Abstract
Purpose
A subgroup of HER2 overexpressing breast tumors co-expresses p95HER2, a truncated HER2 receptor that retains a highly functional HER2 kinase domain but lacks the extracellular domain and results in intrinsic trastuzumab resistance. We hypothesized that lapatinib, a HER2 tyrosine kinase inhibitor, would be active in these tumors. We have studied the correlation between p95HER2 expression and response to lapatinib, both in preclinical models and in the clinical setting
Experimental design
Two different p95HER2 animal models were used for preclinical studies. Expression of p95HER2 was analyzed in HER2 overexpressing breast primary tumors from a first line lapatinib monotherapy study (EGF20009) and a second line lapatinib in combination with capecitabine study (EGF100151). p95HER2 expression was correlated with overall response rate (complete + partial response), clinical benefit rate (complete response + partial response + stable disease ≥ 24 weeks) and progression-free survival using logistic regression and Cox-proportional hazard models.
Results
Lapatinib inhibited tumor growth and HER2 downstream signaling of p95HER2 expressing tumors. A total of 68 and 156 tumors from studies EGF20009 and EGF100151 were evaluable, respectively, for p95HER2 detection. The percentage of p95HER2 positive patients was 20.5% in the EGF20009 study and 28.5% in the EGF100151 study. In both studies there was no statistically significant difference in progression-free survival, clinical benefit rate and overall response rate between p95HER2-positive and p95HER2-negative tumors.
Conclusions
Lapatinib as a monotherapy or in combination with capecitabine appears to be equally effective in patients with p95HER2-positive and p95HER2-negative HER2-positive breast tumors.
Keywords: lapatinib, p95HER2, breast cancer, trastuzumab, HER2
Introduction
The HER family of receptors is composed of four closely related tyrosine kinase receptors: HER1, HER2, HER3 and HER4. All HER receptors consist of an N-terminal extracellular ligand-binding domain, a short transmembrane region, and an intracellular tyrosine kinase (TK) domain (1-3).
Dimerization of HER receptors, induced by ligand binding or receptor overexpression in the case of HER2, leads to the interaction between the intracellular kinase domain of the receptors and subsequent transphosphorylation of several tyrosine residues located in the C-terminal tails (4-5). These phosphorylation events trigger the recruitment of several adaptor proteins that mediate the activation of downstream signaling pathways. Among them, the PI3K-Akt-mammalian target of rapamycin (mTOR) and the mitogen activated protein kinases (MAPKs) pathways promote cell proliferation, transformation and survival (6-7).
Overexpression/amplification of HER2 occurs in approximately 20% of human breast cancers and is associated with a more aggressive phenotype and worse prognosis (8-9). HER2 overexpressing tumors are sensitive to monoclonal antibodies (mAb) and small molecule tyrosine kinase inhibitors (TKIs) that interfere with HER2 function and signaling (10-14). Trastuzumab, a humanized mAb directed against the extracellular domain (ECD) of the receptor was the first approved therapy for the treatment of HER2-positive breast cancer. Trastuzumab in combination with chemotherapy has demonstrated a robust improvement in disease-free and progression-free survival in addition to overall survival in the advanced disease (15-16) as well as in the early (adjuvant) setting (17-19). Despite the considerable clinical benefit provided, a large fraction of HER2-positive tumors display primary or acquired resistance to trastuzumab (for review see (20)). Among the potential mechanism of resistance to trastuzumab, the presence of truncated forms of HER2 lacking the trastuzumab-binding extracellular domain [collectively known as p95HER2 or HER2 C-terminal fragments (CTF)] (14) has been extensively studied.
