Abstract
Recent studies indicate that membrane cholesterol can associate with G protein-coupled receptors (GPCRs) and affect their function. Previously, we reported that manipulation of membrane cholesterol affects ligand binding and signal transduction of the type 1 cholecystokinin receptor (CCK1R), a Class A GPCR. We now demonstrate that the closely related type 2 cholecystokinin receptor (CCK2R) does not share this cholesterol sensitivity. The sequences of both receptors reveal almost identical cholesterol interaction motifs in analogous locations in transmembrane segments two, three, four, and five. The disparity in cholesterol sensitivity between these receptors, despite their close structural relationship, provides a unique opportunity to define the possible structural basis of cholesterol sensitivity of CCK1R. To evaluate the relative contributions of different regions of CCK1R to cholesterol sensitivity, we performed ligand binding studies and biological activity assays of wild-type and CCK2R/CCK1R chimeric receptor-bearing Chinese hamster ovary cells after manipulation of membrane cholesterol. We also extended these studies to site-directed mutations within the cholesterol interaction motifs. The results contribute to a better understanding of the structural requirements for cholesterol sensitivity in CCK1R and provides insight into the function of other cholesterol-sensitive Class A GPCRs.
Keywords: receptor binding, biological activity, chimeric receptors, G protein-coupled receptors, perfringolysin
Cholesterol is an important lipid component of the eukaryotic plasma membrane that has substantial effects on the physicochemical characteristics of the membrane. These include effects on the membrane rigidity and fluidity, as well as its dimensions (1–3). It has recently become clear that cholesterol can be tightly associated with membrane proteins, including G protein-coupled receptors (GPCRs) (4–6), with this association capable of modifying the function and regulation of those proteins (7, 8–10). Of note, some sites of association of cholesterol with membrane proteins can be predicted by specific sequence motifs (11–13), although it is likely that these are not the only regions in which this lipid interacts with such membrane proteins.
We previously reported that the function of the type 1 cholecystokinin (CCK1) receptor (CCK1R) is affected by the lipid composition of the plasma membrane, with cholesterol having prominent effects (7). This receptor is a member of Class A GPCRs, with important physiologic effects on nutrient homeostasis, stimulating gallbladder contraction, pancreatic exocrine secretion, gastroenteric motility, and even postcibal satiety (14). The type 2 cholecystokinin receptor (CCK2R) is highly homologous with CCK1R and is expressed on the gastric parietal cell, enteric neurons, and many areas of the brain, where it has effects on stimulating gastric acid secretion and has been implicated in anxiety and panic states (15).
Depletion of cholesterol in the membrane has previously been reported to have little or no effect on the function of the CCK2R (8). Because of the close structural relationship between these two receptors, we now examine the effects on the CCK2R of the same broad battery of manipulations, both to reduce and to increase membrane cholesterol, that we had applied to the CCK1R (7). Indeed, this demonstrated no effects of these manipulations on the CCK2R. With this prominent differential sensitivity to cholesterol for these closely related GPCRs, we utilized CCK2R/CCK1R receptor chimeras and site-directed receptor mutants to systematically explore the possible structural basis for the observed cholesterol sensitivity. These studies localized a region of importance within exon three that includes transmembrane (TM) segments three and four of the CCK1R, a sequence that contains two cholesterol recognition motifs.
MATERIALS AND METHODS
Materials
Synthetic cholecystokinin (CCK) octapeptide (CCK-26-33) was purchased from Peninsula Laboratories (Belmont, CA). Alexa Fluor 660-C5-maleimide, Amplex Red reagent, Ham's F-12 medium, DMEM, Lipofectamine LTX Plus reagent, soybean trypsin inhibitor, and Fura-2-acetoxymethyl ester (Fura-2AM) were from Invitrogen (Carlsbad, CA). Fetal clone 2 culture medium supplement was from Hyclone Laboratories (Logan, UT). Molecular biology reagents were from New England Biolabs (Ipswich, MA). G418 was from Research Products International Corporation (Mt. Prospect, IL). Lipoprotein-deficient serum (LPDS) was obtained from Intracel (Frederick, MD). BSA was from Equitech Bio, Inc. (Kerrville, TX). Costar 96-well black wall/clear bottom assay plates and 96-well V-bottom assay plates were from Corning (Corning, NY). CelLytic B, CelLytic IB, mevastatin, DL-mevalonic acid lactone, isopropyl-1-thio- β -d-galactopyranoside (IPTG), LDL, methyl- β -cyclodextrin (M β CD), methyl- β -cyclodextrin-cholesterol (M β CD-cholesterol), and probenecid were from Sigma-Aldrich (St. Louis, MO). All other reagents were analytical grade.
Receptor-bearing cell systems
Chinese hamster ovary (CHO)-K1 cell lines engineered to express the wild-type human type 1 CCK receptor (CHO-CCK1R) and the wild-type human type 2 CCK receptor (CHO-CCK2R) were used as sources of these receptors in normal and cholesterol-modified environments. These cell lines have been previously characterized, and were shown to express fully functional receptors that bind CCK, signal, and undergo agonist-induced internalization in a normal manner (16). CHO cell lines were grown at 37°C in a humidified environment containing 5% carbon dioxide in tissue culture plasticware containing Ham's F-12 medium supplemented with 5% fetal clone 2, and COS-1 cells were grown in DMEM medium under the same conditions. Cells were passaged approximately two times per week.
Previously described cDNA constructs for chimeric human CCK2R/CCK1R receptors in eukaryotic expression vectors were used in this study (17). Stable receptor-expressing cell lines were established by transfecting CHO-K1 cells (American Type Culture Collection; Manassas, VA) using lipofectamine LTX according to the manufacturer's directions. Receptor-expressing clones were selected by G418 selection and limiting dilution. When necessary, the populations of receptor-expressing cells were enriched by flow cytometric sorting prior to clonal selection. Clonal cell lines having levels of surface receptor expression comparable to that of the CHO-CCK1R and CHO-CCK2R cell lines were selected based on radioligand binding assays (described below). For one of the chimeric constructs, CCK2R(ex1-3)/CCK1R(ex4-5), transfection of CHO-K1 cells did not result in stable transfectants with sufficient receptor expression. For that construct, we utilized transfection of COS-1 cells using the DEAE-dextran method (16) and studied transient-expressing cells 48 h later.
Site-directed mutagenesis of the human type 1 and type 2 CCK receptors was also performed. This was designed to disrupt the previously described cholesterol binding motifs present in many GPCRs (11, 12). These included CCK1R(Y140A), CCK1R(W166A), and CCK1R(Y237A) in the background of the CCK1R, and CCK2R(Y153A), CCK2R(W179A), and CCK2R(Y246A) in the background of the CCK2R, interfering with motifs within TM3, TM4, and TM5, respectively. Constructs were prepared using oligonucleotide-directed mutagenesis using the QuikChange mutagenesis kit (Agilient Technologies; Santa Clara, CA), with products verified by dideoxynucleotide chain-termination cDNA sequencing. CHO cell lines expressing the CCK1R constructs were prepared and studied as described above, and the CCK2R constructs were studied as transiently expressed in COS-1 cells.
Modification of membrane cholesterol levels
Cholesterol levels in receptor-bearing CHO cell lines were depleted either chemically, using the cholesterol binding reagent M β CD, or metabolically, by growing the cells in Ham's F-12 medium containing 5% LPDS, 10 μ M mevastatin (a hydroxymethylglutaryl-CoA reductase inhibitor), and 200 μ M DL-mevalonic acid lactone. For chemical depletion, cells were incubated with 7.5 mM M β CD in Krebs-Ringer/HEPES (KRH) medium (25 mM HEPES, pH 7.4, 104 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1.0 mM KH2PO4, 1.2 mM MgSO4) for 30 min at 37°C with gentle shaking. For metabolic depletion, cells were grown in Ham's F-12 medium containing 5% LPDS and 10 μ M mevastatin for 2 days. After membrane cholesterol depletion, cells were washed with KRH medium supplemented with 0.01% soybean trypsin inhibitor and 0.2% BSA before being used for receptor binding and biological activity studies.
Cholesterol levels in receptor-bearing cells were chemically enriched by incubation with 7.5 mM M β CD-cholesterol in KRH medium for 30 min at 37°C with gentle shaking, or by incubating the metabolically cholesterol-depleted cells with 150 μ g/ml LDL in complete Ham's F-12 medium for 24 h (7, 18).
Determination of plasma membrane cholesterol content
For determination of plasma membrane cholesterol, we utilized a morphologic assay with filipin (7), a biochemical assay on isolated membranes with Amplex Red reagent (19), and a new flow cytometric assay on intact cells utilizing a cell impermeant cholesterol binding probe. The filipin staining of membrane cholesterol was performed as previously described (7). The Amplex Red assay was performed on a lipid extract (20) from a particulate fraction (21) derived from the CCK receptor-bearing cells. Membrane protein content was determined in the same particulate fraction using a BCA kit (22), allowing the cholesterol content to be expressed as micrograms per milligram protein.
