Abstract
Objective
To compare the dose effects of long-acting extended-release dexmethylphenidate (ER d-MPH) and ER mixed amphetamine salts (ER MAS) on attention-deficit/hyperactivity disorder (ADHD) symptom dimensions, global and specific impairments, and common adverse events associated with stimulants.
Methods
Fifty-six children and adolescents with ADHD participated in an 8-week, double-blind, crossover study comparing ER d-MPH (10, 20, 25–30 mg) and ER MAS (10, 20, 25–30) with a week of randomized placebo within each drug period. Efficacy was assessed with the ADHD Rating Scale-IV (ADHD-RS-IV), whereas global and specific domains of impairment were assessed with the Clinical Global Impressions Severity and Improvement Scales and the parent-completed Weiss Functional Impairment Scale, respectively. Insomnia and decreased appetite, common stimulant-related adverse events, were measured with the parent-completed Stimulant Side Effects Rating Scale.
Results
Both ER d-MPH and ER MAS were associated with significant reductions in ADHD symptoms. Improvement in Total ADHD and Hyperactivity/Impulsivity symptoms were strongly associated with increasing dose, whereas improvements in Inattentive symptoms were only moderately associated with dose. About 80% demonstrated reliable change on ADHD-RS-IV at the highest dose level of ER MAS compared with 79% when receiving ER d-MPH. Decreased appetite and insomnia were more common at higher dose levels for both stimulants. Approximately 43% of the responders were preferential responders to only one of the stimulant formulations.
Conclusions
Dose level, rather than stimulant class, was strongly related to medication response.
Introduction
Stimulant medications, including immediate release (IR) and extended release (ER) methylphenidate (MPH) and amphetamine (AMP), have been shown to be highly efficacious for improving attention-deficit/hyperactivity disorder (ADHD) symptoms in literally hundreds of placebo-controlled trials (Swanson 1993; Wilens and Spencer 2000). Yet, we know relatively little about the similarities and differences between the two main classes of stimulants due to a paucity of head-to-head studies with newer, long-acting agents. MPH and AMP formulations have equal efficacy and similar side effect profiles according to several reviews, practice guidelines, and algorithms (American Academy of Child and Adolescent Psychiatry 2002; Daughton and Kratochvil 2009). For example, the Texas Algorithm for ADHD recommends using either class of stimulant medication as the initial treatment for ADHD, and switching to the other stimulant class if the first is either not effective or not well tolerated (Pliszka et al. 2000; 2003). Other reviews and a recent meta-analysis of 23 studies have concluded that effect sizes are somewhat greater for AMP (Arnold 2000; Faraone et al. 2002; Faraone and Buitelaar 2010), although reviews and meta-analyses are likely to over-represent data from older studies, many of which utilized older IR stimulant formulations. In addition, early studies did not routinely dose the two medications comparably. Titrating to the optimal dose in crucial, as described in the Multimodal Treatment Study of Children with Attention-Deficit/Hyperactivities Disorder (MTA) and Preschool ADHD Treatment Study (PATS) studies that utilized double-blind procedures to determine the optimal dose level (Greenhill et al. 2001; Greenhill et al. 2006a). In the MTA study, individually titrated doses were higher and more effective than doses of MPH in the community treatment group, which did not systematically evaluate different dose levels.
Currently, ER formulations of MPH and AMP have replaced the IR formulations as first line treatments due to their longer duration of behavioral effects and convenience (Swanson and Hechtman 2005; Olfson et al. 2009). For the two stimulant medications, we chose Dexmethylphenidate Hydrochloride ER (Focalin XR [brand name], here referred to as d-MPH ER) and Adderall XR (mixed AMP salts [MAS] ER). MAS ER is a racemic mixture of dextro- and levo-isomers of AMP salts that contains 50% IR MAS and 50% delivered at a second pulse 4 hours later. MAS ER is one of the most frequently prescribed stimulant medications for ADHD (Olfson, Marcus and Wan 2009) Dose-dependent efficacy versus placebo in childhood has been demonstrated in a large, parallel group study of 584 children treated with 10, 20, or 30 mg (Biederman et al. 2002). Behavioral and cognitive effects were demonstrated to last for 12 hours, with the 30 mg condition associated with the most robust gains. Anorexia, insomnia, and mood lability were more common in children receiving ER MAS compared with placebo, occurring in 21.9%, 16.6%, and 8.6% of subjects, respectively. Dexmethylphenidate hydrochloride (Focalin XR; ER d-MPH) is the pharmacologically active d-threoenantiomer of racemic MPH. Similar to MAS ER, 50% of the drug is released initially with the remaining 50% released approximately 4 hours later. Thus, the MPH and AMP formulations used in this study are formulated in a very similar manner. In a parallel group, 7-week study, 67.3% of children and adolescents receiving ER formulation of d-MPH that was clinically titrated up to 30 mg (mean dose=24 mg) were rated “much improved” or “very much improved” on the Clinical Global Impressions-Improvement (CGI-I) Scale compared with 13.3% of those receiving placebo (Greenhill et al. 2006b). The most common spontaneously occurring adverse event was decreased appetite, which was reported in 30% of the ER d-MPH group versus 8.5% of those taking placebo.
