Abstract
Background and Aims
It has been previously shown that abscission of apple fruitlets is preceded by an increase in ethylene evolution and in the amount of transcripts for 1-aminocyclopropane-1-carboxylate oxidase (ACO), an enzyme catalysing the final step in ethylene biosynthesis. These events are concomitant with shedding induction and chemical thinning. There are several thinners but their mode of action and efficacy is poorly understood. One of them is benzylaminopurine (BA), a cytokinin believed to act by enhancing vegetative activity and stressing the competition between shoots and fruitlets, thus leading to fruitlet shedding. Nevertheless, the specific mechanism of action of BA and the variable effect depending on apple cultivar (easy or difficult to thin) are poorly understood.
Methods
Abscission, the amount of MdACO1 transcripts and other parameters were followed in immature apple fruits during the period of physiological drop. The cultivars studied were ‘Golden Delicious’ and the ‘spur’ type ‘Red Delicious’. BA was used as a thinning agent and was sprayed 14 d after petal fall (DAPF). Fruitlets were divided into central (C) and lateral (L) fruitlet populations.
Key Results
Fruitlet size was significantly different between C and L fruitlets but it did not differ much between the populations within the same cultivar. C fruitlets were characterized by basal ethylene evolution while L fruitlets displayed an increase in hormone biosynthesis during abscission induction. Cluster composition evaluated by the L/C ratio differed in the two varieties, being almost unchanged throughout abscission induction in ‘Golden Delicious’ and progressively decreasing in ‘Red Delicious’. Shoot growth activity evaluated at the end of the season indicated a possible connection with both the ongoing abscission and BA application. MDACO1 transcripts were mainly detected in L fruitlets and the accumulation was related to total abscission in ‘Golden Delicious’, while in ‘Red Delicious’ expression was observed in both C and L fruitlets.
Conclusions
BA probably exerts its thinning effect through vegetative growth. In the ‘spur’ type ‘Red Delicious’ the chemical is ineffective, probably due to a limited action on shoot growth due to genetic characteristics. The amount of MdACO1 transcripts in seeds is a good indicator of abscission.
Key words: Abscission, ACO, benzylaminopurine (BA), branching, ethylene, Malus domestica
INTRODUCTION
Several organs such as flowers, immature and mature fruits, leaves, buds and shoots undergo abscission. The process results in the detachment of the organ from the plant body due to cell separation occurring in a specific cell layer termed the abscission zone (Roberts et al., 2002). The factors affecting this process can be grouped into two main classes, namely those external and internal to the plant (Taylor and Whitelaw, 2001). Several studies have proposed the involvement of the subtending organ part in the shedding induction, leading to activation of the abscission zone (Bangerth, 2000; Drazeta et al., 2004; Else et al., 2004; Alferez et al., 2005; Dal Cin et al., 2005a). Moreover, it is generally accepted that auxin and ethylene are the two main plant growth regulators involved (Uheda and Nakamura, 2000; Dhanalakshmi et al., 2003; Dal Cin et al., 2005a).
Immature fruit drop (termed fruitlet physiological drop) is commonly regarded as a self-regulatory mechanism that avoids an excessive load on the plant that might lead to starvation in later stages of fruit development (Bangerth, 2000). This process is at least in part a consequence of the competition among fruitlets and between fruitlets and shoots. In apple, buds give rise to flowers and a shoot that develops at the side of the twig subtending the five-flower cluster. The central flower is referred to as the ‘king flower’ because it blooms first and is believed to be the dominant one. From the central flower originates a fruitlet that is bigger and less prone than lateral fruitlets to abscise. The phenomenon of abscission resembles a hierarchy that also involves lateral fruitlets within the same cluster, with the severity depending on blooming time difference and pollination success (Bangerth, 2000). In addition, physiological drop is also affected by the position within the plant. Unfavourably located fruitlets, such as those developing from 1-year branches, display a higher level of abscission compared with those located on bourses (Bangerth, 2000).
The self-regulatory mechanism responsible for the shedding of immature apple fruit may be magnified by chemicals whose actions are quite variable and dependent on environmental conditions, and by varietal effects that can make plants either easy or difficult to thin, even though different chemicals or combinations of them are used. For example, ‘Golden Delicious’ trees respond readily to thinners while the group of spur-type ‘Red Delicious’ trees do not (Wertheim, 2000). It is interesting that the former is characterized by a flourishing canopy while the latter has a more reduced habit. Chemical thinners probably act by interfering mainly with the hormonal status of the fruitlet (ethephon and carbaryl), or of the plant as a whole (auxin and cytokinin-like compounds) (Bangerth, 2000; Jones et al., 2000; Wertheim, 2000). Compounds belonging to the second group are particularly interesting because they can be employed to elucidate mechanisms of competition between the fruit cluster and the shoot. Among these, benzylaminopurine (BA) seems the most appealing because its efficacy differs greatly between cultivars. In addition, this bioregulator is commonly used in plant nurseries to increase branching, although at a higher concentration than used for thinning. Although BA is widely used and its efficacy has recently been reviewed (Buban, 2000), its mode of action and the reasons for the variability it its performance still remain elusive. In a recent paper, it was shown that apple fruitlets with greatly diiffering levels of abscission displayed significant differences in ethylene evolution. These fruitlets also displayed major differences in MdACO1 transcript accumulation, above all in the seed and cortex (Dal Cin et al., 2005a).
