Abstract
As the specific composition of lipids is essential for the maintenance of membrane integrity, enzyme function, ion channels, and membrane receptors, an alteration in lipid composition or metabolism may be one of the crucial changes occurring during skeletal and cardiac myopathies. Although the inheritance (autosomal dominant, autosomal recessive, and X-linked traits) and underlying/defining mutations causing these myopathies are known, the contribution of lipid homeostasis in the progression of these diseases needs to be established. The purpose of this review is to present the current knowledge relating to lipid changes in inherited skeletal muscle disorders, such as Duchenne/Becker muscular dystrophy, myotonic muscular dystrophy, limb-girdle myopathic dystrophies, desminopathies, rostrocaudal muscular dystrophy, and Dunnigan-type familial lipodystrophy. The lipid modifications in familial hypertrophic and dilated cardiomyopathies, as well as Barth syndrome and several other cardiac disorders associated with abnormal lipid storage, are discussed. Information on lipid alterations occurring in these myopathies will aid in the design of improved methods of screening and therapy in children and young adults with or without a family history of genetic diseases.
Keywords: phospholipids, Duchenne muscular dystrophy, myotonic muscular dystrophy, limb-girdle myopathic dystrophies, Barth syndrome, cardiomyopathy
Muscle disorders, known as myopathies, are characterized by muscle weakness, often due to fibrous tissue infiltration (1). Although myopathies can be acquired, this review will focus on inherited forms of the disorder. These inherited myopathies are often phenotypically similar, but they differ in their mode of inheritance, age of onset, muscle cell targets, and disease progression (2). Defects/deficiencies in transmembrane- and membrane-linked proteins, such as dystrophin, dysferlin, caveolin-3, and sarcoglycans, are causative of Duchenne and Becker myopathic dystrophies, limb-girdle (LGMD2B) myopathic dystrophy, LGMD1C, and LGMD2C, 2D, 2E, and 2F (Table 1, Fig. 1) (3–7). Defects in the genes coding for calpain-3, choline kinase β, AMP-dependent protein kinase, and desmin have been shown to cause LGMD2A, rostrocaudal MD, myotonic MD, and desminopathies, respectively (Table 1, Table 2) (4, 8–12). The mutations that underlie these skeletal muscle abnormalities also commonly affect cardiac muscle, resulting in a deterioration of heart function (1, 2, 12–15).
TABLE 1.
Lipid alterations reported in various inherited muscular dystrophies
| Muscular Dystrophy | Defective Gene/Protein | Lipid Alterations | Location of Initial Muscle Weakness | Reference |
| Duchenne MDMode of inheritance: XR | Dystrophin | Muscle biopsy: ↓ in Phospholipid/TG ratio and PI; ↑ TG, PC, SM, and cholesterol; no change in CL, PS; ↑ monounsaturated fatty acids and ↓ in C18:2 fatty acids. Mdx mice: ↑ Caveolin-3 expression, PC changes | In lower extremities with involvement of respiratory muscles | 100, 103–105, 129, 130 |
| Becker MDMode of inheritance: XR | Dystrophin | No gross abnormalities observed in muscle and adipose tissue; ↓ [carnitine] in muscle | Symmetric in body and lower extremities | 99, 101, 102 |
| Myotonic MDMode of inheritance: AD | DMPK | RBC: no change in phospholipid composition, fatty acid composition, altered fatty acids in plasmalogens | In feet, hands, lower legs and forearms | 3, 121, 131, 150 |
| DesminopathiesMode of inheritance: 80% AD / 20% AR | Desmin | Cytoplasmic caveolin-3 deposits | Distal leg muscles with early respiratory and cardiac muscle involvement | 101, 102, 111–113 |
| Limb-girdle myopathic dystrophies (LGMD) type 1Mode of inheritance: AD | Lamin A/C (LGMD 1B), caveolin-3 (LGMD 1C) | Decreased caveolin-3 in lipid rafts (LGMD 1C) | Overlap with DMD and BMD; involvement of shoulder and pelvic girdle muscles | 158 |
| Limb-Girdle myopathic dystrophies (LGMD) type 2Mode of inheritance: AR | Calpain 3 (LGMD 2A), Dysferlin (LGMD 2B), α-Sarcoglycan (LGMD-2D), γ -Sarcoglycan (LGMD-2C), β-Sarcoglycan (LGMD-2E), δ-Sarcoglycan (LGMD-2F) | No alteration in fatty acid and lipid composition, decrease in VLCAD in the mitochondrial fractions (LGMD 2A). BIO 14.6 hamster: Age-dependent changes in phospholipid composition; ↑ saturated and ↓ unsaturated fatty acids, ↓ PI and PE (LGMD 2D-E) | Overlap with DMD and BMD; involvement of shoulder and pelvic girdle muscles | 8, 154–157, 159 |
| Dunnigan-type familial partial lipodystrophy (FPLD)Mode of inheritance: AD | Lamin A/C | Inhibition of adipocyte differentiation leads to ↑ lipid accumulation, ↑ TG and ↓ lipoprotein levels. Mouse fibroblasts: ↑ lipid accumulation and TG synthesis Myocytes: ↑ fatty acid influx | Wasting of fat in the extremities and gluteal area | 166–168 |
| Rostrocaudal muscular dystrophy (rmd)aMode of inheritance: AR | Choline kinase β | ↓ in PC content; affects PC biosynthesis and distribution | Rostral to caudal gradient of severity | 10, 174–176 |
AD, autosomal dominant; AR, autosomal recessive; CL; cardiolipin; DMPK, myotonic dystrophy protein kinase gene; MD, muscular dystrophy; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; RBC, red blood cell; SM, sphingomyelin; TG, triglyceride; XR, X-linked recessive.
Only described in animal model. However, a congenital form has been recently described in humans (117).
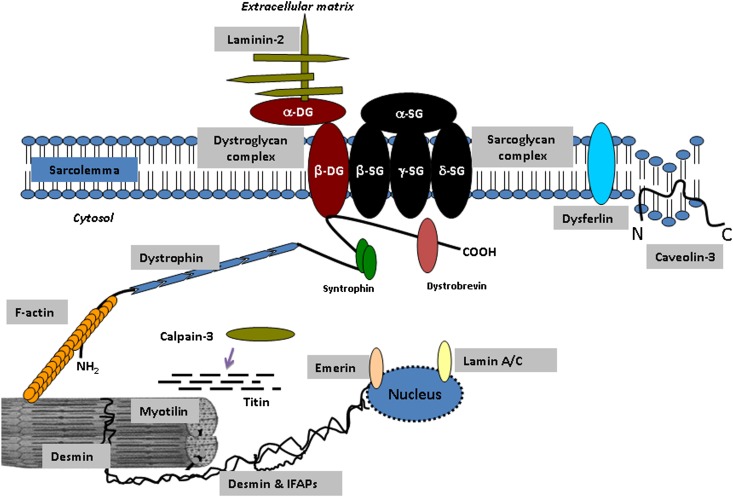
Fig. 1.
Representation of dystrophic-glycoprotein complex (DGC) showing dystroglycan and sarcoglycan as well as other major proteins involved in the development of various skeletal and cardiac muscle disorders. Purple arrow indicates calpain-3 interaction with titin. Refer to Table 1 for the specific skeletal and cardiac muscle disorders and the resulting lipid alterations. DG, dystroglycan; IFAP, intermediate filament accessory protein (desmoplakin, calponin, B-crystallin, and C-flip); SG, sarcoglycan.
TABLE 2.
Primary cardiac muscle disorders with inherited defects and presenting symptoms
| Muscular Dystrophy | Mode of Inheritance | Defective Gene/Protein | Lipid Alterations | Major Symptoms | Reference |
| Primary (systemic) carnitine deficiency | AR | OCTN2/SLC22A5 | ↓ intracellular and plasma carnitine; ↑ lipid accumulation in muscle | Hypertrophic cardiomyopathy, skeletal muscle weakness and hepatic encephalopathy | 181–188 |
| Secondary carnitine deficiency | AR | MCAD | ↑ carnitine esters; ↓ carnitine | Myopathy and hepatomegaly | 189–194 |
| Carnitine palmitoyltransferase deficiency | AR | CPT1 | Carnitine deficiencies | Cardiomyopathy | 196–197 |
| Carnitine palmitoyltransferase deficiency | AR | CPT2 | Carnitine deficiencies | Cardiomyopathy, encephalopathy and hepatomegaly | 198–203 |
| Very long-chain; medium-chain and short-chain acyl-CoA dehydrogenase deficiency | AR | VLCAD, EFTA/EFTB and SCAD | ↓ oxidation of long-chain fatty acid; ↑ butyrylcarnitine and ethylmalonic acid | Hypertrophic cardiomyopathy, rhabdomyolysis; hypotonia, hepatomegaly; hypertonia, myopathy | 204–216 |
| Rhabdomyolysis | AD | Mitochondrial trifunctional protein | ↓ oxidation of long-chain fatty acid | Progressive cardiomyopathy; skeletal myopathy | 212 |
| Familial hypertrophic cardiomyopathy (FHC) and inherited dilated cardiomyopathy (DCM) | FHC: AD; DCM: AD, AR, XR | Contractile proteins ActinCAV3 | ↓ Cardiolipin | Hypertrophic cardiomyopathy, idiopathic dilated cardiomyopathy | 217–232 |
| Barth syndrome | AR | Tafazzin, Xq28.12 | ↓ Cardiolipin | Hypertrophic cardiomyopathy with proximal and distal skeletal muscle weakness and fatigue | 247–252 |
| AMP activated protein kinase α2 deficiency | AD | AMPKα2 | ↓ Cardiolipin | Hypertrophic cardiomyopathy | 250, 264–266 |
AR, autosomal recessive; AD, autosomal dominant; XR, X-linked recessive.
Alterations in lipid metabolism (16) are commonly observed in skeletal and cardiac muscle disorders (Table 2); for example, changes in lipid storage are associated with hypertrophic or dilated cardiomyopathy (Table 3) (17–20). Because cellular integrity (21) is dependent on the interaction of various proteins with one another and with the membrane lipids, any disease process disrupting one or another aspect of these essential functions will have serious consequences. The purpose of this review is to present the current knowledge of fatty acid and lipid metabolism in myocytes and to document the pathogenesis of lipid abnormalities in inherited skeletal muscle disease (Table 1), cardiac muscle disease (Table 2), and lipid storage diseases (Table 3).
TABLE 3.
Primary cardiac muscle and lipid storage disorders with inherited defects and presenting symptoms
| Muscular Dystrophy | Mode of Inheritance | Defective Gene/Protein and Gene Location | Type of Lipids Involved | Age and Cause of Death | Major Symptoms | Reference |
| Fabry disease | XR | GLA, Xq22 | Glycosphingolipid | 58.2 years for males and 75.4 years for females; heart and renal failure | LV hypertrophy, valvular defects, AV conduction defects and arrhythmias | 272–274 |
| Gangliosidoses | AR | GLB1, 3p21.33 | Ganglioside | Child-adulthood; Cardiopulmonary failure | LV hypertrophy, Atria septal defects and valvular defects | 275, 276 |
| Mucolipidosis | AR | GNPTAB, 12q23.2 | Phospholipid-bound vacuole | First decade of life; pneumonia or heart failure | Valvular defects | 270, 277 |
| Gaucher disease | AR | GBA, 1q21 | Glucocerebroside | Child-adulthood; massive hepatosplenomegaly, cardiovascular, and cerebrovascular complications | Restrictive filling and reduced diastolic volume | 271, 279 |
| Neutral lipid storage disease with myopathy | AR | ATGL11p15.5 | TG | Variable, cardiomyopathy | Intimal thickening of coronary arteries and atheromatous lesions | 283, 285 |
| Neutral lipid storage disease with ichthyosis | AR | ABHD5, 3p21 | TG | Variable, hepatomegaly | Mild myopathy and hepatomegaly | 290 |
ABHD, abhydrolase domain-containing gene; AR, autosomal recessive; ATGL, adipose TG deposition lipase gene; GBA, glucosidase β acid; GLA, galactosidase α; GLB, lysosomal galactosidase β; GNPTAB, N-acetylglucosamine-1-phosphate transferase α- and β-subunits; TG, triglyceride; XR, X-linked recessive.
FATTY ACID UPTAKE AND INTRACELLULAR TRANSPORT
Cell types that undergo high levels of fatty acid metabolism are able to transport fatty acids at a higher rate than cell types with lower lipid metabolism (22, 23). For example, in skeletal or cardiac muscle, a specific and saturable uptake pathway for long-chain fatty acids exists (24, 25). A proposed mechanism for fatty acid uptake is an insulin- or muscle contraction-induced translocation of fatty acid transport proteins to the plasma membrane, resulting in a net uptake of circulating fatty acids (26). However, the precise mechanism of long-chain fatty acid uptake into myocytes is still rather uncertain and is split between two prevailing hypotheses: passive diffusion- and protein-specific-mediated transport (22, 26–31) (Fig. 2). It has been proposed that long-chain fatty acid transport at the plasma membrane occurs by dissociation of the fatty acid from plasma albumin, delivery of the free fatty acid to the membrane, its transfer across the membrane to the cytoplasm, and its intracellular metabolism (32). These steps of fatty acid transport and transmembrane movement may be carried out by a protein-independent transport mechanism, with no net expenditure of cellular energy (33–35). Indeed, unilammelar phosphatidylcholine (PC) membrane vesicle studies support this flip-flop mechanism for nonionized long-chain fatty acid transport (36), and the addition of free long-chain fatty acids to these vesicles resulted in a very rapid flip-flop of the fatty acids (36, 37).
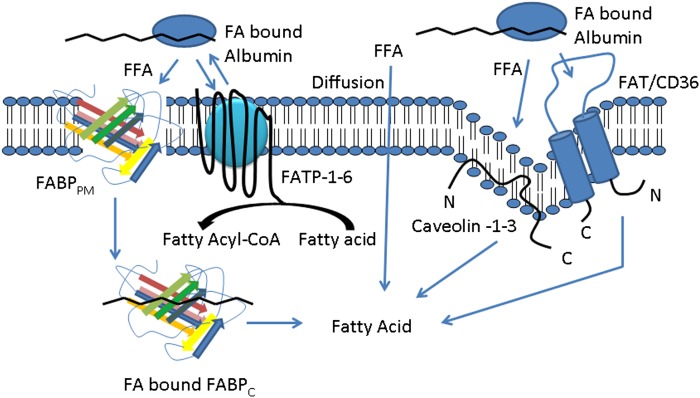
Fig. 2.
Proposed overview of the cellular uptake of free fatty acids. Free fatty acids enter cells either by diffusion or through interaction with one or more fatty acid transport proteins. CoA is added to the free fatty acid via acyl-CoA synthetase once the fatty acid enters into the cell in preparation for β-oxidation, TG synthesis, or phospholipid biosynthesis. FATP couples free fatty acid transport with acyl-CoA synthesis. Predicted protein structures are based on literature and previous representations. FABP curved lines represents α-helices; multicolored arrows represent β-pleated sheets. FABPPM/C, fatty acid binding protein plasma membrane/cytosolic; FATP, fatty acid transport protein; FAT/CD36, fatty acid translocase/CD36; FFA, free fatty acid.
Other studies indicate that transport of fatty acids is facilitated by several classes of specific transporters (Fig. 2) expressed on the cell membrane (22, 35, 38–41). These include fatty acid transport proteins 1-6 (FATP1-6), fatty acid translocase/CD36 (FAT/CD36), fatty acid binding proteins (FABP), plasma membrane fatty acid binding protein (FABPpm), cytosolic fatty acid binding proteins (FABPc) and caveolin (22, 35, 42–45). mRNA expression of these transporters is tissue specific and dependent on the metabolic fatty acid requirements of the cell/tissue (46). For example, FATP-6 is predominantly expressed in the heart (47), whereas FATP-1 is predominantly expressed in adipose tissue (44) but is also significantly expressed in brain tissue and skeletal and cardiac muscles (46–48). Adipose, muscle, and intestinal tissues highly express FAT/CD36, whereas the liver has low expression (49). Regulation of FAT/CD36 is mediated by long-chain fatty acids and insulin, which promote its translocation from intracellular depots to the plasma membrane (50–52). H-FABP (heart) is primarily expressed in the heart but is also expressed in the brain, intestine, and adipose tissues (53). High expression of one or more FABP is directly correlated with the specific tissue's high rate of fatty acid uptake and metabolism (54). In H-FABP null mice, isolated cardiac myocytes displayed decreased fatty acid uptake and metabolism compared with wild-type mice (55).
The three isoforms of the structural protein that constitute caveolae are caveolin-1, caveolin-2, and caveolin-3 (56). Each caveolin is a hairpin-like 22 kDa integral membrane protein that faces the cytosol (56). Caveolin-1 and caveolin-2 are coexpressed in various cell types, whereas caveolin-3 is muscle specific (57, 58). Caveolin-1 and caveolin-3 are capable of forming caveolae on their own; however, caveolin-2 can only form caveolae in the presence of caveolin-1 (57).
FATTY ACID OXIDATION, ACCUMULATION, AND REMODELING
When long-chain fatty acids enter cells, they are esterified to fatty acyl-CoA, transported to the mitochondria to undergo β-oxidation, incorporated into phospholipids, or stored as triacylglycerol (TG) (Fig. 3) (reviewed in Ref. 59). The carnitine-palmitoyl transferase (CPT) system facilitates the oxidation of long-chain fatty acids in the mitochondria (Fig. 4) (60, 61). This system consists of carnitine-palmitoyl transferase-1 (CPT1), carnitine-acylcarnitine translocase (CACT), and carnitine-palmitoyl transferase-2 (CPT2) (60). Because the inner mitochondrial membrane is impermeable to acyl-CoAs, the outer membrane enzyme CPT1 catalyzes the conversion of long-chain fatty acyl-CoAs to acylcarnitines, which then translocate across the inner mitochondrial membrane through the action of carnitine-acylcarnitine translocase (61–63). In the final step, acylcarnitines are converted back to their corresponding acyl-CoAs by CPT2, allowing for β-oxidation to proceed (61–63). The three isoforms of CPT1 are expressed in different tissues: CPT1A in liver, CPT1B in muscle, and CPT1C in brain (64–66). The heart is unique as both the liver and muscle forms are expressed (67). The entire pathway is regulated by malonyl-CoA, which provides a signal to the cell on the availability of lipids for energy needs (60).
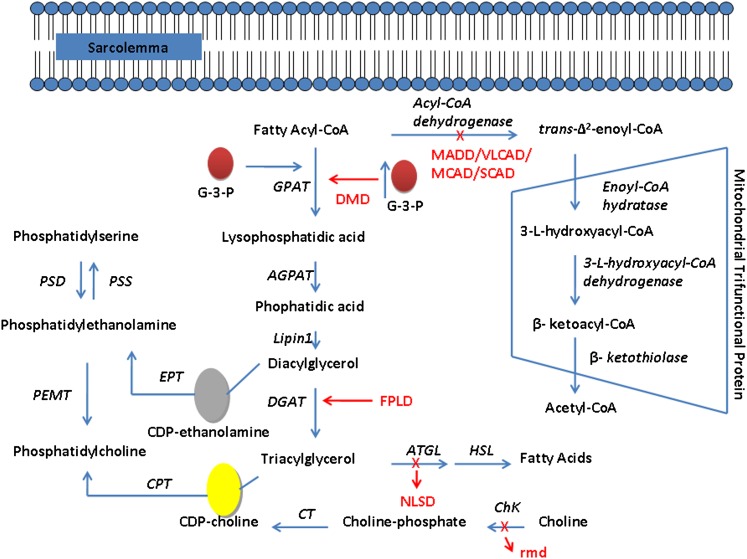
Fig. 3.
Fatty acid β-oxidation, TG synthesis, and phospholipid biosynthesis pathways. In the de novo biosynthesis of phospholipids and TG, esterified fatty acid is condensed with sn-glycerol-3-phosphate to form 1-acyl-sn-glycerol-3-phosphate catalyzed by glycerol-3-phosphate acyltransferase, followed by formation of phosphatic acid (PA) catalyzed by 1-acylglycerolphosphate acyltransferase. Duchenne muscular dystrophy (DMD) patients have reduced channeling of fatty acids toward β-oxidation, leading to reduced phospholipid ratio (Table 1). Increased TG accumulation is observed in familial partial lipodystrophy (FPLD) patients. DMD, FPLD, and rostrocaudal muscular dystrophy (rmd) are described in the section Lipid Abnormalities in Inherited Skeletal Muscle Diseases. Description of the skeletal and cardiac manifestations of multiple acyl-CoA dehydrogenase deficiency (MADD), very long chain acyl-CoA dehydrogenase (VLCAD), medium chain acyl-CoA (MCAD), and short-chain acyl-CoA dehydrogenase (SCAD) are described in the section Inherited Cardiac Muscle Diseases. Neutral lipid storage disease (NLSD) is described in the section Lipid Storage Disorders. A red “X” indicates a defect in the gene and the associated abnormality caused by the defect; a red arrow indicates the biosynthetic pathway affected by the abnormality. AGPAT, 1-acyl-sn-glycerol-3-phosphate acyltransferase; ChK, choline kinase; CPT, choline phosphotransferase; CT, cholinephosphate cytidylyltransferase; EPT, ethanolaminephosphate cytidylyltransferase; DGAT, 1,2-diacylglycerol-acyltransferase; G-3-P, glycerol-3-phosphate; GPAT, glycerol-3-phosphate acyltransferase; Lipin-1, phophatidate phosphohydrolase; PEMT, phosphatidylethanolamine N-methyltransferase; PSD, phosphatidylserine decarboxylase; PSS, phosphatidylserine synthase.
