Abstract
Background and Aims
Introgressive hybridization between two co-existing Betula species in Iceland, diploid dwarf birch B. nana and tetraploid downy birch B. pubescens, has been well documented. The two species are highly variable morphologically, making taxonomic delineation difficult despite stable ploidy levels. Here an analysis is made of morphological variation within each ploidy group with an aim to establishing a reliable means to distinguish the species.
Methods
Plant materials were collected from 14 woodlands in Iceland. The plants were identified based on 2n chromosome numbers. Morphological variation in species-specific characters within each ploidy group was analysed qualitatively and quantitatively. The morphological index was based on eight discrete characters, whereas the multivariate analysis was based on nine leaf variables.
Key Results
Of the 461 plants examined, 9·5 % were found to be triploid hybrids. The three ploidy groups were morphologically distinguishable but their variation overlapped. The diploid, triploid and tetraploid groups had average scores of 1·3, 4·1 and 8·3, respectively, in the morphology index scale from 0 (B. nana) to 13 (B. pubescens). A linear discriminant analysis also revealed significant separation among the three ploidy groups and the model assigned 96 % and 97 % of the B. nana and B. pubescens individuals correctly. The triploid hybrids were difficult to predict since only half of them could be assigned correctly. Leaf length was the most useful variable identifying triploid hybrids. Geographical patterns within the ploidy groups could partly be explained by differences in mean July temperature.
Conclusions
Hybridization between B. nana and B. pubescens is widespread in Iceland. The species can be distinguished from each other morphologically, and from the triploid hybrids. The overlapping morphological variation indicates bidirectional introgression between the two species via triploid hybrids. Iceland could be considered a birch hybrid zone, harbouring genetic variation which may be advantageous in subarctic regions.
Key words: Birch, Betula nana, Betula pubescens, diploid, triploid, tetraploid, morphological variation, morphology index, multivariate data analysis, interspecific hybrid, introgression, introgressive hybridization
INTRODUCTION
Morphological variation in birch (Betula L.) is known to be extensive, due in part to frequent hybridization in this genus (Woodworth, 1929; Johnsson, 1945; Walters, 1964; Furlow, 1997). This has made taxonomy of Betula problematic. However, there are generally acknowledged to be two species of tree birch in most of Europe: silver birch (B. pendula Roth.; B. verrucosa Ehrh., diploid 2n = 28) and downy birch (B. pubescens Ehrh.; B. alba K. & M.; B. odorata Bechst., tetraploid 2n = 56), both belonging to the subsection Albae. Other than these two tree birch species, a smaller birch, so-called dwarf birch (B. nana L., diploid 2n = 28, circumpolar species, subsection Nanae), has its distribution in the Alpine and Arctic regions of Europe. In the areas where species distributions overlap, plants with intermediate morphology (presumed hybrids) have frequently been noted (reviewed in Atkinson, 1992). The taxonomy of birch is further complicated because in many regions of Europe there is no clear morphological borderline between true species and intermediate forms, which is an expected consequence of gene flow via introgressive hybridization or introgression (reviewed in Anamthawat-Jónsson, 2003a).
Two species of Betula co-exist in Iceland: the diploid dwarf birch B. nana and the tetraploid tree birch B. pubescens (Stefánsson, 1924; Gröntved, 1942; Löve and Löve, 1956). Betula nana is a prostrate shrub up to 1 m in height. The species is represented by subspecies nana (Suk.) Hultén in Europe and western Asia; and subspecies exilis (Suk.) Hultén in North America and central and eastern Asia (Hultén and Fries, 1986). Only the subspecies nana is found in Iceland. Betula pubescens is an European species, represented by subspecies pubescens Ehrh., which may grow up to 25 m tall with single or many stems (monocormic or polycormic), and subspecies tortuosa (Ledeb.) Nyman, which is a shrub or low tree found in the mountain regions of northern Europe (Walters, 1964). The latter subspecies, so-called mountain birch, is believed to be the result of introgressive hybridization with B. nana (Vaarama and Valanne, 1973; Kallio et al., 1983). Due to extensive and continuous morphological variation of birch in Iceland (Thórsson et al., 2001; Anamthawat-Jónsson and Thórsson, 2003), this tree birch species is not divided into subspecies, but is treated as Betula pubescens sensu lato.
In the forestry context, birch (i.e. B. pubescens) is the only tree forming woodland in Iceland. Natural birch woodland in Iceland covers only about 1 % of the total land area today, but birch is believed to have had an almost continuous distribution, covering most of the lowland of Iceland before the first settlement in the ninth century (Jónsson, 2005). During the last centuries, deforestation has been a continuous process, resulting in a highly fragmented distribution of birch populations (Fig. 1). Nevertheless, considerable morphological variation exists within the Icelandic tree birch species (B. pubescens), both within and between populations. Although monocormic (single stem) and polycormic trees with crooked stems are found, the latter form dominates Icelandic woodlands. Along the tree-line on the mountainsides, a zone of birch shrubs growing horizontally on the ground can be seen throughout the country. In regions with extreme oceanic climate and heavy coastal storms, birch tends to be in the form of 1–2 m low shrubs. The height of mature trees can reach 10–12 m towards the inland areas, but the majority of natural birch woodlands (over 80 %) are dominated by trees <2 m high (Bjarnason et al., 1977; Jónsson, 2005).
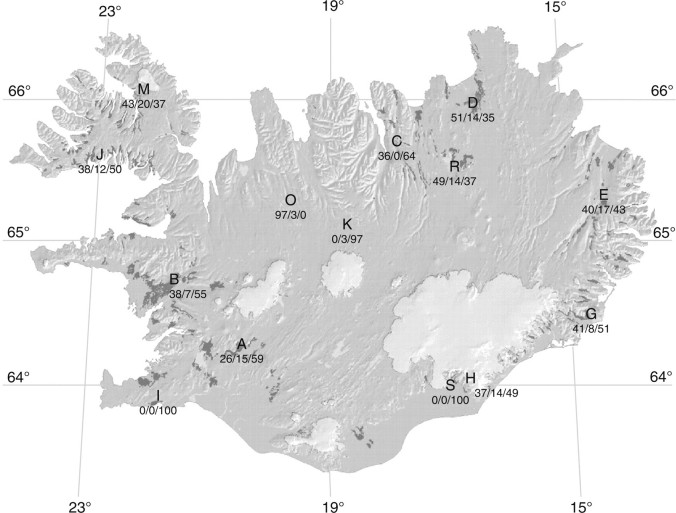
Fig. 1.
Map of Iceland showing sampling locations defined in Table 1. The percentage of diploid/triploid/tetraploid plants is shown at each site. Dark patches represent woodland vegetation, which is made up essentially of Betula pubescens. This birch distribution map is courtesy of B. Kjartansson, generated from the database of the Icelandic Forest Research Division, which is based on the nation-wide survey of birch forest from 1987 to 1991.