In this manuscript data are presented data from in planta experiments. Apple trees were followed from petal fall up to the end of the ‘June drop’ and attention was specifically focused on the time in which abscission is generally accepted to be evoked (2–4 weeks after petal fall). The study involved central and lateral fruitlets borne on both control and BA-sprayed apple trees belonging to ‘Golden Delicious'and the spur-type ‘Red Delicious’ cultivars. Parameters considered were fruitlet growth, abscission, shoot growth, and ethylene evolution and MdACO1 expression in seed, cortex and peduncle. Based on the results, an explanation of the differences between cultivars and a possible shedding mechanism related to cluster-shoot competition is proposed.
MATERIALS AND METHODS
Plant material and treatments
Research was carried out in 2004 on 7-year-old apple (Malus domestica L. Borkh) trees of ‘Golden Delicious’ (‘Golden Delicious/M9’) and the ‘spur-type’ ‘Red Delicious’ (‘Red Chief/M26’) grown at the experimental farm of the Istituto Agrario Sperimentale San Michele all'Adige (Trento, Italy). Forty ‘Golden Delicious’ (‘Golden’) trees of uniform size were grouped into five blocks (eight trees each). All the lateral flowers were removed from the clusters of two blocks at the time of blooming, keeping only the king flowers free-pollinated with compatible pollen of the cultivar ‘Stark Red’ (treatment K). BA at 88·8 nm (commercial form ‘Brancher-Dirado’ A.I. 9·35 %, Agrimport, Bolzano, Italy) was sprayed on trees of two blocks, one with intact clusters (treatment BA) and the other with only the central fruitlet (treatment K + BA), at 14 d after petal fall (DAPF) when the average fruitlet diameter was about 10 mm. On the same date one tree block with intact clusters was sprayed with naphthalene acetic acid (NAA) at 5·4 nm (commercial form ‘Dirager’, A.I. 3·3 %, Gobbi, Genova, Italy). The remaining two blocks, one with intact clusters (CTRL) and the other with laterals removed (K), were sprayed with water to run-off and kept as controls.
In addition to the ‘Golden Delicious’ trial, BA sprays were also performed on ‘Red Delicious’ (‘Red’), a cultivar resistant to the thinning action of the chemical. Sixteen trees were grouped into two blocks (eight trees each), one was treated with BA (BA) and the other was kept as a control (CTRL). Concentration of the chemical and the timing of treatments were as stated above.
Fruit growth was monitored separately in lateral (L) and central (C) fruitlets borne on 40 representative clusters on trees of ‘Golden’ and ‘Red’ from 11 to 24 DAPF.
Ethylene evolution was monitored by the laser-based photoacoustic (LBPA) technique on C and L fruitlets of ‘Golden’ (CTRL, BA and K) and ‘Red’ (CTRL and BA) populations from 11 to 29 DAPF, as previously described (Dal Cin et al., 2005a).
The composition of the cluster, expressed as the L/C ratio, was assessed by counting the number of L and C fruitlets borne on 40 representative clusters of ‘Golden’ (CTRL, BA and NAA) and ‘Red’ (CTRL and BA) populations from 11 to 24 DAPF.
Fruitlet shedding was monitored from 2 to 8 weeks after petal fall on ‘Golden’ (CTRL, BA, NAA and K) and ‘Red’ (CTRL and BA) populations. Percentage abscission per tree was expressed as the ratio between fruitlets present 9 weeks after petal fall and the total number of flowers at bloom. The size of shed fruitlets (cross-diameter) was determined throughout the abscission period.
BA effects on vegetative growth
The effect of BA on shoot growth was evaluated on ‘Golden’ and ‘Red’ trees at the end of the growing season by measuring the diameter and length of central shoots, as well as the number and length of lateral shoots. Differences between variables were tested for significance using Duncan's Multiple Range Test with an alpha value of 0·05.
Molecular analysis
Seed, cortex and peduncle samples were collected from fruitlets of ‘Golden’ (CTRL, BA, NAA and K) and ‘Red’ (CTRL and BA) at 11, 14, 17, 19, 21 and 24 DAPF, frozen in liquid nitrogen and stored at − 80 °C for molecular analysis. Total RNA was obtained following the protocol described by Dal Cin et al. (2005b). Expression analysis of MdACO1 (AB030859) was performed with Northern blotting. In order to compare transcript quantity for the different subpopulations, samples from the same tissue were electrophoresed in the same gel, blotted and hybridized together. Total RNA amounts of 15 µg (cortex and peduncle) and 10 µg (seed) were loaded on a 1 % agarose denaturing gel. Equal total RNA loading was checked by observation under a UV lamp. Blotting was performed with GeneScreen (NEN, Dupont) membranes as described by Sambrook et al. (1989). Membranes were pre-hybridized for 6 h and then hybridized for 16 h at 42 °C in 50 % formamide, 6 × SSPE, 5 × Denhardt's, 25 mm potassium phosphate and 10 mg mL−1 salmon sperm DNA. Hybridizations were carried out using a 500 bp 32P-radiolabelled MdACO1 clone as the probe, which was obtained as described previously (Dal Cin et al., 2005a). Membranes were washed three times in 1 × SSC, 0·1 % SDS, for 15 min each at 42 °C before exposure to X-ray film. Membranes were then stripped according to the manufacturer's instructions and hybridized with apple rRNA–18S cDNA as an internal control, and hybridized as described above; results were similar to those obtained by UV lamp observation and so are not presented here.