We also developed a direct assay of cellular plasma membrane cholesterol content that is applicable to healthy, intact cells using cell cytometry. For this, a probe was prepared based on the observation that θ toxin produced by Clostridium perfringens [Perfringolysin θ (PFO)] binds to cholesterol within the plasma membrane of eukaryotic cells and disrupts them by forming homo-oligomeric pores (23–25). The fourth domain of this toxin has been shown to be responsible for its ability to bind to cholesterol, and this isolated domain does not form pores in the membrane (26). Fluorescent PFO domain 4 has been utilized in flow cytometry and fluorescence microscopy (27, 28). DNA encoding PFO domain 4 was amplified using PCR with the template representing the PFO-containing plasmid, pAH21 (the kind gift of Dr. Alejandro P. Heuck from the University of Massachusetts, Amherst). The reaction product was digested with XhoI and NheI, and was ligated into pET-28a vector, with the DNA sequence verified by the dideoxynucleotide chain-termination method. The endogenous cysteine residue in position 48 (corresponding to position 431 in the intact protein) was mutated to an alanine using the oligonucleotide-directed mutatgenesis method and the QuikChange mutagenesis kit (Agilent Technologies; Santa Clara, CA), and the product was verified by sequence analysis. The resulting plasmid was introduced into competent Escherichia coli strain BL21(DE3)pLysS (Promega; Madison, WI). A 5 ml overnight culture of these cells was used to inoculate 500 ml of Luria broth (Research Products International; Mt. Prospect, IL) supplemented with 0.5 mg/ml kanamycin. The culture was incubated at 37°C with shaking to yield an OD600 of 0.4–0.6, at which time 1 mM IPTG was added and the incubation was continued for 4 h.
The bacteria were collected by centrifugation, with the pellet washed with 0.5 M NaCl, and frozen overnight. Lysis was achieved by resuspending the thawed pellet in 4 ml CelLytic B (Sigma-Aldrich) per gram of pellet, incubating on ice for 30 min, and sonicating on ice using a Branson Sonifier 250 (Danbury, CT) constant duty cycle, output level 6, for 4–10 s intervals. The inclusion body pellet was collected from the lysate by centrifugation at 4°C for 15 min at 16,000 g. The inclusion bodies were solubilized using CelLytic IB (Sigma-Aldrich) following the manufacturer's instructions. Protein induction, size, and purity were verified by SDS-PAGE. The solubilized pellet, containing 95% PFO domain 4, was adjusted to pH 7.0 by addition of 3% triethylamine (Thermo Scientific; Rockford, IL) before being used in derivatization reactions with Alexa Fluor-660-C5-maleimide (Invitrogen), following the manufacturer's instructions. Alexa-conjugated PFO domain 4 was purified by gel filtration over a Sephadex G50 Superfine column (GE Healthcare Life Sciences) using citric acid-Na2HPO4 buffer (pH 5.0). Alexa-PFO domain 4 content of fractions was verified by SDS-PAGE.
Flow cytometry
The plasma membrane cholesterol content of receptor-bearing CHO cells was analyzed by flow cytometry using the Alexa-PFO domain 4 probe. After treatment to deplete or enrich membrane cholesterol, or left untreated, the cells in culture were lifted using Cell Dissociation Solution (Sigma-Aldrich) for 5–10 min, washed with PBS, pH 7.4, and resuspended in PBS supplemented with 0.5% BSA and 0.01% sodium azide at a density of 1 × 106 cells/ml. Ten micrograms per milligram Alexa-PFO domain 4 was added, and the cells were incubated at room temperature for 30 min. Five microliters of 7-aminoactinomycin D (7-AAD) Viability Staining Solution (eBioscience; San Diego, CA) was added to each sample 5 min before the end of the incubation. The cells were pelleted, resuspended in buffer, and then analyzed using a Cyan flow cytometer (Beckman Coulter; Brea, CA). Cells were gated based on 7-AAD exclusion, as well as forward and side scatter, to eliminate dead cells and debris. Particular attention was given to forward scatter gating to ensure that cells being analyzed for Alexa-PFO domain 4 fluorescence were of a limited range of median sizes. An average of 20,000 7-AAD-negative gated events were collected for each sample. Raw data were analyzed using FloJo software (Tree Star; Ashland, OR). Differences in fluorescence intensity relative to that of the untreated cells were analyzed by one-way ANOVA using the Prism 4.0 software package by GraphPad (San Diego, CA).
Radioligand binding
Cells expressing each receptor construct were plated in 24-well tissue culture plates coated with poly l-lysine in Ham's F12 medium (CHO cells) or DMEM (COS-1 cells) supplemented with 5% fetal clone 2 and cultured for 48–72 h at 37°C in a humidified environment containing 5% carbon dioxide to achieve 80–90% confluence. The cells were then incubated with the radioligand, 125I-d-Tyr-Gly-[(Nle28,31)CCK-26-33] (5 pM, ∼ 20,000 cpm/well), in the absence or presence of increasing concentrations of unlabeled CCK in 0.5 ml of KRH medium containing 0.2% BSA and 0.01% soybean trypsin inhibitor at room temperature for 60 min with gentle shaking. Nonspecific binding was defined as the amount of radioactivity bound in the presence of 1 μ M unlabeled CCK. The binding reaction was terminated by washing the cells twice with ice-cold KRH medium. The cells were then lysed with 0.5 M NaOH, and lysates were collected and analyzed for radioactivity in a γ counter. Radioligand binding data were analyzed using the LIGAND program of Munson and Rodbard (29) and were graphed using the nonlinear least-squares curve-fitting routine in the Prism 4.0 software package by GraphPad.
Biological activity
Biological activity was assessed by the measurement of intracellular calcium concentrations in response to CCK in receptor-expressing cells. CHO cells stably expressing the indicated receptor constructs (or COS-1 cells transiently expressing receptor constructs) were plated in a 96-well black-walled plate (Corning) at a density of 15,000–20,000 cells per well and cultured for 24 h in a humidified incubator at 37°C containing 5% carbon dioxide. The cells were then studied without treatment or were treated with 7.5 mM M β CD or 7.5 mM M β CD-cholesterol in KRH medium for 30 min at 37°C with gentle rocking, or left untreated. The cells were then washed and loaded with 1.5 μ M Fura-2AM in KRH medium containing 1.2 mM MgCl2, 0.2% BSA, and 2.5 mM probenecid for 1 h at 37°C. Biological activity was measured by stimulating the cells with various concentrations of CCK at 37°C and monitoring fluorescence over 2 min with a FlexStation 3.0 (Molecular Devices; Sunnyvale, CA) by robotic addition of ligand (1 pM to 10 nM) using Softmax Pro 5.4 software. Emission was measured at 520 nm after excitation at 340 nm and 380 nm. Calcium concentrations were calculated from the ratio of the two fluorescence intensities. Data were graphed using the nonlinear square curve-fitting routine in the Prism 4.0 software package (GraphPad).
RESULTS
Plasma membrane cholesterol composition
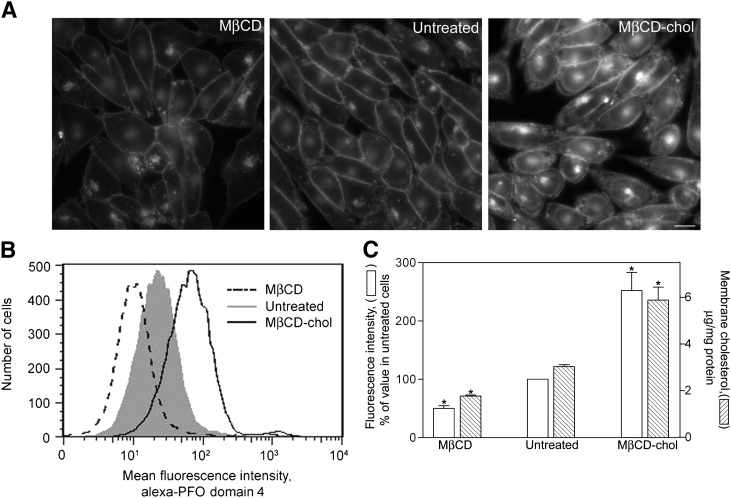
The various chemical and metabolic methods utilized for depleting and enriching membrane cholesterol were effective ( ). Fluorescence microscopy using filipin (Fig. 1A) illustrates the effectiveness of these treatments to deplete and to enrich membrane cholesterol in a CHO cell environment. The fluorescently labeled PFO domain 4 was prepared and utilized to measure membrane cholesterol content of intact receptor-bearing CHO cells by flow cytometry (typical profiles shown in Fig. 1B). The M β CD treatment reduced plasma membrane cholesterol by approximately 50%, while treatment with M β CD-cholesterol increased plasma membrane cholesterol by approximately 2-fold relative to untreated cells. The flow cytometry data correlated well with the quantitative analysis of membrane cholesterol in CHO cells following these treatments using the Amplex Red reagent (Fig. 1C).
Fig. 1.
Cellular cholesterol content. Shown are the epifluorescence images of filipin staining of cholesterol in receptor-bearing CHO-CCK1R cells (bar = 20 μ m) (A), fluorescence profiles from flow cytometry of alexa-PFO domain 4-labeled cells (B), and quantitation of membrane cholesterol based on fluorescence intensities in the flow studies, as well results of Amplex Red assays of cholesterol content (C). Flow cytometry data shown in panel B are reflective of data from four independent experiments, with each histogram including approximately 20,000 gated events in a typical experiment. Fluorescence intensity data graphed in panel C represent means ± SEM relative to the signal from control, untreated cells in these four experiments. These results were confirmed using the biochemical assay of membrane cholesterol; the results of this assay correlated well with those of the flow analysis. The cholesterol content of the membranes from the cholesterol-depleted and cholesterol-enriched cells were significantly different from that of the control untreated cells (* P < 0.05).
