Abstract
Carotid atherosclerotic plaques represent both stable and unstable atheromatous lesions. Atherosclerotic plaques that are prone to rupture owing to their intrinsic composition such as a large lipid core, thin fibrous cap and intraplaque hemorrhage are associated with subsequent thromboembolic ischemic events. At least 15–20% of all ischemic strokes are attributable to carotid artery atherosclerosis. Characterization of plaques may enhance the understanding of natural history and ultimately the treatment of atherosclerotic disease. MRI of carotid plaque and embolic signals during transcranial Doppler have identified features beyond luminal stenosis that are predictive of future transient ischemic attacks and stroke. The value of specific therapies to prevent stroke in symptomatic and asymptomatic patients with severe carotid artery stenosis are the subject of current research and analysis of recently published clinical trials that are discussed in this article.
Keywords: carotid atherosclerosis, carotid endarterectomy, diagnostic studies, medical therapy, stenting, stroke
Stroke is the third leading cause of mortality in the USA and accounts for over 143,579 deaths every year. Approximately 795,000 people experience a new or recurrent stroke every year [1]. Over 15 million people suffer strokes each year worldwide, resulting in 5 million deaths and an additional 5 million who are permanently disabled [2]. Men tend to have more frequent strokes at a younger age (<75 years) but overall, women have more strokes than men. At least 15–20% of all ischemic strokes are attributed to carotid artery atherosclerosis [3]. Furthermore, it is estimated that 5–10% of individuals over 65 years of age have asymptomatic carotid artery atherosclerosis with at least 50% artery stenosis [4,5]. Therefore, differentiating symptomatic from asymptomatic atherosclerotic stenosis is important in determining choice of treatment. In this article we focus on the pathophysiology, clinical imaging, and management of symptomatic and asymptomatic carotid artery stenosis.
Atherosclerotic carotid artery plaque
Carotid atherosclerotic plaque is similar to that found at other arterial sites. The carotid bulb is the major site of involvement in atherosclerotic stenosis. In addition to several known risk factors (i.e., smoking, high blood pressure, high levels of LDLs and genetic predisposition), focal distribution of atherosclerotic lesions has been considered to be dependent, at least in part, on hydrodynamic factors. Vessel segments with low wall shear stress and resultant flow stagnation appear to be at greater risk. However, the combination of low wall shear stress, hypercholesterolemia and turbulent blood flow all contribute to the development of plaque. The so-called ‘vulnerable plaque’ responsible for rupture is characterized by the presence of a lipid-rich necrotic core (LRNC) that is covered by a thin fibrous cap and depleted of smooth muscle cells [6,7]. The plaque also contains inflammatory cells, mainly activated macrophages, mast cells and T lymphocytes.
Atherosclerosis is believed to be initiated by the entry of LDL particles into the subintimal space and becoming trapped by a high affinity to the glycoprotein molecules at that site [8,9]. Under certain metabolic conditions, LDL particles can become oxidized. This leads to signaling and entry of monocytes by activated endothelial surface adhesion molecules into the site to clear up these particles but also initiates a local inflammation. Smooth muscles are also attracted into the area. The monocytes are then converted to macrophages that engorge lipid and transform into foam cells [10]. When these cells die their lipid load with a high content of free cholesterol is released into the extracellular space. This serves to attract additional macrophages into the area, leading to a vicious cycle that leads to the formation of a necrotic core confined between the external and internal elastic membrane of the artery. The core is rich in cellular debris and free cholesterol, which by physical and/or humoral mechanisms can then form cholesterol crystals. Crystals have sharp-tipped edges that can cut through fibrous tissues [11,12]. Once a saturated solution of cholesterol develops in the core, crystallization is initiated and occurs suddenly and irreversibly [13]. When cholesterol is converted from a liquid to a solid crystalline state it expands in volume. Within the confines of the plaque, this can stretch and thin the fibrous cap, causing rupture by perorating crystals (Figure 1). Compromise of the fibrous cap that separates the core form the arterial lumen leads to thrombosis with potential arterial lumen obstruction or distal embolization, causing ischemia and/or infarction. Furthermore, crystals showering into the distal artery can injure the intimal surface causing arterial spasm that further aggravates ischemia [14].
Figure 1. Demonstration of volume expansion of cholesterol during crystallization.
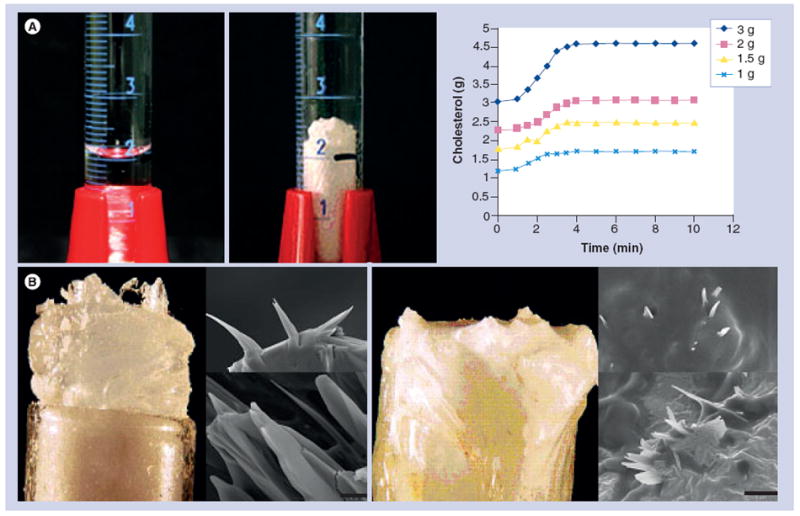
(A) Dissolved cholesterol in graduated cylinder expands in volume above the meniscus line. Graph of volume expansion is greater with increasing amounts of cholesterol (1–3 g). (B) Cholesterol crystals are seen growing above the edge of the test tube after crystallization. Scanning electron micrographs demonstrate sharp-tipped crystal geometries. When a fibrous membrane is placed over the mouth of the test tube, crystals perforate the membrane as noted by scanning electron microscopy (bar = 5 μm).
Reproduced with permission from [18].
The fibrous cap
The fibrous cap represents the portion of the plaque that faces the vascular lumen and maintains the integrity of the plaque. It is composed of vascular smooth muscle cells embedded in a matrix that contains type 1 and 3 collagen fibers. In the American Heart Association classification of atherosclerotic lesions, the fibrous cap appears in type 4 atheromatous lesions [15]. Type 5 lesion represents more extracellular lipids, hematoma or thrombotic deposits, while type 6 lesion represents surface defect, hematoma and thrombosis [16].
Symptomatic carotid stenosis is often associated with type 5 or 6 plaques. Thinning of the fibrous cap is considered to be an independent risk factor for ischemic neurological symptoms. In one study, fibrous cap thinning was found in 95% of symptomatic plaques and 48% of asymptomatic plaques. Plaque rupture was associated with 74% of symptomatic plaques and 32% of asymptomatic plaque [17]. Plaques with thin fibrous caps have been associated with plaque rupture. It has been suggested that the fibrous cap is thinned by autolysis via the action of metalloproteinases released by macrophages [8,10]. However, a direct relationship between metalloproteinases and rupture has not been established. Moreover, the fibrous cap can be thinned by stretching when the LRNC undergoes volume expansion during crystallization [18].
Lipid-rich necrotic core
A large lipid core is the hallmark of a vulnerable plaque and represents the amount of accumulated cholesterol deposited within the arterial wall [19,20]. Cholesterol saturation has been demonstrated to be a potential major trigger for cholesterol crystallization that can lead to volume expansion and plaque rupture [11-13]. Moreover, patients with more frequent neurological symptoms have more dense cholesterol crystal formations within the necrotic core (Figure 2) [12]. Thus, noninvasive imaging, especially with MRI that can detect large lipid cores, may be useful clinically in detecting vulnerable plaques [21]. Furthermore, novel ultrasound parameters such as area reduction and flow patterns may be useful as well. These could help identify high-risk patients for stroke as well as evaluate the effectiveness of medical therapy in plaque stabilization. We have recently demonstrated that statins dissolve cholesterol crystals, thus potentially stabilizing plaques (Figure 3) [22].
Figure 2. Neurological symptoms increase with cholesterol crystal content in plaque.