RESULTS
Fruitlet growth
‘Golden Delicious’ (‘Golden’) C fruitlets cross-diameter increased linearly in all trials, whereas L fruitlets increased in size at a similar rate up until 17 DAPF but thereafter their growth rate diminished relative to C fruitlets (Fig. 1A). ‘Red Delicious’ (‘Red’) fruitlets displayed an almost linear growth in both C and L fruitlets, although they started to diverge around 19 DAPF (Fig. 1B). C fruitlets were on average larger than L fruitlets in all populations of both ‘Golden’ and ‘Red’. Fruitlets showed an increase in cross-diameter variability as the experiment progressed. Fruitlet variability was high among different clusters in ‘Golden’ and within the same cluster in ‘Red’ (results not shown).
Fig. 1.
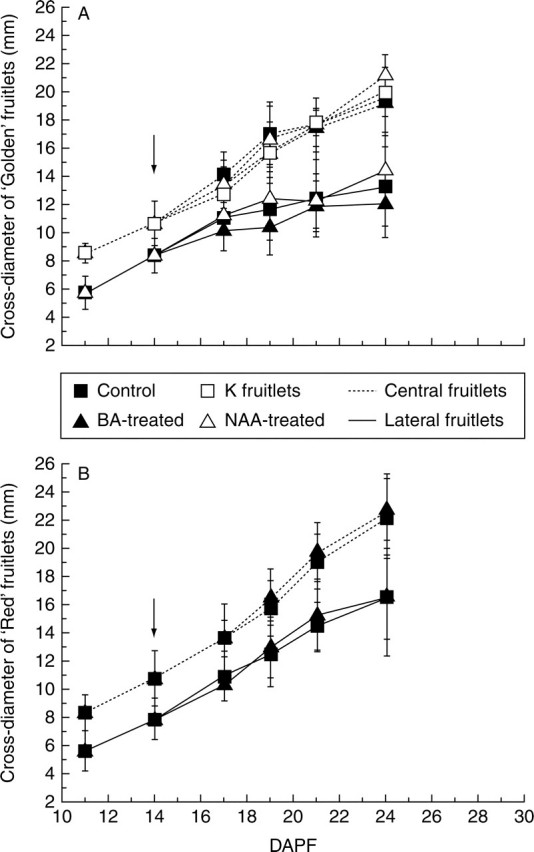
Cross-diameter of fruitlets of (A) ‘Golden Delicious’ and (B) ‘Red Delicious’ monitored throughout the period of abscission induction (11–24 d after petal fall, DAPF). Values are means (±s.d.) of fruitlets from 40 representative clusters per population. Arrows indicate application time for BA and NAA (see text for details).
Ethylene evolution
In ‘Golden’, C fruitlets displayed a lower level of ethylene production compared with that of L fruitlets (Fig. 2A). In BA-treated trees, L fruitlets produced three times more ethylene than those of controls at 17 and 19 DAPF. K fruitlets were characterized by low ethylene production, even though the level was significantly higher than that of C fruitlets of trees with intact clusters at 17 and 19 DAPF. Unlike ‘Golden’, ‘Red’ fruitlets already showed some small differences between C and L fruitlets at 11 DAPF; thereafter the differences became greater (Fig. 2B). Ethylene remained at a low level in C fruitlets whereas it increased in L fruitlets, peaking at 19 DAPF in both CTRL and BA-treated fruitlets, being higher in the latter.
Fig. 2.
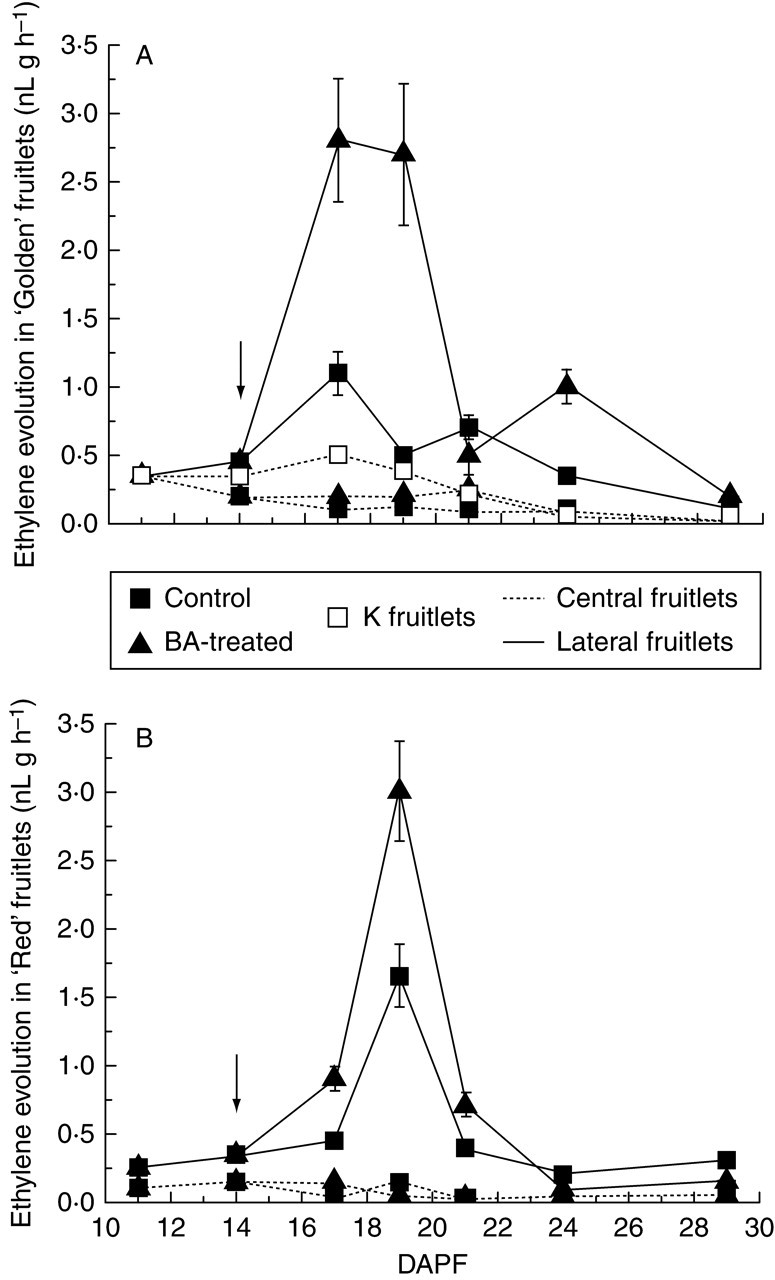
Ethylene evolution of fruitlets of (A) ‘Golden Delicious’ and (B) spur-type ‘Red Delicious’, monitored throughout the period of abscission induction (11–29 DAPF). Values are means (±s.d.) of fruitlets from 15 representative clusters per population. Arrows highlight application time. Arrows indicate application time for BA (see text for details).