Function of CCK receptors in distinct cholesterol environments
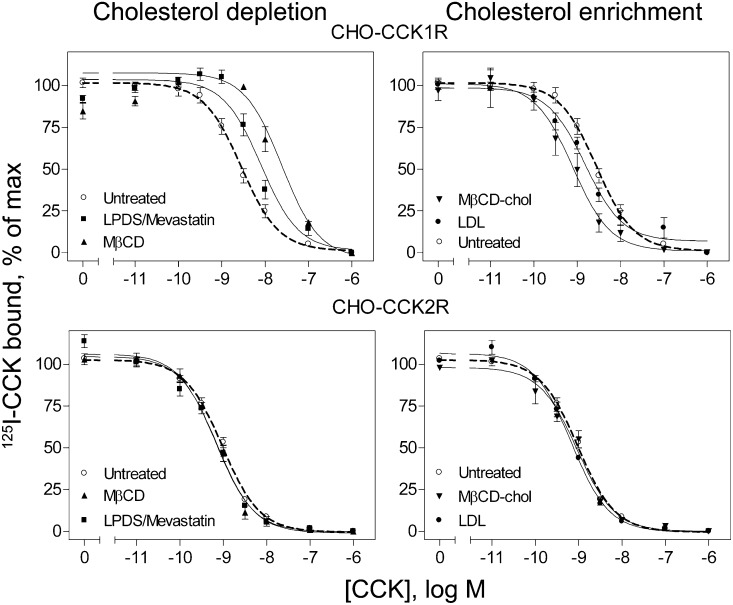
Cholesterol depletion resulted in a shift to lower affinity binding of CCK to the type 1 CCK receptor (P < 0.05), as we have previously reported (7), but it had no effect on CCK binding to the type 2 CCK receptor (P > 0.05) ( Fig. 2, Table 1). Cholesterol depletion using M β CD had a greater effect on decreasing CCK binding affinity in the CCK1R cells than did the metabolic method of depletion; this is consistent with the more-extensive effect of M β CD in reducing the cholesterol content of the plasma membrane in these cells. The effect of cholesterol depletion in reducing CCK binding affinity was fully reversed upon replenishment of the membrane cholesterol. In another series of experiments, the Ki values for CCK binding were 1.9 ± 0.1 nM for the untreated CCK1R-bearing cells, 13.2 ± 4.3 nM for these cells after treatment with M β CD (P < 0.05 relative to control cells), and 2.1 ± 0.1 nM after replenishing the cholesterol in these cells using M β CD-cholesterol (P > 0.05 relative to control cells).
Fig. 2.
CCK radioligand binding to wild-type receptors on cholesterol-modified cells. Shown are CCK competition binding curves for CHO-CCK1R cells in the top panels and CHO-CCK2R cells in the bottom panels. The panels on the left reflect manipulations to decrease cholesterol, whereas those on the right reflect manipulations to increase cholesterol. CCK binding to CCK1R was reduced in affinity after cholesterol depletion and increased in affinity after cholesterol enrichment. CCK binding to CCK2R was unaffected by any of the manipulations studied. Values reflect percentages of maximal saturable CCK radioligand binding in the absence of competing CCK. Data represent means ± SEM of three to nine independent experiments performed in duplicate.
TABLE 1.
Binding and biological activity parameters for wild-type CCK receptors expressed on CHO cell lines after cholesterol manipulations
| Constructs | Condition | Binding Affinities | Binding Sites | Calcium Responses |
| Ki | Sites/cell × 105 | EC5050 | ||
| nM | nM | |||
| CCK1R | Untreated | 4.6 ± 0.7 | 2.3 ± 0.3 | 0.02 ± 0.01 |
| MβCD | 23.4 ± 6.7a | 1.7 ± 0.6 | 0.32 ± 0.06a | |
| LPDS/mevastatin | 13.3 ± 2.5a | 1.5 ± 0.2 | ND | |
| MβCD-cholesterol | 1.5 ± 0.5a | 1.8 ± 0.9 | 0.05 ± 0.01a | |
| LDL | 1.9 ± 0.3a | 1.4 ± 0.2 | ND | |
| CCK2R | Untreated | 0.7 ± 0.1 | 1.7 ± 0.4 | 0.06 ± 0.01 |
| MβCD | 0.4 ± 0.2 | 1.1 ± 0.3 | 0.09 ± 0.02 | |
| LPDS/mevastatin | 0.7 ± 0.1 | 0.7 ± 0.1 | ND | |
| MβCD-cholesterol | 0.8 ± 0.1 | 1.2 ± 0.1 | 0.05 ± 0.02 | |
| LDL | 0.7 ± 0.1 | 0.5 ± 0.1 | ND |
Data are expressed as means ± SEM of values from three to nine independent experiments. ND, not determined.
P < 0.05 significantly different from the control, untreated condition.
Cholesterol enrichment using either method resulted in increases in CCK binding affinity to CCK1R (P < 0.05), as we have previously described (7). In contrast, this manipulation had no effect on CCK binding to CCK2R (Fig. 2, Table 1).
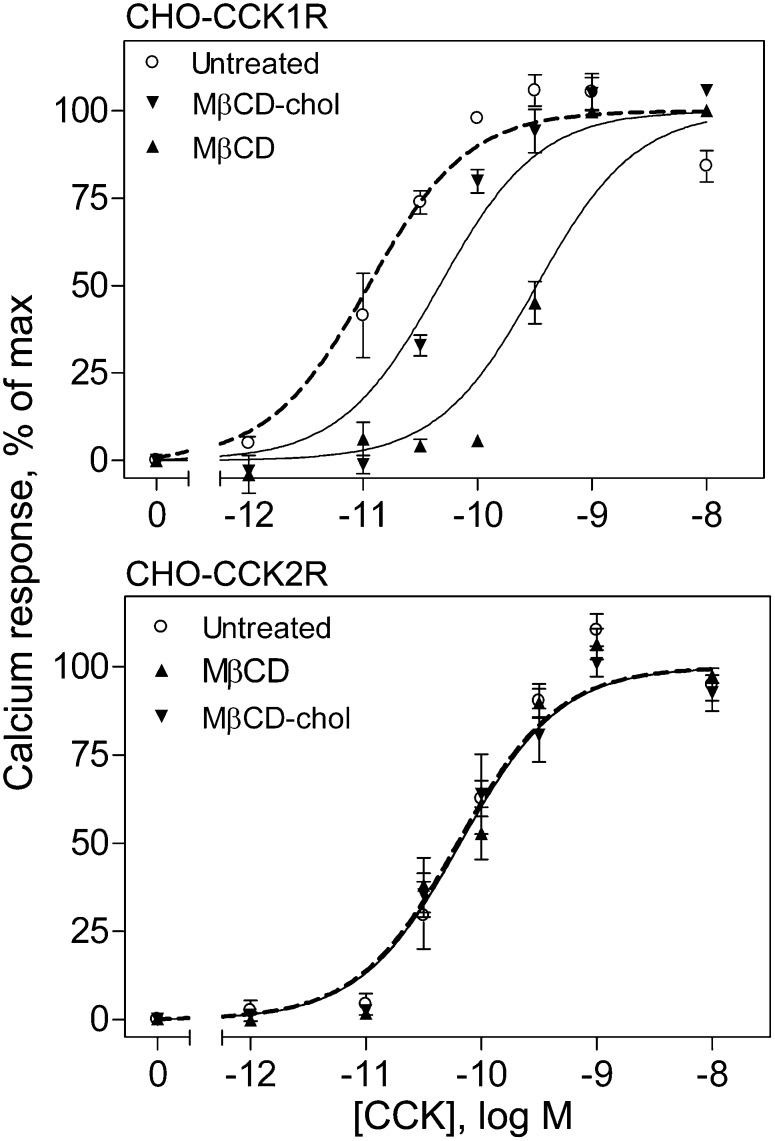
We measured the ability of CCK to stimulate biological activity at the CCK1R and CCK2R in the stable receptor-bearing CHO cell lines by measuring changes in intracellular calcium in response to this hormone. As we previously observed (7), both cholesterol depletion and cholesterol enrichment resulted in reduced calcium responses to CCK in the CCK1R-bearing cells. In contrast, neither of these treatments had any effect on the CCK responses in CCK2R-bearing cells ( , Table 1), consistent with the binding data shown in Fig. 2. Like the reversibility of the effect of cholesterol modification on CCK binding to the CCK1R, the decrease in biological activity in response to CCK at this receptor that is associated with depletion of membrane cholesterol was fully reversible by replenishment of the cholesterol content (7).
Fig. 3.
CCK-stimulated intracellular calcium responses at wild-type receptors. Shown are the CCK concentration-dependent calcium-response curves in CHO-CCK1R cells (top panel) and CHO-CCK2R cells (bottom panel). Each cell type was left untreated, chemically depleted of membrane cholesterol with M β CD, or had membrane cholesterol enriched by M β CD-cholesterol treatment. Intracellular calcium responses are expressed as percentages of maximal responses for each condition. The calcium-response curve was shifted to the right for CCK1R cells after depletion or enrichment of the cholesterol, whereas these manipulations had no effect of the CCK2R cells. Data represent means ± SEM of four to five independent experiments performed in triplicate.
