Our data demonstrated that the inoculation with vaccine derived from the 2009 pandemic influenza raised vigorous neutralization antibodies against both cognate H1N1 and heterotypic influenza viruses. This observation has important implication for vaccine development.
Abstract
Background. A mass vaccination has been implemented to prevent the spread of 2009 pandemic influenza virus in China. Highly limited information is available on whether this vaccine induces cross-reactive neutralization antibodies against other subtypes of influenza viruses.
Methods. We employed pseudovirus-based assays to analyze heterosubtypic neutralization responses in serum samples of 23 recipients of 2009 pandemic influenza vaccine.
Results. One dose of pandemic vaccine not only stimulated good neutralization antibodies against cognate influenza virus 2009 influenza A (H1N1), but also raised broad cross-reactive neutralization activities against seasonal H3N2 and highly pathogenic avian influenza virus H5N1 and lesser to H2N2. The cross-reactive neutralization activities were completely abolished after the removal of immunoglobin G (IgG). In contrast, H1N1 vaccination alone in influenza-naive mice elicited only vigorous homologous neutralizing activities but not cross-reactive neutralization activities.
Conclusions. Our data suggest that the cross-reactive neutralization epitopes do exist in this vaccine and could elicit significant cross-reactive neutralizing IgG antibodies in the presence of preexisting responses. The exposure to H1N1 vaccine is likely to modify the hierarchical order of preexisting immune responses to influenza viruses. These findings provide insights into the evolution of human immunity to influenza viruses after experiencing multiple influenza virus infections and vaccinations.
In 2009, a new pandemic H1N1 influenza virus with a combination of gene segments derived from North American and Eurasian swine lineages was identified [1, 2]. Worldwide mass vaccination has been implemented to prevent its spread and to reduce illness and death from this pandemic influenza. Several lines of evidences were reported on the safety, immunogenicity, and protection effectiveness of this vaccine [3–5]. However, very limited information is available on how the vaccine influences on immune responses to concomitant seasonal or highly pathogenic avian influenza viruses that also have posed considerable threads to public health. Therefore, we initiated experiments to investigate the neutralization activities to heterosubtypic influenza viruses in serum samples collected from the recipients of the 2009 pandemic H1N1 influenza vaccine.
MATERIALS AND METHODS
Participants and Serum Samples
Twenty-three employees of Shanghai Public Health Clinical Center (SHAPHC) received 1 dose (15 μg/0.5 mL) of unadjuvanted inactivated split of 2009 pandemic influenza vaccine (vaccine strain was A/California/07/2009, Shanghai Institute of Biological Products) by intramuscular inoculation on 15 October 2009. Participants in this study were confirmed for the absence of influenza illness in the last 3 months before vaccination. Pregnant and lactating women were also excluded. Table 1 shows the demographic profiles of participants, composed of 18 women and 5 men, born between 1952 and 1989. The baseline samples were collected on the day of and just prior to vaccine administration. The samples of postvaccination were collected on the 21st day after vaccine administration. Written informed consents were obtained from all participants. The overall study was reviewed and proved by the Ethics Committee of SHAPHC.
Table 1.
Demographic Information of Study Participants and Their Hemagglutination-Inhibition Titers Pre- and Postvaccination
| HI titer |
||||
| Subject no. | Year of birth | Gender | Prevaccination | Postvaccination |
| 34 | 1952 | female | 10 | 20 |
| 30 | 1955 | female | 40 | 80 |
| 2 | 1960 | male | 20 | 640 |
| 148 | 1961 | female | 20 | 80 |
| 32 | 1965 | male | 40 | 160 |
| 130 | 1968 | female | 10 | 640 |
| 36 | 1969 | female | 10 | 80 |
| 40 | 1971 | female | 20 | 640 |
| 24 | 1975 | female | 5 | 320 |
| 28 | 1976 | female | 5 | 80 |
| 15 | 1977 | female | 10 | 1280 |
| 51 | 1977 | male | 20 | 640 |
| 10 | 1979 | female | 20 | 320 |
| 41 | 1979 | female | 5 | 320 |
| 29 | 1981 | female | 5 | 40 |
| 120 | 1981 | female | 80 | 320 |
| 127 | 1981 | male | 5 | 20 |
| 93 | 1982 | female | 40 | 320 |
| 121 | 1982 | female | 5 | 160 |
| 9 | 1983 | male | 40 | 80 |
| 39 | 1987 | female | 10 | 160 |
| 35 | 1989 | female | 10 | 40 |
| 123 | 1989 | male | 5 | 20 |
Abbreviation: HI, hemagglutination-inhibition.
