Abstract
The interpretation of the electron ionization mass spectra of straight-chain and methyl-branched saturated and unsaturated wax esters (WEs) is discussed in this study based on the spectra of 154 standards. The most important fragments indicative of the structure of the acid and alcohol chains are identified and summarized for WEs with various number of double bonds in the chains. Briefly, most WEs provide acylium ions allowing structural characterization of the acid part, whereas the alcohol part gives corresponding alkyl radical cations. The elemental composition of selected important fragments is established from a high-resolution accurate mass analysis. The ion abundances are discussed with respect to the length and unsaturation of the aliphatic chains. The interpretation of the spectra of branched or unsaturated WEs requires the recognition of small but important peaks that are difficult to discern among the other fragments. We demonstrate that such fragments are easily detected in differential mass spectra. This approach requires spectra of WE standards (e.g., straight-chain analogs in the case of branched WEs) recorded under the same experimental conditions. The WEs mass spectral database provided in the supplemental data can be used as a reference for the analysis of the GC/EI-MS data.
Keywords: interpretation, neutral lipids, spectral database, waxes
Wax esters (WEs), simple lipids composed of long-chain fatty alcohols esterified to long-chain fatty acids (FAs), are widespread in nature. They serve a variety of functions in living organisms, including surface protection (1, 2), energy storage (3), chemical communication (4), or sound transmission (5). The human body biosynthesizes WEs to play important physiological roles in the complex protection of fetuses and newborns, skin, hair, ears, and eyes (6–10). Dietary WEs are an important source of very long chain fatty alcohols and acids that exert regulatory roles in the cholesterol metabolism (11). Naturally occurring WEs usually form complex mixtures composed of many molecular species. These mixtures contain straight- and branched-chain esters of various chain lengths and numbers of double bonds depending on the biochemical synthetic pathways in particular organisms. WEs are also produced industrially and used in large quantities in cosmetics, polishes, lubricants, surface coating, and other applications (12). Gas chromatography (GC) has frequently been used for analyzing WEs, often after their hydrolysis. The separation of intact WEs has been made possible by the introduction of high-temperature columns. GC coupled with electron ionization mass spectrometry (GC/EI-MS) offers high separation efficiency, ease of use, and mass spectra allowing structure elucidation. Other analytical methods based on liquid chromatography/mass spectrometry or matrix-assisted laser desorption/ionization mass spectrometry (13–15) can also be utilized, especially in the case of thermally unstable or insufficiently volatile WEs.
The research on the mass spectra of FA esters can be traced back to the early days of mass spectrometry. The EI spectra are explained perhaps in all of the textbooks on organic mass spectrometry, but the discussion is mostly limited to methyl or ethyl esters. The EI mass spectra of saturated straight-chain WEs has been studied for more than 40 years using both low- and high-resolution measurements and deuterium-labeling experiments (16–22). The spectra interpretation is usually straightforward in terms of the determination of the molecular weight and acid/alcohol chain length. However, the structure elucidation becomes much more complicated for branched or unsaturated esters, e.g., in case of samples of animal or human origin. Only a few reports closely dealing with the EI spectra of branched or unsaturated WEs exist in the literature (17, 20, 22–24). The application of commercial spectra libraries for spectra interpretation is of limited value because of the low number of WE entries.
The aim of this paper is to review and broaden the knowledge on the EI spectra of WEs in order to provide hints for the interpretation of the GC/MS data. The study is based on the EI mass spectra of 154 WE standards recorded under standardized conditions and follows a previous work focused on GC of straight-chain WEs (25). The main diagnostic fragments of the various types of WEs are identified using high-resolution accurate-mass (HRAM) measurement and their relative intensities are compared. To the best of our knowledge, the mass spectra of most WEs included in this study, particularly those with branched and polyunsaturated chains, have never been described before. A new interpretation strategy based on the use of differential spectra is suggested. The spectrum database is available online in the supplementary data.
MATERIALS AND METHODS
Standards of WEs
WE standards were obtained from Nu-Check-Prep, Inc. (Elysian, MN) or synthesized via acid chlorides (14). The FAs for synthesis were purchased from Sigma-Aldrich (St. Louis, MO), TCI America (Portland, OR), Matreya LLC (Pleasant Gap, PA), and Larodan Fine Chemicals AB (Malmö, Sweden). The alcohols were prepared by the reduction of the corresponding methyl esters (26). The standards were dissolved in hexane at a concentration of 1 mg/ml.
GC/MS
The mass spectra were recorded using a 5975B quadrupole mass spectrometer coupled to a 6890N gas chromatograph (Agilent, Santa Clara, CA). The samples were injected splitless at 240°C. Chromatographic separation was achieved on a HP-5MS capillary column (30 m × 0.25 mm, a film thickness of 0.25 μm, Agilent). The GC was operated in the constant flow mode (1.5 ml/min) with helium as a carrier gas. The temperature program was: 70°C (1 min), then 50°C/min to 240°C, then 1°C/min to 320°C (10 min). The temperatures of the transfer line, ion source, and quadrupole were 250°C, 230°C, and 150°C, respectively. The compounds were ionized by 70 eV electrons. The ion optics was tuned and calibrated with the automatic standard spectra tune procedure using perfluorotri-n-butylamine (PFTBA). The quadrupole was scanned in the 25–675 m/z range. The high-resolution EI spectra were measured using a GCT Premier benchtop orthogonal acceleration time-of-flight mass spectrometer (Waters, Milford, MA; instrument specifications: resolution 7000 FWHM, mass accuracy 0.1 mDa or 5 ppm) coupled to the Agilent 7890A GC System. The chromatographic conditions were the same as described above. The high-resolution mass spectra were internally calibrated with PFTBA.
Data processing
The low-resolution mass spectra were summed across the chromatographic peaks, the background was subtracted, exported to NIST MS Search 2.0 (National Institute of Standards and Technology, Gaithersburg, MD) and saved in the MSP file format. The spectrum database was built from the averaged spectra calculated from five MSP files corresponding to five independent GC/MS runs. The averaging of the MSP files was performed with in-house developed software.
The generic spectra of WEs X:0-Y:1 and WEs X:1-Y:0 were calculated from the spectra of five selected esters of a given type (either containing monounsaturated acid and saturated alcohol, or vice versa). Each spectrum was modified by the removal of fragments characteristic for the compound (i.e., ions diagnostic for an acid or an alcohol chain of a particular length). The modified spectra (MSP file format) were averaged and added to the spectrum library.
The differential spectra of the branched and unsaturated WEs were calculated in the Comparison Window (Subtraction tab) of the NIST MS Search 2.0 program.
Abbreviations and nomenclature of WEs
An abbreviated nomenclature for naming the WEs was adopted (25). The first part of the abbreviations refers to the alcohol segment of the molecule, whereas the second part indicates the FA. Unless stated otherwise, a common methylene interrupted arrangement and/or cis geometry of double bonds is assumed. Thus, for instance, an abbreviation WE 18:0-22:0 is used for octadecyl docosanoate (stearyl behenate), WE 14:0-2Me16:0 for tetradecyl 2-methylhexadecanoate and WE 22:0-18:1(n–9) for docosyl (9Z)-octadecenoate (behenyl oleate). To indicate a group of WEs, X is used for the alcohol chain carbons and Y for the acid chain carbons. Thus, for instance, WEs X:0-Y:1 stands for wax esters with saturated alcohols and monounstaurated acids. The general formula of WEs is considered to be RCOOR’, where R and R’ are the alkyl moieties of the acid and alcohol part, respectively.