Structural basis for differential cholesterol sensitivity of CCK receptors
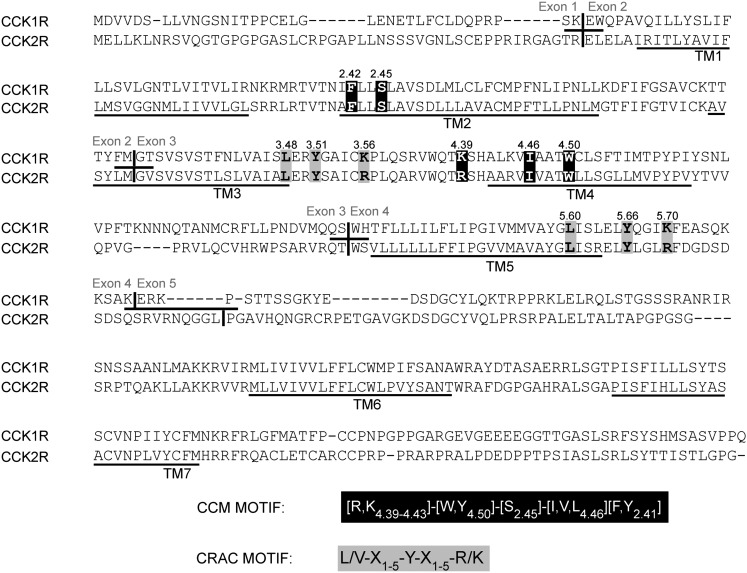
Comparison of the amino acid sequences of human CCK1R and CCK2R illustrates the high degree of homology and identity (53% identity overall, with 69 percent identity within the TM segments) between the two receptors ( ). Sequence motifs previously described as being important for membrane protein interaction with cholesterol are present in three positions in both of these receptors. As highlighted in Fig. 4, TM segments 3 and 5 of both receptors contain the cholesterol recognition/interaction amino acid consensus pattern (CRAC) L/V-X1-5-Y-X1-5-R/K, described by Li and Papadopoulos in 1998 (11), representing CCK receptor residues L 3.48-Y 3.51-K,R 3.56 and L 5.60-Y 5.66-K,R 5.70. Also present in TM4 and TM2 of both receptors is the cholesterol consensus motif (CCM) described by Hanson et al. in 2008 (12) and later expanded by Adamian, Naveed, and Liang in 2011 (13). This motif [4.39-4.43 (R,K)][4.50 (W,Y)][2.45 (S)][4.46 (I,V,L)][2.41 (F,Y)] in the Ballesteros and Weinstein numbering system (30), emerged from analysis of the 2.8 Å structure of the human β 2-adrenergic receptor (31), fitting the CCK receptors at R,K 4.39-W 4.50-S 2.45-I 4.46-F 2.42. It should be noted that, except for conservative variations between lysine and arginine in a given position, these motifs are identical in both types of CCK receptors. Given the close structural similarity between the two receptors, it is possible that residues neighboring one or more cholesterol interaction motifs might contribute to the observed differences in cholesterol sensitivity.
Fig. 4.
Primary sequences and alignment of CCK1 and CCK2 receptors. Shown are aligned sequences of CCK1R and CCK2R, with exon junctions and predicted TM segments marked. The cholesterol-association motifs, CCM (white letters on black background) and CRAC (black letters on gray background) are noted.
To determine the possible structural basis for differential cholesterol sensitivity of these receptors, we began by utilizing a series of chimeric receptors (CCK2R/CCK1R) originally prepared and characterized by Wu et al. (17). These chimeric constructs take advantage of the fact that the genes encoding human CCK1R and CCK2R each contain five exons in a similar organization (17, 32–34). The exons from CCK1R were sequentially replaced by exons from CCK2R, starting from the N terminus. By stably expressing these chimeric receptors in CHO cells (or transiently in COS-1 cells) and characterizing their sensitivity to cholesterol, we attempted to localize the broad regions of these receptors most contributing to their differential cholesterol sensitivity.
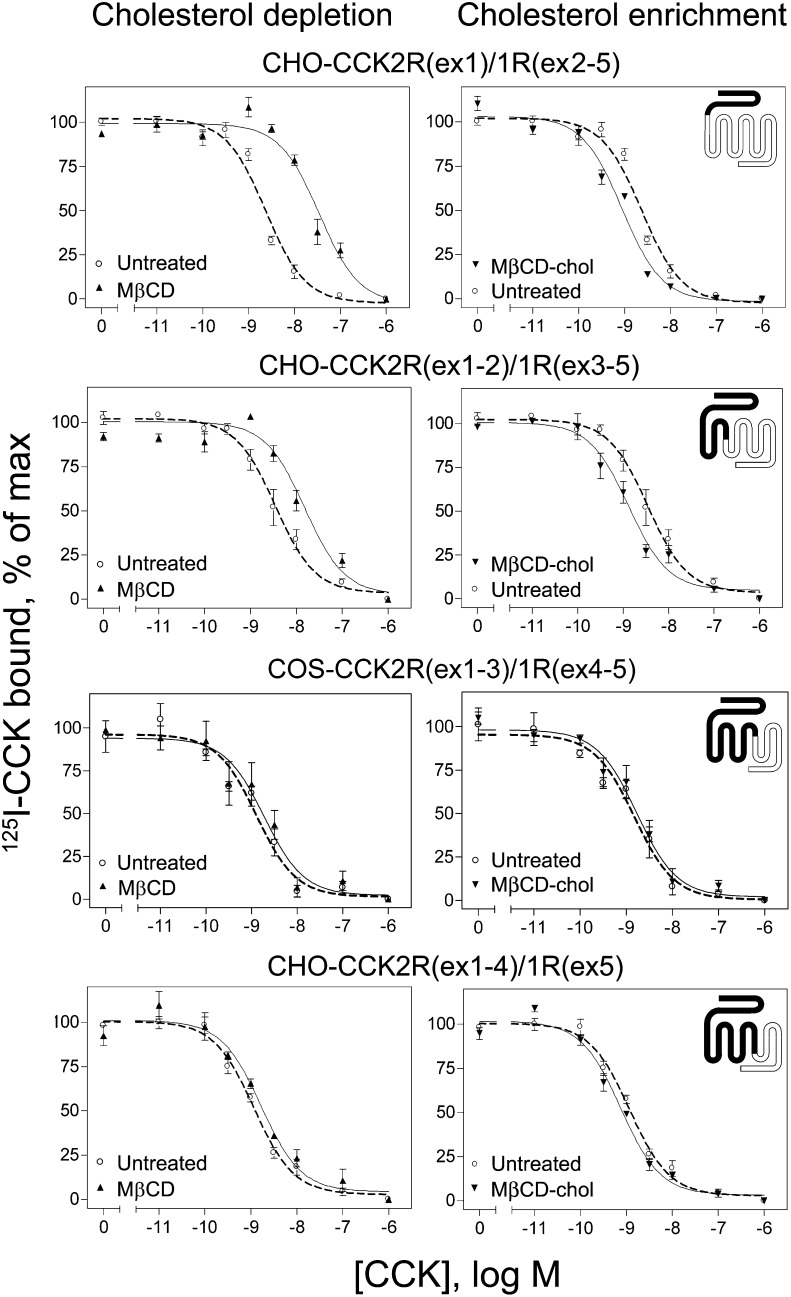
Each of the chimeric receptor-bearing cell lines were studied with unmodified membrane cholesterol and after the depletion or enrichment of the membrane cholesterol (Fig. 5 and Table 2) . After cholesterol depletion using M β CD, chimeras CCK2R(ex1)/CCK1R(ex2-5) and CCK2R(ex1-2)/CCK1R(ex3-5) both exhibited decreased CCK binding affinity (P < 0.05). In contrast, no significant shift in CCK binding affinity was observed in the CCK2R(ex1-3)/CCK1R(ex4-5) or CCK2R(ex1-4)/CCK1R(ex5) chimeric constructs. Metabolic depletion of membrane cholesterol by culture in LPDS/mevastatin gave similar results (data not shown). After membrane cholesterol enrichment using M β CD-cholesterol, increases in CCK binding affinity were observed for chimeras CCK2R(ex1)/CCK1R(ex2-5) and CCK2R(ex1-2)/CCK1R(ex3-5) (P < 0.05). Chimeric constructs CCK2R(ex1-3)/CCK1R(ex4-5) and CCK2R(ex1-4)/CCK1R(ex5) again exhibited no change in CCK binding affinity in the presence of cholesterol modification. Enrichment of membrane cholesterol by culture in LDL-supplemented media gave similar results (data not shown).
Fig. 5.
CCK radioligand binding to chimeric CCK2R/CCK1R receptors on cholesterol-modified cells. Shown are CCK competition binding curves for chimeric receptor constructs after cholesterol depletion with M β CD (left panels) or cholesterol enrichment with M β CD-cholesterol treatment (right panels). Cholesterol depletion decreased and cholesterol enrichment increased the CCK binding affinities for CCK2R(ex1)1R(ex2-5) and CCK2R(ex1-2)1R(ex3-5) chimeric receptors. Neither treatment had any significant effect on the CCK2R(ex1-3)1R(ex4-5) or CCK2R(ex1-4)1R(ex5) chimeric receptors. Data points reflect percentages of maximal saturable CCK radioligand binding in the absence of competing CCK and represent means ± SEM of three to nine independent experiments performed in duplicate.
TABLE 2.
Binding and biological activity parameters of chimeric CCK receptors after cholesterol manipulations
| Constructs | Condition | Binding Affinities | Binding Sites | Calcium Responses |
| Ki | Sites/cell × 105 | EC50 | ||
| nM | nM | |||
| CCK2R(ex1)/1R(ex2-5) | ||||
| Untreated | 2.4 ± 0.1 | 0.9 ± 0.3 | 0.01 ± 0.01 | |
| MβCD | 20.6 ± 4.5a | 0.8 ± 0.3 | 0.12 ± 0.02a | |
| MβCD-cholesterol | 0.8 ± 0.1a | 0.6 ± 0.2 | 0.03 ± 0.01a | |
| CCK2R(ex 1-2)/1R(ex3-5) | ||||
| Untreated | 5.5 ± 0.7 | 2.6 ± 0.6 | 0.16 ± 0.03 | |
| MβCD | 23.2 ± 3.9a | 1.5 ± 0.5 | 1.29 ± 0.50a | |
| MβCD-cholesterol | 1.5 ± 0.4a | 1.5 ± 0.9 | 0.62 ± 0.23a | |
| CCK2R(ex1-3)/1R(ex4-5) | ||||
| Untreated | 8.2 ± 3.4 | 1.2 ± 0.5 | 0.04 ± 0.01 | |
| MβCD | 7.4 ± 2.6 | 1.3 ± 0.6 | 0.04 ± 0.01 | |
| MβCD-cholesterol | 13.5 ± 4.7 | 1.2 ± 0.4 | 0.04 ± 0.01 | |
| CCK2R(ex1-4)/1R(ex5) | ||||
| Untreated | 2.0 ± 0.7 | 0.5 ± 0.1 | 0.09 ± 0.03 | |
| MβCD | 2.7 ± 0.1 | 0.2 ± 0.1 | 0.05 ± 0.03 | |
| MβCD-cholesterol | 1.1 ± 0.2 | 0.3 ± 0.1 | 0.12 ± 0.02 | |
Data are expressed as means ± SEM of values from three to nine independent experiments.
