Abstract
The aim of this study was to investigate the effects of (-)-epigallocatechin-3-gallate (EGCG) on a newly developed high-fat/Western-style diet-induced obesity and symptoms of metabolic syndrome. Male C57BL/6J mice were fed a high fat/Western-style (HFW; 60% energy as fat and lower levels of calcium, vitamin D3, folic acid, choline bitartrate, and fiber) or HFW with EGCG (HFWE; HFW with 0.32% EGCG) diet for 17 wk. As a comparison, two other groups of mice fed a low-fat (LF; 10% energy as fat) and high-fat (HF; 60% energy as fat) were also included. HFW group developed more body weight gain and severe symptoms of metabolic syndrome than the HF group. EGCG treatment significantly reduced body weight gain associated with increased fecal lipids, and decreased blood glucose and alanine aminotransferase (ALT) levels compared to the HFW group. Fatty liver incidence, liver damage and liver triglyceride levels were also decreased by EGCG treatment. Moreover, EGCG treatment attenuated insulin resistance and levels of plasma cholesterol, monocyte chemoattractant protein-1 (MCP-1), C-reactive protein (CRP), interlukin-6 (IL-6), and granulocyte colony-stimulating factor (G-CSF). Our results demonstrate that the HFW diet produces more severe symptoms of metabolic syndrome than the HF diet and EGCG treatment can alleviate these symptoms and body fat accumulation. The beneficial effects of EGCG are associated with decreased lipid absorption and reduced levels of inflammatory cytokines.
Keywords: EGCG, high-fat/Western-style diet, obesity, metabolic syndrome, hepatic steatosis
INTRODUCTION
Obesity, a metabolic disturbance resulting from an imbalance between caloric intake and expenditure, is increasing at an alarming rate worldwide. Obesity plays an important role in promoting the development of metabolic diseases, which includes hyperinsulinemia, hypertension, hyperlipidemia, type 2 diabetes mellitus, and an increased risk of atherosclerotic cardiovascular disease (1, 2). Metabolic syndrome is a cluster of disorders that occur together to increase the risk for type 2 diabetes, coronary artery disease, and stroke. Risk factors for metabolic syndrome include high blood pressure, obesity, hyperlipidemia, and insulin resistance, and is often characterized by chronic inflammation and hepatic steatosis (3).
A defined Western-style diet has been designed to qualitatively and quantitatively mimic dietary risk factors of Western populations in studies on colon carcinogenesis (4). These risk factors include (i) high dietary fat (40% of total calories or 20% of weight of diet); (ii) inadequate dietary calcium (0.5 mg/g diet, equivalent to ~220 mg/day in a human 2000 kcal diet); (iii) inadequate dietary vitamin D3 (0.11 IU/g diet, equivalent to ~50 IU/day in a human 2000 kcal diet) and (iv) inadequate dietary folic acid, L-cysteine, and choline bitartrate (approximately the lower one-quarter of the average human diets in the USA) (5). Feeding the Western-style diet to C57BL/6 mice for 18 to 24 months, resulted in the induction of colonic tumors, induced oxidative stress, and altered expression profiles of lipid metabolism and tricarboxylic acid cycle genes in the flat colonic mucosa (5-7).
Green tea, made from the leaves of Camellia sinensis (fam. Theaceae), is the second most popular beverage worldwide next to water. Green tea and its major polyphenolic components, catechins, have been shown to possess many potential health effects, including antioxidant, anticarcinogenic, hypocholesterolemic, and cardioprotective activities (8-10). (-)-Epigallocatechin-3-gallate (EGCG) is the most abundant catechin found in green tea, and may account for 50–75% of the catechins. Many of the beneficial effects of green tea have been attributed to EGCG (11-14). Several studies have shown the beneficial effects of green tea and EGCG on increasing energy expenditure, fat oxidation, weight loss, fat mass, and helping weight maintenance after weight loss (15-17). TEAVIGO, a green tea extract containing ≥94% EGCG and ≤0.1% caffeine, was reported to significantly reduce body weight and body fat in mice fed a high-fat diet (18, 19). Other studies have also demonstrated the beneficial effects of green tea or TEAVIGO on metabolic syndrome in db/db mice and in a fructose-fed rat model, to improve glucose tolerance and insulin sensitivity (11, 20, 21). Furthermore, EGCG treatment by gavage for 3 wk has been shown to significantly decrease blood pressure and improve insulin sensitivity in spontaneously hypertensive rats (22).
Hepatic steatosis, also known as non-alcoholic fatty liver disease, is one of most common pathological changes in the liver (23) and together with chronic low-grade inflammation are two conditions that are associated with obesity and metabolic syndrome (3, 24). Green tea extract treatment protected against non-alcoholic fatty liver disease by limiting hepatic lipid accumulation and injury in high-fat diet-fed C57BL/6J and leptin-deficient ob/ob obese mice (25). Our previous study also showed that long-term EGCG treatment attenuated the development of obesity, symptoms associated with the metabolic syndrome, and fatty liver via decreased lipid absorption and decreased inflammatory cytokines (26).
In this study, we further investigate the effects of a physiologically applicable dose of EGCG on obesity and symptoms of metabolic syndrome induced by a modified Western-style diet. While maintaining the features of reduced levels of calcium, vitamin D, folate, choline and fiber, we increased the fat content from 40% to 60% of total calories to induce obesity. This diet, named high-fat/Western-style (HFW) diet, induces more severe metabolic syndrome than a high-fat diet. These results and effects of dietary EGCG in alleviating conditions associated with obesity and metabolic syndromes are reported herein.
MATERIALS AND METHODS
Chemicals and Diets
EGCG (94.5% pure) was provided by Dr. Y. Hara of the Mitsui Norin Co. Ltd. (Tokyo, Japan). The diets were modified from the AIN-76A diet and prepared by Research Diets Inc (New Brunswick, NJ), as described previously (5, 26). They were low-fat diet (LF; 10% energy as fat), high-fat diet (HF; 60% energy as fat), high-fat/Western-style diet (HFW; 60% energy as fat, reduced levels of calcium, vitamin D3, choline, folate, and fiber), and high-fat/Western-style plus EGCG diet (HFWE; HFW supplemented with 3.2 g EGCG/kg diet) (Table 1). The fiber in the HFW diet was decreased from 5% in AIN-76A (approximately to 25 g in a human 2000 kcal diet) to 2%. Two methyl transfer-donor components, folic acid and choline, were also decreased in the HFW diet.
Table 1.
