Abstract
Chronic obstructive pulmonary disease (COPD) and inflammatory bowel diseases (IBD) are chronic inflammatory diseases of mucosal tissues that affect the respiratory and gastrointestinal tracts, respectively. They share many similarities in epidemiological and clinical characteristics as well as inflammatory pathologies. Importantly, both conditions are accompanied by systemic co-morbidities that are largely overlooked in both basic and clinical research. Therefore, consideration of these complications may maximise the efficacy of prevention and treatment approaches. Here, we examine both the intestinal involvement in COPD and the pulmonary manifestations of IBD. We also review the evidence for inflammatory organ cross-talk that may drive these associations, and discuss the current frontiers of research into these issues.
Keywords: COPD, IBD, Crohn’s disease, ulcerative colitis, inflammation, cross-talk, smoking, microbiome, lymphocyte, autoimmunity
1. Introduction
Chronic obstructive pulmonary disease (COPD) and inflammatory bowel diseases (IBD) are mucosal inflammatory diseases affecting the respiratory system and gastrointestinal tract, respectively. COPD affects 64 million people worldwide and is the 4th leading cause of death1. IBD has a prevalence of >300/100,000 globally and there has been a dramatic increase in the incidence of IBD over the last 50 years2, 3. COPD and IBD are both chronic diseases, which are driven by recurrent cycles of inflammation that lead to tissue damage and remodelling which progressively worsen symptoms. There are no cures for either disease and both require lifelong health maintenance, for which current therapies are suboptimal4–6. Many of the similarities in the pathological features of COPD and IBD are a result of the common physiology of the respiratory and gastrointestinal systems.
1.1 Common physiology of the respiratory and gastrointestinal tracts
Structurally the respiratory and gastrointestinal tracts have many similarities7, 8 . Both have an extensive, highly vascularised, luminal surface area9–12 which is protected by a selective epithelial barrier13–15 and an overlying mucus-gel layer16, 17 from commensal bacteria, pathogens and foreign antigens. These epithelial surfaces cover a sub-mucosal layer of loose connective tissue and mucosa-associated lymphoid tissue (MALT), comprised of resident lymphocytes. This lymphoid tissue regulates antigen sampling, lymphocyte trafficking and mucosal host defence18, 19. Respiratory and gastrointestinal epithelia share a common embryonic origin in the primitive foregut20, 21, which may account for their similarities. However, it is most likely that it is the similar inflammatory and immune components of these organs that are the cause of the overlap in pathological changes in respiratory and intestinal mucosal diseases.
1.2 COPD
COPD is an umbrella term describing a group of conditions characterized by prolonged airflow obstruction and loss of the functional capacity of the lungs. Patients suffer from chronic bronchitis and emphysema that lead to breathing difficulties (dyspnoea)22. Symptoms are induced by exaggerated and chronic inflammatory responses to the noxious insult of smoke exposure, with periodic exacerbations of disease typically caused by bacterial or viral infection23. Smoking is the major causal risk factor in COPD in westernized countries, but wood smoke and pollution are important in other areas, and there are genetic and epigenetic components24. Recent studies show that exposure to respiratory infections or hyperoxia in early life may also contribute to the development of COPD25, 26.
1.3 IBD
IBD is a term that describes a group of inflammatory diseases of the gastrointestinal tract. Ulcerative colitis (UC) and Crohn’s disease (CD) are the two most common forms of IBD27. Physiologically, UC and CD are quite distinct. UC is characterized by continuous, superficial ulceration of the colon, whereas CD manifests with transmural, sporadic (skip) lesions and may occur at any point along the digestive tract28, 29. Both conditions are associated with excessive daily bowel movements, severe abdominal pain, diarrhoea, weight loss, malnutrition and intestinal bleeding. The causes of IBD are unclear, however several factors are known to contribute to the onset of disease including genetic risk, environmental stress, the intestinal microbiome and inflammatory dysfunction30.
1.4 Inflammatory organ cross-talk in COPD and IBD
It is widely accepted that secondary organ disease occurs in both COPD and IBD 31–37. There is much recent clinical interest in intestinal manifestations of COPD and an increasing number of studies have highlighted the prevalence of pulmonary inflammation in IBD. At an epidemiological level there is a strong association between the incidence of COPD and CD38–40. A population-based cohort study performed by Ekbom et al., showed that the risk of CD in COPD sufferers was 2.72 times higher than in healthy controls and greater than the risk reported for smoking alone39. There is also a familial risk factor, with an increased risk of CD among first-degree blood relatives of COPD sufferers, although shared environmental factors may account for this. Specific intestinal manifestations of COPD include atrophic gastritis and nutritional absorption deficiency in the small intestine34, 41.
Conversely, COPD has been shown to be a significant mortality factor among CD sufferers38, 40, with standardised mortality ratios of 2.5–3.5 for COPD in the CD population. Kuzela et al., demonstrated a high incidence of abnormal pulmonary function in both CD and UC patients, despite a lack of radiological abnormalities42. Similar findings by Tzanakis et al., led them to propose that patients suffering from IBD should undergo pulmonary evaluation including physical examination, chest X-ray and pulmonary function testing43–45. Black et al., performed a literature survey that identified 55 articles citing thoracic disorders in IBD patients, with large airway involvement accounting for 39% of these associations33. Three more specific studies of randomly selected IBD patients showed incidence rates of pulmonary organ involvement at 44%46, 48%47 and 50%48. The symptoms manifested as interstitial lung disease, increased numbers of alveolar lymphocytes and a reduction in the diffusion capacity of the lung. Pulmonary involvement was more likely in UC, but was still significant in CD.
Hence there is a clear but undefined link between inflammatory diseases in the respiratory and intestinal systems. While the associations have been clearly identified in the clinical literature, there have been few basic research studies that have investigated the mechanisms of the inflammatory cross-talk involved.
2. Common risk factors in COPD and IBD
COPD and IBD are multifactorial diseases and share many aspects of the classical “triad” of risk factors; environmental factors, genetic susceptibility and microbial involvement. In addition, both conditions exhibit clear signs of immunological dysfunction in their pathologies. However, while smoke or particulate inhalation is a critical environmental factor for COPD, the corresponding factors for IBD are ill-defined. Conversely, although there is a clear link between the intestinal microbiome and IBD, the potential of an intrinsic lung microbiome as a risk factor in COPD has only recently emerged.
2.1 Smoking as a risk factor for COPD and IBD
Cigarette smoking is the single most important risk factor in COPD. Approximately 80% of people with COPD are past or present smokers. Toxins and particulate matter in inhaled smoke induce acute inflammation in the airways. With repeated insult, inflammation becomes chronic and damages the airway epithelium and lung tissue49–51. Eventually this leads to remodelling of the respiratory epithelium, emphysema and chronic disease. However, only between 15–50% of all smokers develop COPD, indicating that smoke inhalation alone is not sufficient to induce disease52, 53 and that other risk factors are likely contribute to the development of COPD. Twin and familial studies have suggested the involvement of genetic factors, with first-degree relatives of COPD sufferers at increased risk54, 55.
Smoking is also a risk factor for IBD and significantly increases the risk of developing CD by 3-fold56–60. In contrast, and surprisingly, the prevalence of UC among smokers is low, with smoking alleviating symptoms of disease60, 61. This is exemplified by familial studies of siblings who are genetically susceptible to IBD. In these studies smokers were shown to be more likely to develop CD and non-smokers to develop UC62. Nevertheless, ex-smokers appear to be at increased risk of UC than those who have never smoked63–65.
The issue is further complicated when incidences of smokers and IBD are correlated as a whole. Eastern countries tend to have a much higher smoking rate than western countries66, yet western countries have a higher incidence of CD, but not UC compared to eastern countries67, 68. The lack of epidemiological correlation between smoking and CD incidence in the east-west divide suggests that, like COPD, smoking by itself is not sufficient to induce IBD. Studies in animal models of CD-like colitis have demonstrated that smoke-exposure exacerbates existing colitis in wildtype animals69–71. This suggests that smoking can augment existing mucosal inflammation, although no-consensus on mechanism has been achieved. Thus, while smoking has an obvious impact on both respiratory and gastrointestinal health, the nature of these phenomena are poorly understood.
2.2 Genetic risk of COPD and IBD
COPD and IBD have known genetic risk factors. To date, four genetic risk factors have been formally identified for COPD. Deficiency of α1 anti-trypsin (A1AT), an enzyme and a serum trypsin inhibitor that protects against protease remodelling in the airway, accounts for 2% of COPD in the population72, 73. Recently, genes for α-nicotinic acetylcholine receptor (CHRNA3/5)74, hedgehog-interacting protein (HHIP)75, 76 and iron regulatory protein 2 (IREB2)77–79 have been shown to be potential susceptibility loci for COPD. However, functional endpoints have yet to be determined for how these genes influence the development of COPD.
Both CD and UC are known to have genetic risk factors, and both ethnic and familial associations have been shown55, 80, 81. Mutations in genes for nucleotide-binding oligomerization domain containing 2 (NOD2)82–84, autophagy-related protein 16-1 (ATG16L1)85, 86, interleukin-23 receptor (IL23R)87, 88 and immunity-related guanosine-5’-triphosphatase family M protein (IRGM89) have been shown to dramatically increase the risk of CD. A recent study has also identified a NOD2 mutation in COPD populations offering a possible link between this condition and CD90. These genes code for proteins which control responses to infection at the intestinal mucosa and regulate autophagy. Thus a paradigm has developed that a defect in bacterial clearance in CD may be one of the key triggers for disease onset. Polymorphisms of human leukocyte antigen (HLA) class II genes also have a strong association with UC, suggesting that lymphocyte regulation is an important factor in its development91, 92. Recent studies have made substantial progress in understanding gene associations with UC. Among the new susceptibility loci identified are laminin subunit beta-1 (LAMB1)93, extracellular matrix protein 1 (ECM1)94, hepatocyte nuclear factor 4 alpha (HNF4A)93 and Cadherin-1 and -3 (CDH1 and 3)93. These genes are involved in maintaining epithelial barrier integrity81, suggesting that a dysfunction in the epithelial barrier may predispose to UC.
It is possible that genetic risk factors may also contribute to the association between COPD and IBD. HHIP is also important in the development of the intestinal crypt axis95, and further studies are required to identify whether this gene contributes to disease overlap between COPD and IBD. The diversity of gene susceptibility loci for both COPD and IBD suggests that susceptibility to these conditions may involve multiple genes and alleles that couple with environmental triggers to induce disease in some individuals.
2.3 Disruption of the microbiome
Bacterial colonization of the lower respiratory tract, although once controversial, is now known to influence the pathogenesis of COPD96, 97. The controversy was due to the classical view, borne largely from culture-based studies, of healthy lungs as a sterile environment98, 99. Advances in culture-independent techniques for microbial analysis have shown that the healthy lung plays host to its own microbiome, which changes significantly during disease100, 101. Nevertheless, the precise role of the lung microbiome in COPD pathogenesis and the mechanisms that underpin infection-induced COPD exacerbations are poorly understood97.