P < 0.05 significantly different from the control, untreated condition.
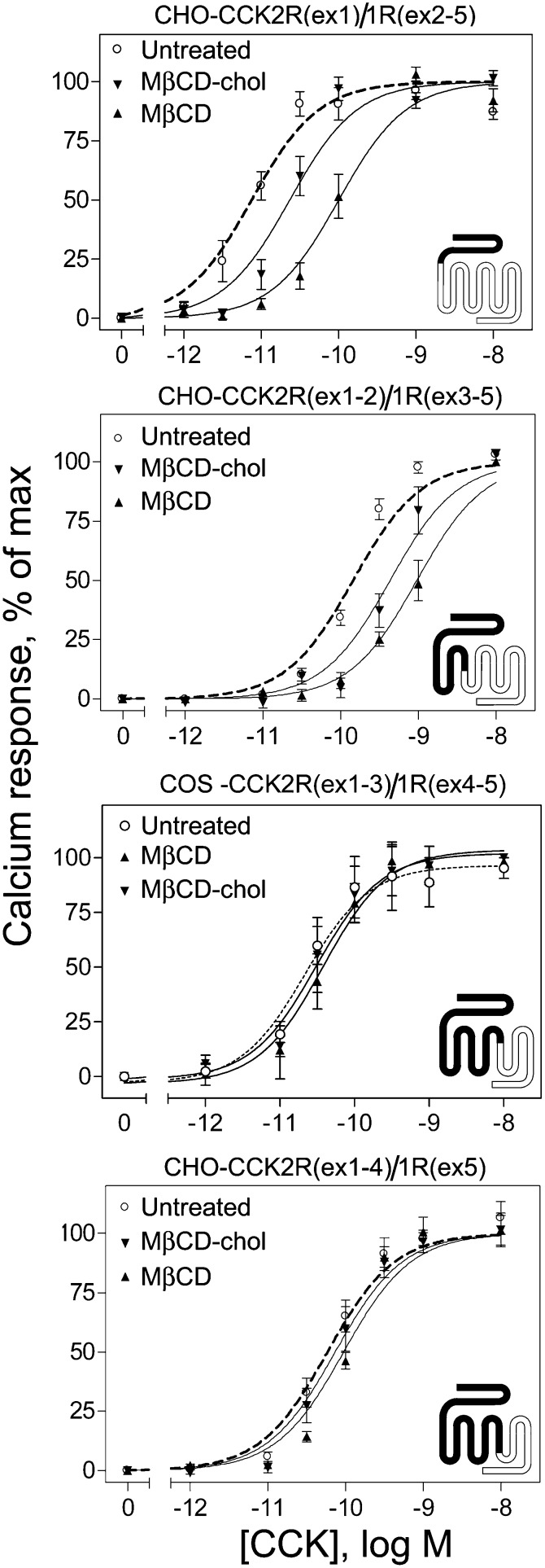
Calcium responses in chimeric receptor-expressing CHO cells to CCK following treatment with M β CD (depletion) or M β CD-cholesterol (enrichment) resulted in types of effects similar to those observed in the ligand binding studies. Both chimeras CCK2R(ex1)/CCK1R(ex2-5) and CCK2R(ex1-2)/CCK1R(ex3-5) displayed decreased calcium responses to CCK, whereas CCK2R(ex1-3)/CCK1R(ex4-5) and CCK2R(ex1-4)/CCK1R(ex5) exhibited no effects of cholesterol modification (Fig. 6, Table 2).
Fig. 6.
CCK-stimulated intracellular calcium responses at chimeric CCK2R/CCK1R receptors. Shown are the CCK concentration-dependent calcium response curves in chimeric receptor constructs after depletion of membrane cholesterol with MβCD and enrichment of membrane cholesterol with MβCD-cholesterol treatment. Both CCK2R(ex1)1R(ex2-5) and CCK2R(ex1-2)1R(ex3-5) constructs exhibited rightward shifts suggesting lower potency of calcium responses, whereas CCK2R(ex1-3)1R(ex4-5) and CCK2R(ex1-4)1R(ex5) exhibited no change in calcium responses. Intracellular calcium responses are expressed as percentages of maximal responses for each condition. Data represent means ± SEM of three to nine independent experiments performed in triplicate.
Thus, the effects of cholesterol modification observed in type 1 CCK receptors continued to be present in the chimeric constructs as N-terminal exons of CCK1R were replaced with those of CCK2R through exon 2, whereas absence of cholesterol sensitivity was observed as soon as CCK1R exon 3 was replaced with that of CCK2R. This suggests that a critical determinant of the cholesterol sensitivity found in CCK1R is within its third exon, which includes most of TM3 and TM4. This region includes one of the CRAC motifs found in TM3 and components of the CCM motif found in TM4.
CCK receptor site mutants
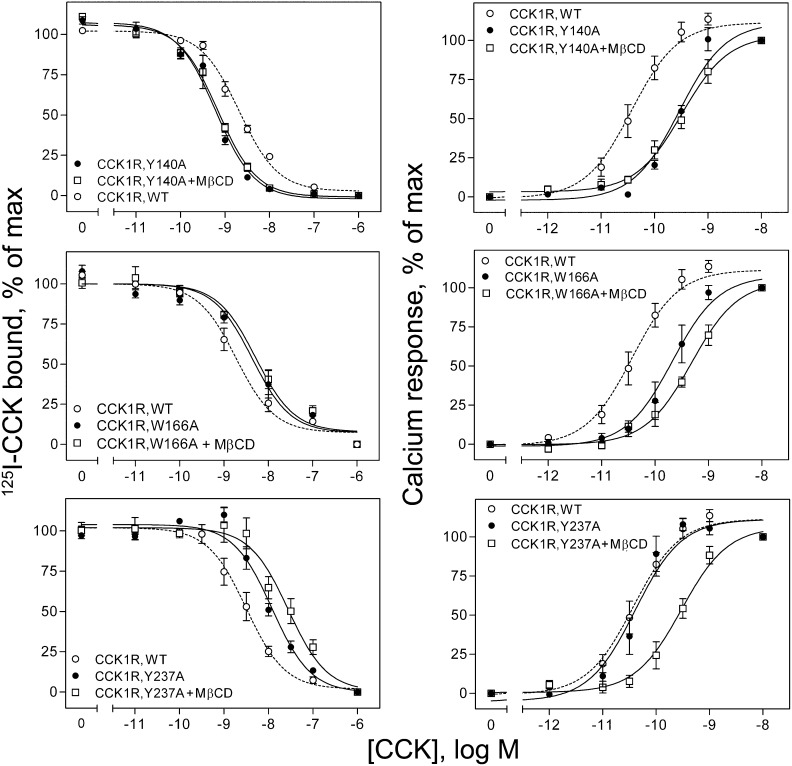
Having found that the third exon of CCK1R probably contains elements essential to cholesterol sensitivity, we next attempted to identify specific residues that may be critical for this activity. As noted above, exon 3 includes both a CRAC motif in TM3 and a CCM motif in TM4. We, therefore, prepared two constructs, representing CCK1R(Y140A) and CCK1R(W166A), that replaced the critical aromatic tyrosine or tryptophan residues in each of these motifs with alanine residues, thus disrupting them. We also prepared a construct, CCK1R(Y237A), that disrupted the TM5 CRAC motif. Data for CCK binding and CCK-stimulated intracellular calcium responses for each of the CCK1R constructs expressed in CHO cell lines are shown in Fig. 7 and Table 3. It is noteworthy that each of the three mutations had negative effects on either CCK binding affinity or CCK-stimulated signaling, compared with wild-type CCK1R. The Y140A mutant exhibited reduced signaling but increased CCK binding affinity, whereas M β CD treatment to reduce membrane cholesterol had no effect on either of these parameters. The W166A mutant exhibited reduced signaling and reduced binding affinity, with reduction in membrane cholesterol further reducing the intracellular calcium responses. The Y237A mutant exhibited no change in signaling, but reduced CCK binding affinity, whereas reducing membrane cholesterol resulted in reductions in both signaling and binding affinities. Corresponding constructs for CCK2R, CCK2R(Y153A), CCK2R(W179A), and CCK2R(Y246A), were also prepared and studied as transiently expressed in COS-1 cells. In contrast to the CCK1R constructs, M β CD treatment had no significant effect on ligand binding affinity for any of the CCK2R constructs (Ki in nM units for untreated and M β CD-treated cells, respectively: WT CCK2R 1.8 ± 0.5, 1.6 ± 0.3; CCK2R(Y153A) 1.1 ± 0.1, 1.1 ± 0.1; CCK2R(W179A) 1.1 ± 0.1, 1.7 ± 1.0; and CCK2R(Y246A) 1.5 ± 0.2, 1.6 ± 0.1).
