Abstract
PURPOSE: Our preliminary report of imatinib mesylate (IM) in gastrointestinal stromal tumor (GIST) patients detailed a high response rate; however, the long-term result is still unknown. We conducted an analysis of Taiwan advanced inoperable/metastatic GIST patients treated on IM regarding survival, pattern of failure, potential prognostic factors, and mutational status. PATIENTS AND METHODS: From 2001 to 2010, patients with pathologically proven advanced inoperable/metastatic GIST receiving IM were enrolled onto this study. Data on KIT mutational status, measurable tumor size, and other potential prognostic factors were prospectively collected. Patients were followed up for a median of 33.6 months. RESULTS: There were 171 patients (106 men and 65 women) with response rate, and their clinical benefit for IM was 57.3% and 87.1%, respectively. Median progression-free survival (PFS) and overall survival (OS) for these 171 patients are 37.6 and 71.0 months, respectively. Of 171 patients, 120 (70.2%) remained on long-term IM use. Poor performance status, tumor larger than 11.5 cm, primary resistance, and the presence of an exon 9 mutation were independently associated with unfavorable PFS. Regarding OS, poor performance status, primary resistance, and tumor larger than 11.5 cm were three independently unfavorable predictors. CONCLUSIONS: The median PFS and OS of 171 GIST patients are 37.6 and 71.0 months, respectively. Poor performance status, tumor size larger than 11.5 cm, primary resistance, and an exon 9 mutation were independently associated with unfavorable PFS. Regarding OS, poor performance status, primary resistance, and tumor size larger than 11.5 cm were three independent unfavorable predictors.
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract. In Taiwan, the annual incidence of GIST is 13.74 per million Taiwanese [1]. Before 2001, no effective systemic treatments existed for GISTs [2]. However, the association between constitutively activated KIT and platelet-derived growth factor receptor A (PDGFRA) signaling and GIST oncogenesis provided justification for testing a small-molecule tyrosine kinase inhibitor in this tumor type [3]. Imatinib mesylate (IM) selectively inhibits certain protein tyrosine kinases: intracellular ABL kinase, chimeric BCR-ABL fusion oncoprotein of chronic myeloid leukemia, transmembrane receptor kit, and PDGFRs [4–7]. GISTs express the cell surface transmembrane receptor kit with a tyrosine kinase activity. There are frequent gain-of-function mutations of kit in GISTs [3]. These mutations result in constitutive activation of kit signaling, which leads to uncontrolled cell proliferation and resistance to apoptosis [3]. IM has shown a promising clinical result for an advanced GIST patient [8], and several trials have shown a promising effect of this target therapy [6,9–11]. As shown in our previous preliminary study, Glivec had a significant impact on survival in patients with advanced GISTs, but the follow-up time is just 16 months and the patient number is limited [12].
This study represented a large single-institute experience on IM use for a decade, including 171 patients with advanced GIST and followed up to a median of 33.6 months. This study examined the long-term impact of IM on patient response, survival, and the correlation of the response rate with the kit gene mutation status. We also tried to elucidate the predictors for favorable PFS and OS of Taiwanese patients with advanced and metastatic GIST receiving IM.
Materials and Methods
Patients, Study Design, and Efficacy Evaluation
From August 2001 to April 2010, 171 adult patients with histologically confirmed, advanced inoperable/metastatic GIST that expressed the CD117 antigen (as a marker of the KIT receptor) and with measurable disease based on Response Evaluation Criteria in Solid Tumors (RECIST) [13] were eligible. Other eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance score of 3 or less and adequate hematologic, renal, and hepatic function. A prospective, nonrandomized, single-center study was conducted to evaluate the effect of IM in inducing an objective response or stable disease in Taiwanese with advanced inoperable/metastatic GIST. Patients were administered 400 mg of IM in 100-mg capsules, taken orally daily with food. Patients had regular physical examinations and evaluations of performance status, body weight, complete blood count, and serum chemistry. Standard computed tomography was performed on each patient every 3 months for the first 3 years and every 6 months for the following 2 years to assess the patient's response. Tumor size was measured in at least five target lesions with the sum of the largest dimension that was used as a response evaluation indicator as described in RECIST [13]. Time to response (TTR = time point of best response - time point of IM administration) was defined as the interval for best drug response during imatinib treatment. Time to progression (TTP = time point of disease progression - time point of IM administration) was defined as the interval for worse drug response with disease progression during imatinib treatment. Progression-free survival (PFS) is defined as no progression after administration of IM. Overall survival (OS) is defined as survival after administration of IM and death as the end point of the study or December 2010. Primary resistance is defined as PFS less than 6 months. Patients underwent regular physical examinations and evaluations of performance status, body weight, complete blood cell count, and serum chemistry. The study was approved by the local institutional review board of Chang Gung Memorial Hospital, and written informed consent for drug administration and analysis of tumor-associated genetic alteration was obtained independently from each patient.
