Background: Dormancy of disseminated cancer cells is a limiting step of cancer metastasis.
Results: A dormant state controls clonogenicity of dispersed prostate cancer cells in culture and is modulated by osmotic pressure, p53, and the activin A signaling pathways.
Conclusion: A dormant state related to stem cell dormancy controls clonogenicity of prostate cancer cells.
Significance: A new model for studying cell dormancy mechanisms is provided.
Keywords: Activin, p53, SMAD Transcription Factor, Tumor Metabolism, Tumor Metastases, Clonogenicity, Dormancy, Hypertonic Stress, Osmotic Pressure, Prostate Cancer
Abstract
Cell dormancy constitutes a limiting step of the metastatic process by preventing the proliferation of isolated cancer cells disseminated at distant sites from the primary tumor. The study of cancer cell dormancy is severely hampered by the lack of biological samples so that the mechanisms that regulate cell dormancy have not been extensively explored. In this work, we describe the rapid induction in vitro of a dormant state in prostate cancer cells by exposure to a slightly hypertonic growth medium. This quiescence is observed only when cells are seeded at low density and, once established, requires additional stimuli besides osmotic pressure to be reversed. Media conditioned by cells grown at high density can partially prevent or reverse dormancy, a phenomenon which can be reproduced with citric acid. In addition to this role of small metabolites, inactivation of the p53 and smad pathways also counters the entry into dormancy, whereas exposure to activin A induces it to some extent. Thus, this easily inducible dormancy reproduces several features associated with the dormancy of stem cells and cancer cells in vivo.
Introduction
Clonogenicity describes the ability of single cells to give rise to clonal populations. This property is of prime importance for stem cells for determining their self-renewal and/or expansion as well as the generation of a differentiated progeny. Clonogenicity is also a feature of cancer cells, both in the primary tumor, which in most cases is clonal in origin, and in metastases. Indeed, the metastatic process is viewed as the clonal expansion of single cancer cells disseminated at distant sites through the blood or the lymphatic circulation. Moreover, a wealth of data suggest that more than the dissemination process itself it is the ability of disseminated cells to give rise to a cell population that is the limiting step in the metastatic process (1, 2). Several studies have shown that patients with prostate or breast cancer but no clinical metastases can harbor numerous cancer cells in their blood circulation for years. In addition, it has been documented that when tumor cells are injected in mice they can be detected for an extended period of time in many organs but that only a small fraction of them will proliferate to give rise to micrometastases (1, 3–5). These experimental data suggest that most of the disseminated cancer cells are in a “dormant” state that, although characterized by the absence of cell proliferation, can nonetheless give rise to a tumor in some cases. The study of cancer cell dormancy is made difficult by the difficulty to investigate these rare cells in vivo or to purify them into a viable population. Consequently, the mechanisms that regulate the entry and/or the maintenance of cell dormancy have not been extensively explored. Even less is known about the internal or external cues that can induce cells to leave dormancy. Some aspects of clonogenicity can be analyzed in cell culture ex vivo through the determination of cloning efficiency: this is a measure of the ability of cells to give rise to distinct clonal cell populations when seeded at low density. We undertook the analysis of the factors modulating the cloning efficiency of a subline derived from LNCaP cells, one of the most studied models of androgen-sensitive prostate cancer cells. In the course of this study, we discovered that osmotic pressure of the culture medium is a key parameter modulating cloning efficiency of prostate cancer cells. Indeed, small variations in osmotic pressure were sufficient to induce a dormant state in cells plated in low density. Once induced into this state, cells will remain quiescent in otherwise permissive conditions but can resume their growth and give rise to colonies when appropriately stimulated.
EXPERIMENTAL PROCEDURES
Cells and Retroviruses
LNCaP and Du 145 cells were provided by Florence Cabon (CNRS FRE 3229, Villejuif, France). They were grown in RPMI1640 medium (containing Glutamax-I and 25 mm Hepes, reference no. 61870, Invitrogen) or DMEM (containing Glutamax-I and 4.5 g/liter glucose without pyruvate, reference no. 61965, Invitrogen), supplemented with 10% fetal calf serum (PAA Laboratories, Pasching, Austria) and penicillin plus streptomycin solution (Invitrogen). Cells were grown at 37 °C in 10% CO2 and passaged by treatment with 0.05% trypsin-EDTA (Invitrogen) every 3–4 days when reaching confluence.
LNCaP* designates a phleomycin-resistant cell population derived from LNCaP by transfection of pBabePhleo-EcoR plasmid DNA (encoding the receptor for ecotropic murine leukemia retroviruses) followed by phleomycin selection. The resistant cells were expanded and aliquots were frozen in liquid nitrogen. Recombinant retroviruses were produced by DNA transfection of 293T cells with the retroviral vectors and complementing expression vectors for gagpol and env retroviral proteins (pVPack-GP and PVPack-Eco, Stratagene, La Jolla, CA). Supernatants were harvested 2 days later, filtered, and used to infect LNCaP* cells without polybrene. After antibiotic selection with puromycin (1 μg/ml for about 1 week in DMEM-FCS) or G418 (1 mg/ml for about 2 weeks in RPMI-FCS), resistant cell populations were expanded for about 1 week of culture in DMEM-FCS, and three aliquots were frozen in liquid nitrogen. Unless otherwise indicated, studies were conducted with low-passage cells. Citric acid was obtained from Carlo Erba (Milano, Italy), glutathione from Sigma-Aldrich (St. Louis, MO), recombinant activin A from R&D Systems (Minneapolis, MN).