Composition of experimental diets
| LF | HF | HFW | HFWE | |
|---|---|---|---|---|
| Macronutrient composition | ||||
| Protein, % of energy | 20.0 | 20.0 | 20.0 | 20.0 |
| Carbohydrate, % of energy | 70 | 20.0 | 20.0 | 20.0 |
| Fat, % of energy | 10.0 | 60.0 | 60.0 | 60.0 |
| Energy, MJ/kg | 15.9 | 21.8 | 23.4 | 23.4 |
| Ingredient | ||||
| Casein, g/kg | 189.6 | 258.4 | 276.9 | 276.0 |
| L-Cystine, g/kg | 2.8 | 3.9 | 4.2 | 4.1 |
| Corn starch, g/kg | 298.6 | — | — | — |
| Maltodextrin, g/kg | 33.2 | 161.5 | 173.1 | 172.5 |
| Surcose, g/kg | 331.7 | 88.9 | 95.3 | 95.0 |
| Soybean oil, g/kg | 23.7 | 32.3 | 34.6 | 34.5 |
| Lard, g/kg | 19.0 | 316.6 | 339.2 | 338.1 |
| Fiber (Cellulose), g/kg | 47.4 | 64.6 | 23.5 | 23.5 |
| Mineral mix1, g/kg | 9.5 | 12.9 | — | — |
| Mineral mix (no added calcium, phosphorus, and potassium), g/kg | — | — | 6.9 | 6.9 |
| Calcium, g/kg | 5.72 | 7.80 | 0.42 | 0.42 |
| Phosphorus, g/kg | 4.33 | 5.90 | 6.29 | 6.27 |
| Potassium, g/kg | 5.67 | 7.73 | 8.24 | 8.21 |
| Vitamin mix1, g/kg | 9.5 | 12.9 | — | — |
| Vitamin mix (no added vitamin D3 and folate), g/kg | — | — | 13.8 | 13.7 |
| Vitamin D3, IU/g | 0.95 | 1.29 | 0.14 | 0.14 |
| Folate, mg/kg | 2.00 | 2.00 | 0.28 | 0.28 |
| Choline butartrate, g/kg | 1.9 | 2.6 | 1.4 | 1.4 |
| EGCG, % | — | — | — | 0.32 |
Research diets; composition of mineral and vitamin mix as described previously by Bose et al. (26)
Animal and Dietary Treatment
Male C57BL/6 mice ages 5 to 6 wk, were purchased from Jackson Laboratories (Bar Harbor, Maine). All animal experiments were carried out under protocol 91-024 approved by the Institutional Animal Care and Use Committee at Rutgers University (Piscatway, NJ). Mice were fed LF, HF, HFW or HFWE diet (n = 32, 22, 43, and 21 per group, respectively) for 17 wk. The dose of 3.2 g EGCG/kg diet in mice is equivalent to ten cups of green tea (2 g tea leaves per cup) per day for an average person requiring 2000 kcal/d based on allometric scaling. Food intake and body weights were monitored weekly throughout the experiment.
Blood Collection and Tissue Harvesting
After 17 wk of treatment, mice were fasted for 6 hours prior to sacrifice (11 mice in LF group and 22 mice in HFW group were kept for further evaluation of colonic cancer formation). Blood was collected and plasma samples were prepared and stored at -80°C. Interscapular brown adipose tissue (BAT) and visceral white adipose tissues (mesenteric, epididymal and retroperitoneal depots) were harvested and weighed. Fatty liver incidence was identified by altered coloration and determined by histological examination. One lobe of the liver was fixed in 10% formalin, and the rest was frozen in liquid nitrogen and stored at -80°C.
Biochemical Analyses of Blood
Blood glucose levels were measured using the Ascensia Contour Blood glucose meter (Bayer Healthcare LLC, Mishawaka, IN), every 4 wk throughout the study. One day before the blood glucose measurement, cage bedding was changed in order to avoid coprophagy. Mice were then fasted for 7 to 8 h and blood samples were collected from the tail vein. For ALT measurement, blood was collected from the submandibular vein every 4 wk, starting from wk 6 of treatment, and serum ALT concentrations were determined using the ALT Discreet Pak Kit (Catachem Inc., Bridgeport, CT). Plasma insulin was measured using a Rat/Mouse Insulin ELISA kit (Millipore Corporation, Billerica, MA), and insulin resistance was calculated according to the homeostasis assessment model (HOMA-IR) as described previously (26). Plasma cholesterol was determined using a Cholesterol E kit (Wako Chemical USA Inc., Richmond, VA), plasma monocyte chemoattractant protein 1 (MCP-1) was determined using a Mouse JE/MCP-1 Immunoassay kit (R&D systems, Minneapolis, MN), plasma C-reactive protein (CRP) was determined using a Mouse CRP ELISA kit (Immunological Consultants Lab Inc., Newberg, OR). Plasma cytokine profile was measured using RayBio Mouse Cytokine Antibody Array C Series 1000 (RayBiotech, Norcross, GA) according to the manufacturer’s instructions. Plasma interleukin-6 (IL-6) was measured using a Mouse IL-6 ELISA kit (R & D Systems, Minneapolis, MN). Plasma granulocyte colony-stimulating factor (G-CSF) was quantified by Mouse G-CSF ELISA kit (RayBiotech, Norcross, GA).
Fecal Lipid Measurement
For total lipid levels in feces, feces were collected over a 24 hour period from cage bedding (at wk 13 and 16 of study). Fecal samples (0.5-1.0g) were then added to 1.5-2 mL deionized water and placed at 4°C overnight. The following day, the fecal mixture was mixed with methanol:chloroform (2:1, v:v) to extract the lipids. The upper lipophilic layer was transferred to a microtube and dried under vacuum. The analysis of total lipid levels was performed by gravimetric measurement.
Liver Triglycerides and Histology
To analyze hepatic triglyceride levels, liver tissues (50-100 mg) were homogenized in 1-2 mL ispropanol using a Polytron disrupter. Following centrifugation at 2000 × g for 10 min, the supernatant was collected and triglyceride levels were measured using a L-Type Triglyceride M Kit (Wako Diagnostics) and values were normalized to liver weight. For histological examination of the liver, paraffin-embedded liver tissue was sectioned at 6 μm and stained with hematoxylin and eosin. A diagnosis of fatty liver was examined based on the presence of macro- and microvesicular fat in >5% of the hepatocytes in a slide sample.
Statistical Analyses
Statistical analyses were conducted using GraphPad Prism software. One-way ANOVA with Tukey’s post hoc test was used for statistical analysis. A chi-square test was performed for statistical analysis of fatty liver incidence. All data are presented as mean ± SEM. Significance was assigned at P < 0.05.