RESULTS AND DISCUSSION
Saturated straight-chain WEs (WEs X:0-Y:0)
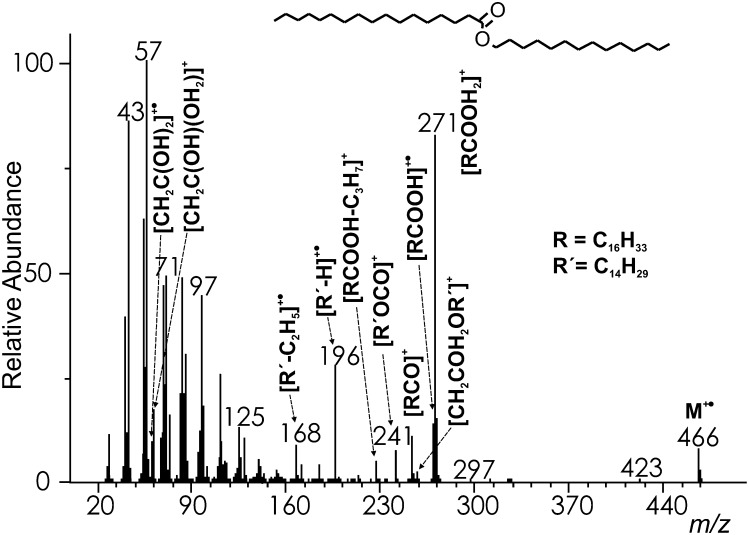
Saturated straight-chain WEs (see the spectrum of WE 14:0-17:0 in Fig. 1) provided molecular ions (M+•) of appreciable intensities (4–9%), sufficient for the reliable assignment of molecular weight. The fragments important for structure elucidation originated from cleavages near the ester bond. The most remarkable fragments (56–100%) were protonated acids [RCOOH2]+ (m/z 271 in WE 14:0-17:0) formed by a rearrangement with a double hydrogen transfer (16–19). The moderately abundant peaks (10–18%) one mass unit lower (m/z 270 in WE 14:0-17:0) were radical cations [RCOOH]+• rationalized by a single hydrogen rearrangement (16, 18). Alpha cleavage at the carbonyl group yielded fairly abundant (5–22%) acylium ions [RCO]+ (m/z 253 in WE 14:0-17:0) (16, 19). The EI spectra also showed fragments allowing a characterization of the alcohol part. Among them, the most abundant were radical cations [R’−H]+•, formally products of the elimination of FA from the molecular ions (m/z 196 in WE 14:0-17:0) formed by at least two distinct mechanisms (16). They were fairly abundant (up to 48%) in WEs containing shorter alcohols, but their intensity decreased down to several relative percent (4–7%) for alcohols with 20 carbons or more. The radical cations readily eliminated ethylene (in analogy with the fragmentation of alkenes) yielding [R’−C2H5]+• (m/z 168 in WE 14:0-17:0; HRAM: measured 168.1860, calculated 168.1878; [C12H24]+•). The C-C bond α-cleavage at the carbonyl group provided small (4–10%) fragments [R'OCO]+ (m/z 241 in WE 14:0-17:0), which were practical for the confirmation of the alcohol chain length (16, 19). Previously described [CH2COH2OR’]+ fragments (16) (m/z 257 in WE 14:0-17:0) were rather small (0–5%). WEs also provided two rearrangement ions, [CH2C(OH)2]+• (m/z 60) and [CH2C(OH)(OH2)]+ (m/z 61) (27). Their intensities ranged from 4–10% and 7–22%, respectively. Interestingly, their intensity ratio (60/61) was found to be almost constant within the group of saturated WEs (0.52 ± 0.04) and, as will be demonstrated later, strongly dependent on the number of double bonds in the WE chains. The peaks of the aliphatic series [CnH2n+1]+ and [CnH2n–1]+ dominated the low mass region. There were also [CnH2n–1O2]+ ions, the fragmentation products of [RCOOH]+•. The ion [RCOOH−C3H7]+ (m/z 227 in WE 14:0-17:0; HRAM: measured 227.2020, calcd. 227.2011; [C14H27O2]+) was more prominent than the others because of the favored loss of the propyl radical.
Fig.1.
The EI mass spectrum of tetradecyl heptadecanoate (WE 14:0-17:0).
Saturated methyl-branched WEs
Whereas the interpretation of the mass spectra of straight-chain saturated WEs is rather straightforward, the recognition and correct interpretation of branched WEs might be a challenging task. The retention parameters are quite important and serve as useful clues for the disclosure of branched WEs in chromatograms, but mass spectrometry is usually needed to deduce the exact position of the branching.
