Background: ARF6 GTPase orchestrates membrane trafficking and actin-based cytoskeleton dynamics in migrating cells.
Results: ACAP4 regulates integrin β1 dynamics in EGF-stimulated cell migration by interaction with Grb2 via Tyr-733 phosphorylation.
Conclusion: These results revealed the function of the ACAP4-Grb2-integrin β1 axis in EGF-elicited cell migration.
Significance: This study sheds light on a better understanding of aberrant tyrosine phosphorylation in tumor metastasis.
Keywords: Cell Invasion, Cell Migration, Cell Motility, GTPase, Phosphorylation, ACAP4, ARF6, EGF, Integrin, Ezrin
Abstract
ARF6 GTPase is an important regulator of membrane trafficking and actin-based cytoskeleton dynamics active at the leading edge of migrating cells. The integrin family heterodimeric transmembrane proteins serve as major receptors for extracellular matrix proteins, which play essential roles in cell adhesion and migration. Our recent proteomic analyses of ARF6 effectors have identified a novel ARF6 GTPase-activating protein, ACAP4, essential for EGF-induced cell migration. However, molecular mechanisms underlying ACAP4-mediated cell migration have remained elusive. Here, we show that ACAP4 regulates integrin β1 dynamics during EGF-stimulated cell migration by interaction with Grb2. Our biochemical study shows that EGF stimulation induces phosphorylation of tyrosine 733, which enables ACAP4 to bind Grb2. This interaction of ACAP4 with Grb2 regulates integrin β1 recycling to the plasma membrane. Importantly, knockdown of ACAP4 by siRNA or overexpression of ACAP4 decreased recycling of integrin β1 to the plasma membrane and reduced integrin-mediated cell migration. Taken together, these results suggest a novel function for ACAP4 in the regulation of cell migration through controlling integrin β1 dynamics.
Introduction
Intracellular membrane trafficking orchestrates diversified cellular dynamics, such as intracellular signal transduction, organelle structure formation, and cytoskeleton reorganization. Recycling endosomal trafficking cooperates with endocytic uptake and disposal to mediate the composition of subcellular compartments. Based on mechanistic characteristics, endocytosis can be divided into two classic patterns: clathrin-dependent endocytosis and clathrin-independent endocytosis (CIE).3 Different from clathrin-dependent endocytosis cargos, CIE cargos are recycled back to the plasma membrane through the tubular recycling endosomes, which is referred to as CIE recycling pathway (1). Over the past several years, a collection of intracellular proteins has been identified for their participation in the clathrin-independent recycling pathway.
Integrins are heterodimers composed of α and β subunits. Different subunits combine together to form distinct variants as the receptors of the copious extracellular matrix (2). β integrins are mediated by the CIE pathway to enter the cell (1). The α5β1 variant is mainly the receptor for fibronectin, and its expression level on the cell surface correlates with cell adhesion, cell division, and cell proliferation. Previous studies have identified that β1 integrin was recycled to the cell surface from the juxtanuclear endocytic recycling compartment through the CIE recycling pathway (1, 3). At the same time, more evidence has shown that small GTPases, including ARF6 and Rab11, play a significant role in tubular endosome-associated recycling pathways. The dominant negative mutant of ARF6, which was locked in the GDP binding form, was found to inhibit integrin β1 recycling stimulated by epidermal growth factor (EGF) (3).
Besides the small GTPases, several regulators of ARF6 have also been demonstrated to be involved in the CIE recycling pathway, such as guanine nucleotide-exchange factors (GEFs), which function to exchange ARF-associated GDP to GTP, and GTPase-activating proteins (GAPs), which mediate the hydrolysis of ARF-bound GTP. Recent studies revealed that knockdown of ARNO, one of ARF6 GEFs, inhibited the recycling of integrin β1 to the membrane surface (4). The Arf6-GAP ACAP1 was reported to interact with endosomal integrin β1 and acted as an effector of ARF6 to regulate the integrin β1 recycling pathway (5–7).
Although only six distinct ARF GTPases have been identified, the list for ARF GAPs is extensive, and their functions are perhaps spatiotemporally oriented. Studies on these accessory proteins indicate that their different subcellular localizations and distinct binding partners help to coordinate their functions (8). ACAP4, which contains the Bin-Amphiphysin-Rvs domain, pleckstrin homology domain, conserved GAP domain, and ankyrin repeats domain has been identified as an ARF GAP and classified into the AZAP family (9, 10). Several lines of biochemical and cellular characterization together with recent crystallographic analyses validate that ACAP4 is a specific GAP for ARF6 GTPase (9, 11). ACAP4 was also found to associate with the focal adhesions in the MDA-MB-231 and U118 cell lines (10). When stimulated with growth factors, ACAP4 was recruited to the plasma membrane ruffles in HeLa and NIH-3T3 cell lines (9, 10). Beyond regulating EGF-stimulated membrane and cytoskeleton remodeling and actin-containing stress fiber formation, ACAP4 is also crucial in the process of cell migration and invasion (9, 10). Moreover, it has been identified that ACAP4 can effectively interact with ezrin, an important membrane-cytoskeleton linker, in a phosphorylation-dependent manner, and this complex formation is a determinant for the acid secretion in the gastric parietal cells by orchestrating H,K-ATPase-containing tubulovesicular trafficking to the apical plasma membrane (12).
Growth factor receptor-binding protein-2 (Grb2) is an important adaptor protein in orchestrating intracellular signal transduction pathways and is involved in cell proliferation, actin-based cell motility, and endocytic trafficking. The 25-kDa protein is composed of two Src homology 3 (SH3) domains separated by one Src homology 2 (SH2) domain, and it functions to mediate the formation of protein complex (13). The SH2 domain can recognize the phosphorylated tyrosine in a specific motif context such as those in activated EGF receptor (EGFR), focal adhesion kinase, and Grb2 associated binder 1 (Gab1) (13–15). The SH3 domains are shown to associate with proline-rich motifs within the targeted protein, including Son of Sevenless 1 (SOS1) and N-Wiskott-Aldrich syndrome protein (N-WASP) (16–20). Thus, Grb2 exerts functions through binding proteins with specific sequence motifs and links the ligand-activated integral membrane receptors to downstream targets.
The cycle between the GTP- and GDP-bound status of ARF6 catalyzed by corresponding GEFs and GAPs is required to complete its functions. Meanwhile, the ARF6-associated tubular endosome is responsible for the CIE recycling of several surface integral membrane proteins, including integrin β1.
In this study, we attempted to test whether ACAP4 was involved in the regulation of integrin β1 recycling. We found that either knockdown of ACAP4 by small interfering RNA (siRNA) or overexpression of ACAP4 induced a decrease in the amount of integrin β1 endocytic recycling to the plasma membrane stimulated by EGF. Overexpression of ACAP4 also had a negative effect on cell adhesion, spreading, and migration on fibronectin. Moreover, Grb2, an adaptor protein downstream of the activated EGF receptor, was characterized to interact with ACAP4, and their interaction was mediated by the phosphorylation of ACAP4 at tyrosine 733 induced by EGF in vivo. Furthermore, attenuation of the ACAP4-Grb2 interaction by mutating the Grb2-binding motif on ACAP4 no longer inhibited the integrin β1 recycling pathway or reduced cell motility on fibronectin. Collectively, our results revealed that ACAP4 regulated integrin β1 recycling in response to the activated EGF signal transduced by the adaptor protein Grb2.
MATERIALS AND METHODS
Reagents
Antibodies against Grb2, integrin β1, and c-Myc were purchased from Santa Cruz Biotechnology. Anti-phosphotyrosine antibody was purchased from Millipore. Anti-ACAP4 antibody was generated as described by Fang et al. (9). FLAG monoclonal antibody M2 was obtained from Sigma. Mouse monoclonal antibody to GFP was purchased from BD Biosciences. AlexaFluor 647-conjugated TS2/16 was generated from BioLegend (San Diego). Lipofectamine 2000 was purchased from Invitrogen. EGF was generated from Calbiochem.
siRNA and Plasmids
siRNA duplex targeted to ACAP4 was obtained as described previously (12). siRNA of Grb2 was purchased from Santa Cruz Biotechnology. As a control, siRNA against scrambled sequence was also used. Human clone of the Grb2 gene was obtained from a brain cDNA library. Point mutations were generated as described previously (9). GFP-Grb2, GFP-ACAP4, and its deletion mutants were constructed by inserting the corresponding gene sequences into the pEGFP-N1 vector (BD Biosciences). To construct FLAG-ACAP4 and its mutants, the cDNA was subcloned into p3×FLAG-myc-CMV-24 vectors (Sigma). Bacterial recombinant proteins were constructed using pGEX-4T-1 and pET-28a vectors (Amersham Biosciences). All constructs were validated by DNA sequencing.
Cell Culture and Transfection
HeLa, COS7, MDA-MB-231, and 293T cells were obtained from the ATCC and cultured in Dulbecco's modified Eagle's growth medium (DMEM, Invitrogen) containing 10% fetal bovine serum (Hyclone, Logan, UT), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). All cell lines were cultured at 37 °C in 5% CO2. Cells were transfected with plasmids or siRNA duplex using Lipofectamine 2000 according to the manufacturer's protocol (Invitrogen).