Mouse Immunization Protocol
Six mice bred under specific-pathogen-free (SPF) circumventions were immunized with 100 μg DNA vaccine expressing HA of pandemic influenza (A/Texas/05/2009[H1N1], designated TE09 H1) by intramuscular route at a 2-week intervals for 4 times, and an additional 6 mice were immunized with 100 μg sham DNA as the mock control group. Serum samples were collected 2 weeks after the final immunization and combined by group for the neutralization assay, which ensured a sufficient volume of serum samples for different pseudovirus and serial dilution.
Hemagglutination-Inhibition Assays
Serum samples were treated with receptor-destroying enzyme (RDE) to inactivate nonspecific inhibitors, and then serially diluted 2-fold into U-bottom 96-well microtiter plates, starting with 1:10 and stopping at 1:1280, in a volume of 50 μL. The hemagglutination-inhibition (HI) titer was determined by the reciprocal of the last dilution that contained nonagglutinated red blood cells. Serum from a 2009 H1N1 infected but recovered subject was used as a positive control, and serum from a naive mouse raised under specific pathogen-free circumventions was used as a negative serum control. Samples with HI titers ≥1:40 were considered as seropositive. The virus strain used was A/Shanghai/37T/2009(H1N1) (SH37T; GenBank accession: ACS27776∼ACS27785), which shares 99.29% homogeneity of hemagglutinin (HA) protein sequence (mutations are P100S, S220T, I338V, and V428I) to A/California/07/2009 (GenBank accession: ACP41953; Table 1; online only).
Generation of Influenza HA/Neuraminidase Pseudotyped Lentivirus
4.5×106 293T packaging cells were cotransfected with 5 μg lentivirus vector pNL4-3.Luc.R-E-, 2.5 μg pVKD-HA, and 2.5 μg pVKD-NA using Lipofectamine 2000 from Invitrogen (Frederick, MD). The HA sequences were humanized from 2009 pandemic influenza TE09 H1 (GenBank accession: ACP41934); seasonal influenza viruses, including A/Brisbane/59/2007(H1N1) (designated as BR07 H1, GenBank accession: ACA28844), A/Singapore/6/1986(H1N1) HA (designated as SI86 H1, GenBank No. ABO38395), A/Japan/305/57(H2N2) (designated as JA57 J2, GenBank accession: AAT66416.1), and A/Moscow/10/1999(H3N2) (designated as MO99 H3, GenBank accession: AAT08002); and highly pathogenic avian influenza A/Viet Nam/1203/2004(H5N1) (designated as VN04 H5, GenBank accession: AAW80717). The sequence of neuraminidase (NA) was derived from A/Shanghai/37T/2009(H1N1) (designated as SH37T, GenBank accession: ACS27784). After overnight incubation, cells were washed once with PBS and cultured in 5 mL complete Dulbecco’s modified Eagle’s medium (DMEM). The pseudotype virus containing supernatants were harvested in 16–20 hours and stored at −80°C in aliquots until used in a neutralization assay. The phylogenetic tree of the amino acid sequences of HAs used in HI and the pseudotyped virus assays are shown in Supplementary Figure 1 (online only).