p95HER2 is expressed in up to 30% of HER2 positive breast cancers and is associated with increased nodal metastasis and shorter disease-free survival when compared to patients that overexpress full length HER2 (21-22). These truncated receptor fragments, produced by either proteolytic shedding of the HER2 receptor ECD (23) or, more frequently, by alternative initiation of translation of the HER2 mRNA (24), retain kinase activity and promote mammary tumor progression and metastasis even more aggressively than full length HER2 (25). Since p95HER2 lacks the extracellular trastuzumab binding domain, we hypothesized that these receptors would not be inhibited by this antibody. This turned out to be the case. We have recently showed that tumor xenografts expressing p95HER2 are refractory to the inhibitory effects of trastuzumab (14, 24). Importantly there was an association between p95HER2 expression and lack of clinical response to trastuzumab in HER2 amplified breast cancer patients (14). On the other hand, p95HER2 retains its kinase activity and, we and others, have demonstrated that p95HER2 expressing cells are sensitive to the antiproliferative activity of lapatinib (14, 26), a dual HER2 and HER1 TKI (27).
Lapatinib binds to the ATP binding site of both HER1 and HER2 preventing receptor phosphorylation and activation of downstream signaling (14, 28-30). Lapatinib when given in combination with capecitabine significantly improved time to progression in HER2-positive breast cancer patients that progressed on trastuzumab-based therapy, compared with capecitabine alone (13). Moreover, lapatinib as monotherapy and in combination with paclitaxel has clinical activity as first-line treatment in HER2-positive breast cancer patients (31-32). Taken together, these observations led to the hypothesis that lapatinib may be active in patients with p95HER2 expressing tumors.
To test this hypothesis we first studied the antitumor activity of lapatinib in two animal models characterized by p95HER2 positive tumors that are either resistant to trastuzumab or depend on p95HER2 to growth. Subsequently, we analyzed the relationship between p95HER2 expression and response to lapatinib in HER2-positive patients who received lapatinib as monotherapy or in combination with capecitabine.
Materials and Methods
Cell lines and animal studies
Four to six week old BALB/c nu/nu athymic female mice were purchased from the NCI Frederick Cancer Center and maintained in pressurized ventilated caging. All studies were performed in compliance with IACUC guidelines.
Fo5 tumors (kindly provided by Gail Lewis Phillips and Mark Sliwkowski) were established by subcutaneously implanting 2×2×2mm-sized tumor pieces in the right flanks of the nude mice. Animals with well established tumors were randomized and treated with 75mg/kg BID 5xweek lapatinib (provided byGlaxoSmithKline,Research Triangle Park, NJ, USA).
Tumor xenografts were measured with calipers and tumor volumes was determined using the formula: (length × width2) × (π/6).
MEFs-3T3 tet-off cell lines, engineered to express the tetracycline-controlled transactivator (tTA) (33), were obtained from Clontech Laboratories (Clontech, Oxford, UK). 106 cells stably transfected with the pUHD10-3h vector encoding the cDNA of p95HER2 starting at methionine 611 (611-CTF(25)) were injected into the right flanks of six-to-eight-week old female BALB/c athymic mice purchased from Charles Rivers Laboratories (Paris, France) and the expression of 611-CTF was induced by doxycycline removal. Animals with well established tumors were randomized and treated with 150mg/Kg daily lapatinib or with 10mg/Kg twice weekly trastuzumab (Herceptin®; kindly provided by F. Hoffmann-La Roche, Basel, Switzerland). Tumor xenografts were measured as described above.
Tumor volumes are plotted as means ±SE.
Immunoblotting
Tumor lysates were prepared by homogenization in SDS lysis buffer (50mM Tris-HCl, (pH7.4) 2% SDS), boiling for 10minutes, followed by brief sonication. Lysates were cleared by centrifugation at 14,000xg for 10min and the supernatants were collected. Protein concentration of each sample was determined using the BCA kit (Pierce) per manufacturer’s instructions. 50 μg protein lysate was loaded onto 7 or 10% SDS-PAGE minigels for immunoblotting. Transfer was onto nitrocellulose membranes followed by incubation with the following primary antibodies: Phosphoinositide 3-kinase (PI3K)-p85 from Upstate Biotechnology (Lake Placid, NY, USA), total HER2 (CB11, Biogenex, San Ramon, CA), phospho-HER2 (p-HER2), phospho-Akt (pAkt, S473 and T308) and phospho mitogen-activated protein kinases (p-MAPKs) from Cell Signaling (Beverly, MA, USA).