Analysis of KIT and PDGFRA Mutations
Sections were prepared from formalin-fixed, paraffin-embedded pretreatment specimens trimmed to enrich tumor cells. Polymerase chain reaction amplification of genomic DNA for KIT and PDGFRA was sent to another hospital and performed by Professor C.Y. Tzen. Amplification was analyzed for mutations as previously described [14].
Statistical Analysis
Data were presented as percentages of patients or means with standard deviation. Numerical data were compared by an independent two-sample t test. Pearson χ2 test and Fisher exact test were used for nominal variables. Time-to-event analyses were performed using Kaplan-Meier methods. The following potential prognostic variables were investigated for their impact on long-term outcomes: age (<65 vs ≥65 years), sex, ECOG performance status (score 0 or 1 vs 2 or 3), summed diameter of five target tumor lesions (<11.5 vs ≥11.5 cm), mutational status (exon 11 vs exon 9 vs wild type), response (complete response [CR] + partial response [PR] vs stable disease [SD] vs progressive disease [PD]), and categories of the following baseline laboratory values: white blood cells, platelets, hemoglobin, albumin, liver function, and renal function. For the prognostic factors evaluation, each potential candidate was initially assessed by univariate analysis. Factors found significant at P < .05 were included in a multivariate Cox proportional hazard model. Thereafter, an enter-selection procedure was applied to select the most relevant prognostic factors. Only factors that remained significant at the .05 level during the selection procedure were included in the final model. All statistical analyses were performed using SPSS computer software package (Version 10.0; SPSS, Inc, Chicago, IL). P < .05 was considered statistically significant.
Results
Clinical Features
Table 1 summarizes the demographic features of 171 patients with advanced inoperable/metastatic GIST treated with IM. There were 106 men and 65 women with a median age of 58 years old (range = 19–89 years old). The median tumor size before IM treatment was 10.0 cm (range = 2.5–30.0 cm). Stomach was the most common site for GISTs treated with IM (60/171, 35.1%), followed by jejunum (31/171, 18.1%), ileum (25/171, 14.6%), and duodenum (20/171, 11.7%) (Table 1).
Table 1.
Demographic Data of the 171 Taiwanese Patients with GIST Receiving IM.
| Age, median (range), y | 58 (19–89) |
| Sex, M/F | 106:65 |
| Origin of GIST, n (%) | |
| Stomach | 60 (35.1) |
| Small bowel | 76 (44.4) |
| Colon-rectum | 19 (11.7) |
| Others | 16 (9.4) |
| Tumor size before IM treatment, median (range), cm | 10 (2.5–30) |
| Genetic spectrum of 122 patients tested, n (%) | |
| Exon 11 mutation | 90 (73.8) |
| Exon 9 mutation | 21 (17.2) |
| PDGFRA (exon 18) | 2 (1.6) |
| Wild type | 9 (7.4) |
Treatment and Outcomes
Since 2001, in Taiwan, IM has been administered to patients with advanced inoperable/metastatic GISTs. 400 mg IM per day was given to all the 171 patients first. All of the 171 patients were followed up after administration of IM at regular intervals until death or until December 2010. Table 2 summarizes antitumor response of IM on 171 Taiwanese with advanced inoperable/metastatic GIST. Overall, 4 patients (2.3%) had CR, 94 (55.0%) had PR, 51 had SD (29.8%), and 22 had PD (12.9%). Of all GIST patients, 87.1% had a clinical benefit. Among the 171 patients, the median TTR for 4 patients who had CR was 13.0 months and that for 94 patients who had PR was 3.2 months. The median PFS for the 94 PR and 51 SD patients was 47.6 and 42.7 months, respectively. The median OS of the 94 PR and 51 SD patients was 71.0 and 80.6 months, respectively. Whereas for the 22 PD patients, the median time to progression was 2.6 months and the median OS was 17.3 months (Table 2). Among the 22 GIST patients with primary resistance, exon 9 is the most common cause (8 patients with exon 9 mutation), followed by exon 11 mutation (6 patients with exon 11). Of 21 GIST patients with exon 9 mutation, 8 (31%) developed primary resistance after IM treatment, whereas of 90 GIST patients with exon 11 mutation, 6 (6.7%) developed primary resistance. Hence, patients with advanced inoperable/metastatic GIST who harbored exon 9 mutation had a significantly higher chance of primary resistance when compared with those who had exon 11 mutation (8/21 exon 9 vs 6/90 exon 11, P = .001).
Table 2.
