Abstract
Target-specific drugs, including natural products, offer promise for the amelioration of cancer and other human ailments. Capsaicin, the pungent ingredient present in chilies (Capsicum annuum L.), and capsazepine, a synthetic analog of capsaicin (collectively referred to as vanilloids), are known to possess a variety of pharmacological and physiological properties. In our continuous effort to discover and characterize cancer chemopreventive agents from natural products, we investigated the effect of vanilloids on nuclear factor κ-light-chain-enhancer of activated B cells (NFκB) activation using stably transfected 293/NFκB-Luc human embryonic kidney cells induced by treatment with tumor necrosis factor-α (TNFα) and on aromatase activity. Capsaicin and capsazepine blocked TNFα-induced NFκB activation in a dose-dependent manner with 50% inhibitory concentration (IC50) values of 0.68 and 4.2 μM, respectively. No significant cytotoxicity was observed at the highest concentrations tested (53.1 μM for capsazepine and 65.5 μM for capsaicin). In addition, these vanilloids inhibited aromatase activity with IC50 values of 13.6 and 8.8 μM, respectively. Computer-aided molecular docking studies showed docking scores indicative of good binding affinity of vanilloids with aromatase and NFκB. The highly conserved residues for capsaicin and capsazepine binding with NFκB p50 were Ser299 and Ile278 (H-bond 2.81Å) and with NFκB p100 were Ser6, Arg82, Val86, Arg90 (H-bond 2.89Å), Gly4, and Ser2 (H-bond 2.81Å). The amino acids Trp224, Arg435, and Val373 (H-bond 2.80Å) were found to be important for the binding of capsaicin and capsazepine with aromatase. Based on these findings, aromatase and NFκB are suggested as valid targets for these compounds; additional investigation of chemopreventive or chemotherapeutic potential is required.
Key Words: cancer chemoprevention, capsaicin, molecular docking, nociceptors, nuclear factor κ-light-chain-enhancer of activated B cells, red pepper, tumor necrosis factor-α, vanilloids
Introduction
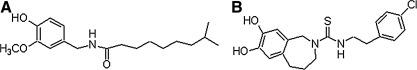
Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is the pungent ingredient in a wide variety of red peppers of the genus Capsicum. Compounds related to capsaicin, including capsazepine (Fig. 1), collectively referred to as vanilloids, bind specifically to a subclass of sensory neurons called nociceptors that carry the sensation of pain.1 Prolonged exposure causes nociceptor terminals to become insensitive to capsaicin, as well as to other noxious stimuli.2 Nociceptor desensitization has led to the use of capsaicin as an analgesic in the treatment of chronic painful disorders.3 In addition to its neuronal effects, several non-neuronal effects of capsaicin have been reported: induction of apoptosis in transformed cells,4 stimulation of prostaglandin formation leading to inhibition of gastric lesions,5 antibacterial activity,6 inhibition of cardiac excitability,7 and platelet aggregation.8 Studies from our laboratory9–13 and other recent reports14,15 have described the variety of pharmacological and physiological properties of capsaicin and its related analogs.
FIG. 1.
Chemical structure of (A) capsaicin and (B) capsazepine.
Nuclear factor κ-light-chain-enhancer of activated B cells (NFκB) consists of homo- and heterodimeric protein complexes that act as transcription factors. NFκB is found in many animal cell types. It is involved in the regulation of immune response and inflammation, cell lineage development, cell apoptosis, cell cycle progression, and oncogenesis in response to stimuli such as stress, cytokines, free radicals, ultraviolet irradiation, oxidized low-density lipoprotein, and microbial antigens and has been shown to regulate the expression of several genes, including bcl-2, bcl-xl, cellular inhibitor of apoptosis protein, survivin, tumor necrosis factor (TNF) signaling pathway-related regulatory factor, cyclooxygenase-2, matrix metalloproteinase peptide-9, and inducible nitric oxide synthase and those for cell cycle–regulatory components whose products are involved in tumorigenesis.16–20 Most carcinogens, pro-inflammatory cytokines such as TNF and interleukin-1, and tumor promoters, including cigarette smoke, phorbol esters, okadaic acid, and H2O2, have been shown to activate NFκB, and resulting tumors have misregulated NFκB activity.21 It is now well established that aberrant regulation of NFκB and the signaling pathways that control its activity are involved in cancer development and progression, as well as in drug resistance, especially during chemotherapy and radiotherapy.22 Blocking NFκB can cause tumor cells to stop proliferating, go to apoptosis, or to become more sensitive to the action of antitumor agents.23,24 Thus, agents that can suppress NFκB activation have the potential to suppress carcinogenesis or tumorigenesis.
Aromatase, a key cytochrome P450 enzyme, catalyzes the rate-limiting aromatization step in the conversion of androgens (testosterone and androstenedione) to estrogens (estradiol and estrone).25–27 Thus, aromatase inhibitors decrease bioavailable estrogen. Aromatase inhibitors have been shown to have considerable antitumor activity in most estrogen receptor–positive breast cancers,28 and they have been recognized as potential chemopreventive agents with proven efficacy in animal models. Non-steroidal aromatase inhibitors are already in clinical use for the treatment of breast cancer.29 Such compounds ultimately reduce estradiol receptor stimulation and reduce formation of genotoxic estrogen metabolites.30 The aromatase complex is associated with the endoplasmic reticulum and consists of a specific cytochrome P450 heme protein (CYP19) and the flavoprotein NADPH cytochrome P450 reductase.31 In our assay, purified CYP19 hydrolyzes dibenzylfluorescein, and aromatase activity was quantified by measuring the fluorescent intensity of the resultant fluorescein.