Integrin β1 Recycling Assay by Cell Imaging
HeLa cells on coverslips were deprived of serum overnight and cultured in DMEM/BSA (DMEM with 0.01% bovine serum albumin). Antibody against integrin β1 (TS2/16) diluted to a final concentration of 10 μg/ml was added to the cells at 4 °C for 1 h. After washing twice with cold DMEM/BSA, cells were cultured at 37 °C, and integrin β1 antibody was allowed to be internalized for 2 h. Then pre-warmed DMEM/BSA containing 0.1 μg/ml EGF or not was added for the indicated time (3). Cells were then fixed with 3.7% formaldehyde for 5 min and permeabilized with 0.1% Triton X-100 in PBS for 2 min. After blocked using PBS containing 0.05% Tween (TPBS) and 1% BSA, cells were stained for integrin β1 with Texas Red-conjugated goat anti-mouse IgG (Jackson ImmunoResearch). Slides were examined using a Delta Vision wide field deconvolution microscope (Applied Precision).
Immunoprecipitation and Western Blotting
Cells transfected with corresponding plasmids were collected and lysed in buffer as follows: 50 mm HEPES, pH 7.4, 150 mm NaCl, 2 mm EGTA, 0.5% Triton X-100, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml pepstatin A. Cell lysates were clarified using a centrifugation at 13,000 rpm for 20 min at 4 °C, and the supernatants were mixed with FLAG-M2 affinity matrix (Sigma). After being washed five times with the lysis buffer, beads were boiled in SDS-PAGE sample buffer. Subsequently, the samples were subjected to the SDS-polyacrylamide gel, transferred onto nitrocellulose membrane, and probed with indicated antibodies followed by detection with ECL (Pierce). The intensity of bands was quantified by ImageJ (National Institutes of Health). Immunoprecipitation of endogenous proteins was carried using anti-ACAP4 mouse antibody followed by protein A/G beads (Pierce) as described above.
Pulldown Assay
Recombinant proteins including GST and His-tag fusion proteins were isolated from bacteria with agarose beads using protocols described previously (12). Pulldown assay was carried out by mixing purified proteins or cell lysates with Sepharose beads associated GST recombinant proteins. After incubation for 2 h, the beads were washed five times with PBS containing 0.5% Triton X-100 and boiled in SDS-PAGE sample buffer. Then the samples were separated with SDS-polyacrylamide gel followed by Coomassie Brilliant Blue staining and Western blotting with appropriate antibodies.
Immunofluorescence Microscopy
HeLa and COS7 cells transfected with corresponding plasmids or siRNA were fixed for 5 min in 3.7% paraformaldehyde and permeabilized for 2 min with 0.1% Triton X-100 in PBS. Coverslips were then blocked with TPBS with 1% BSA for 1 h. Fixed and permeabilized cells were then stained with primary antibodies for 1 h and washed three times with TPBS. Cross-absorbed secondary antibodies were then added, and after washing with TPBS, coverslips were visualized under Delta Vision deconvolution microscopy.
Biochemical Assay of Integrin β1 Recycling
Transfected HeLa cells were subjected to starvation in DMEM/BSA overnight and binding with integrin β1 antibody for 1 h at 4 °C. After quickly washing twice with cold DMEM/BSA, cells were incubated with pre-warmed DMEM/BSA for 2 h to induce internalization. Cells were then washed with glacial acid wash buffer (0.5% acetic acid, 0.5 m NaCl, pH 3.0) to remove the excess integrin β1 antibody at the cell surface. After stimulation with 0.1 μg/ml EGF for 5 min, cells were washed with acid buffer again on ice. The lysis buffer was then added to the cells, and antibody-bound integrin β1 was pulled out using protein A/G (4). Western blotting against integrin β1 was carried out after samples were fractionated using SDS-polyacrylamide gel.
Flow Cytometry
The steady-state levels of integrin β1 on the cell surface were measured using flow cytometry (4). Transfected HeLa cells were detached using 1 mm EGTA, 4 mm EDTA in PBS and fixed in cold 3.7% paraformaldehyde. Then the cells were labeled with AlexaFluor 647-conjugated integrin β1 antibody, and the median surface integrin β1 levels in GFP-positive cells were quantified by flow cytometry.
Cell Adhesion, Cell Spreading, and Cell Migration Assay
For cell adhesion assay, HeLa cells were transiently transfected and cultured for 24 h. Cells were detached by incubating with 1 mm EGTA, 4 mm EDTA in PBS at 37 °C for 10 min and dissociated into single cells. After washing once with serum-free medium, the cells were aliquoted into plates coated with different concentrations of fibronectin. After adhesion for 1 h, nonadherent cells were washed with DMEM/BSA, and adherent cells were fixed with 3.7% paraformaldehyde. To quantify the adhesion efficiency, fixed cells were first incubated with 5 mg/ml crystal violet in 20% ethanol for 15 min and were then allowed to be extensively washed by water. After plates completely dried, 2% SDS was added to the plates for 30 min. The dye extraction was then quantified with an ELISA reader at 570 nm.
For the cell spreading assay, COS7 cells were treated similarly as described above for the adhesion assay by plating cells on coverslips coated with 10 μg/ml fibronectin. After 1 h, COS7 cells were fixed and imaged with microscopy. The areas of cell spreading were then quantified by software ImageJ (National Institutes of Health). Statistic significance was determined by t test.
For cell migration assay, transfected MDA-MB-231 cells were seeded on a glass bottom culture dish (MatTek, MA) coated with 10 μg/ml fibronectin. After addition of 0.1 μg/ml EGF into Leibovitz's L-15 medium (Invitrogen), the routes of cell migration were recorded by a Delta Vision DVI system at 37 °C every 30 s for 1 h.
RESULTS
ACAP4 Functions at Integrin β1 Recycling in EGF Stimulation
Our recent proteomic study has identified the function of ACAP4 in EGF-stimulated volatile membrane remodeling and cell migration (9). To delineate the ACAP4 molecular machinery underlying EGF-elicited cell migration, we carried out mass spectrometric analyses of ACAP4 protein complex isolated from EGF-stimulated HeLa cells as described previously (9).4 Mass spectrometric analyses and Western blotting experiments confirm the presence of integrin β1 and several other proteins presented in the ACAP4 immunoprecipitates.
Integrin β1 constantly undergoes endocytosis and recycling, which are crucial for cell motility (21, 22). Previous studies showed that ARF6 activation was indispensable for integrin β1 recycling, and ACAP4 played an important role in cell migration and invasion as a potential GAP for ARF6 (3, 6, 7, 9–11). These studies indicated that ACAP4 might regulate cell motility by serving as the ARF6 catalyst to influence integrin β1 recycling.
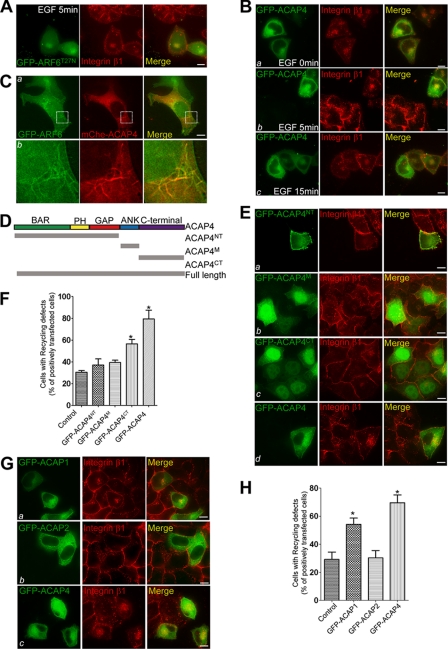
To validate our hypothesis, integrin β1 recycling was analyzed by immunocytochemical staining in HeLa cells transfected with GFP-ARF6T27N, which was revealed to inhibit the integrin β1 recycling, or GFP-ACAP4 (3). After starvation and labeling by integrin β1 antibody, cells were made to internalize the antibody-bound integrins for 2 h. Subsequently, cells were stimulated with EGF (0.1 μg/ml) and fixed at different time points as follows: 0, 5, and 15 min. Within 5 min of EGF stimulation, most of the endosomal pool of integrin β1 redistributed to the cell surface in nontransfected cells, whereas both GFP-ARF6T27N and GFP-ACAP4 were found to block integrin β1 recycling to the cell periphery (Fig. 1, A, 2nd panel, and B, panel b). Integrin β1 was focally accumulated, which was reminiscent of that under starvation conditions (Fig. 1B, panel a). We also observed significant co-localization of the dominant negative form of ARF6 and ACAP4 with integrin β1 at the juxtanuclear endocytic recycling compartment. Therefore, we concluded that overexpressed ACAP4 co-localized with internal integrin β1 and inhibited its recycling stimulated by EGF.
FIGURE 1.
