Abstract
Background and Aims
Tuberculate ectomycorrhizae are a unique form of ectomycorrhiza where densely packed clusters of mycorrhizal root tips are enveloped by a thick hyphal sheath to form a tubercle. The functional significance of such a unique structure has not previously been established. The purpose of the present study was to investigate and measure the potential nitrogenase activity associated with Suillus tomentosus/Pinus contorta tuberculate ectomycorrhizae in two stand ages, young and old, and across a range of nitrogen-poor soil conditions.
Methods
Short roots were compared with other mycorrhizae and non-mycorrhizal secondary roots using tuberculate ectomycorrhizae. Assessment of nitrogenase activity was determined and quantitative measurements were taken on tuberculate ectomycorrhizae in situ in a variety of different circumstances, by using an adaptation of the acetylene reduction assay.
Key Results
Significant nitrogenase activity was measured associated with S. tomentosus/P. contorta tuberculate ectomycorrhizae whereas no nitrogenase activity was measured with non-tuberculate mycorrhizae or secondary roots without mycorrhizae. Average nitrogenase activity ranged from undetectable to 5696·7 nmol C2H4 g−1 tubercle 24 h−1. Maximum nitrogenase activity was 25 098·8 nmol C2H4 g−1 tubercle 24 h−1. Nitrogenase activity was significantly higher in young stands than in old stands of P. contorta. Season or some covariate also seemed to affect nitrogenase activity and there was suggestion of a site effect.
Conclusions Suillus tomentosus/P. contorta
tuberculate ectomycorrhizae are sites of significant nitrogenase activity. The nitrogenase activity measured could be an important contribution to the nitrogen budget of P. contorta stands. Season and stand age affect levels of nitrogenase activity.
Key words: Nitrogenase activity, Suillus tomentosus, tuberculate ectomycorrhizae, Pinus contorta, nitrogen budgets, acetylene reduction assay
INTRODUCTION
Nitrogen is the most limiting nutrient in most ecosystems and largely determines the growth of various plant species including many conifer tree species (Johnson et al., 1982; Weetman et al., 1988; Preston and Mead, 1994). A great deal of research and knowledge has accumulated on the nitrogen-fixing capabilities of legumes and other nodulated nitrogen-fixing genera, for example Alnus spp., Casuarina spp. and Parasponia spp. (Ekblad and Huss-Danell, 1995; Webster et al., 1998; Tjepkema et al., 2000). There are numerous combinations of nitrogen-fixing symbionts, suggesting multiple parallel evolutions of nodulated nitrogen-fixing structures. Many leguminous plants are typically associated with bacteria from the genera Rhizobium, Bradyrhizobium and relatives and are often able to fix substantial quantities of nitrogen (Vance, 1998; Graham and Vance, 2000). The nitrogen-fixing endosymbiont for both Alnus spp. and Casuarina spp. is the bacterial genus Frankia, whereas the endosymbiont of Parasponia spp. is a Rhizobium spp. (Trinick and Hadobas, 1995; Webster et al., 1995).
Although the study of nodule-associated nitrogen fixation is well established, recent research on the symbiotic relationships of nodulated plants has revealed a third organism involved in the symbiosis that appears to be significant in terms of overall nitrogen-fixing capability (Singh, 1996; Das et al., 1997; Barea et al., 2002, 2005). It has long been known that many leguminous plants are mycorrhizal, most commonly with species of arbuscular mycorrhizal fungi. It has much more recently been shown that combined inoculation with arbuscular mycorrhizal fungi and either Rhizobium spp. or Bradyrhizobium spp. on to leguminous plants significantly increases the amount of nodulation, nodule biomass, overall plant growth and, most importantly, level of nitrogen fixation compared with plants inoculated with bacteria alone (Azcón et al., 1991; Singh, 1996; Das et al., 1997; Barea et al., 2002, 2005). This mycorrhizal influence is not limited to legumes but has also been observed with non-leguminous plants such as Casuarina colonized by Frankia spp. (Vasanthakrishna et al., 1994; Ravichandran and Balasubramanian, 1999). Additionally, it has also been observed that ectomycorrhizal fungi show a similar influence when combined with Frankia spp. inoculated on to Alnus spp. (Miller et al., 1992; Yamanaka et al., 2003, 2005), and when combined with Bradyrhizobium spp. inoculated on to Acacia mangium (Jayakumar and Tan, 2005).
Associative nitrogen fixation is also well established as an important nitrogen input for many plant ecosystems. Associative nitrogen fixation is the phenomenon whereby nitrogen-fixing bacteria are not encased within a nodule of the host root tissue but instead are found in the rhizosphere or mycorrhizosphere (van Berkum and Bohlool, 1980). Li and Hung (1987) also reported nitrogen-fixing Clostridium spp. and Azospirillum spp. isolated from surface-sterilized ectomycorrhizal root tips, which suggests that associative nitrogen-fixing bacteria may reside within the ectomycorrhizae but, again, not within a nodulated structure. Associative nitrogen fixation proximal to mycorrhizal roots was first suggested by Richards and Voigt (1964) and its existence (if not importance) has been firmly established during the last 20 years (Chatarpaul and Carlisle, 1983; Dawson, 1983; Florence and Cook, 1984; Amaranthus et al., 1990; Tsimilli-Michael et al., 2000). In these types of relationships, bacterial species other than those of the genera Rhizobium, Bradyrhizobium and Frankia have been shown to fix nitrogen which is then taken up by the mycorrhizal fungus and, ultimately, by the host plant. Li and Hung (1987) proposed that phosphorus uptake by the ectomycorrhizal fungus increased and enhanced the nitrogen-fixing capabilities of the associative nitrogen-fixing bacteria.