It is also known that changes in the intestinal microbiome are associated with IBD30, 102, 103, however again the nature of the shift in commensal populations is not well established. Indeed, it is certain that the microbiome contributes to both the initial inflammation and chronic nature of IBD, but it is unclear if commensals are the initiating factor104. Regardless of the role in the initiation of IBD, chronic inflammation contributes to a loss of diversity in the microbiome, which appears to perpetuate the disease102, 104, 105. In both COPD and IBD the microbiome of the lung and intestine have changes in the dominant species and a reduction in diversity106, without decreases in microbial numbers107. Whether these changes are a mechanism or consequence of inflammation is not understood, but clearly a healthy microbiome is important to both respiratory and gastrointestinal health.
2.4 Epithelial barrier dysfunction
Maintenance of epithelial barrier function is critical for maintaining the healthy state of the respiratory and gastrointestinal mucosa. This is because the epithelial barrier separates the interstitium and underlying tissues from the milieu of antigenic material in the mucosal lumen. Consequently, loss of barrier function as a result of mucosal inflammation contributes to the chronic nature of these conditions, although it is not yet understood if loss of function is a causative factor or a consequence of disease. COPD patients are particularly susceptible to bronchitis (inflammation of the bronchial mucosa), which develops as smoke exposure damages the airway epithelial barrier. Shaykhiev et al., have shown that smoking leads to down-regulation of genes coding for tight junction and adherence proteins, which was more pronounced in smokers with COPD108. In vitro studies examining the effect of cigarette smoke extract on primary bronchial epithelial cells have shown that the endogenous protease calpain, mediates degradation of tight junction complexes109. Thus, smoking, the major environmental risk factor for COPD, promotes the dysregulation of the pulmonary epithelial barrier.
Epithelial barrier dysfunction is a common feature of IBD110. However, although this is well established, like COPD, it is unknown if barrier dysfunction is a causative or consequential factor111, 112. Certainly, in IBD, increased epithelial permeability promotes the progression of chronic inflammation. Soderholm et al., demonstrated that the epithelial tight junctions of non-inflamed intestinal tissue from CD patients were more susceptible to breakdown upon luminal antigenic stimulation113. Epithelial breakdown allows the establishment of invasive bacterial infections, which are more characteristic of CD than UC114. However, both UC and CD patients have high IgG titres against intestinal microbes115, and both diseases show histopathologic evidence for the loss of tight-junctional integrity 116–118, suggesting that epithelial dysfunction is important in both conditions.
2.5 Pattern recognition receptors (PRRs)
PRRs are a family of highly conserved proteins that are expressed by cells of the innate immune system. They recognize components termed pattern associated molecular patterns (PAMPs) of microorganisms, cellular stress signals and damaged tissue. They may be membrane-bound or cytoplasmic and, when activated, induce the production and secretion of inflammatory mediators and signalling molecules. Two PRR families known to be important in the mucosal inflammatory response are the cytoplasmic NOD family of receptors and the membrane-bound Toll-like receptor (TLR) family119–121.
COPD patients are known to be at an increased risk of pulmonary infection, leading to inflammatory exacerbations of their disease, however the mechanisms that underlie this increased risk are not well understood122. Kinose et al., have recently identified increases in the prevalence of the NOD2 rs1077861 single nucleotide polymorphism (SNP) in COPD patients90. NOD2 recognizes muramyl dipeptide (MDP), an element of peptidoglycan, which is an important component of the cell wall of virtually all bacteria. This SNP causes a conformational change in NOD2 and leads to a series of downstream interactions that culminate in NFκB activation and an enhanced inflammatory cytokine response upon stimulation. Although baseline NOD2 expression was unaltered in COPD patients, the SNP was associated with increased COPD disease severity measured by reduced lung function90. The mechanism for the involvement of the SNP in COPD pathology has yet to be fully characterized.
NOD2 is also strongly associated with CD, whereby a defect in NOD2 signalling leads to impaired epithelial barrier function, increased IL-1β and an overcompensating TLR2 response, and promotes increases in serum IL-12120, 123. NOD2 mutations are present in 15% of the CD population and a NOD2 SNP has recently been associated with smoking and CD124. Although Kinose et al., did not examine TLR2 or IL-12 in the COPD study, IL-12 has been shown to be associated with increased CD8 cytotoxic T cell and natural killer (NK) cell activation in COPD patients and mouse models125, 126, although whether this is related to NOD2 polymorphisms, requires further investigation. NOD2 may therefore be a common link between COPD and CD, with polymorphisms identified in COPD and CD populations, including an association with smoking and CD.
TLRs that recognize viral and bacterial proteins maintain mucosal homeostasis, and genetic varients of TLRs have been identified in COPD and IBD121, 127–130. Certainly, infection plays a prominent role in COPD pathogenesis and TLR2, which recognises a range of bacterial and yeast proteins, has reduced expression and responsiveness to LPS in alveolar macrophages from COPD patients and smokers131. This suggests that there is a defect in the mucosal innate response in COPD. Conversely, TLR2 was shown to be upregulated in peripheral blood monocytes from COPD patients compared to healthy controls128, perhaps indicating the presence of systemic inflammation in these patients. While certain TLR2 polymorphisms are linked with increased infection, they do not appear to be associated with COPD132. Thus the exact nature of and defects of TLR2 responses in COPD remain unclear. TLR4, which recognizes LPS, promotes COPD pathogenesis, although the pathways involved appear to be complex130. Investigation of murine models indicates that TLR4 is involved in the development of smoke-induced inflammatory responses133. This inflammatory response was driven by IL-1β secretion from macrophages and neutrophil recruitment to lung tissue. Smoke exposure also drives TLR4-dependent IL-8 production in monocyte-derived macrophages134. In both of these studies, smoke-induced TLR4 activation was independent of LPS.
Both TLR2 and TLR4 were found to be induced in the colonic mucosa of pediatric IBD patients135. Furthermore, Canto et al., identified an increase in TLR2 expression on peripheral blood monocytes, which was associated with elevated circulating TNF-α concentrations in active UC and CD136. This suggests that, like COPD, systemic inflammation may be involved is IBD pathogenesis. The D299G and T399I SNPs of TLR4 have been shown to be associated with both UC and CD137–139, while T399I has also been identified in COPD patients140, suggesting a possible common link. While the functional consequences of these gene variants are not yet fully appreciated, it is known that inflammatory cytokine signalling results in increased TLR4 expression on macrophages from the intestinal epithelium and lamina propria in both UC and CD resulting in increased responsiveness to LPS141, 142. Thus, TLR4 may play a common role in mucosal inflammatory disease whereby an inflammatory insult coupled with TLR4 gene variations results in hypersensitivity to LPS and an exaggerated immune response in the lung or intestine.
3. Potential mechanisms of organ cross-talk
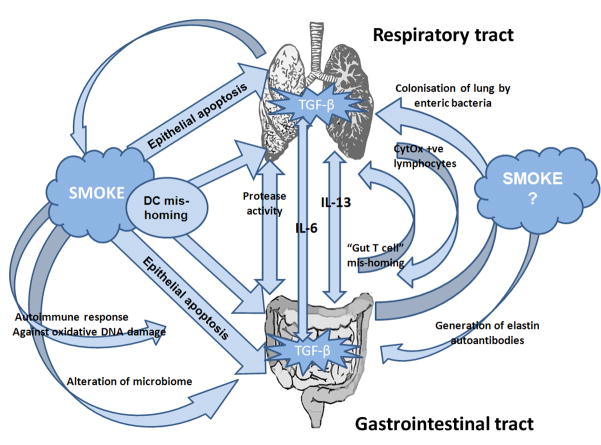
Despite the similarities in the physiology of the respiratory and gastrointestinal mucosal organs, the common risk factors involved in the development of COPD and IBD and the incidences of inflammatory cross-talk between the two organs in disease, no mechanism has been identified for pulmonary-intestinal organ cross-talk. While the respiratory and gastrointestinal tracts both share components of the common mucosal immune system, the pathways involved in cross-talk may be multi-factorial, like COPD and IBD themselves (Figure 1).
Figure 1.
Possible mechanisms of respiratory-gastrointestinal cross-talk include: overproduction of proteases during excessive inflammation, changes in immune cell function, including increases in cytochrome oxidase (CytOx) expressing lymphocytes and gut originating T cell mis-homing. Cigarette smoke exposure may play a role in organ cross-talk by affecting these processes, and/or by causing mis-homing of dendritic cells (DC) and epithelial cell apoptosis in respiratory or gastrointestinal tissues. Smoke exposure may also lead to changes in the microbiome, promoting growth of enteric bacteria in the lung or altering the microbiome in the intestine that induces inflammatory responses. Inflammation may lead to the production of autoimmune antibodies against the ubiquitous mucosal protein elastin or autoimmune responses against antigens produced during smoke-induced oxidative DNA damage. Systemic IL-6, in conjunction with localized TGF-β, may drive cross-organ Th17 polarized inflammation. Systemic IL-13 may drive aberrant NKT and macrophage responses across organs.
3.1 Protease regulation in COPD and IBD
There is evidence that dysregulation of protease activity may play a role in both COPD and IBD. Increased levels of the proteases that break down connective tissue components have been identified in COPD patients and modelled in animals143. Of particular interest are the matrix metalloproteinase (MMP) family of proteases, which play a role in the digestion of collagen, elastin, fibronectin and gelatin, key components in mucosal structural integrity144. Increased levels of epithelial and leukocyte MMP-2, -9 and -12 have been associated with the pathogenesis of COPD143, 145, 146 and IBD147–150, which may contribute to a “runaway remodelling” process.
The role of A1AT in COPD is established, however the prevalence of A1AT in IBD is debatable. A1AT neutralizes proteases involved in tissue remodelling, such as neutrophil elastase151 and MMP-12152. Deficiencies in A1AT production promotes extensive tissue damage during mucosal inflammation as the tissue remodelling process progresses unchecked. Deficiency of A1AT leads to the development of emphysema and COPD153, 154. Because of its role in the remodelling of inflamed tissue, faecal A1AT levels are commonly used as a marker for disease severity in CD patients155, 156. This suggests that lack of A1AT is does not promote the development of CD. While some studies have suggested higher levels of A1AT in UC patients157, 158, there is a higher prevalence of the allele linked to A1AT deficiency (PiZ) among the UC population157 and UC patients with this allele develop more severe forms of colitis158. Further work is required to address this divergence.
3.2 Immune cell homing and systemic factors
Both COPD and IBD are considered to be systemic inflammatory diseases and peripheral lymphocyte activity may contribute to pathogenesis36, 159–162. During inflammation, the bronchus associated lymphoid tissue (BALT) regulates lymphocyte trafficking from lung tissue through the general circulation18. This mirrors the role of the gut associated lymphoid tissue (GALT) and both lung and intestinal lymphocytes migrate to other mucosal sites as part of the common mucosal immune system163. It is possible that this trafficking, while functioning primarily as a common host mucosal defence, may be responsible for extra-organ inflammation associated with COPD and IBD.