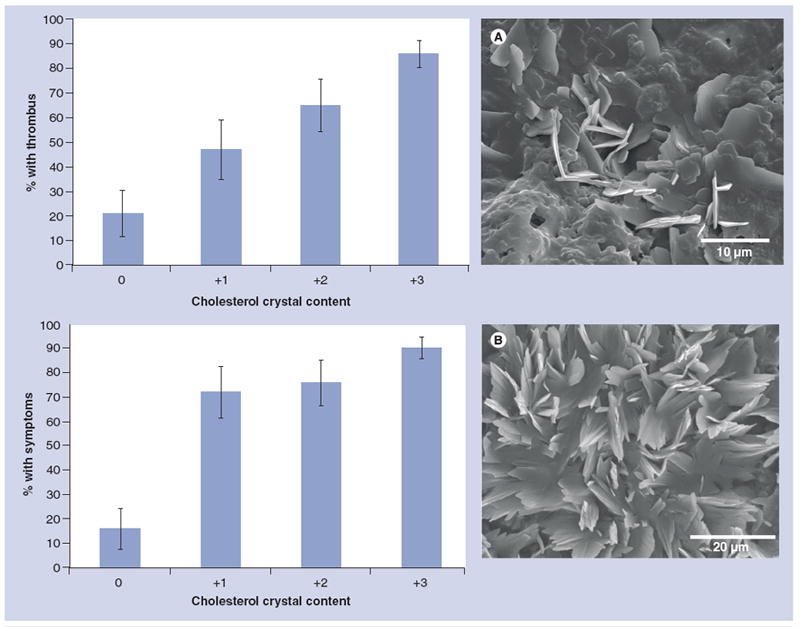
(A) Both neurological symptoms and thrombus are increased with increasing cholesterol crystal content in plaques. (B) Examples of carotid plaques from patients with transient ischemic attack.
Reproduced with permission from [12].
Figure 3. Dissolving cholesterol crystals in patients on oral statins.
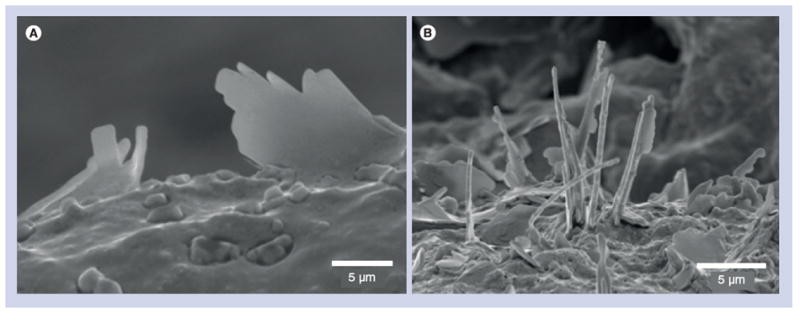
(A) Scanning electron micrograph of carotid plaque from carotid endarterectomy of a patient with transient ischemic attack not on statins demonstrating intact cholesterol crystals. (B) Scanning electron micrograph of carotid plaque from a patient with transient ischemic attack on statins demonstrating dissolving crystals.
Reproduced with permission from [22].
The LRNC constitutes the main portion of the plaque. A larger LRNC has been associated with thin or ruptured fibrous cap in both symptomatic and asymptomatic carotid atherosclerotic disease [23]. The volume of LRNC by MRI has also been found to be a strong predictor of future surface disruption in asymptomatic individuals with 50–79% stenosis [24]. Furthermore, a larger LRNC provides an environment that is conducive to macrophage activation, which releases proteolytic enzymes that degrade collagen. Furthermore, several tissue factors are found in the necrotic core that when exposed to blood can lead to acute thrombosis, embolization and stroke [25]. A large LRNC has a higher content of free cholesterol and thus a greater potential for volume expansion with crystallization [18]. Moreover, tearing of the plaque cap has been associated with cholesterol crystals aligned in parallel formations that reflect high pressure build-up within the plaque and may serve as a marker for plaque rupture [26].
Inflammatory cell infiltrate
Another feature of vulnerable plaques is the presence of inflammatory cell infiltrates often noted in high-grade carotid stenosis [27]. Symptomatic patients have a larger percentage of macrophages and T lymphocytes compared with asymptomatic patients [28]. Macrophages produce proteolytic enzymes, matrix metalloproteinases that are thought to be responsible for collagen breakdown and lesion instability [29]. T lymphocytes also contribute to plaque instability by inducing macrophages to secrete matrix metalloproteases (MMPs) via CD40 [30]. In addition, T lymphocytes secrete IFN-γ, which results in impaired collagen synthesis by smooth muscle cells in both resting and stimulated states [31]. These inflammatory processes as well as expansion of cholesterol with crystallization in the LRNC will result in perforation of the fibrous cap leading to rupture. Moreover, cholesterol crystals have been found to develop very early in atherogenesis, leading to activation of the NLRP3 inflammasome and IL-1β that activates local and systemic inflammation with production of C-reactive protein (CRP) [32].
Recent studies have shown a strong association between the neutrophil numbers and rupture-prone plaques [33]. Furthermore, higher neutrophil count in the plaque is associated with large lipid necrotic core.
Intraplaque hemorrhage
Barger et al. [34] and recently others [35,36] have demonstrated that most mature plaques have a rich network of small vessels, known as ‘vasa vasorum’, that interweave into the plaque matrix. Moreover, intraplaque hemorrhage (IPH) has been shown to predict the risk of future neurological events [37]. Carr et al. showed that IPH had a higher incidence in symptomatic patients compared with asymptomatic patients (84 vs 56% asymptomatic patients) but the difference was only of marginal significance (p = 0.06) [17]. Furthermore, there was a strong correlation between IPH and plaque rupture in the same study. Symptomatic patients with moderate stenosis also have a higher prevalence of IPH than patients with mild stenosis. In coronary atherosclerotic plaques, IPH has been considered to trigger a vulnerable plaque to rupture. In addition, repeated IPH leads to increased free cholesterol content in the LRNC from red blood cell membranes [38]. Furthermore, it is conceivable that the formation and expansion of sharp-tipped cholesterol crystals within the necrotic core could readily cut through the vasa vasorum network causing IPH (Figure 4). Therefore, in addition to macrophage cell death, IPH plays an important role in necrotic core development.
Figure 4. Mechanism of plaque hemorrhage, rupture and/or erosion induced by cholesterol crystallization with volume expansion of the necrotic core.
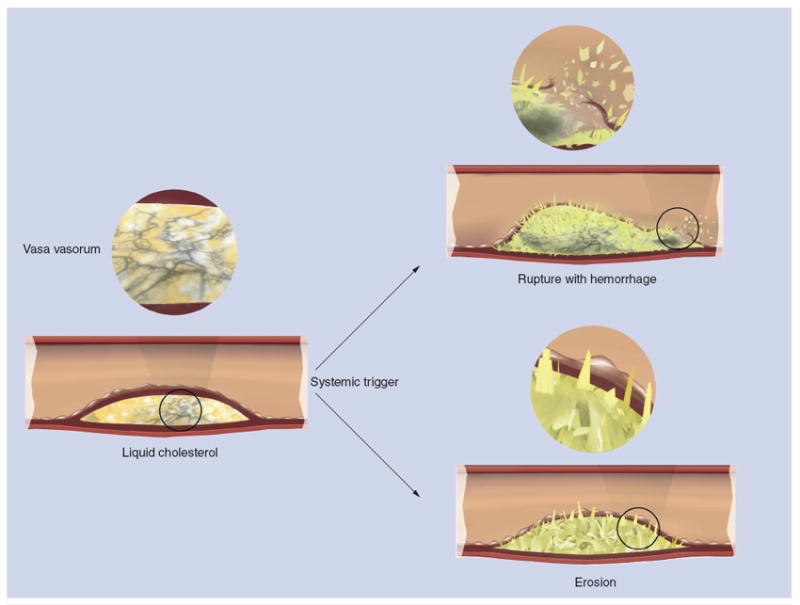
In the case of a large necrotic core, the plaque cap is torn leading to rupture, whereas in the case of a small necrotic core, it leads to erosion. Furthermore, trauma to the vasa vasorum by expanding cholesterol crystals within the plaque causes intraplaque hemorrhage.
Modified with permission from [18].
Factors that influence plaque morphology
Age
Plaque instability seems unrelated to age. The Oxford plaque study compared the characteristics of 526 carotid plaques obtained from carotid endarterectomy (CEA) and found that younger patients had a greater proportion of inflammatory cell infiltration, while older patients had a large lipid core and plaque calcification, but there was no age-related trend towards plaque instability [39]. This suggests that elevated stroke risk in elderly patients with symptomatic carotid stenosis may be due to other factors.
Gender
Plaque phenotype is influenced by gender differences. In asymptomatic patients with greater than 50% carotid stenosis, high-risk plaque features are found more commonly in men than in women [40]. Furthermore, by MRI men were found to have thinner fibrous caps and larger LRNC. In fact, for the same degree of carotid stenosis men have a larger lipid core than women. These features may help explain gender differences in stroke incidence and prevention. Larger LRNC are more likely to cause plaque rupture while smaller LRNC are more likely to erode (Figure 4) [18].
Smoking
Smoking is associated with younger age at CEA, suggesting that it may play a role in accelerating the development and/or progression of atherogenesis. However, plaque morphology appears to be similar in ex-/never smokers, which suggests that the mechanism of plaque instability seems to be unrelated to smoking [39]. However, this does not rule out the possibility of altered plaque physiology as a cause.