Removal of Immunoglobin G
Four serum samples with highest cross-reactive neutralization activities against VN04 H5 were selected for the depletion of immunoglobin G (IgG). IgG was removed using Protein G Sepharose Beads. Briefly, 200 μL Protein G Sepharose Beads was washed 3 times with ice-cooled phosphate-buffered saline (PBS). At the final wash, the supernatant was aspired after centrifuging, and 100 μL pooled serum was added. The serum-beads mixture was incubated at 4°C under rotary agitation for 1 hour, centrifuged, and the supernatant was kept as IgG-depleted serum. All steps were repeated 3 times to remove abundant IgG.
Pseudotype-Based Neutralization Assay
Human serum samples or IgG-depleted serum samples were 2- or 3-fold serially diluted and incubated with indicated amounts of pseudotyped virus at the final volume of 150 μL at 37°C for 1 hour, then the mixture was added to cultures of Madin-Darby canine kidney (MDCK) cells. After overnight incubation, cells were then washed with PBS and cultured in complete DMEM for 48 hours. Luciferase activity (RLA) was measured by a BrightGlo Luciferase assay according to the manufacturer’s instruction (Promega, Madison, WI). In the assay, the neutralizing titer of human serum samples was calculated according to the inhibition of MDCK cells by the pseudotyped viruses’ infection. Inhibition percentage was calculated as follows: (RLA in medium control – RLA in serum at a given dilution)/RLA in medium control. The 50% inhibitory concentration (IC50) was reported as the dilutions of a given serum that resulted in a 50% reduction of RLA. For a pseudovirus-based assay, <50% inhibition was considered as negative, and the final dilution of serum samples able to reach ≥50% inhibition was considered as the serum sample titer. For mice experiments, serum samples collected 2 weeks after the final immunization and combined by group were used in the neutralizing activity test.
Statistical Analysis
Geometric mean titers (GMTs) were calculated by assigning a titer of 5 to samples with no detectable HI antibodies at the starting dilution (titer < 10). Wilcoxon signed rank test was used to calculate P value when compared with GMT between paired data. Pearson product moment correlation was used to calculate the correlation. Figures were plotted using GraphPad Prism software.
RESULTS
Antibody Responses to the Homologous Pandemic H1N1 Influenza Virus
We evaluated the immunogenicity of the pandemic H1N1 influenza vaccine in recipients. As expected, 18 of 23 participants displayed undetectable or low levels of HI antibodies against the homologous pandemic H1N1 influenza virus before immunization, whereas the other 5 participants had a level of HI with the titer higher than 40, though they reported no influenza illness or symptoms in the last 3 months (Table 1). Consistent with other reports, the HI titer significantly increased in all participants with a varied extent on day 21 post vaccination (GMT 13.12 vs 150.6, P < .0001, Table 1), and 87.0% (20 out of 23) participants had a titer higher than 40 [3, 5].
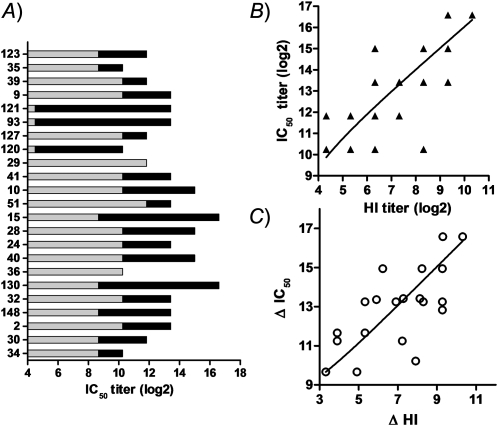
We also employed a more sensitive neutralization assay, a luciferase and pseudovirus–based assay, to measure the neutralization activity against the pandemic H1N1 influenza virus. Unexpectedly, we observed significant neutralization activities in the serum samples of 20 subjects at the baseline (Figure 1A, gray bar). Since all participants had no exposure history to 2009 influenza A (H1N1), it is likely those neutralization activities resulted from cross-reactive antibodies developed during previous exposure to other influenza virus infection. This observation may explain why the majority of 2009 influenza A (H1N1) infections were manifested as subclinical infection. Interestingly, despite the presence of significant cross-reactive neutralization activities, 1 dose of 2009 H1N1 vaccine resulted in a significant increase of neutralization activities in 21 out of 23 volunteers (Figure 1A, dark bar). The total neutralization activities (IC50) were closely associated with total HI titers (Figure 1B), and the increase of IC50 from baseline was also correlated to the increase of HI titers from the baseline (Figure 1C), indicating that these 2 assays are comparable and the pseudovirus-based assay is reliable.