In the healthy state, lymphocytes continuously migrate through the circulatory system, entering and exiting the tissue in response to antigen exposure. In order to control trafficking of lymphocytes through tissues, these cells express unique homing receptors, which are specific for corresponding ligands on their target tissues. Thus, through a combination of homing molecules and specific receptor-ligand interactions, lymphocytes will return to their tissue of origin during an immune response164, 165. The subtype and phenotype of circulating lymphocytes in COPD patients have not been well characterised159. However, there is evidence of abnormal function in peripheral lymphocytes that may contribute to extra-pulmonary disease in COPD patients. Sauleda et al., showed increased cytochrome oxidase (CytOx) activity in the circulating lymphocytes of COPD patients, which correlated with increased CytOx detected in wasting skeletal muscle that is commonly associated with COPD166. Interestingly, this increased oxidative response in circulating lymphocytes is also observed in other chronic inflammatory diseases, such as asthma and rheumatoid arthritis, but whether these same responses occur in IBD is unknown.
For IBD patients the selectivity of lymphocyte-endothelial interaction is lost. Salmi et al., showed that in IBD patients, the expression of homing receptors in intestinal lymphocytes did not confer tissue specificity167. These altered homing properties may contribute to the extra-intestinal manifestations of IBD. It is known that gut-derived lymphocytes possess the capacity to bind to synovial168 and hepatic169 tissue, possibly accounting for the manifestations of IBD observed in these organs. This mis-homing of lymphocytes is thought to contribute to ocular and dermatological extra-intestinal manifestations of IBD165. Whether this same phenomenon contributes to the lung pathologies observed in IBD is unknown. Increased lymphocyte populations have been observed in the BAL of IBD patients170, 171 and analysis of the sputum of IBD patients showed that 65% had an increased CD4/CD8 T cell ratio in lung tissue172. Whether this represents a further example of lymphocyte mis-homing involved in the pulmonary manifestations of IBD has yet to be confirmed.
It is possible that the inhalation of smoke affects gut lymphocyte homing and promotes an inappropriate immune response. Smoke exposure is known to affect T cell trafficking through altered chemotactic chemokine levels173, 174. Smoke inhalation also appears to affect the homing properties and maturation of myeloid dendritic cells (mDCs)175–178, which are key antigen presenting cells in mucosal immune responses. The result is a rapid accumulation of mDCs in the airways of smokers175, which may be a result of a reduced capacity of mDCs to migrate to the lymph node175, 176. A recent animal study has similarly shown that smoke inhalation results in the accumulation of DCs in the intestinal Peyer’s patches of wildtype mice, although unlike the airways, this does not seem to be dependent on changes in expression of the DC homing molecule CCR6179. The increase in DCs was accompanied by a similar accumulation of CD4+ T cells and an apparent increase in apoptosis of the cells overlying the follicle-associated epithelial (FAE) tissue of the intestine.
This loss in epithelial barrier, may lead to increased antigen presentation and promote an intestinal inflammatory response. A caveat to this study was the use of a whole body smoke exposure model, which may not induce the same physiological consequence as inhaled smoke. Erosion of the epithelial layer overlying the FAE has been observed in CD patient biopsies180. While no data on smoking-status of these patients exists, smoke-induced epithelial apoptosis is one possible mechanism for the development of these erosions. Thus smoking may induce an overall increase in antigenic presentation in the intestines, which may contribute to IBD pathogenesis.
Circulating TNF-α has been strongly implicated in co-morbidities associated with COPD52 and plays a central role in the progression of CD181. While, anti-TNF therapies do not appear to provide therapeutic relief in COPD52, they have been relatively successful for inducing remission in CD182–184. Whether this is due to the nature of the damage in COPD or the efficacy of TNF therapy requires further investigation. Studies in transgenic mouse models that over-express TNF-α, the TNFΔARE mouse model, have shown the development of spontaneous Crohn’s-like ileitis and proximal colitis185. While ocular and synovial involvement has been observed, there have been no reports of respiratory disease in this model. However, as with pulmonary manifestations of IBD, the airway involvement may be sub-clinical and histopathological and lung function studies may be required.
IL-6 plays a role in the acute phase response to inflammation and has been implicated in the pathogenesis of both COPD186, 187 and IBD188, 189. IL-6 is systemically elevated in patients with emphysema and has been shown be associated with apoptosis in pulmonary tissue186, 187. Importantly, IL-6, in combination with TGF-β, is a major factor in the development of the Th17 subset of T helper cells121, 190. Th17 cells are a distinct effector T cell subset that secrete IL-17A, IL-17F, IL-21, IL-22, IL-26, and TNF-α and promote neutrophil chemotaxis121, 191–194. Recent work has identified increased peripheral Th17 cells in COPD patients190.
IL-6 and Th17 cells are also associated with both CD and UC189, 195, and high levels of IL-6 and Th17 associated cytokines have been identified in both the blood189 and the inflamed and non-inflamed mucosa195, 196 of IBD patients. Moreover, blockade of the IL-6 pathway is therapeutic in animal models. The fact that IL-6 is elevated in the non-inflamed intestinal mucosa of IBD patients, without causing tissue damage, may suggest that a secondary tissue insult is required. As TGF-β regulates mucosal tissue remodelling and is strongly associated with COPD and IBD, it is conceivable, that increased systemic IL-6, coupled with TGF-β production at the mucosal surface (due to smoke damage in the lungs of an IBD patient or an intestinal infection in an COPD patients), may lead to the development of a Th17 polarized inflammatory response at a secondary organ.
IL-13 is likely to contribute to COPD progression197 and mutations in the IL-13 promoter may promote this pathogenesis 198. T-cell receptor-invariant natural killer cells (iNKCs) or DCs, activated by bacterial or viral infection in the airways, secrete IL-13, which activates macrophages 197, 199–201. This in turn causes further IL-13 production, which leads to STAT6-dependent goblet cell hyperplasia, smooth muscle hyper-responsiveness, and airway remodelling192, 202.
IL-13 also plays a role in the pathogenesis of UC, but does not appear to be involved in CD203. In UC it appears to be the aberrant stimulation of the immune response by the microbiome, that results in direct iNKC cytotoxic action on the epithelium and secretion of IL-13 driving epithelial barrier dysfunction and apoptosis, and the enhancement of NKC toxicity203, 204. Like COPD, STAT6 is an important mediator for the action of IL-13 on the epithelium205, and the STAT6 pathway is a potential therapeutic target in both conditions. Whether these pathways act systemically in COPD and IBD is unknown, although serum IL-13 is increased in COPD198, possibly driving aberrant NKT and macrophage responses across organs.
3.3 Interaction of the respiratory and intestinal microbiomes
COPD sufferers have an altered lung microbiome compared to healthy individuals, including “healthy” smokers106. This does not exclude the possibility that smoking influences the lung microbiome. Smoking has been shown to restrict the ability of alveolar macrophages to phagocytose and kill bacteria206. This suggests that smoking may lead to a defect in immunoregulation of the lung microbiome. There is evidence that components of the enteric microflora, specifically Gram negative bacilli, may also make up a component of the lung microflora207, 208. These bacteria are resistant to cigarette smoke209 and may contribute to severe exacerbations of COPD208. Furthermore, inappropriate immune responses against intestinal microflora are widely accepted to be a critical factor in the ongoing inflammation associated with IBD. Thus there exists the possibility that the immune response against commensal microflora observed in IBD patients, may not be restricted to the gastrointestinal tract, but may also be directed towards enteric bacteria present in the lung microfora.
There have been no definitive studies on the effect of smoking on the respiratory or intestinal microbiome. This is especially surprising given cigarette smoke is known to selectively inhibit bacterial growth, favouring a Gram negative bacilli population209. It is possible that smoke-induced changes to the intestinal microbiome may promote the increased risk of IBD observed in COPD sufferers. There is growing interest in how diet and nutrition may influence the human microbiome and interplay with the immune system and ultimately human health210, 211. Faecal bacteriotherapy, whereby the microflora of a healthy patient is transplanted to a colic patient, has shown promise in case studies, as a treatment for UC105, 212, 213. This suggests that the composition of the microbiome plays an important role in the intestinal inflammation, and restoration of a “healthy microbiome” can promote remission of disease. While ultimately conjecture, it is conceivable that smoking may disrupt the “healthy microbiome” and therefore link, smoking and COPD to IBD. This could also account for the familial link of COPD and IBD observed by Ekbom et al214, since there is a familial link to the make-up of an individuals microbiome and genetics play a role in microbiome development215, 216.
3.4 Autoimmunity
There is some evidence to suggest that COPD has an autoimmune element which leads to disease progression and relapse217. Key to this concept are the observations that only some smokers develop COPD and that the clinical features of COPD continue to increase in severity even after the cessation of smoking. This suggests that ongoing immune responses occur against elements other than cigarette smoke. Smoke-induced emphysema has been shown to generate an autoimmune response against elastins144, 218. In this proposed model, exposure to smoke-antigens promotes an immune response that includes secretion of high levels of elastin proteases (elastases) from neutrophils and macrophages (eg. neutrophil elastase, MMP-9 and -12)219. The elastases degrade and fragment elastin proteins, to which the adaptive immune system mounts a response144. As elastin is a ubiquitous protein in mucosal tissue, an autoimmune response could lead to pathologies outside the lung, and may be a mechanism for intestinal pathologies associated with smoking.
Tzortzaki & Siafakas proposed that smoke-induced oxidative epithelial damage initiates the disease process in COPD through the initiation of autoimmune responses220. In their proposed model, oxidative DNA damage to epithelial cells leads to phenotypic changes and recognition of these cells as “non-self” by pulmonary DCs. This results in a loss of barrier function as a T cell response is initiated against the epithelium. Such autoimmune responses may affect the intestinal epithelium, or may be driven by smoke exposure at the intestinal mucosa.
It is generally accepted that CD is a disease with an autoimmune component. The prevailing hypothesis for the development of CD is that an initial infection or insult leads to an inappropriate immune response against the intestinal mucosa and/or commensal bacterial population30, 57. This leads to the recurring cycles of chronic inflammation that characterise CD. UC also has a clear autoimmune element, albeit different to that of CD221, 222. Recent work has found that isoforms of human tropomyosin (hTM 1–5) are capable of inducing auto-antibodies and T cell responses in UC223. Autoimmunity would also explain some elements of organ cross-talk in inflammatory disease. Immune responses against bacteria or conserved mucosal protein epitopes of the pulmonary and gastrointestinal tracts may explain cross-organ inflammation in COPD and IBD. Expression of hTM on extra-intestinal organs may account for cross-organ inflammatory associations in UC, although hTM5, the trypomyosin with the strongest link to UC, has not been identified in lung tissue223.
4. Summary
COPD and IBD are driven by inflammatory processes, are systemic diseases and are epidemiologically linked. Given the consistent indications of the limited research to date, it is clear that comprehensive studies on the prevalence of intestinal involvement in COPD and pulmonary disease among IBD patients is required. The mechanisms that underpin the development of extra-organ inflammation in COPD and IBD patients are confounded by the complicated aetiologies of these conditions. Both conditions share environmental triggers and have similar immune and physiological involvement. However, the diversity of the mechanisms that may be involved in the development of each condition suggests that crosstalk in these diseases may be a multi-faceted process involving multiple pathways (Figure 1). Our understanding of this area is largely based on epidemiological and clinical observations and there is a need for basic research to elucidate the associations and mechanisms involved. A better understanding of the nature of organ cross-talk in COPD and IBD will contribute to the elucidation of the aetiologies of these conditions and may identify therapeutic strategies for mucosal inflammatory disease.