Plasmids
Plasmids pBabe puro-p53 wild type and pG13luc were provided by Dr Mark Pearson. Plasmid p21-luc was provided by Dr. Christophe Lallemand. Plasmid pBabepuro-p53-R248Q was provided by Dr Luis Martinez. Plasmid pFbneo-p53R175H was constructed upon insertion of a EcoR1-BamH1 fragment excised from plasmid Pc3m-p53R175H (provided by Dr. Luis Martinez) into the pFbneo plasmid (Stratagene) opened by EcoR1 and BamH1. The biological activity of the retroviral vectors expressing mutated p53 was ascertained by infection of primary culture of mouse embryo fibroblasts and measurement of the expected extension of their lifespan (6) (data not shown). Plasmid pBabepuro-smad7 was constructed upon insertion of a EcoR1-Sal1 fragment excised from plasmid pcDNA3-FLAG-smad7 (encoding a FLAG-marked smad7 from murine origin provided by Dr. Peter ten Dijke) into pBabepuro plasmid opened by EcoR1 and Sal1.
Luciferase Assay
106 cells were plated in a 5-cm dish and transfected 1 day later using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions (3 μl of Lipofectamine per μg of DNA). Cells were lysed 2 days post-transfection, and luciferase activities were measured with a dual luciferase kit (Promega, Charbonnières, France).
Western Blot Analysis
Western blot analyses were performed as previously described (7). Immunodetection was performed with an ECL+ detection kit (Amersham Biosciences) using antibodies against smad7 (mouse monoclonal MAB2029, R&D Systems).
Cloning Efficiency Assay
To assay cloning efficiency, we first determined a threshold cellular density that yielded no or very few colonies after 16 days culture in DMEM-FCS. For most serum lots, this density was 5000 cells in a 9-cm dish for LNCaP cells and 10,000 for LNCaP* cells (or 5000 cells in a 5-cm dish). After plating, cells were left to grow for about 15 days, fixed in 2% formaldehyde in phosphate-buffered saline (1×, Invitrogen) for 4 h and stained overnight with crystal violet by addition of half a volume of a solution containing 0.2% crystal violet (Sigma-Aldrich) in 10% glacial acetic acid and 20% methanol. Colonies were counted after washing with water and drying. Errors bars indicate S.D. for the indicated number of averaged experiments.
Flow Cytometry Analysis
Single cell suspensions were obtained from cell culture plates by treatment with trypsin-EDTA solution (Invitrogen) and vigorous pipetting in FCS-supplemented growth medium. Cells pelleted by centrifugation were resuspended in a small volume of PBS 1× and fixed by overnight incubation in a 2-ml volume of 70% ethanol, 30% PBS solution at −20 °C. After centrifugation, cells were resuspended in PBS 1× containing 10 mm EDTA and 0.5 μg/ml RNase A and incubated at 37 °C for 2 h. Propidium iodide (0.5 μg/ml final concentration in PBS/10 mm EDTA) was then added, and cells were analyzed by cytometry after 1 h of incubation at room temperature on a FACScan cytometer (BD Biosciences). Raw data were analyzed with Weasel v3.0 software with the curve data fit function.
RESULTS
Cloning Efficiency of Prostate Cancer Cells Is Inversely Correlated to Osmotic Pressure of the Culture Medium
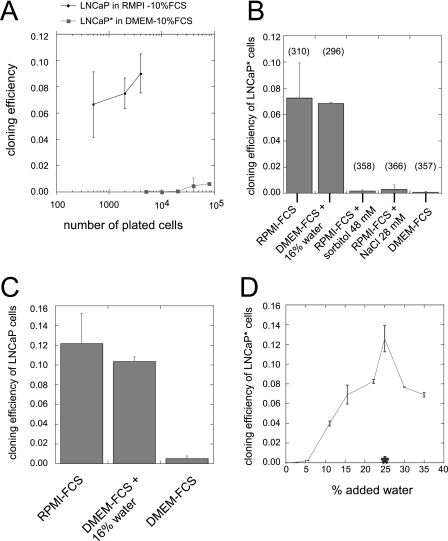
In the course of analyzing the in vitro growth potential of prostate cancer cell lines, we used the LNCaP* cells, a cell population derived from LNCaP cells by transfection with a vector expressing the ecotropic receptor for murine leukemia retroviruses (pBabe-Phleo-EcoR) followed by phleomycin selection. At variance with the parental LNCaP cells that were routinely passaged in RPMI medium supplemented with 10% fetal calf serum (RPMI-FCS), LNCaP* cells were cultured in Dulbecco's modified Eagle's medium containing 4500 mg/liter glucose and supplemented with 10% fetal calf serum (DMEM-FCS). We observed that LNCaP* cells cultured in DMEM-FCS had a markedly reduced cloning efficiency when compared with LNCaP cells grown in RPMI-FCS (Fig. 1A). Indeed, the cloning efficiency of LNCaP cells seeded at 5 × 103 cells per 5-cm plate varied between 0.07 and 0.1, depending upon the experiment. By contrast, the cloning efficiency of LNCaP* was around 2 × 10−4, more than 2 orders of magnitude lower. We investigated whether the choice of growth medium could account for this difference in cloning efficiency. First, we observed that the cloning efficiency of LNCaP cells strongly decreased in DMEM-FCS, whereas the cloning efficiency of LNCaP* cells reached levels similar to that of LNCaP in RPMI-FCS (Fig. 1, B and C). Thus, although there was little difference in the intrinsic cloning efficiencies of LNCaP and LNCaP* cells, they were strongly dependent upon the culture medium. RPMI 1640 and DMEM differ substantially in their composition, and their predicted osmotic pressures are 310 and 363 mOsm (per liter), respectively. To test whether osmotic pressure could influence cloning efficiency, we manipulated the osmotic pressure of both growth media. As displayed in Fig. 1, B and C, dilution of DMEM-FCS with water to an osmotic pressure of 310 mOsm (+16% water) enhanced LNCaP and LNCaP* cloning efficiency. Conversely, increasing osmotic pressure of RPMI-FCS with sorbitol or NaCl to a value similar to that of DMEM-FCS (approximately 360 mOsm) strongly decreased cloning efficiency of LNCaP* cells (Fig. 1B). Moreover, the cloning efficiency of LNCAP* cells varied continuously in response to dilution of DMEM-FCS with water (Fig. 1D). A maximum was reached with an added volume of 25% water, yielding an osmotic pressure of 270 mOsm. Importantly, dilution of DMEM-FCS with a 180 mm saline solution (Posm = 360 mOsm) had no effect on cloning efficiency (Fig. 1D).
