Background: The cation chloride cotransporter NCC is degraded by undefined mechanisms.
Results: NCC requires specific conserved machinery for chaperone-dependent recognition, ubiquitination, and proteasomal routing.
Conclusion: NCC exhibits distinct ERAD requirements, which correlate with its transmembrane topology and distinguish it from other clients.
Significance: These ER quality control components process misfolded conformational intermediates of NCC and other structurally related cotransporters that are vital for human health.
Keywords: Chloride Transport, Endoplasmic Reticulum (ER), Heat Shock Protein, Proteasome, Sodium Transport, Ubiquitin, ERAD, Hsp70, NCC, Thiazide
Abstract
The thiazide-sensitive NaCl cotransporter (NCC, SLC12A3) mediates salt reabsorption in the distal nephron of the kidney and is the target of thiazide diuretics, which are commonly prescribed to treat hypertension. Mutations in NCC also give rise to Gitelman syndrome, a hereditary salt-wasting disorder thought in most cases to arise from impaired NCC biogenesis through enhanced endoplasmic reticulum-associated degradation (ERAD). Because the machinery that mediates NCC quality control is completely undefined, we employed yeast as a model heterologous expression system to identify factors involved in NCC degradation. We confirmed that NCC was a bona fide ERAD substrate in yeast, as the majority of NCC polypeptide was integrated into ER membranes, and its turnover rate was sensitive to proteasome inhibition. NCC degradation was primarily dependent on the ER membrane-associated E3 ubiquitin ligase Hrd1. Whereas several ER luminal chaperones were dispensable for NCC ERAD, NCC ubiquitination and degradation required the activity of Ssa1, a cytoplasmic Hsp70 chaperone. Compatible findings were observed when NCC was expressed in mammalian kidney cells, as the cotransporter was polyubiquitinated and degraded by the proteasome, and mammalian cytoplasmic Hsp70 (Hsp72) coexpression stimulated the degradation of newly synthesized NCC. Hsp70 also preferentially associated with the ER-localized NCC glycosylated species, indicating that cytoplasmic Hsp70 plays a critical role in selecting immature forms of NCC for ERAD. Together, these results provide the first survey of components involved in the ERAD of a mammalian SLC12 cation chloride cotransporter and provide a framework for future studies on NCC ER quality control.
Introduction
The thiazide-sensitive NaCl cotransporter (NCC)2 is expressed at the apical surface of the distal convoluted tubule of the kidney, where it mediates the reabsorption of 5–10% of filtered sodium chloride (1). NCC plays an important role in determining the final sodium chloride content of urine entering the collecting system and, hence, is critical for long term blood pressure regulation. Inhibition of NCC through the administration of thiazide diuretics has been associated with substantial reductions in hypertension-associated morbidity and mortality. Moreover, loss-of-function mutations of NCC cause Gitelman syndrome, an autosomal recessive salt-wasting disorder (2, 3). Recently, it was shown that the carrier state for these loss-of-function mutations confers protection from the development of hypertension, strongly suggesting that factors governing the activity of NCC are key determinants of essential hypertension in the general population (4–6).
NCC is a member of the SLC12 cation chloride cotransporter family of electroneutral transporters; other members include two Na−K+-2Cl− cotransporters (NKCC1 and NKCC2) and four K+-Cl− cotransporters (KCC1–4) (7). These membrane proteins are important for the regulation of cell volume and ion homeostasis in a variety of cell types, including neurons, erythrocytes, and diverse epithelia (7). The genes encoding these transporters are highly homologous and are predicted to share a complex topology consisting of 12 transmembrane domains, a sizable exofacial loop containing N-glycosylation sites, and large cytoplasmic amino and carboxyl termini flanking the transmembrane domains. Many of the mutations that cause Gitelman syndrome are associated with reduced plasma membrane expression and a shift in the equilibrium of NCC expression toward its immature, glycosylated form (8–10). This finding implies that many loss-of-function mutations that give rise to Gitelman syndrome cause the cotransporter to become misfolded within the early biosynthetic pathway, leading to the recognition of NCC by ER quality control mechanisms and degradation. Despite the relevance of NCC biogenesis to hypertension and the pathogenesis of Gitelman syndrome, the molecular mechanisms involved in the quality control of NCC remain undefined.
Both wild type and mutant versions of membrane proteins with complex topologies, such as NCC, are subject to ER quality control and degradation. For example, a significant portion of wild type cystic fibrosis transmembrane conductance regulator (CFTR) is selected for ER-associated degradation (ERAD) (11, 12), as is the epithelial sodium channel, ENaC (13–16). However, the deletion of a phenylalanine at position 508 in CFTR is sufficient to divert nearly all of the protein to the ERAD pathway, which gives rise to cystic fibrosis (11, 12). In general, the selection of these and other proteins for ERAD requires substrate-specific interactions with molecular chaperones, such as members of the heat shock protein 70 (Hsp70) and 40 (Hsp40) families (17). After chaperone-mediated selection in the ER lumen and/or cytoplasm, components of the ubiquitin-conjugation and ligation systems are recruited and append a polyubiquitin chain onto cytoplasmic domains of the substrate. Polyubiquitinated substrates are then extracted from the ER by the Cdc48-p97 complex, which couples ATP hydrolysis to retro-translocation. Ultimately, the ERAD substrate is delivered to the proteasome and degraded. At present, it is impossible to predict a priori which of the many chaperones in mammalian cells might play a role in the selection of a specific ERAD substrate, like NCC. This problem is compounded by the fact that distinct chaperones may be involved in protein folding as well as degradation (18). Nevertheless, a thorough definition of chaperone-substrate interactions for proteins such as NCC is critical; efforts to alter chaperone activity are under way based on their proven ability to alter the conformations and promote the trafficking of disease-causing mutant proteins (19–21).
To begin to define the pathway by which NCC is subject to quality control, we employed yeast as a model expression system to compare mammalian NCC processing in wild type strains and in strains with targeted mutations of select components of the ERAD pathway. We verified that NCC is a bona fide ERAD substrate in yeast, allowing us to co-opt the system to identify components of the ubiquitination machinery and the chaperones that target NCC for degradation. Using genetic and biochemical tools, we find that one of these chaperones, the cytoplasmic Hsp70, facilitates NCC polyubiquitination. We then utilize a mammalian renal cell model for NCC and show that mammalian cytoplasmic Hsp70 selects NCC for ERAD. Together, these data establish NCC as an ERAD substrate and identify Hsp70 as a critical mediator of cation chloride cotransporter quality control.
EXPERIMENTAL PROCEDURES
Yeast Strains and Molecular Methods
A summary of the Saccharomyces cerevisiae strains used in this study is provided in supplemental Table S1.
All NCC clones used in these studies were derived from previously described and characterized cDNAs, including untagged mouse NCC (8), N-terminal hemagglutinin-tagged mouse NCC (HA-NCC) (22), and a mouse NCC construct containing a double HA tag in the cotransporters second extracellular loop (2XHA-NCC) (22). To express NCC in yeast, the coding sequence was excised from pcDNA3.1 (see below) with EcoRI and ligated into the same site in the multicopy, uracil-selectable plasmid, pRS426GPD (23), in which expression is driven from a modest, constitutive promoter. Yeast were transformed with the plasmid using a standard lithium acetate procedure, and colonies were selected on synthetic complete media lacking uracil (24). For experiments in mammalian cells, untagged NCC was subcloned from pgh19 (8) into the EcoRI site of pcDNA3.1. pRK5-HA-ubiquitin (25) was a gift of Paul Welling (University of Maryland School of Medicine, Baltimore, MD), and human Hsp70 (Hsp72, HspA1A) in pcDNA3 was a gift from Ron Rubenstein (University of Pennsylvania School of Medicine, Philadelphia, PA). An in-frame N-terminal myc epitope was added to the Hsp70 construct via PCR and subcloned into pMO-myc (22). Site-directed mutagenesis was performed using a PCR-based strategy with PfuTurbo DNA polymerase (QuikChange; Agilent).