Timing of ischemic symptoms
Plaque instability is related to the nature and timing of ischemic symptoms. The Oxford plaque study concluded that plaque instability tends to persist after a transient ischemic attack (TIA) and then decreases with time after a stroke. Plaques removed 180 days after an event had greater inflammation and hence instability after a TIA than after a stroke [39]. Symptomatic carotid lesions tend to remodel with time into more stable plaques after a stroke. This has been associated with a decrease in the number of macrophages as well as a decrease in the expression of IL-6, IL-8 and caspase activity [41].
Imaging of atherosclerotic carotid artery stenosis
Imaging of plaques may enhance the understanding of the natural history of atherosclerotic disease. Currently, a number of noninvasive and invasive imaging modalities are used to study atherosclerotic carotid artery stenosis. These include Doppler-ultrasound; MRI; computer tomographic angiography (CTA); transcranial Doppler (TCD) and positron emission tomography (PET).
Surface ultrasound
Ultrasound is the initial imaging modality of choice in evaluating carotid artery stenosis [42]. High-resolution (<0.4 mm axial), gray-scale, real-time B-mode ultrasound with Doppler flow imaging is frequently utilized to image carotid arteries (Figure 5). Data supporting the routine use of Doppler ultrasound to screen for carotid stenosis in an asymptomatic population is rather weak, as there is low overall prevalence of treatable disease in the general asymptomatic population [43]. However, Doppler ultrasound should be used in symptomatic patients presenting with signs and symptoms of retinal and/or cerebral ischemia, as well as in patients found to have a carotid bruit on physical examination [44]. In addition to evaluation for severity of stenosis, Doppler ultrasound can help characterize the plaque and hence can identify plaques at high risk of embolization [45]. However, a major limitation is that it is user dependent.
Figure 5. Ultrasound and Doppler of left carotid artery in a patient with an asymptomatic stenosis demonstrating a complex intramural lesion suggestive of plaque hemorrhage (arrow).
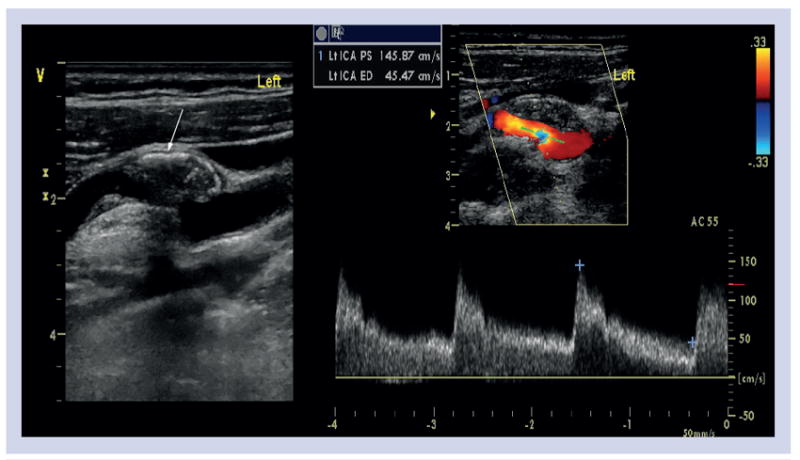
The measurement of intima media thickness (IMT) on carotid ultrasound has been widely used as a marker of systemic atherosclerosis and a surrogate marker for coronary artery disease [46]. However, the Framingham Heart Study demonstrated low correlation (<0.3) between the carotid IMT and coronary calcification [47] while the Atherosclerosis Risk In Communities (ARIC) study suggested that the carotid IMT data may enhance cardiovascular risk assessment, particularly among individuals classified as intermediate risk by use of conventional risk factors [48]. At present, the use of carotid IMT measurement to guide treatment for patients is not recommended.
Magnetic resonance imaging
High-resolution MRI has been widely used to evaluate characteristics of atherosclerosis. The in vivo MRI depiction of carotid plaque hemorrhage, LRNC and thinning of the overlying fibrous cap have been validated by histological correlation [49-51]. These features make the plaque prone to rupture with subsequent thromboembolic ischemic events [52]. The clinical significance of these MR-depicted features have been confirmed in multiple prospective studies of asymptomatic patients with moderate carotid stenosis that lead to subsequent strokes or TIA (Figure 6) [53,54]. In addition, men were found to have these higher risk carotid plaque features compared with women after controlling for potential confounders. These MR-depicted carotid plaque differences may help explain sex differences in stroke incidence and prevention [40].
Figure 6. Same case as in Figure 5 (with 500-μm resolution contrast-enhanced MR angiogram) of left carotid artery confirming intraplaque hemorrhage by black-blood T1-weighted cross-sectional images using 3D magnetization-prepared rapid acquisition gradient echo sequence, where the intraplaque hemorrhage is bright.
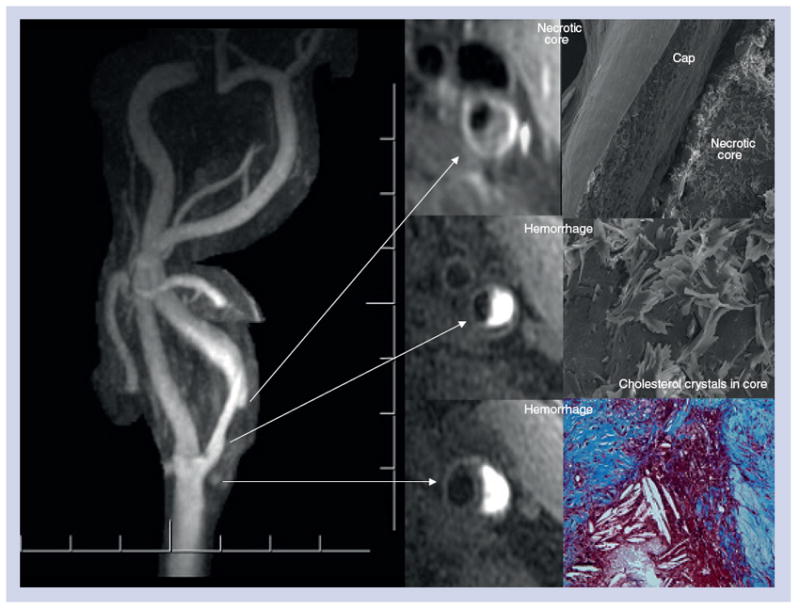
Along the inferior aspect of the intraplaque hemorrhage there is a 1-mm thick fibrous cap between the dark lumen and the bright deep intraplaque hemorrhage. Superiorly there is a well-defined fibrous cap (<500 μm) between the lumen and lipid core. Following endarterectomy, light and scanning electron microscopy demonstrate extensive cholesterol crystals with intraplaque hemorrhage and thin fibrous cap.
In a subset of patients enrolled in the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) with severe ipsilateral carotid stenosis, the presence of IPH demonstrated a trend towards a higher incidence of repeat stroke/TIA in the subsequent 9 months compared with acute symptomatic patients without ipsilateral carotid IPH (18.7 vs 3%; p = 0.051) [55]. In a separate study of acutely symptomatic patients with ipsilateral severe carotid stenosis, the presence of IPH was associated with increased spontaneous microembolic activity by TCD imaging and cerebral ischemic lesion patterns suggestive of recurrent embolic events on brain MRI studies [37]. Thus, carotid plaque MRI has been shown to identify features beyond luminal stenosis that are predictive of future TIA and stroke. Data from these studies indicate that patients with LRNC with or without hemorrhage may represent the desired phenotype for monitoring treatment effects in the individual [56]. While stroke-related mortality has declined over the past four decades, data indicate that the mortality rate has begun to plateau. This change may be attributable to individual response to therapy derived from population-based studies. Thus, carotid plaque MRI may be well suited to guide future therapeutic strategies by directly monitoring changes in this high-risk carotid plaque phenotype.
Computed tomography
Computer tomographic angiography along with MRI are the most accurate noninvasive methods in evaluating morphological characteristics of carotid plaque [57]. Compared with conventional CTA, dual-energy CTA has been shown to identify vulnerable plaques with greater accuracy. The evaluation of degree of carotid stenosis using CTA has been studied with variable results. CTA has compared favorably with catheter angiography for evaluation of patients with extracranial vascular disease, with 100% sensitivity and 63% specificity (95% CI: 25–88%); the negative-predictive value of CTA demonstrating 70% carotid artery stenosis was 100% [58]. However, the sensitivity and specificity has varied widely, ranging from 65 to 100% and 63 to 100%, respectively. CTA underestimates the degree of stenosis more often than overestimating, but careful assessment of images helps reduce misclassification [59].
Transcranial Doppler ultrasound
Transcranial Doppler ultrasound has been an important imaging technique in the management of stroke patients. It can be used in the detection of both intra- and extra-cranial arterial disease. TCD has been used to assess hemodynamic effects on intracranial circulation of carotid artery stenosis. The flow velocity in the intracranial circulation is often reduced depending upon on the severity of internal carotid artery stenosis [60].