Antitumor Response of IM on 171 Taiwanese with Advanced GIST.
| Response | n (%) | Median IM Administration Duration (Months) | Median TTR/PFS (Months) | Median Post-IM OS (Months) |
| CR | 4 (2.3) | 56.2 | 13.0/NA | NA |
| PR | 94 (55) | 34.0 | 3.2/47.6 | 71.0 |
| SD | 51 (29.8) | 29.1 | 3.9/42.7 | 80.6 |
| PD | 22 (12.9) | 7.2 | 2.6* | 17.3 |
Means median time for time to progression.
Spectrum of Mutations in 171 Patients with Advanced Inoperable/Metastatic GIST
Table 3 summarizes the correlation between antitumor response and mutation status of 171 Taiwanese with advanced GIST treated with IM. Tumor specimens suitable for genetic analysis were available from 122 (71.3%) of the 171 patients with advanced GIST. Overall, 111 (90.9%) of the 122 examined GISTs had activated mutations of kit exons 9 and 11. Nine (7.4%) had no mutation of KIT, and only two patients with PDGFRA mutation (exon 18 V840). Of 122 GISTs, 21 (17.2%) expressed exon 9 mutation and 90 (73.8%) had exon 11 mutation. In 90 patients with GISTs harboring kit exon 11 mutations, the CR and PR rates (overall response rate = 74.4% [4 CR and 63 PR]) are significantly higher than those of 9 of 21 patients with tumors containing a kit exon 9 mutation (overall response rate = 40.9% [9 PR]) (P = .0005; Table 3). Regarding clinical benefit, GIST patients harboring kit 11 mutations also had a significantly higher clinical benefit than those harboring kit 9 mutations (P =.0005).
Table 3.
Correlation between Antitumor Response and Mutation Status of 171 Taiwanese with Advanced GIST Treated with IM.
| CR | PR | SD | PD | P | |
| Exon 9 (n = 21) | 0 | 9 (42.9%) | 4 (19.0%) | 8 (30.1%) | .0005 |
| Exon 11 (n = 90) | 4 (4.4%) | 63 (70%) | 17 (18.9%) | 6 (6.7%) | |
| Wild type (n = 9) | 0 | 2 (22.2%) | 5 (55.6%) | 2 (22.2%) |
| CR + PR + SD | PD | P | |
| Exon 9 (n = 21) | 13 (61.9%) | 8 (30.1%) | .0005 |
| Exon 11 (n = 90) | 84 (93.3%) | 6 (6.7%) | |
| Wild type (n = 9) | 7 (77.8%) | 2 (22.2%) |