Cluster composition
The L/C ratio in ‘Golden’ clusters remained fairly constant (around 4) throughout the experimental period in all treatments (Fig. 3A). This would indicate that BA, on a single fruitlet basis, is equally effective in determining L or C fruitlet shedding. In ‘Red’ the situation was different: the L/C ratio, around 3 at 11 DAPF, declined during the experiment to around 2·5 (Fig. 3B). In BA-treated trees the ratio showed a slightly lower level than in the CTRL trees at 14 DAPF. These data would indicate that in ‘Red’ the L fruitlets are more prone to shed than the C ones, and that BA reinforces this tendency.
Fig. 3.
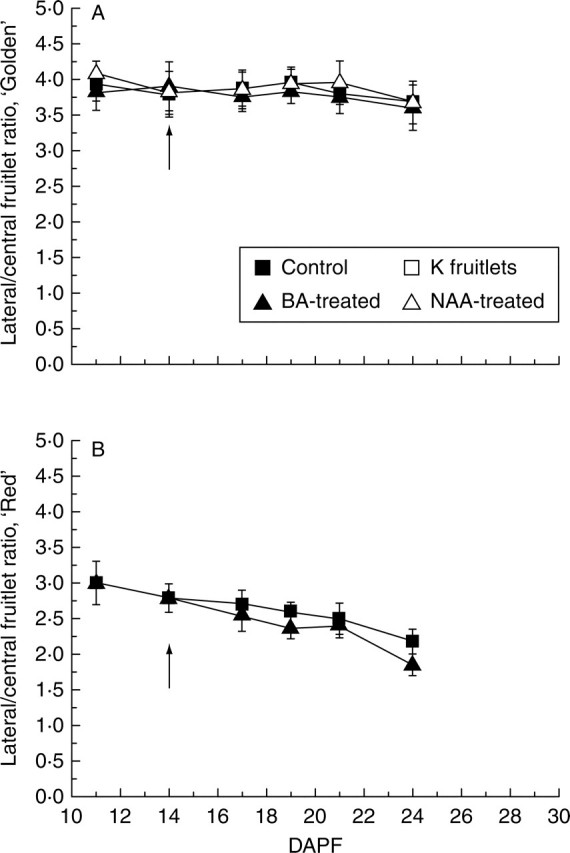
Ratio between number of lateral and central fruitlets per cluster for (A) ‘Golden Delicious’ and (B) ‘Red Delicious’ monitored during abscission induction (11–24 DAPF). Values are means (±s.d.) of fruitlets from 40 representative clusters per population. Arrows indicate application time for BA and NAA (see text for details).
Abscission
Total abscission was generally high in both cultivars (Fig. 4). In ‘Golden’, the highest drop was observed in the BA- and NAA-treated trees (90 % and 82 %, respectively. In CTRL and K treatments the abscission level was 75 and 20 %, respectively. In ‘Red’, the abscission was 91 % in CTRL trees and around 86 % in BA-treated ones.
Fig. 4.
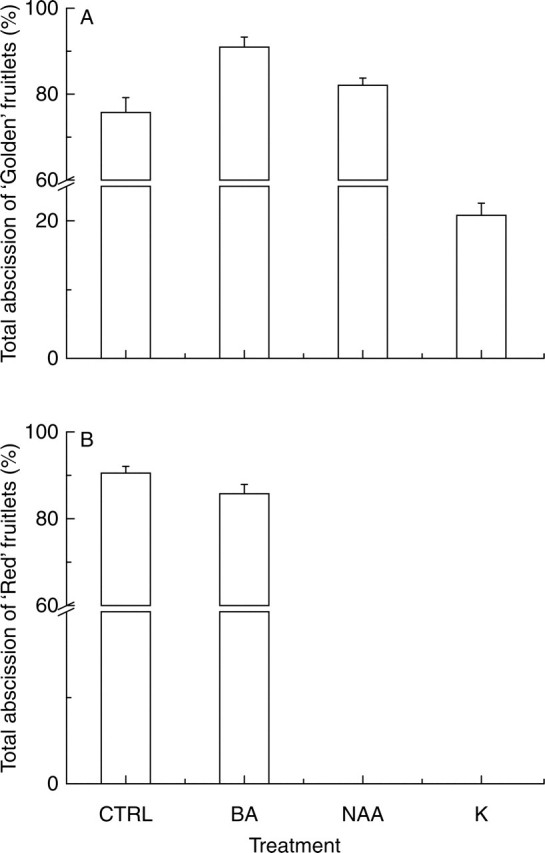
Total percentage abscission of fruitlets of (A) ‘Golden Delicious’ and (B) ‘Red Delicious’ monitored at the end of the June drop in control trees (CTRL), BA-treated trees (BA), NAA-treated trees (NAA), and king-fruitlet-bearing trees (K). Values are the mean (±s.d.) of total abscission per tree (n = 8).