Because capsaicin and its related analogs have been reported to show diverse biological activity, we have targeted NFκB and aromatase activity to determine the chemopreventive potential of capsaicin and capsazepine in an effort to understand the mechanism of action of vanilloids. Our preliminary observation of the non-neuronal activity of capsaicin led us to investigate other vanilloids for further biological testing. This report describes NFκB and aromatase inhibitory activity by vanilloids. The effect of vanilloids on NFκB and aromatase activity has been compared with that of N-tosyl-L-phenylalanyl-chloromethyl ketone (TPCK) and naringenin, known inhibitors of NFκB and aromatase, respectively. In addition, molecular docking studies of NFκB and aromatase in complex with capsaicin and capsazepine are described. These studies provide insight into the mechanism of NFκB and aromatase inhibition by capsaicin and related compounds.
Materials and Methods
Chemicals
Capsaicin, capsazepine, dimethyl sulfoxide, sulforhodamine B, NADP, glucose 6-phosphate, glucose 6-phosphate dehydrogenase, MgCl2, and dibenzylfluorescein were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). Dulbecco's modified Eagle's medium, antibiotic-antimycotic, hygromycin B, and minimal essential medium sodium pyruvate were purchased from Invitrogen Corp. (Carlsbad, CA, USA). Reporter lysis buffer and the luciferase assay system were purchased from Promega (Madison, WI, USA). TNFα was purchased from Calbiochem (Gibbstown, NJ, USA).
NFκB luciferase assay
The NFκB inhibition assay was conducted using luciferase expression as previously described.32,33 Human embryonic kidney cells (293/NFκB-Luc) were used to monitor alteration of the NFκB pathway. This cell line is stably transfected with a luciferase reporter construct regulated by the NFκB response element. Transcription factor can bind to the response element when stimulated by TNFα, allowing transcription of the luciferase gene. Luciferase reacts with substrate, emitting light that was detected using a luminometer. Cells were seeded (105 cells/mL) on sterile white-walled 96-well plates and grown to approximately 80% confluence by incubating for 48 hours. Capsaicin and capsazepine were tested at a concentration of 20 μg/mL followed by 1:3 serial dilutions for dose-dependent studies. After treatment with test compounds for 10 minutes, cells were incubated for an additional 6 hours with or without TNFα (10 ng/mL). The cells were gently washed twice with phosphate-buffered saline and kept at −80°C with cell lysis buffer (Promega). The luciferase assay was performed using the Luc assay system from Promega following the manufacturer's instructions. Luciferase activity was monitored using a LUMIstar Galaxy luminometer (BMG, Offenburg, Germany), dose–response curves were constructed, and results were expressed as values for the concentration required to inhibit TNFα-activated NFκB activity by 50% (IC50).
Aromatase assay
Aromatase activity was assayed as previously reported29 with the necessary modifications to perform the test in 384-well plates. The test substance (3.5 μL at 20 μg/mL) was preincubated with 30 μL of NADPH regenerating system (2.6 mM NADP+, 7.6 mM glucose 6-phosphate, 0.8 U/mL glucose 6-phosphate dehydrogenase, 13.9 mM MgCl2, and 1 mg/mL albumin in 50 mM potassium phosphate buffer, pH 7.4) for 10 minutes. The enzyme and substrate mixture (33 μL of 1 μM enzyme [165 μU of CYP19, BD Biosciences, San Jose, CA, USA], 0.4 μM dibenzylfluorescein, and 4 mg/mL albumin in 50 mM potassium phosphate, pH 7.4) was added, and the plate was incubated for 30 minutes at 37°C before quenching with 25 μL of 2 N NaOH. After termination of the reaction and shaking for 5 minutes, the plate was further incubated for 2 hours at 37°C. This enhances the ratio of signal to background. Fluorescence was measured using a BioTek (Winooski, VT, USA) Synergy 2 plate reader at 485 nm (excitation) and 530 nm (emission). IC50 values and dose–response curves were based on three independent experiments performed in duplicate using five concentrations of tested substance.
Cytotoxicity assay
LNCaP (androgen-sensitive human prostate carcinoma, ATCC number CRL-1740), MCF7 (human mammary gland adenocarcinoma, ATCC number HTB-22), and Hepa 1c1c7 (murine hepatoma, ATCC number CRL-2026) cell lines were acquired from the American Type Culture Collection (Manassas, VA, USA). LU-1 (lung cancer carcinoma) was provided by the Department of Surgical Oncology, University of Illinois Chicago, Chicago, IL, USA. Evaluation of cytotoxic potential was performed by the method of Skehan et al.34 In brief, using 96-well plates, cells were seeded at a density of 5.0×104 cells/mL and treated with concentrations of each compound ranging from 0 to 20 μg/mL (10 μL of solution in 10% dimethyl sulfoxide). A day 0 control plate was incubated for 30 minutes at 37°C; all other plates were incubated for 72 hours. After incubation, cells were fixed by addition of 50 μL of cold 50% trichloroacetic acid, incubated at 4°C for 30 minutes, washed with tap water, and air-dried. Cells were then stained with 100 μL of 0.4% sulforhodamine B in 1% acetic acid for 30 minutes at 23°C. After washing with 1% acetic acid (four times) to remove excess dye and air-drying, bound dye was solubilized by addition of 200 μL of 10 mM Tris base (pH 10). Finally, the plates were shaken for 5 minutes, and absorption at 515 nm was determined with a BioTek ELx800 plate reader. All determinations were performed in triplicate, and the IC50 values of active samples were assessed by linear regression analyses of dose–response data.