Fig. 7.
CCK binding and biological activity at cholesterol-association motif site mutants of CCK1R. Shown in the left column are CCK radioligand competition binding curves, and in the right column are CCK-stimulated intracellular calcium response curves for each of the three site mutants of CCK1R. Binding data points reflect percentages of maximal saturable CCK radioligand binding in the absence of competing CCK, and intracellular calcium responses are expressed as percentages of maximal responses for each condition. Data represent means ± SEM of three to seven independent experiments performed in duplicate.
TABLE 3.
Binding and biological activity parameters of CCK1R mutants
| Constructs | Binding Affinities | Binding Sites | Calcium Responses | |||
| Ki | Sites/cell × 105 | EC50 | ||||
| nM | nM | |||||
| Untreated | + MβCD | Untreated | +MβCD | Untreated | +MβCD | |
| CCK1R, WT | 4.3 ± 1.0 | 58.7 ± 10.5b | 3.4 ± 0.5 | 2.1 ± 0.3 | 0.05 ± 0.01 | 0.24 ± 0.03b |
| CCK1R(Y140A) | 0.6 ± 0.1a | 0.8 ± 0.2 | 3.3 ± 0.6 | 2.3 ± 0.9 | 0.40 ± 0.08a | 0.42 ± 0.11 |
| CCK1R(W166A) | 9.6 ± 2.4a | 7.4 ± 1.5 | 2.6 ± 0.4c | 3.0 ± 0.7c | 0.29 ± 0.06a | 0.50 ± 0.09b |
| CCK1R(Y237A) | 37.5 ± 3.8a | 65.9 ± 5.1b | 3.8 ± 0.3 | 3.2 ± 0.2 | 0.05 ± 0.02 | 0.32 ± 0.07b |
Data are expressed as means ± SEM of values from three to seven independent experiments.
P< 0.05 significantly different from wild-type CCK1R construct.
P< 0.05 significantly different from the same construct in the absence of MβCD treatment.
Values are expressed as number of sites/mg protein.
DISCUSSION
Reports over several years have documented functional effects of alteration of membrane cholesterol on particular GPCRs (7, 8, 10, 35–37). With the well-described effects of cholesterol on membrane fluidity and rigidity, and even on the dimensions of the bilayer (1–3), this type of lipid modification could have indirect effects on receptor conformation that could affect its ligand binding characteristics, on lateral mobility that is critical for G protein coupling, and on receptor trafficking and sequestration that contribute to desensitization. A series of studies in which the functions of GPCRs, including receptors for oxytocin, galanin-2, CXCR4, and CCR5, were affected by the modification of cholesterol by treatment with cholesterol oxidase to change its biochemical properties, presumably independent of gross changes in the organization of the lipid bilayer, have been interpreted to suggest the possibility of more direct effects of cholesterol on GPCR function (8, 38, 39). This was further supported by the recent report that cholesterol is present in distinct positions within the crystal structure of the β 2-adrenergic receptor (12). Indeed, several other GPCR crystal structures subsequently solved, including the CXCR4 and A2A adenosine receptors, also demonstrate associated cholesterol molecules (40–42).
Included among the GPCRs in which modification of cholesterol has been shown to have functional effects is the CCK1R (7). Both reducing and increasing membrane cholesterol content with chemical and metabolic manipulations resulted in changes in CCK binding affinity and in CCK-stimulated signaling at the CCK1R (7). Indeed, analogous effects of cholesterol on this receptor have been described in natural CCK1R-bearing cells (43, 44). These studies were performed with prairie dog gallbladder smooth-muscle cells from animals fed a high-cholesterol diet resulting in elevated membrane cholesterol (44). Those cells exhibited signaling that was reduced relative to that observed in the same cells from animals fed a normal diet. The effect of abnormal cholesterol in those natural cells, like the model cell system studied in the current work, was reversible upon normalization of the cholesterol content (44).
Another natural CCK1R-expressing cell system, human gallbladder smooth-muscle cells, has also been reported to exhibit the same types of cholesterol sensitivity (45–50). Smooth-muscle cells from gallbladders of patients having cholesterol gallstones have been shown to have elevated membrane cholesterol, whereas those from patients having pigment gallstones have normal levels of cholesterol (46). The cells from patients with cholesterol gallstones, in contrast to those from patients with pigment gallstones, exhibit higher CCK binding affinity and reduced signaling (46, 48–50), results that are comparable to those in the gallbladder cells from prairie dogs fed a high-cholesterol diet and in the CHO-CCK1R cells with elevated cholesterol in the current report. This observation was attributed to nonproductive binding of ligand, with CCK not inducing the active conformation of this receptor that would expose the cytosolic surface that normally couples with its G protein (48, 49). In human gallbladder cells, the abnormalities of CCK binding and signaling were also reversed upon extraction of cholesterol to achieve normal levels (46).
In the present report, we have applied the same experimental manipulations previously applied to the type 1 CCK receptor (7) to the structurally closely related type 2 CCK receptor. In marked contrast to the CCK1R, CCK binding and signaling at the CCK2R was not affected by modification of the membrane cholesterol content. This provided a unique opportunity to explore the structural basis of the difference in cholesterol sensitivity between these two receptors.
In addition to the extensive sequence homology between CCK1R and CCK2R, these receptors contain the consensus GPCR cholesterol-association motif proposed by Hanson et al. (12), as well as the more-general membrane protein cholesterol-association motif earlier recognized by Li and Papadopoulos (11). These sequence patterns are present in analogous locations in both receptors. The fact that two receptors that are so similar in structure and function, and that are expressed in identical membrane environments, can express differing sensitivities to membrane cholesterol offers an opportunity to look for a possible structural basis for the differential effect of cholesterol. In the current work, we have explored this using chimeric CCK2R/CCK1R receptor constructs as well as site-directed mutants to determine the receptor regions and specific amino acids most critical for the cholesterol sensitivity of the CCK1R.
Of note, the chimeric receptor constructs including the portion of the CCK2R encoded by the first two exons and the portion of the CCK1R encoded by exons three through five continued to exhibit the cholesterol sensitivity typical of the CCK1R. In contrast, the construct incorporating exons one through three of the CCK2R and exons four and five of the CCK1R did not exhibit cholesterol sensitivity. The observation that substitution of exon 3 from CCK2R into CCK1R abrogates the cholesterol sensitivity of that receptor suggests a structural basis for this lipid effect, with a key determinant or determinants within this exon. Of note, this exon contains two sequence motifs previously described as important for binding cholesterol.
It is noteworthy that elimination of one of these CCK1R exon three motifs by site-directed mutagenesis [Y140A disrupting the CRAC motif in TM3 described by Li and Papadopoulos (11)] resulted in a receptor that no longer exhibited cholesterol sensitivity. However, this mutant receptor exhibited reduced CCK-stimulated intracellular calcium responses and increased CCK binding affinity relative to the wild-type receptor. This raised the concern that the conformation of this receptor might not be normal, particularly because this mutation alters the conserved (D/E)3.49-R3.50-Y3.51 motif, and thus its value for localizing the differential effects of cholesterol in the two structurally related CCK receptors might be less than optimal. However, studies of Y3.51A mutations in other Class A GPCRs have shown no effect of this mutation on ligand binding affinity or receptor trafficking, and no or marginal effect on receptor signaling (51–53). Therefore, the suggestion that the observed effects of Y140A are due to a role of this residue in differential cholesterol effects cannot be ruled out at this time. Also of note, all three of the cholesterol binding motif site mutants exhibited negative functional effects on CCK binding affinity and/or CCK-stimulated signaling. Disruption of the second cholesterol binding motif within exon three [W166A disrupting the CCM motif in TM4 described by Hanson et al. (12)] and the motif within exon four (Y237A disrupting the CRAC motif within TM5) both continued to be sensitive to the cholesterol composition of the membrane.
These data may suggest that the TM3 CRAC cholesterol-binding motif could be responsible for the cholesterol sensitivity of the CCK1R. However, this motif is essentially the same in both the CCK1R and the CCK2R, differing only in the presence of a lysine in CCK1R and an arginine in the CCK2R, both residues having similar properties and both being recognized as part of the consensus pattern of this motif. Even the sequences surrounding this motif are quite similar for these two receptors, although minor differences do exist and could contribute to the observed differential cholesterol sensitivity observed. Indeed, such an effect of surrounding residues has been previously recognized (11, 12).
The importance of a CRAC motif in cholesterol interaction with a GPCR has recently been reported for the human type 1 cannabinoid receptor (CB1R). Like the CCK1R, manipulations of membrane cholesterol content affect ligand binding and signaling of CB1R, as well as its localization to membrane rafts (54, 55) and reviewed in (56). Comparable to the observations of CCK1R and CCK2R, the closely related CB2R does not share this sensitivity to membrane cholesterol. It was recently reported that CB1R possesses a CRAC motif in TM7 that differs from CB2R sequence by one residue. When this residue, lysine 402, is mutated to the glycine of the CB2R sequence, the resulting receptor behaves like CB2R and is insensitive to changes in membrane cholesterol (56). The situation is more complicated with the CCK receptors, inasmuch as the TM3 CRAC motif is present in both receptors and nearly identical in sequence. However, the example of CB1R does illustrate that cholesterol binding motifs outside of the CCM can have a significant influence on receptor signaling and trafficking.
In addition to the possibility that differences in cholesterol sensitivity between the CCK1R and the CCK2R are the result of differences in the direct interaction of cholesterol with the two receptors, there is also the possibility that the cholesterol may contribute indirectly by having different impacts on processes that are normally distinct between the two receptors. Mechanisms of binding their natural ligands and mechanisms of receptor trafficking are two examples of such processes that could be affected by changes in membrane lipids.