Such variation may be influenced by environmental factors, but previous studies (see review by Anamthawat-Jónsson, 2003a) have clearly supported genetic explanations. Morphological characters, especially in leaf shape, are very useful for species identification as they are relatively independent of environmental changes. Leaves of B. pubescens have been taxonomically described as being cordate with dentate margins, whereas B. nana leaves are orbicular with crenate margins (Walters, 1964). Based on species-specific characters, the polycormic and procumbent growth habit in B. pubescens is believed to have come from B. nana along with several features of B. nana leaf shape, as a result of introgressive hybridization between the two birch species (Stefánsson, 1924; Gröntved, 1942; Elkington, 1968; Thórsson et al., 2001). Hybridization between Betula species is thought to occur more frequently in the subarctic regions due to the overlap in distribution and flowering times, and possibly because of a less efficient incompatibility system at lower temperatures (Hagman, 1971; Eriksson and Jonsson, 1986). These interspecific hybrids, which are not totally sterile, can mediate gene flow between the parental species via backcrossing of the hybrids. This introgressive hybridization process in Icelandic Betula has been supported by data from crossing experiments (Anamthawat-Jónsson and Tómasson, 1990). Triploid hybrids from crosses between diploid B. nana and tetraploid B. pubescens were backcrossed with B. pubescens pollen donor. Triploids and tetraploids were discovered among the backcrossed progeny. Aneuploids have never been found among birch plants in nature, or from crosses. We therefore hypothesized that triploid plants must have produced only two types of gametes, n = 14 and n = 28, and all other numbers made aborted gametes.
A previous study on introgressive hybridization in birch from two locations in Iceland (Thórsson et al., 2001) revealed bidirectional gene flow between the two species via triploid interspecific hybrids. Here, the study has been extended by analysing morphological (phenotypic) variation in birch from all major woodlands in Iceland. Chromosome counts are used to unambiguously assign each individual plant to one of the three genome classes: diploid (2n = 28) B. nana, tetraploid (2n = 56) B. pubescens and triploid (2n = 42) hybrids, after which the morphological variation within each ploidy group is assessed qualitatively and quantitatively.
MATERIALS AND METHODS
Sampling sites
Fourteen natural woodlands from all regions of the country were investigated during the study period from 1998 to 2004 (Fig. 1 and Table 1). They represented the overall topographical and geographical diversity of Iceland. The Brekkuskógur (A) and Bifröst (B) woodlands, in western Iceland, are part of a very large and continuous woodland area still in existence today. In the north-western fjords, the woodland area at Kjálkafjördur (J) is defined by narrow fjords with steep hills and limited flatland, and therefore the woodlands are often small and patchy. The woodland at Kaldalón (M) stretches from the very deep and mountainous fjord Isafjardardjup towards the glacier Drangajökull. In the north, locations representing different altitudes and vegetation types were selected. At the highest site Blöndulón (O) only the dwarf birch B. nana was found. Although both species occur together at Fagrahlíd (K), the highest site for tree birch in this study, samples were collected only from B. pubescens. Two sites in the north, Mývatn (R) and Vagla-Hálsskógur (C), are part of hill woodland that was probably continuous in the past. The woodland at Ásbyrgi (D) is part of the northernmost lowland vegetation in Iceland. In the east where the climate is more continental, a woodland was selected at Eidar (E). In the east and south-east, the selected woodlands are close to Vatnajökull, Iceland's largest glacier. Birch in the Jökulsá í Lóni woodland (G) grows along glacial streams and often on sandy soil. In the Skaftafell National Park (H), two sites were investigated. On the Skaftafell hills, the birch is small and shrub-like, and on the exposed face and high up towards the hill top some plants grow horizontally over a rocky surface. On the other hand, birch in the Bæjarstadaskógur forest (S), which is situated across a narrow glacial flood plain from the Skaftafell hills, is a unique monocormic birch, so-called ‘Bæjarstadarbirki’. This birch has become the most sought-after cultivar for amenity planting and for birch breeding programmes. At the south-western coastal site Reykjanes (I), no B. nana was found among the polycormic shrub-like B. pubescens.
Table 1.
Sampling locations in Iceland (Fig. 1), total number of plants studied (n) and number of diploid (2x = 28), triploid (3x = 42) and tetraploid (4x = 56) plants from each site
| Location | Label | Location (°N/°W) | Altitude (m)* | T (°C)† | n | Diploid | Triploid | Tetraploid |
|---|---|---|---|---|---|---|---|---|
| Brekkuskógur | A | 64·27/20·51 | 129 | 11·57 | 27 | 7 | 4 | 16 |
| Bifröst | B | 64·76/21·59 | 62 | 11·10 | 42 | 16 | 3 | 23 |
| Vagla-Hálsskógur | C | 65·72/17·89 | 131 | 10·59 | 33 | 12 | 0 | 21 |
| Ásbyrgi | D | 66·01/16·50 | 41 | 11·12 | 35 | 18 | 5 | 12 |
| Eidar | E | 65·32/14·36 | 49 | 10·64 | 35 | 14 | 6 | 15 |
| Jökulsá í Lóni | G | 64·43/14·90 | 36 | 9·95 | 39 | 16 | 3 | 20 |
| Skaftafell | H | 64·03/16·98 | 267 | 10·47 | 35 | 13 | 5 | 17 |
| Bæjarstadaskógur | S‡ | 64·05/17·04 | 125 | 10·47 | 15 | 0 | 0 | 15 |
| Reykjanes | I‡ | 63·89/21·72 | 20 | 11·09 | 30 | 0 | 0 | 30 |
| Kjálkafjördur | J | 65·62/22·94 | 14 | 10·63 | 34 | 13 | 4 | 17 |
| Fagrahlíd | K | 65·22/18·66 | 402 | 10·54 | 30 | 0 | 1 | 29 |
| Kaldalón | M | 66·10/22·39 | 36 | 9·94 | 35 | 15 | 7 | 13 |
| Blöndulón | O§ | 65·36/19·80 | 452 | 8·36 | 36 | 35 | 1 | 0 |
| Mývatn | R | 65·58/16·85 | 304 | 10·23 | 35 | 17 | 5 | 13 |
* Metres above sea level (average for woodland).
† Average temperature for July, from 1998 to 2005.
‡ Only B. pubescens was found in this area.
§ Only B. nana was in this area.
Plant materials
In the woodlands where both B. nana and B. pubescens occur, the plants grow most often side by side, although B. nana tends to be more dominant on wet ground, whereas B. pubescens occupies drier land. The plants were selected in pairs where possible, each pair consisting of one B. nana-like and the nearest B. pubescens-like plant or vice versa. The distance between plants or pairs was about 50 m. In total, 461 plants were selected (see sample size/woodland in Table 1). The plants were visited during the growing season (June–August). The birches have a single, sustained period of leaf production, from bud burst in the beginning of the growing season to the end of the season when all leaves have been shed. The first visit was to select and mark the plants. Their growth form and growth habit was recorded on site. Young leaf buds (for chromosome isolation) were collected at the beginning of the growing season. Mature leaves were collected for morphological analysis, most often in July.
