Background: Polarization of cells along an anterior-posterior axis involves biogenesis of the posterior pole.
Results: Cells target proteins to the posterior pole on the basis of higher order oligomerization and plasma membrane binding.
Conclusion: The exosome/microvesicle biogenesis pathway also mediates posterior pole biogenesis.
Significance: Cells use an internal, signal-independent mechanism for posterior pole biogenesis.
Keywords: Cell Migration, Cell Polarity, Dictyostelium, Exosomes, HIV, Retrovirus, EMV, Microvesicle, Uropod, Vesicle Secretion
Abstract
Biogenesis of the posterior pole is critical to directed cell migration and other polarity-dependent processes. We show here that proteins are targeted to the posterior pole on the basis of higher order oligomerization and plasma membrane binding, the same elements that target proteins to exosomes/microvesicles (EMVs), HIV, and other retrovirus particles. We also demonstrate that the polarization of the EMV protein-sorting pathway can occur in morphologically non-polarized cells, defines the site of uropod formation, is induced by increased expression of EMV cargo proteins, and is evolutionarily conserved between humans and the protozoan Dictyostelium discoideum. Based on these results, we propose a mechanism of posterior pole biogenesis in which elevated levels of EMV cargoes (i) polarize the EMV protein-sorting pathway, (ii) generate a nascent posterior pole, and (iii) prime cells for signal-induced biogenesis of a uropod. This model also offers a mechanistic explanation for the polarized budding of EMVs and retroviruses, including HIV.
Introduction
Cell polarity is generated by the polarized sorting of proteins and lipids (1, 2) and is required for directed cell migration, epithelial function, organ morphogenesis, and development (1–9). In mammalian leukocytes and social amoeba (e.g. Dictyostelium discoideum), polarized protein sorting must be particularly robust, as these cells target distinct sets of molecules to anterior and posterior poles, do so in the absence of any obvious barriers to lipid and protein diffusion, and switch rapidly between morphologically non-polarized and highly polarized states (2, 3, 10).
Although the mechanisms of directed cell migration are still under investigation, much has been learned about how proteins are selectively enriched at the anterior pole (leading edge) of rapidly migrating cells (2, 3, 8, 9). In both mammalian leukocytes and D. discoideum, exposure to chemoattractants activates G-protein-coupled receptors, leading to a localized accumulation of phosphatidylinositol (3,4,5)-trisphosphate (PIP3)2 in the inner leaflet of the plasma membrane (PM), proximal to the highest extracellular concentration of chemoattractant (2, 3, 11, 12). This leads to the selective trafficking of PIP3-binding effector molecules to the anterior pole of the cell (13, 14). Ras is also activated at the leading edge, and this recruits additional proteins to the anterior pole (2, 15–19).
Stimulation of chemoattractant receptors also induces the biogenesis of a uropod at the posterior pole of the cell, via a signaling cascade involving the Rho family GTPase RhoA (20, 21). The uropod is a large protrusion (>1 μm) that mediates cell retraction during chemotaxis and plays key roles in signal transduction and endocytosis (22). Numerous proteins are selectively targeted to uropods, and in mammalian cells these include PM proteins (e.g. CD43, CD44, and others) and cytosolic scaffolding proteins (e.g. ezrin, radixin, and moesin (ERM proteins)) that bind to filamentous actin and generate highly oligomeric, PM-anchored protein complexes (22, 23). Although much is known about the molecular composition and function of the uropod, there is as yet no general paradigm for how cells establish a posterior pole, selectively target proteins to this pole, or convert it into a functional uropod.
Here we report on the relationship between cell polarity and the protein-sorting pathway that targets proteins to secreted vesicles (exosomes and microvesicles, or EMVs). EMVs are small vesicles (∼50 to 250 nm diameter) that bud from animal cells and transmit signals, proteins, lipids, and nucleic acids to neighboring cells (24–34). In T cells and several other leukocytes, it has been shown that EMV proteins, lipids, and their bound carbohydrates bud from discrete domains of the plasma membrane (30, 35–38). This differential sorting of proteins and lipids to distinct domains of the plasma membrane is reminiscent of cell polarity, and we show here that the EMV protein-sorting pathway plays several important roles in the biogenesis of the posterior pole.
EXPERIMENTAL PROCEDURES
Cell Lines, Culture Conditions, and Transfections
CCRF-CEM and Hsb-2 T cells were obtained from ATCC and cultured in RPMI medium (Invitrogen/BRL) containing 10% fetal calf serum and penicillin/streptomycin. HL60 cells were obtained from ATCC and cultured in Iscove's modified Dulbecco's medium containing 20% FBS and penicillin/streptomycin. For differentiation of HL60 cells, 190 μl of DMSO was added to 106 cells in 15 ml of Iscove's modified Dulbecco's medium containing 20% FBS for a period of 5–7 days. Jurkat T cells were a gift of Dr. James Hildreth (Meharry Medical College) and were grown in serum-free medium (AIM-V, Invitrogen/BRL). Transfections of CCRF-CEM, Hsb-2, Jurkat T cells, and HL60 cells were by nucleofection as recommended by the manufacturer (Amaxa Technologies).
D. discoideum cells (AX2 strain) were cultured in HL5 medium at 22 °C. The D. discoideum cell lines expressing EIAV Gag-GFP or HIV Gag-GFP were generated by electroporation of AX2 cells with the plasmids pCV5/EIAV Gag-GFP or pCV5/HIV Gag-GFP, followed by selection on G418-containing medium for 7–10 days (39). G418-resistant colonies were collected, and expressing clones were identified by fluorescence microscopy. D. discoideum cells stably expressing EIAV Gag-GFP or HIV Gag-GFP were grown in HL5 medium containing 20 μg/ml G418.
Reagents
N-Rh-PE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl)) and N-F-PE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(carboxyfluorescein)) were obtained from Avanti Polar Lipids. Antibodies specific for CD43, pERM proteins, CD44, and ICAM-2 were obtained from commercial sources. The antibody to myosin II was a generous gift from J. Yang (Johns Hopkins University). The fMLP peptides, DAPI, and Y-27632 were from Sigma.
Plasmids
The HIV Gag-GFP and HIV Gag expression vectors were described previously (35), as were the plasmids designed for mammalian cell expression of EIAV Gag-GFP, MLV Gag-GFP, and HTLV-1 Gag-GFP TyA-GFP, AcylTyA-GFP, AcylTyA, HIV Gag(p49)-GFP, HIV Gag(p41)-GFP, HIV Gag(p41)-LZ-GFP, HIV Gag(p41)-p6-GFP, HIV Gag(p39*)-GFP, HIV Gag(p39*)-LZ-GFP, HIV Gag(p39*)-LZ-DsREDm, HIV Gag(p39*)-LZ-DsRED, HIV Gag-DsRED (30), and for HIV Gag(G2A)-GFP, MusD-GFP, AcylMusD-GFP, AKT-TyA-GFP, AKT-GFP, CD43-GFP, and CD43-TyA-GFP (35). To make the Lys-282 → Asn, Arg-283 → Gly, and Arg-284 → Gly mutations (40) in the CD43-GFP ORF, we amplified the CD43 ORF with sets of nested primers designed to generate the desired mutations, and then cloned the mutant CD43 ORF between the Asp-718 and BamHI sites of pcDNA3-GFP. The same fragment was amplified using primers designed to append HindIII and Asp-718 sites on the 5′ and 3′ ends of the ORF, respectively, and cloned between the HindIII and Asp-718 sites of pcDNA3-TyA-GFP (35).
The plasmid designed to express centrin-DsREDm was synthesized by amplifying the ORF of human centrin (41) using oligonucleotides that appended an Asp-718 site immediately upstream of the start codon and a BamHI site in place of its stop codon, cleaving the product with Asp-718 and BamHI, and cloning the resulting fragment between the Asp-718 and BamHI sites of pcDNA3-DsREDm. The CFP-Golgi expression vector was from Clontech.
To express EIAV Gag-GFP and HIV Gag-GFP in D. discoideum, we amplified the ORF with a ribosome binding site (AAATAAAA) upstream of the start codon, and Asp-718 and BamHI sites on the 5′ and 3′ ends of the fragment, respectively. These were then inserted between the Asp-718 and BamHI sites of pcDNA3-GFP to generate intermediate plasmids. The Gag-GFP ORFs were then excised with Asp-718 and XhoI and cloned between the Asp-718 and XhoI sites of a modified form of pCV5 with unique Asp-718 and XhoI sites downstream of the promoter.