FIGURE 1.
Cloning efficiencies of LNCaP* and native LNCaP cells are regulated by the osmotic pressure of the growth medium. A, cloning efficiency as a function of the number of plated cells per 9-cm plate. Native LNCaP cells were cultured in RPMI-10% FCS, whereas LNCaP* cells were cultured in DMEM-FCS. B, regulation of the cloning efficiency of LNCaP* cells by osmotic pressure. 104 LNCaP* cells were plated in a 9-cm-diameter culture plate with the indicated growth medium. The number in parentheses indicates the osmotic pressure (mOsm) of the corresponding medium. C, regulation of the cloning efficiency of LNCaP by osmotic pressure. 4 × 103 cells were plated in a 9-cm-diameter culture plate with the indicated growth medium as in B. D, dilution of DMEM-FCS with water increases the cloning efficiency of LNCaP* cells. The final percentage of added water is indicated in the abscissa and cloning efficiency in the ordinate. Data are averages of two independent experiments. The asterisk in D indicates the cloning efficiency measured after a 25% dilution of DMEM-FCS with a 180 mm saline solution (360 mOsm).
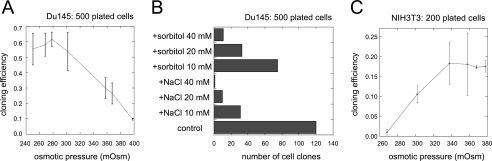
The effect of osmotic pressure on cloning efficiency was also examined with a different prostate cancer cell line, Du145, which is androgen-independent and more tumorigenic. Although Du145 cells were routinely cultured in DMEM-FCS, they exhibited a high cloning efficiency under these conditions (0.2–0.3, Fig. 2A). This difference was in line with the idea that the tumorigenic and metastatic potentials are reflected in the cloning efficiency in vitro. Nonetheless, decreasing the osmotic pressure of the growth medium by adding water further increased the cloning efficiency (Fig. 2A), a maximum being reached at around 270 mOsm, as for LNCAP*. Conversely, increasing the osmolarity of DMEM with sodium chloride or sorbitol reduced the cloning efficiency (Fig. 2, A and B). As illustrated with NIH3T3 fibroblasts (Fig. 2C), not all cell lines exhibited a maximal cloning efficiency at low osmotic pressure. Quite to the contrary, the cloning efficiency of NIH3T3 cells increased more than 10-fold when the osmotic pressure was raised from 270 mOsm to 360 mOsm.
FIGURE 2.
Cloning efficiency of Du145 prostate cancer cells and NIH3T3 fibroblasts is differentially regulated by osmotic pressure. A, cloning efficiency of Du145 cells in function of osmotic pressure. 500 Du145 cells were plated in a 9-cm-diameter plate with DMEM-FCS supplemented with water or 5 m NaCl up to the indicated osmotic pressure. B, increasing osmotic pressure of DMEM-FCS decreases Du145 cloning efficiency. Osmotic pressure was increased by addition of sorbitol (20 mm sorbitol = 20 mOsm) or NaCl (10 mm NaCl = 20 mOsm). C, cloning efficiency of NIH3T3 cells is maximal in hypertonic growth medium. Experimental conditions were as in A, except that only 200 cells were seeded per 9-cm-diameter culture plate. A and C are the average of two experiments. B is one representative experiment from a set of two independent experiments.
Hypertonic Medium Rapidly Induces Quiescence of Prostate Cancer Cells at Clonal Cell Density
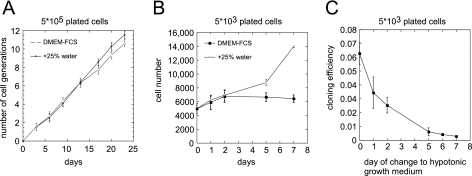
Osmotic pressure could act on the proliferation of LNCaP* cells or, more specifically, on their cloning potential. When regularly passaged at high density (at 5 × 105 cell per 5-cm plate) for 23 days, LNCaP* cells grew equally well in DMEM-FCS as in DMEM-FCS + 25% water (Fig. 3A). Thus, the effects of osmotic pressure on cell proliferation were restricted to cells plated at low cell density (clonogenic conditions). This prompted us to investigate in more detail the behavior of cells plated at low cell density in hypertonic medium (DMEM-FCS). Microscopic examination revealed that although some cell divisions occurred during the first 3 days, the number of cells subsequently remained constant or slowly decreased toward the end of the assay (14–16 days). By usual criteria, such as trypan blue exclusion, these cells were still alive (data not shown and see below). By contrast, when plated at low density in hypotonic medium (DMEM-FCS + 25% water), a subset of cells regularly divided so that by the seventh day of culture the difference between cells plated in DMEM-FCS and DMEM-FCS + 25% water was conspicuous. To quantitate these observations, cells were numerated after trypsinization at different times after plating. As shown in Fig. 3B, these data confirmed that during the first few days there was no major difference between the two growth conditions, but that afterward a net increase in cell number was observed only at low osmotic pressure. On average, the total number of cells doubled during the first 5–6 days. By contrast, in hypertonic medium, the number of living cells remained close to the number of seeded cells during the first week. Beyond 7 days, cell death occurred in hypertonic medium, bringing more variability to the data (by day 10, the number of cells was about 40% of that initially seeded).
FIGURE 3.
