Abstract
Fgf signaling is required for many biological processes involving the regulation of cell proliferation and maintenance, including embryonic patterning, tissue homeostasis, wound healing, and cancer progression. Although the function of Fgf signaling is suggested in several different regeneration models, including appendage regeneration in amphibians and fin and heart regeneration in zebrafish, it has not yet been studied during zebrafish photoreceptor cell regeneration. Here we demonstrate that intravitreal injections of FGF-2 induced rod precursor cell proliferation and photoreceptor cell neuroprotection during intense light damage. Using the dominant-negative Tg(hsp70:dn-fgfr1) transgenic line, we found that Fgf signaling was required for homeostasis of rod, but not cone, photoreceptors. Even though fgfr1 is expressed in both rod and cone photoreceptors, we found that Fgf signaling differentially affected the regeneration of cone and rod photoreceptors in the light-damaged retina, with the dominant-negative hsp70:dn-fgfr1 transgene significantly repressing rod photoreceptor regeneration without affecting cone photoreceptors. These data suggest that rod photoreceptor homeostasis and regeneration is Fgf-dependent and that rod and cone photoreceptors in adult zebrafish are regulated by different signaling pathways.
Keywords: Retina, Zebrafish, Fgf, Fgfr1, Regeneration, hsp70:dn-fgfr1, Homeostasis, Rod Precursor
1. Introduction
Zebrafish, like other teleost fish, have the ability to regenerate multiple tissues and organs as adults, including appendages (i.e., fins) (Geraudie and Singer, 1992; Johnson and Weston, 1995; Morgan, 1901), cardiac tissue (Poss et al., 2002), spinal cord (Becker et al., 1997), and retina (Bernardos et al., 2007; Cameron, 2000; Vihtelic and Hyde, 2000). For example, following photoreceptor cell ablation by constant intense light treatment, Müller glia reenter the cell cycle to produce neuronal progenitors that continue to proliferate and migrate to the photoreceptor layer, where they ultimately differentiate into new rod and cone photoreceptors (Vihtelic and Hyde, 2000). Although a few proteins were recently shown to be required at various stages in the retinal regeneration process (Craig et al., 2010; Fausett et al., 2008; Qin et al., 2009; Thummel et al., 2010; Thummel et al., 2008b), relatively little is known about the signaling pathways that mediate the regenerative response in the retina.
One candidate for regulating adult retinal regeneration is the fibroblast growth factor (Fgf) signaling pathway. Fgfs are a family of secreted small polypeptides that bind to specific transmembrane receptor tyrosine kinases (Fgfrs). Ligand binding induces receptor dimerization and activation. Depending on the cellular context, activated receptors stimulate downstream signaling pathways that lead to cell proliferation, differentiation, migration, or survival (Turner and Grose, 2010). Fgf signaling has been implicated in many biological processes such as induction and patterning events during embryonic development (Crossley et al., 1996; Martin, 1998; Ohuchi et al., 1997; Peters and Balling, 1999; Reifers et al., 1998; Vogel et al., 1996; Zhu et al., 1996), tissue maintenance (Campochiaro et al., 1996; Stone et al., 1999), wound healing (Ortega et al., 1998), and cancer pathogenesis (Turner and Grose, 2010).
Fgf signaling was first shown to be important in regenerating the amphibian limb. Components of the Fgf signaling pathway were necessary for normal regeneration of newt limbs (Boilly et al., 1991; Poulin et al., 1993; Zenjari et al., 1997), and fgf8 expression was associated with successful hindlimb regeneration in Xenopus tadpoles (Christen and Slack, 1997). Blocking Fgf signaling by application of specific Fgfr inhibitors to Xenopus tadpoles suppressed premetamorphic hindlimb regeneration (D'Jamoos et al., 1998), whereas implanting Fgf2-soaked beads rescued the regeneration of de-innervated axolotl limbs (Mullen et al., 1996). More recent studies have also implicated a role for Fgf signaling in tissue regeneration in adult zebrafish. Fgf signaling has been shown to be necessary for proper regeneration of the adult zebrafish caudal fin (Lee et al., 2005; Poss et al., 2000; Thummel et al., 2006; Whitehead et al., 2005) and heart (Lepilina et al., 2006).
To our knowledge, this is the first work to describe the role of Fgf signaling in adult zebrafish retinal regeneration. Here we show that intravitreal injections of FGF-2 induce rod precursor cell proliferation and neuroprotection during intense light damage to photoreceptors. Using the dominant-negative Tg(hsp70:dn-fgfr1) transgenic line (Lee et al., 2005), we found that Fgf signaling was required for homeostasis of rod, but not cone, photoreceptors. Even though fgfr1 is expressed in both rod and cone photoreceptors, we found that Fgf signaling differentially affected the regeneration of cone and rod photoreceptors following light damage, with the dominant-negative hsp70:dn-fgfr1 transgene significantly repressing rod photoreceptor regeneration without affecting cone photoreceptors.
2. Materials and Methods
2.1 Zebrafish
Four zebrafish (Danio rerio) lines were used in this study: wild-type AB, albino, Tg(hsp70:dn-fgfr1) (Lee et al., 2005), and Tg(gfap:GFP)mi2002 (Bernardos and Raymond, 2006). Fish were maintained according to standard rearing protocols. All procedures using animals are in compliance with the ARVO statement for the use of animals in vision research and have been approved by the appropriate university committee on use and care of animals. Heat-shock induction of the hsp70:dn-fgfr1 transgene was performed by placing the fish in an automated heating unit, which exposed the fish to a daily heat shock of 38°C for 1 hour (Lee et al., 2005). Photoreceptors were destroyed in adult zebrafish using one of two previously published methods shown to destroy both rod and cone photoreceptors: a 30-minute exposure to very intense light (>100,000 lux) (Bernardos et al., 2007) or a four-day exposure to constant bright light (∼8,000 lux; Vihtelic and Hyde, 2000). The 30-minute exposure is fast, but only small groups can be treated at a time, whereas the four-day exposure takes more time, but larger numbers of fish can be treated simultaneously. Importantly, both treatments have been shown to destroy rod and cone photoreceptors, while leaving inner retinal neurons intact (Bernardos et al., 2007; Kassen et al., 2007; Kassen et al., 2008; Qin et al., 2009; Raymond et al., 2006; Thummel et al., 2010; Thummel et al., 2008a; Thummel et al., 2008b; Vihtelic and Hyde, 2000; Vihtelic et al., 2006). To detect effects of Fgf signaling on photoreceptor regeneration, light-treated fish were exposed daily to heat shock (Lee et al., 2005).
2.2 Immunohistochemistry
Zebrafish were euthanized by an anesthetic overdose of either Tricaine or 2-phenoxyethanol at 2.0 mg/ml in tank water and the eyes were harvested. Depending on which antisera was used, eyes were fixed overnight in either 9:1 ethanolic formaldehyde (100% ethanol; 37% formaldehyde) or 4% paraformaldehyde in 0.1 M phosphate buffer. Eyes were cryopreserved, sectioned, and immunohistochemistry was performed as previously described (Bernardos et al., 2007; Thummel et al., 2008a; Vihtelic and Hyde, 2000). The primary antibodies used in this study include a mouse monoclonal antibody specific for red-green double cones, zpr-1 (1:400; Zebrafish International Resource Center, ZIRC), anti-Proliferating Cell Nuclear Antigen (PCNA) mouse monoclonal antibody (1:1000, clone PC10, Sigma-Aldrich, St. Louis, MO), rabbit anti-Rhodopsin polyclonal antiserum (1:5000; (Vihtelic et al., 1999)), ROS-1 monoclonal antibody (1:1000), which labels rod photoreceptor outer segments (Raymond et al., 1995), and rabbit anti-GFP polyclonal antiserum (1:500, Abcam, Cambridge, MA). Secondary antibodies included AlexaFluor-conjugated 488 and 594 goat anti-primary antibody (Molecular Probes, Eugene, OR) and Cy3-conjugated rabbit anti-mouse IgG (1:100; Jackson ImmunoResearch, West Grove, PA). Some sections were also stained with the nuclear marker, 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma-Aldrich) or TO-PRO-3 (1:750, Molecular Probes).
