Abstract
Spine-associated Rap-specific GTPase-activating protein (SPAR) is a postsynaptic protein that forms a complex with postsynaptic density (PSD)-95 and N-methyl-D-aspartate receptors (NMDARs), and morphologically regulates dendritic spines. Mild intermittent hypoxia (IH, 16.0% O2, 4 h/day for 4 weeks) is known to markedly enhance spatial learning and memory in postnatal developing mice. Here, we report that this effect is correlated with persistent increases in SPAR expression as well as long-term potentiation (LTP) in the hippocampus of IH-exposed mice. Furthermore, an infusion of SPAR antisense oligonucleotides into the dorsal hippocampus disrupted elevation of SPAR expression, preventing enhanced hippocampal LTP in IH-exposed developing mice and also reducing LTP in normoxic mice, without altering basal synaptic transmission. In SPAR antisense-treated mice, acquisition of the Morris water maze spatial learning task was impaired, as was memory retention in probe trails following training. This study provides the first evidence that SPAR is functionally required for synaptic plasticity and contributes to the IH-induced enhancement of spatial learning and memory in postnatal developing mice.
Keywords: hypoxia, learning and memory, LTP, postnatal, SPAR
The cellular basis of learning and memory is thought to require long-lasting changes in synapses involving both functional and morphological plasticity. The hippocampus has an essential role in spatial learning (Morris et al., 1982), and stress is a powerful modulator of hippocampal function (Fenoglio et al., 2006; Bangasser and Shors, 2007; Koenigs et al., 2008). The relationship between stress and hippocampal synaptic plasticity is an inverted-U function (Diamond et al., 1992) and is dependent on stress intensity as well as developmental stage. For example, hippocampus-dependent behavior in adulthood is sensitive to the effects of hypoxia during postnatal development. In our previous study, neonatal exposure to intermittent hypoxia (IH), a mild stress, was found to enhance spatial learning and memory in mice tested at later stages of development (Zhang et al., 2005). This finding provides a powerful model for investigating the mechanisms underlying the effects of postnatal hypoxia on adult synaptic plasticity and behavior.
Excitatory amino acid receptors, especially N-methyl-D-aspartate receptors (NMDARs), play important roles in learning and memory. Overexpression of the N-methyl-D-aspartate receptor subunit 2B (NR2B) in the forebrain of transgenic mice leads to increased NMDAR activity, and enhanced performance in learning and memory task (Tang et al., 1999). At excitatory synapses, NMDARs are integrated into postsynaptic densities (PSDs) that contain a variety of scaffolding and signaling proteins involved in synaptic plasticity and learning (Sheng and Kim, 2002). In mutant mice lacking the prototypical scaffold protein PSD-95, NMDAR-mediated synaptic plasticity is altered and learning is impaired (Migaud et al., 1998). Another PSD protein, spine-associated Rap-specific GTPase-activating protein (SPAR), interacts with the guanylate kinase–like domain of PSD-95 and forms a complex with PSD-95 and NMDAR in brain (Pak et al., 2001; Roy et al., 2002). SPAR causes enlargement of dendritic spine heads, a process that depends on its RapGAP and actin-interacting domains (Pak et al., 2001), and may be involved in the maturation of spines. Alterations in the expression or function of these postsynaptic proteins may be important for mediating NMDAR-dependent synaptic plasticity and learning.
Based on our finding that postnatal exposure to mild IH improves spatial learning and memory in mice, we were interested in the molecular changes that occur at the postsynaptic specializations of hippocampal neurons during this process. We focused on SPAR, a potent regulator of dendritic spine morphology in vitro, due to the known ability of hypoxia to trigger morphological plasticity in dendritic spines (Jourdain et al., 2002, 2003). Thus, we proposed that SPAR acts as a crucial molecule involved in the synaptic plasticity of spines and IH-induced spatial learning enhancement. We found that SPAR expression was significantly and persistently upregulated in the hippocampus of mice following IH treatment. To test this idea further, SPAR antisense oligonucleotides were infused into the dorsal hippocampus in vivo to disrupt SPAR protein expression, and we measured long-term potentiation (LTP) in the CA1 area of the hippocampus as well as the performance of mice in spatial learning tasks.
EXPERIMENTAL PROCEDURES
Animals and hypoxia
For breeding, adult male and female ICR mice were purchased from the Zhejiang Province Experimental Animal Center (approval No. SCXK2003-0001) and bred in our animal room. Following birth, the newborn mice with their mothers were immediately exposed to hypoxia simulating an altitude of 2 km (16.0% O2 at sea level) in a well-ventilated hypobaric chamber. Control newborn mice were set at sea level (21.0% O2) in another similar chamber.
All pups were maintained with their mothers on a 12-h light/dark cycle (lights on 07:00–19:00 h) at 22±1 °C, and had free access to food and water. The IH lasted for 4 weeks (for eight litters), and was performed from 08:00 to 12:00 h daily (4 h/day). After weaning, the mothers were removed from the home cages, and only male offspring were randomly assembled to form three groups for drug administration and behavioral tests at ages up to postnatal day (P) 35. One pup per litter was used to make up each group, which consisted of members from eight litters. At P14, P28, and P35, male offspring were taken for Western blotting (Fig. 1). The experimental conditions and procedures were approved by the Local Institutional Animal Care and Use Committee and were carried out in close agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Fig. 1.
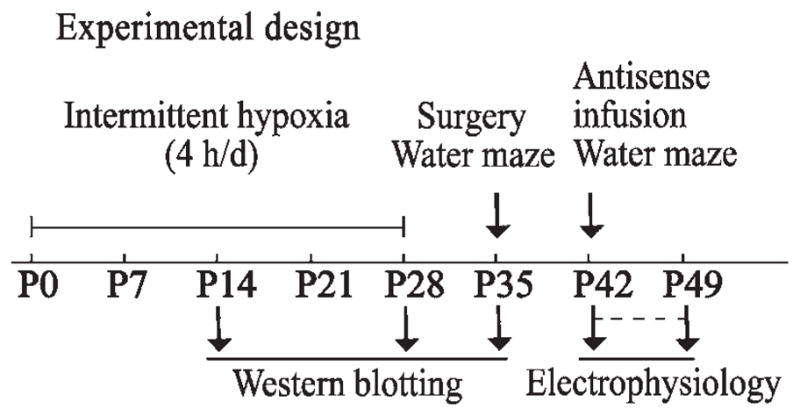
Experimental design. The IH paradigm (16% O2) lasted for 4 weeks (for eight litters), for 4 h/day. At P14, P28, and P35, male offspring were taken for Western blotting. After weaning, the mothers were removed from the home cages, and only male offspring were randomly assembled to form new groups for antisense infusion, behavioral tests, and electrophysiology.
Morris water maze
A 1 m diameter, 0.5 m high, circular black tank filled with water (21 °C) made opaque by the addition of ink, was positioned underneath a video camera to track animal activity. An 11 cm diameter black platform, lying 1 cm below the water surface, was placed in the center of one of the four imaginary quadrants and remained constant for each mouse. Prominent visual cues (a pole, the door, and researchers) surrounding the maze provided spatial cues. Data were analyzed using a tracking program (DigBehv-MWM; Jiliang Software Technology Co. Ltd., Shanghai, China).