An important context for this study comparing the effects of ER formulations of MPH and AMP is the current national interest in Comparative Effectiveness Research (CER) (Clancy 2009). The objective of CER is to compare frequently used treatments to determine comparative effectiveness and tolerability, to identify factors that can potentially aid in matching treatments to individual patient characteristics, and to obtain relevant measures in samples that are likely to generalize to clinical practice. Given the uncertainty regarding dose comparability of ADHD preparations and the potential for ascertainment bias when selecting subjects on the basis of previous response, CER studies employing multiple doses and utilizing samples similar to clinic populations (i.e., either stimulant naïve, partial responders, or nonresponders) would aid physicians in (1) choosing an ADHD medication; (2) titrating to an appropriate dose; and (3) estimating the likelihood of differential response if the response to the first medication is not effective. It is hoped that eventually information from CER studies can identify potential predictors of individual response that can guide treatment (Garber and Tunis 2009). With these three questions in mind, we sought to compare the dose–response effects of ER d-MPH and ER MAS, on ADHD symptoms, global and specific domains of impairment, and two common adverse effects of stimulants: insomnia and decreased appetite.
Methods
Subjects
Eligible participants were recruited from the investigators' practices, clinic referrals, and radio advertisements. All subjects met Diagnostic and Statistical Manual of Mental Disorders, 2nd edition (DSM-II) (American Psychiatric Association 1994) criteria for ADHD based upon the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) (Kaufman et al. 1997), administered by a licensed child and adolescent psychiatrist or psychologist. The Child Behavior Checklist (Achenbach 1991) was also completed to obtain a dimensional measure of child psychopathology.
All subjects received a physical examination, and laboratory studies to rule out medical exclusionary criteria. Each child received a pre- and poststudy ECG to screen for cardiac abnormalities. Each ECG was read by a pediatric cardiologist and scored using the recent recommendations of the American Heart Association (Vetter et al. 2008). Youth with mental retardation, autism, severe mood disorders, Tourette's disorder, seizure disorders, or other medical disorders that were contraindications of stimulant treatment or that mimic ADHD (e.g., thyroid disorder) were excluded.
Procedures
A parent or guardian was required to give signed informed consent and all participating children provided assent. The consent and assent forms, study protocol, and advertisements for recruitment were reviewed and approved by the Institutional Review Board of University of Illinois at Chicago (Clinical Trials Registration: ClinicalTrials.gov identifier: NCT00393042).
Experimental design
Children previously taking stimulant medications completed a two-day washout period before beginning a placebo-controlled, double-blind, two-period, 8-week crossover design, with three dose conditions of ER d-MPH and ER MAS (10, 20, and 25–30 mg) administered sequentially from lowest dose to highest dose with a randomized week of placebo in each period. Order of drug was randomized so that 50% started with ER d-MPH and 50% started with ER MAS. The maximum dose was 25 mg in smaller children (i.e., <35 kg) to minimize potential side effects. There was no washout period between treatment periods.
The research pharmacist developed a randomization schedule for order of study drug and randomization of the placebo weeks, and prepared weekly blister packs for each subject containing capsules of study drug, which were indistinguishable from each other.
Weekly procedures and measures
During each weekly visit, children and their parents met with the investigators and research staff to assess medication effects and monitor safety. Children's weight, height, blood pressure, pulse, and temperature were obtained each week. In cases of intolerability occurring after the first 2 weeks on a particular medication, the child could skip to the next drug period.
At the weekly medication visits, the following measures were obtained: (1) ADHD Parent Rating Scale-IV [ADHD-RS-4; (DuPaul et al. 1998)], which measured severity of Total ADHD symptoms, Inattention and Hyperactivity/Impulsivity symptoms; (2) CGI-Severity (CGI-S) and CGI-I Scales (Guy 1976), for clinician ratings of overall severity and improvement completed by a Child and Adolescent Psychiatrist or Child Psychologist; (3) Weiss Functional Impairment Rating Scale (WFIRS) (Weiss et al. 2007), a parent-rating scale designed to assess functional impairment in the following domains: Family, learning and school, life skills, self-concept, social activities, and risky activities; and (4) Stimulant Side Effects Rating Scale (SERS); (Barkley et al. 1990), a 17-item scale that parents rate on a 10-point (0–9) scale ranging from absent to serious.
Statistical plan
Dose–response effects were examined via Hierarchical Linear Models (Bryk and Raudenbush 1992). Specifically, for each of the outcome variables we added main effect and interaction terms into the Linear Mixed Modeling equation in the following order: Sex, age, weight, ADHD Subtype, stimulant naïve status, stimulant drug type, dose, and then the interaction of dose with each of these variables in turn. The variables other than dose and drug type were entered as main effects to serve as covariates (i.e., to control for their contribution to explaining variance in the outcome variables and to prevent any potential bias in estimates of the effects of the focal explanatory variables dose and drug type).
In a set of exploratory analyses, these variables' interactions with dose were also entered to test whether they moderated the effects of dose or drug type on the outcome variables. Due to the exploratory nature of these analyses, statistical significance of each explanatory variable was evaluated using a nominal p-value threshold of α=0.05.