For the morphological analysis (characters in Table 2), 30 leaves were collected randomly from each plant and from as many branches of the plant as possible. The leaves were always late-leaves from long-shoots (see definitions in Clausen and Kozlowski, 1965; Atkinson, 1992). The leaves collected were normally from the third to the fifth positions from the shoot tip. Visually, a panel of leaf samples from each tree/shrub could be recognized as if it were a fingerprint of that particular plant. Leaf morphology was not known to be directly influenced by environmental factors, and in this study leaves of the same type and developmental stages were used. The adequacy of the leaf sampling method employed in our study was supported by the variance analysis of leaf variables (the first five variables in Table 2B), by means of partitioning of the variance components into three levels: within plants, among plants within woodland and among woodlands or sampling sites. Only 23 % of the variance was due to variation within plants. In the 77 % of the total variance by species, 44 % and 55 % was due to differences among plants within populations of B. nana and B. pubescens, respectively, whereas 33 % and 22 % was due to the variation among woodlands.
Table 2.
(A) Species-specific discrete characters used to construct the morphology index; (B) list of quantitative variables of leaf size and shape used in the linear discriminant analysis
| (A) Species-specific discrete characters | |
| Tree or shrub | Tree higher than 1 m (1), shrub shorter than 1 m (0) |
| Growth habit | Procumbent (0), erect (1) |
| Leaf tip | Obtuse >125° (0), sub-acute 95–125° (1), acute <95° (2) |
| Leaf base | Rounded (0), cordate (1), cuneate (2) |
| Leaf margin | Crenate (0), serrate (1), dentate (2) |
| Leaf shape | Orbicular (0), obovate (1), ovate (2) |
| Leaf teeth | Single (0), multi-toothed (1) |
| Petiole | Sessile 0–3 mm (0), intermediate 3–7 mm (1), non-sessile 7–15 mm (2) |
| (B) Quantitative variables | |
| 1 (cm2) | Leaf area |
| 2 (cm) | Total (vertical) leaf length including petiole |
| 3 (cm) | Maximum (horizontal) leaf width |
| 4 (cm) | Length of leaf blade (from leaf tip to the point connecting petiole) |
| 5 (cm) | Maximum width of leaf blade perpendicular to the blade length |
| 6 (cm) | Position (% of blade length) where maximum width has been measured |
| 7 (°) | Lobe angle (angle at base) |
| 8 (cm) | Length of petiole |
| 9 (cm2) | Petiole area |
As birch shrubs and trees in Iceland are mostly multicormic, whereby stems are often derived from buds below ground, it was not practical to determine axis type and position or age of every stem or branch of a birch plant. Therefore the plants were selected based on overall size, i.e. they were within the most common size range of mature birch plants which was equivalent to being about 30–50 years old (Jónsson, 2004). Based on one population in this study (E, Eidar), the means for plant size were 3·5 × 2·6 m (width × height) in B. pubescens and 1·9 × 0·4 m for B. nana. The extensive width of individual plants, relative to height, was because most plants were multicormic as described above.
Morphological analysis
Discrete, species-specific morphological characters were used to determine the morphological variation of B. nana and B. pubescens (Clapham et al., 1962; Elkington, 1968; Kenworthy et al., 1972; Pelham et al., 1988). Eight characters including growth form, growth habit and characteristics of leaf shape were examined qualitatively and scored according to Table 2A, whereby each character was given two or three possible scores from zero (B. nana) to one or two (B. pubescens). Scores for leaf characters of each plant were assigned using average values from 30 leaves that had been pressed and sealed in plastic and scanned. The scores of all characters were then combined for each plant into a single value, called a morphology index. This was assigned to place B. nana at the lowest rank (zero) and B. pubescens at the highest (maximum 13). Quantitative analysis of leaf characters was performed for nine variables (Table 2B), using the leaf morphology analysis program WinFolia (Regent Instruments, Quebec, Canada). Most of these variables have been used for differentiating populations and species of Betula (e.g. Atkinson and Codling, 1986; DeHond and Campbell, 1989; Kovacic and Simic, 2001).
Chromosome analysis
Mitotic chromosomes were isolated from young leaf buds using the protoplast dropping method of Anamthawat-Jónsson (2003b). Briefly, the buds were collected in iced water and treated for 24 h to arrest metaphases before fixing in a mixture of three parts absolute ethanol and one part of glacial acetic acid. The fixed buds were digested in an enzyme mixture of 2·5 % w/v cellulase Onozuka R10 (Merck) and 2·5 % v/v pectinase (Sigma-Aldrich) for 16–20 h at room temperature. After hypotonic treatment with 75 mm KCl solution for 5–10 min, and repeated fixation to clear cytoplasm, the protoplasts were dropped onto microscopic slides. The chromosomes were stained with the fluorescent dye DAPI (4,6-diaminophenylindole) and visualized in a Nikon epifluorescence microscope (Eclipse 800) using ×1000 magnification. The images were captured with a Nikon digital camera (DXM 1200F) using 12·5 million pixels. Chromosomes were counted from 10–20 metaphases from each plant.
Statistical analysis
Multivariate analysis of variance (MANOVA) was used to test the differentiation among ploidy groups and among sites within each group, whereas linear discriminant analysis (LDA) was conducted to evaluate how the variables can be used to classify the different individuals. The principle of both methods is based on a linear combination of variables that maximizes the ratio of between-groups variance to within-groups variance (Quinn and Keough, 2002). The homogeneity of variances of each variable was tested with the Bartlett test (Sokal and Rohlf, 1995). Based on the results from the Bartlett test, log-transformation was applied to all measurements to reduce the scatter in variances and to normalize the data. The contribution of different variables to the discrimination observed with the LDA analysis was studied by correlating each variable with the first two discriminants. Probability of the observed correlations was adjusted using the Bonferroni method (Quinn and Keough, 2002). To explain the differences among populations, population averages of the LDA analysis were correlated with differences among mean temperatures in July and December, with differences in altitudes and geographic distances. The pairwise correlations were tested with the Mantel test (Sokal and Rohlf, 1995). All statistical methods were performed using the statistical software R (http://www.r-project.org).
