Abstract
INTRODUCTION: P21-activated kinase 4 (PAK), a subfamily of serine/threonine kinases originally known as a regulator of cytoskeletal dynamics and cell motility, has recently been revealed to play a key role in oncogenic signaling pathways. We studied the frequency and clinical features of PAK4-overexpressed metastatic gastric cancer. PATIENTS AND METHODS: PAK4 overexpression was screened by Western blot in 18 human gastric cancer cell lines. Immunohistochemical staining of PAK4 protein was performed in tumor specimens of 49 metastatic gastric cancer patients who received palliative capecitabine/cisplatin as first-line treatment. RESULTS: PAK4 protein overexpression was detected strongly in five gastric cell lines (AGS, MGK-28, MKN-74, SNU-216, SNU-601) and weakly in four cell lines (KATOIII, MKN-1, SNU-620, and SNU-719). PAK4 knockdown by small interfering RNA induced apoptosis in PAK4-overexpressed AGS gastric cancer cells. Immunohistochemical staining revealed PAK4 overexpressions in 4 (8.1%) of 49 metastatic gastric cancer specimens. None of the four patients with PAK4(+) responded to capecitabine/cisplatin chemotherapy, and PAK4(+) gastric cancer patients had a trend of poorer survival compared with PAK(-)(P = .876). CONCLUSIONS: We demonstrated PAK4 overexpression in a subset of gastric cancer patients, implicating a role in gastric cancer tumorigenesis. Its prognostic significance and efficacy as a drug target should be further studied.
Introduction
Gastric cancer is the most common cancer type and the major cause of cancer death in Korea [1]. Although most of the newly diagnosed gastric cancer patients undergo curative resection, more than 50% of the patients with advanced gastric cancer develop recurrence after surgery. Thus, novel treatment strategies should be actively sought in advanced gastric cancer. P21-activated kinases (PAKs), conserved serine/threonine kinases that have recently been found to be key regulators of cancer cell signaling networks, play fundamental roles in a wide spectrum of cancer cellular mechanisms, including cancer cell motility, survival, apoptosis, and metastasis [2–5]. PAKs were firstly identified as the effectors for the small GTPases Cdc42 and Rac [6] and associated with cytoskeletal dynamics and actin depolymerization [6,7]. Six mammalian Paks have been identified and classified into groups 1 (PAKs1-3) and 2 (PAKs4-6) [8]. Previous studies have confirmed the overexpression of PAK4 in an array of cancer cell lines [2]. Recently, Pak4 overexpression and activation were associated with cancer metastasis, reduced patient survival, advanced stage, and increased resistance to chemotherapy in ovarian cancer [9]. PAK4 is thought to promote cancer cell progression through the regulation of c-Src, MEK-1/ERL1/2, MMP2, and c-Src/EGFR [9]. There is an increasing body of evidence that Pak4 may be a novel therapeutic target in many cancer types including gastric cancer [10]. The importance of PAK4 has been rehighlighted with the development of PAK4 kinase inhibitors as anticancer agents [6,7,11]. We undertook this study to survey the proportion of metastatic gastric cancer patients harboring Pak4 overexpression and to correlate the PAK4 positivity with treatment outcome to first-line capecitabine/cisplatin chemotherapy.
Patients and Methods
Cell Culture and Reagents
Eighteen human gastric cancer cell lines (AGS of moderately to poorly differentiated primary gastric adenocarcinoma; MKN-45 from poorly differentiated adenocarcinoma; MKN-28 and MKN-74 from gastric tubular adenocarcinoma; MKN-1 from gastric adenosquamous cell carcinoma; SNU-719 from moderately differentiated adenocarcinoma; SNU-216 and NCI-N87 from metastatic gastric adenocarcinoma; SNU-1, SNU-484, and SNU-520 from poorly differentiated primary gastric adenocarcinoma; KATOIII, SNU-601, and SNU-668 from metastatic signet ring cell carcinoma; and SNU-16, SNU-5, SNU-620, and SNU-638 from ascites of poorly differentiated adenocarcinoma) were purchased from Korea Cell Line Bank (Seoul, South Korea). All of the cell lines were grown in RPMI-1640 medium (PAA Laboratories GmbH, Pasching, Austria) supplemented with 10% heat-inactivated fetal bovine serum and antibiotic/antimycotic. Cells were incubated at 37°C in 5% CO2, and the medium was changed twice a week. After confluence, cells were subdivided into new flasks until the end of the experiment.