Testing took place between 08:00 and 16:00 h. To test the IH-induced enhanced spatial learning, the mice underwent a pre-training trial (2 min free-swimming with no platform present) in order to introduce them to the water maze at P35. For training, the offspring were given three trials on each day for four consecutive days and a block consisted of three trials corresponding to different randomized release points. On each trial, the mouse was released into the maze facing the sidewall, starting from a different position in one of the three quadrants not containing the platform. Each position was used once in each block of three trials in a pseudorandom manner. The trial ended when the mouse climbed onto the platform, where it was allowed to remain for 15 s before removal from the arena. If the mouse did not find the escape platform within 60 s, it was guided to it, and left there for 15 s. A 30 min interval was imposed before the beginning of the next trial. The time mice spent in finding the submerged platform (escape latency) and the swimming speed were recorded. One day after the training period, the mice were submitted to a single 60 s probe test in which the platform was removed from the maze. The time that mice spent swimming around the former location of the platform (defined as within a 12.5 cm radius of the original location) and the swimming speed were analyzed. In all groups, a visual acuity test was performed after the last probe test. The time taken to climb onto a raised visible platform placed in the quadrant opposite to the training position was recorded to assess any visual impairment.
The maze training protocol was modified for the SPAR anti-sense study, to ascertain whether or not SPAR antisense might be associated with an impairment of water maze performance and to superimpose the time course of the significant depression in SPAR expression with the training or probe test period. For training, PBS, missense, or antisense was infused bilaterally into the dorsal hippocampus before the day of pretraining. Training was started on the second day after infusion and the offspring were given six trials on each day for two consecutive days. A probe test was performed once each day on the third and fourth days after training. Hippocampal infusion of PBS, missense, or antisense was carried out after six training trials on each day for three consecutive days.
Eight-arm maze
The eight-arm radial maze was conducted as previously reported (Zhang et al., 2005). SPAR antisense-treated mice 7 weeks of age were trained on an eight-arm maze, which was constructed with a 25 cm middle platform and 20 cm long arms. Food cups were located near the distal end of each arm. Mice were food-restricted to 80% of their normal body weight, which was maintained throughout the experiment. In the first two days, the same cage mates were placed on the maze for five minutes on two consecutive days and allowed to explore with food to habituate to the maze. On the third day, SPAR antisense or missense was infused bilaterally into the hippocampus. On the fourth day, mice received two trials/day in which they were given up to five minutes (300 s) to find the food reward (peanut) in four of eight baited arms. Four-hour intervals were allowed between two trials and training took place between 08:00 and 18:00 h daily. We recorded the time to complete the task, working memory errors (reentry into a previously visited arm), and reference memory errors (entry into an arm without bait).
Western blotting
Mice were decapitated 20 h after the last day of IH at P14 and P28. After 28 days of IH, animals were decapitated at P35. Control mice were killed at the same time points. Hippocampi were dissected on ice for Western blotting. Tissues were lysed at 4 °C in a buffer composed of 20 mM Hepes, 1.5 mM MgCl2, 0.2 mM EDTA, 100 mM NaCl, 0.2 mM DTT, 0.5 mM sodium orthovanadate, and 0.4 mM PMSF (pH 7.4). The lysate was then centrifuged at 10,000×g for 30 min. The protein concentration of the supernatant was measured in each soluble fraction by using the Bradford method, and samples were subjected to SDS-PAGE (7.5% acrylamide gel) and transferred to PVDF (Pall, NY, USA). Membranes were blocked for 1 h in a 10% non-fat dry milk solution in TBS-Tween. After 1.5 h incubation with antibodies to NR2A, NR2B, PSD-95, or SPAR, membranes were washed and incubated for 1 h with HRP-conjugated secondary antibodies. Proteins were visualized by enhanced chemiluminescence (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and semiquantitative analysis was performed by scanning densitometry. To normalize data across experiments, all densitometric values were expressed as a ratio in which the actin densitometric reading served as the denominator. The SPAR antibody (Daniel T Pak prepared, 1:400 dilution) has been described (Pak et al., 2001). The following antibodies were purchased from commercial sources: monoclonal anti-NR2B (1:500 dilution; Millipore, Bedford, MA, USA); NR2A (1:1000 dilution; Abcam, Cambridge, UK); PSD-95 (1:4000 dilution; Affinity BioReagents, Golden, CO, USA); actin (1:3000 dilution; Santa Cruz Biotechnology).
Immunofluorescence
Representative coronal brain sections (20 μm) from control and IH-exposed animals were fixed with 4% paraformaldehyde and 0.2% picric acid in 0.1 M phosphate buffer, pH 7.4, for 30 min at 4 °C, and then washed three times in PBS. Sections were permeabilized with 0.4% Triton X-100 (Sigma, St. Louis, MO, USA) in PBS for 15 min at room temperature (RT). After washing with PBS, sections were blocked with 5% normal goat serum in PBS containing 0.4% Triton X-100 for 30 min at RT, and then incubated overnight with rabbit anti-SPAR antibody (1:100 dilution) at 4 °C. Negative controls were performed under identical conditions by incubating with an equal amount of nonimmune IgG instead of primary antibody. Sections were washed three times with PBS and incubated with Cy3-conjugated goat anti-rabbit secondary antibody (Millipore) for 1 h at RT. Images were collected on a Nikon TE2000 microscope using a 20× objective. The same settings for brightness and contrast were used within each experiment.
Surgery
At P35, mice were implanted with bilateral cannulae aimed at the dorsal hippocampus. Animals were anesthetized by i.p. injection of sodium pentobarbital (80 μg/g) and mounted into a Kopf stereotaxic frame, which was used to position the 24-gauge stainless steel guide cannulae in the dorsal hippocampi (AP −1.8 mm, ML ± 1.5 mm, DV −1.8 mm). The coordinates were chosen based on a mouse brain atlas (Franklin and Paxinos, 1997). The cannulae were fixed with dental acrylic and fitted with 33-gauge obturators to maintain cannula patency. Mice were housed individually and allowed at least 7 days of postoperative recovery before being used in behavioral experiments. At P42, PBS, SPAR antisense, or missense controls were infused into the dorsal hippocampus, then the water maze test was performed and the electrophysiological properties were measured. At P14, P28, and P35, hippocampal tissues were removed for Western blotting.
Antisense treatment
SPAR antisense was designed against the translation start codon of mouse SPAR (GenBank accession no. NM172579). SPAR missense has the same base composition as SPAR antisense but the sequence is different. The sequences of the SPAR antisense and SPAR missense oligonucleotides are 5′-gatcacttaaatccctgc-3′ and 5′-ctagtcacctatgaatcc-3′, respectively. Because of the low stability of phosphodiester-linked oligonucleotides and the high toxicity of completely phosphorothioated oligonucleotides, these oligonucleotides were synthesized with “end caps” in which the terminal three nucleotides at both the 5 and 3′ ends were phosphorothioate-linked. The unique nature of these sequences was confirmed by searching the GenBank database.
For infusions, the injectors were inserted into the guide cannulae and left in place for 1 min followed by a 1 min infusion of 1.5 nmol/side of antisense or missense (0.5 μl/per side, all dissolved in 500 nl PBS) or 500 nl of PBS. The injectors were left in place for an additional minute after the infusion and then replaced with the obturators. Following water maze testing, mice were rapidly decapitated and the brains were immediately removed, frozen in liquid nitrogen, and stored at −80 °C. The brains were sectioned at 20 μm and stained with Methylene Blue. Cannula placements were determined by light microscopy.