Lipid Labeling, Antibody Labeling, and Microscopy
Lipid labeling was carried out by chilling cells to 4 °C, incubating them with N-Rh-PE or N-F-PE (1 μm final concentration) at 4 °C, washing the cells three times with PBS, then incubating the cells in growth medium for 14–24 h at 37 °C. DAPI labeling was as described previously (35). Antibody labeling of cell surface proteins CD43, CD44, CD63, and ICAM2 was performed by fixing cells in 4% p-formaldehyde/PBS, incubating them with unlabeled primary antibodies, washing extensively in PBS, incubating cells with fluorescently labeled anti-mouse IgG antibodies, then washing again in PBS. Antibody labeling of pERM was carried out by fixing cells in 4% p-formaldehyde/PBS, permeabilizing the cells with 1% Triton X-100/PBS, then incubating the cells with unlabeled primary antibodies/PBS, washing the cells in PBS, incubating the cells with fluorescently labeled polyclonal secondary antibodies, washing again with PBS, then examining the cells by fluorescence and phase-contrast microscopy. For lipid-labeled cells, the samples were fixed in 4% p-formaldehyde/PBS, washed in PBS, and then examined by fluorescence and phase-contrast microscopy. Microscopy was performed at 20–25 °C using a fluorescence microscope (BH-2, Olympus) equipped with a 60× numerical aperture 1.4 lens. Images were captured with a charge-coupled device camera (Sensicam QE, Cooke) linked to a Mac G4 computer using IPlab software (Scanalytics). In all experiments we examined >20 expressing cells, and the EMV cargoes were sorted to the uropod in >90% of the cells.
Migrating cells were examined using an inverted Zeiss fluorescence microscope (LSM 510 Meta, Carl Zeiss MicroImaging, Inc.) with a 63× numerical aperture 1.4 lens and dedicated imaging software (LSM image browser, Carl Zeiss MicroImaging, Inc.). Chemotaxis was initiated by exposure of differentiated HL60 cells to 10 μm fMLP peptide, or by exposure of differentiated D. discoideum cells to 1 μm cAMP, each introduced through a micropipette.
Immunogold surface labeling of Jurkat T cells was performed essentially as described (42). In brief, cells were incubated at 4 °C with anti-CD43 monoclonal antibody/PBS for 1 h, washed with PBS at 4 °C, incubated at 4 °C with polyclonal rabbit anti-mouse IgG antibodies in PBS, then incubated in growth medium for 1 h at 37 °C. Cells were then fixed for 1 h at room temperature in 2% p-formaldehyde in 100 mm PO4, pH 7.4, washed, blocked in 10% fetal calf serum in 100 mm PO4, pH 7.4, for 20 min, and incubated overnight with donkey anti-rabbit IgG 18-nm gold conjugate, washed, and fixed a second time for 1 h in 2% glutaraldehyde, 2.5% sucrose (w/v), and 100 mm cacodylate, pH 7.4. The cells were spun into a tight pellet, fixed in Palade's osmium for 1 h, en bloc stained overnight in Kellenberger's uranyl acetate, dehydrated through a graded series of ethanol, and embedded in Epon. 80 nm Ultrathin sections were cut on an ultramicrotome (model UCT, Leica) collected onto 400-mesh high transmission nickel grids, post-stained in uranyl acetate and lead citrate, and observed on a transmission electron microscope (EM 410, Philips). Transmission electron microscopy was performed using the same procedure except that antibody incubations were omitted.
Higher Order Oligomerization of CD43
For antibody-induced, higher order oligomerization of CD43, cells were brought to 4 °C and incubated with FITC-conjugated primary monoclonal antibodies specific for CD43, in PBS, for 1 h at 4 °C. Cells were washed in PBS at 4 °C, then either incubated in PBS at 4 °C for 1 h or in PBS containing polyclonal rabbit anti-mouse IgG antibodies for 1 h, washed, all at 4 °C, and then incubated in growth medium for 1 h at 37 °C to allow CD43 trafficking to occur.
RESULTS
EMV Cargoes Are Sorted to the Posterior Pole of Human T Cells
EMV cargoes are targeted to discrete domains of the plasma membrane in morphologically non-polarized T cells (30, 35, 38). However, there is a distinct heterogeneity in regard to the distribution of EMV budding sites in these cells, with most cells (∼70%) possessing several EMV budding sites dispersed over the cell surface, and a smaller percentage (∼25%) clustering their EMV budding sites at a single pole (35). The occasionally polarized distribution of EMV cargoes in morphologically non-polarized leukocytes raised the possibility that the EMV protein-sorting pathway might play a role in cell polarity. We explored this idea by following the distribution of EMV test proteins in morphologically polarized T cells, primarily the Hsb-2 and CEM T-cell lines.
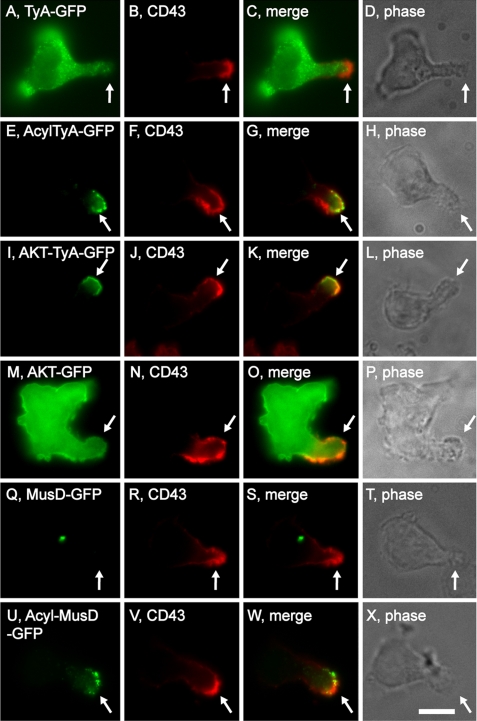
We previously developed matched pairs of EMV test proteins, such as (i) the yeast TyA protein, a highly oligomeric, cytoplasmic protein, and (ii) AcylTyA, a PM-anchored derivative that is secreted from the cells in EMVs (30, 38). GFP-tagged forms of these two proteins were expressed in the polarized T-cell lines Hsb-2 and CEM cells, and localized relative to the uropod marker proteins CD43 and CD44 (22, 43). TyA-GFP accumulated in the cytoplasm of Hsb-2 T cells (Fig. 1, A–D). In contrast, AcylTyA-GFP was selectively targeted to their uropod (Fig. 1, A–H), indicating that the EMV protein-sorting pathway might play a role in protein trafficking to the posterior pole. Similar results were observed in CEM T cells (supplemental Fig. S1, A–H).
FIGURE 1.
Polarized T cells target EMV test proteins to their posterior pole. Fluorescence and phase microscopy images of Hsb-2 T cells that had been transfected with plasmids designed to express various EMV test proteins, incubated 6–8 h, fixed, and stained with antibodies specific for the uropod marker protein CD43. A–D, TyA-GFP; E–H, AcylTyA-GFP; I–L, AKT-TyA-GFP; M–P, AKT-GFP; Q–T, MusD-GFP; and U–X, AcylMusD-GFP. Arrows denote the position of the uropod. Bar, 10 μm.
To determine whether the posterior pole trafficking of AcylTyA-GFP was due to its specific type of membrane anchor, or whether other PM anchors would target TyA-GFP to uropods, we followed the sorting of AKT-TyA-GFP, which is secreted from the cell in EMVs and binds the PM via a PIP3-binding domain (from the protein kinase AKT) (38). AKT-TyA-GFP was highly enriched at the uropod of both Hsb-2 cells (Fig. 1, I–L) and CEM T cells (supplemental Fig. S1, I–L), providing additional evidence that EMV cargoes are targeted to the posterior pole. These results also show that proteins can be targeted to the uropod by very different types of PM anchors. The enrichment of AKT-TyA-GFP at the uropod is unlikely to reflect the relative distribution of PIP3 in the PM, because PIP3 is typically enriched at the anterior pole (3, 11), the PIP3 phosphatase phosphatase and tensin homolog is highly enriched at the uropod (44), and an AKT-GFP fusion protein is not enriched at the uropod of Hsb-2 cells (Fig. 1, M–P).
Another pair of EMV test proteins are (i) MusD-GFP, which accumulates in intracytoplasmic protein particles, and (ii) AcylMusD-GFP, which is sorted to sites of EMV budding and secreted from the cell in vesicles (38). Following their expression in Hsb-2 T cells, we observed that MusD-GFP accumulated in intracellular particles, whereas AcylMusD-GFP was localized to their uropod (Fig. 1, Q–X). Similar results were observed in CEM T cells (supplemental Fig. S1, M–T), and together, these results provide additional evidence that highly oligomeric, cytoplasmic proteins can be targeted to the uropod by a PM anchor.