Figure 1.
Homologous immune responses in serum samples of 2009 pandemic vaccine recipients. Twenty-three volunteers received 1 dose of 2009 pandemic vaccine. Serum samples were collected before and 21 days after vaccination. A, The individual IC50 titers (reciprocal 50% inhibitory concentration) before (gray) and after (dark) vaccination. B, Correlation between the IC50 titers and hemagglutination-inhibition (HI) titers in serum samples post vaccination. C, Correlation between the increase of HI titer from baseline (HI post – HI pre) and the change in magnitude of IC50 titer from baseline (IC50 post – IC50 pre).
Overall, our data proved that the pandemic H1N1 influenza vaccine is immunogenic, and is able to elicit both HI and neutralization antibodies.
2009 Pandemic Influenza Vaccine Boosts Cross-reactive Responses to Other Subtypes of Influenza Viruses
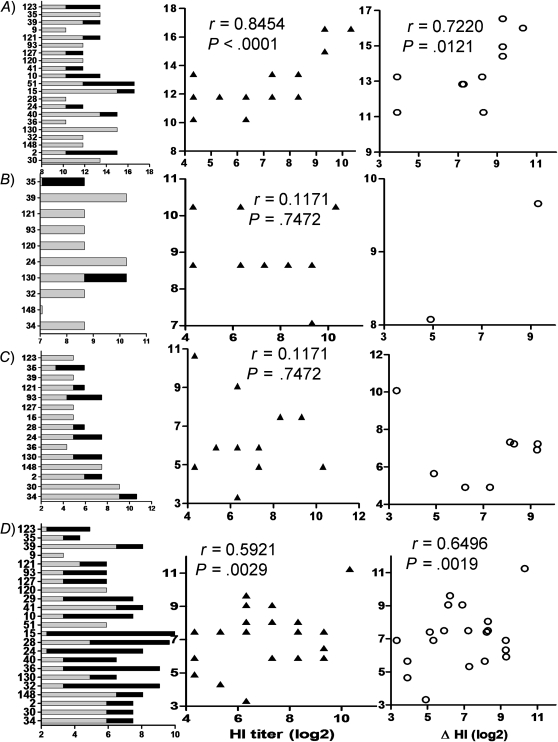
We next evaluated whether the neutralization antibodies elicited by the 2009 pandemic vaccine had an impact on other influenza A viruses by testing the cross-reactive neutralization activities against heterosubtypes H1N1, H2N2, H3N2, and H5N1. We first tested the neutralization activities against HA pseudoviruses derived from the contemporary human seasonal influenza A viruses, including BR07 H1 and MO99 H3. Remarkably, the baseline serum samples already had very high neutralization titers to these 2 seasonal influenza viruses, indicating that prior infection or vaccination imprinted the immune system and developed the preexisting immunity. Again, despite the presence of high neutralization activities against MO99 H3, the IC50 titers increased markedly in 11 of 21 participants following vaccination, whereas only 2 out of 10 subjects increased their titers to BR07 H1. In addition, the IC50 titers to MO99 H3 in postvaccination serum samples were significantly correlated with the titer of HI to TE09 H1. Furthermore, the increases of IC50 titers to MO99 H3 from baseline were also significantly correlated to the increases of HI titers to TE09 H1 from the baseline (Figure 2A and 2B). These data indicate that 2009 pandemic vaccine boosted cross-reactive responses to seasonal influenza MO99 H3 but not BR07 H1.
Figure 2.
