regeneration compared with that of control uncoated fibrin matrix in vitro. Noteworthy was the synergistic effect of multiple peptides on neurite outgrowth, where the combined effect of four individual laminin peptides was greater than the sum of neurite extension for individual peptide alone. In an in vivo study, using a 4 mm dorsal root model, an NGC filled with a peptide-loaded fibrin matrix was successfully implanted. The incorporation of individual peptides within a fibrin matrix showed no significant difference versus fibrin alone; however the synergistic effects of the four laminin peptides showed a significant increase in nerve regeneration versus control fibrin matrices [71]. Similarly, in a work carried out by Yao et al. [60], a human laminin five peptide (PPFLMLLKGSTR (Pro–Pro–Phe–Leu–Met–Leu–Leu–Lys–Gly–Ser–Thr–Arg)) coating was shown to exhibit similar levels of neurite outgrowth to that of a collagen-coated substrate in vitro and successfully used in combination with micro-structured templates, enhancing neurite growth and alignment.
These peptides have been used in a number of similar studies and results have shown levels of regeneration equivalent to whole proteins [44,67,86]. Itoh et al. [44] successfully coated collagen intraluminal fillers with either laminin or the YIGSR peptide, and compared regeneration with uncoated collagen fibres. It was shown that both the laminin-coated and peptide-coated fibres indicated a significant increase in nerve regeneration versus uncoated collagen fibres. In particular, there was no significant difference in nerve regeneration between the whole glycoprotein and the peptide mimetic [44]. In more recent studies, Santiago et al. [67] modified the inner surface of a PCL scaffold with a peptide sequence (RGD) as a means to enhance axonal interactions as well as SC adhesion, and to increase adhesion of implanted adipose-derived stem cells (ASCs). Wang & Huang [86] incorporated a CYIGSR (Cys–Tyr–Ile–Gly–Ser–Arg) peptide (YIGSR peptide with a glycine spacer) with a bi-layered micro/nanofibrous conduit, resulting in increased nerve regeneration. These surface modifications, including large glycoproteins or their peptide memetics, can be seen as key factors for enhancing structural features of current NGCs.
3. Molecular delivery therapies: the creation of a conductive microenvironment
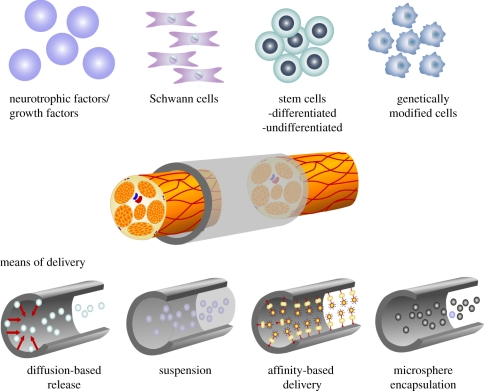
The addition of structural features to hollow NGCs is one approach to improve nerve regeneration—in particular, across critical nerve gap [36,43,45,49,51]. The addition of these features alone is insufficient to increase functional recovery. In efforts to improve functional regeneration in both critical and non-critical gaps, the creation of a more conductive microenvironment is of high importance. The reduction in functional nerve regeneration over these challenging nerve gaps can be attributed to a variety of factors. These include inadequate ECM formation (mentioned earlier), insufficient neurotrophic support, inadequate Schwann numbers, reduction in SC migration and proliferation, and possible reduction in the neurotrophic effects of the distal nerve stump [1,7,31]. In efforts to enhance functional nerve regeneration, advances have been made to create a more conductive environment for repair. Strategies include the use of exogenous growth factors (e.g. vascular endothelial growth factor (VEGF), fibroblast growth factor (bFGF)), neurotrophic factors (e.g. neurotrophin-3 (NT3), NGF) or cell-based therapies (e.g. SCs, stem cells; figure 3) [9,78–85,87–91].
Figure 3.
Schematic of cellular and molecular-based therapies used for the creation of a more conductive nerve microenvironment. Examples of molecular therapies include growth factors (VEGF and bFGF) and neurotrophic factors (NT3 and NGF). Likewise cell therapies involve the use of SCs, stem cells (ASCs and MSCs) and genetically modified cells (SCs overexpressing GDNF). These can be delivered by a number of means including: (i) suspension within solution or a biomaterial matrix (hydrogel, sponge), (ii) released via a diffusion-based systems (controlled released via cross-linking, slow degrading polymer coatings etc. from luminal wall), (iii) the use of affinity-based delivery systems (factors conjugated to a fibrin matrix), and (iv) microsphere (e.g. collagen, fibrin) encapsulation which can either be suspended within the lumen or released from the luminal wall.
Neurotrophic factors enhance functional regeneration, by supporting axonal growth, SC migration and proliferation, and increasing neuroprotection through receptor-mediated activation of specific intrinsic signalling pathways [92]. These neurotrophic factors primarily belong to three distinct families: (i) the neurotrophins; (ii) the glial-cell-line-derived neurotrophic factor family ligands (GFLs); and (iii) the neuropoeitic cytokines [93]. Each family has distinct functional characteristics with some overlapping cellular responses [93]. Neurotrophins include NGF, brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT3) and neurotrophin-4 (NT4) [94]; GFLs include GDNF, and neuropoeitic cytokines include cilary neurotrophic factor (CNTF) [93]. These neurotrophic factors have been used alone or in combination so as to harness the most effective response for nerve regeneration (table 4).
An ethylene vinyl acetate (EVA) conduit used for the release of either NGF or GDNF bridged a 15 mm nerve gap through the addition of these respective neurotrophic factors alone [87]. The GDNF conduit group exhibited four times the number of myelinated axons than that of the NGF group, showing its potential for peripheral nerve repair [87]. Neurotrophic factors delivered alone however have shown limited functional recovery, and efforts have been made recently for the synergistic delivery of these neurotrophic factors [9]. One such study, carried out by Madduri et al. [9], involved co-delivery of both NGF and GDNF, using a luminal diffusion-based delivery system. This study argued that co-delivery was essential for increased functional regeneration, as peripheral nerve contained different neuronal and glial subpopulations (both motor and sensory) [4,9]. NGF, which acts through the high-affinity TrkA receptor, is primarily found on sensory neurons, shown to promote axon regeneration and re-innervate sympathetic axons following nerve injury [9].
The failure of single growth factor delivery may also be attributed to poor release kinetics, with some delivery systems exhibiting a high initial burst release [88]. In efforts to improve release, delivery systems that alter these release kinetics are being considered. One such strategy involves the use of physical cross-linking methods used in combination with a polymer coating [9]. This combination was shown to limit the initial burst release of growth factors, indicating a significant increase in nerve regeneration versus a PLGA polymer coating alone. The effects of this system on late stage functional recovery remain to be seen, but early results seem promising.
One alternative, for controlled delivery of neurotrophic factors, is the use of an affinity-based delivery system [83,89]. This system encloses growth factors within a fibrin-based matrix for intraluminal delivery of growth factors. This avoids the initial burst release seen in some diffusion-based systems and allows the controlled release of growth factors by cell-based degradation of the delivery system and the surrounding fibrin matrix. Using this system, GDNF or NGF were successfully delivered within the lumen of a silicone conduit, increasing the early stage regenerative response and successfully bridging a 13 mm gap in a rat sciatic nerve model [83]. For functional recovery, GDNF, in combination with this diffusion-based delivery system, exhibited a higher level functional recovery than that of the control allograft groups. This may be partially attributable to an increase in the number of large myelinated axons, increased early stage regeneration and the ability of the fibrin matrix to act as an intraluminal guidance structure for early stage cell migration. This study successfully incorporates a fibrin-based intraluminal guidance structure for enhanced contact guidance while synergistically creating a more conductive microenvironment for functional nerve regeneration [83]. GDNF, in particular, contributes to this significant increase in functional recovery, owing to its ability to act on both motor and sensory neurons [90]. This same combinatorial effect was seen in the use of nanofibrous constructs that were successfully combined with GDNF delivery, resulting in a similar increase in functional recovery [40]. However, the nerve regeneration response as a whole is stimulated by a number of factors that act synergistically to improve nerve repair. If the development of the nervous system is considered in its entirety, there is a defined synergy between angiogenesis and neurogenesis [91]. The addition of VEGF indicated a significant increase in angiogenesis and also exhibited a similar increase in overall nerve regeneration [79]. Although the addition of neurotrophic factors has shown advantages for nerve repair, their use has some limitations, including unintentional activation of multiple signalling pathways resulting in undesired biological effects, e.g. aberrant sprouting associated with the use of NGF, and short half-lives and poor stability—lasting literally minutes upon release in serum conditions [94–96]. These limitations may be overcome through increasing our knowledge of neurotrophic factor and growth factor delivery reducing unintentional effects. Similarly, by optimizing the release kinetics of neurotrophic factors delivery (using new biomaterial technologies) the disadvantage of their limited half-lives may be overcome [94–96]. New emerging delivery approaches being developed in our own laboratory include the use of biological collagen/fibrin microspheres, microfibres and hydrogels, as well as synthetic polymeric carriers for the creation of a sustained system for viable growth factor delivery [97–99].