Electrophysiology
Both IH-treated and normoxic mice were used for LTP experiments at P42–P49. After decapitation of mice receiving PBS, SPAR antisense, or missense, hippocampal slices 400 μm thick were cut with a vibratome (World Precision Instruments, Sarasota, FL, USA) and kept submerged at 27–28 °C. Slices were perfused (at 2–2.5 ml/min) with oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (ACSF) containing (in mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, and 11 glucose, pH 7.3. For each experimental condition, hippocampal slices from four to seven animals were used. Synaptic responses were induced in stratum radiatum of CA1 next to area CA3 by stimulating Schaffer collaterals with 0.1-ms pulses. Extracellular field excitatory postsynaptic potentials (fEPSPs) were recorded in the stratum radiatum of CA1 using glass microelectrodes filled with ACSF (10–20 MΩ). The amplitude of the fEPSP was set to 30%–40% of maximum. For baseline recordings, slices were stimulated at 0.033 Hz for 20 min for E-LTP and 30 min for L-LTP. Tetanic LTP was induced by high-frequency stimulation in brief trains (100 Hz, 1 s), applied either as a single train or four trains delivered 5 min apart. Paired-pulse facilitation (PPF) was assessed by applying two pulses separated by inter-stimulus-intervals ranging from 10 to 160 ms. PPF was expressed as the increase in leading slope of the second fEPSP relative to the first. Electrophysiological data were sampled at 10 kHz on a PC using PowerLab (ADInstruments, Castle Hill, Australia). The leading slope of the evoked fEPSP was calculated as an indicator of synaptic strength. The experiments were carried out and analyzed in a blind pattern.
Statistical analysis
Results are presented as mean±SEM. All data were analyzed by one-way or repeated-measures analysis of variance (ANOVA) with SPSS (version 13.0, Chicago, IL, USA), followed by the least significant difference (LSD) post hoc test to compare individual groups or group effects over time. In all cases, P<0.05 was considered statistically significant.
RESULTS
Expression patterns of postsynaptic proteins following IH
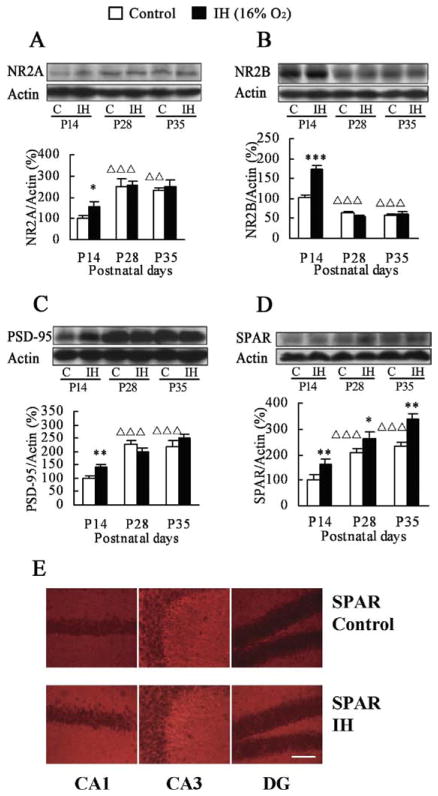
Many postsynaptic proteins are highly dynamic within spines, permitting rapid formation and remodeling of synaptic connections in developing brain (Marrs et al., 2001; Charych et al., 2006; Lamprecht et al., 2006). To study the expression patterns of postsynaptic proteins induced in mouse hippocampus by IH, newborn mice were immediately exposed to hypoxia simulating an altitude of 2 km (16.0% O2), 4 h/day for 28 days. This procedure is known to enhance learning and memory (Zhang et al., 2005; see also Fig. 4A, 4B). The hippocampi were dissected out at P14, P28, and P35 (Fig. 1), and Western blotting was performed to compare protein expression levels of NR2A, NR2B, PSD-95, and SPAR in hippocampal tissues between IH-treated mice and controls. In the control offspring, one-way ANOVA analysis showed that expression of the NMDAR subunit NR2A increased from P14 to P28 (P<0.001), while NR2B expression decreased from P14 to P28 (P<0.001; Fig. 2A, 2B), consistent with previous reports (Monyer et al., 1994; Sheng et al., 1994). No significant change in the expression of NR2A and NR2B was observed between P28 and P35. Mice subjected to IH had higher NR2A and NR2B expression than did controls, but only at P14 (NR2A: control P14 vs. IH P14, P=0.041; NR2B: control P14 vs. IH P14, P<0.001). There was no IH-induced change in NR2A and NR2B expression at any other time point examined during postnatal development.
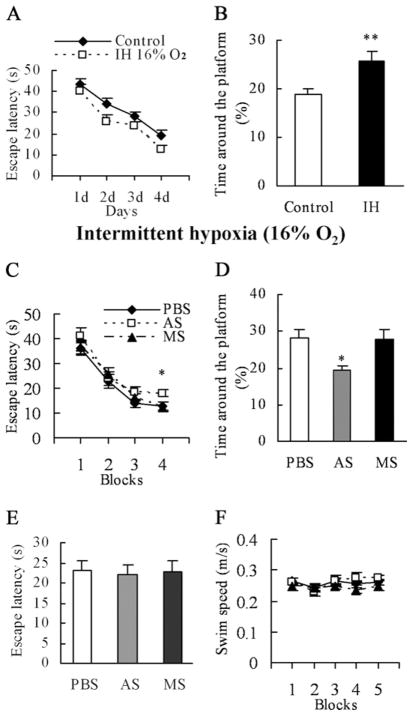
Fig. 4.
SPAR AS impairs performance in the water maze task. Before the behavioral test, the mice underwent 4 h of hypoxia (16% O2) per day for 4 weeks starting from birth. We confirmed the effects of IH on spatial learning. (A) Escape latencies to find the platform during the training phase in the water maze (n=14 per group), were shorter for IH-treated mice than controls. (B) IH-treated mice showed preference for the former location of the platform in the probe test compared with controls. At P42, mice received infusion of PBS (n=11), SPAR AS (n=10), or missense (n=11) to measure the effects of IH-induced higher SPAR expression on spatial learning. (C) Average escape latencies to find the platform were similar in all three groups for the training blocks as a whole. In contrast, on the last block, the overall escape latency in AS-treated mice was higher than that of PBS- and MS-treated controls. (D) SPAR AS mice spent less time around the former location of the platform during the 60-s duration of the probe trial compared with controls. (E) Escape latencies to locate the visible platform were the same for each group during the visual acuity test. (F) Swimming speeds did not differ among the three groups during training and retention testing. ** P<0.01; * P<0.05. AS, antisense; MS, missense.
Fig. 2.
Effects of IH on expression levels of postsynaptic proteins. Expression changes of hippocampal NR2A (A), NR2B (B), PSD-95 (C), and SPAR (D) in the IH-treated mice and controls at P14, P28, and P35. Actin was used as loading control and for normalization. (E) Representative examples of hippocampal SPAR immunofluorescence in the IH-treated groups and controls at P35. Increased staining for SPAR occurred in the neuropil of CA1, CA3, and dentate gyrus (DG). * P<0.05; ** P<0.01; *** P<0.001 vs. controls at the same postnatal day. Δ ΔP<0.01; ΔΔΔP<0.001 vs. controls at P14. C, control. Scale bar=100 μm.