High osmotic pressure inhibits cell growth only at low cell density. A, active growth of LNCaP* cells plated at high cell density in hyperosmotic medium. Cells were passaged every 3–4 days at 5 × 105 cells per 5-cm-diameter culture plate in DMEM-FCS or DMEM-FCS + 25% water. Cumulative numbers of cell generations are displayed in function of days in culture. B, survival of LNCaP* cells plated at low cell density in hypertonic DMEM-FCS medium. Cells were plated at 5000 cells per 5-cm-diameter culture plate in DMEM-FCS or DMEM-FCS + 25% water. At the indicated times, adherent cells were harvested by trypsin treatment and counted with a Nageotte hemocytometer under a phase contrast microscope. During the 7 days of culture, cells with abnormal morphology (e.g. of very small size) were only seen occasionally. This growth curve is the average of two experiments, each performed in duplicates. C, exposure to high osmotic pressure decreases the cloning efficiency in permissive conditions. 5000 LNCaP* cells per 5-cm culture plate were incubated in DMEM-FCS for the indicated period before changing the growth medium to DMEM-FCS + 25% water. Macroscopic colonies were numbered 14 days thereafter. Data are averages of three independent experiments.
This survival of LNCaP* cells plated at low cell density in DMEM-FCS led us to investigate whether they could resume growth when the medium was changed to a hypotonic one (DMEM-FCS + 25% water). Remarkably, a few days of exposure to hypertonic medium was sufficient to strongly reduce the number of colonies growing in hypotonic conditions. This reduction was 2-fold after 24 h and 10-fold after 5 days (Fig. 3C). As the cells were still alive but not able to proliferate, this indicated that a stable quiescence was rapidly induced by culture in DMEM-FCS at low cell density.
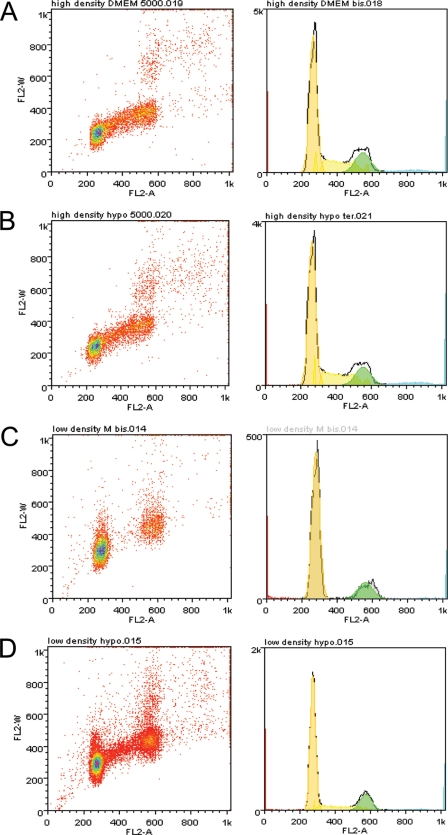
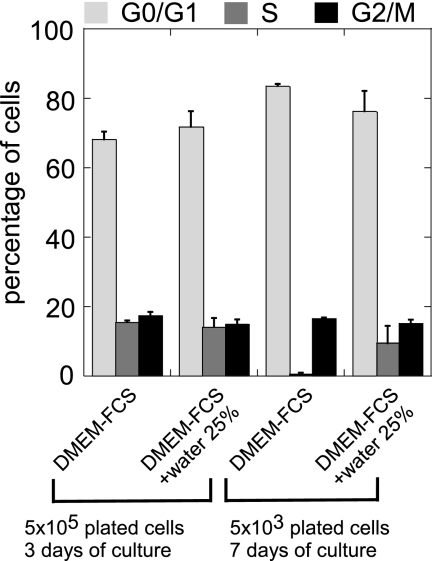
To characterize this quiescent state, we first analyzed the cell cycle distribution of LNCaP* cells by flow cytometry. When cells were grown at high density, similar distributions within the cell cycle were observed in hyper- or hypotonic medium (DMEM-FCS or DMEM-FCS + 25% water, Figs. 4, A and B and 5). When plated at low density and kept for 7 days in hypotonic medium, cells were actively growing and distributed among the different phases of the cell cycle, although the percentage in S phase was smaller than for cells at high density (9% versus 15%), in agreement with an active proliferation of some of the initially seeded cells. By contrast, cells plated at low density and kept in hypertonic medium were almost completely excluded from the S-phase (0.3%, Fig. 4C and 5) and were distributed between the G0/G1 and G2/M phases. Together, these data suggested that the quiescent state induced at low cell density in DMEM-FCS results from a blockage of cells in the G0/G1 or G2/M phase of the cell cycle.
FIGURE 4.
Cell cycle analysis of LNCaP* cells grown under different conditions. LNCaP* cells under different growth conditions were analyzed by flow cytometry after propidium iodide staining. A, high cell density in DMEM-FCS (5 × 105 cells were seeded per 5-cm plate and grown for 3 days). B, high density in DMEM-FCS + 25% water (same protocol as in A). C, low density in DMEM-FCS (5 × 103 cells were seeded per 5-cm plate and grown for 7 days). D, low density in DMEM-FCS+ 25% water (same protocol as in C). The left panels are dot plots with pulse area (FL2-A) in the abscissa versus pulse width (FL2-W) fluorescence in the ordinate. The right panels are the corresponding histograms of cell cycle distribution with FL2-A in the abscissa.
FIGURE 5.
Effect of osmotic pressure and cell density on cell cycle distribution of LNCaP* cells. These histograms are derived from the quantification of two independent experiments performed and analyzed as in Fig. 4.
