INTRODUCTION
The phenomenon of desiccation tolerance in bryophtes is well known: many species can withstand drying to water contents of 5–10 % of their dry weight, in which state effectively no liquid phase remains in the cells, and return to normal metabolism and growth following rehydration (Alpert, 2006). The essential facts of bryophyte desiccation tolerance are well documented (Irmscher, 1912; Höfler, 1946; Clausen, 1952; Abel, 1956; Hosokawa and Kubota, 1957; Hinshiri and Proctor, 1971; Bewley, 1973; Dilks and Proctor, 1974, 1976, 1979; Schonbeck and Bewley, 1981a, b). However, we are still far from understanding fully the mechanisms of desiccation tolerance in either bryophytes or vascular ‘resurrection’ plants (Bewley, 1979; Bewley and Krochko, 1982; Stewart, 1990; Ingram and Bartels, 1996; Oliver and Bewley, 1997; Hartung et al., 1998; Kappen and Valladares, 1999; Scott, 2000; Proctor and Pence, 2002; Proctor and Tuba, 2002; Oliver et al., 2005). In particular, there has been disagreement about whether desiccation tolerance in such mosses as Tortula (Syntrichia) ruralis is constitutive, with recovery essentially a matter of reassembly and reactivation of components conserved intact through a drying–rehydration cycle, or non-constitutive, the latter involving processes variously described as acclimation, hardening or ‘repair’ (Mayaba et al., 2001). The idea of a repair-based mechanism of desiccation tolerance in bryophytes was introduced by Bewley in the context of the recovery of normal function by cell membranes, which are leaky to ions and metabolites immediately following rehydration (Bewley, 1979; Bewley and Krochko, 1982). It was extended by Oliver and Bewley (1984b) to take account of the fine-structural changes (including swelling of chloroplasts and mitochondria, and varying degrees of apparent disruption of the thylakoids and mitochondrial cristae) seen in electron micrographs of cells of freshly rehydrated bryophytes, and has since been reiterated in a succession of reviews (Bewley and Oliver, 1992; Oliver, 1996; Oliver and Bewley, 1997; Oliver et al., 1998). However, these reviews include no new cytological data, and what ‘repair’ might embrace remained largely undefined. Alpert and Oliver (2002) discuss evidence from recent physiological work, and Oliver et al. (2005) envisage a substantial role for constitutive tolerance in bryophytes. An ultrastructural study using freeze-fractured preparations (Platt et al., 