Cross-reactive neutralization activities to heterosubtypes of influenza viruses in serum samples of 2009 pandemic vaccine recipients. Neutralization activities against A/Moscow/10/1999(H3N2) (A), A/Brisbane/59/2007(H1N1) (B), A/Japan/305/57(H2N2) (C), and A/Viet Nam/1203/2004(H5N1) (D). Left, individual 50% inhibitory concentration (IC50) titers; middle, correlation between the hemagglutination-inhibition (HI) titers to pandemic influenza and IC50 titers to tested virus in sera postvaccination; right, correlation between the increase of HI titers to pandemic influenza from baseline and the change in magnitude of IC50 titers to tested virus.
We next tested whether the vaccine-elicited neutralization antibodies were capable of cross-reacting to JA57 H2, a virus that led to the epidemic of Asian influenza in Japan that began in May 1957 [6]. Unexpectedly, the neutralization activities against JA57 H2 were elevated in half of recipients following vaccination; however, the neutralization activities against this H2 were reversely associated with HI titers to TE09 H1, though it did not reach statistical significance (Figure 2C, middle panel). These data may indicate that cross-reactive neutralization antibodies against JA57 H2 may compete with HI responses to TE09 H1. In addition, we also quantified the cross-reactive neutralization activities to highly pathogenic avian influenza VN04 H5. The H5 neutralization activity was readily presented in prevaccination serum samples at relatively lower titers compared with that for H3 or H1. Interestingly, following vaccination, the H5 neutralization activities were profoundly increased in 21 out of 23 vaccinees, and significant correlations were observed in both total titers and the increase from the baseline in magnitude of IC50 with HI to TE09 H1, indicating that the elevation of H5 neutralization activity was attributed to the administration of the 2009 pandemic vaccine (Figure 2D). However, no associations between the levels of enhanced heterotypic neutralization activities and ages were observed in this small group (data not shown).
Profound Decrease of Cross-reactive Neutralizing Activities After the Depletion of IgG Antibodies
To understand the nature of this cross-reactivity, we determined the cross-reactive neutralizing activities of the serum samples after the depletion of IgG. Four serum samples, including subjects 15, 28, 32, and 36, were selected for the depletion as those samples gave rise to the highest cross-reactive neutralization activities in our above experiments. As expected, the depletion of IgG resulted in a dramatic decrease in the neutralization activities against both homologous TE09 H1 and heterosubtypic VN04 H5 to varied extents (Table 2). Interestingly, while the cross-reactive neutralization activities against VN04 H5 were completely abolished after the depletion of IgG, the neutralization activities against homologous TE09 H1 were only partially removed though a profound decrease was observed in 3 out of 4 patients (Table 2). Surprisingly, the neutralization activities remained intact in 1 patient (subject 36) after the removal of IgG from serum samples although the depletion had resulted in the complete removal of cross-reactive neutralization activities against H5. In addition, these observations coincided with the failure to enhance the neutralization activities in this subject after vaccination with 2009 pandemic influenza vaccine. Overall, these data suggest that the cross-reactive neutralization activities are largely mediated by IgG antibodies, whereas homologous neutralization activities may be mediated by both IgG and non-IgG antibodies.
Table 2.
Profound Decrease of Cross-reactive Neutralizing Activities After the Depletion of Immunoglobin G Antibodies
| Virus strain | Subject ID | IgG+a | IgG–b |
| TE09 H1 | |||
| 15 | 32805 | 1215 | |
| 28 | 32805 | 405 | |
| 32 | 32805 | 405 | |
| 36 | 1215 | 1215 | |
| VN04 H5 | |||
| 15 | 2430 | <20 | |
| 28 | 810 | <20 | |
| 32 | 540 | <20 | |
| 36 | 540 | <20 |
Abbreviations: IgG, immunoglobin G; TE09 H1, A/Texas/05/2009(H1N1); VN04 H5, A/Viet Nam/1203/2004(H5N1).
IgG+ indicates the serum samples containing IgG antibodies.