We next assessed hippocampal PSD-95 and SPAR expression under the same conditions (Fig. 2C, 2D). In control offspring, PSD-95 and SPAR levels increased from P14 to P28 (PSD-95: P<0.001; SPAR: P<0.001, one-way ANOVA), leveling off at P35. These findings, together with the report that PSD-95 and synaptophysin expression increase in this time period (Glantz et al., 2007), likely reflect synapse maturation during postnatal development. As with NR2A and NR2B, an IH-induced increase in PSD-95 expression was seen only at P14 (P=0.004). However, in IH-treated mice, hippocampal SPAR expression was higher at all time points compared with normoxic controls (P14: P=0.001; P28: P=0.042; P35: P=0.007). Thus, among the postsynaptic markers examined, the persistent upregulation of SPAR following IH appears relatively specific.
Immunofluorescence was used to confirm the expression of SPAR in the IH-exposed mouse hippocampus. Indeed, more intense SPAR immunoreactivity was detected at P35 after IH than in controls (Fig. 2E). Increased hippocampal SPAR immunofluorescence was localized in the oriens layer, stratum radiatum, stratum lucidum, and molecular layer of the dentate gyrus, where the dendrites of hippocampal neurons reside. However, the pyramidal cell layer and granule cell layer of the dentate gyrus showed only very light staining. Taken together, these results suggest that prolonged induction of SPAR in hip-pocampal dendrites may have unique functions as part of a long-term response to IH.
SPAR knockdown with antisense
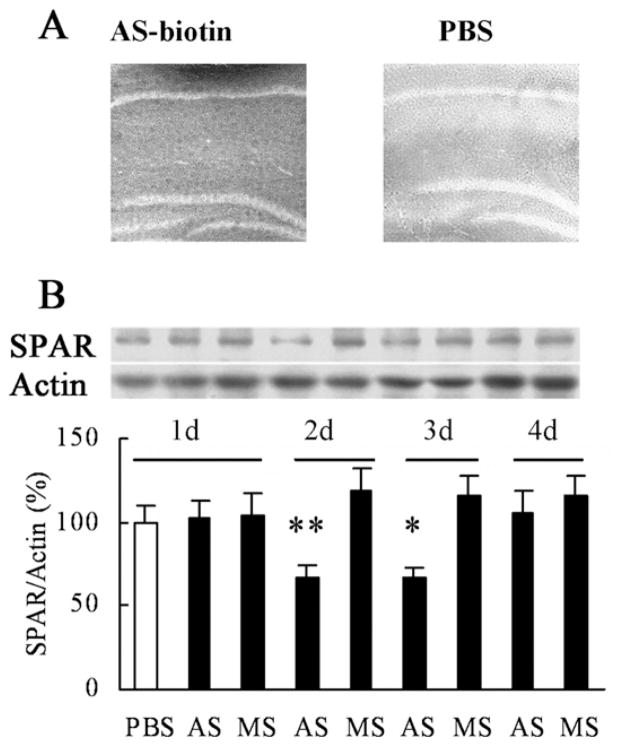
To causally link the IH-induced increase in SPAR expression to IH-induced enhancement in water maze performance, we used antisense technology to knock down hippocampal SPAR expression in vivo. To test the efficacy of the antisense oligonucleotides, we first performed stereotaxic administration of 5′ biotinylated SPAR antisense, delivered by cannula bilaterally (0.5 μl, 1.5 nmol/per side) into the dorsal hippocampus of P42 control mice. The antisense-biotin staining were tested immunohistochemically only in hippocampus, other brain regions were not stained (Fig. 3A). This treatment led to a time-dependent reduction in SPAR protein expression (Fig. 3B). One-way ANOVA conducted on data from Western blotting revealed a significant effect of SPAR antisense treatment (day 2: antisense vs. missense, P=0.007; day 3: antisense vs. missense, P=0.039), with loss of effect by day 4. Importantly, the degree of knockdown brought SPAR expression levels in IH-induced mice back down approximately to the level of normoxic controls, thereby allowing us to test the specific requirement of heightened SPAR expression in enhanced learning and memory.
Fig. 3.
Time-dependent effects of SPAR AS. PBS, SPAR AS, or MS was infused bilaterally into the dorsal hippocampus (1.5 nmol/side). (A) Biotinylated SPAR antisense was present in the dorsal hippocampus 2 h after injection but not in PBS control. (B) SPAR AS injection led to a time-dependent decrease in the hippocampal SPAR protein level at day 2 and day 3 following injection (n=5 mice per group). Day 2 ** P<0.01 AS vs. MS; Day 3 * P<0.05 AS vs. MS. AS, antisense; MS, missense.
SPAR antisense impairs spatial learning in IH-treated mice
We confirmed IH-induced enhancement of learning and memory using the Morris water maze, a well-validated hippocampal-dependent behavioral paradigm to assess spatial learning in animals (Morris et al., 1982; Eichenbaum et al., 1990). Fig. 4A, 4B illustrates the mean escape latencies during the training phase and the time spent in the vicinity of the previous platform location after the platform was removed during the probe trials. As anticipated from previous studies (Zhang et al., 2005), repeated measures ANOVA revealed a significant treatment effect (F(1,26) = 8.762, P=0.006) during the training phase, demonstrating that IH led to faster acquisition of the platform location. Moreover, one-way ANOVA showed a significant treatment effect (F(1,26) = 9.036, P=0.006) during the probe trials.
We next infused PBS, SPAR antisense, or missense control into the dorsal hippocampus of IH-treated mice at P42. After 48 h, mice were trained in the water maze with six trials per day (three trials per block) for 2 days. Memory retention and visual acuity tests were conducted 24 h after training.
Fig. 4C shows the average escape latencies across the four blocks of trials for each group. Repeated measures ANOVA revealed a significant training effect (F(3,87) = 73.480, P<0.001) but no treatment effect (F(2,29) = 2.100, P=0.141) and no significant treatment×time interaction (F(6,87) = 0.623, P=0.711) for all of the training blocks as a whole. However, escape latencies in the last block of trials (block 4, day 2) were significantly longer in antisense-treated mice than in PBS- and missense-treated mice (F(2,29) = 3.482, P=0.044; antisense vs. PBS: P=0.031; antisense vs. missense: P=0.027).
During the probe test, the hidden platform was removed, each mouse was placed into the maze for 60 s, and the percentage of time spent swimming to locate the former platform was measured in PBS-, antisense-, and missense-treated mice (Fig. 4D). One-way ANOVA showed a significant treatment effect (F(2,29) = 4.909, P=0.015). Post hoc analysis showed that the antisense-treated group spent less time around the former location of the platform than did the PBS- and missense-treated groups (antisense vs. PBS: P=0.009; antisense vs. missense: P=0.012). Antisense treatment had no effect on the time taken to reach a raised visible platform (F(2,29) = 0.046, P=0.955), indicating no long-term effect of any treatment on visual acuity (Fig. 4E). Furthermore, the similar swimming speeds in the training and retention trials (F(2,29) = 1.569, P=0.225) revealed that the impaired memory in the antisense group was not due to deficits in motor ability (Fig. 4F). Interestingly, the performance of antisense-treated IH mice was similar to that of untreated normoxic mice, suggesting that the loss of enhanced learning was specifically linked to the reduction of SPAR expression back to normal levels.