In silico studies
In order to gain a better understanding of the interactions of capsaicin and capsazepine with NFκB and aromatase, docking studies were carried out. The three-dimensional structures of capsaicin (CID_1548943), capsazepine (CID_2733484), naringenin (CID_932), letrozole (CID_3902), and TPCK (CID_9824) were retrieved through the PubChem database at the National Center for Biotechnology Information web server (pubchem.ncbi.nlm.nih.gov). X-ray crystallographic three-dimensional structures of NFκB (PDB ID 1BFS for NFκB p-50; PDB ID 2D96 for NFκB p-100) and aromatase (PDB ID 3EQM) were retrieved from the Brookhaven Protein DataBank (www.pdb.org).
Docking was carried out using GOLD (Genetic Optimization for Ligand Docking) software (version 3.2; Cambridge Crystallographic Data Centre, Cambridge, United Kingdom), which uses the Genetic Algorithm (GA). This method allows for partial flexibility of the protein and full flexibility of the ligand (gold.ccdc.cam.ac.uk). All water molecules and heteroatoms were removed from the targets to evaluate the scoring functions. The default parameters to run the software were as follows: population size, 100; selection-pressure, 1.1; number of operations, 10,000; number of islands, 1; niche size, 2; and operator weights for migrate of 0, mutate of 100, and crossover of 100 were applied. The active site was defined within 15 Å, and the ligand-binding interactions were analyzed using scoring functions (i.e., Goldscore [GS]). For each of the lead–target interactions, independent GA runs were performed on a set of two groups with a population size of 100 individuals. When the top three solutions attained root mean square deviation (RMSD) values within 1.5 Å, GA docking was terminated. The RMSD values for the docking calculations are based on the RMSD matrix of the ranked solutions. We observed that the best-ranked solutions were always among the first 10 GA runs, and the conformation of molecules based on the best fitness score was further analyzed.
GS performs a force field–based scoring function and is made up of four components: (1) protein–ligand hydrogen bond energy (external H-bond); (2) protein–ligand van der Waals energy (external vdw); (3) ligand internal van der Waals energy (internal vdw); and (4) ligand intramolecular hydrogen bond energy (internal H-bond). The external vdw score is multiplied by a factor of 1.375 when the total fitness score is computed. This is an empirical correction to encourage protein–ligand hydrophobic contact. The fitness function has been optimized for the prediction of ligand binding positions: GS=S(hb_ext)+S(vdw_ext)+S(hb_int)+S(vdw_int), where S(hb_ext) is the protein–ligand hydrogen bond score, S(vdw_ext) is the protein–ligand van der Waals score, S(hb_int) is the score from intramolecular hydrogen bonds in the ligand, and S(vdw_int) is the score from intramolecular strain in the ligand.
Both capsaicin and capsazepine, along with the positive controls (TPCK and naringenin), were analyzed using docking studies, and the IC50 values of each are described in detail in Results.
Estimation of binding affinity
Binding affinity (Ki) values were calculated by using Autodock version 4.0 (autodock.scripps.edu). Polar hydrogen atoms were added to the macromolecules, and all rotatable bonds present in the ligand were set as rotatable bond for experiments. A grid box was created for macromolecule (dimensions of the grid box was 40×40×40 with grid spacing of 0.381). A GA was used to run Autodock. Population sizes of 150 and 10 million energy were used for the 100 times search, with 40×40×40 dimensions of grid box size and 0.381 Å grid spacing around the catalytic triad. The best conformation was selected from the lowest docking energy identified among the different pockets of the macromolecule for each ligand, with not more than 1.5 Å RMSD. The formula for calculation is as follows: Ki=exp(ΔG/RT) (http://autodock.scripps.edu/faqs-help/faq/how-autodock-4-converts-binding-energy-kcal-mol-into-ki).
Statistical analysis
All the determinations were performed in duplicate, and data are mean±SD values of three independent analyses. IC50 values were calculated by using Table Curve 2D Windows version 4.07 (SPSS Inc., Chicago, IL, USA). Results were subjected to analysis of variance, and differences between means at the P<.05 level were considered significant compared with the control in Student's t test.
Results
We report here aromatase inhibition and suppression of TNFα-induced NFκB activation determined with stably transfected 293/NFκB-Luc human embryonic kidney cells by vanilloids. Capsaicin and capsazepine both demonstrated significant inhibition of NFκB activation, with IC50 values of 0.68 μM and 4.2 μM, respectively. TPCK was adopted as the positive control for the NFκB assay (IC50 =5 μM) (Table 1). Both compounds were also found to inhibit aromatase: capsazepine with an IC50 of 8.8 μM and capsaicin with an IC50 of 13.6 μM. This inhibition is comparable to that of the positive control, naringenin (IC50 =1 μM). At the highest concentration tested (53.1 μM for capsazepine and 65.5 μM for capsaicin), the vanilloids did not affect cell viability.
Table 1.