Antibodies
The following commercial antibodies were used: mouse monoclonal anti-HA (HA-11, Covance), mouse monoclonal anti-myc (4A6, Millipore), rabbit polyclonal anti-c-myc (A-14, Santa Cruz), mouse monoclonal anti-human Hsp70 (Hsp72) (C92F3A-5, Stressgen/Enzo Life Sciences), rabbit polyclonal anti-tubulin (Sigma), horseradish peroxidase (HRP)-conjugated goat anti-mouse and goat anti-rabbit antibodies (Jackson ImmunoResearch), rabbit polyclonal anti-ubiquitin antibody (FL-76, Santa Cruz), and HRP-conjugated rat monoclonal anti-HA high affinity (3F10, Roche Applied Science). Polyclonal rabbit anti-mouse NCC antibody (26) was a gift from David Ellison (Oregon Health and Science University, Portland OR), polyclonal rabbit anti-Pdi1 was provided by Vlad Denic (Harvard University, Cambridge, MA), and polyclonal rabbit anti-Pma1 was a gift from Amy Chang (University of Michigan, Ann Arbor, MI). Polyclonal rabbit anti-Sec61 (27) and polyclonal rabbit anti-Kar2/BiP (28) were previously described.
Yeast Cycloheximide Chase Assay
Yeast cells transformed with the NCC expression plasmid were grown in synthetic complete media lacking uracil at 26 °C to an A600 of 0.8–1.0. These log-phase cultures were then transferred to a water bath at either 30 °C or 37 °C (for assays using temperature-sensitive mutant strains). To stop protein translation, cycloheximide was added to a final concentration of 100 μg/ml, and aliquots were removed at 0, 15, 30, and 60 min. Cells were isolated by centrifugation at 4 °C, quick-frozen, and stored at −80 °C, the cell pellets were thawed on ice, and total protein was TCA-precipitated as described (29). The protein samples were analyzed by SDS-PAGE and immunoblot analysis with HRP-conjugated anti-HA antibody (Roche Applied Science). NCC was visualized using ECL (Pierce) and a Kodak 440CF image station, and the data were quantified with Kodak 1D software. The amount of protein at the start of the chase (time 0) was set to 100%, and the amounts of protein at subsequent time points were expressed as a percent of the starting material.
Protein Localization Studies in Yeast
The integration of NCC into the yeast ER membrane was analyzed by carbonate extraction (30). In brief, yeast microsomes were prepared as previously described (28) and incubated with ice-cold 100 mm Na2CO3, pH 13, or buffer 88 (20 mm HEPES, pH 6.8, 150 mm KOAc, 5 mm MgOAc, 250 mm sorbitol) on ice for 30 min. After incubation, the membranes were centrifuged at ∼100,000 × g in a Sorvall RC M120EX centrifuge for 60 min at 4 °C. The supernatant was removed, and the pellets were washed with buffer and centrifuged again for 15 min. The final pellets were solubilized in SDS sample buffer, and the pellet and supernatant fractions were subjected to SDS-PAGE and immunoblot analysis with anti-HA antibody to detect NCC and anti-Sec61 and anti-Pdi1 antibodies to detect integral membrane and soluble ER proteins, respectively.
The intracellular residence of NCC was determined by sedimentation in a sucrose gradient essentially as described (31). A 30-ml culture was grown to an A600 of 0.8, and the cells were harvested by centrifugation and then disrupted with agitation on a Vortex mixer with glass beads. The cell lysates were cleared of debris by low speed centrifugation, and the resulting lysate (300 μl) was layered on the top of an 11-ml 30–70% sucrose gradient and centrifuged at 100,000 × g in a Beckman SW41 rotor for 14 h at 4 °C. Where indicated, the gradients either contained EDTA or Mg2+ to release or maintain, respectively, the associated ribosomes. Fractions were collected by pipeting from the top of the tube, and proteins were analyzed by SDS-PAGE and immunoblotting for NCC with anti-HA antibody. Specific antisera against the ER (Kar2/BiP) and the plasma membrane (Pma1) resident proteins were also used (see above).
NCC Ubiquitination in Yeast
Yeast cells expressing NCC were grown to A600 of 0.8 at 26 °C. For experiments employing E3 ubiquitin ligase mutant strains, the cells were isolated at room temperature in a clinical centrifuge, washed 1 time in sterile water, and quick-frozen for storage at −80 °C. For experiments in the Hsp70/Ssa1 mutant strains, the cells were shifted to 37 °C for 15 min and then immediately cooled in an ice-water bath, and a final concentration of 10 mm NaN3 was added. The cells were then isolated in a clinical centrifuge and quick-frozen and stored at −80 °C. The preparation of cell lysates, immunoprecipitation of NCC, and analysis of conjugated ubiquitin were performed essentially as described for CFTR (32). In brief, cells were disrupted by glass bead lysis, and membranes were pelleted by centrifugation. Membranes were treated with SDS buffer to liberate the integral membrane proteins, and the solution was diluted into a Triton X-100-containing buffer. Anti-HA antibody conjugated to agarose beads was added, and solutions were incubated overnight with rocking at 4 °C. The beads were washed 3 times, and the bound proteins were liberated in SDS sample buffer at 37 °C for 30 min. Finally, the released proteins were subjected to SDS-PAGE and immunoblot analysis with anti-HA antibody to detect total NCC or anti-ubiquitin antibody to detect the ubiquitinated NCC fraction.
Cell Culture and Transfection
Madin Darby canine kidney (MDCK) cells were provided by Rebecca Hughey (University of Pittsburgh, Pittsburgh PA), and HEK-293T cells were donated by Kenneth Hallows (University of Pittsburgh, Pittsburgh, PA). Cells were cultured in high glucose Dulbecco's modified Eagle's medium supplemented with 10% FBS, l-glutamine, and penicillin/streptomycin at 37 °C. Transient transfections were performed using either FuGENE 6 (Roche Applied Science) or Lipofectamine 2000 (Invitrogen) per the instructions of the manufacturer, and cells were subjected to analysis 24–48 h post-transfection.
Preparation of Mammalian Cell Lysates and Immunoblot Analysis
Cells were washed twice with phosphate-buffered saline (PBS), scraped, collected, and isolated by centrifugation at 1000g for 5 min. Post-nuclear lysate supernatants were obtained by passing the pellets 25 times through a 20–200-μl pipette tip in 1 of 3 lysis buffers depending on the experiment: cell lysis buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm Na2EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, and 1 μg/ml leupeptin, 1 mm PMSF), radioimmune precipitation assay buffer (identical to cell lysis buffer except Triton X-100 was replaced with 1% Nonidet P-40 and 1% sodium deoxycholate), or detergent solution (50 mm Tris-HCl, pH 8.5, 1% Nonidet P-40, 0.4% sodium deoxycholate, and 62.5 mm EDTA supplemented with 1 tablet Roche Complete Protease Inhibitor Mixture and 1 mm PMSF). The samples were incubated on ice for 15 min, and insoluble material was removed by centrifugation at 16,000 × g for 5 min. Protein concentrations were determined by the Bradford method (Bio-Rad protein assay kit). Lysates were diluted according to the instructions of the manufacturer, so that the detergents in the lysis buffers would not interfere with the protein assay. For samples subjected to SDS-PAGE, lysates were denatured in Laemmli buffer, maintained at room temperature for 30 min, and loaded in a 4 °C cold room onto 10% polyacrylamide gels preincubated with chilled SDS buffer, and the proteins were resolved at 4 °C on the polyacrylamide gels. Immunoblot analysis was performed as described previously (22).
Metabolic Labeling and Pulse-Chase Studies in Mammalian Cell Lines
HEK293T or MDCK cells expressing N-terminal HA-tagged NCC in 6-well plates were assayed 24 h post-transfection. Cells were washed twice with prewarmed starving medium (cysteine/methionine-free high-glucose DMEM supplemented with 10% dialyzed FBS, penicillin/streptomycin, and glutamine) and incubated in fresh starving medium in a 5% CO2 incubator at 37 °C for 30 min. The cells were then washed twice on ice with ice-cold PBS and metabolically labeled at 37 °C in the CO2 incubator in starving medium containing 150 μCi/ml Translabel (MP Biomedicals) for 30 min. The radiolabel was aspirated from the samples, and the cells were washed twice in prewarmed chasing medium (complete high glucose DMEM supplemented with 10 mg/ml each of unlabeled methionine and cysteine). The cells were then “chased” in the incubator at 37 °C for the indicated times and washed on ice 3 times with ice-cold PBS. The cells were then lysed in 400 μl of radioimmune precipitation assay buffer/well by adding the lysis buffer directly to the plates and rocking the cells at room temperature for 1 h. Lysates were collected into microcentrifuge tubes and clarified by centrifugation at 1000 − g for 5 min. HA-NCC was then immunoprecipitated from the cleared lysates by adding 1 μg of HA-11 antibody and incubating the solution overnight at 4 °C with end-over-end rotation. NCC-antibody complexes were subsequently precipitated from the sample by adding 30 μg of protein A/G agarose slurry and rotating the mixture at room temperature for 1 h. The beads were washed five times in 500 μl of PBS, and the immunoprecipitates were eluted from the beads in 5× Laemmli buffer and fractionated by SDS-PAGE. The gels were dried (Dry-Ease gel drying kit, Invitrogen), and radiolabeled NCC was detected with a Personal Molecular Imager system (Bio-Rad) using a TR phosphorimaging screen.