Survival Analysis for 171 Patients with Advanced Inoperable/Metastatic GIST Receiving Imatinib
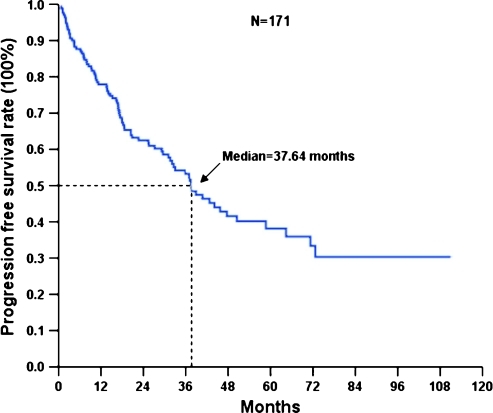
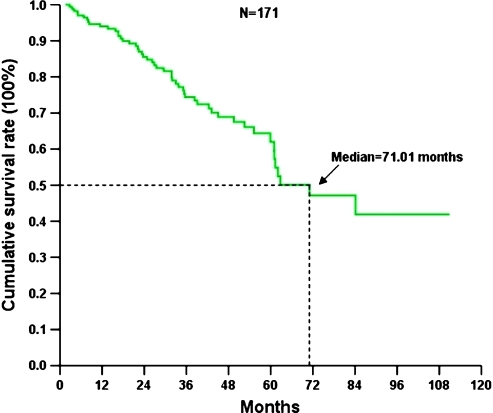
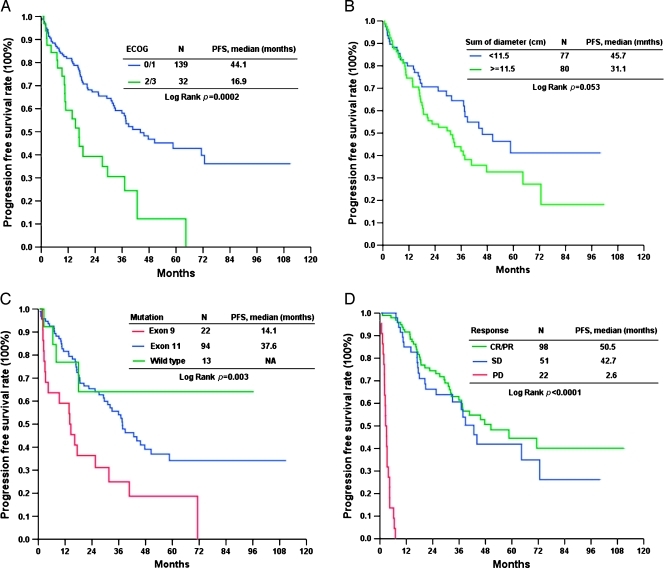
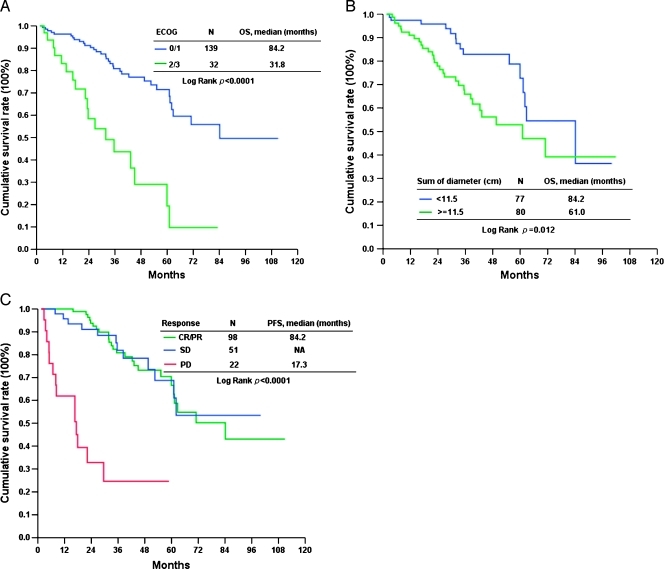
The median follow-up period after IM was 33.6 months (range = 1.6–110.9 months). Of these 171 patients, 87 developed progression (50.9%) and 51 (29.8%) died because of GIST. The 171 Taiwanese with advanced inoperable/metastatic GIST had a median PFS of 37.64 months and OS of 71.0 months (Figures 1 and 2). Tables 4 and 5 summarize the survival analysis of PFS and OS of these 171 GIST patients after IM treatment, including clinical features, tumor size, mutational status, and laboratory data. Univariate survival analysis revealed that age older than 65 years, poor performance with ECOG 2 or 3, exon 9 mutation, tumor size larger than 11.5 cm, and primary resistance are associated with inferior PFS of GIST patients receiving IM treatment (Figure 3). Multivariate Cox proportional hazard analysis demonstrated that poor performance status, exon 9 mutation, tumor size larger than 11.5 cm, and primary resistance are associated with an inferior PFS of GIST patients receiving IM treatment. Regarding OS, univariate survival analysis revealed that age older than 65 years, poor performance with ECOG 2 or 3, poor nutritional status, and tumor size larger than 11.5 cm are associated with inferior OS of GIST patients receiving IM treatment (Figure 4). However, multivariate Cox proportional hazard analysis demonstrated that good performance status, tumor size less than 11.5 cm, and good response were the only three independent prognostic factors that favorably affected OS of patients with advanced inoperable/metastatic GIST after IM treatment (Table 5).
Figure 1.
PFS of 171 Taiwanese with advanced GIST treated with IM.
Figure 2.
OS of 171 Taiwanese with advanced GIST treated with IM.
Table 4.
Prognostic Factors for PFS Based on Univariate Analyses and Final Multivariate Model.
