Abstract
Biosynthesis of 5,15-dihydroxyeicosatetraenoic acid (5,15-diHETE) in leukocytes involves consecutive oxygenation of arachidonic acid by 5-lipoxygenase (LOX) and 15-LOX in either order. Here, we analyzed the contribution of cyclooxygenase (COX)-2 to the biosynthesis of 5,15-diHETE and 5,11-diHETE in isolated human leukocytes activated with lipopolysaccharide and calcium ionophore A23187. Transformation of arachidonic acid was initiated by 5-LOX providing 5S-HETE as a substrate for COX-2 forming 5S,15S-diHETE, 5S,15R-diHETE, and 5S,11R-diHETE as shown by LC/MS and chiral phase HPLC analyses. The levels of 5,15-diHETE were 0.45 ± 0.2 ng/106 cells (mean ± SEM, n = 6), reaching about half the level of LTB4 (1.3 ± 0.5 ng/106 cells, n = 6). The COX-2 specific inhibitor NS-398 reduced the levels of 5,15-diHETE to below 0.02 ng/106 cells in four of six samples. Similar reduction was achieved by MK-886, an inhibitor of 5-LOX activating protein but the above differences were not statistically significant. Aspirin treatment of the activated cells allowed formation of 5,15-diHETE (0.1 ± 0.05 ng/106 cells, n = 6) but, as expected, abolished formation of 5,11-diHETE. The mixture of activated cells also produced 5S,12S-diHETE with the unusual 6E,8Z,10E double bond configuration, implicating biosynthesis by 5-LOX and 12-LOX activity rather than by hydrolysis of the leukotriene A4-epoxide. Exogenous octadeuterated 5S-HETE and 15S-HETE were converted to 5,15-diHETE, implicating that multiple oxygenation pathways of arachidonic acid occur in activated leukocytes. The contribution of COX-2 to the biosynthesis of dihydroxylated derivatives of arachidonic acid provides evidence for functional coupling with 5-LOX in activated human leukocytes.
Keywords: 5-lipoxygenase, leukotriene, prostaglandin, 15-lipoxygenase, aspirin
Oxygenation of arachidonic acid at the 5-carbon by 5-lipoxygenase (5-LOX) gives rise to 5S-hydroperoxyeicosatetraenoic acid (5S-HPETE). 5S-HPETE undergoes further transformation by 5-LOX to the leukotriene epoxide LTA4 or reduction to the hydroxy derivative, 5S-HETE (1). 5-LOX is expressed in neutrophils, eosinophils, macrophages, and mast cells, and requires support by the accessory membrane protein 5-LOX activating protein (FLAP) for enzymatic activity in intact cells (2). Whereas 5-LOX is the only enzyme capable of oxygenating the 5-position of arachidonic acid, several enzymes can oxygenate the 15-carbon (3): two 15-lipoxygenases, 15-LOX-1 (the 12/15-LOX in the mouse) and 15-LOX-2 (4, 5), COX-1 and COX-2, both forming 15-HETE and 11-HETE as by-products of prostaglandin biosynthesis (6, 7), and aspirin-acetylated COX-2, a pure 15R-oxygenase (8–11).
Insertion of oxygen at one end of arachidonic acid does not impinge on the reactive pentadiene system at the other end, and, therefore, double oxygenation at both the 5- and 15-carbons of arachidonic acid is readily achievable. For example, formation of 5,15-diHETE in rat mononuclear cells and in human leukocytes is catalyzed by consecutive transformation of arachidonic acid by 5-LOX and 15-LOX in either order (12). Consecutive oxygenation of arachidonic acid by 5-LOX and 15-LOX is also instrumental in the biosynthesis of lipoxins. Lipoxin formation, however, additionally requires that one of the enzymes executes LTA-synthase activity, i.e., the dehydration of a hydroperoxide to an epoxide. Hydrolysis of the epoxide intermediate furnishes the final trihydroxylated arachidonic acid derivative (13, 14). The 15R-oxygenating activity of aspirin acetylated COX-2 can substitute for the 15-LOX activity in lipoxin biosynthesis, resulting in the formation of aspirin-triggered lipoxins (15).
Our interest in the biosynthesis of 5,15-diHETE stemmed from its formation as a byproduct of the oxygenation of 5S-HETE by COX-2 in vitro (16). This finding implicated crossover of the 5-LOX and COX-2 pathways as an alternative biosynthetic route of 5,15-diHETE in vivo with 5-LOX forming 5S-HETE as the first step, followed by COX-2 catalysis as the second step. The main product of the reaction is a di-endoperoxide that is structurally similar to the arachidonic acid-derived endoperoxide of prostaglandin biosynthesis (17). The catalytic byproducts include 5,15-diHETE (as a ∼4:1 mixture of the 5S,15S- and 5S,15R-diastereomers) and 5S,11R-diHETE (16). The diHETEs are the 5S-hydroxy analogs of the 15- and 11-HETE byproducts of the COX-1 and COX-2 reactions with arachidonic acid (18, 19).
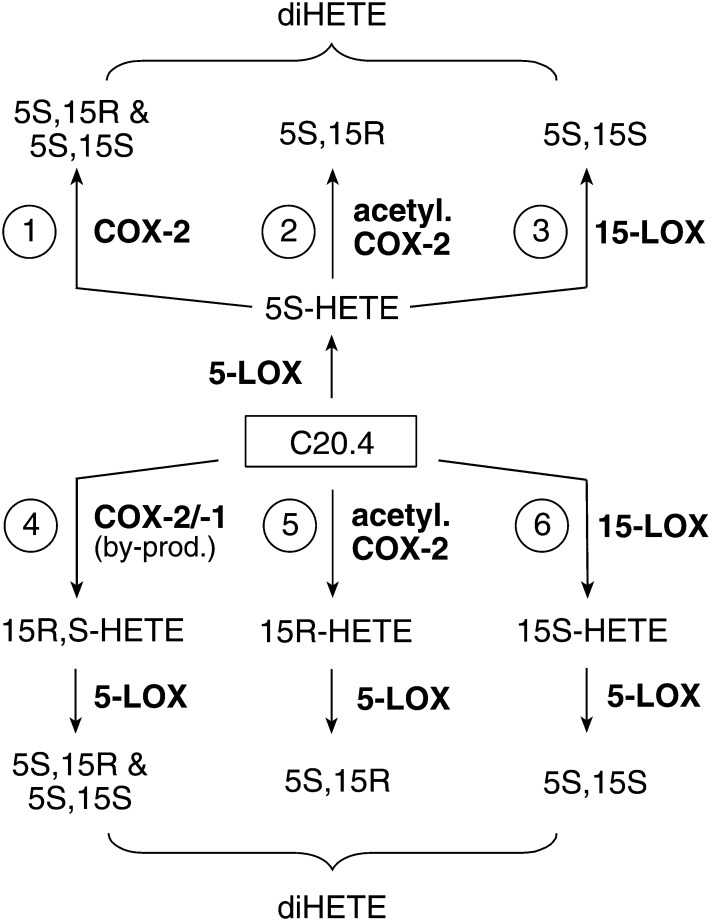
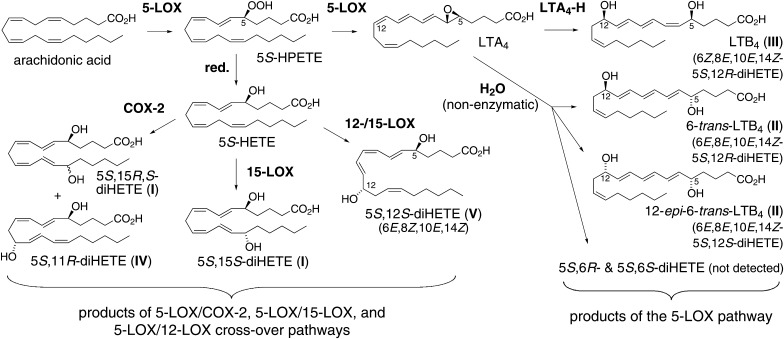
Thus, 5,15-diHETE may be biosynthesized through a multitude of pathways that involve reactions of 5-LOX, 15-LOX, COX-1, and COX-2, or acetylated COX-2 as illustrated in Fig. 1. Here, we analyzed the biosynthesis of 5,15-diHETE and 5,11-diHETE in activated human leukocytes. The enzymatic reactions involved were elucidated by the use of inhibitors of 5-LOX and COX-2 activities, deuterated HETE substrates, and stereochemical analysis of the diHETE products.