Results
Diagnostic and descriptive information
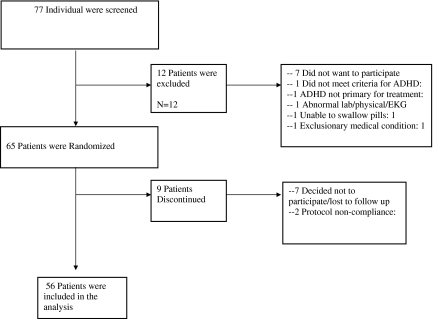
A total of 65 children and adolescents met the inclusion criteria for the study and were enrolled between January 2007 and January 2009. Participants ranged in age from 9 to 17 (mean=11.7, standard deviation [SD]=2.24); 47 were boys and 17 were girls. The disposition of all enrolled subjects is described in Figure 1. Since we were interested in evaluating efficacy and adverse events at all dose conditions, the study sample included all participants who received at least 2 weeks of study drug to insure that all participants had been exposed to at least 1 week of active drug (n=56). Demographic and descriptive data of the study population as well as those who dropped out of the study prematurely are presented in Table 1. There were no significant differences between the study completers and drop-outs in ADHD symptoms, CGI-S, age, or weight. Thirty percent of the analyzed study sample were stimulant naïve, as compared with 56% of those who discontinued.
FIG. 1.
Patient disposition. ADHD=attention-deficit/hyperactivity disorder; EKG=electrocardiogram.
Table 1.
Demographics and Clinical Characteristics
| |
Completersa(n=56) |
Dropouts (n=9) |
||
|---|---|---|---|---|
| Variables | Mean | SD | Mean | SD |
| Age | 11.78 | 2.24 | 12.28 | 2.24 |
| Weight | 50.98 | 21.32 | 50.28 | 20.62 |
| ADHD-RS-IV | 39.05 | 9.74 | 38.55 | 10.67 |
| CGI-S | 4.98 | 0.72 | 4.88 | 0.60 |
| CBCL internalizing | 58.83 | 10.69 | 61.66 | 9.94 |
| CBCL externalizing | 60.07 | 10.41 | 61.33 | 13.17 |
| Total | 63.98 | 8.49 | 65.22 | 8.59 |
| N | % | N | % | |
|---|---|---|---|---|
| Gender | ||||
| Female | 15 | 27 | 2 | 22 |
| Male | 41 | 73 | 7 | 78 |
| Race | ||||
| African American | 23 | 41 | 4 | 50 |
| Asian | 1 | 2 | 0 | 0 |
| Caucasian | 23 | 41 | 2 | 25 |
| Hispanic/Latino | 4 | 7 | 2 | 25 |
| Native American | 1 | 2 | 0 | 0 |
| Biracial/mixed race | 4 | 7 | 0 | 0 |
| Stimulant Use | ||||
| No previous use | 17 | 30 | 5 | 56 |
| Previously treated with MPH | 24 | 43 | 2 | 22 |
| Previously treated with AMPH | 3 | 5 | 0 | 0 |
| Previously treated with both | 12 | 22 | 2 | 22 |
| ADHD | ||||
| Combined type | 38 | 67 | 7 | 78 |
| Inattentive type | 18 | 33 | 2 | 22 |
At least 2 weeks of study drug administration.
ADHD-RS IV=Attention-Deficit/Hyperactivity Disorder Rating Scale IV; CGI-S=Clinical Global Impressions-Severity; CBCL=Child Behavior Checklist; MPH=methylphenidate; AMPH=amphetamine; SD=standard deviation.
Similar to other ADHD clinic-referred samples, the population was predominantly male (73%; 47 boys and 17 girls), with a higher prevalence of the Combined subtype (67%). The sample was ethnically diverse, with equivalent percentages of children from African-American and Caucasian, or European-American ethnic backgrounds (41% each); in addition, 7% were Latino, 7% biracial, 2% Asian, and 2% Native American.
Dose–response effects on ADHD symptoms and global and specific impairment
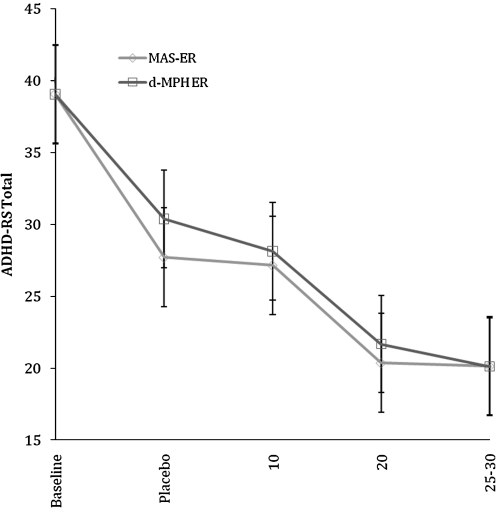
Decreases in ADHD-RS Total score by dose level for both ER MAS and ER d-MPH are shown in Figure 2. There were significant and substantial dose-related decreases in Total and Hyperactive-Impulsive symptom scores (p<0.001, R2=0.59 and p<0.001, R2=0.46, respectively) that did not differ by type of stimulant, as well as significant dose-related decreases for Inattention symptoms (p<0.001, R2=0.11) that were more modest in magnitude but which also did not differ by type of stimulant.