4. Schwann cells: the gold standard for cell-based repair
During nerve regeneration, SC migration and proliferation can be seen as prerequisites for successful nerve repair, seen in the cellular phase of successful nerve repair [1,7,28,31]. SCs that remain after Wallerian degeneration migrate and proliferate to form aligned glial bands of Büngner, during the cellular phase of NGC repair [7,28,29,31]. At this stage, SCs have switched to a regenerative phenotype, are actively secreting neurotrophic factors, and laying down basal lamina, and importantly their numbers have increased to 4–17 times the original number seen in normal nerve (approx. 20–106 cells ml−1) [28,37]. However, as gap length increases, SC migration, proliferation and alignment decrease and SC numbers may be deemed insufficient for the creation of a conductive nerve environment [1]. In attempts to aid the regenerative cellular response to injury, cellular-based therapies are being considered as an alternate means for repair (table 5) [33,51,61,67,90,100–102].
Table 5.
Examples of cell-based approaches for peripheral nerve repair.
| cell type | NGC | method of delivery | model | gap | significant results | references |
|---|---|---|---|---|---|---|
| SCs + US | hollow PLGA/ silicone NGC | wall release (3 × 105 cells ml−1) | rat sciatic | 10 mm | in PLGA NGCs + SCS + US nerve regeneration significantly increased. | Chang et al. [61] |
| SCs dBMSCs | PHB conduit + fibrin matrix | fibrin matrix (8 × 107 cells ml−1) | rat sciatic | 10 mm | increased nerve regeneration distance versus a hollow PHB and fibrin alone. | Kalbermatten et al. [33] |
| human uASCs | PCL conduit + RGD peptide | suspension (2 × 108 cells ml−1) | rat sciatic | 6 mm | increased functional recovery and regeneration versus control groups. | Santiago et al. [67] |
| SCs | cellulose conduit + BD hydrogel | suspension (8 × 107 cells ml−1) | rat sciatic | 10 mm | SCs + hydrogel increased regeneration distance. Effects lost at 16 weeks. | McGrath et al. [90] |
| uBMSCs | PCL conduit | injection into lumen (5 × 108 cells ml−1) | mouse median | 3 mm | increased number of myelinated fibres and angiogenesis versus control group | Oliveira et al. [100] |
| uBMSCs | chitosan conduit + PLGA fibres | injection into lumen (8 × 107cells ml−1) | dog sciatic | 50 mm | increased regeneration and functional recovery versus non BMSC group. | Ding et al. [51] |
| SCs, dASCs dBMSCs | fibrin glue conduit | injection into lumen (4 × 107 cells ml−1) | rat sciatic | 10 mm | dBMSCs/dASCs increase regeneration distance and SC migration versus hNGC. SC remained superior to all groups. | Di Summa et al. [101] |
| dBMSCs SCs | collagen conduit (Neuragen) | wall release (8 × 105 cells ml−1) | rat sciatic | 12 mm | increase in nerve regeneration in SC dBMSC NGCs versus hollow group. | Ladak et al. [102] |
One suggested approach to improve functional recovery and nerve regeneration, and as an alternative to neurotrophic factor delivery, is the use of autologous or allogenic SCs [37,90,101]. If autograft is taken as the current gold standard for peripheral nerve repair, similarly the addition of autologous SCs to NGCs can be taken as the current gold standard of cellular-based therapies. The use of SCs have the advantage of producing a number of neurotrophic factors, building their own basal lamina, expressing cell adhesion molecules and at a later stage are actively involved in the re-myelination of regenerating nerve fibres [90,101]. The introduction of additional SCs would therefore assist in the creation of a conductive nerve microenvironment, especially across a critical nerve gap [50,90].
SCs may be introduced into the conduit via a number of methods. These include injection, suspension within an intraluminal hydrogel, distributed along intraluminal guidance structures or released from the luminal wall [33,61,67,101]. The implanted SCs can be successfully incorporated into the regenerative process and is nicely shown through the use of retrovirally labelled allogenic SCs (harvested from neonatal rats) [37]. At the optimum concentration (80 × 106 cells ml−1), these labelled cells were shown to be successfully incorporated into the host regenerative process, and furthermore doubled the rate of regeneration versus that of control hollow silicone NGCs [37]. On the basis of these studies, SCs have been successfully implanted in a number of studies with varying effects on nerve regeneration and functional recovery. In a study by Di Summa et al. [101], SCs were seeded within a hollow fibrin conduit and implanted in a 10 mm rat sciatic nerve model. These fibrin-SC conduits showed a significant increase in nerve regeneration, versus control hollow conduits, conduits seeded with differentiated bone marrow-derived mesenchymal stem cells (dBMSCs), or conduits seeded with differentiated adipose-derived mesenchymal stems cells (dADSCs) [101]. Recently, this same system, was used to bridge a 10 mm rat sciatic nerve gap over a period of 16 weeks [103]. This later study highlighted the benefits of alternate cell therapies, showing functional recovery levels comparable to that of autograft (discussed later). A similar study using a polyhydroxybuturate (PHB) conduit filled with a fibrin matrix and seeded with SCs or dBMSCS was shown to increase early nerve regeneration (two weeks), unlike that of control hollow PHB conduits and conduits filled with matrix alone [33]. However effects on late stage functional recovery remain to be seen.
The use of autologous SCs has a number of disadvantages associated with their use: culture times are long and difficult; the extraction of SCs from the host is often painful and requires sacrifice of host nerve tissue [101]. The sacrifice of this tissue has the same disadvantages as those of autograft, i.e. donor site morbidity, and the need for a secondary surgical site. Similarly for the use of allogenic SCs, an extensive immune response, requiring further immune suppression, similar to that associated with the use of allografting, is exhibited [104]. Alternative extraction methods and cellular therapies are now being considered, including stem cells and the use of gene therapy approaches.
4.1. Stem cells: a possible alternative to autologous Schwann cells
One cellular-based alternative is the use of stem cells to enhance the host regenerative response. In a number of studies, these cell types have been considered to enhance nerve regeneration (table 5). These stem cells come from numerous sources but many studies are concentrating on the use of either BMSCs or ASCs [51,67,101]. Autologous BMSCs can be easily derived by aspirating from the bone marrow of patients [51]. Likewise, ASCs can be easily extracted using conventional liposuction techniques [101]. These cells conform to the criteria for ideal transplantable cells: are easily extracted, proliferate rapidly in culture, have a relatively low cost, raise no ethical issues associated with their use and have the ability to differentiate along multiple cells lines, in particular neural and associated glial cell lineages [51,100,101,105]. Both ASCs and BMSCs have the advantage of exhibiting the ability to secrete multiple neurotrophic factors, including GDNF, NGF, NT-3 and BDNF [106–108]. These cells have been used in a number of studies in both the differentiated and undifferentiated states in order to investigate their effect on peripheral nerve regeneration (table 5).
The advantage of using mesenchymal stem cells (MSCs) in their undifferentiated state in vivo allows these multi-potent cells to be stimulated by advancing axons and native SCs, differentiating the MSCs along multiple pathways. This can aid in the creation of a conductive environment for nerve regeneration [100,101]. This differentiation in vivo may be caused by the fusion of implanted MSCs with host cells, rather than by directly differentiating into known cell types [51]. These MSCs have the capacity to differentiate directly or indirectly into glial-like cells, possibly secreting a variety of neurotrophic factors. Alternatively, the implanted MSCs have showed the capacity to differentiate into other supportive cells, such as endothelial-like cells, smooth muscle cells or pericytes [100]. These endothelial-like cells can produce a variety of growth factors, such as VEGF, which has been shown to have a simultaneous effect on angiogenesis, neuritogenesis and neuroregeneration, which translates to positive effects on nerve regeneration in vivo [79,91,100].
In a very interesting study by Oliveira et al. [100], the addition of undifferentiated BMSCs were shown to significantly increase functional recovery in a mouse median nerve model. Using the earlier mentioned model, a PCL NGC with suspended undifferentiated BMSCs was successfully implanted and regeneration was evaluated up to 12 weeks post implantation. At the defined endpoint, there was a significant increase in the number of myelinated fibres and angiogenesis, versus control conduits [100]. The authors hypothesized that this could be attributed to the multi-potent nature of the BMSCs and their known ability to secrete multiple neurotrophic factors. Similarly, in a study carried out by Ding et al. [51] a combination of intraluminal fillers and undifferentiated BMSCs showed that a significant increase in functional recovery across a critical nerve gap of approximately 50 mm in a canine nerve model versus that of the control group. This functional recovery approached that of autografts.
However, the mechanisms of the enhanced regenerative response are largely unknown, with very few BMSCs seen differentiating along an SC-like lineage [100]. This variability was highlighted in an early study by Santiago et al., where the use of undifferentiated ASCs showed no trans-differentiation to an SC-like phenotype. A significant increase in nerve thickness was seen in the cell-based group versus that of hollow NGCs [67]. The variability of using these undifferentiated MSCs may limit their future clinical applications, and further characterization is needed to assess their suitability for peripheral nerve repair.
