Abstract
We investigated the influence of the HIV infection on serum paraoxonase-3 (PON3) concentration and assessed the relationships with lipoprotein-associated abnormalities, immunological response, and accelerated atherosclerosis. We studied 207 HIV-infected patients and 385 healthy volunteers. Serum PON3 was determined by in-house ELISA, and PON3 distribution in lipoproteins was investigated by fast-performance liquid chromatography (FPLC). Polymorphisms of the PON3 promoter were analyzed by the Iplex Gold MassArrayTM method. PON3 concentrations were increased (about three times) in HIV-infected patients with respect to controls (P < 0.001) and were inversely correlated with oxidized LDL levels (P = 0.038). Long-term use of nonnucleoside reverse transcriptase inhibitor (NNRTI)-based antiretroviral therapy was associated with a decrease of PON3 concentrations. In a multivariate linear regression analysis, these relationships were still strong when the main confounding covariates were considered. PON3 was mainly found in HDL in HIV-infected patients, but a substantial amount of the protein was detected in LDL particles. This study reports for the first time an important increase in serum PON3 concentrations in HIV-infected patients that is associated with their oxidative status and their treatment with NNRTI. Long-term, prospective studies are needed to confirm the possible influence of this enzyme on the course of this disease and its possible utility as an analytical biomarker.
Keywords: antioxidants, atherosclerosis, high-density lipoproteins, HIV-infection, oxidative stress, paraoxonases
Human immunodeficiency virus (HIV)-infected patients often develop long-term metabolic alterations and concomitant atherosclerosis (1, 2). This association has acquired clinical relevance since the introduction of effective therapeutic measures that have modified HIV infection to a chronic disease and as the consequences of metabolic derangements become more evident over time. In the course of HIV infection, there are several key changes in lipoprotein metabolism, including increased lipid peroxidation, hypertriglyceridemia, and low high-density lipoprotein (HDL) concentration (3). Among them, changes in HDL are particularly relevant, as HIV-infected patients with higher HDL-cholesterol concentrations appear to have a better HIV disease course than those with lower HDL concentrations (4). In addition, we have previously documented that serum paraoxonase-1 (PON1) activity and concentration are influenced by HIV infection (5) and that PON1 gene polymorphisms are related to the presence of subclinical atherosclerosis and CD4+ T-cell recovery following treatment (6).
The PON enzyme family comprises three members, PON1, PON2, and PON3, whose genes are located adjacent to each other on chromosome 7q21-22 (7). In mammals, the PON1 and PON3 genes are expressed in many cell types (8), and their protein products are found in the bloodstream bound to HDL (9). Conversely, PON2 is an intracellular enzyme that is not found in the bloodstream (10). All these enzymes are able to delay low-density lipoprotein (LDL) oxidation and cellular oxidative stress (11). In addition, data obtained from a variety of mouse models of atherosclerosis have consistently shown that human PON1, 2, or 3 expression inhibits or reverses the development of atherosclerosis via mechanisms involving the reduction of oxidative stress, the promotion of cholesterol efflux from macrophages, and the normalization of vascular endothelium function (12–15). Moreover, recent studies showed that PON2 expression is increased in cultured hematopoietic cells and mouse thymocytes after HIV-1 infection (16). The PON family also plays a role in innate immunity and can prevent bacterial infection (17).
Although knowledge on PON1 and PON2 structure and function is rapidly expanding, data about the PON3 protein remain elusive. Its gene was identified in 1996 when Primo-Parmo et al. (7) detected a large number of cDNA sequences in the Genome Data Base with significant similarity to, but not identical with, human PON1. The percentage identity among human PON1, PON2, and PON3 genes is high (about 70%), and the genes are believed to derive from a common precursor (11). Clinical research on PON3 has been hampered by the lack of methods for measurement, but we recently described a high-throughput, reliable enzyme-linked immunosorbent assay (ELISA) to analyze PON3 concentration in human serum (18). The main objective of the present study was to investigate whether serum PON3 concentration may provide new information to improve our understanding of metabolic complications associated with HIV infection.
MATERIALS AND METHODS
Study participants
From among the HIV-infected patients attending our clinic, 207 (139 men, 68 women; mean age, 38 years; range, 22-66 years) accepted an invitation to participate in the present study. Of these patients, 122 were coinfected by the hepatitis C virus (HCV). All patients were undergoing antiretroviral therapy with protease inhibitor (PI)-based or nonnucleoside reverse transcriptase inhibitor (NNRTI)-based schemes. The antiretroviral adjuvant drugs were zidovudine, stavudine, didanosine, or lamivudine. The exclusion criteria were age under 18 years, or renal function impairment defined as creatinine levels higher than 106 μmol/l, or having an AIDS-related opportunistic disease at the time of the study. Twenty-five patients had subcutaneous lipoatrophy, defined as the presence of hollow cheeks, prominent superficial veins in the limbs, or flattening of the buttocks (19). Carotid and femoral ultrasound measurements were performed in 178 patients and the intima-media thickness (IMT) was measured as an estimate of the presence of subclinical atherosclerosis, as previously described (20). Patients were considered to have subclinical atherosclerosis when IMT was ≥ 0.8 mm or when an atheromatous plaque was seen in the analyzed areas of the arteries. The main clinical characteristics of these patients are summarized in supplementary Table I. The control group consisted of 385 healthy volunteers (153 men, 232 women; mean age, 47 years; range, 19-75 years) who participated in an ongoing epidemiological study conducted in our geographic area, the details of which have been previously reported (21). All the volunteers had been invited to attend a clinical examination and to provide a fasting blood sample. There was no clinical or analytical evidence of renal insufficiency, liver damage, neoplasia, or neurological disorders.