Immunohistochemistry
Xenografts samples were prepared as described earlier (34). Primary antibody was p-MAPKs from Cell Signaling and secondary anti-rabbit antibody was from Amersham (Amersham Biosciences, Uppsala, Sweden). As a negative control, primary antibody was omitted. Slides were scanned with ScanScope CS system (Aperio, Vista, CA, USA). Quantification of p-MAPKs was obtained by scoring two slides each from five different tumors, from both placebo and lapatinib-treated groups, by a qualified pathologist (LP) and displayed as H score plotted as means ±SE.
Patients
Women participating in one of 2 clinical trials provided written informed consent allowing for biomarker research to be performed on tumor tissue obtained at the time of their diagnosis or at surgery. The details for both trials, including efficacy and safety outcome, have been previously reported (13, 31, 35). Briefly, in study EGF20009, 138 women with HER2-positive (FISH-positive) treatment-naïve, advanced breast cancer randomized in a 1:1 ratio to receive oral lapatinib 1500mg once daily or oral lapatinib 500mg twice daily. In study EGF100151, 399 women with HER2-positive (IHC 3+ and/or FISH-positive) advanced breast cancer previously treated with anthracycline-, taxane- and trastuzumab-based therapy were randomized in a 1:1 ratio to receive capecitabine 2500mg/m2 (days 1-14 of a 21 day cycle) or capecitabine 2000mg/m2 (days 1-14 of a 21 day cycle) with lapatinib 1250mg once daily (continuously). In both clinical studies, efficacy analyses were conducted based on both investigator and blinded independent central review (BICR) assessment of tumor response and progression using Response Criteria in Solid Tumor (RECIST) guidelines (36) in the intent-to-treat (ITT) patient population.
Immunofluorescence Detection of p95HER2 in Breast Tumors
Patients with sufficient tumor tissue available (i.e., at least 2 slides/sections) and with a clear invasive cellular component were included in the evaluation of p95HER2 expression with the aim of obtaining a more robust estimation of the incidence of p95HER2 expression.
The expression of N-terminally truncated HER2 was evaluated by Immunofluorescence as previously described and scored by two pathologists (L.P. and C.A.) (14). The investigators at our Institution, including the pathologists, were blinded to the study or the therapy arm of the analyzed slides.
To increase the stringency of the analyses tumors were scored positive for p95HER2 expression if ≥50% of tumoral cells showed any cytoplasmic staining detected with the anti-HER2 ICD antibody (recognizing both membrane and cytoplasmic HER2). Cytoplasmic staining was confirmed by co-localization with the anti- cytokeratin antibody, as observed by a yellow (red and green merged) signal (Fig 1). In addition, HER2 ICD staining was compared with the pure membrane staining observed with the anti-HER2 ECD antibody that does not co-localize with cytokeratin staining. All fluorescence assays were performed using a Dako Autostainer.
Figure 1.
p95HER2 staining in breast tumors. HER2 ICD staining (red) co-localizes with cytokeratin staining (green) only in p95HER2 positive tumors (B). Co-localization appears as a yellow-orange signal.
Fluorescent signals were analyzed with a FluoView FV1000 Olympus Confocal Microscope and evaluated by 2 independent pathologists (L.P. and C.A.) blinded to clinical information.
Statistical Methods
For nude mice experiments, comparisons between groups were made using a two-tailed Student’s t-test. Differences for which P was less than 0.05 were considered statistically significant.