FIG. 2.
Effects of extended release of mixed amphetamine salts and extended release dexmethylphenidate on ADHD Rating Scale Total Score (ADHD-RS) by dose. ER, extended release; d-MPH, dexmethyphenidate; MAS, mixed amphetamine salts.
Similarly, there were significant dose-related decreases in CGI-S scores (p<0.001, R2=0.48) that did not differ by type of stimulant. There were also significant effects of dose on the WFIRS Total Score (p=0.008, R2=0.02), on the Family (p=0.010, R2=0.02), Learning (p=0.002, R2=0.03), Social Activities (p=0.018, R2=0.13), and Risk Taking (p=0. 050, R2=0.01) subscales, but not on the Living Skills or Self-Esteem subscales. There were no drug-by-dose interactions, indicating similar dose effects of the two medications on WFIRS-P scores.
Safety and tolerability
Six participants did not complete the study due to adverse events. There was one serious adverse event, which occurred when a child was taking 20 mg of ER d-MPH. The child was hospitalized one night for observation after an abnormal ECG following a possible seizure. The ECG normalized, and the child subsequently continued stimulant treatment outside the study. Otherwise, there were no clinically significant changes in ECG, nor were there significant dose-response effects on systolic blood pressure (p=0.182, R2=0.04), diastolic blood pressure (p=0.877, R2=0.00), or pulse (p=0.420, R2=0.00). However, there was a significant decrease in weight by dose level (p=0.012, R2=0.13), irrespective of stimulant type.
The frequency of “severe” side effects (i.e., rated 7 or above on a 9 point scale) on the SERS is reported in Table 2. The most common severe side effects, occurring in >10% of the sample at any dose except placebo, were insomnia, loss of appetite, irritable, and nail biting. There were significant stimulant dose effects for SERS ratings of insomnia (p=0.045, R2=0.01) and decreased appetite (p=0.005, R2=0.16), indicating increased severity of adverse events with increasing dose.
Table 2.
Percent and Frequency of Severe Side Effects at Each Dose of Extended Release Dexmethyphenidate and Extended Release Mixed Amphetamine Salts
| |
Percent severe ER d-MPH |
Percent severe ER-MAS |
||||||
|---|---|---|---|---|---|---|---|---|
| Side effect | Placebo (N=45) | 10 mg (N=50) | 20 mg (N=47) | 25–30 mg(N=42) | Placebo (N=52) | 10 mg (N=55) | 20 mg (N=50) | 25–30 mg (N=47) |
| Insomnia or trouble sleeping | 4.55 (2) | 4.08 (2) | 10.64 (5) | 7.14 (3) | 5.77 (3) | 12.73 (7) | 12.00 (6) | 8.51 (4) |
| Nightmare | 0 | 2.04 (1) | 0 | 2.38 (1) | 1.92 (1) | 0 | 2.00 (1) | 0 |
| Stares | 0 | 0 | 0 | 0 | 1.92 (1) | 3.64 (2) | 2.00 (1) | 2.13 (1) |
| Talk less | 4.55 (2) | 0 | 4.26 (2) | 4.76 (2) | 0 | 1.82 (1) | 0 | 6.38 (3) |
| Uninterested | 0 | 0 | 2.13 (1) | 2.38 (1) | 1.92 (1) | 3.64 (2) | 0 | 2.13 (1) |
| Loss of Appetite | 2.22 (1) | 10.20 (5) | 14.89 (7) | 7.14 (3) | 3.85 (2) | 9.26 (5) | 14.00 (7) | 12.77 (6) |
| Irritable | 6.67 (3) | 2.04 (1) | 14.89 (7) | 5.77 (3) | 7.27 (4) | 10.00 (5) | ||
| Stomach Ache | 2.22 (1) | 4.08 (2) | 0 | 11.90 (5) | 1.92 (1) | 1.82 (1) | 2.00 (1) | 2.13 (1) |
| Head ache | 0 | 4.08 (2) | 0 | 2.38 (1) | 3.85 (2) | 1.82 (1) | 2.00 (1) | 6.38 (3) |
| Drowsiness | 2.22 (1) | 0 | 0 | 0 | 1.92 (1) | 0 | 0 | 4.26 (2) |
| Sadness | 0 | 0 | 2.13 (1) | 0 | 0 | 0 | 0 | 4.26 (2) |
| Crying | 2.22 (1) | 0 | 2.13 (1) | 0 | 1.92 (1) | 1.82 (1) | 0 | 4.26 (2) |
| Anxiety | 0 | 2.04 (1) | 0 | 0 | 0 | 0 | 0 | 0 |
| Nail biting | 6.67 (3) | 12.24 (6) | 19.15 (9) | 0 | 9.62 (4) | 16.36 (9) | 14.00 (7) | 0 |
| Euphoric | 4.55 (2) | 0 | 0 | 14.28 (6) | 1.92 (1) | 3.64 (2) | 0 | 10.64 (5) |
| Dizziness | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tics | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.13 (1) |
| 0 | 0 | |||||||
ER d-MPH=extended release dexmethyphenidate; ER MAS=extended release mixed amphetamine salts.