RESULTS
Mouse Body Weight, Food Intake and Body Fat
There was no significant difference in food intake among the four groups of mice throughout the course of this study (data not shown). After starting the test diets for 1 wk, the HFW mice had a significantly higher body weight than the LF mice (HFW: 21.6 ± 0.3 g; LF: 20.2 ± 0.3 g; P < 0.05), whereas HF mice were significantly heavier than LF mice after 3 wk of HF diet treatment (Figure 1A). HFW mice weighed significantly higher than the HF mice after 11 wk of treatment, and these trends remained throughout the course of the treatment period. After 9 wk of EGCG treatment, a significantly lower body weight gain in HFWE mice was observed (P < 0.05; Figure 1A), and the HFWE mice weighed 9% less than the HFW mice at the end of the 17-wk experimental period.
Figure 1.
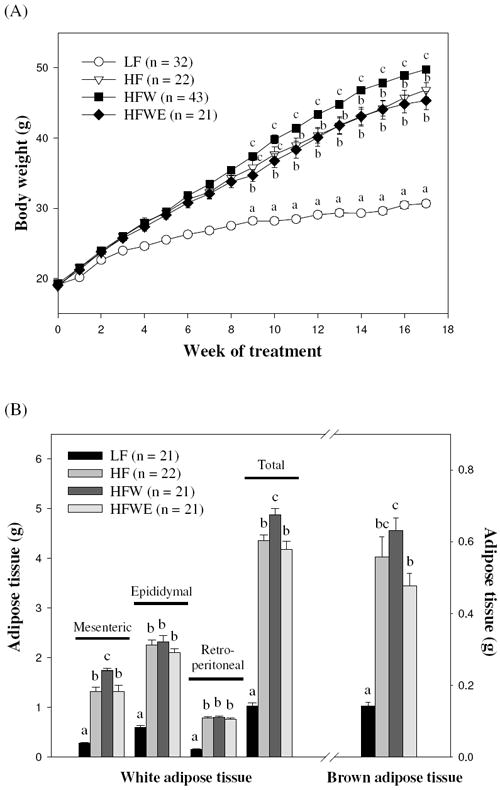
Effects of dietary treatments on body weight (A), and adipose tissue weight (B) in C57BL/6J mice. Values are expressed as mean ± SEM. Different letters indicate statistical difference, P < 0.05.
Consistent with the body weight results, adipose mass in HF and HFW mice was significantly higher compared to LF mice both in BAT and total visceral adipose tissue (mesenteric, epididymal, and retroperitoneal adipose tissue) (P < 0.05; Figure 1B). As compared with HFW mice, HFWE mice had a lower BAT weight (0.48 ± 0.04 g vs. 0.63 ± 0.03 g) and total visceral adipose weight (4.18 ± 0.16 g vs. 4.87 ± 0.14 g). EGCG treatment significantly reduced the weight of the BAT depot (24% decrease; P < 0.05) and the mesenteric adipose depot (24% decrease; P < 0.05).
Blood Glucose, Plasma Insulin, and HOMA-IR Index
The fasting-state blood glucose levels of the mice are shown in Figure 2. HF and HFW mice had significantly higher blood glucose levels compared with LF mice by wk 8 of treatment, and levels of the HF group were higher than those of the HFW group (P < 0.05). At the end of the experiment, however, the blood glucose levels of the HF and HFW groups were both 40% higher than those of the LF group. EGCG treatment significantly decreased the blood glucose levels elevated by the HFW diet (P < 0.05; Figure 2), and this trend remained to the end of the treatment period. The blood glucose levels of HFWE mice exhibited a 12.3% decrease compared with HFW mice at the end of the study (P < 0.05).
Figure 2.
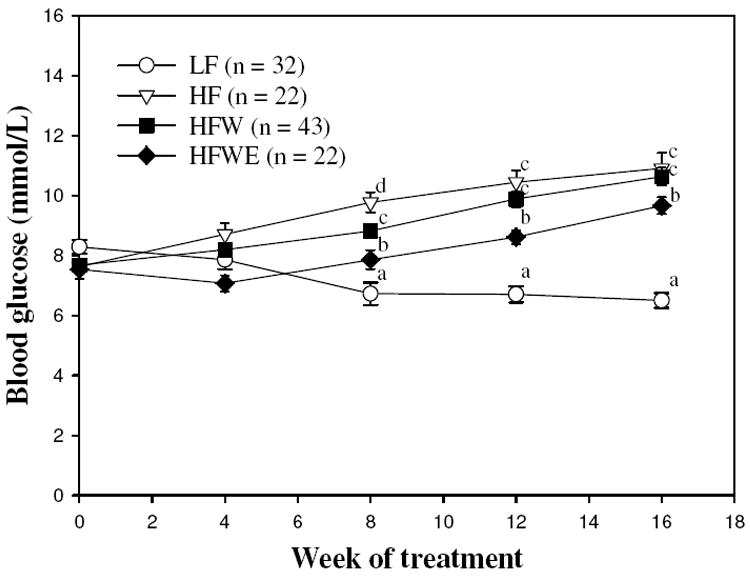
Effects of dietary treatments on blood glucose levels in C57BL/6J mice during the experimental period. Values are expressed as mean ± SEM. Different letters indicate statistical difference, P < 0.05.
The effect of EGCG on the plasma insulin concentrations of mice fed the HFW diet was also determined at the end of the experiment. HFW and HF diet treatment increased plasma insulin concentrations of mice compared with those of the LF group (P < 0.05; Table 2), and HFWE mice had significantly reduced plasma insulin concentrations compared with HFW mice (34.1% decrease; Table 2). HF and HFW mice had significantly higher HOMA-IR index, calculated using the final blood glucose and insulin concentrations, than LF mice (P < 0.05; Table 2), and EGCG treatment decreased HOMA-IR index by 42.6% in HFW mice (P < 0.05).
Table 2.