IgG– indicates the IgG-depleted serum samples.
Vaccination With 2009 Pandemic Influenza–Derived HA Immunogen Mainly Elicited Homologous Neutralization Antibodies in Influenza-Naive Mice
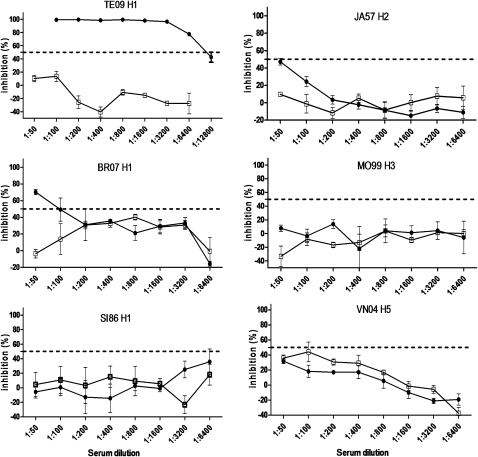
We proposed that the cross-reactive neutralization antibodies observed at baseline were developed by the preexposure to seasonal influenza A infection or vaccination, and the 2009 pandemic influenza vaccine (H1N1) might contain cross-reactive neutralization epitopes, thereby enhancing the cross-reactive neutralization activities against H5N1, H3N2, and H2N2. We tested this hypothesis in mice models. SPF breeding mice naive to influenza were vaccinated 4 times with DNA expressing HA of 2009 pandemic influenza H1N1, and neutralization activities to different subtypes of influenza viruses were quantified in an identical pseudovirus-based assay as described above. As shown in Figure 3, there was no preexisting immunity to any subtype of influenza in the mock control mouse. Unexpectedly, vigorous neutralization activities were only observed against homologous pseudovirus TE09 H1 in 2009 pandemic influenza vaccinated serum samples, whereas only marginal activities were identified for BR07 H1 and JA57 H2, and no detectable activities were observed against SI86 H1, MO99 H3, and VN04 H5. These data suggest that homologous neutralization epitopes are highly immunogenic in 2009 pandemic influenza vaccine (H1N1) and thereby capable of raising vigorous neutralization antibodies, whereas cross-reactive neutralization epitopes are too weak in their immunogenicity to compete with homologous epitopes, and fail to raise significant cross-reactive neutralization activities when this vaccine is administrated alone.
Figure 3.
Vaccination with 2009 pandemic influenza–derived hemagglutinin (HA) immunogen mainly elicited homologous neutralization antibodies in influenza-naive mice. Six mice per group bred in specific pathogen-free environment received 4× DNA vaccine-expressing HA of pandemic vaccine, or sham DNA as mock control group. Two weeks after final inoculation, serum samples were collected and combined by group. The neutralization activities against homologous, seasonal, and highly pathogenic avian influenza viruses in both vaccinated mice (▴) and mock-control mice (Δ) were shown in serial 2-fold dilutions, triplicates were set for each dilution, and the mean value/standard error bar were used in plot. Less than 50% inhibition was considered as negative, the final dilution of serum samples able to reach ≥50% inhibition was considered as the serum sample titer. Since serum samples from 6 mice were combined for this assay, the value for each serum sample dilution represents the mean value from 6 mice.
DISCUSSION
The outbreak of influenza is driven by coevolution between the virus and human immunity. To prevent the outbreak of influenza and reduce the severity of related illness and mortality, World Health Organization (WHO) yearly recommends as vaccine candidates those strains most likely to emerge based on surveillance of circulating viruses. But this strategy does not always work, exemplified by the outbreak of 2009 pandemic influenza (H1N1). It only took 3 months for the newly emerged virus to spread worldwide due to a lack of preexisting immunity in a subpopulation, because recent seasonal influenza vaccines induce little or no cross-reactive antibody responses to pandemic influenza [7]. Mass vaccination has been implemented to prevent its further spread. To understand and prepare for influenza epidemics, it is important to question what influences the pandemic vaccine has on the immunity to seasonal or highly pathogenic avian influenza viruses.