Relief of Dormancy by Small Molecular Weight Compounds
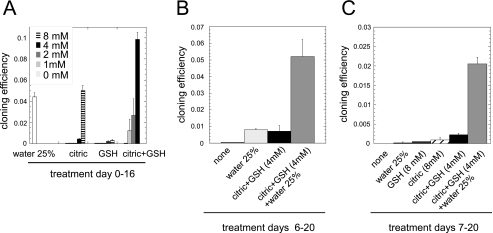
Because changing the medium to a hypotonic one was not enough to efficiently rescue cells from quiescence, we searched for additional stimuli that could do so. When LNCaP* cells were seeded at low density in DMEM-FCS medium conditioned by prostate cancer cells maintained at high density, the cloning efficiency was comparable with those observed in RPMI-FCS. As this activity could be recovered in the small molecular weight fraction obtained by ultrafiltration with a 2-kDa exclusion limit (see supplemental Fig. S1), we screened various small molecular weight metabolites for their ability to stimulate clonal cell growth of LNCaP* in DMEM-FCS. This approach enabled us to identify citric acid as a potent inducer of clonal cell growth in hypertonic medium (Fig. 6A). The enhancement of cloning efficiency achieved with 8 mm citric acid was comparable with that observed by the optimal dilution of DMEM-FCS (25% water). This activity was shared by sodium citrate but not fumaric or maleic acids (data not shown). Although GSH alone had little effect on the cloning efficiency, a strong synergy was observed at 4 mm of each compound (Figs. 6A and 8), equaling or exceeding the maximal response generated by dilution of DMEM.
FIGURE 6.
Citric acid and glutathione cooperate with low osmotic pressure to reverse cell dormancy. A, prevention of dormancy by citric acid and glutathione. Cloning efficiency of LNCaP* cells was measured in DMEM-FCS, supplemented as indicated (5000 cells were seeded per 5-cm dish and cultured for 16 days). Diagrams are derived from two independent experiments. B, reversion of dormancy after a 6-day exposure to DMEM-FCS. Cloning efficiency of LNCaP* cells were measured in DMEM-FCS, supplemented as indicated, after a 6-day exposure to DMEM-FCS (same culture conditions as in A). C, reversion of dormancy after a 7-day exposure to DMEM-FCS. The culture conditions were as in B, except for the 7-day period in DMEM-FCS.
FIGURE 8.
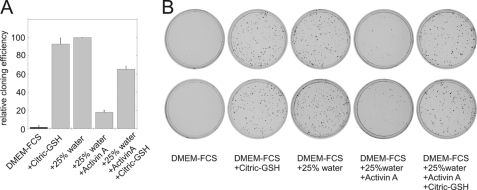
Inhibition of cloning efficiency by activin A. LNCaP* cells (1000 cells per 5-cm plate) were plated in DMEM-FCS, supplemented as indicated. Activin A was added at 50 ng/ml, citric acid and glutathione at 4 mm, and water at 25%. A, graphic representation of two averaged independent experiments. Cloning efficiencies were normalized to that observed in 25% water (taken as 100%). B, cell culture plates from one representative experiment stained with crystal violet.
These data indicated that citric acid and glutathione could prevent the entry into quiescence. To test whether these compounds could reverse the quiescence induced by DMEM-FCS at low cell density, cells were plated at low cell density in DMEM-FCS and cultured for 6 days before changing the growth conditions for another 14 days. In this assay, citric acid plus glutathione (4 mm each) rescued cells from quiescence to the same extent as DMEM-FCS + 25% water (Fig. 6B). Importantly, the association of both treatments produced the same number of colonies as the direct plating in hypotonic medium, indicating a complete reversion of quiescence (compare Fig. 6B to Figs. 6A and 1B). Similar results were obtained when treatments were operated on the 7th day after plating (Fig. 6C), whereas at this stage changing to an hypotonic medium alone was insufficient to resume cell growth. Thus, the quiescent state induced by exposure to a hypertonic medium was both stable (i.e. changing growth conditions to optimum hypotonic medium did not reversed it in a majority of cells) and fully reversible by additional stimuli, and therefore qualified as a dormant state.
Modulation of Cell Dormancy by the p53 Tumor Suppressor and smad Signaling Pathways
We have shown above that some of the dormant cells are blocked in the G2 phase of the cell cycle. Such a blockage in G2 was reminiscent of the action of the p53 tumor suppressor (8) and/or of growth factors of the TGFβ family (9). To investigate the role of these pathways, LNCaP* cells were infected with retroviral vectors (see “Experimental Procedures”) expressing p53 alleles carrying dominant negative mutations or smad7, an inhibitory smad that blocks the action of smads activated by receptors of the TGFβ family cytokines (10). Cell populations were derived upon antibiotic selection and expression of the transduced gene verified by transient transfections of luciferase reporter genes for p53 activity and by Western blot analysis for smad7 expression (supplemental Fig. S2). We measured both cloning efficiency of the cells in hypertonic (DMEM-FCS) or hypotonic (DMEM-FCS +25% water) medium and the reversibility of dormancy following a transient exposure to hypertonic medium. Notably, control populations (infected with an empty vector) had about a 5-fold higher cloning efficiency than the initial LNCaP* cells (reaching 0.1%). Thus, the selection procedure of infected cells induced or selected for slightly altered growth properties with a less efficient induction of dormancy by exposure to DMEM-FCS (compare Figs. 6 and 7). Nonetheless, the dynamic range of the assay remained sufficient (a difference of 2 orders of magnitude in the cloning efficiency between restrictive and permissive conditions) to test for the entry into and rescue from dormancy.
FIGURE 7.
