Abstract
Until 2004, the skin disease known as Buruli ulcer, caused by Mycobacterium ulcerans, could only be treated by surgery and skin grafting. Although this worked reasonably well on early lesions typically found in patients in Australia, the strategy was usually impractical on large lesions resulting from diagnostic delay in patients in rural West Africa. Based on promising preclinical studies, treatment trials in West Africa have shown that a combination of rifampin and streptomycin administered daily for 8 weeks can kill M. ulcerans bacilli, arrest the disease, and promote healing without relapse or reduce the extent of surgical excision. Improved treatment options are the focus of research that has increased tremendously since the WHO began its Global Buruli Ulcer Initiative in 1998.
Keywords: antibiotics, Buruli ulcer, clinical trials, mouse footpad model, Mycobacterium ulcerans, treatment
Background & early descriptions of the disease
Mycobacterium ulcerans causes a progressively destructive skin disease that may have been recognized as early as 1897 by Albert Cook, a medical missionary, who spent his entire career in colonial Uganda. His meticulous records maintained in the Mengo Hospital library in Kampala and photographed by the Australian pathologist, John Hayman [1,2], indicate by drawings and notes that the disease was most likely present in Uganda at that time. It was eventually noted by Belgian physicians working in the Democratic Republic of the Congo and then by workers in Australia. Owing to a faulty incubator, the Australian researchers found that the organism, isolated from lesions of patients in Bairnsdale, southeastern Australia, grew at 32°C rather than 37°C, and produced the first published description of the disease in 1948 [3]. Reports from the Congo appeared in the 1950s [4,5], but it was only after the publication in the Lancet by British and Ugandan medical scientists reporting a focus of cases 2 h drive northeast of Kampala in Buruli (now Nakasongola), a swampy area near the Nile and Lake Kyoga, that the disease acquired the name, Buruli ulcer, by which it is known today [6].
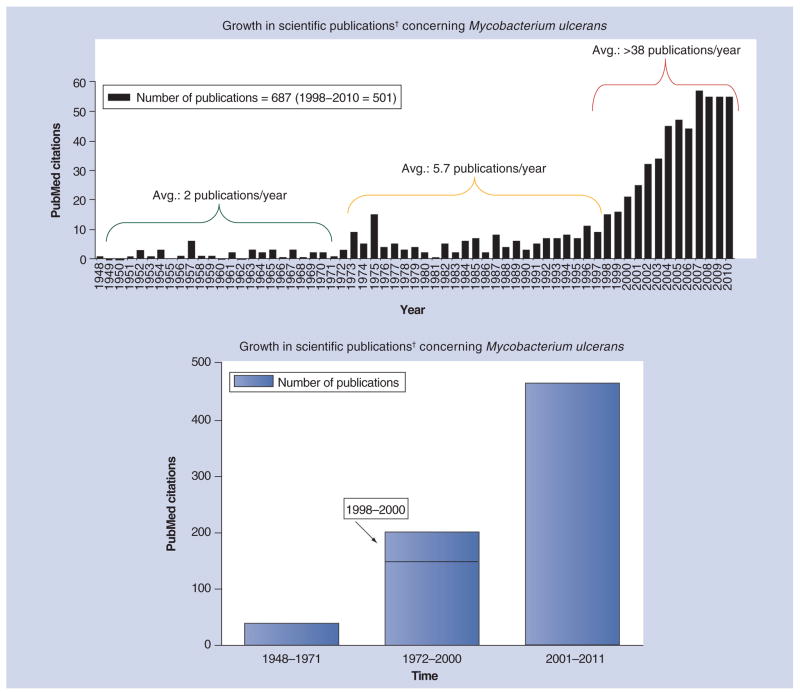
There were approximately three dozen publications concerning M. ulcerans from 1951 to 1971 (Figure 1) and were primarily authored by Belgian, British, Australian and American scientists, largely reflecting the areas where the disease was seen. Among the key insights were the following: that there is an association of cases with riverine, swampy areas [1,7]; the disease is not contagious [7]; the organism, uniquely among mycobacteria, likely produces a toxin that destroys subcutaneous fat cells [8,9]; mice, when infected in the footpad, permit bacterial growth with concomitant swelling [10]; and a number of drugs have activity against M. ulcerans in vitro and in mice [11–14].
Figure 1. Growth in scientific publications concerning Mycobacterium ulcerans has accelerated since the late 1990s.
For the first 22 years after the discovery of the causative organism of what is now called Buruli ulcer, publications were rare (approximately two per year) but outlined much of the research that has answered important questions about pathogenesis, epidemiology and treatment. There was a slight increase in publication frequency (nearly six per year) following publication of the Ugandan studies in the 1970s. However, with increased involvement from the WHO and definitive characterization of the mycolactone toxin, there have been over 38 publications per year since 1998.
†Based on a search of PubMed using Mycobacterium ulcerans and publication year.
Avg.: Average.
In retrospect, these insights were more or less conclusive or have been since confirmed while others were tantalizingly close to what is known or practiced today. Most remarkably, treatment with antibiotics, when attempted, was almost always provided as monotherapy and was always somewhat disappointing.
The basic facts currently known regarding Buruli ulcer based on research through 1971
Although early reports [3,4,6] gave some description of the area where patients lived and acquired the disease, the first thorough environmental study was carried out by Lunn et al. in northwestern Uganda [1]. They were able to confirm that patients live along the Nile in small crowded huts with other unaffected persons. They proposed that water contact was important but could not isolate the organism from water or any aquatic animal. The reservoir remains unknown but riverine, swampy environments seem to be universally found in areas of transmission in West Africa, Australia and Latin America. In 2008, the first cultivation and characterization of M. ulcerans from a nonbiting aquatic bug obtained in Benin was reported [15] and Marion et al. were able to detect acid-fast bacilli (AFB) and M. ulcerans DNA from biting insects obtained from endemic riverine areas disrupted by intensive agricultural activity, but not from nonendemic still-forested river areas in Cameroon [16]. M. ulcerans DNA was also detected in mosquitoes using the IS2404 probe after a large outbreak in the Point Lonsdale beach resort in southeastern Australia [17]. Risk of acquiring the disease was reduced in those regularly using insect repellent or wearing long trousers [18]. In Australia, evidence of M. ulcerans DNA in aquatic environments was found in an endemic coastal resort town but remarkably also in the feces of a high proportion of possums (tree-dwelling marsupials) in one outbreak area, some of whom have had active Buruli ulcer [19].
Although the principal mycobacterial diseases, tuberculosis and leprosy, nearly always involve transmission from one human, or other animal host, to another, Buruli ulcer is almost always found in association with an environmental source. This was perhaps most clearly demonstrated in refugees from Rwanda who had never experienced the disease in their native country but endured an epidemic affecting 9% of the community in the Kinyara refugee settlement on the banks of the Nile. Within 2–3 months after moving to a new camp 150 miles away near Lake Albert, no further cases were observed over a 2-year period [7]. All evidence indicated that there was a nonhuman, environmental source of infection.
Studies of the pathology of M. ulcerans disease were carried out in early investigations in Australia and The Democratic Republic of the Congo [3,4]. The studies reported by Connor and Lunn in 1965 included a patient with a preulcerative lesion that was excised and carefully examined [8]. It was noted that bacilli were concentrated in the center of the necrotic zone and there was extensive necrosis most likely caused by a diffusible toxic substance. Attempts were made to characterize this substance in subsequent decades [20–22]. It was shown that the histopathology observed in human skin was reproducible in guinea pig skin. However, it turns out that the toxin is not a protein but in fact an immunosuppressive macrolide, now designated mycolactone, purified and characterized in the laboratory of Pamela Small [23–25].
Although MacCallum et al. infected mice with M. ulcerans intraperitoneally and observed edema in subcutaneous tissue and ulceration of the scrotum, it took 3–6 months to appear [3]. While on sabbatical at the Rockefeller Institute in New York in 1949, Australian virologist, Frank Fenner, first found that progressive swelling occurred after inoculation of bacilli in mouse footpads, with lesions appearing 1–16 week later in a dose-dependent manner (Box 1). Fenner estimated the generation time of M. ulcerans in the mouse foot to be approximately 3–4 days [10], whereas in vitro with Dubos medium the generation time was 48 h. Leach and Fenner went on to evaluate available antimycobacterial compounds – streptomycin (STR), para-amino-salicylic acid (PAS), thiosemicarbazone and isoniazid – in vitro and in the footpad model [12]. Although PAS showed some activity in vitro, only STR was active in vivo (i.e., prevented swelling) and only if administered either on the day of footpad inoculation or at the onset of visible swelling. The drug did not appear to have activity against M. ulcerans in advanced lesions, although intercurrent infections compromised this arm of the experiment. Using an intravenous mouse infection model, Feldman and Karlson acknowledged the limitations of the model but did support the findings of the effectiveness of STR treatment as well as suggesting some benefit with dapsone [11].
Box 1. Reflections on the mouse footpad model.
Developed by Australian virologist Frank Fenner in the 1950s [10].
Mycobacterium ulcerans bacilli are injected just under the surface of the skin into a relatively cool area of the mouse body, mimicking the peripheral location of most human lesions.
Gross pathology is progressive swelling of the footpad that often leads to ulceration and loss of the foot and leg if let untreated, with no or limited pain, and is easily monitored for both worsening infection and response to antibiotic treatment. A reduction in swelling is observed with effective curative therapy (for examples see [77,95]). Prevention of swelling can be seen in mice treated early after infection (for an example [97]).