Here we report the effects of a pandemic vaccine on humoral immune responses to other subtypes of influenza virus in vaccine recipients. The pandemic vaccine not only raises neutralization antibodies to cognate H1N1, but also elicits broadly heterosubtypic neutralization responses—the implications of which would affect recommendations regarding the composition of influenza vaccine immunogens. These findings are consistent with the observation in pandemic virus–infected patients [8]. We proposed from these data that cross-reactive neutralization epitopes may exist in this vaccine and thereby has the ability to raise broad cross-reactive neutralization antibodies against different subtypes of influenza viruses. Unexpectedly, when we tested this idea in mice experiments, we observed that the inoculation of 2009 pandemic influenza HA alone was only able to elicit high neutralization activities to cognate H1N1 virus, but only marginal neutralization activities were observed to BR07 H1 and JA57 H2, and none were observed to SI86 H1, MO99 H3, and VN04 H5. The observational discrepancy between humans and mice may be explained by the different neutralization activities at baseline, vigorous cross-reactive neutralization activities were identified in human samples at baseline, whereas no activities were detected in mouse samples in the absence of vaccination, as shown in mock-control-immunized mice. These data demonstrated that the preexisting immunity, as observed in our study, plays an important role in finalizing the composition of immunity, and that the inoculation of the 2009 pandemic vaccine served as a boost and was able to enhance the preexisting cross-reactive neutralization activities but not for de novo cross-reactive neutralization activities. In other words, the cross-reactive neutralization epitopes do exist in the 2009 pandemic influenza vaccine; their immunogenicity was only manifested in the presence of their preexisting cross-reactive neutralizing activities.
Existence of cross-reactive immunity between previous influenza infections and the 2009 pandemic influenza has been observed in several reports, and demonstrated that the preexposure to 1918 pandemic influenza H1N1 or to 2007 seasonal influenza H1N1 might have enhanced the cross-reactive neutralizing antibodies against the 2009 pandemic influenza [9, 10]. Similarly, repeated infection with seasonal influenza viruses improves protection and clearance of 2009 pandemic influenza in ferrets[11]. Interestingly, vaccination with 1976 “swine flu” coincided with the increased neutralizing activities against the 2009 pandemic influenza [7, 12]. In addition, immunization with inactivated trivalent seasonal influenza vaccines induced cross-reactive neutralizing antibodies against the 2009 pandemic influenza in humans [13] and against highly pathogenic H5N1 influenza virus in mice models [14]. The cross-reactive epitopes may be less immunogenic, thus a potent adjuvant was required to manifest the cross-protective effect of an inactivated H1N1 influenza vaccine against an H3N2 virus [15]. A recent report demonstrated that it is widely prevalent for the existence of heterosubtypic broadly neutralizing human anti-influenza antibodies[16]. All these observations together with our data largely support the veracity of the hypothesis of original antigenic sin proposed by Thomas Francis in 1960 [17], and the exposure to an influenza strain distinct from the previous one not only resulted in development of immunity to the current strain, but also boosted the immune response to the previous virus strain; therefore, the exposure history to different influenza viruses dictates the hierarchy of cross-reactive immunity, which greatly emphasizes the importance of employing mismatched influenza HA immunogens for priming and boosting in order to raise broad cross-reactive neutralization antibodies in the context of a vaccine development strategy. However, the phenomenon of original antigenic sin results in the competition between preexisting and newly developed immunity and thereby attenuates the immune responses to current viral infection, and consequently may blunt the protection from current influenza infection.
The preexisting cross-reactive neutralization antibodies raised by preexposure to seasonal influenza viruses may explain why the majority of 2009 influenza A (H1N1) infections were subclinical infection in adult populations. Our data inform us that the pandemic of influenza will not only influence the spread of its cognate influenza virus but also the heterosubtypic influenza virus. The understanding of cross-reactive influences between influenza viruses will help us to learn about existing immunity in populations, to predict the next possible wave of pandemic influenza, and to gain new insight into established cross-reactive neutralization immunity.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
The authors thank all study participants for their donation of blood samples, and Yassir F. Abubakar for his editorial help.