1994) showed that organelles and membranes in Tortula ruralis and the ‘resurrection’ plant Selaginella lepidophylla are not disorganized or disrupted in the desiccated state. Further work on dry, partially and fully hydrated S. lepidophylla, using freeze-substitution and conventional fixations with buffers of different osmotic strengths, showed that, in this pteridophyte at least, what has been described as desiccation-induced damage is almost certainly an artefact of inadequate fixation (Platt et al., 1997). These authors indicated a pressing need to re-examine the cytological changes in de- and rehydrating mosses using improved fixation protocols. A recent study of the effects of desiccation and rehydration on the food-conducting leptoids and meristematic cells in Polytrichum formosum using both conventional and anhydrous fixations gave no evidence of damage to organelles but showed major changes in the endomembrane domains, microtubular cytoskeleton and in the shapes of the plastids and mitochondria (Pressel et al., 2006).
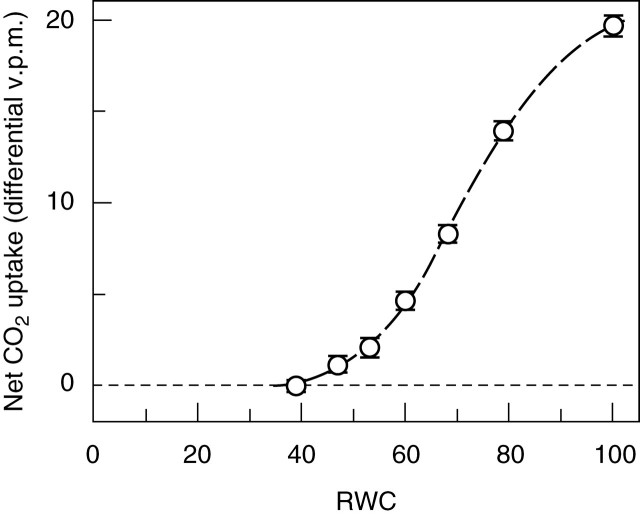
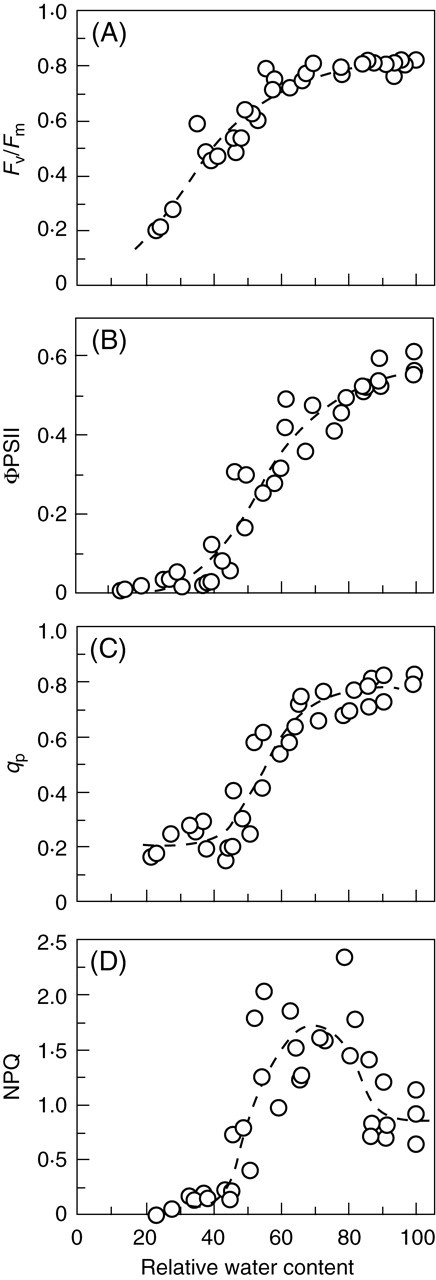
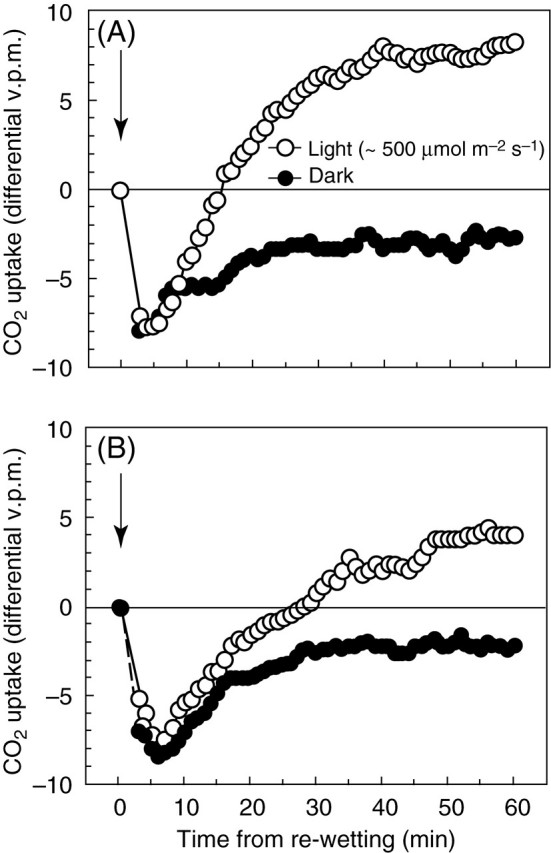
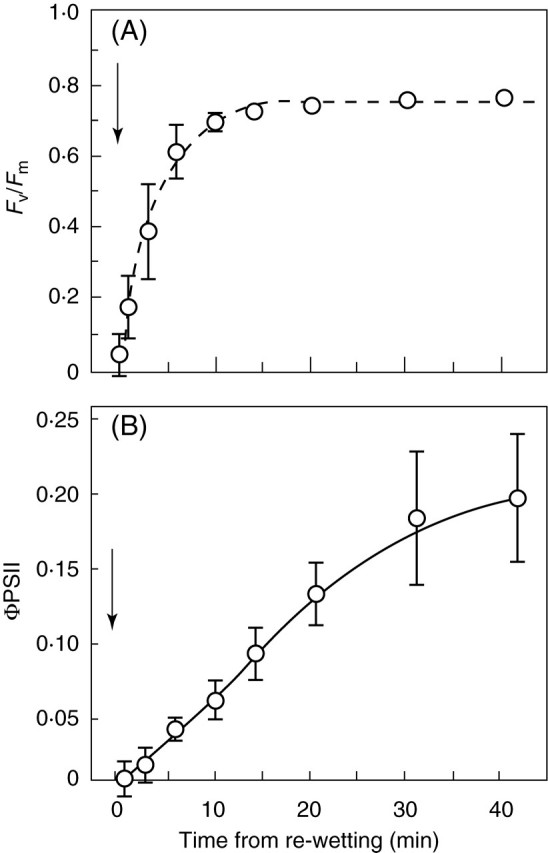
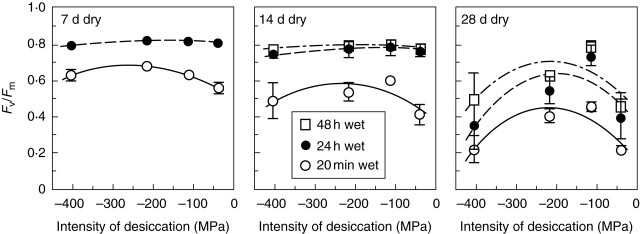
Physiological data show that the rates of recovery on remoistening vary significantly between different systems, but that recovery of some major functions of bryophyte cells may be very rapid. Infrared gas analysis shows almost instant reactivation of respiration, and return to substantially normal rates of net photosynthesis within 30–60 min in such species as Tortula (Syntrichia) ruralis, Grimmia pulvinata and Andreaea rothii (Tuba et al., 1996; Proctor and Pence, 2002), and 14CO2 uptake experiments show that photosynthesis begins within a few minutes of rehydration (Proctor and Smirnoff, 2000). Recovery of the photosystems is also very rapid (Proctor and Smirnoff, 2000; Proctor, 2001); in the initial stages, half-recovery times for Fv/Fm [a measure of the maximum quantum efficiency of photosystem II (PSII)] may be as short as 20–40 s. This suggests that the integrity of the thylakoids is preserved throughout the events of desiccation and re-moistening. On the other hand, the fact that complete recovery of photosynthesis clearly takes much longer (up to 24 h or more) suggests the involvement in recovery of other features of cell biology, as yet unknown. Also unexplained is why excessively rapid drying is often fatal.