RESULTS
Classification of the plants by ploidy
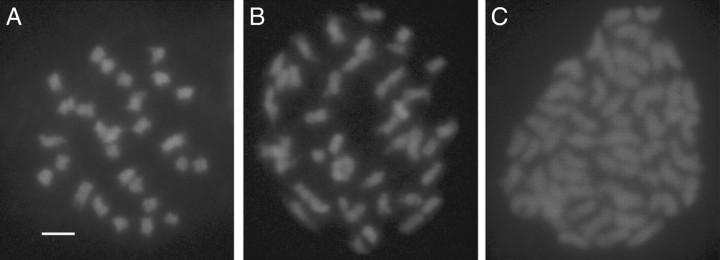
Of the 461 plants examined in this study, 176 plants (38·2 %) were confirmed with accurate chromosome counts as being diploid (2n = 2x = 28), 241 plants (52·3 %) as tetraploid (2n = 4x = 56) and 44 plants (9·5 %) as triploid with 42 chromosomes (Table 1, and Figs 1 and 2). No aneuploid cells or aneuploid plants were detected. The majority of the plants that were recorded as B. nana and B. pubescens in the field were diploid and tetraploid, respectively. Triploid individuals, on the other hand, had the appearance of either species as much as they appeared as morphological intermediates. Triploid hybrids were found in all parts of Iceland and in all of the woodlands examined except for Vagla-Hálsskógur (C), Bæjarstadaskógur (S) and Reykjanes (I). Two of these three woodlands, i.e. S and I, had no B. nana (Table 1), and this may be the reason for not detecting any triploid hybrids. On the other hand, there were plenty of diploid B. nana plants growing side by side with the tetraploid plants in the Vagla-Hálsskógur woodland (C), and yet there was no triploid hybrid. In this study the opposite situation was also found–one triploid plant (out of 36) was identified in Blöndulón (O), despite the absence of B. pubescens. Pollen of this tetraploid species could have originated in other parts of the country, but this requires further investigation. Triploid hybrids were most frequently found in Kaldalón (M, 20 %), Eidar (E, 17 %), Brekkuskógur (A, 15 %), Ásbyrgi (D, 14 %), Skaftafell (H, 14 %) and Mývatn (R, 14 %). These woodlands are distributed all around the country and are situated in different geographical and climatic environments.
Fig. 2.
Chromosome number of diploid Betula nana (A: 2n = 2x = 28), triploid hybrid (B: 2n = 3x = 42) and tetraploid B. pubescens (C: 2n = 4x = 56). The chromosomes were isolated from leaf buds and stained with DAPI. Scale bar = 2 µm.
Morphology index versus ploidy
It is possible to morphologically differentiate between the two Betula species, i.e. the diploid B. nana and the tetraploid B. pubescens (Fig. 3). Most diploid plants had B. nana-like morphology and most tetraploid plants had several characters typical of B. pubescens, as expected. The triploid hybrids were highly variable; while some resembled B. nana or B. pubescens, the majority of triploid plants were morphological intermediates.
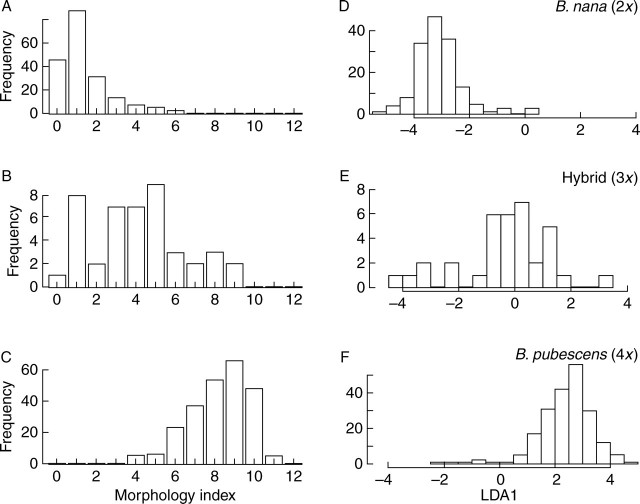
Fig. 3.
Distribution of morphological variation within diploid Betula nana, tetraploid B. pubescens and triploid samples: (A–C) showing number of plants having morphology index values from 0 to 12 based on species-specific characters (Table 2A); (D–F) showing variances from the first axis of the linear discriminant analysis (LDA1) based on leaf variables in Table 2B.
Based on the qualitative analysis of species-specific leaf characters (Table 2A and Fig. 3A–C), diploid and tetraploid plants formed separate peaks of morphology index distribution representing B. nana and B. pubescens, respectively (Fig. 3A vs. C). The B. nana peak (Fig. 3A) covered the scores from 0 to 6, with an average score of 1·3. About 80 % of diploid plants had the morphology index of 0–1 and only 23 % of this had the minimum score of zero, which is taxonomically equivalent to being pure B. nana. The scores 2–6 extended into the intermediate regions of the distribution, meaning that about 20 % of the diploid plants looked much less like B. nana. A broader peak of morphological indices was obtained from B. pubescens, whereby the tetraploid plants had scores of 4–12, with the average of 8·3 (Fig. 3C). About 83 % of these plants had scores of 7–10, about 15 % had scores of 4–6, and some 2 % had scores higher than ten. Taxonomically, the maximum score for B. pubescens should be 13 (Table 2A), but none of our tetraploid plants had this morphology index. Only one B. pubescens individual had a score of 12. Based on the average score of 8·3, most of the Icelandic tetraploid birch plants looked more like hybrids than typical European B. pubescens.
The 44 triploid plants, which were confirmed by chromosome counts, had an even wider distribution of scores, from 0 to 9, with an average value of 4·1 (Fig. 3B). Only 43 % of them were positioned in the intermediate region of the morphology distribution (scores 4–6). A further 41 % was found within the B. nana peak, and 50 % thereof scored at the lowest ranks of 0–1. However, no triploid scored higher than 9, while the B. pubescens distribution reached 12. In conclusion, 84 % of the triploid plants were either morphological intermediates or had B. nana morphology. The intermediate region, where the species peaks connected (scores 4–6), included a total of 67 individuals, which was equivalent to 14·5 % of all plants examined. About half of the plants having intermediate (hybrid) morphology could predictably be triploid, while the other half would be diploid or tetraploid.
When the morphology at each sampling site was analysed separately, four sites in the northern, western and north-western regions (i.e. B, R, M and O) had diploid B. nana individuals with morphology distribution reaching up to 5 or 6, meaning that they looked more like hybrids than B. nana. Interestingly, woodlands M, O and R are cold sites regarding average temperatures in July, and the latter two are at high altitudes. However, woodland B is neither a cold site nor at a high altitude. The morphology index distribution of B. nana at all other sites is confined to the 0–3 range, except at site J where it reached 4. No specific geographical pattern for B. pubescens could be drawn from these morphological data.
Quantitative variables versus ploidy
The linear discriminant analysis (LDA) of the quantitative variables clustered the different ploidy levels, i.e. diploid, triploid and tetraploid plants, into distinct groups (Fig. 3D–F) and this was also supported by MANOVA (P = 2·2–16). The first linear discriminant LDA1 accounted for almost all of the variance among plants (99·2 %), while the rest (0·8 %) was due to the second linear discriminant LDA2. The model assigned 96 % and 97 % of the B. nana and B. pubescens individuals correctly. The triploids were more difficult to predict: only about half of the individuals (49 %) was assigned correctly, as the distribution of their LDA1 values overlapped with those of the two Betula species (Fig. 3D–F). Variances of the LDA1 for the diploids, triploids and tetraploids were 0·699, 2·472 and 0·981, respectively. Pairwise comparisons of the LDA1 for the three ploidy groups showed that variance among the triploid plants was significantly larger than that detected in either of the two species (P < 0·0001), whereas the difference between the variances of B. nana and B. pubescens was not statistically significant.