Acknowledgments
SK has been supported by a Crohn’s and Colitis Fellowship of America and is currently supported by funding from the National Health and Medical Research Council of Australia. NJT is supported by funding from the National Health and Medical Research Council of Australia and the National Institute of Health of the United States of America. PMH is supported by funding from the National Health and Medical Research Council of Australia and the Australian Research Council.
Contributor Information
Simon Keely, School of Biomedical Sciences and Pharmacy and Hunter Medical Research Institute, The University of Newcastle, NSW, Australia.
Nicholas J. Talley, Faculty of Health and Hunter Medical Research Institute, The University of Newcastle, NSW, Australia
Philip M. Hansbro, Centre for Asthma and Respiratory Disease, School of Biomedical Sciences and Pharmacy and Hunter Medical Research Institute, The University of Newcastle, NSW, Australia.
References
- 1.WHO. The global burden of disease: 2004 update. World Health Organisation; 2008. [Google Scholar]
- 2.Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol. 2006;12(38):6102–6108. doi: 10.3748/wjg.v12.i38.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 4.Association ACsaC. The economic costs of Crohn's disease and ulcerative colitis. Access Economics Pty Limited; 2007. [Google Scholar]
- 5.Kamm MA. Review article: chronic active disease and maintaining remission in Crohn's disease. Alimentary pharmacology & therapeutics. 2004;20 (Suppl 4):102–105. doi: 10.1111/j.1365-2036.2004.02052.x. [DOI] [PubMed] [Google Scholar]
- 6.Holguin F, Folch E, Redd SC, Mannino DM. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest. 2005;128(4):2005–2011. doi: 10.1378/chest.128.4.2005. [DOI] [PubMed] [Google Scholar]
- 7.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7(4):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 8.Mestecky J, McGhee JR, Michalek SM, Arnold RR, Crago SS, Babb JL. Concept of the local and common mucosal immune response. Adv Exp Med Biol. 1978;107:185–192. doi: 10.1007/978-1-4684-3369-2_22. [DOI] [PubMed] [Google Scholar]
- 9.Kuebler WM. Inflammatory pathways and microvascular responses in the lung. Pharmacol Rep. 2005;57 (Suppl):196–205. [PubMed] [Google Scholar]
- 10.Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason KL, Huffnagle GB, Noverr MC, Kao JY. Overview of gut immunology. Adv Exp Med Biol. 2008;635:1–14. doi: 10.1007/978-0-387-09550-9_1. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi I, Kiyono H. Gut as the largest immunologic tissue. JPEN J Parenter Enteral Nutr. 1999;23(5 Suppl):S7–12. doi: 10.1177/014860719902300503. [DOI] [PubMed] [Google Scholar]
- 13.Keely S, Glover LE, Weissmueller T, MacManus CF, Fillon S, Fennimore B, et al. Hypoxia-inducible factor-dependent regulation of platelet-activating factor receptor as a route for gram-positive bacterial translocation across epithelia. Mol Biol Cell. 2010;21(4):538–546. doi: 10.1091/mbc.E09-07-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kominsky DJ, Keely S, MacManus CF, Glover LE, Scully M, Collins CB, et al. An endogenously anti-inflammatory role for methylation in mucosal inflammation identified through metabolite profiling. J Immunol. 2011;186(11):6505–6514. doi: 10.4049/jimmunol.1002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthay MA. Function of the alveolar epithelial barrier under pathologic conditions. Chest. 1994;105(3 Suppl):67S–74S. doi: 10.1378/chest.105.3_supplement.67s. [DOI] [PubMed] [Google Scholar]
- 16.Keely S, Rawlinson LA, Haddleton DM, Brayden DJ. A tertiary amino-containing polymethacrylate polymer protects mucus-covered intestinal epithelial monolayers against pathogenic challenge. Pharm Res. 2008;25(5):1193–1201. doi: 10.1007/s11095-007-9501-3. [DOI] [PubMed] [Google Scholar]
- 17.Keely S, Rullay A, Wilson C, Carmichael A, Carrington S, Corfield A, et al. In vitro and ex vivo intestinal tissue models to measure mucoadhesion of poly (methacrylate) and N-trimethylated chitosan polymers. Pharm Res. 2005;22(1):38–49. doi: 10.1007/s11095-004-9007-1. [DOI] [PubMed] [Google Scholar]
- 18.Holt PG. Development of bronchus associated lymphoid tissue (BALT) in human lung disease: a normal host defence mechanism awaiting therapeutic exploitation? Thorax. 1993;48(11):1097–1098. doi: 10.1136/thx.48.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forchielli ML, Walker WA. The role of gut-associated lymphoid tissues and mucosal defence. Br J Nutr. 2005;93 (Suppl 1):S41–48. doi: 10.1079/bjn20041356. [DOI] [PubMed] [Google Scholar]
- 20.Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134(10):1991–2000. doi: 10.1242/dev.02846. [DOI] [PubMed] [Google Scholar]
- 21.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127(12):2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 22.Roth M. Pathogenesis of COPD. Part III. Inflammation in COPD. Int J Tuberc Lung Dis. 2008;12(4):375–380. [PubMed] [Google Scholar]
- 23.Vestbo J, Hogg JC. Convergence of the epidemiology and pathology of COPD. Thorax. 2006;61(1):86–88. doi: 10.1136/thx.2005.046227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang IV, Schwartz DA. Epigenetic control of gene expression in the lung. Am J Respir Crit Care Med. 2011;183(10):1295–1301. doi: 10.1164/rccm.201010-1579PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Reilly M, Hooper SB, Allison BJ, Flecknoe SJ, Snibson K, Harding R, et al. Persistent bronchiolar remodeling following brief ventilation of the very immature ovine lung. Am J Physiol Lung Cell Mol Physiol. 2009;297(5):L992–L1001. doi: 10.1152/ajplung.00099.2009. [DOI] [PubMed] [Google Scholar]
- 26.Horvat JC, Starkey MR, Kim RY, Phipps S, Gibson PG, Beagley KW, et al. Early-life chlamydial lung infection enhances allergic airways disease through age-dependent differences in immunopathology. J Allergy Clin Immunol. 2010;125(3):617–625. 625 e611–625 e616. doi: 10.1016/j.jaci.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. The Lancet. 2007;369(9573):1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 28.Allez M, Modigliani R. Clinical features of inflammatory bowel disease. Curr Opin Gastroenterol. 2000;16(4):329–336. doi: 10.1097/00001574-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Palnaes Hansen C, Hegnhoj J, Moller A, Brauer C, Hage E, Jarnum S. Ulcerative colitis and Crohn's disease of the colon. Is there a macroscopic difference? Ann Chir Gynaecol. 1990;79(2):78–81. [PubMed] [Google Scholar]
- 30.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3(7):390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 31.Tzanakis NE, Tsiligianni IG, Siafakas NM. Pulmonary involvement and allergic disorders in inflammatory bowel disease. World J Gastroenterol. 2010;16(3):299–305. doi: 10.3748/wjg.v16.i3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basseri B, Enayati P, Marchevsky A, Papadakis KA. Pulmonary manifestations of inflammatory bowel disease: case presentations and review. J Crohns Colitis. 2010;4(4):390–397. doi: 10.1016/j.crohns.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Black H, Mendoza M, Murin S. Thoracic manifestations of inflammatory bowel disease. Chest. 2007;131(2):524–532. doi: 10.1378/chest.06-1074. [DOI] [PubMed] [Google Scholar]
- 34.Fedorova TA, Spirina L, Chernekhovskaia NE, Kanareitseva TD, Sotnikova TI, Zhidkova NV, et al. The stomach and duodenum condition in patients with chronic obstructive lung diseases. Klin Med (Mosk) 2003;81(10):31–33. [PubMed] [Google Scholar]
- 35.Benard A, Desreumeaux P, Huglo D, Hoorelbeke A, Tonnel AB, Wallaert B. Increased intestinal permeability in bronchial asthma. J Allergy Clin Immunol. 1996;97(6):1173–1178. doi: 10.1016/s0091-6749(96)70181-1. [DOI] [PubMed] [Google Scholar]
- 36.Levine JB, Lukawski-Trubish D. Extraintestinal considerations in inflammatory bowel disease. Gastroenterol Clin North Am. 1995;24(3):633–646. [PubMed] [Google Scholar]
- 37.Decramer M, Rennard S, Troosters T, Mapel DW, Giardino N, Mannino D, et al. COPD as a lung disease with systemic consequences--clinical impact, mechanisms, and potential for early intervention. COPD. 2008;5(4):235–256. doi: 10.1080/15412550802237531. [DOI] [PubMed] [Google Scholar]
- 38.Duricova D, Pedersen N, Elkjaer M, Gamborg M, Munkholm P, Jess T. Overall and cause-specific mortality in Crohn's disease: a meta-analysis of population-based studies. Inflamm Bowel Dis. 2010;16(2):347–353. doi: 10.1002/ibd.21007. [DOI] [PubMed] [Google Scholar]
- 39.Ekbom A, Brandt L, Granath F, Lofdahl CG, Egesten A. Increased risk of both ulcerative colitis and Crohn's disease in a population suffering from COPD. Lung. 2008;186(3):167–172. doi: 10.1007/s00408-008-9080-z. [DOI] [PubMed] [Google Scholar]
- 40.Jess T, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Tremaine WJ, Melton LJ, 3rd , et al. Survival and cause specific mortality in patients with inflammatory bowel disease: a long term outcome study in Olmsted County, Minnesota, 1940–2004. Gut. 2006;55(9):1248–1254. doi: 10.1136/gut.2005.079350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beloborodova EI, Akimova LA, Burkovskaia BA, Asanova AV, Semenenko EV. Activity of systemic inflammatory reaction in patients with chronic obstructive pulmonary disease in regard to small intestinal absorption function. Ter Arkh. 2009;81(3):19–23. [PubMed] [Google Scholar]
- 42.Kuzela L, Vavrecka A, Prikazska M, Drugda B, Hronec J, Senkova A, et al. Pulmonary complications in patients with inflammatory bowel disease. Hepatogastroenterology. 1999;46(27):1714–1719. [PubMed] [Google Scholar]
- 43.Tzanakis N, Bouros D, Samiou M, Panagou P, Mouzas J, Manousos O, et al. Lung function in patients with inflammatory bowel disease. Respir Med. 1998;92(3):516–522. doi: 10.1016/s0954-6111(98)90301-8. [DOI] [PubMed] [Google Scholar]
- 44.Tzanakis N, Samiou M, Bouros D, Mouzas J, Kouroumalis E, Siafakas NM. Small airways function in patients with inflammatory bowel disease. Am J Respir Crit Care Med. 1998;157(2):382–386. doi: 10.1164/ajrccm.157.2.97-04075. [DOI] [PubMed] [Google Scholar]