2.3 Histology
Following 60 days of daily heat-shocks, Tg(hsp70:dn-fgfr1) zebrafish and wild-type sibling controls were euthanized by an anesthetic overdose and the eyes were harvested and fixed overnight in 4% paraformaldehyde. Hematoxylin and eosin staining was performed on 14 μm cryosections as described (Poss et al., 2002). Images were obtained using a Leica DM6000 microscope fitted with a Retiga EXi camera (Q-IMAGING).
2.4 Cell death analysis
Terminal Transferase dUTP Nick End Labeling (TUNEL) assay was performed on frozen eye sections using the ApoAlert DNA fragmentation kit (Clonetech, Mountain View, CA), with some minor modifications. Eyes were fixed overnight in ethanolic formaldehyde, processed and sectioned as previously described (Bernardos et al., 2007; Thummel et al., 2008a; Vihtelic and Hyde, 2000). The tissue was permeabilized in ice-cold NaCitrate buffer (0.1%NaCitrate, 0.1% Triton X-100). TdT reaction was performed at 37°C for 1 hour per manufacturer's suggestion with the exception of using biotinylated dNTPs (New England Biolabs, Ipswich, MA), followed by AlexaFluor-conjugated StrepAvidin labeling (Molecular Probes). Analysis was performed using confocal microscopy. Quantification of TUNEL-positive nuclei is described below.
2.5 in situ hybridization
Eyes from adult Tg(gfap:GFP)mi2002 fish were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer and prepared for cryosectioning as previously described (Bernardos et al., 2007). For in situ hybridization on cryosections, digoxigenin (DIG)-labeled cRNA probe for fgfr1 was prepared and hybridized at 5 μg/ml as described (Raymond et al., 2006).
2.6 FGF-2 injections
Intravitreal injections of FGF-2 or saline were performed on wild-type or dark-adapted albino zebrafish for three consecutive days; this protocol is based on work previously described in the chick retina (Fischer et al., 2002). Briefly, fish were anesthetized and a small incision was made in the cornea with a sapphire blade (Thummel et al., 2008b). A Hamilton syringe was used to inject 0.5 μl of 2μg/μl FGF-2 in PBS (phosphate-buffered saline) or vehicle control (PBS) into the vitreous of the left eye, while right eyes were uninjected. For wild-type animals that were not light-treated, eyes were harvested for immunohistochemistry 24 hours after each injection for three consecutive days. For dark-adapted albino zebrafish, the fish were placed in constant light treatment (∼8,000 lux) immediately following the third injection and eyes were harvested after 24 hours.
2.7 Imaging
Fluorescent microscopy was performed with either a Leica TCS SP5 confocal microscope or an AxioImager epifluorescent compound microscope equipped with an AxioCam mRM digital camera (Carl Zeiss Microimaging). Images were processed with Adobe Photoshop and all adjustments were equally applied to the entire image.
2.8 Quantitative analysis
All quantitative analysis was performed on retinal cryosections through the dorsoventral axis in the plane of the optic disc from the eyes of Tg(hsp70:dn-fgfr1) fish and wild-type siblings. A statistical difference between two groups was assessed using the standard Student's t-test. Error bars represent standard error of the mean (SEM) and values are given as “Average ± SEM.”
Maintenance studies
Tg(hsp70:dn-fgfr1) fish and wild-type siblings were heat-shocked daily for 3, 5, 7, 10, 14, or 60 days. Ten eyes from each group were harvested and processed for immunolabeling, cell death analysis or histology. PCNA-positive nuclei in the ONL were quantified across a 740 μm linear distance in the dorsal retina prior to heat shock (0 d) and at 3, 5, 7, and 10 days of heat-shock. TUNEL-positive nuclei were quantified across the entire retinal section at 0 and 10 days of heat shock. After 60 days of heat shock, the thickness of the ONL (in the central dorsal retina) was measured on histological sections. Unpaired Student's t-test was used for all statistical analysis.
FGF2 intravitreal injections
To assess whether FGF2 promotes neuroprotection of photoreceptors, Tg(hsp70:dn-fgfr1) fish and wild-type siblings were intravitreally-injected with FGF2 or saline, exposed to constant light treatment, and then assayed for cell death at 24 hours post light (hpl). TUNEL-positive nuclei were quantified in 8 fish per group; all TUNEL-positive nuclei were counted in retinal sections cut through the center of the eye along the dorsal-ventral axis. To assess whether FGF2 promotes proliferation of rod precursors, Tg(hsp70:dn-fgfr1) fish and wild-type siblings were intravitreally-injected with FGF2 or saline and then immunolabeled for PCNA. PCNA-positive nuclei were quantified in 6-8 fish per group; all PCNA-positive nuclei were counted in retinal sections through the center of the eye along the dorsal-ventral axis. Unpaired Student's t-test was used for all statistical analysis.
Light lesion studies
Tg(hsp70:dn-fgfr1) fish and wild-type siblings were exposed to 30-minutes of very intense light as described above and heat-shocked daily for 14 days. To analyze ONL thickness and cone cell density at 14 dpl, 8 eyes from each group were harvested and processed for immunohistochemistry. All zpr-1-positive double cone cells were counted within a linear length of 100 μm, and the thickness of the rod nuclear layer was measured. Unpaired Student's t-test was used for statistical analysis.
3. Results
3.1 Endogenous fgfr1 is expressed in photoreceptors and inner nuclear layer nuclei in the adult zebrafish retina
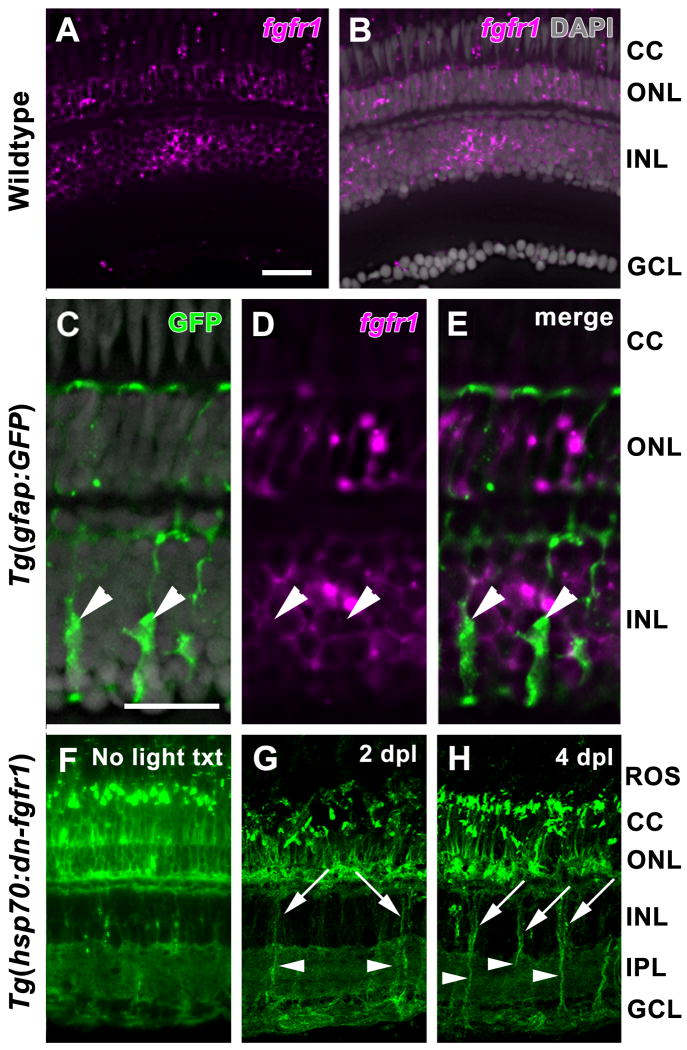
Four Fgf receptors (Fgfr1-4) mediate Fgf signaling via homo- and hetero-dimerization (Shi et al., 1993). The expression of all four fgfr subtypes was examined in the adult zebrafish retina by in situ hybridization. While probes for fgfr2, fgfr3, and fgfr4 showed no signal above background (data not shown), fgfr1 was detected in both rod and cone photoreceptors and in the inner nuclear layer (INL), but not in the ganglion cell layer (GCL) (Fig. 1A, B). This expression pattern did not change during retinal regeneration (data not shown). In the INL, fgfr1 did not colabel with the GFP-positive Müller glia in the Tg(gfap:GFP)mi2002 line (Fig. 1C-E) (Bernardos and Raymond, 2006). Based on cell morphology and location within the INL, fgfr1 was expressed by a subset of amacrine and bipolar cells (Fig. 1C-E).