Effect of dietary treatment on plasma biomarkers and liver pathology1
| Liver pathology | LF | HF | HFW | HFWE |
|---|---|---|---|---|
| n | 10-21 | 10-22 | 10-21 | 10-21 |
| Plasma biomarkers | ||||
| Insulin3, pmol/L | 93.5 ± 14.1a | 647.5 ± 60.9bc | 761.0 ± 53.2c | 501.6 ± 51.4b |
| HOMA-IR3 | 4.5 ± 0.7a | 43.6 ± 4.6c | 50.7 ± 4.9c | 29.1 ± 3.0b |
| Cholesterol3, mmol/L | 2.6 ± 0.2a | 3.8 ± 0.1c | 5.4 ± 0.3d | 3.2 ± 0.1b |
| MCP-12, pmol/L | 9.2 ± 0.9a | 11.4 ± 1.3ab | 13.3 ± 0.8b | 9.0 ± 0.7a |
| CRP2, ng/mL | 23.1 ± 0.6b | 23.1 ± 0.7b | 22.5 ± 0.5b | 20.0 ± 0.5a |
| Liver pathology | ||||
| Liver wt/BW3, g/g | 0.038 ± 0.001b | 0.038 ± 0.002b | 0.040 ± 0.002b | 0.029 ± 0.001a |
| Fatty liver incidence (%)3 | 0/21 (0)a | 19/22 (86)c | 21/21 (100)c | 11/21 (52)b |
| Liver triglyceride, μmol/g tissue | 18.3 ± 1.3a | 127.7 ± 9.4c | 178.1 ± 7.2d | 84.8 ± 12.6b |
| Plasma ALT3, U/L | 81.4 ± 12.5a | 200.3 ± 22.9b | 289.4 ± 24.6c | 96.0 ± 13.2a |
C57BL/6J mice were fed the experimental diets for 17 wk. Data are presented as mean ± SEM. Means in a row with different letters in superscripts indicate statistical differences, P < 0.05.
n = 10 per grop.
n = 21-22 per group.
Hepatic Pathology
ALT levels were measured in blood samples collected at wk 6, 10, 13, and 16 and at the end of the experiment (wk 17). HFW mice had significantly elevated serum ALT levels compared with LF and HF mice by wk 10 and 13 (P < 0.05; Figure 3E). This trend remained throughout the course of the treatment period. At the end of study, the plasma ALT concentrations of HFW group were 3.6-fold and 1.4-fold higher than the LF and HF groups, respectively (P < 0.05; Table 2). EGCG treatment significantly reduced the serum ALT levels by wk 10 of treatment (P < 0.05; Figure 3E), and there was no significant difference in plasma ALT levels between HFWE and LF groups at the end of study (P > 0.05; Table 2).
Figure 3.
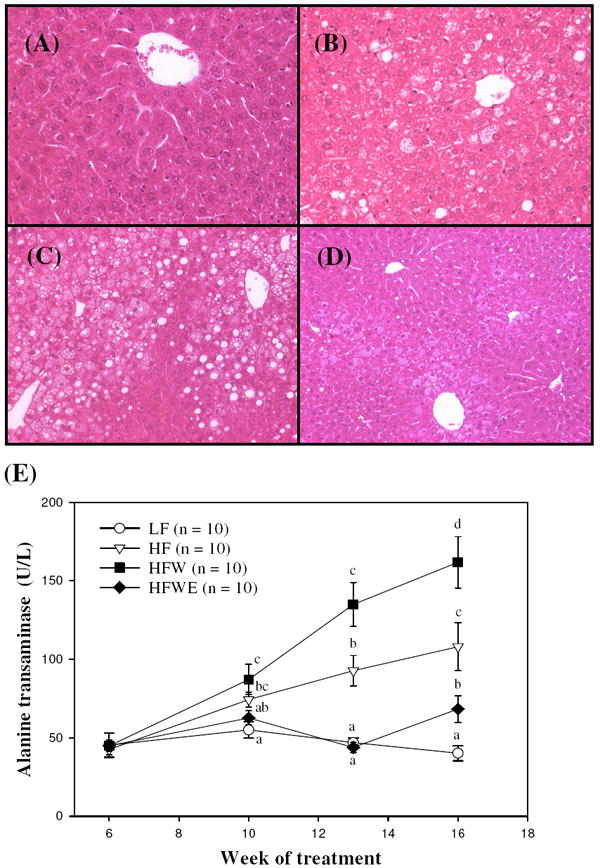
Effects of dietary treatments on liver pathology in C57BL/6J mice. Histological examination of liver samples from LF (A), HF (B), HFW (C), and HFWE (D) mice, shown at 20X (A, B) and 10X (C, D) magnification. (E) Serum alanine aminotransferase (ALT) levels in C57BL/6J mice during the experimental period. Values are expressed as mean ± SEM. Different letters indicate statistical difference, P < 0.05.
There were no significant differences in liver:body weight ratios among the LF, HF, and HFW groups, but the HFWE group had a significantly lower liver:body weight ratio compared to the HFW group (P < 0.05; Table 2). Furthermore, HFW mice had a significantly higher liver triglyceride concentration compared with HF mice, and even higher than LF mice (Table 2). EGCG treatment significantly reduced the liver triglyceride concentrations (52% decrease; Table 2).
By light microscopy, there was no evidence of fatty liver observed in LF mice (Figure 3A). In contrast, all mice fed with the HFW diet demonstrated fatty liver and the incidence of fatty liver appeared to be higher than HF mice (100% vs. 86%; Table 2), although there was no statistical difference between two groups. EGCG treatment significantly reduced the incidence of fatty liver (P < 0.05; Table 2). Histological analysis of liver sections from the four groups of mice was carried out. Livers from mice administered the HF diet demonstrated moderate centrilobular hepatic steatosis with sparing of the periportal zone (Figure 3B). The cytoplasm of the centrilobular hepatocytes showed both microvesicular vacuolation (the presence of numerous small lipid droplets) as well as large, clear locular lipid droplets. Livers from mice given HFW diet showed moderate to severe fatty degeneration, which was most extensive in the centrilobular zone, but often extending to involve the edges of the periportal zone (Figure 3C). This fatty change included both microvesicular and locular fat, with the latter occasionally displacing the nucleus from its central position. Hepatocytes immediately surrounding the portal triad did not contain fat, but revealed more extensive hydropic change than those from the HF group. Livers from the HFWE group demonstrated considerable histological variability. Livers of several mice from this group showed a markedly attenuated degree of fatty change when compared to the HF or HFW groups, with the fatty change primarily consisting of the accumulation of widely scattered large lipid droplets in the centrilobular zone, with little evidence of microvesicular involvement. Livers of other mice from the HFWE group had widespread, focal, pericentral fatty change, including microvesicular steatosis. In all livers examined from the HFWE group, the extent of fatty change was markedly less than that seen in the HF or HFW groups (Figure 3D).