Small Interfering RNA Treatment of AGS Cells
PAK4 targeting small interfering RNA (siRNA) and control siRNA were obtained from Dharmacon (Lafayette, CO). AGS cells were transfected with siRNA with Effectine transfection reagent as per the manufacturer's protocol (Qiagen, Hilden, Germany).
Western Blot Analysis
Total cell extracts were obtained using lysis buffer containing 20 mM HEPES (pH 7.4), 150 mM NaCl, 1 mM MgCl2,1mM EDTA,2mM EGTA,10% glycerol,1%TritonX-100, 1 µg/ml leupeptin, and 1 µg/ml aprotinin. Equal amounts (30 µg) of cell lysates were dissolved in 8% or 12% Tris-glycine gels with TrisGly running buffer (Invitrogen, Novex, Carlsbad, CA), transferred onto nitrocellulose membrane, and incubated with following specific antibodies: polyclonal rabbit PAK4 antibody (ab62509; Abcam plc, Cambridge, United Kingdom), cleaved caspase-3 antibody (9664; Cell Signaling Technology, Boston, MA), cleaved caspase-9 antibody (9501; Cell Signaling Technology), cleaved PARP antibody (9541; Cell Signaling Technology), and rabbit polyclonal tubulin (sc9104; Santa Cruz Biotechnology, Santa Cruz, CA). Immune complexes were visualized by enhanced chemiluminescence (Novex ECL, Invitrogen).
Patients
From February 2009 to July 2010, 49 metastatic gastric cancer patients who received palliative capecitabine/cisplatin chemotherapy had tumor specimens available for immunohistochemical staining. Formalin-fixed paraffin sections were stained with polyclonal rabbit anti-Pak4 antibody (ab62509; Abcam plc) with 1:500 dilution. (All tumor slides were reviewed by one pathologist who is an expert in gastrointestinal pathology [K.-M.K.].) All clinical and pathologic variables were collected. Treatment response to capecitabine/cisplatin chemotherapy was evaluated by RECIST 1.0 criteria [12]. The protocol has been approved by the institutional review board at Samsung Medical Center.
Statistical Analyses
Overall survival was measured from the first date of palliative chemotherapy to the date of death. Overall survival was calculated using the Kaplan-Meier method. Correlation analyses of the expression of PAK4 with clinical and pathologic variables were performed using the two-sided χ2 test or Fisher exact test. Difference in overall survival according to expressions of PAK4 was compared using log-rank test. P < .05 was considered statistically significant.
Results
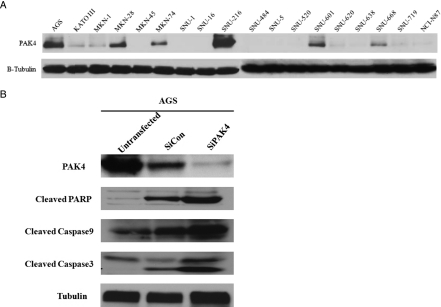
PAK4 overexpression was screened in 18 gastric cancer cell lines in vitro by immunoblot analysis. As shown in Figure 1A, PAK4 protein overexpression was strongly detected in five cell lines (AGS, MKN-28, MKN-74, SNU-216, and SNU-601) and weakly in four cell lines (KATOIII, MKN-1, SNU-620, and SNU-719). AGS gastric cancer cells with high levels of PAK4 protein expression were treated with PAK4 targeting siRNA (Figure 1B). siPAK4 treatment induced apoptosis in AGS cells as confirmed by caspase 3, caspase 9, and PARP cleavages.
Figure 1.
(A) Results of PAK4 expression level test using by Western blot. (B) Results of PAK4 knockdown by siRNA treatment in PAK4-overexpressed AGS cancer cell lines.