Saturated monomethyl-branched WEs
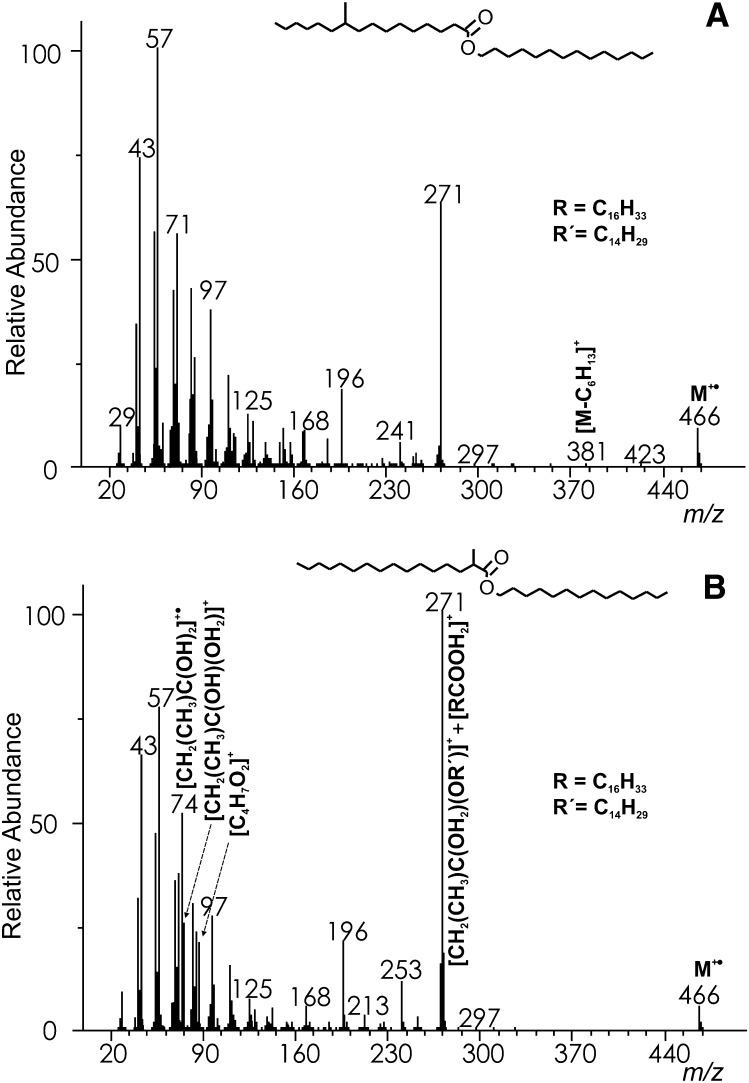
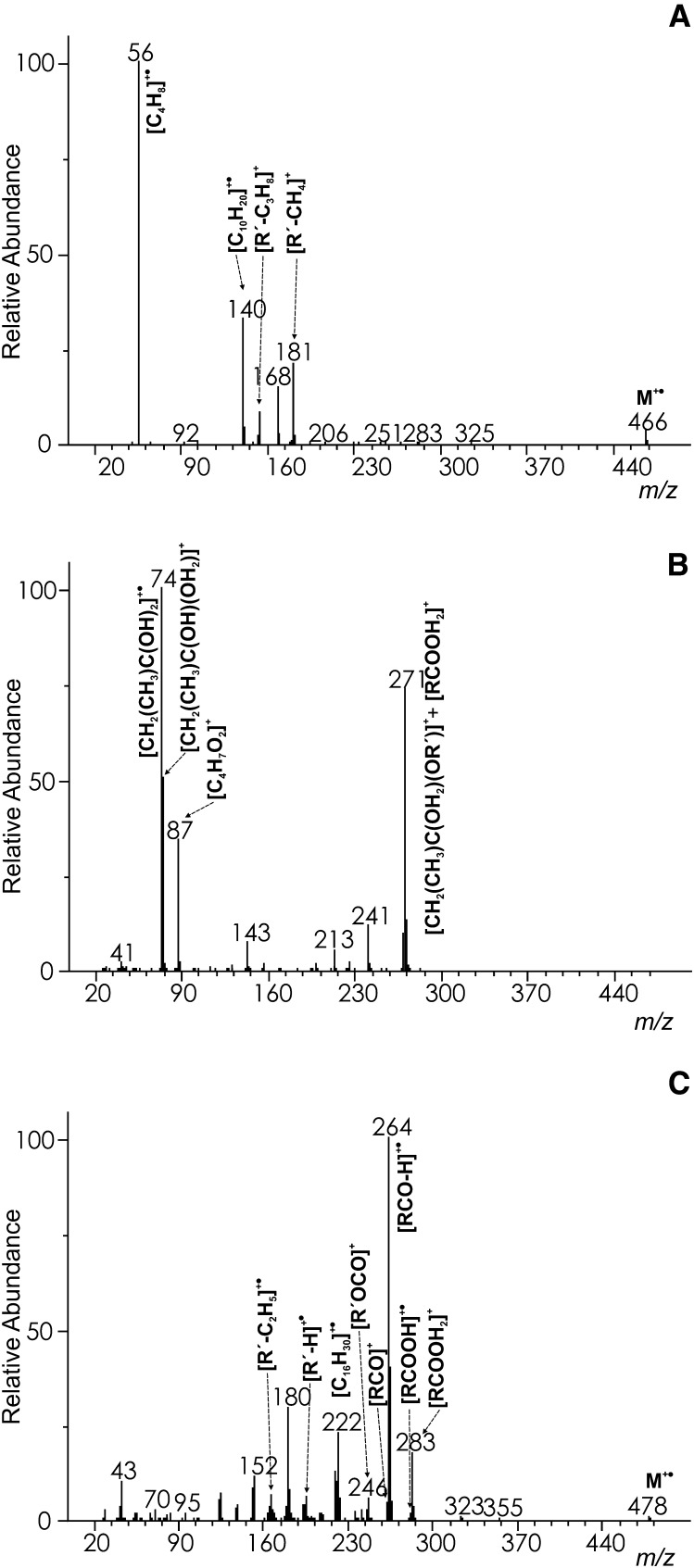
The mass spectra of WEs with monomethyl branching near the end or in the middle of the FA chain closely resembled those of straight-chain esters and showed only slight variations in the peak intensities (cf. Figs. 1, 2A). The molecular ions were as abundant as in the spectra of straight-chain analogs (6–9%). The iso- and anteiso- methyl branched esters eliminated methyl and ethyl radical yielding [M−CH3]+ and [M−C2H5]+, respectively, but these fragments were too small for a reliable localization of the branching position. Similarly, the σ-bond cleavage at the branching point in the middle of the acid chain provided only tiny peaks; e.g., the intensity of [M−C6H13]+ in WE 14:0-10Me16:0 (m/z 381 in Fig. 2A; HRAM: measured 381.3749, calcd. 381.3733; [C25H49O2]+) did not exceed 0.5%. The appearance of the spectrum changed significantly when the branching point was next to the ester bond, e.g., in WE 14:0-2Me16:0 (Fig. 2B). The rearrangement ions shifted to m/z 74 ([CH2(CH3)C(OH)2]+•) and m/z 75 ([CH2(CH3)C(OH)(OH2)]+) and their intensities increased. The spectrum base peak m/z 271 was protonated 2-methylhexadecanoic acid [RCOOH2]+, likely with the contribution of [CH2(CH3)C(OH2)(OR’)]+. A fairly abundant m/z 87 (HRAM: measured 87.0450, calcd. 87.0446; [C4H7O2]+) was another fragment indicating methyl branching in position 2 in the acid chain.
Fig.2.
The EI mass spectrum of tetradecyl 10-methylhexadecanoate (WE 14:0-10Me16:0) (A) and tetradecyl 2-methylhexadecanoate (WE 14:0-2Me16:0) (B). For a description of the unmarked ions, see Fig. 1.
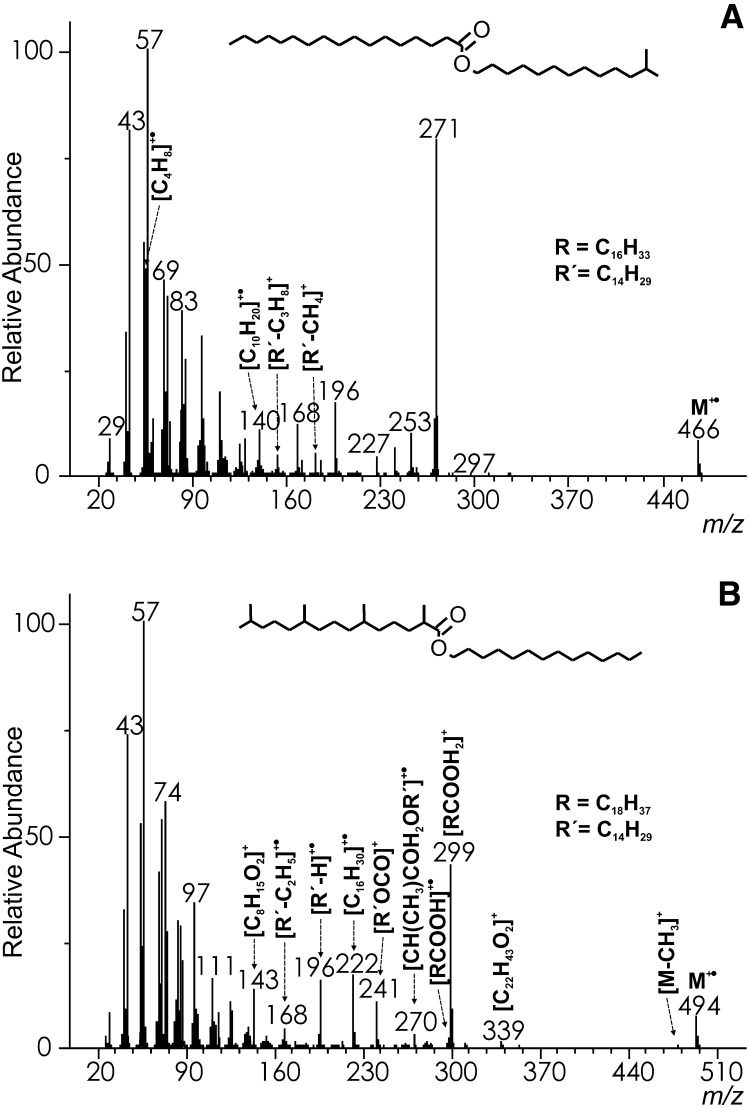
The spectra changed to a larger extent when methyl branching was located in the alcohol chains (see the spectrum of WE 12Me13:0-17:0 in Fig. 3A). The intensities of molecular ions remained in the same range (6–9%), but they did not lose any radicals related to the branched site. However, radicals were eliminated from [R’−H]+• and [R’–C2H5]+• ions yielding fragments indicative of the branching position. For instance, they appeared at m/z 181 ([R’−CH4]+; (the accurate mass was not determined because of an overlap with a peak of the internal calibrant) and m/z 153 ([R’–C3H8]+; HRAM: measured 153.1638, calcd. 153.1643; [C11H21]+), respectively in WE 12Me13:0-17:0). The rearrangement fragment m/z 140 (HRAM: measured 140.1590, calcd. 140.1565; [C10H20]+•) was presumably formed from branched [R’–H]+• after the elimination of C4H8. The most useful fragments for disclosing iso- and anteiso- branched alcohols were markedly increased butene ([C4H8]+•; m/z 56) and pentene ([C5H10]+•; m/z 70) rearrangement ions, respectively.
Fig.3.
The EI mass spectrum of 12-methyltridecyl heptadecanoate (WE 12Me13:0-17:0; for a description of the unmarked ions, see Fig. 1.) (A) and pristanyl myristate (WE 2,6,10,14-tetraMe15:0-14:0) (B).