Fig. 1.
Pathways for biosynthesis of 5,15-diHETE. Different combinations of 5-LOX, 15-LOX, COX-1, COX-2, or aspirin-acetylated COX-2 can lead to the formation of 5S,15S- and 5S,15R-diHETE from arachidonic acid (C20.4).
Experimental Procedures
Materials
Arachidonic acid was purchased from NuChek Prep. d8-Arachidonic acid, NS-398, and MK-886 were from Cayman Chemical. Lipopolysaccharide (LPS) (serotype 0111:B4) was from Calbiochem, d4-LTB4 was from Biomol/Enzo. 5S-HETE and [5,6,8,9,11,12,14,15]d8-5-HETE were prepared by chemical synthesis from arachidonic acid or d8-arachidonic acid as described (20). 5-HETE was resolved into enantiomers using chiral-phase HPLC (21). d8-5-HETE could not be resolved into the enantiomers on the columns available (Chiralpak AD, Chiralpak AD-RH) and was used as the racemic mixture. [5,6,8,9,11,12,14,15]d8-15S-HETE was prepared using soybean LOX and reduction of the hydroperoxide with triphenylphosphine (22). 6E,8Z,10E-5S,12S-diHETE was prepared by reaction of 5S-HETE with recombinant platelet 12-LOX expressed in the baculovirus/Sf9 insect cell system.
Leukocyte preparation and extraction
Leukocytes were isolated from peripheral blood from healthy human subjects. The study was approved by the Vanderbilt University Medical Center Institutional Review Board (protocol #091243), and informed consent was obtained from the donors. Venous blood (45 ml) was collected into a syringe containing 5 ml of citric acid and 10 ml of 6% dextran (250 kDa average molecular weight). The blood was allowed to settle for 1 h and the top layer was collected and centrifuged. The pellet was washed with PBS, and remaining red cells were lysed by treatment with a 10-fold excess of deionized water for 30 s. The leukocytes were centrifuged, washed, and diluted in PBS+ containing 5 mM glucose to a concentration of 1.5 × 107 cells/ml. 1.0 × 107 cells were diluted to 1 ml, LPS (10 μg/ml) was added, and the cells were incubated at 37°C for 6 h. After addition of calcium ionophore A23187 (5 μM), the cells were incubated for an additional 15 min before the addition of 10 ng of d4-LTB4 standard, acidification to pH 4 using acetic acid, and extraction (Waters HLB cartridge). Products were eluted from the HLB cartridge with methanol, evaporated under a stream of nitrogen, and dissolved in 10 μl acetonitrile and 40 μl of LC-MS column solvent A.
Leukocyte treatment
The inhibitors NS-398 (10 μM), AA-861 (10 μM), or MK-886 (5 μM) were added to the cells 30 min before the end of LPS treatment. Aspirin was added at 2 mM final concentration (11). The deuterated substrates, d8-5S-HETE and d8-15S-HETE, respectively, were added at 50, 100, and 500 ng/106 cells (0.75, 1.5, and 7.5 μM, respectively) at the time of addition of A23187 and incubated for 15 min before acidification and extraction.
Resolution of 5,15-diHETE diastereomers
Resolution of the 5S,15S- and 5S,15R-diHETE diastereomers was achieved after derivatization to the pentafluorobenzyl (PFB) esters using a Chiralpak AD-RH column (4.6 × 150 mm; 5 μm) eluted with a solvent of acetonitrile/ethanol (90/10, by vol.) at a flow rate of 1 ml/min. The ∼77:23 mixture of 5S,15S- and 5S,15R-diHETE formed by reaction of recombinant COX-2 with 5S-HETE (16) was used to determine the elution order of the diastereomers. For analysis of 5,15-diHETE formed by leukocytes the cells stimulated with A23187 were extracted using an HLB cartridge, evaporated, and dissolved in 40 μl of pentafluorobenzyl bromide (10% in acetonitrile) and 20 μl of diisopropylethylamine. After 1 h incubation at room temperature, the samples were evaporated and reconstituted in 50 μl of acetonitrile for LC/MS analysis. The samples were analyzed using LC/MS with atmospheric pressure chemical ionization (APCI) (23). The same ion transition as in ESI analysis (m/z 335 to 201) was used. Instrument parameters were optimized by direct liquid infusion of a standard of 15-HETE-PFB ester dissolved in column solvent.
LC/MS and NMR analysis
Samples were analyzed using a ThermoFinnigan Quantum Access triple quadrupole mass spectrometer equipped with an electrospray interface and operated in the negative ion mode. Instrument specific parameters (sheath and auxiliary gas pressures, temperature, and interface voltage) were optimized using direct infusion of a solution of PGE2. A Waters Symmetry Shield C18 column (2.1 × 150 mm; 3 μm) was eluted with a linear gradient of acetonitrile/water, 10 mM NH4OAc from 5/95 (by vol.) to 95/5 (by vol.) at 0.2 ml/min within 10 min. The following transitions were recorded in the selected reaction monitoring (SRM) mode: 5,15-diHETE: m/z 335 to 201; d8-5,15-diHETE: m/z 343 to 208; 5,11-diHETE: m/z 335 to 183; LTB4: m/z 335 to 195; d4-LTB4: m/z 339 to 197; 5-HETE: m/z 319 to 115; d8-5-HETE: m/z 327 to 116; 15-HETE: m/z 319 to 175. The analysis was split into two segments monitoring diHETEs from 0 to 8 min and HETEs from 8 to 15 min. Products were quantified by comparison of their peak area versus the internal standard d4-LTB4 (6,7,14,15-d4-6Z,8E,10E-5S,12R-diHETE).
NMR spectra were recorded on a Bruker AV-II 600 MHz spectrometer equipped with a cryoprobe. CDCl3 was used as solvent (δ 7.25 ppm).