Inhibition of Aromatase Activity and Nuclear Factor κB Activation by Capsaicin and Capsazepine
| |
Aromatase |
NFκB |
||
|---|---|---|---|---|
| IC50 (μM) | Docking score (GS) | IC50 (μM) | Docking score (GS) | |
| Capsaicin | 13.6±0.14 | 46.14 | 0.68±0.03 | 37.31 (p50), 38.59 (p100) |
| Capsazepine | 8.8±0.33 | 51.25 | 4.2±0.08 | 31.67 (p50), 36.77 (p100) |
| Naringenin | 1.0±0.18 | 45.64 | ND | ND |
| TPCK | ND | ND | 5.1±2.1 | 35.71 (p50), 34.63 (p100) |
Data are mean±SD 50% inhibitory concentration (IC50) values (in μM). Naringenin and N-tosyl-l-phenylalanyl-chloromethyl ketone (TPCK) were used as positive controls for the aromatase and nuclear factor κ-light-chain-enhancer of activated B cells (NFκB) assays, respectively. Docking scores (Goldscore [GS]) of vanilloids and positive controls with aromatase, NFκB p50, and NFκB p100 receptors were calculated.
ND, not determined.
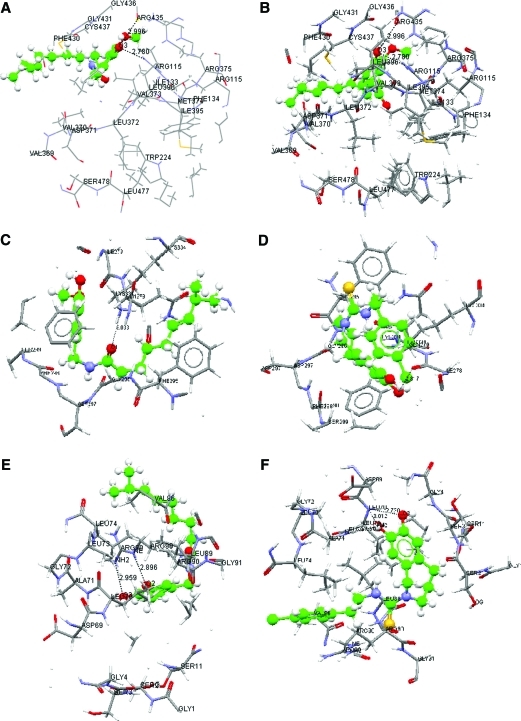
In silico docking results of capsaicin and capsazepine indicated strong binding compared with TPCK and naringenin (Table 1 and Fig. 2) and are in keeping with the in vitro biological activity.
FIG. 2.
Binding domains of aromatase, NFκB p50, and NFκB p100 receptors: (A) capsaicin docked on aromatase, (B) capsazepine on aromatase, (C) capsaicin on NFκB p50, (D) capsazepine on NFκB p50, (E) capsaicin on NFκB p100, and (F) capsazepine on NFκB p100. Color images available online at www.liebertonline.com/jmf
The important amino acids participating in binding pocket formation for NFκB were Phe295, Lys334 (H-bond 3.03Å), Gln279, Phe298 (H-bond 2.84Å), Asp297, Gly296 (NFκB p50), Leu74, Gly72, Ala71, Asp69, Leu70 (H-bond 3.0Å), Gly91, Ser11, Gly1, and Ser3 (NFκB p100). The unique and highly conserved residues in capsaicin and capsazepine for NFκB p50 were Ser299 and Ile278 (H-bond 2.81Å) and for NFκB p100 were Ser6, Arg82, Val86, Arg90 (H-bond 2.89Å), Gly4, and Ser2 (H-bond 2.81Å). Specific residues interacting with TPCK were Ile280, Thr313, Lys315, Pro314, Gly293, and Arg281 for NFκB p50, whereas no unique amino acids were found with NFκB p100. Four of these residues (Thr313, Lys315, Glu293, and Arg281) are hydrophilic in nature, which could reduce binding affinity.
The important amino acids participating in binding pocket formation for aromatase were Phe134, Arg115 (H-bond 2.78Å), Ile133, Trp224, Arg376, Met374, Leu477, Ile395, Val373, Ser479, Leu396, Leu372, Val370, Val369, Pro429 (H-bond 2.1Å), Phe430, Gly431 (H-bond 2.69Å), Gly436, Cys437, and Asp371. Trp224, Arg435, and Val373 (H-bond 2.80Å) were found to be unique for both capsaicin and capsazepine, whereas no unique amino acids were found with naringenin.
For comparison, in addition to naringenin, we performed docking experiments using the well-known pharmacologic inhibitor letrozol. The GS for the interaction was 50.37. Trp224 was found as a unique and common amino acid among the capsazepine, capsaicin, and letrozole binding pockets. The important amino acids participating in binding pocket formation for aromatase have been discussed, including a few uncommon amino acids like Thr310, Gly439, Phe148, Met127, Phe221, Glu302, Ala306, Asp309, Met311, and Ala306. These extra amino acids may be the reason for the high GS for letrozole compared with naringenin, capsazepine, and capsaicin.
For analyzing viability and biological activity of the target molecule, about 11 structural properties were calculated through the project leader module of Fujitsu Computer Systems (Westwood, MA, USA) Scigress Explorer™ version 7.7 (e.g., conformation minimum energy [in kcal/mol], dipole moment [in debye], electron affinity [in eV], dielectric energy [in kcal/mol], steric energy [in kcal/mol], total energy [in Hartree units], heat of formation [in kcal/mol], highest occupied molecular orbital energy [in eV], lowest unoccupied molecular orbital energy [in eV], ionization potential [in eV], polarizability, etc.) (Table 2). Drug-like properties of the compounds were analyzed using Lipinski's Rule of Five: the molecule should have not more than five hydrogen bond donors (OH and NH groups), not more than 10 hydrogen bond acceptors (notably N and O), a molecular weight under 500, and a partition coefficient log P of <5.35
Table 2.