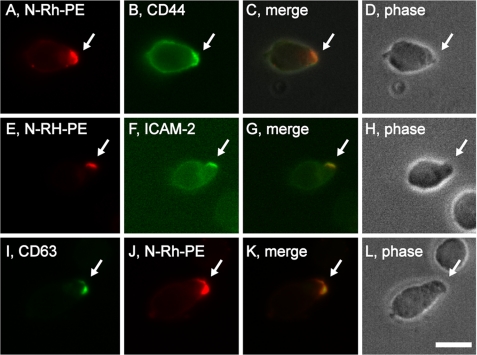
We also followed the subcellular distribution of classic exosomal markers. Many cells selectively target N-modified forms of phosphatidylethanolamine (PE) to sites of EMV budding and secrete them from the cell in EMVs (35, 45). We therefore pulse-labeled CEM T cells with the EMV lipid marker N-Rh-PE (a rhodamine-labeled form of PE), incubated them overnight, and stained them with antibodies to uropod marker proteins. CEM T cells selectively targeted N-Rh-PE to uropods where it co-localized with both CD44 (Fig. 2, A–D) and ICAM-2 (Fig. 2, E–H). N-Rh-PE-labeled CEM cells were also stained for the exosomal marker protein CD63 (26), and it too was highly enriched at uropods (Fig. 2, I–L). Taken together, these data demonstrate that the EMV protein-sorting pathway targets proteins to the uropod of polarized T cells.
FIGURE 2.
Polarized T cells target exosome lipids and proteins to the posterior pole. Fluorescence and phase microscopy images of CEM T cells that had been pulse-labeled with N-Rh-PE, incubated overnight, then fixed and stained with antibodies specific for: A–D, CD44; E–H, ICAM-2; or I–L, CD63. Bar, 10 μm.
The EMV Protein-sorting Pathway Can Drive the Polarized Sorting of the Uropod Protein CD43
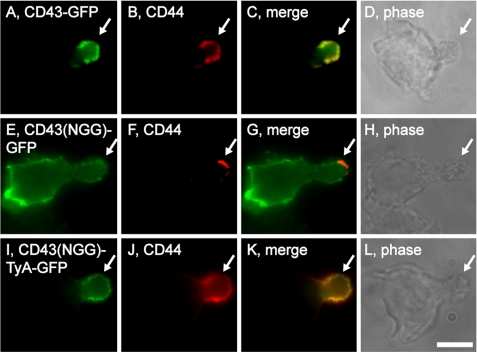
The polarized sorting of CD43 is modeled as a simple consequence of CD43 binding to ERM proteins (22, 23). Consistent with this hypothesis, we found that CD43-GFP was localized to the uropod of Hsb-2 cells (Fig. 3, A–D), whereas the CD43(NGG)-GFP protein, which carries a triple mutation that disrupts ERM-CD43 binding (Lys-282 → Asn, Arg-283 → Gly, and Arg-284 → Gly (40, 46), abbreviated here as NGG), was not enriched at the uropod and instead was distributed over the entire PM (Fig. 3, E–H). To test whether CD43(NGG)-GFP could be redirected back to the uropod by the EMV pathway, we fused it to the highly oligomeric cytoplasmic protein TyA, which targets CD43-GFP into the EMV protein-sorting pathway (38). The resulting protein, CD43(NGG)-TyA-GFP, was selectively targeted to uropods (Fig. 3, I–L). These results demonstrate that the polarized trafficking of CD43 does not require interaction with ERM proteins per se, so long as CD43 is targeted into the EMV protein-sorting pathway.
FIGURE 3.
Polarized sorting of CD43 by the EMV protein-sorting pathway. Fluorescence and phase microscopy images of Hsb-2 T cells that had been transfected with plasmids designed to express: A–D, CD43-GFP; E–H, CD43(NGG)-GFP; or I–L, CD43(NGG)-TyA-GFP. The arrows denote the position of the uropod. Bar, 10 μm.
Polarized EMV Cargoes Represent a Nascent Posterior Pole
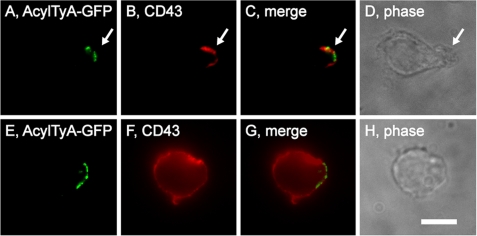
To determine whether the EMV protein-sorting pathway might play an early role in the biogenesis of the posterior pole, we followed the sorting of both EMV cargoes and classic uropod markers following the drug-induced elimination of uropods. Uropod biogenesis requires Rho kinase activity, and addition of the Rho kinase inhibitor Y-27632 to polarized leukocytes causes them to lose their uropod and redistribute uropod markers across the PM (47). To determine whether Rho kinase activity was also required for the polarized distribution of EMV marker proteins, we treated Hsb-2 cells expressing AcylTyA-GFP with 20 μm Y-27632 for 30 min, fixed them, stained them using antibodies specific for CD43, and examined them by epifluorescence microscopy (Fig. 4, A–H). Inhibition of Rho kinase caused the loss of morphological polarity and the redistribution of CD43 over the entire plasma membrane. However, it did not eliminate the polarized distribution of AcylTyA-GFP, demonstrating that Rho kinase is required for uropod biogenesis but not for the polarized distribution of EMV cargoes.
FIGURE 4.
Inhibition of Rho kinase blocks uropod biogenesis but not EMV cargo polarization. Fluorescence and phase microscopy images of Hsb-2 T cells that had been transfected with a plasmid designed to express AcylTyA-GFP and then either fixed (A–D) or incubated for 30 min in the presence of the ROCK inhibitor Y-27632 (E–H). Bar, 10 μm.
These data are consistent with a hypothesis of posterior pole biogenesis in which (i) polarization of the EMV protein-sorting pathway generates a nascent posterior pole and (ii) uropods form at these nascent poles by the Rho kinase-induced targeting of uropod proteins into the EMV protein-sorting pathway. To test this hypothesis we took advantage of the fact that microtubule-depolymerizing agents (e.g. nocodazole, colchicine, and others) induce Rho kinase activity and uropod biogenesis in morphologically non-polarized leukocytes (48–52). Nocodazole-induced uropod biogenesis is more specific than chemoattractant-induced uropod biogenesis, because it does not involve the other signaling pathways that are typically activated by physiological chemoattractants (e.g. activation of heterotrimeric G proteins, phosphatidylinositol 3-kinase, AKT/PKB, MAPK, and others (51)).
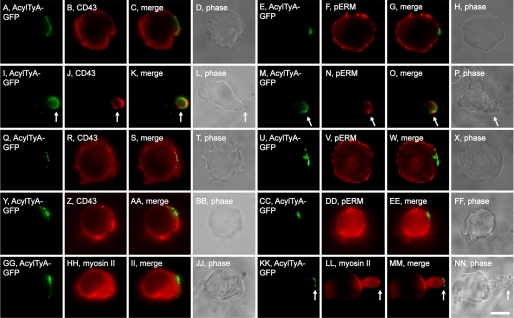
More specifically, we transfected Jurkat T cells with a plasmid designed to express AcylTyA-GFP, exposed them to 1 μm nocodazole for 5 min, then fixed the cells, stained them with antibodies specific for uropod markers, and examined them by epifluorescence microscopy. Prior to addition of nocodazole, Jurkat T cells lacked morphological polarity and showed no evidence of polarized sorting for either CD43 (Fig. 5, A–D) or phosphorylated forms of ERM proteins (pERM) (Fig. 5, E–H), even in those cells that displayed a highly polarized distribution of the EMV cargo AcylTyA-GFP. In contrast, exposure of these cells to 1 μm nocodazole for just 5 min led to the trafficking of uropod proteins to the region of the PM that had been enriched for EMV cargoes, as well as the formation of the uropod at this domain (Fig. 5, I–P). In all cells, uropods formed at the domains enriched for EMV cargoes. This process was blocked by Y-27632 (Fig. 5, Q–X), consistent with the Rho kinase-dependent nature of uropod biogenesis.
FIGURE 5.
