Abstract
Nature 479 7372, 189–193 (2011); published online October 09 2011
Mammary stem cells (MaSCs) have been prospectively isolated and shown to reconstitute a fully functional mammary gland in the mouse at the single cell level. These cells are self-renewing and can differentiate into all mature mammary epithelial cell types, thus fulfilling the defining characteristics of a stem cell. In a recent issue of Nature, Blanpain and colleagues (Van Keymeulen et al, 2011) add a new layer of complexity to the prevailing model of the mammary epithelial hierarchy through the recognition of novel unipotent stem cells that appear to maintain normal homeostasis of postnatal mammary epithelium. These data point to the existence of an unexpectedly complex hierarchy of stem cells that include multipotent as well as more restricted stem cells.
The mammary gland is composed of a ductal epithelial tree that comprises an inner layer of luminal cells and an outer layer of myoepithelial cells. During pregnancy, alveolar cells arise and undergo terminal differentiation into milk-producing cells. MaSCs, highly enriched in the CD29hiCD49fhiCD24+Sca-1− subset, have been shown to regenerate entire mammary glands following their transplantation into cleared mammary fat pads that are divested of their endogenous epithelium but provide an intact stromal environment. Furthermore, the regenerated epithelial trees contain daughter cells with the same in vivo repopulating activity as the original implanted stem cell (Shackleton et al, 2006; Stingl et al, 2006). A second type of multipotent stem cell is dramatically expanded during pregnancy and likely drives the massive increase in alveolar epithelium (Asselin-Labat et al, 2010). This stem cell subset has less self-renewing capability than MaSCs from young virgin mammary glands and displays a distinct gene expression signature, suggesting that it may be a short-term repopulating cell.
Using a series of elegant lineage-tracing studies, Van Keymeulen et al present evidence that the mammary gland is maintained by two novel unipotent stem-like cells. They utilized reporter mice in which a cre-activated fluorescent marker gene was controlled by one of a number of different lineage-specific promoters in a temporal manner, by either tamoxifen or doxycycline induction. Activation of the YFP reporter by a basal cytokeratin 14 (K14)–driven cre in late embryogenesis resulted in the labelling of myoepithelial and luminal cells in mice at puberty, suggesting that a bipotent primordial stem cell yields both epithelial lineages early in development. In postnatal and adult luminal epithelium, however, K14 expression in luminal cells was largely extinguished. Cells labelled via another basal-specific promoter (K5) also contributed exclusively to the myoepithelial lineage. The proportion of labelled cells remained relatively constant over the developmental stages, thus indicating that these cells are long-lived.
Parallel findings were made for luminal cells labelled by virtue of K8-cre-mediated activation of the YFP reporter. These cells only contributed to the luminal lineage and exhibited clonal expansion and differentiation into milk-producing cells during pregnancy. Serial transplantation of large cell numbers indicated that the basal/myoepithelial and luminal populations contained self-renewing unipotent stem cells. Interestingly, in co-transplantation experiments of YFP-labelled myoepithelial cells with a limiting number of unmarked luminal cells, the myoepithelial stem cells adopted bipotent cellular properties, suggesting that they are capable of dedifferentiation. Thus, a hierarchy of stem cells appears to exist within the mammary gland, including unipotent and multipotent cells that likely play different roles in the morphogenesis and maintenance of the mammary epithelium. Pertinently, retroviral-mediated clonal tracking studies have revealed considerable heterogeneity within the hematopoietic stem cell compartment.
The report by Van Keymeulen et al challenges the role of the prospectively isolated multipotent MaSC in the adult mammary gland. In all likelihood, this stem cell lies upstream of the newly identified unipotent MaSCs. One implication of the study is that the multipotent MaSC may only be recruited in regeneration or transplantation assays, and does not normally contribute to homeostasis of the mammary gland. Notably, transplantation assays of stem cells resident in the bulge have demonstrated that they have the potential to repopulate all the main structures of the skin, whereas in vivo genetic tagging of the same bulge cells revealed that they essentially only contribute to maintenance of the hair follicle (Morris et al, 2004). Thus, multipotency may be necessary in the case of wound healing or tissue regeneration but not for organ homeostasis.