Plasma Cholesterol and Inflammatory Markers
The plasma cholesterol levels of mice were determined at the end of the experiment. The plasma cholesterol levels of HF mice were higher than those of LF mice, and the HFW mice had higher values than HF mice (P < 0.05; Table 2). EGCG treatment significantly decreased the elevation in plasma cholesterol levels (P < 0.05). The levels of inflammatory markers, MCP-1, CRP, and IL-6, were also measured. The results showed that the HFW group had higher levels of MPC-1, but not of CRP, as compared to the LF group (Table 2). HFWE mice had significantly lower plasma MCP-1 and CRP concentrations than HFW mice (P < 0.05). Cytokine antibody arrays were used to screen for differences in cytokine levels of different groups. As shown in Figure 3A-D, signals corresponding to IL-6 and G-CSF were visibly increased on the array for the HFW group as compared to those for the LF and HF groups, and the signals were significantly attenuated by EGCG treatment (P < 0.05). The increased IL-6 and G-CSF levels and their attenuation by EGCG were confirmed by ELISA assay (Figure 4E and 4F). Leptin was also increased in HF, HFW, and HFWE group as compared to those in LF group. On the other hand, the treatment did not affect the levels of other 37 cytokines on the array.
Figure 4.
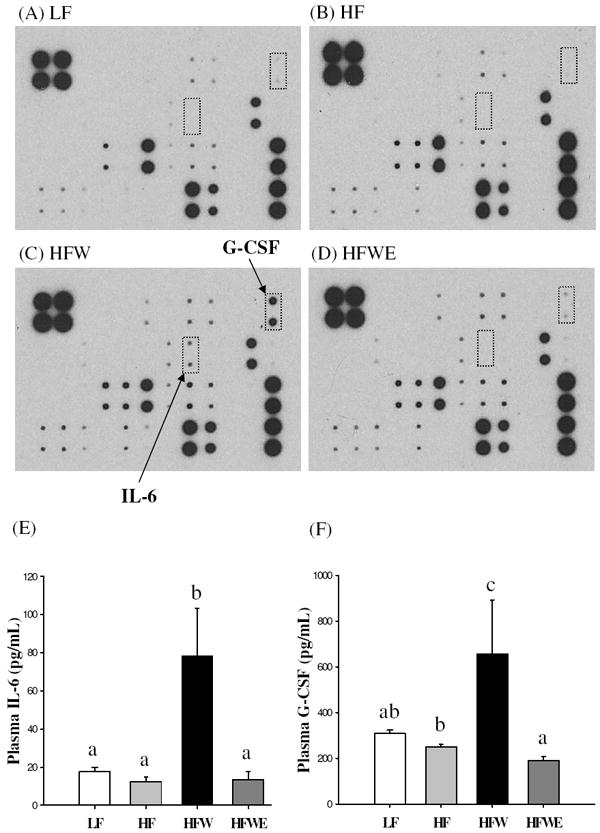
Effects of dietary treatments on plasma cytokines in C57BL/6J mice at the end of the experimental period. Cytokine levels were determined in 3 pooled samples (each was pooled from 7 mice) by protein array (A-D) or by ELISA (E and F), in which values are expressed as mean ± SEM. Different letters indicate statistical difference, P < 0.05.
Fecal Lipids
Fecal samples in each group were collected at wk 13 and 16 of the study, and fecal lipid levels were measured. Mice fed the HF diet had significantly higher fecal lipid concentrations compared with LF mice, and the levels of the HFW group were higher than those of the HF group (Figure 5). The lipid levels of fecal samples collected from the HFWE group were significantly higher than those from the LF, HF, and HFW groups (P < 0.05), suggesting that EGCG decreased the absorption of dietary diet.
Figure 5.
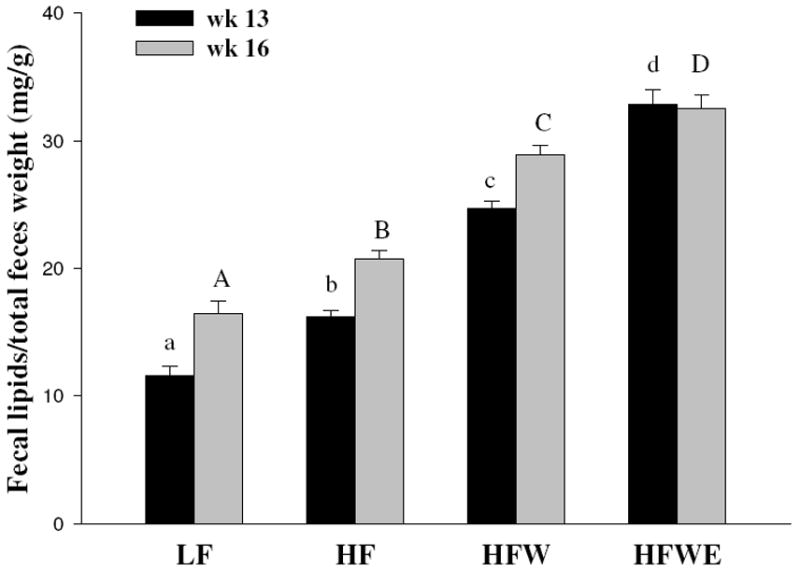
Effects of dietary treatments on lipid levels of feces at wk 13 and wk 16 in C57BL/6J mice. Fecal samples (n = 3) were randomly collected over a 24 hour period from the bedding of each cage. Values are expressed as mean ± SEM. Different letters indicate statistical difference, P < 0.05.
DISCUSSION
The objective of the present study was to examine the effect of dietary EGCG (3.2 g/kg diet) treatment on obesity, symptoms of metabolic syndrome, and fatty liver. Our results demonstrate that dietary supplementation with EGCG significantly decreased body weight gain, percent body fat, visceral fat weight, insulin resistance, blood glucose and cholesterol levels, hepatic steatosis, and inflammatory cytokines even in mice fed the HFW diet, a high-fat diet compounded with deficiencies in calcium and several vitamins. To the best of our knowledge, this study is the first to show that EGCG can prevent body weight gain, metabolic syndrome and hepatic steatosis in a “Western-style diet” model.