Transcranial Doppler ultrasound can also be used to evaluate the presence of microembolic signals in patients with carotid artery stenosis. Embolic signals may independently predict stroke risk, recurrence during acute stroke, symptomatic carotid lesion and postoperatively after carotid CEA. Embolic signals are consistently found to be more frequent in patients with large artery disease, less frequent in cardioembolic stroke, and minimal or absent in patients with lacunar stroke [61]. This can be used to determine the etiology of stroke and also identify patients at risk for further stroke who require more aggressive antiplatelet therapy. The Asymptomatic Carotid Emboli Study (ACES) concluded that embolic signals on TCD in asymptomatic carotid stenosis were associated with higher risk of stroke and TIA [62]. Therefore, it is possible that these asymptomatic patients may benefit from revascularization. In a recent study we conducted simultaneous carotid ultrasound and TCD to demonstrate that unstable plaques would release particulate materials, presumed to be cholesterol crystals. This release of particulates occurred during the test by even the gentle manipulation of the ultrasound probe over carotid artery [63].
PET with CT scanning
Positron emission tomography with CT scanning (PET/CT) has been shown to detect ‘inflamed plaque’ by the presence of macrophage cell accumulation that may indicate plaque instability [64-66]. These studies were demonstrated both in atherosclerotic rabbit models as well as in human sites of plaque with dense macrophage accumulation signaling areas of active inflammation. In fact, these data may provide an effective noninvasive technique for detecting vulnerable lesions. Furthermore, this may be used to evaluate the effect of medical therapies on reducing plaque inflammation.
Serum biomarkers & carotid artery plaque
Serum biomarkers reflecting molecular processes such as inflammation, lipid accumulation, apoptosis, proteolysis and thrombosis have shown to be highly related with plaque vulnerability and may help distinguish unstable from stable carotid artery plaques. Serum biomarkers that have shown to be highly associated with the presence of vulnerable carotid artery plaques are mainly inflammatory and proteolytic markers such as high-sensitivity C-reactive protein, IL-6, MMP-9, MMP-2, and tissue inhibitors of metalloproteinases (TIMP)-1 and -2. This noninvasive way to identify high-risk patients may be a very promising tool in the future selection of patients for carotid surgery. Furthermore, use of combination of biomarkers with radiological findings (i.e., enhanced fluordeoxyglucose uptake by PET/CT scanning) may provide additional insights into plaque instability [64]. A comprehensive review of this topic is beyond the scope of this article and readers interested in further details in this area are referred to an excellent review article by Hermus et al. [67].
Natural history of carotid artery atherosclerosis
Carotid atherosclerosis may progress as in other arterial beds, but the relationship between plaque growth and TIA or stroke is complex. In the North American Symptomatic Carotid Endarterectomy Trial (NASCET) there was strong correlation between severity of stenosis and risk of stroke [68]. However, this relationship is less clear in asymptomatic patients. In the Asymptomatic Carotid Atherosclerotic Study (ACAS) [69], 5-year follow-up demonstrated 5 versus 11% ipislateral stroke or death among patients with ≥60% stenosis when treated with CEA and medical therapy versus medical therapy alone. Furthermore, in the Asymptomatic Carotid Surgery Trial (ACST), similar benefits were reported in favor of CEA [70]. However, it is important to note that since 2001 the average annual rates of ipsilateral stroke among patients receiving medical therapy alone has fallen below those of patients who received CEA in ACAS [71]. Thus, medical therapy as currently used seems to have a greater impact.
Management of atherosclerotic carotid artery plaque
A clear distinction between symptomatic and asymptomatic atherosclerotic carotid artery disease is important. Asymptomatic severe carotid stenosis generally refers to atherosclerotic narrowing of the proximal internal carotid artery exceeding 50–60% in the absence of previous referable symptoms of stroke or TIA. This lesion, at least in Westernized communities, becomes increasingly prevalent from the fifth decade. Asymptomatic patients are mostly diagnosed after a routine carotid artery ultrasound either due to high index of suspicion for carotid artery disease or secondary to nonspecific symptoms. Patients with multiple vascular risk factors such as cigarette smoking, hypertension and coronary artery disease have a higher pretest probability of carotid atherosclerosis [72]. A physical examination finding such as the presence of carotid bruit is often a poor predictor of carotid artery stenosis. In one study, one-third of 331 consecutive patients referred to a neurology clinic had a carotid bruit, but the positive-predictive value was only 37% for the presence of moderate-to-severe stenosis [73].
Atherosclerotic extracranial carotid artery disease usually results in symptoms caused by embolic events. This is in contrast to acute coronary syndromes, where a minority of ischemic strokes is caused by thrombotic occlusions. These symptoms are hemispheric as they refer to single carotid distribution, typically ipsilateral visual loss (amarurosis fugax), contralateral weakness or numbness of the body, dysarthria, aphasia and visual field defects.
Asymptomatic carotid artery atherosclerosis
Medical therapy
Aggressive treatment of vascular risk factors must be initiated promptly as it can reduce both coronary and cardiovascular events in patients with carotid artery disease. This includes treatment of hypertension with a target blood pressure of ≤140/90 mmHg and the addition of lipid-lowering agents such as statins with a goal of decreasing the LDL-cholesterol value to less than 100 mg/dl, triglyceride levels to less than 150 mg/dl and increasing HDL levels to more than 40 mg/dl. There is good evidence that treatment with statins lowers stroke risk by approximately 30% [74]. Other specific studies have shown a reduction in the risk of first stroke with statin treatment among diabetics [75], hypertensives [76] and the elderly [77]. Although the reduction in carotid intimal thickening is very small as a result of statin therapy, the important effect is most likely to be plaque stabilization, possibly by dissolving cholesterol crystals [22,78]. However, statins appear to increase the risk of intracerebral hemorrhage [79], perhaps due to punctures in the arterial wall by cholesterol crystals that are dissolved by statins leaving behind a channel, as has been recently demonstrated [22]. Exercise, weight loss, dietary precautions and smoking cessation are discussed in detail with patients. This should be preceded by stress testing prior to initiation of a moderate-intensity exercise program.
Antiplatelet therapy in the form of aspirin is recommended for all patients with asymptomatic carotid artery stenosis. There is some evidence that the combination of aspirin and clopidogrel may be more effective than aspirin alone, but larger trials (CHARISMA [80] and MATCH [81]) have not confirmed the benefit of adding clopidogrel to aspirin. Moreover, the risk of moderate-to-severe bleeding was increased with combination therapy.
Invasive therapy
This includes carotid artery stenting (CAS) and CEA. According to the American Heart Association and American Stroke Association recommendations these options can be utilized for high-grade (>60%) asymptomatic carotid artery stenosis (Table 1) [82].
Table 1.
American Heart Association and American Stroke Association recommendations on carotid endarterectomy for carotid artery stenosis.
| Stenosis (%) | Recommendations | Level of recommendations |
|---|---|---|
| Symptomatic stenosis | ||
|
| ||
| High grade (≥70%) | CEA by a surgeon with perioperative mortality rate <6% | Class I |
| Level of evidence A | ||
|
| ||
| Moderate (≥50 and <70%) | CEA, depending on patient-specific factors such as age, sex, comorbidities and severity of symptoms | Class I |
| Level of evidence A | ||
|
| ||
| Mild (<50%) | No indications for CEA | Class I |
| Level of evidence A | ||
|
| ||
| Asymptomatic stenosis | ||
|
| ||
| High grade (≥60%) | CEA when performed by a surgeon with a perioperative mortality rate of <3% | Class I |
| Level of evidence A | ||
CEA: Carotid endarterectomy.
Data taken from [82].
Carotid endarterectomy
Two large trials, ACST [70] and ACAS [69], including 4782 patients with asymptomatic carotid artery stenosis of 60–99% or 70–99%, respectively, have shown reduced rates of ipsilateral strokes at 5 years with CEA versus medical therapy. However, the goal of preventing a disabling or fatal stroke with CEA was achieved only in ACST at an overall reduction of 0.5% per year. This means that 40 patients would need to be treated to prevent one major stroke over a period of 5 years. Furthermore, women seemed to have less benefit from CEA in the ACAS trial. The benefits attributed to CEA should be calculated on the basis of prevention of large artery strokes. Approximately half of the strokes that occur in the territory of asymptomatic carotid artery stenosis are not from large arteries. Moreover, CEA cannot prevent strokes of cardioembolic source and lacunar strokes that are unlikely to be from large arteries. This is in contrast to symptomatic carotid artery disease where the risk of stroke is much higher for the same degree of stenosis. Thus, it is possible to risk-stratify patients with asymptomatic carotid artery stenosis who will benefit the most from invasive techniques by using a combination of clinical and ultrasonic plaque features. In a prospective multicenter trial, Nicolaides et al. has proposed combinations of severity of stenosis, age, systolic blood pressure, increased serum creatinine, smoking history of more than 10 pack-years and increased plaque area as few predictors of increased risk of ipsilateral events in asymptomatic patients [83].