It is well established that the CCK1R and the CCK2R bind the same CCK peptide ligand differently (21, 57, 58). The peptide ligand binds to extracellular loops and the N-terminal tail of the CCK1R, whereas in the CCK2R, the carboxyl-terminal end of the peptide ligand may dip into the helical bundle (57, 59). It is intriguing to suggest the possibility that a peptide within the helical bundle could stabilize that bundle and, thereby, overcome the negative impact of abnormal membrane cholesterol content on the function of the CCK2R. In contrast, the physical separation of the extracellular receptor regions key for the binding of CCK to the CCK1R from the cytosolic receptor regions that are involved in G protein coupling could explain the dissociation of the effects of membrane cholesterol modification on CCK binding and on CCK-stimulated biological activity, as has been observed (7).
Although we normally think of the internalization of the CCK1R and the CCK2R as being similar, with both predominantly following agonist-stimulated entry into the clathrin-mediated endocytic pathway (60, 61), there is certainly the possibility that these two receptors have differences in their trafficking. More than 80% of the type 1 CCK receptors present on the surface of CHO-CCK1R cells promptly internalize via this pathway after CCK stimulation, whereas the remainder have been shown to internalize via potocytosis into caveolae (61). However, the CCK1R has been described as occupying a unique plasma membrane compartment after CCK stimulation of rat pancreatic acinar cells (60). This was described as a site of “insulation” that is relatively devoid of G proteins, in which the lateral mobility of the CCK receptor was markedly reduced as another highly specialized cellular mechanism for desensitization (62). Nothing is known about the possibility that the CCK2R might utilize a similar mechanism. Similarly, direct evaluation of CCK receptor entry into cellular lipid rafts in natural receptor-bearing cells has not been reported.
In the current work, we have established that the closely related types 1 and 2 CCK receptors are differentially affected by membrane cholesterol, with the CCK1R negatively impacted by abnormal membrane cholesterol content, whereas the CCK2R is resistant to a broad range of membrane cholesterol composition. A series of chimeric CCK2R/CCK1R constructs localized the effect to exon three of the CCK1R that includes two recognized cholesterol binding motifs, and disruption of the motif within TM3 by site-directed mutagenesis resulted in a CCK1R that had lost its cholesterol sensitivity. In addition to these possible direct effects of cholesterol interaction, other possible contributions to the differential effects of cholesterol on these receptors might reflect differences in modes of ligand binding and trafficking.
Acknowledgments
The authors thank A. M. Ball, D. I. Pinon, and M. L. Augustine for their excellent technical assistance, Dr. Q. Chen for performing some of the early experiments leading to this project, and Dr. M. Dong for help in preparing the Alexa-PFO domain 4 probe and for his helpful discussions.
Footnotes
Abbreviations:
- 7-AAD
- 7-aminoactinomycin D
- CB1R
- type 1 cannabinoid receptor
- CCK
- cholecystokinin
- CCK1R
- type 1 cholecystokinin receptor
- CCK2R
- type 2 cholecystokinin receptor
- CCM
- cholesterol consensus motif
- CHO
- Chinese hamster ovary
- CRAC
- cholesterol recognition/interaction amino acid consensus pattern
- Fura-2AM
- Fura-2-acetoxymethyl ester
- GPCR
- G protein-coupled receptor
- IPTG
- isopropyl-1-thio-β-d-galactopyranoside
- KRH
- Krebs-Ringer/HEPES
- LPDS
- lipoprotein-deficient serum
- MβCD
- methyl-β-cyclodextrin
- MβCD-cholesterol
- methyl-β-cyclodextrin-cholesterol complex
- PFO
- Perfringolysin θ
- TM
- transmembrane
This work was supported by Grant DK-32878 from the National Institutes of Health and by the Mayo Clinic-Kinney Career Development Award. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Brown D. A., London E. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275: 17221–17224. [DOI] [PubMed] [Google Scholar]
- 2.Edidin M. 2001. Shrinking patches and slippery rafts: scales of domains in the plasma membrane. Trends Cell Biol. 11: 492–496. [DOI] [PubMed] [Google Scholar]
- 3.Pike L. J. 2003. Lipid rafts: bringing order to chaos. J. Lipid Res. 44: 655–667. [DOI] [PubMed] [Google Scholar]
- 4.Barrantes F. J. 2010. Cholesterol effects on nicotinic acetylcholine receptor: cellular aspects. Subcell. Biochem. 51: 467–487. [DOI] [PubMed] [Google Scholar]
- 5.Gimpl G., Fahrenholz F. 2000. Human oxytocin receptors in cholesterol-rich vs. cholesterol-poor microdomains of the plasma membrane. Eur. J. Biochem. 267: 2483–2497. [DOI] [PubMed] [Google Scholar]
- 6.Paila Y. D., Chattopadhyay A. 2009. The function of G-protein coupled receptors and membrane cholesterol: specific or general interaction? Glycoconj. J. 26: 711–720. [DOI] [PubMed] [Google Scholar]
- 7.Harikumar K. G., Puri V., Singh R. D., Hanada K., Pagano R. E., Miller L. J. 2005. Differential effects of modification of membrane cholesterol and sphingolipids on the conformation, function, and trafficking of the G protein-coupled cholecystokinin receptor. J. Biol. Chem. 280: 2176–2185. [DOI] [PubMed] [Google Scholar]
- 8.Gimpl G., Burger K., Fahrenholz F. 1997. Cholesterol as modulator of receptor function. Biochemistry. 36: 10959–10974. [DOI] [PubMed] [Google Scholar]
- 9.Pike L. J., Casey L. 2002. Cholesterol levels modulate EGF receptor-mediated signaling by altering receptor function and trafficking. Biochemistry. 41: 10315–10322. [DOI] [PubMed] [Google Scholar]
- 10.Pucadyil T. J., Chattopadhyay A. 2004. Cholesterol modulates ligand binding and G-protein coupling to serotonin(1A) receptors from bovine hippocampus. Biochim. Biophys. Acta. 1663: 188–200. [DOI] [PubMed] [Google Scholar]
- 11.Li H., Papadopoulos V. 1998. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology. 139: 4991–4997. [DOI] [PubMed] [Google Scholar]
- 12.Hanson M. A., Cherezov V., Griffith M. T., Roth C. B., Jaakola V. P., Chien E. Y., Velasquez J., Kuhn P., Stevens R. C. 2008. A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure. 16: 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adamian L., Naveed H., Liang J. 2011. Lipid-binding surfaces of membrane proteins: evidence from evolutionary and structural analysis. Biochim. Biophys. Acta. 1808: 1092–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ondetti M. A., Rubin B., Engel S. L., Pluscec J., Sheehan J. T. 1970. Cholecystokinin-pancreozymin: recent developments. Am. J. Dig. Dis. 15: 149–156. [DOI] [PubMed] [Google Scholar]
- 15.Dufresne M., Seva C., Fourmy D. 2006. Cholecystokinin and gastrin receptors. Physiol. Rev. 86: 805–847. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Z. J., Harikumar K. G., Holicky E. L., Miller L. J. 2003. Heterodimerization of type A and B cholecystokinin receptors enhance signaling and promote cell growth. J. Biol. Chem. 278: 52972–52979. [DOI] [PubMed] [Google Scholar]
- 17.Wu V., Yang M., McRoberts J. A., Ren J., Seensalu R., Zeng N., Dagrag M., Birnbaumer M., Walsh J. H. 1997. First intracellular loop of the human cholecystokinin-A receptor is essential for cyclic AMP signaling in transfected HEK-293 cells. J. Biol. Chem. 272: 9037–9042. [DOI] [PubMed] [Google Scholar]
- 18.Puri V., Watanabe R., Dominguez M., Sun X., Wheatley C. L., Marks D. L., Pagano R. E. 1999. Cholesterol modulates membrane traffic along the endocytic pathway in sphingolipid-storage diseases. Nat. Cell Biol. 1: 386–388. [DOI] [PubMed] [Google Scholar]
- 19.Amundson D. M., Zhou M. 1999. Fluorometric method for the enzymatic determination of cholesterol. J. Biochem. Biophys. Methods. 38: 43–52. [DOI] [PubMed] [Google Scholar]
- 20.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 21.Harikumar K. G., Clain J., Pinon D. I., Dong M., Miller L. J. 2005. Distinct molecular mechanisms for agonist peptide binding to types A and B cholecystokinin receptors demonstrated using fluorescence spectroscopy. J. Biol. Chem. 280: 1044–1050. [DOI] [PubMed] [Google Scholar]
- 22.Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150: 76–85. [DOI] [PubMed] [Google Scholar]
- 23.Flanagan J. J., Tweten R. K., Johnson A. E., Heuck A. P. 2009. Cholesterol exposure at the membrane surface is necessary and sufficient to trigger perfringolysin O binding. Biochemistry. 48: 3977–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heuck A. P., Hotze E. M., Tweten R. K., Johnson A. E. 2000. Mechanism of membrane insertion of a multimeric beta-barrel protein: perfringolysin O creates a pore using ordered and coupled conformational changes. Mol. Cell. 6: 1233–1242. [DOI] [PubMed] [Google Scholar]
- 25.Rossjohn J., Feil S. C., McKinstry W. J., Tweten R. K., Parker M. W. 1997. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell. 89: 685–692. [DOI] [PubMed] [Google Scholar]