- 45.Tzanakis NE. Pulmonary involvement and allergic disorders in inflammatory bowel disease. World Journal of Gastroenterology. 2010;16:299. doi: 10.3748/wjg.v16.i3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Songur N, Songur Y, Tuzun M, Dogan I, Tuzun D, Ensari A, et al. Pulmonary function tests and high-resolution CT in the detection of pulmonary involvement in inflammatory bowel disease. J Clin Gastroenterol. 2003;37(4):292–298. doi: 10.1097/00004836-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Douglas JG, McDonald CF, Leslie MJ, Gillon J, Crompton GK, McHardy GJ. Respiratory impairment in inflammatory bowel disease: does it vary with disease activity? Respir Med. 1989;83(5):389–394. doi: 10.1016/s0954-6111(89)80070-8. [DOI] [PubMed] [Google Scholar]
- 48.Ceyhan BB, Karakurt S, Cevik H, Sungur M. Bronchial hyperreactivity and allergic status in inflammatory bowel disease. Respiration. 2003;70(1):60–66. doi: 10.1159/000068407. [DOI] [PubMed] [Google Scholar]
- 49.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American journal of respiratory and critical care medicine. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 50.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. The New England journal of medicine. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 51.Stampfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9(5):377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 52.Sevenoaks MJ, Stockley RA. Chronic Obstructive Pulmonary Disease, inflammation and co-morbidity--a common inflammatory phenotype? Respir Res. 2006;7:70. doi: 10.1186/1465-9921-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lundback B, Lindberg A, Lindstrom M, Ronmark E, Jonsson AC, Jonsson E, et al. Not 15 but 50% of smokers develop COPD?--Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2003;97(2):115–122. doi: 10.1053/rmed.2003.1446. [DOI] [PubMed] [Google Scholar]
- 54.Hallberg J, Iliadou A, Anderson M, de Verdier MG, Nihlen U, Dahlback M, et al. Genetic and environmental influence on lung function impairment in Swedish twins. Respir Res. 2010;11:92. doi: 10.1186/1465-9921-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandford AJ, Joos L, Pare PD. Genetic risk factors for chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2002;8(2):87–94. doi: 10.1097/00063198-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Somerville KW, Logan RF, Edmond M, Langman MJ. Smoking and Crohn's disease. Br Med J (Clin Res Ed) 1984;289(6450):954–956. doi: 10.1136/bmj.289.6450.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Danese S, Fiocchi C. Etiopathogenesis of inflammatory bowel diseases. World journal of gastroenterology : WJG. 2006;12:4807–4812. doi: 10.3748/wjg.v12.i30.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Birrenbach T, Böcker U. Inflammatory bowel disease and smoking: a review of epidemiology, pathophysiology, and therapeutic implications. Inflammatory bowel diseases. 2004;10:848–859. doi: 10.1097/00054725-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 59.Cosnes J, Nion-Larmurier I, Afchain P, Beaugerie L, Gendre JP. Gender differences in the response of colitis to smoking. Clin Gastroenterol Hepatol. 2004;2(1):41–48. doi: 10.1016/s1542-3565(03)00290-8. [DOI] [PubMed] [Google Scholar]
- 60.Cosnes J. Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice. Best Pract Res Clin Gastroenterol. 2004;18(3):481–496. doi: 10.1016/j.bpg.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Logan RF, Edmond M, Somerville KW, Langman MJ. Smoking and ulcerative colitis. Br Med J (Clin Res Ed) 1984;288(6419):751–753. doi: 10.1136/bmj.288.6419.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bridger S, Lee JC, Bjarnason I, Jones JE, Macpherson AJ. In siblings with similar genetic susceptibility for inflammatory bowel disease, smokers tend to develop Crohn's disease and non-smokers develop ulcerative colitis. Gut. 2002;51(1):21–25. doi: 10.1136/gut.51.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beaugerie L, Massot N, Carbonnel F, Cattan S, Gendre JP, Cosnes J. Impact of cessation of smoking on the course of ulcerative colitis. Am J Gastroenterol. 2001;96(7):2113–2116. doi: 10.1111/j.1572-0241.2001.03944.x. [DOI] [PubMed] [Google Scholar]
- 64.Silverstein MD, Lashner BA, Hanauer SB. Cigarette smoking and ulcerative colitis: a case-control study. Mayo Clin Proc. 1994;69(5):425–429. doi: 10.1016/s0025-6196(12)61637-1. [DOI] [PubMed] [Google Scholar]
- 65.Boyko EJ, Koepsell TD, Perera DR, Inui TS. Risk of ulcerative colitis among former and current cigarette smokers. N Engl J Med. 1987;316(12):707–710. doi: 10.1056/NEJM198703193161202. [DOI] [PubMed] [Google Scholar]
- 66.WHO. Smoking statistics. WHO (World Health Organisation); 2002. [Google Scholar]
- 67.Yang SK, Loftus EV, Sandborn WJ. Epidemiology of inflammatory bowel disease in Asia. Inflammatory bowel diseases. 2001;7:260–270. doi: 10.1097/00054725-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 68.Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis. 2010;11(3):134–147. doi: 10.1111/j.1751-2980.2010.00429.x. [DOI] [PubMed] [Google Scholar]
- 69.Sun YP, Wang HH, He Q, Cho CH. Effect of passive cigarette smoking on colonic alpha7-nicotinic acetylcholine receptors in TNBS-induced colitis in rats. Digestion. 2007;76(3–4):181–187. doi: 10.1159/000112643. [DOI] [PubMed] [Google Scholar]
- 70.Galeazzi F, Blennerhassett PA, Qiu B, O'Byrne PM, Collins SM. Cigarette smoke aggravates experimental colitis in rats. Gastroenterology. 1999;117(4):877–883. doi: 10.1016/s0016-5085(99)70346-x. [DOI] [PubMed] [Google Scholar]
- 71.Guo X, Ko JK, Mei QB, Cho CH. Aggravating effect of cigarette smoke exposure on experimental colitis is associated with leukotriene B(4) and reactive oxygen metabolites. Digestion. 2001;63(3):180–187. doi: 10.1159/000051887. [DOI] [PubMed] [Google Scholar]
- 72.de Serres FJ, Blanco I, Fernandez-Bustillo E. Estimating the risk for alpha-1 antitrypsin deficiency among COPD patients: evidence supporting targeted screening. COPD. 2006;3(3):133–139. doi: 10.1080/15412550600829257. [DOI] [PubMed] [Google Scholar]
- 73.Stein PK, Nelson P, Rottman JN, Howard D, Ward SM, Kleiger RE, et al. Heart rate variability reflects severity of COPD in PiZ alpha1-antitrypsin deficiency. Chest. 1998;113(2):327–333. doi: 10.1378/chest.113.2.327. [DOI] [PubMed] [Google Scholar]
- 74.Pillai SG, Ge D, Zhu G, Kong X, Shianna KV, Need AC, et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet. 2009;5(3):e1000421. doi: 10.1371/journal.pgen.1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Durme YM, Eijgelsheim M, Joos GF, Hofman A, Uitterlinden AG, Brusselle GG, et al. Hedgehog-interacting protein is a COPD susceptibility gene: the Rotterdam Study. Eur Respir J. 2010;36(1):89–95. doi: 10.1183/09031936.00129509. [DOI] [PubMed] [Google Scholar]
- 76.Wilk JB, Chen TH, Gottlieb DJ, Walter RE, Nagle MW, Brandler BJ, et al. A genome-wide association study of pulmonary function measures in the Framingham Heart Study. PLoS Genet. 2009;5(3):e1000429. doi: 10.1371/journal.pgen.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chappell SL, Daly L, Lotya J, Alsaegh A, Guetta-Baranes T, Roca J, et al. The role of IREB2 and transforming growth factor beta-1 genetic variants in COPD: a replication case-control study. BMC Med Genet. 2011;12:24. doi: 10.1186/1471-2350-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chappell SL, Daly L, Lotya J, Alsaegh A, Guetta-Baranes T, Roca J, et al. The role of IREB2 and transforming growth factor beta-1 genetic variants in COPD: a replication case-control study. BMC Med Genet. 12:24. doi: 10.1186/1471-2350-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DeMeo DL, Mariani T, Bhattacharya S, Srisuma S, Lange C, Litonjua A, et al. Integration of genomic and genetic approaches implicates IREB2 as a COPD susceptibility gene. Am J Hum Genet. 2009;85(4):493–502. doi: 10.1016/j.ajhg.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bengtson MB, Solberg C, Aamodt G, Jahnsen J, Moum B, Sauar J, et al. Clustering in time of familial IBD separates ulcerative colitis from Crohn's disease. Inflamm Bowel Dis. 2009;15(12):1867–1874. doi: 10.1002/ibd.20978. [DOI] [PubMed] [Google Scholar]
- 81.Cho JH, Brant SR. Recent Insights Into the Genetics of Inflammatory Bowel Disease. Gastroenterology. 2011;140:1704–1712.e1702. doi: 10.1053/j.gastro.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Strober W, Kitani A, Fuss I, Asano N, Watanabe T. The molecular basis of NOD2 susceptibility mutations in Crohn's disease. Mucosal Immunol. 2008;1 (Suppl 1):S5–9. doi: 10.1038/mi.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411(6837):603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 84.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 85.Prescott NJ, Fisher SA, Franke A, Hampe J, Onnie CM, Soars D, et al. A nonsynonymous SNP in ATG16L1 predisposes to ileal Crohn's disease and is independent of CARD15 and IBD5. Gastroenterology. 2007;132(5):1665–1671. doi: 10.1053/j.gastro.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 86.Cummings JR, Cooney R, Pathan S, Anderson CA, Barrett JC, Beckly J, et al. Confirmation of the role of ATG16L1 as a Crohn's disease susceptibility gene. Inflamm Bowel Dis. 2007;13(8):941–946. doi: 10.1002/ibd.20162. [DOI] [PubMed] [Google Scholar]
- 87.Cotterill L, Payne D, Levinson S, McLaughlin J, Wesley E, Feeney M, et al. Replication and meta-analysis of 13,000 cases defines the risk for interleukin-23 receptor and autophagy-related 16-like 1 variants in Crohn's disease. Can J Gastroenterol. 2010;24(5):297–302. doi: 10.1155/2010/480458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yano T, Kurata S. An unexpected twist for autophagy in Crohn's disease. Nat Immunol. 2009;10(2):134–136. doi: 10.1038/ni0209-134. [DOI] [PubMed] [Google Scholar]
- 89.Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat Genet. 2007;39(7):830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kinose D, Ogawa E, Hirota T, Ito I, Kudo M, Haruna A, et al. A NOD2 gene polymorphism is associated with the prevalence and severity of chronic obstructive pulmonary disease in a Japanese population. Respirology (Carlton, Vic) 2011 doi: 10.1111/j.1440-1843.2011.02069.x. [DOI] [PubMed] [Google Scholar]
- 91.Cottone M, Bunce M, Taylor CJ, Ting A, Jewell DP. Ulcerative colitis and HLA phenotype. Gut. 1985;26(9):952–954. doi: 10.1136/gut.26.9.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nahir M, Gideoni O, Eidelman S, Barzilai A. Letter: HLA antigens in ulcerative colitis. Lancet. 1976;2(7985):573. doi: 10.1016/s0140-6736(76)91820-1. [DOI] [PubMed] [Google Scholar]