Quantitative Data and Physicochemical Properties of Capsaicin, Capsazepine, Naringenin, and TPCK
| Molecule | Conformation minimum energy (kcal/mol) | Dipole moment (debye) | Electron affinity (eV) | Dielectric energy (kcal/mol) | Steric energy (kcal/mol) | Total energy (Hartree) | Heat of formation (kcal/mol) | HOMO energy (eV) | Ionization potential (eV) | LUMO energy (eV) | Polarizability |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Capsaicin | −22.498 | 2.123 | −0.020 | −0.424 | −23.128 | −165.864 | −124.702 | −9.123 | 9.118 | 0.026 | 35.355 |
| Capsazepine | −17.838 | 4.209 | 0.517 | −0.937 | −17.860 | −184.890 | −27.060 | −8.439 | 8.440 | −0.513 | 40.057 |
| Naringenin | −16.833 | 4.171 | 0.607 | −0.568 | −16.795 | −153.053 | −158.860 | −9.271 | 9.272 | −0.610 | 29.335 |
| TPCK | 2.028 | 4.612 | 0.828 | −0.816 | 2.108 | −174.804 | −67.797 | −9.559 | 9.560 | −0.827 | 35.102 |
HOMO, highest occupied molecular orbital; LUMO, lowest unoccupied molecular orbital.
We observed that Autodock results showing low binding energy have low Ki values as well. To address this, we used Autodock version 4.0 for the estimation of Ki. GOLD is a well-known docking software, but it has limitations in calculating Ki. On the basis of the estimated Ki value, we have categorized our compounds in descending order (i.e., for aromatase, capsazepine>naringenin>capsaicin; for NFκB p100, TPCK>caspazepine>capsaicin; and for NFκB p50, TPCK>capsazepine>capsaicin) (Table 3).
Table 3.
Binding Energy and Constant Inhibition of Tested Compounds
| |
Estimated binding energy for |
|
||
|---|---|---|---|---|
| Receptor/ligand | Aromatase | NFκB (p50) | NFκB (p100) | Estimated Ki(μM) |
| Naringenin | −7.22 | ND | ND | 4.87031×10−6 |
| Capsaicin | −6.42 | ND | ND | 1.88883×10−5 |
| Capsazepine | −7.95 | ND | ND | 1.41392×10−6 |
| Letrozole | −6.41 | ND | ND | 1.92110×10−5 |
| Capsaicin | ND | ND | −5.22 | 1.44260×10−4 |
| Capsazepine | ND | ND | −6.33 | 2.19996×10−5 |
| TPCK | ND | ND | −6.49 | 1.67759×10−5 |
| Capsaicin | ND | −5.13 | ND | 1.68023×10−4 |
| Capsazepine | ND | −6.02 | ND | 3.71973×10−5 |
| TPCK | ND | −6.48 | ND | 1.70626×10−5 |
Ki, inhibition constant; ND, not determined.
Discussion
The study of natural products can reveal interesting biology and generate leads pertaining to specific cellular targets, activities, and therapeutic manipulations. Capsicum is extensively used as a natural food additive for flavoring, seasoning, coloring, and antiseptic properties. Its use is especially popular in hot climates where consumption may be as high as 2.5 g/day.36 The ubiquitous human consumption of foods containing capsaicin and other vanilloids suggests that these agents may also be important as dietary antioxidants and/or anti-inflammatory mediators.37
Recently, disruption of mitochondrial transmembrane potential, bioenergetics, and redox state, apoptosis by activation of peroxisome proliferator-activated receptor-γ, generation of reactive oxygen species, and antioxidant activity of a group of vanilloid compounds were described, as well as structure–activity relationships.38 It is interesting that capsaicin has been reported to inhibit NADH oxidase activity in transformed cells39,40 and in rat plasma membranes.41–44 The inhibitory effect of capsaicin causes reactive oxygen species generation and oxidative stress in transformed cells. However, in erythrocytes, capsaicin acts as an antioxidant. Several studies show that capsaicin protects against lipid peroxidation, plasma membrane redox system function, -SH group oxidation, and protein carbonyl formations in erythrocytes.10,12,13,38
Among inhibitors of NFκB, salicylates, aspirin,45 apigenin, and curcumin46,47 were reported to suppress activation by preventing the degradation of the NFκB inhibitor (IκB), and therefore NFκB was retained in the cytosol. The serine protease inhibitor TPCK can block NFκB activation completely. TPCK inhibits the processing of NFκB subunits as well as IκB degradation.48 It has also been demonstrated that TPCK inhibits DNA replication by G0–G1 growth arrest and thus promotes apoptosis by other cytotoxic stimuli.49 The present study provides evidence that vanilloids can act as inhibitors of NFκB and aromatase. The inhibitory actions of vanilloids do not appear to result from nonspecific cellular toxicity but rather are specific for NFκB-dependent gene transcription. In other studies, it has been demonstrated that capsaicin inhibits phorbol ester–induced activation of NFκB and activator protein-1 by blocking the signal transducer and activator of transcription 3 signaling pathway.50–52 In addition, we have found that capsazepine, a vanilloid receptor antagonist, also inhibits TNFα-induced NFκB activation and aromatase activity. Our findings on capsazepine are in agreement with a previous report on inhibition of nitric oxide and inducible nitric oxide synthase protein synthesis induced by lipopolysaccharide in RAW 264.7 macrophages; this inhibition occurred via suppression of mRNA expression through inactivation of NFκB53 and inhibition of IκB-α degradation in lipopolysaccharide-stimulated peritoneal macrophages54 by capsaicin. However, to our knowledge, this is the first report to demonstrate inhibition of aromatase activity by vanilloids.