Although the inoculum is probably higher than that occurring in human infection, histopathological analysis of both experimentally infected footpads and clinical lesions typically results in the observation of large numbers of acid-fast bacilli. The acid-fast bacilli degenerate and become beaded in appearance in response to treatment in both mice [Converse PJ et al., Unpublished Observations] and in humans [83,90].
Unlike human or guinea pig skin, there is relatively little subcutaneous fat in mice. Therefore, the action of the mycolactone toxin on adipocytes is not as marked in mice.
Microbiological analysis of footpads allows for culture of the entire lesion and the evaluation of bactericidal and bacteriostatic drug activity.
Disseminated disease and edematous lesions occur in humans. Dissemination of M. ulcerans infection, with contralateral foot involvement is rarely observed in mice. Immunocompetent mice may lose feet after ulceration of lesions in the absence of treatment [Converse PJ et al., Unpublished Observations]. Immunodeficient mice reveal dissemination to the thigh and the blood as determined by both culture and histopathology. Similarly, reports of HIV-coinfected patients with Buruli ulcer also indicate that the disease may be more severe in these patients [102–105].
The mouse footpad model is a useful tool for the evaluation of drugs that are active against M. ulcerans in vivo. Mice do not always metabolize drugs in the same manner as humans. A dose that is potent in humans may not be in mice, although rifampin and streptomycin are identical or very similar. The clarithromycin dose is quite different and therefore requires different dosing regimens in mice to avoid interference with the activity of rifampin. The equipotent dose has to be determined for any new potential drug.
Although the mouse footpad model does have some resemblance to human lesions, there are forms of the human disease (e.g., edema) that are not reproduced. Remarkably, rifampin plus streptomycin treatment appears to be effective in most presentations of disease in humans.
Remarkably, the mouse footpad model was rarely used in the next 40 years of albeit limited research on M. ulcerans. Parenthetically, at Fenner’s suggestion [Morrison N, Pers. Comm.], Charles Shepard at the Centers for Disease Control, adapted the method for the first successful in vivo model of the otherwise uncultivable leprosy bacillus [26]. The model was used in centers around the world to evaluate the activity of new compounds and to monitor patient isolates for drug resistance. Lunn and Rees observed some activity against M. ulcerans in the mouse with clofazimine but no activity in humans [27,28]. Pattyn and Royackers confirmed the activity of STR using the footpad model [13]. Stanford and Phillips also showed that rifampin (RIF) had some benefit [14]. The authors mentioned clinical trials in progress with RIF monotherapy. The results of such trials have never been fully published but have been alluded to in reviews and the WHO reports by Meyers [29,30]. Working in the Congo in the early 1970s, he found that daily RIF alone at 600 mg/day (adult dose; adjusted down for children) for approximately 6–8 weeks, healed most preulcerative and some early small, ulcerated lesions, but not advanced ulcers. Grange and Meyers noted the limited availability of RIF [Grange J, Pers. Comm.], which was also very expensive, and recalled significant delays in customs in Kinshasa. These factors effectively precluded evaluating sufficient Buruli ulcer patients. In fact, RIF was at that time formally reserved for drug-resistant tuberculosis and, later, for leprosy [Meyers, Pers. Comm.]. A RIF monotherapy trial was also apparently initiated concurrently in Uganda [31] by the late W David Revill [Barker DJP, Morrow R, Pers. Comm.] who never published his findings. J Stanford stated that the trial, conducted in two Ugandan sites, located to the north and south of Lake Kyoga, was never completed [Stanford J, Pers. Comm.]. Those data have apparently not been recoverable. In 1975, the benefit of RIF in the mouse model was confirmed by Havel and Pattyn [32]. These authors noted that almost all testing had used monotherapy and proposed that a rational approach required combined therapeutic regimens. However, their discouraging conclusion was that surgery or an unspecified preventive measure would likely be more economical. Of course, RIF was still a very expensive drug until the 1990s. Unlike the more prevalent, better known and more feared mycobacterial disease, leprosy, there may have been no charitable organizations that could have funded research and, eventually, funded treatment of patients with Buruli ulcer. In any case, the potential of RIF in Buruli ulcer therapy was a missed opportunity, although one might also argue that its potential was not lost by using it as a single drug at that time rather than in the combination therapy regimens used in recent years.
Further expansion of knowledge from 1971–2000
From 36 publications concerning M. ulcerans and Buruli ulcer over the first two decades (from 1948 to 1971), the number of papers increased to approximately 150 over the next 25 years (from 1972 until 1997) (Figure 1). Some efforts were made to identify better treatment methods [33–35] and the action of the suspected toxin [20–22], including observations that the pathology seen in human skin can be reproduced in guinea pig skin. The major advances in that period were improved diagnostic testing possibilities through the application of PCR [36–40] and documentation of the emergence of the disease in West Africa [41–47]. At the very end of this period, the toxin and its structure were fully identified [23–25,48], preliminary genome assessments were made together with the recognition of the relationship between M. ulcerans and Mycobacterium marinum [49], potential drug treatment options were evaluated with the systematic use of the mouse footpad model [50], and, very significantly, the WHO made a commitment to battle M. ulcerans disease in 1997 [201], followed by the establishment of the Global Buruli Ulcer Initiative in 1998 and a first international conference on the disease in Côte d’Ivoire and the issuance of the Yamoussoukro Declaration [202]. Thorough reviews documenting existing knowledge as well as audiovisual materials were also produced [51–55]. The decade or so that has followed has been highly productive in terms of research in epidemiology [56], natural history [16,57], diagnostic methods [58,59], immunology [60–63] and vaccine testing [64,65], pathology [66–68], and treatment of this bacterial disease with antibiotics rather than surgery. Very significantly, the M. ulcerans genome was deciphered along with the description of a giant plasmid containing the genes encoding mycolactone [69–71]. From 2001 through to early 2011, there have been more than 465 publications concerning M. ulcerans. Much of this work has been well reviewed elsewhere [72,73] and we will now focus on Buruli ulcer treatment.
Antibiotic treatment for Buruli ulcer: in vitro culture, mouse models & human trials, or are drugs mightier than the knife?
Using the mouse footpad model as developed by Fenner [10,12] and adapted for screening antileprosy compounds in a kinetic manner by Shepard [74], Grosset and colleagues showed that clarithromycin (CLR), which was reported to have good in vitro activity against M. ulcerans [75], only had bacteriostatic activity in vivo [50]. However, RIF, rifabutin, and amikacin (AMK), but not sparfloxacin or minocycline, demonstrated bactericidal activity and apparent cure. The results with AMK essentially confirmed early studies from the 1950s [11,12] and 1970s [14,32], in which the very closely related drug, STR, was shown to be active against M. ulcerans. A follow-up study [76] confirmed this prediction, as well as the other findings, and, for the first time, used a combination therapy of RIF and AMK. Telithromycin and levofloxacin had no activity as tested, while azithromycin and moxifloxacin had limited bacteriostatic activity. Even with a prolonged follow-up period of approximately 7 months, 50% of mice preventively treated with the combination of RIF plus AMK had no lesions after treatment of 5 days a week for 4 weeks. This finding led to the testing of RIF plus AMK in a curative mouse model with 12 weeks of treatment starting after the onset of inflammatory footpad swelling (Figure 2). Swelling regressed during the first 4 weeks of treatment and did not reappear from week 4 through to the end of the experiment 28 weeks after infection and 14 weeks after treatment completion. By contrast, an all-oral drug regimen of RIF plus CLR plus sparfloxacin initially failed to prevent progressive inflammation and swelling and the mice eventually relapsed with swelling extending from the footpad to the entire foot [77].
Figure 2. The mouse footpad model of Mycobacterium ulcerans disease.
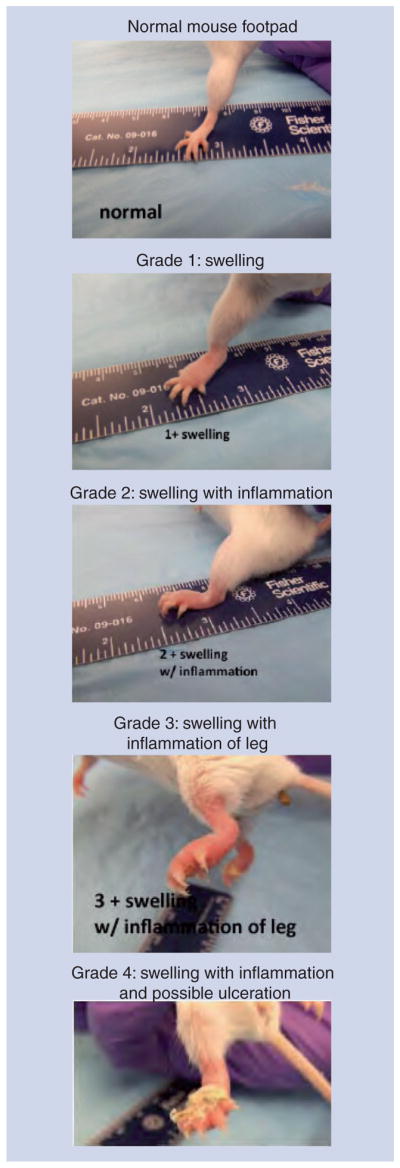
Grade 1 swelling of the footpad is easily distinguished from the normal footpad 4–8 weeks after infection. Swelling progresses to the grade 2 stage with increasing inflammation. At grade 3, inflammation is apparent further up the leg. If mice are not sacrificed, there can be manifestations of ulceration with cage bedding sticking to the foot. See also Box 1 discussing the model.