Financial support.
This work was supported by the National Grand Program on Key Infectious Disease Control (grants 2012ZX10001-006 and 2009ZX10602-11), the China Ministry of Health, and the Key Basic Research Project (10JC1413600) and Shanghai-Canada joint program (09540701100), Science and Technology Commission of Shanghai Municipality, Shanghai.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Garten RJ, Davis CT, Russell CA, et al. Antigenic and genetic characteristics of swine-origin 2009 A (H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith GJ, Vijaykrishna D, Bahl J, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–5. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 3.Zhu FC, Wang H, Fang HH, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–23. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Xu F, Lu L, et al. Safety and effectiveness of a 2009 H1N1 vaccine in Beijing. N Engl J Med. 2010;363:2416–23. doi: 10.1056/NEJMoa1006736. [DOI] [PubMed] [Google Scholar]
- 5.Liang XF, Wang HQ, Wang JZ, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375:56–66. doi: 10.1016/S0140-6736(09)62003-1. [DOI] [PubMed] [Google Scholar]
- 6.Fukumi H. Summary report on the Asian influenza epidemic in Japan, 1957. Bull World Health Organ. 1959;20:187–98. [PMC free article] [PubMed] [Google Scholar]
- 7.Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 8.Wrammert J, Koutsonanos D, Li GM, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181–93. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizzo C, Rota MC, Bella A, et al. Cross-reactive antibody responses to the 2009 A/H1N1v influenza virus in the Italian population in the pre-pandemic period. Vaccine. 2010;28:3558–62. doi: 10.1016/j.vaccine.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Lemaitre M, Leruez-Ville M, De Lamballerie XN, et al. Seasonal H1N1 2007 influenza virus infection is associated with elevated pre-exposure antibody titers to the 2009 pandemic influenza A (H1N1) virus. Clin Microbiol Infect. 2011;17:732–7. doi: 10.1111/j.1469-0691.2010.03352.x. [DOI] [PubMed] [Google Scholar]
- 11.Laurie KL, Carolan LA, Middleton D, Lowther S, Kelso A, Barr IG. Multiple infections with seasonal influenza A virus induce cross-protective immunity against A(H1N1) pandemic influenza virus in a ferret model. J Infect Dis. 2010;202:1011–20. doi: 10.1086/656188. [DOI] [PubMed] [Google Scholar]
- 12.McCullers JA, Van De Velde LA, Allison KJ, Branum KC, Webby RJ, Flynn PM. Recipients of vaccine against the 1976 “swine flu” have enhanced neutralization responses to the 2009 novel H1N1 influenza virus. Clin Infect Dis. 2010;50:1487–92. doi: 10.1086/652441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee VJ, Tay JK, Chen MI, et al. Inactivated trivalent seasonal influenza vaccine induces limited cross-reactive neutralizing antibody responses against 2009 pandemic and 1934 PR8 H1N1 strains. Vaccine. 2010;28:6852–7. doi: 10.1016/j.vaccine.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Ichinohe T, Tamura S, Kawaguchi A, et al. Cross-protection against H5N1 influenza virus infection is afforded by intranasal inoculation with seasonal trivalent inactivated influenza vaccine. J Infect Dis. 2007;196:1313–20. doi: 10.1086/521304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quan FS, Compans RW, Nguyen HH, Kang SM. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J Virol. 2008;82:1350–9. doi: 10.1128/JVI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sui J, Sheehan J, Hwang WC, et al. Wide prevalence of heterosubtypic broadly neutralizing human anti-influenza a antibodies. Clin Infect Dis. 2011;52:1003–9. doi: 10.1093/cid/cir121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis T. On the doctrine of original antigenic sin. Proc Am Philos Soc. 1960;104:572–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.