Abstract
Glioblastoma multiforme (GBM) overexpresses interleukin 13 receptor α2 (IL-13Rα2), a tumor-restricted receptor that is not present in normal brain. We and others have created targeted therapies that specifically eradicate tumors expressing this promising tumor-restricted biomarker. As these therapies head toward clinical implementation, it is critical to explore mechanisms of potential resistance. We therefore used a potent IL-13Rα2-targeted bacterial cytotoxin to select for naturally occurring “escapee” cells from three different IL-13Rα2-expressing GBM cell lines. We found that these side populations of escapee cells had significantly decreased IL-13Rα2 expression. We examined clinically relevant biologic characteristics of escapee cell lines compared to their parental cell lines and found that they had similar proliferation rates and equal sensitivity to temozolomide and radiation, the standard therapies given to GBM patients. In contrast, our escapee cell lines were less likely to form colonies in culture and migrated more slowly in wound healing assays. Furthermore, we found that escapee cells formed significantly less neurospheres in vitro, suggesting that IL-13Rα2-targeted therapy preferentially targeted the “stem-like” cell population and possibly indicating decreased tumorigenicity in vivo. We therefore tested escapee cells for in vivo tumorigenicity and found that they were significantly less tumorigenic in both subcutaneous and intracranial mouse models compared to matching parental cells. These data, for the first time, establish and characterize the clinically relevant biologic properties of IL-13Rα2-targeted therapy escapees and suggest that these cells may have less malignant characteristics than parental tumors.
Introduction
High-grade astrocytomas including anaplastic astrocytomas (World Health Organization grade 3) and glioblastoma multiforme (GBM; World Health Organization grade 4) are invariably fatal malignancies despite the current standard of care that includes surgery, chemotherapy, and radiation therapy [1,2]. Molecular targeted therapies have emerged as an innovative approach to eradicate tumors that express tumor-restricted biomarkers without harming normal brain or tissue. In past works, we found that interleukin 13 receptor α2 (IL-13Rα2) is a high-grade astrocytoma-restricted receptor for IL-13 that is overexpressed in greater than 70% of GBMs but not significantly expressed in brain or normal tissues except in the testes [3–7]. Thus, we and others have developed a number of therapeutic strategies that potently and specifically target GBM cells expressing IL-13Rα2 [4,5,8–17]. For example, Mintz et al. [17] and Debinski et al. [18–20] used powerful bacterial toxins genetically fused to targeted derivatives of IL-13 to eradicate IL-13Rα2-expressing tumors. Importantly, a first generation IL-13-based cytotoxin, IL-13-PE38QQR, has shown clinical activity in phase 1 and 2 clinical trials for GBM patients when administered locally with convection-enhanced delivery (CED) [21–26]. Of significance, the phase 3 Randomized Evaluation of CED of IL-13-PE38QQR with Survival End points (PRECISE) trial demonstrated increased overall survival of patients treated by neurosurgeons with experience performing CED [27], although it did not reach its clinical end points owing to a number of factors, including use of early generation sub-optimized CED, lack of biomarker expression screening, and possibly affinity toward the physiologically abundant IL-13Rα1/IL4Rα heterodimer that is expressed on healthy brain [27–29]. However, we and others are building on the positive findings of the PRECISE trial by developing advanced platforms that exploit IL-13Rα2-specific ligands, such as targeted mutants of IL-13, peptides and antibodies, which only target the tumor-associated IL-13Rα2 [14,15,30]. Other advances in IL-13Rα2 targeting include systemically administered therapies [11,12,16] and diagnostic technologies that can quantitate, a priori, IL-13Rα2 expression [31]. Furthermore, significant improvements to CED promise to effectively increase the volume exposed to locally delivered IL-13Rα2-targeted therapies [7,32] as it has been estimated by new software developed after the PRECISE trial completion that only 21.1% of the highest-risk 2-cm region surrounding the resection cavity was covered on average in the trial [32]. Thus, as these improved platforms are developing, it is important to examine mechanisms and clinically relevant biologic characteristics of cells that escape targeted IL-13Rα2 therapy.
As with other therapies, development of therapeutic resistance remains a major challenge because, as cells either become or are inherently resistant to a therapy, they may also become more aggressive and be less sensitive toward standard therapeutics [33,34]. Therapeutic resistance can be actively induced in cancer cells on exposure to a specific therapeutic agent, such as when anticancer drugs are pumped out through drug efflux pumps that can be present before therapy and amplified directly in response to therapeutic administration. In contrast, cancer cells may naturally not be sensitive to a particular agent because of expression alterations that undermine a drug's action [35]. Examples of this passive resistance include cancer cells that are naturally not susceptible to taxanes due to lost expression of β2-tubulin, a protein to which they must bind to exert their anticancer action [36], and intrinsic resistance to tumor necrosis factor-related apoptosis-inducing ligand that is sometimes encountered due to absent expression of functional DR4 and DR5, the death receptors that bind tumor necrosis factor-related apoptosis-inducing ligand and induce apoptosis in cancer cells [37]. In this work, for the first time, we selected subpopulations of GBM cells that are naturally not susceptible to IL-13Rα2-targeted therapy because of the lack of receptor expression. Furthermore, we examine clinically relevant biologic properties of these “escapee” cells as well as their susceptibility to the current standard therapies used in the clinic to treat GBM patients.