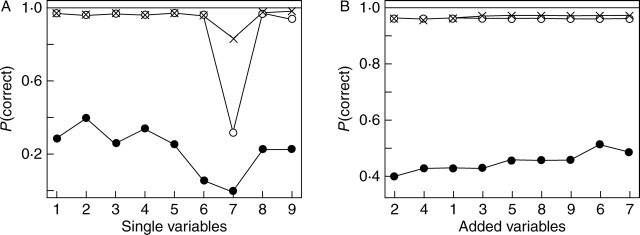
Leaf variables examined in this study were significantly correlated with the first and second linear discriminants and all variables combined supported the classification based on ploidy levels. A strong correlation was found between all variables and LDA1 (r > 0·94, P < 0·001), except for variable no. 7 (r = –0·209). This variable (lobe angle) was the only one showing significant correlation with LDA2. A further analysis of how well the different variables could be used to assign the plants to different ploidy levels correctly is presented (Fig. 4). The approach based on single variables was able to assign the diploids and the tetraploids correctly in >94 % of the cases (Fig. 4A), and this was the case for all variables except, once again, variable no. 7. Variable no. 2 (total leaf length) was most useful for identifying the triploid hybrids, and this variable alone could be used to assign 40 % of the hybrids correctly. The variables were then ranked according to how well they could be used to predict the triploids and were added accumulatively one by one to the analysis (Fig. 4B). This procedure led to a slight improvement in the classification (the ploidy grouping): when all variables apart from no. 7 were used it predicted 51·4 % of the triploids correctly (Fig. 4B) compared with 49 % by LDA1 without the ranking (Fig. 3E).
Fig. 4.
Probability of leaf variables in assigning a sample to its ploidy group correctly. Analysis of single variables (A) and accumulative effect (B) support the classification based on ploidy. The symbols open circles, crosses and closed circles denote Betula nana, B. pubescens and triploid hybrids, respectively.
The contribution of different variables to the separation within groups varied considerably. For the diploid B. nana, all leaf variables except variable no. 7 were correlated with the first and second discriminants, with the correlation coefficients ranging from 0·27 to 0·58 (P ≤ 0·001). No significant relationships were found for the triploid data. The situation was different for B. pubescens, whereby all variables except no. 7 showed a strong correlation with the LDA2 (P ≤ 0·001) and variables no. 7 and no. 8 were significantly correlated with the LDA1 (P ≤ 0·001). Discrimination along the first axis appears therefore to result from the combination of different trait values rather than the contribution of single traits.
Geographical pattern within ploidy groups
A clear indication of geographical structure was found among the woodlands investigated when the ploidy groups were analysed separately. The P-values for the MANOVA test were 2·2−16, 7·99−09 and 2·2−16 for the diploid B. nana, the triploid hybrids and the tetraploid B. pubescens, respectively. Applying the linear discriminant analysis to the two species and the triploids, the first two axes explained 78–93 % of the total variation in all cases. In B. pubescens there was still some variation unexplained: axis no. 3 accounted for 10 % of the data, while axes no. 4 and no. 5 accounted for about 4 % each.
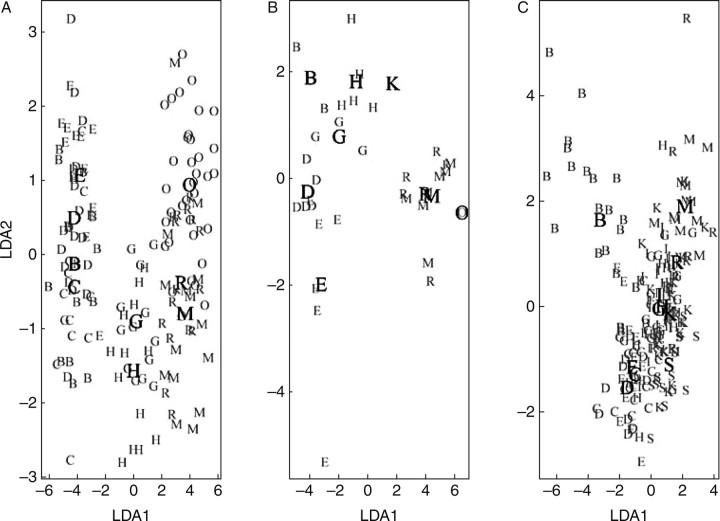
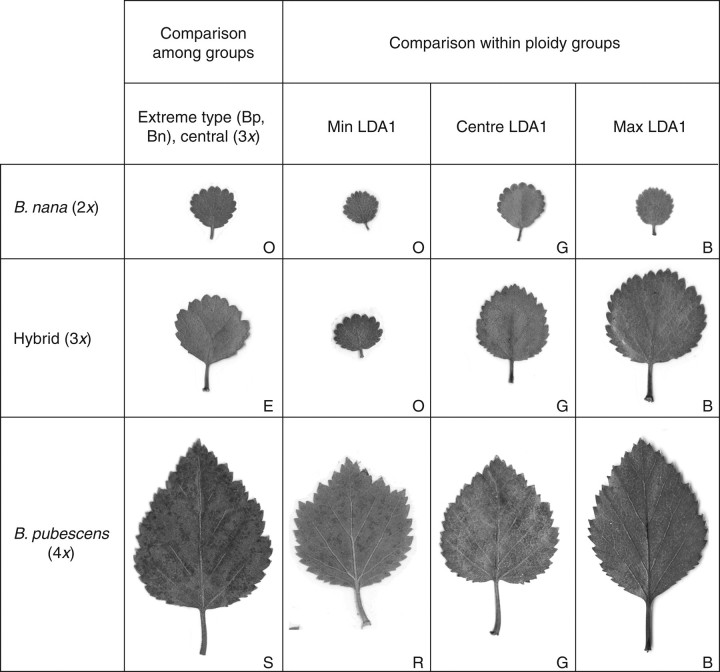
Individual plants found at the extremes in the LDA analysis (Fig. 5) included a tetraploid B. pubescens tree sampled in the birch forest Bæjarstadarskógur (S) and a diploid B. nana shrub from the dwarf birch woodland Blöndulón (O). The mean LDA1 value for B. pubescens at Bæjarstadarskógur was 3·72, being the sample most different from B. nana. The morphology index of this tree was also the highest among the tetraploid plants discovered in this study, i.e. the only sample having the index of 12. Its leaf morphology (Fig. 6) taxonomically most resembled European B. pubescens, whereas the extreme B. nana type had the morphology index of zero as expected. The leaf morphological variation shown in Fig. 6 provided evidence that the maximum and the central LDA1 types in different ploidy groups originated from the same locations (B and G, respectively). Samples of B. nana and triploids having the minimum LDA1 were from Blöndulón (O), but the B. pubescens were from Mývatn (R). Interestingly, both locations (O and R) are from the interior of the country and are at high altitudes. Similar patterns (i.e. geographical differentiation) were shown by the LDA scatter plots for the different ploidy groups (Fig. 5). Woodlands B, C, D and E were clustered to the left, G and H in the centre, but R, M and O (and K) to the right. This pattern does not reflect geographical distance among locations. However, the difference among the LDA values for the different areas sampled showed a strong correlation with mean temperature in July. The difference among average LDA values for B. nana samples and the difference in mean temperature was significantly correlated (r = 0·555, P ≤ 0·000), as was also the case for the triploids and B. pubescens (r = 0·606, P ≤ 0·006 and r = 0·430, P ≤ 0·049, respectively). The individuals found to be at the extremes of the LDA1 axis were sampled at locations with lowest (min LDA1) and highest (max LDA1) July mean temperatures. Correlations with other variables such as mean temperatures in December, altitudes and wind were much weaker and generally non-significant.