A fasting venous blood sample was obtained from all the participants. CD4+ T cells and CD8+ T cells were analyzed immediately, and serum, plasma, and leukocytes were stored at −80°C in our biological sample bank until the other measurements were performed. We employed independent aliquots that were never thawed before this investigation, although participants in this study partially coincided with those reported in previous investigations (5, 6). All the participants provided fully informed consent to participation in the study on the understanding that anonymity of all data is guaranteed. The study was approved by the Institutional Review Board of the Hospital Universitari de Sant Joan de Reus.
Biochemical and serological measurements
Serum PON3 concentrations were determined by in-house ELISA using rabbit polyclonal antibodies generated against a synthetic peptide with a sequence specific to mature PON3. Details of this method have been previously reported (18, 22). Plasma viral load was measured with the COBAS® TaqMan® HIV-1 assay (Roche, Basel, Switzerland), and CD4+ T-cell and CD8+ T-cell counts were measured by flow cytometry (Coulter Epics XL-MLC, Beckman Coulter, Fullerton, CA). Antibodies against HCV, serum β-2-microglobulin [a marker of lymphocyte destruction and progression of HIV-infection (23)], and serum cholesterol, triglycerides, HDL-cholesterol, and apolipoprotein (apo)A-I were measured in an automated analyzer (UniCelTM DxI 800, Beckman Coulter, Fullerton, CA). Oxidized LDL levels were measured by ELISA (Mercodia, Uppsala, Sweden).
FPLC lipoprotein fractionation
PON3 distribution in lipoproteins was assessed by FPLC (Bio-Rad BioLogic DuoFlow 10 system, Bio-Rad Laboratories, Hercules, CA). Sera from three HIV-infected patients and three noninfected participants were pooled separately. To maximize the possible differences between groups, sera from the HIV-infected patients were chosen to have a PON3 concentration > 20 mg/l. Two-hundred microliters from each pool were injected into a Superose 6/300 GL column (GE Healthcare Europe, Glattbrugg, Switzerland), and 500 μl fractions were collected. Cholesterol, triglycerides, and PON3 in each fraction were measured as described.
PON3 promoter genotyping
Genomic DNA was obtained from leukocytes (Puregene DNA Isolation reagent set, Gentra Systems, Minneapolis, MN). Selected single nucleotide polymorphisms (SNP) of the PON3 promoter were analyzed by the Iplex Gold MassArrayTM method (Sequenom, San Diego, CA) at the Spanish National Genotyping Center (Centro Nacional de Genotipado, Universitat Pompeu Fabra, Barcelona, Spain).
Statistical analysis
The normality of distributions was determined with the Kolmogorov-Smirnov test. Differences between two groups were assessed with the Student t-test (parametric) or the Mann-Whitney U test (nonparametric). Differences between multiple groups were analyzed by the Kruskal-Wallis test. Pearson or Spearman correlation coefficients were used to evaluate the degree of association between variables. Each SNP was tested for Hardy-Weinberg equilibrium using Haploview 4.0 software (24). Estimates of linkage disequilibrium between SNPs were calculated using Fisher's test. Diagnostic accuracy for the measurement of serum PON3 concentration was calculated with ROC analysis (25). A multiple linear regression model was fitted to evaluate the factors that were independently associated with PON3 concentrations in HIV-infected patients. Results are shown as means and SD (parametric) or as medians and 95% confidence interval (CI; nonparametric). The SPSS 18.0 package was used for all statistical calculations.
RESULTS
Relationships among serum PON3 concentrations and lipoprotein abnormalities
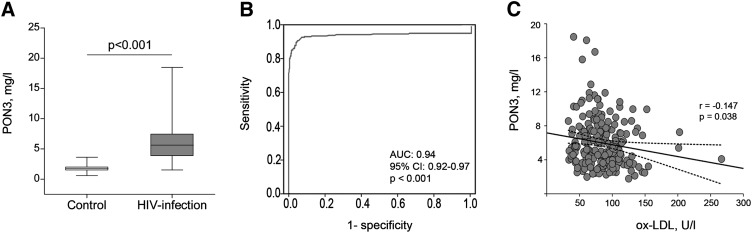
Serum PON3 concentrations were significantly increased in HIV-infected patients with respect to the control group [5.5 (1.2-10.8) versus 1.8 (1.0-2.5) mg/l, respectively; P < 0.001; Fig. 1A]. The results of the ROC analysis for serum PON3 concentration measurement are shown in Fig. 1B. The area under the curve (AUC) was 0.94 (95% CI: 0.92-0.97; P < 0.001), which highlights the remarkable differences in serum PON3 concentrations between patients and controls. We observed a significant inverse relationship (r = −0.147; P = 0.038) between serum PON3 concentration and oxidized LDL levels (Fig. 1C) in HIV-infected patients but not in the control group (r = 0.024; P = 0.786). HIV-infected patients were characterized by raised serum triglyceride concentration and decreased cholesterol values in HDL and LDL (Table 1); there were no significant associations among serum PON3 concentrations, cholesterol, and triglycerides (supplementary Table II).