Although there was a trend for higher rates of severe insomnia for ER MAS at the 10 mg dose (p=0.058), there were no stimulant medication differences detected at the higher dose levels. Similarly, rates of decreased appetite did not differ between the two stimulants (p=0.103).
Clinical improvement and differential response
We first examined clinician-rated global improvement via the CGI-I Scale (i.e., CGI-I), and then calculated clinically significant change in ADHD RS using the Reliable Change Index (RCI) (Jacobson and Truax 1991). Approximately half the participants at the highest dose level for each stimulant were rated as “Much” or “Very much” improved on the CG-I (Table 3), whereas 80% demonstrated reliable change on ADHD RS at the highest dose level of ER MAS compared with 79% when receiving d-MPH. RCI did not differ by stimulant formulation (p=0.855, R2=0.00).
Table 3.
Percent and Frequency Achieving Clinical Global Impressions-Improvement “Much” or “Very Much” Improved
| |
ER d-MPH |
ER MAS |
||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (N=46) | 10 mg(N=50) | 20 mg(N=51) | 25–30 mg(N=49) | Placebo (N=53) | 10 mg(N=55) | 20 mg(N=51) | 25–30 mg (N=49) | |
| Responders | 19.57 (9) | 18 (9) | 45.10 (23) | 48.98 (24) | 24.53 (13) | 27.27 (15) | 45.10 (23) | 51.02 (25) |
To examine differential response to the two stimulant medications using a measure of global impairment, positive response was defined by those attaining a CGI-S score of 1–2 during any week, or a score of 3 (“mildly ill”), which represented a change from baseline of at least two points. The majority of children (73%) responded similarly to both stimulant formulations, 21/56 (37.5%) did not have a positive response to either drug, and 20/56 (35.7%) responded positively to both medications. An additional 8/56 (14.3%) subjects displayed a positive response to ER d-MPH only and 7/56 (12.5%) responded to ER MAS only. Thus, 26.8% of the total sample had a preferential response to one or the other stimulant. However, approximately 43% of the responders were preferential responders to only one stimulant class. The magnitude of response displayed by children who responded differentially to only one stimulant was robust, as evidenced by a mean decrease in ADHD RS scores from baseline of 28.2 (SD=9.5) for those who responded to ER d-MPH and 27.7 (SD=10.2) for those who responded to ER MAS.
Moderators of dose–response effects: Exploratory analyses
We next conducted a set of exploratory analyses testing whether dose–response varied as a function of children's ADHD Subtype, sex, age, weight, or stimulant naïve status. Inattention symptoms responded to lower stimulant doses (p=0.018, R2=0.02). Dose–response for Inattention symptoms also differed by sex (p=0.040, R2=0.01), such that Inattention symptoms decreased more consistently for females.
Discussion
Choosing a long-acting MPH or AMP medication
At the group level of analysis, treatment with either ER MAS or d-MPH resulted in rapid reductions in ADHD symptoms and improvements in both global and many specific domains of impairment. There were no significant differences between the two stimulant formulations. Per parent report on the WFIRS, symptomatic improvement was associated with improved functioning in multiple domains, including family, school, and social functioning. This suggests that the impact of treatment with ER MAS and d-MPH extends to functioning beyond the school setting. Although improvement was not reported in daily living skills or self-esteem, this is not surprising as acute stimulant treatment is unlikely to be sufficient to remediate the often chronic deficits in adaptive functioning and self-perceptions that characterize youth with ADHD (Stein et al. 1995). Therefore, either AMP or MPH can be selected as the initial choice of medication.
Implications for titrating ER MAS and d-MPH dosing
Improvement in behavior was strongly related to dose, and in contrast to recent reviews and meta-analyses suggesting slightly greater efficacy with AMP, the present study demonstrates equipotency of the two classes of stimulants when comparable doses of long-acting formulations are used. Both higher dose levels and more time were required to achieve superiority over placebo improvement rates, suggesting the importance of careful titration until optimal response is obtained. Although 10 mg and placebo were both associated with mild improvement, normalization of ADHD symptoms (e.g., ADHD RS<18) required higher dose levels. Indeed, the field appears to be moving toward increasing the intensity and duration of ADHD pharmacotherapy to target reductions in impairment in addition to symptom reduction as a goal of ADHD treatment (Steele et al. 2006; Buitelaar et al. 2009).
Improvement in inattentive symptoms was only moderately associated with increasing dose, whereas hyperactivity and total score reductions were more strongly related to higher stimulant dosing. This is consistent with several (e.g., Stein et al. 2003; Newcorn et al. 2010), but not all (i.e., Solanto et al. 2009), previous studies that examined dose–response by ADHD symptoms domains by dose.
Surprisingly, the magnitude of the placebo response on ADHD symptoms was similar to the 10 mg dose of either ER d-MPH or ER MAS. The relatively robust placebo effect in this study highlights the importance of using a placebo comparison in ADHD treatment studies, rather than looking at change scores from baseline, which would not correct for expectancy and time effects. The present findings are consistent with previous literature on placebo effects in ADHD trials (Newcorn et al. 2009), suggesting that higher placebo rates occur in research participants with the inattentive type, stimulant naive status, and with African American ethnicity.