Results
Formation of diHETEs in activated leukocytes
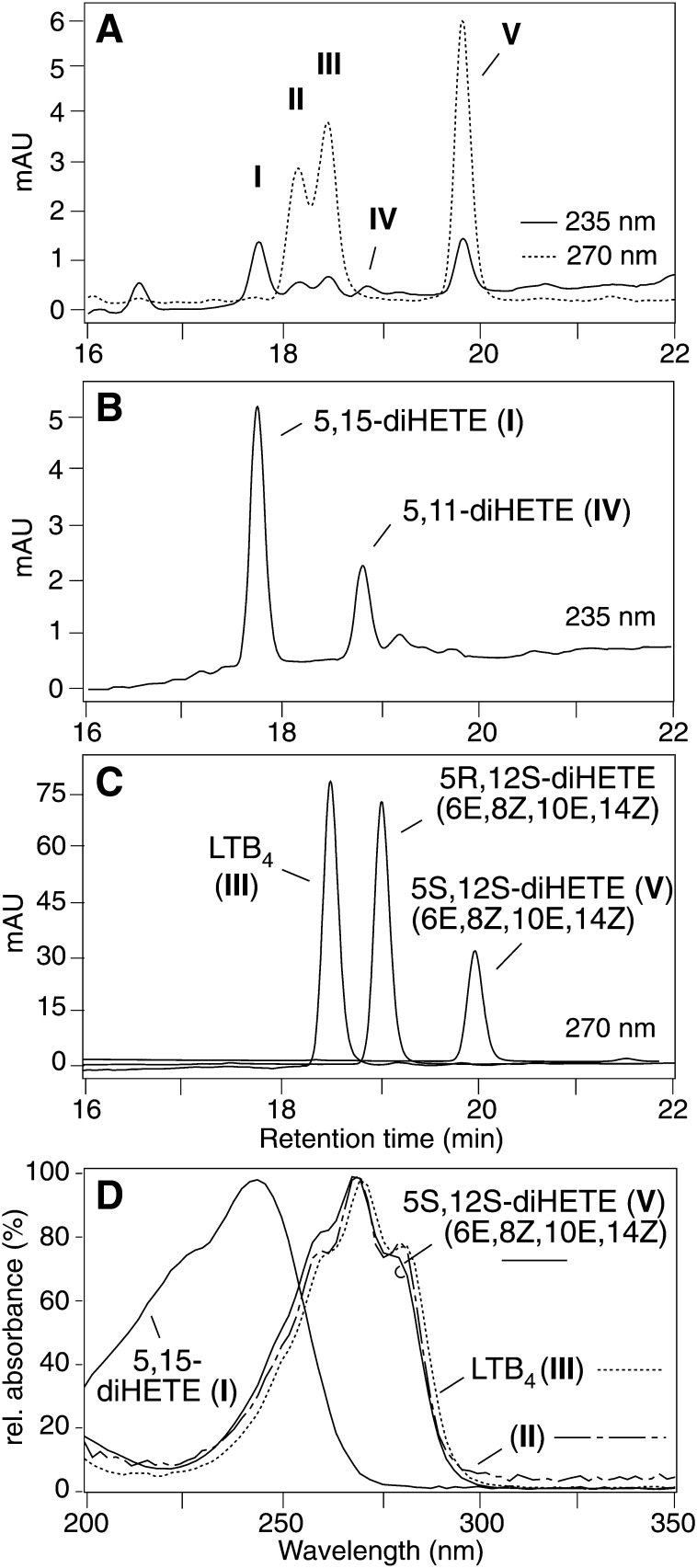
Reversed-phase-HPLC analysis of human leukocytes activated with LPS for 6 h followed by A23187 for 15 min showed formation of five products (I–V) with retention times and UV spectra that were characteristic of diHETE derivatives of arachidonic acid (Fig. 2A). Products I and IV were identified as 5,15-diHETE and 5,11-diHETE, respectively, by comparison of HPLC retention times and UV spectra with authentic standards (Fig. 2B) prepared by reaction of recombinant COX-2 with 5S-HETE (16). Product III was identified as LTB4 by coelution with an authentic standard and UV analysis (Fig. 2C, D). Product II was tentatively identified as 6E,8E,10E,14Z-5S,12R,S-diHETE (i.e., a mixture of 6-trans-LTB4 and 6-trans-12-epi-LTB4) based on retention time relative to LTB4 and UV spectrum (Fig. 2A, D) (24). The diastereomers of II are the major products formed by nonenzymatic hydrolysis of the LTA4 epoxide.
Fig. 2.
RP-HPLC analysis of diHETEs in activated human leukocytes. A: Leukocytes activated with LPS (6 h) and A23187 (15 min) form five distinct diHETEs. The absorbance of products I and IV at 235 nm indicates conjugated diene diHETEs, absorbance at 270 nm (II, III, and V) indicates conjugated triene diHETEs. (B) Elution of the standards 5,15-diHETE and 5,11-diHETE. (C) The chromatograms of separate analyses of the standards of LTB4 (III), 6E,8Z,10E,14Z-5R,12S-diHETE, and 6E,8Z,10E,14Z-5S,12S-diHETE (V), respectively, are combined in the same panel. (D) UV spectra of 5,15-diHETE (I), II (tentatively identified as a mixture of 6-trans-LTB4 and 6-trans-12-epi-LTB4), LTB4 (III), and 6E,8Z,10E,14Z-5S,12S-diHETE (V) recorded during the HPLC-diode array analysis in panel A. A Waters Symmetry C18 column was eluted with acetonitrile/water/acetic acid (37.5/62.5/0.01, by vol.) at 1.0 ml/min and online diode array detection. The chromatograms shown were recorded at 235 nm (panels A, B) and 270 nm (panels A, C).
Product V was identified as 6E,8Z,10E,14Z-5S,12S-diHETE by comparison of retention time and UV spectrum with an authentic standard (Fig. 2C, D). Peak V coeluted with the 5S,12S-diastereomer while the 5R,12S-diastereomer was resolved by about 1 min (Fig. 2C). The configuration of the double bonds of the authentic standard was confirmed by NMR analysis (J6,7 = 15.2 Hz (trans), J8,9 = 10.6 Hz (cis), J10,11 = 15.3 Hz (trans), J14,15 = 9.4 Hz (cis)). The double bond configuration of the 6E,8Z,10E triene implied that V was formed by consecutive oxygenation of arachidonic acid by 5-LOX and 12-LOX in either order, rather than by hydrolysis of the LTA4 epoxide. The 12-LOX activity was likely due to platelet 12-LOX. It has been shown that in vitro mixtures of platelets and neutrophils form 6E,8Z,10E,14Z-5S,12S-diHETE in response to stimulation with A23187 (25).
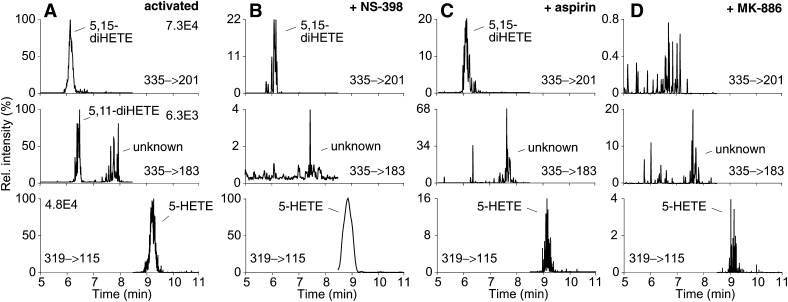
Analysis of 5,15-diHETE and 5,11-diHETE formation
We hypothesized that 5,15-diHETE (I) and 5,11-diHETE (IV) are products of COX-2 catalyzed oxygenation of 5S-HETE in activated leukocytes (Fig. 1). The role of COX-2 and 5-LOX in the biosynthesis of 5,15-diHETE and 5,11-diHETE was probed using inhibitors and LC/MS analysis in the SRM mode (Fig. 3A). Treatment of LPS-activated leukocytes with the COX-2 selective inhibitor NS-398 or with aspirin 30 min prior to stimulation with A23187 decreased 5,15-diHETE and completely inhibited 5,11-diHETE (Fig. 3B, C). The lesser effect on 5,15-diHETE was likely due to 15-LOX activity that would not have been affected by the inhibitors. In addition, aspirin treatment of COX-2 was expected to induce formation of 5S,15R-diHETE that coelutes with the 5S,15S-diastereomer (16). The FLAP inhibitor MK-886 inhibited formation of both 5,15-diHETE and 5,11-diHETE (Fig. 3D).