SPAR antisense impairs spatial learning and memory in normoxic mice
SPAR antisense was further used for knocking down SPAR expression in normoxic mice (Fig. 5A). Repeated measures ANOVA that compared the escape latencies across all groups indicated a significant training effect (F(3,84) = 35.145, P<0.001) but no treatment effect (F(2,28) = 1.065, P=0.358) and no significant treatment×time interaction (F(6,84) = 0.479, P=0.822) for all of the training blocks as a whole (Fig. 5B). However, escape latencies in the last block of training were longer for antisense-treated mice than PBS- and missense-treated mice (F(2,28) = 4.036, P=0.029; antisense vs. PBS: P=0.014; antisense vs. missense: P=0.036).
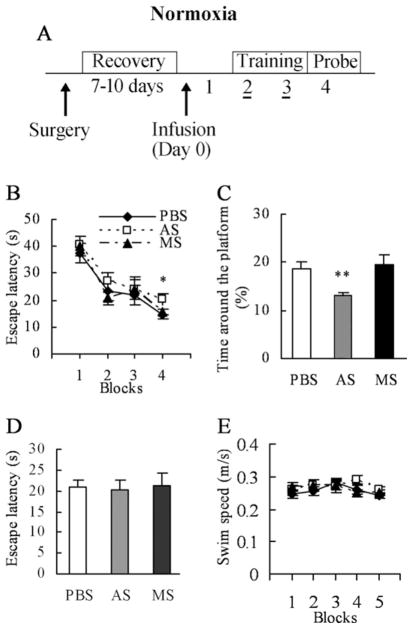
Fig. 5.
In normoxic mice, infusion of SPAR antisense impairs performance in the water maze task. (A) Animals were bilaterally implanted with guide cannulas into the dorsal hippocampus and allowed to recover for 7–10 days before receiving a single injection of PBS, SPAR antisense, or missense. Animals were trained in the water maze for two consecutive days, followed by a probe test. (B) No significant differences were seen for the blocks of trials as a whole for the training phase in mice treated with PBS (n=11), SPAR antisense (n=11), or missense (n=9). However, in the fourth block, the antisense-treated mice took more time to locate the platform than controls. (C) SPAR antisense mice crossed the former location of the platform fewer times than the two control groups in the probe trials. All three groups had similar escape latencies to locate the visible platform during the visual acuity test (D), and similar swimming speeds during training and retention testing (E). * P<0.05; ** P<0.01.
The time spent around the former location of the platform was next measured for all groups in the probe test (Fig. 5C). One-way ANOVA showed a significant treatment effect (F(2,28) = 6.152, P=0.006). Post hoc analysis revealed that mice treated with PBS and missense spent roughly equal times exploring the former location of the platform, while mice given the antisense spent significantly less time exploring this area (antisense vs. PBS: P=0.007; antisense vs. missense: P=0.004). The time taken to reach a raised visible platform showed a similar profile in all groups, irrespective of the treatment received (F(2,27) = 0.568, P=0.573; Fig. 5D). The average swimming speed of all three treatment groups in the training and retention trials was equivalent (F(2,28) = 0.611, P=0.550; Fig. 5E).
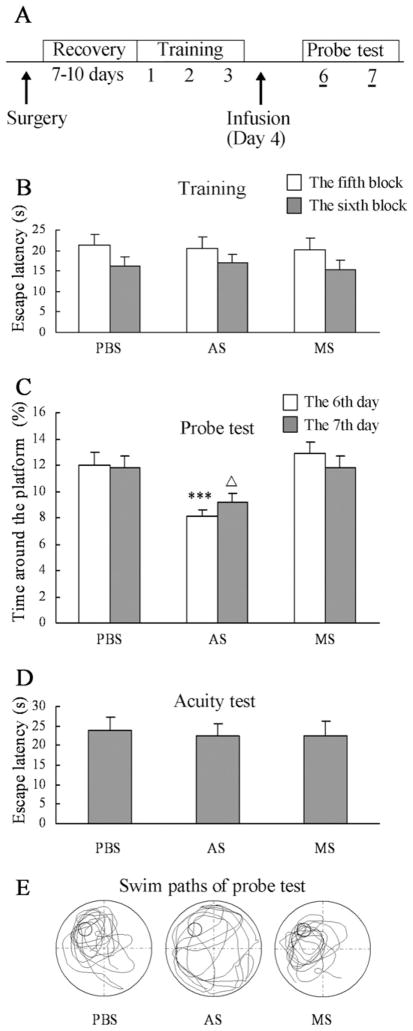
To further measure the direct effect of SPAR knockdown on memory retention, animals were divided into three groups in a pseudorandom manner after three consecutive days of training in the water maze (Fig. 6A). The cannulated mice spent a short time to find the platform on the last training day (Fig. 6B). After training, SPAR anti-sense was infused into the dorsal hippocampus to correlate the time course of significant SPAR knockdown with the probe test period. On the first day of probe testing (6th day), the average time around the former location of the platform was decreased in mice treated with SPAR anti-sense compared with controls (F(2,31) = 10.668, P<0.001; antisense vs. PBS: P<0.002; antisense vs. missense: P<0.001; Fig. 6C, 6E). The memory retention in SPAR antisense mice was still markedly impaired on the second day of probe testing (7th day; F(2,31) = 3.743, P=0.035; antisense vs. PBS: P=0.03; antisense vs. missense: P=0.024; Fig. 6C). The acuity test showed similar escape latencies for mice to find the platform by vision in all three groups (F(2,31) = 0.047, P=0.954; Fig. 6D).
Fig. 6.
Effect of SPAR AS infusion on memory retention after water maze acquisition. (A) After training for three consecutive days in the water maze, animals received an infusion of PBS (n=10), SPAR AS (n=13), or MS (n=11) on day 4, followed by probe tests on days 6 and 7. (B) In the two blocks of the third training day, the escape latencies for animals to find the platform were similar in all three groups before infusion. (C) SPAR knockdown mice spent less time around the former location of the platform during the probe tests compared with controls. (D) There was no significant difference in the performance of the visual acuity test in all three groups. (E) Representative swim paths during a 60-s probe test carried out with the animals are shown in (C). ΔP<0.05; *** P<0.001. AS, antisense; MS, missense.
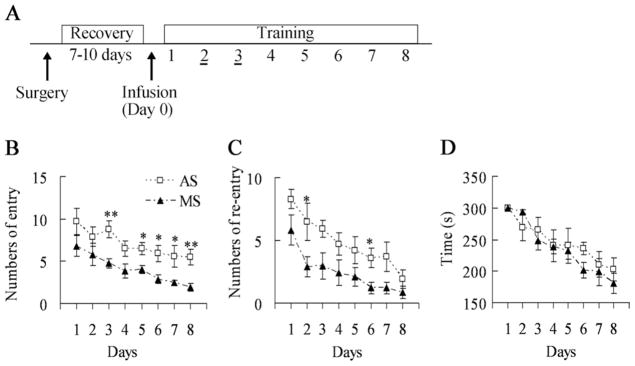
Similar to the water maze, the eight-arm maze is another hippocampus-dependent spatial learning task (Olton and Papas, 1979). Since both spatial reference and working memory can be measured in the eight-arm maze, we further employed it to analyze the effect of SPAR knockdown on spatial working memory (Fig. 7A). Mice treated with SPAR antisense made many more reference memory errors (F(1,10) = 14.033, P=0.004; Fig. 7B). The knockdown of hippocampal SPAR in mice also impaired working memory in this maze (F(1,10) = 9.024, P=0.013; Fig. 7C). Both antisense and missense groups showed improved eight-arm maze performance over days (F(1,10) = 67.878, P<0.001). There was no significant time difference to clear the baited arms between antisense and missense groups for eight days of training (F(1,10) = 0.317, P= 0.586; Fig. 7D).