BICR was the protocol-defined assessment of the primary endpoint in both studies and thus used in analyses with p95HER2. Overall response rate (ORR; confirmed complete [CR] or partial response [PR]) and clinical benefit rate (CBR; confirmed CR or PR, or stable disease [SD] for > 6 months) were compared between p95HER2-positive and p95HER2-negative groups using chi-square tests. Progression-free survival (PFS) was compared between p95HER2-positive and p95HER2-negative groups using Cox proportional hazards models and Kaplan-Meier analyses. SAS was used for statistical analysis and S-Plus was used for graphical display. In study EGF20009, the data from the 2 lapatinib monotherapy arms (2 dose cohorts) were combined as no significant differences in the clinical outcome parameters were observed, and both patient as well as tumor characteristics were balanced (31).
Results
Lapatinib efficacy in mice bearing trastuzumab-resistant p95HER2 positive breast tumors
We recently demonstrated that MCF-7 cells stably expressing p95HER2 and resistant to trastuzumab remained sensitive to the antiproliferative effects of the TKI lapatinib, both in vitro and in vivo (14). To expand these results in a model that more closely resembles the situation encountered in the clinic, we tested the growth inhibitory activity of lapatinib in MMTV-HER2 Fo5 mammary tumor transplants, a trastuzumab-refractory breast tumor that co-expresses ,as in clinical specimens, both full length HER2 and p95HER2 (37). These cells were isolated from breast tumors derived from the MMTV-HER2 transgenic mouse lineage 5 that spontaneously became insensitive to trastuzumab therapy (38-39). In Fig 2A we show that lapatinib (75mg/kg BID 5xweek) markedly reduces tumor growth in mice bearing Fo5 tumors compared to placebo treated controls. In a pharmacodynamic study we treated Fo5 bearing mice with 5 consecutive doses of lapatinib (100mg/kg BID) and then sacrificed at the indicated times after the final dose (2 mice per time point are shown). Immunoblots of lysates of these tumors demonstrate that phosphorylation of p95-HER2 and HER2 is downregulated three and six hours after the last lapatinib administration (Fig 2B). Inhibition of HER2 and p95-HER2 phosphorylation is associated with downregulation of phosphorylated Akt (S473 and T308) and MAPKs (Fig 2B). p85-PI3K blots serve as loading controls. These results are representative of two independent experiments.
Figure 2.
Antitumor activity of lapatinib on Fo5 tumors. (A) Mice with established Fo5 tumors (5 mice/group) were randomized to receive either placebo or treatment with lapatinib 75 mg/Kg BID Monday-Friday. Treatment was discontinued after day 35 post injection. *P < 0.01 placebo versus lapatinib.
(B) Mice with established Fo5 tumors were treated with 5 doses (75 mg/Kg BID × 2.5 days) of lapatinib, and tumors collected at indicated times after the 5th dose. Immunoblotting was performed on tumor lysates using the listed antibodies.
Lapatinib activity in targeting p95HER2-dependent tumors
To assess the exquisite activity of lapatinib in inhibiting p95HER2-dependent tumor growth we used an experimental model based on mouse embryonic fibroblasts (MEFs) stably transfected with the most active fragment of p95HER2 (611-CTF), produced by alternative initiation of translation starting at methionine 611 of the HER2 sequence (25). The expression of this form of p95HER2 is tightly controlled by a tet-off inducible system (see Materials and Methods) and the tumorigenicity of these MEFs stable clones stringently depend on the expression/activity of the oncogene. As expected, tumors derived from MEFs expressing 611-CTF were refractory to trastuzumab (10mg/Kg twice weekly, Supplementary Fig 1). Lapatinib treatment (150mg/Kg daily) strongly inhibits tumor growth of MEFs expressing 611-CTF (Fig 3A).
Figure 3.