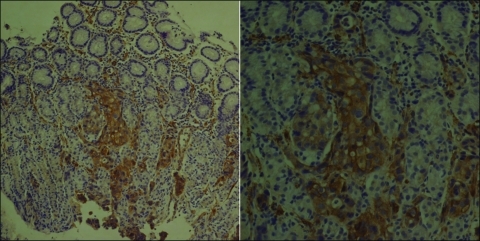
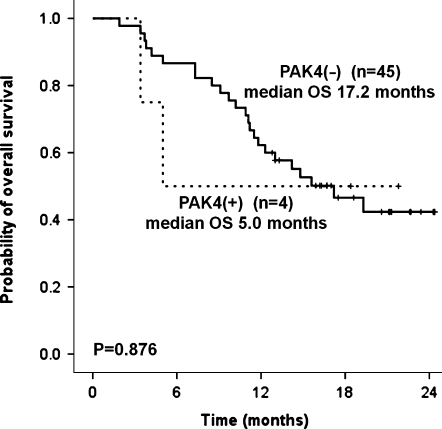
Next, we surveyed the PAK4 overexpression in 49 tumor specimens patients with from metastatic, unresectable gastric cancer who received first-line capecitabine/cisplatin chemotherapy. Twenty-one patients (42.9%) had poorly differentiated adenocarcinoma and 16 patients (32.7%) had signet ring cell carcinoma. At the time of first-line palliative chemotherapy, the most common sites of metastases were as follows in the order of frequency: 27 (55.1%) peritoneal seeding, 15 (30.6%) distant lymph node metastases, 5 (10.2%) ovarian metastases, and 5 (10.2%) liver metastases (Table 1). A representative photomicrograph of immunohistochemical staining for PAK4 is provided in Figure 2. In positive cases, PAK4 was stained deep brown in the cytoplasm of tumor cells. All four cases positively stained for PAK4 were poorly differentiated tubular adenocarcinomas. None of differentiated-type adenocarcinomas or signet ring cell carcinomas were positive for PAK4. Correlative analyses between PAK4 and clinical parameters demonstrated no significant relationship with age, Lauren type, lymphovascular invasion, histologic diagnosis, and tumor extent (Table 2). Of note, none of the four PAK4(+) gastric cancer patients demonstrated response to capecitabine/cisplatin chemotherapy. One patient initially responded to two cycles of capecitabine/cisplatin but did not maintain the response for more than 4 weeks and developed disseminated disease. The remaining three patients did not respond to chemotherapy. Hence, although statistical significance could not be reached because of the small number of gastric carcinomas with PAK4(+), the response rate to capecitabine/cisplatin was low (0%) in PAK4(+) patients when compared with PAK4(-) (24.4%) patients. In line with this, patients with PAK4(+) gastric cancer had a tendency toward poorer survival when compared with PAK(-) gastric cancer patients (P = .876; Figure 3).
Table 1.
Patients' Characteristics.
| Characteristics | |
| Age (years) | |
| Median, range | 53, 28–76 |
| Sex, n (%) | |
| Male | 35 (71.4) |
| Female | 14 (28.6) |
| Timing of distant metastasis, n (%) | |
| Synchronous | 42 (85.7) |
| Metachronous | 5 (10.2) |
| Unresectable | 2 (4.1) |
| Type of gastrectomy, n (%) | |
| Subtotal | 4 (8.1) |
| Total | 12 (24.4) |
| None | 31 (63.3) |
| Palliative bypass surgery | 2 (4.0) |
| Location of tumor, n (%) | |
| Distal 1/3 | 14 (28.6) |
| Middle 1/3 | 16 (32.7) |
| Proximal 1/3 | 11 (22.4) |
| Diffuse | 8 (16.3) |
| Histologic diagnosis, n (%) | |
| Well differentiated adenocarcinoma | 0 (0) |
| Moderately differentiated adenocarcinoma | 10 (20.4) |
| Poorly differentiated adenocarcinoma | 21 (42.9) |
| Signet ring cell carcinoma | 16 (32.7) |
| Others | 2 (4.0) |
| Extent of disease at the time of diagnosis of distant metastasis, n (%) | |
| Confined to peritoneum (including peritoneal seeding) | 27 (55.1) |
| Confined to intra-abdominal solid organ | 11 (22.4) |
| Extra-abdominal metastasis | 9 (18.4) |
| Locally advanced, unresectable | 2 (4.0) |
| Metastatic site, n (%) | |
| Peritoneum or omentum | 27 (55.1) |
| Lymph node | 15 (30.6) |
| Ovary | 5 (10.2) |
| Liver | 5 (10.2) |
| Ascites | 4 (8.1) |
| Lung | 3 (6.1) |
| Bone | 3 (6.1) |
Figure 2.