- 26.Shimada Y., Maruya M., Iwashita S., Ohno-Iwashita Y. 2002. The C-terminal domain of perfringolysin O is an essential cholesterol-binding unit targeting to cholesterol-rich microdomains. Eur. J. Biochem. 269: 6195–6203. [DOI] [PubMed] [Google Scholar]
- 27.Iwamoto M., Morita I., Fukuda M., Murota S., Ando S., Ohno-Iwashita Y. 1997. A biotinylated perfringolysin O derivative: a new probe for detection of cell surface cholesterol. Biochim. Biophys. Acta. 1327: 222–230. [DOI] [PubMed] [Google Scholar]
- 28.Waheed A. A., Shimada Y., Heijnen H. F., Nakamura M., Inomata M., Hayashi M., Iwashita S., Slot J. W., Ohno-Iwashita Y. 2001. Selective binding of perfringolysin O derivative to cholesterol-rich membrane microdomains (rafts). Proc. Natl. Acad. Sci. USA. 98: 4926–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munson P. J., Rodbard D. 1980. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 107: 220–239. [DOI] [PubMed] [Google Scholar]
- 30.Ballesteros J. A., Weinstein H. 1992. Analysis and refinement of criteria for predicting the structure and relative orientations of transmembranal helical domains. Biophys. J. 62: 107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenbaum D. M., Cherezov V., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Yao X. J., Weis W. I., Stevens R. C., et al. 2007. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science. 318: 1266–1273. [DOI] [PubMed] [Google Scholar]
- 32.Miller L. J., Holicky E. L., Ulrich C. D., Wieben E. D. 1995. Abnormal processing of the human cholecystokinin receptor gene in association with gallstones and obesity. Gastroenterology. 109: 1375–1380. [DOI] [PubMed] [Google Scholar]
- 33.Miyake A. 1995. A truncated isoform of human CCK-B/gastrin receptor generated by alternative usage of a novel exon. Biochem. Biophys. Res. Commun. 208: 230–237. [DOI] [PubMed] [Google Scholar]
- 34.Song I., Brown D. R., Wiltshire R. N., Gantz I., Trent J. M., Yamada T. 1993. The human gastrin/cholecystokinin type B receptor gene: alternative splice donor site in exon 4 generates two variant mRNAs. Proc. Natl. Acad. Sci. USA. 90: 9085–9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levitt E. S., Clark M. J., Jenkins P. M., Martens J. R., Traynor J. R. 2009. Differential effect of membrane cholesterol removal on mu- and delta-opioid receptors: a parallel comparison of acute and chronic signaling to adenylyl cyclase. J. Biol. Chem. 284: 22108–22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niu S. L., Mitchell D. C., Litman B. J. 2002. Manipulation of cholesterol levels in rod disk membranes by methyl-beta-cyclodextrin: effects on receptor activation. J. Biol. Chem. 277: 20139–20145. [DOI] [PubMed] [Google Scholar]
- 37.Pontier S. M., Percherancier Y., Galandrin S., Breit A., Gales C., Bouvier M. 2008. Cholesterol-dependent separation of the beta2-adrenergic receptor from its partners determines signaling efficacy: insight into nanoscale organization of signal transduction. J. Biol. Chem. 283: 24659–24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen D. H., Taub D. D. 2003. Inhibition of chemokine receptor function by membrane cholesterol oxidation. Exp. Cell Res. 291: 36–45. [DOI] [PubMed] [Google Scholar]
- 39.Pang L., Graziano M., Wang S. 1999. Membrane cholesterol modulates galanin-GalR2 interaction. Biochemistry. 38: 12003–12011. [DOI] [PubMed] [Google Scholar]
- 40.Cherezov V., Rosenbaum D. M., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Kuhn P., Weis W. I., Kobilka B. K., et al. 2007. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 318: 1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu B., Chien E. Y., Mol C. D., Fenalti G., Liu W., Katritch V., Abagyan R., Brooun A., Wells P., Bi F. C., et al. 2010. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 330: 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu F., Wu H., Katritch V., Han G. W., Jacobson K. A., Gao Z. G., Cherezov V., Stevens R. C. 2011. Structure of an agonist-bound human A2A adenosine receptor. Science. 332: 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Q., De Petris G., Yu P., Amaral J., Biancani P., Behar J. 1997. Different pathways mediate cholecystokinin actions in cholelithiasis. Am. J. Physiol. 272: G838–G844. [DOI] [PubMed] [Google Scholar]
- 44.Yu P., Chen Q., Biancani P., Behar J. 1996. Membrane cholesterol alters gallbladder muscle contractility in prairie dogs. Am. J. Physiol. 271: G56–G61. [DOI] [PubMed] [Google Scholar]
- 45.Amaral J., Xiao Z. L., Chen Q., Yu P., Biancani P., Behar J. 2001. Gallbladder muscle dysfunction in patients with chronic acalculous disease. Gastroenterology. 120: 506–511. [DOI] [PubMed] [Google Scholar]
- 46.Chen Q., Amaral J., Biancani P., Behar J. 1999. Excess membrane cholesterol alters human gallbladder muscle contractility and membrane fluidity. Gastroenterology. 116: 678–685. [DOI] [PubMed] [Google Scholar]
- 47.Chen Q., Amaral J., Oh S., Biancani P., Behar J. 1997. Gallbladder relaxation in patients with pigment and cholesterol stones. Gastroenterology. 113: 930–937. [DOI] [PubMed] [Google Scholar]
- 48.Xiao Z. L., Chen Q., Amaral J., Biancani P., Behar J. 2000. Defect of receptor-G protein coupling in human gallbladder with cholesterol stones. Am. J. Physiol. Gastrointest. Liver Physiol. 278: G251–G258. [DOI] [PubMed] [Google Scholar]
- 49.Xiao Z. L., Chen Q., Amaral J., Biancani P., Jensen R. T., Behar J. 1999. CCK receptor dysfunction in muscle membranes from human gallbladders with cholesterol stones. Am. J. Physiol. 276: G1401–G1407. [DOI] [PubMed] [Google Scholar]
- 50.Yu P., Chen Q., Harnett K. M., Amaral J., Biancani P., Behar J. 1995. Direct G protein activation reverses impaired CCK signaling in human gallbladders with cholesterol stones. Am. J. Physiol. 269: G659–G665. [DOI] [PubMed] [Google Scholar]
- 51.Gáborik Z., Jagadeesh G., Zhang M., Spat A., Catt K. J., Hunyady L. 2003. The role of a conserved region of the second intracellular loop in AT1 angiotensin receptor activation and signaling. Endocrinology. 144: 2220–2228. [DOI] [PubMed] [Google Scholar]
- 52.Ohyama K., Yamano Y., Sano T., Nakagomi Y., Wada M., Inagami T. 2002. Role of the conserved DRY motif on G protein activation of rat angiotensin II receptor type 1A. Biochem. Biophys. Res. Commun. 292: 362–367. [DOI] [PubMed] [Google Scholar]
- 53.Proulx C. D., Holleran B. J., Boucard A. A., Escher E., Guillemette G., Leduc R. 2008. Mutational analysis of the conserved Asp2.50 and ERY motif reveals signaling bias of the urotensin II receptor. Mol. Pharmacol. 74: 552–561. [DOI] [PubMed] [Google Scholar]
- 54.Bari M., Battista N., Fezza F., Finazzi-Agro A., Maccarrone M. 2005. Lipid rafts control signaling of type-1 cannabinoid receptors in neuronal cells. Implications for anandamide-induced apoptosis. J. Biol. Chem. 280: 12212–12220. [DOI] [PubMed] [Google Scholar]
- 55.Bari M., Paradisi A., Pasquariello N., Maccarrone M. 2005. Cholesterol-dependent modulation of type 1 cannabinoid receptors in nerve cells. J. Neurosci. Res. 81: 275–283. [DOI] [PubMed] [Google Scholar]
- 56.Oddi S., Dainese E., Fezza F., Lanuti M., Barcaroli D., De Laurenzi V., Centonze D., Maccarrone M. 2011. Functional characterization of putative cholesterol binding sequence (CRAC) in human type-1 cannabinoid receptor. J. Neurochem. 116: 858–865. [DOI] [PubMed] [Google Scholar]
- 57.Dong M., Liu G., Pinon D. I., Miller L. J. 2005. Differential docking of high-affinity peptide ligands to type A and B cholecystokinin receptors demonstrated by photoaffinity labeling. Biochemistry. 44: 6693–6700. [DOI] [PubMed] [Google Scholar]
- 58.Silvente-Poirot S., Wank S. A. 1996. A segment of five amino acids in the second extracellular loop of the cholecystokinin-B receptor is essential for selectivity of the peptide agonist gastrin. J. Biol. Chem. 271: 14698–14706. [DOI] [PubMed] [Google Scholar]
- 59.Harikumar K. G., Pinon D. I., Miller L. J. 2006. Fluorescent indicators distributed throughout the pharmacophore of cholecystokinin provide insights into distinct modes of binding and activation of type A and B cholecystokinin receptors. J. Biol. Chem. 281: 27072–27080. [DOI] [PubMed] [Google Scholar]
- 60.Roettger B. F., Rentsch R. U., Hadac E. M., Hellen E. H., Burghardt T. P., Miller L. J. 1995. Insulation of a G protein-coupled receptor on the plasmalemmal surface of the pancreatic acinar cell. J. Cell Biol. 130: 579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roettger B. F., Rentsch R. U., Pinon D., Holicky E., Hadac E., Larkin J. M., Miller L. J. 1995. Dual pathways of internalization of the cholecystokinin receptor. J. Cell Biol. 128: 1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roettger B. F., Pinon D. I., Burghardt T. P., Miller L. J. 1999. Regulation of lateral mobility and cellular trafficking of the CCK receptor by a partial agonist. Am. J. Physiol. 276: C539–C547. [DOI] [PubMed] [Google Scholar]