Saturated WEs monomethyl-branched in both chains
The features characteristic for methyl-branched alcohols and acids were combined in the spectra of the WEs with methyl branching in both chains. Iso- or anteiso- branched alcohols esterified by methyl-branched FAs yielded abundant rearrangement fragments m/z 56 or m/z 70 and at the same time, barely noticeable cations formed by the loss of radicals from the branched acid chains. For instance, fragments indicating branching in the acid part of WE 12Me13:0-2Me16:0 (supplementary data, Spectrum #42) were found at m/z 74, m/z 75 and m/z 87 whereas iso-branched alcohol manifested itself by the more abundant m/z 56.
Saturated multiply methyl-branched WEs
The mass spectra of WEs with multiply-branched chains provided fragments allowing for at least a partial localization of the branching sites. The clearly visible molecular peak of pristanic acid tetradecyl ester (WE 14:0-2,6,10,14-tetraMe15:0) m/z 494 was accompanied by a tiny m/z 479 ([M−CH3]+) largely formed by the fragmentation of the iso-branched site (Fig. 3B). The [RCOOH2]+ peak (m/z 299) was of moderate intensity, but other acid-related fragments [RCOOH]+• (m/z 298) and [RCO]+ (m/z 281) were barely detected. The ions related to alcohol, [R'OCO]+, [R’−H]+•, [R’−C2H5]+• appeared at m/z 241, m/z 196, and m/z 168, respectively. The remarkable rearrangement peak m/z 222 (HRAM: measured 222.2347, calcd. 222.2342, [C16H30]+•) is known from the spectra of pristanic acid methyl ester (28). Another ion m/z 270 (HRAM: measured 270.2567, calcd. 270.2553; [C17H34O2]+•) was consistent with the structure of [CH(CH3)COH2OR’]+•. Rearrangement ions of this type have been described for straight-chain WEs (16). The ion intensity was considerably increased in the pristanic acid ester because of the methyl branching in position 2. Branching in the same position was evident also from abundant m/z 74, m/z 75 and m/z 87, the same ions as in WE 14:0-2Me16:0 (see above). Methyl branching in position 6 manifested itself by m/z 143 (HRAM: measured: 143.1075, calcd. 143.1067; [C8H15O2]+) produced by a σ-bond cleavage of [RCOOH]+•, and m/z 339 (HRAM: measured 339.3274, calcd. 339.3258, [C22H43O2]+) formed by an analogous cleavage of [M]+•. The only fragment evidencing methyl branching in position 10 was the hardly noticeable m/z 213 (the σ-bond cleavage of [RCOOH]+•). The esters of pristanic acid and iso or anteiso branched alcohols closely resembled the spectrum explained above.
Unsaturated WEs
The mass spectra of the unsaturated WEs tended to be uninformative. The extensive fragmentation resulted in small or even missing molecular ions and rather low abundant diagnostic fragments. In general, the more double bonds present, the more difficult it was to interpret the spectra. An interpretation strategy based on converting unsaturated WEs to saturated ones using reduction with deuterium has been suggested (16), but this approach is not always applicable for WE mixtures. Direct interpretation of the spectra of unsaturated WEs is then the only way to obtain at least partial structural information.
WEs with one double bond in the acid part (WEs X:0-Y:1)
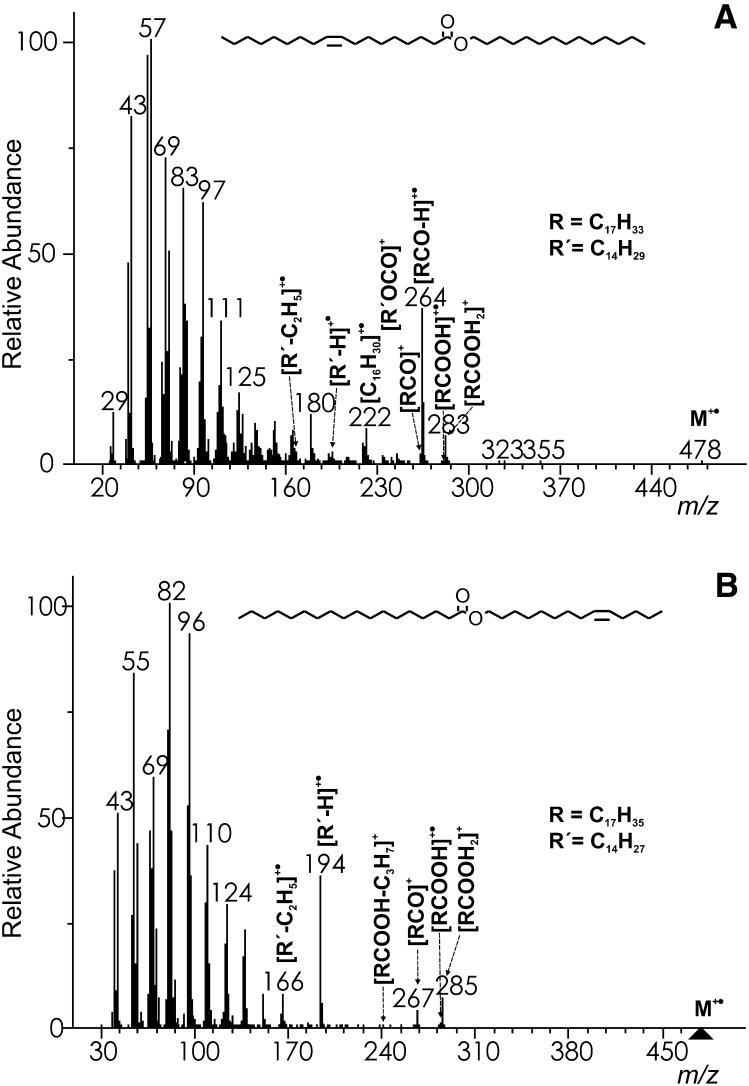
The molecular ion of myristyl oleate [WE 14:0-16:1(n–9)] was barely visible (Fig. 4A); its intensity was <1%, like in all of the spectra of WEs of this type. The most important diagnostic fragments were related to the acid chains. Among them, radical cations [RCO–H]+• [m/z 264 in WE 14:0-16:1(n–9)] (17, 23) corresponding to the neutral loss of alcohol were the most abundant (24–46%). Other fragments, [RCO]+ [m/z 265 in WE 14:0-16:1(n–9)] and [RCOOH2]+ [m/z 283 in WE 14:0-16:1(n–9)], were considerably smaller (6–15%) and [RCOOH]+• fragments [m/z 282 in WE 14:0-16:1(n–9)] were less important (<2%) (17, 23). An appreciably abundant ion m/z 222 in the spectrum of WE 14:0-16:1(n–9) was explained by a hydrogen rearrangement with a charge retention on the hydrocarbon (HRAM: measured 222.2346, calcd. 222.234; [C16H30]+•) (17). Further elimination of alkenes C3H6, C4H8, C5H10 provided relatively prominent m/z 180, 166, and 152, respectively, like in the spectra of shorter esters (methyl, ethyl, or propyl esters) (29). An effect of the double bond position and geometry has been studied in a series of WEs containing octadecenoic acid [WE 16:0-18:1; (n–12), (n–9), trans(n–9), (n–7); supplementary data, Spectra #109–112]. As expected, the effect was very small because of the extensive isomerization prior to or during fragmentation. The only differences were tiny changes in the ion intensities, which cannot be used for distinguishing isomers.
Fig.4.
The EI mass spectrum of myristyl oleate [WE 14:0-18:1(n−9)] (A) and myristoleyl stearate (WE 14:1(n–5)-18:0) (B).
Only small fragments diagnostic for the alcohol part of WEs were detected. The relative abundances of [R’–H]+• (m/z 196 in WE 14:0-16:1(n–9); HRAM: measured 196.2198, calcd. 196.2191; [C14H28]+•) and [R’−C2H5]+• [m/z 168 in WE 14:0-16:1(n–9)] were up to 5% in esters with shorter alcohols, but they dropped almost to zero for longer ones. The abundances of [R'OCO]+ [m/z 241 in WE 14:0-16:1(n–9)] were up to 2%. In the low mass region, the peaks of the aliphatic [CnH2n–1]+ and [CnH2n+1]+ series were mostly present.