Percutaneous interventions
Recent advances in percutaneous catheterization techniques have led to the development of CAS as a proposed alternative to CEA. Several trials have investigated carotid artery angioplasty with and without stents and with and without devices to capture distal emboli. In the Stenting and Angioplasty with Protection in Patients with High Risk for Endarterectomy (SAPPHIRE) trial [84], 334 patients who had asymptomatic stenosis of more than 80% and symptomatic patients with more than 50% stenosis at high surgical risk were randomly assigned to undergo either CAS with embolic protection device or CEA. In asymptomatic patients, fewer primary end points of death, myocardial infarction and stroke occurred after CAS (9.9 vs 21.5%). On the basis of this study, the US FDA granted approval in 2005 for carotid stenting for both symptomatic and asymptomatic high-risk surgical candidates. More recently, the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) also randomized symptomatic and asymptomatic patients (≥60% stenosis by angiography or ≥80% stenosis by CTA or MRI) to CAS versus CEA. Of these, 47% were asymptomatic [85]. The results were similar for the composite end point of periprocedural stroke, death or myocardial infarction (i.e., within 30 days of procedure) as well as long-term stroke. However, analysis of the individual components of the composite end point showed significant differences between the two treatments. The risk of periprocedural stroke was higher with stenting, but there was no difference in long-term ipsilateral stroke. Furthermore, the risk of myocardial infarction was higher in the CEA group. One important finding is that rates of long-term strokes in this trial are similar to those observed with current medical therapy (~1%) [71]. Such results have introduced new discussion regarding the necessity of performing invasive procedures for asymptomatic patients and necessitate new studies to compare the current medical therapy to intervention.
Symptomatic carotid artery stenosis
Cerebrovascular events are classified as TIA if they completely resolve within 24 h or as strokes if they cause deficit lasting longer than 24 h. Patients with TIA have 5% chance of stroke within 30 days and almost 25% will have a recurrent cerebrovascular event within 1 year. Johnston et al. proposed and validated a point score system to estimate the short-term risk of ipsilateral stroke after TIA [86]. The system is derived from five components used to calculate the score including age, blood pressure, clinical features, duration of symptoms and diabetes mellitus (acronym: ABCD). A patient with an ABCD score of 0–3 has 1.2% risk of stroke in 7 days as opposed to a 59% risk with a score of 4–5 [86]. Owing to this observation that the risk of ipsilateral stroke is much higher in the first few weeks after a TIA, physicians should entertain intensive early medical and surgical/invasive therapeutic options. An update of the ABCD score predicting early stroke risk after TIA was published recently as ABCD2 [86]. The new guidelines recommend CEA within 2 weeks for patients presenting with a TIA or minor stroke [87].
Medical therapy
In the period immediately following the onset of ischemic symptoms, cautious blood pressure management is advised. Precipitous drops in blood pressure should be avoided and gradual blood pressure reduction (i.e., over a period of few days) is recommended. Benefit of treatment to a specific target blood pressure (e.g., below 140/90 mmHg) has not been established in relation to the risk of exacerbating cerebral ischemia in the hyperacute period. AHA/ASA guidelines for prevention of stroke recommend antihypertensive treatment beyond the immediate period for patients who have experienced ischemic stroke or TIA. A systemic review comprising seven trials with a combined sample size of 15,527 patients with ischemic stroke or TIA showed a significant reduction in all recurrent strokes (24%) and nonfatal recurrent stroke (21%) with the use of antihypertensive drugs [88]. Use of statins is recommended with a goal of decreasing LDL-cholesterol serum levels to less than 100 mg/dl (class I) [82]. The Stroke Prevention by Aggressive Reduction in Cholesterol Level (SPARCL) trial was designed to determine whether a daily dose of 80 mg of atorvastatin would reduce the risk of stroke in patients with no known coronary heart disease who had had a stroke or TIA within the past 6 months [79]. A primary end point (fatal or nonfatal stroke) occurred in 265 patients in the atorvastatin group and 311 in the placebo group. On the basis of these data, 46 patients would be treated for 5 years to prevent one stroke. Secondary prevention with antithrombotic therapy is also recommended by AHA/ASA guidelines [82]. For initial treatment, aspirin (50–325 mg/day) or a combination of aspirin and extended-release dipyridamole or clopidogrel (class I) is indicated. Administration of clopidogrel in combination with aspirin is not recommended within 3 months after stroke or TIA.
Invasive therapy
Carotid endarterectomy
Carotid endarterctomy is an established and widely practiced invasive treatment in selected patients with symptomatic carotid artery stenosis of 50% or greater provided that the perioperative combined risk of stroke and death is less than 6% [82]. Two large trials have described different subsets of patients that benefit from this procedure. The NASCET randomized 2226 patients with symptomatic stenosis to medical treatment or CEA [68]. Surgery was associated with a significantly lower rate of ipsilateral cerebral events in patients with greater than 70% stenosis. Surgery was also found to be superior in patients with moderate stenosis (50–69%), while patients with less than 50% stenosis did not benefit. The European Carotid Surgery Trial (ECST) [89] randomized 3024 patients with high-grade stenosis (≥80%) and found significantly better results with CEA. Each trial used different methods of measurement of the degree of stenosis on prerandomization angiograms; the NASCET method underestimates stenosis compared with the ECST method. Stenoses reported to be 70–99% in the NASCET trial were equivalent to 80–99% by ECST and stenoses reported to be 70–99% by the ECST trialists were 50–99% by NASCET. In ECST the absolute reduction in risk of major stroke or death at 3 years was 11.6% (p < 0.001) [89]. This was consistent with the 10.1% (p < 0.01) reduction in major stroke or death at 2 years reported for patients in NASCET (70–99% stenosis) [68]. NASCET also demonstrated a 6.9% (p = 0.03) absolute reduction in risk of disabling stroke or death in patients with 50–69% stenosis (ECST: 70–80% stenosis). This benefit was not seen in ECST (Table 2).
Table 2.
Overview of carotid endarterectomy versus medical therapy trials.
| Trial (year) | Stenosis (%) | Treatment | Results |
|---|---|---|---|
| Symptomatic patients | |||
| NASCET (1991) | 70–99 | CEA + medical therapy versus medical therapy | 65% lower rate of ipsilateral cerebral events with CEA |
| ECST (1991) | 70–99 | CEA + medical therapy versus medical therapy | Incidence of ipsilateral ischemic stroke 2.8 versus 16.8% with aspirin alone |
| VA (1991) | 50–99 | CEA + medical therapy versus medical therapy | Death or stroke 7.7% with CEA versus 19.4% with medical therapy |
| Asymptomatic patients | |||
| ACAS (1995) | 60–99 | CEA + medical therapy versus medical therapy | Relative risk reduction of 53% with CEA |
| ACST (2004) | 60–99 | CEA + medical therapy versus medical therapy | 5-year stroke risk 6.4% with CEA versus 11.8% with medical management |
| VA (1993) | 50–99 | CEA + medical therapy versus medical therapy | 61% lower risk of TIA or stroke with CEA |
ACAS: Asymptomatic Carotid Atherosclerotic Study; ACST: Asymptomatic Carotid Surgery Trial; CEA: Carotid endarterectomy; ECST: European Carotid Surgery Trial; NASCET: North American Symptomatic Carotid Endarterectomy Trial; TIA: Transient ischemic attack; VA: The Veterans Affairs Cooperative Study Group.
Carotid artery stenting
In both NASCET [68] and ECST [89], patients were carefully selected. In the NASCET, patients were excluded if they were 79 years of age or older, had organ failure or cancer likely to cause death in 5 years or had a cardiac valvular or rhythm abnormality. In addition, patients were ‘temporarily ineligible’ if they had uncontrolled hypertension or diabetes, or had experienced unstable angina or myocardial infarction in the preceding 6 months. Only one-third of the patients that were operated on in participating institutions were enrolled in the trial. In addition, CEA has been associated with cranial nerve paralysis, wound complications and cardiovascular events. This has led to the development of less invasive percutaneous approaches for patients with significant comorbidities.
Two large trials have been recently conducted and are discussed here (Table 3). The SAPPHIRE trial compared CAS with distal protection versus CEA [84]. Criteria for high risk included clinically significant cardiac disease (congestive heart failure, abnormal stress test results or need for open-heart surgery), severe pulmonary disease, contralateral carotid artery occlusion, contralateral laryngeal nerve palsy, previous neck surgery or neck radiation therapy, recurrent stenosis after CEA, or age older than 80 years. The primary end point of the study was the cumulative incidence of a major cardiovascular or neurological event at 1 year. A total of 334 patients were equally randomized to carotid stenting and CEA. In patients with symptomatic stenosis, the occurrence of the primary end point was similar with both CAS and CEA (16.8 vs 16.5%). The primary end point occurred in 20 patients randomly assigned to undergo stenting and in 32 patients assigned to undergo CEA. Based on these results, the US FDA approved the use of stenting with distal protection for high-risk patients.
Table 3.