In order to investigate if the chemicals affected the timing of shedding, abscission dynamics were followed in ‘Golden’ up to the end of June drop (Fig. 5A). Abscission dynamics in the CTRL, BA and NAA fruitlets were similar, with peaks at around 30 DAPF. Most of the drop occurred between 24–35 DAPF. In treatment K, fruitlet drop was at a low level and the trend was almost linear. The cross-diameter of ‘Golden’ abscised fruitlets, monitored throughout shedding, displayed an increasing, linear trend and was not affected by treatment (Fig. 5B).
Fig. 5.
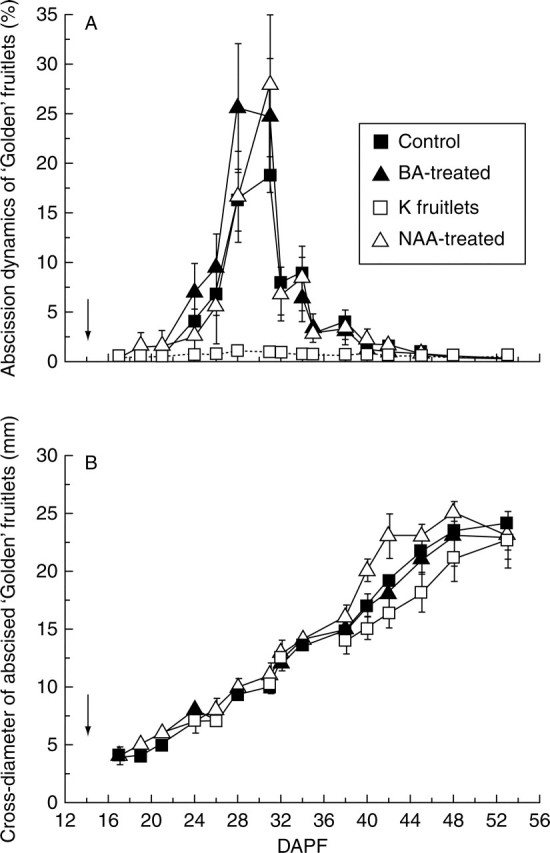
(A) Abscission dynamics of ‘Golden Delicious’ expressed as a percentage of total abscission and (B) cross-diameter of abscised fruitlets during abscission (12–56 DAPF). Values are means (±s.d.) of fruitlets from eight different plants per population. Arrows indicate application time for BA and NAA (see text for details).
Vegetative activity
The effect of BA on vegetative growth was evaluated for all treatments applied to ‘Golden’ and ‘Red’ (Table 1). In ‘Golden’, central shoot diameter was significantly increased by BA, whereas only small differences were observed between CTRL and K trees. For central shoot length, no significant differences were observed between BA and CTRL trees, whereas in K trees BA significantly reduced shoot elongation. The number of lateral shoots was increased by BA in trees with intact clusters compared with K + BA, whereas few differences were observed among CTRL, BA and K treatments. The mean length of lateral shoots was higher in K + BA compared to CTRL, whereas slight differences were observed between BA and K. The total length of lateral shoots was increased by BA in both CTRL and K trees (Table 1). No significant effect of BA on vegetative activity in ‘Red’ was observed (data not presented).
Table 1.
Vegetative activity monitored at the end of the growing season in ‘Golden Delicious’ control trees (CTRL), BA-treated trees (BA), king-fruitlet-bearing trees (K) and king-fruitlet-bearing trees treated with BA (K + BA)
| CTRL | BA | K | K + BA | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | Group | Mean | Group | Mean | Group | Mean | Group | |
| Central shoot diameter (mm) | 13·167 | B | 13·917 | A | 13·417 | A/B | 13·667 | A |
| Central shoot length (mm) | 154·17 | A/B | 160·83 | A/B | 197·5 | A | 124·17 | B |
| Number of lateral shoots | 16·167 | A/B | 17·583 | A | 15·083 | A/B | 14·333 | B |
| Lateral shoot mean length (mm) | 46·072 | B | 52·28 | A/B | 50·958 | A/B | 67·356 | A |
| Lateral shoot total length (mm) | 766·7 | B | 931·7 | A | 723·8 | B | 1013·8 | A |
Values are means, and divide into two distinct groups, labelled ‘A’ and ‘B’, which are significantly different at P < 0·05 (Duncan's Multiple Range Test), and an intermediate group, labelled ‘A/B’.
MdACO1 expression analysis
The overall expression of MdACO1 was higher in the cortex and seeds than in the peduncle (Fig. 6). The MdACO1 expression in ‘Golden’ C seeds of K, CTRL and BA- and NAA-treated trees remained at undetectable levels, whereas in L fruitlets transcripts accumulated mainly at 24 DAPF in CTRL, from 19 to 24 DAPF in BA, and from 21 to 24 DAPF in NAA. The highest induction was observed in L fruitlets treated with BA. In ‘Red’, MdACO1 expression occurred at a significantly lower level and was mainly detected in L fruitlets from 19 to 24 DAPF in CTRL and from 19 to 21 DAPF in BA-treated fruitlets. In ‘Red’ C fruitlets, some transcript accumulation occurred at 11 and 19 DAPF in both CTRL and BA fruitlets.