In another study, Li et al. (1992) proposed that nitrogen-fixing bacteria reside within the tuberculate (nodule-like) structure of some kinds of ectomycorrhizae, but were unable to verify this and concluded that the bacteria were only associated with the tubercle surface. They estimated the overall contribution of nitrogen fixed by Rhizopogon vinicolor/Pseudotsuga menziesii tuberculate ectomycorrhizae (TEM) to the nitrogen nutrition of P. menziesii and found it was of minor significance.
Recently, the culturable nitrogen-fixing bacteria Paenibacillus pabuli, Paenibacillus amylolyticus and Methylobacterium mesophilicum were shown to reside within TEM formed by Suillus tomentosus on Pinus contorta var. latifolia roots (Paul, 2002). These bacteria were isolated from inner tissue and were not found on the surface of the tubercles (Paul, 2002). The presence of nitrogen-fixing bacteria exclusively within a nodular structure – the TEM – suggests an evolutionary parallel to the other types of nodulated loci of significant levels of nitrogen fixation. Therefore, the TEM is a logical place to look for concentrated nitrogen fixation. TEM, composed of a combination of bacteria, fungus and plant tissue, are not as dissimilar from other nitrogen-fixing nodules as was once assumed.
The objective of the present study was to measure the nitrogenase activity associated with S. tomentosus/P. contorta TEM in situ and to evaluate the potential importance of the nitrogenase activity to the nitrogen budget of P. contorta stands in the study area. Work was done at three sites with a range of soil conditions, but at all sites, nitrogen availability was low. In addition, two stand ages were compared: young and old. Work was done in two seasons: spring and summer.
MATERIALS AND METHODS
Study sites
The potential nitrogenase activity of S. tomentosus/P. contorta var. latifolia TEM, via acetylene reduction, was measured at three sites (Alex Graham, Nimpo Lake, Puntzi Lake) located across the Sub Boreal Pine Spruce xeric cold (SBPSxc) biogeoclimatic subzone in the Chilcotin Forest district, 150 km west of Williams Lake, British Columbia, Canada. DNA sequencing confirmed morphotype evaluation that the TEM on these study sites were exclusively from S. tomentosus, which is abundant in the area (Paul, 2002). The SBPSxc is characterized by cold, dry winters and hot, dry summers. Forest floors are typically thin (<4 cm) and decomposition is slow. The soils are nutrient-deficient with relatively low-productivity (Steen and Demarchi, 1991; Steen and Coupé, 1997).
At each of the study sites, except for Alex Graham, samples were collected in two different stand age classes. The stand ages were class 2 (<40 years old) and class 8 (>140 years old). At Alex Graham there was no suitable woody debris in the age class 8 site and therefore samples were only collected in the class 2 site. The soil at Puntzi Lake consisted of sandy loam derived from basaltic parent material with a mesic hygrotope (wet basaltic), while at the Alex Graham site the soils were characterized as dry (submesic) with sandy loam texture and derived from basaltic parent material (referred to as dry basaltic). At Nimpo Lake, the soil was sandy loam textured derived from granitic parent material (granitic) with a dry hygrotope (submesic).
Evaluation of nitrogenase activity
Quantitative assessment of nitrogenase activity was performed on S. tomentosus/P. contorta TEM in situ by using an adaptation of the acetylene reduction assay (ARA) (Hardy et al., 1968; Li et al., 1992). Measurements were conducted once in spring (May–June) and once in late summer (August–September) in 1997 and 1998. To conduct the ARA, TEM were located in randomly selected rotting logs in each stand at each site. The reason for conducting the procedure on TEM in rotting logs was that TEM are abundant and easy to find there (Paul et al., 2006). Appropriate roots were gently excavated from the woody material without breaking connection to the main root system, and cleaned of excess debris (Fig. 1A). It is very difficult to clear sufficiently around roots in mineral soil to allow them to be inserted into the apparatus without accidentally detaching them from the secondary roots.
Fig. 1.

(A) Pinus contorta root with Suillus tomentosus tuberculate ectomycorrhizae cleaned of woody material ready for insertion into incubation tube. (B) Acetylene reduction assay incubation tube showing plunger assembly (P) inserted, gas retention vial (RV) within incubation tube, and retention vial septum (IS) placed in the vial opening. (C) Acetylene reduction assay incubation tube being flushed with inert argon gas via the injection line (AI) and being vented by the evacuation needle (EV). (D) Acetylene reduction assay incubation system showing evacuation syringe (ES), argon injection syringe (AS) and incubation tube (IT).