Uropod formation at sites of EMV cargo polarization. A–H, fluorescence and phase microscopy images of Jurkat T cells expressing AcylTyA-GFP, fixed, and stained using antibodies specific for CD43 (A–D) and pERM (E–H) proteins. I–P, fluorescence and phase microscopy images of Jurkat T cells expressing AcylTyA-GFP, incubated for 5 min in nocodazole, fixed, and stained using antibodies specific for: I–L, CD43 and M–P, pERM proteins. Arrows denote the position of the uropod. Q–X, fluorescence and phase microscopy images of Jurkat T cells expressing AcylTyA-GFP, incubated for 5 min in the presence of Y-27632, then 5 min in the presence of nocodazole and Y-27632, fixed, and stained using antibodies specific for: Q–T, CD43 and U–X, pERM proteins. Y–FF, fluorescence and phase microscopy images of Jurkat T cells expressing AcylTyA-GFP, incubated for 5 min in the presence of blebbistatin, then 5 min in the presence of nocodazole and blebbistatin, fixed, and stained using antibodies specific for: Y–BB, CD43 and CC–FF, pERM proteins. GG–NN, fluorescence and phase microscopy images of Jurkat T cells expressing AcylTyA-GFP, incubated in the absence of nocodazole (GG–JJ) or presence of nocodazole (KK–NN), fixed, and stained using antibodies specific myosin II. Arrows denote the position of the uropod. Bar, 10 μm.
Myosin II is a major target of Rho kinase and is inhibited by blebbistatin (53, 54). Not surprisingly, blebbistatin also blocked nocodazole-induced uropod biogenesis (Fig. 5, Y–FF). Interestingly, myosin II is not present at sites of polarized EMV proteins prior to addition of nocodazole, but is recruited to the uropod and the uropod neck during nocodazole-induced uropod formation (Fig. 5, GG–NN).
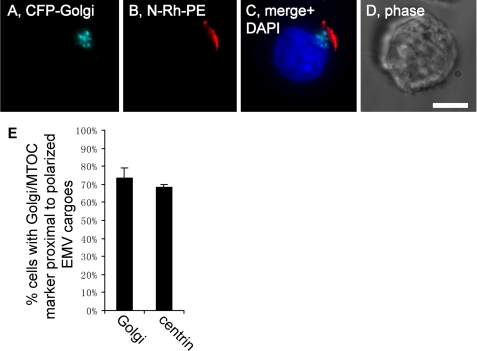
If the pole enriched for EMV cargoes represents a nascent posterior pole, it should have other properties of the posterior pole, even in morphologically non-polarized cells. One such property is the positioning of the Golgi apparatus and microtubule-organizing center (MTOC) proximal to the posterior pole in polarized lymphocytes (22, 43, 55, 56). We therefore localized these intracellular structures elative to the pole enriched for EMV cargoes. Jurkat T cells were transfected with plasmids designed to express either CFP-Golgi (a Golgi marker) or centrin-DsREDm (a marker of the MTOC), pulse-labeled with N-Rh-PE to mark the domain of polarized EMV cargoes, and examined by epifluorescence microscopy. These experiments revealed that the Golgi was positioned proximal to EMV cargoes in ∼70% of cells that had polarized their EMV protein-sorting pathway (Fig. 6, A–E), similar to the percentage of polarized T cells that position their Golgi proximal to the uropod. The results observed for cells expressing centrin-DsREDm were virtually the same (Fig. 6E), demonstrating that the MTOC is also localized proximal to domains of the PM enriched for EMV cargoes.
FIGURE 6.
EMV cargoes polarize proximal to the Golgi and MTOC. A–D, fluorescence and phase microscopy images of N-Rh-PE-labeled Jurkat T cells expressing CFP-Golgi that had been fixed and stained with DAPI. Bar, 10 μm. E, bar graph showing the percentage (average ± 1 S.D.; n = 3) of N-Rh-PE-labeled Jurkat T cells that had been transfected with either CFP-Golgi or centrin-DsREDm and also displayed a highly polarized distribution of EMV cargoes that was located proximal to either (left bar) the Golgi apparatus or (right bar) the MTOC.
Expression of EMV Cargo Proteins Polarizes the EMV Protein-sorting Pathway
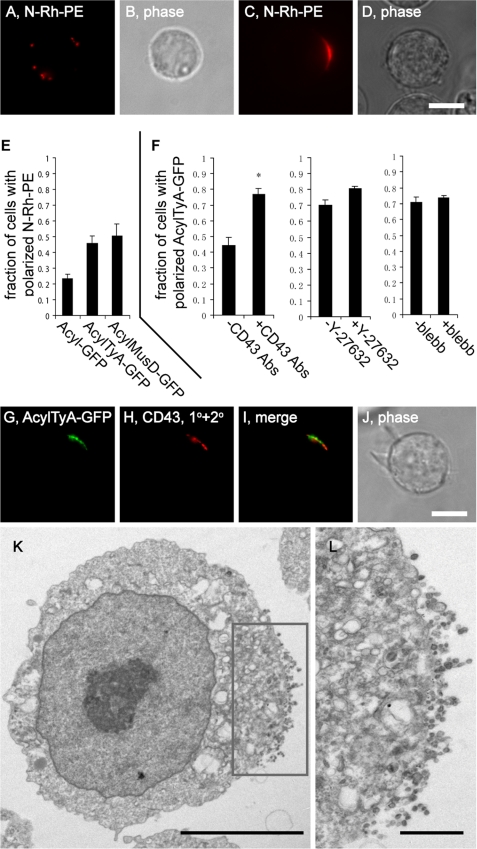
Given that the polarization of the EMV protein sorting pathway appears to generate a nascent posterior pole, we next sought to identify factors that induce the polarization of the EMV protein-sorting pathway. As noted previously, morphologically non-polarized T cells have a bimodal distribution of phenotypes in regard to EMV protein polarization, with most (∼70%) showing a distribution of EMV cargoes over the cell surface, and ∼25% showing a pronounced enrichment of EMV cargoes at one pole of the cell. These two phenotypes are shown here for N-Rh-PE-labeled Jurkat T cells (Fig. 7, A–D). During the course of our studies we became aware that increased expression of an EMV cargo tended to increase the percentage of cells with a polarized distribution of EMV cargoes. To quantify this effect we transfected Jurkat T cells with plasmids designed to express either a non-EMV cargo (Acyl-GFP) or an EMV cargo protein (AcylTyA-GFP or AcylMusD-GFP). These cells were then pulse-labeled with N-Rh-PE, incubated overnight, and examined by fluorescence microscopy, and the percentages of cells that displayed a non-polarized or a polarized distribution of the EMV lipid N-Rh-PE were calculated. We detected a polarized distribution of N-Rh-PE in 23 ± 3% of cells expressing the non-EMV protein Acyl-GFP (average ± 1 S.D.), similar to the percentage of T cells that spontaneously polarize their EMV cargoes (35). In contrast, the expression of AcylTyA-GFP resulted in an increase of ∼2-fold in the percentage of cells with a highly polarized distribution of N-Rh-PE, to 46 ± 5% of (n = 3; p = 0.0094). A similar effect was seen in cells expressing AcylMusD-GFP, which displayed a polarized distribution in 51 ± 7% of cells (n = 3; p = 0.034) (Fig. 7E).
FIGURE 7.
Expression of EMV cargoes induces molecular polarization of the cell. A–D, fluorescence and phase microscopy images of N-Rh-PE-labeled Jurkat T cells. Bar, 10 μm. E, bar graph showing the fraction (average ± 1 S.D.; n = 3) of N-Rh-PE-labeled Jurkat T cells with a highly polarized distribution of EMV cargoes in cells expressing AcylGFP, AcylTyA-GFP, or AcylMusD-GFP. F, bar graphs showing (left graph) the fraction (average ± 1 S.D.; n = 3) of Jurkat T cells with a highly polarized distribution of AcylTyA-GFP prior to and after the antibody-induced targeting of CD43 into the EMV protein-sorting pathway, and (right graphs) the fraction of Jurkat T cells with a highly polarized distribution of AcylTyA-GFP after the antibody-induced targeting of CD43 into the EMV protein-sorting pathway, in the absence or presence of Y-27632, and in the absence of presence of blebbistatin (blebb). G–J, fluorescence and phase microscopy images of Jurkat T cells expressing AcylTyA-GFP after the antibody-induced targeting of CD43 into the EMV protein-sorting pathway. Bar, 10 μm. K and L, immunoelectron micrographs of N-Rh-PE-labeled Jurkat T cells expressing AcylTyA-GFP, following the antibody-induced targeting of CD43 into the EMV protein-sorting pathway. K, a low magnification image shows the absence of pronounced anterior-posterior polarity, except in the sites of EMV production, and the inset shows the region magnified further in L. Bar, 1 μm. L, higher magnification image of the pole enriched for EMV cargoes and sites of vesicle production. Immunogold conjugates (∼18 nm) were directed against CD43. Bar, 100 nm.