Deglycosylation Studies
Endoglycosidase H and peptide N-glycosidase F were obtained from New England Biolabs. HEK293 cells expressing HA-NCC were metabolically labeled for 2 h in starving medium containing 150 μCi/ml Translabel, cell lysates were prepared, and NCC was immunoprecipitated as described above. For both reactions, immunoprecipitates were eluted from the anti-HA immunoaffinity resin by adding 10 μl of 1× glycoprotein denaturing buffer (New England Biolabs, included with the endoglycosidase H and peptide N-glycosidase F enzymes) and heating the samples at 90 °C for 2 min. The eluates were cooled to room temperature and adjusted to a final volume of 20 μl containing either 2500 units of endoglycosidase H or 1000 units of peptide N-glycosidase F and their requisite buffers per the instructions of the manufacturer. Samples were incubated at 37 °C with endoglycosidase H for 3 h or peptide N-glycosidase F for 1 h before SDS-PAGE and autoradiography as described above.
NCC Co-immunoprecipitation Analysis
Cells transiently expressing the indicated constructs were lysed in cell lysis buffer as described above. A total of 300 μg of the lysate was diluted to a total of 300 μl and precleared with 30 μl of protein A/G-agarose slurry (Calbiochem) by end-over-end rotation at 4 °C for 2 h. Next, the indicated amount of antibody was added to the precleared lysates, and the samples were rotated in fresh 30-μl aliquots of protein A/G beads overnight at 4 °C. The samples were then centrifuged at low speed, and the beads were washed once in 500 μl of PBS, twice in 500 μl of PBS containing 0.5% Triton X-100, and once more in 500 μl of PBS. Immunoprecipitated proteins were eluted by incubating the beads at room temperature for 30 min in 5× Laemmli buffer, separated by SDS-PAGE, and analyzed by immunoblotting as described above.
Data Analysis
For Western blot quantification, densitometry was carried out with NIH ImageJ software. Bio-Rad QuantityOne was used for analysis of phosphorimaging data. GraphPad Prism software was used for statistical analyses. Comparisons between two groups were determined by a Student's t test.
RESULTS
NCC Can Be Expressed in the Yeast, S. cerevisiae, and Resides Predominantly in the ER Membrane
We previously developed yeast expression systems to define and characterize the ERAD requirements for several clinically relevant mammalian ion channels, including the chloride channel CFTR (29, 32–34) and the trimeric sodium channel, ENaC (13, 14). The yeast system can then be exploited to identify the unique degradation requirements for each substrate by examining the protein fate in cells mutated for specific genes, such as those required for ERAD (35). These include both cytosolic and ER luminal chaperones, E3 ubiquitin ligases, and components required to retrotranslocate substrates from the ER to the cytoplasm for delivery to the proteasome. Results from the yeast system can subsequently be confirmed in mammalian cells, thus establishing this model organism as a tool to expedite gene discovery. For example, we identified the contributions of small heat shock proteins (Hsps) during the ERAD of CFTR and ENaC in both yeast and vertebrate/mammalian cell systems (13, 32). Recently, we identified a role for two ER luminal Hsp40s, Scj1 and Jem1, during the selection and ERAD of ENaC in yeast; the homologous human luminal Hsp40s were similarly required to facilitate the destruction of the ENaC subunits, as assessed in an Xenopus oocyte expression system (14).
Previous observations of NCC biogenesis in heterologous expression systems, including Xenopus laevis oocytes and mammalian cells, indicate that the cotransporter exists in two glycosylated species at steady state: a core (high mannose) endoglycosidase H-sensitive band that migrates at 110 kDa and a mature endoglycosidase H-resistant band that resolves more broadly between 110–150 kDa (8, 36, 37). Both of these species have also been observed in the mammalian kidney (8). Because high mannose glycospecies reside within the biosynthetic pathway proximal to the pre-medial Golgi (38), these observations suggest that NCC is substantially ER-localized and subject to ER quality control mechanisms that monitor and facilitate its folding or degradation. To test this hypothesis and to identify and characterize the factors underlying NCC ERAD, we developed a yeast expression system to study NCC processing. To facilitate protein detection, an NCC construct with a double HA tag incorporated into the second extracellular loop (2XHA-NCC) was employed. Notably, the tagged and untagged versions of NCC are equally efficient at facilitating Na+ uptake, demonstrating that the inclusion of the epitope does not alter NCC transport characteristics (22). Therefore, this 2XHA-NCC cDNA was subcloned into a yeast expression vector, and NCC expression was verified in various yeast strains (see “Experimental Procedures”; data not shown).
To determine the subcellular localization of NCC in yeast, we subjected whole cell extracts to sucrose gradient centrifugation (see “Experimental Procedures”). In the absence of Mg2+ and presence of EDTA, this technique separates ER-derived membranes from plasma membranes. As seen in Fig. 1A, the ER resident chaperone Kar2/BiP resides in less dense fractions, whereas the plasma membrane resident, Pma1, migrates to denser fractions at the bottom of the gradient. We observed that essentially all of the NCC in the gradient mirrored Kar2/BiP residence, strongly suggesting that NCC is predominately in the yeast ER. To confirm ER localization, we then performed the sucrose gradient centrifugation in the presence of Mg2+ and absence of EDTA, which retains ribosomes on the ER membrane and leads to a characteristic shift of this organelle to denser regions of the gradient. As displayed in Fig. 1B, both Kar2/BiP and NCC exhibited a Mg2+-dependent shift, further supporting ER residence.
FIGURE 1.

NCC is an integral membrane protein and resides in the ER when expressed in yeast. Yeast lysates containing NCC were subjected to sucrose gradient centrifugation in the absence (A) or presence (B) of 2 mm MgCl2 in the buffer. The gradients were fractionated from the top (fraction 1) and analyzed for ER and plasma membrane (PM) containing fractions by immunoblotting for Kar2/BiP (ER) and Pma1 (PM). C, yeast membrane fractions were treated with Na2CO3 or a buffer control and subjected to high speed centrifugation. Pellet (P) and supernatant (S) fractions were immunoblotted for NCC and for the integral membrane protein Sec61 and the soluble, peripheral ER protein, Pdi1.
To confirm that NCC is integrated into the ER membrane and is not peripherally associated with the lipid bilayer, we isolated ER-enriched membranes and subjected them to a high pH, Na2CO3 wash (see “Experimental Procedures:). Under these conditions, peripherally associated proteins are liberated from the membrane and reside in the supernatant after centrifugation, whereas integral membrane proteins pellet with the membranes (30). After Na2CO3 treatment, NCC remained in the pellet, as did an integral membrane component of the translocation channel, Sec61 (Fig. 1C). In contrast, a soluble protein in the ER, Pdi1, was released into the supernatant. Together, these data indicate that NCC integrates into the ER membrane when expressed in yeast.
NCC Is an ERAD Substrate in Yeast
To determine whether NCC is targeted for ERAD, we performed cycloheximide chase assays to measure the rate of protein degradation after protein synthesis was arrested. We found that NCC was quite unstable in yeast, such that <10% of the starting material remained after 60 min. To determine if the degradation of NCC is proteasome-dependent, which is a hallmark of ERAD, we first introduced the NCC expression vector into a pdr5Δ strain, which lacks a multidrug pump and facilitates the uptake of the proteasome-specific inhibitor, MG-132 (39). As seen in Fig. 2A, NCC was significantly stabilized after cycloheximide addition in cells treated with MG-132 when compared with cells treated with the vehicle, DMSO. Interestingly, stabilization was most dramatic at early time points. Because MG-132 inhibits only one of the three protease activities of the proteasome (40), it is possible that the two uninhibited proteases act secondarily during degradation but eventually can compensate for the inhibited activity. It is also possible that NCC is shunted to the vacuole for degradation over time. To test this hypothesis, we examined whether there was vacuole-dependent degradation by measuring NCC stability in a pep4Δ strain, which lacks nearly all vacuole-associated protease activity (41). However, NCC degradation was robust in this strain (supplemental Fig. S1).