Fig. 1.
(A) Serum PON3 concentrations in control subjects and HIV-infected patients. (B) ROC plot for serum PON3 concentration measurement in HIV-infected and noninfected subjects. AUC, Area-under-the curve; CI, Confidence interval. (C) Relationship between serum PON3 concentrations and oxidized LDL levels (ox-LDL) in HIV-infected patients.
TABLE 1.
Selected biochemical variables in the control group and in HIV-infected patients
| Parameter | Control group(n = 385) | HIV-infected patients(n = 207) | P |
| Cholesterol (mmol/l) | 5.28 (0.98) | 4.89 (1.23) | <0.001 |
| Triglycerides (mmol/l) | 1.1 (0.5-2.6) | 1.5 (0.6-8.5) | <0.001 |
| HDL-cholesterol (mmol/l) | 1.48 (0.39) | 1.18 (0.45) | <0.001 |
| LDL-cholesterol (mmol/l) | 3.20 (0.95) | 2.75 (0.96) | <0.001 |
| Apolipoprotein A-I (g/l) | 1.69 (0.28) | 1.38 (0.31) | <0.001 |
| Oxidized LDL (U/l) | 84.5 (81.8-88.7) | 81.5 (40.5-145.9) | 0.951 |
Results are presented as means and SD in parentheses (parametric) or as medians and 95% CI in parenthesis (nonparametric).
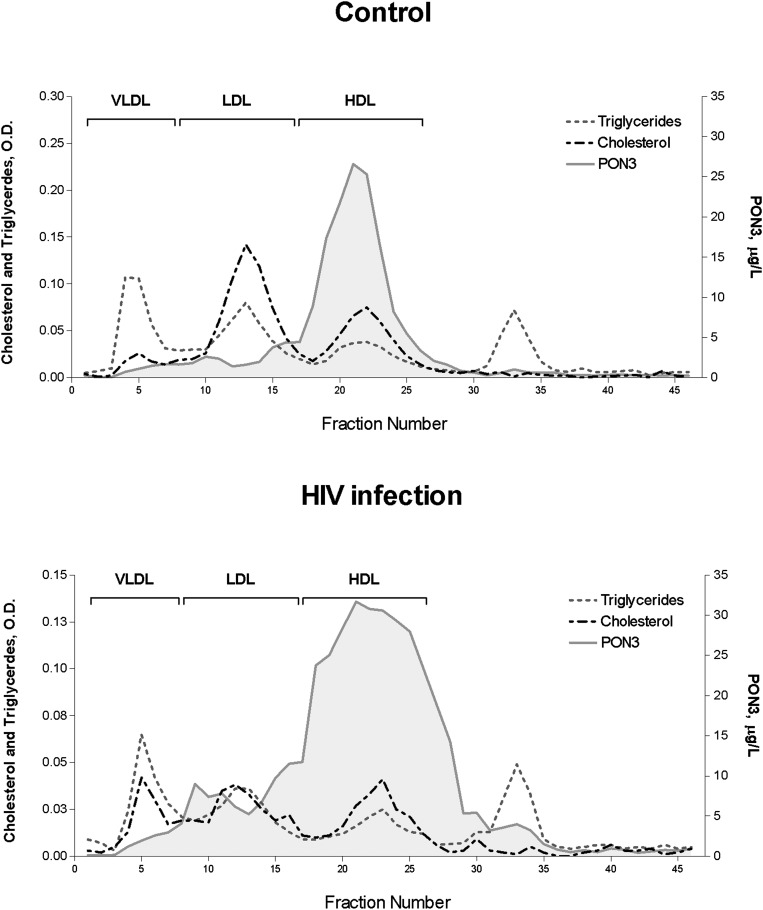
FPLC lipoprotein fractionation
In noninfected participants, PON3 immunoreactivity was observed almost exclusively in HDL fractions. However, in the HIV-infected pool, a substantial amount of this protein eluted with the smallest HDL and with LDL particles (Fig. 2).
Fig. 2.
FPLC lipoprotein fractionation and PON3 lipoprotein distribution in the control and the HIV-infected pools.
Influence of genotype on serum PON3 concentrations
The frequency distributions of the selected PON3 promoter gene polymorphisms are shown in Table 2. There were no significant differences between control subjects and HIV-infected patients. These polymorphisms moderately influenced serum PON3 concentrations in the control subjects but not in the patient group.
TABLE 2.