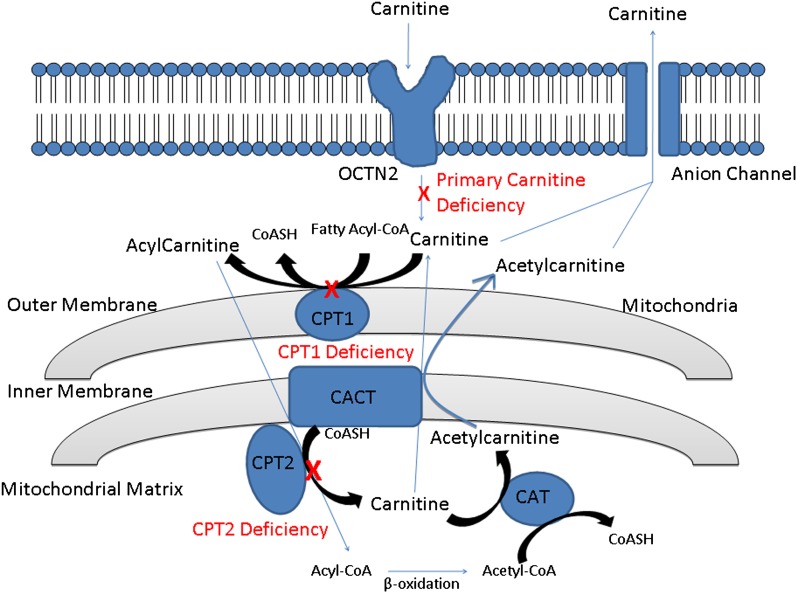
Fig. 4.
Overview of carnitine transport across the plasma and mitochondrial membranes. Carnitine is transported across the plasma membrane via the OCTN2/SLC22A5 transporter. CPT1 catalyzes the conversion of long-chain fatty acyl-CoAs to acylcarnitines, which then translocate across the inner mitochondrial membrane through the action of CACT. Acylcarnitines are converted back to their corresponding acyl-CoAs by CPT2, allowing for β-oxidation to proceed. The released carnitine can leave the mitochondria via CACT and may exit the cell via volume-sensitive anion channels. Carnitine acetyltransferase (CAT) converts short- and medium-chain acyl-CoAs into acetylcarnitines and then leaves the mitochondrial matix via CACT. A mutation in the OCTN2 transporter results in decreased accumulation of intracellualr carnitine. Carnitine and carnitine palmitoyl transferase deficiencies are described in the section Inherited Cardiac Muscle Diseases. A red “X” indicates a defect in the gene and the associated abnormality caused by this defect. CACT, carnitine-acylcarnitine translocase; CPT1, carnitine-palmitoyl transferase-1; CPT2, carnitine-palmitoyl transferase-2.
Regulation of CPT1 alone cannot explain the observed changes in mitochondrial fatty acid import when there is an increased cellular energy demand (68, 69). FAT/CD36 was shown to colocalize with CPT1 on the outer mitochondrial membrane and may play a role in fatty acid import (62). The exact mechanism of action of FAT/CD36 on the mitochondrial membrane is not well established, but it may be acting as an acceptor of long-chain fatty acids, presenting them for activation by long-chain acyl-CoA synthetase, which in turn are converted to acylcarnitines by CPT1 (62). Although FAT/CD36 knockout mice exhibited normal fatty acid oxidation (70), several studies implicate FAT/CD36 as a crucial mitochondrial fatty acid importer, especially during exercise or muscle contraction (22, 71, 72). FATP-1 has been shown to be localized in the mitochondria of skeletal muscle cells and thus may play a role in fatty acid import (63). Overexpression of both FATP-1 and CPT1 in mitochondrial fractions resulted in an additive effect on fatty acid oxidation, suggesting that FATP-1 cooperates with CPT1 in fatty acid import to the mitochondria (63). FATP-1 and CPT1 physically interact in the mitochondria, as both proteins coimmunoprecipitate in mitochondrial fractions of L6E9 myotubes and rat skeletal muscle in vivo (63). The mechanistic process defining this interaction and cooperation between FATP-1 and CPT1 remains unknown.
Fig. 3 depicts a brief summary of the de novo biosynthesis of phospholipids and TG. There are two isoforms of glycerol-3-phosphate acyltransferase (GPAT) and several isoforms of 1-acylglycerolphosphate acyltransferase (AGPAT); these are reviewed elsewhere (73). In both skeletal and cardiac myocytes, over 90% of GPAT activity is attributed to GPAT1 (74). Similarly, AGPAT1 is the most highly expressed AGPAT isoform in skeletal and cardiac muscles (75). Phosphatidic acid (PA) lies at a branch point where it may be converted by PA phosphohydrolase (lipin-1) to form 1,2-diacyl-sn-glycerol for the formation of TG (76), a reaction catalyzed by 1,2-diacyl-sn-glycerol acyltransferase. There are two isoforms of 1,2-diacyl-sn-glycerol acyltransferase (77). 1,2-diacyl-sn-glycerol acyltransferase-1 is widely expressed in all tissues, with high expression levels in adipose tissue, skeletal muscle, and intestine, whereas 1,2-diacyl-sn-glycerol acyltransferase-2 is solely expressed in the liver and adipose tissue (78, 79). 1,2-diacyl-sn-glycerol acyltransferase-1 exhibits similar activity in both skeletal muscle and adipose tissue in rats (80).
The 1,2-diacyl-sn-glycerol formed from PA may be utilized in the de novo biosynthesis of PC or phosphatidylethanolamine (PE) (81, 82). In addition, PA can be converted by CDP-DG synthetase to CDP-1,2-diacyl-sn-glycerol and used in the synthesis of phosphatidylinositol (PI) and the polyglycerophospholipids, phospatidylglycerol and cardiolipin (CL) (83). Newly formed phospholipids may be remodeled by a phospholipase A2-mediated deacylation, followed by a lysophospholipid acyltransferase-mediated reacylation (84). This remodeling is required to allow for the incorporation of unsaturated fatty acids into the sn-2 position of the phospholipid and thus allows for the differing enzymatic functions determined by the different sn-1 and sn-2 acyl chain substrates (84). This remodeling is essential for cell viability. For example, in mutant E. coli strains capable of synthesizing only saturated fatty acids, a termination in DNA replication and cell division occurred as a result of the unsaturated fatty acids being diluted from membrane phospholipids (84). Similarly, in rats fed a high trans-acyl fatty acid diet, liver and heart cell membrane assembly became compromised and resulted in cell death, demonstrating the importance of acyl-chain availability to the synthesizing enzymes (84).
Newly formed as well as stored TG may be hydrolyzed to release fatty acid for energy production via the hierarchical action of adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) (85). The accumulation of fatty acids in the heart has been documented in obese individuals, type II diabetic patients, and in those who suffer from metabolic syndrome (86). This accumulation of fatty acids may arise by increased hydrolysis of endogenous muscle TG stores (Fig. 3), by uptake from exogenous sources, such as nonesterified fatty acid bound to albumin in the blood (Fig. 2), or by TG in lipoproteins. The net result is an increased cardiac lipid content that is associated with a lipotoxic cardiomyopathy that contributes to cardiac dysfunction (86). The mechanism for the cardiac dysfunction may be related to a fatty acid-mediated increase in expression of lipogenic transcription factors, such as the sterol response element binding protein-1c (SREBP-1c) and peroxisome proliferator-activated receptor γ (PPARγ), both of which promote lipogenesis during episodes of excess nutrition (87). Finally, imbalance between fatty acid uptake and β-oxidation has the potential to contribute to insulin resistance in muscle (88).
LIPID ABNORMALITIES IN INHERITED SKELETAL MUSCLE DISEASES
Muscular dystrophies
Duchenne muscular dystrophy (DMD; OMIM no. 301200), is the most common type of inherited skeletal muscle disorder, affecting 1 in 3,500 young males (89–91) (Table 1). Progressive muscle weakness is evident at 3–5 years of age, followed by the inability to walk by 10–12 years of age, and culminating in death in early adulthood, with life expectancy rarely beyond 20–30 years of age (92, 93). Genetic studies have indicated that mutations of DMD lead to complete deficiency of the full-length 3685 amino acid dystrophin protein (3, 94, 95). Dystroglycan and sarcoglycans are the major proteins of DGC, and they link the cortical cytoskeleton to the extracellular matrix (2, 96–98) (Fig. 1). Therefore, the lack of dystrophin causes structural disorganization of the sarcolemma, necrosis of myofibrils, fibrosis, inflammation, and vascular dysfunction in DMD patients (4).
Becker muscular dystrophy (BMD; OMIM no. 300376) is a milder form of DMD with a decrease in dystrophin content as opposed to the complete absence in DMD (Table 1). The incidence of BMD is 1 in 18,450 male births. Most patients with BMD develop musculoskeletal symptoms at a slower rate compared with DMD patients and remain ambulatory up to the third or fourth decade of life (99). As shown in Table 1, no gross lipid abnormalities in BMD patients’ muscles have been identified; however, reduced carnitine concentrations have been observed. The percentage of sphingomyelin (SM) was unaltered in the gastrocnemius muscle of BMD patients compared with controls (100). In addition, no change was observed in the fatty acid composition of muscle phospholipids in patients with BMD (101). Finally, no alterations in the activities of selected enzymes involved in phospholipid metabolism, including CDP-choline:diglyceride-P-cholinetransferase (Fig. 3), CDP-choline:ceramide-P-cholinetransferase, and those involved in the degradation of PC, were observed in the biopsies of quadriceps femoris muscle obtained from BMD patients (102).
Lipid mapping analysis of human striated muscle samples from male control and DMD-affected children revealed accumulation of intact PC, cholesterol (CH), SM, TG, and an abundance of monounsaturated fatty acid species within the most-damaged areas of the dystrophic muscles (103) (Table 1, Fig. 3). Conversely, a decrease in phosphatiylinositol (PI) was observed in the most-damaged regions. An increase in [3H]glycerol incorporation into PC, phosphatidylserine (PS), PI, and TG was observed in skeletal muscles of DMD patients (104). Additionally, the phospholipid-to-TG ratio was decreased in DMD patients, indicating a perturbation in the lipid bilayer (104). An increase in the C18:1 fatty acid (oleate) in PC of human dystrophic muscle was associated with a decrease in C18:2 fatty acid (linoleate) (7, 100). In biopsies of rectus abdominus and gastrocnemius muscle in 3- to 5-year-old males diagnosed with DMD, an increase in SM and cholesterol content was coupled with a decrease in PC and choline plasmalogen (105). However, PS, monophosphoinositide, CL, and total phospholipid content were not altered in biopsies from DMD patients (105). These results suggest interference in the muscle maturation of PC in DMD patients at different stages of disease development.
In dystrophic mouse muscle, changes in whole-tissue phospholipid composition (with less PC but more PE, SM, and cholesterol) was directly linked with changes in the muscle microsomal fraction in contrast to myofibrillar and mitochondrial fractions (106). Similar alterations in phospholipid and cholesterol content have been observed in the dystrophic skeletal muscles of the dystrophin-deficient mdx mouse (a model for hereditary muscular dystrophy), indicating a common pathological mechanism. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and tandem mass spectrometry analysis of the phospholipid composition in skeletal muscle of mdx mice indicated that an inversion of intensity ratio of m/z 758.6 (hexadecanoyl, [cis-9,12]octadecadienoyl-glycero-3-phosphocholine, PC (C16:0/C18:2) over m/z 760.6 (hexadecanoyl, [cis-9]octadecadienoyl-glycero-3-phosphocholine, PC (C16:0/C18:1) in mdx mice structured and destructured areas, suggests that PC alteration is an early event in the muscle degeneration-regeneration process (107). Similar changes have been observed in dystrophic muscle tissue sections isolated from 12- to 14-year-old children (103). In destructured areas of muscle, the less unsaturated PC species (C16:0/C18:1) were more abundant than the unsaturated PC species (C16:0/C18:2) compared with the control regions, which indicates a reduction in membrane flexibility in DMD muscle (108).
Muscle degeneration (reduced muscle weight and tension output) occurring in mdx mice is thought to be a result, in part, of increased calcium entry through sarcolemmal cationic channels. A recent study showed that iPLA2 localized in the vicinity of the sarcolemma hydrolyzed PC and that the resultant lysophosphatidylcholine increased calcium entry through sarcolemmal channels (109). These data implicate iPLA2 as a possible mediator of the alteration in PC composition in DMD. Introduction of an exogenous nitric oxide donor into mdx mice led to restoration of membrane lipid composition to levels very similar to those of wild-type mice (110). Nitric oxide synthase null mice exhibited a 4.1-fold increased expression of peroxiredoxin-6 (111), a protein that exhibits a second function as an iPLA2 (112). These studies suggest that inhibition of iPLA2 could serve as a potential therapeutic target in dystrophic muscle. Nonetheless, the alterations in PC fatty acid composition need further investigation in the developmental and later stages of the disease.
As previously stated, production of energy in muscle from long-chain fatty acid oxidation is dependent upon the presence of carnitine (113). Carnitine levels in muscle biopsies from patients with DMD, BMD, limb-girdle dystrophy, and polymyositis/dermatomyositis are low/reduced compared with histologically normal muscle (114–116). The low values of carnitine seen in these dystrophic patients may be a nonspecific effect, possibly related to the severe muscle damage. Proton NMR analysis in biopsied skeletal muscle from DMD patients demonstrated, in addition to reduced levels of choline-containing lipids indicative of membrane abnormalities, a decrease in acetate-containing lipids that is indicative of reduced transport of fatty acids into mitochondria due to decreased carnitine concentrations (115). Indeed, in approximately 50% of DMD patients examined, a reduction in myocardial fatty acid metabolism was indicated by reduced uptake and metabolism of a radioiodinated branched fatty acid (118). As depicted in Fig. 2, caveolae may participate in uptake of fatty acids. Increased caveolin-3, an important component of the lipid rafts in cardiac, skeletal, and smooth muscle and a modulator of DGC function (Fig. 1) (119, 120), has been observed in skeletal muscle of DMD patients (121). The increase in caveolin-3 expression may be a compensatory mechanism to increase fatty acid uptake into cells in DMD. Similar changes in caveolin-3 expression in skeletal muscle in mdx mice have been observed (122, 123). Transgenic mice overexpressing caveolin-3 revealed a similar phenotype to human DMD, exhibiting downregulation of dystrophin, necrosis, and regeneration of skeletal muscle fibers with central nuceli (123). Overexpression of caveolin-1 resulted in no discernable phenotype, suggesting the DMD phenotype is caveolin-3 specific (123).
To investigate cardiac involvement in DMD, studies have utilized the UM-X7.1 and BIO 14.6 strains of hamster, well-known animal models of DMD with congestive cardiomyopathy (124, 125). In UM-X7.1 animals, total phospholipid content was significantly decreased in cardiac muscle without any alteration in skeletal muscle phospholipids. However, PC content and the cholesterol-to-phospholipid ratio were increased in both cardiac and skeletal muscle of UM-X7.1 myopathic hamsters (126). These animals exhibited lower activity and expression of fatty acid synthase and stearoyl-CoA desaturase activities in the liver, as well as lowered plasma insulin levels compared with age-matched control animals fed the same diet (127). The insulin deficiency may in part lead to the observed altered muscle fatty acid metabolism. BIO 14.6 animals also exhibited an increase in cholesterol-to-phospholipid ratio in cardiac tissue similar to that observed in UM-X7.1 animals (128). This increase in cholesterol-to-phospholipid ratio seems to be a common feature of DMD lesions (105, 129, 130). It has been proposed that the decrease in phospholipids may be due to the release of phospholipids during the degeneration of muscle as well as the increased activity of phospholipase A in DMD tissue. During muscle regeneration, increased cholesterol synthesis is required, which may be responsible for the elevated cholesterol levels in DMD patients (130).
Myotonic muscular dystrophy (dystrophia myotonica, Steinert's disease; OMIM no. 160900) is a systemic genetic disorder inherited as an autosomal-dominant trait with an expansion mutation of an unstable trinucleotide repeat (CTG) at the 3′ UTR of the myotonic dystrophy protein kinase (DMPK) gene (11, 131) (Table 1). This variable repeat results in a 50% reduction of the DMPK protein in skeletal muscle and is associated with the inability to relax muscles, cardiac arrhythmias, and insulin resistance (132). It is the most common form of adult onset muscular dystrophy, with a prevalence of 1 in 8,000 and an average life expectancy of 53.2 years in male patients (108, 133). It has been suggested that a primary enzyme abnormality in 3β-hydroxysterol Δ24-reductase, which converts desmosterol to cholesterol, may be one of the underlying mechanisms involved in the development of the disease. An early study observed a significant amount of sterol accumulation in skeletal muscle of myotonic patients (134). This, however, seems unlikely as defects in this enzyme result in desmosterolosis (OMIM no. 602398), a very serious autosomal-recessive disorder of cholesterol biosynthesis (135). Subsequent studies utilizing more sensitive techniques revealed no alteration in desmosterol levels in experimental animal models (136), myotonic muscular dystrophy patients (137), or cultured skin fibroblasts (138). In addition, no alteration in [3H]glycerol incorporation into PC, PS, PI, or TG were observed in skeletal muscles of myotonic muscular dystrophy patients compared with control subjects (103). Fatty acid analysis of plasmalogens revealed reduced unsaturation levels of PE in myotonic muscular dystrophy erythrocyte membranes compared with controls (139). Dmpk−/− mice exhibited decreased insulin sensitivity in cardiac and skeletal muscle, resulting in increased circulating glucose and lipid levels (140). Recently, it was suggested that the CUG-binding protein 1 may play a major role in myotonic muscular dystrophy pathogenesis, as overexpression of CUG-binding protein 1 lead to severe muscle wasting in transgenic mice (141).
Desminopathies
Desminopathies are a heterogeneous group of inherited disorders caused by mutations in the desmin gene (Table 1), as well as by mutations in α-B-crystallin and synemin (142, 143). As reviewed in Ref. 12, 80% of patients with desminopathies exhibit autosomal-dominant inheritance, whereas the remainder displays autosomal-recessive inheritance. Desmin is the major intermediate filament protein surrounding the Z-disc of myofibrils that interact with other cytoskeletal proteins, including intermediate filament-associated proteins, to maintain the intracellular integrity and mediate force transmission and mechanochemical signaling (141) (Fig. 1).
Wang et al. (142) provided the first functional link between the human p.Arg173_Glu179del desmin (D7-des) mutation and desminopathy in a transgenic mice model in which they observed an accumulation of chimeric intracellular aggregates containing desmin and other cytoskeleton proteins in isolated cardiomyocytes. A mutation in desmin at p.L345P has been observed in mice, which resulted in abnormal Ca2+ handling by the mitochondria, leading to a significant defect in mitochondrial function associated with left ventricular hypertrophy (144). Transfection of mutant desmin into a human epithelial cell line that does not express type III intermediate filaments, SW13(vim-) cells, as well as in mouse myoblasts that express muscle-specific endogenous type III intermediate filaments, C2C12 cells, demonstrated the importance of desmin in filament assembly and revealed that any mutation in desmin will lead to a significant alteration in the preexisting endogenous intrafilamenous network, resulting in myopathy (145, 146). A number of mitochondrial protein differences have been identified between control and desmin null mice (147), including an increase in β-oxidation, acetate, and ketone body metabolizing enzymes in desmin null mice reminiscent of changes that occur in the diabetic heart (147, 148).
As intermediate filaments provide the mechanochemical links between the contractile apparatus and mitochondria, nucleus, and the sarcolemma and coordinate efficient protein and lipid trafficking (149), a defect in desmin may affect all of these processes. Deposits of caveolin-3 have been observed in muscle cytoplasm of patients with primary desminopathy (150). Caveolin-3 is normally present in sarcolemmal lipid rafts; however, its presence in the muscle cytoplasm of these patients may be a result of reduced caveolar trafficking (151). Although lipid rafts are known to be enriched in SM and gangiosides (152), no information regarding the phospholipid composition is available in the literature in the case of primary desminopathies.