The secondary root, while still connected to the tree and with attached mycorrhizae (where the treatment indicated), was gently inserted into a rubber septum collar. The roots with attached septum were then gently inserted through a hole cut into the rubber plunger component of a 30-cm3 plastic syringe. Before the plastic syringe (incubation tube) was completely assembled, a 6-mL gas chromatograph retention vial, with an inert butyl rubber septum placed partially in the opening of the vial, was positioned at the bottom of each incubation tube to allow collection of the gas sample for analysis once the incubation was complete (Fig. 1B). The incubation tubes were then completely sealed by tightly fitting the rubber plunger assembly with the root attached into the mouth of the incubation tubes and applying a silicon caulking grease around the root where it passed through the slot in the septum (Fig. 1B). The incubation tubes were immediately flushed with inert argon gas to lower the oxygen concentration in the tubes to between 1 and 3 % (Fig. 1C). This step was necessary because many N2-fixing bacteria are microaerophilic or anaerobic (Aho et al., 1974; Silvester et al., 1982; Amaranthus et al., 1990; Li et al., 1992) and the nitrogenase enzyme undergoes rapid oxidative damage in atmospheric air. Once the incubation tubes were flushed, 10 % of the gas headspace in the tubes was extracted using a gas-tight syringe (Chromatographic Specialists, Brockville, ON, Canada), and replaced with pure acetylene (Fig. 1D). This created an incubation environment of 10 % acetylene with microaerobic conditions (87–89 % argon, 1–3 % oxygen).
The main treatments for the ARA were secondary roots with TEM, secondary roots with non-tuberculate mycorrhizae (SRM) and secondary non-mycorrhizal roots (SR). It has been reported that ethylene oxidation is inhibited by acetylene so that any ethylene produced by root tissues will accumulate (Bont, 1976; Nohrstedt, 1976; Witty, 1979) and may result in an overestimation of nitrogenase activity. To test for this possibility, additional incubation tubes containing roots with TEM were incubated with a very low concentration (0·05 %) of acetylene (ARTEM). This concentration is sufficient to repress the oxidation of ethylene in the absence of ARA activity and allows measurement of ethylene production by the root system (Nohrstedt, 1976). Background ethylene production from the incubation apparatus was estimated by including a treatment that consisted of incubation tubes assembled with no roots, no mycorrhiza and no tubercles and incubated with 10 % acetylene (87–89 % argon, 1–3 % oxygen) (AR). Treatment AR was also used to correct for air-space variations in the incubation tubes and for gas leakage (McNabb and Geist, 1979).
It has been reported that prolonged incubations (>12 h) of excised roots, with acetylene, overestimates nitrogenase activity due to bacterial growth during the assay, which depletes root mineral N, a repressor of N2-fixation (van Berkum and Sloger, 1985). To avoid this possibility, during the main sample collecting periods (spring and summer, 1997, 1998), incubation lasted only 10 h. All results are reported on a 24-h basis.
After incubation, gas samples were collected by pushing the rubber plunger down into the syringe, thereby closing the seal on the gas retention vial (Fig. 1B). Once closed, the retention vials were airtight and contained a 6-mL sample of gases from within the apparatus.
A 1-mL gas sample was extracted from each of the chromatograph sample vials and was analysed for ethylene concentration using an HP 5400 gas chromatograph fitted with a 80–100 mesh Porapak R column. The column temperature was maintained at 70 °C with nitrogen as the carrier gas. The injection temperature and flame ionization detector temperature was set at 105 °C, and the flow rate of the carrier gas set at 40 cm3 min−1 (Li et al., 1992). Five replicate root systems were tested for each of the treatment types per sample period in both years.
Tubercle biomass measurements and nitrogenase calculations
Once incubations were complete, all tubercles in the incubation tubes were detached from the secondary roots and tubercles and secondary roots were dried at 70 °C for 8 h and weighed separately. Nitrogenase activity per gram of TEM was calculated by first correcting for endogenous and background ethylene production. The corrected values were then multiplied by the total gas volume in the incubation tubes to determine net ethylene production for each complete TEM system. The resulting value was divided by the mass of tubercles in each incubation tube to obtain a measure of nitrogenase activity per gram of TEM.
Statistical analysis
A two-way analysis of variance (ANOVA) was conducted to evaluate the effects of the three sites (dry basaltic, wet basaltic and granitic) and the two stand age classes on the nitrogenase activity of S. tomentosus/P. contorta TEM. Post-hoc tests were used to evaluate significant main effects and interactions. The least significant difference (LSD) method was used for variables with equal variances and the Dunnett's C method for variables with unequal variance. For the data sets in this study, most of the variables were close to normally distributed, and had, for the most part, equal variances and were independent of each other. Statistical analyses were performed using SPSS© 10·1 for Windows.
RESULTS
The ARAs revealed an overall significant difference among treatments (ANOVA, F5,683 = 22·97, P < 0·001). Post-hoc LSD analysis revealed significant differences between the TEM samples and all of the other treatment types (Table 1).
Table 1.
Least significant difference results for comparisons of acetylene reduction (AR) values between Suillus tomentosus tuberculate ectomycorrhizal (TEM) samples and the other four treatment types
| Treatment type | Designation | Mean AR value (nmol C2H4 mL−1) | P value |
|---|---|---|---|
| TEM | TEM | 2·113 | –* |
| Mycorrhizal roots | SRM | 1·115 | 0·004 |
| Non-mycorrhizal roots | SR | 1·028 | 0·001 |
| 0·05 % acetylene and TEM roots | ARTEM | 0·636 | <0·001 |
| AR apparatus with no roots | AR | 0·776 | <0·001 |
*P < 0·01.
The site average amounts of ethylene (C2H4) produced per gram of S. tomentosus/P. contorta TEM for a 24-h period ranged from 0·0 to 5696·7 nmol. This includes results from all sites, for both years (Table 2). Average C2H4 levels for summer 1997 ranged from 237·9 to 1623·3 nmol g−1 TEM 24 h−1, whereas values for summer 1998 ranged from 129·7 to 5696·7 nmol g−1 TEM 24 h−1 (Table 2). Ethylene production in the spring of both 1997 and 1998 was lower than summer production. Average C2H4 amounts for spring 1997 ranged from 32·8 to 242·8 nmol g−1 TEM 24 h−1 whereas none was detected in spring 1998 (Table 2).