Fig. 6.
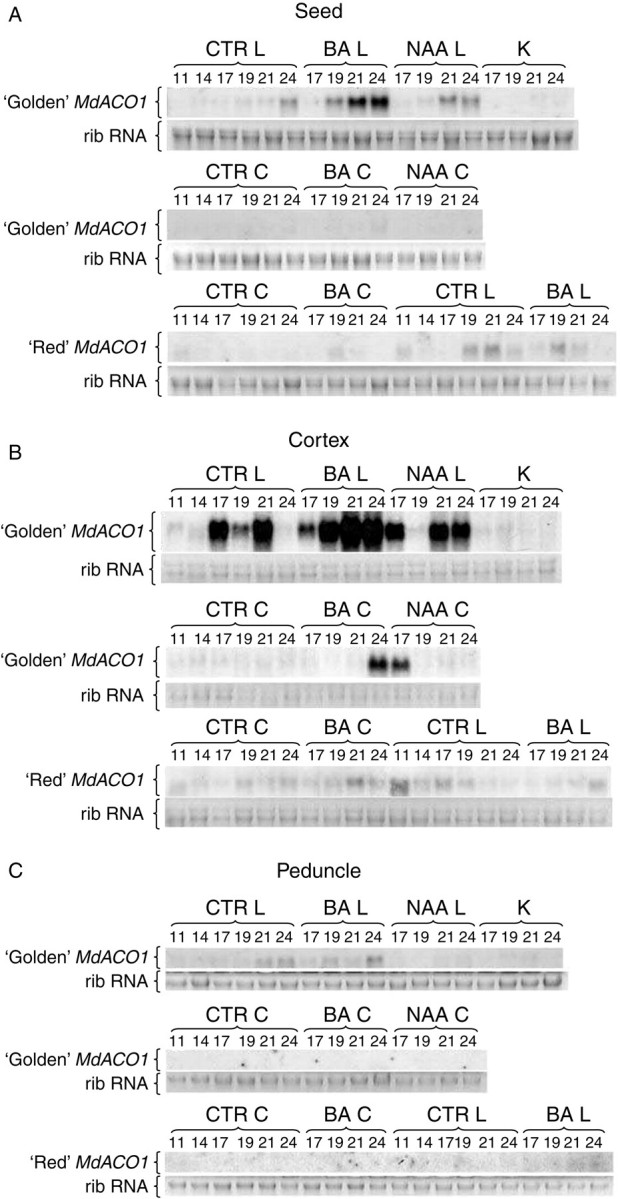
MdACO1 expression of ‘Golden Delicious’ (‘Golden’) and ‘Red Delicious’ (‘Red’) in (A) seeds, (B) cortex and (C) peduncle from representative fruitlets collected during abscission induction (11, 14, 17, 19, 21 and 24 DAPF) in control trees (CTRL), BA-treated trees (BA), NAA-treated trees (NAA), and king-fruitlet-bearing trees (K), in either central (C) or lateral (L) fruitlets. Ribosomal RNA (rib RNA) indicates equal loading of total RNA.
Expression in the cortex of ‘Golden’ was mainly detected in L fruitlets, although some transcript accumulation occurred in BA- and NAA-treated C fruitlets at 17 and 24 DAPF, respectively (Fig. 6B). In L, accumulation was observed at a later stage and the pattern differred in CTRL, BA and NAA treatments. In CTRL a pronounced induction was observed at 17 and 21 DAPF. In BA the trend was for a smooth increase from 19 to 24 DAPF, whereas in NAA a transient upregulation was detected 17 DAPF followed by a downregulation at 19 DAPF and increases at 21 and 24 DAPF. Analysis of transcript accumulation in ‘Red’ showed that the gene was expressed at similar levels in C and L fruitlets. In C, transcripts were detected at early (11–14 DAPF) as well as at late stages (19–24 DAPF) both in CTRL and BA. In L, MdACO1 was mainly expressed at an early stage (11–14 DAPF), after which CTRL and BA fruitlets differed; in the former the transcript amount declined and in the latter increased only at 24 DAPF.
MdACO1 expression in the peduncle remained at a basal level in all treatments and in both cultivars. A slight accumulation was observed at the end of the experimental period in peduncles of ‘Golden’ CTRL and BA L fruitlets (Fig. 6C).
DISCUSSION
It is generally believed that fruitlet abscission is a process with a correlative basis in which the strongest fruitlets are positively selected against the weakest ones (Bangerth, 2000). Thus, within a population, the fruitlets undergoing abscission are the smallest and less developed ones. The data presented in this study clearly indicated that central (C) fruitlets were consistently larger than lateral (L) ones throughout the experimental period. As previously reported (Dal Cin et al., 2005a), and as verified in this trial (data not shown), abscission almost exclusively affects L fruitlets, although to different degrees depending on cultivar. In ‘Golden Delicious’ (‘Golden’) the L/C ratio changed only slightly throughout the experiment, having a value around 4 without any significant difference among treatments. BA treatment in this cultivar stimulated shedding compared with controls (90 % vs 75 %) without affecting the fruitlet size. In ‘Red Delicious’ (‘Red’), the L and C fruitlet growth rates were closer to each other than in ‘Golden’, the natural abscission was high (90 %) and the shedding was even slightly reduced by BA. These findings, along with the fact that the L/C value in ‘Red’ was around 3 at the beginning of the experiment, indicate that abscission-induction dynamics are different in ‘Golden’ and ‘Red’ fruitlets. In the former, abscission induction occurs mainly between lateral homogenous fruitlets, whereas in the latter it involves fruitlets that are progressively weaker within the cluster. This hypothesis is consistent with the blooming date and flower abscission observations. All these facts, along with the differential effects of BA on vegetative growth in the two cultivars, would indicate that in ‘Red’ fruitlet shedding is the consequence of competition occurring mainly among fruitlets that are progressively weaker within the cluster, whereas in ‘Golden Delicious’ the competition between shoots and fruitlets plays a major role. Thus the promoting effect of BA on fruitlet abscission in ‘Golden’ might be due to a strengthening of the shoot dominance over the cluster.