ER MAS was associated with slightly higher rates of severe insomnia compared with ER d-MPH at the 10 mg dose. This finding is consistent with a previous study of IR preparations that reported higher rates of insomnia with twice daily AMP (0.15 mg/kg/dose) compared with MPH (0.5 mg/kg/dose) (Efron et al. 1997). In the present study of multiple dose effects, there were no stimulant differences in insomnia rates at higher dose levels. Adverse events were more common at the second highest dose level as opposed to the highest dose level, presumably due to more time to accommodate to the medication as well as several participants who did not receive the highest dose level due to previous adverse events. There is clear evidence of a dose-dependent relationship for insomnia and decreased appetite when the entire range of doses and placebo are considered, although in the vast majority these events were not viewed as severe.
How common is differential response?
Differential response is of most relevance in cases where there is poor, partial, or nonresponse to the first stimulant tried. In the present study, the majority of children responded similarly to both stimulants. However, nearly half of the responders (i.e., 43%) achieved their response preferentially with one or the other stimulant class; 14.3% of the total samples were responders to ER d-MPH only, and 12.5% responded only to ER MAS. These findings resemble those of Elia and colleagues (1991), who compared the efficacy and tolerability of twice-daily IR MPH and dextroamphetamine in 48 boys with ADHD in a day-hospital setting. Both medications produced similar improvement on behavioral ratings of ADHD symptoms at the group level; however, there was considerable differential response in that 14 out of 46 responders responded to only one drug based upon CGI ratings. Similarly, in a large, parallel group comparator study of OROS MPH and the nonstimulant, atomoxetine (Newcorn et al. 2008), approximately one-third of subjects were preferential responders to one or the other medication. Taken together, the results of these studies illustrate the importance of examining response to different ADHD treatments, even when there seems to be a response to the first medication tried. Regardless, the rather large group of selective responders to the different classes of stimulants is provocative, and raises important questions for future research. It is hoped that future CER studies with larger samples may identify specific clinical or genetic predictors of differential response, which can then be studied in prospective effectiveness trials.
As in most studies of clinic-referred children, the sample was heterogeneous with respect to previous stimulant experience, ethnicity, and ADHD subtype. However, the study has several limitations that impact generalizability to clinical practice, including the short duration of time children were maintained on each dose and the fixed dose titration. Although there were no significant differences in tolerability detected between the two stimulant formulations, it should be noted that our sample was not adequately powered to detect small or modest effects. Hence the possibility of small differences in response associated with type of stimulant cannot be ruled out. Further, improvement could occur at any week/dose condition and; therefore, the responder data may be subject to chance fluctuations. An additional limitation is that, whereas we studied two highly representative ER formulations of MPH and AMP, we did not study all the formulations of these two medication classes. It is tempting to suggest that findings for other MPH and AMP formulations would parallel those reported here, but this cannot be assumed. Finally, measures of efficacy and tolerability were based primarily on parent report. However, it is possible that teacher reports might have produced different findings. For example, in the MTA study, teacher ratings were able to detect placebo drug differences during dose ranging trials and were associated with larger effects sizes (i.e., 0.8–1.3) than parent ratings (i.e., 0.4–0.6). Nonetheless, parent ratings have demonstrated sensitivity and clinical utility in numerous studies of long-acting medications (Biederman et al. 2006).
Conclusion
Both ER d-MPH and ER MAS were associated with significant, dose-dependent reductions in ADHD symptoms. Decreased appetite and insomnia were more common at higher dose levels for both stimulants. Dose level, rather than stimulant class, was strongly related to medication response. Although the majority of children responded similarly to both stimulants, 14.3% of the total samples were responders to ER d-MPH only, and 12.5% responded only to ER MAS. Future comparative effectiveness studies with multiple informants and larger samples over longer time periods are necessary to develop a data-driven, personalized approach to ADHD treatment.
Clinical Significance
Study findings support the importance of individually titrating to optimal efficacy and tolerability, and are consistent with virtually all treatment algorithms to try a different class of ADHD medication when the first medication selected fails. However, it is still to be determined whether it is best to use another stimulant formulation or a nonstimulant such as atomoxetine, clonidine, or guanfacine as the second choice, and whether there are clinical or biological predictors of response.
Disclosures
Dr. Stein received an investigator-initiated grant from Novartis to conduct this study. He participates in the ADHD Speakers' Bureau for Novartis and Shinogi, and is an advisor to Novartis, Shinogi, and Shire. Dr. Newcorn is a recipient of grants for research support from Eli Lilly, McNeil, and Shire. He is also a consultant and/or advisor for Alcobra, Eli Lilly, and Shire. Drs. Waldman, Charney, Aryal, Sable, and Gruber have no conflicts of interest to disclose.
Acknowledgments
Financial Support: Investigator-initiated study sponsored by Novartis Pharmaceuticals, with additional support provided by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS), Award Number UL1RR029879 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
The authors would like to acknowledge James McGough, M.D., and Thomas Owley, M.D. for co-leading the Data Safety Monitoring Board. They also wish to thank Lauren Maul, Michael Pacini, PharmD., Esperanza Salinas, M.D., Tanya Froehlich, M.D., and Robert Gibbons Ph.D.