To increase the levels of EMV cargoes even further we used antibodies to target CD43 into the EMV biogenesis pathway (30). Following this treatment, we observed that the percentage of Jurkat T cells with a highly polarized distribution of AcylTyA-GFP rose significantly (Fig. 7, F–J), from 44 ± 5% to 77 ± 4% (n = 3; p = 0.02). This cargo-induced polarization of the EMV protein-sorting pathway occurred independent of Rho kinase, because the percentage of these cells with highly polarized EMV cargoes was essentially the same, 70 ± 4% in the absence of Y-27632 and 80 ± 2% in the presence of Y-27632 (n = 3; p = 0.7) (Fig. 7F). Blebbistatin also had no effect on cargo-induced polarization of EMV cargo proteins (Fig. 7F).
We next tested whether the increased levels of EMV cargoes had any substantive effect on cell ultrastructure. Jurkat T cells were transfected with a plasmid designed to express AcylTyA (30) and subjected to antibody-induced higher order oligomerization of CD43 to target CD43 into the EMV protein-sorting pathway. The cells were then fixed and processed for electron microscopy. Numerous cell profiles were examined, and we were unable to detect any significant alteration in cell morphology, with one exception: the sites of EMV budding were concentrated at a single pole of the PM (Fig. 7, K and L).
Polarized Sorting of Retroviral Gag Proteins by the EMV Biogenesis Pathway
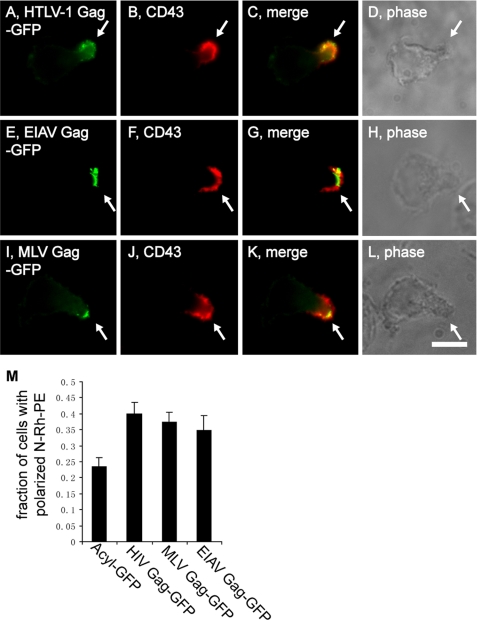
Several lines of evidence indicate that HIV and other retroviruses bud via the EMV biogenesis pathway and that retroviral Gag proteins are EMV cargo proteins (27, 30, 35, 36, 38, 57, 58). Therefore, if cells target proteins to the posterior pole via the EMV protein-sorting pathway they should also target retroviral Gag proteins to their posterior pole. To test this prediction we transfected polarized T cells with plasmids designed to express Gag proteins from human T-lymphotropic virus-1, equine infectious anemia virus (EIAV), and murine leukemia virus (MLV), incubated them a few hours to allow for Gag protein expression, then fixed the cells and processed them for immunofluorescence microscopy using antibodies specific for a uropod marker proteins. Hsb-2 cells sorted human T-lymphotropic virus-1 Gag-GFP, EIAV Gag-GFP, and MLV Gag-GFP to their posterior pole, shown here by their localization at uropods and co-localization with CD43 (Fig. 8, A–L). Similar results were observed in CEM cells, the only difference being that we used CD44 as the uropod marker (supplemental Fig. S2, A–L).
FIGURE 8.
T cells target retroviral Gag proteins to their posterior pole. A–L, fluorescence and phase microscopy images of Hsb-2 T cells that had been transfected with plasmids designed to express: A–D, HTLV1 Gag-GFP; E–H, EIAV Gag-GFP; or I–L, MLV Gag-GFP, incubated 6–8 h, fixed, and stained with antibodies specific for the uropod marker protein CD43. In all images, the arrows denote the position of the uropod. M, bar graph showing the percentage (average ± 1 S.D.; n = 3) of N-Rh-PE-labeled Jurkat T cells with a highly polarized distribution of EMV cargoes in cells expressing AcylGFP, HIV Gag-GFP, MLV Gag-GFP, or EIAV Gag-GFP. Bar, 10 μm.
Given that other EMV cargoes induce the polarization of the EMV protein-sorting pathway, we transfected morphologically non-polarized Jurkat T cells with plasmids designed to express the non-EMV protein Acyl-GFP, or the retroviral Gag proteins HIV Gag-GFP, MLV Gag-GFP, or EIAV Gag-GFP, pulse-labeled them with N-Rh-PE, and examined the cells to determine the percentage of cells with a polarized distribution of N-Rh-PE (Fig. 8M). In cells that expressed the non-EMV cargo, AcylGFP, N-Rh-PE was highly polarized in 23 ± 3% of cells, essentially the same (∼25%) that was reported for untreated T cells (35). In contrast, expression of HIV Gag-GFP induced the polarization of the EMV protein-sorting pathway, increasing the percentage of cells with a single pole of EMV cargoes to 40 ± 3% (n = 3; p = 0.0016). The polarization of the EMV protein-sorting pathway was also induced by expression of MLV Gag-GFP (37 ± 3%; n = 3; p = 0.044) and by expression of EIAV Gag-GFP (35 ± 5%; n = 3; p = 0.031).
HIV Gag Is Targeted to the Posterior Pole by the Same Signals That Target It to EMVs
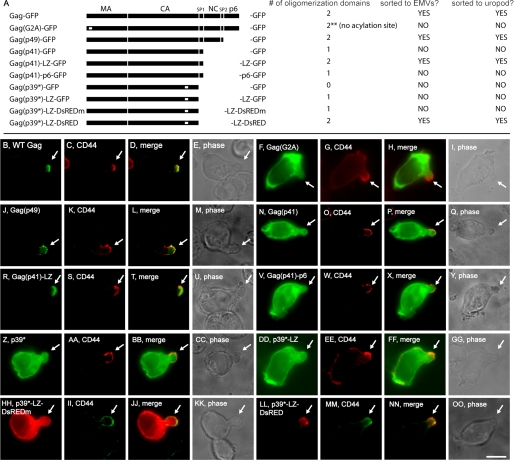
The hypothesis that proteins are targeted to the posterior pole on the basis of higher order oligomerization and PM-binding predicts that budding-competent forms of HIV Gag should also be targeted to the uropod of polarized T cells. To test this prediction we transfected CEM T cells with plasmids that express HIV Gag proteins (Fig. 9A) that vary in their (i) plasma membrane-binding ability, (ii) number of oligomerization domains, and (iii) targeting to EMVs (30, 38, 58).
FIGURE 9.
The uropod targeting information in HIV Gag is the same as its EMV targeting information. A, line diagram showing the general structure of HIV Gag proteins, together with a description of their membrane anchor, number of oligomerization domains, sorting to EMVs, and sorting to uropods. B–OO, fluorescence and phase microscopy of CEM T cells stained for CD44 and expressing: B–E, HIV Gag-GFP; F–I, HIV Gag(G2A)-GFP; J–M, HIV Gag(p49)-GFP; N–Q, HIV Gag(p41)-GFP; R–U, HIV Gag(p41)-LZ-GFP; V–Y, HIV Gag(p41)-p6-GFP; Z–CC, HIV Gag(p39*)-GFP; DD–GG, HIV Gag(p39*)-LZ-GFP; HH–KK, HIV Gag(p39*)-LZ-DsREDm; and LL–OO, HIV Gag(p39*)-LZ-DsRED. White arrows show the position of the uropod in each cell. Bar, 10 μm.
HIV Gag-GFP was selectively targeted to the uropod of polarized T cells (Fig. 9, B–E), as shown recently by Ono and colleagues (59). HIV budding requires that Gag bind the PM, and Gag does so primarily via attachment of a myristoyl group at glycine 2 of the protein. Substituting alanine for this glycine blocks HIV budding (60) and prevents HIV Gag-GFP from entering the EMV protein-sorting pathway (38). This mutation also prevented the polarized sorting of HIV Gag-GFP, because CEM cells failed to target HIV Gag(G2A)-GFP to their uropod (Fig. 9, F–I).