Table 2.
Average and maximum ethylene production of Suillus tomentosus tuberculate ectomycorrhizae (TEM) at three sites within the Sub Boreal Pine Spruce xeric cold biogeoclimatic zone for spring and summer 1997 and summer 1998
| Season/year | Site/stand class | Average TEM activity* (nmol C2H4 g−1 24 h−1) (± s.e.) | Maximum TEM activity† (nmol C2H4 g−1 24 h−1) |
|---|---|---|---|
| Spring 1997 | AG 2 | 242·9 (138·5) | 724·5 |
| PL 2 | 199·1 (94·4) | 504·0 | |
| NL 2 | 129·0 (72·3) | 328·4 | |
| AG 8 | N/A | N/A | |
| PL 8 | 75·8 (45·5) | 222·5 | |
| NL 8 | 32·9 (14·7) | 75·8 | |
| Summer 1997 | AG 2 | 1623·4 (457·3) | 2580·8 |
| PL 2 | 1143·1 (306·3) | 2082·9 | |
| NL 2 | 292·0 (181·6) | 977·3 | |
| AG 8 | N/A | N/A | |
| PL 8 | 461·5 (147·1) | 1032·1 | |
| NL 8 | 237·9 (118·3) | 676·0 | |
| Summer 1998 | AG 2 | 4725·5 (3970·1) | 20577·0 |
| PL 2 | 5696·7 (4859·1) | 25098·8 | |
| NL 2 | 564·5 (181·1) | 1033·0 | |
| AG 8 | N/A | N/A | |
| PL 8 | 413·9 (150·4) | 842·8 | |
| NL 8 | 129·7 (90·7) | 483·7 |
AG, Alex Graham mountain; PL, Puntzi Lake; NP, Nimpo Lake. s.e., standard error, n = 5 for each average.
* Averages based on five root systems in each stand.
† Maximum measured from one root system.
There were significant differences in C2H4 production between the stand ages in spring 1997 and summer of both 1997 and 1998. The difference between young, class 2 stands and old, class 8 stands was significant for all seasons and both years combined (ANOVA, F1,72 = 8·965, P = 0·004) with young stands exhibiting greater nitrogenase activity (Table 3). The sites (soil types) were not significantly different (Table 3).
Table 3.
Two-way ANOVA (3 × 2) results for comparisons between sites (Alex Graham, Puntzi Lake, Nimpo Lake) and between stand ages (class 2, class 8) for Suillus tomentosus tuberculate ectomycorrhizae of average acetylene reduction assay results (nmol C2H4 g−1 TEM 24 h−1)
| Year/Season | Variable | Factor | d.f. | F statistic | P-value |
|---|---|---|---|---|---|
| Spring 97 | nmol C2H4 g−1 | Site | 2 | 1·238 | 0·309 |
| TEM 24 h−1 | Stand age | 1 | 3·388 | 0·039* | |
| Summer 97 | nmol C2H4 g−1 | Site | 2 | 5·878 | 0·509 |
| TEM 24 h−1 | Stand age | 1 | 5·172 | 0·032* | |
| Summer 98 | nmol C2H4 g−1 | Site | 2 | 0·938 | 0·406 |
| TEM 24 h−1 | Stand age | 1 | 5·155 | 0·034* |
* Significant at P < 0·05.
Maximum amounts of C2H4 produced per g TEM 24 h−1 showed similar trends as those for average values based on soil types and stand age class comparisons (Fig. 2). The highest amount of C2H4 produced (25 098·8 nmol g−1 TEM 24 h−1) occurred in young P. contorta stands in summer 1998 (Fig. 2F).
Fig. 2.
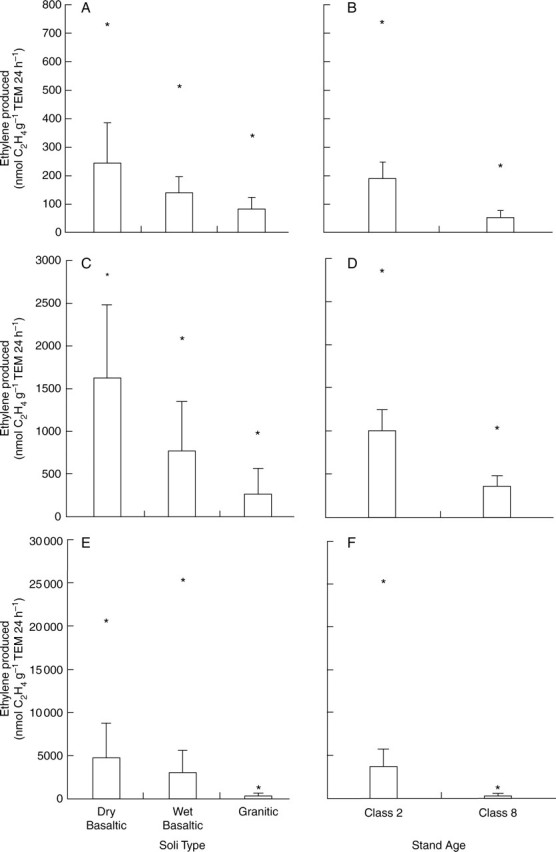
Data showing average nmol C2H4 g−1 Suillus tomentosus/Pinus contorta tubercle 24 h−1 based on soil type and stand age classes: (A) soil types from spring 1997, (B) stand age classes from spring 1997, (C) soil types from summer 1997, (D) stand age class from summer 1997, (E) soil types from summer 1998 and (F) stand age class from summer 1998. Maximum values are represented by asterisks. Dry basaltic = Alex Graham, Wet basaltic = Puntzi Lake, Granitic = Nimpo Lake; Class 2 = young stands (<40 years), Class 8 = old stands (>140 years).