Lapatinib-specific targeting of p95HER2. (A) Established xenografts (5 mice/group) derived from MEFs expressing 611-CTF were randomized to receive either placebo or treatment with lapatinib 150 mg/Kg daily. Treatment was discontinued after day 25 post injection. *P < 0.01 placebo versus lapatinib. (B) Six mice bearing MEFs 611-CTF xenografts with a volume ≥ 500mm3 received 1g/l doxycycline in the drinking water. Complete tumor shrinkage was achieved in 11 days. 611-CTF expression in absence or presence (48 hours) of doxycycline is shown. (C) Representative p-MAPKs staining in 611-CTF MEFs tumors receiving either placebo (control) or lapatinib (LAP). Tumors were collected at the end of the experiment shown in panel A. (D) Quantification of the p-MAPKs staining (H score) was obtained by scoring two slides each from five different tumors from both placebo and lapatinib-treated groups. Results are expressed as means ±SE. *P < 0.01 placebo versus lapatinib.
To confirm that the tumorigenesis of these cells is dependent on 611-CTF expression we switched off 611-CTF expression (by adding doxycycline) in a separate group of animals with xenografts volume ≥ 500mm3. In these mice, doxycycline administration in the drinking water rapidly abolishes 611-CTF expression and, consequently, leads to tumor shrinkage (Fig 3B).
Inhibition of MAPKs phosphorylation is shown (Fig 3C and D) as a readout of lapatinib inhibition of 611-CTF signaling (25). These results are representative of two independent experiments.
p95HER2 Expression in Tumor Tissue from Studies EGF20009 and EGF100151
Tumor tissue was evaluable for p95HER2 analysis in 68 out of 138 patients from study EGF20009 and in 156 out of 399 patients from study EGF100151. Although we were able to obtain a higher tumor number (105 and 223, for EGF20009 and EGF100151, respectively), not all tumor sections were evaluable. In EGF20009, the first line lapatinib single agent study, 20.5% (14/68) had tumors that were p95HER2 positive whereas 79.5% (54/68) were negative. In EGF100151, the capecitabine plus lapatinib study, 28.5% (45/156) tumors were p95HER2 positive and 71.5% (111/156) were negative. Overall, 26% of HER2-positive tumors were positive for p95HER2 expression, an incidence consistent with previous reports (14, 21-22). In those patients with p95HER2 evaluable results (Table 1), the patient and disease characteristics were generally well representative of the corresponding study populations as a whole (13, 31).
Table 1.
Patient Demographic and Disease History
| EGF20009 | EGF100151 | EGF100151 | |
|---|---|---|---|
| L+C | C | ||
| N=68 | N=76 | N=80 | |
| Median (Q1, Q3) Age, Yrs | 50 (45, 63) | 53 (47, 60) | 51 (43, 57) |
| ECOG PS | |||
| 0, N (%) | 23 (34) | 49 (64) | 45 (56) |
| ≥1, N (%) | 45 (66) | 27 (36) | 33 (41) |
| Visceral Metastasis, N (%) | 42 (62) | 62 (82) | 60 (75) |
| No Metastatic Sites | |||
| <3, N (%) | 30 (44) | 44 (58) | 46 (57.5) |
| ≥3, N (%) | 38 (56) | 32 (42) | 34 (42.5) |
| Stage | |||
| IIIB or C, N (%) | 16 (24) | 4 (5) | 2 (2.5) |
| IV, N (%) | 52 (76) | 72 (95) | 78 (97.5) |
Abbreviations: L=lapatinib; C=capecitabine; N=sample number; ECOG PS: Eastern Cooperative Oncology Group Performance Status; No=number-
Relationship of p95HER2 Status with Clinical Outcome
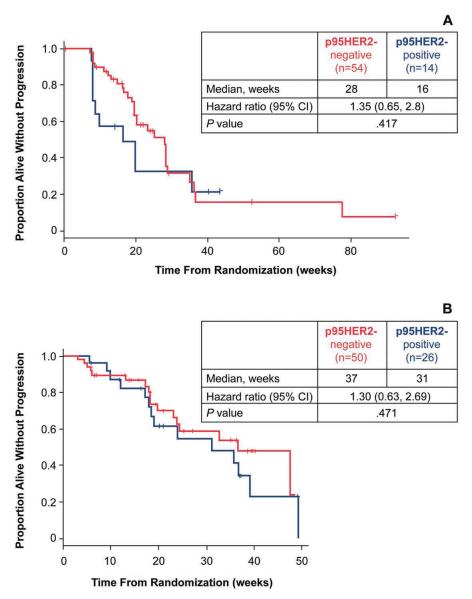
To determine whether the presence of p95HER2 affected the efficacy of lapatinib in the clinic, progression-free survival (PFS) was analyzed in lapatinib-treated patients sub-grouped according to the p95HER2 status of their tumor. In patients receiving lapatinib as first-line treatment, no statistically significant difference in PFS was observed between the p95HER2-positive and p95HER2-negative subgroups [hazard ratio (HR) = 1.35; 95% CI = 0.64, 2.8; P = 0.417; Fig 4A]. Similarly, PFS as a result of lapatinib plus capecitabine treatment was not significantly different between the two p95HER2 subgroups (HR = 1.30; 95% CI = 0.63, 2.69; P = 0.471; Fig 4B).