FIGURE 2.
NCC degradation is proteasome-dependent and requires ER associated E3 ubiquitin ligases. Cycloheximide chase reactions were performed as described under “Experimental Procedures” at 30 °C, and lysates were immunoblotted with anti-HA antibody to measure NCC stability over time. A, a pdr5Δ yeast strain was treated for 20 min with 50 μm MG-132 (closed squares) or DMSO (open squares) before a cycloheximide chase reaction. Data represent the means ± S.E. of three experiments. B, cycloheximide chase reactions were performed in wild type, hrd1Δ, doa10Δ, or hrd1Δdoa10Δ yeast strains. Data represent the mean ± S.E. for 7–9 experiments. In both A and B, representative immunoblots are also shown in the bottom panels. C, NCC expressed in either wild type or hrd1Δdoa10Δ mutant yeast strains was immunoprecipitated (IP) from lysates with anti-HA-agarose beads and analyzed by immunoblotting (WB) with either anti-HA or anti-ubiquitin antibody. The result from an immunoprecipitation using cells containing an empty expression vector is shown as a negative control.
To provide further evidence that NCC is an ERAD substrate in yeast, cycloheximide chase assays were performed in strains lacking one or both of the two ERAD-requiring ubiquitin ligases, Hrd1 and Doa10. As shown in Fig. 2B, NCC turn-over was identical in the wild type and doa10Δ strains but was significantly slowed in the hrd1Δ strain. Moreover, NCC was stabilized further in the hrd1Δdoa10Δ mutant, suggesting that Doa10 plays a secondary role during NCC degradation, possibly due to greater misfolding of the cytoplasmic domains of NCC, as it accumulates in the ER. Of note, a reciprocal effect was observed when Ste6* degradation was measured; here, ERAD was primarily Doa10-dependent and largely Hrd1-independent, but more pronounced stabilization was observed when both E3s were absent (42). To confirm that the E3-deficient strains exhibited the expected phenotypes, we repeated these experiments using a different protein, a mutated form of carboxypeptidase Y known as CPY* (supplemental Fig. S2), a well characterized ERAD substrate that shows Hrd1-dependent degradation (43). As anticipated, CPY* carboxypeptidase Y was exclusively Hrd1-dependent. We also established that the presence of the epitope tag did not lead to an artificial degradation signal by comparing the degradation rate of an HA-tagged to -untagged NCC in cycloheximide chase reactions. Notably, the degradation rates of both forms of NCC were identical, and both NCC species exhibited E3-dependent degradation (supplemental Fig. S3).
Formally, the deletion of the E3s may have led to secondary effects, which might grossly alter ER and/or cellular homeostasis and slow the degradation rate of NCC. To exclude this possibility, we measured NCC ubiquitination after its immunoprecipitation from wild type and hrd1Δdoa10Δ yeast strains. As shown in Fig. 2C, NCC ubiquitination was essentially absent in the hrd1Δdoa10Δ mutant, whereas a strong signal corresponding to NCC was observed in wild type cells. Based on the fact that NCC is ER-retained, is ubiquitinated, and is degraded in a ubiquitin-proteasome-dependent manner, we can conclude that this protein is an ERAD substrate in yeast. In further support of this conclusion, we also found that NCC degradation required the function of Cdc48, the AAA+ ATPase that extracts ubiquitinated substrates from the ER before their proteasome-dependent degradation (17) (supplemental Fig. S4).
The Cytoplasmic Hsp70, Ssa1, Facilitates NCC Degradation
Because the selection of ERAD substrates usually requires specific chaperones, we next sought to identify the Hsp70s and/or Hsp40s that facilitate NCC degradation. Both cytoplasmic and ER luminal chaperones have been identified as being important for the degradation of various ERAD substrates in yeast; however, the exact make-up of the required chaperones is substrate-specific and is often influenced by a substrate membrane topology (17). NCC contains 12 transmembrane segments, 2 large cytoplasmic domains, and a large ER-exposed exofacial loop. Given the fact that the cotransporter contains domains in the ER and cytoplasm, NCC could potentially interact with chaperones on either side or on both sides of the ER membrane.
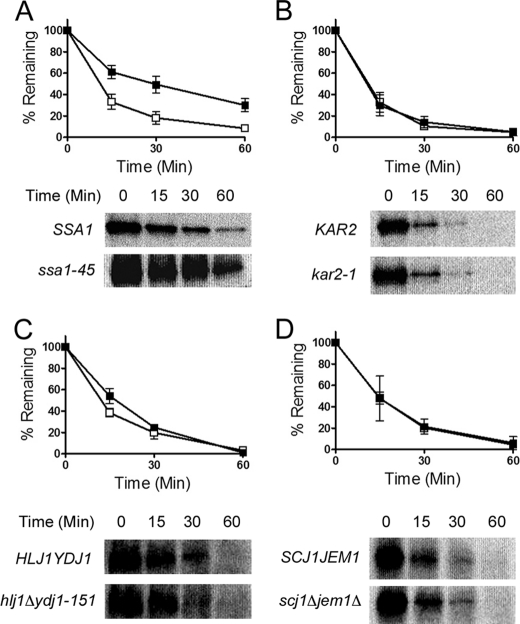
By utilizing temperature sensitive and deletion mutants, we analyzed NCC degradation when the functions of the major Hsp70 and Hsp40 chaperones in the cytoplasm and in the ER were ablated. The outcome of these experiments is presented in Fig. 3. Overall, we found that the only chaperone that affected NCC degradation was the cytoplasmic Hsp70, Ssa1. Specifically, after temperature shift to 37 °C, NCC was moderately stabilized in the ssa1–45 mutant compared with the wild type SSA1 strain (Fig. 3A). Somewhat surprisingly, two functionally redundant cytoplasmic Hsp40s, Hlj1 and Ydj1 (33), were dispensable for NCC degradation (Fig. 3C). These results suggest that Ssa1 action is Hsp40-independent or may require members of another chaperone class to promote maximal rates of NCC degradation. As an initial test of this hypothesis, we examined whether the deletion of the genes encoding the small Hsps, Hsp26 and Hsp42, which contributed to CFTR and ENaC ERAD (13, 32), had an effect on NCC turnover; however, NCC degradation was unchanged between the wild type and hsp26Δhsp42Δ strains (supplemental Fig. S5). Finally, in contrast to the ability of a cytoplasmic chaperone to facilitate NCC turnover, neither the ER luminal Hsp70 Kar2/BiP nor the functionally redundant luminal Hsp40s, Scj1 and Jem1, contributed to NCC degradation (Fig. 3, B and D). As a control and consistent with published results (44), we confirmed that carboxypeptidase Y was significantly stabilized in these strains (data not shown).
FIGURE 3.
The cytoplasmic Hsp70, Ssa1, is required for efficient NCC degradation. Cycloheximide chase reactions were performed as described under “Experimental Procedures,” and lysates were immunoblotted with anti-HA antibody. Cells were grown at 26 °C and shifted to 37 °C for 10 min before cycloheximide was added. A, wild type cytoplasmic Hsp70 SSA1 or ssa1–45 mutant yeast is shown. B, wild type ER luminal Hsp70 KAR2 or kar2–1 mutant yeast is shown. C, wild type cytoplasmic Hsp40 HLJ1YDJ1 or hlj1Δydj1–151 mutant yeast is shown. D, wild type ER luminal Hsp40 SCJ1JEM1 or scj1Δjem1Δ mutant yeast is shown. Closed squares represent data from mutant strains, and open squares represent data from the isogenic wild type strains. Data represent the mean ± S.E. for 6–11 experiments for each strain. In each part representative immunoblots are also shown in the bottom panels.