DISCUSSION
Occurrence of nitrogen fixation in relation to TEM
Suillus tomentosus/P. contorta TEM exhibited in situ nitrogenase activity as indicated by the ARA. Ethylene was only produced in amounts above background levels in the treatments that included TEM as opposed to non-TEM mycorrhizae or non-mycorrhizal roots. Acetylene reduction was quite variable depending upon the season of sampling, stand age and possibly other variables (soil). Any of a number of covariates such as soil moisture or temperature could have actually been responsible for the variation. Nevertheless, substantial acetylene reduction was found on TEM on numerous occasions from a variety of locations within this study. The experiment conducted here was well controlled with checks for ethylene evolution from the apparatus, ethylene oxidation inhibition by acetylene and prolonged incubation effects. The finding of acetylene reduction was repeated with consistent patterns in relation to treatments. We are confident that the nitrogenase activity detected was associated with the TEM and not from some other source.
Li et al. (1992) found minor levels of acetylene reduction on the TEM system they studied. It is possible that the high spatial and temporal variability observed herein may also have been a factor in the Li et al. (1992) findings. In addition, mineral nitrogen availability is known to reduce nitrogen fixation rates (Sougoufara et al., 1990; Zuberer, 1998; Dianda and Chalifour, 2002) and therefore TEM occurring in stands with high nitrogen availability may display lower nitrogenase activity. The soils of the SBPSxc where this study was undertaken are consistently nutrient-poor, especially in nitrogen.
Levels of nitrogenase activity
The nitrogenase activity measured from S. tomentosus/P. contorta TEM in situ is substantial when compared with values of associative nitrogenase activity measured from Rhizopogon vinicolor/Pseudotsuga menziesii TEM in situ (Li et al., 1992). In their study, Li et al. (1992) report an average value of 39·1 nmol C2H4 g−1 TEM 24 h−1. In comparison, the average nitrogenase activity for S. tomentosus/P. contorta TEM is 5696·7 nmol C2H4 g−1 TEM 24 h−1. In contrast to Li et al. (1992), who only found N2-fixing bacteria on the surface of Rhizopogon vinicolor/Pseudotsuga menziesii TEM, N2-fixing bacteria reside within S. tomentosus/P. contorta TEM (Paul, 2002). The bacterial species found in S. tomentosus/P. contorta TEM were identified [using a combination of BIOLOG carbon utilization analysis (BIOLOG, Hayward, CA, USA), gas chromatography of fatty acid methyl esters and PCR amplification and sequencing] as Paenibacillus amylolyticus and Methylobacterium mesophilicum (Paul, 2002). The combination of high nitrogenase activity and N2-fixing bacteria residing within S. tomentosus/P. contorta TEM strongly suggests that S. tomentosus/P. contorta TEM may be functioning as symbiotic nitrogen-fixing structures similar to other nodular loci of nitrogen fixation.
Effects of stand age, season and site
The intention behind sampling in different ages of stands, seasons and sites, for the purpose of the present study, was not to determine if these factors had significant effects on nitrogenase activity, but rather to increase the odds of having at least some measurements in a period and place where nitrogenase activity was high. Nevertheless, this study found some interesting relationships between these variables and acetylene reduction activity.
There were significant differences in acetylene reduction with stand age. There are logical arguments why this might be a real effect. For example, the significantly higher values for the young stands over the old stands could be related to a higher demand for nitrogen in young stands (Weetman, 1988; Kimmins, 1997; Olsson et al., 1997) and this could be reflected in higher rates of fixation of nitrogen by TEM in the young stands. As Dakora and Phillips (2002) have discussed, there is a link between soil nutrient status and root exudates to short roots, which may mediate nutrient uptake. In the case of a nitrogen-fixing TEM, it is probable that increased root exudation in a stand with high nutrient demand on nutrient-poor soils would increase rates of nitrogen fixation.
These data strongly suggest that there are seasonal variations in nitrogenase activity. It was surprising that spring was not a season of peak activity because summer in the study area is typically quite dry, which it was anticipated would have reduced biological activity in and around roots. Other ecosystems, or this ecosystem under different weather patterns, could behave differently. However, rotting wood does act as a good reservoir for moisture (Stevens, 1997), which may lessen the relevance of summer dryness to N-fixation in rotting wood. Although the sites (soil types) were not significantly different (Fig. 2A, C, E) there appears to be a trend that could be verified by a trial expressly designed to determine site effects.