The new Western-style diet developed by Newmark et al. (5, 6) mimics intake levels of nutrients that are major dietary risk factors for human colon cancer and other diseases in Western countries (higher fat combined with lower calcium, vitamin D3, fiber, and one-carbon donors). In the present study, we increased the dietary fat content from 40% to 60% of total calories to promote the development of obesity and metabolic syndrome and to evaluate a possible role for increased fat intake in colonic tumor formation. We observed that HFW mice had significantly higher body weight gain, plasma cholesterol, plasma ALT, liver triglyceride, and fatty liver incidence and severity than HF mice. The HFW diet was designed to have of inadequate calcium, vitamin D3, and methyl-donor nutrients. Deficiency of methyl-donor nutrients such as choline may promote the development of hepatic steatosis (27, 28). Choline is needed for the biosynthesis of phosphatidylcholine, which is essential for the structural integrity and signaling functions of cell membranes, as well as for lipid transport and metabolism (29). Lack of phosphatidylcholine limits the export of excess triglyceride from the liver (30, 31). In addition, deficiency of choline can induce hepatocytes apoptosis and result in ALT to leak out from the liver to the blood (27, 32, 33). Our results are consistent with these reports and demonstrate that these deficiencies further enhance the high-fat induced obesity and metabolic syndrome, especially hepatic steatosis. The contribution of the low levels of calcium and vitamin D to metabolic syndrome is not known. The HFW diet may be useful in future studies on hepatic steatosis. After 17 wk of diets treatment, some mice in the LF (n = 11) and HFW (n = 22) groups were kept for a total of 56 wk for evaluation of colonic tumor formation, however, there were no adenoma or adenocarcinoma observed.
The present results provide direct evidence that dietary EGCG treatment significantly reduced body weight gain in mice fed with HFW diet. We found that the visceral fat weight in HFW mice was significantly higher than those in HF mice, and EGCG treatment significantly decreased BAT weight and visceral adipose tissue weight. Previous studies showed that treatment with tea catechins significantly decreased BAT weight in high-fat-fed mice (34). It has been demonstrated that visceral adipose tissue is more metabolically active than subcutaneous adipose, and that visceral fat reduction induced greater beneficial effects on parameters of metabolic syndrome than subcutaneous fat reduction (35, 36). Our study confirms the effects of EGCG on BAT and visceral adipose depots, and this also provides a basis for the beneficial effect of EGCG against metabolic syndrome. The prevention of fat accumulation by EGCG may be due to reduction of lipid absorption (Figure 5) or enhancement of fatty oxidation (25, 26).
Metabolic syndrome is a cluster of disorders of pathological conditions related to obesity, insulin resistance, and dyslipidemia. Our results show that EGCG treatment significantly reduced blood glucose, insulin, and insulin resistance (HOMA-IR) in mice fed HFW diet. A previous study reported by Lin et al. (37) showed that EGCG alleviated insulin resistance in human HepG2 hepatoma cells under high glucose conditions. There are also reports suggesting that EGCG has several direct effects on the improvement of glucose homeostasis. Green tea polyphenols treatment has been shown to improve glucose transport and lipid metabolism in insulin-resistant rats (38). Another study revealed that the expression of hepatic gluconeogenic enzymes, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase, were significantly reduced in mice by treatment with EGCG for 7 days (39).
Our histological and biochemical analyses show that supplementation with EGCG reduced the development of hepatic steatosis and injury induced by the HFW diet. The decrease of microvesicular fat determined by histopathological analysis and the reduction of hepatic triglyceride levels demonstrate that supplementation with EGCG reduced the fat accumulation in the liver. Also, serum ALT levels were significantly reduced in HFWE mice throughout the experiment, reflecting decreased hepatic injury.
The decrease in hepatic steatosis as a result of EGCG supplementation may help alleviate insulin resistance. Hepatic insulin resistance is associated with hepatic steatosis, which is a common feature of type 2 diabetes in humans. A possible molecular mechanism for hepatic steatosis induced hepatic insulin resistance is that accumulated of hepatocellular diacylglycerol activates protein kinase C-epsilon which catalyzes serine/threonine phosphorylation, resulting in reduced insulin-stimulated tyrosine phosphorylation, of the insulin receptor substrates-2 (40). Studies in rats have also demonstrated that hepatic steatosis decreased hepatic insulin sensitivity by stimulating gluconeogenesis and activating protein kinase C and Jun N-terminal kinase 1, which in turn interferes with the phosphorylation of insulin receptor substrate and impairs the ability of insulin to activate glycogen synthase (41). EGCG has been reported to both inhibit protein kinase C and increase insulin receptor substrate mediated downstream signaling in cells (42).
Similar to our previous results (26), we found that dietary EGCG intake significantly increased the fecal lipid content. There is a report that green tea decreases intestinal lipid absorption (43-46). Tea catechins were shown to significantly reduce the lymphatic absorption of triglyceride, cholesterol, and α-tocopherol in ovariectomized rats (45-47), and the mechanism may involve the inhibition of pancreatic lipolytic enzymes, such as lipase and phospholipase A2. Another possibility is the physical binding of catechins to lipids, including cholesterol, decreasing their absorption in the intestine. Indeed, we found that EGCG treatment also decreased plasma cholesterol levels. The decreased absorption of lipids by EGCG may be a major factor for its effect in reducing body weight gain and hepatic lipid accumulation.
EGCG treatment decreased plasma MCP-1 in mice fed the HFW diet. Moreover, we found that IL-6 and G-CSF, which are proinflammatory cytokines secreted by adipose tissue (48, 49), were both increased in the plasma of HFW mice. It has been demonstrated that many proinflammatory cytokines are concomitantly up-regulated in adipose tissue of obese subjects (50). MCP-1 has been shown to be involved in the recruitment of macrophages into the adipose tissue and results in chronic inflammation (51). It is widely accepted that obesity-induced inflammation plays an important role in the development of metabolic syndrome. MCP-1 levels were induced in mice fed HF and HFW diets and the elevated MCP-1 may be a result of the increased body fat. Surk et al. (52) recently reported that there were significant associations between the IL-6 and G-CSF levels and insulin-stimulated Akt/PKB phosphorylation. These proinflammatory factors reduced insulin-stimulated Akt/PKB phosphorylation in human skeletal muscle cells and may contribute to the pathogenesis of obesity-associated insulin resistance. The reduction of the levels of MCP-1, IL-6, and G-CSF is likely to contribute to the decrease of insulin resistance by EGCG treatment. Our results also showed that EGCG attenuated CRP levels in mice fed the HFW diet. CRP is an inflammatory factor and associated with long-term cardiovascular morbidity (53). A previous study by Aronson et al. (54) indicated that obesity is the major factor associated with elevated CRP levels in individuals with metabolic syndrome. The decrease of CRP levels by EGCG in HFW mice suggests that EGCG may possess potential beneficial effects in the prevention of cardiovascular diseases.