Distribution of PON3 genotypes in the control group and HIV-infected patients
| Genotype frequency (%) |
PON3 (mg/l) |
||||
| Polymorphism | Control | HIV | Control | HIV | |
| PON3-567a | CC | 59.8 | 61.7 | 1.83 (0.45) | 5.97 (0.21) |
| CT | 36.4 | 32.1 | 1.68 (0.41) | 6.19 (0.31) | |
| TT | 3.8 | 6.3 | 1.56 (0.28) | 5.51 (0.55) | |
| PON3-665a | AA | 59.6 | 61.7 | 1.83 (0.45) | 5.97 (0.21) |
| AG | 36.6 | 32.1 | 1.68 (0.40) | 6.19 (0.31) | |
| GG | 3.8 | 6.3 | 1.56 (0.28) | 5.51 (0.55) | |
| PON3-746a | CC | 59.7 | 61.5 | 1.83 (0.44) | 5.98 (0.21) |
| CT | 36.5 | 31.8 | 1.67 (0.41) | 6.21 (0.32) | |
| TT | 3.8 | 6.6 | 1.56 (0.27) | 5.45 (0.52) | |
| PON3-4105 | GG | 61.7 | 64.8 | 1.82 (0.45) | 5.95 (0.20) |
| GA | 35.3 | 30.0 | 1.68 (0.40) | 6.18 (0.32) | |
| AA | 3.0 | 5.2 | 1.56 (0.32) | 5.89 (0.66) | |
| PON3-4970 | TT | 62.7 | 64.5 | 1.82 (0.45) | 5.99 (0.20) |
| TG | 34.8 | 30.7 | 1.69 (0.39) | 6.14 (0.32) | |
| GG | 2.5 | 4.9 | 1.51 (0.31) | 5.54 (0.60) | |
| PON3-4984 | AA | 62.4 | 64.6 | 1.81 (0.46) | 5.97 (0.20) |
| AG | 34.8 | 30.5 | 1.69 (0.39) | 6.08 (0.32) | |
| GG | 2.8 | 4.9 | 1.51 (0.31) | 5.54 (0.60) | |
Polymorphisms associated with significant changes (P < 0.05) in the control group but not in HIV-infected patients.
All PON3 promoter polymorphisms were strongly linked in a single haplotype, and we did not observe any significant differences between patients and controls (supplementary Fig. I).
Relationships among serum PON3 concentrations and the immunological and virological outcomes
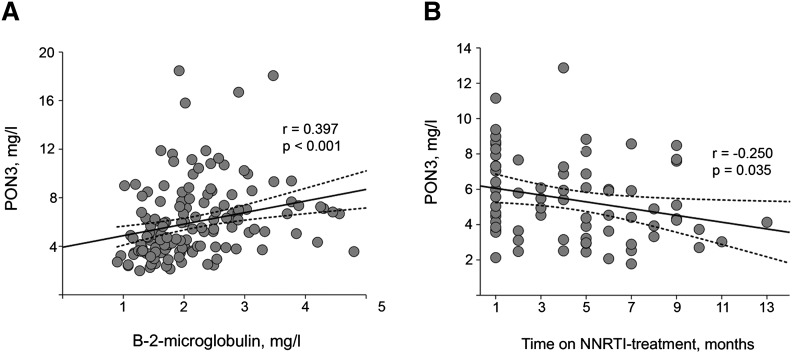
Coinfection with HCV was associated with a significantly higher PON3 concentration [5.8 (2.6-11.1) versus 4.5 (2.4-11.6) mg/l, respectively; P = 0.024]. There were not any significant associations between serum PON3 concentrations and CD4+ T-cell and CD8+ T-cell counts, the CD4+/CD8+ ratio, or the plasma HIV-1 viral load (supplementary Table III). There was a significant direct linear relationship (r = 0.397; P < 0.001) between serum PON3 and β-2-microglobulin concentrations (Fig. 3A). There were no significant differences in serum PON3 concentrations between HIV-infected patients with or without lipoatrophy [5.5 (2.0-12.8) versus 5.1 (2.4-10.3) mg/l, respectively; P = 0.644].
Fig. 3.
Relationships among serum PON3 concentrations, β-2-microglobulin concentrations, and the duration of nonnucleoside reverse transcriptase inhibitors (NNRTI) in HIV-infected patients.
Influence of treatments on serum PON3 concentrations
We observed a significant inverse relationship between serum PON3 concentrations and the duration of the antiretroviral therapy in patients under the NNRTI-based scheme (r = −0.250; P = 0.035; Fig. 3B) but not in patients receiving a PI-based scheme (r = −0.059; P = 0.408).
PON3 and subclinical atherosclerosis
When patients were classified according to the presence (n = 137) or absence (n = 41) of subclinical atherosclerosis, no significant differences in serum PON3 concentrations were found [5.2 (2.5-11.2) versus 5.4 (2.5-11.1) mg/l, respectively; P = 0.959]. Furthermore, there was no significant association between serum PON3 concentration and the quantitative value of the IMT (r = 0.047; P = 0.544).
Multivariate analysis for PON3 concentration in HIV-infected patients
A multiple linear regression analysis showed significant and independent relationships of serum PON3 concentrations with oxidized LDL and with the use of NNRTI as the backbone of the antiretroviral therapy (Table 3). These results were confirmed when the use of antiretroviral treatment was substituted by its duration [NNRTI: B = −0.186 (0.080), β = −0.249, t = −2.083, P = 0.040; PI: B = −0.023 (0.050), β = −0.055, t = −0.419, P = 0.677].