| Factors | Univariate Analysis | Multivariate Analysis | |||||
| Total No. | No. Events | 5-Year PFS (%) | Cumulative Hazard Ratio | Log-rank, P | P | Hazard Ratio (95% CI) | |
| Age, y | |||||||
| ≤65 | 124 | 61 | 44.86 | 1 | 1 | ||
| >65 | 47 | 26 | 13.26 | 2.52 | .049 | .471 | 1.24 (0.70–2.19) |
| Sex | |||||||
| Male | 106 | 54 | 37.84 | 1 | |||
| Female | 65 | 33 | 38.33 | 0.99 | .981 | ||
| ECOG | |||||||
| 0, 1 | 139 | 65 | 42.77 | 1 | 1 | ||
| 2, 3 | 32 | 22 | 12.23 | 2.47 | .0002 | .048 | 1.93 (1.02–4.07) |
| Genetic status | |||||||
| Exon 9 | 22 | 18 | 18.70 | 1 | 1 | ||
| Exon 11 | 94 | 50 | 34.18 | 0.64 | .042 | 0.52 (0.28–0.98) | |
| Wild type | 13 | 4 | 64.10 | 0.27 | .003 | .036 | 0.29 (0.09–0.92) |
| Sum of tumor, cm | |||||||
| <11.5 | 77 | 32 | 41.18 | 1 | 1 | ||
| ≥11.5 | 80 | 48 | 32.67 | 1.26 | .043 | .004 | 2.18 (1.29–3.69) |
| WBC, x109/L | |||||||
| <10 | 136 | 70 | 38.30 | 1 | |||
| ≥10 | 19 | 10 | 24.56 | 1.46 | .706 | ||
| RBC, x109/L | |||||||
| <4.0 | 58 | 32 | 28.61 | 1 | |||
| ≥4.0 | 76 | 40 | 30.91 | 0.94 | .915 | ||
| Hemoglobin, g/dl | |||||||
| <12 | 93 | 50 | 31.17 | 1 | |||
| ≥12 | 61 | 29 | 41.03 | 0.76 | .328 | ||
| HCT, % | |||||||
| <36 | 71 | 41 | 23.79 | 1 | |||
| ≥36 | 64 | 32 | 36.64 | 0.70 | .391 | ||
| MCV, fl | |||||||
| <80 | 23 | 11 | 29.11 | 1 | |||
| ≥80 | 111 | 61 | 29.67 | 0.98 | .792 | ||
| Platelet, x109/L | |||||||
| <150 | 19 | 10 | 33.39 | 1 | |||
| ≥150 | 136 | 70 | 34.99 | 0.96 | .922 | ||
| Albumin, g/dl | |||||||
| <3.5 | 35 | 20 | 35.56 | 1 | |||
| ≥3.5 | 79 | 38 | 34.36 | 1.03 | .316 | ||
| INR | |||||||
| ≤1.2 | 70 | 38 | 20.94 | 1 | |||
| >1.2 | 10 | 6 | 35.00 | 0.67 | .832 | ||
| BUN, mg/dl | |||||||
| ≤21 | 110 | 58 | 30.32 | 1 | |||
| >21 | 14 | 8 | 40.41 | 0.76 | .820 | ||
| Creatinine, mg/dl | |||||||
| ≤1.03 | 99 | 54 | 32.69 | 1 | |||
| >1.03 | 52 | 25 | 37.34 | 0.88 | .239 | ||
| AST, U/L | |||||||
| <34 | 114 | 56 | 37.50 | 1 | |||
| ≥34 | 27 | 15 | 32.75 | 1.14 | .563 | ||
| ALT, U/L | |||||||
| <36 | 115 | 59 | 32.04 | 1 | |||
| ≥36 | 22 | 13 | 34.34 | 0.94 | .245 | ||
| ALK-P, U/L | |||||||
| <94 | 98 | 45 | 39.95 | 1 | |||
| ≥94 | 26 | 17 | 31.33 | 1.27 | .310 | ||
| Bil, T | |||||||
| <1.0 | 94 | 49 | 37.72 | 1 | |||
| ≥1.0 | 25 | 10 | 34.52 | 1.09 | .458 | ||
| Sodium, mEq/L | |||||||
| <139 | 55 | 32 | 23.13 | 1 | |||
| >139 | 48 | 24 | 43.80 | 0.56 | .818 | ||
| Potassium, mEq/L | |||||||
| ≤4 | 58 | 32 | 25.60 | 1 | |||
| >4 | 45 | 24 | 33.15 | 0.81 | .819 | ||
| Response | |||||||
| CR/PR | 98 | 41 | 44.53 | 1 | 1 | ||
| SD | 51 | 24 | 41.91 | 1.07 | .083 | 1.75 (0.93–3.29) | |
| PD | 22 | 22 | 0.00 | NA | <.0001 | <.0001 | 186.88 (37.15–940.09) |
95% CI indicates 95% confidence interval; ALK-P, alkaline phosphatase; ALT, alanine ainotransferase; AST, aspartate aminotransferase; Bil, T, total bilirubin; BUN, blood urea nitrogen; CR, complete response; ECOG, Eastern Cooperative Oncology Group; HCT, hematocrit; INR, international normalized ratio; PD, progressive disease; PFS, progression free survival; PR, partial response; SD, stable disease; y, years.
Table 5.