Unsaturated WEs with a double bond in the alcohol part (WEs X:1-Y:0)
The spectra of WEs with a double bond in the alcohol part were markedly different, showing also ions diagnostic for the alcohol part of esters [see the spectrum of WE 14:1(n–5)-18:0, Fig. 4B]. The molecular ions were absent. The main fragments indicating an acid chain were relatively abundant protonated acids [RCOOH2]+ [m/z 285 in WE 14:1(n–5)-18:0; 11–20%] and acylium ions [RCO]+ [m/z 267 in WE 14:1(n–5)-18:0; 4–18%] (17, 23). Other acid-related fragments, [RCOOH]+• [m/z 284 in WE 14:1(n–5)-18:0] and [RCOOH−C3H7]+ [m/z 241 in WE 14:1(n–5)-18:0] were up to 2%. The most important alcohol-related fragments were abundant (20–44%) radical cations [R’–H]+• [m/z 194 in WE 16:1(n–7)-18:0] and appreciably abundant (4–12%) products of ethylene elimination [R’−C2H5]+• [m/z 166 in WE 14:1(n–5)-18:0] (17). An effect of the double bond position in the alcohol chain on the low mass ions intensities was noticed, especially when the double bond was close to the ester group. For instance, fragment m/z 82 (HRAM: measured 82.0782, calcd. 82.0783; [C6H10]+•) in WE 18:1(n–12)-16:0 was markedly higher than in the other isomers because of preferential cleavage of the alcohol chain in the position of the double bond. The differences were rather small for unambiguous identification of isomers with a different double bond position, but a library search should score the correct isomers higher. The mass spectra of cis/trans n–9 isomers were practically indistinguishable. The low mass spectra region of WEs with unsaturated alcohol was dominated by [CnH2n–2]+ fragments, as is the case in the spectra of monounsaturated acetates (29). Other fragments were ions of the [CnH2n–1]+ and [CnH2n+1]+ aliphatic series.
Unsaturated WEs with a double bond in the acid and alcohol part (WEs X:1-Y:1)
WEs with one double bond in the acid chain and one double bond in the alcohol chain provided spectra with diagnostic peaks of low intensities. Small molecular peaks (1%) were present. The acid-related fragments were [RCO]+ (6–9%), [RCO–H]+• (3–5%), [RCOOH2]+ (1–4%), (17) and [RCO–3H]+• (2–3%; HRAM of WE 16:1(n–9)-14:1(n–5): measured 206.1686, calcd. 206.1671, [C14H22O]+•; supplementary data, Spectrum #72). The ions disclosing the alcohol part of WEs were of similar abundances; [R’−H]+• (5–9%),[17] [R’−3H]+• (1-5%; HRAM of WE 16:1(n–9)-14:1(n–5): measured 248.2480, calcd. 248.2504, [C18H32]+•) and [R’−C2H5]+• (1–6%). The low mass ions were fragments of aliphatic series [CnH2n–1]+, [CnH2n–2]+ and [CnH2n+1]+ with C4H7+ (m/z 55) being the base peak.
Unsaturated WEs: esters of linoleic acid and saturated alcohols (WEs X:0-Y:2)
The esters of linoleic acid and saturated alcohols provided appreciably abundant molecular ions (2–3%). As in WEs with monounsaturated acids, acid-related fragments were relatively abundant, whereas alcohol-related diagnostic fragments were missing. The most important fragments were [RCO–H]+• (18–30%), [RCOO]+ (10–13%), [RCO]+ (9–10%), and [RCOOH]+• (6–9%), which in linoleic acid esters appeared at m/z 262, m/z 263, m/z 279, and m/z 280. The ion [RCOO]+ (HR of WE 14:0-18:2(n–6): measured 279.2379, calcd. 279.2319, [C18H31O2]+), which corresponds to the elimination of the hydrocarbon radical from the molecular ion, was not important in the previous types of WEs. A rearrangement ion m/z 220 (HRAM of WE 14:0-18:2(n–6): measured 220.2235, calcd. 220.2186, [C16H28]+•; supplementary data, Spectrum #87) was formed by an analogical mechanism as m/z 222 in WE 14:0-16:1(n–9). The alcohol-related ions were missing for long-chain alcohols, but shorter ones provided [R’−H]+• (up to 3%) and [R’−C2H5]+• (up to 4%). The fragments in the low mass region belonged mostly to the [CnH2n–1]+, [CnH2n+1]+, [CnH2n–3]+, and [CnH2n–2]+• ion series.
Unsaturated WEs: esters of saturated acids and linoleyl alcohol (WEs X:2-Y:0)
The esters of saturated acids with linoleyl alcohol provided small but distinguishable molecular ions (∼1%). Fragments allowing the characterization of the acid and alcohol chains were present. The most abundant acid-related fragments were [RCO]+ (6–25%) and [RCOOH2]+ (7–13%); the alcohol parts manifested themselves by [R’–H]+• (15–43%), [R'O]+ (4–5%), and [R’–C2H5]+• (2–3%) fragments. These fragments appeared in linoleyl alcohol esters at m/z 183, m/z 201, m/z 248, and m/z 265, respectively. The [R'O]+ fragments [HRAM of m/z 265 in of WE 18:2(n–6)-14:0 (supplementary data, Spectrum #85) was not determined because of an overlap with a peak of the internal calibrant] were rationalized by an α cleavage in the carbonyl group followed by the loss of CO. The low mass region contained fragments of the [CnH2n–3]+, [CnH2n–1]+, [CnH2n–2]+•, [CnH2n–5]+, and [CnH2n–4]+• aliphatic ion series.
Unsaturated WEs: esters of alpha-linolenic acid and saturated alcohols (WEs X:0-Y:3)
Molecular ions were the most abundant diagnostic peaks (3–5%) in the spectra of α-linolenic acid esters. The molecular ions eliminated C2H5•, C3H7•, C5H9• radicals and neutral butene, which is typical for the n–3 arrangement of double bonds, e.g., in α-linolenic acid methyl and ethyl esters (29). Gama-linolenic acid esters provide different fragments which can be used for distinguishing between the isomeric chains. As in other esters with unsaturated acids and saturated alcohols, only the acid-related diagnostic fragments were present, namely [RCOO]+ (4–5%) and [RCO]+ (2–3%). These fragments appeared at m/z 277 and m/z 261, respectively, in α-linolenic acid esters. The low mass region was crowded with many ions of aliphatic series, mainly [CnH2n–1]+, [CnH2n–3]+, [CnH2n–5]+, [CnH2n+1]+, [CnH2n–4]+•, and [CnH2n–7]+. The most prominent peak of the [CnH2n–4]+• series m/z 108 (HRAM of WE 14:0-18:3(n–3): measured 108.0938, calcd. 108.0939, [C8H12]+•; supplementary data, Spectrum #83) also indicated the n–3 polyenic chain (30).
Unsaturated WEs: esters of saturated acids and alpha-linolenyl alcohol (WEs X:3-Y:0)
Esters of saturated FAs and α-linolenyl alcohol also provided appreciably abundant (3–4%) molecular peaks. Again, the n–3 arrangement of double bonds appeared by the loss of C2H5•, C3H7•, C5H9• radicals and butene. Fragments indicative of both ester parts were detected: [RCO]+ (4–19%), [RCOOH2]+ (9–18%), and less intense [R’–H]+• (< 2%) and [R'O]+ (3–5%) (HRAM of WE 18:3(n–3)-14:0: measured 263.2386, calcd. mass 263.2375, [C18H31O]+; supplementary data, Spectrum #81). Abundant fragments of the [CnH2n–5]+, [CnH2n–3]+, [CnH2n–1]+, [CnH2n–4]+•, [CnH2n+1]+, and [CnH2n–7]+ ion series were detected. As in the previous case, the increased peak m/z 108 indicated the n–3 double bond arrangement (30).