Fig. 3.
Effect of COX-2 and 5-LOX inhibitors on the formation of 5,15-diHETE, 5,11-diHETE, and 5-HETE by activated leukocytes. Leukocytes were activated with LPS and A23187 (A) without inhibitor, and (B) in the presence of NS-398, (C) aspirin, and (D) MK-886 and analyzed using LC-ESI-MS. The ion traces for the transitions of 5,15-diHETE (m/z 335 to 201, top), 5,11-diHETE (m/z 335 to 183, middle), and 5-HETE (m/z 319 to 115, bottom) are shown. The signal intensities are given across each row relative to the uninhibited sample in (A) set to 100%, and corrected using d4-LTB4 as internal standard. The sample in panel B was derived from a different subject and analyzed at a different time than the samples in panels A, C, and D.
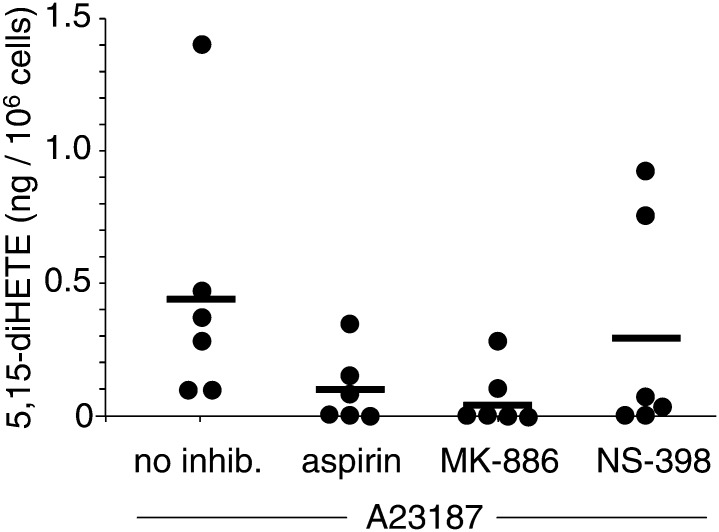
We used d4-LTB4 as internal standard to quantify 5-HETE, 5,15-diHETE, and LTB4 in activated leukocytes isolated from six healthy human subjects (Fig. 4). The levels of 5,15-diHETE were 0.45 ± 0.2 ng/106 cells (mean ± SEM, n = 6) compared with 5-HETE ranging between 0.4 and 2.4 ng/106 cells (mean 1.0 ± 0.36 ng/106 cells, n = 6) and LTB4 0.7 – 3.6 ng/106 cells (mean 1.3 ± 0.5 ng/106 cells, n = 6).
Fig. 4.
Quantification of 5,15-diHETE in activated human leukocytes. Samples from 6 healthy volunteers in the absence of or treated with aspirin, MK-886, or NS-398 30 min prior to 15-min incubation with A23187 were analyzed using LC-ESI-MS in the SRM mode. The mean of the values is indicated with a horizontal bar.
MK-886 reduced formation of 5,15-diHETE to below detectable levels in four out of six samples, indicating the crucial role of 5-LOX in biosynthesis (0.07 ± 0.04 ng/106 cells, n = 6) (Fig. 4). A possible explanation why MK-886 failed to completely inhibit formation of 5,15-diHETE in two samples is that the cells were partially broken, which renders the inhibitor ineffective. MK-886 inhibits FLAP rather than 5-LOX and is only effective in intact cells (26). Treatment with NS-398 (0.30 ± 0.17 ng/106 cells, n = 6) reduced the levels of 5,15-diHETE between 5- and 10-fold in four out of six samples whereas in one sample it had only a small effect and in the other increased the level of 5,15-diHETE. Inhibition by NS-398 indicates a major although not exclusive role of COX-2 in the biosynthesis of 5,15-diHETE. The portion of 5,15-diHETE that was not inhibited by NS-398 was likely formed by 15-LOX expressed by eosinophils in the leukocyte preparations. Unexpectedly, NS-398 also reduced the levels of 5-HETE and LTB4 in some of the samples (not shown). This could have been due to the ability of NS-398 to inhibit leukocyte lipid body formation independent of COX-2 inhibition leading to the suppression of LOX-derived eicosanoids (27). Aspirin did not completely inhibit formation of 5,15-diHETE (0.10 ± 0.05 ng/106 cells, n = 6), compatible with the expected formation 5S,15R-diHETE from 5S-HETE (16). None of the differences between the means of the treatment groups were statistically significant using a two-tailed unpaired t-test.
In addition, formation of 5,11-diHETE is highly indicative of the reaction of COX-2 with 5-HETE. Besides COX-2, COX-1 is the only other mammalian enzyme capable of oxygenating the 11-carbon of arachidonic acid, but COX-1 does not react with 5-HETE (17). The inverse reaction (of 5-LOX with 11R-HETE) is very inefficient (16) and thus unlikely to be relevant in cells. The levels of 5,11-diHETE were between 0.01 and 0.04 ng/106 cells. 5,11-DiHETE was reduced below the detectable limit (1 pg/106 cells) upon treatment with aspirin, MK-886, or NS-398, respectively.
Stereochemical analysis of 5,15-diHETE
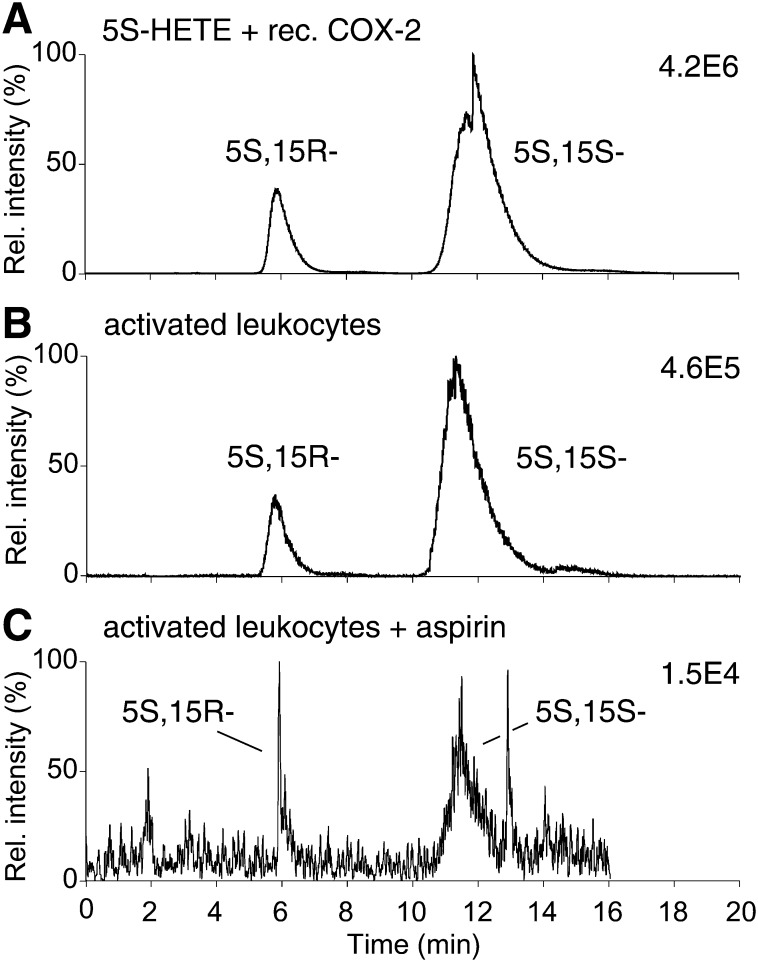
The configuration of C-15 of 5,15-diHETE can give an indication to the enzymes involved in its biosynthesis (cf. Fig. 1). 5,15-DiHETE formed by consecutive action of 5-LOX and 15-LOX (in either order) is purely 5S,15S (12). In contrast, oxygenation of 5S-HETE by COX-2 in vitro leads to formation of a ∼1:4 mixture of the 5S,15R- and 5S,15S-diHETE diastereomers, i.e., about 20% of 5,15-diHETE has the 5S,15R configuration. Acetylation of recombinant COX-2 by aspirin leads to exclusive formation of the 5S,15R diastereomer (16). Analysis of the configuration of the 5- and 15-carbons in 5,15-diHETE is challenging because the 5S,15S- and 5S,15R-diastereomers do not separate using RP-HPLC (12), and only marginally resolve using straight-phase-HPLC (16). Therefore, we developed a method for separation of the diastereomers using a Chiralpak AD-RH chiral phase column coupled with LC/MS detection. 5,15-DiHETE was derivatized to the PFB ester in order to enhance sensitivity by employing APCI with detection of the [M-PFB]− molecular ion (23).