Ecological significance of the observed activity
The highest average nitrogenase activity of S. tomentosus/P. contorta TEM from this study was 5696·7 nmol C2H4 g−1 TEM 24 h−1 and the maximum nitrogenase activity was 25 098·8 nmol C2H4 g−1 TEM 24 h−1. These values are very high relative to nitrogenase activity from associative and non-symbiotic nitrogen-fixing bacteria in other conifer systems. It has been reported that in a model ecosystem of Pinus resinosa Ait. and Pinus rigida Mill., the maximum nitrogenase activity of mycorrhizal roots was 44·4 nmol C2H4 g−1 mycorrhizal root 24 h−1 (Bormann et al., 1993). This is approximately 120 times lower than the highest average nitrogenase activity of S. tomentosus/P. contorta TEM. Additionally, the maximum average level of non-symbiotic nitrogenase activity in coarse woody material (CWM) of P. contorta, Pseudotsuga menziesii, Tsuga heterophylla (Raf.) Sarg. and Thuja plicata Donn ex D. Don stands ranges from 7·3 to 32·6 nmol C2H4 g−1 CWM 24 h−1 (Silvester et al., 1982; Jurgensen et al., 1987; Crawford et al., 1997; Wei and Kimmins, 1998). These levels have been considered to be significant inputs of nitrogen for the forests where they were measured. In comparison, the highest average nitrogenase activity levels obtained in this study for S. tomentosus/P. contorta TEM are approximately 150–800 times these values. There could be many reasons for higher levels of nitrogenase activity in this study compared with studies that looked at associative or non-symbiotic nitrogenase activity in CWM. The most probable explanation for the difference is that nitrogen fixation in TEM is not equivalent to free-living or associative fixation. High nitrogenase activity in TEM is most probably the result of a structure that facilitates nitrogen fixation.
The highest average nitrogenase activity of S. tomentosus/P. contorta TEM from this study is approximately 10 % of the average activity reported from root nodules on Alnus rubra (51 000 nmol C2H4 g−1 nodule 24 h−1) and 15·7 % of the activity of Alnus sinuata (36 100 nmol C2H4 g−1 nodule 24 h−1) (Binkley, 1980; Lee and Son, 2005). Alnus is widely recognized as making biologically significant contributions of fixed nitrogen to the ecosystems where it is abundant and TEM have nitrogenase activity levels of the same order of magnitude as Alnus. Whether the standard of comparison is known rates of symbiotic or associative nitrogenase activity, the present findings indicate that TEM have nitrogenase activity levels that are biologically significant. This may be particularly important in ecosystems such as the SBPSxc where P. contorta is the dominant tree species in both early and late seres. The next phase of this work will be to quantify the levels of fixation on an area or unit biomass basis, and this will be the critical step in establishing the full importance of this newly recognized nitrogen-fixing structure.
Supplementary Material
ACKNOWLEDGEMENTS
Our sincere gratitude goes to Sheldan Myers for his outstanding patience and help during the duration of this experiment. We also wish to thank Susanne Nordstöm and Karen Myers for their generous contributions and support towards the completion of this work. Finally, our gratitude goes to Dr Andy Taylor for his most helpful comments on the manuscript. Financial support for this work was provided by Forest Renewal British Columbia, BC Forest Service, NSERC Canada and BC Science Council.
LITERATURE CITED
- Aho PE, Seidler RJ, Evans HJ, Rajau PN. Distribution, enumeration and identification of nitrogen-fixing bacteria associated with decay in living white fir trees. Phytopathology. 1974;64:1413–1420. [Google Scholar]
- Amaranthus MP, Li CY, Perry DA. Influence of vegetation type and madrone soil inoculum on associative nitrogen fixation in Douglas-fir rhizospheres. Canadian Journal of Forest Research. 1990;20:368–371. [Google Scholar]
- Azcón R, Rubio R, Barea JM. Selective interactions between different species of mycorrhizal fungi and Rhizobium meliloti strains, and their effects on growth, N2-fixation (15N) and nutrition of Medicago sativa L. New Pythologist. 1991;117:399–404. doi: 10.1111/j.1469-8137.1991.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Barea JM, Toro M, Orozco MO, Campos E, Azcón R. The application of isotopic 32P and 15 N-dilution techniques to evaluate the interactive effect of phosphate-solubilizing rhizobacteria, mycorrhizal fungi and Rhizobium to improve the agronomic efficiency or roch phosphate for legume crops. Nutrient Cycling in Agroecosystems. 2002;63:35–42. [Google Scholar]
- Barea JM, Azcón R, Azcón-Aguilar C. Microorganisms in soils: roles in genesis and functions. Heidelberg: Springer-Verlag; 2005. Interactions between mycorrhizal fungi and bacteria to improve plant nutrient cycling and soil structure; pp. 195–212. [Google Scholar]