The objectives of the work described in this paper were (a) to explore further the basic physiological features and time relations of the recovery of photosynthesis and photosynthetic CO2 uptake, (b) to provide a comprehensive account of the full temporal sequence of the cytological changes associated with recovery to the normal hydrated state, (c) to seek a reconciliation of the apparently conflicting published physiological and cytological evidence on recovery, and (d) to seek explanations for the slow return of metabolic activities to full pre-desiccation values, and for death following excessively rapid dehydration.
MATERIALS AND METHODS
The desiccation-tolerant moss Polytrichum formosum Hedw. was chosen for ease of handling in physiological experiments, and because optimal fixation and sectioning protocols for ultrastructural studies had been worked out in earlier studies (Ligrone and Duckett, 1994; Pressel et al., 2006).
Sources of material
Material for the physiological experiments was collected from Quercus petraea (Mattuschka) Liebl. coppice in Stoke Woods, Exeter, UK (50°45′N, 3°31′W, OSGB grid reference SX 925961) on 21 February 2002. Material for electron microscopy was from mixed Quercus/Fagus woodland, Box Hill, Surrey, UK (51°15′N, 0°18′W, OSGB grid reference TQ 182513).
Desiccation treatments
Unless otherwise stated, batches of eight cut shoots (2-cm apical lengths) were allowed to dry naturally in the laboratory atmosphere (40–50 % RH, 20–22 °C); water loss from a representative sample of eight shoots closely fitted an exponential decay curve, with a half-desiccation time of 17 min.
Cytological protocols
The controls were freshly collected fully hydrated shoots, while the desiccated sample consisted of shoots dried out naturally and kept in this condition for 18 d.
For light microscopy, hydrated and rehydrated samples were mounted in water, dehydrated samples in immersion oil. Both were examined with a Leitz Ortholux microscope using differential interference-contrast optics.
For electron microscopy, leaves from control, and shoots allowed to rehydrate for 0·5, 2·0, 12, 24 and 48 h, were cut into 2-mm lengths and fixed at room temperature for 3 h at pH 7 in 3 % (v/v) glutaraldehyde, 1 % formaldehyde (freshly prepared from paraformaldehyde) and 0·75 % tannic acid in 0·05 m Na-phosphate buffer. Leaf samples from dry shoots and shoots rehydrated for 0·5 h were fixed with that same protocol detailed above but in 0·1 m Na-phosphate buffer. Preliminary experiments using buffers of lower osmolarity produced extensive swelling of the thylakoids in dehydrated and freshly rehydrated samples, as described previously in Selaginella (Platt et al., 1997), and were discontinued. After rinsing in buffer the samples were post-fixed overnight with 1 % osmium tetroxide in 0·1 m buffer, pH 6·8, dehydrated and embedded in Spurr's resin via propylene oxide, as described by Ligrone and Duckett (1994). Thin sections, cut with a diamond knife, were sequentially stained with methanolic uranyl acetate and basic lead citrate and examined with a JEOL 1200EX2 electron microscope.
CO2-exchange measurements
Measurements of net CO2 exchange were made in the laboratory with a portable infrared gas analyser (IRGA) (LCA-2; ADC Ltd, Hoddesdon, Herts, UK), with a Parkinson leaf chamber (broad-leaf type), in the differential mode. Eight 2-cm apical cut lengths of moss shoot were used in the leaf chamber for each measurement. The light source was a quartz-halogen fibre-optic illuminator (Schott), set to give a photosynthetic photon flux density (PPFD) of 500 µmol m–2 s–1.
Chlorophyll-fluorescence measurements
Chlorophyll fluorescence was measured with a modulated chlorophyll fluorometer (FMS1; Hansatech Ltd, King's Lynn, UK). Definitions and calculation of fluorescence parameters follow Maxwell and Johnson (2000). Measurements of ΦPSII (effective quantum yield of PSII), qP (a measure of the oxidation state of the first electron acceptor QA) and NPQ (non-photochemical quenching) were made using the internal actinic light source of the instrument. The measuring sequence for these (starting with dark-relaxed material) was: saturating flash (0·8 s) to measure Fv/Fm, actinic light on for 6 min, saturating flash, actinic light off followed by far-red pulse and measurement of F0′. Unless otherwise stated, the actinic light source was set to give approx. 150 µmol m–2 s–1 PPFD.