Owing to these possible limitations of undifferentiated MSCs, one interesting alternative is the use of their differentiated counterparts. BMSCs and ASCs can be differentiated in vitro through combinations of various neurotrophic and growth factors, into a more glial or neural cell lineage [102,104–106,108]. These differentiated MSCs (dMSCs) have the advantage of being differentiated in a controlled manner, with both bone marrow and adipose-derived cells showing the ability to differentiate into SC-like cells [101,102,104,106]. These SC-like cells have a positive effect on neurite outgrowth on sensory dorsal root ganglion neurons in vitro [104,106] and, in recent studies, have been shown to have beneficial effects in vivo [102]. In the case of dASCs, these positive effects on neurite outgrowth may be attributed to increased level of BDNF and NGF versus that of their undifferentiated counterparts [108]. The controlled differentiation of MSCs may also reduce concerns of adverse effects associated with undifferentiated MSCs, effects such as differentiation along a tumourigenic cell line [101]. Similarly, this controlled differentiation allows the creation of a less variable cellular-based treatment for peripheral nerve repair.
In a study by Di Summa et al. [101] differentiated ASCs and BMSCs were tested in a 10 mm rat sciatic nerve model where they exhibited a significant increase in nerve regeneration versus that of control hollow conduits at an early stage of nerve regeneration (two weeks). In a subsequent study, dASCs showed a significant increase in functional recovery and similar nerve morphometry to that of autograft 16 weeks post implantation [103]. This study emphasizes the benefits of pre-differentiating MSCs to an SC-like phenotype before implantation. It may yet prove a valid alternative for autologous SC implantation.
Ladak et al. [102] in a later study showed dBMSCs implanted within a hollow collagen NGC, have a similar increase in levels of regeneration, with similar increases in vitro neurite outgrowth. Despite promising in vitro results and an increase in levels of nerve regeneration in vivo, functional recovery remained poor and significantly lower than that of autograft groups [102]. This contrary result underlines the importance of correct cell–material combinations. Future studies in this area may consider the use of combinatorial approaches, e.g. using structural contact guidance in the earlier mentioned intraluminal guidance structures and variations in conduit design. These structures may be used to control the distribution of the implanted cells within the NGC and simultaneously interacting with native SCs. In addition, these same differentiated and undifferentiated MSCs may be subjected to ex vivo modulation discussed later.
4.2. Genetically modified cells
Current modification of SC cultures is primarily carried out ex vivo and a relatively small amount has been implanted in vivo. In an initial study by Timmer et al. [109], SCs were transfected to overexpress basic high-molecular-weight fibroblast growth factor (FGF-2). The transfected cells were enclosed within a Matrigel-filled silicone tube and implanted in a 15 mm rat sciatic nerve model. The transfected cell group showed a significant increase in the number of myelinated axons versus the control group. Later, a similar study was carried out by Haastert et al. [110] using two isoforms of FGF-2 (either high or low molecular weight FGF-2). SCs were transfected to overexpress each isoform of FGF-2 and similarly enclosed within a Matrigel-filled silicone tube and implanted in a 15 mm rat sciatic nerve model. The high molecular weight FGF-2 supported functional sensory recovery; however, the low molecular weight FGF-2 was shown to have an inhibitory effect on myelination [110].
In a study by Li et al. [111], allogenic rat SCs were transfected with a retrovirus encoding for enhanced expression of GDNF and implanted within a rat sciatic nerve model. The use of these GDNF modified SCs showed a significant increase in myelination, nerve regeneration and functional recovery compared with that of hollow conduits or similarly conduits seeded with unmodified SCs. It was also reported that this enhanced expression could be maintained for up to six weeks post injury, peaking roughly at four weeks post-transduction [111]. The use of genetically modified SCs requires further in vivo studies to be carried out to assess their potential benefit for functional nerve regeneration, and another interesting alternative may be the ex vivo modification of undifferentiated or differentiated MSCs, possibly increasing their potential for peripheral nerve repair.
Of note was a study carried out by Schmitte et al. [112] suggesting that the use of adult canine Schwann cells (cSCs) is a more clinically relevant model for translational research—in particular, for the translation of cellular-based therapies, this cell type—unlike those of rodent cells—displays characteristics that are similar to primate cells. These characteristics include the stable expression of the low-affinity binding receptor, p75NTR; the ability to grow for long periods in the absence of mitogens and no spontaneous immortalization of cultured SCs. These cSCs were successfully transfected by nucleofection, an alternative means to retrovirus transduction and showed the ability to express enhanced green fluorescent protein in vitro and in vivo. However, transfection by non-viral plasmids was seen as quite transient and expression levels became minimal after a period of one week in vivo. This suggests the need for prolonged release of this non-viral plasmid within the cell, if it is to show potential for peripheral nerve repair.
5. Conclusions and future perspectives for nerve guidance conduit repair
Despite advances in peripheral nerve repair, the best combination of materials has yet to be realized. Current approaches have a number of limitations (e.g. short half-lives, slow degradation rates, areas devoid of growth, beneficiary effects being lost at later stages of nerve regeneration). Each of these limitations needs to be addressed in order for these technologies to reach the clinic. Overcoming these limitations and expanding our current knowledge is a key step towards the creation of the next generation of NGCs.
5.1. Improvements in current intraluminal guidance structures
One possible limitation is the use of slow degrading intraluminal guidance structures. Current intraluminal guidance structures are made of slow degrading polymers and this often results in inhibition of regenerating axons or the formation of axonal and SC-depleted zones [39,41,47,113]. One interesting alternative to this approach is the use of a faster degrading polymer within the lumen of the conduit [13,114]. In a study by Nichterwitz et al. [114], poly-p-dioxanone (PDO) filaments enclosed within a host epineural tube were implanted in a rat sciatic nerve model and assessed after a period of six weeks for SC alignment and axonal regeneration. This study highlights the capacity of SCs to form glial bands of Büngner and to maintain these bands despite the fact that the underlying PDO filaments had begun to degrade. These regenerating axons were shown to follow these glial cell bands during repair. This can be seen to match the regeneration in the conduit over a relatively short gap. Noteworthy, during the weeks of the cellular and axonal phase, the fibrin cable which has a degradation time of approximately two weeks, has most likely degraded having fulfilled its role for cellular migration and has been replaced with a trophically and topographically aligned tissue cable which is a key factor for successful nerve repair [33,34]. This short degradation time could be seen as an essential prerequisite for the use of intraluminal fillers. This could be seen as an essential prerequisite for the use of intraluminal fillers. However increases in toxicity levels must be kept in mind. A study that supports this claim was carried by Wood et al. [83]. This study used a fibrin matrix which degraded after a four week period and despite its relatively short residence time showed beneficial effects on nerve regeneration. This further supplements the idea that intraluminal fillers are beneficial, but only at the early stages of growth, after which it can be hypothesized that they become an inhibitory molecule.
5.2. Neurotrophic factor mimetics: future molecular therapies
Molecular therapies using neurotrophic factor-based therapies have shown potential for enhancing functional recovery, as well as for increasing nerve regeneration. These therapies may also hold the potential for functional critical nerve repair. The use of these factors has a number of limitations as mentioned earlier. One alternative to the use of large neurotrophic factor is the use of small molecule mimetics [94,96,115,116]. These neurotrophic factor mimetics have the advantages of low immunogenicity, low molecular mass with relatively low manufacturing costs, compared with the use of whole proteins [94]. Research in this area concentrates primarily on the creation of mimetics of the neurotrophin factor, in particular mimetics of NGF, NT-3 and BDNF [94,96,115]. These mimetics have primarily focused on creation of ligands of these neurotrophins with specific receptor targets [94,96]. In the case of NGF mimetics, one example is the use of a NGF mimetic that selectively binds and activates the high-affinity TrkA receptor, but not that of the low-affinity p75 receptor. The activation of these signalling pathways is associated with quite different responses. TrkA activation has effects on neuronal survival and differentiation, and p75 activation has been associated with apoptosis [94,96]. These mimetics overcome the limitation of the larger neurotrophic factors as they have increased stability and controlled activation of known cellular pathways. However, these neurotrophin mimetics are currently in the early stages of research and are not as of yet being considered for peripheral nerve repair. Future strategies may include controlled release of these neurotrophin mimetics as a substitute for whole protein therapies, allowing for more controlled targeting of cellular responses.
5.3. Reaching the clinic: future design considerations and objectives
In attempts to reach the clinic, a number of large animal studies and clinical trials have been carried out [117–119]. Lessons are continuously being learned from these studies and a number of design criteria are emerging that must be taken into consideration for future clinic use. In particular, the influence of nerve diameter on NGC repair has recently come to attention [6]. Current FDA-approved conduits have failed over increased nerve diameters, a failure primarily caused by incorrect NGC conduit design [6]. This problem highlights another key hurdle that needs to be surmounted in NGC repair, and, as clinical trials progress, more may become apparent. At this stage however, the next generation of NGCs should have a clear and well-defined objective, if they are to show benefit when brought to clinical practice. These objectives may possibly include some of the following criteria for nerve repair:
— show levels of regeneration equivalent or superior to that of autograft, the current gold standard for repair;
— extend regeneration beyond that of the current critical nerve gap of current NGCs and/or autograft;
— attain superior functional recovery, approaching that of the original uninjured nerve; and
— act as a superior replacement to other tissue engineering approaches.