Figure 1. Expression of fgfr1 and hsp70:dn-fgfr1 in the adult zebrafish retina.
A. Expression of fgfr1 transcripts in both rod and cone photoreceptors and in the inner nuclear layer is detected by in situ hybridization. B. Overlay of panel A with DAPI nuclear staining. C. GFP expression in Müller glia (arrowheads) in the Tg(gfap:GFP)mi2002 retina. D. Expression of fgfr1 in the same retinal section shown in panel C. E. Expression of fgfr1 in the inner nuclear layer does not colabel with GFP-positive Müller glia (arrowheads). F. Ubiquitous GFP expression driven by the hsp70 promoter in a Tg(hsp70:dn-fgfr1) retina two days after daily heat shock. G. Strong expression of dn-fgfr1-GFP fusion protein in Müller glia soma (arrows) and cell processes (arrowheads) at 2 days of constant light treatment (2 dpl). H. Strong expression of dn-fgfr1-GFP fusion protein in Müller glia soma (arrows) and cell processes (arrowheads) at 4 days of constant light treatment (4 dpl). ROS = rod outer segments; CC = cone cells; ONL = outer nuclear layer; INL = inner nuclear layer; IPL = inner plexiform layer; GCL = ganglion cell layer. Scale bar: Panel A = 50 μm (A-B, F-H); Panel C = 25 μm (C-E).
3.2 The hsp70:dn-fgfr1-gfp transgene is expressed in the adult zebrafish retina following heat-shock induction
To inhibit Fgf signaling, we used the Tg(hsp70:dn-fgfr1) line, harboring a dominant-negative fgfr1 fused with gfp under the control of the heat shock promoter hsp70 (Lee et al., 2005). The fusion protein is expected to form heterodimers with endogenous Fgfrs upon ligand binding and thus block the downstream signaling of all Fgfr subtypes (Lee et al., 2005). To confirm the dn-fgfr1-gfp transgene is heat-inducible in the adult retina, GFP expression was examined in the transgenic fish retinas after 2 days of daily heat shock. GFP fluorescence was observed in all retinal layers, with weak expression observed in the inner nuclear layer (INL; Fig. 1F).
To test the role of Fgf signaling in retinal regeneration, two photolytic lesion models were utilized; either continuous exposure to very bright light for several days (Vihtelic and Hyde, 2000) or exposure to extremely intense light for 30 minutes (Bernardos et al., 2007). Although each has a practical advantage (see Materials and Methods), both protocols have been shown to selectively destroy rod and cone photoreceptors while leaving the other retinal neurons intact (Bernardos et al., 2007; Kassen et al., 2007; Kassen et al., 2008; Qin et al., 2009; Raymond et al., 2006; Thummel et al., 2010; Thummel et al., 2008a; Thummel et al., 2008b; Vihtelic and Hyde, 2000; Vihtelic et al., 2006). Following retinal injury and cell loss, a subset of Müller glia reenter the cell cycle and become the source of retinal progenitor cells (Fausett and Goldman, 2006; Fimbel et al., 2007; Kassen et al., 2007; Raymond et al., 2006; Thummel et al., 2010; Yurco and Cameron, 2005). Following light lesions, retinal progenitors migrate to the photoreceptor layer, proliferate and differentiate into new photoreceptors.
To examine whether expression of the dn-fgfr1-gfp transgene persisted during these critical stages of photoreceptor regeneration, adult Tg(hsp70:dn-fgfr1) fish were exposed to constant bright light treatment and subjected to daily heat shock. Strong GFP expression was observed in the degenerating photoreceptors and in Müller glial cell somata and processes at 2 and 4 days after the onset of light treatment (days post light = dpl; Fig. 1G, H, respectively).
3.3 Fgf signaling regulates rod photoreceptor regeneration without affecting Müller glia or progenitor cell proliferation
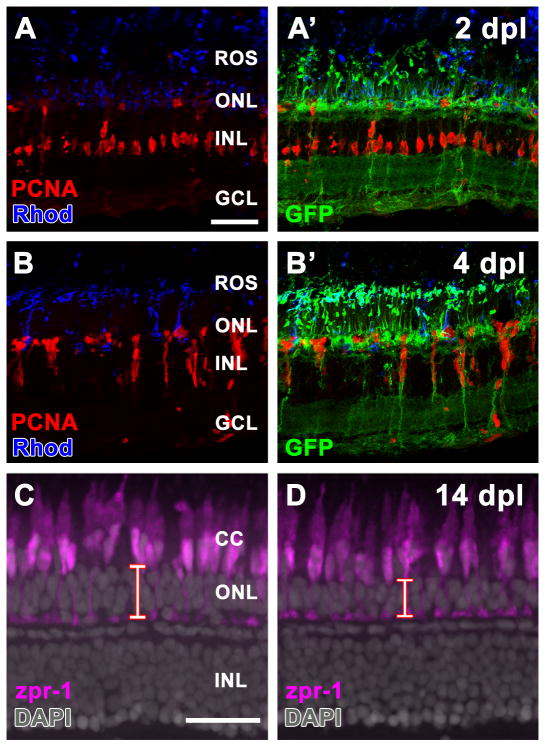
Functional studies have shown that factors such as the proneural transcription factor Ascl1a and Proliferating Cell Nuclear Antigen (PCNA) are essential for proper Müller glia proliferation during retinal regeneration (Fausett et al., 2008; Ramachandran et al., 2010; Thummel et al., 2008b), whereas the eye determination transcription factor Pax6 and the chaperone protein Hspd1 are required for persistent proliferation of the retinal progenitors (Qin et al., 2009; Thummel et al., 2010). To test whether Fgf signaling is required for either Müller glia or progenitor cell proliferation, adult Tg(hsp70:dn-fgfr1) fish and their wild-type siblings were exposed to constant bright light and heat-shocked daily. Eyes were harvested at 2 and 4 dpl and immunolabeled for PCNA, a widely-used marker for cell proliferation in retinal regeneration and other systems (Fimbel et al., 2007; Kassen et al., 2007; Kassen et al., 2010; Leung et al., 2005; Mahler and Driever, 2007; Thummel et al., 2006; Thummel et al., 2010; Thummel et al., 2008a; Thummel et al., 2008b; Vihtelic and Hyde, 2000; Vihtelic et al., 2006), and Rhodopsin, which labels rod photoreceptor outer segments (ROS). At 2 dpl, transgenic animals were indistinguishable from wild-type siblings (Fig. 2; wild-type retinas not shown), with photoreceptor degeneration (loss of ROS) and PCNA-positive Müller glia (Fig. 2A, A'). Similarly, 4 dpl, transgenic retinas exhibited large clusters of PCNA-positive retinal progenitors migrating to the ONL (Fig. 2B, B'), indicating that Fgf signaling is not required for these early proliferative events in retinal regeneration.
Figure 2. Fgf signaling is required for rod, but not cone, photoreceptor cell regeneration.
A. Retinal section from a Tg(hsp70:dn-fgfr1) adult zebrafish following daily heat shock and 2 days of constant light treatment (2 dpl). Müller glia cell proliferation (PCNA immunolabeling; red) and rod outer segment degeneration (Rhodopsin immunolabeling; blue) are unaffected by loss of Fgf signaling. A'. Overlay of panel A showing immunolocalization of the dn-fgfr1-GFP fusion protein. B. Retinal section from a Tg(hsp70:dn-fgfr1) adult zebrafish following daily heat shock and 4 days of constant light treatment (4 dpl). The continual proliferation of inner nuclear layer progenitors (PCNA immunolabeling; red) and their migration to the outer nuclear layer is unaffected by loss of Fgf signaling. B'. Overlay of panel B showing immunolocalization of the dn-fgfr1-GFP fusion protein. C – D. Retinal sections from wild-type (C) and Tg(hsp70:dn-fgfr1) zebrafish (D) 14 days after intense light damage (14 dpl) and daily heat shock. Double cones are immunolabeled with zpr-1 (magenta). DAPI nuclear stain shows retinal layers. C. Wild-type retinas contain zpr-1+ double cones and thick outer nuclear layer (red bar), which primarily houses rod photoreceptor nuclei. D. The Tg(hsp70:dn-fgfr1) retinas contain zpr-1+ double cones and a thinner than normal outer nuclear layer (red bar). ROS = rod outer segments; CC = cone cells; ONL = outer nuclear layer; INL = inner nuclear layer; GCL = ganglion cell layer. Scale bar: Panel A = 50 μm (A-B'); Panel C = 50 μm (C-D).