To substantiate these responses, we performed docking experiments using capsaicin and capsazepine (ligands) with NFκB and aromatase (receptors). All the targets showed an acceptable minimum docking score. Docking with NFκB showed higher binding affinities for capsaicin and capsazepine compared with TPCK, possibly because of interaction with the hydrophobic residues Ile278, Leu73, and Val86. Interaction with Trp224 and Val373 may be responsible for the higher binding affinity of capsaicin and capsazepine with aromatase.
Results were analyzed to find common and unique amino acids. Amino acids common in binding pocket formation for test compounds as well as control compounds as in the case of NFκB were Phe295, Lys334 (H-bond 3.03Å), Gln279, Phe298 (H-bond 2.84Å), Asp297, and Gly296 for NFκB p50 and Leu74, Gly72, Ala71, Asp69, Leu70 (H-bond 3.0Å), Gly91, Ser11, Gly1, and Ser3 for NFκB p100. Other than these common amino acids, there were a few unique amino acids that were present in binding pockets of capsaicin and capsazepine for NFκB p50 (Ser299 and Ile278 [H-bond 2.81Å]) and NFκB p100 (Ser6, Arg82, Val86, Arg90 [H-bond 2.89Å], Gly4, and Ser2 [H-bond 2.81Å]). The important amino acids participating in binding pocket formation for aromatase were Phe134, Arg115 (H-bond 2.78Å), Ile133, Trp224, Arg376, Met374, Leu477, Ile395, Val373, Ser479, Leu396, Leu372, Val370, Val369, Pro429 (H-bond 2.1Å), Phe430, Gly431 (H-bond 2.69Å), Gly436, Cys437, and Asp371. Trp224, Arg435, and Val373 (H-bond 2.80Å) were found to be unique for both capsaicin and capsazepine, whereas no unique amino acids were found with naringenin. In the case of aromatase, the IC50 of naringenin is low compared with those of capsaicin and capsazepine. This may be due to differences in chemical configurations or bioavailability. Naringenin is a flavonoid, whereas capsaicin/capsazepine are vanilloids.
Lipinski's “Rule of 5” test for drug-likeness indicates that capsaicin is a viable drug candidate. For capsazepine, the log P (octanol/water partition coefficient) value was greater than 5, which could indicate low intestinal absorption (Table 4). The analysis of NFκB and aromatase using the X-ray structure as an alignment template led to the identification of important amino acids involved in the ligand-binding interactions. Hence, vanilloids, including capsaicin, could be used as a pharmacophore model for designing new cancer chemopreventive agents.
Table 4.
Evaluation of Capsaicin, Capsazepine, Naringenin, and TPCK for Lipinski's Rule
| |
|
|
Group count for H-bond donors (<5) |
Atom count for H-bond acceptors (<10) |
|
|||
|---|---|---|---|---|---|---|---|---|
| Molecule | Molecular weight<500 | Log P<5 | Amine | Secondary amine | Hydroxyl | Nitrogen | Oxygen | Lipinski's Rule of 5 violations |
| Capsaicin | 305.416 | 3.530 | 0 | 1 | 1 | 1 | 3 | 0.000 |
| Capsazepine | 376.900 | 5.699* | 0 | 1 | 2 | 2 | 2 | 1.000 |
| Naringenin | 272.257 | 1.987 | 0 | 0 | 3 | 0 | 5 | 0.000 |
| TPCK | 351.847 | 4.183 | 0 | 1 | 0 | 1 | 3 | 0.000 |
Violates Lipinski's Rule by 1 (Log P<5).
Based on these encouraging responses and molecular interaction studies, we suggest that the suppression of NFκB activation and aromatase activity by vanilloids could play a chemopreventive role in carcinogenesis. Further investigations into the actions of vanilloids, especially those related to the NFκB signaling pathway, aromatase inhibition, and in vivo models, could provide new insights into the medicinal value of these molecules.
Acknowledgments
This work was supported by program project P01 CA48112 awarded by the National Cancer Institute. S.L. acknowledges the Indo-US Science and Technology Forum, New Delhi, for a Research Fellowship.
Author Disclosure Statement
No competing financial interests exist. All the authors have substantially participated in the investigation, data analysis, and the preparation of the manuscript and accept full responsibility for its content.