These promising preclinical results led to the design of a clinical trial in Ghana [78]. This trial involved 21 patients, all with preulcerative lesions, all of whom were studied extremely carefully. All patients who received 4, 8, or 12 weeks of the RIF (10 mg/kg) plus STR (15 mg/kg) combination therapy daily had lesions that were culture negative (in vitro and in the mouse), positive by PCR, and positive for AFB on treatment completion. By contrast, all patients who received treatment for only 2 weeks, or no treatment at all, were culture positive. Lesion surface area decreased by approximately 30–50% in all treatment groups [78].
The results of the trial in Ghana provided the basis for the issuance of Provisional Guidelines for Buruli ulcer treatment by the WHO in 2004 [203] and were first applied in Benin. Over 200 patients eligible for treatment at the Buruli Ulcer Center in rural Pobè in 2003 and 2004 were treated according to the WHO recommendation. In fact, most of these patients came from riverside villages located between two towns, only 8 km apart. Of the patients, three quarters had ulcers and 83% of those were larger than 5 cm. A majority of patients with ulcerated lesions (60%) were AFB-positive and 31% of the AFB-negative patients had a positive PCR result. Almost all patients were successfully treated either with antibiotics (RIF plus STR) for the full 8 weeks, or with antibiotics plus surgery (more likely in patients with larger, i.e., >15 cm, lesions) and skin grafting after 4 weeks. Notably, no patient discontinued therapy owing to side effects from the antibiotics. After 1 year, only three patients had recurrence of M. ulcerans disease. The authors concluded that the WHO-recommended STR plus RIF combination regimen is highly efficacious for treating M. ulcerans disease [79].
By contrast, a study published a few years earlier comparing treatment outcomes at two Ghanaian hospitals after surgery – often multiple surgeries – showed that over one third of patients were not healed [80]. Better outcomes were associated with more surgeries and longer hospital stay as well as the use of RIF and a history of BCG vaccination. A randomized, partially placebo-controlled, but not blinded, trial of RIF plus dapsone in the Côte d’Ivoire demonstrated some benefit in terms of reduction in ulcer size and appearance, although the placebo group also improved, making the results difficult to interpret [81]. The initial lesion size was also smaller in the placebo group. A retrospective study in Australia found that use of RIF plus ciprofloxacin significantly improved the outcome from surgical treatment [82]. Use of antibiotics alone in four patients was not successful. Treatment with AMK alone was associated with toxicity. From these studies, it seems reasonable to conclude that neither surgery, nor RIF plus dapsone (or ciprofloxacin), can be considered comparable to treatment with RIF plus STR.
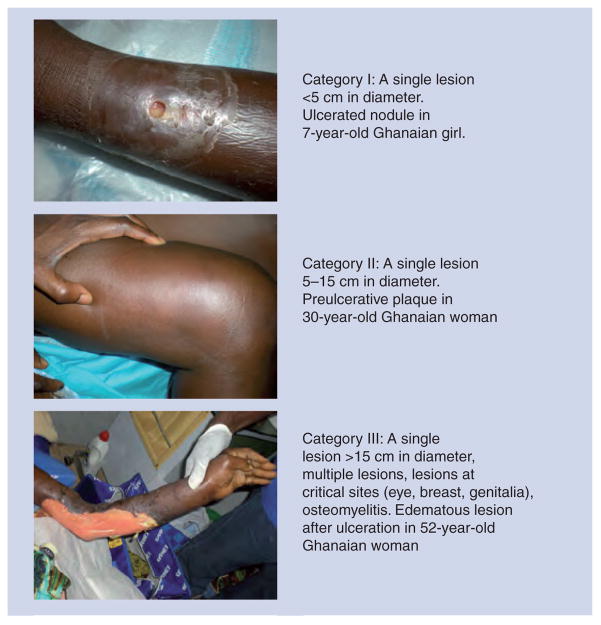
The 2004 provisional treatment guidance from the WHO established three categories to facilitate documentation of cases and a form to be used by Buruli ulcer disease control workers [204]. The initial lesion in humans may be a nodule, plaque, or edema that eventually breaks down or ulcerates without treatment. The ulcer is painless and has undermined edges. It may enlarge considerably from a small lesion to involve an entire limb or trunk. The categories for human disease are shown in Figure 3. The lesion index system used for evaluating treatment response and disease progression in the mouse is indicated in Figure 2. Diagnosis in humans is by clinical criteria supported by acid-fast smear microscopy, ideally confirmed by culture (≥8 week at 32°C), PCR at central laboratories, and/or histopathology. Failure to treat can result in self-healing or, more likely, amputation or contractures with functional limitations.
Figure 3. Clinical manifestations of Mycobacterium ulcerans infection.
The earliest manifestations of Buruli ulcer range from small nodules to firm plaques to edematous lesions. These may prompt patients to seek medical attention before the occurrence of ulceration as in both the Category I and Category II patients shown here. Before treatment could be administered the small nodule ulcerated on the lower leg of the girl. The Category III patient had first noticed her symptoms 2 weeks before coming to hospital. For the WHO’s classification system see reference [202].
Studies attempting to discern the mechanism of improved outcomes following RIF plus STR treatment have found improved inflammatory and cellular immune responses in patients following treatment. In untreated patients, the histopathology is notable for a poor inflammatory response and growing regions of necrosis in dermal and adipose tissue, with clusters of extracellular mycolactone-producing AFB. The epidermis eventually collapses. Following antibiotic treatment, it was noted that bacteria were located primarily intracellularly rather than extracellularly, and that there was a development of organized cellular infiltration in the lesions. This was considered to be a reversal of local immune suppression and indicative of a healing process [67,83]. Analysis of whole blood cells stimulated overnight with M. ulcerans sonicate indicated that during antibiotic treatment there was also a more general enhancement of the immune response with a significant increase in IFN-γ secretion [84]. Both studies suggest that the antibiotic treatment may inhibit mycolactone production. Unpublished studies in mice from our laboratory before and after, and in the absence of, treatment, support the histopathological findings in humans. The effect of antibiotic treatment on toxin production is being tested [Converse PJ et al., Unpublished Data].
Although RIF plus STR treatment generally appears to be beneficial and toxicities observed relatively rarely in West African patients, some patients appeared to have a worsening of their condition after initial improvement. This phenomenon has come to be known as the ‘paradoxical reaction’, first described for M. ulcerans disease in Australian patients who underwent surgery accompanied by RIF and ciprofloxacin [85]. The phenomenon has been recognized elsewhere [86,87] but may have also played a role along with the slow and continuing response after treatment completion in discouraging earlier investigations of drug treatment [88].
The continuing response and a very encouraging study of both 8 week RIF plus STR treatment (i.e., an oral and an injectable drug) or 4 week RIF plus STR treatment followed by a 4 week RIF plus CLR course (both oral drugs) was documented in a randomized clinical trial carried out at two sites in Ghana [89]. The CLR dose was 7.5 mg/kg. Randomization minimized differences in study sites and lesion types using a computer-generated assignment sent via text message to the study coordinator. The intent-to-treat analysis found that 96% of participants in the RIF plus STR only group and 91% in the other arm had healed lesions at 1 year after the start of treatment. Vestibulotoxicity was observed in only one patient in the 8 week RIF plus STR group and in two patients in the 4 week RIF plus STR/4 week RIF plus CLR group. Only four patients were lost to follow-up, but all of these had healed. There were no lesion recurrences. The trial was confined to patients with early, limited lesions and showed that the regimens had similar efficacy, possibly allowing for a reduction of injections.
Efficacy in small as well as large lesions was documented in 160 PCR-positive patients in a trial also based in Ghana [87]. Only eight (5%) patients required skin grafting after completion of the 2-month course of RIF plus STR treatment and there were no recurrences among the 158 patients reviewed at the 1-year follow-up. There was a wide range of times to complete healing and the healing rate, as measured by reduction in ulcer diameter. Remarkably, of the 24 patients with edematous lesions, 80% of which were category III lesions, 21 were reported to achieve complete healing. There were only three adverse events: two of these were attributed to STR and the patients were successfully switched to moxifloxacin; the third appeared to have a reaction to RIF and was successfully managed by stopping RIF administration and managing the ulcer by daily wound dressing. Paradoxical reactions (i.e., new, inflammatory, fluctuant lesions) also occurred in three patients, two during and one following treatment. All lesions healed after aspiration of sterile, culture-negative pus. Two of ten patients who were offered surgery and skin grafts declined but nevertheless healed completely. The authors concluded that an 8-week course of RIF plus STR was highly successful and practical in treating M. ulcerans disease [87].
A less encouraging report from Zaire, where only patients with large (i.e., ≥10 cm longest diameter) lesions were studied, concluded that delaying surgery might be harmful to patients [86]. However, all 91 patients received surgery and skin grafts after 4 weeks of RIF plus STR treatment and then received another 8 weeks of antibiotic treatment. The data showed that 98.4% of PCR-positive patients had a successful clinical outcome, as did 83.3% of the PCR-negative patients. It is difficult to see the harm overall in these outcomes. On the basis of negative acid-fast staining and culture as well as a lack of histopathological compatibility with Buruli ulcer, the authors, in spite of clinical diagnoses, speculated that the PCR-negative patients were not true cases, although specimen sampling errors offer another explanation [86]. The authors also brought up the important issue of HIV coinfection, which could not be established and for which care was not available at that time. The response of immunocompromised patients to RIF plus STR may lag behind compared with HIV-negative patients. In addition, it will be important to evaluate the interactions of antiretrovirals and drugs used for treating Buruli ulcer.