PAK4 overexpression in gastric cancer by immunohistochemical staining.
Table 2.
Comparison of Clinical Features According to PAK4 Expression.
| No. cases (N = 49) | PAK4 Expression | |||
| Positive (n = 4) | Negative (n = 45) | P | ||
| Age (years) | ||||
| ≤60 | 35 (70.8%) | 3 (75.0%) | 32 (70.5%) | N-C |
| >60 | 14 (29.2%) | 1 (25.0%) | 13 (29.5%) | |
| Lauren type | ||||
| Intestinal | 7 (14.3%) | 0 (0%) | 7 (15.6%) | N-C |
| Diffuse | 7 (14.3%) | 1 (25.0%) | 6 (13.3%) | |
| Mixed | 2 (4.1%) | 0 (0%) | 2 (4.4%) | |
| Unknown | 33 (67.3%) | 3 (75.0%) | 30 (66.7%) | |
| Lymphovascular invasion | ||||
| Presence | 14 (28.6%) | 1 (25.0%) | 13 (28.9%) | N-C |
| Absence | 1 (2.0%) | 0 (0%) | 1 (2.2%) | |
| Unknown | 34 (69.4%) | 3 (75.0%) | 31 (68.9%) | |
| Histologic diagnosis | ||||
| Adenocarcinoma | 33 (67.3%) | 3 (75.0%) | 30 (66.7%) | N-C |
| Signet ring cell carcinoma | 16 (32.7%) | 1 (25.0%) | 15 (33.3%) | |
| Best response to capecitabine/cisplatin chemotherapy | ||||
| CR/PR | 12 (24.5%) | 0 (0.0%) | 11 (24.4%) | N-C |
| SD | 28 (57.1%) | 3 (75.0%) | 25 (55.6%) | |
| PD | 7 (14.3%) | 1 (25.0%) | 7 (15.6%) | |
| Not evaluated | 2 (4.1%) | 0 (0%) | 2 (4.4%) | |
| Metastatic sites | ||||
| Confined to intraperitoneuthem (peritoneum, omentum, ascites, ovary, intra-abdominal lymph node) | 36 (73.5%) | 3 (75.0%) | 33 (73.3%) | N-C |
| Extraperitoneal organs (lung, liver, bone) | 13 (26.5%) | 1 (25.0%) | 12 (26.7%) | |
CR indicates complete remission; N-C, not contributable; PD, progressive disease; PR, partial remission; SD, stable disease.
Figure 3.
Comparison of overall survival according to PAK4 overexpression status in 49 gastric cancer patients.
Discussion
The survival outcome of metastatic gastric cancer patients despite palliative chemotherapy is still unsatisfactory. On the basis of our recent retrospective analysis on 1455 unresectable, metastatic gastric cancer patients who were treated palliative chemotherapy from 1994 to 2005, the median survival time was only 8.6 months (95% confidence interval [CI], 8.1–9.1 months) [13]. Recently, the first molecular target with significant survival benefit was identified in gastric cancer. In TOGA trial, median survival time was 13.8 months (95% CI, 12–16 months) in HER2(+) patients assigned to trastuzumab plus 5-fluorouracil/cisplatin or capecitabine/cisplatin when compared with 11.1 months (95% CI, 10–13 months; P = .0046) [14]. Through this trial, the potential survival benefit from molecularly targeted agents in specific segments of gastric patient population has been strongly suggested, which warrants further investigation for molecular targets. Recently, PAK4 has been highlighted as a potential novel target for solid tumors [7,10,15]. PAK4 inhibitors effectively inhibited PAK4-dependent pathways leading to tumor shrinkage in multiple human tumor xenografts including colon, breast, lung, and melanoma [7]. Nevertheless, PAK4 inhibitors or PAK4 over-expression have not been extensively surveyed in gastric cancer yet. The expression of PAK4 has been reported in one small study, which reported a significant and positive correlation between PAK4 expression and lymph node metastasis [16].
