Abstract
EMBO J 30 22, 4665–4677 (2011); published online September 30 2011
β-Actin mRNA requires the RNA-binding protein ZBP1 for trafficking to distal regions of the cytoplasm. In this issue of The EMBO Journal, Donnelly et al show that ZBP1 is a limiting and essential factor for adult axonal regeneration in vivo, via trafficking of additional mRNAs. These findings highlight a complex web of interactions between RNA-binding proteins and cargo mRNAs.
Intracellular trafficking and localization of mRNA is a fundamental feature of living cells. This is of particular relevance in highly polarized cells where the site of transcription is far removed from the final location of the protein. Localized translation enables a rapid response to different stimulations and ensures fast and effective adjustment to novel environmental conditions (Andreassi and Riccio, 2009). For example, maintenance of a latent signalling complex in the form of axonal mRNAs can allow regulated long-range signalling to the cell body in injured peripheral neurons upon local translation of specific components of the system (Perry and Fainzilber, 2009). Targeting of neuronal transcripts into dendrites or axons is typically dependent on specific sequences, most often located in the 3′ UTR region and recognized by RNA-binding proteins (RBPs) (Yoo et al, 2010).
β-Actin is one of the best-characterized localizing mRNAs in neurons, and is transported into both axons and dendrites in diverse neuronal populations (Zheng et al, 2001; Tiruchinapalli et al, 2003). A number of in-vitro culture studies have suggested that local translation of β-actin is required for neurite outgrowth or guidance decisions (Welshhans and Bassell, 2011 and references cited therein). The localization of β-actin mRNA requires a 54-nucleotide 3′ UTR element, termed a zip code, that is recognized by the zip code binding protein 1 (ZBP1). ZBP1 has been implicated in both localization and translational control of β-actin mRNA. The latter is regulated by Src-mediated phosphorylation of Tyr396 in ZBP1, which reduces its affinity for the zip code motif thereby releasing the mRNA for translation (Huttelmaier et al, 2005). Very recently, Willis et al (2011) have used a transgenic approach to demonstrate that the 3′ UTR of β-actin also drives axonal mRNA localization in vivo. A diffusion-limited GFP reporter fused to the 3′ UTR of β-actin was expressed in both peripheral and central processes of sensory neurons in mice. Now Donnelly et al take advantage of the same transgenic lines to demonstrate that ZBP1 is a limiting factor for adult sensory axon regeneration after nerve injury.
Donnelly and colleagues measure endogenous β-actin mRNA levels in adult axons expressing exogenous GFP reporters fused to the 3′ UTR of β- or γ-actin. Transgenic or virally transduced sensory neurons expressing reporters containing β-actin 3′ UTR had reduced levels of endogenous β-actin, an effect not seen in neurons expressing reporters containing γ-actin 3′ UTR. Strikingly, the decreases in endogenous axonal transcript were paralleled by reductions in axonal outgrowth capacity upon nerve injury (Figure 1). Development of sensory neurons in β-actin 3′ UTR transgenic mice was normal in all parameters tested, indicating that their reduced regeneration capacity is most likely due to a dominant negative effect of the transgene in adulthood.
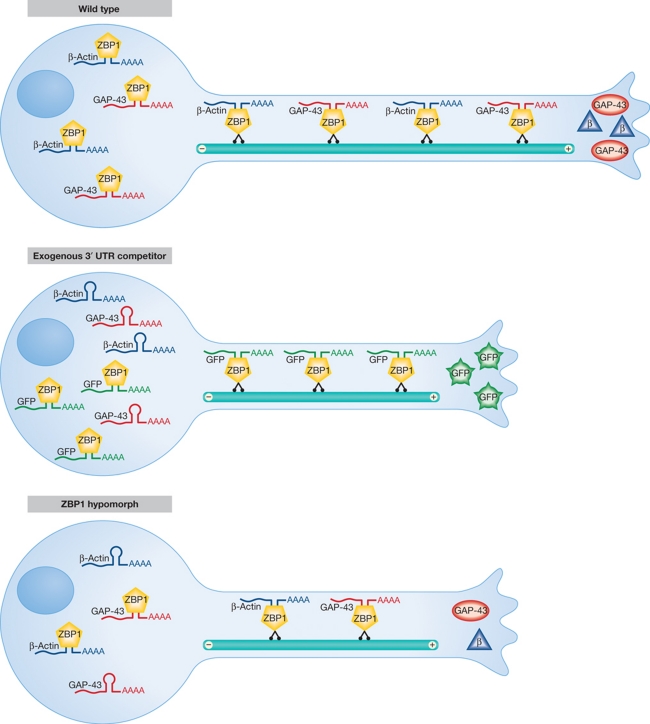
Figure 1.
A limiting role for the RNA localization machinery in axonal regeneration. (Upper schematic) ZBP1 is required for the axonal localization of β-actin, GAP-43, and likely additional mRNAs. (Middle) Introduction of an exogenous mRNA that competes with endogenous transcripts for binding to ZBP1 reduces the supply of endogenous mRNAs to the axon and attenuates axon regeneration after injury. (Lower) Reductions in ZBP1 levels in the neuron have the same effect.