- 93.Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41(12):1330–1334. doi: 10.1038/ng.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fisher SA, Tremelling M, Anderson CA, Gwilliam R, Bumpstead S, Prescott NJ, et al. Genetic determinants of ulcerative colitis include the ECM1 locus and five loci implicated in Crohn's disease. Nat Genet. 2008;40(6):710–712. doi: 10.1038/ng.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132(2):279–289. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 96.Murphy TF, Sethi S. Bacterial infection in chronic obstructive pulmonary disease. The American review of respiratory disease. 1992;146(4):1067–1083. doi: 10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- 97.Zalacain R, Sobradillo V, Amilibia J, Barron J, Achotegui V, Pijoan JI, et al. Predisposing factors to bacterial colonization in chronic obstructive pulmonary disease. Eur Respir J. 1999;13(2):343–348. doi: 10.1034/j.1399-3003.1999.13b21.x. [DOI] [PubMed] [Google Scholar]
- 98.Baughman RP, Thorpe JE, Staneck J, Rashkin M, Frame PT. Use of the protected specimen brush in patients with endotracheal or tracheostomy tubes. Chest. 1987;91(2):233–236. doi: 10.1378/chest.91.2.233. [DOI] [PubMed] [Google Scholar]
- 99.Kahn FW, Jones JM. Diagnosing bacterial respiratory infection by bronchoalveolar lavage. J Infect Dis. 1987;155(5):862–869. doi: 10.1093/infdis/155.5.862. [DOI] [PubMed] [Google Scholar]
- 100.Harris JK, De Groote MA, Sagel SD, Zemanick ET, Kapsner R, Penvari C, et al. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc Natl Acad Sci U S A. 2007;104(51):20529–20533. doi: 10.1073/pnas.0709804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang YJ, Kim E, Cox MJ, Brodie EL, Brown R, Wiener-Kronish JP, et al. A persistent and diverse airway microbiota present during chronic obstructive pulmonary disease exacerbations. OMICS. 2010;14(1):9–59. doi: 10.1089/omi.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frank DN, Robertson CE, Hamm CM, Kpadeh Z, Zhang T, Chen H, et al. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2010;17(1):179–184. doi: 10.1002/ibd.21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sartor RB. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology. 2010;139(6):1816–1819. doi: 10.1053/j.gastro.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 104.Salzman NH, Bevins CL. Negative interactions with the microbiota: IBD. Adv Exp Med Biol. 2008;635:67–78. doi: 10.1007/978-0-387-09550-9_6. [DOI] [PubMed] [Google Scholar]
- 105.Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37(1):42–47. doi: 10.1097/00004836-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 106.Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One. 2011;6(2):e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schultsz C, Van Den Berg FM, Ten Kate FW, Tytgat GN, Dankert J. The intestinal mucus layer from patients with inflammatory bowel disease harbors high numbers of bacteria compared with controls. Gastroenterology. 1999;117(5):1089–1097. doi: 10.1016/s0016-5085(99)70393-8. [DOI] [PubMed] [Google Scholar]
- 108.Shaykhiev R, Otaki F, Bonsu P, Dang DT, Teater M, Strulovici-Barel Y, et al. Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell Mol Life Sci. 2011;68(5):877–892. doi: 10.1007/s00018-010-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heijink IH, Brandenburg SM, Postma DS, van Oosterhout AJ. Cigarette smoke impairs airway epithelial barrier function and cell-cell contact recovery. Eur Respir J. 2011 doi: 10.1183/09031936.00193810. [DOI] [PubMed] [Google Scholar]
- 110.Rask-Madsen J, Hammersgaard EA, Knudsen E. Rectal electrolyte transport and mucosal permeability in ulcerative colitis and Crohn's disease. J Lab Clin Med. 1973;81(3):342–353. [PubMed] [Google Scholar]
- 111.McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15(1):100–113. doi: 10.1002/ibd.20539. [DOI] [PubMed] [Google Scholar]
- 112.Yu Y, Sitaraman S, Gewirtz AT. Intestinal epithelial cell regulation of mucosal inflammation. Immunol Res. 2004;29(1–3):55–68. doi: 10.1385/IR:29:1-3:055. [DOI] [PubMed] [Google Scholar]
- 113.Soderholm JD, Olaison G, Peterson KH, Franzen LE, Lindmark T, Wiren M, et al. Augmented increase in tight junction permeability by luminal stimuli in the non-inflamed ileum of Crohn's disease. Gut. 2002;50(3):307–313. doi: 10.1136/gut.50.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martin HM, Campbell BJ, Hart CA, Mpofu C, Nayar M, Singh R, et al. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology. 2004;127(1):80–93. doi: 10.1053/j.gastro.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 115.Furrie E, Macfarlane S, Cummings JH, Macfarlane GT. Systemic antibodies towards mucosal bacteria in ulcerative colitis and Crohn's disease differentially activate the innate immune response. Gut. 2004;53(1):91–98. doi: 10.1136/gut.53.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lunardi C, Bason C, Dolcino M, Navone R, Simone R, Saverino D, et al. Antiflagellin antibodies recognize the autoantigens Toll-Like Receptor 5 and Pals 1-associated tight junction protein and induce monocytes activation and increased intestinal permeability in Crohn's disease. J Intern Med. 2009;265(2):250–265. doi: 10.1111/j.1365-2796.2008.02013.x. [DOI] [PubMed] [Google Scholar]
- 117.Marin ML, Greenstein AJ, Geller SA, Gordon RE, Aufses AH., Jr A freeze fracture study of Crohn's disease of the terminal ileum: changes in epithelial tight junction organization. Am J Gastroenterol. 1983;78(9):537–547. [PubMed] [Google Scholar]
- 118.Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116(2):301–309. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 119.Bauer S, Muller T, Hamm S. Pattern recognition by Toll-like receptors. Adv Exp Med Biol. 2009;653:15–34. doi: 10.1007/978-1-4419-0901-5_2. [DOI] [PubMed] [Google Scholar]
- 120.Eckmann L, Karin M. NOD2 and Crohn's disease: loss or gain of function? Immunity. 2005;22(6):661–667. doi: 10.1016/j.immuni.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 121.Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123(3):326–338. doi: 10.1111/j.1365-2567.2007.02719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Crim C, Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34(3):641–647. doi: 10.1183/09031936.00193908. [DOI] [PubMed] [Google Scholar]
- 123.Strober W, Kitani a, Fuss I, Asano N, Watanabe T. The molecular basis of NOD2 susceptibility mutations in Crohn's disease. Mucosal immunology. 2008;1 (Suppl 1):S5–9. doi: 10.1038/mi.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van der Heide F, Nolte IM, Kleibeuker JH, Wijmenga C, Dijkstra G, Weersma RK. Differences in genetic background between active smokers, passive smokers, and non-smokers with Crohn's disease. Am J Gastroenterol. 2010;105(5):1165–1172. doi: 10.1038/ajg.2009.659. [DOI] [PubMed] [Google Scholar]
- 125.Freeman CM, Han MK, Martinez FJ, Murray S, Liu LX, Chensue SW, et al. Cytotoxic potential of lung CD8(+) T cells increases with chronic obstructive pulmonary disease severity and with in vitro stimulation by IL-18 or IL-15. J Immunol. 2010;184(11):6504–6513. doi: 10.4049/jimmunol.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Motz GT, Eppert BL, Wortham BW, Amos-Kroohs RM, Flury JL, Wesselkamper SC, et al. Chronic cigarette smoke exposure primes NK cell activation in a mouse model of chronic obstructive pulmonary disease. J Immunol. 2010;184(8):4460–4469. doi: 10.4049/jimmunol.0903654. [DOI] [PubMed] [Google Scholar]
- 127.Kathrani A, House A, Catchpole B, Murphy A, German A, Werling D, et al. Polymorphisms in the TLR4 and TLR5 gene are significantly associated with inflammatory bowel disease in German shepherd dogs. PLoS One. 2010;5(12):e15740. doi: 10.1371/journal.pone.0015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pons J, Sauleda J, Regueiro V, Santos C, Lopez M, Ferrer J, et al. Expression of Toll-like receptor 2 is up-regulated in monocytes from patients with chronic obstructive pulmonary disease. Respir Res. 2006;7:64. doi: 10.1186/1465-9921-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sabroe I, Whyte MK, Wilson AG, Dower SK, Hubbard R, Hall I. Toll-like receptor (TLR) 4 polymorphisms and COPD. Thorax. 2004;59(1):81. [PMC free article] [PubMed] [Google Scholar]
- 130.Sarir H, Henricks PA, van Houwelingen AH, Nijkamp FP, Folkerts G. Cells, mediators and Toll-like receptors in COPD. Eur J Pharmacol. 2008;585(2–3):346–353. doi: 10.1016/j.ejphar.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 131.Droemann D, Goldmann T, Tiedje T, Zabel P, Dalhoff K, Schaaf B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res. 2005;6:68. doi: 10.1186/1465-9921-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pabst S, Yenice V, Lennarz M, Tuleta I, Nickenig G, Gillissen A, et al. Toll-like receptor 2 gene polymorphisms Arg677Trp and Arg753Gln in chronic obstructive pulmonary disease. Lung. 2009;187(3):173–178. doi: 10.1007/s00408-009-9144-8. [DOI] [PubMed] [Google Scholar]
- 133.Doz E, Noulin N, Boichot E, Guenon I, Fick L, Le Bert M, et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008;180(2):1169–1178. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- 134.Sarir H, Mortaz E, Karimi K, Kraneveld AD, Rahman I, Caldenhoven E, et al. Cigarette smoke regulates the expression of TLR4 and IL-8 production by human macrophages. J Inflamm (Lond) 2009;6:12. doi: 10.1186/1476-9255-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Szebeni B, Veres G, Dezsofi A, Rusai K, Vannay A, Mraz M, et al. Increased expression of Toll-like receptor (TLR) 2 and TLR4 in the colonic mucosa of children with inflammatory bowel disease. Clin Exp Immunol. 2008;151(1):34–41. doi: 10.1111/j.1365-2249.2007.03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Canto E, Ricart E, Monfort D, Gonzalez-Juan D, Balanzo J, Rodriguez-Sanchez JL, et al. TNF alpha production to TLR2 ligands in active IBD patients. Clin Immunol. 2006;119(2):156–165. doi: 10.1016/j.clim.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 137.Hong J, Leung E, Fraser AG, Merriman TR, Vishnu P, Krissansen GW. TLR2, TLR4 and TLR9 polymorphisms and Crohn's disease in a New Zealand Caucasian cohort. Journal of gastroenterology and hepatology. 2007;22(11):1760–1766. doi: 10.1111/j.1440-1746.2006.04727.x. [DOI] [PubMed] [Google Scholar]