References
- 1.Jansco G. Kiraly E. Jansco-Gabor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurons. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- 2.Szallasi PM. Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- 3.Szallasi PM. Blumberg PM. Vanilloid receptor: new insights enhance potential as a therapeutic target. Pain. 1996;68:195–208. doi: 10.1016/s0304-3959(96)03202-2. [DOI] [PubMed] [Google Scholar]
- 4.Macho A. Calzado MA. Muñoz-Blanco J, et al. Selective induction of apoptosis by capsaicin in transformed cells: the role of reactive oxygen species and calcium. Cell Death Differ. 1999;6:155–165. doi: 10.1038/sj.cdd.4400465. [DOI] [PubMed] [Google Scholar]
- 5.Uchida M. Yano S. Watanabe K. The role of capsaicin sensitive afferent nerves in protective effect of capsaicin against absolute ethanol-induced gastric lesions in rats. Jpn J Pharmacol. 1991;55:279–282. doi: 10.1254/jjp.55.279. [DOI] [PubMed] [Google Scholar]
- 6.Cichewicz RH. Thorpe PA. The antimicrobial properties of chile peppers (Capsicum species) and their uses in Mayan medicine. J Ethnopharmacol. 1996;52:61–70. doi: 10.1016/0378-8741(96)01384-0. [DOI] [PubMed] [Google Scholar]
- 7.Franco-Cereceda A. Lundberg JM. Actions of calcitonin gene-related peptide and tachykinins in relation to the contractile effects of capsaicin in the guinea-pig and rat heart in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1988;337:649–655. doi: 10.1007/BF00175791. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan MR. Satyanarayana MN. Effect of capsaicin on lipoprotein lipase in rats fed high fat diet. Indian J Exp Biol. 1989;27:910–912. [PubMed] [Google Scholar]
- 9.Rizvi SI. Luqman S. Capsaicin-induced activation of erythrocyte membrane acetyl cholinesterase activity. Med Chem Res. 2001;10:502–506. [Google Scholar]
- 10.Rizvi SI. Luqman S. Anti-oxidative property of capsaicin. Med Chem Res. 2002;11:301–307. [Google Scholar]
- 11.Rizvi SI. Luqman S. Capsaicin-induced changes in erythrocyte membrane adenosine triphosphatases activity. Cell Mol Biol Lett. 2003;8:919–926. [PubMed] [Google Scholar]
- 12.Luqman S. Rizvi SI. Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phytother Res. 2006;20:303–306. doi: 10.1002/ptr.1861. [DOI] [PubMed] [Google Scholar]
- 13.Rizvi SI. Luqman S. Capsicum: a medically important spice. In: De AK, editor. Spices: The Elixir of Life. Originals; New Delhi: 2011. pp. 77–86. [Google Scholar]
- 14.Materska M. Perucka I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L) J Agric Food Chem. 2005;53:1750–1756. doi: 10.1021/jf035331k. [DOI] [PubMed] [Google Scholar]
- 15.Kempalah RK. Srinivasan K. Influence of dietary curcumin, capsaicin and garlic on the antioxidant status of red blood cells and the liver in high-fat-fed rats. Ann Nutr Metab. 2004;48:314–320. doi: 10.1159/000081198. [DOI] [PubMed] [Google Scholar]
- 16.Melisi D. Chiao PJ. NFκB as a target for cancer therapy. Expert Opin Ther Targets. 2007;11:133–144. doi: 10.1517/14728222.11.2.133. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal BB. Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Chen F. Castranova V. Shi X. New insights into the role of nuclear factor κB in cell growth regulation. Am J Pathol. 2001;159:387–397. doi: 10.1016/s0002-9440(10)61708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thanos D. Maniatis T. NFκB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 20.Luqman S. Pezzuto JM. NFκB: a promising target for natural products in cancer chemoprevention. Phytother Res. 2010;24:949–963. doi: 10.1002/ptr.3171. [DOI] [PubMed] [Google Scholar]
- 21.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NFκB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue J. Gohda J. Akiyama T. Semba K. NFκB activation in development and progression of cancer. Cancer Sci. 2007;98:268–274. doi: 10.1111/j.1349-7006.2007.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frydrych I. Mlejnek P. Serine protease inhibitors N-tosyl-l-lysinyl-chloromethylketone (TLCK) and N-tosyl-l-phenylalaninyl-chloromethylketone (TPCK) are potent inhibitors of activated caspase proteases. J Cell Biochem. 2008;103:1646–1656. doi: 10.1002/jcb.21550. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto Y. Gaynor RB. Therapeutic potential of inhibition of the NFκB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mamett LJ. Aspirin and the potential role of prostaglandins in colon cancer. Cancer Res. 1992;52:5575–5589. [PubMed] [Google Scholar]
- 26.Czajka-Oraniec I. Simpson ER. Aromatase research and its clinical significance. Endokrynol Pol. 2010;61:126–134. [PubMed] [Google Scholar]
- 27.Balunas MJ. Kinghorn AD. Natural compounds with aromatase inhibitory activity: an update. Planta Med. 2010;76:1087–1093. doi: 10.1055/s-0030-1250169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabian CJ. Kimler BF. Mayo MS. Grizzle WE. Masood S. Ursin G. Clinical approaches to discovering and testing new breast cancer prevention drugs. In: Kelloff GJ, editor; Hawk ET, editor; Sigman CC, editor. Cancer Chemoprevention. Vol. 2. Humana Press; Totowa, NJ: 2005. pp. 213–237. [Google Scholar]
- 29.Maiti A. Cuendet M. Croy VL. Endringer DC. Pezzuto JM. Cushman M. Synthesis and biological evaluation of (±)-abyssinone II and its analogues as aromatase inhibitors for chemoprevention of breast cancer. J Med Chem. 2007;50:2799–2806. doi: 10.1021/jm070109i. [DOI] [PubMed] [Google Scholar]