- van Berkum P, Bohlool BB. Evaluation of nitrogen fixation by bacteria in association with roots of tropical grasses. Microbiology Reviews. 1980;44:491–517. doi: 10.1128/mr.44.3.491-517.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berkum P, Sloger C. A critical evaluation of the characteristics of associative nitrogen fixation in grasses. Proceedings of the International Symposium on Nitrogen and the Environment; Faisalabad, Pakistan: Nuclear Institute for Agriculture and Botany; 1985. pp. 124–136. [Google Scholar]
- Binkley D. Nodule biomass and acetylene reduction rates of red alder and Sitka alder on Vancouver Island, B.C. Canadian Journal of Forestry Research. 1980;11:281–286. [Google Scholar]
- Bont JA. Bacterial degradation of ethylene and the acetylene reduction test. Canadian Journal of Microbiology. 1976;22:1060–1062. doi: 10.1139/m76-155. [DOI] [PubMed] [Google Scholar]
- Bormann BT, Bormann FH, Bowden RS, Pierce RS, Hamburg SP, Wang D, et al. Rapid N2-fixation in pines, alder and locust: evidence from the sandbox ecosystem study. Ecology. 1993;74:583–598. [Google Scholar]
- Chatarpaul L, Carlisle A. Nitrogen fixation: a biotechnological opportunity for Canadian forestry. Forest Chronicle. 1983;59:249–250. [Google Scholar]
- Crawford RH, Li CY, Floyd M. Nitrogen fixation in root-colonized large woody residue of Oregon coastal forests. Forest Ecology and Management. 1997;92:229–234. [Google Scholar]
- Dakora FD, Phillips DA. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant and Soil. 2002;245:35–47. [Google Scholar]
- Das PK, Sahoo PN, Jena MK. Effect of VA-mycorrhiza and Rhizobium inoculation on nutrient uptake, growth attributes and yield of greengram (Vigna radiata L.) Environment and Ecology. 1997;15:830–833. [Google Scholar]
- Dawson JO. Dinitrogen fixation in forest ecosystems. Canadian Journal of Microbiology. 1983;29:979–992. [Google Scholar]
- Dianda M, Chalifour FP. Effect of mineral nitrogen and plant genotype on the growth and nodulation of Faidherbia albida. Canadian Journal of Botany. 2002;80:241–254. [Google Scholar]
- Ekblad A, Huss-Danell K. Nitrogen fixation by Alnus incana and nitrogen transfer from A. incana to Pinus sylvestris influenced by macronutrients and ectomycorrhiza. New Phytologist. 1995;131:453–459. doi: 10.1111/j.1469-8137.1995.tb03082.x. [DOI] [PubMed] [Google Scholar]
- Florence LZ, Cook FD. Asymbiotic N-fixing bacteria associated with three boreal conifers. Canadian Journal of Forest Research. 1984;14:595–597. [Google Scholar]
- Graham PH, Vance CP. Nitrogen fixation in perspective: an overview of research and extension needs. Field Crops Research. 2000;65:93–106. [Google Scholar]
- Hardy RWF, Holsten RD, Jackson EK, Burns RC. The acetylene-ethylene assay for N2-fixation: laboratory and field evaluation. Plant Physiology. 1968;43:1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar P, Tan TK. Growth performance and nodulation response of Acacia mangium co-inoculated with Bradyrhizobium sp and Pisolithus tinctorius. Symbiosis. 2005;40:109–114. [Google Scholar]
- Johnson DW, Cole DW, Bledsoe CS, Cromack K, Edmonds RL, Gessel SP, et al. Nutrient cycling in forests of the Pacific Northwest. In: Edmonds RL, editor. Analysis of coniferous forest ecosystems in the western United States. Stroudsberg, PA: Hutchinson and Ross; 1982. pp. 186–232. [Google Scholar]
- Jurgensen MF, Larsen MJ, Graham RT, Harvey AE. Nitrogen fixation in woody residue of northern Rocky Mountain conifer forests. Canadian Journal of Forestry Research. 1987;17:1283–1288. [Google Scholar]
- Kimmins JP. Forest ecology. 2nd edn. New Jersey: Prentice-Hall; 1997. [Google Scholar]
- Lee YY, Son Y. Diurnal and seasonal patterns of nitrogen fixation in an Alnus hirsuta plantation of central Korea. Journal of Plant Biology. 2005;48:332–337. [Google Scholar]
- Li CY, Hung LL. Nitrogen-fixing (acetylene-reducing) bacteria associated with ectomycorrhizae of Douglas-fir. Plant and Soil. 1987;98:425–428. [Google Scholar]
- Li CY, Massicotte HB, Moore LVH. Nitrogen fixing Bacillus sp. associated with Douglas-fir tuberculate ectomycorrhizae. Plant and Soil. 1992;140:35–40. [Google Scholar]
- McNabb DH, Geist JM. Acetylene reduction assay of symbiotic N2-fixation under field conditions. Ecology. 1979;60:1070–1072. [Google Scholar]
- Miller SL, Koo CD, Molina R. Early colonization of red alder and Douglas-fir by ectomycorrhizal fungi and Frankia in soils from the Oregon coast range. Mycorrhiza. 1992;2:53–61. [Google Scholar]
- Nohrstedt HO. Natural formation of ethylene in forest soils and methods to correct results given by the acetylene reduction assay. Soil Biology and Biochemistry. 1976;15:281–286. [Google Scholar]
- Olsson U, Binkley D, Smith FW. Nitrogen supply, nitrogen use, and production in an age sequence of lodgepole pine. Forest Science. 1997;44:454–457. [Google Scholar]
- Paul LR. Vancouver, BC, Canada: University of British Columbia; 2002. Nitrogen fixation associated with tuberculate ectomycorrhiza on lodgepole pine (Pinus contorta) PhD thesis. [Google Scholar]
- Paul LR, Chapman BK, Chanway CP. Suillus tomentosus tuberculate ectomycorrhizal abundance and distribution in Pinus contorta woody debris. Canadian Journal of Forest Research. 2006;36:460–466. [Google Scholar]