WEs with monounsaturated alcohol and polyunsaturated acid
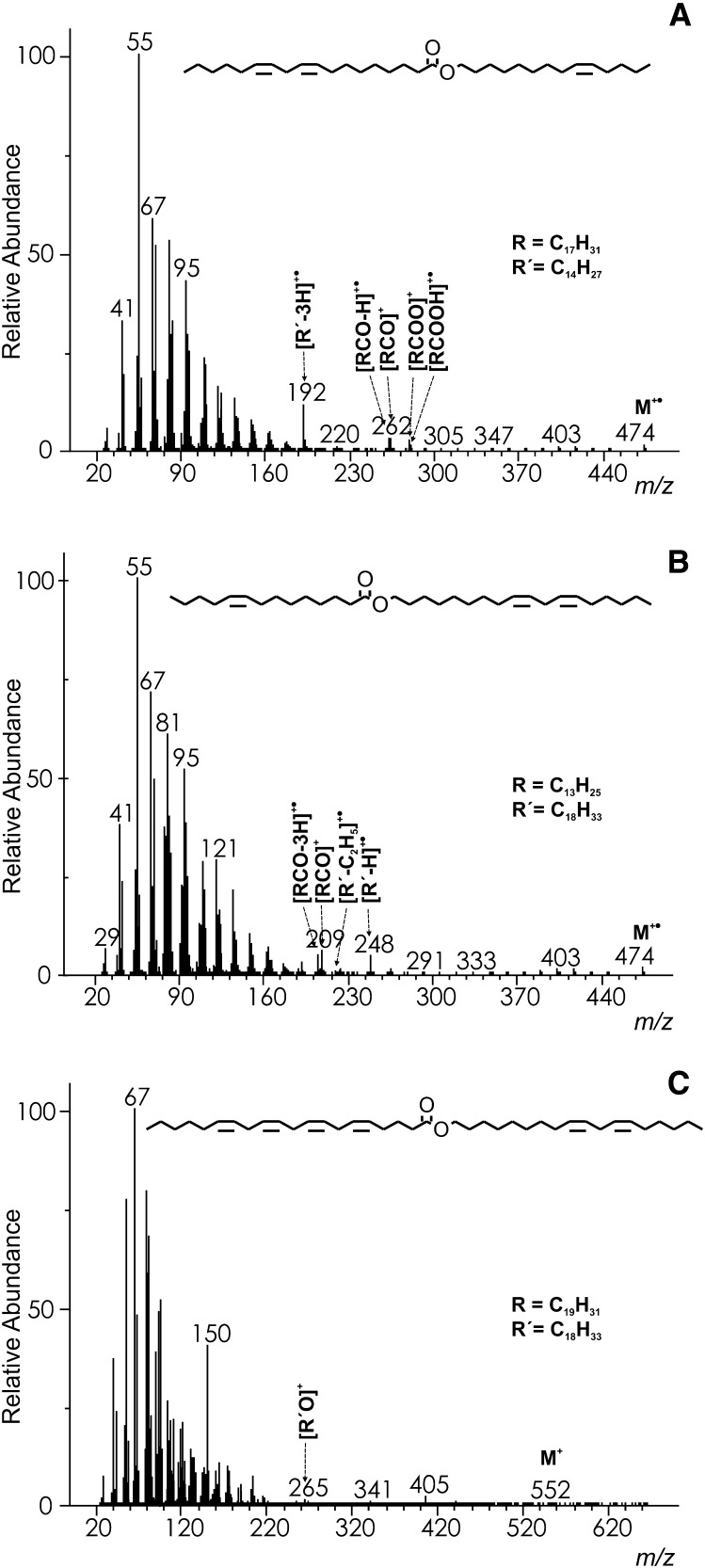
Esters of linoleic or α-linolenic acid with singly unsaturated alcohols provided low molecular and diagnostic fragments, typically in the range of 3–4%. The acid-related [RCO]+ and [RCOO]+ fragments were observed and linoleic acid esters also showed similarly abundant [RCO–H]+• and less pronounced [RCOOH]+• (1–2%).Whereas α-linolenic acid did not yield any fragments related to the alcohol part, the spectra of linoleic acid esters (Fig. 5A) contained an appreciably abundant (6–12%) fragment [R’–3H]+• (HRAM of WE 14:1(n–5)-18:2(n–6): measured 192.1896, calcd. 192.1878, [C14H24]+•; supplementary data, Spectrum #84). Esters of arachidonic acid provided molecular ions and [RCOO]+ fragments close to the noise level. The polyunsaturated methylene-interrupted double bond arrangement in the acid chains was easily disclosed based on the pronounced m/z 108 (an n–3 arrangement in an α-linolenoic acid chain) and m/z 150 (an n–6 arrangement in an arachidonic acid chain) (30). The most pronounced low mass ions belonged to the [CnH2n–1]+, [CnH2n–3]+, [CnH2n–5]+, and [CnH2n–4]+• aliphatic series; the base peak was m/z 55.
Fig.5.
The EI mass spectrum of myristoleyl linolate [WE 14:1(n–5)-18:2(n–6)] (A), linoleyl myristoleate [WE 18:2(n–6)-14:1(n–5)] (B) and linoleyl arachidonate [WE 18:2(n–6)-20:4(n–3)] (C).
WEs with monounsaturated acid and polyunsaturated alcohol
The mass spectra of esters composed of monounsaturated FA and linoleyl or α-linolenyl alcohol contained molecular ions (2–3%) as well as fragments allowing the characterization of both ester parts. The linoleyl alcohol esters (Fig. 5B) yielded [RCO]+ (m/z 209; 6–8%), [RCO–3H]+• (4–5%) (HRAM of WE 18:2(n–6)-14:1(n–5): measured mass 206.1686, calcd. mass 206.1671, [C14H22O]+•), [R’–H]+• (m/z 248; 5–7%), and [R’–C2H5]+• (m/z 220; 1–3%), whereas α-linolenyl alcohol esters provided [RCO]+ (6–7%) and [R'O]+ (2–3%). As in the previous cases, the n–3 methylene-interrupted polyunsaturated system manifested itself by m/z 108 and the loss of small radicals and butene from the molecular ions. The most abundant ion series were [CnH2n–1]+, [CnH2n–3]+, [CnH2n–2]+•, and [CnH2n–4]+•; the base peak was m/z 55.
WEs polyunsaturated in both chains
The molecular peaks of highly unsaturated WEs were either absent or up to 1%. Tiny acylium ions [RCO]+ (up to 3%) were usually the only ions usable for characterizing the esters. These fragments allowed distinctions to be made among the similar spectra of WE 18:2(n–6)-18:3(n–3) and WE 18:3(n–3)-18:2(n−6) (m/z 261 and m/z 263, respectively). Some other fragments appearing close to noise level, e.g., [RCOO–2H]+ in WE 18:2(n–6)-18:2(n–6) (HRAM: measured 277.2172, calcd. 277.2162; [C18H29O2]+; supplementary data, Spectrum #124) or [R'O]+ in WE 18:2(n–6)-20:4(n–3) (Fig. 5C) were noticed. The most prominent low mass ions were those of the aliphatic series [CnH2n–3]+, [CnH2n–5]+ and [CnH2n–1]+ with m/z 67 [C6H7+] or m/z 79 [C6H7+] being the base peaks. The n–3 and n–6 polyunsaturated chains provided typical fragments as described above. Although the spectra of highly polyunsaturated WEs did not provide prominent diagnostic peaks, they were usually distinct enough to identify them correctly based on the WE library search.
This section is summarized in an overview of the important diagnostic fragments provided in Table I in the supplementary data.
Differential mass spectra
The correct interpretation of the spectra of WEs bearing specific structural features, such as branching or double bonds, often requires recognizing small peaks that are difficult to discern among the other fragments. Relatively small differences in the ion intensities might also be important for structure elucidation. However, such small nuances are easy to overlook. For example, WEs with iso or anteiso branching in the alcohol chain yielded even electron fragments after the elimination of radicals from [R’–H]+• and [R’–C2H5]+• and elevated fragments m/z 56 or m/z 70, respectively. When compared with straight-chain analogs, the spectra were almost undistinguishable at first sight. However, when the spectra of the branched WEs and their straight-chain analogs were subtracted, all the above-mentioned features became clearly visible. An example of the differential spectra of WE 12Me13:0-17:0 and WE 14:0-2Me16:0 calculated in this way is given in Fig. 6A, B. Having both spectra measured under the same experimental conditions is an important prerequisite for calculating the differential spectra correctly. When analyzing WE mixtures by GC/MS, the peaks of straight-chain WEs are usually present and their spectra are thus recorded under the same conditions. The subtraction can easily be performed using standard library search software such as NIST MS Search.