Although RIF plus STR treatment of M. ulcerans infection for 8 weeks results in the elimination of viable bacilli, lesion healing, and a reversal of local immunosuppression caused by mycolactone, new skin lesions occasionally appear, possibly caused by immune response-mediated paradoxical reactions [85]. Ruf et al. described, by histopathological and immunohistochemical analysis, two young patients who, 12–409 days after completion of antibiotic treatment for a single category III lesion, developed multiple new PCR-positive, culture-negative skin lesions (four each, six nodules and two ulcers, 5–30 cm from primary lesion) resembling Buruli ulcer (e.g., fat cell ghosts) [90]. However, the AFB had a degenerated appearance and there were massive leukocyte (monocytes/macrophages, T and B cells) infiltrates, also typical of cured lesions [83]. Although reinfection could not be excluded, the new infection foci were removed by limited debridement or excision and spontaneously resolved without additional antibiotic therapy, apparently by adaptive immune responses, probably primed by antibiotic treatment of the primary lesions.
Can STR be replaced by an oral antibiotic? Or, are pills yet mightier than the syringe? Future treatments
Despite the high efficacy of RIF plus STR treatment, there is a desire in the Buruli ulcer community to have an all-oral treatment regimen, which means replacing STR, the most bactericidal drug in the combination. An oral replacement could be less costly and safer without the requirement for sterile needles and providers to administer the injections. It could also reduce the potential toxicity, although as noted earlier, this seems to be rare in West African patients. On the other hand, the advantage of an injected drug is that patients and providers must both comply with the treatment. Attempts to resolve this issue have focused on two options, replacement of STR with CLR, or moxifloxacin (MXF), and replacement of RIF with the long-acting rifamycin, rifapentine.
As protocols are developed and pursued for all-oral regimens, it is important to bear in mind that these drugs also have side effects. CLR, and all macrolides, are associated with gastrointestinal discomfort, which is somewhat more frequent in children. Although Buruli ulcer affects all age groups, children represent the majority of patients. It would be important to assure compliance despite nausea, stomach pain, and diarrhea that patients may associate with a particular drug. Newer formulations, such as an extended release CLR, may be more tolerable, be taken once daily, and result in greater compliance [91], but may be somewhat more costly. MXF, and fluoroquinolones in general, are not recommended for pregnant women and children because of a possible negative impact on articular cartilage. Again, gastrointestinal adverse effects are also associated with this class of antibiotics [92]. Finally, it is important that whatever drugs are recommended need to be available to avoid the use of untested alternatives. Patient and provider compliance will continue to be critical in the treatment of this mycobacterial disease as is also the case in tuberculosis and leprosy.
Preclinical studies in the mouse footpad model have indeed offered grounds for hope in this regard. Initial studies showed that a number of antimycobacterial compounds, but not PA-824, are at least somewhat bactericidal for M. ulcerans in mice. Combinations of RIF plus linezolid or R207910 (TMC207) or MXF were as active as RIF plus STR [93]. Owing to considerations of cost and toxicity, subsequent experiments focused on RIF plus MXF. Six groups of mice with swelling of grade 2 or 3, were treated for 8 weeks with oral monotherapy (RIF, MXF or CLR) or a combination of the oral drugs. RIF plus STR-treated mice and untreated mice were controls. Two groups received RIF plus STR for the first 2 or 4 weeks, followed by RIF plus MXF for the remainder of the treatment period. By 4 weeks all combination therapy treated groups had a reduction of ~5log10 CFU compared with untreated controls with approximately 6log10 CFU/footpad. By week 8, all the combinations achieved culture negativity. Of the oral combinations, RIF plus CLR performed the best and was, in this experiment, superior to RIF plus STR [94]. A follow-up study that also tested combinations with rifapentine substituted for RIF found that all regimens, except for MXF plus CLR, achieved culture negativity after 8 weeks of treatment and an absence of relapse during 28 weeks of monitoring for swelling and cultivable organisms [95]. Ji et al. concluded that the oral drugs complement one another and help prevent the development of RIF resistance [95,96]. An alternative to the aforementioned curative model used by Ji et al. is the kinetic model in which drug activity is assessed before footpad swelling and the onset of the adaptive immune response [74]. Using this model for a shortened treatment period, 4 weeks, in order to assure bacterial survival and eventual relapse, Almeida et al. found that CLR has only bacteriostatic activity, and that in mice, there is a reduction of RIF activity owing to reduced absorption of RIF, when it is coadministered with a dose of CLR that is equipotent to that used in humans [97].
Case reports from Benin [98] and Australia [99,100] are also encouraging for the achievement of an all-oral treatment regimen. The first case report from Benin concerned a woman who delayed treatment for her Buruli ulcer lesion (AFB-positive, PCR-positive and histopathologically consistent) for 7 months, by which time she was also 6 months pregnant. Given that STR is contraindicated in pregnancy, she was treated with RIF plus CLR for 8 weeks. CLR (500 mg) was given twice daily. The treatment was well tolerated, resulted in significant reductions in lesion size, and complete healing following skin grafting 6 weeks after treatment completion or 4 weeks after giving birth to a healthy son [98]. In Australia, a case report showed that a 45-year-old farmer in northern tropical Australia presented with an edematous lesion that was AFB-positive, PCR-positive, and culture positive [99]. Such lesions are unusual in Australia. Excision appeared likely to have left M. ulcerans in the forearm. Antimycobacterial therapy with RIF, ethambutol, and CLR was administered for 2 months after surgery and subsequent examinations were negative for AFB or culturable M. ulcerans. The key findings of a second study in Australia [100] and concerning four culture- and PCR-positive cases were that RIF-based oral antibiotics (the second drug was either CLR, approximately 250 mg twice daily, or MXF, 400 mg daily) for limited Buruli ulcer cases prior to surgery resulted in culture-negative specimens but that clinical healing is slow, possibly owing to immunologically mediated paradoxical reactions. Nevertheless, the oral antibiotic therapy followed by delayed surgery appears to simplify management by allowing excision and wound closure in one step without relapse.
A pilot study reported in early 2011 from Benin [101] involved 30 patients (19 of whom were ≤15 years of age) with lesions ≤10 cm in diameter. All patients were successfully treated, without adverse events, for 8 weeks with RIF (10 mg/kg, as done in all studies) and CLR (12 mg/kg once daily, higher than the 7.5 mg/kg used in the mixed regimen Ghana trial). Limited surgery was carried out on 11 patients while four patients had extensive surgery, (i.e., major excision followed by skin grafting). Tissue specimens from these 15 patients were all culture negative but 13 were PCR positive. There were no relapses or recurrences 18 months after treatment completion. It is clear that there should be a randomized controlled trial to compare RIF plus STR with RIF plus CLR to determine if the latter combination is indeed efficacious and can lead to simpler, less invasive, and less painful treatment. Such a trial, to be conducted under the auspices of the WHO, is under consideration [Grosset J, Pers. Comm.]. Surgery is likely to remain necessary for extensive lesions but most patients can be cured through antibiotics alone.
Conclusion
The disease caused by M. ulcerans was not fully characterized by medical science until the first publication in 1948. Research was slow to accumulate as shown by the paucity of publications over the first three decades with a slight increase over the next three, and a relative explosion of interest and progress in the last 14 years. In regards to treatment, the lessons from first tuberculosis, and later leprosy, that it is imperative in mycobacterial diseases, in particular, to use multiple drug therapy were slowly appreciated. Systematic evaluation of drugs in the mouse model only began in the late 1990s. That effort led to trials of RIF plus STR treatment that have given increased confidence to clinicians and public health workers to apply and persevere with the treatment. Further mouse studies have led to the development and testing of all oral regimens [94,95,97]. Should the early results with the latter be confirmed in well-controlled trials, and if compliance can be assured, it will be a boon to health workers and patients alike, reducing the amount of time required for hospitalization and clinic visits and time away from productive economic activity, school, families and children. The ‘ifs’ are not negligible, but there is good reason for optimism in the treatment of this long neglected disease.
Future perspective
In the short term, one can expect that all-oral regimens, on the basis of the early observational studies described earlier, will indeed be shown in appropriate clinical trials to be at least as good as, if not superior to, the currently recommended regimen of RIF plus STR. Conditions in trials tend to be superior to conditions of routine care. If drugs are procured and dispensed appropriately in field conditions, if both providers and patients adhere to effective treatment protocols, if there are, in other words, no shortcuts, new oral regimens can succeed. Patients coming to treatment early when lesions are smaller and less likely to require hospitalization should help to significantly reduce the amount of surgical intervention and skin grafting required for Buruli ulcer treatment. It will also be important to continue to train medical professionals regarding appropriate treatment for the disease and the consequences of inadequate treatment. If there is sustained effort, in this regard, it should be possible to greatly reduce the burden of M. ulcerans disease.