Overexpression of ACAP4 inhibits EGF-stimulated integrin β1 recycling. A, ARF6T27N overexpression inhibits integrin β1 recycling from endocytic recycling compartment to the plasma membrane. HeLa cells grown on coverslips were transiently transfected with GFP-ARF6T27N. After 24 h of expression, HeLa cells were deprived of serum overnight and allowed to internalize antibody-bound integrin β1 for 2 h. Subsequently, HeLa cells were treated with 0.1 μg/ml EGF for 5 min, fixed, permeabilized, and stained for GFP-ARF6T27N (green) and integrin β1 (red). Overexpression of GFP-ARF6T27N suppresses the normal recycling of integrin β1 shown in nontransfected HeLa cells. In GFP-ARF6T27N-transfected cells, integrin β1 is mainly internally accumulated, which co-localizes with GFP-ARF6T27N. Bar, 10 μm. B, ACAP4 overexpression similarly suppresses the EGF-stimulated integrin β1 recycling. This set of optical images was collected from GFP-ACAP4 overexpressed HeLa cells that were subjected to integrin β1 endocytic recycling assay as indicated in A and stained for GFP-ACAP4 (green) and integrin β1 (red). In transfected cells, integrin β1 is focally accumulated in the pericentriolar endosomes together with GFP-ACAP4. However, integrin β1 is recycled and has a diffuse surface distribution in nontransfected cells within 5 min of EGF addition. Bars, 10 μm. C, ACAP4 co-localizes with ARF6 to the tubular endosomes. HeLa cells were co-transfected with GFP-ARF6 and mCherry-ACAP4. After transfection for 24 h, the subcellular distributions of ACAP4 (red) and ARF6 (green) were visualized with real time imaging. Enlarged images are also shown in panel b. Bar, 10 μm. D, schematic drawing of ACAP4 structure features and its truncation mutants. ACAP4NT, residues 1–550 of ACAP4; ACAP4M residues 560–660 of ACAP4; ACAP4CT, residues 660–903 of ACAP4; Full-length, full-length ACAP4. E, this montage represents images collected from GFP-ACAP4 and its deletion mutant (green)-transfected HeLa cells. After 24 h of transfection, the integrin β1 (red) recycling of transfected cells was tested as indicated in A. Bars, 10 μm. F, quantitative analyses of the effect of ACAP4 and its deletion mutants on EGF-stimulated integrin β1 recycling. The number of transfected cells whose EGF-stimulated integrin β1 recycling was decreased was compared with that of nontransfected cells shown in E. Values represent the means ± S.E. of 100 cells from three different preparations. The error bars represent S.E. * indicates significant difference from that of GFP-ACAP4 (p < 0.05 by t test). G, this set of optical images was collected from HeLa cells transfected with GFP-ACAP1, GFP-ACAP2, and GFP-ACAP4, respectively. After 24 h of transfection, cells were starved, labeled with integrin β1 antibody, and subjected to the EGF-stimulated integrin β1 recycling assay. Cells were then fixed and stained for ACAPs (green) and integrin β1 (red). Bars, 10 μm. H, quantitation of the influence of ACAP1, ACAP2, and ACAP4 overexpression on EGF-stimulated integrin β1 recycling in G. Values represent the means ± S.E. of 100 cells from three different preparations. The error bars represent S.E. * indicates significant difference from that of GFP-ACAP4 (p < 0.05 by t test).
To ascertain the function of ACAP4 in ARF6-mediated integrin β1 recycling, we co-transfected HeLa cells with GFP-ARF6 and mCherry-ACAP4. As shown in Fig. 1C, real time imaging of transfected cells demonstrated that ACAP4 co-localized to the juxtanuclear and tubular recycling compartment with ARF6. This observation confirmed the role of ACAP4 in ARF6-mediated clathrin-independent cargo recycling.
To further determine the functional domain of ACAP4 regulating EGF-stimulated integrin β1 recycling, the full-length ACAP4 cDNA was split based on the structural feature of ACAP4 and inserted into the GFP vector (Fig. 1D). The truncated versions were GFP-ACAP4NT (1–550 amino acids), GFP-ACAP4M (560–660 amino acids), and GFP-ACAP4CT (660–903 amino acids). These deletion mutants and full-length ACAP4 were transfected into HeLa cells for 24 h and then subjected to integrin β1 recycling assay with EGF stimulation for 5 min. As shown in Fig. 1, E and F, integrin β1 clustered at juxtanuclear endosomes in cells overexpressing GFP-ACAP4 and GFP-ACAP4CT. However, integrin β1 in cells transfected with GFP-ACAP4NT or GFP-ACAP4M recycled to the cell periphery normally. Thus, these results suggest that the C-terminal region of ACAP4 contributes to the recycling of integrin β1 upon EGF addition.
Because ACAP1 overexpression was reported to suppress endocytic recycling of integrin β1 (7), we carried out experiments to examine the influence of several ARF6 GAPs on EGF-stimulated integrin β1 recycling. GFP-ACAP1, GFP-ACAP2, and GFP-ACAP4 were transfected into HeLa cells. After 24 h of transfection, HeLa cells were deprived of serum overnight and labeled with integrin β1 antibody. EGF was then added to stimulate integrin β1 recycling. Results showed that both ACAP1 and ACAP4 blocked the integrin β1 recyling from the juxtanuclear recycling compartments to the cell membrane upon EGF stimulation for 5 min (Fig. 1, G and H, and data not shown). Thus, we conclude that ACAP4 functions to regulate the integrin β1 recycling downstream of EGF.
Grb2 Physically Interacts with ACAP4 in Cell Migration
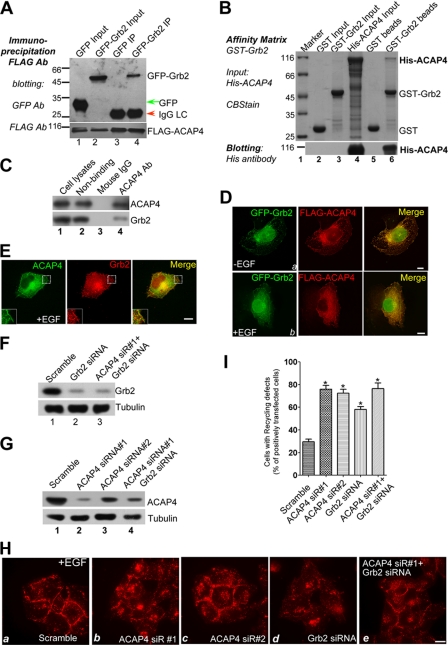
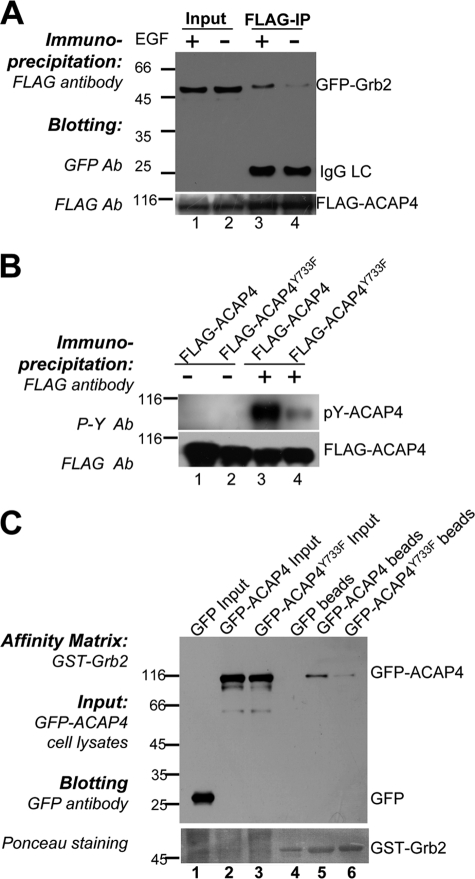
To further explore the underlying mechanism of integrin β1 recycling regulated by ACAP4, we performed yeast two-hybrid assay with full-length ACAP4 as the bait. Several positive hits pointed to Grb2, an important adaptor protein of growth factor receptors (14, 23, 24), as an interacting partner for ACAP4. To confirm the physical interaction between ACAP4 and Grb2, we performed a co-immunoprecipitation assay using anti-FLAG-M2 affinity beads. As shown in Fig. 2A, Western blotting with FLAG and GFP antibody revealed a successful co-precipitation of GFP-Grb2 with FLAG-ACAP4 from 293T cell lysates. To assess whether ACAP4 directly interacts with Grb2, we carried out a pulldown assay using bacterially recombinant proteins. Glutathione S-transferase (GST)-Grb2 fusion protein was generated and immobilized on agarose beads and mixed with purified His tag-fused ACAP4. After extensive washing, bound proteins were eluted from the beads and fractionated by SDS-PAGE. Coomassie Brilliant Blue staining as well as Western blotting with His antibody showed that GST-Grb2 readily bound His-ACAP4, although GST alone did not (Fig. 2B, lanes 5 and 6). The above observations indicated that Grb2 adaptor protein acted as a binding partner of ACAP4. To test if ACAP4 forms a cognate complex with Grb2, aliquots of HeLa cell lysates were incubated with anti-ACAP4 mouse antibody and mouse IgG. The mouse antibodies were then captured by protein A/G-agarose beads followed by extensive washes to remove nonspecific binding proteins. As shown in Fig. 2C, Grb2 was pulled down by ACAP4 antibody but not control mouse IgG (lanes 4 and 3, respectively), validating the biochemical interaction between ACAP4 and Grb2.