Fig. 5.
Scatter plots of the two major functions from the linear discriminant analysis (LDA1 and LDA2), showing variances of different populations for each ploidy group: diploid Betula nana (A), triploid (B) and tetraploid B. pubescens (C). Mean values for the sampling locations are shown in bold.
Fig. 6.
Leaves representing variation in the first axis of the linear discriminant analysis. The extreme types include the most Betula nana-like leaf from Blöndulón (O), the most B. pubescens-like leaf from the Bæjarstadarskógur woodland (S), and the most hybrid-like leaf from the Eidar woodland (E). Leaves representing variation within ploidy group are also shown, from the lowest (min LDA1), the middle/central, to the highest (max LDA1) values.
DISCUSSION
Natural hybridization in birch
Natural hybridization between the diploid and the tetraploid Betula species in Iceland is clearly not a rare event. Although the average frequency of natural triploid hybrids is 9·5 % of all plants examined, the occurrence is variable among locations, from being undetectable (C, Vagla-Hásskógur, northern) up to 20 % (M, Kaldalón, north-western fjord). An extensive morphological assessment of birch plants in the north-western fjord of Iceland (Elkington, 1968) provided an indication that hybridization was likely to be common, at least in the woodlands investigated. In a garden situation where B. nana plants were open pollinated, triploid progeny were produced with surprisingly high frequency (about 70 % of seedlings) (Anamthawat-Jónsson and Tómasson, 1999). Natural hybridization is certainly not restricted, but the hybrids themselves may not be competitive in natural conditions or the new seedlings are simply not able to establish themselves in the woodland areas occupied by older plants. In Icelandic conditions, birch seedlings are rarely found within undisturbed woodlands (Aradóttir et al., 2001). In the present study, triploid plants have often been found at edges of birch fields, or in marginal, open or disturbed ground. The frequency of triploid plants is obviously higher in more open woodlands with eroded soil and gravel road nearby (M 20 %; E 17 % and H 14 % triploids) in contrast to the woodlands with dense vegetation and little possibility of new seed germination (C 0 %, B 7 % and G 8 % triploids). Jetlund (1994) observed that B. pubescens specimens with intermediate morphology were more often seen in open areas above the productive forest. Birch hybridization in Iceland may be driven by habitats as described for other plant species (Rieseberg and Ellstrand, 1993; Bobola et al., 1996), and such habitats can probably support repeated hybridization as well as introgression through different periods characterized by the changing environments.
Interspecific triploid hybrids, if they are not totally sterile, can mediate gene flow between the parental species via back-crossing of the hybrids. It has been shown previously that introgression (introgressive hybridization) has occurred both in natural birch woodlands in Iceland and in controlled crosses (see Anamthawat-Jónsson, 2003a). Triploid hybrids identified in this study are not totally sterile: some triploid individuals produce a high proportion of viable pollen grains and others produce viable seeds (K. Anamthawat-Jónsson, University of Iceland, unpubl. res.). Such hybrids are certainly capable of mediating gene flow via back-crossing with the parental species, and possibly together with hybridization among the triploids themselves.
A significantly larger variance (of the LDA1) found among the triploid hybrids than those of the two species may reflect an increased segregational variance (Lynch and Walsh, 1997) following reproduction among the hybrids. If the triploids are offspring of a cross between the diploid and tetraploid parents, the variance expected in the F1 generation ought to be equal to the mean of the parental generations. In the F2 generation, additional variance is expected due to segregational variance. Non-additive variance, such as that due to epistasis and dominance could further contribute to the variation in the F2 generation. Other possible causes of the increased variance include back-crossing between the triploids and either of the parental species. To evaluate such variance components, and the impact of ploidy levels, crosses between the two species and with the F1 hybrids would be required (Wu, 1995). In the present investigation, whereby natural populations are studied directly, the variance found among the triploid hybrids is more than double those of the diploid and the tetraploid species. These natural triploid hybrids are evidently more than just the F1 generation. The release of a hidden, non-additive genetic variation might be of adaptive significance in the fluctuating environment, for the segregating hybrids as well as for the two species via back-crossing.
The scattering of the triploids is also observed, with regards to the place of origin of individual triploid plants. Among nine triploid plants that were classified as B. pubescens based on morphology, two are from Kaldalón (M, north-west) and two from Skaftafell (H, south-east). Similarly, among the six triploids that were classified as B. nana, there are two from Kaldalón (M) and one from Skaftafell (H). This large morphological variation within sites suggests that the gene flow (via back-crossing) must have had a major contribution to the variance among the triploid hybrids. Furthermore, the rare occurrence of birch plants that have all of the species-specific morphological characters, especially those of B. pubescens, can only indicate that the back-crossing of the hybrids (i.e. introgressive hybridization) has been happening for a long time. Palynological study is under way, with the aim of constructing the history of birch introgression in Iceland since the early Holocene by using morphometric characters specific to pollen of triploid plants. The studies of pollen deposits and palaeoclimatic records in Iceland (Caseldine, 2001) indicate a phase when there was considerable hybridization between the well-established B. nana and the incoming form of tree birch B. pubescens. Similar palynological and macrofossil analysis provides evidence for the occurrence of birch hybrids in the phases of subarctic conditions during the Late Glacial Period in large parts of north-west Europe (Wagner et al., 2000).
Morphological variation and introgression
The multivariate data analysis performed, together with a number of statistical tests, has confirmed the occurrence of bidirectional introgression between the two Betula species in Iceland. Both species have most likely been modified (introgressed) via back-crossing of the triploid interspecific hybrids. As a consequence, the differences between the two species are less than that expected botanically and the species do not contain all of their species-specific characters. However, both the morphology index method and the multivariate data analysis provide a convincing case of a morphological separation of the three ploidy levels, whereby the triploid hybrids show intermediate morphology. The triploid group forms its own peak, indicating that the group may function in the same way as a hybrid swarm or hybrid zone found in other species. Remington (1968) described it originally as a suture zone of hybrid interaction between recently joined biotas: a possibility of speciation in these secondary contacts. For European species, this has been seen as interfaces between regional biotas where many divergent genomes met and hybridized, especially after the last Ice Age (Hewitt, 2001). It is quite possible that considerable hybridization took place between well-established circumpolar species B. nana and the incoming European tree birch B. pubescens after the last ice retreats, as Caseldine (2001) has suggested. In this context, Iceland could be considered a birch hybrid zone, which has the function of maintaining genetic variation of the woodland birch species by the introgression process. Such variation is likely to be advantageous in cold climates and the environments found in Iceland and in other subarctic regions.