There have been reports that concurrent HIV infection, or other causes of immunodeficiency, can exacerbate the presentation of Buruli ulcer and lead to severe complications [102–105]. How well immune-compromised patients respond to treatment is a nearly unexplored area where both mouse and, urgently, clinical studies will be required. With the expansion of the availability of antiretroviral treatment, the complications of drug interactions that exist for RIF and antiretroviral drugs will also need to be considered and evaluated for Buruli ulcer treatment.
As for most diseases, prevention is preferable to cure. However, early vaccination studies indicated that protection afforded by BCG vaccination is short term [106,107], but severe forms of the disease such as osteomyelitis may be less likely [108]. A recent study using two different mouse strains and two different virulent, mycolactone-producing strains, revealed that not all hosts can be protected, even on a short-term basis, and that protection may not be equally strong against all M. ulcerans strains [65]. There may, of course, be better vaccination methods or better vaccines [109]. However, globally, Buruli ulcer is not a very common disease and development of an effective, specific vaccine may not be a cost-effective procedure. As long as BCG is routinely used at birth to protect against childhood tuberculosis and against leprosy, any protective benefit against M. ulcerans is nearly cost free but may not be very great. It may also be conjectured that BCG, Mycobacterium vaccae [Grange J, Stanford J, Pers. Comm.], or other enhancers of immunity to mycobacteria could augment chemotherapeutic effects and eliminate residual bacteria.
There may be other methods, instead of, or in addition to vaccination that may achieve prevention. At least one study in Australia, where mosquitoes are suspected to be involved in transmission, found that use of insect repellent reduced the risk of developing Buruli ulcer [18]. In Africa, where bed nets are used to prevent malaria transmission, transmission of M. ulcerans could be reduced by this barrier if flying and biting bugs are involved in transmission [57]. Defining the mode of transmission continues to be an active area of research in Buruli ulcer. Relatively simple methods may potentially reduce the frequency of M. ulcerans infections or reduce exposure of communities to sources of the organism. The role of disrupted environments has often been mentioned as a risk factor for M. ulcerans transmission [16,57,110]. In fact, the large number of cases in Uganda in the 1960s to 1970s may have been initiated by the serious Nile flooding in 1962–1964, which increased the range of environments favorable to M. ulcerans survival and transmission [110]. The subsequent decrease may have resulted from a return to an unfavorable environment for the organism as well as greater community awareness and early ‘nodulectomy’ [Morrow R, Pers. Comm.].
The advantages of the current treatment approach for Buruli ulcer is that it employs drugs that are active and already approved for human use and that have well-known safety and toxicity profiles. Nevertheless, drug resistance is a potential issue and it is tempting to speculate on a novel scheme. The host target for mycolactone is unknown as yet. If a compound could be identified that interferes with that interaction or the resulting action, it could provide a very disease-specific treatment, probably without great risk of resistance. Experiments are in progress to characterize the timing of mycolactone production after infection. Data from ongoing experiments confirm that bacterial multiplication occurs rather steadily until a plateau in bacterial numbers and just after the onset of footpad swelling in mice. In humans the plateau may occur just after the appearance of a nodule or plaque. One could postulate that mycolactone production may occur rather abruptly, in response to the achievement or sensing of a quorum [111,205] and it is at that point that toxin expression is induced. Interference with the sensing process could be another tactic to control the devastation wrought by M. ulcerans.
Less conventional methods have been, and continue to be, explored but have not been shown to be clearly practical in endemic settings [112,113]. Hyperbaric oxygen treatment was reported to be no better than RIF monotherapy in mice and it was acknowledged to be unavailable in the field [35]. Heat may also be beneficial on the basis of the same study but it is difficult to apply evenly to lesions.
Unlike M. leprae, the leprosy bacillus, M. ulcerans is cultivable in vitro. However, the time required (≥8 week at 32°C) is much greater than that for M. tuberculosis, making the testing of both drugs and vaccines even more time consuming. Recently, Zhang et al. demonstrated that it is possible to generate and use a fully virulent, toxin-producing, luminescent strain of M. ulcerans that can allow the testing of drugs and other interventions in a near-real-time manner [114]. The application of such a tool should markedly enhance the identification of better compounds or better combinations of existing drugs to get better and possibly more rapid treatments of Buruli ulcer.
Executive summary.
Mycobacterium ulceransdisease (Buruli ulcer)
Buruli ulcer is a neglected tropical disease as designated by the WHO.
It was first definitely described in the medical literature in 1948.
The mode of transmission is unknown; an aquatic reservoir is likely and (biting) arthropods are likely to be involved.
The disease typically starts with a nodule that eventually ulcerates and may progress to involve the entire limb or trunk if untreated.
Pathology is mediated by a macrolide toxin, termed mycolactone.
There have been cases reported from every continent. However, most cases are reported, at this time, among poor farmers in rural West Africa and people living in, or visiting, beach resorts in southeast Australia.
Buruli ulcer treatment
Until 2004, surgical excision and skin grafting were the only methods used for treatment of this bacterial disease.
Currently, most patients can be effectively treated with rifampin, an orally administered drug, and streptomycin, an injectable drug.
Oral-only regimens are currently being sought.
Human treatment regimens have been, and will continue to be, firstly evaluated in mouse footpad models.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors’ work on Buruli ulcer was supported by the Fondation Raoul Follereau from 2007 to 2009. Currently the authors are supported by NIH grant R01-AI-82612 (2009–2014). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
- 1.Lunn HF, Connor DH, Wilks NE, et al. Buruli (mycobacterial) ulceration in Uganda (a new focus of Buruli ulcer in Madi district, Uganda): report of a field study. East Afr Med J. 1965;42:275–288. [PubMed] [Google Scholar]
- 2.Stinear T. PhD thesis. Department of Microbiology, Monash University; Clayton, Australia: 2002. Molecular and environmental aspects of Mycobacterium ulcerans. [Google Scholar]
- 3.MacCallum P, Tolhurst JC, Buckle G, Sissons HA. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93–122. [PubMed] [Google Scholar]
- 4.Janssens PG, Quertinmont MJ, Sieniawski J, Gatti F. Necrotic tropical ulcers and mycobacterial causative agents. Trop Geogr Med. 1959;11:293–312. [PubMed] [Google Scholar]
- 5.Van Oye E, Ballion M. Faudra-t-il tenir compte d’une nouvelle affection à bacilles acido-resistants en Afrique? Note preliminaire. [Is it necessary to take into account a new infection from acid-resistant bacilli in Africa? Preliminary note.] Ann Soc Belg Med Trop. 1950;30(3):619–627. [PubMed] [Google Scholar]
- 6.Clancey JK, Dodge OG, Lunn HF, Oduori ML. Mycobacterial skin ulcers in Uganda. Lancet. 1961;2:951–954. doi: 10.1016/s0140-6736(61)90793-0. [DOI] [PubMed] [Google Scholar]
- 7.Uganda Buruli Group. Epidemiology of Mycobacterium ulcerans infection (Buruli ulcer) at Kinyara, Uganda. Trans R Soc Trop Med Hyg. 1971;65(6):763–775. doi: 10.1016/0035-9203(71)90090-3. [DOI] [PubMed] [Google Scholar]
- 8▪.Connor DH, Lunn HF. Mycobacterium ulcerans infection (with comments on pathogenesis) Int J Lepr. 1965;33(Suppl 3):698–709. Insightful analysis of histopathological sections that inferred the presence of a novel mycobacterial toxin. [PubMed] [Google Scholar]
- 9.Connor DH, Lunn HF. Buruli Ulceration: a clincopathologic study of 38 Ugandans with Mycobacterium ulcerans ulceration. Arch Pathol. 1966;81:183–199. [Google Scholar]
- 10▪.Fenner F. The pathogenic behavior of Mycobacterium ulcerans and Mycobacterium balnei in the mouse and the developing chick embryo. Am Rev Tuberc. 1956;73(5):650–673. doi: 10.1164/artpd.1956.73.5.650. First description of mouse footpad model for Mycobacterium ulcerans animal and preclinical studies. [DOI] [PubMed] [Google Scholar]