Limb-girdle myopathic dystrophies
Limb-girdle myopathic dystrophies (LGMD), which constitute a heterogeneous group of muscular dystrophies characterized by progressive muscle weakness, are responsible for up to one third of all muscular dystrophy cases (153) (Table 1). LGMD has been split into two different types based upon mode of inheritance. Typically, type 1 LGMD has autosomal-dominant inheritance and onset occurs in early adulthood; type 2 LGMD has autosomal-recessive inheritance and onset occurs during childhood. Due to the presence of a diverse number of mutations representing the autosomal-dominant and autosomal-recessive forms of LGMDs (9), direct measurements of different phospholipids and fatty acid composition in both experimental animal models and patients is an immense challenge. Further work is needed to understand how mutations in these diverse genes result in membrane lipid alterations. Because of the overlap between the development of dystrophinopathies and sarcoglycanopathies (LGMD-2D-E), the BIO 14.6 hamster with a specific mutation in the δ-sarcoglycan gene has been used as a model of sarcinopathy (154, 155). Isolated heart membranes of BIO 14.6 hamsters of different age groups exhibited an age-dependent effect on phospholipid composition (156). Although no difference in lipid composition and microdynamics was observed between 4-week-old BIO 14.6 and control F1b hamsters, a significant decrease in 20:0, 20:2, and 32:4 fatty acids and an increase in saturated fatty acids 18:0 and 22:0 were observed at 18 weeks of age. In addition, at 31 weeks of age, a decrease in the content of unsaturated fatty acids 20:4 and 22:6 was observed compared with controls (156). A significant decrease in membrane phospholipids, such as PI and PE, was observed in the later stages of life in BIO 14.6 hamsters compared with controls. In addition, an increase in the PI-specific phospholipase C activity in hearts of these cardiomyopathic hamsters was observed (157).
Interestingly, a specific deletion of a tripeptide [ΔTFT (63–65)] in the caveolin-3 gene (within the region that affects the oligomerization and scaffolding of caveolin-3) in primary myoblasts of neonatal C57Bl/6 mice mimicked the autosomal-dominant LGMD1C (OMIM no. 607801) form of the disease (158). This mutation in the caveolin-3 gene has also been described previously in humans (152). The tripeptide deletion results in caveolin-3 being maintained in the Golgi complex, and the reduced localization of caveolin-3 to the sarcolemmal lipid rafts may result in the perinuclear accumulation of the proto-oncogene Src, which may lead to increased apoptosis and muscle degeneration in LGMD1C patients (158).
A significant decrease in very long-chain acyl-CoA dehydrogenase (VLCAD) activity in the mitochondrial fractions of skeletal muscle of calpain-3 knockout mice, which represent an autosomal-recessive form of LGMD2A (OMIM no. 253600), has been observed and points to a secondary defect in fatty acid oxidation in this type of LGMD (8). Because VLCAD plays an important role in maintaining phospholipid homeostasis and reesterification in the membrane (159), the above study (8) provides only indirect evidence for phospholipid alterations in LGMD2A.
Dunnigan-type familial partial lipodystrophy
Novel missense mutations in lamin A and C proteins, which are encoded by the LMNA gene, have been identified in individuals with Dunnigan-type familial partial lipodystrophy (FPLD; OMIM no. 151660) (160–163) (Table 1). These missense mutations, R28W and R62G, affect the two different domains of the lamin A/C gene, the N-terminal head domain and the α-helical rod domain (163). Lamin A and C mRNA are primarily expressed in heart, skeletal muscle, and the placenta, as well as in a variety of other tissues, including lung, kidney, and pancreas (164). LMNA−/− mice exhibit dilated cardiomyopathy due to the disruption of the desmin cytoskeleton network in cardiomyocytes, resulting in altered nuclear function, such as reduced PPARγ expression and decreased SREBP1 import (165). Typical FPLD has autosomal-dominant laminopathy, which is characterized by wasting of fat in the extremities and gluteal area starting around puberty and accompanied by excess fat deposition in the face, neck, back, and labia majora (166, 167). In addition, females have hirsutism, menstrual abnormalities, and polycystic ovarian disease (166–168).
The biochemical hallmark in affected individuals is development of metabolic syndrome with diabetes mellitus; dyslipidemia associated with hypertriglyceridemia and depressed high density lipoprotein levels; hypertension; and early coronary artery disease (166, 168, 169). FPLD individuals may not develop the typical biochemical profile described above; these nondiabetic carriers of FPLD develop metabolic changes that include high levels of insulin, nonesterified free fatty acids, C-reactive protein, and mean TG levels with lower HDL cholesterol, adiponectin, and leptin (170). Hepatic steatosis with elevated levels of liver enzymes are included as part of the FLPD clinical phenotype (171).
FPLD patients do not present with muscle disorders until after puberty, when these patients display hypertrophy of skeletal muscle fibers (20). Recent studies have demonstrated an excessive skeletal muscle fatty acid β-oxidation both in vitro and in vivo due to a defect in lamin A/C (20). However, this fatty acid oxidation is largely incomplete due to a reduction in glucose oxidation, which results in an imbalance in skeletal muscle energy substrate metabolism (20). Studies in mouse 3T3-L1 preadipocytes, which differentiate in vitro to adipocytes, have demonstrated that the overexpression of mutant lamin A inhibited lipid accumulation, TG synthesis, and overexpression of adipogenic markers. These changes were associated with inhibition of overexpression of PPARγ2 and GLUT-4. On the other hand, mouse embryonic fibroblasts lacking lamin A accumulated intracellular lipids rapidly and exhibited increased de novo TG synthesis with elevated basal phosphorylation of the insulin signaling molecule AKT1. This study concluded that lamin A acts as an inhibitor of adipocyte differentiation and that mutations in lamin A leads to lipid accumulation as observed in FPLD (172) (Fig. 3). Increased fatty acid flux from adipose tissue to skeletal and cardiac myocytes and impaired lipid oxidation may contribute to the hypertriglyceridemia seen in these patients (20). Although treatment of FPLD patients with low-dose recombinant methionyl human leptin has shown some promise in decreasing the levels of TG and total cholesterol with improvement in fasting glucose and insulin sensitivity in a small group of FPLD patients (173), further studies targeting a larger number of patients are needed to establish the therapeutic role of leptin in dyslipidemia and adipocyte differentiation in FPLD.
Rostrocaudal muscular dystrophy
A spontaneous mouse mutation which leads to progressive muscular dystrophy with rostral to caudal gradient of severity starting from day 6 to 2–3 months of age was identified (10) (Table 1). It was observed that degenerated fibers were in the hindlimb along with variation in fiber size with progressive muscle wasting and extreme fatty acid infiltration in the hindlimb compared with the forelimbs of rostrocaudal muscular dystrophy (rmd)/rmd mice. An intragenic 1.6 kb deletion within the Chkb gene is responsible for the lipid defect in these mutant mice. Chk enzyme exists in two isoforms (α and β), and Chkb catalyses the phosphorylation of choline to phosphocholine for the biosynthesis of PC (Fig. 3). Both phosphocholine and PC play important roles in cell growth, signaling, and proliferation, and they are essential for cell survival (174–176). A decrease in the content of PC in rmd/rmd mice skeletal muscle or alterations in the lipid composition, represented by changes in the PC/PE ratio, may lead to muscular dystrophy in this mouse model (10). This spontaneous recessive mutation in Chkb in rmd/rmd mice shares several phenotypic characteristics with the dysferlin null mouse, which is a model for limb-girdle dystrophy type 2B myopathy (177). Dysferlin is a sarcolemma-associated protein that is important in skeletal membrane repair using a Ca2+-dependent resealing mechanism (178). However, the membrane disruptions are milder than dysferlinopathies (179), as determined by the lowered increase in serum creatine kinase levels, lack of significant uptake of Evans blue dye by skeletal muscle, and unaltered levels of proteins, such as dystrophin, α-dystroglycan, β-dystrogylcan, emerin, dysferlin, α-sarcoglycan, and β-sarcoglycan in the DGC (Fig. 1). These minor membrane changes suggest membrane fusion defects as an underlying mechanism of this type of muscular dystrophy (10). Although a shift in mitochondrial fission and fusion toward the formation of megamitochondria was observed in the hindlimbs of rmd/rmd mice, no direct link between apoptosis and muscle degeneration was observed.
Wu et al. (180) observed a larger percentage of total muscle PC in mitochondria of Chkb−/− mice. They suggested that the redistribution of PC in affected hindlimb muscle is due to PC channeling into mitochondria instead of into the sarcolemma; thus, PC is diverted away from sarcolemmal repair. In addition, the authors indicated that elimination of Chkb significantly causes impairment of PC biosynthesis in hindlimb muscle that is coupled with increased PC catabolism. This conclusion was supported by the observation that administration of CDP-choline to Chkb−/− mice decreased the plasma level of creatine kinase and increased the PC content of skeletal muscle. However, the occurrence of muscular dystrophy due to a defect in Chkb needs to be further investigated in humans, especially those with idiopathic muscular dystrophy. The therapeutic value of CDP-choline administration in some forms of muscular dystrophy with impaired sarcolemmal integrity needs to be established (180). A human congenital muscular dystrophy with choline kinase β deficiency was recently identified in 15 patients (117).
INHERITED CARDIAC MUSCLE DISEASES
Carnitine deficiency
One of the major inherited cardiomyopathies is associated with carnitine, which plays an important role in the transport of long-chain fatty acids through the inner mitochondrial membrane (113). Primary (systemic) carnitine deficiency (OMIM no. 212140) is an inherited metabolic disorder that gives rise to cardiomyopathy and lipid accumulation in the muscle (181) (Table 2). Primary carnitine deficiency is an autosomal-recessive disorder due to the lack of the functional organic cationic/carnitine transporter 2 (OCTN2/SLC22A5) (Fig. 4). Mutations in OCTN2 affect carnitine transport by impairing the maturation of the transporters to the plasma membrane (182). This results in low carnitine plasma levels due to decreased intracellular carnitine accumulation and increased carnitine excreted in the urine. Homozygous deletion of 17081C of the SLC22A5 gene results in a frameshift mutation at R282D, which leads to a premature stop codon in the OCTN2 carnitine transporter, as observed in Hungarian Roma (Gypsy) infants who present with cardiomyopathy and decreased plasma carnitine levels (183). The affected children present with feeding difficulties, upper respiratory tract infections, hepatomegaly with abnormal liver function tests, hypoglycemia, and mildly elevated creatine kinase (170). A residual carnitine transport activity has been observed in some OCTN2 mutations, and in such situations, life-long replacement of L-carnitine and decreased intake of dietary fat are considered the major treatment options for primary carnitine deficiency (182–184). In untreated patients, the deficiency of carnitine can progress to hypertrophic cardiomyopathy and weakness of skeletal muscles (185). Acute episodes of hepatic encephalopathy are the major reasons of sudden infant death (186). Hypertrophic cardiomyopathy is common in older patients with myofibrillar deorganization and mitochondrial abnormalities (187); however, in adolescence, OCTN2 mutation can present with ventricular fibrillation without overt cardiomyopathy (188).
Secondary carnitine deficiency can occur due to fatty acid oxidation defects, including medium chain acyl-CoA dehydrogenase deficiency (189) (Fig. 3, Table 2). This defect in fatty acid oxidation occurs in skeletal and cardiac myocytes and results in myopathy and hepatomegaly (189). Excretion of carnitine esters due to the accumulation of nonmetabolized acyl-CoA intermediates and followed by subsequent transesterification of the fatty acyl moiety to carnitine is a major cause of the carnitine deficiency (190, 191). In addition, homocysteineuria due to methylenetetrahydrofolate reductase deficiency, 3-hydroxy-3-methylglutaryl-CoA deficiency, and cytochome C oxidase deficiency are associated with low carnitine levels. Acquired disorders, such as alcoholic cirrhosis, renal failure as a result of chronic hemodialysis, and epilepsy treated with valproic acid, also have been associated with secondary carnitine deficiency (192–194). However, the exact mechanism for carnitine deficiency and the possible benefits of carnitine replacement under these conditions need further study (183, 189, 190, 195).
Carnitine palmitoyltransferase deficiency
In addition to carnitine itself, deficiencies of carnitine palmitoyltranferases 1 (CPT1; OMIM no. 255120) and 2 (CPT2; OMIM no. 255110) and carnitine-acylcarnitine translocase (CACT; OMIM no. 212138) have been described (Fig. 4, Table 2). CPT1 deficiency is a rare disease, and thus far only CPT1A (liver-type CPT1) gene mutations have been identified (196, 197). CPT1-B enzyme function is crucial for normal heart function, and its loss may be incompatible with life (197). Interestingly, only a few cases of liver-type CPT1 deficiency without muscle involvement have been reported (197). On the other hand, in CPT2 deficiency, at least 15 unique autosomal recessively inherited mutations (198, 199) have been shown to be responsible for systemic neonatal, infantile/childhood, and adult myopathic forms of this phenotypically heterogeneous disease (196, 199).
Neonatal CPT2 deficiency presents with cardiomyopathy, encephalopathy, and hepatomegaly. Death usually occurs during the first few months of life (200). The infantile or childhood type occurs after the first month of life and is triggered by fasting. It has a rigorous presentation with attacks of hypoketotic hypoglycemia with cardiomegaly, hepatomegaly, and sudden death before the age of 1 year (201, 202). The adult (classic or benign form) myopathic type is characterized by recurrent episodes of rhabdomyolysis triggered by physical stresses, with the first episode occurring between 1 and 12 years of age or sometimes during adolescence (203). Avoidance of precipitating factors, such as prolonged fasting or exercise for more than 30 min, and introduction of a high-carbohydrate/low-fat diet, as well as regular monitoring of renal function during the attacks of myoglobinuria, have been suggested as the main treatment (196). Due to the heterogeneous presentation of the disease and large number of mutations in the CPT2 gene, systematic large clinical trials are needed to check the validity of these treatment options.
Other inherited fatty acid oxidation disorders resulting in cardiac myopathy
Other frequently noted inherited cardiac myopathies occur due to very long-chain acyl-CoA dehydrogenase deficiency (OMIM no. 201475) (204), multiple acyl-CoA dehydrogenase deficiency (OMIM no. 231680), mitochondrial myopathies, and abnormalities of myocardial proteins (205, 206) (Fig. 3, Table 2). Deficiency of very long chain acyl-CoA dehydrogenase, which catalyses the first step in β-oxidation of long-chain fatty acids, has been divided into three phenotypes with infantile, childhood, and adolescent/adult onset (207–209). The infantile onset form has cardiac defects with hypertrophic cardiomyopathy, the childhood onset form has repeated episodes of hypoglycemia, and the adolescent/adult onset has intermittent rhabdomyolysis (207–209). The mutational spectrum of VLCAD deficiency is very wide, with over 80 mutations identified in the VLCAD gene and none of them being prevalent (210, 211). To find a possible genotype-phenotype relationship, these mutations have been grouped into two categories: null mutations and missense mutations (210). Null mutations that result in no residual VLCAD enzyme activity have generally been associated with the infantile phenotype, whereas missense mutations that result in a residual enzyme activity have been associated with the childhood and early adulthood phenotypes (210, 211). However, recently an overlap between these phenotypes was observed as a novel compound-heterozygote missense mutation of exons 9 and 10 in VLCAD that has been associated with the perinatal-onset form and repeated episodes of rhabdomyolysis during infancy and early childhood.
In addition, rhabdomyolysis has been shown with a rare deficiency of mitochondrial trifunctional protein with a mutation at R235W in the α-subunit, which was observed in cultured skin fibroblasts of the affected patient (212) (Table 2).
Multiple acyl-CoA dehydrogenase deficiency (MADD; OMIM no. 231680), also known as glutaric acidemia type II, is associated with deficiency of several mitochondrial dehydrogenases that utilize flavin adenine dinucleotide as a cofactor and includes the aforementioned acyl-CoA dehydrogenases involved in mitochondrial fatty acid oxidation and enzymes that degrade glutaric acid (glutaryl-CoA dehydrogenase); isovaleric acid; and a precursor to glycine, sarcosine (Fig. 3). MADD is caused by mutations in the electron transfer flavoprotein (encoded by two genes, ETFA and ETFB) or in the electron transfer flavoprotein dehydrogenase gene (ETFDH) (213). ETF is a dimeric mitochondrial matrix protein composed of α- and β-subunits, and a mutation in either subunit or in ETFDH will result in MADD (213). Null mutations in either ETFB or ETFDH appear to be associated with the neonatal onset (type 1) of the disease (213, 214). Its mode of inheritance follows an autosomal-recessive pattern. Two different newborn presentations are seen: one with congenital abnormalities; including dysmorphic facial features and dysplastic, cystic kidneys; and one without these congenital abnormalities. Both have a broad clinical phentotype displaying hypotonia, hepatomegaly, severe nonketotic hypoglycemia, and metabolic acidosis. If newborns survive the first weeks, they frequently die during the following month from hypertrophic cardiomyopathy, displaying characteristic fatty infiltration of the heart, kidneys, and liver. The third presentation is late onset, which is a milder form with variable symptoms, including lipid storage myopathy (215).
Another disorder of mitochondrial fatty acid oxidation is due to a deficiency of short-chain acyl-CoA dehydrogenase (OMIM no. 201470), which has autosomal-recessive inheritance and leads to the accumulation of butyrylcarnitine and ethylmalonic acid in blood and urine (216) (Fig. 3, Table 2). The clinical phenotype of short-chain acyl-CoA dehydrogenase deficiency includes developmental delay, feeding difficulties, metabolic acidosis, seizures, myopathy, and hypertonia (216). However, no consistent relationship between a definable clinical phenotype and mutations in the short-chain acyl-CoA dehydrogenase gene has been established, and in recent years, it has been suggested that this disorder may be a nondisorder (216).
Familial hypertrophic cardiomyopathy and inherited dilated cardiomyopathy
The prevalence of familial hypertrophic cardiomyopathy (FHC; OMIM no. 115197) is approximately 1 in 500 with autosomal-dominant transmission and a heterogeneous clinical presentation (217–220). More than 100 mutations have been documented, with most of these occurring in the genes encoding contractile proteins (217–220) (Table 2). A separate set of mutations in the actin gene at R312H and E361G have been associated with inherited dilated cardiomyopathy (DCM; OMIM no. 115200) (221) (Table 2). The prevalence of DCM is 1 in 2,500 with autosomal-dominant, autosomal-recessive, and X-linked modes of inheritance (222). About one half of the patients with DCM have idiopathic dilated cardiomyopathy (223). Patients with FHC undergo transition from hypertrophy to heart failure. However, the alterations in membrane phospholipids during this transition are not completely understood in human FHC and DCM.
On the basis of similarities between findings in humans with respect to the mutations in different functional domains of the actin gene, which can lead to either FHC or idiopathic dilated cardiomyopathy (223), and findings in the mouse model that exhibit features of both diseases (224), it has been suggested that the underlying pathophysiological mechanism in both of these diseases appears to be the same (223). The spontaneously hypertensive heart failure (SHHF) rat model presents all of the signs of inherited DCM and has shown some promise in this area. Sparagna et al. (225) have observed that progressive changes in cardiac CL molecular acyl composition occur in the mitochondria during the pathogenesis of heart failure in these rats. These changes are characterized by a marked loss of the (C18:2)4-CL species and an increase in minor species containing highly unsaturated acyl chains (e.g., 20:4 and 22:6), with a concurrent loss of CL mass (225). Decreases in (C18:2)4-CL preceded the development of heart failure in the SHHF rats by several months and correlated with a loss of mitochondrial cytochrome oxidase activity (226). Recently, we showed that these alterations in CL content are linked with differential alterations in various enzymes in CL de novo biosynthesis (CTP:PA cytidylyltransferase, phosphatidylglycerolphosphate synthase, and CL synthase) as well as CL remodeling enzymes (MLCL AT and TAZ) (227).
Studies have shown that a mutation in human caveolin-3 is present in patients with familial FHC (228). The missense T63S mutation in the CAV3 gene resulted in decreased cell surface expression of caveolin-3 (228). Recently, another missense T78M mutation in the CAV3 gene has been identified in a patient suffering from both DCM and LGMD-1C (229). Interestingly, these patients do not display skeletal muscle anomalies that are common in LGMD with underlying caveolin-3 mutations (228, 230). This difference may be explained by the severity of the functional changes in caveolin-3 due to the mutations. The LGMD-associated mutation exerted a more severe dysfunction as the mutant caveolin-3 underwent ubiquination and proteasomal degradation, further reducing the cell-surface expression of caveolin-3 (228). The caveolin-3 mutation affects cardiac muscles more than skeletal muscles. Skeletal muscle contracts on demand, whereas the continuous contraction and relaxation of cardiac muscle requires more caveolin-3 to deliver a larger amount of fatty acid to mitochondria for sustained energy production (228). Moreover, caveolin-3 may be regulated by different mechanisms in skeletal and cardiac muscles (229). Because hypertrophy occurs as a secondary response to other conditions, such as valvular disease, hypertension, and ischemic heart disease (231, 232), delineation of inherited FMC and DCM changes from other noninherited conditions has become quite a challenge.
Alterations in cardiolipin homeostasis
Barth syndrome.