This introgressed birch can have certain advantages; for example, the ability to spread vegetatively forming a large multicormic shrub could ensure survival of the plant in extreme environments. A molecular study on alpine sedge has shown that genotype integrity is maintained in optimal habitats, whereas introgressed individuals are favoured in marginal habitats (Choler et al., 2004). Birch woodlands in Iceland are rather patchy or discontinuous today and the selected woodlands for this study reflect the highly variable habitats around the country. Environmental factors such as soil conditions, temperatures, snow load, wind and other weather conditions, which can differ very much among locations, can affect tree morphology. Davy and Gill (1984) reported a correlation between soil wetness and morphological characters of B. pendula and B. pubescens in eastern England. Verwijst (1988) also found a correlation between soil pH and growth form of B. pubescens: the polycormic type being more prominent in soil with low pH. Both Sulkinoja (1990) and Pelham et al. (1988) anticipated the effect of environmental and weather conditions on leaf morphology and growth form of B. pubescens. In the present study, significant correlation was found between the LDA values of leaf morphology and mean July temperatures, but not with other factors such as geographical distance, altitude or wind. In the woodlands experiencing cold summer (often associated with glacial sites or the interior highlands), the leaf morphology in all ploidy groups tends to be closer to the minimum LDA values and low morphology index. On the other hand, the woodlands in lowland areas tend to have morphology closer to the maximum LDA values and high morphology index. Shrub-like birch with intermediate or hybrid-like morphology is also known to be common in regions characterized by cold climates such as Fennoscandia, the highland areas of Scandinavia, other mountain regions of Europe, and southern Greenland (e.g. Kallio et al., 1983; Gardiner, 1984; Sulkinoja, 1990; Jetlund, 1994). Such morphological differentiation is likely to be driven by the introgressive hybridization process, if the introgressant types are more adaptable to (or more tolerant of) environmental pressure and habitats such as those found in Iceland and elsewhere in the subarctic regions.
The present study has also tested the impact of several leaf characters on the classification of Betula species and identification of the hybrids. The analysis of quantitative traits is evidently more powerful than the hybrid index as the LDA model can distinguish the three ploidy groups with better resolution. In addition to predicting the two Betula species correctly >94 % of the time, the model has predicted 51 % of the triploid hybrids correctly, while the hybrid index analysis identifies only 42 % of the cases. As the LDA model can quantify the effect of different variables on the classification, the model has depicted variable no. 2 (total leaf length) as being a single variable that has the most impact, i.e. it can identify up to 40 % of the triploid hybrids correctly. This is not surprising as total leaf length includes at least two of the most critical characters that can differentiate B. nana from B. pubescens qualitatively: the former has a sessile petiole and rounded (obtuse) leaf tip but the latter species has a non-sessile petiole and pointed (acute) leaf tip. Other variables have contributed very little: five leaf variables combined add merely 11 %, and variable no. 7 (lobe angle) has a negative effect on the differentiation of the ploidy groups. Nevertheless, this variable seems to be useful for characterizing variation within B. pubescens, hence it could be applied in further studies in which other species of Betula are involved or a wider range of distribution is investigated.
ACKNOWLEDGEMENTS
We are very grateful to Snædís H. Björnsdóttir for assistance with the sample collection and morphological measurements, and Dr Thröstur Eysteinsson for discussion of the results. The Icelandic Forest Research at Mógilsá provided facilities and vehicles for the field work. Financial support from the Icelandic Research Centre (Rannís research project no. 040238021 and PhD research grant) is greatly appreciated.
LITERATURE CITED
- Anamthawat-Jónsson K. Hybrid introgression in Betula. In: Sharma AK, Sharma A, editors. Plant genome–biodiversity and evolution. Enfield, USA/Plymouth, UK: Science Publishers; 2003a. pp. 249–265. Vol. 1. Phanerogams. [Google Scholar]
- Anamthawat-Jónsson K. Preparation of chromosomes from plant leaf meristems for karyotype analysis and in situ hybridisation. Methods in Cell Science. 2003b;25:91–95. doi: 10.1007/s11022-004-5620-y. [DOI] [PubMed] [Google Scholar]
- Anamthawat-Jónsson K, Thórsson ÆTh. Natural hybridisation in birch: triploid hybrids between Betula nana and B. pubescens. Plant Cell Tissue and Organ Culture. 2003;75:99–107. [Google Scholar]
- Anamthawat-Jónsson K, Tómasson T. Cytogenetics of hybrid introgression in Icelandic birch. Hereditas. 1990;112:65–70. [Google Scholar]
- Anamthawat-Jónsson K, Tómasson T. High frequency of triploid birch hybrid by Betula nana seed parent. Hereditas. 1999;130:191–193. [Google Scholar]
- Aradóttir AL, Thorsteinsson I, Sigurdsson S. Distribution and characteristics of birch woodlands in North Iceland. In: Wielgolaski FE, editor. Nordic mountain birch ecosystems. Paris: UNESCO and the Parthenon Publishing Group; 2001. pp. 51–61. [Google Scholar]
- Atkinson MD. Biological flora of the British Isles No. 175: Betula pendula Roth. (B. verrucosa Ehrh.) and B. pubescens Ehrh. Journal of Ecology. 1992;80:837–870. [Google Scholar]
- Atkinson MD, Codling AN. A reliable method for distinguishing between Betula pendula and B. pubescens. Watsonia. 1986;16:75–76. [Google Scholar]
- Bjarnason H, Sigurdsson S, Jörundsson H. Reykjavík: Skógrækt ríkisins & Skógræktarfélag Íslands; 1977. Athuganir á stærd thess og ástandi [Woodlands of Iceland. Inventory of areas and conditions] [in Icelandic] [Google Scholar]
- Bobola M, Eckert R, Klein A, Stapelfeldt K, Hillenberg K, Gendreau S. Hybridization between Picea rubens and Picea marina: differences observed between montane and coastal island populations. Canadian Journal of Forestry Research. 1996;26:444–452. [Google Scholar]