Although several lines of evidence support the hypothesis that HIV budding follows the EMV biogenesis pathway, there is an alternative hypothesis, which proposes that the C-terminal p6 domain of Gag is the primary mediator of HIV budding (61, 62). Removing the p6 domain from HIV Gag does not affect its PM-binding domain (MA), major oligomerization domains (CA and NC), or its ability to bud from human T cells (30, 58). Loss of p6 also had no effect on Gag trafficking to the posterior pole, shown here by the uropod localization of HIV Gag(p49)-GFP (Fig. 9, J–M).
HIV Gag(p41)-GFP lacks the NC domain of Gag, one of its two major oligomerization domains. HIV Gag(p41)-GFP is not secreted in EMVs (30) and was not targeted to the posterior of polarized T cells (Fig. 9, N–Q). However, we previously reported that replacing the NC domain with a heterologous oligomerization domain (a synthetic leucine zipper) rescued its vesicular secretion, allowing HIV Gag(p41)-LZ-GFP to bud from cells (30) and be sorted to uropods in polarized T cells (Fig. 9, R–U). The p6 domain had no such effect, because HIV Gag(p41)-p6-GFP was unable to bud from human T cells (30) and was not targeted to the posterior pole (Fig. 9, V–Y).
HIV Gag(p39*) lacks both of its major oligomerization domains due to (i) the absence of the NC domain and (ii) a point mutation in its capsid (CA) domain that disrupts Gag-Gag binding (63). HIV Gag(p39*) does not oligomerize, is not secreted from the cell in vesicles (30), and was not targeted to the cell posterior (Fig. 9, Z–CC). Adding back a synthetic leucine zipper does not restore its EMV secretion (30) and also failed to restore its uropod trafficking (Fig. 9, DD–GG). Similar results were observed for HIV Gag(p39*)-LZ-DsREDm (30), which carries a monomeric form of DsRED in place of the GFP tag (Fig. 9, HH–KK). In contrast, HIV Gag(p39*)-LZ-DsRED has the tetrameric form of DsRED, is able to form higher order oligomeric complexes, and is targeted to EMVs (30). This protein was highly enriched at uropods of polarized T cells (Fig. 9, LL–OO). These data provide yet another line of evidence that higher order oligomerization and PM binding target proteins to the posterior pole.
Polarized Sorting of EMV Cargoes during Rapid Cell Migration
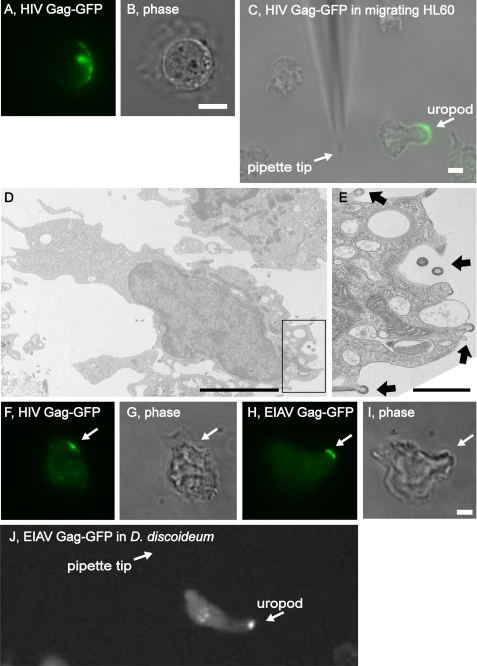
To test whether the polarized sorting of EMV cargoes also occurs in actively migrating cells we explored their trafficking in HL60 cells, a human neutrophil-like cell line (20). Prior to differentiation, suspension-grown HL60 resemble suspension-grown T cells in that they lack morphological polarity and occasionally polarize their EMV cargoes, shown here by the polarized distribution of HIV Gag-GFP (Fig. 10, A and B). The polarized sorting of EMV cargoes was even more apparent in migrating HL60 cells, shown here by the uropod localization of HIV Gag-GFP in differentiated HL60 that was migrating up along a gradient of fMLP peptide (Fig. 10C). We also examined the ultrastructure of differentiated HL60 cells expressing HIV Gag, which revealed the presence of Gag-containing budding intermediates at the posterior pole (Fig. 10, D and E).
FIGURE 10.
The EMV protein-sorting pathway targets proteins to the uropod in migrating HL60 human neutrophils and the protozoan D. discoideum. A and B, fluorescence and phase microscopy images of HL60 T cells that had been transfected with a plasmid designed to express HIV Gag-GFP. Bar, 10 μm. C, merged fluorescence and phase microscopy image of a differentiated HL60 T-cell that had been transfected with a plasmid designed to express HIV Gag-GFP, migrating toward a pipette emitting fMLP peptide. Arrows show the position of the uropod and pipette tip. Bar, 10 μm. D and E, transmission electron micrographs of differentiated HL60 cells that had been transfected with a plasmid designed to express HIV Gag, exposed to 1 μm fMLP peptide for 5 min, then fixed and processed for electron microscopy. D, note the emergent vesicles in the boxed area, and the absence of such structures at the PM of the pseudopod. Bar, 10 μm. E, higher magnification of inset showing the presence of budding and budded Gag-containing vesicles. Arrows show the position of Gag-containing vesicles that have been released or are in the process of budding from the cell (Gag can be identified on the basis of its unique electron-dense appearance that it acquires during the budding process). Bar, 1 μm. F–I, fluorescence and phase microscopy images of undifferentiated D. discoideum AX2 cells expressing (F and G, HIV Gag-GFP or H and I, EIAV Gag-GFP. Arrows show the position of the polarized EMV cargoes. Bar, 10 μm. J, merged fluorescence and phase microscopy image of a differentiated AX2 cell expressing EIAV Gag-GFP and migrating toward a pipette emitting cAMP. Arrows show the position of the uropod and pipette tip. Bar, 10 μm.
Polarized Sorting of EMV Cargoes in the Protozoan D. discoideum
To determine whether the EMV protein-sorting pathway participates in posterior pole biogenesis in an evolutionarily distant organism, we examined the localization of EMV cargoes in the social amoeba D. discoideum. Like human T cells and neutrophils, D. discoideum can polarize along an anterior-posterior axis and selectively targets distinct proteins to their front and rear poles (2, 3). Undifferentiated D. discoideum AX2 cells trafficked EMV cargoes to a discrete domain of the PM (Fig. 10, F–I). When differentiated, AX2 cells polarized along an anterior-posterior axis, migrated toward a pipette emitting cAMP, and targeted EMV cargoes to their posterior pole, shown here by the targeting of EIAV Gag-GFP to the posterior pole in these rapidly migrating cells (Fig. 10J). The targeting of EMV cargoes to the posterior pole of D. discoideum is even more apparent in movies generated by live cell video microscopy (supplemental Fig. S3). These data indicate that the EMV protein-sorting pathway targets proteins to the posterior pole in organisms as divergent as protozoans and humans.
DISCUSSION
The generation of anterior-posterior cell polarity is critical for rapidly migrating cells such as mammalian leukocytes and social amoeba. The data presented here demonstrate that the EMV protein-sorting pathway plays important roles in posterior pole biogenesis, does so in evolutionary distant eukaryotes, and mediates the polarized budding of EMVs and retroviruses.
Implications for Protein Trafficking to the Posterior Pole
Our data show that proteins are trafficked to the posterior pole on the basis of higher order oligomerization and plasma membrane binding, the same elements that target proteins to EMVs and retrovirus particles. The evidence for this conclusion comes from the pronounced enrichment of EMV test proteins at the uropod of polarized leukocytes and protozoans, including classic exosome markers, synthetic EMV test proteins, and diverse retroviral Gag proteins (30, 38, 58). This means that virtually any protein can be targeted to the posterior pole merely by increasing its relative binding to the plasma membrane and/or its higher order oligomerization. These properties are notable for their lack of correlation with any specific amino acid sequence, ability to be changed in a post-translational manner, and their ability to affect one another (targeting a protein to membranes will increase its effective concentration and tendency to oligomerize, and oligomerization of a membrane-binding protein will increase its avidity for the membrane).
This model for “posterior” protein trafficking is consistent with much of what is already known about uropod proteins. Uropod proteins lack shared peptide motifs, indicating that their localization to the posterior pole is driven by less-defined properties. Furthermore, uropod proteins are known to form highly oligomeric, PM-bound protein complexes, and these complexes might be diverting them into the EMV protein-sorting pathway. In addition, the targeting of uropod proteins to the posterior pole is rapidly reversible and must, therefore, be driven by post-translational mechanisms rather than their primary structure. Also, many uropod proteins have been detected in purified EMVs, including ERM proteins (64–67), CD43 (34), CD44 (68), phospholipid scramblase (69, 70), and flotillin (68, 69, 71). Finally, our model of polarized protein sorting is consistent with the decades-old phenomenon of ligand-induced “capping” and “shedding” of cell surface proteins (72).