Barth syndrome (BTHS; OMIM no. 302060) is a rare X-linked recessive disease caused by mutations in the tafazzin gene (TAZ), which presents at birth or early in life (233, 234) (Table 2). Over 100 mutations have been described, including frameshifts, nonsense, splice-site, and missense mutations. To date, no correlation between genotype and disease severity in BTHS has been observed (235–238). In some patients, symptoms seem to partially resolve for a defined period of time (236), known as the “honeymoon period,” which occurs between the ages of 5 and 12 years (239). The incidence of the disease is currently unknown but may be as high as 1 in 300,000. BTHS is a multisystem disorder characterized by hypertrophic cardiomyopathy associated with proximal and distal skeletal muscle weakness and fatigue, growth retardation, cognitive defects, cyclic neutropenia, mild hypocholesterolemia, and an elevated urinary excretion of 3-methyglutaconic acid (234, 240).
BTHS is the first known inborn error that affects CL, a polyglycerophospholipid essential for the function of the mitochondrial respiratory chain. CL is synthesized and remodeled in the inner mitochondrial membrane in a highly organized manner, as previously described (241–243) (Fig. 5). On the basis of the sequence homology of the TAZ gene with acyltransferases, Neuwald (244) suggested that BTHS may occur due to a defect in phospholipid metabolism. Subsequent studies by several laboratories have supported reduced levels of tetralinoleoyl-CL [(C18:2)4-CL] as the principal biochemical abnormality in BTHS patients. In cultured skin fibroblasts of BTHS patients, Vreken et al. (245) demonstrated a 75% reduction in the CL pool size with a reduced incorporation of [1-14C]linoleic acid (C18:2n-6) into CL and phosphatidylglycerol, despite no alteration in the biosynthetic rate of these polyglycerophospholipids. In addition, incorporation of [1-14C]palmitate (C16:0), stearate (C18:0), oleate (C18:1n-9), and arachidonate (C20:4n-6) into CL did not differ between control and BTHS fibroblasts (245). Valianpour et al. (246) observed a significant decrease in (C18:2)4-CL in cultured skin fibroblasts of BTHS patients using normal phase high-performance liquid chromatography-electrospray mass spectrometry. A similar decrease in (C18:2)4-CL content was also observed in platelets (247) and in biopsies of skeletal muscle, as well as in left and right ventricles of hearts obtained from BTHS patients (248, 249). In lymphoblasts, a 50% reduction and in fibroblasts, a 75% reduction of (C18:2)4-CL content was observed in this disease (249). In another study, the fatty acid composition of all major mitochondrial phospholipids, including PC, PE, and CL, were altered in lymphoblasts from BTHS patients; however, these changes were most prominent in CL (250). In particular, palmitoleic (16:1) and linoleic (18:2) acids were replaced by palmitic (16:0) and stearic (18:0) acids, and there was a shift from oleic acid (18:1ω9) to vaccenic acid (18:1ω7). Due to these alterations, vaccenic acid became the dominant acyl group in CL in lymphoblasts isolated from BTHS patients. In addition, analysis of the PC molecular species showed an increase in palmitoleic acid (16:0–16:1-PC) with a decrease in oleic acid (16:0–18:1-PC) in BTHS lymphoblasts. Only minor alterations in PE with a small increase in palmitoleic acid were observed.
Fig. 5.
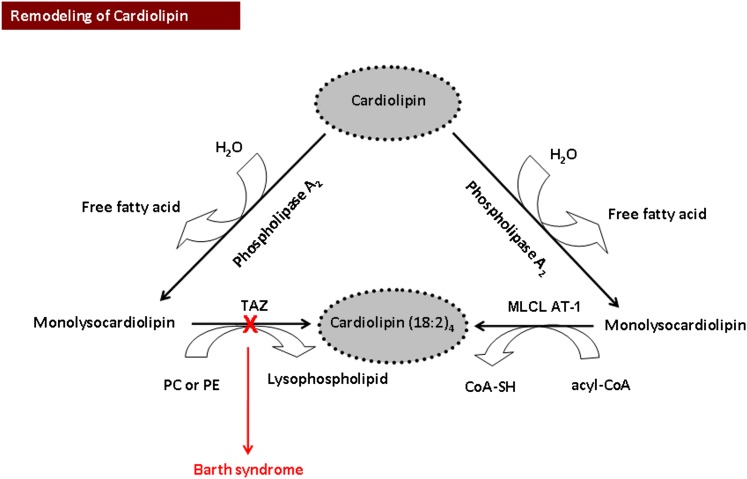
Overview of the pathway of mitochondrial cardiolipin remodeling. Cardiolipin is hydrolyzed by phospholipase A2 to produce monolysocardiolipin and then resynthesized to tetralinoleoyl-cardiolipin [Cardiolipin-(18:2)4] by monolysocardiolipin acyltransferase (MLCL AT) or by the cardiolipin transacylase tafazzin (TAZ). A red “X” indicates a mutation in the TAZ gene resulting in Barth syndrome.
To maintain its unique unsaturated fatty acyl composition, remodeling of CL is essential. Phospholipase A2 and monolysocardiolipin acyltransferase (MLCL AT-1) play important roles in this process (251–253) (Fig. 5). In addition, it is well documented that the human gene TAZ encodes a transacylase, tafazzin, which is involved in the remodeling of CL (254, 255). In this context, an increased accumulation of intermediates of remodeling, including MLCLs, were observed in heart, skeletal muscle, lymphocytes, and cultured lymphoblasts of BTHS patients (256, 257). Knockdown of taz in zebrafish and mouse models of BTHS resulted in a cardiac phenotype that resembled human BTHS heart failure (258, 259). In the zebrafish model, injection of wild-type taz mRNA restored normal cardiac function (258). Drosophila melanogaster taz mutants exhibited a BTHS-related phenotype, with the triad of abnormal cardiolipin, pathologic mitochondria, and motor weakness; this phenotype was suppressed by inactivation of the gene encoding type VIA calcium-independent phospholipase A2 (260). We demonstrated that expression of MLCL AT-1 in human BTHS lymphoblasts resulted in increased MLCL AT protein, [1-14C]linoleate incorporation into CL, CL mass, and mitochondrial function, measured in terms of succinate dehydrogenase (mitochondrial complex II) activity compared with mock-transfected BTHS lymphoblasts (253). HPLC-MS has confirmed the validity of MLCL and CL analysis as a diagnostic tool for BTHS (261). Nonetheless, it has been shown that the clinical presentation and severity of BTHS is not specifically linked to the CL deficiency (249). Thus, caution must be exercised when correlating alterations in CL with the progression of the disease.
Spencer et al. (262) examined the serum lipid profile in a cohort of BTHS males compared with their unaffected siblings. The cohort was small, and only 24% showed a reduction in total serum cholesterol below 2.84 mmol/l (110 mg/dl). Further analysis indicated that 56% of the cohort exhibited a reduced LDL profile of 1.55 mmol/l (<60 mg/dl). We investigated cholesterol biosynthesis in lymphoblasts from a normal individual and an age-matched Barth syndrome patient, ΔTAZ1 (263). Although total cholesterol levels were similar under standard culture conditions, ΔTAZ1’s lymphoblasts had a diminished capacity to respond to increased demand for cholesterol biosynthesis due to a reduced ability to increase hydroxymethylglutaryl-CoA reductase activity. The impact of hypocholesterolemia in BTHS will need to be investigated in the major cholesterol-producing cell type, the hepatocyte, and in more patients, particularly in response to times of increased cholesterol demand, especially developmental periods outside the “honeymoon period.” We hypothesize that these are the periods when defects may become apparent and potentially most detrimental to the patients.
AMP-activated protein kinase alpha2 deficiency.
AMPK, a ubiquitous serine/threonine protein kinase, is activated by various pathological and physiological processes (264). It has a catalytic α-subunit with two isoforms, α1 and α2. AMP-activated protein kinase α2 (AMPKα2) is expressed primarily in the heart and contributes 70–80% of the total AMPK catalytic activity (265). Athea et al. (266) have shown that AMPKα2 deficiency affects CL homeostasis and mitochondrial function (Table 2). In AMPKα2−/− mice, a 25% decrease in mitochondrial complex I activity was observed. In addition, a 13% decrease in mitochondrial linoleic acid (18:2), the main fatty acid of cardiac CL, was observed in these animals. The authors suggested that a decrease in CL content may be associated with mRNA downregulation of the rate-limiting enzyme of CL biosynthesis, CTP:PA cytidylyltransferase-2, and the CL remodeling enzyme, acyl-CoA:lysocardiolipin acyl transferase 1. However, the mRNA content of taz remained unaltered. It was interesting to note that the ultrastructural changes in the mitochondria of AMPKα2−/− mice resembled to some extent the abnormalities in mitochondria observed in lymphoblasts of BTHS patients (250). However, further studies in human subjects are needed to define a direct relationship between AMPKα2 and BTHS.
LIPID STORAGE DISORDERS
Cardiac disorders associated with abnormal lipid storage
Lipid storage disorders are often associated with hypertrophic or dilated cardiomyopathy (17, 267) as shown in Table 3. These include Fabry disease (268), gangliosidoses (269), mucolipidosis (270), mucopolysaccharidosis, and neuronal ceroid lipofuscinosis. Cardiomyopathy of the three functional types include congestive, as in Fabry disease; hypertrophic, as in glycogen storage disease, type II; and restrictive, as in Gaucher disease (271). Valvular and myocardial involvement occur predominantly in the glycogen storage diseases, types II–IV, mucolipidoses, sphingolipidoses, and neuronal ceroid lipofuscinosis (267).
Fabry disease.
Fabry disease (OMIM no. 301500) is a rare (1 in 50,000) X-linked disorder caused by a deficiency of lysosomal α-galactosidase A, leading to accumulation of globotriaosylceramide and related glycosphingolipids in various tissues including the heart (268) (Table 3). In 60% of males, the associated cardiac abnormalities include left ventricular hypertrophy, which may mimic hypertrophic cardiomyopathy, valvular dysfunction, various atrioventricular conduction system defects, and arrhythmias. These cardiac abnormalities are the most common cause of death in affected individuals (272). Preclinical diagnosis is key for treatment of the disease, and the use of human recombinant α-galactosidase A is highly effective (273, 274).
Gangliosidoses.
GM1 gangliosidosis (OMIM no. 230500) is an autosomal recessively inherited lysosomal storage disorder characterized by accumulation of GM1 gangliosides, oligosaccharides, and the mucopolysaccharide keratan sulfate due to a deficiency of lysosomal hydrolase β-galactosidase (GLB1) (269, 275) (Table 3). The human GLB1 gene produces two alternatively spliced transcripts that encode the lysosomal enzyme β-galactosidase (GLB1) and the elastin binding protein (269, 275). GLB1 mutations may affect both proteins or only GLB1. Elastin binding protein is essential for early elastogenesis and the formation of elastic fibers in blood vessel walls by functioning as a molecular chaperone to tropoelastin, which covalently cross-links and forms elastin (276). When elastin binding protein mutation is involved, it leads to the development of a range of infantile cardiomyopathic phenotypes, including left ventricular hypertrophy, atrial septa defects, and mitral, tricuspid, and arotic valvular insufficiencies linked to impaired elastogenesis (269).
Mucolipidosis.
Mucolipidosis II α/β (OMIM no. 252500) and III α/β (OMIM no. 252600) are autosomal-recessive diseases caused by a deficiency of the α- and/or β-subunit of the enzyme N-acetylglucosamine-1-phosphotransferase, which is encoded by the GNPTAB gene (270) (Table 3). The deficiency in N-acetylglucosamine-1-phosphotransferase results in an inability to internalize enzymes into lysosomes. In mucolipidosis II (I-cell disease), accumulation of membrane-bound vacuoles in mesenchymal (foam) cells occurs in myocardial fibers (277). In children with this fatal disorder, the accumulation of foam cells in the myocardium is associated with thickened and retracted aortic, mitral, and tricuspid valves that may lead to aortic regurgitation and valvular collapse (278).
Gaucher disease.
Gaucher disease (OMIM no. 230800), the most common of the lysosomal storage diseases (1 in 40,000 live births), is an autosomal-recessive disorder defined by mutations in the glucosidase β acid gene, which codes for β-glucosidase, an enzyme involved in the hydrolysis of glucosylceramide (271, 279) (Table 3). The result is an accumulation of glucocerebrosides in the lysosomes of reticuloendothelial cells. Infiltration of the heart by nongranulomatous masses of histiocytes results in a cardiomyopathy characterized by a restrictive filling and reduced diastolic volume of either or both ventricles with normal or near-normal systolic function and wall thickness (280, 281). Enzyme-replacement therapy is now the standard treatment for patients with type 1 (nonneuronopathic) Gaucher disease (282).
Neutral lipid storage disorders
Neutral lipid storage disease (triglyceride deposit cardiomyopathy).
Neutral lipid storage disease (NLSD; OMIM no. 610717) is an autosomal-recessive disorder characterized by accumulation of neutral lipids in multiple organs (reviewed in Ref. 283) (Table 3). Mutations in adipose triglyceride lipase (ATGL/PNPLA2) and comparative gene identification-58 (CGI-58/ABHD5) result in NLSD (Fig. 3). Although mutations in either the ATGL or the CGI-58 gene result in TG accumulation, the resulting disease severities are not identical. Mutations in ATGL result in a more severe myopathy (NLSDM) than do mutations in CGI-58. In addition, patients with CGI-58 mutations exhibit ichthyosis (NLSDI). These observations indicate an ATGL-independent function of CGI-58. ATGL specifically hydrolyzes the first fatty acid from TG, and CGI-58/ABHD5 stimulates ATGL activity by an unknown mechanism.
A point mutation in exon 7 of the ATGL/PNPLA2 gene, which encodes an essential intracellular TG lipase, has been shown to be associated with TG deposit cardiomyovasculopathy (284) (Table 3). This mutation was identical to that observed in neutral lipid storage disease with myopathy (NLSDM) (285). It was noted that individuals with NLSDM were not obese, in spite of the severe defect in TG degradation in fibroblasts and the marked TG storage in liver, muscles, and other visceral cells (285). However, in the explanted heart of a 41-year-old patient with TG deposit cardiomyovasculopathy, the TG content compared with cholesterol content was significantly higher than that in control subjects (284). Levels of plasma lipids, carnitine, and TG were all normal. Diffuse intimal thickening of the coronary arteries and fibroatheromatous lesions were also observed.
In a recent study in ATGL knockout mice (ATGL−/−), alterations in TG metabolism due to the deletion of ATGL severely reduced lipolysis in adipose tissue and thereby reduced the availability of free fatty acid in the plasma (286). This in turn enhanced the carbohydrate oxidation during fasting and resulted in depletion of muscle and liver glycogen stores and reduced blood glucose. The exercise-induced increase in plasma free fatty acid and glycerol was decreased due to ATGL deletion, indicating impairment in exercise-induced lipolysis in these mice. ATGL deficiency has also been shown to alter insulin sensitivity by affecting intramyocellular lipids as well as serum factors involved in the insulin signaling pathway that are distinct from different insulin target tissues. The skeletal muscles of ATGL−/− mice primarily contribute to enhanced insulin sensitivity in vivo despite TG accumulation, and both local (changes in intracellular lipid phenotype) and systemic (circulating adipokines and RBP4) factors contribute to tissue-specific effects of total ATGL deficiency on insulin action (287).
Chanarin-Dorfman syndrome.
Chanarin-Dorfman syndrome (CDS; OMIM no. 275630) is an autosomal-recessive disease due to mutations in the ABHD5 gene (also known as CGI-58) (Table 3) (288). It is defined as a NLSD with congenital ichthyosis. It is a multisystem disorder with mild myopathy, hepatomegaly, intestinal involvement, various ophthalmologic symptoms, frequent deafness, mild mental retardation, and growth retardation (288–292). Muscle involvement occurs in about 60% of cases (288) and is characterized by slowly progressive weakness of proximal limb muscles sparing axial muscles, raised muscle creatine kinase enzymes, and electromyographic evidence of myopathy (293, 294). Due to the differential diagnosis of CDS, peripheral blood smears are used to detect the lipid vacuoles in neutrophils to diagnose patients with ischthyosiform erythroma (295).
Some individuals with NLSD present with atypical CDS-like features with myopathy but without ichthyosis and without mutations in ABHD5. Thus, it remains questionable whether ichthyosis occurs in CDS but not in NLSD with myopathy. Although both NLSD with myopathy and CDS have similar systemic storage of TG due to mutations in ABHD5 and PNPLA2, the lipid accumulation in the epidermis is lower in NLSD with myopathy than in CDS (295). It is suggested that CGI-58 colocalizes with ATGL to the surface of cytoplasmic lipid droplets, and this could have an additional metabolic function required for normal skin physiology in NLSD with myopathy patients (285). Additional cases and further genetic and functional studies are needed to better characterize the phenotype/genotype correlations and the pathogenic mechanism of this disorder (294).
CONCLUSIONS
A significant number of lipid composition and metabolic alterations have been observed in skeletal muscle disorders, such as DMD, BMD, myotonic muscular dystrophies, desminopathies, LGMDS, rmd, and FPLD. In addition, lipid alterations are important components of inherited cardiac muscle diseases, including inborn errors of fatty acid metabolism, Barth syndrome, FHC, and DCM, as well as lipid storage disorders. However, a large gap still exists between the molecular pathology of these muscular dystrophies and downstream biomechanical events regulating lipid metabolism during the progression of disease.
In DMD, cardiac defects cannot be diagnosed in the early stages of the disease, as there is no clinical evidence of cardiac disease, whereas in the later stages, cardiac insufficiency with pulmonary infection is the most frequent cause of mortality in these patients (205, 235). Dilated cardiomyopathy seems to be a common feature in patients with BMD (296); however, the severity and onset of cardiac symptoms are not in proportion to the development of skeletal myopathy (296, 297). Although all three major forms of myotonic muscular dystrophies (congenital, classic, and minimal) have common symptoms, such as progressive muscle weakness and stiffness with difficulty in relaxation after voluntary contractions, a heterogeneous group of defects, including insulin resistance, dyslipidemia, hormone dysfunction, hypogonadism, alopecia, reduced alertness, erectile dysfunction, and presenile cataract, have been observed in myotonic muscular dystrophy patients at different stages of disease development (298, 299). Depending on the rate of progression of the disease, first-degree heart block associated with cardiac dysfunction and conduction defects has been considered as a frequent cause of sudden death in myotonic muscular dystrophy patients (300). In addition, LGMDs predominantly affect proximal muscles around the scapular and the pelvic girdles. Some LGMDs are severe, some are benign, and others exhibit a broad spectrum of severity; the appearance of symptoms can occur anytime from childhood to adulthood (97, 153).
Variables such as age, diet, coexisting illness, including ischemic heart disease and hypertension (in case of cardiomyopathies), as well as an undetermined pattern of inheritance make it much more difficult to decipher the primary and secondary causes of these skeletal and cardiac myopathies. The intricate relationship between the muscle membrane and subcellular organelles, such as sarcoplasmic reticulum, myofibrils, nucleus, and mitochondria, under these pathological conditions needs further investigation. Additional identification of genes and mutations will aid genetic counseling of patients and are important parameters to improve the management of these diseases. It is important to know that distribution of lipids on the extracellular and intracellular membrane may be crucial in heralding changes during various pathological conditions. It can be argued that changes in lipid composition may be associated with alterations in different enzymes involved in these processes or that lipid changes can lead to the underlying pathological phenomenon.
With the advancement of technology, better measurements of various lipid species (lipidomics) in isolated cells and purified membranes will provide more insight on disease progression. Methods, including lipid mapping, imaging mass spectrometry, and in particular, time-of flight secondary ion mass spectrometry imaging, have the capability of analyzing a number of lipid anomalies in a variety of biological samples (301). For instance, time-of flight secondary ion mass spectrometry imaging has been used in the study of striated muscle samples from DMD-affected children. Differences in fatty acid, phospholipid, diacylglycerol, and TG distribution in healthy and dystrophic muscle tissue sections have been identified (103, 302). The major advantage with this technique is its ability to resemble physiological conditions, as it allows the identification and localization of lipids directly without disturbance to biological tissues. This is in contrast to previous techniques used for lipid identification, such as thin-layer chromatography and high-performance liquid chromatography, in which solvent extraction protocols had the potential to modify lipids (303). For example, in Fabry disease studies, traditional diagnostic techniques are insufficient and often result in false negatives. The use of matrix-assisted laser desorption ionization time-of-flight mass spectrometry allowed detection of differences in glycosphingolipid composition in patients presenting with clinical signs of Fabry disease (304). As these emerging techniques become more mainstream, the ability to detect, identify, and characterize lipid changes/differences in various skeletal and cardiac muscle disorders will enhance our understanding of the important role these biomarkers play in human disease.