Materials and Methods
Materials
Tissue culture equipment was from Corning Glass (Corning, NY). SNB-19 and A-172 cell lines were purchased from American Type Culture Collection (Manassas, VA). U-251 malignant glioma (MG) cell line was a gift from Dr. Jay Dorsey. MTS/PMS (3-(4,5-dimethylthiazol-2yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt/phenazine methosulfate was purchased from Promega (Madison, WI). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot transferring equipment were from Bio-Rad (Hercules, CA) and Invitrogen (Carlsbad, CA). Primary anti-IL-13Rα2 antibody (AF146) was obtained from R&D Systems (Minneapolis, MN), and secondary antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). IODO-GEN reagents for 125I labeling were purchased from Pierce (Rockford, IL).
Selection of IL-13Rα2-Targeted Therapy Escapee Cells
To obtain subpopulations of GBM cells that are not sensitive to IL-13Rα2-targeted therapy, we treated three different IL-13Rα2-expressing GBM cell lines (SNB-19, A-172, and U-251) with high doses of IL-13-based cytotoxins (10-100 ng/ml) that we previously demonstrated to potently kill IL-13Rα2-expressing cells. Importantly, we alternated the selection with IL-13-based Diphtheria (DT) and IL-13-based Pseudomonas exotoxin (PE) derivatives to prevent resistance to a specific type of bacterial toxin [15,38]. We also performed similar selection on two early-passage tumor cell lines derived from patients diagnosed with GBM that we obtained from the Wake Forest University Brain Tumor Center of Excellence tumor depository (BTCOE 4673 and 4682, both at passage 6).
Cytotoxicity Assay
Cytotoxicity assays were performed as described previously [15]. Briefly, 1 x 103 cells were plated on each well of a 96-well plate in 150 µl of medium and allowed to adhere overnight. The following day, 25 µl of blocker (8 µg/ml), for example, IL-13 derivatives in 0.1% bovine serum albumin/PBS buffer, or buffer alone, was added. After an hour of incubation at 37°C, 25 µl of varying concentrations of IL-13-based PE or DT was added in quadruplicate and incubated at 37°C in 5% CO2/95% O2 for 48 hours. After 48 hours, MTS/PMS cell proliferation assay was performed as instructed by the manufacturer (Promega). Ten microliters of dye was added to each well and incubated for 2 to 4 hours. Plates were then read using a microplate reader at an absorbance of 490 nm. Cyclohexamide-treated, cell-containing wells served as the background for the assay. Background was subtracted from each data point and was divided by the value from the wells that were not treated with cytotoxin to obtain the fraction of cells remaining. The fraction of cells remaining was multiplied by 100 to obtain the percent of control.
Western Blot
Western blot analysis was done as described previously [39]. Membranes were incubated with primary antibody overnight at 4°C and with secondary antibody conjugated with HRP for 1 hour at room temperature. Detection was done using the enhanced chemiluminescence plus Western Blot Analysis Detection System (GE Healthcare, Piscataway, NJ) with the LAS-3000 imaging system (Fujifilm) and images compiled using Adobe Photoshop Elements 5.0 (Adobe Systems, San Jose, CA). Lysates of early-passage cells were harvested at passage 8.
Clonogenic Assay
Cells (100 cells per well) were plated in six-well plates in triplicate in growth medium, which was replaced with fresh medium every 3 days. Colonies were microscopically imaged at low power (x4 magnification) after 14 days. Plates were then washed with PBS, fixed with methanol, stained with crystal violet, and photographed.
Cell Proliferation Assay
A total of 1 x 103 cells per well were plated in 96-well plates in quadruplicate for each cell line. After 24, 48, and 72 hours in culture, proliferation was measured using a colorimetric MTS/PMS cell proliferation assay as instructed by manufacturer (Promega). Cyclohexamide-treated cell-containing wells served as the background for the assay. Background was subtracted from each data point and was divided by the values obtained after 24 hours to represent fold proliferation.
Temozolomide Sensitivity Assay
A total of 1 x 103 cells per well were plated in 96-well plates in growth medium and allowed to adhere overnight. The following day, varying concentrations of temozolomide (TMZ; Sigma-Aldrich, St Louis, MO) were added in quadruplicate and incubated at 37°C in 5% CO2/95% O2 for 72 hours. After 72 hours, MTS/PMS cell proliferation assay was performed as instructed by the manufacturer (Promega). Cyclohexamide-treated cell-containing wells served as the background for the assay. Background was subtracted from each data point and was divided by the value from the wells that were not treated with TMZ to obtain the fraction of cells remaining. The fraction of cells remaining was multiplied by 100 to obtain the percent of control.
Radiation Sensitivity Assay
A total of 1 x 103 cells per well were plated in 96-well plates in growth medium and allowed to adhere overnight. The following day, plates were subjected to varying doses of external beam irradiation or sham control and incubated at 37°C in 5% CO2/95%O2 for 72 hours. After 72 hours, MTS/PMS cell proliferation assay was performed as instructed by the manufacturer (Promega). Cyclohexamide-treated, cell-containing wells served as the background for the assay. Background was subtracted from each data point and was divided by the value from the wells that received sham irradiation to obtain the fraction of cells remaining. The fraction of cells remaining was multiplied by 100 to obtain the percent of control.