To determine the role of Fgf signaling in rod and cone photoreceptor regeneration, Tg(hsp70:dn-fgfr1) fish and wild-type siblings were exposed to extremely intense light for 30 minutes and then harvested at 14 dpl. The fish were heat-shocked daily throughout the experiment. Within the light-damaged region, the number of red-green double cones in the Tg(hsp70:dn-fgfr1) fish was not significantly different from that in light-treated wild-type siblings (average of 21.8 ± 0.5 and 22.1 ± 0.5 red-green cones per 100 μm of linear length of retina, respectively; n = 8; p = 0.68; Fig. 2C, D). However, the thickness of the ONL, which provides an index of number of rod photoreceptors, was approximately 30% less in the Tg(hsp70:dn-fgfr1) fish compared with the wild-type siblings (10.9 ± 0.3 μm versus 15.3 ± 0.3 μm, respectively; n = 8: p < 0.001; Fig. 2C, D). These results suggest that Fgf signaling is required for the regeneration of rod, but not cone, photoreceptors.
3.4 FGF-2 induces rod precursor proliferation and provides neuroprotection
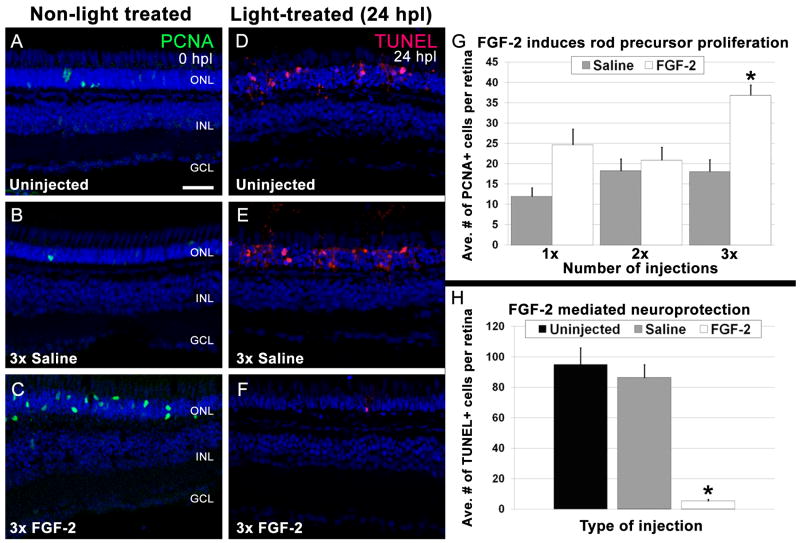
In the growing adult teleost fish retina, rod photoreceptors slowly accumulate in number by proliferation of committed rod precursor cells residing in the ONL that are derived from INL progenitors produced by Müller glia (Bernardos et al., 2007; Otteson et al., 2001). To test whether exogenous application of FGF is sufficient to induce proliferation of Müller glia, INL progenitors, and/or rod precursors in the undamaged retina, wild-type zebrafish were intravitreally injected with either saline or FGF-2 for up to three consecutive days. Eyes were harvested 24 hours after each injection and the total number of PCNA-positive nuclei per retinal section was quantified. PCNA-positive nuclei were found only in the ONL. Uninjected, unlesioned retinas, had an average of 16.5 ± 4.9 PCNA-positive, ONL nuclei per retinal section, which represents the normal, continuous production of rod photoreceptors in the adult zebrafish (Fig. 3A). There was no significant increase in the number of PCNA-positive, ONL nuclei compared with uninjected retinas following 1, 2, or 3 daily saline injections (Fig. 3B, G; p = 0.33, 0.77, 0.78, respectively). Similarly, there was no significant increase in the number of PCNA-positive ONL nuclei after either 1 or 2 daily FGF-2 injections (Fig. 3G; p = 0.02, 0.5, respectively). However, 3 injections of FGF-2 significantly increased the number of PCNA-positive ONL rod precursor cells relative to the saline-injected controls (Fig. 3C, G; p < 0.001), indicating that Fgf signaling is sufficient to induce rod precursor cell proliferation.
Figure 3. FGF-2 induces rod precursor proliferation and provides neuroprotection.
A - C. Retinal sections from undamaged eyes that were either uninjected (A), or injected three consecutive days with Saline (B) or FGF-2 (C). PCNA immunolocalization (green) shows proliferating cells. Nuclei are stained with TO-PRO-3 (blue). A. A few PCNA-positive rod precursors are present in the outer nuclear layer. B. Saline injections did not significantly increase the number of PCNA-positive rod precursors. C. Large numbers of PCNA-positive rod precursors are observed in the outer nuclear layer in FGF-2-injected retinas. D - F. Retinal sections of 24-hour light-treated zebrafish that were either uninjected (D) or injected three consecutive days with Saline (E) or FGF-2 (F). TUNEL immunolocalization (red) shows cells undergoing apoptosis. Nuclei are stained with TO-PRO-3 (blue). D. Large numbers of TUNEL-positive cells are observed in the outer nuclear layer of uninjected retinas. E. Saline injections did not significantly alter the number of TUNEL-positive nuclei. F. Eyes injected three consecutive days with FGF-2 exhibited significantly fewer TUNEL-positive cells. G. Graphic representation of the average number of PCNA-positive cells in the outer nuclear layer in non-light treated eyes injected with either Saline or FGF-2. H. Graphic representation of the average number of TUNEL-positive cells in 24-hour light-treated retinas that were either uninjected, or injected for three consecutive days with Saline or FGF-2.
Data from several different neuronal damage models in a variety of species implicate Fgf signaling in promoting neuroprotection (Faktorovich et al., 1990, 1992; LaVail et al., 1991). To test whether Fgf signaling could play a similar role in adult zebrafish photoreceptors, dark-adapted adult albino zebrafish were intravitreally injected with either saline or FGF-2 prior to constant light exposure. Twenty-four hours after light onset, uninjected, saline-injected, and FGF-2-injected eyes were analyzed for cell death. In uninjected and saline-injected light-damaged retinas, an average of 94.6 ± 11.2 and 86.1 ± 8.7 TUNEL-positive nuclei were observed in the ONL, respectively (Fig. 3D, E, H). In contrast, significantly fewer TUNEL-positive cells were observed in FGF-2-injected retinas (5.1 ± 1.1; p < 0.0001) relative to saline-injected controls (Fig. 3F, H), indicating Fgf signaling is neuroprotective for rod photoreceptors in the adult zebrafish retina.
3.5 Fgf signaling is required for rod photoreceptor maintenance
As our data indicated that Fgf signaling promoted rod precursor proliferation and protected photoreceptors from photolytic damage, we asked if Fgf signaling also played a role in photoreceptor homeostasis by daily heat-shocking adult Tg(hsp70:dn-fgfr1) fish and their wild-type siblings and analyzing their retinas at several different time points post-heat shock.