- Preston CM, Mead DJ. Growth response and recovery of 15N-fertilizer one and eight growing seasons after application to lodgepole pine in British Columbia. Forest Ecology and Management. 1994;65:219–229. [Google Scholar]
- Ravichandran VK, Balasubramanian TN. Growth response of Casuarina equisetifolia seedlings to dual inoculation of Frankia and VAM (Glomus fasciculatum) Journal of Ecobiology. 1999;11:175–179. [Google Scholar]
- Richards BN, Voigt GN. Role of mycorrhizae in nitrogen fixation. Nature. 1964;201:310–311. [Google Scholar]
- Silvester WB, Sollins P, Verhoeven T, Cline SP. Nitrogen fixation and acetylene reduction in decaying conifer boles: Effects of incubation time, aeration and moisture content. Canadian Journal of Forest Research. 1982;12:646–652. [Google Scholar]
- Singh CS. Arbuscular mycorrhiza (AM) in association with Rhizobium sp. improves nodulation, N2 fixation, and N utilization of pigeon pea (Cajanus cajan), as assessed with a 15N technique, in pots. Microbiological Research. 1996;151:87–92. [Google Scholar]
- Sougoufara B, Danso SKA, Diem HG, Dommergues YR. Estimating N2-fixation and N derived from soil by Casurina equisetifolia using labelled 15N fertilizer: some problems and solutions. Soil Biology and Biochemistry. 1990;22:695–701. [Google Scholar]
- Steen O, Coupé RA. Victoria: B.C. Ministry of Forests; 1997. A field guide to forest site identification and interpretation for the Cariboo Forest Region. [Google Scholar]
- Steen O, Demarchi DA. Sub-boreal pine – spruce zone. In: Meidinger D, Pojar J, editors. Ecosystems in British Columbia. Victoria: B.C. Ministry of Forests; 1991. pp. 195–207. [Google Scholar]
- Stevens V. British Columbia, Ministry of Forests, Research Program: Victoria, B.C.; 1997. The ecological role of coarse woody debris: an overview of the ecological importance of CWD in B.C. forests. [Google Scholar]
- Tjepkema JD, Schwintzer CR, Burris RH, Johnson GV, Silvester WB. Natural abundance of 15N in actinorhizal plants and nodules. Plant and Soil. 2000;219:285–289. [Google Scholar]
- Trinick MJ, Hadobas PA. Formation of nodular structures on the non-legumes Brassica napus, B. campestris, B. juncea and Arabidopsis thaliana with Bradyrhizobium and Rhizobium isolated from Parasponia spp. or legumes grown in tropical soils. Plant and Soil. 1995;172:207–219. [Google Scholar]
- Tsimilli-Michael M, Eggenberg P, Biro B, Koves-Pechy K, Voros I, Strasser RJ. Synergistic and antagonistic effects of arbuscular mycorrhizal fungi and Azospirillum and Rhizobium nitrogen-fixers on the photosynthetic activity of alfalfa, probed by the polyphasic chlorophyll a fluorescence transient O-J-I-P. Applied Soil Ecology. 2000;15:169–182. [Google Scholar]
- Vance CP. The Rhizobiaceae. Dordrecht: Kluwer Academic Publishers; 1998. Legume symbiotic nitrogen fixation: agronomic aspects; pp. 509–530. [Google Scholar]
- Vasanthakrishna M, Bagyaraj DJ, Nirmalnath PJ. Responses of Casuarina equisetifolia to inoculation with Glomus fasciculatum and/or Frankia. Forest Ecology and Management. 1994;68:399–402. [Google Scholar]
- Webster G, Davey MR, Cocking EC. Parasponia with rhizobia: a neglected non-legume nitrogen-fixing symbiosis. Agbiotech News and Information. 1995;7:119–124. [Google Scholar]
- Webster G, Davey MR, Cocking EC. Tree biotechnology: towards the millennium. Nottingham: Nottingham University Press; 1998. Parasponia: a nitrogen-fixing non-legume of the Ulmaceae; pp. 77–86. [Google Scholar]
- Weetman GF. Nutrition and fertilization of lodgepole pine. Proceedings Future Forests of the Mountain West: A Stand Culture Symposium, 1986; Washington, USA: USDA Forest Service General Technical Report; 1988. pp. 231–239. INT-243. [Google Scholar]
- Weetman GF, Fournier RM, Schnorbus E. Lodgepole pine fertilization screening trials: four-year growth response following initial predictions. Soil Science Society of America Journal. 1988;52:833–839. [Google Scholar]
- Wei X, Kimmins JP. Asymbiotic nitrogen fixation in harvested and wildfire killed lodgepole pine forests in the central interior of British Columbia. Forest Ecology and Management. 1998;109:343–353. [Google Scholar]
- Witty JF. Acetylene reduction assay can over-estimate nitrogen fixation in soil. Soil Biology and Biochemistry. 1979;11:209–210. [Google Scholar]
- Yamanaka T, Li CY, Bormann BT, Okabe H. Tripartite associations in an alder: effects of Frankia and Alpova diplophloeus on the growth, nitrogen fixation and mineral acquisition of Alnus tenuifolia. Plant and Soil. 2003;254:179–186. [Google Scholar]
- Yamanaka T, Akama A, Li CY, Okabe H. Growth, nitrogen fixation and mineral acquisition of Alnus sieboldiana after inoculation of Frankia together with Gigaspora margarita and Pseudomonas putida. Journal of Forest Research. 2005;10:21–26. [Google Scholar]
- Zuberer DA. Principles and applications of soil microbiology. New Jersey: Prentice-Hall; 1998. Biological dinitrogen fixation: introduction and non-symbiotic; pp. 295–321. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