Elution with acetonitrile/ethanol (90/10, by vol.) achieved baseline resolution of the 5S,15R-diHETE-PFB and 5S,15S-diHETE-PFB diastereomers. Using recombinant COX-2, the peak areas showed the expected ratio of 20:80 for 5S,15R to 5S,15S (Fig. 5A). Analysis of 5,15-diHETE formed by LPS/A23187-activated leukocytes showed a ratio of ∼15:85 of the 5S,15R- to the 5S,15S-diastereomer (Fig. 5B). Thus, compared with the ratio formed by recombinant COX-2 the proportion of the 5S,15R diastereomer in the leukocytes was largely retained implying a major contribution of COX-2 to the synthesis of 5,15-diHETE. The slight enrichment (∼10–20%) of the 5S,15S-diastereomer was likely due to synthesis by a 15-LOX enzyme. In a second sample, <5% of 5,15-diHETE was the 5S,15R-diastereomer, implicating a lesser contribution of COX-2 and higher contribution of 15-LOX. Aspirin treatment of activated leukocytes led to a smaller than expected increase in the proportion of 5S,15R-diHETE, possibly due to incomplete acetylation of COX-2. A representative sample (Fig. 5C) showed that about 25% of total 5,15-diHETE was the 5S,15R-diastereomer.
Fig. 5.
Stereochemical analysis of 5,15-diHETE. LC-MS analysis of (A) 5,15-diHETE formed by reaction of recombinant COX-2, (B) 5,15-diHETE formed by activated leukocytes, and (C) 5,15-diHETE formed by aspirin-treated activated leukocytes. The samples were derivatized to the PFB esters and analyzed using a Chiralpak AD-RH column eluted with acetonitrile/ethanol (90/10, by vol.) at 1 ml/min and detection by LC-APCI-MS operated in the SRM mode monitoring m/z 335 to 201.
Transformation of exogenous 5-HETE and 15-HETE to 5,15-diHETE
We analyzed the ability of activated leukocytes to utilize either 5-HETE or 15-HETE in the biosynthesis of 5,15-diHETE by measuring formation of octadeuterated 5,15-HETE from exogenously added d8-5-HETE and d8-15S-HETE, respectively. d8-5-HETE was used as a racemic mixture. The enantiomers did not resolve on SP or RP Chiralpak AD chiral phase HPLC columns although both give exceptional resolution of unlabeled HETEs (21, 28).
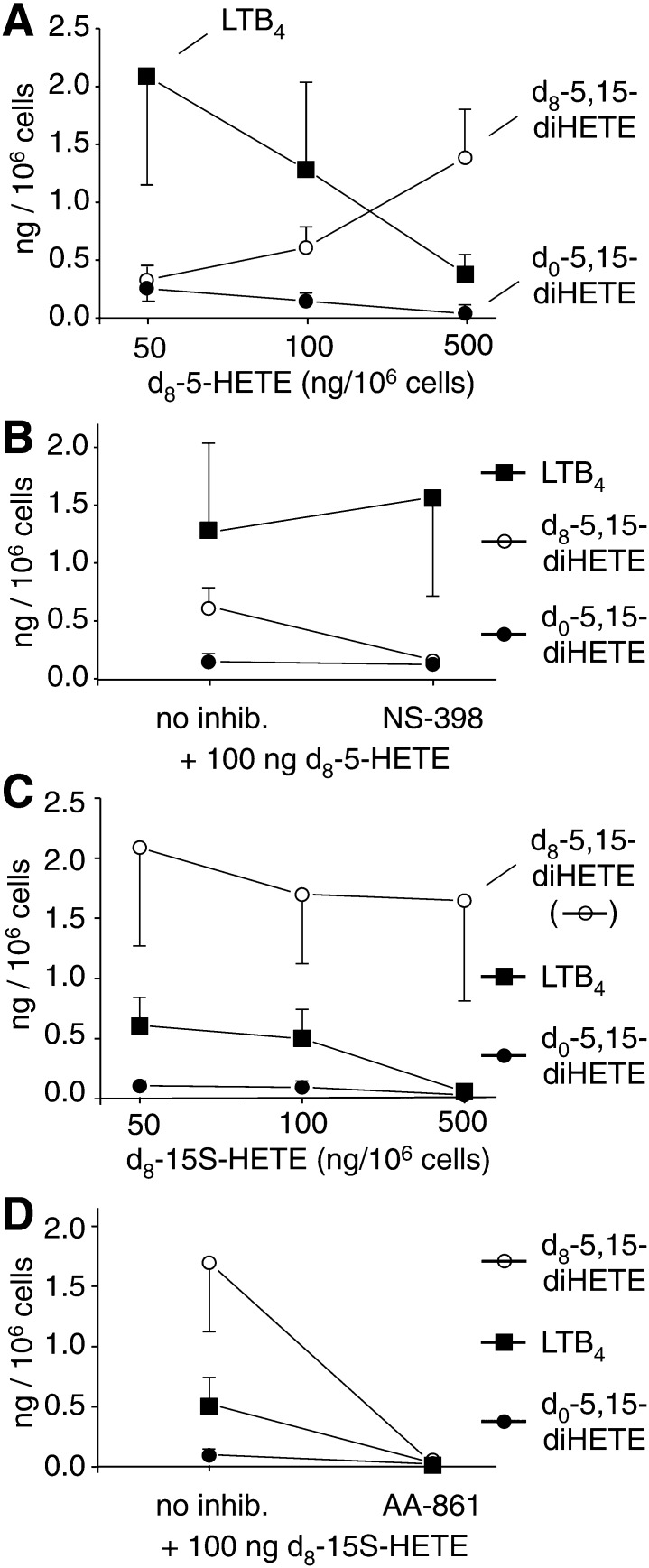
Three different concentrations of d8-5-HETE (50, 100, and 500 ng/106 cells, equivalent to 0.75, 1.5, and 7.5 μM) were added to LPS-activated leukocytes together with A23187 and incubated for 15 min. Formation of d8-5,15-diHETE, d0-5,15-diHETE, and LTB4 was analyzed using LC/MS in the SRM mode. With increasing concentration of exogenous d8-5-HETE, the levels of d8-5,15-diHETE increased, and this occurred partly at the expense of formation of d0-5,15-diHETE (Fig. 6A). This provided evidence for an activity that catalyzes 15-oxygenation of 5-HETE in the leukocytes. The 15-oxygenase activity was in part due to COX-2 because NS-398 reduced the formation of d8-5,15-diHETE (Fig. 6B). Additional indication that 15-oxygenation of 5-HETE was catalyzed by COX-2 came from the low levels of 15-LOX activity in the activated leukocytes. The levels of 15-HETE were about 1-2% of the levels of 5-HETE.