Overview of carotid artery stenting versus carotid endarterectomy trials.
| Trial (year) | Stenosis (%) | Treatment | Results |
|---|---|---|---|
| EVA-3S (2006) | ≥60 symptomatic | CEA versus CAS | Terminated early due to higher deaths and strokes in the stenting group |
|
| |||
| SAPPHIRE (2004) | >50 symptomatic | CEA versus CAS | Stenting noninferior to CEA in high-risk patients |
| ≥80 asymptomatic | |||
|
| |||
| SPACE (2008) | ≥50 symptomatic | CEA versus CAS | Results were not similar enough to prove noninferiority of stenting |
|
| |||
| ICSS (2010) | ≥50 symptomatic | CEA versus CAS | Risk of any stroke was higher with stenting 7.7% versus 4.1% with surgery |
|
| |||
| CREST (2010) | ≥50 symptomatic | CEA versus CAS | Periprocedural stroke, MI, or death or ipsilateral stroke 7.2% with stenting versus 6.8% with surgery |
| ≥60 asymptomatic | |||
CAS: Carotid artery stenting; CEA: Carotid endarterectomy; CREST: Carotid Revascularization Endarterectomy Versus Stenting Trial; EVA-3S: Endarterectomy Versus Stenting in Patients With Symptomatic Severe Carotid Stenosis Trial; ICSS: International Carotid Stenting Study; MI: Myocardial infarction; SAPPHIRE: Stenting and Angioplasty With Protection in Patients at High Risk for Endarterectomy; SPACE: Stent-Protected Angioplasty Versus Carotid Endarterectomy in Symptomatic Patients Trial.
The second and most recently published trial, CREST, is the largest randomized prospective study to date comparing CAS versus CEA. Unlike NASCET, CREST focused on low-risk surgical patients [85]. The overall safety and efficacy of the two procedures was largely the same for patients who had a prior stroke and for those who had not. However, more heart attacks occurred with the CEA group compared with CAS (2.3 vs 1.1%) and more strokes in the CAS group compared with the CEA group (4.1 vs 2.3%) in the weeks following the procedure. The study also found that in patients who were less than 69 years of age, CAS was better while in patients more than 70 years of age, CEA was superior.
Several other trials have been conducted. However, owing to major limitations and criticism, these trials, the Stent-Protected Angioplasty Versus Carotid Endarterectomy in Symptomatic Patients (SPACE) trial, Endarterectomy Versus Stenting in Patients With Symptomatic Severe Carotid Stenosis (EVA-3S) trial and the International Carotid Stenting Study (ICSS), are only described briefly in Table 3.
Conclusion
In conclusion, extracranial carotid artery atherosclerotic plaque is a common diagnosis in general medical practice. Despite considerable progress in understanding the pathophysiology of atherosclerosis, the practical utility of morphological and biochemical assessments in predicting stroke requires validation by prospective studies. Advancements in noninvasive imaging technology have accelerated the acquisition of data and greatly improved diagnostic accuracy to rival that of conventional angiography for evaluation of patients with extracranial atherosclerosis. The value of specific therapies to prevent stroke in symptomatic and asymptomatic patients with severe carotid artery stenosis is the subject of current research. Further analysis of the ongoing and recently published trials will help to clarify the role of medical, interventional and surgical therapies available to patients with carotid atherosclerosis.
Expert commentary
Stroke is the second most common cause of death worldwide and at least 15–20% of all ischemic strokes are attributed to carotid artery atherosclerosis. Advanced atherosclerotic plaque detected at the carotid bifurcation reveals characteristic features of unstable plaque in both symptomatic and asymptomatic patients. Early lesion development is initiated by intimal accumulation of lipoprotein particles. These particles undergo oxidative modification and activate cytokines, which facilitates uptake and migration of monocytes into the artery wall. These monocytes become lipid-laden macrophages or foam cells that release additional cytokines, oxidants and matrix metalloproteinases. Smooth muscle cells migrate from the media to the intima and proliferate. Free cholesterol accumulates in the plaque, forming a necrotic core that is contained by a fibrous cap. Increasing free cholesterol saturation leads to crystal formation and expansion of the core, thinning out and perforating an already weakened fibrous cap. Crystal formation also triggers both a local and systemic inflammatory reaction. Characterization of plaques by various imaging methods has enhanced the understanding of natural history of atherosclerotic disease. The in vivo MRI depiction of carotid plaque hemorrhage, LRNC and thinning of the overlying fibrous cap have been validated with histological correlation. Atherosclerotic plaque that is prone to rupture owing to its intrinsic composition such as IPH and a large lipid core are associated with subsequent thromboembolic ischemic events. In addition, carotid plaque MRI may be well suited to guide future therapeutic strategies by directly monitoring changes in asymptomatic patients with high-risk carotid plaque phenotype. Clinically, carotid ultrasonography or CTA most often provides the information needed to guide the choice of medical, endovascular or surgical treatment. Moreover, the combined approach of carotid ultrasound with TCD may provide a unique method to detect unstable plaque by the release of cholesterol crystals and other particles during the study. Aggressive treatment of vascular risk factors must be initiated promptly as it can reduce both coronary and cardiovascular events in patients with carotid artery disease. For many asymptomatic patients with carotid artery stenosis, intensive medical treatment may be the most appropriate therapeutic option. Trials of carotid revascularization must be interpreted in the context of the evolution of medical therapy. Although pharmacotherapy aimed at risk reduction was incorporated in most trials, guidelines and strategies have changed and more effective measures have enhanced the medical armamentarium. The value of specific therapies in preventing stroke in both symptomatic and asymptomatic patients with severe carotid artery stenosis is the subject of current research. Further analysis of the ongoing and recently published trials will further help to clarify the role of medical, interventional and surgical therapies available to these patients.
Five-year view
Future studies are required for understanding the pathophysiology of atherosclerosis and the practical utility of these morphological and biochemical assessments in predicting stroke. Another major problem to overcome is the lack of good evidence with which to target asymptomatic patients above specific risk thresholds for detection of hemodynamically significant carotid stenosis and to identify those who may benefit from therapeutic intervention. Future development in vascular contract agents and 3D vascular imaging may improve the role of ultrasound. Furthermore, specific MRI algorithms that characterize plaques (i.e., IPH) could become more valuable in risk assessment. Further work is also needed to determine the value of measuring the IMT of the carotid artery wall as a way of directing preventive therapies. It is critical to define optimal antithrombotic therapy for patients who experience recurrent cerebral ischemia during antiplatelet therapy. However, novel therapeutic approaches targeting cholesterol crystallization could help in plaque stabilization-enhancing medical therapy. Important questions about the value of CAS relative to CEA have been answered recently but many more controversies have risen. Age, gender and ethnic-based studies focusing on asymptomatic patients and comparative trials of both methods of revascularization versus current medical management will be areas of interest in coming years.
Key issues.
Extracranial carotid artery atherosclerotic plaque is a common diagnosis in general medical practice and differentiation between asymptomatic and symptomatic carotid artery stenosis is important in tailoring proper therapy.
Advancements in noninvasive imaging technology have accelerated the acquisition of data and greatly improved diagnostic accuracy to rival that of conventional angiography for evaluation of patients with this entity.
Elucidation by the novel mechanism of plaque rupture via cholesterol crystallization could help enhance methods to stabilize plaques.
The value of specific therapies to prevent stroke in symptomatic and asymptomatic patients with severe carotid artery stenosis has improved in recent years but continues to be the subject of active medical research. Opportunities exist for further understanding the pathophysiology of atherosclerosis and the practical utility of these morphological and biochemical assessments in predicting stroke.
Combined carotid ultrasound and transcranial Doppler could provide a novel noninvasive method of detecting unstable plaque in asymptomatic patients.
Recent large trials such as Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) make it clear that with adequate training, physicians can perform carotid artery stenting and carotid endarterctomy with low complication rates and outcomes with stenting are comparable to those with surgery in both the short term and the long term, and that the choice of management should be individualized.
Acknowledgments
George Abela is a speaker for Merck, Abbott, CardioNet, GlaxoSmithKline and Takeda; he has received major grants from Merck and Novartis. Majid Mughal is a speaker for Gilead Sciences, United Therapeutics and Actelion. Arshad Majid is a speaker for Boeringher Ingelheim. J Kevin DeMarco is a speaker for ABC Medical Education and is is a subcontractor on William Kerwin, PhD (NIH grant no. R44-HL070576).