Fig. 7.
Radial arm maze performance in SPAR AS- and MS-treated mice (MS, n=7; AS, n=5). (A) Animals were trained in the radial arm maze after infusing SPAR AS or MS into the dorsal hippocampus. (B) Mice receiving SPAR AS made more arm entry errors (reference memory) than controls (F(1,10) = 14.033, P=0.004). (C) SPAR AS-treated mice made more arm re-entry errors (working memory) than controls (F(1,10) = 9.024, P=0.013). (D) The average times of both treatment groups in the training trials were equivalent. * P<0.05; ** P<0.01. AS, antisense; MS, missense.
SPAR is required for LTP in hippocampal CA3–CA1 of normoxic mice
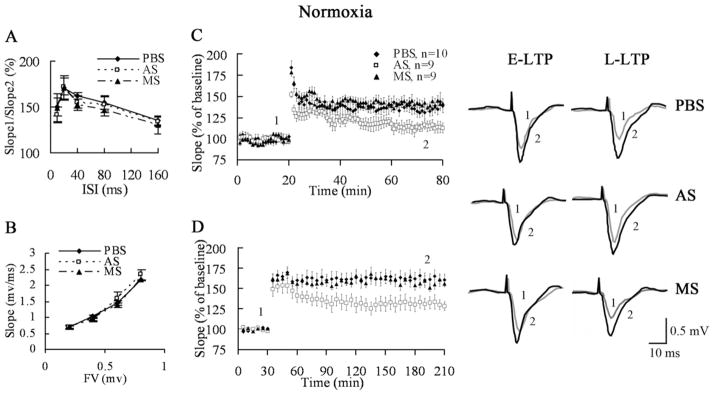
To further explore whether the high SPAR expression affected synaptic plasticity that is thought to underlie learning and memory, we performed electrophysiological studies in hippocampal slices from mice treated with PBS, SPAR antisense, and missense (0.5 μl/side, bilateral intrahippocampal infusion 2 days before hippocampal slices were prepared). Before investigating long-term changes of synaptic plasticity, we analyzed basal transmission in mice treated with PBS, antisense, and missense. Extracellular recordings from the CA3–CA1 pathway in SPAR antisense mice did not show significant changes in PPF and the size of the presynaptic fiber volley (Fig. 8A, 8B), suggesting that partial knockdown of SPAR does not compromise basic synaptic transmission or functionally disrupt normal dendritic structure.
Fig. 8.
SPAR is required for hippocampal LTP in normoxic mice. (A) Paired pulse facilitation of the fEPSP at various interstimulus intervals (ISI) from mice receiving PBS (n=5), SPAR AS (n=6), or MS (n=5). (B) Fiber volley (FV) of fEPSP at various stimulus intensities. The volley was in the range of 0.2–0.8 mV for all three groups (PBS, n=6, AS, n=5, MS, n=7). (C) LTP was induced by stimulation with HFS. SPAR AS mice (n=9) showed a significant deficit in E-LTP compared to PBS (n=10) and MS mice (n=9). (D) Slices from SPAR AS mice (n=8) displayed a marked defect in late-phase LTP after 4× HFS compared with the PBS (n=10) and MS groups (n=8). AS, antisense; MS, missense.
To investigate the role of SPAR in CA3–CA1 LTP, high frequency stimulation (HFS, 100 Hz) was used in acute hippocampal slices to induce early-phase long-term potentiation (E-LTP). After stable baseline recording for 20 min, a HFS was given to fibers of the CA3 presynaptic neurons and LTP was recorded from CA1 neurons for 1 h after stimulation. Compared with PBS- and SPAR missense-treated controls, SPAR antisense showed a reduction in E-LTP (PBS: 138.5%± 8.3%, antisense: 114.6%± 5.5%, missense: 142.5%± 4.3% in the last 5 min of LTP; one-way ANOVA, P=0.012, PBS vs. antisense: P=0.014, missense vs. antisense: P=0.006; Fig. 8C).
LTP has at least two distinct temporal phases: the E-LTP does not require new protein synthesis, while the late phase long-term potentiation (L-LTP) requires protein and RNA synthesis (Frey et al., 1988; Nguyen et al., 1994; Kandel, 2001). We further investigated whether knockdown of SPAR had an effect on L-LTP induced by stronger stimulation (4× HFS). The results showed that downregulation of SPAR expression led to a marked deficit of L-LTP in CA3–CA1 (PBS: 160.6%±7.4%, antisense: 128.5%±5.6%, missense: 155.9%±3.2% at 175–180 min after stimulation; one-way ANOVA, P=0.002, PBS vs. antisense: P=0.001, missense vs. antisense: P=0.005; Fig. 8D). These results suggest that SPAR is involved in both early and late phases of LTP.
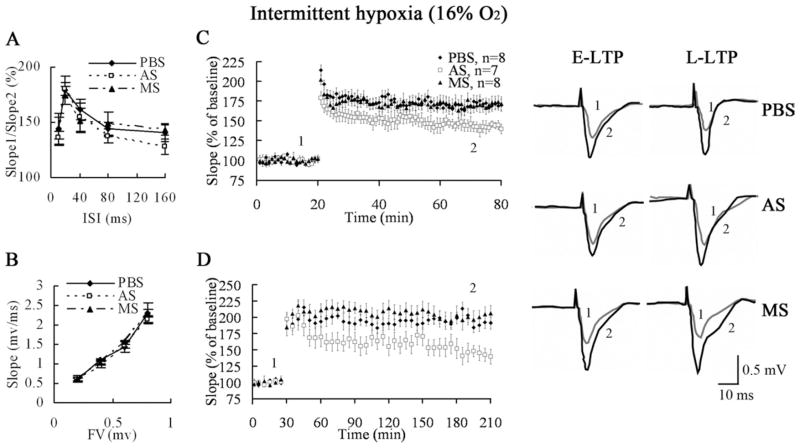
SPAR is required for enhanced LTP in hippocampal CA3–CA1 of IH-treated mice
The E-LTP in hippocampal CA3–CA1 significantly increases in IH-treated mice compared with normoxic mice (Zhang et al., 2005). We next investigated the specific role of elevated SPAR levels in the enhanced LTP of IH-treated mice. As with normoxic mice, antisense-mediated knockdown of SPAR showed normal basal synaptic transmission in IH-treated mice (Fig. 9A, 9B), suggesting no gross derangements of overall synaptic structure. The PPF was no significant difference between normoxic and IH-treated mice. We analyzed the CA3–CA1 pathway of IH-treated mice that received infusion of PBS and SPAR missense controls as well as SPAR antisense to measure whether LTP was affected. Compared with controls, slices from SPAR antisense mice showed a significant reduction in E-LTP (PBS: 169.1%±5.7%, antisense: 143.3%±4.2%, missense: 172.5%±7.6%, one-way ANOVA, P=0.008, PBS vs. antisense: P=0.009, missense vs. antisense: P=0.004; Fig. 9C). Furthermore, in comparison to PBS and SPAR missense controls, SPAR antisense mice showed a marked reduction in L-LTP as well (PBS: 191.5%±9.2%, antisense: 139.8%±11.4%, missense: 206.1%±11.7%, one-way ANOVA, P=0.001, PBS vs. antisense: P=0.003, missense vs. antisense: P<0.001; Fig. 9D). Importantly, the degree of LTP observed in mice treated with IH and SPAR antisense quantitatively resembled the LTP levels in control mice, suggesting that the IH-induced increase in SPAR expression was largely responsible for the increased LTP following IH. Taken together, these findings indicate that SPAR is a key functional protein induced by IH, which contributes to the enhancement of LTP as well as hippocampus-dependent learning and memory.