Figure 4.
Association of clinical outcome as a result of lapatinib-based treatment and p95HER2 status in patients with HER2-positive MBC. (A) Analysis of PFS sub grouped by p95HER2 status in patients treated with lapatinib monotherapy (EGF20009) or (B) with lapatinib in combination with capecitabine (EGF100151). Among patients with HER2-positive tumors exhibiting co-expression of p95HER2 versus those with HER2-positive tumors that were p95HER2-negative no significant difference in PFS was observed in either study.
An analysis was conducted to determine whether the presence of p95HER2 had an effect on CBR or ORR as an outcome of lapatinib-based treatment. Clinical benefit rate and overall response rate were not significantly associated with p95HER2 expression (Table 2). In patients treated with monotherapy lapatinib (EGF20009), the CBR for the p95HER-positive group was 29% (95% CI: 2, 56) versus 43% (95% CI: 29, 57) for the p95HER2-negative group (P = 0.379). In patients treated with lapatinib plus capecitabine (EGF100151), a CBR of 38% (95% CI: 13, 68) versus 40% (95%CI: 25, 57) was observed in the p95HER2-positive versus p95HER2-negative groups, respectively (P =0.999). Additionally, ORR was not significantly different between the two p95HER2 subgroups regardless of whether patients were treated with lapatinib alone or in combination with capecitabine (EGF20009: P =0.7580 ; EGF100151: P =0.999). The ORR and CBR in both p95HER2 populations in each trial are consistent with the tumor response rates reported in the respective overall study populations (31-32).
Table 2.
Tumor Response in Lapatinib Treated Patients Subgrouped by p95HER2 Status
| EGF20009 | EGF100151 | |
|---|---|---|
| L | L plus C | |
| p95HER2-positive, N | 14 | 26 |
| ORR, % | 29 | 31 |
| (95% CI, %) | (5, 53) | (13, 49) |
| CBR, % | 29 | 38 |
| (95% CI, %) | (2, 56) | (13, 63) |
| p95HER2-negative, N | 54 | 50 |
| ORR, % | 35 | 34 |
| (95% CI, %) | (22, 48) | (21, 47) |
| CBR, % | 43 | 40 |
| (95% CI, %) | (29, 57) | (25, 57) |
Abbreviations: L, lapatinib; C, capecitabine, N, number; ORR, overall response rate; CBR, clinical benefit response rate; CI, confidence interval
Discussion
Resistance to trastuzumab remains a challenge in the therapy of HER2 overexpressing breast cancer and efforts are being directed at identifying potential underlying mechanisms (40). A potential leading cause of trastuzumab resistance is the co-expression in HER2 amplified tumors of p95HER2, a truncated form of the HER2 receptor that lacks the trastuzumab-binding extracellular domain but that retains a functional HER2 kinase domain and is highly tumorigenic (41). Given the high HER2 kinase activity of p95HER2, a potential approach to treat these tumors would be with HER2 receptor tyrosine kinase inhibitors. (14, 26) In this study, we have demonstrated that trastuzumab-refractory breast tumors that co-express, as in clinical specimens, both full length HER2 and p95HER2 are sensitive to the TKI lapatinib. We also demonstrate that tumors co-expressing p95HER2 appear to respond similarly to lapatinib as tumors expressing only the full length HER2 receptor; this result needs further validation due to the small sample sizes. In clinical samples from 2 large lapatinib studies in patients with advanced HER2-positive breast cancer we observed that the presence of p95HER2 occurs in ~25% of HER2 amplified breast tumors. In these 2 studies tumors expressing p95HER2 were sensitive to lapatinib. In fact, both the PFS and clinical benefit rate in both studies were not statistically different in the p95HER2 positive and negative groups. Taking in consideration that p95HER2 tumors are resistant to trastuzumab (14), our results suggest that lapatinib, as well as other TKIs, may be a preferred therapeutic option for these tumors.