In conclusion, dietary supplementation with EGCG significantly reduces the development of obesity, hyperglycemia, insulin resistance, hypercholesterolemia and hepatic steatosis in mice fed the HFW diet, which mimics many dietary risk factors in the Western society. These results suggest that the beneficial effects of EGCG may be mediated by decreased lipid absorption and decreased levels of inflammatory cytokines such as MCP-1, IL-6, and G-CSF as well as CRP. The possible beneficial effects of EGCG in diabetic or prediabetic patients need to be further investigated.
Acknowledgments
This work was supported by grants from the U.S. National Institutes of Health (RO1 CA120915, CA120915-S2, RO1 CA122474 and RO1 CA133021) and by shared Facilities funded by National Cancer Institute Cancer Center Support Grant (CA72720) and National Institute of Environmental Health Center Grant (ES05022).
ABBREVIATIONS USED
- ALT
alanine aminotransferase
- BW
body weight
- CRP
C-reactive protein
- EGCG
(-)-epigallocatechin-3-gallate
- G-CSF
granulocyte colony-stimulating factor
- HF
high-fat
- HFW
high-fat/Western-style
- HFWE
high-fat/Western-style plus EGCG
- IL-6
interlukin-6
- LF
low-fat
- MCP-1
monocyte chemoattractant protein-1
LITERATURE CITED
- 1.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 2.Ross R, Despres JP. Abdominal obesity, insulin resistance, and the metabolic syndrome: contribution of physical activity/exercise. Obesity (Silver Spring) 2009;17(Suppl 3):S1–2. doi: 10.1038/oby.2009.381. [DOI] [PubMed] [Google Scholar]
- 3.Daskalopoulou SS, Mikhailidis DP, Elisaf M. Prevention and treatment of the metabolic syndrome. Angiology. 2004;55:589–612. doi: 10.1177/00033197040550i601. [DOI] [PubMed] [Google Scholar]
- 4.Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57BL/6 mice. Carcinogenesis. 2001;22:1871–1875. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- 5.Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30:88–92. doi: 10.1093/carcin/bgn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang K, Kurihara N, Fan K, Newmark H, Rigas B, Bancroft L, Corner G, Livote E, Lesser M, Edelmann W, Velcich A, Lipkin M, Augenlicht L. Dietary induction of colonic tumors in a mouse model of sporadic colon cancer. Cancer Res. 2008;68:7803–7810. doi: 10.1158/0008-5472.CAN-08-1209. [DOI] [PubMed] [Google Scholar]
- 7.Erdelyi I, Levenkova N, Lin EY, Pinto JT, Lipkin M, Quimby FW, Holt PR. Western-style diets induce oxidative stress and dysregulate immune responses in the colon in a mouse model of sporadic colon cancer. J Nutr. 2009;139:2072–2078. doi: 10.3945/jn.108.104125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sae-Tan S, Grove KA, Lambert JD. Laboratory studies on weight control and prevention of metabolic syndrome by green tea. Pharmacol Res. 2010 doi: 10.1016/j.phrs.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hininger-Favier I, Benaraba R, Coves S, Anderson RA, Roussel AM. Green tea extract decreases oxidative stress and improves insulin sensitivity in an animal model of insulin resistance, the fructose-fed rat. J Am Coll Nutr. 2009;28:355–361. doi: 10.1080/07315724.2009.10718097. [DOI] [PubMed] [Google Scholar]
- 11.Wolfram S, Raederstorff D, Preller M, Wang Y, Teixeira SR, Riegger C, Weber P. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J Nutr. 2006;136:2512–2518. doi: 10.1093/jn/136.10.2512. [DOI] [PubMed] [Google Scholar]
- 12.Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003;133:3262S–3267S. doi: 10.1093/jn/133.10.3262S. [DOI] [PubMed] [Google Scholar]
- 13.Wolfram S, Wang Y, Thielecke F. Anti-obesity effects of green tea: from bedside to bench. Mol Nutr Food Res. 2006;50:176–187. doi: 10.1002/mnfr.200500102. [DOI] [PubMed] [Google Scholar]
- 14.Thielecke F, Boschmann M. The potential role of green tea catechins in the prevention of the metabolic syndrome - a review. Phytochemistry. 2009;70:11–24. doi: 10.1016/j.phytochem.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Westerterp-Plantenga MS, Lejeune MP, Kovacs EM. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res. 2005;13:1195–1204. doi: 10.1038/oby.2005.142. [DOI] [PubMed] [Google Scholar]
- 16.Nagao T, Komine Y, Soga S, Meguro S, Hase T, Tanaka Y, Tokimitsu I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am J Clin Nutr. 2005;81:122–129. doi: 10.1093/ajcn/81.1.122. [DOI] [PubMed] [Google Scholar]
- 17.Kao YH, Hiipakka RA, Liao S. Modulation of obesity by a green tea catechin. Am J Clin Nutr. 2000;72:1232–1234. doi: 10.1093/ajcn/72.5.1232. [DOI] [PubMed] [Google Scholar]
- 18.Klaus S, Pultz S, Thone-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int J Obes (Lond) 2005;29:615–623. doi: 10.1038/sj.ijo.0802926. [DOI] [PubMed] [Google Scholar]
- 19.Wolfram S, Raederstorff D, Wang Y, Teixeira SR, Elste V, Weber P. TEAVIGO (epigallocatechin gallate) supplementation prevents obesity in rodents by reducing adipose tissue mass. Ann Nutr Metab. 2005;49:54–63. doi: 10.1159/000084178. [DOI] [PubMed] [Google Scholar]
- 20.Wu LY, Juan CC, Ho LT, Hsu YP, Hwang LS. Effect of green tea supplementation on insulin sensitivity in Sprague-Dawley rats. J Agric Food Chem. 2004;52:643–648. doi: 10.1021/jf030365d. [DOI] [PubMed] [Google Scholar]
- 21.Tsuneki H, Ishizuka M, Terasawa M, Wu JB, Sasaoka T, Kimura I. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol. 2004;4:18. doi: 10.1186/1471-2210-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, Kim JA, Quon MJ, Montagnani M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab. 2007;292:E1378–1387. doi: 10.1152/ajpendo.00698.2006. [DOI] [PubMed] [Google Scholar]
- 23.Sass DA, Chang P, Chopra KB. Nonalcoholic fatty liver disease: a clinical review. Dig Dis Sci. 2005;50:171–180. doi: 10.1007/s10620-005-1267-z. [DOI] [PubMed] [Google Scholar]
- 24.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 25.Bruno RS, Dugan CE, Smyth JA, DiNatale DA, Koo SI. Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. J Nutr. 2008;138:323–331. doi: 10.1093/jn/138.2.323. [DOI] [PubMed] [Google Scholar]