Prognostic Factors for OS Based on Univariate Analyses and Final Multivariate Model.
| Factors | Univariate Analysis | Multivariate Analysis | |||||
| Total No. | No. Events | 5-Year PFS (%) | Cumulative Hazard Ratio | Log-rank, P | P | Hazard Ratio (95% CI) | |
| Age, y | |||||||
| ≤65 | 124 | 33 | 65.50 | 1 | 1 | ||
| >65 | 47 | 18 | 49.43 | 1.67 | .024 | .531 | 0.72 (0.26–2.02) |
| Sex | |||||||
| Male | 106 | 32 | 62.39 | 1 | |||
| Female | 65 | 19 | 61.94 | 1.02 | .840 | ||
| ECOG | |||||||
| 0, 1 | 139 | 33 | 71.50 | 1 | 1 | ||
| 2, 3 | 32 | 18 | 19.39 | 4.89 | <.0001 | <.001 | 5.17 (2.10–12.75) |
| Genetic status | |||||||
| Exon 9 | 22 | 8 | 50.59 | 1 | |||
| Exon 11 | 94 | 32 | 65.70 | 0.62 | |||
| Wild type | 13 | 2 | 70.00 | 0.85 | .557 | ||
| Sum of tumor, cm | |||||||
| <11.5 | 77 | 15 | 72.71 | 1 | 1 | ||
| ≥11.5 | 80 | 30 | 52.88 | 2.00 | .012 | .027 | 3.21 (1.14–9.04) |
| WBC, x109/L | |||||||
| <10 | 136 | 39 | 58.34 | 1 | |||
| ≥10 | 19 | 6 | 81.16 | 0.39 | .906 | ||
| RBC, x109/L | |||||||
| <4.0 | 58 | 22 | 51.97 | 1 | |||
| ≥4.0 | 76 | 20 | 63.47 | 0.69 | .177 | ||
| Hemoglobin, g/dl | |||||||
| <12 | 93 | 31 | 50.48 | 1 | 1 | ||
| ≥12 | 61 | 14 | 75.30 | 0.42 | .054 | .157 | 0.29 (0.05–1.62) |
| HCT, % | |||||||
| <36 | 71 | 26 | 46.67 | 1 | 1 | ||
| ≥36 | 64 | 16 | 72.89 | 0.41 | .072 | .729 | 1.31 (0.29–6.01) |
| MCV, fl | |||||||
| <80 | 23 | 8 | 49.90 | 1 | |||
| ≥80 | 111 | 34 | 61.19 | 0.71 | .685 | ||
| Platelet, x109/L | |||||||
| <150 | 19 | 6 | 73.0 | 1 | |||
| ≥150 | 136 | 39 | 59.3 | 1.66 | .938 | ||
| Albumin, g/dl | |||||||
| <3.5 | 35 | 14 | 45.10 | 1 | 1 | ||
| ≥3.5 | 79 | 17 | 60.82 | 0.62 | .049 | .370 | 0.67 (0.28–1.60) |
| INR | |||||||
| ≤1.2 | 70 | 20 | 60.14 | 1 | |||
| >1.2 | 10 | 3 | 63.00 | 0.91 | .896 | ||
| BUN, mg/dl | |||||||
| ≤21 | 110 | 30 | 63.55 | 1 | |||
| >21 | 14 | 6 | 38.69 | 2.09 | .261 | ||
| Creatinine, mg/dl | |||||||
| ≤1.03 | 99 | 29 | 53.99 | 1 | |||
| >1.03 | 52 | 15 | 71.61 | 0.54 | .542 | ||
| AST, U/L | |||||||
| <34 | 114 | 30 | 59.52 | 1 | |||
| ≤34 | 27 | 9 | 62.36 | 0.91 | .610 | ||
| ALT, U/L | |||||||
| <36 | 115 | 35 | 59.75 | 1 | |||
| ≥36 | 22 | 5 | 67.03 | 0.78 | .664 | ||
| ALK-P, U/L | |||||||
| <94 | 98 | 25 | 58.66 | 1 | |||
| ≥94 | 26 | 8 | 59.33 | 0.98 | 0.634 | ||
| Bil, T | |||||||
| <1.0 | 94 | 24 | 60.47 | 1 | |||
| ≥1.0 | 25 | 7 | 44.33 | 1.62 | 0.741 | ||
| Sodium, mEq/L | |||||||
| <139 | 55 | 22 | 58.22 | 1 | |||
| >139 | 48 | 10 | 63.95 | 0.83 | 0.179 | ||
| Potassium, mEq/L | |||||||
| ≥4 | 58 | 22 | 54.35 | 1 | |||
| >4 | 45 | 10 | 71.90 | 0.54 | 0.244 | ||
| Response | |||||||
| CR/PR | 98 | 25 | 66.53 | 1 | 1 | ||
| SD | 51 | 12 | 68.69 | 0.92 | .160 | 2.12 (0.75–5.97) | |
| PD | 22 | 14 | 24.62 | 1.40 | <0.0001 | <.0001 | 17.65 (5.21–59.87) |
Figure 3.