Footnotes
Abbreviations:
- ACTC
- α-actin gene
- AMPKα2
- AMP-activated protein kinase alpha 2
- ATGL
- adipose triglyceride deposition lipase gene
- BMD
- Becker muscular dystrophy
- BTHS
- Barth syndrome
- CDS
- Chanarin-Dorfman syndrome
- Chkb
- choline kinase β
- CL
- cardiolipin
- CPT
- carnitine palmitoyltranferase
- DCM
- dilated cardiomyopathy
- DGC
- dystrophin-glycoprotein complex
- DMD
- Duchenne muscular dystrophy
- FHC
- familial hypertrophic cardiomyopathy
- FPLD
- Dunnigan-type familial partial lipodystrophy
- GLB1
- lysosomal hydrolase β-galactosidase
- LGMD
- limb-girdle muscular dystrophy
- LMNA
- lamin A/C
- MADD
- multiple acyl-CoA dehydrogenase deficiency
- MLCL AT
- monolysocardiolipin acyltransferase
- NLSD
- neutral lipid storage disorder
- OCTN2/SLC22A5
- organic cationic/carnitine transporter 2
- PA
- phosphatidic acid
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PI
- phosphatidylinositol
- iPLA2
- calcium-independent phospholipase A2
- PPAR
- peroxisome proliferator-activated receptor
- PS
- phosphatidylserine
- rmd
- rostrocaudal muscular dystrophy
- SHHF
- spontaneously hypertensive heart failure
- SM
- sphingomyelin
- TAZ
- tafazzin
- TG
- triglyceride
- VLCAD
- very long chain acyl-CoA dehydrogenase
This work was supported by grants from the Heart and Stroke Foundation of Manitoba, the Canadian Institutes of Health Research (CIHR), and the Barth Syndrome Foundation (Canada and USA) to G.M.H.; and by a grant from the Winnipeg Rh Institute Foundation to T.Z. R.W.M. was the recipient of a Manitoba Health Research Council Studentship. H. K. S-C was a post-doctoral fellow of the CIHR-IMPACT Strategic Training Program and Heart and Stroke Foundation of Canada. G.M.H. is a Canada research chair in Molecular Cardiolipin Metabolism and a Manitoba Institute of Child Health scientist.
REFERENCES
- 1.Emery A. E. 2002. The muscular dystrophies. Lancet. 359: 687–695. [DOI] [PubMed] [Google Scholar]
- 2.Cohn R. D., Campbell K. P. 2000. Molecular basis of muscular dystrophies. Muscle Nerve. 23: 1456–1471. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman E. P., Kunkel L. M. 1989. Dystrophin abnormalities in Duchenne/Becker muscular dystrophy. Neuron. 2: 1019–1029. [DOI] [PubMed] [Google Scholar]
- 4.Blake D. J., Weir A., Newey S. E., Davies K. E. 2002. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev. 82: 291–329. [DOI] [PubMed] [Google Scholar]
- 5.Kohlschutter A., Wiesmann U. N., Herschkowitz N. N., Ferber E. 1976. Phospholipid composition of cultivated skin fibroblasts in Duchenne's muscular dystrophy. Clin. Chim. Acta. 70: 463–465. [DOI] [PubMed] [Google Scholar]
- 6.Plishker G. A., Appel S. H. 1980. Red blood cell alterations in muscular dystrophy: the role of lipids. Muscle Nerve. 3: 70–81. [DOI] [PubMed] [Google Scholar]
- 7.Kunze D., Reichmann G., Egger E., Olthoff D., Dohler K. 1975. Fatty acid pattern of lipids in normal and dystrophic human muscle. Eur. J. Clin. Invest. 5: 471–475. [DOI] [PubMed] [Google Scholar]
- 8.Kramerova I., Kudryashova E., Wu B., Germain S., Vandenborne K., Romain N., Haller R. G., Verity M. A., Spencer M. J. 2009. Mitochondrial abnormalities, energy deficit and oxidative stress are features of calpain 3 deficiency in skeletal muscle. Hum. Mol. Genet. 18: 3194–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laval S. H., Bushby K. M. 2004. Limb-girdle muscular dystrophies–from genetics to molecular pathology. Neuropathol. Appl. Neurobiol. 30: 91–105. [DOI] [PubMed] [Google Scholar]
- 10.Sher R. B., Aoyama C., Huebsch K. A., Ji S., Kerner J., Yang Y., Frankel W. N., Hoppel C. L., Wood P. A., Vance D. E., et al. 2006. A rostrocaudal muscular dystrophy caused by a defect in choline kinase beta, the first enzyme in phosphatidylcholine biosynthesis. J. Biol. Chem. 281: 4938–4948. [DOI] [PubMed] [Google Scholar]
- 11.Brook J. D., McCurrach M. E., Harley H. G., Buckler A. J., Church D., Aburatani H., Hunter K., Stanton V. P., Thirion J. P., Hudson T. 1992. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 68: 799–808. [DOI] [PubMed] [Google Scholar]
- 12.Goldfarb L. G., Dalakas M. C. 2009. Tragedy in a heartbeat: malfunctioning desmin causes skeletal and cardiac muscle disease. J. Clin. Invest. 119: 1806–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakefield S. E., Dimberg E. L., Moore S. A., Tseng B. S. 2009. Dystrophinopathy presenting with arrhythmia in an asymptomatic 34-year-old man: a case report. J. Med. Case Reports. 3: 8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokota R., Shirotani M., Kouchi I., Hirai T., Uemori N., Ohta Y., Mitsui Y., Hattori R. 2004. Subclinical Becker's muscular dystrophy presenting with severe heart failure. Intern. Med. 43: 204–208. [DOI] [PubMed] [Google Scholar]
- 15.Finsterer J., Bittner R. E., Grimm M. 1999. Cardiac involvement in Becker's muscular dystrophy, necessitating heart transplantation, 6 years before apparent skeletal muscle involvement. Neuromuscul. Disord. 9: 598–600. [DOI] [PubMed] [Google Scholar]
- 16.Di Mauro S., Trevisan C., Hays A. 1980. Disorders of lipid metabolism in muscle. Muscle Nerve. 3: 369–388. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert E. F. 1987. The effects of metabolic diseases on the cardiovascular system. Am. J. Cardiovasc. Pathol. 1: 189–213. [PubMed] [Google Scholar]
- 18.Chen J., Hong D., Wang Z., Yuan Y. 2010. A novel PNPLA2 mutation causes neutral lipid storage disease with myopathy (NLSDM) presenting muscular dystrophic features with lipid storage and rimmed vacuoles. Clin. Neuropathol. 29: 351–356. [DOI] [PubMed] [Google Scholar]
- 19.Akman H. O., Davidzon G., Tanji K., Macdermott E. J., Larsen L., Davidson M. M., Haller R. G., Szczepaniak L. S., Lehman T. J., Hirano M., et al. 2010. Neutral lipid storage disease with subclinical myopathy due to a retrotransposal insertion in the PNPLA2 gene. Neuromuscul. Disord. 20: 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boschmann M., Engeli S., Moro C., Luedtke A., Adams F., Gorzelniak K., Rahn G., Mähler A., Dobberstein K., Krüger A., et al. 2010. LMNA mutations, skeletal muscle lipid metabolism, and insulin resistance. J. Clin. Endocrinol. Metab. 95: 1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeagle P. L. 1989. Regulation of membrane function through composition, structure, and dynamics. Ann. N. Y. Acad. Sci. 568: 29–34. [DOI] [PubMed] [Google Scholar]
- 22.Abumrad N., Harmon C., Ibrahimi A. 1998. Membrane transport of long-chain fatty acids: evidence for a facilitated process. J. Lipid Res. 39: 2309–2318. [PubMed] [Google Scholar]
- 23.Ibrahimi A., Bonen A., Blinn W. D., Hajri T., Li X., Zhong K., Cameron R., Abumrad N. A. 1999. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J. Biol. Chem. 274: 26761–26766. [DOI] [PubMed] [Google Scholar]
- 24.Sorrentino D., Stump D., Potter B. J., Robinson R. B., White R., Kiang C. L., Berk P. D. 1988. Oleate uptake by cardiac myocytes is carrier mediated and involves a 40-kD plasma membrane fatty acid binding protein similar to that in liver, adipose tissue, and gut. J. Clin. Invest. 82: 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stremmel W. 1989. Transmembrane transport of fatty acids in the heart. Mol. Cell. Biochem. 88: 23–29. [DOI] [PubMed] [Google Scholar]
- 26.Koonen D. P., Glatz J. F., Bonen A., Luiken J. J. 2005. Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle. Biochim. Biophys. Acta. 1736: 163–180. [DOI] [PubMed] [Google Scholar]
- 27.Brunaldi K., Huang N., Hamilton J. A. 2010. Fatty acids are rapidly delivered to and extracted from membranes by methyl-beta-cyclodextrin. J. Lipid Res. 51: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton J. A., Johnson R. A., Corkey B., Kamp F. 2001. Fatty acid transport: the diffusion mechanism in model and biological membranes. J. Mol. Neurosci. 16: 99–108. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton J. A., Guo W., Kamp F. 2002. Mechanism of cellular uptake of long-chain fatty acids: do we need cellular proteins? Mol. Cell. Biochem. 239: 17–23. [PubMed] [Google Scholar]
- 30.Abumrad N., Coburn C., Ibrahimi A. 1999. Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells: CD36, FATP and FABPm. Biochim. Biophys. Acta. 1441: 4–13. [DOI] [PubMed] [Google Scholar]
- 31.Storch J., Kleinfeld A. M. 1986. Transfer of long-chain fluorescent free fatty acids between unilamellar vesicles. Biochemistry. 25: 1717–1726. [DOI] [PubMed] [Google Scholar]
- 32.Black P. N., DiRusso C. C. 2003. Transmembrane movement of exogenous long-chain fatty acids: proteins, enzymes, and vectorial esterification. Microbiol. Mol. Biol. Rev. 67: 454–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamp F., Hamilton J. A., Westerhoff H. V. 1993. Movement of fatty acids, fatty acid analogues, and bile acids across phospholipid bilayers. Biochemistry. 32: 11074–11086. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton J. A. 1998. Fatty acid transport: difficult or easy? J. Lipid Res. 39: 467–481. [PubMed] [Google Scholar]
- 35.Schaffer J. E. 2002. Fatty acid transport: the roads taken. Am. J. Physiol. Endocrinol. Metab. 282: E239–E246. [DOI] [PubMed] [Google Scholar]
- 36.Kamp F., Zakim D., Zhang F. L., Noy N., Hamilton J. A. 1995. Fatty acid flip-flop in phospholipid bilayers is extremely fast. Biochemistry. 34: 11928–11937. [DOI] [PubMed] [Google Scholar]
- 37.Thompson B. R., Lobo S., Bernlohr D. A. 2010. Fatty acid flux in adipocytes: the in's and out's of fat cell lipid trafficking. Mol. Cell. Endocrinol. 318: 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frohnert B. I., Bernlohr D. A. 2000. Regulation of fatty acid transporters in mammalian cells. Prog. Lipid Res. 39: 83–107. [DOI] [PubMed] [Google Scholar]
- 39.Hui T. Y., Bernlohr D. A. 1997. Fatty acid transporters in animal cells. Front. Biosci. 2: d222–d231. [DOI] [PubMed] [Google Scholar]
- 40.Storch J., Corsico B. 2008. The emerging functions and mechanisms of mammalian fatty acid- binding proteins. Annu. Rev. Nutr. 28: 73–95. [DOI] [PubMed] [Google Scholar]
- 41.Dutta-Roy A. K. 2000. Cellular uptake of long-chain fatty acids: role of membrane associated fatty-acid-binding/transport proteins. Cell. Mol. Life Sci. 57: 1360–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stremmel W., Lotz G., Strohmeyer G., Berk P. D. 1985. Identification, isolation, and partial characterization of a fatty acid binding protein from rat jejuna microvillous membranes. J. Clin. Invest. 75: 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaffer J. E., Lodish H. F. 1994. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell. 79: 427–436. [DOI] [PubMed] [Google Scholar]
- 44.Schaffer J. E. 1996. A novel adipocyte long chain fatty acid transport protein. Eur. J. Med. Res. 1: 176–180. [PubMed] [Google Scholar]
- 45.Harmon C. M., Abumrad N. A. 1993. Binding of sulfosuccinimidyl fatty acids to adipocyte membrane proteins: isolation and aminoterminal sequence of an 88-kD protein implicated in transport of long-chain fatty acids. J. Membr. Biol. 133: 43–49. [DOI] [PubMed] [Google Scholar]
- 46.Binnert C., Koistinen H. A., Martin G., Andreelli F., Ebeling P., Koivisto V. A., Laville M., Auwerx J., Vidal H. 2000. Fatty acid transport protein-1 mRNA expression in skeletal muscle and in adipose tissue in humans. Am. J. Physiol. Endocrinol. Metab. 279: E1072–E1079. [DOI] [PubMed] [Google Scholar]
- 47.Gimeno R. E., Ortegon A. M., Patel S., Punreddy S., Ge P., Sun Y., Lodish H. F., Stahl A. 2003. Characterization of a heart-specific fatty acid transport protein. J. Biol. Chem. 278: 16039–16044. [DOI] [PubMed] [Google Scholar]
- 48.Fitscher B. A., Riedel H. D., Young K. C., Stremmel W. 1998. Tissue distribution and cDNA cloning of a human fatty acid transport protein (hsFATP4). Biochim. Biophys. Acta. 1443: 381–385. [DOI] [PubMed] [Google Scholar]
- 49.Abumrad N. A., el-Maghrabi M. R., Amri E. Z., Lopez E., Grimaldi P. A. 1993. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 268: 17665–17668. [PubMed] [Google Scholar]
- 50.Luiken J. J., Dyck D. J., Han X. X., Tandon N. N., Arumugam Y., Glatz J. F., Bonen A. 2002. Insulin induces the translocation of the fatty acid transporter FAT/CD36 to the plasma membrane. Am. J. Physiol. Endocrinol. Metab. 282: E491–E495. [DOI] [PubMed] [Google Scholar]
- 51.Luiken J. J., Koonen D. P., Willems J., Zorzano A., Becker C., Fischer Y., Tandon N. N., Van Der Vusse G. J., Bonen A., Glatz J. F. 2002. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes. 51: 3113–3119. [DOI] [PubMed] [Google Scholar]
- 52.Sfeir Z., Ibrahimi A., Amri E., Grimaldi P., Abumrad N. 1997. Regulation of FAT/CD36 gene expression: further evidence in support of a role of the protein in fatty acidbinding/transport. Prostaglandins Leukot. Essent. Fatty Acids. 57: 17–21. [DOI] [PubMed] [Google Scholar]
- 53.Veerkamp J. H., Maatman R. G. 1995. Cytoplasmic fatty acid-binding proteins: their structure and genes. Prog. Lipid Res. 34: 17–52. [DOI] [PubMed] [Google Scholar]
- 54.Storch J., McDermott L. 2009. Structural and functional analysis of fatty acid-binding proteins. J. Lipid Res. 50(Suppl): S126–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaap F. G., Binas B., Danneberg H., van der Vusse G. J., Glatz J. F. 1999. Impaired long-chain fatty acid utilization by cardiac myocytes isolated from mice lacking the heart-type fatty acid binding protein gene. Circ. Res. 85: 329–337. [DOI] [PubMed] [Google Scholar]
- 56.McFarland M. J., Porter A. C., Rakhshan F. R., Rawat D. S., Gibbs R. A., Barker E. L. 2004. A role for caveolae/lipid rafts in the uptake and recycling of the endogenous cannabinoid anandamide. J. Biol. Chem. 279: 41991–41997. [DOI] [PubMed] [Google Scholar]
- 57.Razani B., Woodman S. E., Lisanti M. P. 2002. Caveolae: from cell biology to animal physiology. Pharmacol. Rev. 54: 431–467. [DOI] [PubMed] [Google Scholar]
- 58.Park D. S., Woodman S. E., Schubert W., Cohen A. W., Frank P. G., Chandra M., Shirani J., Razani B., Tang B., Jelicks L. A., et al. 2002. Caveolin-1/3 double-knockout mice are viable, but lack both muscle and non-muscle caveolae, and develop a severe cardiomyopathic phenotype. Am. J. Pathol. 160: 2207–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopaschuk G. D., Ussher J. R., Folmes C. D., Jaswal J. S., Stanley W. C. 2010. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 90: 207–258. [DOI] [PubMed] [Google Scholar]
- 60.McGarry J. D., Brown N. F. 1997. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur. J. Biochem. 244: 1–14. [DOI] [PubMed] [Google Scholar]
- 61.Kerner J., Hoppel C. 2000. Fatty acid import into mitochondria. Biochim. Biophys. Acta. 1486: 1–17. [DOI] [PubMed] [Google Scholar]
- 62.Campbell S. E., Tandon N. N., Woldegiorgis G., Luiken J. J., Glatz J. F., Bonen A. 2004. A novel function for fatty acid translocase (FAT)/CD36: involvement in long chain fatty acid transfer into the mitochondria. J. Biol. Chem. 279: 36235–36241. [DOI] [PubMed] [Google Scholar]
- 63.Sebastián D., Guitart M., García-Martínez C., Mauvezin C., Orellana-Gavaldà J. M., Serra D., Gómez-Foix A. M., Hegardt F. G., Asins G. 2009. Novel role of FATP1 in mitochondrial fatty acid oxidation in skeletal muscle cells. J. Lipid Res. 50: 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Esser V., Britton C. H., Weis B. C., Foster D. W., McGarry J. D. 1993. Cloning, sequencing, and expression of a cDNA encoding rat liver carnitine palmitoyltransferase I. Direct evidence that a single polypeptide is involved in inhibitor interaction and catalytic function. J. Biol. Chem. 268: 5817–5822. [PubMed] [Google Scholar]
- 65.Yamazaki N., Shinohara Y., Shima A., Terada H. 1995. High expression of a novel carnitine palmitoyltransferase I like protein in rat brown adipose tissue and heart: isolation and characterization of its cDNA clone. FEBS Lett. 363: 41–45. [DOI] [PubMed] [Google Scholar]
- 66.Price N., van der Leij F., Jackson V., Corstorphine C., Thomson R., Sorensen A., Zammit V. 2002. A novel brain-expressed protein related to carnitine palmitoyltransferase I. Genomics. 80: 433–442. [DOI] [PubMed] [Google Scholar]
- 67.Brown N. F., Hill J. K., Esser V., Kirkland J. L., Corkey B. E., Foster D. W., McGarry J. D. 1997. Mouse white adipocytes and 3T3–L1 cells display an anomalous pattern of carnitine palmitoyltransferase (CPT) I isoform expression during differentiation. Inter-tissue and inter-species expression of CPT I and CPT II enzymes. Biochem. J. 327: 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Odland L. M., Heigenhauser G. J., Lopaschuk G. D., Spriet L. L. 1996. Human skeletal muscle malonyl-CoA at rest and during prolonged submaximal exercise. Am. J. Physiol. 270: E541–E544. [DOI] [PubMed] [Google Scholar]
- 69.Dean D., Daugaard J. R., Young M. E., Saha A., Vavvas D., Asp S., Kiens B., Kim K. H., Witters L., Richter E. A., et al. 2000. Exercise diminishes the activity of acetyl-CoA carboxylase in human muscle. Diabetes. 49: 1295–1300. [DOI] [PubMed] [Google Scholar]
- 70.King K. L., Stanley W. C., Rosca M., Kerner J., Hoppel C. L., Febbraio M. 2007. Fatty acid oxidation in cardiac and skeletal muscle mitochondria is unaffected by deletion of CD36. Arch. Biochem. Biophys. 467: 234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bezaire V., Bruce C. R., Heigenhauser G. J., Tandon N. N., Glatz J. F., Luiken J., Bonen A., Spriet L. L. 2006. Identification of fatty acid translocase on human skeletal muscle mitochondrial membranes: essential role in fatty acid oxidation. Am. J. Physiol. Endocrinol. Metab. 290: E509–E515. [DOI] [PubMed] [Google Scholar]
- 72.Schenk S., Horowitz J. F. 2006. Coimmunoprecipitation of FAT/CD36 and CPT I in skeletal muscle increases proportionally with fat oxidation after endurance exercise training. Am. J. Physiol. Endocrinol. Metab. 291: E254–E260. [DOI] [PubMed] [Google Scholar]
- 73.Wendel A. A., Lewin T. M., Coleman R. A. 2009. Glycerol-3-phosphate acyltransferases: rate limiting enzymes of triacylglycerol biosynthesis. Biochim. Biophys. Acta. 1791: 501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park H., Kaushik V. K., Constant S., Prentki M., Przybytkowski E., Ruderman N. B., Saha A. K. 2002. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J. Biol. Chem. 277: 32571–32577. [DOI] [PubMed] [Google Scholar]
- 75.Takeuchi K., Reue K. 2009. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am. J. Physiol. Endocrinol. Metab. 296: E1195–E1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Donkor J., Sariahmetoglu M., Dewald J., Brindley D. N., Reue K. 2007. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J. Biol. Chem. 282: 3450–3457. [DOI] [PubMed] [Google Scholar]
- 77.Yamashita A., Sugiura T., Waku K. 1997. Acyltransferases and transacylases involved in fatty acid remodeling of phospholipids and metabolism of bioactive lipids in mammalian cells. J. Biochem. 122: 1–16. [DOI] [PubMed] [Google Scholar]