TABLE 3.
Multiple regression analysis of the determinants of serum PON3 concentration in HIV-infected patients
| Unstandardized coefficients |
Standardized coefficients |
||||
| B | Standard error | Beta | t | P | |
| Age (years) | −0.0.36 | 0.047 | −0.079 | −0.753 | 0.454 |
| Gender (male) | 0.528 | 0.772 | 0.076 | 0.684 | 0.496 |
| BMI (kg/m ) | 0.098 | 0.105 | 0.102 | 0.932 | 0.354 |
| Use of NNRTI | −2.276 | 0.875 | −0.329 | −2.600 | 0.011 |
| Use of PI | −0.815 | 0.447 | −0.230 | −1.821 | 0.072 |
| Lipoatrophy | 1.035 | 0.870 | 0.144 | 1.190 | 0.238 |
| Hepatitis C virus coinfection | 0.486 | 0.798 | 0.072 | 0.609 | 0.544 |
| HIV-1 viral load (<200 copies/ml) | 0.932 | 0.821 | 0.136 | 1.136 | 0.260 |
| CD4+ T-cell count (cells/mm ) | −0.001 | 0.001 | −0.130 | −1.170 | 0.246 |
| Total cholesterol (mmol/l) | 0.457 | 0.322 | 0.179 | 1.418 | 0.160 |
| HDL-cholesterol (mmol/l) | −0.529 | 0.831 | −0.074 | −0.636 | 0.526 |
| Oxidized LDL (mmol/l) | −0.037 | 0.013 | −0.340 | −2.833 | 0.006 |
| β-2-microglobulin (mg/l) | 0.176 | 0.292 | 0.176 | 0.550 | 0.550 |
| Dependent variable: PON3 (mg/l) | |||||
BMI, body mass index; NNRTI, nonnucleoside analogs reverse transcriptase inhibitor; PI, protease inhibitor.
DISCUSSION
Viral replication and some clinical manifestations of HIV infection involve a misbalance in reduction-oxidation (redox) status and free radical production (26). Moreover, oxidative stress may be induced by antiretroviral treatments (27). PON3 is an enzyme with lactonase activity (28), the physiological function of which is not completely understood, but evidence suggests that it has an antioxidant role by hydrolyzing oxidized lipid peroxides, similar to PON1 and PON2. Purified human and rabbit PON3 and recombinant PON3 have been shown to decrease macrophage oxidative stress and inhibit the in vitro oxidation of LDL (29–32). The present study revealed a remarkable increase (about three times) in serum PON3 concentration in HIV-infected patients that may be clinically relevant. ROC analysis showed an AUC very close to 1.0, demonstrating a high sensitivity and specificity of serum PON3 measurement in distinguishing between HIV-infected and noninfected subjects. Interestingly, oxidized LDL levels were not significantly increased, but a significant inverse relationship was observed between their serum levels and those of PON3. These data support the concept that PON3 plays a protective role against oxidative stress and increased lipid peroxidation in HIV infection. Whether this increase in circulating PON3 is related to a higher cellular expression is, at present, unknown. However, a recent study reported a similar increase (five times) in Pon3 mRNA expression in late gestation, a physiological state with high oxidative stress (33). Contrary to what we previously observed for PON1 in HIV-1 infection (5), serum PON3 concentrations were not significantly related with markers of lymphocyte recovery. We did not observe any association with CD4+ T-cell counts, and the correlation observed with β-2 microglobulin in the bivariate analysis disappeared in the multiple regression analysis. An interesting observation was the association of HCV coinfection with higher PON3 concentrations. These results confirm a recent report from our group describing increased serum PON3 concentrations in patients with chronic liver disease, mainly secondary to HCV infection (34).
In the present study, treatment of HIV-infected patients with NNRTI, but not with PI, was associated with a significant decrease of serum PON3 concentrations. The use of some nucleoside reverse transcriptase inhibitors (NRTI) and PIs is associated with an atherogenic lipoprotein profile (35), but NNRTIs, such as efavirenz, promote antiatherogenic changes in HDL particles and function, including normalization of size and lipid composition, enhancement of reverse cholesterol transport, and improvement of antioxidant capacity (36). A previous study from our group showed that NNRTI treatment was associated with a relative normalization of serum apoA-I and apoA-II, as well as HDL-cholesterol concentrations, in HIV-infected patients (37), which is consistent with the reported beneficial effects of this compound on HDL composition and function. However, an alternative explanation is that the influence of NNRTI fades with time. Similar effects have been described for some antiretroviral drugs. Shlay et al. (38) reported that NRTI are associated with positive changes in subcutaneous tissue distribution during the early periods and negative changes in the late periods of treatment.