Metabolic inhibitors
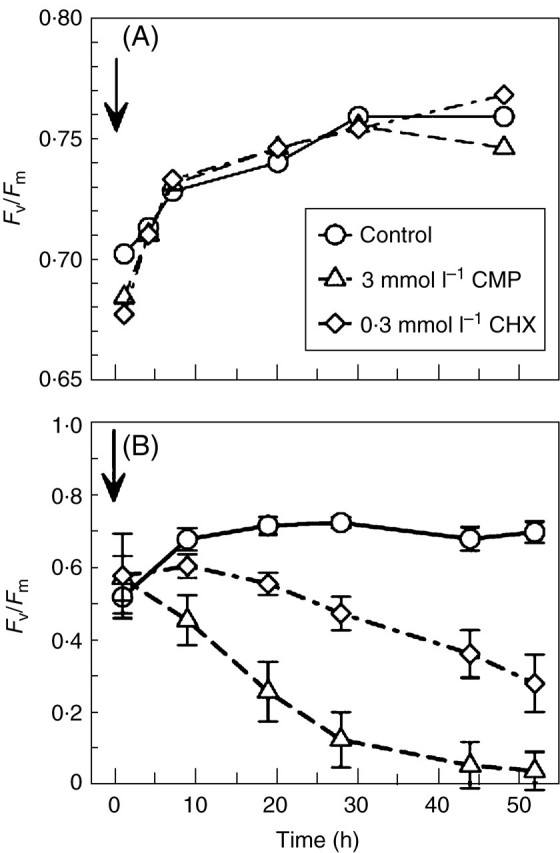
Chloramphenicol (CMP, here used at 3 mmol L–1), inhibits synthesis of chloroplast-encoded proteins; cycloheximide (CHX, here used at 0·3 mmol L–1) is an inhibitor of nuclear-encoded protein synthesis (Bottomley and Bohnert, 1982; Galling, 1982). The concentrations used were determined from previous experiments to give substantially complete inhibition without significant side effects (Proctor and Smirnoff, 2000).
Desiccation experiments
Batches of eight cut shoots (approx. 2 cm) were maintained at different intensities of desiccation in glass desiccators, over salts in equilibrium with their saturated solutions: sodium acetate, CH3COONa·3H2O (74 % RH, –41 MPa); potassium carbonate, K2CO3·2H2O (43 % RH, –114 MPa); potassium acetate, CH3COOK (20 % RH, –218 MPa), or a 75 % w/w sulfuric acid/water mixture (5 % RH, –412 MPa).
DISCUSSION
The physiological and cytological results set out above present a nicely consistent picture of events through a drying and re-wetting cycle in Polytrichum formosum. There is no indication of damage to membranes or organelles in the course of either desiccation or rehydration, but there are major morphological changes on a time scale that is reflected in physiological events. Certain basic processes (respiration, photosynthetic charge separation, protein synthesis) start very rapidly on rehydration. Others, including photosynthetic carbon fixation recover more slowly in a manner that suggests dependence on progressive re-establishment of the spatial relationships of the fully hydrated cell. The physiological and electron microscope data both suggest that recovery on re-wetting is primarily a matter of reassembly and reactivation of components which have been conserved undamaged through the desiccation – rehydration cycle. In particular, it seems clear from physiological experiments that recovery in itself involves little or no de novo protein synthesis; in the dark, recovery appears indifferent to the presence of protein-synthesis inhibitors. In the light, protein synthesis, especially of chloroplast-encoded proteins, is clearly essential for the repair of ongoing photo-damage (Proctor and Smirnoff, 2000; Proctor, 2001).
The present study shows that desiccation and rehydration involve remarkable cytological changes in the photosynthetic cells in the leaves of Polytrichum formosum. The changes recorded here are closely consistent with those described previously in the leptoids of P. formosum (Pressel et al., 2006), in the leaves of other mosses and in Selaginella lepidophylla (Platt et al., 1997), and provide a ready explanation for the rapid resumption of respiration and photosynthesis following rehydration and the much slower return of these processes to pre-desiccation rates. A few cytological features are unchanged through the drying and re-wetting cycle, some recover within minutes, but others take many hours to regain their normal morphology.
Unfortunately, Polytrichum formosum has proved unsuitable for high pressure freeze-substitution (Schmid, 1998), which would have avoided any question of possible artefacts from conventional aqueous chemical fixation. Dehydrated specimens fixed anhydrously using hexane produce results wholly consistent with standard aqueous fixation (Pressel et al., 2006). The cell volumes recorded in dehydrated and rehydrating specimens (Table 1) show that these do not swell as a result of imbibition of excess water during aqueous fixation; this was also the case in dry leaves of Selaginella lepidophylla when freeze-substitution images were compared with those from conventional fixation (Platt et al., 1997). Indeed the latter study revealed remarkably few ultrastructural differences between freeze-substitution and conventional fixation apart from the higher electron-opacity of the mitochondrial matrix and chloroplast stroma using the former protocol. The statement by Platt et al. (1997) that conventional fixation produced significant swelling of the mitochondria and chloroplasts is not borne out by direct measurements of their published micrographs. It therefore seems reasonable to conclude that the present observations on changes in the ultrastructure of Polytrichum leaves are not fixation artefacts.
The finding by Platt et al. (1997), borne out by preliminary work on P. formosum by ourselves, that newly rehydrated cells are much more sensitive to the osmolarity of the fixative than fully hydrated controls, now provides an explanation for the damaged organelles described in earlier studies that lent weight to the concept of damage and repair. These results indicate that, immediately following rehydration, bryophyte cells appear to be extremely fragile. The recent discovery that dehydration causes depolymerization of the microtubule cytoskeleton in both leptoids and meristematic cells of P. formosum (Pressel et al., 2006) provides a likely explanation. Until rehydrated cells have reassembled the cytoskeletal network that forms the framework supporting the cytoplasmic organization they may well be particularly prone to damage from osmotic stress.