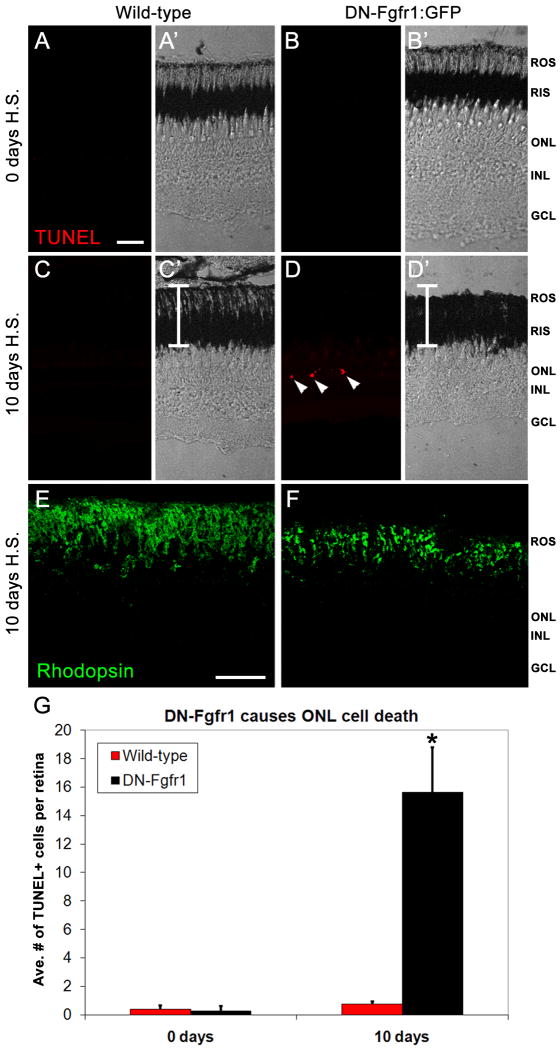
We first analyzed whether continuous daily heat-shock resulted in increased cell death in wild-type animals. We found no difference in the number of TUNEL-positive nuclei in non-heat shock animals (0.38 ± 0.26) compared with wild-type zebrafish at 10 days post-heat shock (0.25 ± 0.16; Fig. 4A, C, G; p = 0.69). In contrast, at 10 days post-heat shock, the transgenic retinas possessed significantly more TUNEL-positive nuclei compared with their wild-type siblings (Fig. 4D, C, G; p < 0.002). In addition, TUNEL-positive nuclei were observed only in the ONL (Fig. 4D), which is primarily comprised of rod photoreceptor nuclei in the adult zebrafish retina. A corresponding decrease was observed in both rod outer segment (ROS) length (compare Fig. 4D' to 4C&') and Rhodopsin organization (compare Fig. 4F to 4E). Thus, the loss of Fgf signaling resulted in significant rod photoreceptor cell death and disorganization by 10 days post-heat shock.
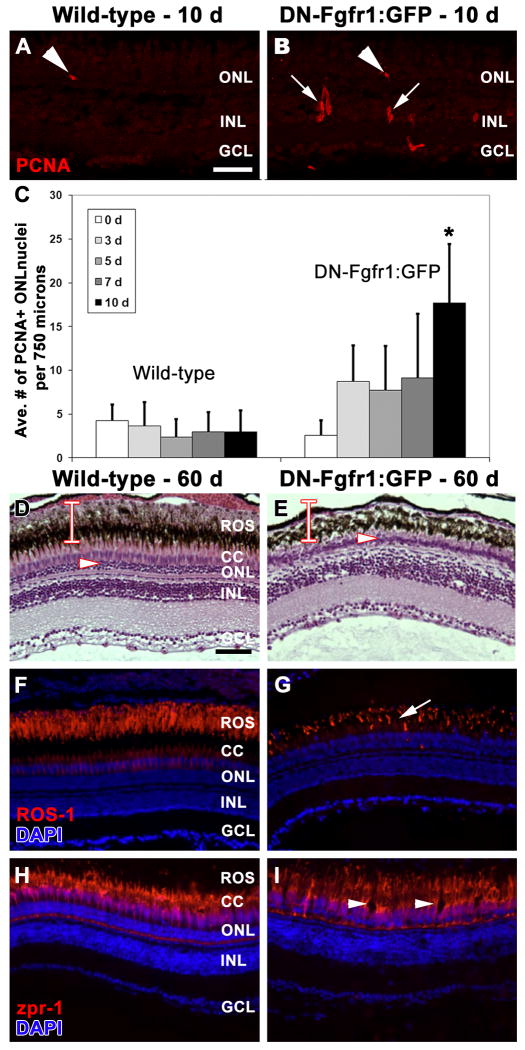
Figure 4. Loss of Fgf signaling causes rod photoreceptor apoptosis and outer segment degeneration.
A-A'. Fluorescent and phase contrast images showing the absence of any TUNEL labeling (red) in a wild-type retina prior to heat-shock. B-B'. Fluorescent and phase contrast images showing the absence of TUNEL labeling (red) in a Tg(hsp70:dn-fgfr1) retina prior to heat-shock. C-C'. Fluorescent and phase contrast images showing the absence of TUNEL labeling (red) in a wild-type retina after 10 days of daily heat-shock. The white bar depicts the normal thickness of rod inner and outer segments. D-D'. Fluorescent and phase contrast images showing TUNEL-positive nuclei in the outer nuclear layer (red) of a Tg(hsp70:dn-fgfr1) retina after 10 days of daily heat-shock. The white bar depicts the normal thickness of rod inner and outer segments, showing rod outer segment degeneration in transgenic retinas. E. Rhodopsin immunolocalization to rod outer segments in a wild-type retina after 10 days of daily heat-shock. F. Rhodopsin immunolocalization to rod outer segments in a Tg(hsp70:dn-fgfr1) retina after 10 days of daily heat-shock, showing degenerated and disorganized outer segments. G. Graphic depiction of the average number of TUNEL-positive nuclei in wild-type and Tg(hsp70:dn-fgfr1) retinas after either 0 or 10 days of daily heat shock. ROS = rod outer segments; RIS = rod inner segments; ONL = outer nuclear layer; INL = inner nuclear layer; GCL = ganglion cell layer. Scale bar: Panel A = 50 μm (A-D'); Panel E = 50 μm (E-F).
Loss of photoreceptors from constant intense light treatment induces proliferation of two types of progenitor cells in the adult zebrafish retina: rod precursors in the ONL, which give rise exclusively to rod photoreceptors (Hitchcock et al., 2004; Otteson et al., 2001; Raymond et al., 2006; Thummel et al., 2010), and activated Müller glia, which can yield any retinal cell type (Fimbel et al., 2007; Raymond et al., 2006; Vihtelic and Hyde, 2000). To test whether the rod photoreceptor death resulting from loss of Fgf signaling activated a regeneration-like response from activated Müller glia, adult Tg(hsp70:dn-fgfr1) fish and their wild-type siblings were examined for PCNA immunolocalization at 0, 3, 5, 7, and 10 days post-heat shock. In wild-type retinas, an average of 4.3 ± 1.8, 3.6 ± 2.7, 2.4 ± 2.1, 3.0 ± 2.2, and 3.0 ± 2.4 PCNA-positive nuclei were detected in the ONL (within a 750 micron linear distance in the central, dorsal retina) at each time point, respectively (Fig. 5C). This represents the normal, continuous production of rod photoreceptors in the adult zebrafish. We found no significant increase in PCNA-positive nuclei at any of these time points, indicating that continuous heat-shock does not result in a proliferative response in rod precursors or Müller glial cells (Fig. 5A). In contrast, clusters of Müller glia-derived, PCNA-positive, INL nuclei were observed in the transgenic retinas (Fig. 5B), along with significantly higher numbers of PCNA-positive rod precursors in the ONL at 10 days post-heat shock (Fig. 5C; p < 0.001). This suggested that the rod photoreceptor cell death resulting from loss of Fgf signaling triggered a proliferation response similar to that induced by intense light treatment.
Figure 5. Long-term defect in Fgf signaling results in significant loss of rod, but not cone, photoreceptors.
A. PCNA immunolocalization (red) in a wild-type retina after 10 days of daily heat-shock shows isolated rod precursor cell proliferation in the ONL (arrowhead). B. PCNA immunolocalization (red) in a Tg(hsp70:dn-fgfr1) retina after 10 days of daily heat-shock shows increased proliferation in rod precursor cells (arrowhead; Panel C) and in inner nuclear layer progenitor cells (arrows). C. Graphic depiction comparing the average number of PCNA-positive cells observed in the outer nuclear layer in wild-type and Tg(hsp70:dn-fgfr1) retinas following daily heat-shock over multiple time points. D-I. Wild-type (D, F, H) and Tg(hsp70:dn-fgfr1) (E, G, I) retinas following 60 days of daily heat-shock. D. Histological section of a wild-type retina showing the normal outer nuclear layer (arrowhead) and the thickness of the rod inner and outer segments (bar). E. Histological section of a Tg(hsp70:dn-fgfr1) retina, showing a thin outer nuclear layer (arrowhead) and a near complete loss of rod outer segments (compare the bar in D and E). F. ROS-1 immunolabeling (red) overlaid with DAPI nuclear stain (blue) in a wild-type retina, showing normal rod outer segment thickness and organization. G. A corresponding Tg(hsp70:dn-fgfr1) retinal section, showing ROS-1 immunolocalization (red) to truncated and disorganized rod outer segments (arrow). H. Immunolocalization of zpr-1 to double cones (red) overlaid with DAPI nuclear stain (blue) in a wild-type retina. I. A corresponding Tg(hsp70:dn-fgfr1) retinal section, showing zpr-1 immunolocalization to double cones (red), which are present, but exhibit areas of disorganization (arrowheads). ROS = rod outer segments; CC = cone cells; ONL = outer nuclear layer; INL = inner nuclear layer; GCL = ganglion cell layer. Scale bar: Panel A = 50 μm (A-B'); Panel D = 50 μm (D-I).