- 11.Feldman WH, Karlson AG. Mycobacterium ulcerans infections; response to chemotherapy in mice. Am Rev Tuberc. 1957;75(2):266–279. doi: 10.1164/artpd.1957.75.2.266. [DOI] [PubMed] [Google Scholar]
- 12.Leach RH, Fenner F. Studies on Mycobacterium ulcerans and Mycobacterium balneiIII Growth in the semi-synthetic culture media of Dubos and drug sensitivity in vitro and in vivo. Aust J Exp Biol Med Sci. 1954;32(6):835–852. [PubMed] [Google Scholar]
- 13.Pattyn SR, Royackers J. [Treatment of experimental infection by Mycobacterium ulcerans and Mycobacterium balnei in mice] Ann Soc Belg Med Trop. 1965;45:31–38. [PubMed] [Google Scholar]
- 14.Stanford JL, Phillips I. Rifampicin in experimental Mycobacterium ulcerans infection. J Med Microbiol. 1972;5(1):39–45. doi: 10.1099/00222615-5-1-39. [DOI] [PubMed] [Google Scholar]
- 15.Portaels F, Meyers WM, Ablordey A, et al. First cultivation and characterization of Mycobacterium ulcerans from the environment. PLoS Negl Trop Dis. 2008;2(3):e178. doi: 10.1371/journal.pntd.0000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marion E, Eyangoh S, Yeramian E, et al. Seasonal and regional dynamics of M ulcerans transmission in environmental context Deciphering the role of water bugs as hosts and vectors. PLoS Negl Trop Dis. 2010;4(7):e731. doi: 10.1371/journal.pntd.0000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson PD, Azuolas J, Lavender CJ, et al. Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg Infect Dis. 2007;13(11):1653–1660. doi: 10.3201/eid1311.061369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quek TY, Athan E, Henry MJ, et al. Risk factors for Mycobacterium ulcerans infection, southeastern Australia Mycobacterium ulcerans in mosquitoes captured during outbreak of Buruli ulcer, southeastern Australia. Emerg Infect Dis. 2007;13(11):1661–1666. doi: 10.3201/eid1311.061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fyfe JA, Lavender CJ, Handasyde KA, et al. A major role for mammals in the ecology of Mycobacterium ulcerans. PLoS Negl Trop Dis. 2010;4(8):e791. doi: 10.1371/journal.pntd.0000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieg RE, Hockmeyer WT, Connor DH. Toxin of Mycobacterium ulcerans Production and effects in guinea pig skin. Arch Dermatol. 1974;110(5):783–788. doi: 10.1001/archderm.110.5.783. [DOI] [PubMed] [Google Scholar]
- 21.Read JK, Heggie CM, Meyers WM, Connor DH. Cytotoxic activity of Mycobacterium ulcerans. Infect Immun. 1974;9(6):1114–1122. doi: 10.1128/iai.9.6.1114-1122.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pimsler M, Sponsler TA, Meyers WM. Immunosuppressive properties of the soluble toxin from Mycobacterium ulcerans. J Infect Dis. 1988;157(3):577–580. doi: 10.1093/infdis/157.3.577. [DOI] [PubMed] [Google Scholar]
- 23▪.George KM, Barker LP, Welty DM, Small PL. Partial purification and characterization of biological effects of a lipid toxin produced by Mycobacterium ulcerans. Infect Immun. 1998;66(2):587–593. doi: 10.1128/iai.66.2.587-593.1998. First of a series of papers from Pamela Small’s laboratory characterizing the major virulence factor of Mycobacterium ulcerans, mycolactone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George KM, Chatterjee D, Gunawardana G, et al. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science. 1999;283(5403):854–857. doi: 10.1126/science.283.5403.854. [DOI] [PubMed] [Google Scholar]
- 25.Pahlevan AA, Wright DJ, Andrews C, George KM, Small PL, Foxwell BM. The inhibitory action of Mycobacterium ulcerans soluble factor on monocyte/T cell cytokine production and NF-κB function. J Immunol. 1999;163(7):3928–3935. [PubMed] [Google Scholar]
- 26.Shepard CC. The experimental disease that follows the injection of human leprosy bacilli into foot-pads of mice. J Exp Med. 1960;112:445–457. doi: 10.1084/jem.112.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lunn HF, Rees RJ. Treatment of mycobacterial skin ulcers in Uganda with a Riminophenazine Derivative (B.663) Lancet. 1964;39:247–249. doi: 10.1016/s0140-6736(64)92351-7. [DOI] [PubMed] [Google Scholar]
- 28.Revill WD, Morrow RH, Pike MC, Ateng J. A controlled trial of the treatment of Mycobacterium ulcerans infection with clofazimine. Lancet. 1973;2(7834):873–877. doi: 10.1016/s0140-6736(73)92005-9. [DOI] [PubMed] [Google Scholar]
- 29.Meyers WM. Mycobacterial infections of the skin. In: Doerr W, Seifert G, editors. Tropical Pathology. Springer-Verlag; Berlin, Germany: 1995. pp. 291–377. [Google Scholar]
- 30.Meyers WM, Aguiar J, Portaels F. Buruli ulcer (Mycobacterium ulcerans infection). 3rd WHO Advisory Group Meeting on Buruli ulcer.; 2000. pp. 64–73. [Google Scholar]
- 31.Stanford JL, Hutt MSR, Phillips I, Revill WDL. Antibiotic treatment in Mycobacterium ulcerans infection. Ain Shams Med J. 1973;25(Suppl):258–261. [Google Scholar]
- 32.Havel A, Pattyn SR. Activity of rifampicin on Mycobacterium ulcerans. Ann Soc Belg Med Trop. 1975;55(2):105–108. [PubMed] [Google Scholar]
- 33.Meyers WM, Shelly WM, Connor DH. Heat treatment of Mycobacterium ulcerans infections without surgical excision. Am J Trop Med Hyg. 1974;23(5):924–929. doi: 10.4269/ajtmh.1974.23.924. [DOI] [PubMed] [Google Scholar]
- 34.Krieg RE, Wolcott JH, Confer A. Treatment of Mycobacterium ulcerans infection by hyperbaric oxygenation. Aviat Space Environ Med. 1975;46(10):1241–1245. [PubMed] [Google Scholar]
- 35.Krieg RE, Wolcott JH, Meyers WM. Mycobacterium ulcerans infection: treatment with rifampin, hyperbaric oxygenation, and heat. Aviat Space Environ Med. 1979;50(9):888–892. [PubMed] [Google Scholar]
- 36.Portaels F, Agular J, Fissette K, et al. Direct detection and identification of Mycobacterium ulcerans in clinical specimens by PCR and oligonucleotide-specific capture plate hybridization. J Clin Microbiol. 1997;35(5):1097–1100. doi: 10.1128/jcm.35.5.1097-1100.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross B, Marino L, Oppedisano F, Edwards R, Robins-Browne R, Johnson P. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J Clin Microbiol. 1997;35(7):1696–1700. doi: 10.1128/jcm.35.7.1696-1700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross BC, Johnson PD, Oppedisano F, et al. Detection of Mycobacterium ulcerans in environmental samples during an outbreak of ulcerative disease. Appl Environ Microbiol. 1997;63(10):4135–4138. doi: 10.1128/aem.63.10.4135-4138.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stinear T, Ross BC, Davies JK, et al. Identification and characterization of IS2404 and IS2606: two distinct repeated sequences for detection of Mycobacterium ulcerans by PCR. J Clin Microbiol. 1999;37(4):1018–1023. doi: 10.1128/jcm.37.4.1018-1023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stinear T, Davies JK, Jenkin GA, et al. A simple PCR method for rapid genotype analysis of Mycobacterium ulcerans. J Clin Microbiol. 2000;38(4):1482–1487. doi: 10.1128/jcm.38.4.1482-1487.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyers WM, Connor DH, Mccullough B, Bourland J, Moris R, Proos L. Distribution of Mycobacterium ulcerans infections in Zaire, including the report of new foci. Ann Soc Belg Med Trop. 1974;54(3):147–157. [PubMed] [Google Scholar]
- 42.Oluwasanmi JO, Solanke TF, Olurin EO, Itayemi SO, Alabi GO, Lucas AO. Mycobacterium ulcerans (Buruli) skin ulceration in Nigeria. Am J Trop Med Hyg. 1976;25(1):122–128. doi: 10.4269/ajtmh.1976.25.122. [DOI] [PubMed] [Google Scholar]
- 43.Monson MH, Gibson DW, Connor DH, Kappes R, Hienz HA. Mycobacterium ulcerans in Liberia: a clinicopathologic study of 6 patients with Buruli ulcer. Acta Trop. 1984;41(2):165–172. [PubMed] [Google Scholar]
- 44.van der Werf TS, van der Graaf WT, Groothuis DG, Knell AJ. Mycobacterium ulcerans infection in Ashanti region, Ghana. Trans R Soc Trop Med Hyg. 1989;83(3):410–413. doi: 10.1016/0035-9203(89)90521-x. [DOI] [PubMed] [Google Scholar]
- 45.Muelder K, Nourou A. Buruli ulcer in Benin. Lancet. 1990;336(8723):1109–1111. doi: 10.1016/0140-6736(90)92581-2. [DOI] [PubMed] [Google Scholar]
- 46.Josse R, Guedenon A, Darie H, Anagonou S, Portaels F, Meyers WM. Mycobacterium ulcerans cutaneous infections: Buruli ulcers. Med Trop (Mars) 1995;55(4):363–373. [PubMed] [Google Scholar]
- 47.Meyers WM, Tignokpa N, Priuli GB, Portaels F. Mycobacterium ulcerans infection (Buruli ulcer): first reported patients in Togo. Br J Dermatol. 1996;134(6):1116–1121. [PubMed] [Google Scholar]