Fig. 6.
Transformation of exogenous d8-5-HETE and d8-15S-HETE into d8-5,15-diHETE by activated human leukocytes. (A) Levels of d8-5,15-diHETE, d0-5,15-diHETE, and LTB4 generated by incubation of leukocytes activated with LPS and A23187 with 50, 100, or 500 ng of d8-5R,S-HETE. (B) Activated leukocytes were incubated with 100 ng d8-5-HETE in the absence or presence of 10 μM NS-398. (C) Formation of d8-5,15-diHETE, d0-5,15-diHETE, and LTB4 upon incubation of the cells with 50, 100, or 500 ng of d8-15S-HETE. (D) Activated leukocytes were incubated with 100 ng d8-15S-HETE in the absence or presence of 10 μM AA-861. Data points are the mean ± S.E.M. from three different donors.
When d8-15S-HETE was added to the LPS-activated leukocytes together with A23187, high incorporation of the labeled substrate into 5,15-diHETE was observed, even at the lowest concentration of d8-15S-HETE used (Fig. 6C). At all three concentrations (50, 100, and 500 ng d8-15S-HETE /106 cells), the amount of d8-5,15-diHETE was between 10- and 20-fold higher than d0-5,15-diHETE, indicating that exogenous 15S-HETE was efficiently converted by the 5-LOX activity in the leukocytes. At the highest concentration of d8-15S-HETE added (7.5 μM), formation of endogenous (d0-) 5,15-diHETE was almost completely suppressed. As expected, the 5-LOX inhibitor AA-861 reduced the amount of both d8-5,15-diHETE and d0-5,15-diHETE as well as LTB4 below detectable levels (Fig. 6D).
The levels of LTB4 (which cannot be formed from 5-HETE or 15-HETE) were reduced about 10-fold at the highest concentration of either of the exogenous HETEs added (Fig. 6A, C). It is not clear why exogenous 5-HETE inhibited LTB4 formation but inhibition by 15-HETE could have been due to outcompeting arachidonic acid as a substrate for 5-LOX (29). Inhibition of endogenous 5,15-diHETE by the highest concentration of 15-HETE could partially be due to inhibition of COX-2 by 15S-HETE (30).
Discussion
Eicosanoid biosynthesis in vivo occurs in an environment that enables transcellular biosynthesis (31). The exchange of arachidonic acid substrate and its oxygenated products between phagocytic, immune, and endothelial cells is used in the biosynthesis of leukotrienes, lipoxins, and other lipid autacoids. Thus, analysis of mixtures of leukocytes can lead to identification of eicosanoids that are absent or less prominent in monotypic cell populations. A major goal of this study was to provide evidence for a functional biosynthetic coupling of 5-LOX expressed in granulocytes and COX-2 expressed in monocytes.
The main product of the transformation of 5-HETE by COX-2 in vitro has been identified as an unstable di-endoperoxide (17); 5,11- and 5,15-diHETE are reaction by-products (16). The chemical instability makes direct identification of the di-endoperoxide in vivo a difficult task. We hypothesized that, instead, formation of 5,15-diHETE and 5,11-diHETE could provide evidence for the oxygenation of 5S-HETE by COX-2 in vivo. While these studies were in progress, we identified two hemiketal eicosanoids as the major transformation products of the unstable di-endoperoxide. The hemiketals are present in activated human leukocytes and induce tubulogenesis of endothelial cells (32).
Biosynthesis of 5,15-diHETE through crossover of the 5-LOX and 15-LOX pathways has been documented in leukocytes from normal volunteers (12) and from patients with eosinophilia (33) and asthma (34). Consecutive oxygenation of arachidonic acid by the 5-LOX and 15-LOX enzymes is illustrated as routes 3 and 6 in Fig. 1. Alternative pathways could entail the 15-oxygenase activities of COX-1 and COX-2 (routes 1 and 4 in Fig. 1), and the 15R-oxygenase activity of aspirin-acetylated COX-2 (routes 2 and 5). Since testing of the alternative pathways required 5-LOX and COX-2 activities we used a crude fraction of human leukocytes containing neutrophils and eosinophils in order to provide 5-LOX activity, and monocytes/macrophages for COX-2 activity. COX-2 activity was induced by LPS (35), followed by stimulation of 5-LOX activity with calcium ionophore (36). Involvement of COX-2 in the biosynthesis of 5,15-diHETE in activated human leukocytes was evident from three independent findings: 1) significant formation of the 5S,15R-diastereomer (about 15% of total 5,15-diHETE) was indicative of COX-2 activity, resembling the ∼20:80 ratio formed by the recombinant enzyme (16) [and no 15R-LOX enzyme exists in humans or any other mammal (37)]; 2) the levels of 5,15-diHETE were markedly decreased in the presence of the COX-2 specific inhibitor NS-398; and 3) aspirin treatment of the leukocytes increased the ratio of 5S,15R-diHETE versus 5S,15S-diHETE (while decreasing overall synthesis of 5,15-diHETE) and abolished formation of 5,11-diHETE. In addition, biosynthesis of 5,11-diHETE was due to COX-2 reaction with 5-HETE because it was blocked by NS-398, and no 11-LOX activity has been reported in mammals (3).
An interesting facet of the double oxygenation of arachidonic acid at carbons 5 and 15 is that the reactions can occur in either order. Routes 4, 5, and 6 in Fig. 1 are essentially the inverse of routes 1, 2, and 3, respectively. What is the order of reaction in the biosynthesis of 5,15-diHETE in the activated leukocytes? Because inhibitors are not a suitable tool to distinguish the order of events, we added octadeuterated 5-HETE or 15-HETE to the activated leukocytes and determined their transformation to 5,15-HETE relative to the endogenous pathway. We found that exogenous 5-HETE was transformed to 5,15-diHETE, and inhibition by NS-398 proved that the transformation was catalyzed by COX-2. This confirmed that 5,15-diHETE in the activated leukocytes was, not to the least part, formed via route 1 in Fig. 1, i.e., by crossover of the 5-LOX and COX-2 pathways.