PFS of 171 Taiwanese with advanced GIST treated with IM in ECOG performance status (A), tumor size (B), mutation status (C), and response (D).
Figure 4.
OS of 171 Taiwanese with advanced GIST treated with IM in ECOG performance status (A), tumor size (B), and response (C).
Discussion
This is one of the largest series in a single center that dealt with patients with advanced inoperable/metastatic GIST treated with IM for a decade. Several issues of interest emerged with the longer follow-up of Taiwanese patients with advanced inoperable/metastatic GIST on this study.
First, IM, a semiselective inhibitor of uncontrolled kinase activity of KIT and PDGFRA, can control advanced GIST in a large proportion of patients for more than 5 years. The 171 Taiwanese patients with advanced inoperable/metastatic GIST had a median PFS of 37.64 months and OS of 71.0 months after a median follow-up of up to 33.6 months. Of 171 patients, 87 (50.9%) developed progression and 51 (29.8%) died because of GIST. These results are substantially superior to the B2222 study [11] but similar to reports on Poland [15] and Korea [16] (median PFS was 40.5 and 48.0 months, respectively). Because a substantial fraction of patients (87/171, 50.9%) on this study eventually faced disease progression, this finding may imply that salvage therapy (potentially including increased imatinib doses [17], alternative tyrosine kinase inhibitors [18], or surgery [19]) is effective. Multimodality therapy is paramount for progression of GIST after IM treatment and to delay of death from GIST.
Second, similar to the B2222 study, long-term PFS and OS on IM were equivalent between patients with GIST achieving an objective response and even complete response by RECIST and those whose disease merely stabilized [11]. This study showed that the most definitive unfavorable factor for PFS and OS of patients with advanced inoperable/metastatic GIST is primary resistance. The reason for primary resistance as the most definitive unfavorable factor for PFS is obvious because primary resistance is defined as PFS of less than 6 months. However, it is still of note that the estimated median OS of patients with primary resistance was quite short (17.3 months), which suggests that salvage therapy for this population is less effective and/or that the mechanisms of resistance to tyrosine kinase inhibition between this population and those with secondary imatinib resistance are quite different.
Third, tumor size assessments based on RECIST demonstrated that tumor size is the other important predictor for PFS as well as OS in this study. Although response assessment systems using density and/or smaller changes in size are much more precise [20], it is likely that they would yield information on potential responses more quickly. Different from the B2222 study, tumor bulk was represented by quantifying five measurable largest lesions as depicted by RECIST [13]. Similar to the B2222 study, patients with the bulkiest tumors had significantly inferior PFS and OS compared to those who had smaller tumors. This supported the hypothesis that a larger number of tumor cells should be quantitatively proportional to a greater likelihood of harboring more resistant clones.
Fourth, similar to the B2222 study, our study showed that performance status and mutational status are independently associated with PFS [11]. Exon 9 mutation had the poorest PFS after IM treatment. Only performance status is also independently associated with OS in this study. Contrary to the B2222 study, this study did not show that sex, neutrophil count, albumin level, as well as other laboratory data were independently associated with either PFS or OS.
Finally, GISTs particularly present a variety of genomic mutations across two different receptor tyrosine kinase genes. KIT or PDGFRA mutation in Taiwanese patients with clinically advanced inoperable/metastatic GIST was examined in this study. In response rate for IM, similar to our previous report, IM induced a sustained objective response in more than half of the Taiwanese patients with advanced GISTs (96/171, 57.3%). Gain-of-function mutations of PDGFRA were only recently discovered in GISTs [21], and studies have reported that PDGFRA and kit mutations are mutually exclusive [14]. However, the incidence of PDGFR mutation in Taiwanese patients with advanced inoperable/metastatic GIST is very low (1.6%) compared with series from the west. Racial difference may be the reason for the difference in genetic alteration between the west and Taiwanese patients. A subset of GIST tumors in this study lacked detectable kit or PDGFRA mutations. Although such GISTs lack apparent genomic mutations, they can express phosphorylated kit or PDGFRA proteins that likely contribute to tumor proliferation or survival [14]. Contrary to the observation of Heinrich et al. [22], GISTs lacking a detectable kinase mutation had a similar clinical benefit for imatinib to tumor with an exon 11 mutation or an exon 9 mutation (77.8% vs 93.9% and 77.8% vs 61.9%, P = .154 and P = .675, respectively). Regarding the relationship between response rate and kinase mutation, kit exon 11 and exon 9 mutations predict a favorable response to IM. In this study, activated mutations of kit exons 11 and 9 are found in most patients (90.9%) with GIST. Similar to the study of Heinrich et al. [22], the clinical benefit did differ between the groups of patients whose GISTs had KIT exons 9 and 11 mutation (61.9% vs 93.3%, P = .0005). This observation confirmed metaGIST that the KIT oncoproteins encoded by exon 9 should be escalated to a higher dose of IM, although the number of cases is still limited.