Hence, the presence of p95HER2 could determine the choice of anti-HER2 therapy if the results are further validated. We are prospectively studying the role of p95HER2 in the neoadjuvant study NeoALTTO as well in the adjuvant study ALTTO as both compare the clinical benefit with trastuzumab versus lapatinib versus the 2 agents given in combination. In addition, we are planning a prospective clinical trial with lapatinib in patients with tumors selected for p95HER2 expression. There is also a need to develop a user-friendly and reproducible method of p95HER2 detection. Immunofluorescence detection of p95HER2, as used here, is complex, time demanding and requires a confocal microscope which would limit its widespread implementation. We have recently developed a mAb directed against an epitope uniquely present in p95HER2 and not full-length HER2. The mAb performs well in paraffin embedded tissues (data not shown) and is in the process of being evaluated in clinical samples. If validated, the presence of p95HER2 could be detected via immunohistochemistry, the same method widely used to detect HER2 overexpression.
There are additional questions that deserve to be explored. At this time we do not know whether p95HER2 is solely responsible for primary resistance to trastuzumab or if, on the contrary, acquired expression can occur as a result of clonal selection in tumors treated with trastuzumab over prolonged periods of time. If this were the case, upfront therapy with a combination of trastuzumab and lapatinib could potentially delay its appearance. We also do not know if p95HER2 co-exists with other potential mechanism of resistance to anti-HER2 therapies such as PI3K mutations or PTEN loss of function.
As with other breast cancer subtypes, there will be an increasing need to further classify HER2 tumors into different subsets that may have different clinical outcomes and benefit from anti-HER2 therapies. While this will undoubtedly add complexity to our daily clinical practice it will also result in an improved management of patients with HER2 positive breast cancer and a better choice of therapy.
Supplementary Material
Statement of Translational Relevance.
A subgroup of HER2 overexpressing breast tumors co-expresses p95HER2, a truncated form of the HER2 receptor that lacks the extracellular domain but retains a highly functional HER2 kinase domain. We have previously shown that p95HER2 expressing tumors are intrinsically resistant to trastuzumab. In the present study, we have demonstrated that p95HER2 expressing breast tumors are sensitive to the tyrosine kinase inhibitor lapatinib. Most importantly, we provide evidence that breast cancer patients co-expressing both HER2 and p95HER2 appear to respond similarly to lapatinib as patients with tumors expressing only the full length HER2 receptor. Our work suggests that p95HER2 expressing tumors should be treated preferentially with a tyrosine kinase inhibitor and that further subclassification of HER2 positive tumors based on the presence or absence of p95HER2 will result in a better choice of therapy and improved management of this disease.