We did not find any significant differences in genotype or haplotype of PON3 promoter gene polymorphisms between patients and controls, suggesting that genotype does not influence the course of the disease. In our previous report, we observed a moderate influence of some polymorphisms on serum PON3 concentration in the general population (18), but this is not the case in HIV-infected patients. Possibly, the upregulation of PON3 expression secondary to the infection masks the small effect of these polymorphisms. Unlike PON1 (6), PON3 seems not to be associated with the presence of subclinical atherosclerosis in HIV-infected patients. Although lipid peroxidation and atherosclerosis are known to be strongly linked phenomena (39), the pathophysiology of atherosclerosis is complex, and our results suggest that the protective effects of these enzymes differ under certain situations. Perhaps PON1 is more efficient in protecting against the alterations leading to atherosclerosis and PON3 is in some way involved in protection against infection. This is, to the best of our knowledge, the first in vivo evidence suggesting such a hypothesis, and it warrants further investigation. Another interesting point is the wider lipoprotein distribution of PON3 in HIV-infected patients. PON3 is observed in substantial amounts in the smallest HDL and in LDL particles. The accepted concept to-date is that PON1 and PON3 are exclusively transported in the bloodstream by HDL (12) and are only associated with other lipoproteins in exceptional circumstances (40). Perhaps the excess PON3 produced in HIV infection cannot be properly packed in the HDL particles, as the conformation of HDL-associated apoA-I leaves little free surface area for other proteins to bind (41). This may have resulted in some PON3 redistributing to LDL. The physiological implications of this observation require further investigation.
In conclusion, this study reports for the first time an important increase in serum PON3 concentrations in HIV-infected patients that is associated with their oxidative status and that could be partially attenuated by treatment with some antiretroviral agents. Long-term, prospective studies are needed to further confirm the possible influence of this enzyme on the course of this disease and its possible utility as an analytical biomarker.
Supplementary Material
Acknowledgments
The authors thank Dr. John Teiber (University of Texas, Dallas, TX) and Drs. Dan Tawfik, Olga Khersonsky, and Leonid Gaidukov (Weizmann Institute of Science, Rehovot, Israel) for the generous gifts of purified PON3 and the TBBL reagent, respectively. The authors are indebted to Dr. Blai Coll (Vascular Medicine Unit, Hospital Universitari de Sant Joan) for his help in IMT measurements.
Footnotes
Abbreviations:
- AIDS
- acquired immunodeficiency syndrome
- FPLC
- fast-performance liquid chromatography
- HCV
- hepatitis C virus
- HIV
- human immunodeficiency virus
- IMT
- intima-media thickness
- NNRTI
- nonnucleoside reverse transcriptase inhibitor
- NRTI
- nucleoside reverse transcriptase inhibitor
- PI
- protease inhibitor
- PON
- paraoxonase
- SNP
- single nucleotide polymorphism
- TBBL
- 5-thiobutyl butyrolactone
This study was supported by grants PI 05/1607, 08/1175, and 08/1032 from the Instituto de Salud Carlos III, Madrid, Spain.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures and one table.
REFERENCES
- 1.Boyd M., Reiss P. 2006. The long-term consequences of antiretroviral therapy: a review. J. HIV Ther. 11: 26–35. [PubMed] [Google Scholar]
- 2.Bergersen B. M. 2006. Cardiovascular risk in patients with HIV infection: impact of antiretroviral therapy. Drugs. 66: 1971–1987. [DOI] [PubMed] [Google Scholar]
- 3.Rose H., Woolley I., Hoy J., Dart A., Bryant B., Mijch A., Sviridov D. 2006. HIV infection and high-density lipoprotein: the effect of disease vs. the effect of treatment. Metabolism. 55: 90–95. [DOI] [PubMed] [Google Scholar]
- 4.Alonso-Villaverde C., Segués T., Coll-Crespo B., Pérez-Bernalte R., Rabassa A., Gomila M., Parra S., Gozález-Esteban M. A., Jiménez-Expósito M. J., Masana L. 2003. High-density lipoprotein concentrations relate to the clinical course of HIV viral load in patients undergoing antiretroviral therapy. AIDS. 17: 1173–1178. [DOI] [PubMed] [Google Scholar]
- 5.Parra S., Alonso-Villaverde C., Coll B., Ferré N., Marsillach J., Aragonès G., Mackness M., Mackness B., Masana L., Joven J., et al. 2007. Serum paraoxonase-1 activity and concentration are influenced by human immunodeficiency virus infection. Atherosclerosis. 194: 175–181. [DOI] [PubMed] [Google Scholar]
- 6.Parra S., Marsillach J., Aragonès G., Beltrán R., Montero M., Coll B., Mackness B., Mackness M., Alonso-Villaverde C., Joven J., et al. 2010. Association of paraoxonase-1 gene haplotypes with the immunologic outcome of and metabolic disturbances and atherosclerosis in HIV-infected patients. J. Infect. Dis. 201: 627–634. [DOI] [PubMed] [Google Scholar]