5.4. Proteomic and genomic analysis of nerve guidance conduit repair: filling in the blanks
There remains a gap in knowledge of some of the key molecular responses to the NGC repair. In particular, as gap length increases, levels of regeneration and functional recovery have been shown to decrease [41,47,53]. The reasons for this decrease in regeneration have been discussed in detail earlier and similarly it has been shown that the level of regeneration and functional recovery can be enhanced through the addition of structure and the creation of a more conductive microenvironment (tables 2–5). However, the mechanisms by which these factors influence nerve regeneration are not clearly understood, and the missing components for complete functional nerve regeneration are yet to be realized. A number of excellent studies have characterized the response of peripheral nerve to injury, using both crush and transection injuries, using either proteomic or genomic approaches or a combination of the two [120–126]. This area of research ranges from analysis of the entire sciatic nerve to studies focusing on more specific areas such as extracted SCs and dorsal root ganglion, detailing both repair and developmental mechanisms associated with the cells. However, research to date has not taken into account the addition of an NGC and its effects on nerve repair. Similarly, the addition of an individual or combinatorial approaches have unknown effects on nerve regeneration and need to be carefully understood. We are currently exploring the biological and cellular response to NGC repair, from the most basic non-critical nerve gap to the more challenging critical gaps, and defining the missing components for increased functional nerve regeneration. This detailed analysis will define the cellular responses from the base genomic level to higher proteomic level at each stage of nerve regeneration and will provide a thorough and deep understanding of NGC repair as a function of gap length. It will further highlight the key repair processes needed for optimal functional nerve regeneration and ultimately where current NGCs fail to meet these criteria. This extensive back-to-roots study will enable us to not only understand the problem, but will also help us to address it.
6. Conclusion
There are many promising approaches being considered to improve existing hollow NGCs. Optimal regeneration will not be achieved using single factor strategies however, and an appropriate combinatorial approach should be considered. Although a number of studies do propose some interesting combination, the best combination of factors has yet to be realized. The decision of the best possible combination should take into consideration the natural sequence of events that occur during regeneration and from this understanding, decide how best to modulate the process. The field however is rapidly approaching, achieving a biomaterial-based alternative for nerve repair; it will be interesting to see if this next generation of NGCs can provide the optimal regenerative microenvironment.
Acknowledgements
This material is based upon works supported by the Science Foundation Ireland under Grant No. 07/SRC/B1163 and Science Foundation Ireland Research Frontiers Program (08/RFP/ ENM1218).
References
- 1.Mukhatyar V., Karumbaiah L., Yeh J., Bellamkonda R. 2009. Tissue engineering strategies designed to realize the endogenous regenerative potential of peripheral nerves. Adv. Mat. 21, 4670–4679 [Google Scholar]
- 2.Siemionow M., Brzezicki G. 2009. Chapter 8: current techniques and concepts in peripheral nerve repair. In International review of neurobiology (eds Stefano G., Pierluigi T., Bruno B.), pp. 141–172 San Diego, CA: Academic Press; [DOI] [PubMed] [Google Scholar]
- 3.Jiang X., Lim S. H., Mao H.-Q., Chew S. Y. 2010. Current applications and future perspectives of artificial nerve conduits. Exp. Neurol. 223, 86–101 10.1016/j.expneurol.2009.09.009 (doi:10.1016/j.expneurol.2009.09.009) [DOI] [PubMed] [Google Scholar]
- 4.Madduri S., Papaloïzos M., Gander B. 2010. Trophically and topographically functionalized silk fibroin nerve conduits for guided peripheral nerve regeneration. Biomaterials 31, 2323–2334 10.1016/j.biomaterials.2009.11.073 (doi:10.1016/j.biomaterials.2009.11.073) [DOI] [PubMed] [Google Scholar]
- 5.Siemionow M., Bozkurt M., Zor F. 2010. Regeneration and repair of peripheral nerves with different biomaterials: review. Microsurgery 30, 574–588 10.1002/micr.20799 (doi:10.1002/micr.20799) [DOI] [PubMed] [Google Scholar]
- 6.Pabari A., Yang S. Y., Seifalian A. M., Mosahebi A. 2010. Modern surgical management of peripheral nerve gap. J. Plast. Reconstr. Aesthet. Surg. 63, 1941–1948 10.1016/j.bjps.2009.12.010 (doi:10.1016/j.bjps.2009.12.010) [DOI] [PubMed] [Google Scholar]
- 7.Belkas S. J., Shoichet S. M., Midha R. 2004. Peripheral nerve regeneration through guidance tubes. Neurol. Res. 26, 151–160 10.1179/016164104225013798 (doi:10.1179/016164104225013798) [DOI] [PubMed] [Google Scholar]
- 8.Bozkurt A., et al. 2009. In vitro cell alignment obtained with a Schwann cell enriched microstructured nerve guide with longitudinal guidance channels. Biomaterials 30, 169–179 10.1016/j.biomaterials.2008.09.017 (doi:10.1016/j.biomaterials.2008.09.017) [DOI] [PubMed] [Google Scholar]
- 9.Madduri S., Di Summa P., Papaloïzos M., Kalbermatten D., Gander B. 2010. Effect of controlled co-delivery of synergistic neurotrophic factors on early nerve regeneration in rats. Biomaterials 31, 8402–8409 10.1016/j.biomaterials.2010.07.052 (doi:10.1016/j.biomaterials.2010.07.052) [DOI] [PubMed] [Google Scholar]
- 10.Lee S. K., Wolfe S. W. 2000. Peripheral nerve injury and repair. J. Am. Acad. Orthop. Surg. 8, 243–252 [DOI] [PubMed] [Google Scholar]
- 11.Brenner M. J., Hess J. R., Myckatyn T. M., Hayashi A., Hunter D. A., Mackinnon S. E. 2006. Repair of motor nerve gaps with sensory nerve inhibits regeneration in rats. Laryngoscope 116, 1685–1692 10.1097/01.mlg.0000229469.31749.91 (doi:10.1097/01.mlg.0000229469.31749.91) [DOI] [PubMed] [Google Scholar]
- 12.Nichols C. M., Brenner M. J., Fox I. K., Tung T. H., Hunter D. A., Rickman S. R., Mackinnon S. E. 2004. Effects of motor versus sensory nerve grafts on peripheral nerve regeneration. Exp. Neurol. 190, 347–355 10.1016/j.expneurol.2004.08.003 (doi:10.1016/j.expneurol.2004.08.003) [DOI] [PubMed] [Google Scholar]
- 13.Koh H. S., Yong T., Teo W. E., Chan C. K., Puhaindran M. E., Tan T. C., Lim A., Lim B. H., Ramakrishna S. 2010. In vivo study of novel nanofibrous intra-luminal guidance channels to promote nerve regeneration. J. Neural Eng. 7, 046003. 10.1088/1741-2560/7/4/046003 (doi:10.1088/1741-2560/7/4/046003) [DOI] [PubMed] [Google Scholar]
- 14.Kiernan J. 1998. The human nervous system: an anatomical viewpoint, 7th edn, pp. 16–42, Philadelphia, PA: Lippincott Williams & Wilkins [Google Scholar]
- 15.Alluin O., Wittmann C., Marqueste T., Chabas J.-F., Garcia S., Lavaut M.-N., Guinard D., Feron F., Decherchi P. 2009. Functional recovery after peripheral nerve injury and implantation of a collagen guide. Biomaterials 30, 363–373 10.1016/j.biomaterials.2008.09.043 (doi:10.1016/j.biomaterials.2008.09.043) [DOI] [PubMed] [Google Scholar]
- 16.Pollard J. D., Fitzpatrick L. 1973. An ultrastructural comparison of peripheral nerve allografts and autografts. Acta Neuropathologica 23, 152–165 10.1007/BF00685769 (doi:10.1007/BF00685769) [DOI] [PubMed] [Google Scholar]