Migration Assay
Cells were cultured in six-well plates in triplicate for each cell line until approximately 90% confluent. Wounds were made in each confluent monolayer of cells with a sterile 200-µl pipette tip, and fresh growth medium was replaced. Phase-contrast microscopic pictures were taken of the same field at 0, 4, 8, and 24 hours or until the wounds were completely healed, whichever came first.
Neurosphere Formation Assay of U-251 Parental and Escapees
U-251 parental cells or escapees were resuspended in neurobasal medium (Invitrogen) containing B27 (1x), N2 (1x), epidermal growth factor (5 ng/ml), basic fibroblast growth factor (5 ng/ml), and l-glutamine (5 ng/ml). Cells were then plated in 6-cm dishes at 5 x 105 cells per dish for 7 days with fresh medium replenished every 3 days [40–42]. Luciferin-D substrate was added (150 µg/dish), and plates were imaged with IVIS-100 Imaging System (Caliper Life Sciences, Hopkinton, MA). Luminescence signal was quantified using LivingImage software accompanied the imaging system.
In Vivo Tumorigenicity of U-251 Parental and Escapees
U-251 parental and escapee cells were implanted subcutaneously into male athymic nude (nu/nu) mice at 1 x 106 cells per mouse in 200 µl of PBS/Matrigel (BD Biosciences) as recommended by the manufacturer. Tumor measurement was obtained weekly using a digital caliper. When tumors reached a volume exceeding 1500 mm3, mice were killed and tumors were removed then snap frozen.
Actively growing U-251 parental and escapee cells were injected at a concentration of 1 x 105 cells in 5 µl of PBS into the right frontal lobe of male athymic nude (nu/nu) mice (6–8 weeks old) as previously described [43]. Briefly, mice were anesthetized with a ketamine/xylazine mixture (114/17 mg/kg), and a 0.5-mm burr hole was made 1 to 1.5 mm lateral of the midline and 0.5 to 1 mm posterior to the coronal suture through a scalp incision. Stereotaxic injection at a rate of 2 µl/min was performed on a Just For Mice stereotaxic apparatus (Harvard Apparatus, Holliston, MA) using a 10-µl syringe (Hamilton, Reno, NV) with a 30-gauge needle, inserted through the burr hole to a depth of 3 mm. Animals losing ≥20% of their body weight or having trouble ambulating or feeding were killed by transcardial perfusion.
Autoradiography
Recombinant IL-13 derivatives were labeled with 125I using IODO-GEN method as recommended by the manufacturer (Pierce). Autoradiography was performed as previously described [5]. Digital detection was done using a storage phosphor screen with the Typhoon Trio (GE Healthcare), and images were compiled using Adobe Photoshop Elements 5.0 (Adobe Systems).
Statistical Analyses
Statistical significance was determined by Student's t test. Error bars represented mean ± SD.
Results
Selection of IL-13Rα2-Targeted Therapy Escapee Cells and Their Characterization
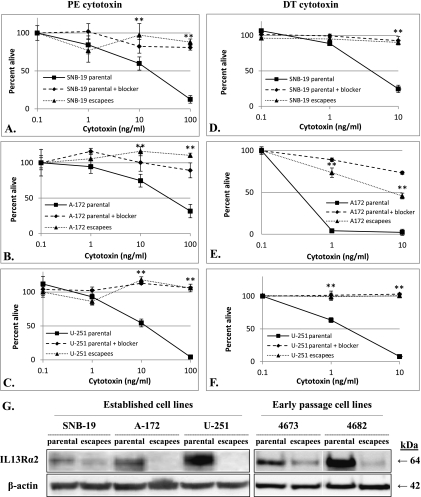
The escapee cell lines were established by continual passage of resistant cells in growth medium supplemented with cytotoxin. After the initial treatment, less than 5% to 10% of cells survived (Figure 1, A–F), but as we continued selecting these nonsensitive cells, they became less responsive to either PE or DT based toxins. After passaging for at least 8 weeks under selection conditions, all escapee cell lines were not at all sensitive to the cytotoxin effects of both IL-13-based PE and DT toxins (Figure 1, A–F), suggesting an IL-13Rα2-based resistance mechanism as opposed to resistance to an individual toxin payload. We subsequently examined the expression of IL-13Rα2 and found significantly decreased or absent IL-13Rα2 expression in all three escapee cell lines compared with parental cells (Figure 1G). To ensure that our escapee cells were not an artifact of long-term cell culture, we performed a similar selection strategy on two early-passage tumor cell lines derived from patients diagnosed with GBM (BTCOE 4673 and 4682) and found that we were also able to select for subpopulations of cells that had significantly decreased susceptibility to the IL-13Rα2-targeted cytotoxin and decreased receptor expression (Figure 1G).
Figure 1.