Callow et al. [2] analyzed the roles of PAK4 and PAK6 have been tested in a panel of cell lines derived from leukemias, melanomas, breast cancer, colon cancer, lung cancer, liver cancer, ovarian cancer, and renal cancer. They found that the PAK4 gene localizes at a region of chromosome 19, which is commonly amplified in a number of human pancreatic cancer, colon cancer, and ovarian cancers. The PAK4 messenger RNA and gene amplification were not tested in our series; however, there are several reports that support a strong correlation between amplification and protein expression in multiple cancer types [9,10].
In line with our study, the association between chemoresistance and PAK4 overexpression has been suggested in ovarian cancer. They surveyed PAK4 overexpression in relation to response to paclitaxel/platinum in 70 ovarian cancer patients and found that patients with high scores of total PAK protein were significantly correlated with poor response to first-line chemotherapy [9]. Although any conclusion is limited from small sample size, none of the PAK4(+) patients responded to first-line capecitabine/cisplatin chemotherapy. The prognostic significance of PAK4 needs to be validated in a larger patient cohort. Of note, our preclinical in vitro test demonstrated that PAK4 knockdown by siRNA-induced apoptosis in PAK4(+) gastric cancer cell line. Whether PAK4 overexpression will represent another subgroup of gastric cancer patients such as HER2(+) with effective druggable target should be evaluated in future clinical trials.
References
- 1.Bae JM, Won JY, Jung KW, Park JG. Annual report of the Korean central cancer registry program 2000. Cancer Res Treat. 2002;34:77–83. doi: 10.4143/crt.2002.34.2.77. [DOI] [PubMed] [Google Scholar]
- 2.Callow MG, Clairvoyant F, Zhu S, Chryver B, Whyte DB, Bischoff JR, Jallal B, Smeal T. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J Biol Chem. 2002;277(1):550–558. doi: 10.1074/jbc.M105732200. [DOI] [PubMed] [Google Scholar]
- 3.Qu J, Cammarano MS, Shi Q, Ha KC, de Lanerolle P, Minden A. Activated PAK4 regulates cell adhesion and anchorage-independent growth. Mol Cell Biol. 2001;21(10):3523–3533. doi: 10.1128/MCB.21.10.3523-3533.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci. 2005;118(pt 9):1861–1872. doi: 10.1242/jcs.02313. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Xiao H, Tian Y, Nekrasova T, Hao X, Lee HJ, Suh N, Yang CS, Minden A. The Pak4 protein kinase plays a key role in cell survival and tumorigenesis in athymic mice. Mol Cancer Res. 2008;6(7):1215–1224. doi: 10.1158/1541-7786.MCR-08-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, Peterson JR. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15(4):322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray BW, Guo C, Piraino J, Westwick JK, Ahzng C, Lamerdin J, Dagostino E, Knighton D, Loi CM, Zager M, et al. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci USA. 2010;107(20):9446–9451. doi: 10.1073/pnas.0911863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar R, Gururaj AE, Barnes CJ. p21-Activated kinases in cancer. Nat Rev Cancer. 2006;6(6):459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- 9.Siu MK, Chan HY, Kong DS, Wong ES, Wong OG, Ngan HY, Tam KF, Zhang H, Li Z, Chan QK, et al. p21-Activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients. Proc Natl Acad Sci USA. 2010;107(43):18622–18627. doi: 10.1073/pnas.0907481107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Liu F, Li F. PAK as a therapeutic target in gastric cancer. Expert Opin Ther Targets. 2010;14(4):419–433. doi: 10.1517/14728221003642019. [DOI] [PubMed] [Google Scholar]
- 11.Sahai E. Mechanisms of cancer cell invasion. Curr Opin Genet Dev. 2005;15(1):87–96. doi: 10.1016/j.gde.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Lim T, Uhm JE, Park KW, Park SH, Lee SC, Park JO, Park YS, Lim HY, Sohn TS, et al. Prognostic model to predict survival following first-line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol. 2007;18(5):886–891. doi: 10.1093/annonc/mdl501. [DOI] [PubMed] [Google Scholar]
- 14.Bang YJ, Van Custem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 15.Molli PR, Li DQ, Murray BW, Rayala SK, Kumar R. PAK signaling in oncogenesis. Oncogene. 2009;28(28):2545–2555. doi: 10.1038/onc.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Hu X, Li F. Expression of PAK4 and its significance in gastric cancer. Chin J Oncol. 2008;30(1):45–48. [Google Scholar]