- 17.Moradzadeh A., Borschel G. H., Luciano J. P., Whitlock E. L., Hayashi A., Hunter D. A., Mackinnon S. E. 2008. The impact of motor and sensory nerve architecture on nerve regeneration. Exp. Neurol. 212, 370–376 10.1016/j.expneurol.2008.04.012 (doi:10.1016/j.expneurol.2008.04.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siemionow M., Sonmez E. 2008. Nerve allograft transplantation: a review. J. Reconstr. Microsurg. 23, 511–520 [DOI] [PubMed] [Google Scholar]
- 19.Ijpma F. F. A., Van De Graaf R. C., Meek M. F. 2008. The early history of tubulation in nerve repair. J. Hand Surg. (European Volume) 33, 581–586 10.1177/1753193408091349 (doi:10.1177/1753193408091349) [DOI] [PubMed] [Google Scholar]
- 20.Lundborg G. 2000. A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J. Hand Surg. 25, 391–414 10.1053/jhsu.2000.4165 (doi:10.1053/jhsu.2000.4165) [DOI] [PubMed] [Google Scholar]
- 21.de Ruiter G. C. W., Malessy M. J. A., Yaszemski M. J., Windebank A. J., Spinner R. J. 2009. Designing ideal conduits for peripheral nerve repair. Neurosurg. FOCUS 26, E5. 10.3171/FOC.2009.26.2.E5 (doi:10.3171/FOC.2009.26.2.E5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiono V., Tondaturo C., Ciardelli G. 2009. Chapter 9: artificial scaffolds for peripheral nerve reconstruction. In International review of neurobiology (eds Stefano G., Pierluigi T., Bruno B.), pp. 173–198 San Diego, CA: Academic Press; [DOI] [PubMed] [Google Scholar]
- 23.Griffin J., Carbone A., Delgado-Rivera R., Meiners S., Uhrich K. E. 2010. Design and evaluation of novel polyanhydride blends as nerve guidance conduits. Acta Biomater. 6, 1917–1924 10.1016/j.actbio.2009.11.023 (doi:10.1016/j.actbio.2009.11.023) [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro-Resende V. T., Koenig B., Nichterwitz S., Oberhoffner S., Schlosshauer B. 2009. Strategies for inducing the formation of bands of Büngner in peripheral nerve regeneration. Biomaterials 30, 5251–5259 10.1016/j.biomaterials.2009.07.007 (doi:10.1016/j.biomaterials.2009.07.007) [DOI] [PubMed] [Google Scholar]
- 25.Park S. C., Oh S. H., Seo T. B., Namgung U., Kim J. M., Lee J. H. 2010. Ultrasound-stimulated peripheral nerve regeneration within asymmetrically porous PLGA/pluronic F127 nerve guide conduit. J. Biomed. Mater. Res. B Appl. Biomater. 94B, 359–366 [DOI] [PubMed] [Google Scholar]
- 26.Xie J., Macewan M. R., Schwartz A. G., Xia Y. 2010. Electrospun nanofibers for neural tissue engineering. Nanoscale 2, 35–44 10.1039/b9nr00243j (doi:10.1039/b9nr00243j) [DOI] [PubMed] [Google Scholar]
- 27.Chang C.-J., Hsu S.-H. 2006. The effect of high outflow permeability in asymmetric poly(dl-lactic acid-co-glycolic acid) conduits for peripheral nerve regeneration. Biomaterials 27, 1035–1042 10.1016/j.biomaterials.2005.07.003 (doi:10.1016/j.biomaterials.2005.07.003) [DOI] [PubMed] [Google Scholar]
- 28.Evans G. R. D., et al. 2002. Bioactive poly(-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials 23, 841–848 10.1016/S0142-9612(01)00190-9 (doi:10.1016/S0142-9612(01)00190-9) [DOI] [PubMed] [Google Scholar]
- 29.Deumens R., Bozkurt A., Meek M. F., Marcus M. A. E., Joosten E. A. J., Weis J., Brook G. A. 2010. Repairing injured peripheral nerves: bridging the gap. Prog. Neurobiol. 92, 245–276 10.1016/j.pneurobio.2010.10.002 (doi:10.1016/j.pneurobio.2010.10.002) [DOI] [PubMed] [Google Scholar]
- 30.Nomura H., Tator C. H., Shoichet M. S. 2006. Bioengineered strategies for spinal cord repair. J. Neurotrauma 23, 496–507 10.1089/neu.2006.23.496 (doi:10.1089/neu.2006.23.496) [DOI] [PubMed] [Google Scholar]
- 31.Williams L. R., Longo F. M., Powell H. C., Lundborg G., Varon S. 1983. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: parameters for a bioassay. J. Comp. Neurol. 218, 460–470 10.1002/cne.902180409 (doi:10.1002/cne.902180409) [DOI] [PubMed] [Google Scholar]
- 32.Hoffman-Kim D., Mitchel J. A., Bellamkonda R. V. 2010. Topography, cell response, and nerve regeneration. Annu. Rev. Biomed. Eng. 12, 203–231 10.1146/annurev-bioeng-070909-105351 (doi:10.1146/annurev-bioeng-070909-105351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalbermatten D. F., Kingham P. J., Mahay D., Mantovani C., Pettersson J., Raffoul W., Balcin H., Pierer G., Terenghi G. 2008. Fibrin matrix for suspension of regenerative cells in an artificial nerve conduit. J. Plast. Reconstr. Aesthet. Surg. 61, 669–675 10.1016/j.bjps.2007.12.015 (doi:10.1016/j.bjps.2007.12.015) [DOI] [PubMed] [Google Scholar]
- 34.Pettersson J., Kalbermatten D., Mcgrath A., Novikova L. N. 2010. Biodegradable fibrin conduit promotes long-term regeneration after peripheral nerve injury in adult rats. J. Plast. Reconstr. Aesthet. Surg. 63, 1893–1899 10.1016/j.bjps.2009.11.024 (doi:10.1016/j.bjps.2009.11.024) [DOI] [PubMed] [Google Scholar]
- 35.Winter J., Schmidt C. 2002. Biomimetic strategies and applications in the nervous system. In Biomimetic materials and design (eds Dillow A. K., Lowman A. M.), pp. 375–415 Boca Raton, FL: CRC Press [Google Scholar]
- 36.Matsumoto K., Ohnishi K., Kiyotani T., Sekine T., Ueda H., Nakamura T., Endo K., Shimizu Y. 2000. Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)-collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 868, 315–328 10.1016/S0006-8993(00)02207-1 (doi:10.1016/S0006-8993(00)02207-1) [DOI] [PubMed] [Google Scholar]
- 37.Mosahebi A., Woodward B., Wiberg M., Martin R., Terenghi G. 2001. Retroviral labeling of Schwann cells: in vitro characterization and in vivo transplantation to improve peripheral nerve regeneration. Glia 34, 8–17 10.1002/glia.1035 (doi:10.1002/glia.1035) [DOI] [PubMed] [Google Scholar]
- 38.Bellamkonda R. V. 2006. Peripheral nerve regeneration: an opinion on channels, scaffolds and anisotropy. Biomaterials 27, 3515–3518 [DOI] [PubMed] [Google Scholar]
- 39.Clements I. P., Kim Y.-T., English A. W., Lu X., Chung A., Bellamkonda R. V. 2009. Thin-film enhanced nerve guidance channels for peripheral nerve repair. Biomaterials 30, 3834–3846 10.1016/j.biomaterials.2009.04.022 (doi:10.1016/j.biomaterials.2009.04.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chew S. Y., Mi R., Hoke A., Leong K. W. 2007. Aligned protein–polymer composite fibers enhance nerve regeneration: a potential tissue-engineering platform. Adv. Funct. Mater. 17, 1288–1296 10.1002/adfm.200600441 (doi:10.1002/adfm.200600441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y.-T., Haftel V. K., Kumar S., Bellamkonda R. V. 2008. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials 29, 3117–3127 10.1016/j.biomaterials.2008.03.042 (doi:10.1016/j.biomaterials.2008.03.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshii S., Oka M. 2001. Collagen filaments as a scaffold for nerve regeneration. J. Biomed. Mater. Res. 56, 400–405 (doi:10.1002/1097-4636(20010905)56:3<400::AID-JBM1109>3.0.CO;2-7) [DOI] [PubMed] [Google Scholar]
- 43.Yoshii S., Oka M., Shima M., Taniguchi A., Akagi M. 2003. Bridging a 30-mm nerve defect using collagen filaments. J. Biomed. Mater. Res. A 67A, 467–474 10.1002/jbm.a.10103 (doi:10.1002/jbm.a.10103) [DOI] [PubMed] [Google Scholar]
- 44.Itoh S., Takakuda K., Samejima H., Ohta T., Shinomiya K., Ichinose S. 1999. Synthetic collagen fibers coated with a synthetic peptide containing the YIGSR sequence of laminin to promote peripheral nerve regeneration in vivo. J. Mater. Sci. Mater. Med. 10, 129–134 10.1023/A:1008977221827 (doi:10.1023/A:1008977221827) [DOI] [PubMed] [Google Scholar]
- 45.Yoshii S., Oka M. 2001. Peripheral nerve regeneration along collagen filaments. Brain Res. 888, 158–162 10.1016/S0006-8993(00)03025-0 (doi:10.1016/S0006-8993(00)03025-0) [DOI] [PubMed] [Google Scholar]
- 46.Toba T., et al. 2001. Regeneration of canine peroneal nerve with the use of a polyglycolic acid-collagen tube filled with laminin-soaked collagen sponge: a comparative study of collagen sponge and collagen fibers as filling materials for nerve conduits. J. Biomed. Mater. Res. 58, 622–630 10.1002/jbm.1061 (doi:10.1002/jbm.1061) [DOI] [PubMed] [Google Scholar]