- 78.Cases S., Smith S. J., Zheng Y. W., Myers H. M., Lear S. R., Sande E., Novak S., Collins C., Welch C. B., Lusis A. J., et al. 1998. Idenification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacyllycerol synthesis. Proc. Natl. Acad. Sci. USA. 95: 13018–13023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cases S., Stone S. J., Zhou P., Yen E., Tow B., Lardizabal K. D., Voelker T., Farese R. V., Jr 2001. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 276: 38870–38876. [DOI] [PubMed] [Google Scholar]
- 80.Roorda B. D., Hesselink M. K., Schaart G., Moonen-Kornips E., Martínez-Martínez P., Losen M., De Baets M. H., Mensink R. P., Schrauwen P. 2005. DGAT1 overexpression in muscle by in vivo DNA electroporation increases intramyocellular lipid content. J. Lipid Res. 46: 230–236. [DOI] [PubMed] [Google Scholar]
- 81.Xu F. Y., Kardami E., Nemer M., Choy P. C., Hatch G. M. 2000. Elevation in phosphatidylethanolamine is an early but not essential event for cardiac cell differentiation. Exp. Cell Res. 256: 358–364. [DOI] [PubMed] [Google Scholar]
- 82.Holub B. J. 1977. Regulation of selectivity of CDPcholine: 1,2-diacyl-sn-glycerol cholinephosphotransferase in rat liver microsomes towards different molecular species of 1,2-diacyl-sn-glycerols. Can. J. Biochem. 55: 700–705. [DOI] [PubMed] [Google Scholar]
- 83.Hatch G. M. 1994. Cardiolipin biosynthesis in the isolated heart. Biochem. J. 297: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lands W. E. 2000. Stories about acyl chains. Biochim. Biophys. Acta. 1483: 1–14. [DOI] [PubMed] [Google Scholar]
- 85.Watt M. J., Steinberg G. R. 2008. Regulation and function of triacylglycerol lipases in cellular metabolism. Biochem. J. 414: 313–325. [DOI] [PubMed] [Google Scholar]
- 86.Brindley D. N., Kok B. P., Kienesberger P. C., Lehner R., Dyck J. R. 2010. Shedding light on the enigma of myocardial lipotoxicity: the involvement of known and putative regulators of fatty acid storage and mobilization. Am. J. Physiol. Endocrinol. Metab. 298: E897–E908. [DOI] [PubMed] [Google Scholar]
- 87.Chinetti-Gbaguidi G., Fruchart J. C., Staels B. 2005. Role of the PPAR family of nuclear receptors in the regulation of metabolic and cardiovascular homeostasis: new approaches to therapy. Curr. Opin. Pharmacol. 5: 177–183. [DOI] [PubMed] [Google Scholar]
- 88.Zhang L., Keung W., Samokhvalov V., Wang W., Lopaschuk G. D. 2010. Role of fatty acid uptake and fatty acid beta-oxidation in mediating insulin resistance in heart and skeletal muscle. Biochim. Biophys. Acta. 1801: 1–22. [DOI] [PubMed] [Google Scholar]
- 89.Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. 1987. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 50: 509–517. [DOI] [PubMed] [Google Scholar]
- 90.Love D. R., Forrest S. M., Smith T. J., England S., Flint T., Davies K. E., Speer A. 1989. Molecular analysis of Duchenne and Becker muscular dystrophies. Br. Med. Bull. 45: 659–680. [DOI] [PubMed] [Google Scholar]
- 91.Worton R. G., Molnar M. J., Brais B., Karpati G. 2001. The muscular dystrophies. The Metabolic and Molecular Bases of Inherited Disease. Eighth edition. Scriver C. R., Beaudet A. L., Valle D., Sly W. S., McGraw-Hill, New York, NY: 5492–5523. [Google Scholar]
- 92.Anand R. 1983. Cellular membranes in Duchenne muscular dystrophy. Int. J. Biochem. 15: 1211–1217. [DOI] [PubMed] [Google Scholar]
- 93.Eagle M., Baudouin S. V., Chandler C., Giddings D. R., Bullock R., Bushby K. 2002. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul. Disord. 12: 926–929. [DOI] [PubMed] [Google Scholar]
- 94.Hoffman E. P., Brown R. H., Jr, Kunkel L. M. 1987. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 51: 919–928. [DOI] [PubMed] [Google Scholar]
- 95.Magri F., Del Bo R., D'Angelo M. G., Govoni A., Ghezzi S., Gandossini S., Sciacco M., Ciscato P., Bordoni A., Tedeschi S., et al. 2011. Clinical and molecular characterization of a cohort of patients with novel nucleotide alterations of the Dystrophin gene detected by direct sequencing. BMC Med. Genet. 12: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rando T. A. 2001. The dystrophin-glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies. Muscle Nerve. 24: 1575–1594. [DOI] [PubMed] [Google Scholar]
- 97.Sciandra F., Bozzi M., Bianchi M., Pavoni E., Giardina B., Brancaccio A. 2003. Dystroglycan and muscular dystrophies related to the dystrophin-glycoprotein complex. Ann. Ist. Super. Sanita. 39: 173–181. [PubMed] [Google Scholar]
- 98.Watchko J. F., O'Day T. L., Hoffman E. P. 2002. Functional characteristics of dystrophic skeletal muscle: insights from animal models. J. Appl. Physiol. 93: 407–417. [DOI] [PubMed] [Google Scholar]
- 99.Bushby K. M., Thambyayah M., Gardner-Medwin D. 1991. Prevalence and incidence of Becker muscular dystrophy. Lancet. 337: 1022–1024. [DOI] [PubMed] [Google Scholar]
- 100.Pearce P. H., Johnsen R. D., Wysocki S. J., Kakulas B. A. 1981. Muscle lipids in Duchenne muscular dystrophy. Aust. J. Exp. Biol. Med. Sci. 59: 77–90. [DOI] [PubMed] [Google Scholar]
- 101.Kuhn E., Fiehn W., Seiler D., Schroder J. M. 1979. The autosomal recessive (Becker) form of myotonia congenita. Muscle Nerve. 2: 109–117. [DOI] [PubMed] [Google Scholar]
- 102.Kunze D., Rustow B., Olthoff D. 1980. Studies of selected enzymes of phospholipids metabolism in the dystrophic human muscle. Clin. Chim. Acta. 108: 211–218. [DOI] [PubMed] [Google Scholar]
- 103.Tahallah N., Brunelle A., De La Porte S., Laprevote O. 2008. Lipid mapping in human dystrophic muscle by cluster-time-of-flight secondary ion mass spectrometry imaging. J. Lipid Res. 49: 438–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ionasescu V., Monaco L., Sandra A., Ionasescu R., Burmeister L., Deprosse C., Stern L. Z. 1981. Alterations in lipid incorporation in Duchenne muscular dystrophy. Studies of fresh and cultured muscle. J. Neurol. Sci. 50: 249–251. [DOI] [PubMed] [Google Scholar]
- 105.Hughes B. P. 1972. Lipid changes in Duchenne muscular dystrophy. J. Neurol. Neurosurg. Psychiatry. 35: 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Owens K., Hughes B. P. 1970. Lipids of dystrophic and normal mouse muscle: whole tissue and particulate fractions. J. Lipid Res. 11: 486–495. [PubMed] [Google Scholar]
- 107.Touboul D., Piednoel H., Voisin V., De La Porte S., Brunelle A., Halgand F., Laprevote O. 2004. Changes of phospholipid composition within the dystrophic muscle by matrix-assisted laser desorption/ionization mass spectrometry and mass spectrometry imaging. Eur. J. Mass Spectrom. (Chichester, Eng.). 10: 657–664. [DOI] [PubMed] [Google Scholar]
- 108.Harper P. S. 1986. Myotonic disorders. Myology. Engel A. G., Banker B. Q., McGraw-Hill, New York, NY: 1267–1311. [Google Scholar]
- 109.Boittin F. X., Shapovalov G., Hirn C., Ruegg U. T. 2010. Phospholipase A2-derived lysophosphatidylcholine triggers Ca2+ entry in dystrophic skeletal muscle fibers. Biochem. Biophys. Res. Commun. 391: 401–406. [DOI] [PubMed] [Google Scholar]
- 110.Benabdellah F., Yu H., Brunelle A., Laprévote O., De La Porte S. 2009. MALDI reveals membrane lipid profile reversion in MDX mice. Neurobiol. Dis. 36: 252–258. [DOI] [PubMed] [Google Scholar]
- 111.Da Silva-Azevedo L., Jahne S., Hoffmann C., Stalder D., Heller M., Pries A. R., Zakrzewicz A., Baum O. 2009. Up-regulation of the peroxiredoxin-6 related metabolism of reactive oxygen species in skeletal muscle of mice lacking neuronal nitric oxide synthase. J. Physiol. 587: 655–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manevich Y., Reddy K. S., Shuvaeva T., Feinstein S. I., Fisher A. B. 2007. Structure and phospholipase function of peroxiredoxin 6: identification of the catalytic triad and its role in phospholipid substrate binding. J. Lipid Res. 48: 2306–2318. [DOI] [PubMed] [Google Scholar]
- 113.Gilbert E. F. 1985. Carnitine deficiency. Pathology. 17: 161–171. [DOI] [PubMed] [Google Scholar]
- 114.Borum P. R., Broquist H. P., Roelops R. J. 1977. Muscle carnitine levels in neuromuscular disease. J. Neurol. Sci. 34: 279–286. [DOI] [PubMed] [Google Scholar]
- 115.Sharma U., Atri S., Sharma M. C., Sarkar C., Jagannathan N. R. 2003. Skeletal muscle metabolism in Duchenne muscular dystrophy (DMD): an in-vitro proton NMR spectroscopy study. Magn. Reson. Imaging. 21: 145–153. [DOI] [PubMed] [Google Scholar]
- 116.Anichini A., Fanin M., Vianey-Saban C., Cassandrini D., Fiorillo C., Bruno C., Angelini C. 2011. Genotype-phenotype correlations in a large series of patients with muscle type CPT II deficiency. Neurol. Res. 33: 24–32. [DOI] [PubMed] [Google Scholar]
- 117.Mitsuhashi S., Ohkuma A., Talim B., Karahashi M., Koumura T., Aoyama C., Kurihara M., Quinlivan R., Sewry C., Mitsuhashi H., et al. 2011. A congenital muscular dystrophy with mitochondrial structural abnormalities caused by defective de novo phosphatidylcholine biosynthesis. Am. J. Hum. Genet. 88: 845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Momose M., Iguchi N., Imamura K., Usui H., Ueda T., Miyamoto K., Inaba S. 2001. Depressed myocardial fatty acid metabolism in patients with muscular dystrophy. Neuromuscul. Disord. 11: 464–469. [DOI] [PubMed] [Google Scholar]
- 119.Tang Z., Scherer P. E., Okamoto T., Song K., Chu C., Kohtz D. S., Nishimoto I., Lodish H. F., Lisanti M. P. 1996. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J. Biol. Chem. 271: 2255–2261. [DOI] [PubMed] [Google Scholar]
- 120.Song K. S., Scherer P. E., Tang Z., Okamoto T., Li S., Chafel M., Chu C., Kohtz D. S., Lisanti M. P. 1996. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J. Biol. Chem. 271: 15160–15165. [DOI] [PubMed] [Google Scholar]
- 121.Repetto S., Bado M., Broda P., Lucania G., Masetti E., Sotgia F., Carbone I., Pavan A., Bonilla E., Cordone G., et al. 1999. Increased number of caveolae and caveolin-3 overexpression in Duchenne muscular dystrophy. Biochem. Biophys. Res. Commun. 261: 547–550. [DOI] [PubMed] [Google Scholar]
- 122.Vaghy P. L., Fang J., Wu W., Vaghy L. P. 1998. Increased caveolin-3 levels in mdx mouse muscles. FEBS Lett. 431: 125–127. [DOI] [PubMed] [Google Scholar]
- 123.Galbiati F., Volonte D., Chu J. B., Li M., Fine S. W., Fu M., Bermudez J., Pedemonte M., Weidenheim K. M., Pestell R. G., et al. 2000. Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc. Natl. Acad. Sci. USA. 97: 9689–9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Homburger F., Nixon C. W., Eppenberger M., Baker J. R. 1966. Hereditary myopathy in the Syrian hamster: studies on pathogenesis. Ann. N. Y. Acad. Sci. 138: 14–27. [DOI] [PubMed] [Google Scholar]
- 125.Homburger F. 1972. Disease models in Syrian hamsters. Prog. Exp. Tumor Res. 16: 69–86. [DOI] [PubMed] [Google Scholar]
- 126.Borowski I. F., Harrow J. A., Pritchard E. T., Dhalla N. S. 1974. Changes in electrolyte and lipid contents of the myopathic hamster (UM-X7.1) skeletal and cardiac muscles. Res. Commun. Chem. Pathol. Pharmacol. 7: 443–451. [PubMed] [Google Scholar]
- 127.Vecchini A., Binaglia L., Bibeau M., Minieri M., Carotenuto F., Di Nardo P. 2001. Insulin deficiency and reduced expression of lipogenic enzymes in cardiomyopathic hamster. J. Lipid Res. 42: 96–105. [PubMed] [Google Scholar]
- 128.Owens K., Weglicki W. B., Sonnenblick E. H., Gertz E. W. 1972. Phospholipid and cholesterol content of ventricular tissue from the cardiomyopathic Syrian hamster. J. Mol. Cell. Cardiol. 4: 229–236. [DOI] [PubMed] [Google Scholar]
- 129.Hsu Q. S., Kaldor G. 1971. Studies on the lipid composition of the fragmented sarcoplasmic reticulum of normal and dystrophic chickens. Proc. Soc. Exp. Biol. Med. 138: 733–737. [DOI] [PubMed] [Google Scholar]
- 130.Srivastava N. K., Pradhan S., Mittal B., Gowda G. A. 2010. High resolution NMR based analysis of serum lipids in Duchenne muscular dystrophy patients and its possible diagnostic significance. NMR Biomed. 23: 13–22. [DOI] [PubMed] [Google Scholar]
- 131.Theerasasawat S., Papsing C., Pulkes T. 2010. CTG repeat lengths of the DMPK gene in myotonic dystrophy patients compared to healthy controls in Thailand. J. Clin. Neurosci. 17: 1520–1522. [DOI] [PubMed] [Google Scholar]
- 132.Salvatori S., Fanin M., Trevisan C. P., Furlan S., Reddy S., Nagy J. I., Angelini C. 2005. Decreased expression of DMPK: correlation with CTG repeat expansion and fibre type composition in myotonic dystrophy type 1. Neurol. Sci. 26: 235–242. [DOI] [PubMed] [Google Scholar]
- 133.Aslanidis C., Jansen G., Amemiya C., Shutler G., Mahadevan M., Tsilfidis C., Chen C., Alleman J., Wormskamp N. G., Vooijs M. 1992. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature. 355: 548–551. [DOI] [PubMed] [Google Scholar]
- 134.Wakamatsu H., Nakamura H., Ito K., Anazawa W., Okajima S. 1970. Serum desmosterol and other lipids in myotonic dystrophy--a possible pathogenesis of myotonic dystrophy. Keio J. Med. 19: 145–149. [DOI] [PubMed] [Google Scholar]
- 135.Waterham H. R., Koster J., Romeijn G. J., Hennekam R. C., Vreken P., Andersson H. C., FitzPatrick D. R., Kelley R. I., Wanders R. J. 2001. Mutations in the 3beta-hydroxysterol Delta24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am. J. Hum. Genet. 69: 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peter J. B., Dromgoole S. H., Campion D. S., Stempel K. E., Bowman R. L., Andiman R. M., Nagatomo T. 1975. Experimental myotonia and hypocholesterolemic agents. Exp. Neurol. 49: 115–122. [DOI] [PubMed] [Google Scholar]
- 137.Andiman R. M., Peter J. B., Dhopeshwarkar G. 1974. Myotonic dystrophy and myotonia congenital: ATPase and lipid composition of erythrocyte membranes and serum lipids with special reference to desmosterol. Neurology. 4: 352–353. [Google Scholar]
- 138.Thomas N. S., Harper P. S. 1978. Myotonic dystrophy: studies on the lipid composition and metabolism of erythrocytes and skin fibroblasts. Clin. Chim. Acta. 83: 13–23. [DOI] [PubMed] [Google Scholar]
- 139.Antoku Y., Sakai T., Tsukamoto K., Goto I., Iwashita H., Kuroiwa Y. 1985. A study on erythrocyte membrane plasmalogen in myotonic dystrophy. J. Neurochem. 44: 1667–1671. [DOI] [PubMed] [Google Scholar]
- 140.Llagostera E., Catalucci D., Marti L., Liesa M., Camps M., Ciaraldi T. P., Kondo R., Reddy S., Dillmann W. H., Palacin M., et al. 2007. Role of myotonic dystrophy protein kinase (DMPK) in glucose homeostasis and muscle insulin action. PLoS ONE. 2: e1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ward A. J., Rimer M., Killian J. M., Dowling J. J., Cooper T. A. 2010. CUGBP1 overexpression in mouse skeletal muscle reproduces features of myotonic dystrophy type 1. Hum. Mol. Genet. 19: 3614–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang X., Osinska H., Dorn G. W., 2nd, Nieman M., Lorenz J. N., Gerdes A. M., Witt S., Kimball T., Gulick J., Robbins J. 2001. Mouse model of desmin-related cardiomyopathy. Circulation. 103: 2402–2407. [DOI] [PubMed] [Google Scholar]
- 143.Paulin D., Huet A., Khanamyrian L., Xue Z. 2004. Desminopathies in muscle disease. J. Pathol. 204: 418–427. [DOI] [PubMed] [Google Scholar]
- 144.Goudeau B., Rodrigues-Lima F., Fischer D., Casteras-Simon M., Sambuughin N., de Visser M., Laforet P., Ferrer X., Chapon F., Sjoberg G., et al. 2006. Variable pathogenic potentials of mutations located in the desmin alpha-helical domain. Hum. Mutat. 27: 906–913. [DOI] [PubMed] [Google Scholar]
- 145.Bar H., Goudeau B., Walde S., Casteras-Simon M., Mucke N., Shatunov A., Goldberg Y. P., Clarke C., Holton J. L., Eymard B., et al. 2007. Conspicuous involvement of desmin tail mutations in diverse cardiac and skeletal myopathies. Hum. Mutat. 28: 374–386. [DOI] [PubMed] [Google Scholar]
- 146.Kostareva A., Sjoberg G., Bruton J., Zhang S. J., Balogh J., Gudkova A., Hedberg B., Edstrom L., Westerblad H., Sejersen T. 2008. Mice expressing L345P mutant desmin exhibit morphological and functional changes of skeletal and cardiac mitochondria. J. Muscle Res. Cell Motil. 29: 25–36. [DOI] [PubMed] [Google Scholar]
- 147.Fountoulakis M., Soumaka E., Rapti K., Mavroidis M., Tsangaris G., Maris A., Weisleder N., Capetanaki Y. 2005. Alterations in the heart mitochondrial proteome in a desmin null heart failure model. J. Mol. Cell. Cardiol. 38: 461–474. [DOI] [PubMed] [Google Scholar]
- 148.Turko I. V., Murad F. 2003. Quantitative protein profiling in heart mitochondria from diabetic rats. J. Biol. Chem. 278: 35844–35849. [DOI] [PubMed] [Google Scholar]
- 149.Capetanaki Y., Bloch R. J., Kouloumenta A., Mavroidis M., Psarras S. 2007. Muscle intermediate filaments and their links to membranes and membranous organelles. Exp. Cell Res. 313: 2063–2076. [DOI] [PubMed] [Google Scholar]
- 150.Shinde A., Nakano S., Sugawara M., Toyoshima I., Ito H., Tanaka K., Kusaka H. 2008. Expression of caveolar components in primary desminopathy. Neuromuscul. Disord. 18: 215–219. [DOI] [PubMed] [Google Scholar]
- 151.Allen J. A., Halverson-Tamboli R. A., Rasenick M. M. 2007. Lipid raft microdomains and neurotransmitter signalling. Nat. Rev. Neurosci. 8: 128–140. [DOI] [PubMed] [Google Scholar]
- 152.Minetti C., Sotgia F., Bruno C., Scartezzini P., Broda P., Bado M., Masetti E., Mazzocco M., Egeo A., Donati M. A., et al. 1998. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat. Genet. 18: 365–368. [DOI] [PubMed] [Google Scholar]
- 153.Daniele N., Richard I., Bartoli M. 2007. Ins and outs of therapy in limb girdle muscular dystrophies. Int. J. Biochem. Cell Biol. 39: 1608–1624. [DOI] [PubMed] [Google Scholar]
- 154.Nigro V., Okazaki Y., Belsito A., Piluso G., Matsuda Y., Politano L., Nigro G., Ventura C., Abbondanza C., Molinari A. M., et al. 1997. Identification of the Syrian hamster cardiomyopathy gene. Hum. Mol. Genet. 6: 601–607. [DOI] [PubMed] [Google Scholar]
- 155.Sakamoto A., Ono K., Abe M., Jasmin G., Eki T., Murakami Y., Masaki T., Toyo-oka T., Hanaoka F. 1997. Both hypertrophic and dilated cardiomyopathies are caused by mutation of the same gene, delta-sarcoglycan, in hamster: an animal model of disrupted dystrophin-associated glycoprotein complex. Proc. Natl. Acad. Sci. USA. 94: 13873–13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Okamoto H., Kawaguchi H., Sano H., Kageyama K., Kudo T., Koyama T., Kitabatake A. 1994. Microdynamics of the phospholipid bilayer in cardiomyopathic hamster heart cell membrane. J. Mol. Cell. Cardiol. 26: 211–218. [DOI] [PubMed] [Google Scholar]
- 157.Kawaguchi H., Shoki M., Sano H., Kudo T., Sawa H., Okamoto H., Sakata Y., Yasuda H. 1992. The studies of cell damaging and cell growth factors which induce cardiomyopathy. Jpn. Circ. J. 56: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 158.Smythe G. M., Eby J. C., Disatnik M. H., Rando T. A. 2003. A caveolin-3 mutant that causes limb girdle muscular dystrophy type 1C disrupts Src localization and activity and induces apoptosis in skeletal myotubes. J. Cell Sci. 116: 4739–4749. [DOI] [PubMed] [Google Scholar]
- 159.de Windt L. J., Cox K., Hofstra L., Doevendans P. A. 2002. Molecular and genetic aspects of cardiac fatty acid homeostasis in health and disease. Eur. Heart J. 23: 774–787. [DOI] [PubMed] [Google Scholar]
- 160.Cao H., Hegele R. A. 2000. Nuclear lamin A/C R482Q mutation in canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum. Mol. Genet. 9: 109–112. [DOI] [PubMed] [Google Scholar]