Fig. 9.
IH-induced increase in SPAR expression is critical for LTP. (A, B) Slices from IH-induced SPAR antisense mice showed normal paired pulse facilitation (PBS, n=6; antisense, n=6; missense, n=7) and no significant changes in the slope of the fiber volley (FV: PBS, n=5; antisense, n=6; missense, n=7). (C) SPAR antisense slices showed a major defect in E-LTP (n=7) compared to controls (PBS, n=8) following HFS stimulation. (D) After 4× HFS, late-phase LTP in SPAR antisense slices (n=8) was strongly reduced compared to controls (PBS, n=7).
DISCUSSION
In our mouse model of improvement of spatial learning induced by chronic mild IH from 1 to 28 days after birth, we observed a variety of dynamic changes in postsynaptic protein expression. We showed that neonatal exposure to IH led to a marked upregulation of the postsynaptic proteins NR2A, NR2B, PSD-95, and SPAR in the hippocampus at P14. Strikingly, among the proteins examined, the expression of SPAR was uniquely elevated in the hippocampus at P28 and P35 after IH treatment. Expression levels of NMDARs and PSD-95 are sensitive to hypoxia (Izumi et al., 1998; Bickler et al., 2003; Jiang et al., 2003; Chen et al., 2006, 2007). However, we found that the expression of NMDARs and PSD-95 in the hippocampus of IH-treated mice was similar to untreated controls at older ages. The marked increase in the expression of NR2A and NR2B in hippocampus induced by IH at P14, but not at P28 and P35, may reflect a transient modification of the number of neurotransmitter receptors that would provide greater potential plasticity and enhance spatial learning in the early period following IH. This heightened plasticity may be related to the greater motility of dendritic spines observed in response to hypoxia (Jourdain et al., 2002, 2003; Meller et al., 2008). The intriguing and distinct pattern of upregulation of SPAR at all postnatal times examined following IH led us to focus on its role specifically in long-term IH-enhancement of synaptic plasticity and spatial learning.
Effect of SPAR on learning and memory
What is the functional consequence of increased SPAR expression? The behavioral data revealed that upregulation of SPAR is involved in the enhanced performance of spatial learning paradigms in IH-treated mice. It is important to emphasize that knockdown of SPAR brought IH-treated mice back to control performance rather than simply impairing learning in general. These findings are consistent with the immunoblotting results showing that antisense treatment of IH-exposed mice reduced SPAR expression to control levels, rather than eliminating expression completely.
Our data indicate that SPAR not only participates in the acquisition of spatial learning but also in memory retention. SPAR knockdown during the training period caused significant impairment in memory retention during probe testing. Furthermore, knocking down SPAR expression during the probe test period by infusing SPAR antisense after three consecutive days of training also led to impaired memory retention. Thus, SPAR is important for acquiring new information as well as longer-term encoding of memory.
Effect of SPAR on LTP
In electrophysiological studies, we found that partial SPAR knockdown did not affect basal synaptic transmission, PPF, or the presynaptic fiber volley in the mature neural network. These data are consistent with the previous report that, in cultured neurons, SPAR overexpression does not increase spine density (Pak et al., 2001), and strongly suggest that knockdown did not impair overall synaptic function. However, SPAR knockdown did significantly diminish both hippocampal CA3–CA1 E-LTP and L-LTP. As with SPAR expression levels and performance in memory tasks, LTP in IH-treated mice was reduced to control levels by SPAR knockdown, thus correlating SPAR upregulation with enhanced function in multiple independent assays.
Mechanism of SPAR action
It is still unclear how SPAR influences synaptic plasticity. Overexpression of SPAR causes an enlargement and an increase in the complexity of spine shape in vitro (Pak et al., 2001). Thus, our finding of upregulated expression of SPAR in hippocampus may indicate more mature spines which could be maintained in the adult brain by persistently increased SPAR expression.
At the molecular level, one potential signaling pathway could involve the Rap-specific GAP activity of SPAR (Pak et al., 2001). Rap is an important molecule for AMPAR internalization in vitro (Zhu, 2003). Thus, SPAR may be involved in synaptic plasticity by antagonizing Rap and thereby promoting synaptic insertion of AMPARs. The SPAR expression is highly regulated in glutamate-induced excitotoxicity in cultured hippocampal neurons (Wu et al., 2007). This RapGAP pathway also lies downstream of EphA4 and Cdk5 to regulate the morphological plasticity of dendritic spines (Richter et al., 2007; Seeburg et al., 2008), which underlies memory storage (Kandel, 2001). Alternatively, enhanced F-actin dynamics within the spine is known to be essential for L-LTP maintenance (Fukazawa et al., 2003) and this may require the ability of SPAR to reorganize F-actin in dendritic spines (Pak et al., 2001). Other anchoring proteins, such as calcineurin and CaMKII, regulate the SPAR function in the PSD. Calcineurin activation contributes to shrinkage of dendritic spines (Zhou et al., 2004); and CaMKII activation is required for the maintenance of the spine change (Lee et al., 2009). The SPAR might be the downstream target of calcineurin and CaMKII.
Effect of mild stressors on learning and memory
Severe hypoxia (alternating 21% and 10% O2 every 90 s) in postnatal development leads to impaired spatial learning and memory (Row et al., 2002). Our finding that neonatal exposure to IH (16.0% O2 for 4 h/day for 4 weeks) enhanced spatial learning may seem at odds with these previous reports, but the discrepancy may be because of the different intensity and period of hypoxia used (Koritzinsky et al., 2006) Evolutionarily, enhancement of spatial learning in a hypoxic environment could contribute to the ability of an animal to form more precise spatial maps in the context of avoidance of relatively inhospitable environments. In addition to mild hypoxia, several other forms of stress are known to enhance learning and memory. For example, fasting increases expression of ghrelin, which enters into the hippocampus and enhances spatial learning and memory (Diano et al., 2006). In addition, restraint and brief intermittent tail-shocks facilitate learning and memory in male animals (Wood and Shors, 1998; Hodes and Shors, 2005). These findings suggest that mild stress factors can be considered as auxiliary therapy for enhancement of learning and memory according to individual conditions.
Postnatal exposure to IH enhances the expression of hippocampal SPAR, which is positively correlated with the performance of spatial learning and memory tasks. We also revealed that SPAR plays an important role in spatial learning in mice without IH exposure. These results suggest that SPAR is a crucial molecule in the hippocampus and regulates the improved performance of spatial learning in the “IH-enhanced mouse” model. Further investigation of this model will shed additional light on hypoxia-induced learning and memory processes during development.