- 47.Ngo T.-T. B., Waggoner P. J., Romero A. A., Nelson K. D., Eberhart R. C., Smith G. M. 2003. Poly(l-lactide) microfilaments enhance peripheral nerve regeneration across extended nerve lesions. J. Neurosci. Res. 72, 227–238 10.1002/jnr.10570 (doi:10.1002/jnr.10570) [DOI] [PubMed] [Google Scholar]
- 48.Wang X., Hu W., Cao Y., Yao J., Wu J., Gu X. 2005. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain 128, 1897–1910 10.1093/brain/awh517 (doi:10.1093/brain/awh517) [DOI] [PubMed] [Google Scholar]
- 49.Lee D.-Y., Choi B.-H., Park J.-H., Zhu S.-J., Kim B.-Y., Huh J.-Y., Lee S.-H., Jung J.-H., Kim S.-H. 2006. Nerve regeneration with the use of a poly(l-lactide-co-glycolic acid)-coated collagen tube filled with collagen gel. J. Cranio-Maxillofac. Surg. 34, 50–56 10.1016/j.jcms.2005.07.011 (doi:10.1016/j.jcms.2005.07.011) [DOI] [PubMed] [Google Scholar]
- 50.Sierpinski P., Garrett J., Ma J., Apel P., Klorig D., Smith T., Koman L. A., Atala A., Van Dyke M. 2008. The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 29, 118–128 10.1016/j.biomaterials.2007.08.023 (doi:10.1016/j.biomaterials.2007.08.023) [DOI] [PubMed] [Google Scholar]
- 51.Ding F., Wu J., Yang Y., Hu W., Zhu Q., Tang X., Liu J., Gu X. 2010. Use of tissue-engineered nerve grafts consisting of a chitosan/poly(lactic-co-glycolic acid)-based scaffold included with bone marrow mesenchymal cells for bridging 50-mm dog sciatic nerve gaps. Tissue Eng. A 16, 3779–3790 10.1089/ten.tea.2010.0299 (doi:10.1089/ten.tea.2010.0299) [DOI] [PubMed] [Google Scholar]
- 52.Stang F., Fansa H., Wolf G., Reppin M., Keilhoff G. 2005. Structural parameters of collagen nerve grafts influence peripheral nerve regeneration. Biomaterials 26, 3083–3091 10.1016/j.biomaterials.2004.07.060 (doi:10.1016/j.biomaterials.2004.07.060) [DOI] [PubMed] [Google Scholar]
- 53.Cai J., Peng X., Nelson K. D., Eberhart R., Smith G. M. 2005. Permeable guidance channels containing microfilament scaffolds enhance axon growth and maturation. J. Biomed. Mater. Res. A 75A, 374–386 10.1002/jbm.a.30432 (doi:10.1002/jbm.a.30432) [DOI] [PubMed] [Google Scholar]
- 54.Kim H., Tator C. H., Shoichet M. S. 2008. Design of protein-releasing chitosan channels. Biotechnol. Prog. 24, 932–937 [DOI] [PubMed] [Google Scholar]
- 55.Rutkowski G., Miller C., Jeftinija S., Mallapragada S. 2004. Synergistic effects of micropatterned biodegradable conduits and Schwann cells on sciatic nerve regeneration. J. Neural Eng. 1, 151–157 10.1088/1741-2560/1/3/004 (doi:10.1088/1741-2560/1/3/004) [DOI] [PubMed] [Google Scholar]
- 56.Hu X., Huang J., Ye Z., Xia L., Li M., Lv B., Shen X., Luo Z. 2009. A novel scaffold with longitudinally oriented microchannels promotes peripheral nerve regeneration. Tissue Eng. A 15, 3297–3308 10.1089/ten.tea.2009.0017 (doi:10.1089/ten.tea.2009.0017) [DOI] [PubMed] [Google Scholar]
- 57.Wang C.-Y., Zhang K.-H., Fan C.-Y., Mo X.-M., Ruan H.-J., Li F.-F. 2011. Aligned natural-synthetic polyblend nanofibers for peripheral nerve regeneration. Acta Biomater. 7, 634–643 10.1016/j.actbio.2010.09.011 (doi:10.1016/j.actbio.2010.09.011) [DOI] [PubMed] [Google Scholar]
- 58.Yao L., De Ruiter G. C. W., Wang H., Knight A. M., Spinner R. J., Yaszemski M. J., Windebank A. J., Pandit A. 2010. Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit. Biomaterials 31, 5789–5797 10.1016/j.biomaterials.2010.03.081 (doi:10.1016/j.biomaterials.2010.03.081) [DOI] [PubMed] [Google Scholar]
- 59.Mahoney M. J., Chen R. R., Tan J., Mark Saltzman W. 2005. The influence of microchannels on neurite growth and architecture. Biomaterials 26, 771–778 10.1016/j.biomaterials.2004.03.015 (doi:10.1016/j.biomaterials.2004.03.015) [DOI] [PubMed] [Google Scholar]
- 60.Yao L., Wang S., Cui W., Sherlock R., O'connell C., Damodaran G., Gorman A., Windebank A., Pandit A. 2009. Effect of functionalized micropatterned PLGA on guided neurite growth. Acta Biomater. 5, 580–588 10.1016/j.actbio.2008.09.002 (doi:10.1016/j.actbio.2008.09.002) [DOI] [PubMed] [Google Scholar]
- 61.Chang C.-J., Hsu S.-H. 2004. The effects of low-intensity ultrasound on peripheral nerve regeneration in poly(-lactic acid-co-glycolic acid) conduits seeded with Schwann cells. Ultrasound Med. Biol. 30, 1079–1084 10.1016/j.ultrasmedbio.2004.06.005 (doi:10.1016/j.ultrasmedbio.2004.06.005) [DOI] [PubMed] [Google Scholar]
- 62.Panseri S., Cunha C., Lowery J., Del Carro U., Taraballi F., Amadio S., Vescovi A., Gelain F. 2008. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol. 8, 39. 10.1186/1472-6750-8-39 (doi:10.1186/1472-6750-8-39) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oh S. H., Kim J. H., Song K. S., Jeon B. H., Yoon J. H., Seo T. B., Namgung U., Lee I. W., Lee J. H. 2008. Peripheral nerve regeneration within an asymmetrically porous PLGA/pluronic F127 nerve guide conduit. Biomaterials 29, 1601–1609 10.1016/j.biomaterials.2007.11.036 (doi:10.1016/j.biomaterials.2007.11.036) [DOI] [PubMed] [Google Scholar]
- 64.Hadlock T., Sundback C., Hunter D., Cheney M., Vacanti J. P. 2000. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 6, 119–127 10.1089/107632700320748 (doi:10.1089/107632700320748) [DOI] [PubMed] [Google Scholar]
- 65.Yang Y., De Laporte L., Rives C. B., Jang J.-H., Lin W.-C., Shull K. R., Shea L. D. 2005. Neurotrophin releasing single and multiple lumen nerve conduits. J. Control. Release 104, 433–446 10.1016/j.jconrel.2005.02.022 (doi:10.1016/j.jconrel.2005.02.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Ruiter G. C., Spinner R. J., Malessy M. J., Moore M. J., Sorenson E. J., Currier B. L., Yaszemski M. J., Windebank A. J. 2008. Accuracy of motor axon regeneration across autograft, single-lumen, and multichannel poly(lactic-co-glycolic acid) nerve tubes. Neurosurgery 63, 144–153 10.1227/01.NEU.0000335081.47352.78 (doi:10.1227/01.NEU.0000335081.47352.78) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santiago L. Y. C.-A., Clavijo-Alvarez J., Brayfield C., Rubin J. P., Marra K. G. 2009. Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplant. 18, 145–158 10.3727/096368909788341289 (doi:10.3727/096368909788341289) [DOI] [PubMed] [Google Scholar]
- 68.Rangappa N., Romero A., Nelson K. D., Eberhart R. C., Smith G. M. 2000. Laminin-coated poly(l-lactide) filaments induce robust neurite growth while providing directional orientation. J. Biomed. Mater. Res. 51, 625–634 (doi:10.1002/1097-4636(20000915)51:4<625::AID-JBM10>3.0.CO;2-U) [DOI] [PubMed] [Google Scholar]
- 69.Wang S., Yaszemski M. J., Knight A. M., Gruetzmacher J. A., Windebank A. J., Lu L. 2009. Photo-crosslinked poly(ε-caprolactone fumarate) networks for guided peripheral nerve regeneration: material properties and preliminary biological evaluations. Acta Biomater. 5, 1531–1542 10.1016/j.actbio.2008.12.015 (doi:10.1016/j.actbio.2008.12.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Itoh S., Takakuda K., Ichinose S., Kikuchi M., Schinomiya K. 2001. A study of induction of nerve regeneration using bioabsorbable tubes. J. Reconstr. Microsurg. 17, 115–124. 10.1055/s-2001-12700 (doi:10.1055/s-2001-12700) [DOI] [PubMed] [Google Scholar]
- 71.Schense J. C., Bloch J., Aebischer P., Hubbell J. A. 2000. Enzymatic incorporation of bioactive peptides into fibrin matrices enhances neurite extension. Nat. Biotechnol. 18, 415–419 10.1038/74473 (doi:10.1038/74473) [DOI] [PubMed] [Google Scholar]
- 72.Chung H. J., Park T. G. 2007. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 59, 249–262 10.1016/j.addr.2007.03.015 (doi:10.1016/j.addr.2007.03.015) [DOI] [PubMed] [Google Scholar]