FIGURE 2.
ACAP4-Grb2 interaction is required for EGF-induced integrin β1 endocytic recycling. A, interaction of ACAP4 with Grb2 in vivo. Cells transiently transfected with FLAG-ACAP4 and GFP or FLAG-ACAP4 and GFP-Grb2 were lysed and incubated with FLAG antibody-associated affinity matrix (lanes 3 and 4). Immunoprecipitates were fractionated by SDS-PAGE and transferred onto nitrocellulose membrane followed by probing with GFP (upper panel) and FLAG (lower panel) antibody. B, interaction of ACAP4 with Grb2 in vitro. GST-Grb2 was purified and immobilized on glutathione-agarose beads to absorb purified recombinant His-ACAP4. Samples were separated by SDS-PAGE followed by Coomassie Brilliant Blue stain (lower panel) and immunoblotting with His antibody (upper panel). C, endogenous interaction of ACAP4 with Grb2. After EGF stimulation, HeLa cells were lysed and incubated with ACAP4 antibody-associated affinity matrix (lane 4). Immunoprecipitates were fractionated by SDS-PAGE and transferred onto nitrocellulose membrane followed by probing with ACAP4 (upper panel) and Grb2 (lower panel) antibody. D, ACAP4 and Grb2 co-localize at the cell periphery. COS7 cells grown on coverslips were co-transfected with GFP-Grb2 and FLAG-ACAP4. After transfection for 24 h, cells were starved overnight followed by a 5-min treatment of EGF (0.1 μg/ml). Subsequently, cells were fixed, permeabilized, and stained for GFP-Grb2 (green) and FLAG-ACAP4 (red). Merged images on the right show in cells without stimulation that Grb2 and ACAP4 are co-localized to the plasma membrane. In EGF-stimulated cells, the co-distribution of Grb2 and ACAP4 is found more obviously on the membrane ruffles. Bars, 10 μm. E, endogenous localization of ACAP4 and Grb2. COS7 cells grown on coverslips were starved overnight followed by a 5-min treatment of EGF (0.1 μg/ml). Subsequently, cells were fixed, permeabilized, and stained for Grb2 (red) and ACAP4 (green). Bars, 10 μm. F, efficiency of Grb2 siRNA oligonucleotides. HeLa cells were transfected with the Grb2 siRNA oligonucleotides and scramble oligonucleotides for 48 h. Aliquots of the samples were subjected to Western blotting using Grb2 and tubulin antibodies (upper panel, Grb2 blot; lower panel, tubulin blot). G, efficiency of ACAP4 siRNA oligonucleotides. HeLa cells were treated with the ACAP4 siRNA oligonucleotides for 48 h and subjected to Western blotting using ACAP4 and tubulin antibodies (upper panel, ACAP4 blot; lower panel, tubulin blot). H, suppression of ACAP4 and Grb2 expression inhibits the EGF-stimulated integrin β1 recycling. This montage was collected from HeLa cells transfected with siRNA against mock, ACAP4, or Grb2. 48 h after transfection, cells were subjected to the integrin β1 recycling assay with 5 min of EGF stimulation. Stimulation of scrambled siRNA-transfected cells induces a redistribution of the endosomal accumulated integrin β1 to the plasma membrane, and suppression of ACAP4 and Grb2 by siRNA oligonucleotides decreases the levels of intracellular integrin β1 returning to the cell surface. Bar, 10 μm. I, percentage of cells in which focally accumulated integrin β1 fails to recycle to the cell surface within 5 min of EGF stimulation. Values represent the means ± S.E. of 200 cells from three separate experiments. The error bars represent S.E. * indicates significant difference from that of GFP-ACAP4 (p < 0.05 by t test).
We further examined the subcellular distribution of ACAP4 with respect to Grb2 upon EGF stimulation. FLAG-ACAP4 and GFP-Grb2 were co-transfected into COS7 cells, and the resulting phenotypes were detected for ACAP4 and Grb2 labeling with an immunofluorescence microscope. In the serum-starved cells, co-localization of the two proteins was found on the plasma membranes (Fig. 2D, panel a). After stimulation with EGF for 5 min, the level of super-imposition of two signals was strengthened in the EGF-stimulated cells when their distribution to the plasma membrane ruffling becomes readily apparent (Fig. 2D, panel b). The co-distribution profile of endogenous ACAP4 and Grb2 in EGF-stimulated HeLa cells was also examined. As shown in Fig. 2E, the distribution pattern of ACAP4 largely overlapped with that of Grb2 localization, supporting the notion that ACAP4 interacts with Grb2. These results demonstrate that ACAP4 was recruited to the membrane ruffles together with Grb2.
ACAP4 and Grb2 Coordinate the Recycling Process of Integrin β1
To evaluate the effects of endogenous ACAP4 on integrin β1 intracellular traffic, we reduced the ACAP4 expression with siRNA oligonucleotide duplex (siRNA1 and siRNA2 targeted to two different regions) and tested its effects on integrin β1 recycling in HeLa cells (Fig. 2, G and H). Interestingly, integrin β1 recycling in these cells was also disrupted reminiscent of that with ACAP4 overexpression (Fig. 2H, panels a and b). As described above, Grb2 readily bound ACAP4 in vivo. Moreover, Grb2 was originally identified as an adaptor protein recruiting downstream effectors to EGFR (13). Thus, it was expected that Grb2 might function downstream of EGFR to transduce the activation signal to ACAP4 and regulate the recycling of integrin β1. To test this, we transfected Grb2-targeted siRNA into HeLa cells for 48 h, and Western blotting with Grb2 antibody revealed a suppression of the Grb2 protein level (Fig. 2F). Meanwhile, the immunofluorescence results showed that reduced Grb2 expression effectively abrogated the normal integrin β1 recycling. Most of the integrin β1 was still restricted to the perinuclear regions in cells transfected with Grb2 targeting siRNA (Fig. 2H, panels a and d). Quantitative analysis revealed that with ACAP4 and Grb2 knockdown, the percentage of cells whose integrin recycling was disrupted was increased by 157 and 97% respectively (Fig. 2I; p < 0.05). Another siRNA oligonucleotide (siRNA2) targeted to a different sequence of ACAP4 gave a similar suppression profile for ACAP4 protein accumulation (Fig. 2G, lane 3) and integrin β1 recycling perturbation (Fig. 2, H, panel c, and I). Dual suppression of ACAP4 and Grb2 gave a suppression profile similar to that of ACAP4 knockdown alone. These data suggested that ACAP4 and Grb2 were required for the intracellular traffic of integrin β1, and their effects were linked.
Mapping the ACAP4-Grb2 Interaction Interfaces
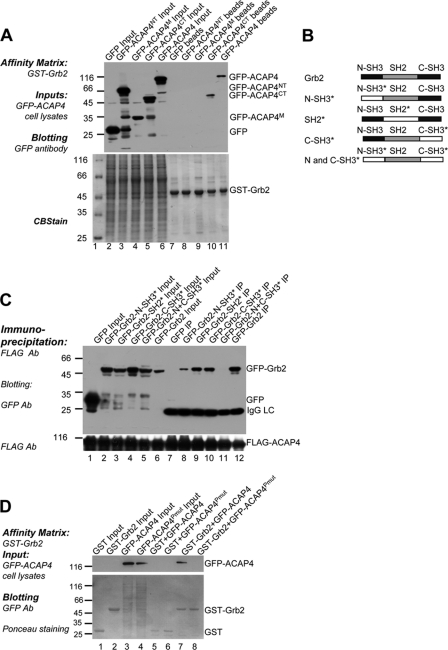
To figure out the region on ACAP4 mediating the interaction with Grb2, the full-length ACAP4 and its different truncations as shown in Fig. 1D were transiently transfected into 293T cells. The cell lysates were then incubated with purified GST-Grb2 on GST affinity beads. After extensive washing, the full-length GFP-ACAP4 and its C-terminal region GFP-ACAP4CT were found to be absorbed by the GST-Grb2 (Fig. 3A, lanes 10 and 11).
FIGURE 3.
Biochemical characterization of ACAP4-Grb2 interacting domains. A, Grb2 associates with the region of 660–903 residues on ACAP4. GFP-ACAP4 and its truncations as shown in Fig. 1D were transfected into cells for 24 h. GST-Grb2 affinity matrix was incubated with the aliquots of transfected cell lysates followed by washing five times and Western blotting with GFP antibody (upper panel, GFP blot; lower panel, Coomassie Brilliant Blue staining). B, schematic illustration of Grb2 motifs and chimeric Grb2 with point mutations corresponding to loss-of-function of its domains. N-SH3*, P49L; SH2*, R86K; C-SH3*, G203R; N and C-SH3*, P49L, and G203R. C, both of the SH3 domains and the SH2 domain of Grb2 contribute to the ACAP4-Grb2 association. FLAG-ACAP4 was co-transfected with GFP, GFP-Grb2, or its point mutants described in B into HeLa cells for 24 h. After being deprived of serum overnight, HeLa cells were subjected to EGF stimulation for 5 min followed by immunoprecipitation with FLAG-M2 beads. Subsequently, Western blotting with antibody against GFP revealed that both C-SH3* and SH2* mutants reduce the interaction of Grb2 with ACAP4, whereas the N-SH3* mutant abolishes their association the most. D, polyproline domain on ACAP4 is responsible for its interaction with Grb2. GST-Grb2 affinity matrix isolates GFP-ACAP4 (lane 7) but not GFP-ACAP4Pmut (lane 8) from 293T cell extracts (upper panel, GFP blot; lower panel, Coomassie Brilliant Blue staining).