- Caseldine C. Changes in Betula in the Holocene record from Iceland–a palaeoclimatic record or evidence for early Holocene hybridisation? Review of Palaeobotany and Palynology. 2001;117:139–152. [Google Scholar]
- Choler P, Erschbamer B, Tribsch A, Gielly L, Taberlet P. Genetic introgression as a potential to widen a species' niche: insights from alpine Carex curvula. Proceedings of the National Academy of Sciences of the USA; 2004. pp. 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham AR, Tutin TG, Warburg EF. Flora of the British Isles. 2nd edn. Cambridge: Cambridge University Press; 1962. [Google Scholar]
- Clausen JJ, Kozlowski T. Heterophyllus shoots in Betula papyrifera. Nature. 1965;205:1030–1031. [Google Scholar]
- Davy AJ, Gill JA. Variation due to environment and heredity in birch transplanted between heath and bog. New Phytologist. 1984;97:489–505. [Google Scholar]
- DeHond PE, Campbell CS. Multivariate analyses of hybridisation between Betula cordifolia and B. populifolia (Betulaceae) Canadian Journal of Botany. 1989;67:2252–2260. [Google Scholar]
- Elkington TT. Introgressive hybridisation between Betula nana L. and B. pubescens Ehrh. in north-west Iceland. New Phytologist. 1968;67:109–118. [Google Scholar]
- Eriksson G, Jonsson A. A review of the genetics of Betula. Scandinavian Journal of Forest Research. 1986;1:421–434. [Google Scholar]
- Furlow JJ. Flora of North America, north of Mexico. Vol. 3. New York: Oxford University Press; 1997. Betulaceae Gray. Birch family; pp. 507–538. Flora of North America Committee eds. [Google Scholar]
- Gardiner AS. Taxonomy of infraspecific variation in Betula pubescens Ehrh., with particular reference to the Scottish Highlands. Proceedings of the Royal Society of Edinburgh; 1984. pp. 13–26. [Google Scholar]
- Gröntved J. The Pteridophyta and Spermatophyta of Iceland: Betulaceae. The Botany of Iceland. 1942;4:206–209. [Google Scholar]
- Hagman M. On self- and cross-incompatibility shown by Betula verrucosa Ehrh. and Betula pubescens Ehrh. Communicationes Instituti Forestalis Fenniae. 1971;73:1–125. [Google Scholar]
- Hewitt GM. Speciation, hybrid zones and phylogeography–on seeing genes in space and time. Molecular Ecology. 2001;10:537–549. doi: 10.1046/j.1365-294x.2001.01202.x. [DOI] [PubMed] [Google Scholar]
- Hultén E, Fries M. Atlas of North European vascular plants. Köningstein: Koeltz Scientific Books; 1986. [Google Scholar]
- Jetlund S. Introgressive hybridisation between the birch species (Betula pubescens ssp. tortuosa) and Betula nana in the mountains in ‘Gudbrandsdalen’, Norway. Norwegian Journal of Agricultural Sciences. 1994;18:15–18. [Google Scholar]
- Johnsson H. Interspecific hybridisation within the genus Betula. Hereditas. 1945;31:163–176. doi: 10.1111/j.1601-5223.1945.tb02752.x. [DOI] [PubMed] [Google Scholar]
- Jónsson TH. Stature of sub-arctic birch in relation to growth rate, lifespan and tree form. Annals of Botany. 2004;94:753–762. doi: 10.1093/aob/mch200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jónsson TH. Útbreidsla birkis á Íslandi [Distribution of birch in Iceland] The Journal of the Icelandic Forestry Association. 2005;2:60–71. [in Icelandic] [Google Scholar]
- Kallio P, Niemi S, Sulkinoja M. The Fennoscandian birch and its evolution in the marginal forest zone. Nordicana. 1983;47:101–110. [Google Scholar]
- Kenworthy JB, Aston D, Bucknall SA. A study of hybrids between Betula pubescens Ehrh. and Betula nana L. from Sutherland–an integrated approach. Transactions of the Botanical Society of Edinburgh. 1972;42:517–539. [Google Scholar]
- Kovacic S, Simic D. Intrapopulational and interpopulational relations of Betula pendula Roth (Betulaceae) in Croatia, based on leaf morphology. Acta Biologica Cracoviensia, Series Botanics. 2001;43:87–96. [Google Scholar]
- Löve Á, Löve D. A cytotaxonomical conspectus of the Icelandic flora. Acta Horti Gothobergensis. 1956;20:65–290. [Google Scholar]
- Lynch M, Walsh B. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates; 1997. [Google Scholar]
- Pelham J, Gardner AS, Smith RI, Last FT. Variation in Betula pubescens Ehrh. (Betulaceae) in Scotland: its nature and association with environmental factors. Botanical Journal of the Linnaean Society. 1988;96:217–234. [Google Scholar]
- Quinn GP, Keough MJ. Experimental design and data analysis for biologists. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Remington C. Suture-zones of hybrid interaction between recently joined biotas. Evolutionary Biology. 1968;2:321–428. [Google Scholar]
- Rieseberg LH, Ellstrand NC. What can morphological and molecular markers tell us about plant hybridisation? Critical Review in Plant Science. 1993;12:213–241. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. 3rd edn. New York, NY: W. H. Freeman; 1995. [Google Scholar]
- Stefánsson S. 18. Betuláceæ. In: Möller SL, editor. Flóra Íslands. 2nd edn. Copenhagen: Hin Íslenzka Bókmenntafjelag; 1924. pp. 83–85. [Google Scholar]
- Sulkinoja M. Hybridisation, introgression and taxonomy of the Mountain birch in SW Greenland compared with related results from Iceland and Finnish Lapland. Meddelelser om Grönland Bioscience. 1990;33:21–29. [Google Scholar]
- Thórsson Æ, Salmela E, Anamthawat-Jónsson K. Morphological, cytogenetic, and molecular evidence for introgressive hybridisation in birch. Journal of Heredity. 2001;92:404–408. doi: 10.1093/jhered/92.5.404. [DOI] [PubMed] [Google Scholar]
- Vaarama A, Valanne T. On the taxonomy, biology and origin of Betula tortuosa Ledeb. Reports of the Kevo Subarctic Research Station. 1973;10:70–84. [Google Scholar]
- Verwijst T. Environmental correlates of multiple-stem formation in Betula pubescens ssp. tortuosa. Vegetatio. 1988;76:29–36. [Google Scholar]
- Wagner F, Neuvonen S, Kurschner WM, Visseher H. The influence of hybridisation on epidermal properties of birch species and the consequences for palaeoclimatic interpretations. Plant Ecology. 2000;148:61–69. [Google Scholar]
- Walters SM. Betulaceae. In: Tutin TG, Heywood VH, Burges NA, et al., editors. Flora Europaea. Vol. 1. Cambridge: Cambridge University Press; 1964. pp. 57–59. [Google Scholar]
- Woodworth RH. Cytological studies in the Betulaceae. I. Betula. The Botanical Gazette. 1929;87:331–363. [Google Scholar]
- Wu RL. A quantitative genetic model for mixed diploid and triploid hybrid progenies in tree breeding and evolution. Theoretical and Applied Genetics. 1995;90:683–690. doi: 10.1007/BF00222134. [DOI] [PubMed] [Google Scholar]