- 73.Armstrong S. J., Wiberg M., Terenghi G., Kingham P. J. 2007. ECM molecules mediate both Schwann cell proliferation and activation to enhance neurite outgrowth. Tissue Eng. 13, 2863–2870 10.1089/ten.2007.0055 (doi:10.1089/ten.2007.0055) [DOI] [PubMed] [Google Scholar]
- 74.Yu X., Bellamkonda R. V. 2003. Tissue-engineered scaffolds are effective alternatives to autografts for bridging peripheral nerve gaps. Tissue Eng. 9, 421–430 10.1089/107632703322066606 (doi:10.1089/107632703322066606) [DOI] [PubMed] [Google Scholar]
- 75.Yu X., Dillon G. P., Bellamkonda R. V. 1999. A laminin and nerve growth factor-laden three-dimensional scaffold for enhanced neurite extension. Tissue Eng. 5, 291–304 10.1089/ten.1999.5.291 (doi:10.1089/ten.1999.5.291) [DOI] [PubMed] [Google Scholar]
- 76.Lee J. H., Yu H.-S., Lee G.-S., Ji A., Hyun J. K., Kim H.-W. Collagen gel three-dimensional matrices combined with adhesive proteins stimulate neuronal differentiation of mesenchymal stem cells. J. R. Soc. Interface 8, 998–1010 10.1098/rsif.2010.0613 (doi:10.1098/rsif.2010.0613). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Itoh S., Yamaguchi I., Suzuki M., Ichinose S., Takakuda K., Kobayashi H., Shinomiya K., Tanaka J. 2003. Hydroxyapatite-coated tendon chitosan tubes with adsorbed laminin peptides facilitate nerve regeneration in vivo. Brain Res. 993, 111–123 10.1016/j.brainres.2003.09.003 (doi:10.1016/j.brainres.2003.09.003) [DOI] [PubMed] [Google Scholar]
- 78.Barras F. M., Pasche P., Bouche N., Aebischer P., Zurn A. D. 2002. Glial cell line-derived neurotrophic factor released by synthetic guidance channels promotes facial nerve regeneration in the rat. J. Neurosci. Res. 70, 746–755 10.1002/jnr.10434 (doi:10.1002/jnr.10434) [DOI] [PubMed] [Google Scholar]
- 79.Hobson M. I. 2002. Increased vascularisation enhances axonal regeneration within an acellular nerve conduit. Ann. R. Coll. Surg. Engl. 84, 47–53 [PMC free article] [PubMed] [Google Scholar]
- 80.Xu X., et al. 2003. Peripheral nerve regeneration with sustained release of poly(phosphoester) microencapsulated nerve growth factor within nerve guide conduits. Biomaterials 24, 2405–2412 10.1016/S0142-9612(03)00109-1 (doi:10.1016/S0142-9612(03)00109-1) [DOI] [PubMed] [Google Scholar]
- 81.Piquilloud G., Christen T., Pfister L. A., Gander B., Papaloïzos M. Y. 2007. Variations in glial cell line-derived neurotrophic factor release from biodegradable nerve conduits modify the rate of functional motor recovery after rat primary nerve repairs. Eur. J. Neurosci. 26, 1109–1117 10.1111/j.1460-9568.2007.05748.x (doi:10.1111/j.1460-9568.2007.05748.x) [DOI] [PubMed] [Google Scholar]
- 82.Chang C.-J. 2009. Effects of nerve growth factor from genipin-crosslinked gelatin in polycaprolactone conduit on peripheral nerve regeneration—in vitro and in vivo. J. Biomed. Mater. Res. A 91A, 586–596 10.1002/jbm.a.32252 (doi:10.1002/jbm.a.32252) [DOI] [PubMed] [Google Scholar]
- 83.Wood M. D., Macewan M. R., French A. R., Moore A. M., Hunter D. A., Mackinnon S. E., Moran D. W., Borschel G. H., Sakiyama-Elbert S. E. 2010. Fibrin matrices with affinity-based delivery systems and neurotrophic factors promote functional nerve regeneration. Biotechnol. Bioeng. 106, 970–979 10.1002/bit.22766 (doi:10.1002/bit.22766) [DOI] [PubMed] [Google Scholar]
- 84.Shen H., Shen Z.-L., Zhang P.-H., Chen N.-L., Wang Y.-C., Zhang Z.-F., Jin Y.-Q. 2010. Ciliary neurotrophic factor-coated polylactic-polyglycolic acid chitosan nerve conduit promotes peripheral nerve regeneration in canine tibial nerve defect repair. J. Biomed. Mater. Res. B Appl. Biomater. 95B, 161–170 10.1002/jbm.b.31696 (doi:10.1002/jbm.b.31696) [DOI] [PubMed] [Google Scholar]
- 85.Liu J.-J., Wang C.-Y., Wang J.-G., Ruan H.-J., Fan C.-Y. 2010. Peripheral nerve regeneration using composite poly(lactic acid-caprolactone)/nerve growth factor conduits prepared by coaxial electrospinning. J. Biomed. Mater. Res. A 96, 13–20 10.1002/jbm.a.32946 (doi:10.1002/jbm.a.32946) [DOI] [PubMed] [Google Scholar]
- 86.Wang D.-Y., Huang Y.-Y. 2008. Fabricate coaxial stacked nerve conduits through soft lithography and molding processes. J. Biomed. Mater. Res. A 85A, 434–438 10.1002/jbm.a.31568 (doi:10.1002/jbm.a.31568) [DOI] [PubMed] [Google Scholar]
- 87.Fine E. G., Decosterd I., Papaloïzos M., Zurn A. D., Aebischer P. 2002. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur. J. Neurosci. 15, 589–601 10.1046/j.1460-9568.2002.01892.x (doi:10.1046/j.1460-9568.2002.01892.x) [DOI] [PubMed] [Google Scholar]
- 88.Madduri S., Feldman K., Tervoort T., Papaloïzos M., Gander B. 2010. Collagen nerve conduits releasing the neurotrophic factors GDNF and NGF. J. Control. Release 143, 168–174 10.1016/j.jconrel.2009.12.017 (doi:10.1016/j.jconrel.2009.12.017) [DOI] [PubMed] [Google Scholar]
- 89.Wood M. D., Moore A. M., Hunter D. A., Tuffaha S., Borschel G. H., Mackinnon S. E., Sakiyama-Elbert S. E. 2009. Affinity-based release of glial-derived neurotrophic factor from fibrin matrices enhances sciatic nerve regeneration. Acta Biomater. 5, 959–968 10.1016/j.actbio.2008.11.008 (doi:10.1016/j.actbio.2008.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mcgrath A. M., Novikova L. N., Novikov L. N., Wiberg M. 2010. BDTM PuraMatrixTM peptide hydrogel seeded with Schwann cells for peripheral nerve regeneration. Brain Res. Bull. 83, 207–213 10.1016/j.brainresbull.2010.07.001 (doi:10.1016/j.brainresbull.2010.07.001) [DOI] [PubMed] [Google Scholar]
- 91.Greenberg D. A., Jin K. 2005. From angiogenesis to neuropathology. Nature 438, 954–959 10.1038/nature04481 (doi:10.1038/nature04481) [DOI] [PubMed] [Google Scholar]
- 92.Madduri S., Gander B. 2010. Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J. Peripher. Nerv. Syst. 15, 93–103 10.1111/j.1529-8027.2010.00257.x (doi:10.1111/j.1529-8027.2010.00257.x) [DOI] [PubMed] [Google Scholar]
- 93.Deister C., Schmidt C. E. 2006. Optimizing neurotrophic factor combinations for neurite outgrowth. J. Neural Eng. 3, 172. 10.1088/1741-2560/3/2/011 (doi:10.1088/1741-2560/3/2/011) [DOI] [PubMed] [Google Scholar]
- 94.Peleshok J., Saragovi H. U. 2006. Functional mimetics of neurotrophins and their receptors. Biochem. Soc. Trans. 34, 612–617 [DOI] [PubMed] [Google Scholar]
- 95.Lietz M., Dreesmann L., Hoss M., Oberhoffner S., Schlosshauer B. 2006. Neuro tissue engineering of glial nerve guides and the impact of different cell types. Biomaterials 27, 1425–1436 10.1016/j.biomaterials.2005.08.007 (doi:10.1016/j.biomaterials.2005.08.007) [DOI] [PubMed] [Google Scholar]
- 96.Longo F. M., Xie Y., Massa S. M. 2005. Neurotrophin small molecule mimetics: candidate therapeutic agents for neurological disorders. Curr. Med. Chem. Cent. Nerv. Syst. Agents 5, 29–41 [Google Scholar]
- 97.Abu-Rub M. T., Billiar K. L., Van Es M. H., Knight A., Rodriguez B. J., Zeugolis D. I., Mcmahon S., Windebank A. J., Pandit A. 2011. Nano-textured self-assembled aligned collagen hydrogels promote directional neurite guidance and overcome inhibition by myelin associated glycoprotein. Soft Matter 7, 2770–2781 10.1039/c0sm01062f (doi:10.1039/c0sm01062f) [DOI] [Google Scholar]
- 98.Dash B. C., Mahor S., Carroll O., Mathew A., Wang W., Woodhouse K. A., Pandit A. 2011. Tunable elastin-like polypeptide hollow sphere as a high payload and controlled delivery gene depot. J. Control. Release 152, 382–392 10.1016/j.jconrel.2011.03.006 (doi:10.1016/j.jconrel.2011.03.006) [DOI] [PubMed] [Google Scholar]
- 99.Réthoré G., Pandit A. 2010. Use of templates to fabricate nanoscale spherical structures for defined architectural control. Small 6, 488–498 10.1002/smll.200901253 (doi:10.1002/smll.200901253) [DOI] [PubMed] [Google Scholar]