Grb2 contains two SH3 domains separated by one SH2 domain. To delineate the motif on Grb2 interacting with ACAP4, we generated several mutants of Grb2 that disrupted the functional domains of Grb2 by key residue point mutations as reported previously (17, 25, 26). These mutants included GFP-Grb2-N-SH3* (P49L), GFP-Grb2-SH2* (R86K), GFP-Grb2-C-SH3* (G203R), and GFP-Grb2-N and C-SH3* (P49L and G203R) (Fig. 3B). Co-immunoprecipitation experiment was carried out using lysates from EGF-stimulated HeLa cells transfected with FLAG-ACAP4 and the GFP-Grb2 mutations, respectively. Western blotting results showed that all of the mutations listed above weakened the ACAP4-Grb2 interaction. However, the point mutations of GFP-Grb2-N-SH3* and GFP-Grb2-N and C-SH3* impaired its ACAP4 binding activity the most (Fig. 3C). Generally, the aforementioned results showed that Grb2 N-SH3 domain is a major contributor to the Grb2-ACAP4 interaction, whereas their optimal association also required SH2 and C-SH3 domains of Grb2.
Given the fact that SH3 domains often bind the proline-rich region, we attempted to characterize this motif on ACAP4. The C terminus of ACAP4 contained a polyproline domain (752–756 amino acids) suggesting that the interaction between ACAP4 and Grb2 might be mediated by this motif. To test this hypothesis, we generated the GFP-ACAP4Pmut mutant by replacing the residues polyproline domain on ACAP4 with AAAA and performed pulldown assay between Grb2 and ACAP4. Extracts from cells transfected with GFP, GFP-ACAP4, and GFP-ACAP4Pmut, respectively, were mixed with GST-Grb2 affinity matrix. As expected, GFP-ACAP4Pmut whose polyproline was abolished had an obvious reduced association with Grb2 (Fig. 3D, lanes 7 and 8). Thus, we concluded that the polyproline domain locating on the C terminus of ACAP4 was responsible for its binding to Grb2.
ACAP4-Grb2 Interaction Is Essential for Cell Adhesion and Migration on Fibronectin
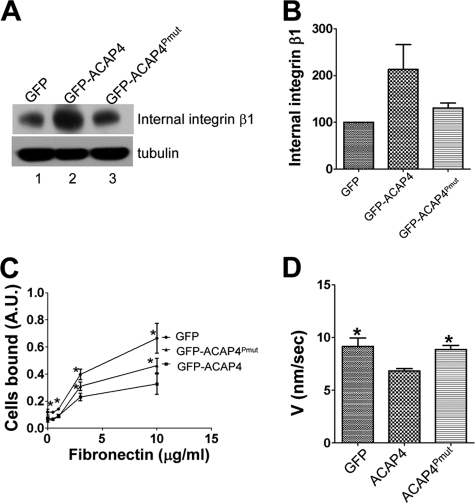
To probe whether the association between ACAP4 and Grb2 was involved in the process of integrin β1 recycling, we perturbed this association using GFP-ACAP4Pmut mutant. The biochemical recycling assay was carried out to quantify the intracellular integrin β1 in GFP-, GFP-ACAP4-, and GFP-ACAP4Pmut-transfected cells (4). After EGF stimulation, internal antibody-bound integrin β1 was isolated and fractionated by SDS-PAGE followed by transferring onto nitrocellulose membranes for anti-integrin β1 Western blotting. As shown in Fig. 4, A and B, the abolishment of ACAP4-Grb2 interaction eliminated the inhibition of integrin β1 recycling caused by GFP-ACAP4 overexpression. To exclude the possibility that ACAP4 influenced integrin expression or degradation, we generated cells overexpressing GFP, GFP-ACAP4, and GFP-ACAP4Pmut, respectively, and probed the whole cell lysates with anti-integrin β1 antibody. Because the amount of integrin β1 remained unchanged,4 we therefore concluded that ACAP4-Grb2 interaction was critical for ACAP4 to regulate integrin β1 trafficking from endocytic recycling compartments to the plasma membranes.
FIGURE 4.
ACAP4-Grb2 interaction regulates EGF-stimulated integrin β1 recycling and cell motility on fibronectin. A, HeLa cells overexpressing GFP, GFP-ACAP4, or GFP-ACAP4Pmut were labeled with integrin β1 antibody and allowed to internalize the antibody-bound integrin β1 for 2 h. After addition of EGF for 5 min, integrin β1 antibody remaining on the cell surface was washed away as described under “Materials and Methods.” Intracellular antibody-bound integrin β1 was isolated by protein A/G and analyzed by Western blotting. B, after EGF stimulation for 5 min, internal integrin β1 in cells overexpressing GFP-ACAP4 or GFP-ACAP4Pmut as shown in A was quantified and normalized to that of GFP-transfected cells. C, HeLa cells were transiently transfected with GFP, GFP-ACAP4, or GFP-ACAP4Pmut. After 24 h of expression, transfected cells were collected and seeded on several concentrations of fibronectin for 1 h. Subsequently, adherent cells were fixed and stained with crystal violet. The amount of adherent cells was quantified with ELISA reader as described under “Materials and Methods.” * indicates significant difference from that of GFP-ACAP4 (p < 0.05 by t test). D, instantaneous velocities of MDA-MB-231 cells transiently transfected with GFP, GFP-ACAP4, or GFP-ACAP4Pmut are presented. After cells were stimulated with EGF, paths of the centroids of at least 20 MDA-MB-231 cells from three experiments were recorded. The error bars represent S.E. * indicates significant difference from that of GFP-ACAP4 (p < 0.05 by t test).
Integrin β1 pairs with α5 to form receptor mediating the attachment between the cell and the extracellular matrix fibronectin. The above experiments revealed that ACAP4 mediated EGF-induced integrin β1 intracellular traffic at the aspect of endocytic recycling. We next set to investigate if ACAP4 influenced the cell adhesion activity on fibronectin. To this end, HeLa cells overexpressing GFP, GFP-ACAP4, or GFP-ACAP4Pmut were seeded on different concentrations of fibronectin for 1 h, and then nonadhesion cells were washed away. After cells were stained with crystal violet, we quantified the adhesion cells on fibronectin by an ELISA reader. The results showed that control cell-transfected GFP bound the matrix more efficiently than GFP-ACAP4Pmut-transfected cells on 3 and 10 μg/ml fibronectin-coated plates. The cell adhesion level was further reduced with GFP-ACAP4 overexpressing cells (Fig. 4C).
To directly assess the importance of ACAP4-Grb2 association on cell motility, we carried out cell migration assay. MDA-MB-231 cells were transiently transfected to express GFP, GFP-ACAP4, and GFP-ACAP4Pmut, respectively. MDA-MB231 cells were chosen because of their fast migration rate suitable for microscopic analyses. After EGF stimulation, the routes of cell migration were recorded for 1 h. As shown in Fig. 4D, our statistic analyses show that GFP-ACAP4Pmut-transfected cells migrated faster than GFP-ACAP4-transfected cells (*, p < 0.05). Therefore, we can conclude that the interaction between ACAP4 and Grb2 is not only essential for EGF-stimulated integrin β1 recycling, but also affects cell adhesion and migration as well.
Tyr-733 Phosphorylation Regulates ACAP4-Grb2 Interaction
As described above, ACAP4 plays an important role in EGF-stimulated integrin β1 endocytic recycling. We set to examine if the association of ACAP4 and Grb2 was also altered by EGF stimulation. HeLa cells were co-transfected with FLAG-ACAP4 and GFP-Grb2, and after 6 h of starvation in serum-free culture medium, cells were subjected to 0.1 μg/ml EGF stimulation for 5 min or not. As shown in Fig. 5A, immunoprecipitation assay using anti-FLAG antibody revealed that ACAP4-Grb2 association was strengthened with addition of EGF.
FIGURE 5.
Phosphorylation at tyrosine 733 of ACAP4 induces its association with Grb2. A, EGF stimulation strengthens the ACAP4-Grb2 association. HeLa cells overexpressing FLAG-ACAP4 and GFP-Grb2 were subjected or not to EGF stimulation for 5 min. Extracts of the cells were absorbed by FLAG-M2 antibody, and co-precipitated GFP-Grb2 was detected by immunoblotting (upper panel, GFP blot; lower panel, FLAG blot). B, tyrosine 733 on ACAP4 is phosphorylated after EGF stimulation in HeLa cells. FLAG-ACAP4 and FLAG-ACAP4Y733F were immunoprecipitated from cells with or without EGF stimulation. Samples were then immunoblotted with antibody against phosphotyrosine. C, Y733F mutation of ACAP4 reduces its association with Grb2. GST-Grb2-bound affinity matrix was incubated with lysates of cells transfected with GFP, GFP-ACAP4, and GFP-ACAP4Y733F, respectively. After washing five times, the affinity matrix was boiled in SDS-PAGE buffer and subjected to SDS-polyacrylamide gel followed by Western blotting with GFP antibody (upper panel, GFP blot; lower panel, Ponceau staining).