Implications for Posterior Pole Biogenesis
Four lines of evidence indicate that polarization of the EMV protein-sorting pathway generates a nascent posterior pole. First, increased expression of EMV cargoes induces the polarized distribution of EMV cargo proteins and lipids. Second, uropods formed at sites of polarized EMV cargoes in morphologically non-polarized Jurkat T cells. Third, inhibition of Rho kinase eliminated uropods and the polarized sorting of uropod marker proteins, but had no effect on the polarized sorting of EMV cargo proteins. Fourth, the Golgi and MTOC were localized proximal to sites of polarized EMV cargoes in morphologically non-polarized T cells, just as they are localized proximal to the uropod in polarized lymphocytes. Taken together, these data raise the possibility that posterior pole biogenesis is an internal, signal-independent process in which elevated levels of EMV cargoes (i) polarize the EMV protein-sorting pathway, (ii) generate a nascent posterior pole, and (iii) prime cells for signal-induced morphological polarization.
This hypothetical mechanism of posterior pole biogenesis predicts that cells can shift rapidly between morphologically polarized and morphologically non-polarized states without losing their intrinsic polarity, which is consistent with the rapid formation of uropods following drug-induced activation of Rho kinase, and their retraction upon Rho kinase inhibition, all while maintaining a polarized distribution of EMV cargoes. Similar results have been noted in other systems in the absence of polarity-inducing stimuli such as chemoattractants (2). An internal, signal-independent mechanism has also been proposed to explain the biogenesis and behavior of pseudopods at the anterior pole (8, 73). Continual operation of internally driven, antagonistic mechanisms for the biogenesis of anterior and posterior poles would actually be advantageous to cells, as it would allow them to respond rapidly to “frontness” or “backness” signals merely by slight shifts in the balance between these pathways.
Implications for EMV Biogenesis
The observation that cells target proteins to their posterior pole on the basis of higher order oligomerization and PM binding predicts that morphologically polarized cells should bud EMVs from their posterior pole. In support of this idea, we detected numerous vesicles at the posterior pole of migrating HL60 cells, and also of morphologically non-polarized Jurkat T cells. Our model also predicts that EMVs released by highly polarized leukocytes will be enriched for uropod proteins. Given that many uropod surface proteins are biochemically active, such differences could influence the biological effects of EMVs on neighboring cells.
Implications for Retrovirus Budding
Our observations are also relevant to retrovirus budding. Previous studies indicate that retroviruses, and their Gag proteins, bud from cells via the EMV biogenesis pathway (27, 30, 35, 36, 38, 57, 58). The data presented here provide additional support for this “Trojan exosome” hypothesis of retrovirus budding by showing that polarized leukocytes (i) traffic EMV cargoes to their posterior pole, (ii) target diverse retroviral Gag proteins to their posterior pole, (iii) target HIV Gag to the uropod via the same signals that target it to EMVs, and (iv) bud HIV Gag from their uropod. This hypothesis is also supported by the results of Llewellyn et al. (59), who were the first to report that HIV buds from the uropod of infected T cells.
The implications of polarized retrovirus budding are similar to those for EMVs. Host cell proteins present on the retrovirus surface are likely to vary in accord with the polarization state of the infected cells. In the case of HIV, this is likely to be a common occurrence, because HIV-infected T cells are typically activated and polarized in vivo (59). As for whether polarized budding has any particular relevance to retroviral biology, its likely that the EMV protein-sorting pathway is responsible for the polarized budding of HIV and other retroviruses at sites of cell-to-cell contact (59, 62, 74, 75), a privileged mode of infection that is likely to (i) enhance retroviral replication by directing viral particle release into small spaces that are in close contact with neighboring cells and (ii) reduce immune surveillance by excluding anti-viral factors (antibodies, complement, phagocytes, and others) from these spaces, and by restricting virus-specific antigens to these “protected” regions of the cell surface.
Supplementary Material
Acknowledgments
We thank Doug Robinson, Dan Raben, Michael Caterina, Miho Iijima, and Carole Parent for their comments and suggestions during the course of this study.
This work was supported, in whole or in part, by National Institutes of Health Grant DK45787.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- PIP3
- phosphatidylinositol (3,4,5)-trisphosphate
- EMV
- exosome/microvesicle
- ERM
- ezrin, radixin, and moesin
- pERM
- phosphorylated forms of ERM proteins
- PM
- plasma membrane
- fMLP
- formylmethionylleucylphenylalanine
- PE
- phosphatidylethanolamine
- NGG
- triple mutation that disrupts ERM-CD43 binding (Lys-282 → Asn, Arg-283 → Gly, and Arg-284 → Gly)
- MTOC
- microtubule-organizing center
- EIAV
- equine infectious anemia virus
- N-Rh-PE
- 1, 2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl)
- N-F-PE
- 1, 2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(carboxyfluorescein).
REFERENCES
- 1. Mellman I., Nelson W. J. (2008) Nat. Rev. Mol. Cell Biol. 9, 833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Swaney K. F., Huang C. H., Devreotes P. N. (2010) Annu. Rev. Biophys 39, 265–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rericha E. C., Parent C. A. (2008) Sci. Signal 1, pe26. [DOI] [PubMed] [Google Scholar]
- 4. Macara I. G., Mili S. (2008) Cell 135, 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harada A. (2010) J. Biochem. 147, 619–624 [DOI] [PubMed] [Google Scholar]
- 6. St Johnston D., Ahringer J. (2010) Cell 141, 757–774 [DOI] [PubMed] [Google Scholar]
- 7. Skoglund P., Keller R. (2010) Curr. Opin. Cell Biol. 22, 589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Insall R. H. (2010) Nat. Rev. Mol. Cell Biol. 11, 453–458 [DOI] [PubMed] [Google Scholar]
- 9. Van Haastert P. J. (2011) Sci. Signal 4, pe6. [DOI] [PubMed] [Google Scholar]
- 10. King J. S., Insall R. H. (2009) Trends Cell Biol. 19, 523–530 [DOI] [PubMed] [Google Scholar]
- 11. Parent C. A., Blacklock B. J., Froehlich W. M., Murphy D. B., Devreotes P. N. (1998) Cell 95, 81–91 [DOI] [PubMed] [Google Scholar]
- 12. Parent C. A. (2004) Curr. Opin. Cell Biol. 16, 4–13 [DOI] [PubMed] [Google Scholar]
- 13. Manahan C. L., Iglesias P. A., Long Y., Devreotes P. N. (2004) Annu. Rev. Cell Dev. Biol. 20, 223–253 [DOI] [PubMed] [Google Scholar]
- 14. Franca-Koh J., Kamimura Y., Devreotes P. N. (2007) Nat. Cell Biol. 9, 15–17 [DOI] [PubMed] [Google Scholar]
- 15. Sasaki A. T., Chun C., Takeda K., Firtel R. A. (2004) J. Cell Biol. 167, 505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sasaki A. T., Janetopoulos C., Lee S., Charest P. G., Takeda K., Sundheimer L. W., Meili R., Devreotes P. N., Firtel R. A. (2007) J. Cell Biol. 178, 185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamimura Y., Xiong Y., Iglesias P. A., Hoeller O., Bolourani P., Devreotes P. N. (2008) Curr. Biol. 18, 1034–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cai H., Das S., Kamimura Y., Long Y., Parent C. A., Devreotes P. N. (2010) J. Cell Biol. 190, 233–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Charest P. G., Shen Z., Lakoduk A., Sasaki A. T., Briggs S. P., Firtel R. A. (2010) Dev. Cell 18, 737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu J., Wang F., Van Keymeulen A., Herzmark P., Straight A., Kelly K., Takuwa Y., Sugimoto N., Mitchison T., Bourne H. R. (2003) Cell 114, 201–214 [DOI] [PubMed] [Google Scholar]