Susceptibility of GBM parental and escapee cells to IL-13Rα2-targeted toxins (excess amounts of IL-13Rα2-specific IL-13 mutants served as blockers in these assays and percentage of cells alive was calculated as percent A490 of untreated control). (A–C) Cytotoxicity of IL-13-based PE toxin on (A) SNB-19 parental and escapees, (B) A-172 parental and escapees, (C) U-251 parental and escapees. (D–F) Cytotoxicity of IL-13-based DT toxin on (D) SNB-19 parental and escapees, (E) A-172 parental and escapees, (F) U-251 parental and escapees (*P ≤ .05, **P ≤ .01 comparing parental and escapee cell lines). (G)Western blot for IL-13Rα2 in SNB-19, A-172, U-251, and BTCOE 4673 and 4682 parental and escapee cell lysates. Similar to the established cell lines, BTCOE 4673 and 4682 early-passage escapee cell lines were no longer susceptible to either IL-13-based toxin (not shown).
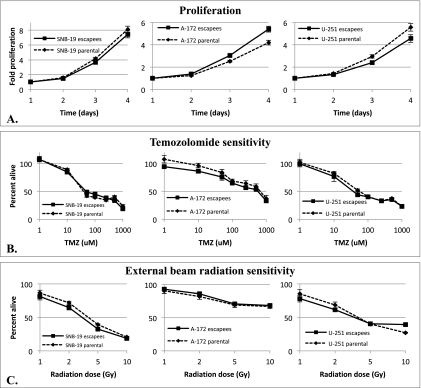
IL-13Rα2-Targeted Therapy Escapees Proliferate Similar to Parental Cells and Remain Sensitive to TMZ and Radiation
We next examined clinically relevant biologic characteristics of escapee cell lines compared to their parental cell lines, including proliferation rates and sensitivity toward TMZ and radiation therapy, the standard of care given to newly diagnosed GBM patients [44,45]. We analyzed the proliferation rates of all escapee cell lines and found no significant difference of all three escapee cell lines compared to their respective parental lines (Figure 2A). Similarly, all three escapee cell lines demonstrated similar sensitivity to either TMZ (Figure 2B) or radiation therapy (Figure 2C) compared to their respective parental lines. Importantly, these data suggest that IL-13Rα2-targeted approaches do not select for more proliferative tumor cells or tumors that are cross resistant to standard GBM therapies, such as TMZ and radiation therapy.
Figure 2.
Proliferation (A), TMZ sensitivity (B), and radiation sensitivity (C) determined in a colorimetric cell assay by SNB-19 parental and escapee cells, A-172 parental and escapee cells and U-251 parental and escapee cells. No significant statistical difference in proliferation and sensitivity to TMZ was observed between matching parental and escapee cells at any time point. No consistent significant difference in sensitivity to radiation was observed across the parental and matching escapee cell lines examined.
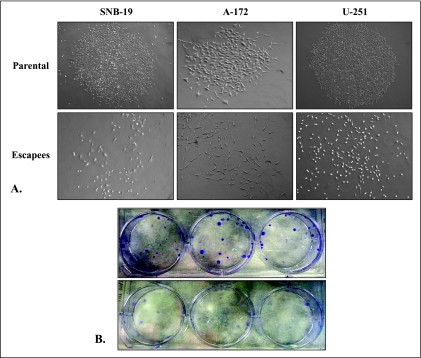
IL-13Rα2-Targeted Therapy Escapees Do Not Form Individual Colonies In Vitro
An in vitro clonogenic assay was performed on each pair of parental-escapee GBM cell lines to compare their colony formation patterns. As expected, all GBM parental cell lines readily formed characteristic colonies in vitro when plated as single cells (Figure 3A). However, the GBM escapees instead followed a disperse growth pattern as demonstrated microscopically in Figure 3A. Similarly, the different colony formation pattern was also evident macroscopically because the escapee cell lines did not demonstrate characteristically discrete colonies when fixed and stained (Figure 3B).
Figure 3.
Colony formation by SNB-19, A-172, and U-251 parental and escapee cells. (A) Representative microscopic appearance of colonies formed by each cell line or lack thereof. (B) Macroscopic appearance of U-251 parental (upper panel) and escapee (lower panel) clonogenic plates.
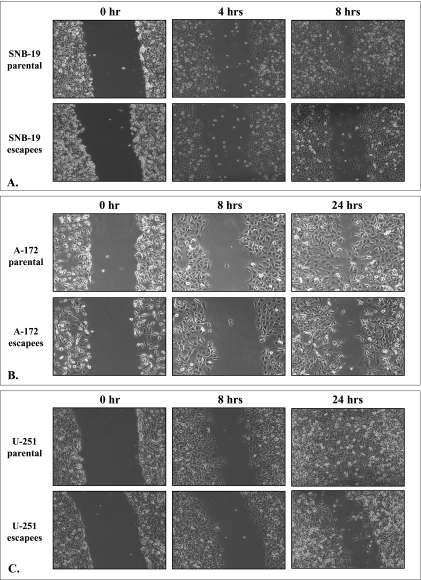
IL-13Rα2-Targeted Therapy Escapees Demonstrate Decreased Migration
One of the most challenging issues in treating GBM is the ability of cells to migrate/invade deep into normal brain tissue. To assess migration rates of escapee cell lines compared to parental cells, we used a wound healing assay. As demonstrated in Figure 4, all three GBM escapee cell lines consistently demonstrated a lag in migration rate compared to their parental counterparts. Because the proliferation rate of the escapee cell lines was demonstrated to be similar to that of their corresponding parental cell lines (Figure 2A), any variation in the wound healing rate on this assay suggests divergence in cell migration capability.