Ssa1 Function Contributes to NCC Ubiquitination in Yeast
The stabilizing effect of compromised Ssa1 function on NCC degradation could be due to a direct effect during substrate ubiquitination. Alternatively or in addition, Ssa1 might act at a post-ubiquitination step, perhaps retaining NCC in a soluble conformation during proteasome delivery (45–47). To determine if Ssa1 acts at a pre- and/or post-ubiquitination step, we immunoprecipitated NCC from wild type SSA1 yeast and the temperature sensitive ssa1–45 mutant strain after a brief temperature shift to inactivate the ssa1–45 protein (48). After only 15 min at 37 °C, the amount of HA-tagged NCC declined in the wild type cells but accumulated in the ssa1–45 mutant, consistent with the stabilization seen in the cycloheximide chase (compare Fig. 4A, IP: HA, WB: HA, to Fig. 3A, 15 min). Even though the amount of ubiquitination is comparable in the wild type and ssa1–45 strains (Fig. 4A, IP: HA, WB: Ub), when this signal is normalized to the total protein (HA) signal, the relative amount of ubiquitinated NCC is 2.3-fold higher in wild type than in ssa1–45 mutant cells (p = 0.004; Fig. 4B). The ssa1-dependent reduction in ubiquitinated NCC is absent if cells are grown exclusively at 26 °C (data not shown), demonstrating the specificity of the ssa1 mutant effect. The reduction in ubiquitination under the examined conditions is consistent with Ssa1 acting primarily at a recognition step during NCC ubiquitination. These results are in accordance with other studies in which the level of ubiquitination of an integral membrane ERAD substrate was shown to be decreased in yeast containing mutant forms of Ssa1 (46, 49).
FIGURE 4.

NCC ubiquitination is compromised in ssa1–45/Hsp70 mutant yeast. A, NCC was immunoprecipitated (IP) from wild type SSA1 and ssa1–45 mutant yeast after a 15-min temperature shift to 37 °C, and the precipitated proteins were immunoblotted (WB) with anti-HA or anti-ubiquitin antibody. A representative blot is shown. B, relative ubiquitination of NCC immunoprecipitated from wild type and ssa1–45 mutant yeast is shown. The graph represents the mean ± S.E. of seven precipitated samples, each from a distinct clone.
NCC Is a Rapidly Degraded ERAD Substrate in Mammalian Cells
To verify the relevance of the observations obtained in the yeast model, we analyzed NCC expression, proteasomal degradation, and ubiquitination in mammalian cells. First, a metabolic labeling pulse-chase assay was employed to specifically examine nascent cotransporters that undergo processing in the ER immediately after biosynthesis. To monitor this newly produced pool, HEK293 cells expressing N-terminal HA-tagged NCC were pulse-labeled with [35S]cysteine and [35S]methionine. The radiolabeled cotransporter was then immunoprecipitated from whole cell extracts with anti-HA antibodies, fractionated by SDS-PAGE, and detected by autoradiography. Using this approach, we identified a radiolabeled band that migrated at 110 kDa (supplemental Fig. S6), consistent with the core-glycosylated, ER-localized immature form of NCC (8, 36, 37). To verify that this band corresponded to core NCC, we performed deglycosylation reactions of radiolabeled NCC in HEK293 cells (supplemental Fig. S6). Cells were pulse-labeled with 35S, and anti-HA-NCC immunoprecipitates were subjected to treatment with the N-glycosidases endoglycosidase H (which selectively cleaves high mannose core N-glycans), or peptide N-glycosidase F, which cleaves both mature and core N-glycans). As predicted, the 110-kDa band shifted slightly in response to treatment with both enzymes, confirming that this band corresponds to core NCC (supplemental Fig. S6, left panel). Of note, we observed that the mature endoglycosidase H-resistant glycosylated species tended to migrate closely to the immature form as a low abundance smear of about 110–130 kDa. This species was visible in both in immunoblots of HEK293 cell lysates (Fig. 5B, right panel) and in metabolic labeling studies after longer exposures of the imaging screen (supplemental Fig. S6, right panel). These findings are compatible with previous reports by other groups that have employed HEK293 cells as an expression system to study NCC expression and regulation (50, 51). These results indicate that when expressed in mammalian cells, NCC predominantly exists in its core glycosylated state, suggesting that the cotransporter undergoes extensive biosynthetic processing in the ER and is strongly sensitive to chaperone-dependent ERAD.
FIGURE 5.
NCC is an ERAD substrate in mammalian cells. A, pulse-chase analysis of NCC proteasomal degradation in HEK293 cells is shown. Top, 24 h post-transfection cells transiently expressing HA-NCC were pretreated with 10 μm MG-132 (+) or DMSO vehicle (−) for 4 h. Cells were then pulsed with 35S for 30 min, and the radiolabeled cotransporter was isolated from cell extracts after the indicated chase period by immunoprecipitation. Treatment with MG-132 or DMSO was maintained through the entire pulse and chase periods. Bottom, quantification of the progressive decay in NCC abundance during the 90-min chase period, expressed as a percentage of NCC abundance at time zero. B, polyubiquitination of NCC is shown. 24 h post-transfection whole cell lysates of HEK293 cells transiently expressing untagged NCC, HA-tagged ubiquitin, or both constructs were subjected to immunoprecipitation (IP) with anti-NCC antibody and immunoblotted (WB) with anti-HA antibody. Immunoprecipitates are shown on the left, and whole cell lysates verifying NCC expression are shown on the right. The molecular weight corresponding to core glycosylated NCC is indicated with an arrowhead. A low abundance 75-kDa proteolytic fragment of NCC that is ubiquitinated is indicated with an asterisk. Oligomeric NCC is indicated with brackets. Representative of five experiments.
Despite the inefficient maturation of NCC in HEK293 cells, we found that the wild type cotransporter was still processed more efficiently than several NCC mutants harboring missense or nonsense mutations known to cause Gitelman syndrome. These NCC mutants (R948X, G738R, R989X, and T997I) were previously characterized in an amphibian expression system and exhibit reduced sodium transport activity due to impaired biosynthetic processing (8–10). We confirmed that these mutant proteins behaved similarly in HEK293 cells cultured at physiologic temperature, as they exhibited a substantial reduction in the steady state abundance of mature NCC as assessed by immunoblot analysis (supplemental Fig. S7). For example, the R989X mutant, which manifested the most mature glycosylation of the four Gitelman mutants tested, exhibited an apparent maturation efficiency of only 12% compared with 31% for the wild type protein. These data indicate that although wild type NCC is inefficiently processed in mammalian cell culture systems, it still undergoes productive folding and maturation to a greater extent than defective loss-of-function mutants known to cause human disease through impaired cotransporter biogenesis.
To determine the effect of proteasome inhibition on the degradation of newly synthesized NCC, HEK293 cells expressing the cotransporter were pulse-labeled in the absence or presence of MG-132 and were subsequently chased with excess unlabeled cysteine and methionine for the times indicated in Fig. 5A. In control (DMSO vehicle-treated) cells transfected with NCC, the cotransporter was rapidly degraded over 90 min. In contrast, the NCC degradation rate was attenuated substantially in cells that were pretreated, pulsed, and chased in the presence of 10 μm MG-132. Notably, the effect of MG-132 on the NCC decay rate was analogous to our observations of NCC degradation in yeast (Fig. 2A), with higher MG-132 effectiveness occurring at earlier points in the time course. These data confirm that the cotransporter is degraded rapidly by the proteasome in mammalian cells, with most newly synthesized NCC undergoing ERAD before export to the Golgi.
To confirm that NCC is polyubiquitinated in mammalian cells, we performed a coimmunoprecipitation experiment in HEK293 cells containing either an empty vector control or expressing an untagged version of NCC with or without a second plasmid encoding an HA epitope-tagged ubiquitin cDNA. Whole cell lysates from these cells were immunoprecipitated with a previously characterized rabbit anti-mouse NCC polyclonal antibody (see “Experimental Procedures”). The samples were subsequently fractionated by SDS-PAGE and subjected to anti-HA immunoblotting. In cells transfected with HA-ubiquitin alone, a faint high molecular weight signal was seen, consistent with the nonspecific precipitation of HA-ubiquitinated proteins by the polyclonal NCC antibody. By contrast, in cells cotransfected with both NCC and HA-ubiquitin, immunoprecipitation with the anti-NCC antibody yielded a robust immunoreactive smear that was strongest at a molecular mass of 110 kDa and higher (Fig. 5B, left panel, arrow). The signal is consistent with the specific covalent attachment of multiple HA-ubiquitin chains to the cotransporter. We also noted a lower-intensity smear from ∼75 kDa, extending up to 110 kDa. Based on an analysis of the NCC migration pattern in the whole cell lysates (Fig. 5B, right panel), this signal corresponded to the ubiquitination of a low abundance proteolytic fragment of the cotransporter (Fig. 5B, asterisk). Collectively, this pattern of immunoreactivity indicates that a substantial fraction of the total steady state NCC pool is polyubiquitinated when expressed in mammalian cells.