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics: 2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics: 2010 update: a report from the American Heart Association. Circulation. 2010;121(12):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Petty G, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. 1999;30(12):2513–2516. doi: 10.1161/01.str.30.12.2513. [DOI] [PubMed] [Google Scholar]
- 4.Mineva PP, Manchev IC, Hadjiev DI. Prevalence and outcome of asymptomatic carotid stenosis: a population-based ultrasonographic study. Eur J Neurol. 2002;9(4):383–388. doi: 10.1046/j.1468-1331.2002.00423.x. [DOI] [PubMed] [Google Scholar]
- 5.Risk of stroke in the distribution of an asymptomatic carotid artery. The European Carotid Surgery Trialists Collaborative Group. Lancet. 1995;345(8944):209–212. [PubMed] [Google Scholar]
- 6.Muller JE, Abela GS, Nesto RW, Tofler GH. Triggers, acute risk factors and vulnerable plaques: the lexicon of a new frontier. J Am Coll Cardiol. 1994;23(3):809–813. doi: 10.1016/0735-1097(94)90772-2. [DOI] [PubMed] [Google Scholar]
- 7.Schaar JA, Muller JE, Falk E, et al. Terminology for high-risk and vulnerable coronary artery plaques. Eur Heart J; Report of a meeting on the vulnerable plaque; June 17 and 18, 2003; Santorini, Greece. 2004. pp. 1077–1082. [DOI] [PubMed] [Google Scholar]
- 8.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi K, Kishi M, Atsumi T, et al. Circulating oxidized LDL forms complexes with β2-glycoprotein I: implication as an atherogenic autoantigen. J Lipid Res. 2003;44(4):716–726. doi: 10.1194/jlr.M200329-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Guyton JR, Klemp KF. Development of the lipid-rich core in human atherosclerosis. Arterioscler Thromb Vasc Biol. 1996;16(1):4–11. doi: 10.1161/01.atv.16.1.4. [DOI] [PubMed] [Google Scholar]
- 11.Abela GS, Aziz K. Cholesterol crystals rupture biological membranes and human plaques during acute cardiovascular events: a novel insight into plaque rupture by scanning electron microscopy. Scanning. 2006;28(1):1–10. doi: 10.1002/sca.4950280101. [DOI] [PubMed] [Google Scholar]
- 12.Abela GS, Aziz K, Vedre A, Pathak DR, Talbott JD, Dejong J. Effect of cholesterol crystals on plaques and intima in arteries of patients with acute coronary and cerebrovascular syndromes. Am J Cardiol. 2009;103(7):959–968. doi: 10.1016/j.amjcard.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Vedre A, Pathak DR, Crimp M, Lum C, Koochesfahani M, Abela GS. Physical factors that trigger cholesterol crystallization leading to plaque rupture. Atherosclerosis. 2009;203(1):89–96. doi: 10.1016/j.atherosclerosis.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Gadeela N, Rubinstein J, Tamhane U, et al. The impact of circulating cholesterol crystals on vasomotor function implications for no-reflow phenomenon. JACC Cardiovasc Interv. 2011;4(5):521–529. doi: 10.1016/j.jcin.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol. 2000;20(5):1177–1178. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 16.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15(9):1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 17.Carr S, Farb A, Pearce WH, Virmani R, Yao JS. Atherosclerotic plaque rupture in symptomatic carotid artery stenosis. J Vasc Surg. 1996;23(5):755–765. doi: 10.1016/s0741-5214(96)70237-9. [DOI] [PubMed] [Google Scholar]
- 18.Abela GS. Cholesterol crystals piercing the arterial plaque and intima triggers local and systemic inflammation. J Clin Lipidol. 2010;4(3):156–164. doi: 10.1016/j.jacl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Davies MJ, Thomas AC. Plaque fissuring: the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J. 1985;53(4):363–373. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loree HM, Kamm RD, Stringfellow RG, Lee RT. Effects of fibrous cap thickness on peak circumferential stress in model atherosclerotic vessels. Circ Res. 1992;71(4):850–858. doi: 10.1161/01.res.71.4.850. [DOI] [PubMed] [Google Scholar]
- 21.Hatsukami TS, Ross R, Polissar NL, Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation. 2000;102(9):959–964. doi: 10.1161/01.cir.102.9.959. [DOI] [PubMed] [Google Scholar]
- 22.Abela GS, Vedre A, Janoudi A, Huang R, Durga S, Tamhane U. Effect of statins on cholesterol crystallization and atherosclerotic plaque stabilization. Am J Cardiol. 2011;107(12):1710–1717. doi: 10.1016/j.amjcard.2011.02.336. [DOI] [PubMed] [Google Scholar]
- 23.Ota H, Yu W, Underhill HR. Hemorrhage and large lipid-rich necrotic cores are independently associated with thin or ruptured fibrous caps: an in vivo 3T MRI study. Arterioscler Thromb Vasc Biol. 29(10):1696–1701. doi: 10.1161/ATVBAHA.109.192179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Underhill HR, Yuan C, Yarnykh VL, et al. Predictors of surface disruption with MR imaging in asymptomatic carotid artery stenosis. AJNR Am J Neuroradiol. 2009;31(3):487–493. doi: 10.3174/ajnr.A1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickson BC, Gotlieb AI. Towards understanding acute destabilization of vulnerable atherosclerotic plaques. Cardiovasc Pathol. 2003;12(5):237–248. doi: 10.1016/s1054-8807(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 26.Frink RJ. Parallel cholesterol crystals: a sign of impending plaque rupture? J Invasive Cardiol. 2010;22(9):406–411. [PubMed] [Google Scholar]
- 27.Carr SC, Farb A, Pearce WH, Virmani R, Yao JS. Activated inflammatory cells are associated with plaque rupture in carotid artery stenosis. Surgery. 1997;122(4):757–763. doi: 10.1016/s0039-6060(97)90084-2. discussion 763–764. [DOI] [PubMed] [Google Scholar]
- 28.Jander S, Sitzer M, Schumann R, et al. Inflammation in high-grade carotid stenosis: a possible role for macrophages and T cells in plaque destabilization. Stroke. 1998;29(8):1625–1630. doi: 10.1161/01.str.29.8.1625. [DOI] [PubMed] [Google Scholar]
- 29.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94(6):2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mach F, Schönbeck U, Bonnefoy JY, Pober JS, Libby P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40: induction of collagenase, stromelysin, and tissue factor. Circulation. 1997;96(2):396–399. doi: 10.1161/01.cir.96.2.396. [DOI] [PubMed] [Google Scholar]
- 31.Del Prete G, De Carli M, Lammel RM, et al. Th1 and Th2 T-helper cells exert opposite regulatory effects on procoagulant activity and tissue factor production by human monocytes. Blood. 1995;86(1):250–257. [PubMed] [Google Scholar]
- 32.Düewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ionita MG, van den Borne P, Catanzariti LM, et al. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of ruptureprone lesions. Arterioscler Thromb Vasc Biol. 2010;30(9):1842–1848. doi: 10.1161/ATVBAHA.110.209296. [DOI] [PubMed] [Google Scholar]
- 34.Barger AC, Beeuwkes R, 3rd, Lainey LL, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984;310(3):175–177. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- 35.Moreno PR, Purushothaman KR, Zias E, Sanz J, Fuster V. Neovascularization in human atherosclerosis. Curr Mol Med. 2006;6(5):457–477. doi: 10.2174/156652406778018635. [DOI] [PubMed] [Google Scholar]
- 36.Abela GS, Maheshwari A, Kantipudi SC. Atherosclerotic vascular disease as a systemic process. In: Abela GS, editor. Peripheral Vascular Disease: Diagnostic and Therapeutic Approaches. Lippincott Williams & Wilkins; NY, USA: 2004. pp. 23–36. [Google Scholar]
- 37.Altaf N, Goode SD, Beech A, et al. Plaque hemorrhage is a marker of thromboembolic activity in patients with symptomatic carotid disease. Radiology. 2011;258(2):538–545. doi: 10.1148/radiol.10100198. [DOI] [PubMed] [Google Scholar]
- 38.Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349(24):2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 39.Redgrave JN, Lovett JK, Rothwell PM. Histological features of symptomatic carotid plaques in relation to age and smoking: the oxford plaque study. Stroke. 2010;41(10):2288–2294. doi: 10.1161/STROKEAHA.110.587006. [DOI] [PubMed] [Google Scholar]
- 40.Ota H, Reeves MJ, Zhu DC, et al. Sex differences in patients with asymptomatic carotid atherosclerotic plaque: in vivo 3.0-T magnetic resonance study. Stroke. 2010;41(8):1630–1635. doi: 10.1161/STROKEAHA.110.581306. [DOI] [PubMed] [Google Scholar]
- 41.Peeters W, Hellings WE, de Kleijn DP, et al. Carotid atherosclerotic plaques stabilize after stroke: insights into the natural process of atherosclerotic plaque stabilization. Arterioscler Thromb Vasc Biol. 2009;29(1):128–133. doi: 10.1161/ATVBAHA.108.173658. [DOI] [PubMed] [Google Scholar]
- 42.Gompels BM. High definition imaging of carotid arteries using a standard commercial ultrasound ‘B’ scanner. A preliminary report. Br J Radiol. 1979;52(620):608–619. doi: 10.1259/0007-1285-52-620-608. [DOI] [PubMed] [Google Scholar]
- 43.Wolff T, Guirguis-Blake J, Miller T, Gillespie M, Harris R. Screening for carotid artery stenosis: an update of the evidence for the U. S. Preventive Services Task Force. Ann Intern Med. 2007;147(12):860–870. doi: 10.7326/0003-4819-147-12-200712180-00006. [DOI] [PubMed] [Google Scholar]
- 44.Kuhn FP, Kramer G, Günther R, Thelen M. B-scan sonography of the carotid artery. Technique, results and indications (author’s translation) Rofo. 1981;135(4):407–411. doi: 10.1055/s-2008-1056905. [DOI] [PubMed] [Google Scholar]