- 138.Rigoli L, Romano C, Caruso RA, Lo Presti MA, Di Bella C, Procopio V, et al. Clinical significance of NOD2/CARD15 and Toll-like receptor 4 gene single nucleotide polymorphisms in inflammatory bowel disease. World J Gastroenterol. 2008;14(28):4454–4461. doi: 10.3748/wjg.14.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shen X, Shi R, Zhang H, Li K, Zhao Y, Zhang R. The Toll-like receptor 4 D299G and T399I polymorphisms are associated with Crohn's disease and ulcerative colitis: a meta-analysis. Digestion. 2010;81(2):69–77. doi: 10.1159/000260417. [DOI] [PubMed] [Google Scholar]
- 140.Speletas M, Merentiti V, Kostikas K, Liadaki K, Minas M, Gourgoulianis K, et al. Association of TLR4-T399I polymorphism with chronic obstructive pulmonary disease in smokers. Clin Dev Immunol. 2009;2009:260286. doi: 10.1155/2009/260286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167(3):1609–1616. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 142.Suzuki M, Hisamatsu T, Podolsky DK. Gamma interferon augments the intracellular pathway for lipopolysaccharide (LPS) recognition in human intestinal epithelial cells through coordinated up-regulation of LPS uptake and expression of the intracellular Toll-like receptor 4-MD-2 complex. Infect Immun. 2003;71(6):3503–3511. doi: 10.1128/IAI.71.6.3503-3511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vlahos R, Bozinovski S, Jones JE, Powell J, Gras J, Lilja A, et al. Differential protease, innate immunity, and NF-kappaB induction profiles during lung inflammation induced by subchronic cigarette smoke exposure in mice. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L931–945. doi: 10.1152/ajplung.00201.2005. [DOI] [PubMed] [Google Scholar]
- 144.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13(5):567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 145.Churg A, Wang R, Wang X, Onnervik PO, Thim K, Wright JL. Effect of an MMP-9/MMP-12 inhibitor on smoke-induced emphysema and airway remodelling in guinea pigs. Thorax. 2007;62(8):706–713. doi: 10.1136/thx.2006.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vernooy JH, Lindeman JH, Jacobs JA, Hanemaaijer R, Wouters EF. Increased activity of matrix metalloproteinase-8 and matrix metalloproteinase-9 in induced sputum from patients with COPD. Chest. 2004;126(6):1802–1810. doi: 10.1378/chest.126.6.1802. [DOI] [PubMed] [Google Scholar]
- 147.Ohkawara T, Nishihira J, Takeda H, Hige S, Kato M, Sugiyama T, et al. Amelioration of dextran sulfate sodium-induced colitis by anti-macrophage migration inhibitory factor antibody in mice. Gastroenterology. 2002;123(1):256–270. doi: 10.1053/gast.2002.34236. [DOI] [PubMed] [Google Scholar]
- 148.Garg P, Vijay-Kumar M, Wang L, Gewirtz AT, Merlin D, Sitaraman SV. Matrix metalloproteinase-9-mediated tissue injury overrides the protective effect of matrix metalloproteinase-2 during colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296(2):G175–184. doi: 10.1152/ajpgi.90454.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Medina C, Santana A, Paz MC, Diaz-Gonzalez F, Farre E, Salas A, et al. Matrix metalloproteinase-9 modulates intestinal injury in rats with transmural colitis. J Leukoc Biol. 2006;79(5):954–962. doi: 10.1189/jlb.1005544. [DOI] [PubMed] [Google Scholar]
- 150.Pender SL, Li CK, Di Sabatino A, MacDonald TT, Buckley MG. Role of macrophage metalloelastase in gut inflammation. Ann N Y Acad Sci. 2006;1072:386–388. doi: 10.1196/annals.1326.019. [DOI] [PubMed] [Google Scholar]
- 151.Yang P, Bamlet WR, Sun Z, Ebbert JO, Aubry MC, Krowka MJ, et al. Alpha1-antitrypsin and neutrophil elastase imbalance and lung cancer risk. Chest. 2005;128(1):445–452. doi: 10.1378/chest.128.1.445. [DOI] [PubMed] [Google Scholar]
- 152.Churg A, Wang X, Wang RD, Meixner SC, Pryzdial EL, Wright JL. Alpha1-antitrypsin suppresses TNF-alpha and MMP-12 production by cigarette smoke-stimulated macrophages. Am J Respir Cell Mol Biol. 2007;37(2):144–151. doi: 10.1165/rcmb.2006-0345OC. [DOI] [PubMed] [Google Scholar]
- 153.Sandford AJ, Weir TD, Spinelli JJ, Pare PD. Z and S mutations of the alpha1-antitrypsin gene and the risk of chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 1999;20(2):287–291. doi: 10.1165/ajrcmb.20.2.3177. [DOI] [PubMed] [Google Scholar]
- 154.Sandford AJ, Weir TD, Pare PD. Genetic risk factors for chronic obstructive pulmonary disease. Eur Respir J. 1997;10(6):1380–1391. doi: 10.1183/09031936.97.10061380. [DOI] [PubMed] [Google Scholar]
- 155.Biancone L, Fantini M, Tosti C, Bozzi R, Vavassori P, Pallone F. Fecal alpha 1-antitrypsin clearance as a marker of clinical relapse in patients with Crohn's disease of the distal ileum. Eur J Gastroenterol Hepatol. 2003;15(3):261–266. doi: 10.1097/00042737-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 156.Meyers S, Wolke A, Field SP, Feuer EJ, Johnson JW, Janowitz HD. Fecal alpha 1-antitrypsin measurement: an indicator of Crohn's disease activity. Gastroenterology. 1985;89(1):13–18. doi: 10.1016/0016-5085(85)90739-5. [DOI] [PubMed] [Google Scholar]
- 157.Elzouki AN, Eriksson S, Lofberg R, Nassberger L, Wieslander J, Lindgren S. The prevalence and clinical significance of alpha 1-antitrypsin deficiency (PiZ) and ANCA specificities (proteinase 3, BPI) in patients with ulcerative colitis. Inflamm Bowel Dis. 1999;5(4):246–252. doi: 10.1097/00054725-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 158.Yang P, Tremaine WJ, Meyer RL, Prakash UB. Alpha1-antitrypsin deficiency and inflammatory bowel diseases. Mayo Clin Proc. 2000;75(5):450–455. [PubMed] [Google Scholar]
- 159.Sinden NJ, Stockley Ra. Systemic inflammation and comorbidity in COPD: a result of 'overspill' of inflammatory mediators from the lungs? Review of the evidence Thorax. 2010;65:930–936. doi: 10.1136/thx.2009.130260. [DOI] [PubMed] [Google Scholar]
- 160.Eagan TML, Aukrust P, Ueland T, Hardie Ja, Johannessen a, Mollnes TE, et al. Body composition and plasma levels of inflammatory biomarkers in COPD. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2010;36:1027–1033. doi: 10.1183/09031936.00194209. [DOI] [PubMed] [Google Scholar]
- 161.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 162.Danese S, Semeraro S, Papa A, Roberto I, Scaldaferri F, Fedeli G, et al. Extraintestinal manifestations in inflammatory bowel disease. World journal of gastroenterology : WJG. 2005;11:7227–7236. doi: 10.3748/wjg.v11.i46.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rothfuss KS, Stange EF, Herrlinger KR. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol. 2006;12(30):4819–4831. doi: 10.3748/wjg.v12.i30.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272(5258):60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 165.Hart AL, Ng SC, Mann E, Al-Hassi HO, Bernardo D, Knight SC. Homing of immune cells: role in homeostasis and intestinal inflammation. Inflammatory bowel diseases. 2010;16:1969–1977. doi: 10.1002/ibd.21304. [DOI] [PubMed] [Google Scholar]
- 166.Sauleda J, Garcia-Palmer FJ, Gonzalez G, Palou A, Agusti AG. The activity of cytochrome oxidase is increased in circulating lymphocytes of patients with chronic obstructive pulmonary disease, asthma, and chronic arthritis. Am J Respir Crit Care Med. 2000;161(1):32–35. doi: 10.1164/ajrccm.161.1.9807079. [DOI] [PubMed] [Google Scholar]
- 167.Salmi M, Granfors K, MacDermott R, Jalkanen S. Aberrant binding of lamina propria lymphocytes to vascular endothelium in inflammatory bowel diseases. Gastroenterology. 1994;106(3):596–605. doi: 10.1016/0016-5085(94)90691-2. [DOI] [PubMed] [Google Scholar]
- 168.Salmi M, Andrew DP, Butcher EC, Jalkanen S. Dual binding capacity of mucosal immunoblasts to mucosal and synovial endothelium in humans: dissection of the molecular mechanisms. J Exp Med. 1995;181(1):137–149. doi: 10.1084/jem.181.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Eksteen B, Grant AJ, Miles A, Curbishley SM, Lalor PF, Hübscher SG, et al. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. The Journal of experimental medicine. 2004;200:1511–1517. doi: 10.1084/jem.20041035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bonniere P, Wallaert B, Cortot A, Marchandise X, Riou Y, Tonnel AB, et al. Latent pulmonary involvement in Crohn's disease: biological, functional, bronchoalveolar lavage and scintigraphic studies. Gut. 1986;27(8):919–925. doi: 10.1136/gut.27.8.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wallaert B, Colombel JF, Tonnel AB, Bonniere P, Cortot A, Paris JC, et al. Evidence of lymphocyte alveolitis in Crohn's disease. Chest. 1985;87(3):363–367. doi: 10.1378/chest.87.3.363. [DOI] [PubMed] [Google Scholar]
- 172.Fireman Z, Osipov a, Kivity S, Kopelman Y, Sternberg a, Lazarov E, et al. The use of induced sputum in the assessment of pulmonary involvement in Crohn's disease. The American journal of gastroenterology. 2000;95:730–734. doi: 10.1111/j.1572-0241.2000.01843.x. [DOI] [PubMed] [Google Scholar]
- 173.Hodge G, Mukaro V, Reynolds PN, Hodge S. Role of increased CD8/CD28(null) T cells and alternative co-stimulatory molecules in chronic obstructive pulmonary disease. Clin Exp Immunol. 2011;166(1):94–102. doi: 10.1111/j.1365-2249.2011.04455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Brozyna S, Ahern J, Hodge G, Nairn J, Holmes M, Reynolds PN, et al. Chemotactic mediators of Th1 T-cell trafficking in smokers and COPD patients. COPD. 2009;6(1):4–16. doi: 10.1080/15412550902724164. [DOI] [PubMed] [Google Scholar]
- 175.Lommatzsch M, Bratke K, Knappe T, Bier a, Dreschler K, Kuepper M, et al. Acute effects of tobacco smoke on human airway dendritic cells in vivo. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2010;35:1130–1136. doi: 10.1183/09031936.00090109. [DOI] [PubMed] [Google Scholar]
- 176.Bratke K, Klug M, Bier A, Julius P, Kuepper M, Virchow JC, et al. Function-associated surface molecules on airway dendritic cells in cigarette smokers. American journal of respiratory cell and molecular biology. 2008;38:655–660. doi: 10.1165/rcmb.2007-0400OC. [DOI] [PubMed] [Google Scholar]
- 177.Tsoumakidou M, Elston W, Zhu J, Wang Z, Gamble E, Siafakas NM, et al. Cigarette smoking alters bronchial mucosal immunity in asthma. American journal of respiratory and critical care medicine. 2007;175:919–925. doi: 10.1164/rccm.200607-908OC. [DOI] [PubMed] [Google Scholar]
- 178.Robbins CS, Dawe DE, Goncharova SI, Pouladi MA, Drannik AG, Swirski FK, et al. Cigarette smoke decreases pulmonary dendritic cells and impacts antiviral immune responsiveness. Am J Respir Cell Mol Biol. 2004;30(2):202–211. doi: 10.1165/rcmb.2003-0259OC. [DOI] [PubMed] [Google Scholar]