- 7.Primo-Parmo S. L., Sorenson R. C., Teiber J., La Du B. N. 1996. The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics. 33: 498–507. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Sanabria F., Rull A., Beltrán-Debón R., Aragonès G., Camps J., Mackness B., Mackness M., Joven J. 2010. Tissue distribution and expression of paraoxonases and chemokines in mouse: the ubiquitous and joint localisation suggest a systemic and coordinated role. J. Mol. Histol. 41: 379–386. [DOI] [PubMed] [Google Scholar]
- 9.Deakin S. P., Bioletto S., Bochaton-Piallat M. L., James R. W. 2011. HDL-associated paraoxonase-1 can redistribute to cell membranes and influence sensitivity to oxidative stress. Free Radic. Biol. Med. 50: 102–109. [DOI] [PubMed] [Google Scholar]
- 10.Ng C. J., Wadleigh D. J., Gangopadhyay A., Hama S., Grijalva V. R., Navab M., Fogelman A. M., Reddy S. T. 2001. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J. Biol. Chem. 276: 44444–44449. [DOI] [PubMed] [Google Scholar]
- 11.Camps J., Marsillach J., Joven J. 2009. The paraoxonases: role in human diseases and methodological difficulties in measurement. Crit. Rev. Clin. Lab. Sci. 46: 83–106. [DOI] [PubMed] [Google Scholar]
- 12.Mackness B., Quarck R., Verreth W., Mackness M., Holvoet P. 2006. Human paraoxonase-1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 26: 1545–1550. [DOI] [PubMed] [Google Scholar]
- 13.Ng C. J., Hama S. Y., Bourquard N., Navab M., Reddy S. T. 2006. Adenovirus mediated expression of human paraoxonase 2 protects against the development of atherosclerosis in apolipoprotein E-deficient mice. Mol. Genet. Metab. 89: 368–373. [DOI] [PubMed] [Google Scholar]
- 14.Shih D. M., Xia Y. R., Wang X. P., Wang S. S., Bourquard N., Fogelman A. M., Lusis A. J., Reddy S. T. 2007. Decreased obesity and atherosclerosis in human paraoxonase 3 transgenic mice. Circ. Res. 100: 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng C. J., Bourquard N., Hama S. Y., Shih D., Grijalva V. R., Navab M., Fogelman A. M., Reddy S. T. 2007. Adenovirus-mediated expression of human paraoxonase 3 protects against the progression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 27: 1368–1374. [DOI] [PubMed] [Google Scholar]
- 16.Yuan J., Devarajan A., Moya-Castro R., Zhang M., Evans S., Bourquard N., Dias P., Lacout C., Vainchenker W., Reddy S. T., et al. 2010. Putative innate immunity of antiatherogenic paraoxonase-2 via STAT5 signal transduction in HIV-1 infection of hematopoietic TF-1 cells and in SCID-hu mice. J. Stem Cells. 5: 43–48. [PubMed] [Google Scholar]
- 17.Camps J., Pujol I., Ballester F., Joven J., Simó J. M. 2011. Paraoxonases as potential antibiofilm agents: their relationship with quorum-sensing signals in gram-negative bacteria. Antimicrob. Agents Chemother. 55: 1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aragonès G., Guardiola M., Barreda M., Marsillach J., Beltran-Debon R., Rull A., Mackness B., Mackness M., Joven J., Simó J. M., et al. 2011. Measurement of serum paraoxonase-3 concentration: method evaluation, reference values and influence of genotypes in a population-based study. J. Lipid Res. 52: 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez E., Mocroft A., García-Viejo M. A., Pérez-Cuevas J. B., Blanco J. L., Mallolas J., Bianchi L., Conget I., Blanch J., Phillips A., et al. 2001. Risk of lipodystrophy in HIV-1 infected patients treated with protease inhibitors: a prospective cohort study. Lancet. 357: 592–598. [DOI] [PubMed] [Google Scholar]
- 20.Alonso-Villaverde C., Coll B., Parra S., Montero M., Calvo N., Tous M., Joven J., Masana L. 2004. Atherosclerosis in patients infected with HIV is influenced by a mutant monocyte chemoattractant protein-1 allele. Circulation. 110: 2204–2209. [DOI] [PubMed] [Google Scholar]
- 21.Ferré N., Camps J., Fernández-Ballart J., Arija V., Murphy M. M., Ceruelo S., Biarnés E., Vilella E., Tous M., Joven J. 2003. Regulation of serum paraoxonase activity by genetic, nutritional, and lifestyle factors in the general population. Clin. Chem. 49: 1491–1497. [DOI] [PubMed] [Google Scholar]
- 22.Marsillach J., Mackness B., Mackness M., Riu F., Beltrán R., Joven J., Camps J. 2008. Immunochemical analysis of paraoxonases-1, 2, and 3 expression in normal mouse tissues. Free Radic. Biol. Med. 45: 146–157. [DOI] [PubMed] [Google Scholar]
- 23.Savès M., Morlat P., Chêne G., Peuchant E., Pellegrin I., Bonnet F., Bernard N., Lacoste D., Salamon R., Beylot J. 2001. Prognostic value of plasma markers of immune activation in patients with advanced HIV disease treated by combination antiretroviral therapy. Clin. Immunol. 99: 347–352. [DOI] [PubMed] [Google Scholar]