To better understand the homeostatic role of Fgf signaling in the retina, we also examined the phenotype produced by persistent long-term loss of Fgf signaling. Adult Tg(hsp70:dn-fgfr1) fish and their wild-type siblings were heat-shocked daily for 60 days. Histological and immunological analysis revealed that the transgenic retinas possessed shorter rod outer segments (compare Fig. 5D, F and 5E, G) and a thinner ONL thickness relative to their wild-type siblings (compare 5D to 5E; average of 3.7 ± 0.5 μm and 11.1 ± 0.9 μm, respectively; p < 0.01). In contrast, the red-green double cones, which were labeled with the zpr-1 monoclonal antibody, were present in a similar number and thickness in both the transgenic and wild-type retinas (Fig. 5I and H, respectively), although they exhibited some disorganization in the transgenic line (Fig. 5I). Together, these data indicate that sustained Fgf signaling is required for rod photoreceptor homeostasis.
4. Discussion
Here we provide evidence that rod photoreceptor homeostasis and regeneration in adult zebrafish is Fgf-dependent but cone photoreceptors do not have the same dependence. First, we show that intravitreal injections of FGF-2 induced rod precursor proliferation and neuroprotection during intense light damage to photoreceptors. Additionally, using the dominant-negative Tg(hsp70:dn-fgfr1) transgenic line, we found that Fgf signaling was required for homeostasis of rod, but not cone, photoreceptors. Finally, we found that Fgf signaling also differentially affected the regeneration of rod photoreceptors in the light-damaged retina.
Fgf signaling has been reported to play a key role in promoting neural retina development and regeneration (Cai et al., 2010; Guillemot and Cepko, 1992; Martinez-Morales et al., 2005; Park and Hollenberg, 1989, 1993; Pittack et al., 1997; Zhao et al., 2001) and in maintaining mammalian photoreceptor homeostasis (Stone et al., 1999). We have shown that intraocular injections of FGF-2 induce rod precursor proliferation, although it is unclear whether these new cells properly differentiate into functional rod photoreceptors and innervate second-order inner neurons. Alternatively, it is possible that the newly generated rods do not function properly or are not needed and therefore targeted for cell death. Future studies combining cell tracing analysis and inducible Fgf-2 transgenes would help elucidate these possible outcomes.
Fgf signaling has been reported to promote neuroprotection (Cai et al., 2011; Faktorovich et al., 1990, 1992; Harada et al., 2000; LaVail et al., 1991; Zhang et al., 2003), and FGF-2 protects photoreceptors from intense light damage in adult rodents (Faktorovich et al., 1992; LaVail et al., 1991). In addition, FGF-2 has been shown to promote photoreceptor survival in adult porcine and chick cell cultures (Frohns et al., 2009; Traverso et al., 2003). Our data strongly suggest a conserved neuroprotective function of Fgf signaling for rod photoreceptors in the zebrafish retina.
Of the four Fgf receptor subtypes, only fgfr1 is expressed in the adult zebrafish retina prior to light damage, and it is expressed in both rod and cone photoreceptors (Fig. 1), which is consistent with a previous report in the adult goldfish retina describing Fgfr1 immunolocalization in both rod and cone photoreceptors (Raymond et al., 1992). Even though fgfr1 is expressed in both rod and cone photoreceptors (Fig. 1B), we found that the dominant-negative hsp70:dn-fgfr1 transgene significantly repressed rod photoreceptor regeneration without affecting regeneration of red-green double cone photoreceptors (Fig. 2C, D). As zebrafish have more than twenty Fgfs, it is possible that rod and cone photoreceptors respond differently to the presence of the different Fgf ligands. Alternatively, the differential response of rods and cones may be mediated through downstream targets. Recently it was reported that the loss of the protein tyrosine phosphatase Shp2, a known downstream target of Fgf signaling (Cai et al., 2010), resulted in severe photoreceptor degeneration of both rod and cone photoreceptors in mice (Cai et al., 2011). Future studies aimed at manipulating individual components of Fgf signaling in a cell-specific manner will be needed to answer these questions.
We found that endogenous fgfr1 is not expressed in the Müller glia of the zebrafish retina prior to light treatment (Fig. 1E) and that loss of Fgf signaling during retinal regeneration did not affect Müller glia proliferation or migration of INL retinal progenitors to the ONL (Fig. 2A-B'). This is in contrast to previous reports on isolated adult bovine Müller glial cells, and exogenous overexpression of FGF-2 in the adult chick retina, in which FGF-2 was shown to be mitogenic to Müller glial cells (Fischer et al., 2002; Mascarelli et al., 1991).
Our data suggest that in the adult zebrafish retina, Fgf signaling is not required for the early stages of retinal progenitor amplification or migration, but instead is involved at a later point when the progenitors are differentiating into rods and cones. However, the cellular mechanism that underlies the rod regeneration defect is not known. One possibility is that Fgf signaling is required for neuronal progenitors to commit to the rod photoreceptor cell fate but not cone photoreceptors. If this were the case, we would expect to observe a greater number of cone photoreceptors in the Tg(hsp70:dn-fgfr1) retinas following regeneration, but we did not. A second possibility is that inhibition of Fgf signaling affects proliferation of rod precursors in the ONL that give rise to differentiated rods. However, PCNA-positive rod precursors were observed in the ONL of transgenic retinas prior to light treatment (Fig. 5C) and at 2 days of light treatment (Fig. 2A, A'), suggesting this hypothesis is also not valid.
Homeostasis data provided evidence in support of a third explanation for the observed requirement for Fgf signaling in rod photoreceptor regeneration: a trophic effect on newly-differentiated rod photoreceptors. When Tg(hsp70:dn-fgfr1) fish and wild-type siblings were heat-shocked daily without light lesion for 10 days, degeneration of rod outer segments and apoptosis of rod photoreceptors was observed in the transgenic retinas, but not in the wild-type retinas (Fig. 4). In the heat-shocked Tg(hsp70:dn-fgfr) retinas, in response to loss of rods, proliferation of rod precursors in the outer nuclear layer was up-regulated (Fig. 5C). Under normal Fgf signaling conditions, these proliferating rod precursors would replenish lost rod photoreceptors (Thummel et al., 2010). However, when sustained inhibition of Fgf signaling for 60 days led to damage and loss of rods, proliferating rod precursors were unable to replenish them. Instead, the thickness of the outer nuclear layer was significantly reduced, reflecting a reduction in number of rods (Fig. 5E, G). These data support the hypothesis that Fgf signaling is required for the survival of existing and newly-differentiated rod photoreceptors. Future studies that combine rod precursor-specific promoters and conditional inhibition of Fgf signaling may better clarify the cellular mechanism that underlies this trophic effect on rod photoreceptors.
In conclusion, this study adds to the growing body of work implicating Fgf signaling as an essential component for tissue regeneration, and is the first, to our knowledge, to provide evidence that Fgf signaling is required for regeneration of photoreceptors in adult retina.
Highlights.
fgfr1 was found to be expressed in the adult zebrafish retina.
FGF-2 induced rod precursor proliferation and photoreceptor neuroprotection
Fgf signaling is not required for retinal progenitor amplification or migration.
Fgf signaling is required for the regeneration of rod, but not cone, photoreceptors.