Cytoplasmic Hsp70 Promotes NCC ERAD in MDCK Epithelia
To further evaluate the relevance of the observations made in yeast, we analyzed the effect of human cytoplasmic Hsp70 (Hsp72) on NCC processing in MDCK epithelial cells. In these cells transient coexpression of Hsp70 with NCC resulted in a >90% reduction in the mature, glycosylated form of NCC (Fig. 6A). These results demonstrate that cytoplasmic Hsp70 overexpression suppresses NCC maturation in the biosynthetic pathway. Closer inspection of the NCC expression pattern in the whole cell lysates revealed that Hsp70 coexpression did not increase the total amount of immature core-glycosylated NCC. Based on this finding, we reasoned that cytoplasmic Hsp70 may not simply act as a holding factor that promotes NCC ER retention in MDCK cells but that it may also interact in a protein complex with NCC to promote its degradation. Indeed, 35S metabolic labeling and pulse-chase studies in MDCK cells confirmed that Hsp70 overexpression accelerated the rate of NCC degradation by ∼50% (Fig. 6B). Because Hsp70 dramatically reduced the abundance of mature NCC without affecting core NCC abundance at steady state, these results suggest that Hsp70 accelerates NCC ERAD to a degree that approximates the rate of NCC biosynthetic maturation in MDCK cells under control conditions. Because these rates appear to match one another, this explains the similarity in the steady state abundance of the core-glycosylated band in the control and experimental groups.
FIGURE 6.
An Hsp70 chaperone complex associates with NCC and accelerates its ERAD in mammalian kidney epithelial cells. A, shown is the effect of Hsp70 expression on NCC maturation. MDCK cells transiently expressing NCC with human Hsp70 (+) or vector pcDNA3.1 (−) (4 μg of DNA total per 10-cm2 well) for 36 h were lysed, and whole cell lysates (20 μg) were subjected to SDS-PAGE and immunoblotting with the indicated antibodies. Core and mature NCC glycoforms are indicated with arrowheads. Note that the Hsp70 antibody is highly specific for the human protein and does not cross-react with the endogenous canine Hsp70 in MDCK cells. Results are representative of five experiments. B, pulse-chase analysis of Hsp70 effects on NCC degradation in MDCK cells is shown. Top, cells transiently expressing HA-NCC in the presence of Hsp70 or pcDNA3.1 vector for 24 h were metabolically labeled for 30 min with [35S]methionine and [35S]cysteine and chased for various times as indicated. Radiolabeled HA-NCC immunoprecipitates were subjected to SDS-PAGE and phosphorimaging analysis as described under “Experimental Procedures.” Bottom, shown is a plot of NCC abundance, measured by densitometry at various points during the time course and expressed as a percent of the starting material at time zero. *, p < 0.018 by Student t test; n = 4. C, reciprocal co-immunoprecipitation of Hsp70 and NCC is shown. Top left, whole cell lysates of MDCK cells transiently expressing HA-NCC with either myc-Hsp70 (+) or pMO-myc vector (−) were immunoprecipitated (IP) with a polyclonal anti-myc antibody and immunoblotted (IB) with anti-HA antibody. Bottom left, 10% of the whole cell lysate inputs for the immunoprecipitation were immunoblotted for HA-NCC and myc-Hsp70 with the indicated antibodies. Results are representative of four experiments. Top right, whole cell lysates of MDCK cells transiently expressing myc-tagged Hsp70 with either HA-NCC (+) or pcDNA3.1 vector (−) were subjected to immunoprecipitation with polyclonal anti-NCC antibody and immunoblotted with anti Hsp70. Bottom right, 10% inputs of the whole cell lysates used in the immunoprecipitation were immunoblotted with the indicated antibodies. Results are representative of three experiments.
To test the hypothesis that Hsp70 resides in a chaperone complex that physically interacts with NCC to promote its degradation, we performed co-immunoprecipitation experiments in MDCK cells expressing tagged versions of NCC and Hsp70. As shown in Fig. 6C, left, an antibody with high specificity for cytoplasmic Hsp70 immunoprecipitated N-terminal HA-tagged NCC only in cell lysates when both NCC and myc-tagged Hsp70 were co-expressed. The immunoprecipitation was specific for the 110-kDa NCC species, indicating that Hsp70 interacted preferentially with the immature ER-localized form of NCC. We also observed co-immunoprecipitation of Hsp70 and NCC in the reciprocal direction. In these experiments low grade Hsp70 background binding to the protein A- and G-Sepharose resin was noted; however, we observed a substantial increase in the Hsp70 signal only in the anti-NCC immunoprecipitates from cell lysates when the chaperone was coexpressed with NCC (Fig. 6C, right). Together these data provide strong evidence that NCC associates with Hsp70 in a protein complex in mammalian cells and that this complex selects immature forms of NCC for ERAD.
DISCUSSION
In this study we used yeast and mammalian expression systems to begin to define the mechanisms underlying NCC ERAD. Yeast were initially employed as a genetically tractable system to identify specific evolutionarily conserved elements involved in NCC degradation. After confirming that the cotransporter is a bona fide ERAD substrate in yeast, we found that NCC is degraded via a pathway that exhibits defined requirements for chaperone-dependent recognition, ubiquitination, membrane extraction, and disposal. Specifically, our data indicate that NCC is recognized by the cytoplasmic yeast Hsp70 Ssa1, marked for degradation primarily by the ER-associated E3 ubiquitin ligase Hrd1, extracted from the membrane by the AAA+ ATPase Cdc48, and degraded via the proteasome. We then verified the relevance of these findings in parallel studies of NCC degradation, ubiquitination, and chaperone interaction in mammalian cells.
Our data represent the first survey of molecular candidates involved in the ER quality control of an SLC12 cation chloride cotransporter. Accordingly, these experiments have delineated some key differences between the mechanisms underlying the ER quality control of cation chloride cotransporters and other integral membrane proteins. For example, the chaperones that participate in the recognition of ENaC for ERAD are different from those involved in NCC recognition. Whereas all ER luminal chaperones tested in this study were found to be dispensable for NCC ERAD, ENaC requires Scj1 and Jem1 to be targeted for proteasomal degradation (14). In contrast, yeast cytoplasmic Hsp70 appears to have a more robust effect on NCC turnover than ENaC (Fig. 3 and Ref. 14). Collectively, these observations are compatible with the transmembrane topologies of the two proteins, as two-thirds of ENaC resides in the ER lumen (14), whereas over half of the NCC polypeptide is positioned in the cytoplasm, with the large NCC C terminus comprising a structured domain that consumes ∼40% of the cotransporter sequence (7).
In our evaluation of components involved in NCC ERAD, we found that the cytoplasmic Hsp70 homolog, Ssa1, was the only chaperone that influenced the cotransporter turnover rate. NCC degradation, however, was not completely suppressed in the ssa1–45 mutant strain (Fig. 4). We envision three explanations that could account for this finding. First, under the conditions of the experiment, only a partial loss of Ssa1 activity may have occurred as ssa1–45 is not a complete loss-of-function allele; thus, the cotransporter could still be recognized, albeit inefficiently, for ERAD by residual Ssa1 function. Second, other chaperones with functions that partially overlap with Ssa1 could participate in NCC ER quality control. For example, the Ssb and Ssz cytoplasmic 70-kDa chaperones could potentially take part in a cotranslational NCC ERAD checkpoint in yeast, as previously work has established their presence on ribosome-bound nascent polypeptide chains (52). The yeast Hsp90s have also been reported to possess some functional overlap with Ssa1 (53), and Hsp90s in general may be biased toward promoting substrate degradation, depending on the cochaperones with which they interact (54). Third, Ssa1 may interact with the cotransporter both during and after ubiquitination. Thus, separate from its role in ERAD substrate recognition, cytoplasmic Hsp70 may help maintain NCC solubility after ubiquitination and during substrate retro-translocation and proteasomal delivery.