It is interesting to note that L and C growth rates started to diverge concurrently with the peak of ethylene evolution in both ‘Golden’ (17 DAPF) and ‘Red’ (19 DAPF). The role of ethylene in plant development has been extensively discussed by Abeles et al. (1992). Ethylene may either play an active role in the growth rate decrease or simply be a response to internal cues that in turn negatively affect fruitlet size. L fruitlets displayed more abundant ethylene biosynthesis than C fruitlets. Nevertheless, the huge stimulation of ethylene evolution induced by BA in ‘Golden’ L fruitlets only partially correlates with the increase in abscission (around 15 %), probably indicating a limit in the mechanism by which ethylene acts in abscission. Moreover, total ethylene and its evolution pattern differed significantly in ‘Golden’ and ‘Red’. The different behaviours may be related to the cluster composition as well as to genetic differences. It has been hypothesized that ethylene sensitivity may play a crucial role during abscission induction (Dal Cin et al., 2005a). However, transcript accumulation of MdERS1 (a gene encoding for an ethylene receptor) as analysed in seed samples (data not shown) showed that there is little difference between cultivars, probably indicating that the cluster composition is the main factor.
As far as the sensitivity of the cultivars to the thinners is concerned, BA and NAA stimulated abscission in ‘Golden’ whereas in ‘Red’ the former seems to produce a decrease in shedding, similarly to that found in other apple varieties as well as in other fruit species (Buban, 2000). With regard to the abscission dynamics in ‘Golden’, it is clear that both BA and NAA increased abscission but did not change the dynamics, nor did they cause differences in abscised fruitlet size or in the growth rate of persisting fruitlets, compared with the control treatment (as discussed above). This is consistent with an action exerted on a correlative basis at the level of competition between shoots and fruitlets, at least for BA. This hypothesis is confirmed by data on vegetative growth, which showed a clear effect of BA on central shoot diameter, and on the number and total length of lateral shoots. What is surprising is that abscission may have some effect on vegetative growth, as shown by the number of lateral shoots in the CTRL and K treatments and, above all, in BA and K + BA. It is likely that during the early development of fruits and shoots a sort of dynamic balance is determined based on competition between apical and lateral shoot meristems and fruitlet cluster (McSteen and Leyser, 2005). Within this physiological context, BA may stimulate fruitlet abscission by strengthening the sink activity of the vegetative part of the plant, the effect of which is clear on total lateral shoot length. However, in the case of ‘Red’, which is characterized by limited growth, BA is not effective even with formulation additives to increase absorption (Dal Cin et al., unpubl. res.), whereas other chemicals, acting mainly on the fruitlet such as carbaryl, can thin (Dal Cin et al., unpubl. res.). BA, which penetrates into the fruit more easily than into the leaf (Greene, 1993), may also affect fruitlet growth directly. It has been shown that BA can actively regulate expression of several genes, some of which are involved in cross-talk with other growth regulators. In general, the role played by cytokinin is to promote growth and cell division; however, it may also increase stability or transcription of some ACS genes (1-aminocyclopropane-1-carboxylate synthase), or decrease the amount of transcript of some ACO genes (Brenner et al., 2005). Indeed, within the cortex a delay in MdACO1 upregulation was observed in L fruitlets treated with BA compared with the CTRL and NAA-treated fruitlets, indicating that BA produces a transient decrease in transcript amount within few hours after application.
The expression of MdACO1 has previously been shown to correlate with abscission (Dal Cin et al., 2005a). Results presented in this study confirm the previous data and strengthen the assertion that its transcript accumulation is not tightly correlated to concurrent ethylene evolution. This might be because a large upregulation of MdACO1 is not necessary to sustain the ethylene burst. The dramatic increase of the gene transcripts occurring after the ethylene peak might be a response to ethylene accumulation, or it might be a consequence of the stress conditions that a fruit undergoing abscission is experiencing. Within this context MdACO1 transcript accumulation may be regarded as a marker of the physiological state, as suggested in other systems (Wagstaff et al., 2005). In addition, at this fruitlet developmental stage MdACO1 displays different transcript accumulation profiles depending on the tissue. It is apparent that the seed was the tissue in which transcript levels best match the level of abscission.