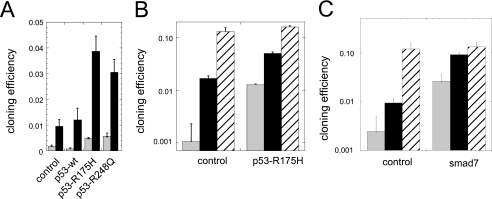
Modulation of cell dormancy by the p53 and smad pathways. Cloning efficiency was measured on LNCAP* cells selected after infection with the indicated retroviral expression vectors. Gray bars, cloning efficiency in DMEM-FCS (4000 cells per 5-cm plate incubated for 16 days; black bars, rescue from dormancy by DMEM-FCS + 25% water after exposure to DMEM-FCS (cells were cultured for 6 (p53) or 7 (smad7) days in DMEM-FCS followed by a 12- to 14-day culture period in DMEM-FCS + 25% water); hatched bars, cloning efficiency in DMEM-FCS + 25% water (cells were directly plated in DMEM-FCS + 25% water and incubated for 16 days). A, cloning efficiency of wild-type or mutated p53 transduced cell populations assayed 10 days after thawing. Results of one representative experiment are shown. In total, four control, one p53-wt, two p53-R175H, and one p53-R248Q independent cell populations were assayed in duplicate with similar results. B, cloning efficiency of p53-R175H-transduced cells assayed 5 weeks after thawing. Shown is the average of two experiments conducted after passaging cells for about 5 weeks in DMEM-FCS. Two independently infected cell populations for both control and p53-R175H-infected cells, in duplicate, were assayed as in A. C, cloning efficiency of control and smad7-transduced cells. Data are the average of two independent experiments. Two independently infected cell populations for both control and smad7-infected cells, in duplicate, were assayed.
Expression of the p53 alleles carrying R175H or R248Q dominant negative mutations, but not of wild-type p53, increased the cloning efficiency in DMEM-FCS 3-fold over the control (Fig. 7A). These mutated p53s also increased 4-fold the escape of dormancy after a 6-day exposure to DMEM-FCS. Interestingly, the cloning efficiency in DMEM-FCS of cells transduced with mutated p53 alleles increased rapidly upon passaging in DMEM-FCS, reaching or exceeding 1% after a few weeks (up to 10-fold over the control, Fig. 7B). Over this period, the cloning efficiency of control populations in DMEM-FCS was unchanged. It is noteworthy that in hypotonic medium the cloning efficiency of the different cell populations were similar (13 and 16%, respectively), suggesting a specific modulation of the dormant phenotype instead of a global enhancement of cloning efficiency by dominant negative p53.
Ectopic expression of smad7 increased cloning efficiency in DMEM-FCS by 10-fold over control (Fig. 7C). Moreover, smad7 expression also increased about 10-fold over control the escape of dormancy induced by a 7-day exposure to DMEM-FCS. In fact, the cloning efficiency measured under these conditions nearly reached that of cells directly plated into hypotonic medium (Fig. 7C). Interestingly, as for dominant negative p53, ectopic expression of smad7 did not significantly increase the cloning efficiency of cells directly plated into hypotonic medium, suggesting a specific action on the dormancy mechanisms. Moreover, the cloning efficiency of smad7-transduced cells did not increase upon cell passaging, in contrast with the phenotypic drift observed with p53 inactivation.
These observations with smad7 suggested an implication of cytokines of the TGFβ family in the establishment and/or maintenance of dormancy induced by hypertonicity. Accordingly, we tested whether some members of this family could induce a dormant state. We found that addition of 25 ng/ml of activin Α (data not shown) or 50 ng/ml (Fig. 8, A and B) reduced about 6-fold the cloning efficiency of LNCaP* in hypotonic growth medium. Although activin A was less effective than hypertonicity in reducing the number of clones, it should be noted that the observed clones had a very small size (Fig. 8B) indicating that a single dose of exogenous activin A profoundly reduced the growth potential of LNCAP* cells. This inhibition by activin A could be partly reversed by addition of citric acid plus glutathione as in our initial observations with DMEM-FCS (Fig. 8). Taken together, these results implicate the TGFβ signaling pathway in the establishment of dormancy by hypertonicity.
DISCUSSION
Induction of a Dormant State in Vitro
In this study we show that the cloning efficiency of prostate cancer cells is very sensitive to the osmotic pressure of the growth medium. Thus, replacing RPMI by DMEM or adjusting their osmotic pressures reduces the cloning efficiency by more than 2 orders of magnitude (Fig. 1). Moreover, exposure to a hypertonic growth medium, like DMEM + 10% FCS (DMEM-FCS), induces a dormant state in prostate cancer cells within a few days. This state is characterized by a long-term survival of dispersed cells with no significant cell death taking place over 7 days (Fig. 3B). These cells are mostly blocked in the G0/G1 and, to a lesser extent, in the G2/M phases of the cell cycle (Figs. 4 and 5). This strong response to DMEM-FCS was unexpected because this growth medium is widely used for growing adherent cells in culture, and its osmotic pressure is nearly optimal for the cloning of NIH3T3 cells (Fig. 2A) and for the culture of primary mouse embryo fibroblasts (data not shown). In fact, LNCaP cells themselves can be easily grown in this medium if they are maintained at high cell density (Fig. 2). Moreover, in contrast with many instances of cell quiescence, this dormant state is not due to a deprivation of growth factors, as it can be prevented by simply diluting DMEM-FCS with 25% water. Thus, a hypertonic medium does not globally block cell growth (see also Ref. 11) but specifically acts on their cloning efficiency.
Stability and Reversion of the Dormant State
Importantly, the growth arrest induced by exposure to a hypertonic medium is maintained even when cells are placed in hypotonic conditions, indicating a stable phenotypic switch (Fig. 3C and 7). For this long-term quiescence to qualify as dormancy, it is critical that cells can resume their growth upon an appropriate stimulation. Taking the lead from the ability of conditioned media from several prostate cancer cell lines to prevent growth arrest (supplemental Fig. S1), we screened small metabolites for a similar activity. We identified two compounds, citric acid and glutathione, that can prevent the entry of LNCaP cells into quiescence and can also cooperate with hypotonic conditions to reverse the dormant state induced by a high osmotic pressure exposure (Fig. 6). Citric acid (or sodium citrate) is efficient at concentrations above 2 mm, which are above the concentration detected in plasma (0.1–0.2 mm) but in the range of citrate concentration in prostate tissue and far below the concentrations measured in semen (12). Citric acid has been proposed to have a key role in prostate cell metabolism (12), and we are investigating the mechanism by which it regulates prostate cancer cell dormancy. Notably, other intermediates of the Krebs cycle (fumaric or maleic acid), chelating agents (EGTA, EDTA), or activators of hypoxia-induced factor 1 (dimethyloxalylglycine or cobalt chloride) cannot substitute for citric acid in our assay (data not shown). As glutathione displayed a synergistic effect with citric acid, the activity of the low molecular weight fraction of conditioned media could result from a synergy between several metabolites present at low concentration.