The recognition steps involved in NCC ERAD are comparable with those previously described for CFTR, which is also Ssa1-dependent in yeast (29). Interestingly, however, the specific cytoplasmic Hsp70s involved in CFTR ERAD in mammalian cells appear to be different from those involved in NCC degradation. Rubenstein and Zeitlin (55) previously showed that the histone deacetylase inhibitor 4-phenylbutryate suppresses CFTR ERAD by down-regulating Hsc70 (HspA8), the constitutively expressed cytoplasmic 70-kDa chaperone in mammals. Under the same conditions, 4-phenylbutryate promotes physical interactions between the stress-inducible chaperone Hsp70 (Hsp72) and CFTR (56), suggesting that Hsc70 stimulates CFTR ERAD, whereas Hsp70 promotes CFTR maturation. We have found that these same cytoplasmic Hsp70 homologs have opposite effects on NCC; Hsp70 is a potent stimulator of NCC ERAD (Fig. 6), and preliminary analyses of the effect of Hsc70 on NCC in MDCK cells indicate that it is less efficient than Hsp70 at promoting NCC degradation (data not shown). These observations are tempered by evidence that 4-phenylbutryate changes the levels of many factors, including several chaperones that may also affect CFTR biogenesis (57). Nevertheless, they reinforce the view that mammalian cytoplasmic Hsp70s are not functionally redundant (58). Our results also support a model in which the relative tendencies of Hsp70 and Hsc70 to stimulate ERAD are not fixed; rather, the abilities of these chaperones to select substrates for folding or degradation vary depending upon the client with which they interact.
In this study we also identified Hrd1 as an important ER-associated E3 ligase that marks NCC for ERAD through ubiquitination. In yeast, Hrd1 is a key participant in the ERAD pathways responsible for the degradation of substrates with folding defects in either the ER luminal (ERAD-L) or transmembrane (ERAD-M) domains (43, 59). Because ER luminal chaperones are dispensable for NCC ERAD in yeast, our observations imply that NCC is at least partially processed for degradation through the ERAD-M pathway. This hypothesis seems reasonable as all cation chloride cotransporters possess 12 transmembrane domains that must pack together into a stable tertiary structure during biogenesis. To a limited extent, NCC turnover was also Doa10-dependent, as a doa10Δhrd1Δ-deficient strain stabilized NCC ERAD to a greater degree than strains in which Hrd1 alone was ablated. Doa10 participates in the degradation of cytoplasmic (ERAD-C) substrates (59, 60). The observation that the Doa10 effect was only seen after ablating Hrd1 suggests that in yeast, ERAD-M processing of NCC may overshadow its quality control by ERAD-C-dependent mechanisms. The mammalian versions of the yeast ER-associated E3 ligases specifically involved in NCC ERAD are currently unknown, but potential candidates for future study include the Doa10 homolog TEB4 (MARCH-VI) (61), the HRD1-SEL1L complex (a homolog of the yeast Hrd1-Hrd3 complex) (62), and the Hrd1-like E3 gp78 (63).
In sum, the work presented in this report outlines a framework for exploring the mechanistic basis of Gitelman syndrome in future studies. In Gitelman syndrome, a defect in NCC function impairs sodium chloride reabsorption in the distal convoluted tubule of the kidney, resulting in renal salt wasting, hypokalemia, and metabolic alkalosis. Nearly all cases of Gitelman syndrome are caused by mutations that reduce NCC plasma membrane expression (64). For many of these mutants, the reduced surface expression correlates with changes in glycosylation, consistent with a reduction in anterograde ER export due to NCC misfolding during biogenesis (Refs. 8–10 and 64 and see supplemental Fig. S7). Our data collectively suggest that chaperone complexes responsible for shunting their clients into ERAD-M or ERAD-C degradation pathways influence NCC turnover. Consequently, we propose that these same pathways sense misfolded conformational intermediates of NCC, targeting the cotransporter for accelerated degradation. We anticipate that the yeast and mammalian expression systems developed here will provide a novel approach to analyze further the processing and ER quality control of wild type and mutant NCC isoforms.
Supplementary Material
Acknowledgments
We thank Karina Pena and Joseph Tran for technical assistance, Ronald Rubenstein, Rebecca Hughey, Ken Hallows, Paul Welling, and Ora Weisz for reagents and helpful technical advice, and Thomas Kleyman for valuable insights during the course of these studies.
This work was supported, in whole or in part, by National Institutes of Health Grants DK79307 (to the Pittsburgh Center for Kidney Research-Model Organisms Core), GM75061 (to J. L. B.), and DK84566 (to A. R. S.). This work was also supported by a Mid-Level Career Development Award from the United States Department of Veterans Affairs (to A. R. S.) and James A. Shaver Fund of the American Heart Association Grant 10BGIA3890010 (to A. R. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S7.
- NCC
- thiazide-sensitive NaCl cotransporter
- SLC
- solute carrier
- ER
- endoplasmic reticulum
- CFTR
- cystic fibrosis transmembrane conductance regulator
- ERAD
- endoplasmic reticulum-associated degradation
- ENaC
- epithelial sodium channel
- Hsp
- heat shock protein
- MDCK
- Madin-Darby canine kidney
- AAA+
- ATPase associated with diverse cellular activities.
REFERENCES
- 1. Ellison D. H., Velázquez H., Wright F. S. (1987) Am. J. Physiol. 253, F546–F554 [DOI] [PubMed] [Google Scholar]
- 2. Simon D. B., Nelson-Williams C., Bia M. J., Ellison D., Karet F. E., Molina A. M., Vaara I., Iwata F., Cushner H. M., Koolen M., Gainza F. J., Gitleman H. J., Lifton R. P. (1996) Nat. Genet. 12, 24–30 [DOI] [PubMed] [Google Scholar]
- 3. Vargas-Poussou R., Dahan K., Kahila D., Venisse A., Riveira-Munoz E., Debaix H., Grisart B., Bridoux F., Unwin R., Moulin B., Haymann J. P., Vantyghem M. C., Rigothier C., Dussol B., Godin M., Nivet H., Dubourg L., Tack I., Gimenez-Roqueplo A. P., Houillier P., Blanchard A., Devuyst O., Jeunemaitre X. (2011) J. Am. Soc. Nephrol. 22, 693–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ji W., Foo J. N., O'Roak B. J., Zhao H., Larson M. G., Simon D. B., Newton-Cheh C., State M. W., Levy D., Lifton R. P. (2008) Nat. Genet. 40, 592–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Subramanya A. R., Welling P. A. (2011) Am. J. Physiol. Renal Physiol. 300, F838–F839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Devuyst O. (2008) Nat. Genet. 40, 495–496 [DOI] [PubMed] [Google Scholar]
- 7. Gamba G. (2005) Physiol. Rev. 85, 423–493 [DOI] [PubMed] [Google Scholar]
- 8. Kunchaparty S., Palcso M., Berkman J., Velázquez H., Desir G. V., Bernstein P., Reilly R. F., Ellison D. H. (1999) Am. J. Physiol. 277, F643–F649 [DOI] [PubMed] [Google Scholar]
- 9. De Jong J. C., Van Der Vliet W. A., Van Den Heuvel L. P., Willems P. H., Knoers N. V., Bindels R. J. (2002) J. Am. Soc. Nephrol. 13, 1442–1448 [DOI] [PubMed] [Google Scholar]
- 10. Sabath E., Meade P., Berkman J., de los Heros P., Moreno E., Bobadilla N. A., Vázquez N., Ellison D. H., Gamba G. (2004) Am. J. Physiol. Renal Physiol. 287, F195–F203 [DOI] [PubMed] [Google Scholar]
- 11. Ward C. L., Omura S., Kopito R. R. (1995) Cell 83, 121–127 [DOI] [PubMed] [Google Scholar]
- 12. Jensen T. J., Loo M. A., Pind S., Williams D. B., Goldberg A. L., Riordan J. R. (1995) Cell 83, 129–135 [DOI] [PubMed] [Google Scholar]