Fig.6.
The EI differential mass spectrum of 12-methyltridecyl heptadecanoate (WE 12Me13:0-17:0) (A), tetradecyl 2-methylhexadecanoate (WE 14:0-2Me16:0) (B) and myristyl oleate [WE 14:0-18:1(n–9)] (C).The spectra were constructed by the subtraction of the spectrum of WE 14:0-17:0 (A,B) or the generic spectrum WEs X:0-Y:1 (C). For details, see Results and Discussion.
Differential spectra were also useful for unsaturated WEs. As double bonds change the fragmentation completely, the spectra of straight-chain esters could not be used for subtractions and special reference spectra had to be created. This approach was based on an observation that low mass fragments were practically the same within a group of WEs with the same number of double bonds either in the alcohol or acid chain. The spectra differed from each other just in a small number of peaks related to the particular acid and alcohol. When removed, “blank” generic spectra were obtained. For instance, the generic spectrum of WEs X:0-Y:1 (supplementary data, Spectrum #155) was constructed from the EI spectra of five esters (WE 22:0-18:1(n–9); WE 18:0-18:1(n–9); WE 16:0-16:1(n–7); WE 14:0-16:1(n–7); WE 12:0-14:1(n–5)). In each spectrum, peaks corresponding to [RCO–H]+•, [RCO]+, [RCOOH2]+ [RCOOH]+•, [R’–H]+•, and [R’–C2H5]+• were deleted and the resulting spectra were summed (averaged). The generic spectrum was used for calculating the differential spectra of WEs monounsaturated in the acid part. When applied for WE 14:0-18:1(n–9) (Fig. 6C), all of the diagnostic fragments were better seen than in the original spectrum. In principle, generic spectra can be calculated for all types of unsaturated esters and used for calculating differential spectra.
Trends in the ion abundances
The abundance of fragment ions is affected by their stability and the stability of the neutral products formed during the decomposition of the molecular ions. Thus, the number of carbons in both chains has an influence on the intensities of the diagnostic ions. Knowing the trends in the fragment ion intensities might be useful for the interpretation process.
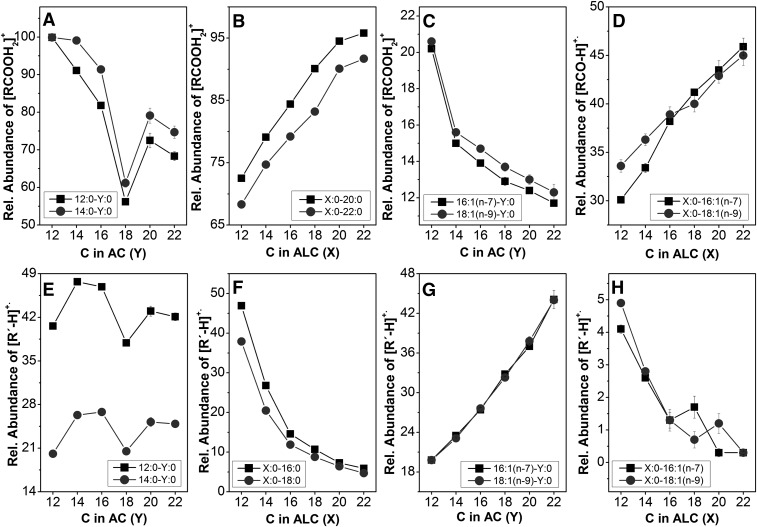
The most distinct fragments of saturated WEs were protonated acids ([RCOOH2]+). In a series of WEs with the same alcohol and increasing acid chain length, the intensity of this particular ion decreased as shown in Fig. 7A. Interestingly, the curves exhibited distinct minima for all WEs composed of stearic acid. In a series with the same acid and increasing alcohol chain length the intensity of [RCOOH2]+ increased, see Fig. 7B, which is likely related to the preferential loss of the larger alkyl radicals at a reactive site. Similar trends were observed in the spectra of WEs with monounsaturated acids; the abundance of [RCOOH2]+ decreased with the acid chain length, whereas the intensities of [RCO–H]+• (the most distinct acid-related fragments in WEs X:0-Y:1) increased with the number of carbons in saturated alcohol (Fig. 7C,D). Radical cations [R’–H]+• were significant fragments allowing the characterization of the alcohol parts of WEs. Their abundances in saturated esters were not significantly affected by acid chain length (Fig. 7E), but decreased with the increasing number of carbons in the alcohol chain (Fig. 7F). It was consistent with the decreasing stability of the alkene radical cations (by analogy with the decreasing intensity of molecular ions of 1-alkenes with the increasing number of carbons). A strong increase of the [R’–H]+• signal with the length of the acid chain was observed for WEs with one double bond in the alcohol chain (Fig. 7G). When a double bond was located in acid, the [R’–H]+• fragments were rather small and further decreased with the alcohol chain length (Fig. 7H), analogously to the saturated WEs.
Fig.7.
The dependences of the fragment ion abundances on the number of carbons in the acid (A,C,E,G) and alcohol (B,D,F,H) chains. The error bars are SDs calculated from five measurements.
The relative abundances of the low mass fragments (ions of aliphatic series) were found to be affected by the number and location (alcohol vs. acid chain) of double bonds. They can serve as a fingerprint for various WE types and can be used for constructing the artificial spectra suitable for calculating differential mass spectra as shown above. We have noticed that the relative intensities of the rearrangement ions m/z 60 and 61 can be used as an empirical measure indicating the number of double bonds. This intensity ratio increased from ca 0.5 (saturated WEs) to ca 5 (polyunsaturated WEs) as shown in Table II in the supplementary data. Such a ratio might be useful for analyzing spectra lacking a molecular peak.
CONCLUSIONS
The EI mass spectra of intact WEs provided information about the molecular weight, number of carbons, and double bonds in the acid and alcohol chains and to some extent also about the positions of methyl branching and double bonds. The molecular peaks were pronounced in saturated and usually recognizable in most unsaturated WEs. Monounsaturated WEs with a double bond in the alcohol chain were an exception; they lacked molecular ions entirely. A number of various fragments utilizable for the characterization of the acid and alcohol chains were formed. Their structures and abundances depended on the number and location (acid vs. alcohol chain) of the double bonds and the chain lengths. Saturated WEs provided sufficiently abundant and diagnostically relevant fragments for establishing the number of carbons in both chains. The methyl branching of the aliphatic chains caused only tiny changes in the spectra appearance, which were usually quite difficult to discern. The spectra features relevant to the branching sites became clearly apparent from differential spectra calculated by the subtraction of the straight-chain analog spectra. Unsaturated WEs provided less abundant diagnostic fragments for interpreting the spectra. The double bond position in monounsaturated WEs had a negligible impact on the spectra; differential spectra showed small variations in the ion intensities only for isomers with a double bond in close proximity to an ester bond. The spectra of the cis/trans isomers were indistinguishable. Methylene-interrupted double bond systems in polyunsaturated WEs were possible to disclose based on their characteristic CnH(2n–4) fragments. With the increasing number of double bonds, the intensities of the diagnostic ion decreased and the spectra of some very highly polyunsaturated esters did not contain any fragments usable for interpretation. However, the low mass fragments were usually characteristic enough for a successful identification by library search. Curiously, the number of double bonds had an impact on the relative intensities of rearrangement ions m/z 60 and 61. Their ratio might help to estimate the number of double bonds if both molecular ions and diagnostic fragments are missing.
When analyzing WE mixtures by GC/MS, combining mass spectral data with retention parameters is the best strategy for characterizing molecular species. The retention logic might suggest e.g., branching, which is then confirmed from the differential mass spectrum. The mass spectra discussed in this work have been recorded with standard ion source tuning. The intensities of the molecular ions and some fragments can be enhanced by changing the ion source parameters or using EI-MS with a supersonic molecular beam (31).