Similar to previous studies (29, 38), we found that addition of exogenous 15-HETE led to inhibition of the formation of LTB4 (Fig. 6). This effect has initially been interpreted as an inhibitory effect of 15-HETE on 5-LOX activity (38) but was later found to be due to efficient utilization of 15-HETE by 5-LOX at the expense of reaction with arachidonic acid (29). Two additional lines of evidence support this explanation: 1) studies with 5-LOX purified from porcine leukocytes showed that 15-HPETE reacted at about 30% of the maximum velocity obtained with arachidonic acid (39); and 2) Mancini et al. (40) showed that recombinant FLAP was able to stimulate oxygenation of 15-HETE about twofold (and oxygenation of 12-HETE almost 200-fold). It appears that in activated leukocytes the availability of arachidonic acid or 15-HETE is limiting the formation of 5,15-diHETE. Taken together, the abundant formation of 5,15-diHETE from 15-HETE implies that in the leukocytes 5-LOX can react efficiently with 15-HETE (route 6 in Fig. 1). This reaction is also a major pathway of biosynthesis of lipoxins (13, 14).
The transformations of arachidonic acid in activated leukocytes analyzed in this study are summarized in Fig. 7. Use of a mixed population of leukocytes revealed efficient exchange of HETEs between LOX isozymes expressed in different cells. 5,15-DiHETE, 5,11-diHETE, and 5,12-diHETE are produced by the cross-over of 5-LOX with 15-LOX, COX-2, and 12-/15-LOX. These three “crossover” diHETEs together with 5-HETE accounted for about half of the observed 5-LOX metabolites, the other half consisting of LTB4 and nonenzymatic hydrolysis products of the LTA4 epoxide. We did not quantify the levels of cysteinyl LTs in the samples.
Fig. 7.
Overview of diHETEs formed in activated leukocytes. The transformation of 15-HETE and 12-HETE by 5-LOX and the reaction of aspirin-acetylated COX-2 have not been included.
Little is known about the biological role of 5,15-diHETE. In line with its biosynthesis by leukocytes, 5,15-diHETE plays a role in the inflammatory response and potentiates the degranulation of human neutrophils in response to platelet activating factor, but not f-Met-Leu-Phe, calcium ionophore A23187, or LTB4 (41). 5S,15S-DiHETE is also a chemoattractant for eosinophils with an ED50 of 0.3 μM (42) and a precursor to the highly potent eosinophil chemoattractant 5-oxo-15-HETE with an ED50 of 5 nM (43).
In summary, formation of 5,11-diHETE and 5,15-diHETE in activated human leukocytes is evidence for a functional biosynthetic cross-over of the 5-LOX and COX-2 pathways. Our studies implicate inhibition of formation of 5,15-diHETE as an additional mechanism of action of NSAIDs and COX-2 selective inhibitors with as of yet incompletely understood biological consequences.
Footnotes
Abbreviations:
- APCI
- atmospheric pressure chemical ionization
- COX
- cyclooxygenase
- FLAP
- 5-lipoxygenase-activating protein
- (di)H(P)ETE
- (di)hydro(pero)xyeicosatetraenoic acid
- LOX
- lipoxygenase
- LPS
- lipopolysaccharide
- LT
- leukotriene
- PFB
- pentafluorobenzyl
- SRM
- selected reaction monitoring
This work was supported by awards R01GM076592, an ARRA administrative supplement (3R01GM076592-03S1), and P50GM015431 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
REFERENCES
- 1.Murphy R. C., Gijon M. A. 2007. Biosynthesis and metabolism of leukotrienes. Biochem. J. 405: 379–395. [DOI] [PubMed] [Google Scholar]
- 2.Miller D. K., Gillard J. W., Vickers P. J., Sadowski S., Leveille C., Mancini J. A., Charleson P., Dixon R. A., Ford-Hutchinson A. W., Fortin R., et al. 1990. Identification and isolation of a membrane protein necessary for leukotriene production. Nature. 343: 278–281. [DOI] [PubMed] [Google Scholar]
- 3.Brash A. R. 1999. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 274: 23679–23682. [DOI] [PubMed] [Google Scholar]
- 4.Sigal E., Craik C. S., Highland E., Grunberger D., Costello L. L., Dixon R. A. F., Nadel J. A. 1988. Molecular cloning and primary structure of human 15-lipoxygenase. Biochem. Biophys. Res. Commun. 157: 457–464. [DOI] [PubMed] [Google Scholar]
- 5.Brash A. R., Boeglin W. E., Chang M. S. 1997. Discovery of a second 15S-lipoxygenase in humans. Proc. Natl. Acad. Sci. USA. 94: 6148–6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamberg M., Samuelsson B. 1967. Oxygenation of unsaturated fatty acids by the vesicular gland of sheep. J. Biol. Chem. 242: 5344–5354. [PubMed] [Google Scholar]
- 7.Xiao G., Tsai A. L., Palmer G., Boyar W. C., Marshall P. J., Kulmacz R. J. 1997. Analysis of hydroperoxide-induced tyrosyl radicals and lipoxygenase activity in aspirin-treated human prostaglandin H synthase-2. Biochemistry. 36: 1836–1845. [DOI] [PubMed] [Google Scholar]
- 8.Mancini J. A., O'Neill G. P., Bayly C., Vickers P. J. 1994. Mutation of serine-516 in human prostaglandin G/H synthase-2 to methionine or aspirin acetylation of this residue stimulates 15-R-HETE synthesis. FEBS Lett. 342: 33–37. [DOI] [PubMed] [Google Scholar]
- 9.Holtzman M. J., Turk J., Shornick L. P. 1992. Identification of a pharmacologically distinct prostaglandin H synthase in cultured epithelial cells. J. Biol. Chem. 267: 21438–21445. [PubMed] [Google Scholar]
- 10.Lecomte M., Laneuville O., Ji C., DeWitt D. L., Smith W. L. 1994. Acetylation of human prostaglandin endoperoxide synthase-2 (cyclooxygenase-2) by aspirin. J. Biol. Chem. 269: 13207–13215. [PubMed] [Google Scholar]
- 11.Schneider C., Brash A. R. 2000. Stereospecificity of hydrogen abstraction in the conversion of arachidonic acid to 15R-HETE by aspirin-treated cyclooxygenase-2. J. Biol. Chem. 275: 4743–4746. [DOI] [PubMed] [Google Scholar]
- 12.Maas R. L., Turk J., Oates J. A., Brash A. R. 1982. Formation of a novel dihydroxy acid from arachidonic acid by lipoxygenase-catalyzed double oxygenation in rat mononuclear cells and human leukocytes. J. Biol. Chem. 257: 7056–7067. [PubMed] [Google Scholar]
- 13.Serhan C. N., Hamberg M., Samuelsson B. 1984. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc. Natl. Acad. Sci. USA. 81: 5335–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kühn H., Wiesner R., Alder L., Fitzsimmons B. J., Rokach J., Brash A. R. 1987. Formation of lipoxin B by the pure reticulocyte lipoxygenase via sequential oxygenation of the substrate. Eur. J. Biochem. 169: 593–601. [DOI] [PubMed] [Google Scholar]