- 13. Kashlan O. B., Mueller G. M., Qamar M. Z., Poland P. A., Ahner A., Rubenstein R. C., Hughey R. P., Brodsky J. L., Kleyman T. R. (2007) J. Biol. Chem. 282, 28149–28156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buck T. M., Kolb A. R., Boyd C. R., Kleyman T. R., Brodsky J. L. (2010) Mol. Biol. Cell 21, 1047–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Staub O., Gautschi I., Ishikawa T., Breitschopf K., Ciechanover A., Schild L., Rotin D. (1997) EMBO J. 16, 6325–6336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valentijn J. A., Fyfe G. K., Canessa C. M. (1998) J. Biol. Chem. 273, 30344–30351 [DOI] [PubMed] [Google Scholar]
- 17. Vembar S. S., Brodsky J. L. (2008) Nat. Rev. Mol. Cell Biol. 9, 944–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cyr D. M., Höhfeld J., Patterson C. (2002) Trends Biochem. Sci. 27, 368–375 [DOI] [PubMed] [Google Scholar]
- 19. Hutt D. M., Powers E. T., Balch W. E. (2009) FEBS Lett. 583, 2639–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balch W. E., Morimoto R. I., Dillin A., Kelly J. W. (2008) Science 319, 916–919 [DOI] [PubMed] [Google Scholar]
- 21. Ong D. S., Kelly J. W. (2011) Curr. Opin. Cell Biol. 23, 231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Subramanya A. R., Liu J., Ellison D. H., Wade J. B., Welling P. A. (2009) J. Biol. Chem. 284, 18471–18480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mumberg D., Müller R., Funk M. (1995) Gene 156, 119–122 [DOI] [PubMed] [Google Scholar]
- 24. Adams A., Kaiser C., and Cold Spring Harbor Laboratory (1998) Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual, 1997 Ed., Cold Spring Harbor Laboratory Press, Plainview, NY [Google Scholar]
- 25. Lim K. L., Chew K. C., Tan J. M., Wang C., Chung K. K., Zhang Y., Tanaka Y., Smith W., Engelender S., Ross C. A., Dawson V. L., Dawson T. M. (2005) J. Neurosci. 25, 2002–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bostanjoglo M., Reeves W. B., Reilly R. F., Velázquez H., Robertson N., Litwack G., Morsing P., Dørup J., Bachmann S., Ellison D. H., Bostonjoglo M. (1998) J. Am. Soc. Nephrol. 9, 1347–1358 [DOI] [PubMed] [Google Scholar]
- 27. Stirling C. J., Rothblatt J., Hosobuchi M., Deshaies R., Schekman R. (1992) Mol. Biol. Cell 3, 129–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brodsky J. L., Hamamoto S., Feldheim D., Schekman R. (1993) J. Cell Biol. 120, 95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y., Nijbroek G., Sullivan M. L., McCracken A. A., Watkins S. C., Michaelis S., Brodsky J. L. (2001) Mol. Biol. Cell 12, 1303–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. (1982) J. Cell Biol. 93, 97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sullivan M. L., Youker R. T., Watkins S. C., Brodsky J. L. (2003) J. Histochem. Cytochem. 51, 545–548 [DOI] [PubMed] [Google Scholar]
- 32. Ahner A., Nakatsukasa K., Zhang H., Frizzell R. A., Brodsky J. L. (2007) Mol. Biol. Cell 18, 806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Youker R. T., Walsh P., Beilharz T., Lithgow T., Brodsky J. L. (2004) Mol. Biol. Cell 15, 4787–4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Y., Michaelis S., Brodsky J. L. (2002) Methods Mol. Med. 70, 257–265 [DOI] [PubMed] [Google Scholar]
- 35. Kolb A. R., Buck T. M., Brodsky J. L. (2011) Am. J. Physiol. Renal Physiol. 301, F1–F11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Jong J. C., Willems P. H., van den Heuvel L. P., Knoers N. V., Bindels R. J. (2003) J. Am. Soc. Nephrol. 14, 2428–2435 [DOI] [PubMed] [Google Scholar]
- 37. Hoover R. S., Poch E., Monroy A., Vázquez N., Nishio T., Gamba G., Hebert S. C. (2003) J. Am. Soc. Nephrol. 14, 271–282 [DOI] [PubMed] [Google Scholar]
- 38. Davidson H. W., Balch W. E. (1993) J. Biol. Chem. 268, 4216–4226 [PubMed] [Google Scholar]
- 39. Lee D. H., Goldberg A. L. (1996) J. Biol. Chem. 271, 27280–27284 [DOI] [PubMed] [Google Scholar]
- 40. Gaczynska M., Osmulski P. A. (2005) Methods Enzymol. 398, 425–438 [DOI] [PubMed] [Google Scholar]
- 41. Jones E. W., Zubenko G. S., Parker R. R. (1982) Genetics 102, 665–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huyer G., Piluek W. F., Fansler Z., Kreft S. G., Hochstrasser M., Brodsky J. L., Michaelis S. (2004) J. Biol. Chem. 279, 38369–38378 [DOI] [PubMed] [Google Scholar]
- 43. Bordallo J., Plemper R. K., Finger A., Wolf D. H. (1998) Mol. Biol. Cell 9, 209–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nishikawa S. I., Fewell S. W., Kato Y., Brodsky J. L., Endo T. (2001) J. Cell Biol. 153, 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carvalho P., Stanley A. M., Rapoport T. A. (2010) Cell 143, 579–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nakatsukasa K., Huyer G., Michaelis S., Brodsky J. L. (2008) Cell 132, 101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sato B. K., Schulz D., Do P. H., Hampton R. Y. (2009) Mol. Cell 34, 212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Becker J., Walter W., Yan W., Craig E. A. (1996) Mol. Cell. Biol. 16, 4378–4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Han S., Liu Y., Chang A. (2007) J. Biol. Chem. 282, 26140–26149 [DOI] [PubMed] [Google Scholar]
- 50. Arroyo J. P., Lagnaz D., Ronzaud C., Vázquez N., Ko B. S., Moddes L., Ruffieux-Daidié D., Hausel P., Koesters R., Yang B., Stokes J. B., Hoover R. S., Gamba G., Staub O. (2011) J. Am. Soc. Nephrol. 22, 1707–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zaarour N., Demaretz S., Defontaine N., Mordasini D., Laghmani K. (2009) J. Biol. Chem. 284, 21752–21764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang P., Gautschi M., Walter W., Rospert S., Craig E. A. (2005) Nat. Struct. Mol. Biol. 12, 497–504 [DOI] [PubMed] [Google Scholar]
- 53. Ahner A., Whyte F. M., Brodsky J. L. (2005) Arch Biochem. Biophys. 435, 32–41 [DOI] [PubMed] [Google Scholar]
- 54. Wang X., Venable J., LaPointe P., Hutt D. M., Koulov A. V., Coppinger J., Gurkan C., Kellner W., Matteson J., Plutner H., Riordan J. R., Kelly J. W., Yates J. R., 3rd, Balch W. E. (2006) Cell 127, 803–815 [DOI] [PubMed] [Google Scholar]
- 55. Rubenstein R. C., Zeitlin P. L. (2000) Am. J. Physiol. Cell Physiol 278, C259–C267 [DOI] [PubMed] [Google Scholar]
- 56. Choo-Kang L. R., Zeitlin P. L. (2001) Am. J. Physiol. Lung Cell Mol. Physiol. 281, L58–L68 [DOI] [PubMed] [Google Scholar]
- 57. Singh O. V., Vij N., Mogayzel P. J., Jr., Jozwik C., Pollard H. B., Zeitlin P. L. (2006) J. Proteome Res. 5, 562–571 [DOI] [PubMed] [Google Scholar]
- 58. Goldfarb S. B., Kashlan O. B., Watkins J. N., Suaud L., Yan W., Kleyman T. R., Rubenstein R. C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 5817–5822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carvalho P., Goder V., Rapoport T. A. (2006) Cell 126, 361–373 [DOI] [PubMed] [Google Scholar]
- 60. Ravid T., Kreft S. G., Hochstrasser M. (2006) EMBO J. 25, 533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hassink G., Kikkert M., van Voorden S., Lee S. J., Spaapen R., van Laar T., Coleman C. S., Bartee E., Früh K., Chau V., Wiertz E. (2005) Biochem. J. 388, 647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mueller B., Klemm E. J., Spooner E., Claessen J. H., Ploegh H. L. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 12325–12330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shmueli A., Tsai Y. C., Yang M., Braun M. A., Weissman A. M. (2009) Biochem. Biophys. Res. Commun. 390, 758–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Knoers N. V., Levtchenko E. N. (2008) Orphanet J. Rare Dis. 3, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.