- 161.Krimm I., Ostlund C., Gilquin B., Couprie J., Hossenlopp P., Mornon J. P., Bonne G., Courvalin J. C., Worman H. J., Zinn-Justin S. 2002. The Ig-like structure of the C-terminal domain of lamin A/C, mutated in muscular dystrophies, cardiomyopathy, and partial lipodystrophy. Structure. 10: 811–823. [DOI] [PubMed] [Google Scholar]
- 162.Verstraeten V. L., Caputo S., van Steensel M. A., Duband-Goulet I., Zinn-Justin S., Kamps M., Kuijpers H. J., Ostlund C., Worman H. J., Briede J. J., et al. 2009. The R439C mutation in LMNA causes lamin oligomerization and susceptibility to oxidative stress. J. Cell. Mol. Med. 13: 959–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Garg A., Vinaitheerthan M., Weatherall P. T., Bowcock A. M. 2001. Phenotypic heterogeneity in patients with familial partial lipodystrophy (dunnigan variety) related to the site of missense mutations in lamin a/c gene. J. Clin. Endocrinol. Metab. 86: 59–65. [DOI] [PubMed] [Google Scholar]
- 164.Lin F., Worman H. J. 1997. Expression of nuclear lamins in human tissues and cancer cell lines and transcription from the promoters of the lamin A/C and B1 genes. Exp. Cell Res. 236: 378–384. [DOI] [PubMed] [Google Scholar]
- 165.Nikolova V., Leimena C., McMahon A. C., Tan J. C., Chandar S., Jogia D., Kesteven S. H., Michalicek J., Otway R., Verheyen F., et al. 2004. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J. Clin. Invest. 113: 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kobberling J., Dunnigan M. G. 1986. Familial partial lipodystrophy: two types of an X linked dominant syndrome, lethal in the hemizygous state. J. Med. Genet. 23: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Garg A., Peshock R. M., Fleckenstein J. L. 1999. Adipose tissue distribution pattern in patients with familial partial lipodystrophy (Dunnigan variety). J. Clin. Endocrinol. Metab. 84: 170–174. [DOI] [PubMed] [Google Scholar]
- 168.Burn J., Baraitser M. 1986. Partial lipoatrophy with insulin resistant diabetes and hyperlipidaemia (Dunnigan syndrome). J. Med. Genet. 23: 128–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bhayana S., Hegele R. A. 2002. The molecular basis of genetic lipodystrophies. Clin. Biochem. 35: 171–177. [DOI] [PubMed] [Google Scholar]
- 170.Hegele R. A., Kraw M. E., Ban M. R., Miskie B. A., Huff M. W., Cao H. 2003. Elevated serum C-reactive protein and free fatty acids among nondiabetic carriers of missense mutations in the gene encoding lamin A/C (LMNA) with partial lipodystrophy. Arterioscler. Thromb. Vasc. Biol. 23: 111–116. [DOI] [PubMed] [Google Scholar]
- 171.Ludtke A., Genschel J., Brabant G., Bauditz J., Taupitz M., Koch M., Wermke W., Worman H. J., Schmidt H. H. 2005. Hepatic steatosis in Dunnigan-type familial partial lipodystrophy. Am. J. Gastroenterol. 100: 2218–2224. [DOI] [PubMed] [Google Scholar]
- 172.Boguslavsky R. L., Stewart C. L., Worman H. J. 2006. Nuclear lamin A inhibits adipocyte differentiation: implications for Dunnigan-type familial partial lipodystrophy. Hum. Mol. Genet. 15: 653–663. [DOI] [PubMed] [Google Scholar]
- 173.Park J. Y., Javor E. D., Cochran E. K., DePaoli A. M., Gorden P. 2007. Long-term efficacy of leptin replacement in patients with Dunnigan-type familial partial lipodystrophy. Metabolism. 56: 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cui Z., Houweling M. 2002. Phosphatidylcholine and cell death. Biochim. Biophys. Acta. 1585: 87–96. [DOI] [PubMed] [Google Scholar]
- 175.Walkey C. J., Yu L., Agellon L. B., Vance D. E. 1998. Biochemical and evolutionary significance of phospholipid methylation. J. Biol. Chem. 273: 27043–27046. [DOI] [PubMed] [Google Scholar]
- 176.Vance D. E. 1990. Boehringer Mannheim Award lecture. Phosphatidylcholine metabolism: masochistic enzymology, metabolic regulation, and lipoprotein assembly. Biochem. Cell Biol. 68: 1151–1165. [DOI] [PubMed] [Google Scholar]
- 177.Millay D. P., Maillet M., Roche J. A., Sargent M. A., McNally E. M., Bloch R. J., Molkentin J. D. 2009. Genetic manipulation of dysferlin expression in skeletal muscle: novel insights into muscular dystrophy. Am. J. Pathol. 175: 1817–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bansal D., Miyake K., Vogel S. S., Groh S., Chen C. C., Williamson R., McNeil P. L., Campbell K. P. 2003. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 423: 168–172. [DOI] [PubMed] [Google Scholar]
- 179.Amato A. A., Brown R. H., Jr 2011. Dysferlinopathies. Handb. Clin. Neurol. 101: 111–118. [DOI] [PubMed] [Google Scholar]
- 180.Wu G., Sher R. B., Cox G. A., Vance D. E. 2009. Understanding the muscular dystrophy caused by deletion of choline kinase beta in mice. Biochim. Biophys. Acta. 1791: 347–356. [DOI] [PubMed] [Google Scholar]
- 181.Engel A. G., Angelini C. 1973. Carnitine deficiency of human skeletal muscle with associated lipid storage myopathy: a new syndrome. Science. 179: 899–902. [DOI] [PubMed] [Google Scholar]
- 182.Amat di San Filippo C., Pasquali M., Longo N. 2006. Pharmacological rescue of carnitine transport in primary carnitine deficiency. Hum. Mutat. 27: 513–523. [DOI] [PubMed] [Google Scholar]
- 183.Melegh B., Bene J., Mogyorósy G., Havasi V., Komlósi K., Pajor L., Oláh E., Kispál G., Sumegi B., Méhes K. 2004. Phenotypic manifestations of the OCTN2 V295X mutation: sudden infant death and carnitine-responsive cardiomyopathy in Roma families. Am. J. Med. Genet. A. 131: 121–126. [DOI] [PubMed] [Google Scholar]
- 184.Wang Y., Kelly M. A., Cowan T. M., Longo N. 2000. A missense mutation in the OCTN2 gene associated with residual carnitine transport activity. Hum. Mutat. 15: 238–245. [DOI] [PubMed] [Google Scholar]
- 185.Treem W. R., Stanley C. A., Finegold D. N., Hale D. E., Coates P. M. 1988. Primary carnitine deficiency due to a failure of carnitine transport in kidney, muscle, and fibroblasts. N. Engl. J. Med. 319: 1331–1336. [DOI] [PubMed] [Google Scholar]
- 186.Rinaldo P., Stanley C. A., Hsu B. Y., Sanchez L. A., Stern H. J. 1997. Sudden neonatal death in carnitine transporter deficiency. J. Pediatr. 131: 304–305. [DOI] [PubMed] [Google Scholar]
- 187.Carroll J. E. 1988. Myopathies caused by disorders of lipid metabolism. Neurol. Clin. 6: 563–574. [PubMed] [Google Scholar]
- 188.Rijlaarsdam R. S., van Spronsen F. J., Bink-Boelkens M. T., Reijngoud D. J., Wanders R. J., Niezen-Koning K. E., Van der Sluijs F. H., Dorland B., Beaufort-Krol G. C. 2004. Ventricular fibrillation without overt cardiomyopathy as first presentation of organic cation transporter 2-deficiency in adolescence. Pacing Clin. Electrophysiol. 27: 675–676. [DOI] [PubMed] [Google Scholar]
- 189.Pons R., De Vivo D. C. 1995. Primary and secondary carnitine deficiency syndromes. J. Child Neurol. 10(Suppl. 2): S8–S24. [PubMed] [Google Scholar]
- 190.Stanley C. A. 1987. New genetic defects in mitochondrial fatty acid oxidation and carnitine deficiency. Adv. Pediatr. 34: 59–88. [PubMed] [Google Scholar]
- 191.Stanley C. A. 2004. Carnitine deficiency disorders in children. Ann. N. Y. Acad. Sci. 1033: 42–51. [DOI] [PubMed] [Google Scholar]
- 192.Ohtani Y., Endo F., Matsuda I. 1982. Carnitine deficiency and hyperammonemia associated with valproic acid therapy. J. Pediatr. 101: 782–785. [DOI] [PubMed] [Google Scholar]
- 193.Bohles H., Richter K., Wagner-Thiessen E., Schafer H. 1982. Decreased serum carnitine in valproate induced Reye syndrome. Eur. J. Pediatr. 139: 185–186. [DOI] [PubMed] [Google Scholar]
- 194.Matsuda I., Ohtani Y., Ninomiya N. 1986. Renal handling of carnitine in children with carnitine deficiency and hyperammonemia associated with valproate therapy. J. Pediatr. 109: 131–134. [DOI] [PubMed] [Google Scholar]
- 195.Kaido M., Fujimura H., Ono A., Toyooka K., Yoshikawa H., Nishimura T., Ozaki K., Narama I., Kuwajima M. 1997. Mitochondrial abnormalities in a murine model of primary carnitine deficiency. Systemic pathology and trial of replacement therapy. Eur. Neurol. 38: 302–309. [DOI] [PubMed] [Google Scholar]
- 196.Cwik V. A. 2000. Disorders of lipid metabolism in skeletal muscle. Neurol. Clin. 18: 167–184. [DOI] [PubMed] [Google Scholar]
- 197.Bonnefont J. P., Djouadi F., Prip-Buus C., Gobin S., Munnich A., Bastin J. 2004. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol. Aspects Med. 25: 495–520. [DOI] [PubMed] [Google Scholar]
- 198.Yasuno T., Kaneoka H., Tokuyasu T., Aoki J., Yoshida S., Takayanagi M., Ohtake A., Kanazawa M., Ogawa A., Tojo K., et al. 2008. Mutations of carnitine palmitoyltransferase II (CPT II) in Japanese patients with CPT II deficiency. Clin. Genet. 73: 496–501. [DOI] [PubMed] [Google Scholar]
- 199.Corti S., Bordoni A., Ronchi D., Musumeci O., Aguennouz M., Toscano A., Lamperti C., Bresolin N., Comi G. P. 2008. Clinical features and new molecular findings in carnitine palmitoyltransferase II (CPT II) deficiency. J. Neurol. Sci. 266: 97–103. [DOI] [PubMed] [Google Scholar]
- 200.North K. N., Hoppel C. L., De Girolami U., Kozakewich H. P., Korson M. S. 1995. Lethal neonatal deficiency of carnitine palmitoyltransferase II associated with dysgenesis of the brain and kidneys. J. Pediatr. 127: 414–420. [DOI] [PubMed] [Google Scholar]
- 201.Thuillier L., Rostane H., Droin V., Demaugre F., Brivet M., Kadhom N., Prip-Buus C., Gobin S., Saudubray J. M., Bonnefont J. P. 2003. Correlation between genotype, metabolic data, and clinical presentation in carnitine palmitoyltransferase 2 (CPT2) deficiency. Hum. Mutat. 21: 493–501. [DOI] [PubMed] [Google Scholar]
- 202.Deutsch M., Vassilopoulos D., Sevastos N., Papadimitriou A., Vasiliou K., Archimandritis A. J. 2008. Severe rhabdomyolysis with hypoglycemia in an adult patient with carnitine palmitoyltransferase II deficiency. Eur. J. Intern. Med. 19: 289–291. [DOI] [PubMed] [Google Scholar]
- 203.Wieser T., Deschauer M., Olek K., Hermann T., Zierz S. 2003. Carnitine palmitoyltransferase deficiency: molecular and biochemical analysis of 32 patients. Neurology. 60: 1351–1353. [DOI] [PubMed] [Google Scholar]
- 204.Gregersen N. 1985. The acyl-CoA dehydrogenation deficiencies. Recent advances in the enzymic characterization and understanding of the metabolic and pathophysiological disturbances in patients with acyl-CoA dehydrogenation deficiencies. Scand. J. Clin. Lab. Invest. Suppl. 174: 1–60. [PubMed] [Google Scholar]
- 205.Reichmann H., Gold R. 1991. Myopathies and cardiomyopathies: histochemical and biochemical analyses. Eur Heart J. 12(Suppl. D): 169–170. [DOI] [PubMed] [Google Scholar]
- 206.Kelly D. P., Strauss A. W. 1994. Inherited cardiomyopathies. N. Engl. J. Med. 330: 913–919. [DOI] [PubMed] [Google Scholar]
- 207.Vianey-Saban C., Divry P., Brivet M., Nada M., Zabot M. T., Mathieu M., Roe C. 1998. Mitochondrial very-long-chain acyl-coenzyme A dehydrogenase deficiency: clinical characteristics and diagnostic considerations in 30 patients. Clin. Chim. Acta. 269: 43–62. [DOI] [PubMed] [Google Scholar]
- 208.Strauss A. W., Powell C. K., Hale D. E., Anderson M. M., Ahuja A., Brackett J. C., Sims H. F. 1995. Molecular basis of human mitochondrial very-long-chain acyl-CoA dehydrogenase deficiency causing cardiomyopathy and sudden death in childhood. Proc. Natl. Acad. Sci. USA. 92: 10496–10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Souri M., Aoyama T., Orii K., Yamaguchi S., Hashimoto T. 1996. Mutation analysis of very-long-chain acyl-coenzyme A dehydrogenase (VLCAD) deficiency: identification and characterization of mutant VLCAD cDNAs from four patients. Am. J. Hum. Genet. 58: 97–106. [PMC free article] [PubMed] [Google Scholar]
- 210.Andresen B. S., Olpin S., Poorthuis B. J., Scholte H. R., Vianey-Saban C., Wanders R., Ijlst L., Morris A., Pourfarzam M., Bartlett K., et al. 1999. Clear correlation of genotype with disease phenotype in very-long-chain acyl-CoA dehydrogenase deficiency. Am. J. Hum. Genet. 64: 479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gobin-Limballe S., Djouadi F., Aubey F., Olpin S., Andresen B. S., Yamaguchi S., Mandel H., Fukao T., Ruiter J. P., Wanders R. J., et al. 2007. Genetic basis for correction of very-long-chain acyl-coenzyme A dehydrogenase deficiency by bezafibrate in patient fibroblasts: toward a genotype-based therapy. Am. J. Hum. Genet. 81: 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Scheuerman O., Wanders R. J., Waterham H. R., Dubnov-Raz G., Garty B. Z. 2009. Mitochondrial trifunctional protein deficiency with recurrent rhabdomyolysis. Pediatr. Neurol. 40: 465–467. [DOI] [PubMed] [Google Scholar]
- 213.Angle B., Burton B. K. 2008. Risk of sudden death and acute life-threatening events in patients with glutaric acidemia type II. Mol. Genet. Metab. 93: 36–39. [DOI] [PubMed] [Google Scholar]
- 214.Olsen R. K., Andresen B. S., Christensen E., Bross P., Skovby F., Gregersen N. 2003. Clear relationship between ETF/ETFDH genotype and phenotype in patients with multiple acyl-CoA dehydrogenation deficiency. Hum. Mutat. 22: 12–23. [DOI] [PubMed] [Google Scholar]
- 215.Lan M. Y., Fu M. H., Liu Y. F., Huang C. C., Chang Y. Y., Liu J. S., Peng C. H., Chen S. S. 2010. High frequency of ETFDH c.250G>A mutation in Taiwanese patients with late-onset lipid storage myopathy. Clin. Genet. 78: 565–569. [DOI] [PubMed] [Google Scholar]
- 216.Jethva R., Bennett M. J., Vockley J. 2008. Short-chain acyl-coenzyme A dehydrogenase deficiency. Mol. Genet. Metab. 95: 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Thaman R., Firoozi S., Hamid M. S., McKenna W. J. 2002. Hypertrophic cardiomyopathy: management issues in the new millennium. Curr. Cardiol. Rep. 4: 226–232. [DOI] [PubMed] [Google Scholar]
- 218.Murphy R. T., Starling R. C. 2005. Genetics and cardiomyopathy: where are we now? Cleve. Clin. J. Med. 72: 465–466, 469–470, 472–473 passim. [DOI] [PubMed] [Google Scholar]
- 219.Ramaraj R. 2008. Hypertrophic cardiomyopathy: etiology, diagnosis, and treatment. Cardiol. Rev. 16: 172–180. [DOI] [PubMed] [Google Scholar]
- 220.Bonne G., Carrier L., Richard P., Hainque B., Schwartz K. 1998. Familial hypertrophic cardiomyopathy: from mutations to functional defects. Circ. Res. 83: 580–593. [DOI] [PubMed] [Google Scholar]
- 221.Olson T. M., Doan T. P., Kishimoto N. Y., Whitby F. G., Ackerman M. J., Fananapazir L. 2000. Inherited and de novo mutations in the cardiac actin gene cause hypertrophic cardiomyopathy. J. Mol. Cell. Cardiol. 32: 1687–1694. [DOI] [PubMed] [Google Scholar]
- 222.Colombo M. G., Botto N., Vittorini S., Paradossi U., Andreassi M. G. 2008. Clinical utility of genetic tests for inherited hypertrophic and dilated cardiomyopathies. Cardiovasc. Ultrasound. 6: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mogensen J., Klausen I. C., Pedersen A. K., Egeblad H., Bross P., Kruse T. A., Gregersen N., Hansen P. S., Baandrup U., Borglum A. D. 1999. Alpha-cardiac actin is a novel disease gene in familial hypertrophic cardiomyopathy. J. Clin. Invest. 103: R39–R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kumar A., Crawford K., Close L., Madison M., Lorenz J., Doetschman T., Pawlowski S., Duffy J., Neumann J., Robbins J., et al. 1997. Rescue of cardiac alpha-actin-deficient mice by enteric smooth muscle gamma-actin. Proc. Natl. Acad. Sci. USA. 94: 4406–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sparagna G. C., Johnson C. A., McCune S. A., Moore R. L., Murphy R. C. 2005. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J. Lipid Res. 46: 1196–1204. [DOI] [PubMed] [Google Scholar]
- 226.Sparagna G. C., Chicco A. J., Murphy R. C., Bristow M. R., Johnson C. A., Rees M. L., Maxey M. L., McCune S. A., Moore R. L. 2007. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J. Lipid Res. 48: 1559–1570. [DOI] [PubMed] [Google Scholar]
- 227.Saini-Chohan H. K., Holmes M. G., Chicco A. J., Taylor W. A., Moore R. L., McCune S. A., Hickson-Bick D. L., Hatch G. M., Sparagna G. C. 2009. Cardiolipin biosynthesis and remodeling enzymes are altered during development of heart failure. J. Lipid Res. 50: 1600–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hayashi T., Arimura T., Ueda K., Shibata H., Hohda S., Takahashi M., Hori H., Koga Y., Oka N., Imaizumi T., et al. 2004. Identification and functional analysis of a caveolin-3 mutation associated with familial hypertrophic cardiomyopathy. Biochem. Biophys. Res. Commun. 313: 178–184. [DOI] [PubMed] [Google Scholar]
- 229.Traverso M., Gazzerro E., Assereto S., Sotgia F., Biancheri R., Stringara S., Giberti L., Pedemonte M., Wang X., Scapolan S., et al. 2008. Caveolin-3 T78M and T78K missense mutations lead to different phenotypes in vivo and in vitro. Lab. Invest. 88: 275–283. [DOI] [PubMed] [Google Scholar]
- 230.Woodman S. E., Park D. S., Cohen A. W., Cheung M. W., Chandra M., Shirani J., Tang B., Jelicks L. A., Kitsis R. N., Christ G. J., et al. 2002. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J. Biol. Chem. 277: 38988–38997. [DOI] [PubMed] [Google Scholar]
- 231.Seidman C. E., Seidman J. G. 2001. Hypertrophic cardiomyopathy. The Metabolic and Molecular Bases of Inherited Disease. Eighth edition. Scriver C. R. Beaudet A. L. Valle D. Sly W. S. McGraw-Hill, New York, NY: 5433–5450. [Google Scholar]
- 232.Dhalla N. S., Saini-Chohan H. K., Rodriguez-Leyva D., Elimban V., Dent M. R., Tappia P. S. 2009. Subcellular remodelling may induce cardiac dysfunction in congestive heart failure. Cardiovasc. Res. 81: 429–438. [DOI] [PubMed] [Google Scholar]
- 233.Barth P. G., Wanders R. J., Vreken P., Janssen E. A., Lam J., Baas F. 1999. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome) (MIM 302060). J. Inherit. Metab. Dis. 22: 555–567. [DOI] [PubMed] [Google Scholar]
- 234.Barth P. G., Valianpour F., Bowen V. M., Lam J., Duran M., Vaz F. M., Wanders R. J. 2004. X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): an update. Am. J. Med. Genet. A. 126A: 349–354. [DOI] [PubMed] [Google Scholar]
- 235.Johnston J., Kelley R. I., Feigenbaum A., Cox G. F., Iyer G. S., Funanage V. L., Proujansky R. 1997. Mutation characterization and genotype-phenotype correlation in Barth syndrome. Am. J. Hum. Genet. 61: 1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Schlame M., Ren M. 2006. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 580: 5450–5455. [DOI] [PubMed] [Google Scholar]
- 237.Chen R., Tsuji T., Ichida F., Bowles K. R., Yu X., Watanabe S., Hirono K., Tsubata S., Hamamichi Y., Ohta J., et al. 2002. Noncompaction study collaborators. Mutation analysis of the G4.5 gene in patients with isolated left ventricular noncompaction. Mol. Genet. Metab. 77: 319–325. [DOI] [PubMed] [Google Scholar]
- 238.Ichida F., Tsubata S., Bowles K. R., Haneda N., Uese K., Miyawaki T., Dreyer W. J., Messina J., Li H., Bowles N. E., et al. 2001. Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation. 103: 1256–1263. [DOI] [PubMed] [Google Scholar]
- 239.Kelley R. 2002, Barth syndrome: X-linked cadiomyopathy and neutropenia. Accessed November 5, 2011, at http://www.barthsyndrome.org/CMFiles/Description_of_Barth_Syndrome_Mar200737QHA-3142007-7837.pdf.