Acknowledgments
Funding sources: This work was funded by the Center for Zebrafish Research at the University of Notre Dame (DRH), National Institutes of Health grants R21EY019401 (RT), R01EY018417 (RT and DRH), P30EY04068 (RT), GM074057 (KDP), R01EY004318 (PAR and ZQ), and start-up funds to RT, including an unrestricted grant from Research to Prevent Blindness to the Wayne State University, Department of Ophthalmology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zhao Qin, Email: qinzhao@umich.edu.
Ambrose R. Kidd, III, Email: kidda@umsl.edu.
Jennifer L. Thomas, Email: jthoma@med.wayne.edu.
Kenneth D. Poss, Email: k.poss@cellbio.duke.edu.
David R. Hyde, Email: dhyde@nd.edu.
Pamela A. Raymond, Email: praymond@umich.edu.
Ryan Thummel, Email: rthummel@med.wayne.edu.
References
- Becker T, Wullimann MF, Becker CG, Bernhardt RR, Schachner M. Axonal regrowth after spinal cord transection in adult zebrafish. J Comp Neurol. 1997;377:577–595. doi: 10.1002/(sici)1096-9861(19970127)377:4<577::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardos RL, Raymond PA. GFAP transgenic zebrafish. Gene Expr Patterns. 2006;6:1007–1013. doi: 10.1016/j.modgep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Boilly B, Cavanaugh KP, Thomas D, Hondermarck H, Bryant SV, Bradshaw RA. Acidic fibroblast growth factor is present in regenerating limb blastemas of axolotls and binds specifically to blastema tissues. Dev Biol. 1991;145:302–310. doi: 10.1016/0012-1606(91)90128-p. [DOI] [PubMed] [Google Scholar]
- Cai Z, Feng GS, Zhang X. Temporal requirement of the protein tyrosine phosphatase Shp2 in establishing the neuronal fate in early retinal development. J Neurosci. 2010;30:4110–4119. doi: 10.1523/JNEUROSCI.4364-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Simons DL, Fu XY, Feng GS, Wu SM, Zhang X. Loss of Shp2-mediated mitogen-activated protein kinase signaling in muller glial cells results in retinal degeneration. Mol Cell Biol. 2011;31:2973–2983. doi: 10.1128/MCB.05054-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DA. Cellular proliferation and neurogenesis in the injured retina of adult zebrafish. Vis Neurosci. 2000;17:789–797. doi: 10.1017/s0952523800175121. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Chang M, Ohsato M, Vinores SA, Nie Z, Hjelmeland L, Mansukhani A, Basilico C, Zack DJ. Retinal degeneration in transgenic mice with photoreceptor-specific expression of a dominant-negative fibroblast growth factor receptor. J Neurosci. 1996;16:1679–1688. doi: 10.1523/JNEUROSCI.16-05-01679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen B, Slack JM. FGF-8 is associated with anteroposterior patterning and limb regeneration in Xenopus. Dev Biol. 1997;192:455–466. doi: 10.1006/dbio.1997.8732. [DOI] [PubMed] [Google Scholar]
- Craig SE, Thummel R, Ahmed H, Vasta GR, Hyde DR, Hitchcock PF. The zebrafish galectin Drgal1-l2 is expressed by proliferating Muller glia and photoreceptor progenitors and regulates the regeneration of rod photoreceptors. Invest Ophthalmol Vis Sci. 2010;51:3244–3252. doi: 10.1167/iovs.09-4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley PH, Minowada G, MacArthur CA, Martin GR. Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell. 1996;84:127–136. doi: 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- D'Jamoos CA, McMahon G, Tsonis PA. Fibroblast growth factor receptors regulate the ability for hindlimb regeneration in Xenopus laevis. Wound Repair Regen. 1998;6:388–397. doi: 10.1046/j.1460-9568.1998.60415.x. [DOI] [PubMed] [Google Scholar]
- Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature. 1990;347:83–86. doi: 10.1038/347083a0. [DOI] [PubMed] [Google Scholar]
- Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Basic fibroblast growth factor and local injury protect photoreceptors from light damage in the rat. J Neurosci. 1992;12:3554–3567. doi: 10.1523/JNEUROSCI.12-09-03554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26:6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J Neurosci. 2008;28:1109–1117. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27:1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, McGuire CR, Dierks BD, Reh TA. Insulin and fibroblast growth factor 2 activate a neurogenic program in Muller glia of the chicken retina. J Neurosci. 2002;22:9387–9398. doi: 10.1523/JNEUROSCI.22-21-09387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohns F, Mager M, Layer PG. Basic fibroblast growth factor increases the precursor pool of photoreceptors, but inhibits their differentiation and apoptosis in chicken retinal reaggregates. Eur J Neurosci. 2009;29:1931–1942. doi: 10.1111/j.1460-9568.2009.06738.x. [DOI] [PubMed] [Google Scholar]
- Geraudie J, Singer M. The fish fin regenerate. Monogr Dev Biol. 1992;23:62–72. [PubMed] [Google Scholar]
- Guillemot F, Cepko CL. Retinal fate and ganglion cell differentiation are potentiated by acidic FGF in an in vitro assay of early retinal development. Development. 1992;114:743–754. doi: 10.1242/dev.114.3.743. [DOI] [PubMed] [Google Scholar]
- Harada T, Harada C, Nakayama N, Okuyama S, Yoshida K, Kohsaka S, Matsuda H, Wada K. Modification of glial-neuronal cell interactions prevents photoreceptor apoptosis during light-induced retinal degeneration. Neuron. 2000;26:533–541. doi: 10.1016/s0896-6273(00)81185-x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P, Ochocinska M, Sieh A, Otteson D. Persistent and injury-induced neurogenesis in the vertebrate retina. Prog Retin Eye Res. 2004;23:183–194. doi: 10.1016/j.preteyeres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Weston JA. Temperature-sensitive mutations that cause stage-specific defects in Zebrafish fin regeneration. Genetics. 1995;141:1583–1595. doi: 10.1093/genetics/141.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassen SC, Ramanan V, Montgomery JE, C TB, Liu CG, Vihtelic TS, Hyde DR. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev Neurobiol. 2007;67:1009–1031. doi: 10.1002/dneu.20362. [DOI] [PubMed] [Google Scholar]
- Kassen SC, Raycroft FJ, Thummel R, Plasschaert R, Hyde DR. Stat3 is required for maximal Müller glial cell proliferation during regeneration of the light-damaged zebrafish retina. J Neurosci. 2010 Under Review, October 2009. [Google Scholar]
- Kassen SC, Thummel R, Burket CT, Campochiaro LA, Harding MJ, Hyde DR. The Tg(cyclinB1:EGFP) transgenic zebrafish line is a marker for proliferation in retinal development and regeneration. Mol Vis. 2008 in press. [PMC free article] [PubMed] [Google Scholar]
- LaVail MM, Faktorovich EG, Hepler JM, Pearson KL, Yasumura D, Matthes MT, Steinberg RH. Basic fibroblast growth factor protects photoreceptors from light-induced degeneration in albino rats. Ann N Y Acad Sci. 1991;638:341–347. doi: 10.1111/j.1749-6632.1991.tb49044.x. [DOI] [PubMed] [Google Scholar]
- Lee Y, Grill S, Sanchez A, Murphy-Ryan M, Poss KD. Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development. 2005;132:5173–5183. doi: 10.1242/dev.02101. [DOI] [PubMed] [Google Scholar]
- Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Leung AY, Leung JC, Chan LY, Ma ES, Kwan TT, Lai KN, Meng A, Liang R. Proliferating cell nuclear antigen (PCNA) as a proliferative marker during embryonic and adult zebrafish hematopoiesis. Histochem Cell Biol. 2005;124:105–111. doi: 10.1007/s00418-005-0003-2. [DOI] [PubMed] [Google Scholar]