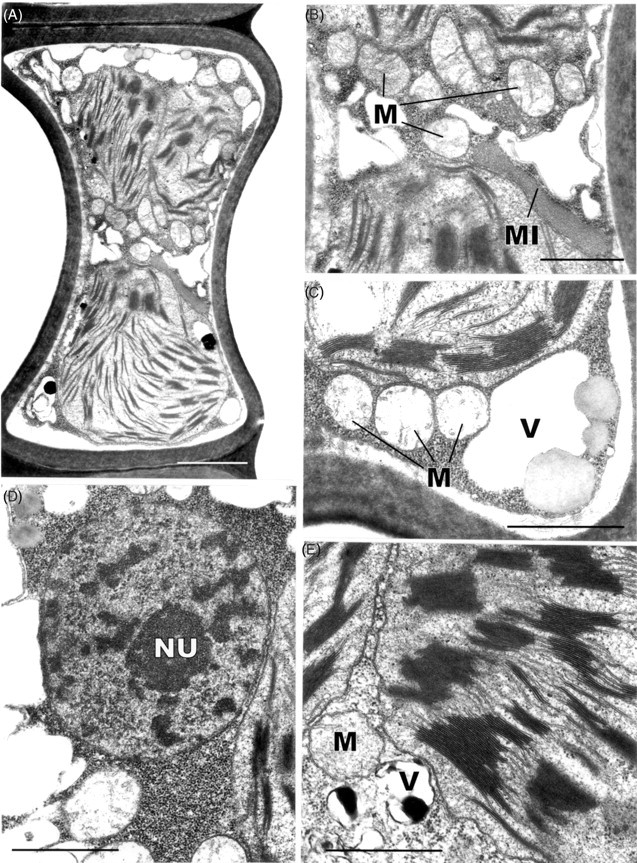
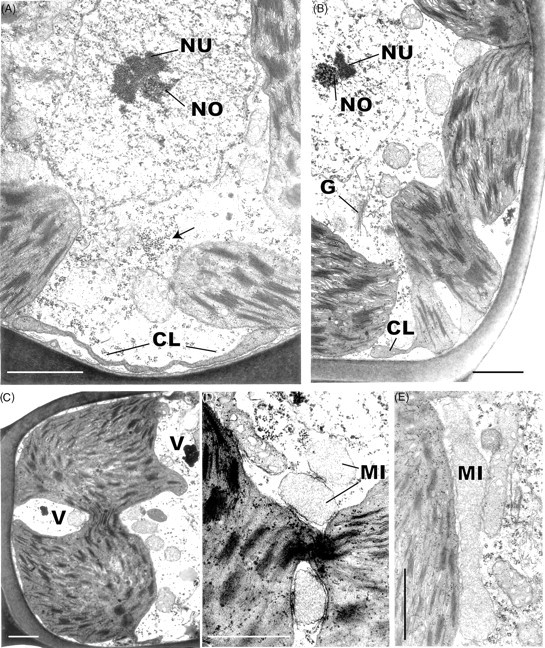
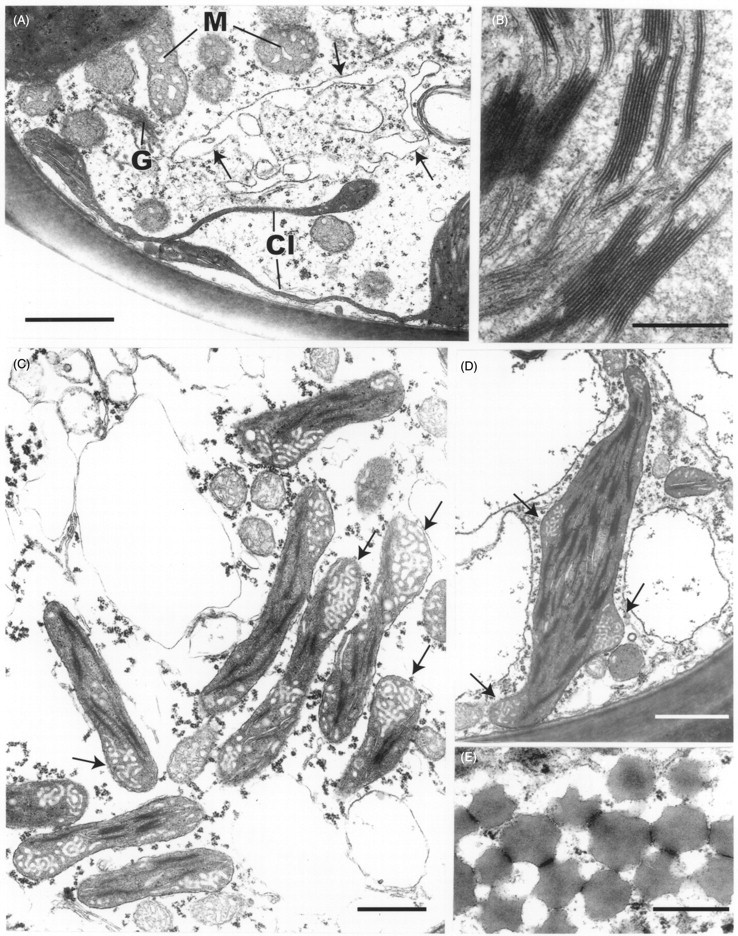
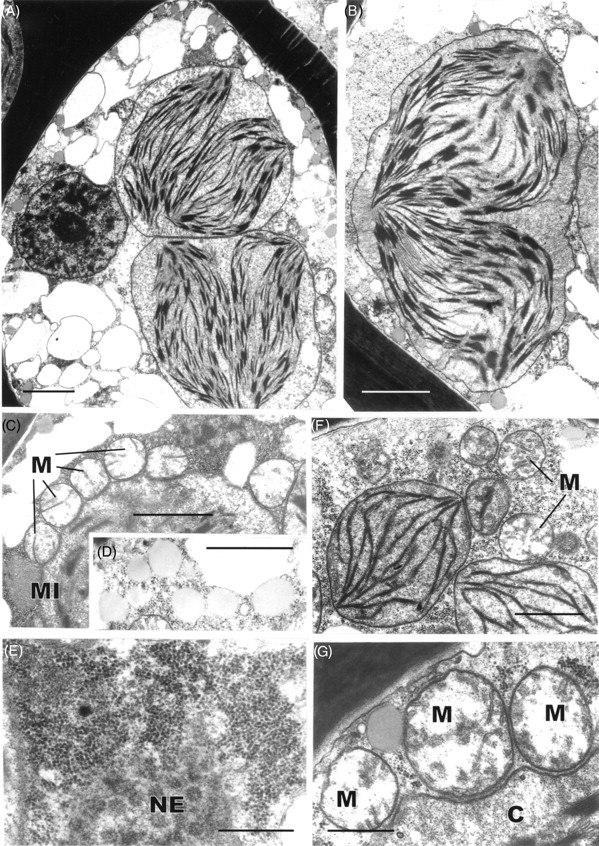
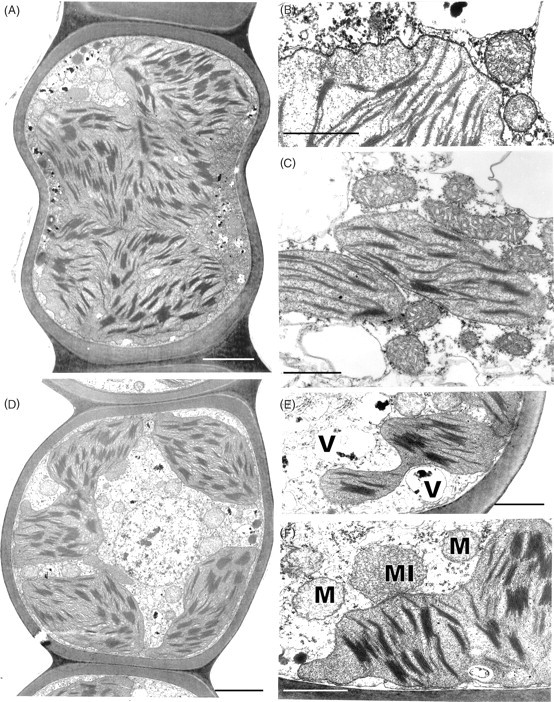
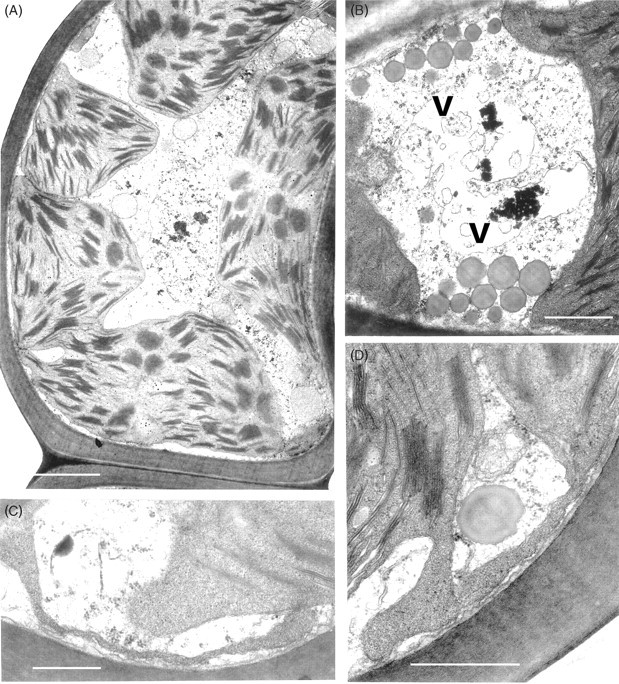
Two features of the leaf cells of Polytrichum that remain virtually unchanged through the drying and re-wetting cycle are the grana – stroma thylakoid networks in the chloroplasts of both the lamella and lamina cells and the mitochondrial cristae, although the latter change from tubular to swollen within the first 2 h after re-wetting. These cytological features are consistent with the rapid resumption of photosynthesis and respiration during rehydration. The microbodies closely associated with the chloroplasts of Polytrichum, and remaining unchanged throughout drying and re-wetting, have a key role in removing the superoxide radicals produced in response to desiccation stress in bryophytes (Smirnoff, 1993; Minibayeva and Beckett, 2001; Mayaba et al., 2002). Similar microbodies have frequently been noted in the vicinity of the choroplasts in a variey of bryophyte tissues (Duckett and Renzaglia, 1988) but their particular prominence in the leaves of Polytrichum and Tortula (Syntrichia) ruralis (Robertson, 1991) may well be associated with desiccation tolerance in both these species. It would interesting to explore relationships between the ‘oxidative bursts’ and microbodies in a range of taxa from contrasting habitats.