In conclusion, the median PFS and OS of or 171 GIST patients are 37.6 and 71.0 months. Poor performance status, tumor size larger than 11.5 cm, primary resistance, and an exon 9 mutation were independently associated with an unfavorable PFS. Regarding OS, poor performance status, primary resistance, and tumor size larger than 11.5 cm were three independent unfavorable predictors.
References
- 1.Tzen CY, Wang JH, Huang YJ, Wang MN, Lin PC, Lai GL, Tzen CY. Incidence of gastrointestinal stromal tumor: a retrospective study based on immunohistochemical and mutational analyses. Dig Dis Sci. 2007;52(3):792–797. doi: 10.1007/s10620-006-9480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanke CD, Corless CL. State-of-the art therapy for gastrointestinal stromal tumors. Cancer Invest. 2005;23:274–280. doi: 10.1081/cnv-200055972. [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 4.Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 5.Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, Lydon NB. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295:139–145. [PubMed] [Google Scholar]
- 6.Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96:925–932. [PubMed] [Google Scholar]
- 7.Wang WL, Healy ME, Sattler M, Verma S, Lin J, Maulik G, Stiles CD, Griffin JD, Johnson BE, Salgia R. Growth inhibition and modulation of kinase pathways of small cell lung cancer cell lines by the novel tyrosine kinase inhibitor STI 571. Oncogene. 2000;19:3521–3528. doi: 10.1038/sj.onc.1203698. [DOI] [PubMed] [Google Scholar]
- 8.Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 9.Tuveson DA, Willis NA, Jacks T, Griffin JD, Singer S, Fletcher CD, Fletcher JA, Demetri GD. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20:5054–5058. doi: 10.1038/sj.onc.1204704. [DOI] [PubMed] [Google Scholar]
- 10.Demetri GD, vonvon Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 11.Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, Corless CL, Fletcher CD, Roberts PJ, Heinz D, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620–625. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 12.Yeh CN, Chen TW, Lee HL, Liu YY, Chao TC, Hwang TL, Jan YY, Chen MF. Kinase mutations and imatinib mesylate response for 64 Taiwanese with metastatic GIST: preliminary experience from Chang Gung Memorial Hospital. Ann Surg Oncol. 2007;14:1123–1128. doi: 10.1245/s10434-006-9288-1. [DOI] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;42:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 15.Rutkowski P, Nowecki ZI, Debiec-Rychter M, Grzesiakowska U, Michej W, Woźniak A, Siedlecki JA, Limon J, vel Dobosz AJ, Kakol M, et al. Predictive factors for long-term effects of imatinib therapy in patients with inoperable/metastatic CD117(+) gastrointestinal stromal tumors (GISTs) J Cancer Res Clin Oncol. 2007;133:589–597. doi: 10.1007/s00432-007-0202-4. [DOI] [PubMed] [Google Scholar]
- 16.Ryu MH, Kang WK, Bang YJ, Lee KH, Shin DB, Ryoo BY, Roh JK, Kang JH, Lee H, Kim TW, et al. A prospective, multicenter, phase 2 study of imatinib mesylate in Korean patients with metastatic or unresectable gastrointestinal stromal tumor. Oncology. 2009;76:326–332. doi: 10.1159/000209384. [DOI] [PubMed] [Google Scholar]
- 17.Zalcberg JR, Verweij J, Casali PG, Le Cesne A, Reichardt P, Blay JY, Schlemmer M, Van Glabbeke M, Brown M, Judson IR, et al. Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005;41(12):1751–1757. doi: 10.1016/j.ejca.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 18.Chen YY, Yeh CN, Cheng CT, Chen TW, Rau KM, Jan YY, Chen MF. Sunitinib for Taiwanese patients with gastrointestinal stromal tumor after imatinib treatment failure or intolerance. World J Gastroenterol. 2011;17(16):2113–2119. doi: 10.3748/wjg.v17.i16.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh CN, Chen TW, Lee HL, Liu YY, Chao TC, Hwang TL, Jan YY, Chen MF. Surgical management in metastatic gastrointestinal stromal tumor (GIST) patients after imatinib mesylate treatment. J Surg Oncol. 2010;102:599–603. doi: 10.1002/jso.21630. [DOI] [PubMed] [Google Scholar]
- 20.Benjamin RS, Choi H, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Charnsangavej C. We should decist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 21.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A. PDGFRA activating mutations in gastrointestinal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 22.Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST), author Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28(7):1247–1253. doi: 10.1200/JCO.2009.24.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]