- 26.Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. 2008;138:1677–1683. doi: 10.1093/jn/138.9.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeisel SH, Da Costa KA, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–2098. [PubMed] [Google Scholar]
- 28.Buchman AL, Dubin MD, Moukarzel AA, Jenden DJ, Roch M, Rice KM, Gornbein J, Ament ME. Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 1995;22:1399–1403. [PubMed] [Google Scholar]
- 29.Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994;14:269–296. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- 30.Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem. 1988;263:2998–3004. [PubMed] [Google Scholar]
- 31.Yao ZM, Vance DE. Head group specificity in the requirement of phosphatidylcholine biosynthesis for very low density lipoprotein secretion from cultured hepatocytes. J Biol Chem. 1989;264:11373–11380. [PubMed] [Google Scholar]
- 32.Albright CD, da Costa KA, Craciunescu CN, Klem E, Mar MH, Zeisel SH. Regulation of choline deficiency apoptosis by epidermal growth factor in CWSV-1 rat hepatocytes. Cell Physiol Biochem. 2005;15:59–68. doi: 10.1159/000083653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeisel SH, da Costa KA, Albright CD, Shin OH. Choline and hepatocarcinogenesis in the rat. Adv Exp Med Biol. 1995;375:65–74. doi: 10.1007/978-1-4899-0949-7_6. [DOI] [PubMed] [Google Scholar]
- 34.Nomura S, Ichinose T, Jinde M, Kawashima Y, Tachiyashiki K, Imaizumi K. Tea catechins enhance the mRNA expression of uncoupling protein 1 in rat brown adipose tissue. J Nutr Biochem. 2008;19:840–847. doi: 10.1016/j.jnutbio.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Park HS, Lee K. Greater beneficial effects of visceral fat reduction compared with subcutaneous fat reduction on parameters of the metabolic syndrome: a study of weight reduction programmes in subjects with visceral and subcutaneous obesity. Diabet Med. 2005;22:266–272. doi: 10.1111/j.1464-5491.2004.01395.x. [DOI] [PubMed] [Google Scholar]
- 36.Zamboni M, Armellini F, Turcato E, Todesco T, Bissoli L, Bergamo-Andreis IA, Bosello O. Effect of weight loss on regional body fat distribution in premenopausal women. Am J Clin Nutr. 1993;58:29–34. doi: 10.1093/ajcn/58.1.29. [DOI] [PubMed] [Google Scholar]
- 37.Lin CL, Lin JK. Epigallocatechin gallate (EGCG) attenuates high glucose-induced insulin signaling blockade in human hepG2 hepatoma cells. Mol Nutr Food Res. 2008;52:930–939. doi: 10.1002/mnfr.200700437. [DOI] [PubMed] [Google Scholar]
- 38.Qin B, Polansky MM, Harry D, Anderson RA. Green tea polyphenols improve cardiac muscle mRNA and protein levels of signal pathways related to insulin and lipid metabolism and inflammation in insulin-resistant rats. Mol Nutr Food Res. 2010;54(Suppl 1):S14–23. doi: 10.1002/mnfr.200900306. [DOI] [PubMed] [Google Scholar]
- 39.Koyama Y, Abe K, Sano Y, Ishizaki Y, Njelekela M, Shoji Y, Hara Y, Isemura M. Effects of green tea on gene expression of hepatic gluconeogenic enzymes in vivo. Planta Med. 2004;70:1100–1102. doi: 10.1055/s-2004-832659. [DOI] [PubMed] [Google Scholar]
- 40.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55(Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 42.Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem. 2002;277:34933–34940. doi: 10.1074/jbc.M204672200. [DOI] [PubMed] [Google Scholar]
- 43.Koo MW, Cho CH. Pharmacological effects of green tea on the gastrointestinal system. Eur J Pharmacol. 2004;500:177–185. doi: 10.1016/j.ejphar.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 44.Koo SI, Noh SK. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J Nutr Biochem. 2007;18:179–183. doi: 10.1016/j.jnutbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loest HB, Noh SK, Koo SI. Green tea extract inhibits the lymphatic absorption of cholesterol and alpha-tocopherol in ovariectomized rats. J Nutr. 2002;132:1282–1288. doi: 10.1093/jn/132.6.1282. [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Noh SK, Koo SI. Green tea catechins inhibit pancreatic phospholipase A(2) and intestinal absorption of lipids in ovariectomized rats. J Nutr Biochem. 2006;17:492–498. doi: 10.1016/j.jnutbio.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Noh SK, Koo SI. Epigallocatechin gallate and caffeine differentially inhibit the intestinal absorption of cholesterol and fat in ovariectomized rats. J Nutr. 2006;136:2791–2796. doi: 10.1093/jn/136.11.2791. [DOI] [PubMed] [Google Scholar]
- 48.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Wijk JP, Rabelink TJ. PPAR-gamma agonists: shifting attention from the belly to the heart? Arterioscler Thromb Vasc Biol. 2004;24:798–800. doi: 10.1161/01.ATV.0000127311.38703.1f. [DOI] [PubMed] [Google Scholar]
- 50.Hauner H. Secretory factors from human adipose tissue and their functional role. Proc Nutr Soc. 2005;64:163–169. doi: 10.1079/pns2005428. [DOI] [PubMed] [Google Scholar]
- 51.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skurk T, Alberti-Huber C, Hauner H. Effect of conditioned media from mature human adipocytes on insulin-stimulated Akt/PKB phosphorylation in human skeletal muscle cells: role of BMI and fat cell size. Horm Metab Res. 2009;41:190–196. doi: 10.1055/s-0028-1093342. [DOI] [PubMed] [Google Scholar]
- 53.Kahn SE, Zinman B, Haffner SM, O’Neill MC, Kravitz BG, Yu D, Freed MI, Herman WH, Holman RR, Jones NP, Lachin JM, Viberti GC. Obesity is a major determinant of the association of C-reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes. 2006;55:2357–2364. doi: 10.2337/db06-0116. [DOI] [PubMed] [Google Scholar]
- 54.Aronson D, Bartha P, Zinder O, Kerner A, Markiewicz W, Avizohar O, Brook GJ, Levy Y. Obesity is the major determinant of elevated C-reactive protein in subjects with the metabolic syndrome. J Obes Relat Metab Disord. 2004;28:674–679. doi: 10.1038/sj.ijo.0802609. [DOI] [PubMed] [Google Scholar]