References
- Achenbach TM. Manual for the Child Behavior Checklist and Revised Child Behavior Prophile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41:26s–48s. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (DSM-IV) 4th. Washington, DC: American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Arnold L. Methylphenidate vs. Amphetamine: Comparative review. J Atten Disord. 2000;3:200–211. [Google Scholar]
- Barkley R. McMurray M. Edelbrock C. Robbins ZK. Side effects of MPH in children with attention deficit hyperactivity disorder: A systematic placebo controlled evaluation. Pediatrics. 1990;86:184–192. [PubMed] [Google Scholar]
- Biederman J. Gao H. Rogers AK. Spencer TJ. Comparison of parent and teacher reports of attention-deficit hyperactivity disorder symptoms from two placebo-controlled studies of atomoxetine in children. Biol Psychiatry. 2006;60:1106–1110. doi: 10.1016/j.biopsych.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Biederman J. Lopez FA. Boellne SW. Chandler MC. A randomized, double-blind, placebo-controlled, parallel-group study of SLI381 (Adderall XR) in children with attention-deficit/hyperactivity disorder. Pediatrics. 2002;110:258–266. doi: 10.1542/peds.110.2.258. [DOI] [PubMed] [Google Scholar]
- Bryk AT. Raudenbush S. Hierarchical Linear Models: Applications, Data Analysis Methods. Newbury Park, Ca: Sage; 1992. [Google Scholar]
- Buitelaar JK. Wilens TE. Zhang S. Ning Y. Feldman PD. Comparison of symptomatic versus functional changes in children and adolescents with ADHD during randomized, double-blind treatment with psychostimulants, atomoxetine, or placebo. J Child Psychol Psychiatry. 2009;50:335–342. doi: 10.1111/j.1469-7610.2008.01960.x. [DOI] [PubMed] [Google Scholar]
- Clancy C. Comparative-effectiveness research—implications of the federal coordinating council's report. N Engl J Med. 2009;361:328–330. doi: 10.1056/NEJMp0905631. [DOI] [PubMed] [Google Scholar]
- Daughton JM. Kratochvil CJ. Review of ADHD pharmacotherapies: Advantages, disadvantages, and clinical pearls. J Am Acad Child Adolesc Psychiatry. 2009;48:240–248. doi: 10.1097/CHI.0b013e318197748f. [DOI] [PubMed] [Google Scholar]
- DuPaul G. Power TJ. Anastopoulos A. Reid R. ADHD Rating Scale-IV: Checklists, Norms, Clinical Interpretations. NY: Guilford Press; 1998. [Google Scholar]
- Efron D. Jarman F. Barker M. Side effects of Methylphenidate and Dextroapmphetamine in children with ADHD: A double blind crossover study. Pediatrics. 1997;100:662–666. doi: 10.1542/peds.100.4.662. [DOI] [PubMed] [Google Scholar]
- Elia J. Borcherding BG. Rapoport J. Keysor C. Methylphenidate and dextroamphetamine treatment of hyperactivity: Are there true nonresponders? Psychiatry Res. 1991;36:141–155. doi: 10.1016/0165-1781(91)90126-a. [DOI] [PubMed] [Google Scholar]
- Faraone SV. Biederman J. Roe C. Comparative efficacy of Adderall and methylphenidate in attention-deficit/hyperactivity disorder: A meta-analysis. J Clin Psychopharmacol. 2002;22:468–473. doi: 10.1097/00004714-200210000-00005. [DOI] [PubMed] [Google Scholar]
- Faraone SV. Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry. 2010;19:353–364. doi: 10.1007/s00787-009-0054-3. [DOI] [PubMed] [Google Scholar]
- Garber A. Tunis SR. Does comparative-effectiveness research threaten personalized medicine? N Engl J Med. 2009;360:1925–1927. doi: 10.1056/NEJMp0901355. [DOI] [PubMed] [Google Scholar]
- Greenhill L. Kollins S. Abikoff H. McCracken J. Riddle M. Swanson J. McGough J. Wigal S. Wigal T. Vitiello B. Skrobala A. Posner K. Ghuman J. Cunningham C. Davies M. Chuang S. Cooper T. Efficacy and safety of immediate-release methylphenidate treatment for preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry. 2006a;45:1284–1293. doi: 10.1097/01.chi.0000235077.32661.61. [DOI] [PubMed] [Google Scholar]
- Greenhill LL. Muniz R. Ball RR. Levine A. Pestreich L. Jiang H. Efficacy and safety of dexmethylphenidate extended-release capsules in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006b;45:817–823. doi: 10.1097/01.chi.0000220847.41027.5d. [DOI] [PubMed] [Google Scholar]
- Greenhill LL. Swanson JM. Vitiello B. Davies M. Clevenger W. Wu M. Arnold LE. Abikoff HB. Bukstein OG. Conners CK. Elliott GR. Hechtman L. Hinshaw SP. Hoza B. Jensen PS. Kraemer HC. March JS. Newcorn JH. Severe JB. Wells K. Wigal T. Impairment and deportment responses to different methylphenidate doses in children with ADHD: The MTA titration trial. J Am Acad Child Adolesc Psychiatry. 2001;40:180–187. doi: 10.1097/00004583-200102000-00012. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDU Assessment Manual for Psychopharmacology, Revised. Bethesda: U.S. Department of Health, Education, and Welfare; 1976. [Google Scholar]