- 179.Verschuere S, Bracke KR, Demoor T, Plantinga M, Verbrugghe P, Ferdinande L, et al. Cigarette smoking alters epithelial apoptosis and immune composition in murine GALT. Laboratory investigation; a journal of technical methods and pathology. 2011;91:1056–1067. doi: 10.1038/labinvest.2011.74. [DOI] [PubMed] [Google Scholar]
- 180.Fujimura Y, Kamoi R, Iida M. Pathogenesis of aphthoid ulcers in Crohn's disease: correlative findings by magnifying colonoscopy, electron microscopy, and immunohistochemistry. Gut. 1996;38(5):724–732. doi: 10.1136/gut.38.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Plevy SE, Landers CJ, Prehn J, Carramanzana NM, Deem RL, Shealy D, et al. A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn's disease. J Immunol. 1997;159(12):6276–6282. [PubMed] [Google Scholar]
- 182.van Deventer SJ. Transmembrane TNF-alpha, induction of apoptosis, and the efficacy of TNF-targeting therapies in Crohn's disease. Gastroenterology. 2001;121(5):1242–1246. doi: 10.1053/gast.2001.29035. [DOI] [PubMed] [Google Scholar]
- 183.Doubremelle M, Bourreille A, Zerbib F, Heresbach D, Metman EH, Beau P, et al. Treatment of Crohn's disease with anti-TNF alpha antibodies (infliximab): results of a multicentric and retrospective study. Gastroenterol Clin Biol. 2002;26(11):973–979. [PubMed] [Google Scholar]
- 184.Antoniu SA. Infliximab for chronic obstructive pulmonary disease: towards a more specific inflammation targeting? Expert Opin Investig Drugs. 2006;15(2):181–184. doi: 10.1517/13543784.15.2.181. [DOI] [PubMed] [Google Scholar]
- 185.Pizarro TT, Arseneau KO, Cominelli F. Lessons from genetically engineered animal models XI. Novel mouse models to study pathogenic mechanisms of Crohn's disease. Am J Physiol Gastrointest Liver Physiol. 2000;278(5):G665–669. doi: 10.1152/ajpgi.2000.278.5.G665. [DOI] [PubMed] [Google Scholar]
- 186.Ruwanpura SM, McLeod L, Miller A, Jones J, Bozinovski S, Vlahos R, et al. Interleukin-6 promotes Pulmonary Emphysema Associated with Apoptosis in Mice. American journal of respiratory cell and molecular biology. 2011 doi: 10.1165/rcmb.2010-0462OC. [DOI] [PubMed] [Google Scholar]
- 187.Xiong Z, Leme AS, Ray P, Shapiro SD, Lee JS. CX3CR1+ lung mononuclear phagocytes spatially confined to the interstitium produce TNF-alpha and IL-6 and promote cigarette smoke-induced emphysema. J Immunol. 2011;186(5):3206–3214. doi: 10.4049/jimmunol.1003221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Danese S, Gao B. Interleukin-6: a therapeutic Jekyll and Hyde in gastrointestinal and hepatic diseases. Gut. 2010;59(2):149–151. doi: 10.1136/gut.2008.173534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30(1):80–89. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- 190.Vargas-Rojas MI, Ramirez-Venegas A, Limon-Camacho L, Ochoa L, Hernandez-Zenteno R, Sansores RH. Increase of Th17 cells in peripheral blood of patients with chronic obstructive pulmonary disease. Respir Med. 2011;105(11):1648–1654. doi: 10.1016/j.rmed.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 191.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 192.Hansbro PM, Kaiko GE, Foster PS. Cytokine/anti-cytokine therapy - novel treatments for asthma? Br J Pharmacol. 2011;163(1):81–95. doi: 10.1111/j.1476-5381.2011.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Horvat JC, Starkey MR, Kim RY, Beagley KW, Preston JA, Gibson PG, et al. Chlamydial respiratory infection during allergen sensitization drives neutrophilic allergic airways disease. J Immunol. 2010;184(8):4159–4169. doi: 10.4049/jimmunol.0902287. [DOI] [PubMed] [Google Scholar]
- 194.Essilfie A-T, Simpson JL, Horvat JC, Preston JA, Dunkley ML, Foster PS, et al. Haemophilus influenzae Infection Drives IL-17-Mediated Neutrophilic Allergic Airways Disease. PLoS Pathog. 2011;7(10):e1002244. doi: 10.1371/journal.ppat.1002244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Olsen T, Rismo R, Cui G, Goll R, Christiansen I, Florholmen J. TH1 and TH17 interactions in untreated inflamed mucosa of inflammatory bowel disease, and their potential to mediate the inflammation. Cytokine. 2011 doi: 10.1016/j.cyto.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 196.Leon AJ, Gomez E, Garrote JA, Bernardo D, Barrera A, Marcos JL, et al. High levels of proinflammatory cytokines, but not markers of tissue injury, in unaffected intestinal areas from patients with IBD. Mediators Inflamm. 2009;2009:580450. doi: 10.1155/2009/580450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14(6):633–640. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liu SF, Chen YC, Wang CC, Fang WF, Chin CH, Su MC, et al. Il13 promoter (-1055) polymorphisms associated with chronic obstructive pulmonary disease in Taiwanese. Exp Lung Res. 2009;35(10):807–816. doi: 10.3109/01902140902893644. [DOI] [PubMed] [Google Scholar]
- 199.Asquith KL, Horvat JC, Kaiko GE, Carey AJ, Beagley KW, Hansbro PM, et al. Interleukin-13 promotes susceptibility to chlamydial infection of the respiratory and genital tracts. PLoS Pathog. 2011;7(5):e1001339. doi: 10.1371/journal.ppat.1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kaiko GE, Phipps S, Hickey DK, Lam CE, Hansbro PM, Foster PS, et al. Chlamydia muridarum infection subverts dendritic cell function to promote Th2 immunity and airways hyperreactivity. J Immunol. 2008;180(4):2225–2232. doi: 10.4049/jimmunol.180.4.2225. [DOI] [PubMed] [Google Scholar]
- 201.Hansbro NG, Horvat JC, Wark PA, Hansbro PM. Understanding the mechanisms of viral induced asthma: new therapeutic directions. Pharmacol Ther. 2008;117(3):313–353. doi: 10.1016/j.pharmthera.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nofziger C, Vezzoli V, Dossena S, Schonherr T, Studnicka J, Nofziger J, et al. STAT6 links IL-4/IL-13 stimulation with pendrin expression in asthma and chronic obstructive pulmonary disease. Clin Pharmacol Ther. 2011;90(3):399–405. doi: 10.1038/clpt.2011.128. [DOI] [PubMed] [Google Scholar]
- 203.Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113(10):1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fuss IJ, Strober W. The role of IL-13 and NK T cells in experimental and human ulcerative colitis. Mucosal Immunol. 2008;1 (Suppl 1):S31–33. doi: 10.1038/mi.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rosen MJ, Frey MR, Washington MK, Chaturvedi R, Kuhnhein LA, Matta P, et al. STAT6 activation in ulcerative colitis: A new target for prevention of IL-13-induced colon epithelial cell dysfunction. Inflamm Bowel Dis. 2011;17(11):2224–2234. doi: 10.1002/ibd.21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.King TE, Jr, Savici D, Campbell PA. Phagocytosis and killing of Listeria monocytogenes by alveolar macrophages: smokers versus nonsmokers. J Infect Dis. 1988;158(6):1309–1316. doi: 10.1093/infdis/158.6.1309. [DOI] [PubMed] [Google Scholar]
- 207.Lode H, Allewelt M, Balk S, De Roux A, Mauch H, Niederman M, et al. A prediction model for bacterial etiology in acute exacerbations of COPD. Infection. 2007;35(3):143–149. doi: 10.1007/s15010-007-6078-z. [DOI] [PubMed] [Google Scholar]
- 208.Soler N, Torres A, Ewig S, Gonzalez J, Celis R, El-Ebiary M, et al. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1498–1505. doi: 10.1164/ajrccm.157.5.9711044. [DOI] [PubMed] [Google Scholar]
- 209.Ertel A, Eng R, Smith SM. The differential effect of cigarette smoke on the growth of bacteria found in humans. Chest. 1991;100(3):628–630. doi: 10.1378/chest.100.3.628. [DOI] [PubMed] [Google Scholar]
- 210.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ehlers S, Kaufmann SH. Infection, inflammation, and chronic diseases: consequences of a modern lifestyle. Trends Immunol. 2010;31(5):184–190. doi: 10.1016/j.it.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 212.Borody TJ, Warren EF, Leis SM, Surace R, Ashman O, Siarakas S. Bacteriotherapy using fecal flora: toying with human motions. J Clin Gastroenterol. 2004;38(6):475–483. doi: 10.1097/01.mcg.0000128988.13808.dc. [DOI] [PubMed] [Google Scholar]
- 213.Grehan MJ, Borody TJ, Leis SM, Campbell J, Mitchell H, Wettstein A. Durable alteration of the colonic microbiota by the administration of donor fecal flora. J Clin Gastroenterol. 2010;44(8):551–561. doi: 10.1097/MCG.0b013e3181e5d06b. [DOI] [PubMed] [Google Scholar]
- 214.Ekbom A, Brandt L, Granath F, Löfdahl C-G, Egesten A. Increased risk of both ulcerative colitis and Crohn's disease in a population suffering from COPD. Lung. 2008;186:167–172. doi: 10.1007/s00408-008-9080-z. [DOI] [PubMed] [Google Scholar]
- 215.Zoetandal EG, Akkermans ADL, Akkermans-van Vliet WM, de Visser JA, de Vos WM. The host genotype affects the bacterial community in the human gastrointestinal tract. Microb Ecol Health Disease. 2001;13:129–134. [Google Scholar]
- 216.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Agusti A, MacNee W, Donaldson K, Cosio M. Hypothesis: does COPD have an autoimmune component? Thorax. 2003;58(10):832–834. doi: 10.1136/thorax.58.10.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Low TB, Greene CM, O'Neill SJ, McElvaney NG. Quantification and evaluation of the role of antielastin autoantibodies in the emphysematous lung. Pulm Med. 2011;2011:826160. doi: 10.1155/2011/826160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shapiro SD. Proteinases in chronic obstructive pulmonary disease. Biochem Soc Trans. 2002;30(2):98–102. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 220.Tzortzaki EG, Siafakas NM. A hypothesis for the initiation of COPD. Eur Respir J. 2009;34(2):310–315. doi: 10.1183/09031936.00067008. [DOI] [PubMed] [Google Scholar]
- 221.Das KM, Bajpai M. Tropomyosins in human diseases: ulcerative colitis. Adv Exp Med Biol. 2008;644:158–167. doi: 10.1007/978-0-387-85766-4_13. [DOI] [PubMed] [Google Scholar]
- 222.Kraft SC, Bregman E, Kirsner JB. Criteria for evaluating autoimmune phenomena in ulcerative colitis. Gastroenterology. 1962;43:330–336. [PubMed] [Google Scholar]
- 223.Mirza ZK, Sastri B, Lin JJ-C, Amenta PS, Das KM. Autoimmunity against human tropomyosin isoforms in ulcerative colitis: localization of specific human tropomyosin isoforms in the intestine and extraintestinal organs. Inflammatory bowel diseases. 2006;12:1036–1043. doi: 10.1097/01.mib.0000231573.65935.67. [DOI] [PubMed] [Google Scholar]