Acknowledgments
This work was supported by the Breast Cancer Research Foundation and GlaxoSmithKline
References
- 1.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–99. doi: 10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 3.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 4.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–67. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson KM, Berger MB, Mendrola JM, Cho HS, Leahy DJ, Lemmon MA. EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol Cell. 2003;11:507–17. doi: 10.1016/s1097-2765(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 6.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–16. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Berezov A, Wang Q, et al. ErbB receptors: from oncogenes to targeted cancer therapies. J Clin Invest. 2007;117:2051–8. doi: 10.1172/JCI32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 9.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 10.Junttila TT, Akita RW, Parsons K, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC 0941. Cancer Cell. 2009;15:429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–37. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 12.Portera CC, Walshe JM, Rosing DR, et al. Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with [corrected] human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin Cancer Res. 2008;14:2710–6. doi: 10.1158/1078-0432.CCR-07-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 14.Scaltriti M, Rojo F, Ocana A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–38. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 15.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 16.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 17.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 18.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 19.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–20. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 20.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–80. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 21.Molina MA, Saez R, Ramsey EE, et al. NH(2)-terminal truncated HER-2 protein but not full-length receptor is associated with nodal metastasis in human breast cancer. Clin Cancer Res. 2002;8:347–53. [PubMed] [Google Scholar]
- 22.Saez R, Molina MA, Ramsey EE, et al. p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin Cancer Res. 2006;12:424–31. doi: 10.1158/1078-0432.CCR-05-1807. [DOI] [PubMed] [Google Scholar]
- 23.Codony-Servat J, Albanell J, Lopez-Talavera JC, Arribas J, Baselga J. Cleavage of the HER2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res. 1999;59:1196–201. [PubMed] [Google Scholar]
- 24.Anido J, Scaltriti M, Serra JJ Bech, et al. Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. EMBO J. 2006;25:3234–44. doi: 10.1038/sj.emboj.7601191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen K, Angelini PD, Laos S, et al. A Naturally Occurring HER2 Carboxy-Terminal Fragment Promotes Mammary Tumor Growth and Metastasis. Mol Cell Biol. 2009 doi: 10.1128/MCB.01803-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia W, Liu LH, Ho P, Spector NL. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW572016. Oncogene. 2004;23:646–53. doi: 10.1038/sj.onc.1207166. [DOI] [PubMed] [Google Scholar]
- 27.Spector NL, Xia W, Burris H, 3rd, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol. 2005;23:2502–12. doi: 10.1200/JCO.2005.12.157. [DOI] [PubMed] [Google Scholar]
- 28.Chu I, Blackwell K, Chen S, Slingerland J. The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res. 2005;65:18–25. [PubMed] [Google Scholar]
- 29.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–9. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 30.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–63. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 31.Gomez HL, Doval DC, Chavez MA, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol. 2008;26:2999–3005. doi: 10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- 32.Di Leo A, Gomez HL, Aziz Z, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol. 2008;26:5544–52. doi: 10.1200/JCO.2008.16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–51. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68:8022–30. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 35.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–43. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 36.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 37.Chandarlapaty S, Scaltriti M, Angelini P, et al. Inhibitors of HSP90 block p95-HER2 signaling in Trastuzumab-resistant tumors and suppress their growth. Oncogene. 2010;29:325–34. doi: 10.1038/onc.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finkle D, Quan ZR, Asghari V, et al. HER2-targeted therapy reduces incidence and progression of midlife mammary tumors in female murine mammary tumor virus huHER2-transgenic mice. Clin Cancer Res. 2004;10:2499–511. doi: 10.1158/1078-0432.ccr-03-0448. [DOI] [PubMed] [Google Scholar]
- 39.Phillips GD Lewis, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–90. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 40.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–75. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen K, Angelini PD, Laos S, et al. A naturally occurring HER2 carboxy-terminal fragment promotes mammary tumor growth and metastasis. Mol Cell Biol. 2009;29:3319–31. doi: 10.1128/MCB.01803-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.