Figure 4.
Migration determined in a wound healing assay by (A) SNB-19 parental and escapee cells, (B) A-172 parental and escapee cells, and (C) U-251 parental and escapee cells.
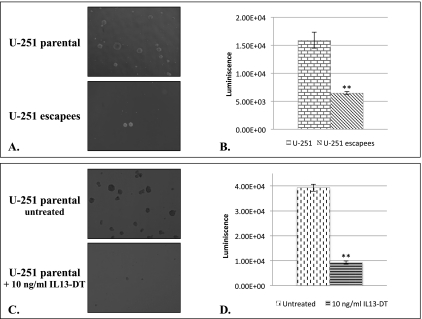
U-251 IL-13Rα2-Targeted Therapy Escapee Cells Have Altered Ability to Form Neurospheres
It has been shown that “stem-like” tumor-initiating cells exist in GBM, which are identified by their ability to form neurospheres in vitro. Furthermore, it has been reported that this population of cells express IL-13Rα2 [46] and thus may be preferentially eradicated by molecular therapies that target IL-13Rα2, resulting in less tumorigenic cells in vivo. We therefore investigated whether our subpopulation of escapee cells is stripped of their ability to effectively form neurospheres in vitro and tumors in vivo. We examined the neurosphere formation potential of U-251 escapee cells compared to their parental cell line because these are the most tumorigenic of the three analyzed parental cell lines. Our data demonstrated that U-251 escapee cells had a significantly decreased ability to form neurospheres in vitro compared to their matching parental cells (Figure 5, A and B). To support the notion that IL-13Rα2-targeted therapy effectively targets tumor-initiating “stem-like” cells, we treated parental U-251 neurospheres with an IL-13-based bacterial toxin that we used in our selection process to obtain the escapee cells. Importantly, we found that the IL-13Rα2-targeted toxin effectively eradicated neurospheres formed from parental U-251 cells (Figure 5, C and D).
Figure 5.
(A) Microscopic appearance (4x) of representative neurospheres formed by U-251 parental and escapee cells. (B) Quantification of luminescent signals from entire dishes of growing neurospheres represented in A. (C) Microscopic appearance of neurospheres formed by U-251 parental cells in the absence and presence of IL-13-based bacterial toxin (IL-13-DT) that targets IL-13Rα2. (D) Quantification of luminescent signals from entire dishes of growing neurospheres represented in C (**P ≤ .01).
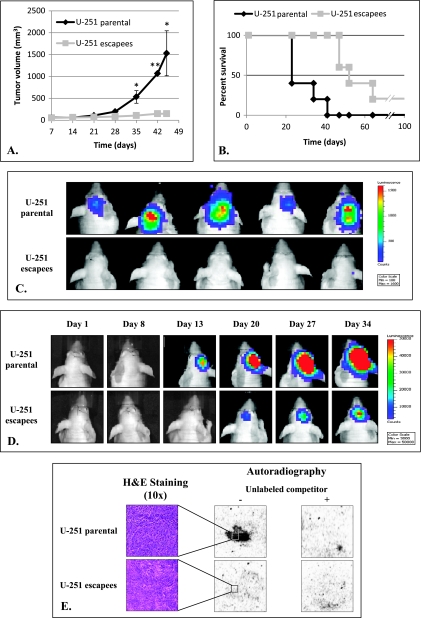
IL-13Rα2-Targeted Therapy Escapees Are Less Tumorigenic In Vivo
On the basis of the differences shown above between IL-13Rα2-targeted therapy escapees and their parental cell lines, we examined the tumorigenicity of the U-251 escapees compared to parental U-251 cells. As expected, parental U-251 cells successfully initiated tumor formation and mice became morbid with tumor burden at approximately 35 days after implantation. In contrast, mice implanted with U-251 escapees cells remained healthy with very small or no palpable tumors present (Figure 6A). In the rare events that the U-251 escapees did initiate a tumor, the formation was significantly delayed.
Figure 6.
In vivo tumorigenicity of U-251 parental and escapee cells. (A) Subcutaneous tumor growth in nude mice (*P ≤ .05, **P ≤ .01). (B) Survival by nude mice after intracranial implantation of U-251 parental and escapee tumors. (C) Bioluminescence image of mice implanted with U-251 parental and escapee cells at day 15 after injection. (D) Bioluminescence image of a representative mouse from each intracranially implanted group from day 1 to day 34 after injection. (E) 125I-labeled IL-13 derivatives binding to U-251 parental and escapee subcutaneous tumor snap-frozen sections via autoradiography.
We further examined the tumorigenicity of escapee cells in an intracranial murine model by stereotactically injecting either U-251 escapee or matching parental cells in the right frontal lobe of athymic nude mice. Similar to the subcutaneous tumors, the U-251 escapee cells were less aggressive, and mice injected with these cells demonstrated prolonged survival compared to those injected with matching parental cells (Figure 6B). This increased survival seemed to be due to both a delay in tumor formation and a decreased proliferation. At day 15 after stereotactic implantation, bioluminescence imaging demonstrated enhanced signals in all mice implanted with parental U-251 cells but not in mice injected with escapee cells (Figure 6C). Furthermore, when following tumor growth in individual mice, we found that in mice implanted with U-251 parental cells, enhanced bioluminescence signal was readily detected as early as day 13 and rapidly progressed in contrast to mice implanted with escapee cells (Figure 6D). Importantly, receptor expression studies using autoradiography on the escapee tumor specimens demonstrated no significant functional IL-13Rα2 expression, similar to the implanted cells and demonstrating that IL-13Rα2 was not reexpressed in these tumors (Figure 6E).