- 21. Meili R., Firtel R. A. (2003) Cell 114, 153–156 [DOI] [PubMed] [Google Scholar]
- 22. Sánchez-Madrid F., Serrador J. M. (2009) Nat. Rev. Mol. Cell Biol. 10, 353–359 [DOI] [PubMed] [Google Scholar]
- 23. Fehon R. G., McClatchey A. I., Bretscher A. (2010) Nat. Rev. Mol. Cell Biol. 11, 276–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schorey J. S., Bhatnagar S. (2008) Traffic 9, 871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Théry C., Ostrowski M., Segura E. (2009) Nat. Rev. Immunol. 9, 581–593 [DOI] [PubMed] [Google Scholar]
- 26. Simons M., Raposo G. (2009) Curr. Opin. Cell Biol. 21, 575–581 [DOI] [PubMed] [Google Scholar]
- 27. Gould S. J., Booth A. M., Hildreth J. E. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10592–10597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trams E. G., Lauter C. J., Salem N., Jr., Heine U. (1981) Biochim. Biophys. Acta 645, 63–70 [DOI] [PubMed] [Google Scholar]
- 29. Pan B. T., Johnstone R. M. (1983) Cell 33, 967–978 [DOI] [PubMed] [Google Scholar]
- 30. Fang Y., Wu N., Gan X., Yan W., Morrell J. C., Gould S. J. (2007) PLoS Biol. 5, e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cocucci E., Racchetti G., Meldolesi J. (2009) Trends Cell Biol. 19, 43–51 [DOI] [PubMed] [Google Scholar]
- 32. Théry C., Zitvogel L., Amigorena S. (2002) Nat. Rev. Immunol. 2, 569–579 [DOI] [PubMed] [Google Scholar]
- 33. Skog J., Würdinger T., van Rijn S., Meijer D. H., Gainche L., Sena-Esteves M., Curry W. T., Jr., Carter B. S., Krichevsky A. M., Breakefield X. O. (2008) Nat. Cell Biol. 10, 1470–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J. J., Lötvall J. O. (2007) Nat. Cell Biol. 9, 654–659 [DOI] [PubMed] [Google Scholar]
- 35. Booth A. M., Fang Y., Fallon J. K., Yang J. M., Hildreth J. E., Gould S. J. (2006) J. Cell Biol. 172, 923–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krishnamoorthy L., Bess J. W., Jr., Preston A. B., Nagashima K., Mahal L. K. (2009) Nat. Chem. Biol. 5, 244–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marsh M., Theusner K., Pelchen-Matthews A. (2009) Biochem. Soc. Trans 37, 185–189 [DOI] [PubMed] [Google Scholar]
- 38. Shen B., Wu N., Yang J. M., Gould S. J. (2011) J. Biol. Chem. 286, 14383–14395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gaudet P., Pilcher K. E., Fey P., Chisholm R. L. (2007) Nat. Protoc. 2, 1317–1324 [DOI] [PubMed] [Google Scholar]
- 40. Yonemura S., Hirao M., Doi Y., Takahashi N., Kondo T., Tsukita S., Tsukita S. (1998) J. Cell Biol. 140, 885–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Errabolu R., Sanders M. A., Salisbury J. L. (1994) J. Cell Sci. 107, 9–16 [DOI] [PubMed] [Google Scholar]
- 42. McCaffery J. M., Farquhar M. G. (1995) Methods Enzymol. 257, 259–279 [DOI] [PubMed] [Google Scholar]
- 43. Sánchez-Madrid F., del Pozo M. A. (1999) EMBO J. 18, 501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu Y., Hannigan M. O., Kotlyarov A., Gaestel M., Wu D., Huang C. K. (2004) Biochem. Biophys. Res. Commun. 316, 666–672 [DOI] [PubMed] [Google Scholar]
- 45. Willem J., ter Beest M., Scherphof G., Hoekstra D. (1990) Eur. J. Cell Biol. 53, 173–184 [PubMed] [Google Scholar]
- 46. Serrador J. M., Nieto M., Alonso-Lebrero J. L., del Pozo M. A., Calvo J., Furthmayr H., Schwartz-Albiez R., Lozano F., González-Amaro R., Sánchez-Mateos P., Sánchez-Madrid F. (1998) Blood 91, 4632–4644 [PubMed] [Google Scholar]
- 47. Fonseca A. V., Freund D., Bornhäuser M., Corbeil D. (2010) J. Biol. Chem. 285, 31661–31671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bourguignon L. Y., Nagpal M. L., Hsing Y. C. (1981) J. Cell Biol. 91, 889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bourguignon L. Y., Hsing Y. C. (1983) Biochim. Biophys. Acta 728, 186–190 [DOI] [PubMed] [Google Scholar]
- 50. Keller H. U., Meier G., Zimmerman A. (1984) Blood Cells 10, 505–509 [PubMed] [Google Scholar]
- 51. Niggli V. (2003) J. Cell Sci. 116, 813–822 [DOI] [PubMed] [Google Scholar]
- 52. Xu J., Wang F., Van Keymeulen A., Rentel M., Bourne H. R. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 6884–6889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Straight A. F., Cheung A., Limouze J., Chen I., Westwood N. J., Sellers J. R., Mitchison T. J. (2003) Science 299, 1743–1747 [DOI] [PubMed] [Google Scholar]
- 54. Matsumura F. (2005) Trends Cell Biol. 15, 371–377 [DOI] [PubMed] [Google Scholar]
- 55. Ratner S., Sherrod W. S., Lichlyter D. (1997) J. Immunol. 159, 1063–1067 [PubMed] [Google Scholar]
- 56. del Pozo M. A., Nieto M., Serrador J. M., Sancho D., Vicente-Manzanares M., Martínez C., Sánchez-Madrid F. (1998) Cell Adhes. Commun. 6, 125–133 [DOI] [PubMed] [Google Scholar]
- 57. Nguyen D. G., Booth A., Gould S. J., Hildreth J. E. (2003) J. Biol. Chem. 278, 52347–52354 [DOI] [PubMed] [Google Scholar]
- 58. Gan X., Gould S. J. (2011) Mol. Biol. Cell 22, 817–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Llewellyn G. N., Hogue I. B., Grover J. R., Ono A. (2010) PLoS Pathog 6, e1001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Göttlinger H. G., Sodroski J. G., Haseltine W. A. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 5781–5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bieniasz P. D. (2009) Cell Host Microbe 5, 550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morita E., Sundquist W. I. (2004) Annu. Rev. Cell Dev. Biol. 20, 395–425 [DOI] [PubMed] [Google Scholar]
- 63. Burniston M. T., Cimarelli A., Colgan J., Curtis S. P., Luban J. (1999) J. Virol. 73, 8527–8540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wubbolts R., Leckie R. S., Veenhuizen P. T., Schwarzmann G., Möbius W., Hoernschemeyer J., Slot J. W., Geuze H. J., Stoorvogel W. (2003) J. Biol. Chem. 278, 10963–10972 [DOI] [PubMed] [Google Scholar]
- 65. Hegmans J. P., Bard M. P., Hemmes A., Luider T. M., Kleijmeer M. J., Prins J. B., Zitvogel L., Burgers S. A., Hoogsteden H. C., Lambrecht B. N. (2004) Am. J. Pathol. 164, 1807–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mears R., Craven R. A., Hanrahan S., Totty N., Upton C., Young S. L., Patel P., Selby P. J., Banks R. E. (2004) Proteomics 4, 4019–4031 [DOI] [PubMed] [Google Scholar]
- 67. Gatti J. L., Métayer S., Belghazi M., Dacheux F., Dacheux J. L. (2005) Biol. Reprod 72, 1452–1465 [DOI] [PubMed] [Google Scholar]
- 68. Choi D. S., Lee J. M., Park G. W., Lim H. W., Bang J. Y., Kim Y. K., Kwon K. H., Kwon H. J., Kim K. P., Gho Y. S. (2007) J. Proteome Res. 6, 4646–4655 [DOI] [PubMed] [Google Scholar]
- 69. Pisitkun T., Shen R. F., Knepper M. A. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 13368–13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mathivanan S., Lim J. W., Tauro B. J., Ji H., Moritz R. L., Simpson R. J. (2010) Mol. Cell Proteomics 9, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gonzales P. A., Pisitkun T., Hoffert J. D., Tchapyjnikov D., Star R. A., Kleta R., Wang N. S., Knepper M. A. (2009) J. Am. Soc. Nephrol. 20, 363–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schreiner G. F., Unanue E. R. (1976) Adv. Immunol. 24, 37–165 [DOI] [PubMed] [Google Scholar]
- 73. Neilson M. P., Veltman D. M., van Haastert P. J., Webb S. D., Mackenzie J. A., Insall R. H. (2011) PLoS Biol. 9, e1000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McDonald D., Wu L., Bohks S. M., KewalRamani V. N., Unutmaz D., Hope T. J. (2003) Science 300, 1295–1297 [DOI] [PubMed] [Google Scholar]
- 75. Jolly C., Kashefi K., Hollinshead M., Sattentau Q. J. (2004) J. Exp. Med. 199, 283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.