In response to growth factor stimulation, a number of protein kinases were activated. The aforementioned results demonstrated that the interaction between ACAP4 and Grb2 was weakened by the point mutation of the Grb2 SH2 domain, which was a conserved motif of 100 amino acids and preferentially bound to the phosphorylated tyrosine-containing motif. Therefore, we assessed whether the ACAP4-Grb2 association was also mediated by the tyrosine phosphorylation within ACAP4.
Using software GPS 2.0, which predicts the potential phosphorylation sites for human protein kinases (27), tyrosine 733 was determined as one of the most likely phosphorylation sites of ACAP4, which was confirmed later by mass spectrum in normally derived human mammary epithelial cell line (28). To elucidate whether the Tyr-733 was also phosphorylated in HeLa cells, we constructed the FLAG-ACAP4Y733F mutant by substituting tyrosine residue with phenylalanine. The cells transfected with FLAG-ACAP4 and FLAG-ACAP4Y733F were starved for 6 h. After being stimulated with EGF for 5 min, cells were subjected to immunoprecipitation with FLAG-M2 antibody. The immunoblotting results with antibody targeting phosphorylated tyrosine revealed that the tyrosine residues on ACAP4 were largely phosphorylated after EGF stimulation. Moreover, the phosphorylation level of FLAG-ACAP4Y733F was lower than that of FLAG-ACAP4, which confirmed that EGF indeed induced Tyr-733 phosphorylation on ACAP4 not only in human mammary epithelial cell but also in HeLa cells (Fig. 5B).
We next examined the effect of ACAP4 Tyr-733 phosphorylation on ACAP4-Grb2 association. To this end, GFP-ACAP4 and GFP-ACAP4Y733F were overexpressed in HeLa cells, and after EGF stimulation, cell lysates were incubated with GST-Grb2-associated affinity matrix. Results showed that the binding activity of GFP-ACAP4Y733F to GST-Grb2 obviously decreased compared with GFP-ACAP4 (Fig. 5C, lanes 5 and 6). Thus, we concluded that EGF stimulation triggered residue Tyr-733 to be phosphorylated, and this modification positively regulated the ACAP4-Grb2 binding. To confirm this notion, we also constructed mutant GFP-ACAP4Pmut and GFP-ACAP4Y733F by double mutating the polyproline region and tyrosine 733 on ACAP4, and we probed for the requirement of these sites for its association with Grb2. Upon EGF stimulation, GFP-ACAP4Pmut and GFP-ACAP4Y733F mutants eliminated the association of ACAP4 and Grb2, whereas GFP-ACAP4Pmut and GFP-ACAP4Y733F reduced the binding to a different extent.4 We therefore conclude that the ACAP4-Grb2 interaction was regulated by the polyproline region and the phosphorylation of tyrosine 733 on ACAP4.
EGF-induced ACAP4 Phosphorylation Promotes the Recycling of Integrin β1 and Cellular Dynamics
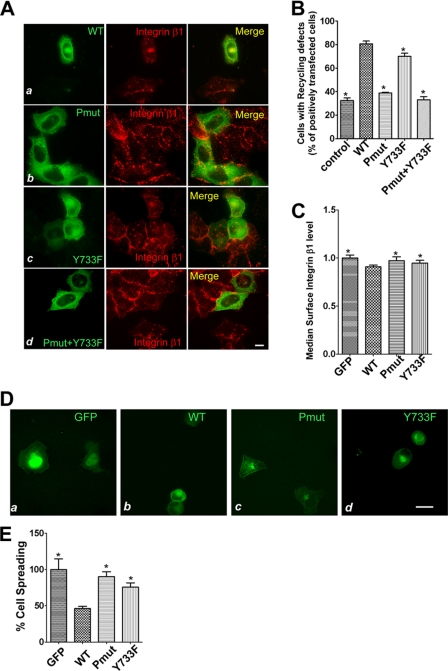
Given the enhanced ACAP4-Grb2 association induced by EGF, we sought to examine whether ACAP4 Tyr-733 phosphorylation affected the recycling of integrin β1. After starvation, cells overexpressing GFP-ACAP4, GFP-ACAP4Pmut, GFP-ACAP4Y733F, or GFP-ACAP4Pmut and GFP-ACAP4Y733F were allowed to recycle the endosomally accumulated integrin β1 under the EGF stimulation. In nontransfected control cells stimulated with EGF for 5 min, only 32% of total cells displayed a pattern of intracellular congregated integrin β1, whereas 68% of the cells showed a diffuse surface distribution of integrin β1. In the meantime, the proportion of cells whose integrin β1 recycling was inhibited was determined as follows: GFP-ACAP4 was about 81%, GFP-ACAP4Pmut reached 39%, GFP-ACAP4Y733F group was about 70%, and GFP-ACAP4Pmut and GFP-ACAP4Y733F was about 33% (Fig. 6, A and B). These results ascertained our hypothesis that the endocytic recycling trafficking of integrin β1 was dependent on the association of ACAP4 with Grb2. Moreover, compared with GFP-ACAP4, GFP-ACAP4Y733F was also found to disrupt the integrin β1 recycling to a less extent.
FIGURE 6.
EGF-elicited ACAP4-Grb2 association governs integrin β1 trafficking and cell spreading on fibronectin. A, optical images were collected from HeLa cells overexpressing GFP-ACAP4, GFP-ACAP4Pmut, GFP-ACAP4Y733F, or GFP-ACAP4Pmut and GFP-ACAP4Y733F. Cells were subjected to the EGF-stimulated integrin β1 recycling assay and stained for GFP-ACAP4 mutants (green) and integrin β1 (red). Bars, 10 μm. B, quantification of the influence of ACAP4 and its mutants on integrin β1 recycling. The data represent the proportions of cells shown in A whose integrin β1 was unable to recycle to the plasma membrane despite EGF stimulation for 5 min. Values represent the means ± S.E. of 100 cells from three different preparations. The error bars represent S.E. * indicates significant difference from that of GFP-ACAP4 (p < 0.05 by t test). C, integrin β1 levels on the cell surface of GFP-, GFP-ACAP4-, GFP-ACAP4Pmut-, and GFP-ACAP4Y733F-transfected cells were determined using flow cytometry. After 24 h of transfection, cells were fixed, stained with AlexaFluor 647-conjugated integrin β1 antibody, and subjected to flow cytometric analysis. Data represent the median intensity of surface integrin ± S.E. of over 10,000 transfected cells. The error bars represent S.E. * indicates significant difference from that of GFP-ACAP4 (p < 0.05 by t test). D, COS7 cells were transfected with GFP, GFP-ACAP4, GFP-ACAP4Pmut, and GFP-ACAP4Y733F for 24 h. Subsequently, cells were harvested by blow-off and then seeded on 10 μg/ml fibronectin for 1 h. This set of optical images was collected after cells were fixed, and the GFP tag proteins are visualized in green. Bar, 10 μm. E, quantitative analysis of the spreading areas of cells in D using ImageJ software. At least 60 cells of each mutant were collected, and their spreading areas were calculated. Data were presented as means ± S.E. of from three separated experiments. * indicates significant difference from that of GFP-ACAP4 (p < 0.05 by t test).
To confirm the influence of ACAP4 and its interaction with Grb2 on integrin β1 trafficking, we examined the levels of integrin β1 on the cell surfaces using flow cytometry. After 24 h of transfection of GFP, GFP-ACAP4, GFP-ACAP4Pmut, or GFP-ACAP4Y733F, HeLa cells were subjected to fixation and labeled with AlexaFluor 647-conjugated integrin β1 antibody. Data determined by flow cytometry showed that the levels of integrin β1 were reduced on the surfaces of GFP-ACAP4-transfected cells. However, cells transfected with GFP-ACAP4Pmut and GFP-ACAP4Y733F elevated the levels of integrin β1 on their surfaces compared with GFP-ACAP4 overexpressed cells (Fig. 6C). Taken together, all these data showed that the polyproline domain and the tyrosine 733 phosphorylation of ACAP4 were crucial for the trafficking of integrin β1 to the cell surface. Furthermore, as demonstrated above, the Y733F mutation weakened the affinity of ACAP4 with Grb2, and this might be the reason for its effects on integrin β1 recycling. To eliminate the possibility that the GAP activity of ACAP4 was changed with GFP-ACAP4Pmut and GFP-ACAP4Y733F mutation, we treated HeLa cells with tetrafluoroaluminate (AlF4). Our previous data demonstrated that the GAP activity of ACAP4 inhibited the formation of protrusions with AlF4 treatment. Neither GFP-ACAP4Pmut nor GFP-ACAP4Y733F reduced the protrusions stimulated with AlF4, which indicated that these mutants did not modulate the GAP activity of ACAP4.5
We also examined the effect of ACAP4 on cell spreading using COS7 cells. To this end, GFP, GFP-ACAP4, GFP-ACAP4Pmut, or GFP-ACAP4Y733F was transiently transfected into COS7 cells. After 24 h, cells were harvested and plated on 10 μg/ml fibronectin for 1 h, and then the extent of cell spreading was calculated by measuring the cell areas using ImageJ. Data showed that cells transfected with GFP-ACAP4 were less well spread compared with GFP-expressing cells (Fig. 6, D and E). We also found that, compared with the effect of GFP-ACAP4 overexpression on cell spreading, GFP-ACAP4Pmut and GFP-ACAP4Y733F overexpression decreased the inhibitions on cell spreading at different degrees.