Discussion
In this work, for the first time, we established and characterized IL-13Rα2-targeted therapy escapee cells from three established GBM cell lines. This new knowledge is increasingly important owing to the spreading interest in developing therapeutic platforms that target IL-13Rα2, including active and passive immunotherapy, gene therapy, targeted viruses, and nanotherapy [8,14,16,30,31]. We therefore isolated escapee cell populations by using IL-13-based bacterial cytotoxins that we and others had previously shown to potently and specifically kill IL-13Rα2-expressing cells [15,17,47]. We found subpopulations of GBM cell lines and early-passage tumor cell cultures that were not susceptible to targeted cytotoxin because they expressed significantly less IL-13Rα2. Of potential clinical significance, all three escapee lines were similar to their parental counterparts in terms of proliferation rate and sensitivity to TMZ and radiation, the standard of care given to patients with newly diagnosed GBM. Importantly, we found that the escapee cell lines had a significantly decreased ability to form colonies or migrate in vitro. Furthermore, escapee cells demonstrated significantly diminished neurosphere formation in vitro, which was concordant with our in vivo data that revealed decreased tumorigenicity of escapee cells in subcutaneous and orthotopic models. We postulate that this lower tumorigenicity can potentially be attributed to the escapees' limited tumor initiation capability, or possibly lack of “stemness,” as demonstrated by their decreased neurosphere formation. This would be in agreement with other published data that indicate IL-13Rα2 expression on CD133+ stem-like cells [46].
We hypothesize that we are selecting for a preexisting population of IL-13Rα2 escapee cells rather than cells that actively downregulate IL-13Rα2 during selection because the toxins that we used in the selection process are highly potent and have been reported to kill even if only a few molecules of bacterial toxin have access to a cell through IL-13Rα2. Such potency would give the cell little chance to downregulate the receptor once it has been exposed to the toxin. Furthermore, the escapee cells remained not susceptible to targeted therapy with significantly decreased IL-13Rα2 expression even months after we stopped the selection process, which argues against a more fluid regulation of IL-13Rα2.
Whereas these initial escapee cells were isolated from early-passage and established cell lines in vitro, our findings suggest for the first time that IL-13Rα2-targeted therapy escapees from a heterogeneous GBM tumor may belong to an independent intrinsic subpopulation that lacks significant expression of the IL-13Rα2 biomarker and demonstrates distinct tumor biology both in vitro and in vivo. Importantly, we are following up this work by confirming our results on IL-13Rα2-negative cells isolated directly from patient's tumor specimens compared to matching IL-13Rα2-positive cells from the same tumor. In addition, we are further evaluating a possible functional role played by IL-13Rα2, which can explain the decreased colony formation and migration patterns in vitro in the escapee population. An alternative scenario is one in which we merely selected for a separate population of cells with different characteristics and receptor expression profiles.
As GBM management remains focused on molecular biomarker-targeted therapies, it is critical to identify mechanisms of potential resistance. This will allow us to develop preventative measures such as combinatorial therapy with other modalities. The evidence presented here suggests that a mechanism of how subpopulations of GBM cells avoid being killed by IL-13Rα2-targeted therapy is through decreased IL-13Rα2 receptor expression. Importantly, these IL-13Rα2-resistant cells remain equally susceptible to chemotherapy and radiation therapy but less tumorigenic in our models. Thus, this first report on the existence of IL-13Rα2-targeted therapy escapee populations suggests the significance of combinatorial approaches to eradicate these resistant but potentially less tumorigenic subpopulation of escapee cells. Our results support the plans for aggressive treatments directed at IL-13Rα2.
Footnotes
This work was supported by the National Institute of Neurological Disorders and Stroke (R21NS067471-0110) and the Dana Foundation Neuro-Immunology Award (both to A.M.) and the Burroughs Welcome Career Award for Medical Scientists (J.D., PI).