- 24.Barrett J. C., Fry B., Maller J., Daly M. J. 2005. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 21: 263–265. [DOI] [PubMed] [Google Scholar]
- 25.Zweig M. H., Campbell G. 1993. Receiver-operating characteristics (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39: 561–577. [PubMed] [Google Scholar]
- 26.Schwarz K. B. 1996. Oxidative stress during viral infection: a review. Free Radic. Biol. Med. 21: 641–649. [DOI] [PubMed] [Google Scholar]
- 27.Manda K. R., Banerjee A., Banks W. A., Ercal N. 2011. Highly active antiretroviral therapy drug combination induces oxidative stress and mitochondrial dysfunction in immortalized human blood-brain barrier endothelial cells. Free Radic. Biol. Med. 50: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Draganov D. I., Teiber J. F., Speelman A., Osawa Y., Sunahara R., La Du B. N. 2005. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J. Lipid Res. 46: 1239–1247. [DOI] [PubMed] [Google Scholar]
- 29.Draganov D. I., Stetson P. L., Watson C. E., Billecke S. S., La Du B. N. 2000. Rabbit serum paraoxonase 3 (PON3) is a high density lipoprotein-associated lactonase and protects low density lipoprotein against oxidation. J. Biol. Chem. 275: 33435–33442. [DOI] [PubMed] [Google Scholar]
- 30.Reddy S. T., Wadleigh D. J., Grijalva V., Ng C., Hama S., Gangopadhyay A., Shih D. M., Lusis A. J., Navab M., Fogelman A. M. 2001. Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler. Thromb. Vasc. Biol. 21: 542–547. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y., Mackness B., Mackness M. 2008. Comparison of the ability of paraoxonases 1 and 3 to attenuate the in vitro oxidation of low-density lipoprotein and reduce macrophage oxidative stress. Free Radic. Biol. Med. 45: 743–748. [DOI] [PubMed] [Google Scholar]
- 32.Shih D. M., Xia Y. R., Yu J. M., Lusis A. J. 2010. Temporal and tissue-specific patterns of Pon3 expression in mouse: in situ hybridization analysis. Adv. Exp. Med. Biol. 660: 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belteki G., Kempster S. L., Forhead A. J., Giussani D. A., Fowden A. L., Curley A., Charnock-Jones D. S., Smith G. C. 2010. Paraoxonase-3, a putative circulating antioxidant, is systematically up-regulated in late gestation in the fetal rat, sheep, and human. J. Clin. Endocrinol. Metab. 95: 3798–3805. [DOI] [PubMed] [Google Scholar]
- 34.García-Heredia A., Marsillach J., Aragonès G., Guardiola M., Rull A., Beltrán-Debón R., Folch A., Mackness B., Mackness M., Pedro-Botet J., et al. 2011. Serum paraoxonase-3 concentration is associated with the severity of hepatic impairment in patients with chronic liver disease. Clin. Biochem. 44: 1320–1324. [DOI] [PubMed] [Google Scholar]
- 35.Tohyama J., Billheimer J. T., Fuki I. V., Rothblat G. H., Rader D. J., Millar J. S. 2009. Effects of nevirapine and efavirenz on HDL cholesterol levels and reverse cholesterol transport in mice. Atherosclerosis. 204: 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira S. A., Batuca J. R., Caixas U., Branco T., Delgado-Alves J., Germano I., Lampreia F., Monteiro E. C. 2009. Effect of efavirenz on high-density lipoprotein antioxidant properties in HIV-infected patients. Br. J. Clin. Pharmacol. 68: 891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aragonès G., Beltrán-Debón R., Rull A., Rodríguez-Sanabria F., Fernández-Sender L., Camps J., Joven J., Alonso-Villaverde C. 2010. Human immunodeficiency virus-infection induces major changes in high-density lipoprotein particle size distribution and composition: the effect of antiretroviral treatment and disease severity. Clin. Chem. Lab. Med. 48: 1147–1152. [DOI] [PubMed] [Google Scholar]
- 38.Shlay J. C., Sharma S., Peng G., Gibert C. L., Grunfeld C. 2008. Long-term subcutaneous tissue changes among antiretroviral-naive persons initiating stavudine, zidovudine, or abacavir with lamivudine. J. Acquir. Immune Defic. Syndr. 48: 53–62. [DOI] [PubMed] [Google Scholar]
- 39.Maskrey B. H., Megson I. L., Whitfield P. D., Rossi A. G. 2011. Mechanisms of resolution of inflammation: a focus on cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 31: 1001–1006. [DOI] [PubMed] [Google Scholar]
- 40.Mackness M., Bouiller A., Hennuyer N., Mackness B., Hall M., Tailleux A., Duriez P., Delfly B., Durrington P., Fruchart J. C., et al. 2000. Paraoxonase activity is reduced by a pro-atherosclerotic diet in rabbits. Biochem. Biophys. Res. Commun. 269: 232–236. [DOI] [PubMed] [Google Scholar]
- 41.Huang R., Silva R. A., Jerome W. G., Kontush A., Chapman M. J., Curtiss L. K., Hodges T. J., Davidson W. S. 2011. Apolipoprotein A-I structural organization in high-density lipoproteins isolated from human plasma. Nat. Struct. Mol. Biol. 18: 416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.