- 15.Clària J., Serhan C. N. 1995. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc. Natl. Acad. Sci. USA. 92: 9475–9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulugeta S., Suzuki T., Tejera Hernandez N., Griesser M., Boeglin W. E., Schneider C. 2010. Identification and absolute configuration of dihydroxy-arachidonic acids formed by oxygenation of 5S-HETE by native and aspirin-acetylated COX-2. J. Lipid Res. 51: 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider C., Boeglin W. E., Yin H., Stec D. F., Voehler M. 2006. Convergent oxygenation of arachidonic acid by 5-lipoxygenase and cyclooxygenase-2. J. Am. Chem. Soc. 128: 720–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey J. M., Bryant R. W., Whiting J., Salata K. 1983. Characterization of 11-HETE and 15-HETE, together with prostacyclin, as major products of the cyclooxygenase pathway in cultured rat aorta smooth muscle cells. J. Lipid Res. 24: 1419–1428. [PubMed] [Google Scholar]
- 19.Setty B. N., Stuart M. J., Walenga R. W. 1985. Formation of 11-hydroxyeicosatetraenoic acid and 15-hydroxyeicosatetraenoic acid in human umbilical arteries is catalyzed by cyclooxygenase. Biochim. Biophys. Acta. 833: 484–494. [DOI] [PubMed] [Google Scholar]
- 20.Griesser M., Boeglin W. E., Suzuki T., Schneider C. 2009. Convergence of the 5-LOX and COX-2 pathways. Heme-catalyzed cleavage of the 5S-HETE-derived di-endoperoxide into aldehyde fragments. J. Lipid Res. 50: 2455–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider C., Yu Z., Boeglin W. E., Zheng Y., Brash A. R. 2007. Enantiomeric separation of hydroxy and hydroperoxy eicosanoids by chiral column chromatography. Methods Enzymol. 433: 145–157. [DOI] [PubMed] [Google Scholar]
- 22.Brash A. R., Song W-C. 1996. Detection, assay, and isolation of allene oxide synthase. Methods Enzymol. 272: 250–259. [DOI] [PubMed] [Google Scholar]
- 23.Lee S. H., Williams M. V., DuBois R. N., Blair I. A. 2003. Targeted lipidomics using electron capture atmospheric pressure chemical ionization mass spectrometry. Rapid Commun. Mass Spectrom. 17: 2168–2176. [DOI] [PubMed] [Google Scholar]
- 24.Borgeat P., Samuelsson B. 1979. Metabolism of arachidonic acid in polymorphonuclear leukocytes. Structural analysis of novel hydroxylated compounds. J. Biol. Chem. 254: 7865–7869. [PubMed] [Google Scholar]
- 25.Marcus A. J., Broekman M. J., Safier L. B., Ullman H. L., Islam N., Sherhan C. N., Rutherford L. E., Korchak H. M., Weissmann G. 1982. Formation of leukotrienes and other hydroxy acids during platelet-neutrophil interactions in vitro. Biochem. Biophys. Res. Commun. 109: 130–137. [DOI] [PubMed] [Google Scholar]
- 26.Dixon R. A., Diehl R. E., Opas E., Rands E., Vickers P. J., Evans J. F., Gillard J. W., Miller D. K. 1990. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 343: 282–284. [DOI] [PubMed] [Google Scholar]
- 27.Bozza P. T., Pacheco P., Yu W., Weller P. F. 2002. NS-398: cyclooxygenase-2 independent inhibition of leukocyte priming for lipid body formation and enhanced leukotriene generation. Prostaglandins Leukot. Essent. Fatty Acids. 67: 237–244. [DOI] [PubMed] [Google Scholar]
- 28.Schneider C., Boeglin W. E., Brash A. R. 2000. Enantiomeric separation of hydroxy-eicosanoids by chiral column chromatography: effect of the alcohol modifier. Anal. Biochem. 287: 186–189. [DOI] [PubMed] [Google Scholar]
- 29.Petrich K., Ludwig P., Kuhn H., Schewe T. 1996. The suppression of 5-lipoxygenation of arachidonic acid in human polymorphonuclear leucocytes by the 15-lipoxygenase product (15S)-hydroxy-(5Z,8Z,11Z,13E)-eicosatetraenoic acid: structure-activity relationship and mechanism of action. Biochem. J. 314: 911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Setty B. N., Stuart M. J. 1986. 15-Hydroxy-5,8,11,13-eicosatetraenoic acid inhibits human vascular cyclooxygenase. Potential role in diabetic vascular disease. J. Clin. Invest. 77: 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folco G., Murphy R. C. 2006. Eicosanoid transcellular biosynthesis: from cell-cell interactions to in vivo tissue responses. Pharmacol. Rev. 58: 375–388. [DOI] [PubMed] [Google Scholar]
- 32.Griesser M., Suzuki T., Tejera N., Mont S., Boeglin W. E., Pozzi A., Schneider C. 2011. Biosynthesis of hemiketal eicosanoids by cross-over of the 5-lipoxygenase and cyclooxygenase-2 pathways. Proc. Natl. Acad. Sci. USA. 108: 6945–6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turk J., Maas R. L., Brash A. R., Roberts L. J., 2nd, Oates J. A. 1982. Arachidonic acid 15-lipoxygenase products from human eosinophils. J. Biol. Chem. 257: 7068–7076. [PubMed] [Google Scholar]
- 34.Chavis C., Chanez P., Vachier I., Bousquet J., Michel F. B., Godard P. 1995. 5–15-diHETE and lipoxins generated by neutrophils from endogenous arachidonic acid as asthma biomarkers. Biochem. Biophys. Res. Commun. 207: 273–279. [DOI] [PubMed] [Google Scholar]
- 35.Patrignani P., Panara M. R., Greco A., Fusco O., Natoli C., Iacobelli S., Cipollone F., Ganci A., Creminon C., Maclouf J., et al. 1994. Biochemical and pharmacological characterization of the cyclooxygenase activity of human blood prostaglandin endoperoxide synthases. J. Pharmacol. Exp. Ther. 271: 1705–1712. [PubMed] [Google Scholar]
- 36.Borgeat P., Samuelsson B. 1979. Arachidonic acid metabolism in polymorphonuclear leukocytes: effects of ionophore A23187. Proc. Natl. Acad. Sci. USA. 76: 2148–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider C., Pratt D. A., Porter N. A., Brash A. R. 2007. Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem. Biol. 14: 473–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanderhoek J. Y., Bryant R. W., Bailey J. M. 1980. Inhibition of leukotriene biosynthesis by the leukocyte product 15-hydroxy-5,8,11,13-eicosatetraenoic acid. J. Biol. Chem. 255: 10064–10066. [PubMed] [Google Scholar]
- 39.Ueda N., Kaneko S., Yoshimoto T., Yamamoto S. 1986. Purification of arachidonate 5-lipoxygenase from porcine leukocytes and its reactivity with hydroperoxyeicosatetraenoic acids. J. Biol. Chem. 261: 7982–7988. [PubMed] [Google Scholar]
- 40.Mancini J. A., Waterman H., Riendeau D. 1998. Cellular oxygenation of 12-hydroxyeicosatetraenoic acid and 15-hydroxyeicosatetraenoic acid by 5-lipoxygenase is stimulated by 5-lipoxygenase-activating protein. J. Biol. Chem. 273: 32842–32847. [DOI] [PubMed] [Google Scholar]
- 41.O'Flaherty J. T., Thomas M. J. 1985. Effect of 15-lipoxygenase-derived arachidonate metabolites on human neutrophil degranulation. Prostaglandins Leukot. Med. 17: 199–212. [DOI] [PubMed] [Google Scholar]
- 42.Morita E., Schroder J. M., Christophers E. 1990. Identification of a novel and highly potent eosinophil chemotactic lipid in human eosinophils treated with arachidonic acid. J. Immunol. 144: 1893–1900. [PubMed] [Google Scholar]
- 43.Schwenk U., Morita E., Engel R., Schroder J. M. 1992. Identification of 5-oxo-15-hydroxy-6,8,11,13-eicosatetraenoic acid as a novel and potent human eosinophil chemotactic eicosanoid. J. Biol. Chem. 267: 12482–12488. [PubMed] [Google Scholar]