The long-term survival of non-dividing LNCaP cells plated at low density in DMEM-FCS is reminiscent of the in vivo dormancy of solitary cancer cells previously observed in various models of metastasis. Indeed, a wealth of observations shows that tumor cells injected in mice can maintain themselves for long periods as solitary non-dividing cells in colonized organs (13). For instance, 3 days after injection of D2A1 or B16F1 cancer cells in the mesenteric vein, more than 70% of the injected cells survive as extravasated solitary cells in the liver parenchyma. After 10–13 days, more than 30% of the injected cells can still be detected as solitary cells (5). In experiments targeting cancer cells to the lung, 3.5% of the injected cells survived as solitary cells at day 14 post-injection, 97% of them being dormant (non-dividing) as shown by TUNEL and Ki-67 staining (14). Therefore, it has been proposed that metastatic recurrences at distant times are due to the reactivation of these dormant solitary cells (1).
Cell Signaling Modulates Dormancy
Induction of dormancy in hypertonic medium (DMEM-FCS) occurs when, and only when, cells are cultured at a very low density (compare Figs. 1A and 3A). Accordingly, we show that prostate cancer cells cultured at high cell density in DMEM-FCS secrete low molecular weight factors in the medium that can promote clonal cell growth in hypertonic medium (supplemental Fig. S1). Although additional mechanisms could be involved, a threshold effect in an autocrine/paracrine loop would be sufficient to account for the change in cloning efficiency as a function of initial cell density (Figs. 1A and 3A).
The blockage of some dormant cells in G2 was reminiscent of the action of the p53 tumor suppressor (8) and/or cytokines of the TGFβ family (9). Moreover, these pathways have been implicated in stem cell quiescence and tumor cell dormancy (15–18), and although LNCaP has a wild-type p53, cells lines with a high cloning efficiency in DMEM-FCS like Du145 and PC3 (Fig. 2 and data not shown) display p53 alterations. We did not detect an activation of p53 under our slightly hypertonic conditions in contrast with the response to an acute hypertonic stress (19) (data not shown). Nonetheless, expression of dominant negative forms of p53 increases by 3–4-fold both the cloning efficiency under hypertonic conditions and the relief from dormancy by hypotonic medium. Clonogenicity under hypotonic conditions is not affected, suggesting a specific involvement in dormancy. Notably, inactivation of the p53 pathway leads to a progressive reduction in the induction of dormancy by hypertonic medium. This suggests a rapid adaptation and/or selection of cells to hypertonicity, possibly in relation with an increased genomic instability.
Ectopic overexpression of smad7, an inhibitory smad, evoked similar but more complete responses than the inactivation of p53. Moreover, cells overexpressing smad 7 have a stable phenotype, allowing an unambiguous assessment of the role of the smad pathway. Thus, smad7 expression resulted in a 10-fold increase of cloning efficiency under hypertonic conditions, implicating this signaling pathway in the establishment of dormancy. Accordingly, addition of activin A and, to a lesser extent, TGFβ (data not shown) reduced the cloning efficiency under otherwise permissive conditions. This inhibition was antagonized by citric acid plus glutathione, like that induced by hypertonic conditions. However, smad7 and activin A only partially suppressed or mimicked the effect of hypertonic growth conditions, respectively, suggesting the implication of other signaling pathways. In support of this possibility, we have observed, using pharmacological inhibitors, that several signaling pathways (including MAPKs and PI3K, data not shown) were implicated in the establishment of a reversible cell quiescence. Thus, hypertonicity could activate and/or inhibit a combination of signaling pathways, leading to a stable dormant state. Remarkably, in association with a hypotonic medium, smad7 allowed cells to fully escape from dormancy and achieve the same cloning efficiency as if they had never been exposed to hypertonic conditions (Fig. 7C). Thus, in addition to its role in the establishment of dormancy, the smad pathway plays a major role in its maintenance.
Altogether, our data suggest a central role of the smad pathway in hypertonicity-induced dormancy. In this regard, it is noteworthy that expression of smad4, the obligatory partner of activated smads, inversely correlates with the metastatic potential of prostate cancer cells (20, 21). Similarly, the level of serum follistatin, an inhibitor of activin A, positively correlates with the presence of bone metastases in patients with prostate cancer (22). Although these observations are at odds with the ability of TGFβ-related factors to promote invasive properties in malignant tumor cells (23), they do fit with our observations on their implication in the induction of dormancy.
Relevance of Dormancy Modulation by Osmotic Pressure
In conclusion, we describe the induction of a dormant state in prostate cancer cells by exposure to a slightly hypertonic growth medium. This dormancy is similar in several respects to the in vivo cancer cell dormancy, including a stable blockage of cell division specific to dispersed cells that can be reversed by appropriate signals. The smad and p53 pathways are involved in its establishment and maintenance, suggesting common mechanisms with in vivo stem cell or cancer cell dormancy. It is important to stress that our “hypertonic conditions” are very different from those used to induce an osmotic stress. We used a range of osmotic pressures centered on isotonicity (with variations from −40 mOsm to +50 mOsm) for several days, in sharp contrast with the exposure for 15 min to a shift in osmotic pressures of 300 mOsm or more. In addition, because the induction of prostate cancer cell dormancy increases continuously from hypotonicity (270 mOsm) to hypertonicity (360 mOsm), even small changes of osmotic pressure within the physiological range could have a significant biological impact. In comparison, the threshold for thirst is 10–20 mOsm/liter above normal plasma osmolality (24), a level sufficient to impact the clonogenicity of prostate cancer cells if maintained for several days (Figs. 1 and 2).