In conclusion, it may be hypothesized that apple fruitlet physiological drop has a strong correlative basis, governed by the interactions between clusters and shoots. BA application in ‘Golden Delicious’ strengthens the competition between clusters and shoots. In ‘Red Delicious’, a spur-type cultivar in which vegetative activity is genetically limited, BA is ineffective as a thinner because the tree is unable to modify the shoot growth pattern. Thus BA may play a major role in abscission by acting at the level of the shoot. However, some direct effects were observed at the level of the cortex as well. In order to discern the effect of BA on fruit growth uncoupled with that on abscission, fruitlet response to BA is currently being investigated by use of molecular markers. Abscission affects mainly small fruitlets; however, the decrease in growth rate seems to be a result rather than a cause of shedding. Ethylene evolution precedes fruitlet drop but its role still seems unclear and further studies are needed to elucidate its role. Moreover, ethylene may control processes involving other hormones, and its response cascade be influenced by specific tissue sensitivity (Dal Cin et al., 2005a) and/or by a number of other factors as demonstrated by Ludwig et al. (2005). MdACO1 expression levels (above all in seeds) parallel abscission, although its precise regulation probably relies on various other factors besides concurrent ethylene evolution, such as post-transcription regulation and the internal and external conditions associated with the tree.
Supplementary Material
LITERATURE CITED
- Abeles FB, Morgan PW, Salveit ME., Jr . Ethylene in plant biology. New York: Academic Press; 1992. [Google Scholar]
- Alferez F, Singh S, Umbach AL, Hockema B, Burns J. Citrus abscission and Arabidopsis plant decline in response to 5-chloro-3-methyl-4-nitro-1H-pyrazole are mediated by lipid signaling. Plant, Cell & Environment. 2005;28:1436–1449. [Google Scholar]
- Bangerth F. Abscission and thinning of young fruit and their regulation by plant hormones and bioregulators. Plant Growth Regulation. 2000;31:43–59. [Google Scholar]
- Brenner WG, Romanov GA, Kollmer I, Burkle L, Schmulling T. Immediate-early and delayed cytokinin response genes of Arabidopsis thaliana identified by genome-wide expression profiling reveal novel cytokinin-sensitive processes and suggest cytokinin action through transcriptional cascades. The Plant Journal. 2005;44:314–333. doi: 10.1111/j.1365-313X.2005.02530.x. [DOI] [PubMed] [Google Scholar]
- Buban T. The use of benzyladenine in orchard fruit growing: a mini review. Plant Growth Regulation. 2000;32:381–390. [Google Scholar]
- Dal Cin V, Danesin M, Boschetti A, Dorigoni A, Ramina A. Ethylene biosynthesis and perception in apple fruitlet abscission (Malus domestica L. Borkh) Journal of Experimental Botany. 2005a;56:2995–3005. doi: 10.1093/jxb/eri296. [DOI] [PubMed] [Google Scholar]
- Dal Cin V, Danesin M, Rizzini FM, Ramina A. RNA extraction from plant tissues: the use of calcium to precipitate contaminating pectic sugars. Molecular Biotechnology. 2005b;31:113–120. doi: 10.1385/MB:31:2:113. [DOI] [PubMed] [Google Scholar]
- Dhanalakshmi R, Prasad TG, Udayakamur M. Is auxin a diffusible signal mediating abscission of recessive sinks? Plant Science. 2003;164:689–696. [Google Scholar]
- Drazeta L, Lang A, Cappellini C, Hall AJ, Volz RK, Jameson P. Vessel differentiation in the pedicel of apple and the effects of auxin transport inhibition. Physiologia Plantarum. 2004;120:162–170. doi: 10.1111/j.0031-9317.2004.0220.x. [DOI] [PubMed] [Google Scholar]
- Else MA, Stankiewicz-Davies AP, Crisp CM, Atkinson C. The role of polar auxin transport through pedicels of Prunus avium L. in relation to fruit development and retention. Journal of Experimental Botany. 2004;55:2099–2109. doi: 10.1093/jxb/erh208. [DOI] [PubMed] [Google Scholar]
- Greene DW. A review of the use of benzyladenine (BA) as a chemical thinner for apples. Acta Horticulturae. 1993;329:231–236. [Google Scholar]
- Jones KM, Bound SA, Oakford MJ, Gillard P. Modelling thinning of pome fruits. Plant Growth Regulation. 2000;31:75–84. [Google Scholar]
- Ludwig AA, Saitoh H, Felix G, Freymark G, Miersch O, Wasternack C, et al. Ethylene-mediated cross-talk between calcium-dependent protein kinase MAPK signaling control stress responses in plants. Proceedings of the National Academy of Sciences of the USA. 2005;102:10736–10741. doi: 10.1073/pnas.0502954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSteen P, Leyser O. Shoot branching. Annual Review of Plant Biology. 2005;56:353–374. doi: 10.1146/annurev.arplant.56.032604.144122. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Elliott KA, Gonzales-Carranza ZH. Abscission, dehiscence, and other cell separation processes. Annual Review of Plant Biology. 2002;53:131–158. doi: 10.1146/annurev.arplant.53.092701.180236. [DOI] [PubMed] [Google Scholar]
- Uheda E, Nakamura S. Abscission of Azolla branches induced by ethylene and sodium azide. Plant Cell Physiology. 2000;41:1365–1372. doi: 10.1093/pcp/pcd071. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis F. Molecular cloning: a laboratory manual. 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Taylor JE, Whitelaw CA. Signals in abscission. New Phytologist. 2001;151:323–339. [Google Scholar]
- Wagstaff C, Chanasut U, Harren FJM, Laarhoven LJ, Thomas B, Rogers HJ, Stead AD. Ethylene and flower longevity in Alstroemeria: relationship between tepal senescence, abscission and ethylene biosynthesis. Journal of Experimental Botany. 2005;56:1007–1016. doi: 10.1093/jxb/eri094. [DOI] [PubMed] [Google Scholar]
- Wertheim SJ. Developments in the chemical thinning of apple and pear. Plant Growth Regulation. 2000;31:85–100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