- 100.Oliveira J. T., Almeida F. M., Biancalana A., Baptista A. F., Tomaz M. A., Melo P. A., Martinez A. M. B. 2010. Mesenchymal stem cells in a polycaprolactone conduit enhance median-nerve regeneration, prevent decrease of creatine phosphokinase levels in muscle, and improve functional recovery in mice. Neuroscience 170, 1295–1303 10.1016/j.neuroscience.2010.08.042 (doi:10.1016/j.neuroscience.2010.08.042) [DOI] [PubMed] [Google Scholar]
- 101.Di Summa P. G., Kingham P. J., Raffoul W., Wiberg M., Terenghi G., Kalbermatten D. F. 2010. Adipose-derived stem cells enhance peripheral nerve regeneration. J. Plast. Reconstr. Aesthet. Surg. 63, 1544–1552 10.1016/j.bjps.2009.09.012 (doi:10.1016/j.bjps.2009.09.012) [DOI] [PubMed] [Google Scholar]
- 102.Ladak A., Olson J., Tredget E. E., Gordon T. 2011. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp. Neurol. 228, 242–252 [DOI] [PubMed] [Google Scholar]
- 103.Di Summa P. G., Kalbermatten D. F., Pralong E., Raffoul W., Kingham P. J., Terenghi G. 2011. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience 181, 278–291 10.1016/j.neuroscience.2011.02.052 (doi:10.1016/j.neuroscience.2011.02.052) [DOI] [PubMed] [Google Scholar]
- 104.Caddick J., Kingham P. J., Gardiner N. J., Wiberg M., Terenghi G. 2006. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia 54, 840–849 10.1002/glia.20421 (doi:10.1002/glia.20421) [DOI] [PubMed] [Google Scholar]
- 105.Kingham P. J., Kalbermatten D. F., Mahay D., Armstrong S. J., Wiberg M., Terenghi G. 2007. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 207, 267–274 10.1016/j.expneurol.2007.06.029 (doi:10.1016/j.expneurol.2007.06.029) [DOI] [PubMed] [Google Scholar]
- 106.Radtke C., Schmitz B., Spies M., Kocsis J. D., Vogt P. M. 2009. Peripheral glial cell differentiation from neurospheres derived from adipose mesenchymal stem cells. Int. J. Dev. Neurosci. 27, 817–823 10.1016/j.ijdevneu.2009.08.006 (doi:10.1016/j.ijdevneu.2009.08.006) [DOI] [PubMed] [Google Scholar]
- 107.Chen Y.-S., Chang J.-Y., Cheng C.-Y., Tsai F.-J., Yao C.-H., Liu B.-S. 2005. An in vivo evaluation of a biodegradable genipin-cross-linked gelatin peripheral nerve guide conduit material. Biomaterials 26, 3911–3918 10.1016/j.biomaterials.2004.09.060 (doi:10.1016/j.biomaterials.2004.09.060) [DOI] [PubMed] [Google Scholar]
- 108.Kaewkhaw R., Scutt A. M., Haycock J. W. 2011. Anatomical site influences the differentiation of adipose-derived stem cells for Schwann-cell phenotype and function. Glia 59, 734–749 10.1002/glia.21145 (doi:10.1002/glia.21145) [DOI] [PubMed] [Google Scholar]
- 109.Timmer M., Robben S., Uuml Ller-Ostermeyer F., Nikkhah G., Grothe C. 2003. Axonal regeneration across long gaps in silicone chambers filled with Schwann cells overexpressing high molecular weight FGF-2. Cell Transplant. 12, 265–277 [DOI] [PubMed] [Google Scholar]
- 110.Haastert K., Lipokatić E., Fischer M., Timmer M., Grothe C. 2006. Differentially promoted peripheral nerve regeneration by grafted Schwann cells over-expressing different FGF-2 isoforms. Neurobiology of Disease. 21, 138–153 10.1016/j.nbd.2005.06.020 (doi:10.1016/j.nbd.2005.06.020) [DOI] [PubMed] [Google Scholar]
- 111.Li Q., Ping P., Jiang H., Liu K. 2006. Nerve conduit filled with GDNF gene-modified Schwann cells enhances regeneration of the peripheral nerve. Microsurgery 26, 116–121 10.1002/micr.20192 (doi:10.1002/micr.20192) [DOI] [PubMed] [Google Scholar]
- 112.Schmitte R., Tipold A., Stein V. M., Schenk H., Flieshardt C., Grothe C., Haastert K. 2010. Genetically modified canine Schwann cells—in vitro and in vivo evaluation of their suitability for peripheral nerve tissue engineering. J. Neurosci. Methods 186, 202–208 10.1016/j.jneumeth.2009.11.023 (doi:10.1016/j.jneumeth.2009.11.023) [DOI] [PubMed] [Google Scholar]
- 113.Bunting S., Di Silvio L., Deb S., Hall S. 2005. Bioresorbable glass fibres facilitate peripheral nerve regeneration. J. Hand Surg. Br. Eur. Vol. 30, 242–247 10.1016/j.jhsb.2004.11.003 (doi:10.1016/j.jhsb.2004.11.003) [DOI] [PubMed] [Google Scholar]
- 114.Nichterwitz S., Hoffmann N., Hajosch R., Oberhoffner S., Schlosshauer B. 2010. Bioengineered glial strands for nerve regeneration. Neurosci. Lett. 484, 118–122 10.1016/j.neulet.2010.08.028 (doi:10.1016/j.neulet.2010.08.028) [DOI] [PubMed] [Google Scholar]
- 115.Colangelo A. M., et al. 2008. A new nerve growth factor-mimetic peptide active on neuropathic pain in rats. J. Neurosci. 28, 2698–2709 10.1523/JNEUROSCI.5201-07.2008 (doi:10.1523/JNEUROSCI.5201-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Webster N., Pirrung M. 2008. Small molecule activators of the TRK receptors for neuroprotection. BMC Neurosci. 9(Suppl. 2), S1. 10.1186/1471-2202-9-S2-S1 (doi:10.1186/1471-2202-9-S2-S1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fan W., Gu J., Hu W., Deng A., Ma Y., Liu J., Ding F., Gu X. 2008. Repairing a 35-mm-long median nerve defect with a chitosan/PGA artificial nerve graft in the human: a case study. Microsurgery 28, 238–242 10.1002/micr.20488 (doi:10.1002/micr.20488) [DOI] [PubMed] [Google Scholar]
- 118.Gu J., Hu W., Deng A., Zhao Q., Lu S., Gu X. 2011. Surgical repair of a 30 mm long human median nerve defect in the distal forearm by implantation of a chitosan–PGA nerve guidance conduit. J. Tissue Eng. Regen. Med. (doi:10.1002/term.407) [DOI] [PubMed] [Google Scholar]
- 119.Hung V., Dellon A. L. 2008. Reconstruction of a 4-cm human median nerve gap by including an autogenous nerve slice in a bioabsorbable nerve conduit: case report. J. Hand Surg. 33, 313–315 10.1016/j.jhsa.2007.12.008 (doi:10.1016/j.jhsa.2007.12.008) [DOI] [PubMed] [Google Scholar]
- 120.Boeshore K. L., Schreiber R. C., Vaccariello S. A., Sachs H. H., Salazar R., Lee J., Ratan R. R., Leahy P., Zigmond R. E. 2004. Novel changes in gene expression following axotomy of a sympathetic ganglion: a microarray analysis. J. Neurobiol. 59, 216–235 10.1002/neu.10308 (doi:10.1002/neu.10308) [DOI] [PubMed] [Google Scholar]
- 121.Jiménez C. R., Stam F. J., Li K. W., Gouwenberg Y., Hornshaw M. P., De Winter F., Verhaagen J., Smit A. B. 2005. Proteomics of the injured rat sciatic nerve reveals protein expression dynamics during regeneration. Mol. Cell. Proteomics 4, 120–132 10.1074/mcp.M400076-MCP200 (doi:10.1074/mcp.M400076-MCP200) [DOI] [PubMed] [Google Scholar]
- 122.Kubo T., Yamashita T., Yamaguchi A., Hosokawa K., Tohyama M. 2002. Analysis of genes induced in peripheral nerve after axotomy using cDNA microarrays. J. Neurochem. 82, 1129–1136 10.1046/j.1471-4159.2002.01060.x (doi:10.1046/j.1471-4159.2002.01060.x) [DOI] [PubMed] [Google Scholar]
- 123.D'antonio M., Michalovich D., Paterson M., Droggiti A., Woodhoo A., Mirsky R., Jessen K. R. 2006. Gene profiling and bioinformatic analysis of Schwann cell embryonic development and myelination. Glia 53, 501–515 10.1002/glia.20309 (doi:10.1002/glia.20309) [DOI] [PubMed] [Google Scholar]
- 124.Bosse F., Hasenpusch-Theil K., Küry P., Müller H. W. 2006. Gene expression profiling reveals that peripheral nerve regeneration is a consequence of both novel injury-dependent and reactivated developmental processes. J. Neurochem. 96, 1441–1457 10.1111/j.1471-4159.2005.03635.x (doi:10.1111/j.1471-4159.2005.03635.x) [DOI] [PubMed] [Google Scholar]
- 125.Nagarajan R., Le N., Mahoney H., Araki T., Milbrandt J. 2002. Deciphering peripheral nerve myelination by using Schwann cell expression profiling. Proc. Natl Acad. Sci. USA 99, 8998–9003 10.1073/pnas.132080999 (doi:10.1073/pnas.132080999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou F.-Q., Snider W. D. 2006. Intracellular control of developmental and regenerative axon growth. Phil. Trans. R. Soc. B 361, 1575–1592 10.1098/rstb.2006.1882 (doi:10.1098/rstb.2006.1882) [DOI] [PMC free article] [PubMed] [Google Scholar]