References
- 1.Sathornsumetee S, Rich JN, Reardon DA. Diagnosis and treatment of high-grade astrocytoma. Neurol Clin. 2007;25:1111–1139. x. doi: 10.1016/j.ncl.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Davis FG, Freels S, Grutsch J, Barlas S, Brem S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973–1991. J Neurosurg. 1998;88:1–10. doi: 10.3171/jns.1998.88.1.0001. [DOI] [PubMed] [Google Scholar]
- 3.Mintz A, Gibo DM, Slagle-Webb B, Christensen ND, Debinski W. IL-13Rα2 is a glioma-restricted receptor for interleukin-13. Neoplasia. 2002;4:388–399. doi: 10.1038/sj.neo.7900234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debinski W, Gibo DM. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med. 2000;6:440–449. [PMC free article] [PubMed] [Google Scholar]
- 5.Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res. 1999;5:985–990. [PubMed] [Google Scholar]
- 6.Debinski W, Gibo DM, Slagle B, Powers SK, Gillespie GY. Receptor for interleukin 13 is abundantly and specifically over-expressed in patients with glioblastoma multiforme. Int J Oncol. 1999;15:481–486. doi: 10.3892/ijo.15.3.481. [DOI] [PubMed] [Google Scholar]
- 7.Debinski W, Slagle B, Gibo DM, Powers SK, Gillespie GY. Expression of a restrictive receptor for interleukin 13 is associated with glial transformation. J Neurooncol. 2000;48:103–111. doi: 10.1023/a:1006446426611. [DOI] [PubMed] [Google Scholar]
- 8.Candolfi M, Xiong W, Yagiz K, Liu C, Muhammad AK, Puntel M, Foulad D, Zadmehr A, Ahlzadeh GE, Kroeger KM, et al. Gene therapy-mediated delivery of targeted cytotoxins for glioma therapeutics. Proc Natl Acad Sci USA. 2010;107:20021–20026. doi: 10.1073/pnas.1008261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson JP, Nagy A, Schally AV, Debinski W. A small targeted anti-glioma chemotherapeutic based on mutated interleukin 13. Neuro Oncol. 2000;2:298. [Google Scholar]
- 10.Zhou G, Ye GJ, Debinski W, Roizman B. Engineered herpes simplex virus 1 is dependent on IL13Rα2 receptor for cell entry and independent of glycoprotein D receptor interaction. Proc Natl Acad Sci USA. 2002;99:15124–15129. doi: 10.1073/pnas.232588699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160–9166. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 12.Madhankumar AB, Slagle-Webb B, Mintz A, Sheehan JM, Connor JR. Interleukin-13 receptor-targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol Cancer Ther. 2006;5:3162–3169. doi: 10.1158/1535-7163.MCT-06-0480. [DOI] [PubMed] [Google Scholar]
- 13.Mintz A, Dorsey JF, Alavi A, El-Deiry WS. Retargeted cytokine-immunoglobulin fusion proteins to deliver radioisotopes for diagnosis, radio-immunotherapy and TRAIL sensitization of high grade astrocytoma and metastatic breast cancer. J Nucl Med. 2006;47(Suppl 1):504. [Google Scholar]
- 14.Nguyen V, Hollingsworth C, Debinski W, Mintz A. A novel ligand delivery system that targets the IL13Rα2 tumor-restricted biomarker. J Nucl Med Meeting Abstracts. 2010;51:58. [Google Scholar]
- 15.Debinski W, Gibo DM, Obiri NI, Kealiher A, Puri RK. Novel anti-brain tumor cytotoxins specific for cancer cells. Nat Biotechnol. 1998;16:449–453. doi: 10.1038/nbt0598-449. [DOI] [PubMed] [Google Scholar]
- 16.Mintz A, Gibo DM, Madhankumar AB, Cladel NM, Christensen ND, Debinski W. Protein- and DNA-based active immunotherapy targeting interleukin-13 receptor α2. Cancer Biother Radiopharm. 2008;23:581–589. doi: 10.1089/cbr.2008.0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mintz A, Gibo DM, Madhankumar AB, Debinski W. Molecular targeting with recombinant cytotoxins of interleukin-13 receptor α2-expressing glioma. J Neurooncol. 2003;64:117–123. doi: 10.1007/BF02700026. [DOI] [PubMed] [Google Scholar]
- 18.Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin. Clin Cancer Res. 1995;1:1253–1258. [PubMed] [Google Scholar]
- 19.Debinski W, Obiri NI, Pastan I, Puri RK. A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J Biol Chem. 1995;270:16775–16780. doi: 10.1074/jbc.270.28.16775. [DOI] [PubMed] [Google Scholar]
- 20.Debinski W, Gibo DM, Puri RK. Novel way to increase targeting specificity to a human glioblastoma-associated receptor for interleukin 13. Int J Cancer. 1998;76:547–551. doi: 10.1002/(sici)1097-0215(19980518)76:4<547::aid-ijc17>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Kioi M, Husain SR, Croteau D, Kunwar S, Puri RK. Convection-enhanced delivery of interleukin-13 receptor-directed cytotoxin for malignant glioma therapy. Technol Cancer Res Treat. 2006;5:239–250. doi: 10.1177/153303460600500307. [DOI] [PubMed] [Google Scholar]
- 22.Kunwar S, Chang SM, Prados MD, Berger MS, Sampson JH, Croteau D, Sherman JW, Grahn AY, Shu VS, Dul JL, et al. Safety of intraparenchymal convection-enhanced delivery of cintredekin besudotox in early-phase studies. Neurosurg Focus. 2006;20:E15. [PubMed] [Google Scholar]
- 23.Vogelbaum M, Sampson JH, Kunwar S, Chang S, Lang FF, Shaffrey M, Asher A, Croteau D, Parker K, Dul JL, et al. Phase I study final safety results: convection enhanced delivery of cintredekin besudotox (IL13-PE38QQR) followed by radiation therapy without and with temozolomide in newly diagnosed malignant glioma. Abstracts for the Eleventh Annual Meeting of the Society for Neuro-Oncology (SNO) Neuro Oncol. 2006;8(4):453. [Google Scholar]