- 48.George KM, Pascopella L, Welty DM, Small PL. A Mycobacterium ulcerans toxin, mycolactone, causes apoptosis in guinea pig ulcers and tissue culture cells. Infect Immun. 2000;68(2):877–883. doi: 10.1128/iai.68.2.877-883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stinear TP, Jenkin GA, Johnson PD, Davies JK. Comparative genetic analysis of Mycobacterium ulcerans and Mycobacterium marinum reveals evidence of recent divergence. J Bacteriol. 2000;182(22):6322–6330. doi: 10.1128/jb.182.22.6322-6330.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50▪.Dega H, Robert J, Bonnafous P, Jarlier V, Grosset J. Activities of several antimicrobials against Mycobacterium ulcerans infection in mice. Antimicrob Agents Chemother. 2000;44(9):2367–2372. doi: 10.1128/aac.44.9.2367-2372.2000. First of a series of papers systematically evaluating antibiotics alone or in combination against M. ulcerans in the mouse footpad model (see also [94,95,97]) following the WHO commitment to combating Buruli ulcer [52–56,201] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson PD, Stinear TP, Hayman JA. Mycobacterium ulcerans – a mini-review. J Med Microbiol. 1999;48(6):511–513. doi: 10.1099/00222615-48-6-511. [DOI] [PubMed] [Google Scholar]
- 52.Van Der Werf TS, Van Der Graaf WT, Tappero JW, Asiedu K. Mycobacterium ulcerans infection. Lancet. 1999;354(9183):1013–1018. doi: 10.1016/S0140-6736(99)01156-3. [DOI] [PubMed] [Google Scholar]
- 53.Asiedu K, Hayman J. Epidemiology. In: Asiedu K, Scherpbier R, Raviglione M, editors. Buruli ulcer: Mycobacterium ulcerans Infection. WHO; Geneva: 2000. [Google Scholar]
- 54.Asiedu K, Meyers W, Agbenorku P. Clinical features and treatment. In: Asiedu K, Scherpbier R, Raviglione M, editors. Buruli Ulcer: Mycobacterium ulcerans Infection. WHO; Geneva: 2000. pp. 37–38. [Google Scholar]
- 55.Asiedu K, Scherpbier R, Raviglione M. Buruli Ulcer Mycobacterium ulcerans infection. WHO; 2000. [Google Scholar]
- 56.van der Werf TS, Stienstra Y, Johnson RC, et al. Mycobacterium ulcerans disease. Bull World Health Organ. 2005;83(10):785–791. [PMC free article] [PubMed] [Google Scholar]
- 57.Merritt RW, Walker ED, Small PLC, et al. Ecology and transmission of Buruli ulcer disease: a systematic review. PLoS Negl Trop Dis. 2010;4(12):e911. doi: 10.1371/journal.pntd.0000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phillips RO, Sarfo FS, Osei-Sarpong F, et al. Sensitivity of PCR targeting Mycobacterium ulcerans by use of fine-needle aspirates for diagnosis of Buruli ulcer. J Clin Microbiol. 2009;47(4):924–926. doi: 10.1128/JCM.01842-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beissner M, Herbinger KH, Bretzel G. Laboratory diagnosis of Buruli ulcer disease. Future Microbiol. 2010;5(3):363–370. doi: 10.2217/fmb.10.3. [DOI] [PubMed] [Google Scholar]
- 60.Phillips R, Sarfo FS, Guenin-Macé L, et al. Immunosuppressive signature of cutaneous Mycobacterium ulcerans infection in the peripheral blood of patients with Buruli ulcer disease. J Infect Dis. 2009;200(11):1675–1684. doi: 10.1086/646615. [DOI] [PubMed] [Google Scholar]
- 61.Boulkroun S, Guenin-Mace L, Thoulouze M-I, et al. Mycolactone suppresses T cell responsiveness by altering both early signaling and posttranslational events. J Immunol. 2010;184(3):1436–1444. doi: 10.4049/jimmunol.0902854. [DOI] [PubMed] [Google Scholar]
- 62.Pidot SJ, Porter JL, Marsollier L, et al. Serological evaluation of Mycobacterium ulcerans antigens identified by comparative genomics. PLoS Negl Trop Dis. 2010;4(11):e872. doi: 10.1371/journal.pntd.0000872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fraga AG, Cruz A, Martins TG, et al. Mycobacterium ulcerans triggers T-cell immunity followed by local and regional but not systemic immunosuppression. Infect Immun. 2011;79(1):421–430. doi: 10.1128/IAI.00820-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanghe A, Adnet P-Y, Gartner T, Huygen K. A booster vaccination with M. bovis BCG does not increase the protective effect of the vaccine against experimental Mycobacterium ulcerans infection in mice. Infect Immun. 2007;75(5):2642–2644. doi: 10.1128/IAI.01622-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Converse PJ, Almeida DV, Nuermberger EL, Grosset JH. BCG-mediated protection against Mycobacterium ulcerans infection in the mouse. PLoS Negl Trop Dis. 2011;5(3):e985. doi: 10.1371/journal.pntd.0000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goto M, Nakanaga K, Aung T, et al. Nerve damage in Mycobacterium ulcerans-infected mice: probable cause of painlessness in Buruli ulcer. Am J Pathol. 2006;168(3):805–811. doi: 10.2353/ajpath.2006.050375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schütte D, Umboock A, Pluschke G. Phagocytosis of Mycobacterium ulcerans in the course of rifampicin and streptomycin chemotherapy in Buruli ulcer lesions. Br J Dermatol. 2009;160(2):273–283. doi: 10.1111/j.1365-2133.2008.08879.x. [DOI] [PubMed] [Google Scholar]
- 68.Silva MT, Portaels F, Pedrosa J. Pathogenetic mechanisms of the intracellular parasite Mycobacterium ulcerans leading to Buruli ulcer. Lancet Infect Dis. 2009;9(11):699–710. doi: 10.1016/S1473-3099(09)70234-8. [DOI] [PubMed] [Google Scholar]
- 69.Stinear TP, Mve-Obiang A, Small PL, et al. Giant plasmid-encoded polyketide synthases produce the macrolide toxin of Mycobacterium ulcerans. Proc Natl Acad Sci USA. 2004;101(5):1345–1349. doi: 10.1073/pnas.0305877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stinear TP, Pryor MJ, Porter JL, Cole ST. Functional analysis and annotation of the virulence plasmid pMUM001 from Mycobacterium ulcerans. Microbiology. 2005;151(Pt 3):683–692. doi: 10.1099/mic.0.27674-0. [DOI] [PubMed] [Google Scholar]
- 71▪.Stinear TP, Seemann T, Pidot S, et al. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res. 2007;17(2):192–200. doi: 10.1101/gr.5942807. Description and annotation of the complete M. ulcerans genome and the giant plasmid containing genes necessary for mycolactone synthesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Demangel C, Stinear TP, Cole ST. Buruli ulcer: reductive evolution enhances pathogenicity of Mycobacterium ulcerans. Nat Rev Microbiol. 2009;7(1):50–60. doi: 10.1038/nrmicro2077. [DOI] [PubMed] [Google Scholar]
- 73.Walsh DS, Portaels F, Meyers WM. Buruli ulcer: advances in understanding Mycobacterium ulcerans infection. Dermatol Clin. 2011;29(1):1–8. doi: 10.1016/j.det.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Shepard CC. A kinetic method for the study of the activity of drugs against Mycobacterium leprae. Int J Lepr. 1967;35:429–436. [Google Scholar]
- 75.Portaels F, Traore H, De Ridder K, Meyers WM. In vitro susceptibility of Mycobacterium ulcerans to clarithromycin. Antimicrob Agents Chemother. 1998;42(8):2070–2073. doi: 10.1128/aac.42.8.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bentoucha A, Robert J, Dega H, Lounis N, Jarlier V, Grosset J. Activities of new macrolides and fluoroquinolones against Mycobacterium ulcerans infection in mice. Antimicrob Agents Chemother. 2001;45(11):3109–3112. doi: 10.1128/AAC.45.11.3109-3112.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dega H, Bentoucha A, Robert J, Jarlier V, Grosset J. Bactericidal activity of rifampin-amikacin against Mycobacterium ulcerans in mice. Antimicrob Agents Chemother. 2002;46(10):3193–3196. doi: 10.1128/AAC.46.10.3193-3196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Etuaful S, Carbonnelle B, Grosset J, et al. Efficacy of the Combination rifampin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob Agents Chemother. 2005;49(8):3182–3186. doi: 10.1128/AAC.49.8.3182-3186.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chauty A, Ardant M-F, Adeye A, et al. Promising clinical efficacy of streptomycin-rifampin combination for treatment of Buruli ulcer (Mycobacterium ulcerans disease) Antimicrob Agents Chemother. 2007;51(11):4029–4035. doi: 10.1128/AAC.00175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teelken MA, Stienstra Y, Ellen DE, et al. Buruli ulcer: differences in treatment outcome between two centres in Ghana. Acta Trop. 2003;88(1):51–56. doi: 10.1016/s0001-706x(03)00170-0. [DOI] [PubMed] [Google Scholar]
- 81.Espey DK, Djomand G, Diomande I, et al. A pilot study of treatment of Buruli ulcer with rifampin and dapsone. Int J Infect Dis. 2002;6(1):60–65. doi: 10.1016/s1201-9712(02)90138-4. [DOI] [PubMed] [Google Scholar]