- 30.Strasser-Weippl K. Goss PE. Counteracting estrogen as breast cancer prevention. In: Kelloff GJ, editor; Hawk ET, editor; Sigman CC, editor. Cancer Chemoprevention. Vol. 2. Humana Press; Totowa, NJ: 2005. pp. 249–264. [Google Scholar]
- 31.Recanatini M. Cavalli A. Valenti P. Nonsteroidal aromatase inhibitors: recent advances. Med Res Rev. 2002;22:282–304. doi: 10.1002/med.10010. [DOI] [PubMed] [Google Scholar]
- 32.Morais MCC. Luqman S. Kondratyuk TP, et al. Suppression of TNF-α induced NFκB activity by gallic acid and its semi-synthetic alkyl-gallates: possible role in cancer chemoprevention. Nat Prod Res. 2010;20:1–8. doi: 10.1080/14786410903335232. [DOI] [PubMed] [Google Scholar]
- 33.Homhual S. Zhang HJ. Bunyapraphatsara N, et al. Bruguiesulfurol, a new sulfur compound from Bruguiera gymnorrhiza. Planta Med. 2006;72:255–260. doi: 10.1055/s-2005-873171. [DOI] [PubMed] [Google Scholar]
- 34.Skehan P. Storeng R. Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 35.Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44:235–249. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 36.Rumsfield JA. West DP. Topical capsaicin in dermatologic and peripheral pain disorder. DICP. 1991;25:381–387. doi: 10.1177/106002809102500409. [DOI] [PubMed] [Google Scholar]
- 37.Surh YJ. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic compounds. Mutat Res. 1999;428:305–327. doi: 10.1016/s1383-5742(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 38.Luqman S. Rizvi SI. Activation of erythrocyte plasma membrane redox system by capsaicin: a possible mechanism to explain its antioxidative effect. Oxid Commun. 2010;33:430–435. [Google Scholar]
- 39.Morre DJ. Chueh PJ. Morre DM. Capsaicin inhibits preferentially the NADH oxidase and growth of transformed cells in cultures. Proc Natl Acad Sci U S A. 1995;92:1831–1835. doi: 10.1073/pnas.92.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolvetang EM. Larm JA. Moutsoulas P. Lawen A. Apoptosis induced by inhibitors of the plasma membrane NADH oxidase involves Bcl-2 and calceneurin. Cell Growth Differ. 1996;7:1315–1325. [PubMed] [Google Scholar]
- 41.Buck SH. Burks SH. The neuropharmacology of capsaicin—review of some recent observations. Pharmacol Rev. 1986;38:179–226. [PubMed] [Google Scholar]
- 42.Meddings JB. Hogaboam CM. Tran K. Reynolds JD. Wallace JL. Capsaicin effects on non-neuronal plasma membranes. Biochim Biophys Acta. 1991;1070:43–50. doi: 10.1016/0005-2736(91)90144-w. [DOI] [PubMed] [Google Scholar]
- 43.Aranda FJ. Villalain J. Fernandez-Gomez JC. Capsaicin affects the structure and phase organization of phospholipid membranes. Biochim Biophys Acta. 1995;1234:225–234. doi: 10.1016/0005-2736(94)00293-x. [DOI] [PubMed] [Google Scholar]
- 44.Tsuchiya H. Biphasic membrane effects of capsaicin, an active component in Capsicum sp. J Ethnopharmacol. 2001;75:295–299. doi: 10.1016/s0378-8741(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 45.Kopp E. Ghosh S. Inhibition of NFκB by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 46.Singh S. Aggarwal BB. Activation of transcription factor NFκB is suppressed by curcumin (diferulolylmethane) J Biol Chem. 1996;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 47.Gerritsen ME. Carley WW. Ranges GE, et al. Flavonoids inhibit cytokine-induced endothelial cell adhesion protein gene expression. Am J Pathol. 1995;147:278–292. [PMC free article] [PubMed] [Google Scholar]
- 48.Mellitis KH. Hay RT. Goodbourn S. Proteolytic degradation of MAD3 (IκBα) and enhanced processing of the NFκB precursor p105 are obligatory steps in the activation of NFκB. Nucleic Acids Res. 1993;21:5059–5066. doi: 10.1093/nar/21.22.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirillova I. Chaisson M. Fausto N. Tumor necrosis factor induces DNA replication in hepatic cells through nuclear factor κB activation. Cell Growth Differ. 1999;10:819–828. [PubMed] [Google Scholar]
- 50.Oyagbemi AA. Saba AB. Azeez OI. Capsaicin: a novel chemopreventive molecule and its underlying molecular mechanisms of action. Indian J Cancer. 2010;47:53–58. doi: 10.4103/0019-509X.58860. [DOI] [PubMed] [Google Scholar]
- 51.Surh YJ. Han SS. Keum YS. Seo HJ. Lee SS. Inhibitory effects of curcumin and capsaicin on phorbol ester-induced activation of eukaryotic transcription factors, NF-kappaB and AP-1. Biofactors. 2000;12:107–112. doi: 10.1002/biof.5520120117. [DOI] [PubMed] [Google Scholar]
- 52.Han SS. Keum YS. Seo HJ. Surh YJ. Curcumin suppresses activation of NF-kappaB and AP-1 induced by phorbol ester in cultured human promyelocytic leukemia cells. J Biochem Mol Biol. 2002;35:337–342. doi: 10.5483/bmbrep.2002.35.3.337. [DOI] [PubMed] [Google Scholar]
- 53.Oh GS. Pae HO. Seo WG, et al. Capsazepine, a vanilloid receptor antagonist, inhibits the expression of inducible nitric oxide synthase gene in lipopolysaccharide-stimulated RAW264.7 macrophages through the inactivation of nuclear transcription factor-kappa B. Int Immunopharmacol. 2001;1:777–784. doi: 10.1016/s1567-5769(01)00012-1. [DOI] [PubMed] [Google Scholar]
- 54.Kim CS. Kawada T. Kim BS, et al. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-α degradation in LPS-stimulated peritoneal macrophages. Cell Signal. 2003;15:299–306. doi: 10.1016/s0898-6568(02)00086-4. [DOI] [PubMed] [Google Scholar]