- 45.Beletsky VY, Kelley RE, Fowler M, Phifer T. Ultrasound densitometric analysis of carotid plaque composition. Pathoanatomic correlation. Stroke. 1996;27(12):2173–2177. doi: 10.1161/01.str.27.12.2173. [DOI] [PubMed] [Google Scholar]
- 46.Srámek A, Bosch JG, Reiber JH, Van Oostayen JA, Rosendaal FR. Ultrasound assessment of atherosclerotic vessel wall changes: reproducibility of intima–media thickness measurements in carotid and femoral arteries. Invest Radiol. 2000;35(12):699–706. doi: 10.1097/00004424-200012000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Kathiresan S, Larson MG, Keyes MJ, et al. Assessment by cardiovascular magnetic resonance, electron beam computed tomography, and carotid ultrasonography of the distribution of subclinical atherosclerosis across Framingham risk strata. Am J Cardiol. 2007;99(3):310–314. doi: 10.1016/j.amjcard.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 48.Nambi V, Chambless L, Folsom AR, et al. Carotid intima–media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55(15):1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai J, Hatsukami TS, Ferguson MS, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112(22):3437–3444. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 50.Ota H, Yarnykh VL, Ferguson MS, et al. Carotid intraplaque hemorrhage imaging at 3.0-T MR imaging: comparison of the diagnostic performance of three T1-weighted sequences. Radiolog. 2011;254(2):551–563. doi: 10.1148/radiol.09090535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saam T, Cai JM, Cai YQ, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005;25(3):234–239. doi: 10.1161/01.ATV.0000149867.61851.31. [DOI] [PubMed] [Google Scholar]
- 52.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(8 Suppl):C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 53.Singh N, Moody AR, Gladstone DJ, et al. Moderate carotid artery stenosis: MR imaging-depicted intraplaque hemorrhage predicts risk of cerebrovascular ischemic events in asymptomatic men. Radiology. 2009;252(2):502–508. doi: 10.1148/radiol.2522080792. [DOI] [PubMed] [Google Scholar]
- 54.Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI: initial results. Stroke. 2006;37(3):818–823. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 55.Kurosaki Y, Yoshida K, Endo H, Chin M, Yamagata S. Association between carotid atherosclerosis plaque with high signal intensity on T1-weighted imaging and subsequent ipsilateral ischemic events. Neurosurgery. 2011;68(1):62–67. doi: 10.1227/NEU.0b013e3181fc60a8. discussion 67. [DOI] [PubMed] [Google Scholar]
- 56.Underhill HR, Yuan C. Carotid MRI: a tool for monitoring individual response to cardiovascular therapy? Expert Rev Cardiovasc Ther. 2011;9(1):63–80. doi: 10.1586/erc.10.172. [DOI] [PubMed] [Google Scholar]
- 57.Mühlenbruch G, Das M, Mommertz G, et al. Comparison of dual-source CT angiography and MR angiography in preoperative evaluation of intra- and extracranial vessels: a pilot study. Eur Radiol. 2009;20(2):469–476. doi: 10.1007/s00330-009-1547-7. [DOI] [PubMed] [Google Scholar]
- 58.Josephson SA, Bryant SO, Mak HK, Johnston SC, Dillon WP, Smith WS. Evaluation of carotid stenosis using CT angiography in the initial evaluation of stroke and TIA. Neurology. 2004;63(3):457–460. doi: 10.1212/01.wnl.0000135154.53953.2c. [DOI] [PubMed] [Google Scholar]
- 59.Silvennoinen HM, Ikonen S, Soinne L, Railo M, Valanne L. CT angiographic analysis of carotid artery stenosis: comparison of manual assessment, semiautomatic vessel analysis, and digital subtraction angiography. AJNR Am J Neuroradiol. 2007;28(1):97–103. [PMC free article] [PubMed] [Google Scholar]
- 60.Anzola GP, Gasparotti R, Magoni M, Prandini F. Transcranial Doppler sonography and magnetic resonance angiography in the assessment of collateral hemispheric flow in patients with carotid artery disease. Stroke. 1995;26(2):214–217. doi: 10.1161/01.str.26.2.214. [DOI] [PubMed] [Google Scholar]
- 61.Kaposzta Z, Young E, Bath PM, Markus HS. Clinical application of asymptomatic embolic signal detection in acute stroke: a prospective study. Stroke. 1999;30(9):1814–1818. doi: 10.1161/01.str.30.9.1814. [DOI] [PubMed] [Google Scholar]
- 62.Markus HS, King A, Shipley M, et al. Asymptomatic embolization for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol. 2010;9(7):663–671. doi: 10.1016/S1474-4422(10)70120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abela GS, Farooq MU, Berger K, Pathak D, Kassab M. A novel method of identifying unstable plaque by simultaneous carotid Ultrasound and trans-cranial doppler. J Am Coll Cardiol. 2010;55(10 Suppl. A):161. [Google Scholar]
- 64.Aziz K, Berger K, Claycombe K, Huang R, Patel R, Abela GS. Noninvasive detection and localization of vulnerable plaque and arterial thrombosis with computed tomography angiography/positron emission tomography. Circulation. 2008;117(16):2061–2070. doi: 10.1161/CIRCULATIONAHA.106.652313. [DOI] [PubMed] [Google Scholar]
- 65.Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105(23):2708–2711. doi: 10.1161/01.cir.0000020548.60110.76. [DOI] [PubMed] [Google Scholar]
- 66.Tawakol A, Migrino RQ, Hoffmann U, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol. 2005;12(3):294–301. doi: 10.1016/j.nuclcard.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Hermus L, Lefrandt JD, Tio RA, Breek JC, Zeebregts CJ. Carotid plaque formation and serum biomarkers. Atherosclerosis. 2010;213(1):21–29. doi: 10.1016/j.atherosclerosis.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 68.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Eng J Med. 1991;325(7):445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 69.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273(18):1421–1428. [PubMed] [Google Scholar]
- 70.Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 71.Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke. 2009;40(10):e573–e583. doi: 10.1161/STROKEAHA.109.556068. [DOI] [PubMed] [Google Scholar]
- 72.Jacobowitz GR, Rockman CB, Gagne PJ, et al. A model for predicting occult carotid artery stenosis: screening is justified in a selected population. J Vasc Surg. 2003;38(4):705–709. doi: 10.1016/s0741-5214(03)00730-4. [DOI] [PubMed] [Google Scholar]
- 73.Davies KN, Humphrey PR. Do carotid bruits predict disease of the internal carotid arteries? Postgrad Med J. 1994;70(824):433–435. doi: 10.1136/pgmj.70.824.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blauw GJ, Lagaay AM, Smelt AH, Westendorp RG. Stroke, statins, and cholesterol. A meta-analysis of randomized, placebo-controlled, double-blind trials with HMG-CoA reductase inhibitors. Stroke. 1997;28(5):946–950. doi: 10.1161/01.str.28.5.946. [DOI] [PubMed] [Google Scholar]
- 75.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 76.Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo–Scandinavian Cardiac Outcomes Trial: Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;(361)(9364):1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 77.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 78.MacMahon S, Sharpe N, Gamble G, et al. Effects of lowering average of below-average cholesterol levels on the progression of carotid atherosclerosis: results of the LIPID Atherosclerosis Substudy. LIPID Trial Research Group. Circulation. 1998;97(18):1784–1790. doi: 10.1161/01.cir.97.18.1784. [DOI] [PubMed] [Google Scholar]
- 79.Amarenco P, Bogousslavsky J, Callahan A, 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 80.Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 81.Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9431):331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 82.Brott TG, Halperin JL, Abbara S, et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the Management of Patients With Extracranial Carotid and Vertebral Artery Disease: Executive Summary A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery Developed in Collaboration With the American Academy of Neurology and Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2011;57(8):1002–1044. doi: 10.1016/j.jacc.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 83.Nicolaides AN, Kakkos SK, Kyriacou, et al. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg. 2010;52(6):1486–1496.e1–5. doi: 10.1016/j.jvs.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 84.Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351(15):1493–1501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 85.Brott TG, Hobson RW, 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363(1):11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369(9558):283–292. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]
- 87.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;42(1):227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 88.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34(11):2741–2748. doi: 10.1161/01.STR.0000092488.40085.15. [DOI] [PubMed] [Google Scholar]
- 89.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351(9113):1379–1387. [PubMed] [Google Scholar]
- 90.Hobson RW, 2nd, Weiss DG, Fields WS, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med. 1993;328(4):221–227. doi: 10.1056/NEJM199301283280401. [DOI] [PubMed] [Google Scholar]