To conclude, these data not only demonstrated that ACAP4 played a key role in integrin β1 recycling and cell spreading but also ascertained the importance of EGF modulated ACAP4-Grb2 interaction on integrin β1 traffic and cell motility.
DISCUSSION
ACAP4, a GTPase-activating protein for ARF6, was originally identified as transcript that was highly expressed in hepatocellular carcinoma (9, 29). Biochemical and crystallographic analyses demonstrate that ACAP4 is a specific GAP for ARF6 (9, 11). Further research revealed that ACAP4 plays an important role in actin and membrane remodeling, such as growth factor stimulated cytoskeleton regulation, actin stress fiber formation, cell migration, and invasion (9–11). However, the function of ACAP4 in vesicle transport remains elusive. In this study, we demonstrated that ACAP4 orchestrates EGF stimulation-dependent integrin β1 recycling. Our studies showed that ACAP4 executed its function in vesicular trafficking via its interaction with Grb2 in a phospho-Tyr-733-dependent manner. This study demonstrates a novel mechanism underlying ACAP4, which orchestrates EGF-stimulated cellular dynamics via promoting vesicular trafficking of integrin β1.
Integrins are important regulators in the process of cell attachment to the extracellular matrix and have a remarkable impact on several cellular processes such as cell polarity, migration, division, and angiogenesis (31–34). Integrin α5β1, which is a transmembrane receptor for fibronectin, was found to be constantly endocytosed and recycled (35, 36). The spatiotemporally regulated trafficking of integrin β1 is now considered to be a main factor to influence integrin β1-involved cell functions (37). By far, several molecules governing integrin β1 endo-exocytic cycle have been identified, including ARF6 and Rab11. The dominant negative mutant of ARF6T27N was shown to inhibit the recycling of the major histocompatibility complex-I (MHC-I) and integrin β1 (37, 38). Previous studies about ARF GEFs have demonstrated that GEP100/Brag2 and ARNO are involved in integrin β1 endocytosis and recycling, respectively (4, 39). Besides, ACAP1, a member of the AZAP GAP group, has also been elucidated to mediate integrin β1 recycling along the tubular endosomes. Here, we showed that the transient overexpression or siRNA suppression of ACAP4 also led to dysfunctions of integrin β1 endocytic recycling. In fact, we were intrigued and surprised to see that overexpression of ACAP4 perturbs integrin β1 endocytic recycling. We envision that overexpression of ACAP4 may perturb the ARF6 GTPase cycle that slows down the rate of integrin β1 endocytic recycling. However, it is possible that ACAP4 may form a complex with other ARF6 GAPs by which ARF6 GTPase cycle was reduced. In supporting our notion, ACAP4 also functions in the recycling of transferrin receptor, another clathrin-independent recycling pathway, and overexpression of ACAP4 also inhibits the recycling of transferrin receptor. In any event, it would be of great interest down the road to delineate their respective contributions. In addition, it would be necessary to illustrate how ACAP4 regulates integrin β1 recycling as our efforts to evaluate a physical interaction between ACAP4-integrin β1 were unsuccessful.
Furthermore, EGF was elucidated to affect integrin β1 endocytic recycling by redistributing it from the internal membrane compartment to the cell surface (3, 40). The adaptor protein Grb2 was originally isolated as a downstream intermediary for activated EGFR in cell proliferation (13). Interestingly, in our yeast two-hybrid assay searching for ACAP4-associated proteins, Grb2 was demonstrated to directly interact with ACAP4. Furthermore, our experimental results showed that Grb2 was in charge of the signal transduction from the activated EGF in the process of ACAP4-regulated integrin β1 recycling. Moreover, Grb2 specifically interacted with ACAP4 but not ACAP1,4 leading us to postulate that the Grb2-ACAP4 interaction may bring forward a novel pathway to link external stimulation to integrin β1 intracellular trafficking. In addition, the association of ACAP4 and Grb2 was enhanced by the EGF-dependent phosphorylation of ACAP4 at Tyr-733. Previous studies have shown that serine/threonine protein kinases such as PKCα, PKCϵ, and Akt played key roles in integrin trafficking (6, 41, 42). Our data indicated that besides the above kinases, a potential tyrosine kinase responsible for ACAP4 Tyr-733 phosphorylation would also be involved in the β1 integrin endocytic trafficking. It will be of interest to identify the kinase in future studies. Moreover, ARF GEF GEP100/Brag2 was demonstrated to bind activated EGFR and induce tumor invasion in response to EGF stimulation (43). It was also suggested that a potential ARF6 GAP was also recruited to EGFR to help accomplish the normal function of ARF6 and regulate cell motility (44). Given the ACAP4-Grb2 interaction and inhibition of cell invasion with ACAP4 knockdown (10), it is worth further investigation whether ACAP4 forms a complex with activated EGFR and Grb2 and whether the recruitment of ACAP4 to the plasma membrane is required for the EGF-stimulated invasion. Reduced ACAP4 was found to disrupt actin-containing stress fiber formation and decrease myosin serine 19 phosphorylation (10). In the meantime, integrin α5β1 adhesion could activate RhoA, which promoted the formation of stress fiber (45–47). Therefore, our finding may help to explain how the elimination of ACAP4 reduced the levels of actin stress fiber. Knockdown of ACAP4 might disturb the integrin α5β1 adhesion formation by inhibiting integrin β1 recycling, affect RhoA activation, and reduce stress fiber network. Thus, it will be of great importance to evaluate whether ACAP4 functions in RhoA-mediated cytoskeleton reorganization and cellular dynamics.
Nevertheless, it is worth noting that Grb2 is an essential adaptor protein in intracellular signal transduction for various cell surface receptors, and Grb2 knockdown affects a series of cell signaling pathways that may also be involved in the integrin β1 endocytic recycling. For instance, ERK2 downstream of Grb2 also had an influence on trafficking of cargo in the clathrin-independent pathway (48, 49). Therefore, it is hard to exclude the influence of other signaling pathways. By using the mutant that weakened the association of ACAP4 and Grb2, we validated that their interaction was significant for the EGF-stimulated integrin β1 recycling.
Taken together, our findings revealed that ACAP4 plays a critical role in the EGF-stimulated integrin β1 recycling via a functional interaction with the adaptor protein Grb2. This interaction was regulated by the EGF-elicited phosphorylation of ACAP4 at tyrosine 733. Finally, our studies demonstrated that the ACAP4-Grb2 interaction not only regulates the intracellular trafficking of integrin β1 but also orchestrates the subsequent cellular dynamics such as cell adhesion, spreading, and migration. Given the fact that tyrosine phosphorylation is aberrantly regulated in invasive cancers during their progression (50), the challenges and excitements ahead are to delineate whether and how ACAP4 phosphorylation plays a role in tumor progression.
Acknowledgments
We thank members of our groups for insightful discussion during the course of this study.
This work was supported, in whole or in part, by National Institutes of Health Grants DK-56292, U54CA118948, and P20CA132389 (to X. Y.), NCRR Grant UL1 RR025008 from the Clinical and Translational Science Award Program, and NCRR Grant G12RR03034 (for use of facilities). This work was supported in part by Chinese 973 Project Grants 2010CB912103, 2012CB945000, 2007CB914503, and 2002CB713700 (to X. Y.), Chinese Natural Science Foundation Grants 30900497 (to F. W.), 30500183, 30870990, and 91129714 (to X. D.), and 90508002 and 90913016 (to X. Y.), Chinese Academy of Science Grants KSCX1-YW-R-65, KSCX2-YW-H-10, and KSCX2-YW-R-195, International Collaboration Grant 2009DFA31010 (to X. D.), and Anhui Province Project Grant 08040102005 (to X. Y.).
X. Yu, F. Wang, and X. Ding, unpublished observations.
X. Yu and F. Wang, unpublished observations.
- CIE
- clathrin-independent endocytosis
- GEF
- guanine nucleotide-exchange factor
- GAP
- GTPase-activating protein
- EGFR
- EGF receptor.