- Mahler J, Driever W. Expression of the zebrafish intermediate neurofilament Nestin in the developing nervous system and in neural proliferation zones at postembryonic stages. BMC Dev Biol. 2007;7:89. doi: 10.1186/1471-213X-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR. The roles of FGFs in the early development of vertebrate limbs. Genes Dev. 1998;12:1571–1586. doi: 10.1101/gad.12.11.1571. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales JR, Del Bene F, Nica G, Hammerschmidt M, Bovolenta P, Wittbrodt J. Differentiation of the vertebrate retina is coordinated by an FGF signaling center. Dev Cell. 2005;8:565–574. doi: 10.1016/j.devcel.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Mascarelli F, Tassin J, Courtois Y. Effect of FGFs on adult bovine Muller cells: proliferation, binding and internalization. Growth Factors. 1991;4:81–95. doi: 10.3109/08977199109000260. [DOI] [PubMed] [Google Scholar]
- Morgan TH. Regeneration in Teleosts. Entwicklungsmechanik Der Organismen. 1901;10:120–131. [Google Scholar]
- Mullen LM, Bryant SV, Torok MA, Blumberg B, Gardiner DM. Nerve dependency of regeneration: the role of Distal-less and FGF signaling in amphibian limb regeneration. Development. 1996;122:3487–3497. doi: 10.1242/dev.122.11.3487. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Nakagawa T, Yamamoto A, Araga A, Ohata T, Ishimaru Y, Yoshioka H, Kuwana T, Nohno T, Yamasaki M, Itoh N, Noji S. The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development. 1997;124:2235–2244. doi: 10.1242/dev.124.11.2235. [DOI] [PubMed] [Google Scholar]
- Ortega S, Ittmann M, Tsang SH, Ehrlich M, Basilico C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci U S A. 1998;95:5672–5677. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otteson DC, D'Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev Biol. 2001;232:62–76. doi: 10.1006/dbio.2001.0163. [DOI] [PubMed] [Google Scholar]
- Park CM, Hollenberg MJ. Basic fibroblast growth factor induces retinal regeneration in vivo. Dev Biol. 1989;134:201–205. doi: 10.1016/0012-1606(89)90089-4. [DOI] [PubMed] [Google Scholar]
- Park CM, Hollenberg MJ. Growth factor-induced retinal regeneration in vivo. Int Rev Cytol. 1993;146:49–74. doi: 10.1016/s0074-7696(08)60379-4. [DOI] [PubMed] [Google Scholar]
- Peters H, Balling R. Teeth. Where and how to make them. Trends Genet. 1999;15:59–65. doi: 10.1016/s0168-9525(98)01662-x. [DOI] [PubMed] [Google Scholar]
- Pittack C, Grunwald GB, Reh TA. Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos. Development. 1997;124:805–816. doi: 10.1242/dev.124.4.805. [DOI] [PubMed] [Google Scholar]
- Poss KD, Shen J, Nechiporuk A, McMahon G, Thisse B, Thisse C, Keating MT. Roles for Fgf signaling during zebrafish fin regeneration. Dev Biol. 2000;222:347–358. doi: 10.1006/dbio.2000.9722. [DOI] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- Poulin ML, Patrie KM, Botelho MJ, Tassava RA, Chiu IM. Heterogeneity in the expression of fibroblast growth factor receptors during limb regeneration in newts (Notophthalmus viridescens) Development. 1993;119:353–361. doi: 10.1242/dev.119.2.353. [DOI] [PubMed] [Google Scholar]
- Qin Z, Barthel LK, Raymond PA. Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. Proc Natl Acad Sci U S A. 2009;106:9310–9315. doi: 10.1073/pnas.0811186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol. 2010;12:1101–1107. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Curran GA. Developmental patterning of rod and cone photoreceptors in embryonic zebrafish. J Comp Neurol. 1995;359:537–550. doi: 10.1002/cne.903590403. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Rounsifer ME. Immunolocalization of basic fibroblast growth factor and its receptor in adult goldfish retina. Exp Neurol. 1992;115:73–78. doi: 10.1016/0014-4886(92)90225-f. [DOI] [PubMed] [Google Scholar]
- Reifers F, Bohli H, Walsh EC, Crossley PH, Stainier DY, Brand M. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development. 1998;125:2381–2395. doi: 10.1242/dev.125.13.2381. [DOI] [PubMed] [Google Scholar]
- Shi E, Kan M, Xu J, Wang F, Hou J, McKeehan WL. Control of fibroblast growth factor receptor kinase signal transduction by heterodimerization of combinatorial splice variants. Mol Cell Biol. 1993;13:3907–3918. doi: 10.1128/mcb.13.7.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Maslim J, Valter-Kocsi K, Mervin K, Bowers F, Chu Y, Barnett N, Provis J, Lewis G, Fisher SK, Bisti S, Gargini C, Cervetto L, Merin S, Peer J. Mechanisms of photoreceptor death and survival in mammalian retina. Prog Retin Eye Res. 1999;18:689–735. doi: 10.1016/s1350-9462(98)00032-9. [DOI] [PubMed] [Google Scholar]
- Thummel R, Bai S, Sarras MP, Jr, Song P, McDermott J, Brewer J, Perry M, Zhang X, Hyde DR, Godwin AR. Inhibition of zebrafish fin regeneration using in vivo electroporation of morpholinos against fgfr1 and msxb. Dev Dyn. 2006;235:336–346. doi: 10.1002/dvdy.20630. [DOI] [PubMed] [Google Scholar]
- Thummel R, Enright JM, Kassen SC, Montgomery JE, Bailey TJ, Hyde DR. Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp Eye Res. 2010;90:572–582. doi: 10.1016/j.exer.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Kassen SC, Enright JM, Nelson CM, Montgomery JE, Hyde DR. Characterization of Muller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp Eye Res. 2008a;87:433–444. doi: 10.1016/j.exer.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Kassen SC, Montgomery JE, Enright JM, Hyde DR. Inhibition of Muller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev Neurobiol. 2008b;68:392–408. doi: 10.1002/dneu.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverso V, Kinkl N, Grimm L, Sahel J, Hicks D. Basic fibroblast and epidermal growth factors stimulate survival in adult porcine photoreceptor cell cultures. Invest Ophthalmol Vis Sci. 2003;44:4550–4558. doi: 10.1167/iovs.03-0460. [DOI] [PubMed] [Google Scholar]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Doro CJ, Hyde DR. Cloning and characterization of six zebrafish photoreceptor opsin cDNAs and immunolocalization of their corresponding proteins. Vis Neurosci. 1999;16:571–585. doi: 10.1017/s0952523899163168. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol. 2000;44:289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Soverly JE, Kassen SC, Hyde DR. Retinal regional differences in photoreceptor cell death and regeneration in light-lesioned albino zebrafish. Exp Eye Res. 2006;82:558–575. doi: 10.1016/j.exer.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Vogel A, Rodriguez C, Izpisua-Belmonte JC. Involvement of FGF-8 in initiation, outgrowth and patterning of the vertebrate limb. Development. 1996;122:1737–1750. doi: 10.1242/dev.122.6.1737. [DOI] [PubMed] [Google Scholar]
- Whitehead GG, Makino S, Lien CL, Keating MT. fgf20 is essential for initiating zebrafish fin regeneration. Science. 2005;310:1957–1960. doi: 10.1126/science.1117637. [DOI] [PubMed] [Google Scholar]
- Yurco P, Cameron DA. Responses of Muller glia to retinal injury in adult zebrafish. Vision Res. 2005;45:991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Zenjari C, Boilly B, Hondermarck H, Boilly-Marer Y. Nerve-blastema interactions induce fibroblast growth factor-1 release during limb regeneration in Pleurodeles waltl. Dev Growth Differ. 1997;39:15–22. doi: 10.1046/j.1440-169x.1997.00003.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, El-Hodiri HM, Ma HF, Zhang X, Servetnick M, Wensel TG, Jamrich M. Targeted expression of the dominant-negative FGFR4a in the eye using Xrx1A regulatory sequences interferes with normal retinal development. Development. 2003;130:4177–4186. doi: 10.1242/dev.00626. [DOI] [PubMed] [Google Scholar]
- Zhao S, Hung FC, Colvin JS, White A, Dai W, Lovicu FJ, Ornitz DM, Overbeek PA. Patterning the optic neuroepithelium by FGF signaling and Ras activation. Development. 2001;128:5051–5060. doi: 10.1242/dev.128.24.5051. [DOI] [PubMed] [Google Scholar]
- Zhu X, Sasse J, McAllister D, Lough J. Evidence that fibroblast growth factors 1 and 4 participate in regulation of cardiogenesis. Dev Dyn. 1996;207:429–438. doi: 10.1002/(SICI)1097-0177(199612)207:4<429::AID-AJA7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]