- 82.O’brien DP, Hughes AJ, Cheng AC, et al. Outcomes for Mycobacterium ulcerans infection with combined surgery and antibiotic therapy: findings from a southeastern Australian case series. Med J Aust. 2007;186(2):58–61. doi: 10.5694/j.1326-5377.2007.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 83.Schütte D, Um-Boock A, Mensah-Quainoo E, Itin P, Schmid P, Pluschke G. Development of highly organized lymphoid structures in Buruli ulcer lesions after treatment with rifampicin and streptomycin. PLoS Negl Trop Dis. 2007;1(1):e2. doi: 10.1371/journal.pntd.0000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sarfo FS, Phillips RO, Ampadu E, Sarpong F, Adentwe E, Wansbrough-Jones M. Dynamics of the cytokine response to Mycobacterium ulcerans during antibiotic treatment for M. ulcerans disease (Buruli ulcer) in humans. Clin Vaccine Immunol. 2009;16(1):61–65. doi: 10.1128/CVI.00235-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’brien DP, Robson ME, Callan PP, Mcdonald AH. “Paradoxical” immune-mediated reactions to Mycobacterium ulcerans during antibiotic treatment: a result of treatment success, not failure. Med J Aust. 2009;191(10):564–566. doi: 10.5694/j.1326-5377.2009.tb03313.x. [DOI] [PubMed] [Google Scholar]
- 86.Kibadi K, Boelaert M, Fraga AG, et al. Response to treatment in a prospective cohort of patients with large ulcerated lesions suspected to be Buruli ulcer (Mycobacterium ulcerans disease) PLoS Negl Trop Dis. 2010;4(7):e736. doi: 10.1371/journal.pntd.0000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sarfo FS, Phillips R, Asiedu K, et al. Clinical efficacy of combination of rifampin and streptomycin for treatment of Mycobacterium ulcerans disease. Antimicrob Agents Chemother. 2010;54(9):3678–3685. doi: 10.1128/AAC.00299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson PD. Should antibiotics be given for Buruli ulcer? Lancet. 2010;375(9715):618–619. doi: 10.1016/S0140-6736(10)60169-9. [DOI] [PubMed] [Google Scholar]
- 89▪.Nienhuis WA, Stienstra Y, Thompson WA, et al. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet. 2010;375(9715):664–672. doi: 10.1016/S0140-6736(09)61962-0. Randomized clinical trial providing evidence of efficacy of rifampin plus streptomycin treatment (together with [79] and [87]) against ulcerative lesions as well as preulcerative nodules [78] [DOI] [PubMed] [Google Scholar]
- 90.Ruf M-T, Chauty A, Adeye A, et al. Secondary Buruli ulcer skin lesions emerging several months after completion of chemotherapy: paradoxical reaction or evidence for immune protection? PLoS Negl Trop Dis. 2011;5(8):e1252. doi: 10.1371/journal.pntd.0001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Williams KN, Bishai WR. Clarithromycin extended-release in community-acquired respiratory tract infections. Expert Opin Pharmacother. 2005;6(16):2867–2876. doi: 10.1517/14656566.6.16.2867. [DOI] [PubMed] [Google Scholar]
- 92.Lipsky BA, Baker CA. Fluoroquinolone toxicity profiles: a review focusing on newer agents. Clin Infect Dis. 1999;28(2):352–364. doi: 10.1086/515104. [DOI] [PubMed] [Google Scholar]
- 93.Ji B, Lefrancois S, Robert J, Chauffour A, Truffot C, Jarlier V. In vitro and in vivo activities of rifampin, streptomycin, amikacin, moxifloxacin, R207910, linezolid, and PA-824 against Mycobacterium ulcerans. Antimicrob Agents Chemother. 2006;50(6):1921–1926. doi: 10.1128/AAC.00052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ji B, Chauffour A, Robert J, Lefrancois S, Jarlier V. Orally administered combined regimens for treatment of Mycobacterium ulcerans infection in mice. Antimicrob Agents Chemother. 2007;51(10):3737–3739. doi: 10.1128/AAC.00730-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ji B, Chauffour A, Robert J, Jarlier V. Bactericidal and sterilizing activities of several orally administered combined regimens against Mycobacterium ulcerans in mice. Antimicrob Agents Chemother. 2008;52(6):1912–1916. doi: 10.1128/AAC.00193-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marsollier L, Honore N, Legras P, et al. Isolation of three Mycobacterium ulcerans strains resistant to rifampin after experimental chemotherapy of mice. Antimicrob Agents Chemother. 2003;47(4):1228–1232. doi: 10.1128/AAC.47.4.1228-1232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Almeida D, Converse PJ, Ahmad Z, Dooley KE, Nuermberger EL, Grosset JH. Activities of rifampin, rifapentine and clarithromycin alone and in combination against Mycobacterium ulcerans disease in mice. PLoS Negl Trop Dis. 2011;5(1):e933. doi: 10.1371/journal.pntd.0000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dossou AD, Sopoh GE, Johnson CR, et al. Management of Mycobacterium ulcerans infection in a pregnant woman in Benin using rifampicin and clarithromycin. Med J Aust. 2008;189(9):532–533. doi: 10.5694/j.1326-5377.2008.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 99.Jenkin GA, Smith M, Fairley M, Johnson PD. Acute, oedematous Mycobacterium ulcerans infection in a farmer from far north Queensland. Med J Aust. 2002;176(4):180–181. doi: 10.5694/j.1326-5377.2002.tb04350.x. [DOI] [PubMed] [Google Scholar]
- 100.Gordon CL, Buntine JA, Hayman JA, et al. All-oral antibiotic treatment for buruli ulcer: a report of four patients. PLoS Negl Trop Dis. 2010;4(11):e770. doi: 10.1371/journal.pntd.0000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chauty A, Ardant M-Fo, Marsollier L, et al. Oral treatment for Mycobacterium ulcerans infection: results from a pilot study in benin. Clin Infect Dis. 2011;52(1):94–96. doi: 10.1093/cid/ciq072. [DOI] [PubMed] [Google Scholar]
- 102.Johnson RC, Ifebe D, Hans-Moevi A, et al. Disseminated Mycobacterium ulcerans disease in an HIV-positive patient: a case study. Aids. 2002;16(12):1704–1705. doi: 10.1097/00002030-200208160-00027. [DOI] [PubMed] [Google Scholar]
- 103.Toll A, Gallardo F, Ferran M, et al. Aggressive multifocal Buruli ulcer with associated osteomyelitis in an HIV-positive patient. Clin Exp Dermatol. 2005;30(6):649–651. doi: 10.1111/j.1365-2230.2005.01892.x. [DOI] [PubMed] [Google Scholar]
- 104.Johnson RC, Nackers F, Glynn JR, et al. Association of HIV infection and Mycobacterium ulcerans disease in Benin. Aids. 2008;22(7):901–903. doi: 10.1097/QAD.0b013e3282f7690a. [DOI] [PubMed] [Google Scholar]
- 105.Sopoh GE, Dossou AD, Brun LV, et al. Severe multifocal form of Buruli ulcer after streptomycin and rifampin treatment: comments on possible dissemination mechanisms. Am J Trop Med Hyg. 2010;83(2):307–313. doi: 10.4269/ajtmh.2010.09-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Uganda Buruli Group. BCG vaccination against Mycobacterium ulcerans infection (Buruli ulcer) First results of a trial in Uganda. Lancet. 1969;1(7586):111–115. [PubMed] [Google Scholar]
- 107.Smith PG, Revill WD, Lukwago E, Rykushin YP. The protective effect of BCG against Mycobacterium ulcerans disease: a controlled trial in an endemic area of Uganda. Trans R Soc Trop Med Hyg. 1976;70(5–6):449–457. doi: 10.1016/0035-9203(76)90128-0. [DOI] [PubMed] [Google Scholar]
- 108.Portaels F, Aguiar J, Debacker M, et al. Prophylactic effect of Mycobacterium bovis BCG vaccination against osteomyelitis in children with Mycobacterium ulcerans disease (Buruli Ulcer) Clin Diagn Lab Immunol. 2002;9(6):1389–1391. doi: 10.1128/CDLI.9.6.1389-1391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huygen K, Adjei O, Affolabi D, et al. Buruli ulcer disease: prospects for a vaccine. Med Microbiol Immunol. 2009;198(2):69–77. doi: 10.1007/s00430-009-0109-6. [DOI] [PubMed] [Google Scholar]
- 110.Barker DJ. The distribution of Buruli disease in Uganda. Trans R Soc Trop Med Hyg. 1972;66(6):867–874. doi: 10.1016/0035-9203(72)90121-6. [DOI] [PubMed] [Google Scholar]
- 111.Chen G, Swem LR, Swem DL, et al. A strategy for antagonizing quorum sensing. Mol Cell. 2011;42(2):199–209. doi: 10.1016/j.molcel.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pszolla N, Sarkar MR, Strecker W, et al. Buruli ulcer: a systemic disease. Clin Infect Dis. 2003;37:E78–E82. doi: 10.1086/377170. [DOI] [PubMed] [Google Scholar]
- 113.Dossou A, Sopoh G, Leigheb G, et al. Hyperbaric oxygen therapy in the treatment of Buruli ulcer. Presented at: WHO Annual meeting on Buruli ulcer; 22–24 March; Geneva, Switzerland. 2010. [Google Scholar]
- 114.Zhang T, Li S-Y, Converse PJ, Almeida DV, Grosset JH, Nuermberger EL. Using bioluminescence to monitor treatment response in real time in mice with Mycobacterium ulcerans infection. Antimicrob Agents Chemother. 2011;55(1):56–61. doi: 10.1128/AAC.01260-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 201.WHO. WHO joins battle against a new emerging disease, Buruli ulcer. 1997 www.who.int/buruli/information/antibiotics/en/index16.html. [PubMed]
- 202.WHO. Buruli ulcer. www.who.int/buruli/gbui/en/index.html.
- 203.WHO. Geneva: 2004. Provisional guidance on the role of specific antibiotics in the management of Mycobacterium ulcerans disease (Buruli ulcer) www.who.int/buruli/information/antibiotics/en/ [Google Scholar]
- 204.WHO. New recording and reporting forms for Buruli ulcer. www.who.int/Buruli/control/forms_2/en.
- 205.Bassler BL. How bacteria “talk”. TED Talks. 2009 www.ted.com/talks/bonnie_bassler_on_how_bacteria_communicate.html.