In the case of the postnatal mammary gland, it remains to be determined whether the multipotent MaSC serves as a ‘reserve’ stem cell and whether these cells were targeted by the basal cell-specific cre lines. Although lineage tracing is a powerful strategy for clonally tracking cells in vivo, it is reliant on the use of well-defined cell type-specific promoters that faithfully mirror expression of the endogenous gene in a particular cell. Interestingly, the s-SHIP promoter has been recently shown to genetically mark activated MaSCs that specifically localize to the cap cell region of terminal end buds in developing mammary glands and alveolar units (Bai and Rohrschneider, 2010). This work demonstrates the localization of a multipotent MaSC to a region in the postnatal gland that is presumed to be enriched for MaSCs. Moreover, long-term label retaining cells capable of asymmetric division (Smith, 2005), and parity-identified progenitors that are multipotent and self-renewing (Boulanger et al, 2005) have been detected in the mammary gland. The delineation of highly refined stem cell markers will be required to resolve the relationship between these stem-like cells and unipotent stem cells.
The discovery of unipotent stem cells in the mammary gland alters the emerging ‘map’ of the hierarchy. These lineage-restricted precursor cells may be unique stem cells with uni-lineage potential or alternatively could correspond to longer-lived progenitors (Figure 1). Indeed, such long-lived progenitors have been demonstrated to maintain homeostasis of the interfollicular epidermis (Clayton et al, 2007) and to be one ‘cell of origin’ for basal carcinomas (Youssef et al, 2010). In the mammary gland, unipotent stem/progenitor cells rather than multipotent MaSCs may largely be responsible for normal tissue maintenance. A combination of cell-fate mapping studies, together with rigorous cell-sorting and transplantation experiments, will likely be required to fully understand the relationships between the different MaSC and progenitor types. A final conundrum is how the two distinct lineage-restricted stem cells coordinate the formation of a bilayered epithelial tree in the developing mammary gland.
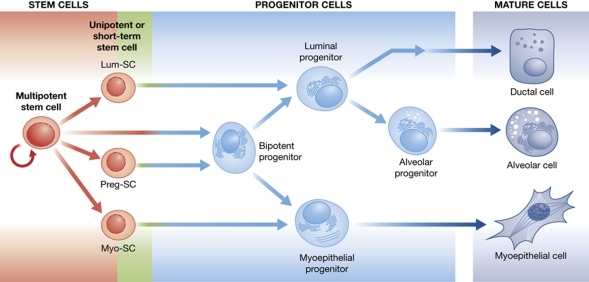
Figure 1.
Model of the epithelial differentiation hierarchy in the mammary gland. There is recent evidence for a heterogeneous compartment of stem cells, in which the stem cell at the apex is multipotent and likely has higher self-renewing capacity than the unipotent stem cells (myoepithelial-stem cell, Myo-SC; luminal-stem cell, Lum-SC) and the ‘shorter-term’ mammary repopulating cell that emerges in pregnancy (Preg-SC). Alternatively, there may be one true stem cell and a hierarchy of descendant progenitor cells (indicated by overlap between the stem and progenitor cell compartments) that include long- and short-lived lineage-restricted progenitors.
Footnotes
The authors declare that they have no conflict of interest.
References
- Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Visvader JE (2010) Control of mammary stem cell function by steroid hormone signalling. Nature 465: 798–802 [DOI] [PubMed] [Google Scholar]
- Bai L, Rohrschneider LR (2010) s-SHIP promoter expression marks activated stem cells in developing mouse mammary tissue. Genes Dev 24: 1882–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CA, Wagner KU, Smith GH (2005) Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene 24: 552–560 [DOI] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH (2007) A single type of progenitor cell maintains normal epidermis. Nature 446: 185–189 [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G (2004) Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22: 411–417 [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE (2006) Generation of a functional mammary gland from a single stem cell. Nature 439: 84–88 [DOI] [PubMed] [Google Scholar]
- Smith GH (2005) Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development 132: 681–687 [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ (2006) Purification and unique properties of mammary epithelial stem cells. Nature 439: 993–997 [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C (2011) Distinct stem cells contribute to mammary gland development and maintenance. Nature 479: 189–193 [DOI] [PubMed] [Google Scholar]
- Youssef KK, Van Keymeulen A, Lapouge G, Beck B, Michaux C, Achouri Y, Sotiropoulou PA, Blanpain C (2010) Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol 12: 299–305 [DOI] [PubMed] [Google Scholar]