Supplementary Material
Acknowledgments
The authors thank Dr. Anna Brˇezinová for help with the sample measurement and Mr. Sean Mark Miller for proofreading the manuscript and making corrections.
Footnotes
Abbreviations:
- calcd
- calculated
- HRAM
- high-resolution accurate-mass
- PFTBA
- perfluorotri-n-butylamine
- WE
- wax ester
The research was supported by the Czech Science Foundation GACR (Project No. 203/09/0139) and Academy of Sciences of the Czech Republic (Research Project Z4 055 0506).
The online version of this article (available at http://www.jlr.org) contains supplementary data.
REFERENCES
- 1.Gunstone F. D., Harwood J. L., Dijkstra A. J. 2007. The Lipid Handbook, CRC Press, Boca Raton, FL. [Google Scholar]
- 2.Riederer M., Müller C. 2006. Biology of the Plant Cuticle, Blackwell Publishing Ltd; Oxford, UK. [Google Scholar]
- 3.Lee R. F., Hagen W., Kattner G. 2006. Lipid storage in marine zooplankton. Mar. Ecol. Prog. Ser. 307: 273–306. [Google Scholar]
- 4.Nelson D. R., Blomquist G. J. 1995. : Insect waxes. Waxes, chemistry, molecular biology and functions. Hamilton R. J., editor The Oily Press, Dundee, Scotland: 1–90. [Google Scholar]
- 5.Varansi U., Feldman H. R., Malins D. C. 1975. Molecular basis for formation of lipid sound lens in echolocating cetaceans. Nature. 255: 340–343. [DOI] [PubMed] [Google Scholar]
- 6.Rissmann R., Groenink H. W. W., Weerheim A. M., Hoath S. B., Ponec M., Bouwstra J. A. 2006. New insights into ultrastructure, lipid composition and organization of vernix caseosa. J. Invest. Dermatol. 126: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 7.Rawlings A. V. 1995. Skin waxes: their composition, properties, structures and biological significance. : Waxes, chemistry, molecular biology and functions. Hamilton R. J., editor The Oily Press, Dundee, Scotland: 223–256. [Google Scholar]
- 8.Masukawa Y., Tsujimura H., Imokawa G. 2005. A systematic method for the sensitive and specific determination of hair lipids in combination with chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 823: 131–142. [DOI] [PubMed] [Google Scholar]
- 9.Koçer M., Guldur T., Akarcay M., Miman M. C., Beker G. 2008. Investigation of age, sex and menstrual stage variation in human cerumen lipid composition by high performance thin layer chromatography. J. Laryngol. Otol. 122: 881–886. [DOI] [PubMed] [Google Scholar]
- 10.Butovich I. A., Uchiyama E., McCulley J. P. 2007. Lipids of human meibum: mass-spectrometric analysis and structural elucidation. J. Lipid Res. 48: 2220–2235. [DOI] [PubMed] [Google Scholar]
- 11.Hargrove J. L., Greenspan P., Hartle D. K. 2004. Nutritional significance and metabolism of very long chain fatty alcohols and acids from dietary waxes. Exp. Biol. Med.(Maywood) 229: 215–226. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton R. J. 1995. Commercial waxes: their composition and applications. : Waxes: Chemistry, Molecular Biology and Functions. Hamilton R. J., editor The Oily Press, Dundee, Scotland: 257–310. [Google Scholar]
- 13.Vrkoslav V., Míková R., Cvacˇka J. 2009. Characterization of natural wax esters by MALDI-TOF mass spectrometry. J. Mass Spectrom. 44: 101–110. [DOI] [PubMed] [Google Scholar]
- 14.Vrkoslav V., Urbanová K., Cvacˇka J. 2010. Analysis of wax ester molecular species by high performance liquid chromatography/atmospheric pressure chemical ionisation mass spectrometry. J. Chromatogr. A. 1217: 4184–4194. [DOI] [PubMed] [Google Scholar]
- 15.Vrkoslav V., Háková M., Pecková K., Urbanová K., Cvacˇka J. 2011. Localization of double bonds in wax esters by high-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry utilizing the fragmentation of acetonitrile-related adducts. Anal. Chem. 83: 2978–2986. [DOI] [PubMed] [Google Scholar]
- 16.Aasen A. J., Hofstett H. H., Iyengar B. T. R., Holman R. T. 1971. Identification and analysis of wax esters by mass spectrometry. Lipids. 6: 502–507. [DOI] [PubMed] [Google Scholar]
- 17.Vajdi M., Nawar W. W., Merritt C. 1981. GC/MS analysis of some long chain esters, ketones and propanediol diesters. J. Am. Oil Chem. Soc. 58: 106–110. [Google Scholar]
- 18.Audisio G., Rossini A., Bianchi G., Avato P. 1987. GC-MS determination of mixtures of long chain aliphatic esters. J. High Resolut. Chromatogr. 10: 594–597. [Google Scholar]
- 19.Arrendale R. F., Severson R. F., Chortyk O. T., Stephenson M. G. 1988. Isolation and identification of the wax esters from the cuticular waxes of green tobacco leaf. Beitrage zur Tabakforschung International. 14: 67–84. [Google Scholar]
- 20.Bianchi G., Tava A., Vlahov G., Pozzi N. 1994. Chemical structure of long-chain esters from “sansa” olive oil. J. Am. Oil Chem. Soc. 71: 365–369. [Google Scholar]
- 21.Reiter B., Lechner M., Lorbeer E., Aichholz R. 1999. Isolation and characterization of wax esters in fennel and caraway seed oils by SPE-GC. J. High Resolut. Chromatogr. 22: 514–520. [Google Scholar]
- 22.Zhang L. X., Yun Y. F., Liang Y. Z., Cao D. S. 2010. Discovery of mass spectral characteristics and automatic identification of wax esters from gas chromatography mass spectrometry data. J. Chromatogr. A. 1217: 3695–3701. [DOI] [PubMed] [Google Scholar]
- 23.Spencer G. F. 1979. Alkoxy-acyl combinations in the wax esters from winterized sperm whale oil by gas chromatography-mass spectrometry. J. Am. Oil Chem. Soc. 56: 642–646. [Google Scholar]
- 24.Pereira A. S., Siqueira D. S., Elias V. O., Simoneit B. R., Cabral J. A., Aquino Neto F. R. 2002. Three series of high molecular weight alkanoates found in Amazonian plants. Phytochemistry. 61: 711–719. [DOI] [PubMed] [Google Scholar]
- 25.Stránský K., Zarevúcka M., Valterová I., Wimmer Z. 2006. Gas chromatographic retention data of wax esters. J. Chromatogr. A. 1128: 208–219. [DOI] [PubMed] [Google Scholar]
- 26.Nystrom R. F., Brown W. G. 1947. Reduction of organic compounds by lithium aluminum hydride. I. Aldehydes, ketones, esters, acid chlorides and acid anhydrides. J. Am. Chem. Soc. 69: 1197–1199. [Google Scholar]
- 27.McFadden W. H., Boggs L. E., Buttery R. G. 1966. Specific rearrangements in mass spectra of butyl hexanoates and similar aliphatic esters. J. Phys. Chem. 70: 3516–3523. [Google Scholar]
- 28.Christie W. W. 2011. The AOCS Lipid Library (Ed. W.W. Christie), Available at http://lipidlibrary.aocs.org. Accessed September 2011.
- 29.National Institute of Standards and Technology (NIST) Library. 2005. Edition, Gaithersburg, MD, USA. [Google Scholar]
- 30.Fellenberg A. J., Johnson D. W., Poulos A., Sharp P. 1987. Simple mass-spectrometric differentiation of the normal-3, normal-6 and normal-9 series of methylene interrupted polyenoic acids. Biomed. Environ. Mass Spectrom. 14: 127–129. [Google Scholar]
- 31.Amirav A., Gordin A., Poliak M., Fialkov A. B. 2008. Gas chromatography-mass spectrometry with supersonic molecular beams. J. Mass Spectrom. 43: 141–163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.