Acknowledgments
This work was supported by grants from the NSFC (Major Project No. 30393130; Project Nos. 30871221) and the National Basic Research Program “973” (No. 2006CB504100). We wish to thank Professor I. C. Bruce, Department of Physiology, Zhejiang University School of Medicine, China, for his help with the English editing of the manuscript.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- E-LTP
early-phase long-term potentiation
- fEPSPs
field excitatory postsynaptic potentials
- HFS
high frequency stimulation
- IH
intermittent hypoxia
- L-LTP
late phase long-term potentiation
- LTP
long-term potentiation
- NMDAR
N-methyl-D-aspartate receptor
- NR2B
N-methyl-D-aspartate receptor subunit 2B
- P (with number)
postnatal day
- PPF
paired-pulse facilitation
- PSD
postsynaptic density
- RT
room temperature
- SPAR
spine-associated Rap-specific GTPase-activating protein
References
- Bangasser DA, Shors TJ. The hippocampus is necessary for enhancements and impairments of learning following stress. Nat Neurosci. 2007;10:1401–1403. doi: 10.1038/nn1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickler PE, Fahlman CS, Taylor DM. Oxygen sensitivity of NMDA receptors: relationship to NR2 subunit composition and hypoxia tolerance of neonatal neurons. Neuroscience. 2003;118:25–35. doi: 10.1016/s0306-4522(02)00763-7. [DOI] [PubMed] [Google Scholar]
- Charych EI, Akum BF, Goldberg JS, Jornsten RJ, Rongo C, Zheng JQ, Firestein BL. Activity-independent regulation of dendrite patterning by postsynaptic density protein PSD-95. J Neurosci. 2006;26:10164–10176. doi: 10.1523/JNEUROSCI.2379-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WF, Chang H, Huang LT, Lai MC, Yang CH, Wan TH, Yang SN. Alterations in long-term seizure susceptibility and the complex of PSD-95 with NMDA receptor from animals previously exposed to perinatal hypoxia. Epilepsia. 2006;47:288–296. doi: 10.1111/j.1528-1167.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- Chen WF, Chang H, Wong CS, Huang LT, Yang CH, Yang SN. Impaired expression of postsynaptic density proteins in the hippocampal CA1 region of rats following perinatal hypoxia. Exp Neurol. 2007;204:400–410. doi: 10.1016/j.expneurol.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschop MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Stewart C, Morris RG. Hippocampal representation in place learning. J Neurosci. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Baram TZ. Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects. Front Neuroendocrinol. 2006;27:180–192. doi: 10.1016/j.yfrne.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149:582–591. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Shors TJ. Distinctive stress effects on learning during puberty. Horm Behav. 2005;48:163–171. doi: 10.1016/j.yhbeh.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Katsuki H, Benz AM, Zorumski CF. Oxygen deprivation produces delayed inhibition of long-term potentiation by activation of NMDA receptors and nitric oxide synthase. J Cereb Blood Flow Metab. 1998;18:97–108. doi: 10.1097/00004647-199801000-00010. [DOI] [PubMed] [Google Scholar]
- Jiang X, Mu D, Sheldon RA, Glidden DV, Ferriero DM. Neonatal hypoxia-ischemia differentially upregulates MAGUKs and associated proteins in PSD-93-deficient mouse brain. Stroke. 2003;34:2958–2963. doi: 10.1161/01.STR.0000102560.78524.9D. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Fukunaga K, Muller D. Calcium/calmodulin-dependent protein kinase II contributes to activity-dependent filopodia growth and spine formation. J Neurosci. 2003;23:10645–10649. doi: 10.1523/JNEUROSCI.23-33-10645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Nikonenko I, Alberi S, Muller D. Remodeling of hippocampal synaptic networks by a brief anoxia-hypoglycemia. J Neurosci. 2002;22:3108–3116. doi: 10.1523/JNEUROSCI.22-08-03108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Raymont V, Cheon B, Solomon J, Wassermann EM, Grafman J. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci. 2008;11:232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koritzinsky M, Magagnin MG, van den Beucken T, Seigneuric R, Savelkouls K, Dostie J, Pyronnet S, Kaufman RJ, Weppler SA, Voncken JW, Lambin P, Koumenis C, Sonenberg N, Wouters BG. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 2006;25:1114–1125. doi: 10.1038/sj.emboj.7600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, Farb CR, Rodrigues SM, LeDoux JE. Fear conditioning drives profilin into amygdala dendritic spines. Nat Neurosci. 2006;9:481–483. doi: 10.1038/nn1672. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs GS, Green SH, Dailey ME. Rapid formation and remodeling of postsynaptic densities in developing dendrites. Nat Neurosci. 2001;4:1006–1013. doi: 10.1038/nn717. [DOI] [PubMed] [Google Scholar]
- Meller R, Thompson SJ, Lusardi TA, Ordonez AN, Ashley MD, Jessick V, Wang W, Torrey DJ, Henshall DC, Gafken PR, Saugstad JA, Xiong ZG, Simon RP. Ubiquitin proteasome-mediated synaptic reorganization: a novel mechanism underlying rapid ischemic tolerance. J Neurosci. 2008;28:50–59. doi: 10.1523/JNEUROSCI.3474-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O’Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17:669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Pak DT, Yang S, Rudolph-Correia S, Kim E, Sheng M. Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron. 2001;31:289–303. doi: 10.1016/s0896-6273(01)00355-5. [DOI] [PubMed] [Google Scholar]
- Richter M, Murai KK, Bourgin C, Pak DT, Pasquale EB. The EphA4 receptor regulates neuronal morphology through SPAR-mediated inactivation of Rap GTPases. J Neurosci. 2007;27:14205–14215. doi: 10.1523/JNEUROSCI.2746-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row BW, Kheirandish L, Neville JJ, Gozal D. Impaired spatial learning and hyperactivity in developing rats exposed to intermittent hypoxia. Pediatr Res. 2002;52:449–453. doi: 10.1203/00006450-200209000-00024. [DOI] [PubMed] [Google Scholar]
- Roy BC, Kohu K, Matsuura K, Yanai H, Akiyama T. SPAL, a Rap-specific GTPase activating protein, is present in the NMDA receptor-PSD-95 complex in the hippocampus. Genes Cells. 2002;7:607–617. doi: 10.1046/j.1365-2443.2002.00546.x. [DOI] [PubMed] [Google Scholar]
- Seeburg DP, Feliu-Mojer M, Gaiottino J, Pak DT, Sheng M. Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008;58:571–583. doi: 10.1016/j.neuron.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–780. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci U S A. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LX, Sun CK, Zhang YM, Fan M, Xu J, Ma H, Zhang J. Involvement of the Snk-SPAR pathway in glutamate-induced excitotoxicity in cultured hippocampal neurons. Brain Res. 2007;1168:38–45. doi: 10.1016/j.brainres.2007.06.082. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Chen XQ, Du JZ, Chen QM, Zhu CY. Neonatal exposure to intermittent hypoxia enhances mice performance in water maze and 8-arm radial maze tasks. J Neurobiol. 2005;65:72–84. doi: 10.1002/neu.20174. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Zhu JJ. Mechanisms of synaptic plasticity: from membrane to intracellular AMPAR trafficking. Mol Interv. 2003;3:15–18. doi: 10.1124/mi.3.1.15. [DOI] [PubMed] [Google Scholar]