- Jacobson N. Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Rao U. Flynn C. Moreci P. Brent D. Williamson D. Ryan N. Schedule for affective disorder and Schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:989–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Newcorn JH. Kratochvil CJ. Allen AJ. Casat CD. Ruff DD. Moore RJ. Michelson D. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: Acute comparison and differential response. Am J Psychiatry. 2008;165:721–730. doi: 10.1176/appi.ajp.2007.05091676. [DOI] [PubMed] [Google Scholar]
- Newcorn JH. Stein MA. Cooper K. Dose-response characteristics in adolescents with ADHD treated with OROS methylphenidate in a 4 week, open-label, dose titration study. J Child Adolesc Psychopharmacol. 2010;20:187–196. doi: 10.1089/cap.2009.0102. [DOI] [PubMed] [Google Scholar]
- Newcorn JH. Sutton V. Zhang S. Wilens T. Kratochvil CJ. Emslie G. Sutton VK. D'Souza DN. Schuh LM. Allen AJ. Characteristics of placebo responders in pediatric clinical trials of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:1165–1172. doi: 10.1097/CHI.0b013e3181bc730d. [DOI] [PubMed] [Google Scholar]
- Olfson M. Marcus S. Wan G. Stimulant dosing for children with ADHD: A medical claims analysis. J Am Acad Child Adolesc Psychiatry. 2009;48:51–59. doi: 10.1097/CHI.0b013e31818b1c8f. [DOI] [PubMed] [Google Scholar]
- Pliszka SR. Greenhill LL. Crismon ML. Sedillo A. Carlson C. Conners CK. McCracken JT. Swanson JM. Hughes CW. Llana ME. Lopez M. Toprac MG. The Texas children's medication algorithm project: Report of the Texas Consensus Conference panel on medication treatment of childhood attention-deficit/hyperactivity disorder. Part II: Tactics. Attention-Deficit/Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:920–927. doi: 10.1097/00004583-200007000-00022. [DOI] [PubMed] [Google Scholar]
- Pliszka SR. Lopez M. Crismon ML. Toprac MG. Hughes CW. Emslie GJ. Lopez M. Boemer C. A feasibility study of the children's medication algorithm project (CMAP) algorithm for the treatment of ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42:279–287. doi: 10.1097/00004583-200303000-00007. [DOI] [PubMed] [Google Scholar]
- Solanto M. Newcorn J. Vail L. Gibert S. Ivanov I. Lara R. Stimulant drug response in predominantly inattentive and combined subtypes of attention deficit/hyperactivity disorder. J Child Adolesc Psychopharmcol. 2009;19:663–671. doi: 10.1089/cap.2009.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele M. Jensen PS. Quinn DM. Remission versus response as the goal of therapy in ADHD: A new standard for the field? Clin Ther. 2006;28:1892–1908. doi: 10.1016/j.clinthera.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Stein MA. Sarampote CS. Waldman IW. Robb A. Conlon C. Pearl P. Black D. Seymour K. Newcorn J. A dose response study of OROS methylphenidate in children with ADHD. Pediatrics. 2003;112:3404–3413. doi: 10.1542/peds.112.5.e404. [DOI] [PubMed] [Google Scholar]
- Stein MA. Szumowski E. Blondis TA. Roizen NJ. Adaptive skills dysfunction in ADD and ADHD children. J Child Psychol Psychiatry. 1995;36:663–670. doi: 10.1111/j.1469-7610.1995.tb02320.x. [DOI] [PubMed] [Google Scholar]
- Swanson J. Effects of stimulant medication on hyperactive children: A review of reviews. Except Child. 1993;160:154–162. [Google Scholar]
- Swanson JM. Hechtman L. Using long-acting stimulants: Does it change ADHD treatment outcome? Can Child Adolesc Psychiatr Rev. 2005;14:2–3. [PMC free article] [PubMed] [Google Scholar]
- Vetter VL. Elia J. Erickson C. Berger S. Blum N. Uzark K. Webb CL. Cardiovascular monitoring of children and adolescents with heart disease receiving stimulant drugs: A scientific statement from the American Heart Association Council on cardiovascular disease in the young congenital cardiac defects committee and the council on cardiovascular nursing. Circulation. 2008;117:2407–2423. doi: 10.1161/CIRCULATIONAHA.107.189473. [DOI] [PubMed] [Google Scholar]
- Weiss M. Brooks BL. Iverson GL. Reliability, Validity of the Weiss Functional Impairment Rating Scale; Presented at the American Academy of Child and Adolescent Psychiatry Annual Meeting; San Diego, CA. Oct;2007 . [Google Scholar]
- Wilens T. Spencer T. The stimulants revisited. Child Adolesc Psychiatr Clin N Am. 2000;9:573–603. [PubMed] [Google Scholar]