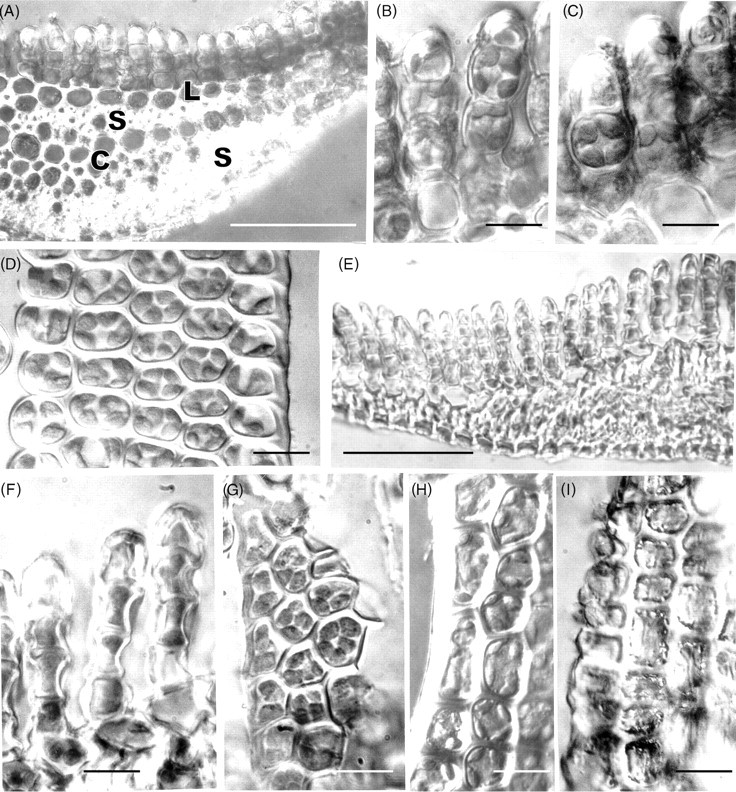
Features of the desiccated leaf lamina cells that can be readily explained as simply due to the physical loss of essentially free water, and which are closely in line with the reduced volume of the lumina (Table 1), include the replacement of the central pleomorphic vacuole system with numerous small vacuoles located at the cell periphery, clustering of the ribosomes, chromatin condensation, and close juxtaposition of the mitochondria and chloroplasts. Some of these features return to the predesiccated condition within 1–2 h of rehydration (dispersal of the ribosomes, separation of chloroplasts and mitochondria) others take much longer to recover (chromatin dispersal, reappearance of pleomorphic vacuoles). The close packing of the ribosomes in the desiccated cells and their separation following rehydration is wholly consistent with biochemical data that the almost immediate resumption of RNA and protein synthesis during rehydration involves existing ribosomes (Oliver and Bewley, 1984a). Similarly the finding that chromatin decondensation may take several hours explains the delayed resumption of transcription following rehydration. There is no evidence from the present and previous studies that desiccation in mosses leads to the formation of crystalline arrays of ribosomes like those described in various animal, vascular plant and prokaryote cells under conditions of stress (Duckett, 1975; Barbieri et al., 1995; Nissen et al., 2000). In the same context, crystals of RUBISCO (the so-called stromacentres) that sometimes appear in the stroma of plastids in stressed homoiohydric plants, were not seen in dehydrated Polytrichum nor are these apparent in micrographs in previous studies. Similarly, crystals are absent from the matrix of dehydrated microbodies in Polytrichum leaves.
Other cytological changes where recovery is more protracted require further explanation. A notable feature of desiccation in the leaf cells of Polytrichum formosum, also seen previously in the food-conducting cells of the same species (Pressel et al., 2006) and in the leaves of Selaginella lepidophylla is the development of numerous small vacuoles replacing the vacuolar system typical of the hydrated state. In every case these take several hours to disappear following rehydration. The origins and possible roles of the small vacuoles are unknown. However, one possibility is that they accumulate soluble carbohydrate moieties, as shown in food-conducting cells (Schmid, 1998), that increase the osmoticum and thus protect the cells against damage from water stress. In Selaginella these vacuoles may be the sites of accumulation of trehalose associated with desiccation (Zantella et al., 1999). The relatively large sizes of the vacuoles occurring in the leaf lamina cells of Polytrichum, both in the dry state and in the first phase of rehydration, might reflect water retention/uptake promoted by the accumulation of osmotically active solutes. In the samples fixed 2 h after rehydration the vacuoles are much smaller, although the overall volume of lamella cells has increased remarkably, which suggests a redistribution of water and osmotically active components between the vacuoles and cytoplasm. The central pleomorphic vacuolar system typical of the hydrated state, however, is fully re-established only after 24–48 h. Such a wide delay suggests that the two vacuolar systems might be ontogenetically unrelated.