REFERENCES
- 1. Grant B. D., Donaldson J. G. (2009) Nat. Rev. Mol. Cell. Biol. 10, 597–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hynes R. O. (2002) Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 3. Powelka A. M., Sun J., Li J., Gao M., Shaw L. M., Sonnenberg A., Hsu V. W. (2004) Traffic 5, 20–36 [DOI] [PubMed] [Google Scholar]
- 4. Oh S. J., Santy L. C. (2010) J. Biol. Chem. 285, 14610–14616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dai J., Li J., Bos E., Porcionatto M., Premont R. T., Bourgoin S., Peters P. J., Hsu V. W. (2004) Dev. Cell 7, 771–776 [DOI] [PubMed] [Google Scholar]
- 6. Li J., Ballif B. A., Powelka A. M., Dai J., Gygi S. P., Hsu V. W. (2005) Dev. Cell 9, 663–673 [DOI] [PubMed] [Google Scholar]
- 7. Li J., Peters P. J., Bai M., Dai J., Bos E., Kirchhausen T., Kandror K. V., Hsu V. W. (2007) J. Cell Biol. 178, 453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inoue H., Randazzo P. A. (2007) Traffic 8, 1465–1475 [DOI] [PubMed] [Google Scholar]
- 9. Fang Z., Miao Y., Ding X., Deng H., Liu S., Wang F., Zhou R., Watson C., Fu C., Hu Q., Lillard J. W., Jr., Powell M., Chen Y., Forte J. G., Yao X. (2006) Mol. Cell. Proteomics 5, 1437–1449 [DOI] [PubMed] [Google Scholar]
- 10. Ha V. L., Bharti S., Inoue H., Vass W. C., Campa F., Nie Z., de Gramont A., Ward Y., Randazzo P. A. (2008) J. Biol. Chem. 283, 14915–14926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ismail S. A., Vetter I. R., Sot B., Wittinghofer A. (2010) Cell 141, 812–821 [DOI] [PubMed] [Google Scholar]
- 12. Ding X., Deng H., Wang D., Zhou J., Huang Y., Zhao X., Yu X., Wang M., Wang F., Ward T., Aikhionbare F., Yao X. (2010) J. Biol. Chem. 285, 18769–18780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lowenstein E. J., Daly R. J., Batzer A. G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E. Y., Bar-Sagi D., Schlessinger J. (1992) Cell 70, 431–442 [DOI] [PubMed] [Google Scholar]
- 14. Schlaepfer D. D., Hanks S. K., Hunter T., van der Geer P. (1994) Nature 372, 786–791 [DOI] [PubMed] [Google Scholar]
- 15. Holgado-Madruga M., Emlet D. R., Moscatello D. K., Godwin A. K., Wong A. J. (1996) Nature 379, 560–564 [DOI] [PubMed] [Google Scholar]
- 16. Buday L., Downward J. (1993) Cell 73, 611–620 [DOI] [PubMed] [Google Scholar]
- 17. Egan S. E., Giddings B. W., Brooks M. W., Buday L., Sizeland A. M., Weinberg R. A. (1993) Nature 363, 45–51 [DOI] [PubMed] [Google Scholar]
- 18. Li N., Batzer A., Daly R., Yajnik V., Skolnik E., Chardin P., Bar-Sagi D., Margolis B., Schlessinger J. (1993) Nature 363, 85–88 [DOI] [PubMed] [Google Scholar]
- 19. Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. (1993) Nature 363, 83–85 [DOI] [PubMed] [Google Scholar]
- 20. Carlier M. F., Nioche P., Broutin-L'Hermite I., Boujemaa R., Le Clainche C., Egile C., Garbay C., Ducruix A., Sansonetti P., Pantaloni D. (2000) J. Biol. Chem. 275, 21946–21952 [DOI] [PubMed] [Google Scholar]
- 21. Caswell P. T., Norman J. C. (2006) Traffic 7, 14–21 [DOI] [PubMed] [Google Scholar]
- 22. Jones M. C., Caswell P. T., Norman J. C. (2006) Curr. Opin. Cell Biol. 18, 549–557 [DOI] [PubMed] [Google Scholar]
- 23. Rozakis-Adcock M., McGlade J., Mbamalu G., Pelicci G., Daly R., Li W., Batzer A., Thomas S., Brugge J., Pelicci P. G., et al. (1992) Nature 360, 689–692 [DOI] [PubMed] [Google Scholar]
- 24. Gale N. W., Kaplan S., Lowenstein E. J., Schlessinger J., Bar-Sagi D. (1993) Nature 363, 88–92 [DOI] [PubMed] [Google Scholar]
- 25. Clark S. G., Stern M. J., Horvitz H. R. (1992) Nature 356, 340–344 [DOI] [PubMed] [Google Scholar]
- 26. Yamazaki T., Zaal K., Hailey D., Presley J., Lippincott-Schwartz J., Samelson L. E. (2002) J. Cell Sci. 115, 1791–1802 [DOI] [PubMed] [Google Scholar]
- 27. Xue Y., Ren J., Gao X., Jin C., Wen L., Yao X. (2008) Mol. Cell. Proteomics 7, 1598–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heibeck T. H., Ding S. J., Opresko L. K., Zhao R., Schepmoes A. A., Yang F., Tolmachev A. V., Monroe M. E., Camp D. G., 2nd, Smith R. D., Wiley H. S., Qian W. J. (2009) J. Proteome Res. 8, 3852–3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okabe H., Furukawa Y., Kato T., Hasegawa S., Yamaoka Y., Nakamura Y. (2004) Int. J. Oncol. 24, 43–48 [PubMed] [Google Scholar]
- 30. Deleted in proof.
- 31. Pellinen T., Tuomi S., Arjonen A., Wolf M., Edgren H., Meyer H., Grosse R., Kitzing T., Rantala J. K., Kallioniemi O., Fässler R., Kallio M., Ivaska J. (2008) Dev. Cell 15, 371–385 [DOI] [PubMed] [Google Scholar]
- 32. Nishimura T., Kaibuchi K. (2007) Dev. Cell 13, 15–28 [DOI] [PubMed] [Google Scholar]
- 33. Reynolds L. E., Wyder L., Lively J. C., Taverna D., Robinson S. D., Huang X., Sheppard D., Hynes R. O., Hodivala-Dilke K. M. (2002) Nat. Med. 8, 27–34 [DOI] [PubMed] [Google Scholar]
- 34. Caswell P., Norman J. (2008) Trends Cell Biol. 18, 257–263 [DOI] [PubMed] [Google Scholar]
- 35. Bretscher M. S. (1989) EMBO J. 8, 1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bretscher M. S. (1992) EMBO J. 11, 405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caswell P. T., Vadrevu S., Norman J. C. (2009) Nat. Rev. Mol. Cell Biol. 10, 843–853 [DOI] [PubMed] [Google Scholar]
- 38. Radhakrishna H., Donaldson J. G. (1997) J. Cell Biol. 139, 49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dunphy J. L., Moravec R., Ly K., Lasell T. K., Melancon P., Casanova J. E. (2006) Curr. Biol. 16, 315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bretscher M. S., Aguado-Velasco C. (1998) Curr. Biol. 8, 721–724 [DOI] [PubMed] [Google Scholar]
- 41. Ng T., Shima D., Squire A., Bastiaens P. I., Gschmeissner S., Humphries M. J., Parker P. J. (1999) EMBO J. 18, 3909–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ivaska J., Whelan R. D., Watson R., Parker P. J. (2002) EMBO J. 21, 3608–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morishige M., Hashimoto S., Ogawa E., Toda Y., Kotani H., Hirose M., Wei S., Hashimoto A., Yamada A., Yano H., Mazaki Y., Kodama H., Nio Y., Manabe T., Wada H., Kobayashi H., Sabe H. (2008) Nat. Cell Biol. 10, 85–92 [DOI] [PubMed] [Google Scholar]
- 44. Valderrama F., Ridley A. J. (2008) Nat. Cell Biol. 10, 16–18 [DOI] [PubMed] [Google Scholar]
- 45. Dubash A. D., Wennerberg K., García-Mata R., Menold M. M., Arthur W. T., Burridge K. (2007) J. Cell Sci. 120, 3989–3998 [DOI] [PubMed] [Google Scholar]
- 46. Lim Y., Lim S. T., Tomar A., Gardel M., Bernard-Trifilo J. A., Chen X. L., Uryu S. A., Canete-Soler R., Zhai J., Lin H., Schlaepfer W. W., Nalbant P., Bokoch G., Ilic D., Waterman-Storer C., Schlaepfer D. D. (2008) J. Cell Biol. 180, 187–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Papusheva E., Heisenberg C. P. (2010) EMBO J. 29, 2753–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Robertson S. E., Setty S. R., Sitaram A., Marks M. S., Lewis R. E., Chou M. M. (2006) Mol. Biol. Cell 17, 645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giubellino A., Burke T. R., Jr., Bottaro D. P. (2008) Expert Opin. Ther. Targets 12, 1021–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sun T., Aceto N., Meerbrey K. L., Kessler J. D., Zhou C., Migliaccio I., Nguyen D. X., Pavlova N. N., Botero M., Huang J., Bernardi R. J., Schmitt E., Hu G., Li M. Z., Dephoure N., Gygi S. P., Rao M., Creighton C. J., Hilsenbeck S. G., Shaw C. A., Muzny D., Gibbs R. A., Wheeler D. A., Osborne C. K., Schiff R., Bentires-Alj M., Elledge S. J., Westbrook T. F. (2011) Cell 144, 703–718 [DOI] [PMC free article] [PubMed] [Google Scholar]