- 24.Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM, Sampson JH, Ram Z, Gutin PH, Gibbons RD, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25:837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 25.Sampson JH, Raghavan R, Provenzale JM, Croteau D, Reardon DA, Coleman RE, Rodriguez Ponce I, Pastan I, Puri RK, Pedain C. Induction of hyperintense signal on T2-weighted MR images correlates with infusion distribution from intracerebral convection-enhanced delivery of a tumor-targeted cytotoxin. AJR Am J Roentgenol. 2007;188:703–709. doi: 10.2214/AJR.06.0428. [DOI] [PubMed] [Google Scholar]
- 26.Vogelbaum MA, Sampson JH, Kunwar S, Chang SM, Shaffrey M, Asher AL, Lang FF, Croteau D, Parker K, Grahn AY, et al. Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: phase 1 study of final safety results. Neurosurgery. 2007;61:1031–1037. doi: 10.1227/01.neu.0000303199.77370.9e. discussion 1037–1038. [DOI] [PubMed] [Google Scholar]
- 27.Sampson JH, Archer G, Pedain C, Wembacher-Schroder E, Westphal M, Kunwar S, Vogelbaum MA, Coan A, Herndon JE, Raghavan R, et al. Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg. 2010;113:301–309. doi: 10.3171/2009.11.JNS091052. [DOI] [PubMed] [Google Scholar]
- 28.Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, Shaffrey M, Ram Z, Piepmeier J, Prados M, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12:871–881. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarboe JS, Johnson KR, Choi Y, Lonser RR, Park JK. Expression of interleukin-13 receptor α2 in glioblastoma multiforme: implications for targeted therapies. Cancer Res. 2007;67:7983–7986. doi: 10.1158/0008-5472.CAN-07-1493. [DOI] [PubMed] [Google Scholar]
- 30.Pandya H, Garg S, Gibo DM, Kridel S, Debinski W. Selection of a linear heptapeptide that binds interleukin 13 receptor α2 at a site distinct from the native ligand. Neuro Oncol. 2009;11:597. [Google Scholar]
- 31.Debinski W, Dickinson P, Gibo DM. Novel monoclonal antibody 13R2.C3 against human and canine interleukin 13 receptor α-2. Neuro Oncol. 2009;11:563–699. [Google Scholar]
- 32.Debinski W, Tatter SB. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother. 2009;9:1519–1527. doi: 10.1586/ern.09.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwamoto FM, Abrey LE, Beal K, Gutin PH, Rosenblum MK, Reuter VE, DeAngelis LM, Lassman AB. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73:1200–1206. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Cusatis G, Brahmer J, Sparreboom A, Robey RW, Bates SE, Hidalgo M, Baker SD. Association of variant ABCG2 and the pharmacokinetics of epidermal growth factor receptor tyrosine kinase inhibitors in cancer patients. Cancer Biol Ther. 2007;6:432–438. doi: 10.4161/cbt.6.3.3763. [DOI] [PubMed] [Google Scholar]
- 35.Zelnak A. Overcoming taxane and anthracycline resistance. Breast J. 2010;16:309–312. doi: 10.1111/j.1524-4741.2010.00911.x. [DOI] [PubMed] [Google Scholar]
- 36.Atalay C, Deliloglu Gurhan I, Irkkan C, Gunduz U. Multidrug resistance in locally advanced breast cancer. Tumour Biol. 2006;27:309–318. doi: 10.1159/000096086. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 38.Debinski W. Local treatment of brain tumors with targeted chimera cytotoxic proteins. Cancer Invest. 2002;20:801–809. doi: 10.1081/cnv-120003545. [DOI] [PubMed] [Google Scholar]
- 39.Wykosky J, Gibo DM, Stanton C, Debinski W. Interleukin-13 receptor α2, EphA2, and Fos-related antigen 1 as molecular denominators of high-grade astrocytomas and specific targets for combinatorial therapy. Clin Cancer Res. 2008;14:199–208. doi: 10.1158/1078-0432.CCR-07-1990. [DOI] [PubMed] [Google Scholar]
- 40.Guerrero-Cazares H, Chaichana KL, Quinones-Hinojosa A. Neurosphere culture and human organotypic model to evaluate brain tumor stem cells. Methods Mol Biol. 2009;568:73–83. doi: 10.1007/978-1-59745-280-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaichana K, Zamora-Berridi G, Camara-Quintana J, Quinones-Hinojosa A. Neurosphere assays: growth factors and hormone differences in tumor and nontumor studies. Stem Cells. 2006;24:2851–2857. doi: 10.1634/stemcells.2006-0399. [DOI] [PubMed] [Google Scholar]
- 42.Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Josiah DT, Zhu D, Dreher F, Olson J, McFadden G, Caldas H. Adipose-derived stem cells as therapeutic delivery vehicles of an oncolytic virus for glioblastoma. Mol Ther. 2010;18:377–385. doi: 10.1038/mt.2009.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 45.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 46.Xu Q, Liu G, Yuan X, Xu M, Wang H, Ji J, Konda B, Black KL, Yu JS. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells. 2009;27:1734–1740. doi: 10.1002/stem.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nash KT, Thompson JP, Debinski W. Molecular targeting of malignant gliomas with novel multiply-mutated interleukin 13-based cytotoxins. Crit Rev Oncol Hematol. 2001;39:87–98. doi: 10.1016/s1040-8428(01)00124-x. [DOI] [PubMed] [Google Scholar]