Probably the most striking and unexpected cytological feature associated with de- and rehydration in the leaf cells of Polytrichum is the change in shape of the chloroplasts. Although the overall volume of the chloroplasts hardly changes between dehydrated and fully hydrated cells, desiccation sees the complete disappearance of all the isthmuses between lobed choroplasts and the lamellar extensions in the cells of the leaf lamellae and peripheral reticulum from the chloroplasts of the leaf lamina. The images of two or even three closely apposed thylakoid systems within the same chloroplast can be interpreted as resulting from the coming together of two thylakoid systems that, prior to desiccation, lay separately within different lobes of the same chloroplast (Fig. 7B and C). Similar close juxtaposition of separate thylakoid systems is also visible in micrographs of dry Selaginella leaves (Platt et al., 1997; Thomson and Platt, 1997). Although multilobed plastids have been described from a variety of tissues in bryophytes, and lamellate plastids are a characteristic feature of spermatogenesis and sporogenesis in mosses (Duckett and Renzaglia, 1988), lamellar extensions from fully differentiated chloroplasts do not appear to have been noted previously and are not apparent in an earlier ultrastructural study of Polytrichum leaves (Paolillo and Reighard, 1967). The disappearance and reappearance of the chloroplast isthmuses and lamellar extensions during drying and re-wetting may be highly significant in the desiccation biology of Polytrichum. In simple physical terms their disappearance clearly minimizes the surface area of the chloroplasts, and the same applies to the mitochondria which are invariably spherical in the dry state (Fig. 9) but are often elongated in the hydrated state (Figs 7E and 11C). In this condition the chloroplasts are likely to be more resistant to stresses associated with dehydration as well as with the rapid osmotic uptake of water immediately following rehydration when the cytoskeleton is absent (see below).
Whereas the unchanged thylakoid systems account for the rapid resumption of photosynthesis following rehydration, the gradual re-formation of the lamellar extensions over a period of up to 48 h provides an explanation for the similarly gradual return of photosythesis to pre-desiccation rates. The increased surface area of the lamellar extensions must certainly facilitate exchanges of metabolites between the chloroplasts and cytoplasm. The disappearance during desiccation of peripheral reticulum, and its slow reappearance following rehydration may also be significant in this context; in vascular plants, peripheral reticulum is thought to be involved in metabolite exchanges (Laetch and Price, 1969).
The lamellar extensions of the chloroplasts invite comparison with the highly dynamic extensions of the plastid envelope known as stromules that have been described from various higher plant tissues. Little is known about stromule function; they are tubular rather than lamellate, and their most likely role is in enhancing the exchange of metabolites with the cytoplasm (Kohler and Hanson, 2000; Kohler et al., 2000; Kwok and Hanson 2004a, b; Waters et al., 2004; Gielwanowska et al., 2005). Experimental data suggest that the shape and motility of stromules are controlled by microfilaments and microtubules, findings closely in line with the report of Pressel et al. (2006) that a key feature of desiccation in Polytrichum is the depolymerization of the microtubule cytoskeleton. This suggests as a working hypothesis that rounding off of the chloroplasts (and mitochondria) during desiccation in Polytrichum leaves may be associated with the loss of the cytoskeleton, and that re-formation of the lamellar extensions depends on its repolymerization.
Neither the physiological nor the fine-structural results of the present work support the kind of simple damage-repair hypothesis of bryophyte desiccation tolerance that seemed reasonable 20 years ago, and was consistent with the evidence available at that time. There seems in fact now to be little evidence that desiccation tolerance in bryophytes is ‘repair based’ in the sense originally envisaged, and a good deal to indicate that the suite of ‘constitutive’ protective substances (including sugars and LEA-like proteins) in bryophytes and the ‘inducible’ desiccation protectants of vascular resurrection plants have important features in common. Oliver et al. (2005) see constitutive protection as playing an important role in the desiccation tolerance of bryophytes. Repair of the essential systems like respiration, light-capture and CO2 fixation, and protein synthesis, now looks to be largely physical, and probably not metabolically costly in terms of either energy or materials. The ‘cost’ may well be mainly in producing (inter alia) LEA-like proteins and high concentrations of sugars beyond the normal needs of metabolism – and maybe in having to run metabolism in a cramped and viscous cell. As Alpert and Oliver (2002) and Alpert (2006) suggest, the difference between bryophytes and vascular plants may essentially reflect their difference in physical size. A bryophyte shoot exposed to dry air will dry to the point where metabolism ceases within a few minutes, while a drying shoot of a vascular resurrection plant may remain metabolically active for many hours. If the bryophyte is to survive at all in a drought-prone habitat, its desiccation tolerance must be constitutive; the vascular plant has time for tolerance to be induced when drought threatens. In this scenario, ‘repair’ in the bryophyte may be not so much repair of damaged structures as repair and maintenance of its ‘constitutive’ tolerance mechanism. There is certainly a less sharp boundary between desiccation tolerance in bryophytes and vascular plants than might appear from contrasting Tortula (Syntrichia) ruralis (or Polytrichum formosum) with Craterostigma plantagineum, or even Selaginella lepidophylla. Some small ferns and Selaginella species withstand similar drying rates to Polytrichum, and many bryophytes show varying degrees of inducibility in their desiccation tolerance (Abel, 1956; Mayaba et al., 2001). The common factors are likely to be more important than the differences.
The findings of the present and previous studies, that de- and rehydration are not simply a matter of physical removal and addition of water but involve a highly organized series of cytological changes taking place over several hours, open the way to exciting advances in understanding the biology of desiccation. Particularly inviting are experiments using drugs that affect the cytoskeleton to discover whether reformation of chloroplast extensions depends on microtubules and/or microfilaments and whether, in the absence of these extensions, photosynthesis fails to return to predesiccation values. Is the absence of a cytoskeleton one reason why newly rehydrated cells remain highly sensitive to the osmolarity of fixatives? Does unnaturally rapid drying lead to death because there is insufficient time for the cytological adjustments (including the formation of a new vacuole system and the retraction of chloroplast extensions) that are critical to survival through desiccation? From the standpoint of functional genomics we are now in a better position to elucidate relationships between de- and rehydrins, cytoskeletal dynamics and membrane stabilization.