Consistent with this hypothesis, ZBP1 transfection rescued the outgrowth deficit in β-actin 3′ UTR transgenic neurons, while ZBP1 mutants lacking an RNA-binding domain or the Tyrosine 396 residue, required for translational derepression, had no such effect. ZBP1+/− heterozygous mice develop without obvious deficits or abnormalities (Hansen et al, 2004), enabling Donnelly et al to conduct regeneration experiments on adult mice with reduced neuronal ZBP1. Similar to the β-actin 3′ UTR transgenic neurons, ZBP1+/− sensory neurons showed decreased axonal outgrowth upon injury, which is rescued by transfection of exogenous ZBP1. Thus, ZBP1 is required for optimal axon outgrowth following injury in adult sensory neurons, and increases in ZBP1 levels can enhance nerve regeneration.
Donnelly et al then examined a number of mRNA candidates in axons of their transgenic and hypomorph mouse models. Axonal levels of GAP-43 mRNA were significantly reduced in both β-actin 3′ UTR transgenic and in ZBP1+/− neurons, and increased in neurons transfected with exogenous ZBP1. Moreover, ZBP1 immunoprecipitates showed reduced amounts of β-actin and of GAP-43 mRNAs in lysates from brain and spinal cord of β-actin 3′ UTR transgenic mice. Thus, introduction of exogenous β-actin 3′ UTR can compete in vivo with other ZBP1 cargo mRNAs such as GAP-43, suggesting that effects of the β-actin 3′ UTR transgene on axonal outgrowth and regeneration may be due to perturbation of other axonal transcripts, and not necessarily β-actin itself. This finding has broad implications for interpretation of phenotypes in transgenic or knockout mice generated to model RNA localization mechanisms.
The studies of Willis et al (2011) and Donnelly et al (2011) demonstrate that axonal mRNA localization occurs in vivo, and moreover provide definitive evidence that a specific RBP is required for efficient axonal regeneration in vivo, due to its role in transport of regeneration-associated axonal mRNAs. Multiple mRNAs are transported by the same RBP and compete for the same binding site. Conversely, other axonal mRNAs clearly require other RBPs for their localization or the same mRNA may utilize different RBPs. These findings reveal a complex web of interactions between RBPs and cargo mRNAs, and show that perturbation of these mechanisms may have both direct and indirect physiological consequences. The essential role of a specific RBP, ZBP1, for robust axonal regeneration in vivo, should spur renewed efforts to identify and characterize axonal RNA targeting mechanisms.
Footnotes
The authors declare that they have no conflict of interest.
References
- Andreassi C, Riccio A (2009) To localize or not to localize: mRNA fate is in 3′UTR ends. Trends Cell Biol 19: 465–474 [DOI] [PubMed] [Google Scholar]
- Donnelly CJ, Willis DE, Xu M, Tep C, Jiang C, Yoo S, Schanen NC, Kim-Safran CB, van Minnen J, English A, Yoon SO, Bassell GJ, Twiss JL (2011) Limited availability of ZBP1 restricts axonal mRNA localization and nerve regeneration capacity. EMBO J 30: 4665–4677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TV, Hammer NA, Nielsen J, Madsen M, Dalbaeck C, Wewer UM, Christiansen J, Nielsen FC (2004) Dwarfism and impaired gut development in insulin-like growth factor II mRNA-binding protein 1-deficient mice. Mol Cell Biol 24: 4448–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttelmaier S, Zenklusen D, Lederer M, Dictenberg J, Lorenz M, Meng X, Bassell GJ, Condeelis J, Singer RH (2005) Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature 438: 512–515 [DOI] [PubMed] [Google Scholar]
- Perry RB, Fainzilber M (2009) Nuclear transport factors in neuronal function. Semin Cell Dev Biol 20: 600–606 [DOI] [PubMed] [Google Scholar]
- Tiruchinapalli DM, Oleynikov Y, Kelic S, Shenoy SM, Hartley A, Stanton PK, Singer RH, Bassell GJ (2003) Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons. J Neurosci 23: 3251–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshhans K, Bassell GJ (2011) Netrin-1-induced local beta-actin synthesis and growth cone guidance requires zipcode binding protein 1. J Neurosci 31: 9800–9813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DE, Xu M, Donnelly CJ, Tep C, Kendall M, Erenstheyn M, English A, Schanen NC, Kirn-Safran CB, Yoon SO, Bassell GJ, Twiss JL (2011) Axonal localization of transgene mRNA in mature PNS and CNS neurons. J Neurosci 31: 14481–14487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S, van Niekerk EA, Merianda TT, Twiss JL (2010) Dynamics of axonal mRNA transport and implications for peripheral nerve regeneration. Exp Neurol 223: 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ, Kelly TK, Chang B, Ryazantsev S, Rajasekaran AK, Martin KC, Twiss JL (2001) A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J Neurosci 21: 9291–9303 [DOI] [PMC free article] [PubMed] [Google Scholar]