- 240.Hauff K. D., Hatch G. M. 2006. Cardiolipin metabolism and Barth syndrome. Prog. Lipid Res. 45: 91–101. [DOI] [PubMed] [Google Scholar]
- 241.Hatch G. M. 2004. Cell biology of cardiac mitochondrial phospholipids. Biochem. Cell Biol. 82: 99–112. [DOI] [PubMed] [Google Scholar]
- 242.Hatch G. M. 1998. Cardiolipin: biosynthesis, remodeling and trafficking in the heart and mammalian cells. Int. J. Mol. Med. 1: 33–41. [DOI] [PubMed] [Google Scholar]
- 243.Hatch G. M. 1996. Regulation of cardiolipin biosynthesis in the heart. Mol. Cell. Biochem. 159: 139–148. [DOI] [PubMed] [Google Scholar]
- 244.Neuwald A. F. 1997. Barth syndrome may be due to an acyltransferase deficiency. Curr. Biol. 7: R465–R466. [DOI] [PubMed] [Google Scholar]
- 245.Vreken P., Valianpour F., Nijtmans L. G., Grivell L. A., Plecko B., Wanders R. J., Barth P. G. 2000. Defective remodeling of cardiolipin and phosphatidylglycerol in Barth syndrome. Biochem. Biophys. Res. Commun. 279: 378–382. [DOI] [PubMed] [Google Scholar]
- 246.Valianpour F., Wanders R. J., Overmars H., Vreken P., Van Gennip A. H., Baas F., Plecko B., Santer R., Becker K., Barth P. G. 2002. Cardiolipin deficiency in X-linked cardioskeletal myopathy and neutropenia (Barth syndrome, MIM 302060): a study in cultured skin fibroblasts. J. Pediatr. 141: 729–733. [DOI] [PubMed] [Google Scholar]
- 247.Valianpour F., Wanders R. J., Barth P. G., Overmars H., van Gennip A. H. 2002. Quantitative and compositional study of cardiolipin in platelets by electrospray ionization mass spectrometry: application for the identification of Barth syndrome patients. Clin. Chem. 48: 1390–1397. [PubMed] [Google Scholar]
- 248.Schlame M., Towbin J. A., Heerdt P. M., Jehle R., DiMauro S., Blanck T. J. 2002. Deficiency of tetralinoleoyl-cardiolipin in Barth syndrome. Ann. Neurol. 51: 634–637. [DOI] [PubMed] [Google Scholar]
- 249.Schlame M., Kelley R. I., Feigenbaum A., Towbin J. A., Heerdt P. M., Schieble T., Wanders R. J., DiMauro S., Blanck T. J. 2003. Phospholipid abnormalities in children with Barth syndrome. J. Am. Coll. Cardiol. 42: 1994–1999. [DOI] [PubMed] [Google Scholar]
- 250.Xu Y., Sutachan J. J., Plesken H., Kelley R. I., Schlame M. 2005. Characterization of lymphoblast mitochondria from patients with Barth syndrome. Lab. Invest. 85: 823–830. [DOI] [PubMed] [Google Scholar]
- 251.Taylor W. A., Xu F. Y., Ma B. J., Mutter T. C., Dolinsky V. W., Hatch G. M. 2002. Expression of monolysocardiolipin acyltransferase activity is regulated in concert with the level of cardiolipin and cardiolipin biosynthesis in the mammalian heart. BMC Biochem. 3: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Danos M., Taylor W. A., Hatch G. M. 2008. Mitochondrial monolysocardiolipin acyltransferase is elevated in the surviving population of H9c2 cardiac myoblast cells exposed to 2-deoxyglucose-induced apoptosis. Biochem. Cell Biol. 86: 11–20. [DOI] [PubMed] [Google Scholar]
- 253.Taylor W. A., Hatch G. M. 2009. Identification of the human mitochondrial linoleoyl-coenzyme a monolysocardiolipin acyltransferase (MLCL AT-1). J. Biol. Chem. 284: 30360–30371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Xu Y., Kelley R. I., Blanck T. J., Schlame M. 2003. Remodeling of cardiolipin by phospholipid transacylation. J. Biol. Chem. 278: 51380–51385. [DOI] [PubMed] [Google Scholar]
- 255.Xu Y., Malhotra A., Ren M., Schlame M. 2006. The enzymatic function of tafazzin. J. Biol. Chem. 281: 39217–39224. [DOI] [PubMed] [Google Scholar]
- 256.Valianpour F., Mitsakos V., Schlemmer D., Towbin J. A., Taylor J. M., Ekert P. G., Thorburn D. R., Munnich A., Wanders R. J., Barth P. G., et al. 2005. Monolysocardiolipins accumulate in Barth syndrome but do not lead to enhanced apoptosis. J. Lipid Res. 46: 1182–1195. [DOI] [PubMed] [Google Scholar]
- 257.Schlame M. 2008. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J. Lipid Res. 49: 1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Khuchua Z., Yue Z., Batts L., Strauss A. W. 2006. A zebrafish model of human Barth syndrome reveals the essential role of tafazzin in cardiac development and function. Circ. Res. 99: 201–208. [DOI] [PubMed] [Google Scholar]
- 259.Acehan D., Vaz F., Houtkooper R. H., James J., Moore V., Tokunaga C., Kulik W., Wansapura J., Toth M. J., Strauss A., et al. 2011. Cardiac and skeletal muscle defects in a mouse model of Barth syndrome. J. Biol. Chem. 286: 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Malhotra A, Edelman-Novemsky I., Xu Y., Plesken H., Ma J., Schlame M., Ren M. 2009. Role of calcium-independent phospholipase A2 in the pathogenesis of Barth syndrome. Proc. Natl. Acad. Sci. USA. 106: 2337–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Houtkooper R. H., Rodenburg R. J., Thiels C., van Lenthe H., Stet F., Poll-The B. T., Stone J. E., Steward C. G., Wanders R. J., Smeitink J., et al. 2009. Cardiolipin and monolysocardiolipin analysis in fibroblasts, lymphocytes, and tissues using high-performance liquid chromatography-mass spectrometry as a diagnostic test for Barth syndrome. Anal. Biochem. 387: 230–237. [DOI] [PubMed] [Google Scholar]
- 262.Spencer C. T., Bryant R. M., Day J., Gonzalez I. L., Colan S. D., Thompson W. R., Berthy J., Redfearn S. P., Byrne B. J. 2006. Cardiac and clinical phenotype in Barth syndrome. Pediatrics. 118: e337–e346. [DOI] [PubMed] [Google Scholar]
- 263.Hauff K. D., Hatch G. M. 2010. Reduction in cholesterol synthesis in response to serum starvation in lymphoblasts of a patient with Barth syndrome. Biochem. Cell Biol. 88: 595–602. [DOI] [PubMed] [Google Scholar]
- 264.Hardie D. G., Scott J. W., Pan D. A., Hudson E. R. 2003. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 546: 113–120. [DOI] [PubMed] [Google Scholar]
- 265.Sambandam N., Lopaschuk G. D. 2003. AMP-activated protein kinase (AMPK) control of fatty acid and glucose metabolism in the ischemic heart. Prog. Lipid Res. 42: 238–256. [DOI] [PubMed] [Google Scholar]
- 266.Athea Y., Viollet B., Mateo P., Rousseau D., Novotova M., Garnier A., Vaulont S., Wilding J. R., Grynberg A., Veksler V., et al. 2007. AMP-activated protein kinase alpha2 deficiency affects cardiac cardiolipin homeostasis and mitochondrial function. Diabetes. 56: 786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Gilbert-Barness E. 2004. Review: metabolic cardiomyopathy and conduction system defects in children. Ann. Clin. Lab. Sci. 34: 15–34. [PubMed] [Google Scholar]
- 268.Linhart A., Magage S., Palecek T., Bultas J. 2002. Cardiac involvement in Fabry disease. Acta Paediatr. Suppl. 91: 15–20. [DOI] [PubMed] [Google Scholar]
- 269.Caciotti A., Donati M. A., Procopio E., Filocamo M., Kleijer W., Wuyts W., Blaumeiser B., d'Azzo A., Simi L., Orlando C., et al. 2007. GM1 gangliosidosis: molecular analysis of nine patients and development of an RT-PCR assay for GLB1 gene expression profiling. Hum. Mutat. 28: 204. [DOI] [PubMed] [Google Scholar]
- 270.Otomo T., Muramatsu T., Yorifuji T., Okuyama T., Nakabayashi H., Fukao T., Ohura T., Yoshino M., Tanaka A., Okamoto N., et al. 2009. Mucolipidosis II and III alpha/beta: mutation analysis of 40 Japanese patients showed genotype-phenotype correlation. J. Hum. Genet. 54: 145–151. [DOI] [PubMed] [Google Scholar]
- 271.Grabowski G. A. 2008. Phenotype, diagnosis, and treatment of Gaucher's disease. Lancet. 372: 1263–1271. [DOI] [PubMed] [Google Scholar]
- 272.MacDermot K. D., Holmes A., Miners A. H. 2001. A natural history of Fabry disease in affected males and obligate carrier females. J. Inherit. Metab. Dis. 24(Suppl. 2): 13–14. [DOI] [PubMed] [Google Scholar]
- 273.Eng C. M., Guffon N., Wilcox W. R., Germain D. P., Lee P., Waldek S., Caplan L., Linthorst G. E., Desnick R. J., and International Collaborative Fabry Disease Study Group. 2001. Safety and efficacy of recombinant alpha-galactosidase A--replacement therapy in Fabry's disease. N. Engl. J. Med. 345: 9–16. [DOI] [PubMed] [Google Scholar]
- 274.Thurberg B. L., Fallon J. T., Mitchell R., Aretz T., Gordon R. E., O'Callaghan M. W. 2009. Cardiac microvascular pathology in Fabry disease: evaluation of endomyocardial biopsies before and after enzyme replacement therapy. Circulation. 119: 2561–2567. [DOI] [PubMed] [Google Scholar]
- 275.Kreutzer R., Kreutzer M., Pröpsting M. J., Sewell A. C., Leeb T., Naim H. Y., Baumgärtner W. 2008. Insights into post-translational processing of beta-galactosidase in an animal model resembling late infantile human G-gangliosidosis. J. Cell. Mol. Med. 12: 1661–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hinek A., Zhang S., Smith A. C., Callahan J. W. 2000. Impaired elastic-fiber assembly by fibroblasts from patients with either Morquio B disease or infantile GM1-gangliosidosis is linked to deficiency in the 67-kD spliced variant of beta-galactosidase. Am. J. Hum. Genet. 67: 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Martin J. J., Leroy J. G., Farriaux J. P., Fontaine G., Desnick R. J., Cabello A. 1975. I-cell disease (mucolipidosis II): a report on its pathology. Acta Neuropathol. 33: 285–305. [DOI] [PubMed] [Google Scholar]
- 278.Satoh Y., Sakamoto K., Fujibayashi Y., Uchiyama T., Kajiwara N., Hatano M. 1983. Cardiac involvement in mucolipidosis. Importance of non-invasive studies for detection of cardiac abnormalities. Jpn. Heart J. 24: 149–159. [DOI] [PubMed] [Google Scholar]
- 279.Brady R. O., Kanfer J. N., Shapiro D. 1965. Metabolism of glucocerebrosides. II. Evidence of an enzymatic deficiency in Gaucher's disease. Biochem. Biophys. Res. Commun. 18: 221–225. [DOI] [PubMed] [Google Scholar]
- 280.Stöllberger C., Finsterer J. 2007. Extracardiac medical and neuromuscular implications in restrictive cardiomyopathy. Clin. Cardiol. 30: 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ferrans V. J., Rodríguez E. R., McAllister H. A., Jr 1985. Granulomatous inflammation of the heart. Heart Vessels Suppl. 1: 262–270. [DOI] [PubMed] [Google Scholar]
- 282.Brady R. O., Schiffmann R. 2004. Enzyme-replacement therapy for metabolic storage disorders. Lancet Neurol. 3: 752–756. [DOI] [PubMed] [Google Scholar]
- 283.Schweiger M., Lass A., Zimmermann R., Eichmann T. O., Zechner R. 2009. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am. J. Physiol. Endocrinol. Metab. 297: E289–E296. [DOI] [PubMed] [Google Scholar]
- 284.Hirano K., Ikeda Y., Zaima N., Sakata Y., Matsumiya G. 2008. Triglyceride deposit cardiomyovasculopathy. N. Engl. J. Med. 359: 2396–2398. [DOI] [PubMed] [Google Scholar]
- 285.Fischer J., Lefevre C., Morava E., Mussini J. M., Laforet P., Negre-Salvayre A., Lathrop M., Salvayre R. 2007. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat. Genet. 39: 28–30. [DOI] [PubMed] [Google Scholar]
- 286.Huijsman E., van de Par C., Economou C., van der Poel C., Lynch G. S., Schoiswohl G., Haemmerle G., Zechner R., Watt M. J. 2009. Adipose triacylglycerol lipase deletion alters whole body energy metabolism and impairs exercise performance in mice. Am. J. Physiol. Endocrinol. Metab. 297: E505–E513. [DOI] [PubMed] [Google Scholar]
- 287.Kienesberger P. C., Lee D., Pulinilkunnil T., Brenner D. S., Cai L., Magnes C., Koefeler H. C., Streith I. E., Rechberger G. N., Haemmerle G., et al. 2009. Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling. J. Biol. Chem. 284: 30218–30229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Pena-Penabad C., Almagro M., Martínez W., García-Silva J., Del Pozo J., Yebra M. T., Sanchez-Manzano C., Fonseca E. 2001. Dorfman-Chanarin syndrome (neutral lipid storage disease): new clinical features. Br. J. Dermatol. 144: 430–432. [DOI] [PubMed] [Google Scholar]
- 289.Dorfman M. L., Hershko C., Eisenberg S., Sagher F. 1974. Ichthyosiform dermatosis with systemic lipidosis. Arch. Dermatol. 110: 261–266. [PubMed] [Google Scholar]
- 290.Chanarin I., Patel A., Slavin G., Wills E. J., Andrews T. M., Stewart G. 1975. Neutral-lipid storage disease: a new disorder of lipid metabolism. BMJ. 1: 553–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Pujol R. M., Gilaberte M., Toll A., Florensa L., Lloreta J., González-Enseñat M. A., Fischer J., Azon A. 2005. Erythrokeratoderma variabilis-like ichthyosis in Chanarin-Dorfman syndrome. Br. J. Dermatol. 153: 838–841. [DOI] [PubMed] [Google Scholar]
- 292.Mela D., Artom A., Goretti R., Varagona G., Riolfo M., Ardoino S., Sanguineti G., Vitali A., Ricciardi S. 1996. Dorfman-Chanarin syndrome: a case with prevalent hepatic involvement. J. Hepatol. 25: 769–771. [DOI] [PubMed] [Google Scholar]
- 293.Di Donato S., Taroni F. 2004. Disorders of lipid metabolism. Myology. Engel A. G., Franzini-Armstrong C., McGraw-Hill, New York, NY: 1587–1621. [Google Scholar]
- 294.Bruno C., Bertini E., Di Rocco M., Cassandrini D., Ruffa G., De Toni T., Seri M., Spada M., Li Volti G., D'Amico A., et al. 2008. Clinical and genetic characterization of Chanarin-Dorfman syndrome. Biochem. Biophys. Res. Commun. 369: 1125–1128. [DOI] [PubMed] [Google Scholar]
- 295.Selimoglu M. A., Esrefoglu M., Gul M., Gungor S., Yildirim C., Seyhan M. 2009. Chanarin-Dorfman syndrome: clinical features of a rare lipid metabolism disorder. Pediatr. Dermatol. 26: 40–43. [DOI] [PubMed] [Google Scholar]
- 296.Nigro G., Comi L. I., Politano L., Limongelli F. M., Nigro V., De Rimini M. L., Giugliano M. A., Petretta V. R., Passamano L., Restucci B. 1995. Evaluation of the cardiomyopathy in Becker muscular dystrophy. Muscle Nerve. 18: 283–291. [DOI] [PubMed] [Google Scholar]
- 297.Kirchmann C., Kececioglu D., Korinthenberg R., Dittrich S. 2005. Echocardiographic and electrocardiographic findings of cardiomyopathy in Duchenne and Becker-Kiener muscular dystrophies. Pediatr. Cardiol. 26: 66–72. [DOI] [PubMed] [Google Scholar]
- 298.Shelbourne P., Johnson K. 1992. Myotonic dystrophy: another case of too many repeats? Hum. Mutat. 1: 183–189. [DOI] [PubMed] [Google Scholar]
- 299.Brisson D., Houde G., St-Pierre J., Vohl M. C., Mathieu J., Gaudet D. 2002. The pleiotropic expression of the myotonic dystrophy protein kinase gene illustrates the complex relationships between genetic, biological and clinical covariates of male aging. Aging Male. 5: 223–232. [PubMed] [Google Scholar]
- 300.Phillips M. F., Harper P. S. 1997. Cardiac disease in myotonic dystrophy. Cardiovasc. Res. 33: 13–22. [DOI] [PubMed] [Google Scholar]
- 301.Brunelle A., Laprévote O. 2009. Lipid imaging with cluster time-of-flight secondary ion mass spectrometry. Anal. Bioanal. Chem. 393: 31–35. [DOI] [PubMed] [Google Scholar]
- 302.Touboul D., Brunelle A., Halgand F., De La Porte S., Laprévote O. 2005. Lipid imaging by gold cluster time-of-flight secondary ion mass spectrometry: application to Duchenne muscular dystrophy. J. Lipid Res. 46: 1388–1395. [DOI] [PubMed] [Google Scholar]
- 303.Magnusson Y., Friberg P., Sjövall P., Dangardt F., Malmberg P., Chen Y. 2008. Lipid imaging of human skeletal muscle using TOF-SIMS with bismuth cluster ion as a primary ion source. Clin. Physiol. Funct. Imaging. 28: 202–209. [DOI] [PubMed] [Google Scholar]
- 304.Touboul D., Roy S., Germain D. P., Baillet A., Brion F., Prognon P., Chaminade P., Laprévote O. 2005. Fast fingerprinting by MALDI-TOF mass spectrometry of urinary sediment glycosphingolipids in Fabry disease. Anal. Bioanal. Chem. 382: 1209–1216. [DOI] [PubMed] [Google Scholar]