Whether or not osmotic pressure plays a direct role in the induction of dormancy in vivo is open to question. Interestingly, the few studies that have attempted to measure the oncotic pressure (colloidal osmotic pressure) of the interstitial fluid of tumors have reported it to be comparable with that of the plasma, in contrast with that of normal tissues (25). It is therefore possible that tumors cells could be subjected to a higher osmotic pressure than normal cells. Further characterization of the pathways and of the soluble factors modulating dormancy in vitro should provide useful insights on the in vivo process of dormancy.
Supplementary Material
Acknowledgments
We thank Dr. Denis Clay for help in flow cytometry analysis, Dr. Luis Martinez for the gift of plasmids pBabepuro-p53-R248Q and Pc3m-p53R175H, Dr. Mark Pearson for the gift of plasmid pBabe puro-p53 wild-type, and Dr. Peter ten Dijke for the gift of plasmid pcDNA3-FLAG-smad7.
Footnotes

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
REFERENCES
- 1. Townson J. L., Chambers A. F. (2006) Cell Cycle 5, 1744–1750 [DOI] [PubMed] [Google Scholar]
- 2. Chaffer C. L., Weinberg R. A. (2011) Science 331, 1559–1564 [DOI] [PubMed] [Google Scholar]
- 3. Chambers A. F., Naumov G. N., Vantyghem S. A., Tuck A. B. (2000) Breast Cancer Res. 2, 400–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guba M., Cernaianu G., Koehl G., Geissler E. K., Jauch K. W., Anthuber M., Falk W., Steinbauer M. (2001) Cancer Res. 61, 5575–5579 [PubMed] [Google Scholar]
- 5. Naumov G. N., MacDonald I. C., Chambers A. F., Groom A. C. (2001) Semin. Cancer Biol. 11, 271–276 [DOI] [PubMed] [Google Scholar]
- 6. Lin A. W., Barradas M., Stone J. C., van Aelst L., Serrano M., Lowe S. W. (1998) Genes Dev. 12, 3008–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tchénio T., Havard M., Martinez L. A., Dautry F. (2006) Mol. Cell. Biol. 26, 580–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taylor W. R., Stark G. R. (2001) Oncogene 20, 1803–1815 [DOI] [PubMed] [Google Scholar]
- 9. Di K., Ling M. T., Tsao S. W., Wong Y. C., Wang X. (2006) Biol. Cell 98, 523–533 [DOI] [PubMed] [Google Scholar]
- 10. Yan X., Chen Y. G. (2011) Biochem. J. 434, 1–10 [DOI] [PubMed] [Google Scholar]
- 11. Junger W. G., Hoyt D. B., Hamreus M., Liu F. C., Herdon-Remelius C., Junger W., Altman A. (1997) J. Trauma 42, 437–445 [DOI] [PubMed] [Google Scholar]
- 12. Costello L. C., Franklin R. B. (1997) Urology 50, 3–12 [DOI] [PubMed] [Google Scholar]
- 13. Chambers A. F., Naumov G. N., Varghese H. J., Nadkarni K. V., MacDonald I. C., Groom A. C. (2001) Surg. Oncol. Clin. N. Am. 10, 243–255, vii [PubMed] [Google Scholar]
- 14. Cameron M. D., Schmidt E. E., Kerkvliet N., Nadkarni K. V., Morris V. L., Groom A. C., Chambers A. F., MacDonald I. C. (2000) Cancer Res. 60, 2541–2546 [PubMed] [Google Scholar]
- 15. Buijs J. T., Henriquez N. V., van Overveld P. G., van der Horst G., ten Dijke P., van der Pluijm G. (2007) Clin. Exp. Metastasis 24, 609–617 [DOI] [PubMed] [Google Scholar]
- 16. Liu Y., Elf S. E., Miyata Y., Sashida G., Liu Y., Huang G., Di Giandomenico S., Lee J. M., Deblasio A., Menendez S., Antipin J., Reva B., Koff A., Nimer S. D. (2009) Cell Stem Cell 4, 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salm S. N., Burger P. E., Coetzee S., Goto K., Moscatelli D., Wilson E. L. (2005) J. Cell Biol. 170, 81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamazaki S., Nakauchi H. (2009) Curr. Opin. Hematol. 16, 255–258 [DOI] [PubMed] [Google Scholar]
- 19. Dmitrieva N. I., Burg M. B. (2005) Mutat. Res. 569, 65–74 [DOI] [PubMed] [Google Scholar]
- 20. Ding Z., Wu C. J., Chu G. C., Xiao Y., Ho D., Zhang J., Perry S. R., Labrot E. S., Wu X., Lis R., Hoshida Y., Hiller D., Hu B., Jiang S., Zheng H., Stegh A. H., Scott K. L., Signoretti S., Bardeesy N., Wang Y. A., Hill D. E., Golub T. R., Stampfer M. J., Wong W. H., Loda M., Mucci L., Chin L., DePinho R. A. (2011) Nature 470, 269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu K. J., Zhang D., Zhu G. F., Cheng H. P., Zeng J., Zhang L. L., Wang X. Y., He D. L. (2009) Zhonghua Nan Ke Xue 15, 41–44 [PubMed] [Google Scholar]
- 22. Tumminello F. M., Badalamenti G., Fulfaro F., Incorvaia L., Crescimanno M., Flandina C., Sepporta M. V., Leto G. (2010) Clin. Exp. Metastasis 27, 549–555 [DOI] [PubMed] [Google Scholar]
- 23. Inman G. J. (2011) Curr. Opin. Genet. Dev. 21, 93–99 [DOI] [PubMed] [Google Scholar]
- 24. Singer G. G., Brenner B. M. (2008) in Harrison's Principles of Internal Medicine, Chapter 17, pp. 274–285, McGraw Hill, New York [Google Scholar]
- 25. Wiig H., Tenstad O., Iversen P. O., Kalluri R., Bjerkvig R. (2010) Fibrogenesis Tissue Repair 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.